Abstract
Immune checkpoint inhibitor monoclonal antibodies allow the host's immune system to attack tumors, which has revolutionized cancer care over the last decade. As the use of immune checkpoint inhibitors has expanded, so have autoimmune-like complications known as immune-related adverse events. These include the infrequent but increasingly more common, potentially deadly neurological immune related adverse events. When feeling acutely ill, patients will often seek care not from their oncologist but from their family physician, clinics, emergency, and urgent care sites, or other available providers. Thus, while assessing acutely ill cancer patients who are experiencing neurological symptoms, non-oncologists should be prepared to recognize, diagnose, and treat neurological immune related adverse events in addition to more familiar conditions. This narrative review is designed to update acute care clinicians on current knowledge and to present a symptom-based framework for evaluating and treating neurological immune related adverse events based on the leading immunotoxicity organizations' latest recommendations.
Keywords: Immune checkpoint inhibitors, Immune-related adverse effects, Nervous system, Diagnosis, Management, Emergency department
1. Introduction
Humans have natural tools to combat immune invaders, including cancer. Monoclonal antibodies called immune checkpoint inhibitors (ICIs) were first shown to help T cells kill cancers in 1996 [1]. There are 10 Food and Drug Administration (FDA) approved ICIs: the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitors ipilimumab and tremelimumab; the programmed cell death protein-1 (PD-1) inhibitors nivolumab, pembrolizumab, cemiplimab, and dostarlimab; the programmed cell death ligand-1 (PD-L1) inhibitors atezolizumab, avelumab, and durvalumab [2,3]; and the recently approved lymphocyte-activation antigen 3 (LAG3) inhibitor relatlimab [4,5]. Initially used in metastatic melanoma [6], checkpoint inhibitor use expanded to non-small cell lung cancer (NSCLC) [7,8], renal cell cancer [9], urothelial cancer [10], Hodgkin's lymphoma [11], and others. Though FDA approved for many cancers, most patients do not respond, thus resistance mechanisms are being researched [12]. ICI-chemotherapy combinations are promising solutions in breast cancer [13], NSCLC [14]; combination with tyrosine kinase inhibitors (TKI) is considered first-line therapy for renal cell cancer [15]. ICI refractory tumors such as castrate resistant prostate cancer may have subsets of ICI responders [16]. As the use of ICIs has expanded, so have autoimmune-like complications, known as immune-related adverse reactions (irAEs); these include the infrequent but potentially deadly neurological irAEs (NirAEs), which are becoming more common.
Non-oncologists who care for acutely ill cancer patients who are experiencing NirAEs should be prepared to recognize and treat them. This narrative review is designed to update acute care clinicians on current knowledge of NirAEs and to present a symptom-based framework for evaluating and treating NirAEs on the basis of the latest recommendations of the leading immunotoxicity organizations (American Society of Clinical Oncology [ASCO] [17], National Comprehensive Cancer Network [NCCN] [18], and the Society for Immunotherapy of Cancer [SITC]) [19].
2. Neurological immune-related adverse events
2.1. Description and pathophysiology
Antigen presenting cells (APCs) process proteins from cancer cells and present them as antigens to T cells. In the priming phase of immune response to cancer cells, APC cell surface protein CD80 stimulates CD28 receptors on naïve T cells that are interacting with the APCs and binding cancer antigens, transforming them into cytotoxic (CD8+) T cells. APC CD80 also competitively binds with T cell checkpoint protein CTLA-4 to decelerate the immune response and promote immunotolerance. Anti-CTLA-4 stops priming deceleration. In the effector phase of immune response, activated CD8+ T cells seek and destroy the cancer cells. PD1 is another inhibitory cell surface receptor on T cells that can down-regulate immune response [20,21]. Some tumors have "learned" to express PDL1 on their cell surface and activate PD1 on the T cells that infiltrate the tumor to shut down the immune response. Anti-PD1 and anti-PDL1 antibodies bind to their respective antigens to prevent the ligand-receptor interaction between PDL1 and PD1, resulting in blockade of this inhibitory signal on the T cells that recognize and attack the cancer cells [21]. LAG3 is another immune checkpoint cell surface receptor of regulatory T cells (Treg) and CD8+ T cells that can induce T cell exhaustion; antibodies that block LAG3 signaling can re-invigorate T cells [22].
Despite promising oncologic outcomes, ICIs can cause illnesses called immune-related adverse events (irAEs) through autoimmunity [[23], [24], [25]]. Though ICIs barely cross the blood-brain barrier [26], T cells do [27]. This likely allows treatment of brain metastases [[28], [29], [30]] but also makes neurotoxicity possible [31]. Immunoenhancement might activate subclinical autoimmune neurological illness or cause flares of established disease. Molecular mimicry or cross-reactivity among tumor antigens and neural antigens may lead to immune attack of the nervous system [32]. Neurological paraneoplastic syndromes (N-PNSs) can resemble NirAEs, and manifest onco-neuronal antibodies that also cross-react with neoplastic and neural antigens to cause damage [[33], [34], [35], [36]]. In contrast with N-PNSs, NirAEs follow ICI treatment of cancer and are not necessarily associated with onco-neuronal autoantibodies [[37], [38], [39]].
2.2. Epidemiology
Most NirAEs occur within 3 months (median: 6 weeks) after initial treatment [40]. NirAE related meningitis, encephalitis, myelitis, and myasthenia present earliest [41]. Cuzzubbo et al. [42] found a 5% incidence in 2017, while a 2022 meta-analysis (including newer PD-L1 inhibitors) showed a 15% incidence (70% from anti-PD-1/PD-L1) [43]. Peripheral nervous system (PNS) irAEs are 2–5 times more common than central nervous system (CNS) toxicity [[44], [45], [46]]. In the largest systematic review of NirAE cases to date, 85% were above age >50 years, and men made up 2/3 [46]. CNS/PNS overlap syndromes occur at a rate of 18% [47].
