Abstract
The most widely prescribed antidepressant, fluoxetine (FLX), is known for its antioxidant and anti-inflammatory effects when administered post-stress. Few studies have evaluated the effects of FLX treatment when chronic stress has induced deleterious effects in patients. Our objective was to evaluate FLX treatment (20 mg/kg/day, i.v.) once these effects are manifested, and the drug's relation to extracellular circulating microRNAs associated with inflammation, a hedonic response (sucrose intake), the forced swim test (FST), and corticosterone levels (CORT) and monoamine concentrations in limbic areas. A group of Wistar rats was divided into groups: Control; FLX; CUMS (for six weeks of exposure to chronic, unpredictable mild stress); and CUMS + FLX, a mixed group. After CUMS, the rats performed the FST, and serum levels of CORT and six microRNAs (miR-16, -21, -144, -155, -146a, -223) were analyzed, as were levels of dopamine, noradrenaline, and serotonin in the prefrontal cortex, hippocampus, and hypothalamus. CUMS reduced body weight, sucrose intake, and hippocampal noradrenaline levels, but increased CORT, immobility behavior on the FST, dopamine concentrations in the prefrontal cortex, and all miRNAs except miR-146a expression. Administering FLX during CUMS reduced CORT levels and immobility behavior on the FST and increased the expression of miR-16, -21, -146a, -223, and dopamine. FLX protects against the deleterious effects of stress by reducing CORT and has an antidepressant effect on the FST, with minimally-modified neurotransmitter levels. FLX increased the expression of miRNAs as part of the antidepressant effect. It also regulates both neuroinflammation and serotoninergic neurotransmission through miRNAs, such as the miR-16.
Keywords: Serotonin, Chronic unpredictable mild stress, Corticosterone, miRNAs, Fluoxetine, Neurotransmitters
Highlights
-
•
CUMS reduced body weight, sucrose preference test, and hippocampal NA levels; increased CORT levels, the immobility behavior in the FST, and DA concentration in the prefrontal cortex.
-
•
CUMS reduced the miR-155, miR-21, miR-16, miR-223, miR-144.
-
•
FLX administration in CUMS reduced CORT levels and immobility behavior in the FST, and increased the miR-146a, miR-21,miR-16 and miR-223 but is not able to restore SPT and alterations in neurotransmitter levels.
-
•
FLX at this dose, time and stress phase has an antidepressant effect through different mechanisms, including the regulation of MIRNAs such as miR-16.
1. Introduction
Depression is a heterogeneous, chronic mental disorder that is among the top five causes of disability in the world and contributes significantly to the global burden of disease by affecting over 264 million people globally, according to the World Health Organization. In severe cases, depression can lead to suicide with an estimated 800,000 people dying from depression annually [1,2]. Depression is etiologically multifactorial, involving genetic and environmental factors, stressful events, and hereditary susceptibility [3,4]. The neurobiology of depression is associated with hypoactivation of the prefrontal cortex and linked limbic structures like the hippocampus and hypothalamus. These factors may have multiple conceptual and physiological overlaps. Stress has been recognized as one of the most important predisposing factors for developing depression but its interaction with, and contribution to, mood disorders are poorly understood [5,6]. Though progress has been made in research and treating depression in recent years, the exact pathogenesis of the disease is still unclear. Stress-based animal models are frequently used to explore the biological mechanisms of depression [7], including CUMS (chronic unpredictable mild stress), a rodent model of depression with high face, theoretical, and predictive validity and great translational potential to reproduce anhedonia, a cardinal symptom of depression [8] that results from a dysfunction of neural reward and motivation circuits [9]. CUMS operates by randomly exposing animals to various stressors in a naturalistic manner to decrease their degree of habituation [10]. It can also reproduce parameters related to clinical depression, such as decreased sucrose consumption, alterations in weight gain, decreased responsiveness to various stimuli, and appropriate responses to antidepressant drugs [8,11,12].
It is now known that miRNAs play a critical role in maintaining normal physiological states [13]. Studies have shown that the dysregulated expression of miRNAs participates in the development of depression [14,15] by affecting neural plasticity [16]. MiRNAs regulate microglia activation [17], and some (miR-16, -21, -126, -155, -146a, -223) have been reported as potential biomarkers for diseases related to inflammatory processes [18]. Specifically, miR-146a has been identified as a key regulator of the innate immune response that blocks the expression of several pro-inflammatory factors (TNF-α, IL-6, IFN-gamma) [[19], [20], [21]]. In contrast, miR-21 has been related to the production of cytokines and pro-inflammatory mediators since it modulates NF-ĸB activation and promotes c-Jun and AKT activity [[21], [22], [23], [24]]. miR-223 and miR-155 are associated with inflammatory processes [23,25], and the latter is implicated in autoimmune diseases such as multiple sclerosis and asthma [23]. It also induces the activation of microglia [26]. miR-16 is a negative regulator of serotonin transport and is also involved in inflammatory processes [27,28]. Recently miRNAs embedded in extracellular vesicles (EVs) have been implicated in depression and treatment responses [29].
The interconnected monoaminergic system has led to the development of adaptive capacities that buffer most genetic-environmental impacts through coping mechanisms [30]. Several studies have shown that alterations in serotonergic (5-HT), dopaminergic (DA), and noradrenergic (NA) neurotransmission, and the associated receptors, may contribute to depression-like behaviors, as these neurotransmitters play key roles in motivation and emotional processing in the brain and inherently influence the risk of developing depression [31]. This theory is the cornerstone of existing pharmacological therapies for depression based primarily on tricyclic antidepressants, selective 5-HT or norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors [32], drugs that directly affect the functional tone of these circuits in limbic and frontal cortical areas [33]. The selective 5-HT reuptake inhibitor, fluoxetine (FLX), is the most widely prescribed drug in the world [9,34]. It acts by inhibiting the serotonin transporter (SERT) with antidepressant effects regulating energy metabolism, synthesis of neurotransmitters, and tryptophan metabolism. It also exerts a protective effect on damaging processes, as it has been shown to attenuate neuronal apoptosis, promote motor functions in damaged tissue (e.g., in cases of cerebral ischemia), and improve cognitive functions [[35], [36], [37], [38]]. Finally, FLX has antitumor, anti-inflammatory, antioxidant, and antiplatelet aggregation effects [39,40].
FLX administration at the onset of stress has a potential anti-inflammatory effect and restores sucrose consumption decreased by CUMS [[41], [42], [43]]. Research has shown that miR-22 and -16 respond to FLX administration [44,45]. However, only a few studies have evaluated the drug's effect during stress to protect organisms from the impact of chronic stress when it begins to have deleterious effects on the body. Since the antidepressant effects of FLX apparently involve several mechanisms, our work was designed to evaluate FLX treatment in the stage when chronic stress has begun to show deleterious effects, focusing on its relation to extracellular circulating microRNAs that have been associated with inflammation, such as SERT regulators, and resilience to stress. To address these issues, we evaluated FLX’ action on hedonic responses (sucrose intake), behavioral responses on the forced swim test (FST), corticosterone levels (CORT) as a reliable indicator of stress impact, and, finally, monoamine concentrations in limbic areas and inflammation related to miRNA concentrations in circulating EVs.
2. Materials and method
Three-month-old male Wistar rats were obtained from the vivarium of the Universidad Autónoma Metropolitana-Iztapalapa. We have carried out all animal experiments in strict accordance with the Official Mexican Standards, NOM-062-ZOO-1999 [46], and the National Institutes of Health Guide for the Care and Use of Laboratory Animals [47]. The experimental protocol was approved by the Ethics Committee of the UAM-I (CBS.310.18). The rats were kept on a 12-h inverted light-dark cycle (lights on 9 p.m.) with food and water ad libitum. They were assigned to the following experimental groups: a) CTRL; b) intact with FLX at a dose of 20 mg/kg/day for three weeks; c) stress group subjected to the CUMS procedure for six weeks; and d) CUMS + FLX with the rats subjected to CUMS for six weeks with FLX (20 mg/kg/day) administered orally for 21 days from the beginning of the fourth week [48,49] (n = 6 per group).
Table 1 describes the stressors applied during the six weeks of CUMS exposure. Body weight and sucrose consumption (SPT) were recorded weekly. The average initial body weights in grams were CTRL = 346, FLX = 345, CUMS = 340, and CUMS + FLX = 341. On the day following the end of CUMS (day 43) or FLX administration, the rats in each group were subjected to the FST for 15 min. After a 24-h period (day 44), the test was repeated for 5 min. Finally, at 72 h after completing CUMS (day 45), the rats were euthanized by decapitation to obtain blood serum and dissect three brain structures: the hypothalamus, hippocampus, and prefrontal cortex, to analyze DA, NA, and 5-HT concentrations. Expression of miR-16, -21, -144, -155, -146a, and -223 and CORT levels were analyzed in plasma and serum, respectively. Fig. 1 describes the experimental design.
Table 1.
Scheme of the types and sequence of the stressors applied to the rats during 6 weeks of subjection to stress. CUMS procedure.
| Day | Stressor | Day | Stressor |
|---|---|---|---|
| 1 | Foreign object in cage 24 h | 22 | Movement restriction 1.5 – 3 h |
| 2 | Overcrowding [15 rats per cage] 24 h | 23 | Social isolation + Persistent light 24 h |
| 3 | Inclined cage of 45° 24 h | 24 | Immersion in cold water [8–10 °C] 15 min |
| 4 | Movement restriction 1.5–3 h | 25 | Cold exposure [4 °C] 1.5–3 h |
| 5 | Persistent light 24 h | 26 | Wet bedding [100 ml water/individual cage] 24 h |
| 6 | Food and water deprivation 24 h | 27 | Food and water deprivation 24 h |
| 7 | Cold exposure [4 °C] 1.5–3 h | 28 | Overcrowding [15 rats per cage] + Cage tilf of 45° 24 h |
| 8 | Immersion in cold water [8–10 °C] 15 min | 29 | Social isolation 24 h |
| 9 | Wet bedding [100 ml water/individual cage] 24 h | 30 | Movement restriction 1.5–3 h |
| 10 | Social isolation | 31 | Persistent light 24 h |
| 11 | Movement restriction + Cold exposure [4 °C] 1.5–3 h | 32 | Immersion in cold water [8–10 °C] 15 min |
| 12 | Inclined cage of 45° + Persistent light 24 h | 33 | Wet bedding + Foreign object in cage 24 h |
| 13 | Food and water deprivation 24 h | 34 | Food and water deprivation 24 h |
| 14 | Immersion in cold water [8–10 °C] 15 min | 35 | Inclined cage of 45° 24 h |
| 15 | Social isolation + Foreign object in cage 24 h | 36 | Overcrowding [15 rats per cage] 24 h |
| 16 | Movement restriction 1.5 – 3 h | 37 | Cold exposure [4 °C] 1.5 – 3 h |
| 17 | Wet bedding [100 ml water/individual cage] 24 h | 38 | Movement restriction 1.5 – 3 h |
| 18 | Overcrowding [15 rats per cage] 24 h | 39 | Social isolation + Foreign object in cage 24 h |
| 19 | Cold exposure [4 °C] 1.5–3 h | 40 | Immersion in cold water [8–10 °C] 15 min + Wet bedding 24 h |
| 20 | Food and water deprivation 24 h | 41 | Food and water deprivation 24 h |
| 21 | Inclined cage of 45° + Foreign object in cage 24 h | 42 | Overcrowding [15 rats per cage] + Persistent light 24 h |
Fig. 1.
