Highlights
-
•
Antimicrobial susceptibility testing (AST) is an invaluable task for selecting appropriate drugs.
-
•
Constant evolution is being observed in methods pertaining to AST in both clinical and research laboratories.
-
•
Conventional phenotypic AST methods have long turnaround time and labour-intensive.
-
•
Genetic and micro/nanotechnology-based AST methods hold optimism but need further validation.
-
•
Novel, faster and point-of-care AST method for routine clinical laboratory use still remains a challenge.
Keywords: Antimicrobial susceptibility testing, Conventional methods, Automations, Genotypic methods, Micro/nanotechnology-based techniques, Advantages and disadvantages
Abbreviations: ADR, Adverse drug reaction; AMR, Antimicrobial resistance; AST, Antimicrobial susceptibility testing; ATCC, American Type Culture Collection; CLSI, Clinical & Laboratory Standards Institute; DOT-MGA, Direct-On-Target Microdroplet Growth Assay; Etest, Epsilometer testing; EUCAST, European Committee on Antimicrobial Susceptibility Testing; CFU, Colony forming units; ID, Identification; MALDI-TOF MS, Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry; MBC, Minimum bactericidal concentration; MDR, Multi drug resistant; MIC, Minimum inhibitory concentration; MHA, Muller Hinton Agar; PCR, Polymerase chain reaction; POC, Point of care; NAAT, Nucleic Acid Amplification Test; PMF, Peptide mass fingerprint; WGS, Whole Genome Sequencing; ZOI, Zone of inhibition
Abstract
Antimicrobial susceptibility testing is an essential task for selecting appropriate antimicrobial agents to treat infectious diseases. Constant evolution has been observed in methods used in the diagnostic microbiology laboratories. Disc diffusion or broth microdilution are classical and conventional phenotypic methods with long turnaround time and labour-intensive but still widely practiced as gold-standard. Scientists are striving to develop innovative, novel and faster methods of antimicrobial susceptibility testing to be applicable for routine microbiological laboratory practice and research. To meet the requirements, there is an increasing trend towards automation, genotypic and micro/nano technology-based innovations. Automation in detection systems and integration of computers for online data analysis and data sharing are giant leaps towards versatile nature of automated methods currently in use. Genotypic methods detect a specific genetic marker associated with resistant phenotypes using molecular amplification techniques and genome sequencing. Microfluidics and microdroplets are recent addition in the continuous advancement of methods that show great promises with regards to safety and speed and have the prospect to identify and monitor resistance mechanisms. Although genotypic and microfluidics methods have many exciting features, however, their applications into routine clinical laboratory practice warrant extensive validation. The main impetus behind the evolution of methods in antimicrobial susceptibility testing is to shorten the overall turnaround time in obtaining the results and to enhance the ease of sample processing. This comprehensive narrative review summarises major conventional phenotypic methods and automated systems currently in use, and highlights principles of some of the emerging genotypic and micro/nanotechnology-based methods in antimicrobial susceptibility testing.
1. Introduction
Emergence of antimicrobial resistance (AMR) has been the most significant problem in the management of infectious diseases, threatening all achievements of the health care settings in the new millennium. For successful treatment of infectious diseases, prior knowledge about an organism’s susceptibility to relevant antimicrobial agents gained through in-vitro testing is a prerequisite. Identification of microbes (ID) and their antimicrobial susceptibility testing (AST) are two key tasks performed by the clinical microbiology laboratories to guide therapeutic choices of antimicrobial agents. Without this knowledge, empirical therapy may lead to treatment failure or emergence of antibiotic-resistant pathogens. First launched in 1929, AST remains invaluable not only to select specific antimicrobial regimen but useful in antibiotic policy, infection epidemiology, drug discovery and resistance monitoring (Bayot and Bragg, 2022). The purposes of AST are many folds (Box 1), but it is primarily intended to check the efficacy of an antimicrobial drug or natural product to maximize the best drug dose regimen. The term “antibiotic” has a more limited meaning compared to antimicrobials that act against all microorganisms including bacteria, viruses, parasites and fungi. After the revolutionary “golden era of antibiotics”, these agents are being used widely not only in healthcare settings but also in food and animal industries because of their versatile nature. Over the time, misuse of antibiotics has escalated the emergence of resistant bacterial pathogens (Fair and Tor, 2014). Currently, the impact of microbial resistance to most available antibiotics, especially emergence of multidrug-resistant (MDR) bacteria has become alarming and threatening the global health. It is predicted that death due to AMR related infections could grow from 1 million of current annual approximation to 10 million by 2050 unless rigorous measures are taken to stop the antibiotic abuse and misuse. To combat the situation and to save patient’s life, there is no alternative to correct identification of pathogens and to select appropriate antibiotic through AST (Nathan and Cars, 2014).
Box 1. Purposes of AST.
-
•
To assist the physician choosing the most suitable antibiotic according to the need of an individual patient (Antibiotic stewardship).
-
•
To guide the empiric use of antibiotics in clinical practice.
-
•
To record epidemiological data on microbial resistance within the community.
-
•
To explore the changing trends in antimicrobial susceptibility.
-
•
To predict the outcome of antibiotic therapy.
-
•
To monitor the resistance mechanisms.
-
•
To detect new resistance mechanisms.
-
•
To compare resistance trends in healthcare facilities from different geographic areas.
-
•
To develop intervention and prevention strategies.
With the inappropriate or improper use of antimicrobials, infectious disease kinetics are changed and can lead to increased adverse drug reactions (ADRs), treatment failures, relapse and the most significant of all, the advent of resistant phenotypes (Christaki et al., 2020). Unfortunately, the pace of discovery of new drug or natural products is very slow to combat the challenge of rapidly growing AMR when the available antimicrobials are constantly losing their efficacy (WHO, 2020). Recent reports on plant-derived compounds such as polyphenolics, alkaloids and other plant extracts proclaim plants as unrealized source of antimicrobial agents (Othman et al., 2019). However, a good number of polyphenolics, alkaloids and flavonoids reported from different plants are still awaiting to be tested for their antimicrobial activity (Ahammed et al., 2021, Islam et al., 2021, Al-Amin et al., 2022, Foyzun et al., 2022, Mustafa et al., 2022, Pawar et al., 2022).
Microorganisms have the inherent or acquired capability of constantly changing susceptibility patterns even to the newest antibiotics. Therefore, it is necessary to ascertain an organism’s antimicrobial susceptibility profile soon after its isolation and identification. This practice not only can help the clinicians choosing the effective antibiotics in treating the patient, but also guides the empirical antibiotic therapy based on current institutional antibiogram data (Burnham et al., 2017). Currently the guidelines provided by the Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) are considered as the reference breakpoint for antimicrobial susceptibility testing worldwide (Maurer et al., 2017).
Phenotypic antimicrobial susceptibility testing refers to a set of observable characteristics or traits of a microbe against a panel of preselected antimicrobial agents, either arrest of growth in the presence of bacteriostatic or death by bactericidal antimicrobial agents. Each clinical microbiology laboratory must establish its own standard battery of antimicrobial agents to be tested routinely for the purpose of AST on clinical isolates. However, there are certain common rules regarding the choice of antibiotics which include pathogen isolated, infection site, type of infection, comorbidities, patient’s age, gender, institution’s formulary agents, physician requests, and the AST methods.
On the contrary, the genotypic methods mostly detect resistance gene(s) within a microbe as causal factor for drug resistance. There are certain essential requirements for phenotypic AST method (Box 2) which are intended to find out the minimum inhibitory concentration (MIC) of an antibiotic. Based on the clinical MIC breakpoints, there are three categorizations of organism, viz., susceptible (S), intermediate (I) or resistant (R) to the antimicrobial agent in question (Box 3) (Wantia et al., 2020). Among the most widely used phenotypic AST methods, agar disc diffusion, broth dilution, gradient diffusion, and commercially available automated and semi-automated systems are exemplary (Balouiri et al., 2016). Recently much attention is being paid to develop rapid phenotypic AST methods, e.g., fluorescence imaging, surface plasmon resonance, and micro/nanotechnology-based devices such as microfluidics, microdroplets to avoid misuse of antibiotics. The main impetus behind automation and nanotechnology-based devices is to minimize the overall turnaround time and to improve sample processing with the eventual goal of early patient diagnosis. Certainly, emergence of AMR is reciprocally linked to the time in getting AST results. Genotypic AST methods use molecular techniques like PCR-based amplification or sequence-based techniques such as DNA microarray and DNA chips to detect bacterial resistance genes, e.g., MRSA (Methicillin resistant Staphylococcus aureus) or MDR-TB (Multidrug-resistant Mycobacterium tuberculosis). Currently, most genotypic methods are being used for drug discovery, resistance monitoring or research, not available for routine clinical microbiology laboratories (Benkova et al., 2020). Regardless of AST methods, certain limitations (Box 4) are always there, and laboratories must be cautious in sampling and testing process so that high levels of accuracy and reliability can be maintained consistently. This narrative review focuses on the major clinical AST methods currently in practice and summarizes the principle of some of the promising emerging techniques.
Box 2. Essential elements of AST.
-
•
Standardized inoculum.
-
•
Use of the correct growth medium.
-
•
divalent cations (Ca2+, Mg2+)
-
•
pH.
-
•
thymidine, thymine.
-
•
Standardized incubation.
-
•
Temperature.
-
•
Time.
-
•
gaseous environment.
-
•
Quality control.
-
•
ATCC (American Type Culture Collection) strains.
Box 3. Interpretations of AST.
-
•
Pathogens are classified as “S - Susceptible, standard dosing regimen”, when the antimicrobial agent has very high chances of being therapeutically successful at a standard dosing regimen.