2.3. Grading and treatment
Severity grading is derived from the National Cancer Institute's Common Terminology Criteria for Adverse Events and is used to determine treatment [48]. The grades are G1: asymptomatic or mild, with no interference with function and symptoms that are not concerning to the patient; G2: moderate, limiting the instrumental activities of daily living; G3: severe, limiting self-care activities of daily living; G4: life threatening; and G5: death. G1 and G2 are low-grade, while G3 to G5 are high-grade, requiring aggressive, often inpatient management. The treatment guidelines are consensus expert recommendations by oncology organizations, including ASCO [17], NCCN [18], and SITC [19]. SITC has specific guidance for NirAE related myasthenia gravis (MG), Guillain-Barre syndrome (GBS), encephalitis, meningitis, and non-GBS peripheral neuropathy [19]. ASCO and NCCN include these plus transverse myelitis [17,18]. The ASCO guidelines also add autonomic neuropathy and several demyelinating irAEs. While these diseases may be more appropriately labeled as mimics of their traditional counterparts (MG-like, GBS-like, MS-like, etc.), the guidelines do not re-name them, assuming that diseases mentioned are the NirAE versions; therefore we may also refer to these NirAE versions by their convenience name. In these guidelines, the first line treatment is usually corticosteroids. Refractory cases are often treated with intravenous immune globulin or plasmapheresis. The latter modality's efficacy may be due to accelerated clearance of ICI or removal of offending antibodies or cytokines [49]. Some serious conditions are also treated with immunosuppressants as specified in the disease specific sections below. Due to the complexity and potential harms and pitfalls of inaccurate diagnosis and treatment, all the organizations strongly encourage involving consultants to help manage high-grade NirAEs. Table 1 summarizes the approaches to treatment of these 3 organizations.
Table 1.
Management of neurologic ICI related adverse events.
| Neurotoxicity | Grade 1 | Grade 2 (moderate) | Grade 3 (severe) | Grade 4 (Life-threatening) |
|---|---|---|---|---|
| Aseptic Meningitis | ||||
| Diagnostic Criteria |
|
|
|
Life-threatening |
| Management |
|
|
Same as grade 3, consider higher level of care. |
|
| Encephalitis | ||||
| Grading criteria |
|
|
|
Life-threatening |
| Management |
|
|
|
Same as grade 3, consider higher level of care. |
| Demyelinating Diseases: Multiple Sclerosis-like CNS Inflammation, Transverse Myelitis, ADEM, ON, NMO | ||||
| Grading criteria |
|
|
|
Life-threatening |
| Management |
|
|
|
Same as Grade 3 with ICU level care |
| Guillain-Barre Like Syndrome | ||||
| Grading criteria |
|
|
|
Life-threatening |
| Management |
|
|
Same as Grade 3 with ICU level care | |
| Peripheral Neuropathy | ||||
| Grading criteria |
|
|
|
Life-threatening |
| Management |
|
|
|
Same as Grade 3 with ICU level care |
| Autonomic Neuropathy | ||||
| Grading criteria |
|
|
|
Life-threatening |
| Management |
|
|
|
Same as Grade 3 with ICU level care |
| Myasthenia Gravis | ||||
| Diagnostic Criteria |
|
|
|
Life-threatening |
| Management |
|
|
Same as Grade 3 with ICU level care | |
Abbreviations: Ab: antibody; ADEM: acute disseminated encephalomyelitis; ADLs: activities of daily living; CSF: cerebrospinal fluid; d: day; g: gram; GBS: Guillain-Barre syndrome; ICI: immune checkpoint inhibitor; ICU: intensive care unit; irAE: immune related adverse events; IV: intravenous; IVIG: intravenous immunoglobulin; kg: kilogram; MGFA: Myasthenia Gravis Foundation of America; NMO: neuromyelitis optica; ON: optic neuritis; PCR: polymerase chain reaction; PFTs: pulmonary function tests; TID: three times a day.
1Symptoms are concerning to patient but not severely limiting such as pain without weakness or difficulty ambulating.
2For severe, progressive symptoms or presence of oligoclonal bands.
3May resume immunotherapy if resolution of symptoms or steroid taper is completed.
4NCCN recommends permanently discontinuing immunotherapy.
5gabapentin, pregabalin or duloxetine for pain.
6NCCN recommends no more than 100 mg per day.
7High-dose steroids (= or > 2 mg/kg/d may exacerbate symptoms.
8Per STC, high-dose pulse steroids particularly warranted in autoimmune myasthenia gravis.
9Per STC, highly recommended.
10STC recommends IVIG, plasmapheresis or rituximab for cases refractory to pulse dose steroids.
aSociety for immunotherapy of Cancer (SITC) Clinical practice guideline. June 2021. Recommends referral to a specialist for all neurologic irAEs.
bNational Comprehensive Cancer Network (NCCN) Guidelines Version May 2021.
cAmerican Society of Clinical Oncology (ASCO) Guideline Update December 2021.
2.4. Prognosis
The mortality rate of NirAEs varies from 8.4% (mostly from MG and myositis) [41] to 11% (seen in multi-national pharmacovigilance data) [50]. This makes it imperative that they be promptly recognized and treated [46,51]. Another study found MG (28%), encephalitis (21%), and GBS (11%) had the highest mortality rates while meningitis, myelitis, and cranial neuropathy had none [45]. Besides death, NirAE patients often have prolonged recovery and deficits [41,[52], [53], [54]].