Experimental design. The rats were assigned to the different experimental groups (CON, FLX, CUMS and CUMS + FLX). Both VCUG and VCUG + FLX groups were subjected to stress for 42 days (6 weeks). One day after the end of CUMS or FLX administration (day 43). The rats in each experimental group underwent FST for 15 min. After a period of 24 h (day 44), the test was repeated for 5 min. Finally, 72 h after completing CUMS (day 45), the rats were sacrificed by decapitation to obtain blood serum and dissect three brain structures: the hypothalamus, the hippocampus and the prefrontal cortex, to analyze the concentrations of DA, NA and 5-HT. The expression of miR-16, -21, -144, -155, -146a and -223 and the levels of CORT were analyzed in plasma and serum, respectively.
2.1. FST
The FST procedure has been adapted from previous studies [2,50,51,53]. Rats were placed individually in glass cylinders (40 cm high × 20 cm in diameter) containing 30 cm of water (maintained at 24 ± 1 °C), not allowing to support themselves by touching the bottom. Two swimming sessions were conducted between 10:00 a.m. and 13:00 p.m.: an initial 15-min test followed 24 h later by a 5-min test. After the two sessions, the rats were dried with towels and returned to their cages. All testing took place during the dark phase of the cycle. All recorded sessions were used for scoring. Fresh water was used for each evaluation to prevent confounding results from interference from urine or feces.
This is a valid reliable method used to assess the effects of various antidepressant drugs [[50], [51], [52]]. During the 5-min test at each 5-s interval, we rated the rats behavior into the following categories: (1) immobility, when rats remained passively floating in the water by only making the movements necessary to keep the head above the water; (2) swimming, when the movements of the four extremities allowed the animals to move around or across the cylinder; and (3) climbing, as active attempts with the forepaws movements in and out of the water, mostly toward the cylinder walls. A previously trained observer blind-sighted to the treatment conditions scored and conducted all behavioral scoring. The total number of counts per 5-min session was scored for each individual behavior. Where the number of times that specific behavior (immobility, swimming, or climbing) represented each count observed during the 5-min test.
2.2. Sucrose preference test (SPT) and body weight
We used the SPT to assess anhedonia by measuring the intake of a 1% sucrose solution once a week on different days (days 0, 7, 14, 21, 28, 35, 42) in a 1-h window after 24 h of water and food deprivation. Two bottles, one with tap water and the other with the sucrose solution were randomly placed (right or left) each week to avoid side preference. Consumption was measured by comparing the volume in ml before and after the 1-h window. The baseline was measured one week before the onset of chronic stress, as described in Ref. [53]. The period of water deprivation preceding the measurement of sucrose intake can be considered a stressor applied in addition to the chronic stress protocol; however, the control rats were also exposed to this period of deprivation as part of the test [7,30,54]. Consumption of water or the sucrose solution was determined by subtracting the residual volume at the end of the test from the initial volume. Sucrose preference (SP) was determined as the percentage of sucrose solution consumed over the total amount of liquid ingested (i.e., water intake plus sucrose intake) as follows: SP = [(sugar solution intake/total liquid intake) × 100]. Body weights were recorded every week.
2.3. Determination of monoamines
The animals of all groups were euthanized by decapitation at the onset of the dark phase, between 9 and 11 a.m. The brain areas of interest were dissected as described in previous reports, with some modifications [[55], [56], [57]]. Immediately after euthanasia, an incision was made along the midline of the head skin. Then a small incision was made in the upper part of the skull, beginning in the caudal area at the point of the parietal bone, taking care not to graze the brain. Next, the dorsal cranial bones and the brain were removed, quickly but gently, from the cranial cavity. The brains were rinsed immediately in Milli-Q water treated with ice-cold DEPC to remove blood from the surface. Once the hypothalamus, hippocampus, and prefrontal cortex were dissected, samples were placed in liquid nitrogen for preservation and stored at −80 °C for later analysis of the monoamines by reversed-phase high-performance liquid chromatography (RP-HPLC).
2.4. Reverse-phase high-performance liquid chromatography (RP-HPLC)
For NA, DA, and 5-HT extraction, 400 μl of buffer containing 5% ascorbic acid, 200 mM sodium phosphate, 2.5 mM l-cysteine, and 2.5 mM EDTA was added. To precipitate the protein 100 μl of 0.4 M perchloric acid was added, with an incubation at 20 °C for 20 min. Then, after centrifugation at 12,000 rpm for 10 min (4 °C) the collected supernatants were filtered by 0.22 μm then used for the evaluation of NA, DA, and 5-HT by RP-HPLC in a system that consisted of a PU-2089-plus pump (Jasco, Inc.), an AS-2057-plus autosampler (Jasco, Inc.), and an X-LC™3120FP fluorescence detector (Jasco, Inc.). ChromNav software (Jasco, Inc.) was used to control all instruments. Chromatographic runs were performed using a Júpiter C18 column (300 Å, 5 μ, 4.6 × 250 mm, Phenomenex®) at 30 °C. The column was equilibrated with mobile phase A containing 0.1% trifluoracetic acid in water. Then a linear gradient was performed from minute 5 to minute 20 with mobile phase B containing 0.1% trifluoroacetic acid in acetonitrile at a flow rate of 0.8 ml/min. The fluorescence detector was set to a gain of 1000, an attenuation of 32, a response time of 20 s, and 280 nm and 315 nm for excitation and emission, respectively. Using 50 μl as sample injection volume.
2.5. RNA isolation from extracellular vesicles
Blood samples were collected, and 350 μL of plasma were centrifuged 12000g for 8 min to further isolate RNA from extracellular vesicles with the exoRNeasy serum/plasma midi kit (adding 1.6 × 108 copies of synthetic cel-miR-39 control in the RNA isolation step) (Qiagen). Immediately after isolation, RNA was retrotranscribed (see below).
2.6. Detection of miRNAs by RT-qPCR
miRNAs levels were measured by RT-qPCR, 1.5 μL of isolated RNA was retrotranscribed with the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems). The RT reaction comprised a single cycle of 30 min at 16 °C, 30 min at 42 °C, and 5 min at 85 °C. Each miRNA was amplified from 2 μL of the RT (using specific primers) with the specific TaqMan probes (Applied Biosystems). Using the LightCycler TaqMan Master Kit reagent, PCR was carried out on a LightCycler 480 II thermocycler (Roche Applied Science, Basel, Switzerland). The PCR conditions included an enzyme activation step of 10 min at 95 °C, followed by 45 cycles of 95 °C for 15 s, at 60 °C for 60 s, and at 72 °C for 1 s. Relative miRNA levels was determined using the 2−Target miRNA Ct− cel-miR−39 Ct) equation.
2.7. Drugs
Fluoxetine hydrochloride (Prozac) was dissolved in a freshly-prepared 0.9% saline solution and administered by gavage [58] at a volume of 0.1 mL/100 g of body weight. This dose was chosen due to its effect in the FST (49).
2.8. Statistical analyses
All results are expressed as mean ± SEM for each group. Between-group differences in monoamines, CORT levels, FST results, and miRNAs were analyzed by a one-way analysis of variance (ANOVA), while body weight and consumption preference on the sucrose test were analyzed using a two-way ANOVA, followed by a Tukey test. A P-value <0.05 indicated statistically-significant differences.
3. Results
3.1. Body weight and SPT
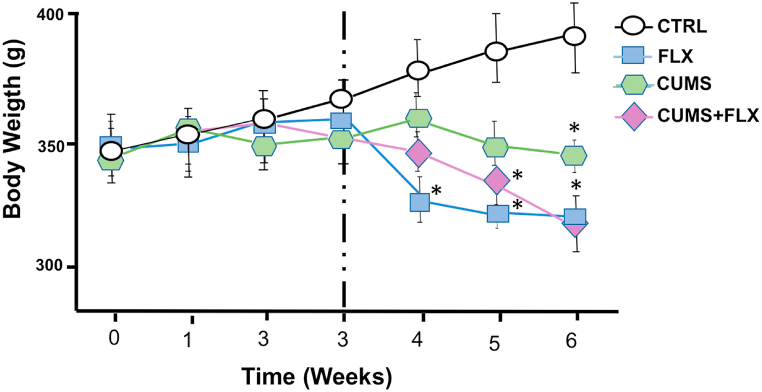
As mentioned above, the average initial body weights of the rats in grams were CTRL = 346, FLX = 345, CUMS = 340, and CUMS + FLX = 341. The FLX group reduced its average body weight by 14.08% from the first week of treatment (p < 0.05). The CUMS and CUMS + FLX groups also significantly reduced their body weights, by 8.97% and 13.77%, respectively, after 5 weeks of stress (p < 0.05), compared to CTRL (Fig. 2).
Fig. 2.
Effects of CUMS and FLX on weekly body weight gain. Body weight gain in the CUMS + FLX, CUMS, and FLX groups was significantly les than in the CTRL group from the 4th week of the study. Data expressed as Means ± Standard Error (n = 6 per group), analyzed by a two-way ANOVA with a post hoc Tukey test. *p < 0.05 compared to CTRL; +p < 0.05 vs. FLX, ap<0.05 vs. CUMS.
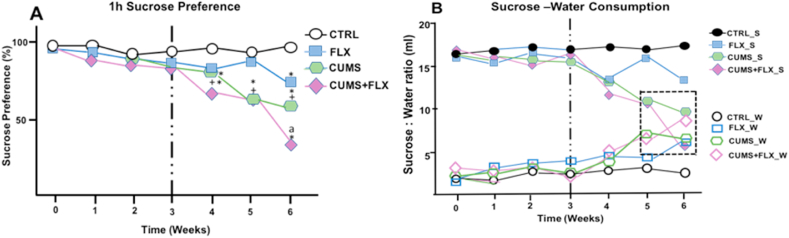
Fig. 3, panels A and B, show the results for sucrose preference (SP) as a percentage of the rats’ consumption of the sucrose solution (Panel A) and water (B) in ml in 1 h. SP decreased in FLX by week 6. In CUMS and CUMS + FLX, SP decreased significantly (p < 0.05) from week 4 of subjection to stress, compared to CTRL. This decrease in sucrose consumption in the CUMS + FLX group was maintained until week 6, compared to CUMS and CTRL (p < 0.01). The reduction in SP revealed an increase in water consumption in the rats in both the CUMS and CUMS + FLX groups, neither one of which discriminated between water and sucrose.