-
•
Pathogens are classified as “I - Susceptible, Increased exposure”, when the antimicrobial agent has very high chances of being therapeutically successful at an adjusted dosing regimen or concentration at the target tissue.
-
•
Pathogens are classified as “R - Resistant” when the antimicrobial agent has very high chances of being therapeutically ineffective even at an elevated exposure. (Wantia et al., 2020)
Box 4. Limitations of AST.
-
•
Susceptibility tests only measure in vitro antimicrobial activity not in the patient.
-
•
It cannot be assured that in vitro killing effect of an antimicrobial agent will be successful treatment.
-
•
Selecting appropriate antimicrobial treatment also involves patient’s personal profile including drug hypersensitivity, clinical condition and any underlying co-morbidities like liver or kidney disease.
-
•
Pharmacokinetics and pharmacodynamics of the antimicrobial agent is also very important consideration.
-
•
Cost and availability of a drug is also needed to be judged.
2. Conventional phenotypic AST methods
The conventional phenotypic AST methods like Kirby-Bauer disc diffusion or broth microdilution determine phenotypic susceptibility of bacteria challenged with antimicrobials by measuring bacterial growth. The phenotypic methods are advantageous over genotypic methods in demonstrating both qualitative and quantitative antimicrobial susceptibility of a pathogen. However, most of the classical manual methods like disc diffusion, agar or broth dilution and concentration gradient method like Etest are time-consuming and require culture inoculum and visual evaluation of growth (Idelevich and Becker, 2019, Benkova et al., 2020). Phenotypic AST methods in common use are briefly discussed below.
2.1. Agar disc diffusion
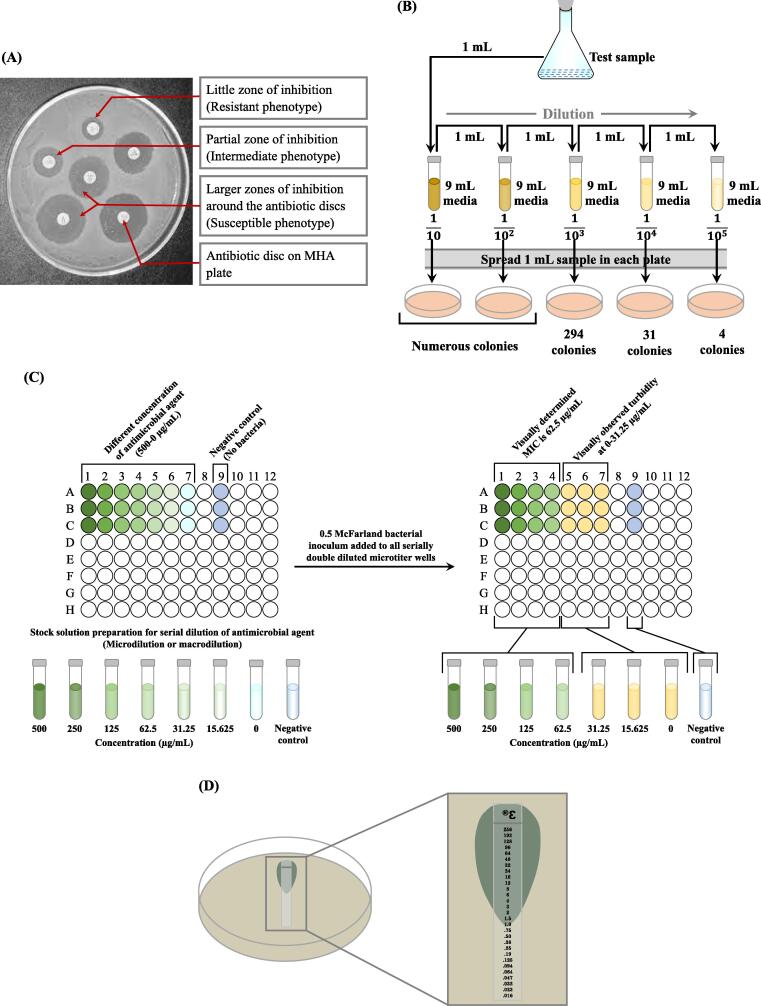
This phenotypic diffusion assay is popularly known as Kirby-Bauer disc diffusion, was developed in 1940 (Heatley, 1944) and is one of the time-tested and widely practiced methods of AST in clinical microbiology laboratories. It still remains as the most accepted manual technique for AST especially suitable for low and medium turnover laboratories (Bauer et al., 1966). In a disc diffusion test, bacterial isolates are tested for their susceptibility to different antibiotics. A clear visible large ring around an antibiotic disc called zone of inhibition (ZOI) indicates growth inhibition by an effective antibiotic (‘susceptible’) while bacterial growth is not affected by an ineffective antibiotic (‘resistant’) (Fig. 1A). In order to promote reproducibility and comparability of results between laboratories, Clinical Laboratory Standard Institute (CLSI) modified Kirby-Bauer disc diffusion technique is recommended by the WHO. In this well-known procedure, a standard inoculum of the test organism that corresponds to 0.5 McFarland turbidity (1.5 × 108 colony forming units/mL) is inoculated onto a 150 mm diameter Muller Hinton Agar (MHA) plate. Then antibiotic filter paper discs (approximately 6 mm in diameter; either commercially available or prepared in-house) containing a defined antibiotic concentration are placed on the lawn of bacteria (12 antimicrobial discs in a 150 mm diameter MHA Petri-plate). Agar plate is incubated at 35–37 °C usually overnight to facilitate the diffusion of antimicrobial agents into the agar to inhibit bacterial growth. The ZOI is measured manually in millimetres using a sliding calliper or a ruler and the diameter of ZOI provides qualitative results for the susceptible, intermediate or resistant bacteria (CLSI-M100, 2022). The CLSI modified Kirby-Bauer disc diffusion method using MHA has been successfully used to determine AST of many fastidious bacteria including Streptococci, Neisseria gonorrhoeae, Neisseria meningitidis, Haemophilus influenzae, Haemophilus parainfluenzae etc. through use of appropriate culture media, incubation atmosphere and elucidative criteria for ZOI to retrieve their AST (Idelevich et al., 2016). For routine susceptibility testing of nonfastidious bacteria, MHA is considered to be the best because of its manifold advantages (Box 5). However, the technique should only be used for well-evaluated bacterial species and not suitable for slow growing bacteria or bacteria that need special nutritional or gaseous requirements (CLSI-M100, 2022).
Fig. 1.
Conventional phenotypic AST methods, (A) Kirby-Bauer agar disc diffusion showing different zones of inhibition on MHA, (B) Schematic of agar dilution for the minimum inhibitory concentration (MIC): Varying concentrations (10-fold dilution) of antimicrobial agents are added to MHA. Then test organism (at a concentration of 104 CFU/mL) is applied directly or using an inoculum replicating apparatus on the agar plate of a particular antibiotic concentration. Results (number of CFU) are observed after incubation at 35℃ for 18–24 h. Number of CFU has been decreased with decreasing concentration of antimicrobial agent. (C) Broth microdilution: Left (stock solution)- Microtiter well nos. 1–7 contain serial double dilution of antimicrobial agent, from higher to lower concentration (500 to 0 µg/mL), well no. 9 is negative control (only antimicrobial agent without bacteria). Right (bacterial inoculum of 0.5 McFarland standard is added to microtiter well nos. 1–7 containing serial double dilution antimicrobial agent and incubated overnight. Visually determined MIC of 62.5 µg/mL is observed in well no. 4 and well nos. 5,6,7 show visual turbidity, indicating growth of bacteria. (D) Schematic of antimicrobial gradient showing zone of inhibition and minimum inhibitory concentration: Left: Petri dish shows image of setup of Etest strip. Right: Zoom in view of schematic representation of the Etest with gradual decreasing concentration of antibiotic from 256 to 0.016 μg/mL and zone of inhibition is shown at 1.0 μg/mL, indicating MIC.
Box 5. Advantages of MHA.
-
•
It supports growth of almost all organisms being a non-selective and non-differential medium.
-
•
Starch in the medium absorbs toxins liberated from bacteria and minimizes the inhibitory action of antibiotics.
-
•
Antibiotics can diffuse better in MHA due to its loose agar.
-
•
It shows reproducible results for each batch of susceptibility testing.
-
•
It markedly reduces the inactivation of sulfonamides and trimethoprim because of its low content of these inhibitors when used for testing the susceptibility of bacterial isolates against these antimicrobials.
There are a number of advantages of this method including its simplicity, reproducibility, screening against numerous isolates, ease in modifying antimicrobial discs and low cost. The disadvantages include inability to differentiate between bactericidal and bacteriostatic effects of antimicrobial agents and lack of determination of MIC of an antimicrobial agent. Recently, with the availability of software that can analyse the image of ZOI captured by camera or scanner has given extra reliability to the disc diffusion interpretations by minimizing the personal interpretation bias. The obtained results are compared with reference database for AST (Le Page et al., 2015).
2.2. Agar dilution
Along with the broth dilution, the agar dilution method is also one of the pioneering AST techniques customary for the researchers to determine the MIC of an antimicrobial agent available since 1940s. The lowest concentration of a drug that inhibits the visible bacterial growth in agar or broth is called MIC and usually expressed in mg/L (μg/mL) (Wheat, 2001). It is still being practised today for quantitative measure in AST especially for the new drugs. For agar dilution, an antimicrobial agent is diluted serially-two-fold and mixed with a molten agar. Upon solidification, 0.5 McFarland turbidity standard of microbial inoculum is implanted over it and incubated at 35-37℃ overnight. Bacterial growth is evidenced by the colony forming units (CFU) and the agar plate containing the lowest antibiotic concentration with no observable bacterial growth indicates its MIC (Fig. 1B) (CLSI-M100, 2022). In usual practice, a single organism with only one concentration of an antibiotic is tested per agar plate, but using inoculum replicators, multiple organisms on a single plate (32–36 inocula per plate) can also be tested on a single plate (Wiegand et al., 2008). The benefits of the agar dilution method are its simplicity, known parameters and cost-effectiveness when using inoculum replicators to test multiple bacteria. However, it is labour-intensive with long turnaround time until the current method is upgraded to full automation. Agar dilution is often recommended as a standard AST method for fastidious organisms like Helicobacter species and strict aerobes (CLSI-M100, 2022).