3. Symptoms
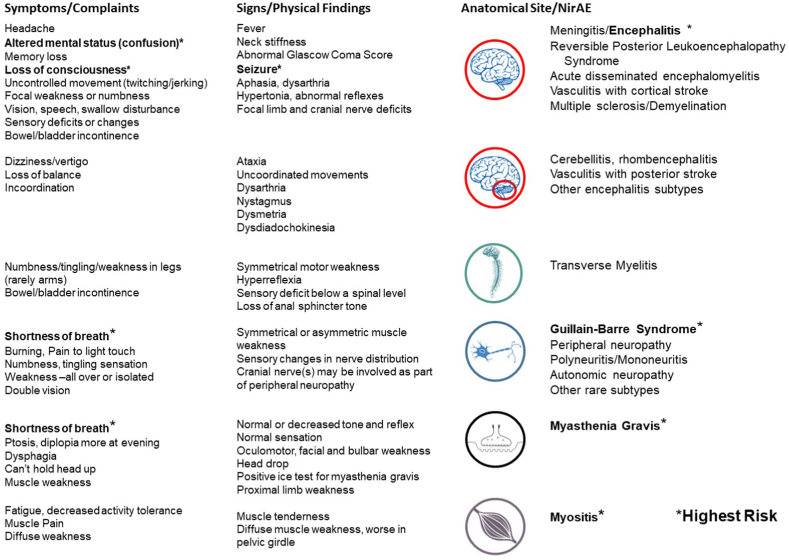
Fortunately, only about 1%–1.5% of NirAEs are high-grade [40,42,47]. Headache, dysgeusia, dizziness, insomnia, and paresthesia are the most frequently reported low-grade symptoms [42,43]. Still, some of these are also seen with high-grade NirAEs. Fig. 1 associates signs and symptoms of NirAEs with their anatomical location and disease entities.
Fig. 1.
Signs and symptoms associated with specific neurological immune related adverse events and their anatomical site.
3.1. Headache
Headaches are typically benign but can be a symptom of severe NirAE meningitis or encephalitis [55,56], as well as brain metastases, stroke/hemorrhage, and viral/bacterial/fungal CNS infection. Fever, meningismus, photophobia, altered mental status, focal deficits, and seizure are high-risk findings. Hypophysitis (acute pituitary irAE inflammation) presents with headache, fatigue (not neuromuscular weakness), and endocrine abnormalities [57]. Some consider this condition a NirAE [31,58]; however, the guidelines classify it as an endocrine toxicity.
3.2. Dizziness
Dizziness is commonly nonspecific, but ataxia or vertigo may be a sign of cerebellitis, NirAE demyelination, or posterior stroke due to irAE vasculitis [38,56]. Strokes in the absence of immune-related vasculitis or metastases may cause the same symptoms and should be in the differential diagnoses. Lightheadedness is often described as dizziness, and may signify orthostasis that occurs with autonomic neuropathy [[59], [60], [61], [62], [63], [64], [65]].
3.3. Confusion with or without seizures
Confusion, lethargy, speech disturbance, and seizures are associated with encephalitis and demyelination. Immune-related thrombotic thrombocytopenic purpura (TTP) may present with neurologic manifestations; ASCO recommends looking for concurrent anemia/thrombocytopenia [17].
3.4. Memory loss, psychiatric disturbances
Anterograde amnesia, delusions, hallucinations, and personality changes are seen with the limbic encephalitis subtype [54].
3.5. Cranial nerve findings
Cranial nerve findings are seen with cavernous sinus, brainstem and cerebellar metastases [66], and posterior strokes, as well as NirAEs. Ocular CN abnormalities (vision loss, diplopia, ptosis, and pupillary abnormalities) are seen with irAE meningitis and encephalitis, demyelination, cranial neuropathy, MG, and myopathy [55,66]. NirAEs that affect the neuromuscular junction (MG) and myopathy may cause ptosis, diplopia, extraocular muscle weakness or incomitant strabismus, which are signs that mimic cranial nerve palsies, but MG and myopathy will present additional symptoms like bulbar palsy and head drop (often preceding limb weakness) [55,67].
3.6. Bowel or bladder incontinence
Bowel or bladder incontinence can be seen with encephalitis, demyelinating brain, or spinal (transverse) myelitis NirAEs [55]. Autonomic neuropathy associated with GBS can also result in these symptoms [[59], [60], [61]].
3.7. Motor weakness with numbness
Numbness and motor loss patterns should be categorized as central, spinal, or peripheral by careful examination. Ascending symmetrical sensory loss, followed by weakness, is associated with peripheral neuropathies. Truncal sensory loss preceding limb numbness indicates spinal cord pathology [68]. Limb weakness can be cortical (hemiparesis) or peripheral (isolated limb or CNS). Quadriplegia/paraplegia can be spinal (transverse myelitis) or peripheral (polyradiculoneuropathy); cervical level myelitis can present similar to GBS. Unlike spinal cord compression, neck or back pain is not typical of myelitis. Neuropathic pain (burning or allodynia) is characteristic of non-GBS peripheral neuropathy [55,56].
3.8. Motor weakness without numbness
MG and myositis can both present as ocular, bulbar, neck (head drop), and limb muscle weakness with normal sensation, which can be mistaken for simple fatigue. CN weakness can be asymmetrical, especially ptosis. Limb weakness is more often symmetrical, with proximal muscle weakness greater than distal. Myositis can be associated with muscle tenderness and pain [17,18,55].
3.9. Shortness of breath, with or without syncope or chest pain
Cardiopulmonary symptoms can be seen with NirAE MG, myositis, and GBS. Respiratory muscle failure or bulbar weakness with aspiration can cause shortness of breath. Rapid shallow breathing with hypercapnia may occur before oxygen desaturation [69]. Pulmonary function tests (PFT) are recommended for early detection of hypoventilation [[17], [18], [19],55]. Myositis/myasthenia/myocarditis overlap syndrome although uncommon can present with shortness of breath, as well as chest pain, heart failure, arrythmias, and syncope [70]. The presence of one condition should prompt a search for the others [53,55,71,72]. Orthostatic hypotension from autonomic neuropathy, and arrhythmias can lead to syncope, and should be differentiated from seizures [[59], [60], [61]].
3.10. Abdominal pain, constipation
Although gastrointestinal irAEs are most commonly non-neurological, rare but deadly myenteric plexus neuropathy can occur and is potentially fatal [62,65,73].