Fig. 3.
Effects of CUMS and FLX on the sucrose preference test showing (A) the percentage of the sucrose solution consumed in 1 h; and (B) consumption of water and sucrose. The preference rate for sucrose and total ingestion of liquid decreased significantly in the CUMS and FLX rats compared to the CTRL and FLX groups. During the final 2 weeks of stress, no difference was observed between water and sucrose consumption in the CUMS and CUMS + FLX groups. Data expressed as Means ± Standard Error (n = 6 per group), analyzed by a two-way ANOVA with a post hoc Tukey test. *p < 0.05 compared to CTRL; +p < 0.05 vs. FLX, ap<0.05 vs. CUMS.
3.2. FST and CORT levels
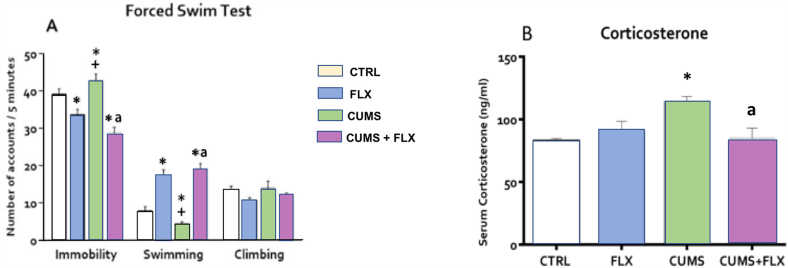
On the FST, the CUMS group significantly increased immobility behavior (p < 0.05) and reduced (p < 0.01) swimming, but with no effect on climbing. The opposite effect was observed in the FLX group, as it reduced immobility but increased swimming, also with no effect on climbing (p < 0.05). The CUMS + FLX group reduced immobility but increased swimming (p < 0.05) (Fig. 4A). Fig. 4 panel B, shows the results of the effect of CUMS, FLX, and CUMS + FLX on plasma CORT levels. The CUMS group experienced a significant increase in CORT concentrations, but this was re-established when FLX was administered at the beginning of week 4 of stress in the CUMS + FLX group (p < 0.05). FLX had no effect on CORT concentrations.
Fig. 4.
Effect of CUMS and FLX on the FST (A) and serum CORT levels (B). CUMS showed an increase in immobility behavior with a decrease in swimming behavior. Treatment with FLX reduced immobility behavior and increased swimming behavior. CORT increased due to the effect of CUMS, and decreased with FLX treatment. Data expressed as Means ± Standard Error (n = 6 per group). ANOVA followed by a Tukey test. *p < 0.05 compared to CTRL; +p < 0.05 vs. FLX, ap<0.05 vs. CUMS.
3.3. Levels of monoamines in the hypothalamus, hippocampus, and prefrontal cortex
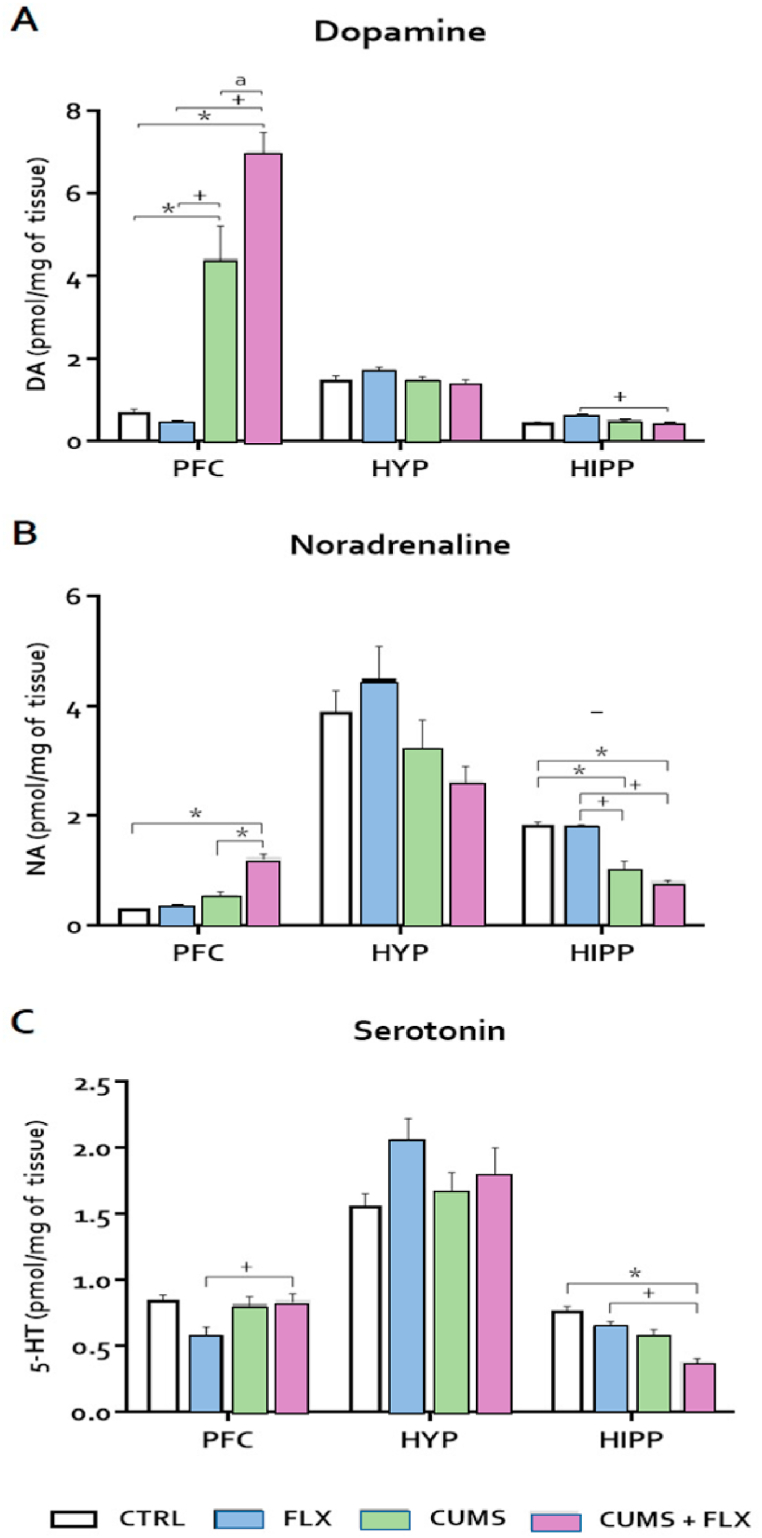
Results show an increased NA concentration in the prefrontal cortex in the CUMS + FLX group compared to CTRL (p < 0.05), though no changes were seen in the hypothalamus. In the hippocampus, NA decreased in the CUMS and CUMS + FLX groups compared to the CTRL and FLX groups (Fig. 5 Panel A) (p < 0.05). DA increased in the prefrontal cortex in the CUMS and CUMS + FLX groups compared to the CTRL and FLX groups (p < 0.05). For the hippocampus, DA decreased only in the CUMS + FLX group with respect to the FLX group (Fig. 5 Panel B). Finally, 5-HT levels increased in the prefrontal cortex in the CUMS + FLX group compared to the FLX group. In the hippocampus, 5-HT decreased in the CUMS + FLX group compared to the CTRL and FLX groups (Fig. 5 Panel C).
Fig. 5.
Effect of CUMS and FLX on DA (A), NA (B), and 5-HT (C) levels. CUMS and FLX induced neurochemical changes in all the brain structures analyzed. An increased in the levels of NA and DA in the PFC in the CUMS and CUMS + FLX groups compared to the CTRL and FLX group. Data expressed as means ± standard error (n = 6 per group). Statistical analysis was performed by an ANOVA followed by a Tukey test. *p < 0.05 compared to CTRL; +p < 0.05 vs. FLX, a p < 0.05 vs. CUMS. NA = noradrenalin; DA = dopamine; 5-HT = serotonin. HYP=Hypothalamus, HIPP= Hipocampus, PFC= Prefrontal Cortex.
3.4. Expression of miRNAs in plasma EVs
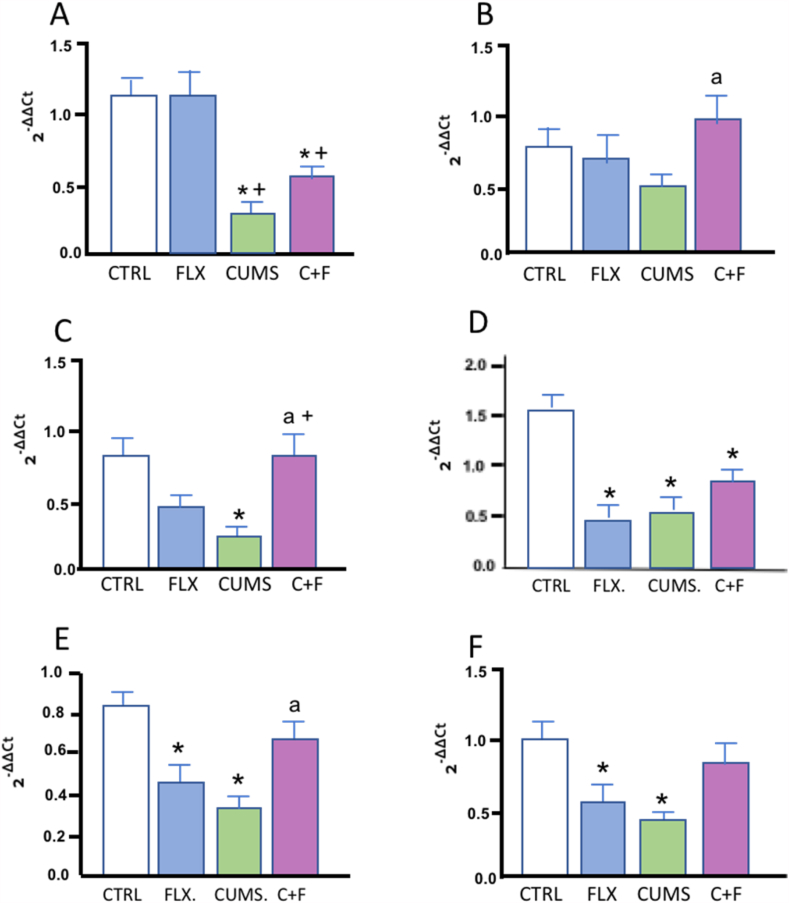
Fig. 6 shows the effect of CUMS, FLX, and CUMS + FLX on the expression of miRNAs. miR-146a was not modified by the effect of CUMS or FLX, but miR-155 decreased in the CUMS (p < 0.01) and CUMS + FLX (p < 0.05) groups compared to the CTRL and FLX groups, respectively (Fig. 6 Panel A and B). Similarly, miR-21 decreased significantly in the CUMS group (p < 0.01) with respect to CTRL, while FLX administration in the CUMS group induced an increase in miR-21 with respect to the CUMS (p < 0.01) and FLX groups (p < 0.05) (Fig. 6 Panel C). MiR-144 and miR-16 (p < 0.0001) decreased in the FLX, CUMS and CUMS + FLX groups (p < 0.05) compared to CTRL (Fig. 6 Panel D and E), but miR-16 increased in the CUMS + FLX group compared to CUMS Likewise, miR-223 decreased significantly in the FLX and CUMS groups compared to CTRL (p < 0.01) (Fig. 6 Panel F).