2.3. Broth dilution
2.3.1. Broth macrodilution
In broth macrodilution, antimicrobial agents are twofold diluted (e.g., 2, 4, 8, and 16 µg/mL) in a liquid medium and disburse in test tubes, hence also called tube-dilution method. This is still in practice as one of the earliest AST methods along with agar dilution. Typically, the volumes dispensed per tube are ≥ 1 mL and hence it is considered a macrodilution method. Non-selective agar plate is used to isolate the bacteria of interest as single colonies which are then suspended in liquid media and turbidity is adjusted to 0.5 McFarland standard. After appropriate dilution, it is transferred to each tube containing twofold dilutions of antimicrobial agent to secure a final concentration of ∼ 5 × 105 CFU/mL, as recommended by the CLSI. All inoculated tubes along with a positive control (tube inoculated with bacteria without antimicrobial agent) are incubated at 35–37 °C for 24 h or more to facilitate optimum bacterial growth (indicated by turbidity). The MIC is indicated by the tube with the lowest concentration of antimicrobial agent where no visible growth of bacteria is observed (CLSI-M100, 2022). As far as the advantages of this method are concerned, both the quantitative MIC values, as well as ‘bactericidal endpoint’ or the minimum bactericidal concentration (MBC) can be obtained. Broth macrodilution can utilize another analytical technique known as the time-kill method, which denotes the differential rate of bacterial killing at varying antimicrobial concentrations (Wiegand et al., 2008). Bacterial viability can be determined by counting the number of colonies on agar plate over 24 h. The time-kill method is specifically useful for evaluating the MBC of an antimicrobial agent gained through progressive interaction between drug and bacteria. It is a standard method as described by the CLSI, but long turnaround time, large space, reagents and labour are limiting factors (CLSI-M100, 2022). To overcome the limitations, broth microdilution method has been developed which utilizes microlitre volume of broth and carried out in a microtiter plate or tray.
2.3.2. Broth microdilution
Microdilution was pioneered in 1977 to miniaturize the dilution method by using microliter volumes (∼100 μL) with an aim to increase the throughput. The broth microdilution habitually tests two-fold dilutions of multiple antimicrobial agents in commercially available and disposable 96-well plastic trays (Fig. 1C). Preparation of microdilution panels is essentially same as that of macrodilution and involve antimicrobial agents serially diluted by twofold. An aliquot of precise volume of preweighed and diluted antimicrobial agent is added to the broth in well from large volume of frozen or dried commercially available microdilution panels vessel by using dispensing instrument. Then bacterial inoculum adjusted to 0.5 McFarland turbidity is added to make a final inoculum concentration of 5 × 105 CFU/mL or 5 × 104 CFU/well. Due to its miniaturization, multiple drugs and/or bacteria can be easily tested simultaneously on a single microtiter well (eight × twofold dilutions in a single tray permits 12 antimicrobials to be tested). After incubation for a minimum period of 12–24 h at 37 °C, the wells are checked for the presence of turbidity visually or through automated reader. The MIC can be determined by measuring the fluorescence intensity or turbidity visually or by a photometric device at 620 nm wavelengths (Puttaswamy et al., 2018). The results are expressed as breakpoints (discriminatory antimicrobial concentrations for susceptibility testing), MICs or combinations of both. The discernible benefits of microdilution are savings of time and reagents, and minimum workspace is required. However, limited availability of commercial antimicrobial panels is a major drawback. Microdilution method is currently considered as gold-standard and reference method with a higher degree of automation as it provides a quantitative objective assessment of in vitro AST (Khan et al., 2019).
2.4. Antimicrobial gradient method
The antimicrobial gradient is a phenotypic method that provides direct quantification of AST combining the principle of both dilution and diffusion of antibiotics in an agar medium (Balouiri et al., 2016). Epsilometer testing (Etest) is a significant development in AST by Bolmström and Eriksson in the late 1980 s. There are a number of commercial preparations of the antimicrobial gradient strips including Etest (bioMerieux AB BIODISK), widely used in the USA, MIC Test Strip (Liofilchem Inc., Waltham, MA), M.I.C. Evaluator (Oxoid, Basingstoke, UK) and Ezy MIC Strip (HiMedia Laboratories Pvt. ltd, Mumbai, India). A strip is created by the impregnation of a predefined increasing concentration gradient of the antimicrobial agent from one end to the other and is deployed on the agar surface, inoculated with the test microorganism. After overnight incubation, the tests are read by looking at the strips from the top of the plate and the MIC is considered at a point where an ellipse shaped zone of inhibition intersects the strip (Fig. 1D) (Sader and Pignatari, 1994). By comparing with the CLSI reference breakpoint values, corresponding MIC value of each antibiotic is interpreted as ‘Susceptible, ‘Intermediate’ or ‘Resistant’. Alike agar disc diffusion, a 150 mm MHA plate supports only 5 to 6 strips placed in a radial fashion on the surface of agar. Ideally, one or two drug strips per plate should be applied to prevent overlapping of inhibition zones. For the purpose of AST of fastidious organisms, enriched medium is used instead of MHA and a special incubation atmosphere is also required. This test is easy to perform for routine bacterial or fungal AST with same turnaround time like that of agar diffusion or dilution methods. Etest results may be affected by individual biasness of visual calculation of MIC and misleading information in case of overlapping zones of inhibition. Further, storage of strip is challenging due to pH-sensitive coated antibiotics and good laboratory set-up and high cost are among other limiting factors for its routine use in AST (Khan et al., 2019).
2.5. Chromogenic agar media for rapid detection of AMR pathogens
Clinical microbiology laboratories are always striving to reduce the turnaround time and the cost in identification and antimicrobial susceptibility testing of pathogens. Since the introduction of chromogenic culture medium in 1990, a wide range of commercially available chromogenic culture media are being used in the diagnostic clinical microbiology. These media detect key microbial enzymes as diagnostic markers for pathogens by exploiting enzyme substrates that release coloured dyes upon hydrolysis by the target pathogen enzymes, resulting in pathogens forming coloured colonies that can easily be identified. Unlike conventional culture media, this reduces the subsequent needs for subculturing and further biochemical testing in identification of an isolate and hence the time until a result is obtained. The principal objective of the development of chromogenic media was to enable rapid identification of resistant microorganisms, thus reduces the time, costs and labour for clinically important resistant pathogens such as MRSA, VRE, and ESBL- and carbapenemases-producing gram-negative bacteria. Additional identification confirmation of the resistant bacteria is sometimes needed depending on the sensitivity and specificity of chromogenic media and the type of microorganism detected. While the cost of chromogenic media is comparable if one considers the downstream processing costs involved in conventional media, the turnaround time varies from 1.4 days to 1.7 days, depending on the confirmatory tests required for each medium (Gajic et al., 2022).
2.6. Quality control in AST
The validity and acceptability of antimicrobial susceptibility testing of an organism is dependent on cross checking with fully characterized control organisms. The American Type Culture Collection (ATCC) is the largest general service culture collection in the world, with collections of all areas of microorganisms as reference standard. ATCC provides for the permanent preservation and availability of these materials to be used by qualified people engaged in research, industry, and academia. Microbial strains are meticulously characterized and preserved by ATCC for reproducibility of results across time and among laboratories around the world. Thus, ATCC provides a variety of microbial quality control strains for use in routine media sterility and growth promotion testing. Each strain preserved by the ATCC is fully authenticated to ensure accurate identification and the highest quality. In a microbiology laboratory for AST, ATCC is used to evaluate the microbial contamination and also to compare the zone of inhibition between test organism and reference organism. Because of issue of public health and safety it is essential that data generated by the microbiological research and testing are accurate and reproducible. Therefore, it is imperative that every AST protocol carried out in the microbiological laboratory undergoes rigorous quality control testing through using ATCC strains. Staphylococcus aureus (S. aureus) ATCC® 25923, Escherichia coli (E. coli) ATCC®25922, and Pseudomonas aeruginosa (P. aeruginosa) ATCC®27853 are frequently used ATCC strains in the clinical microbiology laboratories (Nassar et al., 2019, Käbisch et al., 2021).
3. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS)
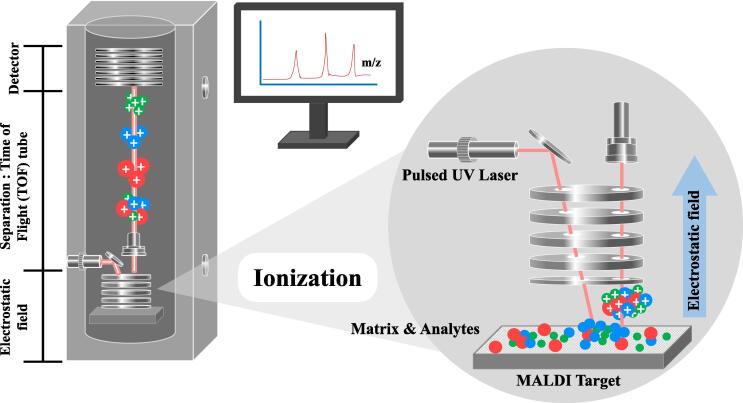
Mass spectrometry is an analytical technique introduced in 1980 s for proteomics. It has been applied as matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOS MS) to clinical microbiology laboratories for the purpose of bacterial identification and antimicrobial susceptibility testing since 2000. It is entrenched on the principle of rapid ionization of abundant ribosomal proteins directly from bacterial isolates or cell pellets using an energy absorbent matrix laser pulse and detected as peptide mass fingerprint (PMF) by the time of flight (TOF) analyzer. The identity of a microorganism is established by measuring the mass to charge (m/z) ratio of its ionizing protein. Particular microbe can be identified down to the genus, and even to the species and strain level by matching its PMF pattern with the extensive open database of PMFs of the ribosomal proteins (Clark et al., 2013). The sample for MALDI-TOF MS is prepared by mixing or coating with a dried matrix solution (derivative of benzoic acid or cinnamic acid) that crystallizes and facilitates co-crystallization of samples entrapped within it. Then using a laser beam, the sample entrapped within the matrix is ionized to generate single protonated ions in an automated mode (Fig. 2). Finally, the charged analytes separated from each other based on their m/z ratio are detected and measured using TOF analyzer. MALDI-TOF MS has been successfully used to identify genes indicative of antibiotics resistance such as vanA and mecA. Although the price of the instrument is high, it is suitable for laboratories handling large number of samples by minimizing the cost of consumables. However, its application for routine rapid AST in clinical microbiology laboratories requires further evaluation in terms of protocols, standard test kits and software. Recently, a novel direct-on-target microdroplet growth assay (DOT-MGA) that utilizes microdroplets for incubation with and without antibiotic has been found as a rapid universal MALDI-TOF MS-based phenotypic AST method (Idelevich et al., 2018). Automated sample processing and improved software analysis have enabled DOT-MGA based MALDI-TOF MS-AST method for testing multiple antibiotics concurrently with a comfortable workflow and increased speed.
Fig. 2.
Schematic of principle of MALDI-TOF mass spectrometry: The sample for MALDI-TOF MS is co-crystallized with the matrix on the sample target and then using a laser beam (e.g., pulsed ultraviolet laser), sample in matrix (analytes and matrix) is ionized to be desorbed by the MALDI ion source. The ion molecules, including the microbial peptides are accelerated by the electrostatic field into the TOF analyzing tube for the separation of ions by TOF according to the m/z ratio and a mass spectrum.
4. Automated AST methods
Automated technologies in antimicrobial susceptibility testing set in motion during 1980 s, have overthrown many conventional phenotypic methods especially for large-scale clinical laboratories. By virtue of their unique features like automation, compactness, rapidness and simplicity, they are widely accepted in clinical microbiology laboratories. Further, integration of computers has facilitated researchers/clinicians to remotely access, share, and analyse data for validation (Richter and Ferraro, 2011, Khan et al., 2019). The automated AST methods currently approved by the ‘US food and drug administration (FDA)’ are MicroScan WalkAway system (Beckman Coulter, Inc. Atlanta, Georgia, USA, 1980), VITEK 2 (bioMe’rieux, Marcy-L’Étoile, France, 2000), BD Phoenix Automated Microbiology System (BD Diagnostics, Franklin Lakes, New jersey, USA, 2001), and Sensititre ARIS 2X (Trek Diagnostic Systems, Oakwood Village, Ohio, USA, 2004). The results are generated faster within 3.5 to 16 h for the first three of these automated methods, while the fourth one takes overnight (Khan et al., 2019).
The automated and semiautomated systems are capable of producing customized test reports of patient through data management systems using computer software. These software systems are designed to usually have two components; an epidemiological data which can be archived for sharing specialized reports (e.g., hospital antibiograms, infection prevention reports, organism trending reports, summary reports, cumulative susceptibility data) and rapid individual patient reports. The integrated data can be transferred to a laboratory information system through a computer interface to produce personalised report. The uncertainty in interpretation of results from manual methods and personal bias are minimized simply with introduction of automated systems and also sample handling time is much reduced. Owing to its accuracy, simplicity and smooth workflow, many of the automated systems are being routinely used in clinical microbiology laboratories across the globe (Khan et al., 2019). However, automated system lacks the ability to demonstrate the mechanisms underlying in the observed susceptibility phenotype.
4.1. Microscan WalkAway system
The MicroScan WalkAway system is an intelligent automatic system based on broth microdilution for streamlining workflow introduced by Beckman-Coulter Diagnostics for identification of bacteria and their AST (ID/AST). The microdilution tray containing 40–96 wells are first hydrated and then samples are inoculated manually to be placed in one of the large self-contained incubator/reader device of the system to analyse the tray. This microbiology system is ideal for mid-to high-volume usage with gold-standard MIC accuracy and proven detection of emerging resistance to the pathogens. It enables the optical detection of bacteria in the reaction wells and interpretations of biochemical results through a photometric or fluorogenic reader with generation of computerised reports that can be interfaced with hospital main frames. AST profiles of rapid-growers can be determined within 4.5–7 h in MicroScan WalkAway system due to high threshold concentration (2 × 107 CFU/mL) of bacteria, while for slow growing organisms it may take up to 18 h (Puttaswamy et al., 2018).
4.2. VITEK 2 systems
VITEK®2 compact and VITEK®2 systems are based on broth microdilution technique to perform all the required steps automatically for the identification of bacteria and their AST was originally developed in the 1970 s by bioMérieux. The system uses “AST cards” and in each card there are 64 microwells that are loaded with dehydrated culture medium and antibiotics at varying concentrations including one positive control well containing only dehydrated medium without antibiotic. After primary isolation of organism, it is emulsified in 0.45 % saline to adjust the inoculum turbidity equivalent to 0.5 McFarland standard. Then the inoculum is placed into the VITEK®2 Cassette at the SMART CARRIER STATION™ to link the VITEK® 2 card and sample virtually. Once the cassette is loaded, all the steps required for ID/AST of bacteria are performed automatically without manual intervention. The card has fluidic connections to automatically fill the prior prepared and standardized samples into multiple wells simultaneously. This fully automated system is able to detect bacterial growth by using attenuation of light measured by an optical scanner that combines multi-channel fluorimeter and photometer to record fluorescence, turbidity and colorimetric signals. The original VITEK has the capacity to process up to 120 test cards at a time, while the VITEK®2 is able to process up to 240 cards simultaneously. The susceptibility results of rapidly growing gram-positive and gram-negative aerobic bacteria can be achieved within 4 to 8 h by using susceptibility cards (Spanu et al., 2003, Biomerieux, 2021). The VITEK®2 compact system is designed to reduce the hands-on time and enhance workflow with rapid reporting.
4.3. BD Phoenix automated microbiology system
The BD Phoenix Automated Microbiology System has been developed by the Becton Dickinson Diagnostic System (BD Diagnostics, Sparks, MD, USA) to provide an automated bacterial ID and AST based on the minimal inhibitory concentration. It is a rapid, accurate and reliable method for the detection of known and emerging antimicrobial resistance. This automated system has disposable panels and broths for microbial identification (ID) and AST, software and a susceptibility testing colorimetric redox indicator. Both fluorogenic and chromogenic substrates are used for the detection of a broad range of gram-positive and gram-negative bacteria. There are 136 microdilution wells present in the disposable test panels, which are available in three different formats, viz., ID-only, AST-only and ID/AST combination panels. The combination panel has an ID side with dried substrates and AST side containing varying concentrations of antimicrobial agents along with growth and fluorescent control panels at appropriate location. The instrument has the capacity to analyse up to 100 ID/AST combination panels at a time, which are read every 20 min. Several antimicrobial agents with twofold doubling dilution concentrations are used in each AST panel. MIC values of each antimicrobial agent is interpreted for identification of organism as Susceptible, Intermediate or Resistant (Wantia et al., 2020, Benkova et al., 2020). The Phoenix system has shown accuracy in detecting drug-resistance gene(s) including extended-spectrum β-lactamases (ESBL), AmpC β-lactamases, vancomycin resistant Enterococcus (VRE), carbapenemases in gram-negative bacteria etc. Its state-of-the-art data management system facilitates communication directly between laboratories and clinicians. While automation of the system has enabled workflow efficiency by inoculum standardization and elimination of drawbacks from manual systems; however, pure culture isolates are still required for the determination and interpretation of AST that takes 6–16 h to generate the MIC results (CLSI-M100, 2022).
4.4. Sensititre ARIS 2X
The Sensititre ARIS 2X System was developed in the 1980 s by Trek Diagnostic Systems and now it is a commercial product by Thermo Fisher Scientific. Similar to VITEK® and the PhoenixTM systems, Sensititre ARIS 2X System also uses broth microdilution and samples are incubated manually but bacterial ID/AST are detected via automation. Maximum up to four modules with a total of 256 plate capacity (each module contains sixty-four plates) can be incubated in each ARIS 2X instrument for 18–24 h, linking to a single computer. The bacterial growth is measured by the hydrolysis of a fluorogenic substrate in each well and determine MIC endpoints. Inbuilt temperature and time control panels are maintained during incubation and robotics is used to transport plates to the reading unit. Inventory is tracked using barcode information present on each plate. There are several appealing features of Sensititre plates including a traditional doubling dilution format, a large selection of antimicrobials and the ability to test both fastidious and nonfastidious bacteria using a single AST system (CLSI-M100, 2022).