4. Clinical approach
Most NirAE literature is organized by specific diagnoses. We feel that it is difficult to remember a checklist of already rare illnesses, so we propose a potentially more useful framework for evaluating acutely ill NirAE patients on the basis of SITC disease definitions, created to resolve variability in NirAE reporting and research [55] and supplement SITC treatment guidelines [19]. Seven core syndromes are named: 4 CNS (meningitis, encephalitis, demyelination, and vasculitis) and 3 PNS (neuropathy, neuro-muscular junction, and myopathy). Because vasculitis and myopathy are classified as musculoskeletal or rheumatologic by treatment guidelines, including by the SITC [[17], [18], [19]], we do not extensively discuss these.
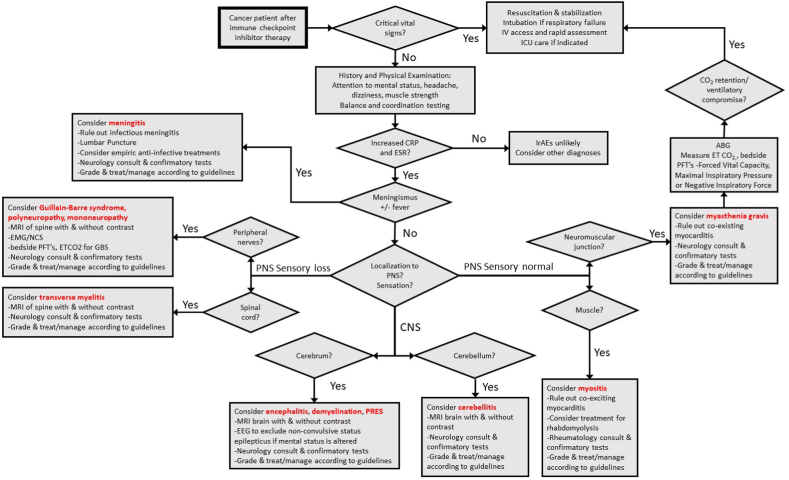
Our approach links symptoms and signs with likely sites of involvement. Some are seen with multiple core syndromes and sites. This symptom-based approach to narrowing the NirAE differential is summarized in Fig. 2.
Fig. 2.
Algorithm for the diagnosis of specific neurological immune related adverse events.
Vital signs and stability should always be assessed first, with resuscitation as necessary. Some patients present with autonomic dysfunction and abnormalities of heart rate or blood pressure, while others have respiratory muscle weakness or myocarditis symptoms [[17], [18], [19]]. Once the patient is stabilized, routine complete blood count, basic metabolic profile, and liver function tests should be obtained; if confusion or fever is present, blood cultures, lactic acid, procalcitonin, ammonia level, and urinalysis should be used to help rule out infectious or metabolic etiologies. Thyroid function tests and cortisol screening can be used for very common coincidental endocrinological irAEs.
We advocate obtaining the C-reactive protein and erythrocyte sedimentation rate. C-reactive protein is consistently elevated with irAEs in small studies [[74], [75], [76], [77], [78], [79]], while the erythrocyte sedimentation rate is less well-studied; both are recommended by NirAE guidelines [[17], [18], [19]]. We believe that if the results for both are normal, alternative diagnoses should be sought. However, if they are abnormal, irAEs should be seriously considered in the differential diagnoses for cancer patients who had been treated with ICIs.
When assessing symptoms and signs as described above, it is important to remember that NirAEs also mimic fatigue, tumor compression, infection, metabolic abnormalities, thromboses, stroke, hemorrhage, brain metastases, and other conditions that must be ruled out. Diagnostic testing recommendations derived from the SITC, ASCO, and NCCN guidelines are designed to help rule out these and unfamiliar conditions like N-PNS [[17], [18], [19]]. A lack of specific NirAE markers often makes them a diagnosis of exclusion [56,66,80].
Suspicion of myositis, myasthenia, or GBS like syndromes mandates bedside pulmonary function tests, including forced vital capacity and negative inspiratory force to evaluate respiratory muscle compromise. If NirAE meningitis or encephalitis is possible, it is important to treat patients empirically with antibiotics and antiviral until infection is ruled out [[17], [18], [19]]. This approach is summarized in Fig. 2. With all of this in mind, in the next section, we discuss NirAEs individually. We do not discuss withholding ICIs because although recommendations are included in the guidelines, non-oncologists will not be stopping or re-challenging these patients with ICIs.
5. Central nervous system neurological immune related adverse events
5.1. Meningitis and encephalitis
5.1.1. Description and pathophysiology
Meningitis and encephalitis are distinct entities; we discuss them together because of their similarities. Meningitis is inflammation of the membranes that surround the brain and spinal cord, usually due to bacteria, viruses, or carcinoma. Encephalitis is inflammation of the brain parenchyma, usually due to herpes simplex virus. ICIs can cause both. The mechanism of ICI-induced meningitis and encephalitis is likely molecular mimicry (anti-tumor/viral immune response cross-reactivity with neural antigens) or unmasking of a previously suppressed autoimmune or paraneoplastic condition, similar to other NirAEs [45,56,81,82].
5.1.2. Epidemiology
Meningitis and encephalitis are 2 of the more frequently reported high-grade NirAEs. In 2 separate systematic reviews, encephalitis comprised 13%–19% of NirAE and 3%–15% of meningitis cases [42,45]. The incidence of meningitis seems to be higher with anti-CTLA-4 (ipilimumab) [41,45,83]. Encephalitis is more often reported with anti-PD-1/PD-L1 [45,54].
5.1.3. Symptoms
Both meningitis and encephalitis can, and usually present with headache. Meningitis causes meningismus, photophobia, and sometimes fever. Encephalitis is more associated with confusion, focal weakness, aphasia, or seizure [84,85]. Meningoencephalitis has features of both meningitis and encephalitis [56,82,86] and is an encephalitis subtype recognized by SITC [55], while other encephalitis subtypes include limbic or extra-limbic encephalitis [54,56,87]. Seizures can include non-convulsive or subclinical variants [54,82].
