Abstract
Background
There are many pharmaceutical interventions available to prevent osteoporotic vertebral fractures in postmenopausal women, but the efficacy and safety of these drugs are unknown. This study aimed to investigate the efficacy and safety of drugs in the prevention of osteoporotic vertebral fractures.
Methods
PubMed, Embase, and the Cochrane Library were comprehensively searched for randomized controlled trials (RCTs) published up to February 15, 2020, including postmenopausal women with osteoporosis. Network meta-analysis was conducted based on the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The relative risk (RR) and 95% confidence interval (CI) were used to report the results. This study was registered with PROSPERO, number CRD42020201167. Main Outcomes were incidences of new vertebral fracture and serious adverse events.
Results
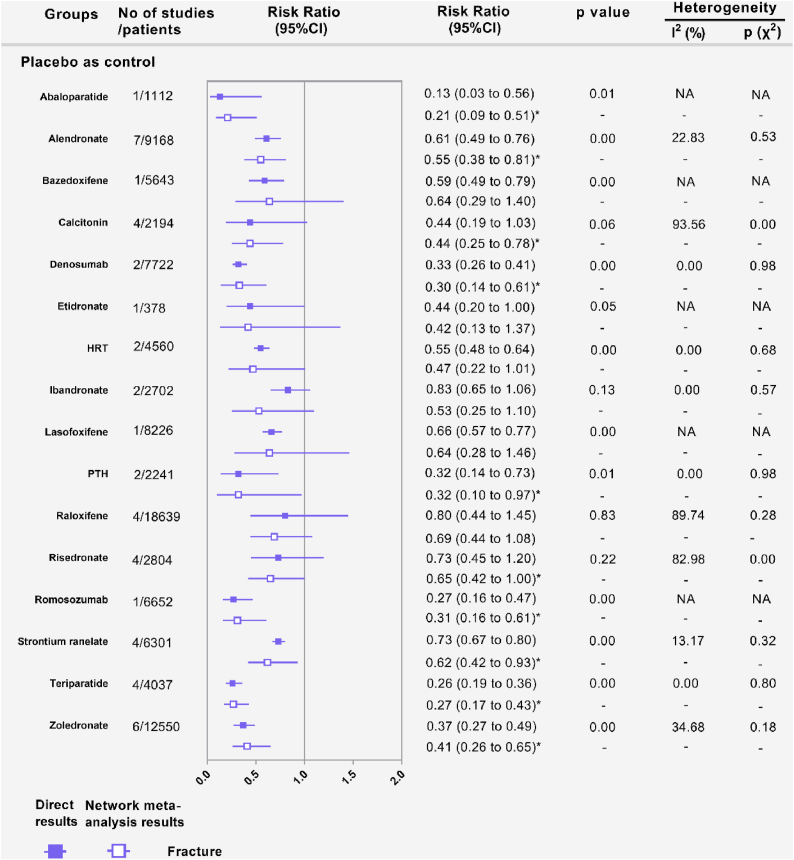
Fifty-five RCTs (n = 104 580) evaluating vertebral fractures of sixteen kinds of pharmacologic therapies were included in the network meta-analysis. Abaloparatide (RR, 0.21; [95% CI, 0.09 to 0.51]), alendronate (RR, 0.55; [95% CI, 0.38 to 0.81]), calcitonin (RR, 0.44; [95% CI, 0.25 to 0.78]), denosumab (RR, 0.33; [95% CI, 0.14 to 0.61]), parathyroid hormone (PTH) (RR, 0.32; [95% CI, 0.10 to 0.97]), risedronate (RR, 0.65; [95% CI, 0.42 to 1.00]), romosozumab (RR, 0.31; [95% CI, 0.16 to 0.61]), strontium ranelate (RR, 0.62; [95% CI, 0.42 to 0.93]), teriparatide (RR, 0.27; [95% CI, 0.17 to 0.43]), and zoledronate (RR, 0.41; [95% CI, 0.93]) were associated with lower vertebral fracture risk compared to placebo. PTH was associated with more adverse event rates. For any two drug treatments, the RR of serious adverse events was not statistically significant. Hormone replacement therapy (HRT) and calcitonin may be slower to work because they have only been shown to reduce the risk of vertebral fractures in long-term (>18 months) follow-up.
Conclusions
A variety of drugs are safe and effective in preventing osteoporotic vertebral fractures. HRT and calcitonin only reduced the risk of vertebral fractures during a follow-up of 21–72 months.
Keywords: Postmenopausal osteoporosis, Vertebral fractures, Safety, Pharmacologic therapy
1. Introduction
Osteoporosis, the most common bone disease, causes 1.5 million osteoporotic fractures in the United States each year, resulting in a total annual cost of 20 billion US dollars [1,2]. Osteoporosis is related to a huge social, economic and public health burden. In addition, spinal fractures are also associated with an increased risk of death and are a powerful predictor of future fractures, which may lead to chronic pain, scoliosis, and loss of self-esteem [3].
To prevent such fractures, a variety of drugs have been developed, and there is evidence that they have the effect of reducing fractures. Drugs for the treatment of osteoporosis can be divided into anti-bone resorption agents (i.e., anti-osteoclast-mediated bone resorption) or anabolic agents (i.e., stimulate osteoblasts to form new bone) [3]. According to the 2017 guidelines of the American College of Physicians [4], the drug treatment of postmenopausal osteoporosis mainly includes bisphosphonates, peptide hormones, receptor modulators of estrogen, new biological agents, calcium and vitamin D supplementation. This guideline is mainly based on a systematic review of randomized controlled trials (RCTs) and does not contain other important evidence, including two new drugs not mentioned in the guideline, abaloparatide (a parathyroid hormone-related protein analog) and romosozumab. However, the 2020 guidelines from the American Association of Clinical Endocrinologists [5] include abaloparatide [6] and romosozumab [7,8] based on only several RCTs, and there is no comprehensive evaluation of their safety. With the increase in the types of anti-osteoporosis drugs, it is very important for patients and physicians to understand the safety and efficacy of fracture prevention.