5. Genotypic AST methods
The genotypic AST is based on identification of specific resistance gene(s) present either on microbial DNA or plasmid or genetic mutations associated with resistant phenotype using DNA-based, amplification-based or sequencing-based molecular approaches. Genotypic methods are used as replacer for AST with subsequent validation of susceptibility with suitable phenotypic test. Different molecular or genomic amplification techniques e.g., polymerase chain reaction (conventional, real-time PCR or sequence specific-PCR), loop-mediated isothermal amplification (LAMP), DNA microarray, DNA chips or whole genome sequencing have been utilized as tools in genomic methods (Cockerill, 1999). All these methods are generally employed for the direct, rapid, sensitive, and specific detection of microbial drug resistance genes. Further, genomic approaches can minimize the tedious bacterial cultures, chances of contamination and the spreading of deadly infections. However, these methods are highly technical and also suffer from limitation in detecting all resistant genes and false positivity other than high cost (Fluit et al., 2001).
5.1. PCR-based methods
PCR is one of the most efficient, rapid and modern molecular tools not only for the detection but also for quantification and profiling of bacterial drug resistance genes. There are various detection systems of PCR-amplified products to confirm the presence of resistance genes including electrophoresis, southern blotting, restriction fragment-length polymorphism (RFLP), single-strand conformation polymorphism (SSCP), DNA fingerprinting, molecular beacons, and other DNA sequencing analysis methods (Fluit et al., 2001). Recently, loop-mediated isothermal amplification (LAMP) technique has been used for the rapid, specific and efficient evaluation of AST that amplifies DNA at a constant temperature of 60–65 °C using a Bst DNA polymerase instead of Taq polymerase (Li et al., 2017). Real-time PCR can quantify the amplified genes for the purpose of AST using hydrolysis probes, hybridization probes, or double-stranded DNA-binding fluorescent dyes (Maugeri et al., 2019). The most intriguing feature of PCR-based techniques is that the samples need not to be sterile and may contain mixtures of bacteria. However, PCR methods are highly technical that need good laboratory facility and incur high cost. Among other limitations, PCR involves complex steps in nucleic acid amplification, there is chance of false positive and negative results, and also enzymatic inhibition from blood and urine components.
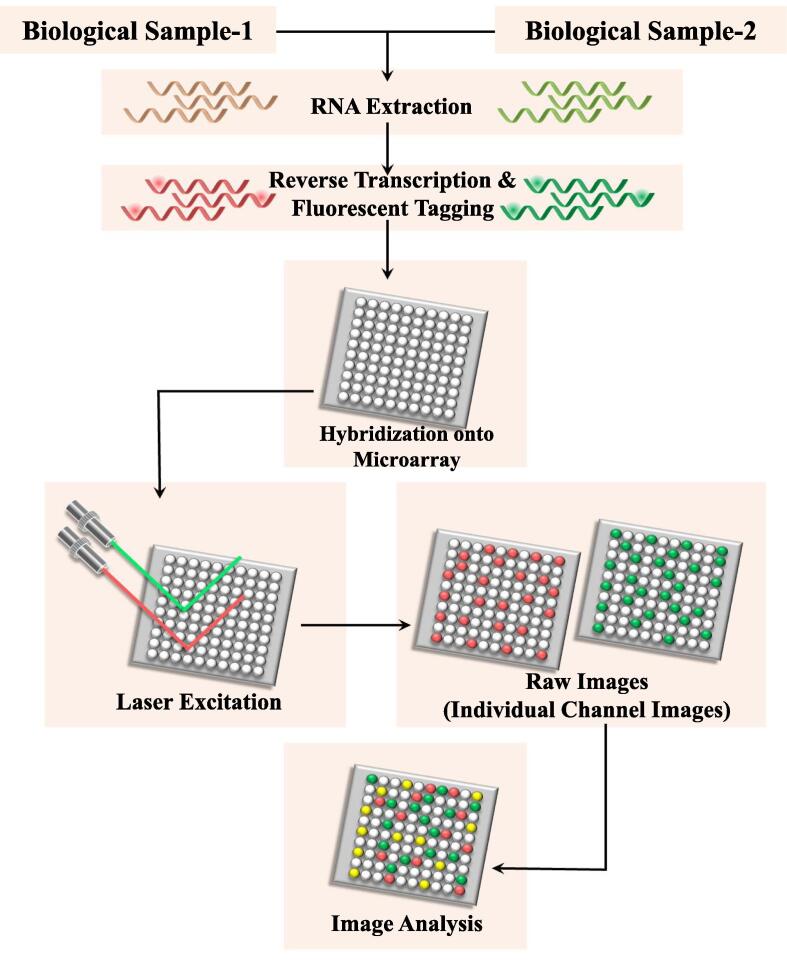
5.2. DNA microarrays and DNA chips
DNA microarrays and DNA chips revolutionized the approach of gene expression profiling with high throughput and hold a great promise for screening susceptibility (Miller and Tang, 2009). A DNA microarray is a collection of thousands of microscopic DNA sequence known as probes attached as spots bounded covalently or noncovalently to a solid surface. Each microarray contains one or a few probe sets for each gene in question. The relative concentrations of unknown nucleic acid in solution (targets) can be measured by using these probes that bind specifically through hybridization. These probes are used to hybridize a target under highly rigorous conditions. Hybridization of probes to their complementary DNA (cDNA) in sample is usually detected and quantified via different detection systems including fluorophore, silver or chemiluminescence-labelled targets, that determine the relative abundance of transcripts in the target sample. DNA chips on the other hand use the glass or silicon platform for binding of probes and resistant gene(s) in the target samples that can be identified through detection of specific hybridization of the labelled probe with the target (Fig. 3).
Fig. 3.
Schematic of principle of DNA microarray and DNA chips: The RNA is extracted from a sample and reverse transcription of mRNA yields cDNA. By integrating with fluorescent dyes, fluorescent-tagged cDNA strands are generated. The labelled cDNAs are then placed on the DNA microarray which permits the hybridization of each cDNA to its complementary strand. The laser excitation of the fluorescent-tagged cDNA strands generates signals which are captured as images by the camera. Image analysis through distinct intensity of the color for each spot yields results instantly.
Each chip can hold hundreds of target nucleotides, ideally suited to bacteria having numerous distinct mechanisms of resistance such as ESBLs in gram-negative bacteria (Cuzon et al., 2012). DNA microarrays and chips have been successfully applied to determine the isoniazid resistance in M. tuberculosis (Huang et al., 2014). Despite having broad-spectrum characteristics, high cost, relatively low accuracy in latent infections and non-specific binding of DNA probes are major limiting factors of the microarray techniques. Moreover it is a time-consuming procedure that requires skilled personnel (Cockerill, 1999).
5.3. Whole genome sequencing (WGS)
Whole genome sequencing is an exhaustive and robust laboratory procedure to determine the order of all nucleotide bases in the genome of an organism at a single time. With the advent of DNA sequencing technology, it has been possible to sequence entire bacterial genomes with extreme rapidity. Coupled with bioinformatic tools, the sequencing methods have widened the possibility of applying these techniques for detecting drug-resistance gene(s) called whole genome sequencing for antimicrobial susceptibility testing (WGS-AST). It has the right potential for more accurate predictions of all known microbial resistant phenotypes with concurrent rich surveillance data. It holds a great promise to overthrow most of the shortcomings associated with phenotypic AST by offering not only a prediction of bacterial resistance genes but also addressing the substantial genetic polymorphisms present in resistant bacteria (Behera et al., 2019). Several methods and tools have been published in recent years that have shown their efficacy in predicting genetic determinants of antimicrobial resistance from whole genome sequencing. It is worth mentioning that sequencing technologies such as next-generation sequencing (NGS) are rapidly expanding the abilities of scientific community to identify and explore antimicrobial resistance. Although in theory, WGS can offer fast pathogen identification including epidemiological typing, and detection of drug resistant genes, however, it still lacks the definite evidence in favour of its application to AST for most bacterial species.
6. Emerging methods
6.1. Isothermal microcalorimetry
Isothermal microcalorimetry (IMC) is a real-time sensitive laboratory technique to identify bacteria in the growing phase by virtue of detection of metabolic activity involving physical or chemical processes by heat flow measurement. For studying bacterial growth kinetics, this isothermal and closed system measures the amount of energy at microwatt levels which is released during growth. Using real-time IMC, the heat flow rate of a given bacteria in suspension in presence of an antimicrobial agent can be measured to monitor the viability which in turn determines its minimum inhibitory concentration (Tellapragada et al., 2020). Apart from determining MIC, bacteriostatic or bactericidal effect of the antibiotic can also be determined by analysing the heat curves. Further, based on its principle this method can predict mechanisms of action of antibiotics (e.g., inhibitor of cell wall, protein or DNA synthesis). Although it may be a useful method for determination of AST especially for high-risk bacteria and classifying new antibiotics based on mechanisms of action, it takes around 24 h to obtain the MIC results, therefore may not be suitable when rapid AST results are requested for targeted antibiotic therapy (Butini et al., 2019).
