Summary
Background
Respiratory viruses have been previously suspected to trigger invasive pneumococcal disease (IPD). After progressive non-pharmaceutical interventions (NPI) lifting, an unusual RSV outbreak has been observed in the Fall 2021, raising concerns about the possible consequences on IPD. We aimed to analyse the evolution of IPD incidence across age-groups since NPI lifting, and its temporal association with respiratory viral infections.
Methods
We conducted a time-series analysis using 1) population-based IPD surveillance data and 2) statistics from the laboratory surveillance network of respiratory viruses in the province of Quebec, Canada, from January 2013 to January 2022. The monthly IPD incidence was analysed by quasi-Poisson regression models across age-groups. The fraction of IPD incidence change potentially attributable to different viruses in 2021–2022 was estimated.
Findings
A total of 7712 IPD cases were included. After a major decrease in IPD incidence from April 2020, IPD rate started to increase in <5-year-old children in October 2021, exceeding the pre-NPI trend (+62%). This was temporally associated with an unusual surge in RSV cases (+53% versus pre-NPI trend). During this 2021–22 surge, the fraction of IPD attributable to RSV dynamics in children was 77% (95% CI [33–100]). By contrast, the IPD incidence in older age-groups remained low, and was temporally associated with influenza dynamics.
Interpretation
These results provide new evidence on the role of respiratory viruses in driving IPD dynamics, with possible differences between children and adults. In the coming future, the potential benefit of interventions targeting RSV, such as vaccines, for IPD prevention should be considered.
Funding
The study was supported by a grant from the Quebec Ministry of Health and Social Services (‘ministère de la Santé et des Services sociaux du Québec’). Publication was supported by a grant from “Fondation de l’Assistance Publique - Hôpitaux de Paris et de l’Alliance « Tous Unis contre le Virus » (Fondation de France/Institut Pasteur/APHP)”. N.O. was supported by the ESPID (European Society of Pediatric Infectious Diseases) 2021–2023 Fellowship Award and the 2022 ISPPD (International Symposium on Pneumococci and Pneumococcal Diseases) Robert Austrian Research award.
Keywords: Invasive pneumococcal disease, Pneumococcal conjugate vaccine, Respiratory viral infection, Time series analysis, Child, Respiratory syncytial virus
Research in context.
Evidence before this study
We searched PubMed for articles published in English up to February 15, 2022, using the terms “Invasive pneumococcal disease” and “respiratory viruses” or “RSV” or “influenza”. Several observational studies suggested that respiratory viruses, including RSV and influenza, may trigger invasive pneumococcal disease (IPD). This link has been reinforced by the strong IPD decrease following the quasi disappearance of respiratory viruses during non-pharmaceutical interventions (NPI) in 2020, while pneumococcal carriage remained unchanged. After progressive NPI lifting, respiratory viruses started to increase, and a major outbreak of RSV has been observed in the Fall 2021 in several countries, raising important concerns about the possible consequences on IPD.
Added value of this study
This time-series analysis of a prospective national surveillance of IPD and a range of respiratory viruses in Quebec from 2013 to 2022 shows a significant increase in IPD incidence in <5-year-old children since October 2021, temporally associated with an unusually early major RSV outbreak. The fraction of IPD increase potentially attributable to RSV in <5-year-old children was major, while other respiratory viruses accounted for a minor part of the IPD increase in this age-group. By contrast, IPD incidence in older age-groups remained low, and was temporally associated with influenza dynamics.
Implications of all the available evidence
This study shows that an unusual RSV outbreak may drive important IPD surge in young children. This strong association provides new evidence of the key role of respiratory viruses in driving IPD epidemiology, with possible differences between children and adults. This may inform further public health interventions targeting respiratory viruses, such as RSV vaccines, to reduce the burden of IPD beyond pneumococcal vaccines.