The computed tomography results for meningitis and encephalitis are usually normal, but it is useful to quickly rule out increased intracranial pressure or bleeding. Encephalitis MRI shows patchy T2 and FLAIR hyperintensities in about 50% of cases, more commonly with focal encephalitis [45,54]. Meningitis will show meningeal enhancement in up to half of cases [45,88]. Lumbar puncture tests include cerebrospinal fluid (CSF) cell count, protein, glucose, gram stain, culture, polymerase chain reaction for herpes simplex virus and other viruses, cytology, oligoclonal bands, autoimmune encephalopathy, and paraneoplastic panels [[17], [18], [19],55]. A CSF analysis in irAE encephalitis and meningitis cases almost always shows pleocytosis with lymphocytic predominance, elevated protein, normal glucose, and a negative culture/Gram stain [54,84,88,89]. An EEG should be obtained if non-convulsive seizures are suspected [17,18]. Peripheral smears should be checked if there is concern that thrombotic thrombocytopenic purpura is causing encephalopathy [17].
5.1.4. Grading and treatment
Empirical antibiotics and acyclovir should be started for meningitis and encephalitis, pending the CSF results. NirAE treatment should start after the bacterial and viral tests are negative [17]. ASCO grades meningitis and encephalitis as G1: mild (note: any cranial nerve problem should be managed as moderate); G2: moderate, some interference with activities of daily living and symptoms that are concerning to patient (i.e., pain but no weakness or gait limitation); and G3/G4: severe, limiting self-care and warranting aid [17]. ASCO recommends follow-up for G1. For G2, oral prednisone (0.5–1 mg/kg/day) should be considered. For G3/G4 meningitis, IV methylprednisolone (1 mg/kg) should be administered and hospitalization should be considered [17]; higher doses are recommended for encephalitis. Methylprednisolone (1–2 mg/kg/day) oral or IV is recommended for G2, and pulse dose IV methylprednisolone (1 g/d) for 3–5 days is recommended for G3/G4 (or worsening despite 2 mg/kg methylprednisolone) plus either IV immunoglobulin (2 g/kg/d) or plasmapheresis [17].
The NCCN and SITC guidelines are similar to the ASCO recommendations but are more aggressive, stating that any grade of encephalitis should receive pulse-dose methylprednisolone (1 g/d) and intravenous immunoglobulin (IVIG) or plasmapheresis [18,19]. Steroid-refractory cases may require immunosuppressive drugs, such as rituximab [17,19] or infliximab [82,88], see Table 1.
5.1.5. Prognosis
In meningitis, there is usually a clinical response within the first 24 h, with few sequelae. One systematic review reported an 85% full recovery rate, and no deaths [45]. Encephalitis has a grimmer prognosis; there was a 21% mortality rate in one systematic review [45], and a 13% fatality rate with only 45% full recovery in another [54].
5.2. Encephalitis variants
5.2.1. Limbic encephalitis
Limbic encephalitis is seen with ICIs [39]. Amnesia and psychosis are characteristic and may be isolated or include other encephalitic features. In 1 systematic review of encephalitis, limbic was the second most common (18 of 82) [54]. In a small series of 8 limbic encephalitis patients, 4 died [38]. MRI usually shows limbic (temporal lobe) T2 hyperintensities. Guidelines all regard the limbic form as an encephalitis subtype; treatment is the same [[17], [18], [19]].
5.2.2. Cerebellitis and rhombencephalitis
NirAE cerebellitis is marked by ataxia, headache, and vomiting. Cerebellitis with brainstem and cranial nerve involvement is called rhombencephalitis. The sequelae include hydrocephalus and tonsillar herniation, sometimes requiring ventriculostomy or surgical decompression [90]. N-PNS cerebellitis is seen with lung, breast, ovarian, and testicular cancers or Hodgkin's lymphoma [91]. In NirAE cases, MRI may show cerebellar edema [92,93], and CSF usually shows pleocytosis and elevated protein [38,[92], [93], [94], [95]]. SITC defines cerebellitis as an encephalitis subtype [55]. None of the treatment guidelines address it separately so it is treated the same as NirAE encephalitis [[17], [18], [19]].
5.2.3. Reversible posterior leukoencephalopathy syndrome
Reversible posterior leukoencephalopathy syndrome, also known as posterior reversible leukoencephalopathy syndrome, presents with headaches, confusion, visual impairment, or seizures. Patients also have hypertension and MRI T2 bilateral posterior symmetrical hyperintensities [[96], [97], [98], [99], [100]]. Reversible posterior leukoencephalopathy syndrome is associated with eclampsia, renal failure, sepsis, autoimmune disease [[98], [99], [100], [101]], immunosuppressants, and chemotherapy [58,97,102,103]. Case reports have implicated ICIs as a cause [[104], [105], [106], [107], [108], [109], [110], [111]]. All cases were treated with standard blood pressure-lowering and seizure control medications. Corticosteroids were used in only 2 cases [104,107]. Reversible posterior leukoencephalopathy syndrome is not clearly an irAE and is not included in any of the guidelines; however, some authors classify it as such [46,56].
6. Demyelination: multiple sclerosis-like CNS inflammation (MS-CI)
6.1. Description and pathophysiology
Multiple sclerosis (MS) is characterized by brain and spinal cord axon demyelination and neurodegeneration, leading to deficits [112,113]. Disease progression is correlated with slowly expanding lesions found in the parenchyma and immune cell follicles in meninges [114]. MS-like CNS inflammation (MS-CI) has been described as an NirAE, mostly due to stimulation of pre-existing or silent disease [42,56,[115], [116], [117], [118]]. NirAE MS shows an enhanced T cell response in the CNS, leading to demyelination, although the mechanism is not clear [117]. There is debate as to whether irAE MS occurs de novo or unmasks clinically silent disease [119]. Since MS is a diagnosis of exclusion without specific biomarkers, its exact correlation to MS-CI is unclear. That may be why SITC and NCCN do not list multiple sclerosis as a specific irAE in their guidelines, although ASCO does [17].