Certain meta-analyses have compared pharmacological interventions for postmenopausal osteoporosis [9–11]. However, some of them only compared a few drugs [10,11] or did not evaluate safety [9]. Moreover, time has a great influence on the effect of drugs, but no study has analyzed the effect of time. Due to the lack of RCTs comparing the efficacy of all drug interventions in preventing fractures, indirect comparison of the treatment of clinical trial evidence through network meta-analysis is an appropriate way to obtain relevant comparative evidence. Therefore, we conducted this network meta-analysis to evaluate the safety and efficacy of 16 pharmacological interventions in preventing new vertebral fractures in postmenopausal women with osteoporosis. To explore the effect of time on drug effects, we also conducted a subgroup analysis.
2. Methods
2.1. Study protocol
We adhered to the Cochrane Handbook for Systematic Reviews of Interventions [12] and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13,14] to conduct this meta-analysis. The protocol was published in PROSPERO (CRD42020201167).
2.2. Data sources and searches
The Cochrane and PROSPERO databases were independently searched by two reviewers to avoid duplicates. Then, electronic databases, including PubMed, Embase and the Cochrane Library, were searched with no language limitations (eTable 1 in the Supplement). RCTs included in the systematic reviews and meta-analyses were confirmed. We conducted additional research to determine the most recent RCTs (from inception through February 15, 2021). The initial searches were updated on May 10, 2021.
2.3. Study selection
Studies were included according to the PICOS criteria (eTable 2 in the Supplement).
2.4. Data extraction and outcomes
Relevant data were independently extracted by two authors, and controversies were resolved through discussion and consensus. We extracted the name of the first author, year of publication, study design, double-blind and open-label duration, age range or mean age, dosage of supplementary calcium and vitamin D, name of therapeutic drugs and their usage and dosage and sample size (incidences of new vertebral fracture, adverse events and serious adverse events).
2.5. Quality and risk-of-bias assessment
The Cochrane Collaboration's risk-of-bias assessment tool [12] was used by two reviewers to independently evaluate the included studies for potential bias. Disagreements between the two investigators were resolved by involving a third investigator. The overall risk of bias was obtained, and was divided into “high risk,” “low risk,” or “unclear risk”.
2.6. Data synthesis and statistical analysis
First, a random effects model was used for pairwise analysis to pool relative risks (RRs) and 95% confidence intervals (CIs). P < 0.05 was considered to be significant. We performed network meta-analyses in STATA software using a frequentist consistency model. Good consistency is the key to reliable results, and is manifested in the consistency between direct and indirect results. A three-step method was used to evaluate inconsistencies [15]. First, we compared the difference between the consistency model and the inconsistency model. Then, the entire network on particular comparisons (nodes) was tested by node splitting analysis; P < 0.05 manifested significant inconsistency. Third, the indirect results (network meta-analysis results) were compared with the pairwise direct results (meta-analysis results) to analyze the source of inconsistency. We used І2 tests to assess heterogeneity between different studies [16]. Sensitivity analysis of zoledronate was conducted based on the frequency of administration. All data were analyzed by STATA 16.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Studies retrieved and characteristics
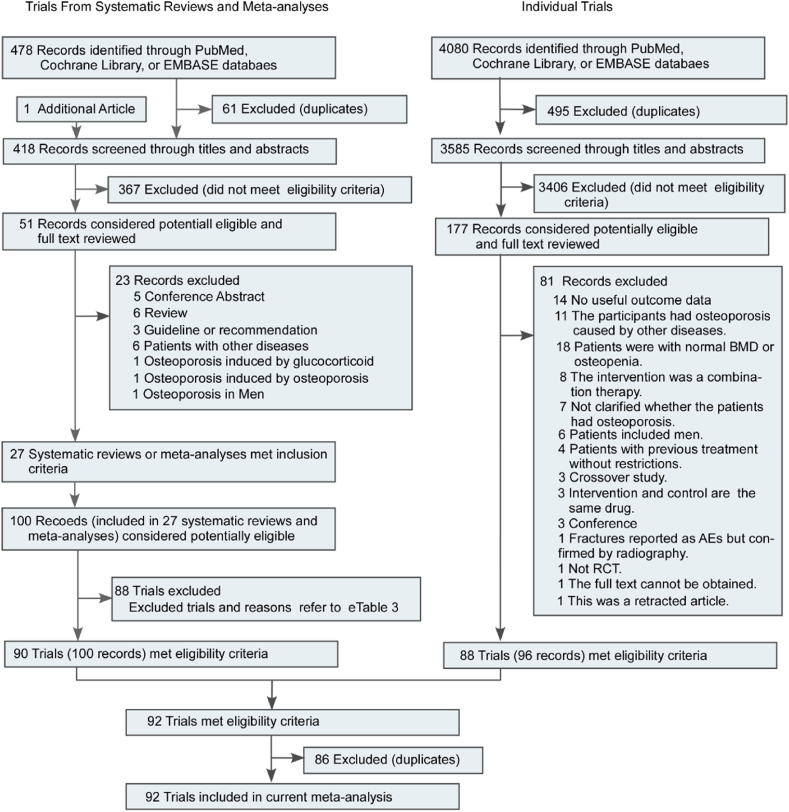
The systematic search identified 418 unique meta-analyses. After exclusion of 367 records, full texts of 51 records were read, and 27 were selected for evaluating pharmacologic therapies for prevention of osteoporotic vertebral fractures in postmenopausal women (Fig. 1 and eTable 3 in the supplement). 90 trials met the inclusion criteria. The RCTs included in the systematic reviews and meta-analyses are summarized in eTable 3 in the Supplement. Eighty-eight trials were excluded for the reasons listed in eTable 4 in the Supplement.
Fig. 1.
Literature search and screening process.