Background
Despite the use of pneumococcal conjugate vaccines, invasive pneumococcal disease (IPD) remains a major cause of morbidity and mortality worldwide especially in young children and older adults, with more than 300,000 deaths in children aged 1–59 months in 2015.1
Pathogenic mechanisms leading to IPD are complex. Viral infections, including respiratory syncytial virus (RSV) and influenza, have been suspected to trigger IPD.2, 3, 4 This hypothesis was mainly based on a similar seasonal pattern,2, 3, 4, 5 and on cohort studies showing an increased risk of IPD in children previously infected by RSV.6 Since 2020, unprecedented COVID-19-related non-pharmaceutical interventions (NPIs) led to a marked decrease in the circulation of most respiratory viruses, including RSV.7 Concomitantly, a major decrease in IPD incidence was observed in many countries.8 Unexpectedly, the pneumococcal carriage rate in children during the same period remained stable, highlighting the potential role of respiratory viruses in driving the IPD dynamics.9, 10, 11
The reduction of respiratory viruses during the implementation of stringent COVID-19-related NPIs raised concerns regarding a possible surge of these viruses following NPI lifting.12 The immune debt theory causing surges in the circulation of respiratory viruses has been supported by simulation studies,13 as well as by recent reports of major RSV outbreaks in the 2021–2022 winter.14 This observation led to concerns regarding a possible concomitant surge in IPD rates as a consequence of respiratory virus outbreaks,15 which may be confirmed by the recent IPD epidemiology in Quebec, Canada.
In this context, we aimed to analyse the recent change in IPD across age groups using an interrupted time-series approach, and the relationship between respiratory viruses and IPD dynamics.
Methods
We conducted an interrupted time series analysis based on a surveillance system covering the entire territory of Quebec, Canada over nine years.
Study population
The reference population consisted of residents in the province of Quebec, Canada (8.6 million in 2022). The study period extended from January 2013, to January 2022.
Pneumococcal vaccination program
Free vaccination with the 23-valent polysaccharide vaccine was introduced for persons 65 years of age and over in 2000, and uptake (at least one dose) is 60% with no major change during the study period.16 The seven-valent pneumococcal conjugate vaccine (PCV7) was offered to all children less than 5 years of age in December 2004, according to a 2 + 1 dose schedule.17 In June 2009, PCV7 was replaced by PCV10, followed by PCV13 in January 2011, and by PCV10 in May 2018, during the study period. In September 2020, a mixed schedule was implemented, with two doses of PCV10 offered at 2 and 4 months of age, followed by a booster dose of PCV13 at 12 months of age. There was no-catch-up program excepted when PCV7 was implemented in 2004. Vaccination coverage (≥3 doses) in children remained >90% from PCV implementation and up to 2019.18
Non-pharmaceutical COVID-19-related interventions
On March 2020, as in many other countries, NPIs were implemented to reduce the spread of COVID-19.19 These measures were progressively lifted in 2021. Details on measures and their timing are provided in Appendix 1. This led us to define three periods for the analysis: the “pre-NPI period” (Period 1) from January 2013 to February 2020, the “full-NPI period” (Period 2) from March 2020 to February 2021, during which stringent measures were implemented, and the “partial-NPI period” (Period 3) from March 2021 to the end of the study period in January 2022, during which control measures were partially lifted.
IPD surveillance data
IPD is a notifiable condition in Quebec since 1996. All IPD cases diagnosed by clinicians and laboratories are mandatorily reported to regional health authorities and, after a validation of diagnosis and epidemiologic investigation, recorded in the provincial registry of notifiable diseases.17 IPD is defined by the isolation of Streptococcus pneumoniae by culture or by a polymerase–chain reaction test from a normally sterile site. IPD cases identified from January 1st, 2013 to January 30th, 2022, were included in the study. In addition, all microbiology laboratories are invited to transmit all IPD strains and/or specimens from children <5 years of age to the provincial reference laboratory (‘Laboratoire de santé publique du Quebec’) for confirmation and serotyping by the Quellung reaction or PCR.17 Monthly IPD incidence rates (per 100,000 persons/month) in <5 years, 5–64 years, and ≥65 years age groups were computed using denominator figures from the Quebec Statistics Institute (‘Institut de la Statistique du Québec’).
Respiratory viruses surveillance data
In Quebec, surveillance of respiratory viruses is conducted throughout a network of hospital laboratories (about 40 laboratories).20 Nasopharyngeal tests from both hospitalized patients and those seen in emergency rooms and outpatient clinics are included, regardless of age. Statistics on the weekly number of tests performed and positive tests for influenza viruses, parainfluenza 1–4 viruses, adenovirus, respiratory syncytial virus (RSV), human metapneumovirus and common coronaviruses (other than SARS-CoV-2) were obtained from the Quebec Public Health Laboratory (‘Laboratoire de santé publique du Québec’) for the period January 2013–January 2022.