6.2. Epidemiology
One Food and Drug Administration adverse events reporting system–based study found 14 cases of irAE MS-CI that were associated with ICI use, 6 with no prior history [115]. A 2020 systematic review found 5 cases of ICI-associated multiple sclerosis-like CNS inflammation, 3 with prior disease and 2 with asymptomatic radiologically isolated syndrome on prior MRI. The median time to onset after ICI treatment was 6.5 weeks (in 1 case, 43 weeks) [117]. The median patient age is mid to late fifties; more men are represented than what is usually seen in non-irAE MS [115,117].
6.3. Symptoms
The 4 classic presentations of irAE MS-CI are unilateral optic neuritis, sensory or motor loss ± ataxia, pure focal sensory loss, and pure brainstem syndromes. Most patients (80%) experience relapsing-remitting MS-CI, and 20% of these patients develop secondary progressive MS-CI. Fifteen percent of patients experience primary progressive MS-CI from the outset [113]. The remaining 5% have a clinically isolated syndrome or radiologically isolated syndrome [112,113].
6.4. Testing
The diagnosis of irAE MS-CI is based on the clinical course and correlated MRI findings: bilateral, often asymmetrical, ovoid T2 hyperintensities, usually in periventricular, juxtacortical, cortical, and infratentorial areas; the corpus collosum, or the spinal cord (usually cervical) [120]. No single test establishes the diagnosis. Oligoclonal bands are found in CSF but not serum in 95% of MS patients. The IgG CSF/serum ratio (IgG index) is elevated in MS [121]. ASCO recommends diagnostic workup, including serum markers of metabolic, paraneoplastic, and infectious diseases, and CSF studies with an autoimmune encephalitis panel, oligoclonal bands, CNS demyelinating disease antibodies (aquaporin 4 [AQP4-IgG] and myelin oligodendrocyte glycoprotein), and viral testing to include John Cunningham virus in order to exclude progressive multifocal leukoencephalopathy [17].
6.5. Grading and treatment
ASCO has standard grading criteria for irAE MS-CI, bundled with other demyelinating NirAEs. The recommended treatments are G1 (asymptomatic): observation; G2 (moderate): prednisone (1 mg/kg daily), and G3/G4 (severe/life-threatening): pulse methylprednisolone (1 g/d) and IVIG or plasmapheresis if no improvement is seen in 3 days [17]. In a refractory case, infliximab resulted in improvement [117].
6.6. Prognosis
In the 2019 Food and Drug Administration adverse events reporting system study, 5 of the 14 irAE MS-CI patients recovered, 3 did not, and 6 had no reported outcome. Two patients died [115]. In a 2020 systematic review, 4 of 5 patients experienced improvement with steroids. None died [117].
7. Demyelination: acute transverse myelitis
7.1. Description and pathophysiology
Acute transverse myelitis is an inflammatory disease of the spinal cord. Autoimmune in nature, several infectious diseases, vaccines and collagen-vascular diseases have been implicated as triggers [122]. The differential diagnosis includes neoplastic, paraneoplastic, vascular, toxic, metabolic, nutritional, rheumatologic, and hereditary etiologies [123]. The resulting nerve damage causes varying degrees of weakness, sensory loss, and autonomic dysfunction. Checkpoint inhibitors have been associated with acute transverse myelitis in case reports and series [73,107,117,[124], [125], [126], [127], [128], [129]].
7.2. Epidemiology
A combined French pharmacovigilance and systematic literature review of 20 patients found 60% were male, mostly with anti-PD-1/PD-L1 treatment. Median age was over 55. 30% had prior radiotherapy [130].
7.3. Symptoms
Patients with ICI-induced acute transverse myelitis typically experience an acute onset of symptoms over the course of hours to days. Almost all patients present with rapidly progressive lower extremity paraparesis, sensory loss and sphincter dysfunction [130]. There are reports of tetraparesis [126,128]. Deep tendon reflexes may be affected, and Babinski sign may be present [124]. There was a case of mixed spinal cord and optic nerve demyelination, similar to neuromyelitis optica (below) without MRI confirmation [127].
7.4. Testing
MRI of the spine should be obtained to rule out compressive lesions and tumors [17,123]. ICI-related acute transverse myelitis presents with both short course (<3 vertebral segments) and long course/extensive disease (>3 segments) [124,128,129]. Lumbar puncture is valuable for ruling out leptomeningeal disease and infectious etiologies [122,123]. CSF viral PCRs are also helpful [17,123]. The ASCO, NCCN, and SITC recommendations include CSF testing for onco-neuronal antibodies and oligoclonal bands [17,18,55]. Serum for HIV, RPR, B12, ANA, thyroid panel, ACTH, cortisol levels, blood and fungal cultures, and paraneoplastic panels should be obtained with a neurology consultant [17,18,55].
7.5. Grading and treatment
The ASCO guidelines [17] recommend close observation in G1 ICI-induced acute transverse myelitis patients, prednisone (1 mg/kg/day) in G2, and pulse dose methylprednisolone (1 g/d) and consideration of IVIG or plasmapheresis in G3/G4; all of these treatments have been used successfully [126]. The NCCN guidelines suggest a more aggressive regimen of 1 g/d of methylprednisolone for 3–5 days for any grade and strong consideration of IVIG or plasmapheresis [18]. Patients whose disease is refractory to these agents have been treated with infliximab, tocilizumab, natalizumab, cyclophosphamide and ruxolitinib [125,131].
7.6. Prognosis
ICI-induced acute transverse myelitis patients tend to respond to steroid treatment [117,124,126,129]. Although death is rare, only some patients achieve a full recovery; most have persistent neurologic deficits [117,130].
8. Other demyelinating diseases
8.1. Optic neuritis
Isolated cases of optic neuritis occur without MS-CI. Non-irAE disease causes unilateral loss of central vison, color vision, and eye pain; only 1/3 of patients have optic disc abnormalities on fundoscopic examination. The NirAE version is often painless and bilateral and is seen with all classes of ICIs (CTLA-4, PD-1, and PD-L1). Two-thirds of patients experience improvement with steroids [132]. The ASCO treatment guidelines are bundled with the guidelines for MS-CI and other demyelinating diseases [17].