The searches identified 3585 unique RCTs. After exclusion of 3406 records, full texts of 177 records were read, and 88 trials were selected (Fig. 1). The assessment of the risk of bias is shown in eTable 5 in the supplement. The characteristics of the included RCTs are reported in eTable in the supplement. Studies had been published between 1990 and 2018. In the included RCTs, the mean age was in the range of 53.5–79.8 years.
3.2. Vertebral fracture based network meta-analysis
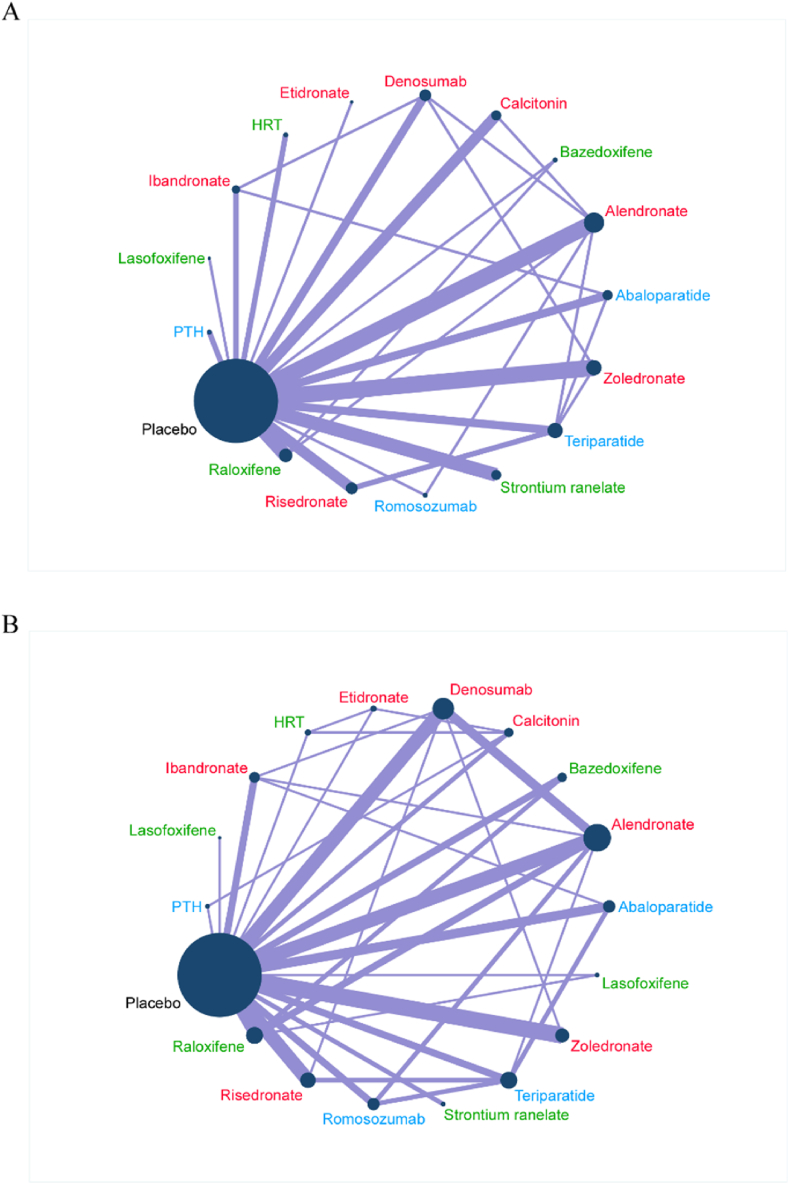
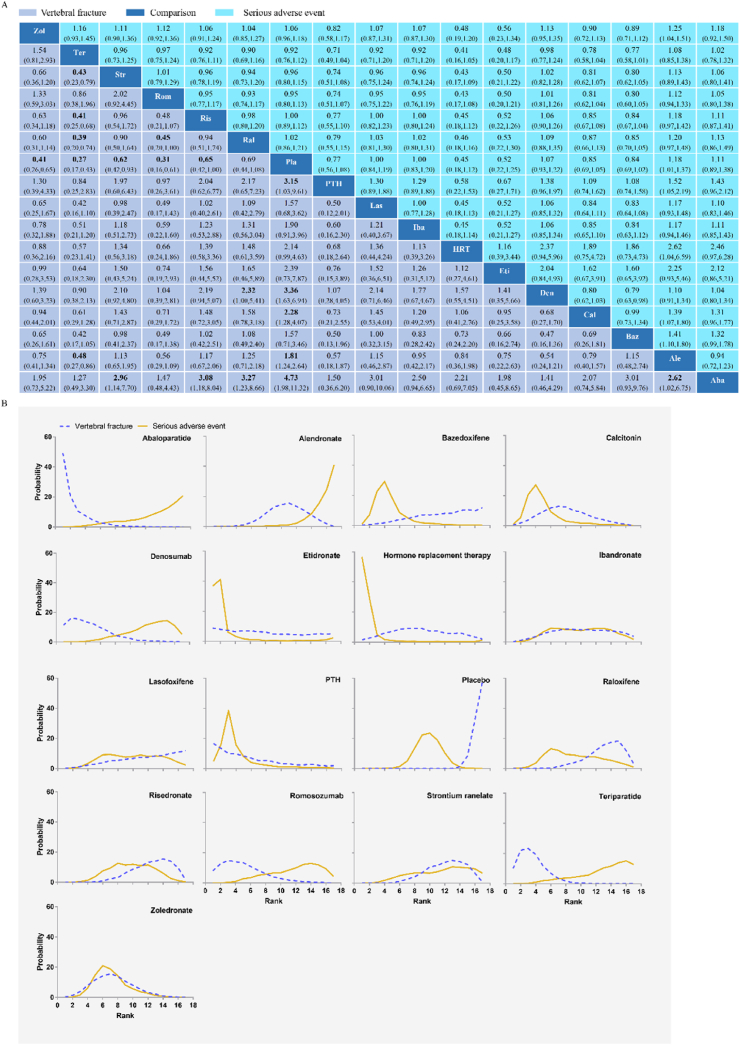
Fig. 2A shows that 55 RCTs (n = 104 580) evaluating vertebral fractures of sixteen kinds of pharmacologic therapies were included in this study. In terms of vertebral fractures (Fig. 3A), abaloparatide (RR, 0.21; [95% CI, 0.09 to 0.51]), alendronate (RR, 0.55; [95% CI, 0.38 to 0.81]), calcitonin (RR, 0.44; [95% CI, 0.25 to 0.78]), denosumab (RR, 0.30; [95% CI, 0.14 to 0.61]), parathyroid hormone (PTH) (RR, 0.32; [95% CI, 0.10 to 0.97]), risedronate (RR, 0.65; [95% CI, 0.42 to 1.00]), romosozumab (RR, 0.31; [95% CI, 0.16 to 0.61]), strontium ranelate (RR, 0.62; [95% CI, 0.42 to 0.93]), teriparatide (RR, 0.27; [95% CI, 0.17 to 0.43]), and zoledronate (RR, 0.41; [95% CI, 0.26 to 0.65]) reduced vertebral fracture risk compared with placebo in the consistency model. Teriparatide was more effective than alendronate (RR, 0.48; [95% CI, 0.27 to 0.86]), raloxifene (RR, 0.39; [95% CI, 0.20 to 0.74]), risedronate (RR, 0.41; [95% CI, 0.25 to 0.68]), and strontium ranelate (RR, 0.43; [95% CI, 0.23 to 0.79]) in preventing vertebral fracture. Abaloparatide was more effective than alendronate (RR, 0.38; [95% CI, 0.15 to 0.98]), raloxifene (RR, 0.31; [95% CI, 0.12 to 0.81]), risedronate (RR, 0.31; [95% CI, 0.12 to 0.81]), and strontium ranelate (RR, 0.34; [95% CI, 0.13 to 0.88]) in preventing vertebral fracture. Denosumab (RR, 0.43; [95% CI, 0.18 to 1.00]) and romosozumab (RR, 0.45; [95% CI, 0.20 to 1.00]) showed a significant reduction in vertebral fracture compared with raloxifene. The results obtained using the consistency model fit well with the results using the inconsistency model; node splitting analyses showed no significant inconsistency (all P > 0.05; eTable 7 