Fig. 6.
Effect of CUMS and FLX on levels of miRNA expression. FLX induced changes in the expression of miRNAs in CUMS rats. Data expressed as Means ± Standard Error (n = 6 per group). Statistical analysis was performed by an ANOVA followed by a Tukey test. *p < 0.05 compared to CTRL; +p < 0.05 vs. FLX, ap<0.05 vs. CUMS.
4. Discussion
In the present study, we analyzed the therapeutic antidepressant effect of FLX on circulating miRNAs in EVs, the FST, corticosterone levels, and sucrose consumption using the CUMS animal depression model. Evidence suggests that stress plays an important role in the development of depression. After 42 days of CUMS, the rats showed reduced sucrose consumption, decreased weight gain, increased swimming behavior on the FST, and higher CORT levels with alterations in monoamine concentrations and reduced expression of miR-16, -223, -21, and -144a, four miRNAs that are modulated in inflammatory states. All these findings support the use of CUMS as an animal model of depression [7,59,60]. FLX at a dose of 20 mg/kg inhibited the increase in stress-induced CORT levels and re-established miRNAs expression and the behavioral response on the FST that had decreased due to the impact of CUMS. However, it did not restore monoamine concentrations or NA and DA levels on the SPT. Moreover, FLX worsened some behavioral characteristics and body weight in the intact and CUMS groups. These results are suggestive of FLX’ therapeutic effect.
4.1. Effect of FLX on body weight and SPT
FLX administration once the stress has begun to damage an organism acts on SERT. Its antidepressant effect involves various mechanisms that seek to improve symptoms of depression [61]. In rats and mice, SPT is recognized as the best method for evaluating anhedonia [62]. Observations of our CUMS-exposed rats showed a significant decrease in sucrose intake compared to CTRL after four weeks that continued through the six weeks of the CUMS protocol, indicating an anhedonic-like state [63]. These results are consistent with previous studies [[64], [65], [66]] and confirm earlier reports [60,64]. Some reports indicate that FLX administration at a dose of 2.1 mg/kg in rats subjected to CUMS improves levels of sucrose consumption on the SPT [67]. This effect, however, was not detected at the dose of 20 mg/kg. Some researchers have suggested that reductions in body weight following CUMS contribute to lower sucrose intake [68,69]. Indeed, a decrease in body weight was observed in the CUMS group. This decrease is a constant finding in stressed animals, one considered a function of immaturity or stress responses. Some studies affirm that the combination of food and water deprivation influences SP and intake following CUMS [7,70,71], arguing that this interacts with exposure stressors that generate anhedonia in previously resilient animals [72]. However, administering FLX to our intact rats had undesirable effects, such as reduced body weight from the first week of administration [73,74].
Studies in humans have also shown reduced body weight due to the effect of FLX [75]. Rats that received different doses of FLX (10, 20, and 40mg/k i.p. for 2, 4, and 12 weeks) also showed decreased body weight from week 2 of administration [[76], [77], [78]], with a dose-dependent effect [79]. This reduction in body weight seems to be attributable to the secondary effects of FLX, such as lower food intake [68,80], a secondary effect that could be due to undesirable impacts on the digestive system, such as diarrhea, nausea, vomiting, dyspepsia, dysphasia, taste confusion, and dry mouth [67], reduced femur length in adult rats [81], and lower weight of the reproductive organs (testes, epididymides, ventral prostate) [77,81,82]. Moreover, at a dose of 20 mg/kg FLX affects the intestinal microbiota that participates in regulating body mass [83]. Finally, the decrease in body weight in the CUMS + FLX group could be the result of the sum of the effects that FLX exerts on body weight plus the effect of stress.
4.2. Effect of FLX on the FST
The FST is one of the best-known screening tests for evaluating depressive-like behaviors and antidepressant agents. On this test, increased immobility behavior by subjects is interpreted as indicative of behavioral despair [84]. Chronic FLX administration resulted in behavioral modifications and improved immobility behavior on the FST, similar to what has been reported previously for this test at a dose of 20 mg/kg [49]. The role of FLX in improving immobility behavior may be due to the modulation of circulating CORT levels. Studies in animals have shown that stress induces CORT to release that, in turn, impairs cortical functions (e.g., in the hippocampus) by suppressing neurogenesis [85], thus leading to depression [86]. Chronic FLX treatment normalizes CORT secretion in experimental models [[87], [88], [89]]. Our results show a similar effect with increased CORT levels induced by CUMS that were re-established when FLX was administered to the CUMS + FLX group. Other evidence suggests that chronic treatment with FLX restores homeostasis in the hypothalamus-pituitary-adrenal (HPA) axis in humans [90] by inducing at least a 50% reduction on the HDRS scale [91]. FLX has also shown efficacy in reducing immobility behavior on the FST in other animal models of depression [49,92]. FLX increases transcript levels of 5-HT1A receptor in the brainstem, frontal cortex, and hippocampus in rats. Its delayed therapeutic action is thought to be mediated by desensitization of the presynaptic somatodendritic 5-HT1A autoreceptors and nerve terminal 5-HT1B autoreceptors, which modulate the firing of serotonergic neurons and 5-HT release, respectively [93]. Long-term FLX administration in rats also produces desensitization of post-synaptic 5-HT1A receptors in the hypothalamus, manifested by reductions in the ACTH and CORT secretory responses to 5-HT1A agonists [93,94].
Disruption of the HPA axis is an established finding in patients with anxiety and/or depression. Hyperactivity of this axis is a particularly powerful factor that triggers depressive disorders [95]. This parameter increased in the CUMS group. It has been reported that stress/depression significantly increases serum CORT levels [96] and results in the occupation of mineralocorticoid and glucocorticoid receptors (GR). Occupation of GR includes a higher response capacity of hippocampal neurons to stimulation by the 5-HT1A receptor, atenuating 5-HT autoinhibition, accompanied by a permissive effect on stress-induced increases in the release of 5-HT in the hippocampus. 5-HT1A receptors are also found in the pyramidal CA1 neurons of the hippocampus [97] so 5-HT can modulate the response capacity of the pyramidal cells bidirectionally [98]. Studies of the rat's hippocampus have shown that long-term exposure to high CORT levels brings an attenuation of responses to the activation of 5-HT1A receptors [[99], [100], [101]] and a reduced function of hippocampal 5-HT1A receptors during stress [102]. Adaptive modifications of the serotonergic modulation of hippocampal neuronal activity are thought to provide an important outcome of different types of antidepressant therapies [103]. In this regard, it has been proposed that FLX acts by increasing the expression of GR while inhibiting the steroid transporters localized on the BBB, in neurons, and PGP, a protein that limits the access of cortisol, thus increasing that of corticosteroids in the brain and glucocorticoid-mediated negative feedback on the HPA axis [104,105]. This also increases the function of the activity of the 5-HT1A receptor by increasing activation of the BNDF receptor (TrkB)) which enhances the capacity of 5-HT1A receptors to activate G proteins in the hippocampus [102]. This reduces the increase in CORT in rats subjected to stress [96]. FLX also counteracts stress-induced changes mainly on presynaptic α2-adrenoceptors expressed in the prefrontal cortex [106], whose noradrenergic activity increases by acting on distinct α2-adrenoceptor subpopulations [106].
4.3. Effect of FLX on monoamines in CUMS rats
Monoamines are involved in the neurobiology of depression and the action mechanisms of antidepressant agents. Monoaminergic neurons in the hypothalamus, hippocampus, and prefrontal cortex have been shown to play a pivotal role in stress response and depression-like behaviors [107,108]. Effects seen on CUMS, suggest that these depression-like behaviors couple with dopaminergic hyperfunction in the NAc and serotonergic hypofunction in the hippocampus, prefrontal cortex, and striatum [[109], [110], [111]], or an increase of 5-HT in the medial prefrontal cortex and striatum [43]. In rodent depression phenotypes, decreased serotonergic activity via 5-HT1A receptors in the prefrontal cortex [112] suggests that CUMS impairs the monoaminergic function in structures involved in both depression and stress. Evidence indicates that 5-HT exerts a modulating effect on DA by increasing or decreasing its activity, depending on actions related to other neurotransmitters that lead to self-regulation; that is, as 5-HT increases, DA decreases [113,114].
In our results, the most significant changes detected in the CUMS group included an increase of DA in the prefrontal cortex, but a decrease in the hippocampus with a tendency towards reducing 5-HT levels. The increase of DA suggests that the modification in depression symptoms may be related to alterations in the neural encoding of action in limbic circuitry, such as the dopaminergic reward circuit that participates in processes like recognizing rewards in an environment and initiating consumption [115], and plays a crucial role in the stress response and mood disorders [116]. In fact, extracellular dopamine levels are elevated in the NAc of humans who are undergoing stress [117]. The 5-HT values, in this case, can be explained by the fact that the animals were not sacrificed at the end of the CUMS protocol, but were evaluated after the FST (that is, 72 and 48 h before sacrifice; see Fig. 1). In this regard, it is well-known that subjection, both unique and repeated, to the FST increases intracellular 5-HT and 5-HIAA levels in various brain structures in rodents [110,118,119]. Presumably, in our study, the stress of FST influenced the response in 5-HT levels in the hippocampus, especially because the rats were sacrificed 72 h after applying the battery of stressful factors and 24 h after the 5-min FST, in contrast to other experimental procedures where subjects were sacrificed upon finalizing the CUMS protocol.