8.2. Neuromyelitis optica
Neuromyelitis optica is a rare demyelinating disease that is characterized by optic nerve and spinal cord involvement. It is associated with aquaporin 4 (AQP4-IgG) and myelin oligodendrocyte glycoprotein antibodies in CSF and serum; CSF oligoclonal bands are much less common [133]. It has been rarely reported with ICIs [[134], [135], [136]]. The AQP4-IgG and myelin oligodendrocyte glycoprotein antibodies can be negative in ICI therapy [134]. The ASCO treatment guidelines are the same as those for MS-CI [17].
8.3. Acute demyelinating encephalomyelitis and acute hemorrhagic encephalomyelitis
Acute demyelinating encephalomyelitis is a classic pediatric post-infectious or post-vaccination demyelinating disease that is similar to MS [137]. Acute hemorrhagic encephalomyelitis, also known as acute hemorrhagic leukoencephalitis or Weston-Hurst disease, is the hemorrhagic version [138]. Diffuse demyelination of the brain, spinal cord, and nerve roots lead to encephalitic and motor/sensory symptoms; myenteric plexus involvement can lead to autonomic dysfunction. There are case reports on ICI treatment [[139], [140], [141]]. Although recognized in SITC disease definitions [55], only ASCO specifies treatment, the same as for MS-CI [17,55].
8.4. Clinically isolated syndrome and radiologically isolated syndrome
As described by the SITC [55], clinically isolated syndrome refers to patients with typical MS-CI symptoms and negative MRI findings, while radiologically isolated syndrome refers to asymptomatic patients with incidental MRI findings of MS. Both conditions can progress to full MS-CI.
9. Peripheral Nervous system
9.1. Guillain-Barré like syndrome (GBS-like NirAE)
9.1.1. Description and pathophysiology
GBS is an inflammatory polyradiculoneuropathy that is usually triggered by recent infection (campylobacter, respiratory pathogens, and others) and a GBS-like NirAE has been seen with ICIs [59,142]. Classic acute inflammatory demyelinating polyneuropathy is characterized by ascending weakness and hyporeflexia. Acute motor axonal neuropathy and acute motor and sensory axonal neuropathy resemble acute inflammatory demyelinating polyneuropathy but progress in days rather than weeks. Miller-Fischer syndrome consists of ophthalmoplegia, ataxia, and areflexia [143]. All of these [59,144,145] plus very slow chronic acute inflammatory demyelinating polyneuropathy [44,146] have been triggered by ICI therapy. GBS-like NirAE likely involves “molecular mimicry” like the non NirAE form; ICIs may induce anti-microbial T cells to cross-react with similar proteins on myelin sheaths or axons [118]. A higher GBS-like NirAE incidence in melanoma is thought to be due to epitopes shared by melanocytes and Schwann cells [147].
9.1.2. Epidemiology
GBS-like NirAE is seen in 0.1%–0.3% of all patients receiving ICIs [51,148]. The syndrome has mostly been described with combination therapy of ipilimumab and nivolumab [142]. In a systemic review from 2021, GBS-like NirAE and other peripheral neuropathies (22%) were the second most common NirAEs [45]. In 2 case series, the mean age was 62 years; it was most common in men and patients with melanoma [44,59].
9.1.3. Symptoms
Most irAE GBS presents as other GBS; however, syndromes also occur [59]. Respiratory muscle involvement can result in intubation [[17], [18], [19]]. Dysesthesias occur in about half of patients; cranial nerve deficits and bulbar symptoms are rare but may be present [44,60]. Autonomic dysfunction is seen in 15% of cases [60]. The time to the onset of symptoms is more variable than with other irAEs, with a median of 8–18 weeks [149].
9.1.4. Testing
Treatment guidelines for ICI-induced GBS-like NirAE recommend evaluating for cord compression or leptomeningeal disease with spinal MRI and lumbar puncture, testing for neurological paraneoplastic syndromes (N-PNS) and other NirAEs (transverse myelitis, myasthenia, myositis, and others) [17,18,55]. The diagnosis is supported by electromyography showing demyelinating polyradiculopathy and CSF demonstrating albuminocytologic dissociation (excess CSF protein relative to the cell count) [147]. Unlike classic GBS, the irAE version more often has CSF pleocytosis [44,59]. Other work-up, such as nerve conduction studies and serum antiganglioside antibody tests, can be considered as recommended by a neurologist [143]. Bedside pulmonary function tests are recommended [[17], [18], [19]].
9.1.5. Grading and treatment
Corticosteroids are not typically beneficial in GBS, and their efficacy in ICI-associated GBS is unclear. There is no Grade 1 for GBS, and Grades 2 through 4 describe a rapid and inevitable progression in limitations of self-care. The most severe grades include severe symptoms such as dysphagia, facial or respiratory muscle weakness. The ASCO guidelines mandate IVIG or plasmapheresis (not both). An optional concurrent trial of (methyl)prednisolone (2–4 mg/kg/d) or pulse methylprednisolone (1 g/d) for G3/4 is considered reasonable [17]. Neurologic consultation and admission to a hospital that is capable of ICU-level care is mandatory. Impending respiratory compromise can be monitored by negative inspiratory force and vital capacity. NCCN and SITC mandate pulse steroids for any grade; otherwise, the recommendations are similar to the ASCO recommendations [18,19]. Many recommend the formation of a multi-disciplinary team with expertise in irAEs to manage these cases [60].
9.1.6. Prognosis
Pharmacovigilance data show that GBS-like NirAE is associated with a 62% hospitalization rate and 23% mortality rate [142]. A literature review of 31 cases showed that 73% of patients responded to combined IVIG and steroid therapy with only mild residual deficits; 29% developed respiratory failure, and 18% died [60]. A 2021 systemic review of 33 cases revealed a similar recovery rate of 73% but a lower mortality rate (9%) for GBS-like NirAE associated respiratory failure [59].