in the Supplement). Fig. 4 shows the direct and indirect results of comparing different interventions. The direct results were prominently consistent with the corresponding indirect results in significance and tendency. The probabilities of pharmacologic therapies for the prevention of osteoporotic vertebral fractures in postmenopausal women, ranking from high to low, were abaloparatide (SUCRA: 0.888), teriparatide (SUCRA: 0.842), denosumab (SUCRA: 0.777), romosozumab (SUCRA: 0.750), PTH (SUCRA: 0.703), and zoledronate (SUCRA: 0. 602), calcitonin (SUCRA: 0. 562), etidronate (SUCRA: 0. 560), Hormone replacement therapy (HRT) (SUCRA: 0. 503), ibandronate (SUCRA: 0. 427), alendronate (SUCRA: 0. 392), lasofoxifene (SUCRA: 0. 321), bazedoxifene (SUCRA: 0. 317), strontium ranelate (SUCRA: 0. 302), risedronate (SUCRA: 0. 278), raloxifene (SUCRA: 0. 239) and placebo (SUCRA: 0. 033) (Fig. 3B).
Fig. 2.
Network plots of comparisons for vertebral fractures (A) and serious adverse event (B)-based network meta-analyses. Each circular node represents a type of treatment. The circle size is proportional to the total number of patients. The width of lines is proportional to the number of studies performing head-to-head comparisons in the same study. HRT: Hormone replacement therapy; PTH: parathyroid hormone. The drugs in red font are anti-osteoclast-mediated bone resorption. The drugs in green font are anti-osteoclast-mediated bone resorption and stimulate osteoblasts to form new bone. The drugs in blue font stimulate osteoblasts to form new bone.
Fig. 3.
Vertebral fractures and serious adverse events (A) according to the drug-based network meta-analysis in the consistency model. Each cell profile contains the pooled RR and 95% CI; significant results are in bold. Ranking curves of vertebral fractures and serious adverse events (B) indicate the probability of the lowest risk of vertebral fractures and serious adverse events, the second lowest risk, the third lowest risk, and so on.
Fig. 4.
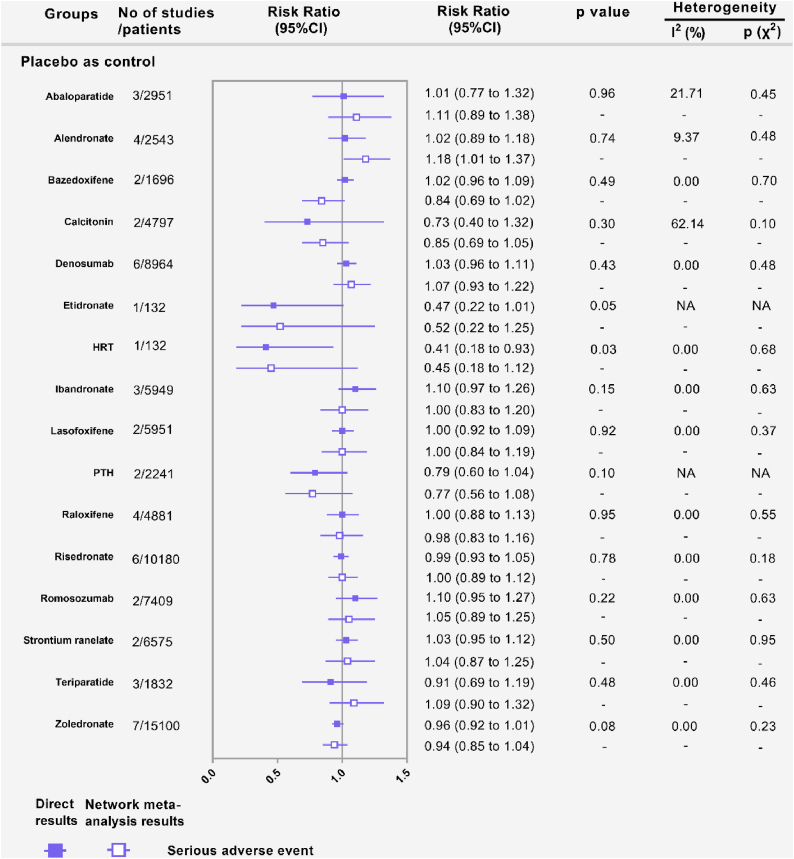
Forest plots depicting the direct and indirect results of vertebral fractures of head-to-head comparisons. HRT: Hormone replacement therapy; PTH: parathyroid hormone. ∗Values in brackets are 95% CI.