Statistical analyses
First, we described the change in IPD incidence after NPI implementation in 2020–21 and partial lifting in 2021–2022 in each age-groups in Quebec, along with evolution of respiratory virus circulation over the same period. Age-group classification for IPD was defined a priori based on previously published articles on this topic.1,17,21
Second, to assess the temporal association between respiratory viruses and the different age-groups of IPD, we fit a quasi-Poisson regression model including seasonality by using harmonic terms (sines and cosines with 6- and 12-month periods),22, 23, 24 temporal trend before and after interventions (NPI implementation in 2020–21 and partial lifting in 2021–2022),22,23 and the monthly number of positive tests for each respiratory virus as explanatory variables.2,10 The time unit was set at 1 month. According to the literature,5 we hypothesized that NPI would have an immediate impact on IPD incidence.25 Therefore, we included dummy variables in the model estimating the immediate change after the interventions.22,26 This model also included a dummy variable to account for the change in the immunization program during the study period (switch from PCV13 to PCV10 in May 2018). In case of re-increase of IPD incidence, to estimate the change in IPD attributable to each respiratory viruses, we estimated the trend of IPD if respiratory viruses would have remained stable by using the same equation and set each respiratory virus to the pre-NPI trend.10 For each time point of the 2021–22 period, we compared the forecasted IPD incidence with the respiratory viruses remaining unchanged to the fitted IPD incidence. The attributable fraction was defined as the respiratory viruses-attributable incidence of IPD divided by the fitted IPD incidence in the 2021–22 period.10
Furthermore, we performed several sensitivity analyses: i) using a segmented linear regression with autoregressive error, including an autoregressive-moving-average term to account for any remaining autocorrelation and an additive model to account for seasonality; ii) using a quasi-Poisson regression model including harmonic terms with only 12-month periods; iii) using the monthly proportion of positive tests for respiratory viruses over time instead of the monthly number of positive tests to assess the temporal association between IPD and respiratory viruses, to account for possible changes in testing practices; iv) using only the pre-NPI period (Period 1) to assess the temporal association between IPD and respiratory viruses; v) including respectively 3, 4 or 5 years before NPI to estimate the pre-intervention IPD trend, to explore whether the number of pre-intervention years included may have influenced the results.
All statistical tests were two sided, with p < 0.05 considered statistically significant. The validity of the segmented regression model was assessed by visual inspection of correlograms and residuals analysis. All statistical analyses were performed using R v4.1.1 (https://www.r-project.org/).
Ethics
The study was conducted under a surveillance and evaluation mandate given to the Quebec National Public Health Institute (‘Institut national de santé publique du Québec’) by the Quebec Ministry of Health and Social Services (‘Ministère de la Santé et des Services sociaux du Québec’). For this reason, no authorization from an ethical research committee nor written informed consents from patients were required, in accordance with Quebec laws.
Role of the funding source
The study sponsors had no role in the design or conduct of the study, collection management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
IPD incidence across age groups
Between January 2013 and January 2022, we included 7712 IPD cases, of which 646 were in children <5 years old, 3493 in 5 to <65 year-old patients, and 3567 in adults ≥65 years old. Characteristics of IPD cases are shown in Table 1.
Table 1.
Characteristics of invasive pneumococcal disease (IPD) in Quebec, January 2013–January 2022.
| Period 1 N = 6755 |
Period 2 N = 434 |
Period 3 N = 523 |
Total N = 7712 |
|
|---|---|---|---|---|
| Age group | ||||
| <5 years | 514 (7.6%) | 47 (10.8%) | 85 (16.3%) | 646 (8.4%) |
| 5 to <65 years | 3046 (45.1%) | 200 (46.1%) | 247 (47.2%) | 3493 (45.3%) |
| ≥65 years | 3191 (47.2%) | 187 (43.1%) | 189 (36.1%) | 3567 (46.3%) |
| Missing | 4 (0.1%) | 0 (0.0%) | 2 (0.4%) | 6 (0.1%) |
| Sex | ||||
| Male | 3630 (53.7%) | 232 (53.5%) | 274 (52.4%) | 4136 (53.6%) |
| Female | 3116 (46.1%) | 201 (46.3%) | 244 (46.7%) | 3561 (46.2%) |
| Missing | 9 (0.1%) | 1 (0.2%) | 5 (1.0%) | 15 (0.2%) |
Variables are described with numbers (percentages).