It is well known that FLX binds to SERT in central neurons, thus increasing serotonin availability in the synaptic cleft [120,121]. Its effect is exerted through SERT and 5-HT1A receptors. Strong experimental evidence suggests that SERT may be regulated [122] and undergo adaptive changes during its therapy that depends on treatment time. Reports on this topic have cited increases, decreases, and the absence of changes in the density of SERT and DRN binding sites, as well as varied results for regions innervated by 5-HT, including the prefrontal cortex [[123], [124], [125], [126], [127]]. In a recent report, chronic FLX treatment induced the internalization of SERT in cellular bodies and cells, such as the axon terminal of 5-HT neurons. A differential effect with the 5-HT1A autoreceptors that is present on the plasma membrane of cell bodies and dendrites is not internalized, but they are desensitized, and their re-sensitization requires several weeks after interruption of chronic FLX treatment [128]. According to our results, FLX did not modify 5-HT. There is some evidence that when FLX treatment is chronic (e.g., three weeks in rats), the increases in extracellular 5-HT concentrations may not persist in the nuclei of origin [[129], [130], [131]] or in the regions of 5-HT projection [[130], [131], [132]]. For example, chronic FLX administration (30 mg/kg, i.p.) for three days did not modify serotonin levels in the frontal cortex [133,134].
4.4. Effect of FLX on miRNA expression
Stress can trigger an inflammatory response in the brain that can induce neurochemical changes and behavioral disturbances that lead to diagnoses of depression [135]. Recently, several miRNAs have been reported to alterated in the brain and peripheral tissues, such as blood, related to stress responses [136,137], depression, and/or treatment with antidepressants [27,138]. Regulating gene expression by miRNAs is considered an important factor in modulating the neurotransmission of the stress response, one that can be used as a biomarker of treatment response [139]. In the present study, the changes in the concentration of circulating miRNAs in EVs may reflect some of the systemic anti-inflammatory effects of FLX and its connection with SERT. Specifically, miR-16 regulates the expression of SERT [27,140], resilience to stress, responds to FLX administration [45], and modifies inflammation [27,28]. 5-HT also regulates key functions in peripheral tissues, such as the immune system. Several immune cells, such as T cells, macrophages, mast cells, dendritic cells, and platelets produce, store, respond, and/or transport serotonin [[141], [142], [143]]). On the other hand, the brain tissue of suicidal patients shows altered expressions of miR-16, as well as down-regulation of its concentration in serum [144], which may also be related to immune cell functions. Chronic FLX treatment increases miR-16 levels in the serotonergic raphe nuclei, thus reducing SERT expression [27]. In this regard, we observed a similar response with a decrease in miR-16 expression in the CUMS group and an increase after FLX treatment. Other studies indicate that the antidepressant effects of FLX appear to involve neurotransmitter synthesis and anti-inflammatory action that may promote hippocampal neuroplasticity by increasing –a protein related to neuronal plasticity in the hippocampus called GAP-43 as seen on a rat model of depression [145]– and that FLX treatment can improve the depressive behavior exhibited.
In our results, CUMS induced a reduction in the miRNAs analyzed that, in other scenarios, has been reported to promote microglial reactivity and produce neuroinflammation [153] in a process that contributes to depression. FLX may also have an effect by reducing the expression of pro-inflammatory cytokines such as IL -1β, IFN -γ, and TNF-α [152] that could regulate the expression of miR-21, -146a, and −155, all of which are involved in microglial activation. This is because all miRNAs, except for −144, were found to increase in the stressed rats with FLX treatment (CUMS + FLX group). Paradoxically, miR-144 decreased in all the experimental groups (FLX, CUMS, CUMS + FLX). It has been reported that miR-144-3b decreased in rats exposed to CUMS but increased due to 7-chloroquinurenic acid, an NMDA receptor antagonist that is a potential rapid antidepressant [153]. miR-144 has been associated with the expression of proteins related to GABAergic, glutamatergic, and dopaminergic synapses [154,155] that control target genes or pathways involved in neurite growth, neurogenesis, and ERK and Wnt/β-catenin signaling [156]. FLX not only inhibits SERT, but simultaneously reduces the release of glutamate and GABA [157], suggesting that these miRNA may be altered upon FLX administration, but remained low in the FLX + CUMS group. It is important to note that our evaluation of miRNAs was performed in plasma vesicles, which may explain the contrasts with other studies that evaluated their presence in the cerebral cortex and hippocampus. It is also well known that numerous stimuli associated with depression can trigger microglial activation, such as peripheral or central inflammatory foci and stress-related conditions due to an increase in glucocorticoids via the HPA axis, which have been reported to activate microglia [143,144] or cause psychological stress that, in turn, promotes microglial activation through the release of brain alarmines [145]. CUMS increases plasma concentrations of IL -6, CRP, and TNF-α and induces depression-like behavior [144], while in animal models, cytokines induce depression-like behaviors [146,147]. In addition, miR-146a increases in microglia and disrupts the normal inflammatory response, including the NF-kB and JAK-STAT signaling pathways, which are activated by both TLR2 and TLR [148] and overexpression of miR-146b-3p, which inhibited the production of inflammatory cytokines in microglia [149]. Moreover, miR-21 and -146a were found to be transferred to the microglia in glioma EVs where they promoted microglial activation [150,151].
5. Conclusion
Stress-induced changes in NA, DA, and 5-HT levels in the hippocampus and prefrontal cortex, structures related to higher CORT levels, decreased body weight, sucrose intake preference, and total fluid intake decreased levels of all the miRNAs evaluated. FLX remains the most important antidepressant treatment, but one that is known to have effects on numerous mechanisms. The therapeutic actions of SERT-targeted antidepressants exert their function by blocking this substance, thereby increasing 5-HT levels in the synapses of serotonergic projection terminals. In our study, FLX reduced corticosterone levels and had an antidepressant effect on the FST, but modified neurotransmitter levels only minimally and produced harmful effects on body weight. Furthermore, because FLX increased the expression of miRNAs as part of its antidepressant effect, it also regulates both neuroinflammation and serotoninergic neurotransmission through miRNAs, as we determined for miR-16.
Author contribution statement
M. Maetzi Estévez-Cabrera; Gilberto Pérez-Sánchez; Adrian Hernández-Diazcouder; J. Luis Cortes-Altamirano: Performed the experiments.
Fausto Sánchez-Muñoz: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Lenin Pavón; Soria-Fregoso; Alfonso Alfaro-Rodríguez: Contributed reagents, materials, analysis tools or data.
Herlinda Bonilla-Jaime: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr Herlinda Bonilla Jaime was supported by Universidad Autónoma Metropolitana unidad Iztapalapa.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
References
- 1.Korte S.M., Prins J., Krajnc A.M., Hendriksen H., Oosting R.S., Westphal K.G., Korte-Bouws G.A., Olivier B. The many different faces of major depression: it is time for personalized medicine. Eur. J. Pharmacol. 2015 Dec;753:88–104. doi: 10.1016/j.ejphar.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 2.WHO . 2020. Depression [WWW document]https://www.who.int/news-room/fact-sheets/detail/depression [Google Scholar]
- 3.Romanczuk-Seiferth N., Pöhland L., Mohnke S., Garbusow M., Erk S., Haddad L., Grimm O., Tost H., Meyer-Lindenberg A., Walter H., Wüstenberg T., Heinz A. Larger amygdala volume in first-degree relatives of patients with major depression. NeuroImage Clin. 2014 Jun;5:62–68. doi: 10.1016/j.nicl.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B.-J., Friston K., Mody M., Wang H.-N., Lu H.-B., Hu D.-W. A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci. Ther. 2018 Nov;24(11):1004–1019. doi: 10.1111/cns.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scopinho A.A., Scopinho M., Lisboa S.F., Correa F.M. de A., Guimarães F.S., Joca S.R.L. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behav. Brain Res. 2010 Dec;214(2):437–442. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Willner P., Bergman J., Vanderschuren L., Ellenbroek B. The behavioural pharmacology of stress. Behav. Pharmacol. 2014 Sep;25(5–6):337–339. doi: 10.1097/FBP.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 7.He L.W., Zeng L., Tian N., Li Y., He T., Tan D.M., Zhang Q., Tan Y. Optimization of food deprivation and sucrose preference test in SD rat model undergoing chronic unpredictable mild stress. Anim. Models Exp. Med. 2020 Mar;3(1):69–78. doi: 10.1002/ame2.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu D., Xie K., Yang X., Gu J., Ge L., Wang X., Wang Z. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav. Brain Res. 2014 May;264:9–16. doi: 10.1016/j.bbr.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Cao B., Zhu J., Zuckerman H., Rosenblat J.D., Brietzke E., Pan Z., Subramanieapillai M., Park C., Lee Y., McIntyre R.S. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019 Jan;92:109–117. doi: 10.1016/j.pnpbp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 10.López-López A.L., Jaime H.B., Escobar Villanueva M., del C., Padilla M.B., Palacios G.V., Aguilar F.J.A. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol. Behav. 2016;161:15–23. doi: 10.1016/j.physbeh.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Katz R.J., Sibel M. Animal model of depression: tests of three structurally and pharmacologically novel antidepressant compounds. Pharmacol. Biochem. Behav. 1982 Jun;16(6):973–977. doi: 10.1016/0091-3057(82)90055-7. [DOI] [PubMed] [Google Scholar]