3.3. Subgroup analysis of vertebral fractures
To explore the effect of time on drug effects, we conducted a subgroup analysis. eFig. 17 shows that 21 RCTs (n = 42 513) evaluating vertebral fractures of ten kinds of pharmacologic therapies in short-term (≤18 months) follow-up were included in the subgroup analysis. Abaloparatide (RR, 0.14; [95% CI, 0.05 to 0.35]), alendronate (RR, 0.34; [95% CI, 0.20 to 0.58]), denosumab (RR, 0.12; [95% CI, 0.03 to 0.56]), raloxifene (RR, 0.45; [95% CI, 0.25 to 0.80]), risedronate (RR, 0.45; [95% CI, 0.34 to 0.61]), romosozumab (RR, 0.22; [95% CI, 0.14 to 0.37]), teriparatide (RR, 0.22; [95% CI, 0.15 to 0.32]), and zoledronate (RR, 0.30; [95% CI, 0.21 to 0.44]) reduced vertebral fracture risk compared with placebo in the consistency model. The probabilities of pharmacologic therapies for the prevention of osteoporotic vertebral fractures in postmenopausal women, ranking from high to low, were abaloparatide (SUCRA: 0.816), denosumab (SUCRA: 0.804), ibandronate (SUCRA: 0. 771), teriparatide (SUCRA: 0.661), romosozumab (SUCRA: 0.646), zoledronate (SUCRA: 0. 461), calcitonin (SUCRA: 0. 460), alendronate (SUCRA: 0. 371), raloxifene (SUCRA: 0. 254), risedronate (SUCRA: 0. 226) and placebo (SUCRA: 0. 031) (eFig. 18).
eFig. 19 shows that 39 RCTs (n = 83 373) evaluating vertebral fractures of ten kinds of pharmacologic therapies in long-term (>18 months) follow-up were included in the subgroup analysis. Abaloparatide (RR, 0.13; [95% CI, 0.03 to 0.65]), alendronate (RR, 0.62; [95% CI, 0.43 to 0.90]), calcitonin (RR, 0.47; [95% CI, 0.27 to 0.82]), denosumab (RR, 0.31; [95% CI, 0.15 to 0.64]), HRT (RR, 0.47; [95% CI, 0.24 to 0.93]), risedronate (RR, 0.53; [95% CI, 0.35 to 0.80]), strontium ranelate (RR, 0.63; [95% CI, 0.44 to 0.88]), teriparatide (RR, 0.24; [95% CI, 0.13 to 0.44]), and zoledronate (RR, 0.37; [95% CI, 0.24 to 0.56]) reduced vertebral fracture risk compared with placebo in the consistency model. The probabilities of pharmacologic therapies for the prevention of osteoporotic vertebral fractures in postmenopausal women, ranking from high to low, were abaloparatide (SUCRA: 0.913), teriparatide (SUCRA: 0.866), denosumab (SUCRA: 0.768), zoledronate (SUCRA: 0. 720), etidronate (SUCRA: 0. 599), PTH (SUCRA: 0.592), calcitonin (SUCRA: 0. 559), HRT (SUCRA: 0. 554), risedronate (SUCRA: 0. 495), alendronate (SUCRA: 0. 374), strontium ranelate (SUCRA: 0. 370), lasofoxifene (SUCRA: 0. 368), bazedoxifene (SUCRA: 0. 324), ibandronate (SUCRA: 0. 225), raloxifene (SUCRA: 0. 204) and placebo (SUCRA: 0. 069) (eFig. 20).