Period 1: Pre-NPI period, from January 2013 to February 2020; Period 2: NPIs fully implemented, from March 2020 to February 2021: Period 3: NPIs partially lifted, from March 2021 to January 2022.
Abbreviation: NPIs: non-pharmaceutical interventions.
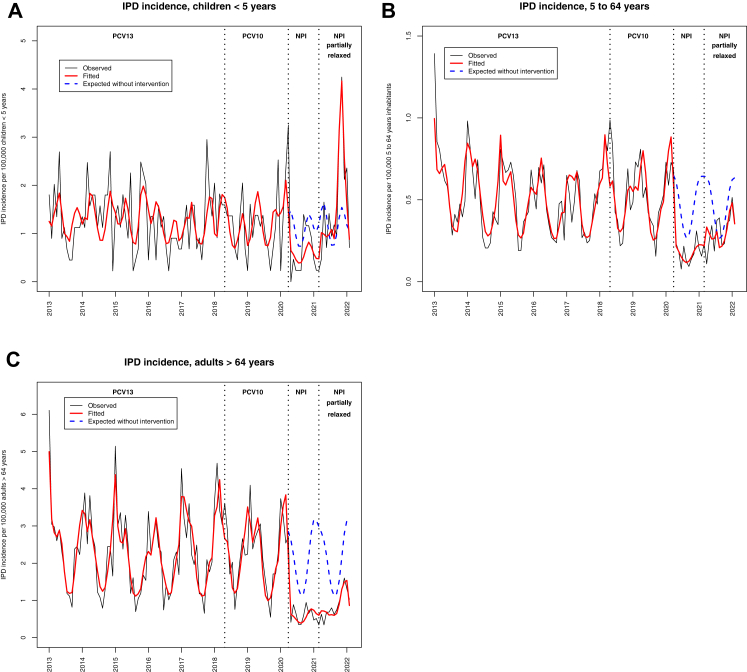
Following full NPI implementation in March 2020, the incidence of IPD decreased in all age groups (Appendix 2, Fig. 1A). Following partial NPI lifting (Period 3), a surge of IPD cases in children <5 years of age starting in October 2021 was observed, which exceeded the pre-NPI baseline level (+62% during Period 3 versus the expected trend based on Period 1, Appendix 2, Fig. 1A). By contrast in older age groups, the IPD incidence remained low, far from the pre-NPI baseline level (Appendix 2, Fig. 1B and C). The main serotype involved in Period 3 surge was serotype 19A representing 25.8% of cases, whereas this serotype represented only 9.6% of cases during Period 1 (Table 2).
Fig. 1.
Evolution of the monthly incidence of IPD across age groups in Quebec, January 2013–January 2022, N = 7712. A) <5 years. B) 5–64 years. C) ≥65 years. The black line shows the observed monthly incidence of IPD, the red line shows the monthly incidence of IPD estimated by the model (quasi-Poisson regression modeling), the dotted blue line shows the expected monthly incidence of IPD if no intervention had occurred (quasi-Poisson regression modeling). Pre-NPI period, PCV13 implemented: from January 2013 to May 2018. Pre-NPI period, PCV10 implemented: from June 2018 to February 2020. Period 2, NPIs implemented: From March 2020 to February 2021. Period 3, NPIs partially relaxed: From March 2021 to January 2022. Abbreviations: IPD: invasive pneumococcal disease. NPIs: non-pharmaceutical interventions.
Table 2.
Evolution of serotypes involved in IPD in children <5 years over time, January 2013–January 2022 (Total = 506 IPD casesa).
| Period 1 During PCV13 use N = 322 |
Period 1 During PCV10 use N = 81 |
Period 2 N = 37 |
Period 3 N = 66 |
|
|---|---|---|---|---|
| PCV7 | 5 (1.6%) | 2 (2.5%) | 1 (2.7%) | 5 (7.6%) |
| PCV10 non-PCV7 | 2 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| PCV13 non-PCV10 | 48 (14.9%) | 16 (19.8%) | 10 (27.0%) | 19 (28.8%) |
| Serotype 3 | 16 (5.0%) | 5 (6.2%) | 4 (10.8%) | 2 (3.0%) |
| Serotype 6A | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Serotype 19A | 31 (9.6%) | 11 (13.6%) | 6 (16.2%) | 17 (25.8%) |
| PCV15 non-PCV13 | 76 (23.6%) | 15 (18.5%) | 10 (27.0%) | 13 (19.7%) |
| PCV20 non-PCV15 | 77 (23.9%) | 17 (21.0%) | 7 (18.9%) | 10 (15.2%) |
| Non-PCV20 | 114 (35.4%) | 31 (38.3%) | 9 (24.3%) | 18 (27.3%) |
Period 1: Pre-NPI period, from January 2013 to February 2020 (PCV13 used from January 2013 to May 2018; PCV10 used from June 2018 to February 2020); Period 2: NPIs fully implemented, from March 2020 to February 2021: Period 3: NPIs partially lifted, from March 2021 to January 2022.