6.2. Microfluidics and microdroplets
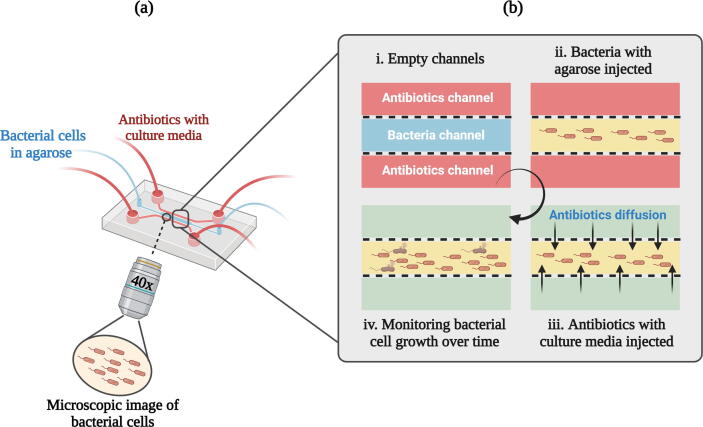
Microfluidics has been emerging as a novel and promising technology in biological research exploiting fluids at the ultra-low volume through engineered manipulation that can overcome the limitations of traditional methods and expedite the AST further. It is based on the principle of physical detention of the bacteria in microchannels at a single-cell level that allows faster ASTs on a time frame corresponding to the generation time of the bacteria. This biomedical lab-on-a-chip system is based on microchannels, in which extremely small volume (10–100 µL) of reagent can be delivered confining bacterial growth at the level of single-cell. It provides the unique opportunity of analysing biophysicochemical changes and effects of nanolitre or picolitre volume of fluid that flow through the channels on microfluidic lab-on-a-chip device (Fig. 4). It has been observed that bacterial division is much quicker when dispensed in small volume with consequent reduction in AST time. The detection methods are generally electrochemical, magnetic or optical/microcalorimetric depending on the device used. Currently miniaturized micro to picolitre chambers, channels and structures such as porous membranes, slits have been fabricated by researchers with the aid of modern nanotechnology and microfabrication techniques (Zhang et al., 2020). The system incorporates microelectrodes and takes a label-free, impedance-based approach for determining the antibiotic susceptibility at the level of single-cell within an hour. Microfluidic platforms have certain advantages including faster performance, accurate screening, efficient transfer of heat and mass and reduced diffusion distances accounting for the prospects of automation and high turnover (Zhu et al., 2018).
Fig. 4.
Schematic representation of the microfluidic agarose channel (MAC) system for antibiotic susceptibility testing: (a) Polydimethylsiloxane (PDMS) fabricated MAC chip is assembled with PDMS-coated glass. Central channel of the chip is loaded with an agarose-bacteria mixture and varying concentrations of antibiotic are added from the side-branched channels. To analyze bacterial growth, each interface between the agarose-bacteria and antibiotic in solutions was monitored using a microscope. (b) Zoom in view of MAC chip (i) The empty channels. (ii) Main channel loaded with the agarose-bacteria mixture (iii) Antibiotic diffusion channels showing a sharp interface generated between the side-branched channels and the main channel. (iv) Microscopic monitoring of bacterial cell growth over time. [Created with BioRender.com.]
The microfluidic droplet method is an important subcategory of microfluidics based on micro or nanodroplets to analyse huge amounts of distinct microdroplets in an immiscible continuous phase with high throughput. Microorganisms and the antibiotics are both encapsulated in ultra-low volume droplets inside a microdroplet reactor in a favourable cellular environment. The device is designed to form automatic droplets and their sequential detection is performed by using optical imaging. Further, by incorporating the fluorescent metabolic marker inside each droplet, the metabolic activity and viability of bacteria within droplets can be determined (Dai et al., 2016). The major leaps of droplet microfluidics are their ability to adjust droplet size, density of bacteria, antibiotic concentrations, and reproducibility to enable its application for faster detection of pathogens and their antimicrobial susceptibility.
6.3. Optical method
Several sophisticated tools incorporated under the optical method have been developed over the years since its first introduction in 1970 s. Generally, optical methods measure bacterial motion and density, molecular vibrations, and fluorescence intensity with the help of light beams. Measurement of optical density (OD) of a suspension containing both test bacteria and antibiotics can differentiate between resistant and sensitive phenotypes when a beam of light is passed through the suspension. Detection of scattered light by a spectrophotometer correlates with the bacterial population in colony-forming units (CFU). The identification of bacterial strains, its growth kinetics as well as quantitative MICs can be measured by optical method. Coupled with an optical image sensor, microfluidic devices are able to detect MIC within a few hours due to real-time analysis and by minimizing the culture dependency. Optical sensor-based nanofluidic (30 nL) analysing single bacterial cell has claimed performing AST within 30 min (Baltekin et al., 2016). Further, it can eliminate all the tedious steps like loading of cells by continuous sample injection and counting-based cell identification by virtue of imaging of a single bacterium (Lu et al., 2017).
6.4. Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) is a specialized type of flow cytometry where optical and fluorescence characteristics are embedded in counting and sorting of cell of particular interest from a heterogeneous mixture of biological cells into two or more containers, one cell at a time. FACS is an invaluable tool currently applied to bacterial analysis from identification and counting of bacteria, to discover changes in cellular and metabolic activity, and even to identify differential gene expressions. In a mixture of bacteria, antibiotics and relevant fluorescent stains, viability of bacteria and their antibiotic susceptibility can be determined by interpreting the spectrum of light emitted due to excitation within 2–3 h. Permissible cells upon binding with fluorescent stains to its nucleic acids, give stronger fluorescent emission than non-permissible cells and greater amount of fluorescence can be observed from completely lysed cells. However, due to inadequate distinction of single cell from cell aggregates by the flow cytometry, viability of the cells does not necessarily correlate with the amount of fluorescence activity. FACS is a rapid process in getting AST results in comparison to conventional methods (∼2h vs ∼ 24 h) as it is based on detection of physiological changes in bacteria caused by antibiotics rather than growth inhibition processes (Hedde et al., 2020). FACS is an automated technique capable of processing thousands of cells per second, accurate, sensitive, and timesaving, however, it struggles with a number of limitations. Apart from cost, other limiting factors include complex samples, inefficient staining, autofluorescence, lack of differentiation of cellular damage by antibiotics and dearth of clinical databases for validation. Further extensive research is necessary to increase its validity especially for rapid AST (Huang et al., 2015).
6.5. ATP bioluminescence assay
Adenosine triphosphate (ATP) bioluminescence assay involves the conversion of luciferin to oxyluciferin by luciferase enzyme. Based on the presence or absence of ATP, it can measure the amount of living or dead cells within a sample. The chemical reaction between luciferin and luciferase would produce a bioluminescent flash in case of a living cell with abundant ATP, which is absent in non-living or dead cells. ATP is the most common compound representing energy transfer from cellular metabolism and its level has a strong positive correlation with the number of bacterial cells. Resistant bacteria when grown in presence of antibiotics, results in bioluminescence, whereas susceptible bacteria remain neutral. The ATP level can be measured by the released photons with a luminometer. Therefore, measuring the ATP level as a reflection of quantitative bacterial growth is more advantageous than the turbidity method used in the conventional broth microdilution. ATP bioluminescence assay has been found to demonstrate the bacteria and their AST in 2 to 4 h (Ihssen et al., 2021).
6.6. SmarticlesTM technology
SmarticlesTM are DNA-delivery pathogen-specific bioparticles and when combined with GeneWEAVE-designed DNA molecules, they allow emission of light from live bacteria. Introduced by Roche, this rapid molecular technique has been linked to antimicrobial susceptibility testing using DNA probes inside a non-replicating bacteriophage that can specifically bind to a particular recombinant bacterium containing plasmid. Luciferase gene inserted into the plasmid gets activated upon contact with drug resistant bacteria and emits light for their detection. This method can be applied to demonstrate the antimicrobial susceptibility patterns directly from positive blood culture samples without prior bacterial isolation and results can be obtained in less than 4 h. Early detection and bypassing the culture are clear advantages of this method which facilitates the best antibiotic course for the patients (Roche, 2015, Puttaswamy et al., 2018).
6.7. Smartphone-based optical spectroscopy
Smartphones can be an ideal tool for diverse biomedical uses including cell phone microscopy, lateral flow assays, cytometric analysis, colorimetric tests and paper-based microfluidic device. They may serve for the rapid identification of pathogens as a suitable point-of-care (POC) platform owing to the facts of having multifunctional features including high-resolution digital cameras, touchscreen interface, high quality computer processors as well as wireless data transfer option into a portable device. Several research groups have demonstrated the biomedical applications of high-resolution smartphones particularly in the optical spectroscopic platforms over the past few years (Ong and Poljak, 2020, Hussain and Bowden, 2021). Such platforms hold a great promise to develop unprecedented POC diagnostics systems, suitable for the resource-limited settings. Further, joining with fluorescent/colorimetric tools, this universal and ever-evolving smartphones will gain the features of point-of-care monitoring, prompting an AST map, and real-time database updates, which will eventually facilitate in gaining in-depth understanding about the geographical prevalence of antimicrobial resistance (Huang et al., 2018). It is reasonably speculated that with continuous advancement and progression, smartphones have the right potentials to foster large-scale implementation of a mobile microbiological laboratory with point-of-care features in the near future, especially in resource-limited countries.
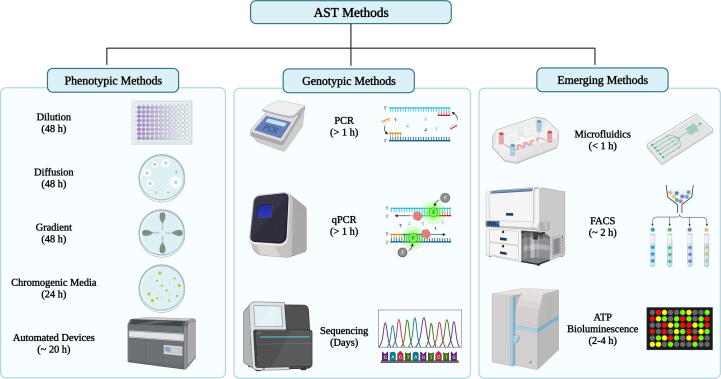
The overview of different AST methods in terms of principles and turnaround time is depicted in Fig. 5.
Fig. 5.