3.4. Adverse events and serious adverse events based network meta-analysis
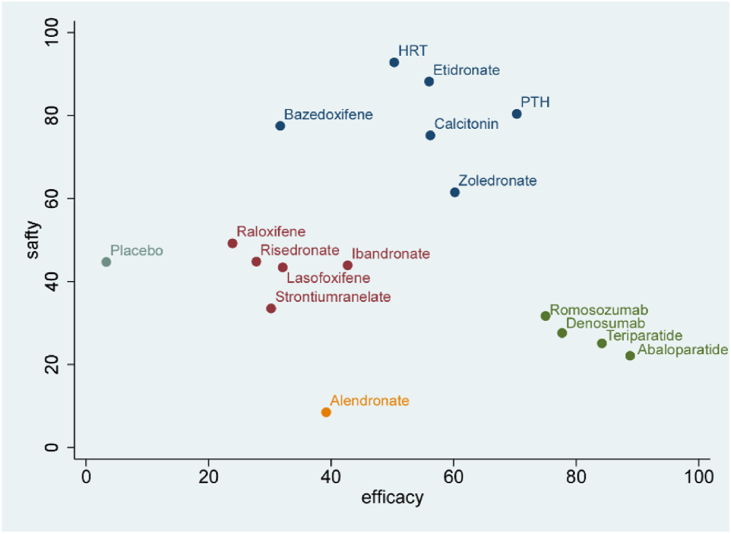
Fig. 2B shows that 57 RCTs (n = 100 195) evaluating serious adverse events of sixteen kinds of pharmacologic therapies were included in this study. For any two drug treatments, including placebo, the RR in serious adverse events was not statistically significant in the consistency model (Fig. 3A). The results obtained using the consistency model fit well with the results using the inconsistency model; node splitting analyses showed no significant inconsistency (all P > 0.05; eTable 7 in the Supplement). Fig. 5 shows the direct and indirect results of comparing different interventions. The direct results were prominently consistent with the corresponding indirect results in significance and tendency. After discussion, the consistency model was finally selected. The probabilities of pharmacologic therapies being associated with serious adverse events, ranking from low to high, were HRT (SUCRA: 0.927), etidronate (SUCRA: 0.879), PTH (SUCRA: 0.802), bazedoxifene (SUCRA: 0. 772), calcitonin (SUCRA: 0. 754) zoledronate (SUCRA: 0. 639), raloxifene (SUCRA: 0. 493), placebo (SUCRA: 0. 445), ibandronate (SUCRA: 0. 443), risedronate (SUCRA: 0. 439), lasofoxifene (SUCRA: 0. 430), strontium ranelate (SUCRA: 0. 340), romosozumab (SUCRA: 0.318), denosumab (SUCRA: 0.277), teriparatide (SUCRA: 0.244), HRT (SUCRA: 0. 503), abaloparatide (SUCRA: 0. 213) and alendronate (SUCRA: 0. 088) (Fig. 3B). The cluster analysis results based on vertebral fractures and serious adverse events are shown in Fig. 6.
Fig. 5.
Forest plots depicting the direct and indirect results of serious adverse events of head-to-head comparisons. HRT: Hormone replacement therapy; PTH: parathyroid hormone. ∗Values in brackets are 95% CI.
Fig. 6.
Clustered ranking plot of drug interventions for vertebral fractures and serious adverse events. HRT: Hormone replacement therapy; PTH: parathyroid hormone.
eFig. 37 in the Supplement shows that 69 RCTs (n = 110 325) evaluating adverse events of sixteen kinds of pharmacologic therapies were included in this study. The results obtained using the consistency model did not fit well with the results using the inconsistency model; node splitting analyses showed no significant inconsistency (not all P > 0.05; eTable 8 in the Supplement). eFig. 54 shows the direct and indirect results of comparing different interventions. The direct results were consistent with the corresponding indirect results in significance and tendency. Therefore, adverse events were pooled in the inconsistency model after discussion. In terms of adverse events (eFig. 38 in the Supplement), alendronate (RR, 0.36; [95% CI, 0.13 to 0.97]), bazedoxifene (RR, 0.23; [95% CI, 0.10 to 0.49]), calcitonin (RR, 0.38; [95% CI, 0.28 to 0.53]), denosumab (RR, 0.26; [95% CI, 0.11 to 0.59]), HRT (RR, 0.18; [95% CI, 0.06 to 0.54]), ibandronate (RR, 0.45; [95% CI, 0.28 to 0.70]), ibandronate (RR, 0.45; [95% CI, 0.28 to 0.70]), lasofoxifene (RR, 0.44; [95% CI, 0.32 to 0.61]), placebo (RR, 0.37; [95% CI, 0.30 to 0.46]), raloxifene (RR, 0.37; [95% CI, 0.13 to 1.00]), risedronate (RR, 0.33; [95% CI, 0.14 to 0.79]), romosozumab (RR, 0.27; [95% CI, 0.10 to 0.74]), strontium ranelate (RR, 0.38; [95% CI, 0.28 to 0.51]), teriparatide (RR, 0.37; [95% CI, 0.23 to 0.61]), and zoledronate (RR, 0.26 [95% CI, 0.11 to 0.63]) were associated with lower rates of adverse events compared with PTH in the inconsistency model.
3.5. Sensitivity analyses based on the frequency of administration
There was no statistically significant difference between 5 mg zoledronate once a year and other frequencies of zoledronate in the prevention of vertebral fractures (eFigs. 55 and 56 in the Supplement). In terms of serious adverse events and all adverse events, there was no significant difference between 5 mg zoledronate once a year and other frequencies of zoledronate (eFigs. 57–60 in the Supplement).
4. Discussion
This network meta-analysis included 92 RCTs involving 145 516 postmenopausal women with osteoporosis treated with sixteen kinds of pharmacologic therapies. This study aimed to investigate the efficacy and safety of drug interventions in preventing osteoporotic vertebral fractures. Several drugs to prevent vertebral fractures have been proven effective, including abaloparatide, alendronate, calcitonin, denosumab, PTH, risedronate, romosozumab, strontium ranelate, teriparatide, and zoledronate. PTH had a higher adverse events rate than most other drugs. This is the first study to explore the effect of time on drug effects. We found that raloxifene can reduce the risk of vertebral fractures in short-term follow-up by 55%. HRT and calcitonin may be slower to work because they have only been shown to reduce the risk of vertebral fractures in long-term follow-up.