- 12.Franceschelli A., Herchick S., Thelen C., Papadopoulou-Daifoti Z., Pitychoutis P.M. Sex differences in the chronic mild stress model of depression. Behav. Pharmacol. 2014 Sep;25(5–6):372–383. doi: 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 13.Lu T.X., Rothenberg M.E. MicroRNA. J. Allergy Clin. Immunol. 2018 Apr;141(4):1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C.-C., Tsai M.-C., Lee C.-T., Sun M.-H., Huang T.-L. Antidepressant treatment increased serum miR-183 and miR-212 levels in patients with major depressive disorder. Psychiatr. Res. 2018 Dec;270:232–237. doi: 10.1016/j.psychres.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Lopizzo N., Zonca V., Cattane N., Pariante C.M., Cattaneo A. miRNAs in depression vulnerability and resilience: novel targets for preventive strategies. J. Neural. Transm. 2019 Sep;126(9):1241–1258. doi: 10.1007/s00702-019-02048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellwig S., Brioschi S., Dieni S., Frings L., Masuch A., Blank T., Biber K. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 2016 Jul;55:126–137. doi: 10.1016/j.bbi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Brites D., Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front. Cell. Neurosci. 2015 Dec;9:1–20. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haider B.A., Baras A.S., McCall M.N., Hertel J.A., Cornish T.C., Halushka M.K. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLoS One. 2014 Feb;9(2):1–11. doi: 10.1371/journal.pone.0089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L., Hua M., Liu C., He N., Li Z., Ma D. The aberrant expression of microRNAs and correlations with T cell subsets in patients with immune thrombocytopenia. Oncotarget. 2016 Nov;7(47):76453–76463. doi: 10.18632/oncotarget.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saba R., Sorensen D.L., Booth S.A. MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ti D., Hao H., Fu X., Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 2016 Dec;59(12):1305–1312. doi: 10.1007/s11427-016-0240-4. [DOI] [PubMed] [Google Scholar]
- 22.Darabi F., Aghaei M., Movahedian A., Pourmoghadas A., Sarrafzadegan N. The role of serum levels of microRNA-21 and matrix metalloproteinase-9 in patients with acute coronary syndrome. Mol. Cell. Biochem. 2016 Nov;422(1–2):51–60. doi: 10.1007/s11010-016-2805-z. [DOI] [PubMed] [Google Scholar]
- 23.Garo L.P., Murugaiyan G. Contribution of MicroRNAs to autoimmune diseases. Cell. Mol. Life Sci. 2016 May;73(10):2041–2051. doi: 10.1007/s00018-016-2167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y.-K., Kim B., Kim V.N. Re-evaluation of the roles of DROSHA, Exportin 5, and DICER in microRNA biogenesis. Proc. Natl. Acad. Sci. USA. 2016 Mar;113(13):E1881–E1889. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenoglio C., Ridolfi E., Galimberti D., Scarpini E. MicroRNAs as active players in the pathogenesis of multiple sclerosis. Int. J. Mol. Sci. 2012 Oct;13(10):13227–13239. doi: 10.3390/ijms131013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thounaojam M.C., Kaushik D.K., Kundu K., Basu A. MicroRNA-29b modulates Japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor alpha-induced protein 3. J. Neurochem. 2014 Apr;129(1):143–154. doi: 10.1111/jnc.12609. [DOI] [PubMed] [Google Scholar]
- 27.Baudry A., Mouillet-Richard S., Schneider B., Launay J.-M., Kellermann O. MiR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010 Sep;329(5998):1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 28.Chao Y.-L., Chen C.-H. An introduction to microRNAs and their dysregulation in psychiatric disorders. Tzu Chi Med. J. 2013 Mar;25(1):1–7. doi: 10.1016/j.tcmj.2012.12.003. [DOI] [Google Scholar]
- 29.Saeedi S., Nagy C., Ibrahim P., Théroux J.F., Wakid M., Fiori L.M., Yang J., Rotzinger S., Foster J.A., Mechawar N., Kennedy S.H., Turecki G. Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol. Psychiatr. 2021 Aug;26:7417–7424. doi: 10.1038/s41380-021-01255-2. [DOI] [PubMed] [Google Scholar]
- 30.Harro J. Animal models of depression: pros and cons. Cell Tissue Res. 2019 Jul;377(1):5–20. doi: 10.1007/s00441-018-2973-0. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Zhao J., Fan X., Guo W. Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Front. Psychiatr. 2019 May;10:1–8. doi: 10.3389/fpsyt.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Block S.G., Nemeroff C.B. Emerging antidepressants to treat major depressive disorder. Asian J. Psychiatry. 2014 Dec;12:7–16. doi: 10.1016/j.ajp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Hamon M., Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013 Aug;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019 Apr;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Khedr L.H., Nassar N.N., Rashed L., El-Denshary E.D., Abdel-Tawab A.M. TLR4 signaling modulation of PGC1-α mediated mitochondrial biogenesis in the LPS-Chronic mild stress model: effect of fluoxetine and pentoxiyfylline. Life Sci. 2019 Dec;239 doi: 10.1016/j.lfs.2019.116869. [DOI] [PubMed] [Google Scholar]
- 36.Lim C.-M., Kim S.-W., Park J.-Y., Kim C., Yoon S.H., Lee J.-K. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J. Neurosci. Res. 2009 Mar;87(4):1037–1045. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- 37.Shumake J., Colorado R.A., Barrett D.W., Gonzalez-Lima F. Metabolic mapping of the effects of the antidepressant fluoxetine on the brains of congenitally helpless rats. Brain Res. 2010 Jul;1343:218–225. doi: 10.1016/j.brainres.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu F., Zhang G., Yin J., Zhang Q., Ge M.Y., Peng L., Wang S., Li Y. Fluoxetine mitigating late-stage cognition and neurobehavior impairment induced by cerebral ischemia reperfusion injury through inhibiting ERS-mediated neurons apoptosis in the hippocampus. Behav. Brain Res. 2019 Sep;370:1–8. doi: 10.1016/j.bbr.2019.111952. [DOI] [PubMed] [Google Scholar]
- 39.Alboni S., van Dijk R.M., Poggini S., Milior G., Perrotta M., Drenth T., Brunello N., Wolfer D.P., Limatola C., Amrein I., Cirulli F., Maggi L., Branchi I. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatr. 2017;22(4):552–561. doi: 10.1038/mp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcinkute M., Afshinjavid S., Fatokun A.A., Javid F.A. Fluoxetine selectively induces p53-independent apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2019 Aug;857:1–11. doi: 10.1016/j.ejphar.2019.172441. [DOI] [PubMed] [Google Scholar]
- 41.Wang J.M., Yang L.H., Zhang Y.Y., Niu C.L., Cui Y., Feng W.S., Wang G.F. BDNF and COX-2 participate in anti-depressive mechanisms of catalpol in rats undergoing chronic unpredictable mild stress. Physiol. Behav. 2015 Nov;151:360–368. doi: 10.1016/j.physbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Caiaffo V., Oliveira B.D.R., de Sá F.B., Evêncio Neto J. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol. Res. Perspect. 2016 Apr;4(3):1–9. doi: 10.1002/prp2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudryashov N.V., Kalinina T.S., Shimshirt A.A., Volkova A.V., Narkevich V.B., Naplekova P.L., Kasabov K.A., Kudrin V.S., Voronina T.A., Fisenko V.P. The behavioral and neurochemical aspects of the interaction between antidepressants and unpredictable chronic mild stress. Acta Naturae. 2020 Jan;12(1):63–72. doi: 10.32607/actanaturae.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurawek D., Kusmider M., Faron-Gorecka A., Gruca P., Pabian P., Kolasa M., Solich J., Szafran-Pilch K., Papp M., Dziedzicka-Wasylewska M. Time-dependent miR-16 serum fluctuations together with reciprocal changes in the expression level of miR-16 in mesocortical circuit contribute to stress resilient phenotype in chronic mild stress – an animal model of depression. Eur. Neuropsychopharmacol. 2016 Jan;26(1):23–36. doi: 10.1016/j.euroneuro.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Miao N., Jin J., Kim S.-N., Sun T. Hippocampal microRNAs respond to administration of antidepressant Fluoxetine in adult mice. Int. J. Mol. Sci. 2018 Feb;19:1–15. doi: 10.3390/ijms19030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agroalimentaria S.N. de S. 2001. Inocuidad y Calidad, n.d. NOM-062-ZOO-1999.http://www.gob.mx/senasica/documentos/nom-062-zoo-1999 [Google Scholar]
- 47.National Research Council (US) The National Academies Collection: Reports Funded by National Institutes of Health. eighth ed. National Academies Press (US); Washington (DC): 2011. Committee for the update of the Guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals; p. 209. [Google Scholar]
- 48.Yang Y., Zhiying H., Xiaoxue D., Henry D., Xue H., Marong F. MiR-16 and Fluoxetine.Both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Front. Neurosci. 2017 Jul;11:428. doi: 10.3389/fnins.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Vázquez-Palacios G., Bonilla-Jaime H., Velázquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with fluoxetine [correction of flouxetine] Pharmacol. Biochem. Behav. 2004;78:165–169. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Detke M.J., Rickels M., Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl.) 1995 Sep;121(1):66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 51.Cryan J.F. Elsevier; 2010. Depression; pp. 382–386. [DOI] [Google Scholar]
- 52.Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. J. Vis. Exp. 2012 Jan;59:1–5. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y.L., Han Q.Q., Gong W.Q., Pan D.H., Wang L.Z., Hu W., Yang M., Li B., Yu J., Liu Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018 Jan;15(1):1–14. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354(1):155–169. doi: 10.1007/s00441-013-1664-0. [DOI] [PubMed] [Google Scholar]
- 55.Heffner T.G., Hartman J.A., Seiden L.S. A rapid method for the regional dissection of the rat brain. Pharmacol. Biochem. Behav. 1980 Sep;13(3):453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- 56.Spijker S. In: Neuroproteomics, Neuromethods. Li K.W., editor. Humana Press; Totowa, New Jersey: 2011. Dissection of Rodent Brain Regions; pp. 13–26. [DOI] [Google Scholar]
- 57.Defazio R., Criado A., Zantedeschi V., Scanziani E. Neuroanatomy-based matrix-guided trimming protocol for the rat brain. Toxicol. Pathol. 2015 Feb;43(2):249–256. doi: 10.1177/0192623314538345. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y., Hu Z., Du X., Davies H., Huo X., Fang M. miR-16 and fluoxetine both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Front. Neurosci. 2017 Jul;11:428. doi: 10.3389/fnins.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Golbidi S., Frisbee J.C., Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015 Jun;308(12):1–53. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 60.Koprdova R., Bögi E., Belovičová K., Sedláčkováet N., Okuliarová M., Ujházy E., Mach M. al. Chronic unpredictable mild stress paradigm in male Wistar rats: effect on anxiety- and depressive-like behavior. Neuroendocrinol. Lett. 2016 Dec;37:103–110. [PubMed] [Google Scholar]
- 61.Martinotti G., Sepede G., Signorelli M., Aguglia E., Di Giannantonio M. Efficacy and safety of fluoxetine monotherapy in bipolar depression: a systematic review. Expet Opin. Pharmacother. 2013 Jun;14(8):1065–1075. doi: 10.1517/14656566.2013.783014. [DOI] [PubMed] [Google Scholar]