Overview of principles and turnaround time of major antimicrobial susceptibility methods: Phenotypic AST methods including dilution & diffusion, gradient, chromogenic and automated platforms have long turnaround times (24–48 h); Genotypic methods such as conventional PCR and qPCR have short turnaround time of hours but sequencing needs days. Emerging techniques like microfluidics, FACS and ATP bioluminescence have very short turnaround time ranging from 30 mins to hours. [Created with BioRender.com.]
7. Conclusion
Antimicrobial susceptibility testing of an organism is an important prerequisite not only for the targeted antimicrobial therapy but also to reduce the chance of development of antimicrobial resistance and its spread. Rapid and reliable selection of antimicrobial agents for the therapeutic success of infectious diseases is the most important outcome of any AST method. Scientific advancement is a continuous process and crucial in any field including healthcare setting where ceaseless innovation can improve patient outcomes and quality of life by providing better compensation. Although advancement in the development of device and technology for AST is commendable, still there is a genuine need for automation especially for the existing manual methods and to develop innovative new technologies for faster and POC diagnosis of infectious diseases. Until recently, clinical microbiology laboratories are using a limited number of phenotypic antimicrobial susceptibility testing methods and surprisingly, the disc diffusion method first published in 1966 is still being accepted as one of these widely practiced methods. Likewise, broth microdilution has gained the reference standard to compare, verify and validate other AST methods. Currently, all the growth-dependent automated systems, such as the BD Phoenix™, the Microscan WalkAway, or VITEK 2 utilize the broth microdilution as the basis. Although the currently available AST platforms are robust and providing improved performances by the diagnostic laboratories, the long turnaround time with consequent delay in providing appropriate targeted treatment are the major limiting factors (Table 1). Moreover, all these methods require relatively large number of viable organisms and multi-step preanalytical processing apart from substantial analytical variability, limited organism spectrum and high cost. To overcome the hurdle, research trend is now moving towards new technological developments that can bypass the need for pure clinical isolates and minimize the long waiting time to result. The rapid advances in molecular technology-based approaches, sequencing tools, and availability of metabolic biomarkers can promote the efficacy of microbial identification and susceptibility testing in a great height. It is anticipated that several genomic approaches and newly emerging micro or nanotechnology-based techniques briefly described herein can assume a role of game changer and make a paradigm shift of future clinical AST. At present, non-phenotypic methods like nucleic acid or nanotechnology-based ASTs are mostly intended for the drug discovery or research, most of them are yet to validate to be adopted for clinical microbiology use. Moreover, they are expensive and have limited range of detection potential of resistance markers. However, detection of resistance determinants by multiplex PCR directly from culture-positive blood samples has been shown to substantially reduce the time and digital PCR has the potential to allow better quantification of target molecules. The miniaturization of sensing devices like microfluidics and its combination with the optical tools, can promote the development of user-friendly and portable devices with future potential to be used at the point of care clinical AST method. Although there are many emerging and rapid AST technologies described conceptually, there is yet to develop any technological breakthrough for a single major or broadly accepted and suitable tool for the clinical microbiology in health-care setting. A number of issues including the costs, optimization of target product profiles, designing new tools, legal and regulatory conducts, hurdles in clinical trials, concerns quality control are worth to consider for any sustainable AST method. Thus, both public and private entities need to understand the issues pertaining to AST platform development and implementation to maximize its availability and use, especially rapid technologies. Clearly, there is a hope that emerging AST technologies along with available proteomics, transcriptome and genome-based sequencing will provide better opportunity not only in antimicrobial resistance prediction but to adopt them in clinical microbiology laboratories with maturation of databases.
Table 1.
Comparison of benefits and limitations among common phenotypic and automated AST methods.
| Methods | Benefits | Limitations | Nature of AST |
|---|---|---|---|
| Disc diffusion | Cheap, flexible in antimicrobial selection, allow visibility of growth, correct inoculum, mixed culture can be detected, easy to interpret even by non-expert, follow CLSI standard and breakpoints available. | Manual setup, tedious, error-prone, needs at least ∼ 105 cells, long turnaround time, MIC cannot be determined. | Qualitative |
| Broth dilution | Economy in reagent and space (for microdilution), reproducible, convenient, CLSI standard and breakpoints available, MIC can be determined. | Manual setup and manual or automated interpretation, laborious and supply intensive, needs at least ∼ 105 cells, inflexibility of drug selections, long turnaround time. | Quantitative |
| Antimicrobial gradient method | Convenient and flexible, cheap and ideal for AST with a few drugs, useful for fastidious organisms. | Manual setup and interpretation, needs at least ∼ 105 cells, interpretation needs expert observation, no CLSI approved breakpoint available, long turnaround time, not cost-effective for multiple drugs. | Quantitative |
| MALDI-TOF MS | High throughput, quick sample preparation, automation. | Expensive, different genetic markers required, needs at least ∼ 105 cells. | Semi-quantitative |
| Automated methods (VITEK, MicroScan) | Automated setup and interpretation, more reliable, large number of samples can be handled, less turnaround time than conventional methods, monitoring of AMR is possible. | Costly, trained personnel required, needs at least ∼ 105 cells, no CLSI approved breakpoint available. | Semi-quantitative |
8. Author's note
The methods and technologies pertaining to antimicrobial susceptibility testing described here are considered randomly without any prior selection. Further, methods have been aligned only on the basis of their applicability to clinical microbiological diagnostics or drug discovery and research.
Author contributions
MAS was responsible for the acquisition, compiling of scientific information and drafting of manuscript. MYA drew all the figures. MAS, MYA, JSP, NA and IBL contributed for editing and revision of text and accepted the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to acknowledge Prof. K.N.S. Sirajudeen, HoD, Basic Medical Sciences department, Kulliyyah of Medicine, International Islamic University Malaysia for his constant encouragement in research and academic activities.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Md. Abdus Salam, Email: abdussalam@iium.edu.my.
Md. Yusuf Al-Amin, Email: amin50@purdue.edu.
Jogendra Singh Pawar, Email: jpawar@purdue.edu.
Naseem Akhter, Email: nakhter2@hfhs.org.
Irine Banu Lucy, Email: lucy@ru.ac.bd.
References
- Ahammed S., Afrin R., Uddin N., Al-Amin Y., Hasan K., Haque U., Islam K.M.M., Alam A., Tanaka T., Sadik G. Acetylcholinesterase inhibitory and antioxidant activity of the compounds isolated from vanda roxburghii. Adv. Pharmacol. Pharm. Sci. 2021 doi: 10.1155/2021/5569054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin M.Y., Lahiry A., Ferdous R., Hasan M.K., Kader M.A., Alam A.K., Saud Z.A., Sadik M.G. Stephania japonica ameliorates scopolamine-induced memory impairment in mice through inhibition of acetylcholinesterase and oxidative stress. Adv. Pharmacol. Pharm. Sci. 2022 doi: 10.1155/2022/8305271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016 doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltekin, Ö., Boucharin, A., Andersson, D.I., Elf, J., 2016. Fast antibiotic susceptibility testing (fastest) based on single cell growth rate measurements. bioRxiv. https://doi.org/10.1101/071407.
- Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966 [PubMed] [Google Scholar]
- Bayot M.L., Bragg B.N. Antimicrobial susceptibility testing. Treasure Island (FL) StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- Behera B., Anil Vishnu G.K., Chatterjee S., Sitaramgupta V.V., Sreekumar N., Nagabhushan A., Rajendran N., Prathik B.H., Pandya H.J. Emerging technologies for antibiotic susceptibility testing. Biosens. Bioelectron. 2019 doi: 10.1016/j.bios.2019.111552. [DOI] [PubMed] [Google Scholar]
- Benkova M., Soukup O., Marek J. Antimicrobial susceptibility testing: currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 2020 doi: 10.1111/jam.14704. [DOI] [PubMed] [Google Scholar]
- Biomerieux, 2021. Vitek® 2: Healthcare. Retrieved May 20, 2022 from https://www.biomerieux-usa.com/vitek-2.
- Burnham C.D., Leeds J., Nordmann P., O'Grady J., Patel J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 2017 doi: 10.1038/nrmicro.2017.103. [DOI] [PubMed] [Google Scholar]
- Butini M.E., Gonzalez Moreno M., Czuban M., Koliszak A., Tkhilaishvili T., Trampuz A., Di Luca M. Real-time antimicrobial susceptibility assay of planktonic and biofilm bacteria by isothermal microcalorimetry. Adv. Exp. Med. Biol. 2019 doi: 10.1007/5584_2018_291. [DOI] [PubMed] [Google Scholar]
- Christaki E., Marcou M., Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J. Mol. Evol. 2020 doi: 10.1007/s00239-019-09914-3. [DOI] [PubMed] [Google Scholar]
- Clark A.E., Kaleta E.J., Arora A., Wolk D.M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013 doi: 10.1128/cmr.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI-M100, 2022. Performance standards for antimicrobial susceptibility testing, 31st edition, clsi document m100. Retrieved Jan 06, 2023 from https://clsi.org/media/z2uhcbmv/m100ed31_sample.pdf.