Abbreviations: IPD: invasive pneumococcal disease. NPCRI: non-pharmaceutical COVID-19-related intervention.
Serotyping was performed for 506/646 (78.3%) IPD cases in children <5 years of age.
Respiratory viruses evolution and temporal association with IPD
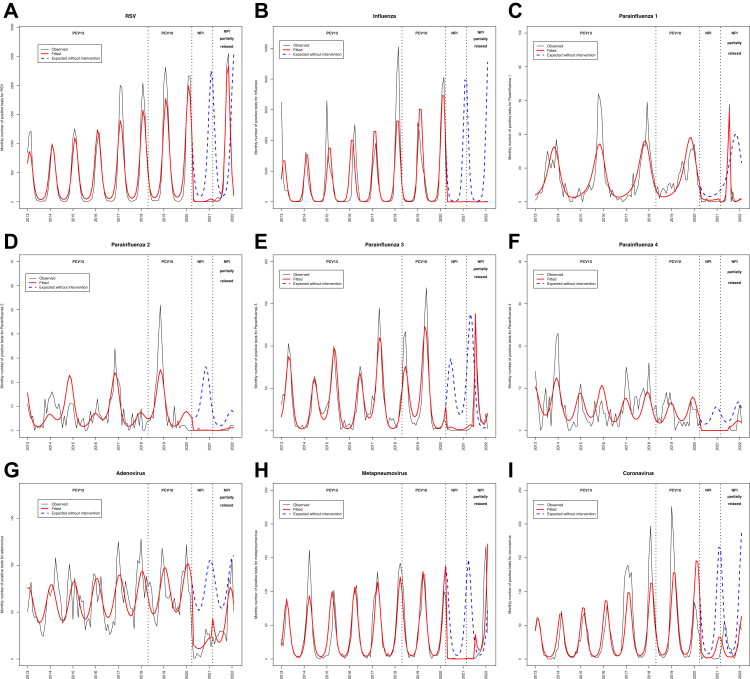
Following full NPI implementation in March 2020, a significant decrease of the monthly number of positive tests for all respiratory viruses was observed (Fig. 2A–I). Following partial NPI lifting, a major unusually early RSV outbreak was observed, starting in July 2021. In Period 3, RSV cases far exceeded the baseline level (+53% versus the expected trend based on Period 1, Fig. 2, Appendix 3). An outbreak was also observed for adenovirus and human metapneumovirus, and to a lesser extent parainfluenza 1 and 3 (Fig. 2). By contrast over the same period, a quasi-absence of influenza epidemics was seen (Fig. 2). The details of each respiratory virus evolution, including the monthly number of tests performed are provided Appendix 4.
Fig. 2.
Evolution of the monthly number of positive tests for respiratory viruses over time, in Quebec, January 2013–January 2022. A) RSV. B) Influenza. C) Parainfluenza 1. D) Parainfluenza 2. E) Parainfluenza 3. F) Parainfluenza 4. G) Adenovirus. H) Human metapneumovirus. I) Common coronavirus (non-SARS-CoV-2). The black line shows the observed monthly incidence of IPD, the red line shows the monthly incidence of IPD estimated by the model (quasi-Poisson regression modeling), the dotted blue line shows the expected monthly incidence of IPD if no intervention had occurred (quasi-Poisson regression modeling). Pre-NPI period, PCV13 implemented: from January 2013 to May 2018. Pre-NPI period, PCV10 implemented: from June 2018 to February 2020. Period 2, NPIs implemented: From March 2020 to February 2021. Period 3, NPIs partially relaxed: From March 2021 to January 2022. Abbreviations: IPD: invasive pneumococcal disease. NPIs: non-pharmaceutical interventions.