We did not include calcium or vitamin D interventions in our study because most patients take vitamin D and calcium on a daily basis, which may affect the results. In addition, many guidelines recommend vitamin D and calcium as the basic treatment for osteoporosis in menopausal women [4, 5]. According to the results of the previous meta-analysis, calcium alone or calcium and vitamin D cannot prevent vertebral fractures [9]. Therefore, we did not recommend taking calcium and vitamin D alone to prevent osteoporotic vertebral fractures. A previous study confirmed that teriparatide is the most effective drug for reducing vertebral and nonvertebral fractures [17]. In our study, we found that abaloparatide and teriparatide were the best for preventing osteoporotic vertebral fractures in elderly women, without a statistically significant difference. Teriparatide was more effective than alendronate, raloxifene, risedronate, and strontium ranelate in preventing vertebral fracture. These results were consistent with those of previous studies [9, 11, 18, 19]. The new Endocrine Society guidelines recommend bone anabolic therapies as first-line therapy for women with severe osteoporosis [20]. Abalaratide and teriparatide (recombinant human parathyroid hormone) are bone-building drugs that stimulate osteoblasts to produce new bone, thus increasing bone mass and strength [21]. Due to the increased incidence of osteosarcomas in rats, abaloparatide and teriparatide should not be used in patients with an increased risk of osteosarcoma (Paget disease of the bone, open epiphyses, history of irradiation involving bones or unknown elevated levels of alkaline phosphatase of skeletal origin) [5]. However, in this study, we did not find that these two drugs have a higher rate of serious complications than placebo during a follow-up of up to 24 months. In an RCT published in 2014, where treatment with romosozumab, alendronate, or teriparatide was administered in postmenopausal women with low bone mass, romosozumab significantly increased the average regional bone density compared with teriparatide [22]. In our study, there was no significant difference between the efficacy of romosozumab and teriparatide in preventing osteoporotic vertebral fractures, and both had good efficacy.
Bisphosphonates are the main drugs used to treat osteoporosis and have been proven to have a reliable effect in preventing osteoporotic fractures [23]. In our study, we found that zoledronic acid can reduce the risk of osteoporotic vertebral fractures compared with placebo by 59%. These results were consistent with previous research [9, 24]. Then, we conducted sensitivity analyses based on the frequency of administration. No statistically significant difference was observed between 5 mg zoledronate once a year and other frequencies of zoledronate in preventing vertebral fractures. This result was consistent with a previous study conducted by McClung et al. [25]. Concerning reports of atypical subtrochanteric fractures and osteonecrosis of the jaw during extended bisphosphonate treatment prompted the Food and Drug Administration (FDA) to make a decision to reassess continued bisphosphonate treatment over 3–5 years of efficacy. The current study did not find that the incidence of serious complications in the zoledronic acid group was higher than that in the control group during a follow-up of up to 72 months, which is consistent with a previous study of long-term (72 months) follow-up [23].
Salmon calcitonin is a peptide containing 32 amino acids that inhibits absorption by binding and activating calcitonin receptors on osteoclasts [26,27]. However, most previous meta-analyses did not evaluate its efficacy and complications [11,24,28]. Although its price is low and it is easy to obtain, the European Medicines Agency (EMA) suspended the marketing of calcitonin nasal spray and restricted the expiration date of other calcitonin products because of a putative association with cancer in 2012 (24 July 2012). Previous meta-studies indicated that a certain link between calcitonin and nasopharyngeal carcinoma cannot be ruled out, but this link is very weak and is unlikely to have a causal relationship [29]. In our study, we confirmed that salmon calcitonin can reduce the risk of vertebral fractures by 56%. This result was consistent with a previous study [30]. Therefore, individual patients need to weigh the risks and benefits of various treatments for osteoporosis.
4.1. Strengths and limitations
First, our search strategy is our advantage. We extracted the relevant RCTs from a previous meta-analysis. Then, we performed an additional search to identify recently published RCTs to ensure that the relevant literature was searched as comprehensively as possible. Second, the indirect results (network meta-analysis results) were compared with the pairwise direct results (meta-analysis results) to analyze the source of inconsistency. Good consistency is the key to reliable results, and is manifested in the consistency between direct and indirect results. Third, in our goal to explore the effect of time on drug efficacy, this study is the first meta-analysis to conduct subgroup analysis based on the length of follow-up time. However, our study inevitably has limitations. First, in the current study, vertebral body fracture was used as the main prognostic indicator because osteoporotic fractures are mainly vertebral body fractures. Second, several studies have small sample sizes (n < 50), which may overestimate the effects of treatment. In addition, prognostic indicators were reported at different time points, which led to heterogeneity.
5. Conclusions
Based on our network meta-analysis, abaloparatide, alendronate, calcitonin, denosumab, PTH, risedronate, romosozumab, strontium ranelate, teriparatide, and zoledronate are effective in preventing vertebral fractures. For any two drug treatments, including placebo, the mean difference in serious adverse events was not statistically significant.
Funding/support
This work was supported by grants from Social Talent Fund of Tangdu Hospital (No. 2021SHRC034) and Tangdu Hospital Seed Talent Program (Fei-Long Wei) and the National Natural Science Foundation of China (No. 81871818). The funding body had no role in the design of the study, data collection, analysis, interpretation or in writing the manuscript.
Author contribution statement
Fei-Long Wei: Contributed reagents, materials, analysis tools or data; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Kai-Long Zhu: Contributed reagents, materials, analysis tools or data; Performed the experiments; Wrote the paper. Cheng-Pei Zhou, Ji-Xian Qian, Tian Li and Quan-You Gao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper. Wei Heng, Ming-Rui Du and Fan Yang: Contributed reagents, materials, analysis tools or data; Wrote the paper. Hao-Ran Gao: Conceived and designed the experiments; Wrote the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Tangdu Hospital, Fourth Military Medical University for supporting our work and Home for Researchers (www.home-for-researchers.com) for a language polishing service.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2022.e11880
Contributor Information
Tian Li, Email: fmmult@foxmail.com.
Ji-Xian Qian, Email: pasmiss2012@163.com.
Cheng-Pei Zhou, Email: zhoucpei@126.com.
Appendix A.