- Cockerill F.R., 3rd Genetic methods for assessing antimicrobial resistance. Antimicrob. Agents Chemother. 1999 doi: 10.1128/aac.43.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon G., Naas T., Bogaerts P., Glupczynski Y., Nordmann P. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (tem, shv and ctx-m), plasmid-mediated cephalosporinases (cmy-2-like, dha, fox, acc-1, act/mir and cmy-1-like/mox) and carbapenemases (kpc, oxa-48, vim, imp and ndm) J. Antimicrob. Chemother. 2012 doi: 10.1093/jac/dks156. [DOI] [PubMed] [Google Scholar]
- Dai J., Hamon M., Jambovane S. Microfluidics for antibiotic susceptibility and toxicity testing. Bioengineering (Basel) 2016 doi: 10.3390/bioengineering3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014 doi: 10.4137/pmc.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluit A.C., Visser M.R., Schmitz F.J. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 2001 doi: 10.1128/cmr.14.4.836-871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyzun T., Mahmud A.A., Ahammed M.S., Manik M.I.N., Hasan M.K., Islam K.M.M., Lopa S.S., Al-Amin M.Y., Biswas K., Afrin M.R., Alam A.K., Sadik G. Polyphenolics with strong antioxidant activity from acacia nilotica ameliorate some biochemical signs of arsenic-induced neurotoxicity and oxidative stress in mice. Molecules. 2022 doi: 10.3390/molecules27031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajic I., Kabic J., Kekic D., Jovicevic M., Milenkovic M., Mitic Culafic D., Trudic A., Ranin L., Opavski N. Antimicrobial susceptibility testing: A comprehensive review of currently used methods. Antibiotics (Basel) 2022 doi: 10.3390/antibiotics11040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatley N.G. A method for the assay of penicillin. Biochem. J. 1944 doi: 10.1042/bj0380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedde P.N., Bouzin M., Abram T.J., Chen X., Toosky M.N., Vu T., Li Y., Zhao W., Gratton E. Rapid isolation of rare targets from large fluid volumes. Sci. Rep. 2020 doi: 10.1038/s41598-020-69315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.L., Hsu Z.J., Chang T.C., Jou R. Rapid and accurate detection of rifampin and isoniazid-resistant mycobacterium tuberculosis using an oligonucleotide array. Clin. Microbiol. Infect. 2014 doi: 10.1111/1469-0691.12517. [DOI] [PubMed] [Google Scholar]
- Huang T.H., Ning X., Wang X., Murthy N., Tzeng Y.L., Dickson R.M. Rapid cytometric antibiotic susceptibility testing utilizing adaptive multidimensional statistical metrics. Anal. Chem. 2015 doi: 10.1021/ac504241x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Xu D., Chen J., Liu J., Li Y., Song J., Ma X., Guo J. Smartphone-based analytical biosensors. Analyst. 2018 doi: 10.1039/c8an01269e. [DOI] [PubMed] [Google Scholar]
- Hussain I., Bowden A.K. Smartphone-based optical spectroscopic platforms for biomedical applications: a review [invited] Biomed. Opt. Express. 2021 doi: 10.1364/boe.416753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelevich E.A., Becker K. How to accelerate antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2019 doi: 10.1016/j.cmi.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Idelevich E.A., Becker K., Schmitz J., Knaack D., Peters G., Köck R. Evaluation of an automated system for reading and interpreting disk diffusion antimicrobial susceptibility testing of fastidious bacteria. PLoS One. 2016 doi: 10.1371/journal.pone.0159183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelevich E.A., Sparbier K., Kostrzewa M., Becker K. Rapid detection of antibiotic resistance by maldi-tof mass spectrometry using a novel direct-on-target microdroplet growth assay. Clin. Microbiol. Infect. 2018 doi: 10.1016/j.cmi.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Ihssen J., Jovanovic N., Sirec T., Spitz U. Real-time monitoring of extracellular atp in bacterial cultures using thermostable luciferase. PLoS One. 2021 doi: 10.1371/journal.pone.0244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.A., Zaman S., Biswas K., Al-Amin M.Y., Hasan M.K., Alam A., Tanaka T., Sadik G. Evaluation of cholinesterase inhibitory and antioxidant activity of wedelia chinensis and isolation of apigenin as an active compound. BMC Complement. Med. Ther. 2021 doi: 10.1186/s12906-021-03373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käbisch L., Schink A.K., Kehrenberg C., Schwarz S. Provisional use of clsi-approved quality control strains for antimicrobial susceptibility testing of mycoplasma ('mesomycoplasma') hyorhinis. Microorganisms. 2021 doi: 10.3390/microorganisms9091829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z.A., Siddiqui M.F., Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics (Basel) 2019 doi: 10.3390/diagnostics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page S., van Belkum A., Fulchiron C., Huguet R., Raoult D., Rolain J.M. Evaluation of the previ® isola automated seeder system compared to reference manual inoculation for antibiotic susceptibility testing by the disk diffusion method. Eur. J. Clin. Microbiol. Infect. Dis. 2015 doi: 10.1007/s10096-015-2424-8. [DOI] [PubMed] [Google Scholar]
- Li Y., Fan P., Zhou S., Zhang L. Loop-mediated isothermal amplification (lamp): a novel rapid detection platform for pathogens. Microb. Pathog. 2017 doi: 10.1016/j.micpath.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Lu H., Caen O., Vrignon J., Zonta E., El Harrak Z., Nizard P., Baret J.C., Taly V. High throughput single cell counting in droplet-based microfluidics. Sci. Rep. 2017 doi: 10.1038/s41598-017-01454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugeri G., Lychko I., Sobral R., Roque A.C.A. Identification and antibiotic-susceptibility profiling of infectious bacterial agents: a review of current and future trends. Biotechnol. J. 2019 doi: 10.1002/biot.201700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer F.P., Christner M., Hentschke M., Rohde H. Advances in rapid identification and susceptibility testing of bacteria in the clinical microbiology laboratory: implications for patient care and antimicrobial stewardship programs. Infect. Dis. Rep. 2017 doi: 10.4081/idr.2017.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.B., Tang Y.W. Basic concepts of microarrays and potential applications in clinical microbiology. Clin. Microbiol. Rev. 2009 doi: 10.1128/cmr.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa, S., Akbar, M., Khan, M.A., Sunita, K., Parveen, S., Pawar, J.S., Massey, S., Agarwal, N.R., Husain, S.A., 2022. Plant metabolite diosmin as the therapeutic agent in human diseases. Current Research in Pharmacology and Drug Discovery. https://doi.org/https://doi.org/10.1016/j.crphar.2022.100122. [DOI] [PMC free article] [PubMed]
- Nassar M.S.M., Hazzah W.A., Bakr W.M.K. Evaluation of antibiotic susceptibility test results: how guilty a laboratory could be? J. Egypt Public Health Assoc. 2019 doi: 10.1186/s42506-018-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cars O. Antibiotic resistance–problems, progress, and prospects. N. Engl. J. Med. 2014 doi: 10.1056/NEJMp1408040. [DOI] [PubMed] [Google Scholar]
- Ong D.S.Y., Poljak M. Smartphones as mobile microbiological laboratories. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2019.09.026. [DOI] [PubMed] [Google Scholar]
- Othman L., Sleiman A., Abdel-Massih R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar J.S., Mustafa S., Ghosh I. Chrysin and capsaicin induces premature senescence and apoptosis via mitochondrial dysfunction and p53 elevation in cervical cancer cells. Saudi J. Biol. Sci. 2022 doi: 10.1016/j.sjbs.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttaswamy S., Gupta S., Regunath H., Smith L., Sengupta S. A comprehensive review of the present and future antibiotic susceptibility testing (ast) systems. Arch. Clin. Microbiol. 2018 doi: 10.4172/1989-8436.100083. [DOI] [Google Scholar]
- Richter, S.S., Ferraro, M.J., 2011. Susceptibility testing instrumentation and computerized expert systems for data analysis and interpretation.
- Roche, 2015. Roche gobbles smarticles. Nat Biotechnol. https://doi.org/10.1038/nbt1015-1012a [DOI] [PubMed]
- Sader H.S., Pignatari A.C. E test: a novel technique for antimicrobial susceptibility testing. Sao Paulo Med. J. 1994 doi: 10.1590/s1516-31801994000400003. [DOI] [PubMed] [Google Scholar]
- Spanu T., Sanguinetti M., Ciccaglione D., D'Inzeo T., Romano L., Leone F., Fadda G. Use of the vitek 2 system for rapid identification of clinical isolates of staphylococci from bloodstream infections. J. Clin. Microbiol. 2003 doi: 10.1128/jcm.41.9.4259-4263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellapragada C., Hasan B., Antonelli A., Maruri A., de Vogel C., Gijón D., Coppi M., Verbon A., van Wamel W., Rossolini G.M., Cantón R., Giske C.G. Isothermal microcalorimetry minimal inhibitory concentration testing in extensively drug resistant gram-negative bacilli: a multicentre study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.01.026. [DOI] [PubMed] [Google Scholar]
- Wantia N., Gatermann S.G., Rothe K., Laufenberg R. New eucast definitions of s, i and r from 2019 - german physicians are largely not aware of the changes. Infection. 2020 doi: 10.1007/s15010-020-01456-x. [DOI] [PubMed] [Google Scholar]
- Wheat P.F. History and development of antimicrobial susceptibility testing methodology. J. Antimicrob. Chemother. 2001 doi: 10.1093/jac/48.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- WHO, 2020. Lack of new antibiotics threatens global efforts to contain drug-resistant infections. World Health Organization Retrieved from https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections#:∼:text=Lack%20of%20new%20antibiotics%20threatens%20global%20efforts%20to%20contain%20drug%2Dresistant%20infections,-17%20January%202020&text=Declining%20private%20investment%20and%20lack,World%20Health%20Organization%20(WHO).
- Wiegand I., Hilpert K., Hancock R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (mic) of antimicrobial substances. Nat. Protoc. 2008 doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Zhang K., Qin S., Wu S., Liang Y., Li J. Microfluidic systems for rapid antibiotic susceptibility tests (asts) at the single-cell level. Chem. Sci. 2020 doi: 10.1039/d0sc01353f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.D., Chu J., Wang Y.H. Advances in microfluidics applied to single cell operation. Biotechnol. J. 2018 doi: 10.1002/biot.201700416. [DOI] [PubMed] [Google Scholar]