The multivariate quasi-Poisson model showed that IPD in the <5-year-old group was temporally associated with RSV (p = 0.0009), while IPD in ≥65 year-old group was temporally associated with Influenza and Parainfluenza 3 (Appendix 5). We also found a similar temporal association between viruses and IPD when limiting the analysis to the pre-NPI period (Period 1), with RSV strongly associated with IPD in children <5 years of age, while influenza was only associated with IPD in older age-groups (Appendix 6).
Attributable fraction of respiratory viruses in IPD increase
The multivariate quasi-Poisson model including all respiratory viruses as explanatory variables found that the fraction of IPD incidence in children <5 years of age attributable to RSV was 77% (95% CI [33; 100]) during the IPD surge in 2021–2022, while the attributable fraction of other viruses was minor (Table 3). Correlograms and residuals analysis were satisfactory (Appendix 7). Similar results were found with the different sensitivity analyses, including analysis where the monthly proportion of positive respiratory tests replaced the number of positive tests as explanatory variables, and a segmented linear regression with autoregressive error (Appendix 8).
Table 3.
Fraction of IPD increase in children <5 years in 2021–22 period attributable to respiratory viruses.
| Period 3 | |
|---|---|
| RSV | 77.2% [33.1; 100] |
| Influenza | 1.5% [−29.3; 32.2] |
| Parainfluenza 1 | 2.5% [−1.1; 6.2] |
| Parainfluenza 2 | 5.5% [−28.1; +39.2] |
| Parainfluenza 3 | 2.0% [−3.3; 7.3] |
| Parainfluenza 4 | −4.1% [−12.1; 3.9] |
| Adenovirus | 6.9% [−30.3; +44.1] |
| Human metapneumovirus | 11.5% [−9.2; 32.2] |
| Common coronaviruses (non-SARS-CoV-2) | −8.3 [−14.2; −2.4] |
Period 3: NPIs partially lifted, from March 2021 to January 2022.
Attributable fraction obtained by multivariate quasi-Poisson regression including all respiratory viruses as explanatory variables.
Abbreviation: NPIs: non-pharmaceutical interventions.
Discussion
This study showed a significant surge of IPD in children <5 years of age, temporally associated with a major RSV outbreak at an unusual moment (early Fall), and with a potentially attributable fraction of the IPD increase to RSV dynamics estimated at 77%. The major RSV outbreak observed in the second part of the year 2021 may be partly related to the immune debt due to the very low circulation of this virus during full NPI period.12, 13, 14 Unlike respiratory viruses, other studies have shown that the ecological niche of S. pneumoniae was almost unchanged during NPI period, with a stable overall carriage rate.9, 10, 11 Thus, no direct immune debt may be expected from pneumococcal carriage.9,10 However, this study shows that a major RSV outbreak can be associated with a surge of IPD cases, as a possible indirect consequence of the respiratory viruses immune debt.
In this study, the pattern of IPD incidence was contrasted across age-groups. Our findings suggest that IPD dynamics in children <5 years were temporally associated with RSV epidemiology, while IPD trends in adults ≥65 years were more associated with the influenza dynamics. A differential role of RSV and influenza on IPD risk according to age has been suggested previously by Watson et al.,5 who found a positive correlation between RSV circulation and IPD in young children, while influenza was less correlated to IPD in this age-group.5 In the latter study, a combination of RSV and influenza circulation was required to explain the IPD pattern in older adults.5 However, because of the partial temporal overlap between RSV and influenza epidemiology, identifying a differential role of RSV and influenza on IPD across age groups was challenging.5 In the present study, the unique epidemiological situation of a major RSV outbreak during a quasi-absence of influenza circulation allowed a disentanglement of these associations, and may pave the road for further public health interventions targeting RSV for young children and influenza for older adults.
Previous studies have suggested that some serotypes may be more often associated with RSV to cause disease, especially less invasive serotypes.27 We could assume that the IPD increase in the fall of 2021 was related to a serotype-specific association with RSV. The main serotype involved in the IPD surge was serotype 19A, in a context of a switch from PCV13 to PCV10 few years before. However, the serotype 19A was not reported to be more often associated with RSV than other serotypes in other studies.27 Thus, the temporal association between RSV and IPD observed in Quebec might not be serotype-dependent but only opportunistic. Investigating the genotype of the serotype 19A may also help better understanding its recent increase in IPD.