- 62.Liu M.Y., Yin C.Y., Zhu L.J., Zhu X.H., Xu C., Luo C.X., Chen H., Zhu D.Y., Zhou Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018 Jul;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 63.Henningsen K., Andreasen J.T., Bouzinova E.V., Jayatissa M.N., Jensen M.S., Redrobe J.P., Wiborg O. Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behav. Brain Res. 2009 Mar;198(1):136–141. doi: 10.1016/j.bbr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 64.Grønli J., Murison R., Fiske E., Bjorvatn B., Sørensen E., Portas C.M., Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav. 2005 Mar;84(4):571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Liu M.Y., Yin C.Y., Zhu L.J., Zhu X.H., Xu C., Luo C.X., Chen H., Zhu D.Y., Zhou Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018 Jul;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 66.Liu X.J., Zhou Y.Z., Li Z.F., Cui J., Li Z.Y., Gao X.X., Sun H.F., Zhang L.Z., Du G.H., Qin X.M. Anti-depressant effects of Xiaoyaosan on rat model of chronic unpredictable mild stress: a plasma metabonomics study based on NMR spectroscopy. J. Pharm. Pharmacol. 2012 Apr;64(4):578–588. doi: 10.1111/j.2042-7158.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 67.Li P., Huang W., Yan Y.N., Cheng W., Liu S., Huang Y., Chen W., Chen Y.P., Gao Y., Lu W., Xu Y., Meng X. Acupuncture can play an antidepressant role by regulating the intestinal microbes and neurotransmitters in a rat model of depression. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2021 May;27:1–12. doi: 10.12659/MSM.929027. https//doi.org/10.12659/MSM.929027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthews K., Forbes N., Reid I.C. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol. Behav. 1995 Feb;57(2):241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 69.Torres-González C., López-Espinoza A., Martínez A.G., Franco K., Díaz F., Sosa G.A., Aguilera V., et al. Consumo de alimento y endulzantes bajo condiciones de estrés crónico en ratas. Rev. Mex. Análisis Conducta. 2009 Sep;35:133–147. [Google Scholar]
- 70.D'Aquila P.S., Newton J., Willner P. Diurnal variation in the effect of chronic mild stress on sucrose intake and preference. Physiol. Behav. 1997 Aug;62(2):421–426. doi: 10.1016/S0031-9384(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 71.Reid M., Hammersley R., Hill A.J., Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br. J. Nutr. 2007 Jan;97(1):193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 72.Remus J.L., Stewart L.T., Camp R.M., Novak C.M., Johnson J.D. Interaction of metabolic stress with chronic mild stress in altering brain cytokines and sucrose preference. Behav. Neurosci. 2015;129(3):321–330. doi: 10.1037/bne0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arndt D.L., Peterson C.J., Cain M.E. Differential rearing alters forced swim test behavior, fluoxetine efficacy, and post-test weight gain in male rats. PLoS One. 2015 Jul;10(7):1–21. doi: 10.1371/journal.pone.0131709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song T., Wu H., Li R., et al. Repeated fluoxetine treatment induces long-lasting neurotrophic changes in the medial prefrontal cortex of adult rats. Behav. Brain Res. 2019 Jun;365:114–124. doi: 10.1016/j.bbr.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Afkhami-Ardekani M., Sedghi H. Effect of fluoxetine on weight reduction in obese patients. Indian J. Clin. Biochem. 2005 Jan;20(1):135–138. doi: 10.1007/BF02893059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uphouse L., Hensler J.G., Sarkar J., Grossie B. Fluoxetine disrupts food intake and estrous cyclicity in Fischer female rats. Brain Res. 2006 Feb;1072(1):79–90. doi: 10.1016/j.brainres.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 77.Bataineh H.N., Daradka T. Effects of long-term use of fluoxetine on fertility parameters in adult male rats. Neuroendocrinol. Lett. 2007 Jun;28(3):321–325. [PubMed] [Google Scholar]
- 78.Aggarwal A., Jethani S.L., Rohatgi R.K., Kalra J. Effects of fluoxetine on testis of albino rats – a histological assessment. Int. J. Sci. Eng. Res. 2012 Jul;3(7):1–5. [Google Scholar]
- 79.Aggarwal A., Jethani S.L., Rohatgi R.K., Kalra J. Selective serotonin Re-uptake inhibitors (SSRIs) induced weight changes: a dose and duration dependent study on albino rats. J. Clin. Diagn. Res. 2016 Mar;10(3):AF01–AF03. doi: 10.7860/JCDR/2016/16482.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Byrd R.A., Markham J.K. Developmental toxicology studies of fluoxetine hydrochloride administered orally to rats and rabbits. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1994 May;22(4):511–518. doi: 10.1006/faat.1994.1058. [DOI] [PubMed] [Google Scholar]
- 81.Westbroek I., Waarsing J.H., van Leeuwen J.P., Waldum H., Reseland J.E., Weinans H., Syversen U., Gustafsson B.I. Long-term fluoxetine administration does not result in major changes in bone architecture and strength in growing rats. J. Cell. Biochem. 2007 May;101(2):360–368. doi: 10.1002/jcb.21177. [DOI] [PubMed] [Google Scholar]
- 82.Aggarwal A., Jethani S.L., Rohatgi R.K., Kalra J. Effect of fluoxetine on epididymis of albino rats: a histological study. Int. J. Sci. Eng. Res. 2013 Aug;4(8):1457–1462. [Google Scholar]
- 83.Lyte M., Daniels K.M., Schmitz-Esser S. Fluoxetine-induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ. 2019 Jan;7:1–19. doi: 10.7717/peerj.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yankelevitch-Yahav R., Franko M., Huly A., Doron R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015 Mar;97:1–7. doi: 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahar I., Bambico F.R., Mechawar N., Nobrega J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014 Jan;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Sakr H.F., Abbas A.M., Elsamanoudy A.Z., Ghoneim F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015 Aug;66(4):515–527. [PubMed] [Google Scholar]
- 87.Filho C.B., Jesse C.R., Donato F., Giacomeli R., Del Fabbro L., da Silva Antunes M., de Gomes M.G., Goes A.T., Boeira S.P., Prigol M., Souza L.C. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015 Mar;289:367–380. doi: 10.1016/j.neuroscience.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 88.Li Q., Huang F., Liu J., Zhao Y.H., Zhang M., Chen Y.J. Psychological stress alters extracellular matrix metabolism in mandibular condylar cartilage. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 2017;20(3):125–135. doi: 10.3290/j.cjdr.a38767. [DOI] [PubMed] [Google Scholar]
- 89.Bonilla-Jaime H., Renata-Márquez S., Arteaga-Silva M., Hernández-González M. Circadian activity of corticosterone in an animal model of depression: response to muscarinic cholinergic stimulation. Physiol. Behav. 2010;100(4):311–315. doi: 10.1016/j.physbeh.2010.03.002. https://10.1016/j.physbeh.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 90.Jazayeri S., Keshavarz S.A., Tehrani-Doost M., Djalali M., Hosseini M., Amini H., Chamari M., Djazayery A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatr. Res. 2010 Jun;178(1):112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 91.Piwowarska J., Chimiak A., Matsumoto H., Dziklińska A., Radziwoń-Zaleska M., Szelenberger W., Pachecka J. Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatr. Res. 2012 Aug;198(3):407–411. doi: 10.1016/j.psychres.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 92.Vázquez-Palacios G., Bonilla-Jaime H., Velázquez-Moctezuma J. Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005 Jan;29(1):39–46. doi: 10.1016/j.pnpbp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 93.Hjorth S., Bengtsson H.J., Kullberg A., Carlzon D., Peilot H., Auerbach S.B. Serotonin autoreceptor function and antidepressant drug action. J. Psychopharmacol. 2000 Jun;14(2):177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- 94.Dremencov E., Gur E., Lerer B., Newman M.E. Subchronic fluoxetine administration to rats: effects on 5-HT autoreceptor activity as measured by in vivo microdialysis. Eur. Neuropsychopharmacol. 2000 Jul;10(4):229–236. doi: 10.1016/s0924-977x(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 95.Wang X.-L., Gao J., Wang X.-Y., Mu X.-F., Wei S., Xue L., Qiao M.Q. Treatment with Shuyu capsule increases 5-HT1AR level and activation of cAMP-PKA-CREB pathway in hippocampal neurons treated with serum from a rat model of depression. Mol. Med. Rep. 2018 Mar;17(3):3575–3582. doi: 10.3892/mmr.2017.8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X.H., Song S.Q., Xu Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatric Dis. Treat. 2017 Oct;13:2727–2736. doi: 10.2147/NDT.S150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roychowdhury S., Haas H., Anderson E.G. 5-HT1A and 5-HT4 receptor colocalization on hippocampal pyramidal cells. Neuropharmacology. 1994;33(3–4):551–557. doi: 10.1016/0028-3908(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 98.Beck S.G., Choi K.C., List T.J. Comparison of 5-hydroxytryptamine 1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Therapeut. 1992;263:350–359. [PubMed] [Google Scholar]
- 99.Mueller N.K., Beck S.G. Corticosteroids alter the 5-HT1A receptor-mediated response in CA1 hippocampal pyramidal cells. Neuropsychopharmacology. 2000 Oct;23(4):419–427. doi: 10.1016/S0893-133X(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 100.Bijak M., Zahorodna A., Tokarski K. Opposite effects of antidepressants and corticosterone on the sensitivity of hippocampal CA1 neurons to 5-HT1A and 5-HT4 receptor activation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001 May;363(5):491–498. doi: 10.1007/s002100000389. [DOI] [PubMed] [Google Scholar]
- 101.Czyrak A., Mackowiak M., Chocyk A., Fijal K., Tokarski K., Bijak M., Wedzony K. Prolonged corticosterone treatment alters the responsiveness of 5-HT1A receptors to 8-OH-DPAT in rat CA1 hippocampal neurons. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002 Oct;366(4):357–367. doi: 10.1007/s00210-002-0586-2. [DOI] [PubMed] [Google Scholar]
- 102.Burke T.F., Advani T., Adachi M., Monteggia L.M., Hensler J.G. 2013 Apr. Sensitivity of Hippocampal 5-HT1A Receptors to Mild Stress in BDNF-Deficient Mice Neuropsychopharmacol; pp. 631–645. [DOI] [PubMed] [Google Scholar]
- 103.Middlemiss D.N., Price G.W., Watson J.M. Serotonergic targets in depression. Curr. Opin. Pharmacol. 2002 Feb;2(1):18–22. doi: 10.1016/s1471-4892(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 104.Pariante C.M., Kim R.B., Makoff A., Kerwin R.W. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br. J. Pharmacol. 2003;139(6):1111–1118. doi: 10.1038/sj.bjp.0705357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pariante C.M., Thomas S.A., Lovestone S., Makoff A., Kerwin R.W. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423–447. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Horrillo I., Ortega J.E., Diez-Alarcia R., Urigüen L., Meana J.J. Chronic fluoxetine reverses the effects of chronic corticosterone treatment on α2-adrenoceptors in the rat frontal cortex but not locus coeruleus. Neuropharmacology. 2019 Nov;158 doi: 10.1016/j.neuropharm.2019.107731. [DOI] [PubMed] [Google Scholar]