References
- 1.Cummings S.R., Melton L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 2.Lorentzon M. Treating osteoporosis to prevent fractures: current concepts and future developments. J. Intern. Med. 2019;285:381–394. doi: 10.1111/joim.12873. [DOI] [PubMed] [Google Scholar]
- 3.Black D.M., Rosen C.J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 4.Qaseem A., Forciea M.A., McLean R.M., Denberg T.D., Barry M.J., Cooke M., Fitterman N., Harris R.P., Humphrey L.L., Kansagara D., et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American college of physicians. Ann. Intern. Med. 2017;166:818–839. doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 5.Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., Harris S.T., Hurley D.L., Kelly J., Lewiecki E.M., et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr. Pract.: Off. J. Am. Col. Endocrinol. Am. Associat. Clin. Endocrinol. 2020;26:1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 6.Miller P.D., Hattersley G., Riis B.J., Williams G.C., Lau E., Russo L.A., Alexandersen P., Zerbini C.A.F., Hu M.-y., Harris A.G., et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 8.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., Maddox J., Fan M., Meisner P.D., Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 9.Barrionuevo P., Kapoor E., Asi N., Alahdab F., Mohammed K., Benkhadra K., Almasri J., Farah W., Sarigianni M., Muthusamy K., et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J. Clin. Endocrinol. Metab. 2019;104:1623–1630. doi: 10.1210/jc.2019-00192. [DOI] [PubMed] [Google Scholar]
- 10.Tan X., Wen F., Yang W., Xie J.-Y., Ding L.-L., Mo Y.-X. Comparative efficacy and safety of pharmacological interventions for osteoporosis in postmenopausal women: a network meta-analysis (Chongqing, China) Menopause. 2019;26:929–939. doi: 10.1097/GME.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 11.Wen F., Du H., Ding L., Hu J., Huang Z., Huang H., Li K., Mo Y., Kuang A. Clinical efficacy and safety of drug interventions for primary and secondary prevention of osteoporotic fractures in postmenopausal women: network meta-analysis followed by factor and cluster analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei F.L., Zhou C.P., Gao Q.Y., Du M.R., Gao H.R., Zhu K.L., Li T., Qian J.X., Yan X.D. Decompression alone or decompression and fusion in degenerative lumbar spondylolisthesis. E. Clinical Med. 2022;51 doi: 10.1016/j.eclinm.2022.101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C., Chen Y.-P., Du X.-J., Liu J.-Q., Huang C.-L., Chen L., Zhou G.-Q., Li W.-F., Mao Y.-P., Hsu C., et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei F.-L., Gao Q.-Y., Heng W., Zhu K.-L., Yang F., Du M.-R., Zhou C.-P., Qian J.-X., Yan X.-D. Association of robot-assisted techniques with the accuracy rates of pedicle screw placement: a network pooling analysis. E. Clinical Med. 2022;48 doi: 10.1016/j.eclinm.2022.101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murad M.H., Drake M.T., Mullan R.J., Mauck K.F., Stuart L.M., Lane M.A., Abu Elnour N.O., Erwin P.J., Hazem A., Puhan M.A., et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 2012;97:1871–1880. doi: 10.1210/jc.2011-3060. [DOI] [PubMed] [Google Scholar]
- 18.Miller P.D., Hattersley G., Riis B.J., Williams G.C., Lau E., Russo L.A., Alexandersen P., Zerbini C.A., Hu M.Y., Harris A.G., et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Pang Y., Shi Y., Xu M., Xu X., Zhang J., Ji L., Zhao D. Indirect comparison of teriparatide, denosumab, and oral bisphosphonates for the prevention of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Menopause. 2015;22:1021–1025. doi: 10.1097/GME.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 20.Eastell R., Rosen C.J., Black D.M., Cheung A.M., Murad M.H., Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society∗ clinical practice guideline. J. Clin. Endocrinol. Metab. 2019;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 21.Canalis E., Giustina A., Bilezikian J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 22.McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A., Langdahl B.L., Reginster J.Y., Zanchetta J.R., Wasserman S.M., et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 23.Reid I.R., Horne A.M., Mihov B., Stewart A., Garratt E., Wong S., Wiessing K.R., Bolland M.J., Bastin S., Gamble G.D. Fracture prevention with zoledronate in older women with osteopenia. N. Engl. J. Med. 2018;379:2407–2416. doi: 10.1056/NEJMoa1808082. [DOI] [PubMed] [Google Scholar]
- 24.Tan X., Wen F., Yang W., Xie J.Y., Ding L.L., Mo Y.X. Comparative efficacy and safety of pharmacological interventions for osteoporosis in postmenopausal women: a network meta-analysis (Chongqing, China) Menopause. 2019;26:929–939. doi: 10.1097/GME.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 25.McClung M., Miller P., Recknor C., Mesenbrink P., Bucci-Rechtweg C., Benhamou C.L. Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet. Gynecol. 2009;114:999–1007. doi: 10.1097/AOG.0b013e3181bdce0a. [DOI] [PubMed] [Google Scholar]
- 26.Davey R.A., Turner A.G., McManus J.F., Chiu W.S., Tjahyono F., Moore A.J., Atkins G.J., Anderson P.H., Ma C., Glatt V., et al. Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J. Bone Miner. Res. 2008;23:1182–1193. doi: 10.1359/JBMR.080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henriksen K., Byrjalsen I., Andersen J.R., Bihlet A.R., Russo L.A., Alexandersen P., Valter I., Qvist P., Lau E., Riis B.J., et al. A randomized, double-blind, multicenter, placebo-controlled study to evaluate the efficacy and safety of oral salmon calcitonin in the treatment of osteoporosis in postmenopausal women taking calcium and vitamin D. Bone. 2016;91:122–129. doi: 10.1016/j.bone.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Liu G.F., Wang Z.Q., Liu L., Zhang B.T., Miao Y.Y., Yu S.N. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J. Cell. Biochem. 2018;119:4469–4481. doi: 10.1002/jcb.26550. [DOI] [PubMed] [Google Scholar]
- 29.Wells G., Chernoff J., Gilligan J.P., Krause D.S. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos. Int. 2016;27:13–19. doi: 10.1007/s00198-015-3339-z. A J. Establi. Result Cooperat. Between European Foundat. Osteoporosis National Osteoporosis Foundation USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overgaard K., Hansen M.A., Jensen S.B., Christiansen C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ. 1992;305:556–561. doi: 10.1136/bmj.305.6853.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.