Our study has several limitations. First, as in any observational study, a definitive causal relationship between respiratory virus epidemiology and IPD dynamics cannot be established. However, the observation of an RSV outbreak occurring at an unusual moment, without any influenza circulation, and directly followed an IPD surge in children <5 years strengthens the link between RSV and IPD dynamics in young children. Population-based surveillance data from other countries are needed to confirm these findings. Second, pneumococcal carriage was not assessed in the study population, and age-specific changes in pneumococcal carriage cannot be ruled out. Further carriage studies are required during IPD surge to better understand the complex relationship between carriage and IPD. Third, to estimate the potentially attributable fraction of IPD increase related to respiratory viruses, we included a limited number of respiratory viruses as explanatory variables. Other pathogens not included in our model, such as rhinovirus or enterovirus may have also played a role, along with unmeasured environmental factors. Fourth, the surveillance of respiratory viruses in Quebec is far from perfect and no age-specific statistics could be obtained. Furthermore, respiratory viruses other than influenza and RSV may be more frequently tested young children than in adults, and our data may reflect virus circulation in children rather than in older age groups. This work is only based on data from Quebec in a specific time frame, and cautions should be taken when extrapolating these findings to other settings or other periods. Finally, the application of NPIs and the intensity of social contacts varied over time and by age-groups in Quebec.28 Thus, a more important application of NPIs in Period 3 in older adults probably played a role in the low IPD incidence in this age-group in Period 3, and may have influenced the temporal association between influenza and IPD in older adults. However, when restricting the analysis to the pre-NPI period, we found very similar results, with a significant temporal association between RSV and IPD only for children <5 years of age, while a significant temporal association between influenza and IPD was only found for older age-groups. Further studies are required to better explore the role of the different NPIs, and the evolution of intergenerational contacts in the current IPD epidemiology.
Conclusion
After an overall decrease in IPD rate starting in April 2020, a marked increase was observed in children <5 years of age in the Fall 2021, following an RSV outbreak. The analysis showed that IPD incidence in children was temporally associated with RSV dynamics. By contrast, IPD incidence in older age-groups remained low, and was associated with influenza dynamics. This unique epidemiological situation, with an RSV outbreak in an unusual moment, without any influenza circulation, and directly followed an IPD surge in young children <5 years, strengthened the link between RSV and IPD dynamics in young children. These findings provide new insights in the role of respiratory viruses in driving IPD dynamics, with possible differences between children and adults. In the future, the potential benefit of interventions targeting RSV, such as vaccines, for IPD prevention should be considered.
Contributors
N.O. and G.D. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and were responsible for the decision to submit the manuscript. All authors made substantial contributions to the conception or design of the work. N.O. and P.D.W. drafted the manuscript. All authors were involved in the acquisition, analysis, or interpretation of data. All authors provided critical revision of the manuscript for important intellectual content.
Data sharing statement
De-identified data that underlie the results reported in this article (text, tables, figures and appendices) and that abide by the privacy rules of the National Public Health Institute of Quebec can be made available to investigators whose secondary data analysis study protocol has been approved by an independent research ethics board.
Declaration of interests
N.O. reports travel grants from Pfizer, Sanofi, and GSK, outside the present work. B.L. received research grants from Pfizer. All other authors report no potential conflicts.
Acknowledgments
The authors thank all the persons who participated to the surveillance system of invasive pneumococcal disease and respiratory viruses in Quebec. The authors thank Mélanie Drolet, Marc Brisson, Maxime Hardy and Guillaume Gingras for their help.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100448.