- 107.Raineki C., Hellemans K.G., Bodnar T., Lavigne K.M., Ellis L., Woodward T.S., Weinberg J. Neurocircuitry underlying stress and emotional regulation in animals prenatally exposed to alcohol and subjected to chronic mild stress in adulthood. Front. Endocrinol. 2014 Feb;5:1–14. doi: 10.3389/fendo.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Willner P. The chronic mild stress (CMS) model of depression: history,evaluation and usage. Neurobiol. Stress. 2016 Aug;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vancassel S., Leman S., Hanonick L., Denis S., Roger J., Nollet M., Bodard S., Kousignian I., Belzung C., Chalon S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J. Lipid Res. 2008;49(2):340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- 110.Yalcin I., Coubard S., Bodard S., Chalon S., Belzung C. Effects of 5,7-dihydroxytryptamine lesion of the dorsal raphe nucleus on the antidepressant-like action of tramadol in the unpredictable chronic mild stress in mice. Psychopharmacology (Berl.) 2008 Nov;200(4):497–507. doi: 10.1007/s00213-008-1227-3. [DOI] [PubMed] [Google Scholar]
- 111.Lu Q., Mouri A., Yang Y., Kunisawa K., Teshigawara T., Hirakawa M., Mori Y., Yamamoto Y., Libo Z., Nabeshima T., Saito K. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 2019;372:1–10. doi: 10.1016/j.bbr.2019.112053. [DOI] [PubMed] [Google Scholar]
- 112.Albert P.R., Vahid-Ansari F., Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front. Behav. Neurosci. 2014 Jun;8:1–13. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo D., Patrick C.J., Kennealy P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008 Dec;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer A.G., Ullsperger M. An update on the role of serotonin and its interplay with dopamine for reward. Front. Hum. Neurosci. 2017 Oct;11:1–10. doi: 10.3389/fnhum.2017.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koob G.F., Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 116.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pruessner J.C., Champagne F., Meaney M.J., Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J. Neurosci. 2004 Mar;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fujino K., Yoshitake T., Inoue O., Ibii N., Kehr J., Ishida J., Nohta H., Yamaguchi M. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci. Lett. 2002 Mar;320:91–95. doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- 119.Renard C.E., Dailly E., David D.J.P., Hascoet M., Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam. Clin. Pharmacol. 2003 Aug;17(4):449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 120.Rutter J.J., Gundlah C., Auerbach S.B. Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine Neurosci. Letture. 1994 Apr;171(1–2):183–186. doi: 10.1016/0304-3940(94)90635-1. [DOI] [PubMed] [Google Scholar]
- 121.Blardi P., De Lalla A., Leo A., Auteri A., Iapichino S., Di Muro A., Dell'Erba A., Castrogiovanni P. Serotonin and fluoxetine levels in plasma and platelets after fluoxetine treatment in depressive patients. Psychopharmacol. 2002 Apr;22(2):131–136. doi: 10.1097/00004714-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Haase J., Killian A.M., Magnani F., Williams C. Regulation of the serotonin transporter by inter- acting proteins. Biochem. Soc. Trans. 2001 Nov;29:722–728. doi: 10.1042/BST0290722. [DOI] [PubMed] [Google Scholar]
- 123.Cheetham S.C., Viggers J.A., Slater N.A., Heal D.J., Buckett W.R. [3H] paroxetine binding in rat frontal cortex strongly correlates with [3H] 5-HT uptake: effect of administration of various antidepressant treat- ments. Neuropharmacology. 1993 Apr;32(8):737–743. doi: 10.1016/0028-3908(93)90181-2. [DOI] [PubMed] [Google Scholar]
- 124.Lesch K.P., Aulakh C.S., Wolozin B.L., Tolliver T.J., Hill J.L., Murphy D.L. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Mol. Brain Res. 1993;17:31–35. doi: 10.1016/0169-328X(93)90069-2. [DOI] [PubMed] [Google Scholar]
- 125.Spurlock G., Buckland P., O'Donovan M., McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5-HT transporter. Neuropharmacology. 1994;33:43–440. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 126.López J.F., Chalmers D.T., Vázquez D.M., Watson S.J., Akil H. Serotonin transporter mRNA in rat brain is regulated by classical antidepressants. Biol. Psychiatr. 1994;35:287–290. doi: 10.1016/0006-3223(94)91262-9. [DOI] [PubMed] [Google Scholar]
- 127.Linnet K., Koed K., Wiborg O., Gregersen N. Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Res. 1995;697:251–253. doi: 10.1016/0006-8993(95)00906-7. [DOI] [PubMed] [Google Scholar]
- 128.Descarries L., Riad M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Phil. Trans. R. Soc. B. 2012 Sep;367:2416–2425. doi: 10.1098/rstb.2011.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bel N., Artigas F. Chronic treatment with flu- voxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse. 1993 Nov;15(3):243–245. doi: 10.1002/syn.890150310. [DOI] [PubMed] [Google Scholar]
- 130.Cremers T.I., Spoelstra E.N., de Boer P., Bosker F.J., Mork A., den Boer J.A., Westerink B.H., Wikstrom H.V. Desensitisation of 5-HT auto- receptors upon pharmacokinetically monitored chronic treatment with citalopram. Eur. J. Pharmacol. 2000 Jun;397(2–3):351–357. doi: 10.1016/S0014-2999(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 131.Riad M., Rbah L., Verdurand M., Aznavour N., Zimmer L., Descarries L. Unchanged density of 5-HT1A autoreceptors on the plasma membrane of nucleus raphe dorsalis neurons in rats chronically treated with fluoxetine. Neurosci. 2008 Feb;151:692–700. doi: 10.1016/j.neuroscience.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 132.Invernizzi R., Bramante M., Samanin R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995 Oct;696(1–2):62–66. doi: 10.1016/0006-8993(95)00730-E. [DOI] [PubMed] [Google Scholar]
- 133.Sarkissian C.F., Wurtman R.J., Morse A.N., Gleason R. Effects of fluoxetine or D-fenfluramine on serotonin release from, and levels in, rat frontal cortex. Brain Res. 1990 Oct;529(1–2):294–301. doi: 10.1016/0006-8993(90)90840-8. [DOI] [PubMed] [Google Scholar]
- 134.Gobert A., Rivet J.M., Cistarelli J.M., Millan M.J. Buspirone enhances duloxetine- and fluoxetine-induced increases in dialysate levels of dopamine and noradrenaline, but not serotonin, in the frontal cortex of freely moving rats. J. Neurochem. 1997 Mar;68(3):1326–1329. doi: 10.1046/j.1471-4159.1997.68031326.x. [DOI] [PubMed] [Google Scholar]
- 135.Straub R.H., Cutolo M. Psychoneuroimmunology-developments in stress research. Wien Med. Wochenschr. 2018 Mar;168(3–4):76–84. doi: 10.1007/s10354-017-0574-2. [DOI] [PubMed] [Google Scholar]
- 136.Chen R.J., Kelly G., Sengupta A., Heydendael W., Nicholas B., Beltrami S., Luz S., Peixoto L., Abel T., Bhatnagar S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015 Oct;305:36–48. doi: 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 137.Andolina D., Di Segni M., Bisicchia E., D'Alessandro F., Cestari V., Ventura A., Concepcion C., Puglisi-Allegra S., Ventura R. Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology. 2016 Aug;107:305–316. doi: 10.1016/j.neuropharm.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bocchio-Chiavetto L., Maffioletti E., Bettinsoli P., Giovannini C., Bignotti S., Tardito D., Corrada D., Milanesi L., Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur. Neuropsychopharmacol. 2013 Jul;23(7):602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 139.O'Connor R.M., Dinan T.G., Cryan J.F. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol. Psychiatr. 2012 Apr;17(4):359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- 140.Launay J.M., Mouillet-Richard S., Baudry A., Pietri M., Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl. Psychiatry. 2011 Nov;1(11):e56. doi: 10.1038/tp.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu H., Denna T.H., Storkersen J.N., Gerriets V.A. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol. Res. 2019 Feb;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 142.Catena-Dell'Osso M., Rotella F., Dell'Osso A., Fagiolini A., Marazziti D. Inflammation, serotonin and major depression. Curr. Drug Targets. 2013 May 1;14(5):571–577. doi: 10.2174/13894501113149990154. [DOI] [PubMed] [Google Scholar]
- 143.Shajib M.S., Khan W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015 Mar;213(3):561–574. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 144.Gheysarzadeh A., Sadeghifard N., Afraidooni L., Pooyan F., Mofid M., Valadbeigi H., et al. Serum-based microRNA biomarkers for major depression: MiR-16, miR-135a, and miR-1202. J. Res. Med. Sci. 2018 Aug;23:69. doi: 10.4103/jrms.JRMS_879_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zavvari F., Nahavandi A., Goudarzi M. Fluoxetine attenuates stress-induced depressive-like behavior through modulation of hippocampal GAP43 and neurogenesis in male rats. J. Chem. Neuroanat. 2020 Jan;103 doi: 10.1016/j.jchemneu.2019.101711. [DOI] [PubMed] [Google Scholar]
- 146.Guo Y., Hong W., Wang X., Zhang P., Körner H., Tu J., Wei W. MicroRNAs in microglia: how do MicroRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front. Mol. Neurosci. 2019 May;12:1–14. doi: 10.3389/fnmol.2019.00125. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao Y., Shang P., Wang M., Xie M., Liu J. Neuroprotective effects of fluoxetine against chronic stress-induced neural inflammation and apoptosis: involvement of the p38 activity. Front. Physiol. 2020;11:1–11. doi: 10.3389/fphys.2020.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu B.-B., Luo L., Liu X.-L., Geng D., Liu Q., Yi L.-T. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology (Berl.) 2015 Feb;232(3):541–550. doi: 10.1007/s00213-014-3690-3. [DOI] [PubMed] [Google Scholar]
- 149.Ma K., Xu A., Cui S., Sun M.-R., Xue Y.-C., Wang J.-H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry. 2016 Oct;6(10) doi: 10.1038/tp.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang X., Song S.-Q., Xu Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr. Dis. Treat. 2017;13:2727–2736. doi: 10.2147/NDT.S150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lazarevic V., Mantas I., Flais I., Svenningsson P. Fluoxetine suppresses glutamate- and GABA-mediated neurotransmission by altering SNARE complex. Int. J. Mol. Sci. 2019 Aug;20(17):1–15. doi: 10.3390/ijms20174247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sorrells S.F., Sapolsky R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007 Mar;21(3):259–272. doi: 10.1016/j.bbi.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maslanik T., Mahaffey L., Tannura K., Beninson L., Greenwood B.N., Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2013 Feb;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 154.Saba R., Gushue S., Huzarewich R.L., Manguiat K., Medina S., Robertson C., Booth S.A. MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo Y., Hong W., Wang X., et al. MicroRNAs in microglia: how do MicroRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front. Mol. Neurosci. 2019 May;12:1–14. doi: 10.3389/fnmol.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou R., Yuan P., Wang Y., Hunsberger J.G., Elkahloun A., Wei Y., Damschroder-Williams P., Du J., Chen G., Manji H.K. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lazarevic V, Mantas I, Flais I, Svenningsson P. Fluoxetine suppresses glutamate- and GABA-mediated neurotransmission by altering SNARE complex. Int. J. Mol. Sci. 2019;20(17):4247. doi: 10.3390/ijms20174247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.