Appendix A. Supplementary data
References
- 1.Wahl B., O'Brien K.L., Greenbaum A., et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger D.M., Klugman K.P., Steiner C.A., Simonsen L., Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernández S., Muñoz-Almagro C., Ciruela P., et al. Invasive pneumococcal disease and influenza activity in a pediatric population: impact of PCV13 vaccination in pandemic and nonpandemic influenza periods. J Clin Microbiol. 2019;57 doi: 10.1128/JCM.00363-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiavenna C., Presanis A.M., Charlett A., et al. Estimating age-stratified influenza-associated invasive pneumococcal disease in England: a time-series model based on population surveillance data. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson M., Gilmour R., Menzies R., Ferson M., McIntyre P., New South Wales Pneumococcal Network The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clin Infect Dis. 2006;42:211–215. doi: 10.1086/498897. [DOI] [PubMed] [Google Scholar]
- 6.Stensballe L.G., Hjuler T., Andersen A., et al. Hospitalization for respiratory syncytial virus infection and invasive pneumococcal disease in Danish children aged <2 years: a population-based cohort study. Clin Infect Dis. 2008;46:1165–1171. doi: 10.1086/529438. [DOI] [PubMed] [Google Scholar]
- 7.Kadambari S., Goldacre R., Morris E., Goldacre M.J., Pollard A.J. Indirect effects of the Covid-19 pandemic on childhood infection in England: population based observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-067519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brueggemann A.B., Jansen van Rensburg M.J., Shaw D., et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the invasive respiratory infection surveillance initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danino D., Ben-Shimol S., Van Der Beek B.A., et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis. 2022;75:e1154–e1164. doi: 10.1093/cid/ciab1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybak A., Levy C., Angoulvant F., et al. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.18959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willen L., Ekinci E., Cuypers L., Theeten H., Desmet S. Infant pneumococcal carriage in Belgium not affected by COVID-19 containment measures. Front Cell Infect Microbiol. 2021;11:825427. doi: 10.3389/fcimb.2021.825427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen R., Ashman M., Taha M.-K., et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51:418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker R.E., Park S.W., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117:30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indolfi G., Resti M., Zanobini A. Associazione Ospedali Pediatrici Italiani Research Group on Bronchiolitis, Outbreak of respiratory syncytial virus bronchiolitis in Italy. Clin Infect Dis. 2022;75:549–550. doi: 10.1093/cid/ciac120. [DOI] [PubMed] [Google Scholar]
- 15.Amin-Chowdhury Z., Bertran M., Sheppard C.L., et al. Does the rise in seasonal respiratory viruses foreshadow the return of invasive pneumococcal disease this winter? Lancet Respir Med. 2022;10:e1–e2. doi: 10.1016/S2213-2600(21)00538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quebec National Public Health Institute Enquête québécoise sur la vaccination contre la grippe saisonnière, le pneumocoque, le zona et sur les déterminants de la vaccination : 2020. INSPQ. 2020. https://www.inspq.qc.ca/publications/2840
- 17.De Wals P., Lefebvre B., Deceuninck G., Longtin J. Incidence of invasive pneumococcal disease before and during an era of use of three different pneumococcal conjugate vaccines in Quebec. Vaccine. 2018;36:421–426. doi: 10.1016/j.vaccine.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 18.Quebec National Public Health Institute Étude sur la couverture vaccinale des enfants québécois âgés de 1 an, 2 ans et 7 ans en 2019. INSPQ. 2019. https://www.inspq.qc.ca/publications/2776
- 19.INSPQ. Quebec National Public Health Institute COVID-19 cases and public health measures over time in Quebec. INSPQ. 2022. https://www.inspq.qc.ca/covid-19/donnees/ligne-du-temps
- 20.Quebec National Public Health Institute Respiratory viruses including influenza surveilance in Quebec. INSPQ. 2022. https://www.inspq.qc.ca/influenza
- 21.Ouldali N., Varon E., Levy C., et al. Invasive pneumococcal disease incidence in children and adults in France during the pneumococcal conjugate vaccine era: an interrupted time-series analysis of data from a 17-year national prospective surveillance study. Lancet Infect Dis. 2021;21:137–147. doi: 10.1016/S1473-3099(20)30165-1. [DOI] [PubMed] [Google Scholar]
- 22.Kontopantelis E., Doran T., Springate D.A., Buchan I., Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jandoc R., Burden A.M., Mamdani M., Lévesque L.E., Cadarette S.M. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol. 2015;68:950–956. doi: 10.1016/j.jclinepi.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angoulvant F., Ouldali N., Yang D.D., et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections-a time series analysis. Clin Infect Dis. 2021;72:319–322. doi: 10.1093/cid/ciaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner A.K., Soumerai S.B., Zhang F., Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg D., Givon-Lavi N., Faingelernt Y., et al. Nasopharyngeal pneumococcal carriage during childhood community-acquired alveolar pneumonia: relationship between specific serotypes and coinfecting viruses. J Infect Dis. 2017;215:1111–1116. doi: 10.1093/infdis/jiw613. [DOI] [PubMed] [Google Scholar]
- 28.Drolet M., Godbout A., Mondor M., et al. Time trends in social contacts before and during the COVID-19 pandemic: the CONNECT study. BMC Public Health. 2022;22:1032. doi: 10.1186/s12889-022-13402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.