9.2. Non-Guillain-Barré syndrome peripheral neuropathy
9.2.1. Description and pathophysiology
Peripheral neuropathy is defined as cranial, spinal (radicular), trunk (plexus), or visceral or peripheral nerve damage. Further categorization includes mononeuropathies, multifocal neuropathies, and polyneuropathies. Pathology is divided into axonal, demyelinating, or mixed. Other descriptors include sensory-predominant, motor-predominant, sensory-motor, length-dependent (starts in the feet and ascends), and length-independent (hands and feet at the same time) [150,151]. While most are slow-onset, some (including NirAE subtypes) are rapid and thus more serious. The spectrum is extremely wide. SITC NirAE neuropathy definitions include classic GBS acute inflammatory demyelinating polyneuropathy and variants. Other neuropathies listed are descriptive, such as polyneuropathy (multiple peripheral nerves), polyradiculoneuropathy (multiple spinal roots and peripheral nerves), and cranial, sensory, and brachial neuritis [55].
9.2.2. Epidemiology
Most series combine GBS-like NirAE with other NirAE peripheral neuropathies; together, they make up 1%–3% of all irAEs [40,[152], [153], [154]]. A systematic review/meta-analysis evaluated them separately and found an overall 5% incidence of non-GBS like peripheral neuropathy, 6.4% sensory neuropathy, and only 0.3% GBS-like NirAE [148]. In contrast, a systematic review of case series totaling 428 patients with NirAEs found a more even split; GBS-like NirAE (69) constituted 16%, and non-GBS like neuropathies (25) and isolated cranial nerves (31) together made up 13% [45].
9.2.3. Symptoms
The signs and symptoms of non-GBS like peripheral neuropathy include muscle weakness, sensory loss (numbness, paresthesia, and absent vibration/proprioception), neuropathic pain (burning, stabbing, and electrical), loss of deep tendon reflexes, and autonomic symptoms (urinary retention, erectile dysfunction, dry mouth, anhidrosis, hiccoughs, gastrointestinal dysmotility, orthostatic hypotension, and sluggish pupils). The distribution can be diffuse and symmetrical, isolated to a limb or another nerve, and may be sensory, motor, or both [150,151]. Many neuropathy subtypes can be painful [63,155].
9.2.4. Testing
HbA1c, TSH, folate, vitamins B6 and B12, SPEP/IFE, and CK should be used to identify reversible metabolic causes of neuropathy. The guidelines recommend determining the erythrocyte sedimentation rate and C-reactive protein. Additional tests to consider, only after neurology consultation, may include ANA, ANCA, anti–smooth muscle, hepatitis B or C, and HIV. Spinal MRI is indicated for acute polyradiculoneuropathies (including non-GBS) to evaluate cord compression, leptomeningeal disease, and transverse myelitis. Some radiculoneuropathies will show T2 hyperintensities of spinal roots. If cranial nerves are involved, a brain MRI is required. With neurology input, lumbar puncture should be considered to rule out other conditions; it can show non-specific elevated protein and occasional mild pleocytosis, even in the absence of other conditions. Electromyography and nerve conduction studies can be used to evaluate demyelination or axonal patterns [[17], [18], [19],56].
9.2.5. Grading and treatment
The ASCO guidelines recommend close follow-up for G1 (mild) peripheral neuropathy. For G2 (moderate), they recommend observation or prednisolone (0.5–1 mg/kg/d) and gabapentin, pregabalin, or duloxetine for pain. For G3/G4 (severe symptoms), hospital admission is necessary; either IV methylprednisolone (2–4 mg/kg/d) or pulse dose methylprednisolone (1 g/d × 3–5 days) should be considered, plus mandatory IVIG or plasmapheresis; and frequent pulmonary function tests should be monitored, as in GBS [17]. The NCCN guidelines are similar, except in that steroids are not optional. In addition, in G2, methylprednisolone should be escalated to 2–4 mg/kg/d in patients who experience disease progression on the lower dose, even if they remain at G2. G3/G4 should be treated similar to GBS with pulse methylprednisolone (1 g/d x 5) days, plus IVIG or plasmapheresis. Frequent pulmonary function tests and monitoring for autonomic dysfunction are recommended [18]. The SITC has similar recommendations [19].
9.2.6. Prognosis
Outcome data on non-GBS neuropathies are sparse. A large systematic review found no deaths from isolated cranial neuropathies, as opposed to an 11% death rate in combined GBS and other neuropathies. The predictors of mortality were mechanical ventilation and intestinal pseudo-obstruction (autonomic) [45]. A systematic review/meta-analysis of randomized controlled trials did not address mortality but found that 90% of non-GBS peripheral neuropathy cases were low-grade, indicating a low fatality rate [148].
9.3. Autonomic neuropathy
Autonomic neuropathy is a very rare complication that is cited in case reports, both associated with GBS [[59], [60], [61]] and as an isolated pathology [[62], [63], [64], [65]]. The exact mechanism of autonomic neuropathy is still not fully understood, as with most irAEs [18]. The symptoms include gastrointestinal dysmotility, urinary retention, erectile dysfunction, dry mouth, anhidrosis, sluggish pupils, temperature dysregulation, and orthostatic hypotension. Of note, transverse myelitis can present with incontinence and urinary retention as a result of spinal cord pathology [73,125,129]. Phrenic nerve palsy is very rare and may lead to respiratory compromise [156,157]. Enteric plexus involvement can also occur; it can lead to intestinal pseudo-obstruction and has a high mortality rate [62,65,73]. The ASCO autonomic neuropathy guidelines recommend testing for other causes of dysfunction (diabetes, adrenal insufficiency, HIV, paraproteinemia, amyloidosis, botulism, Parkinson's, and auto immune and paraneoplastic diseases) in consultation with neurologists or the appropriate organ system specialist. Initial G1 or G2 disease should be monitored closely for 1 week. If disease progresses to G2, prednisone (0.5–1 mg/kg/d) should be started. G3/4 requires pulse methylprednisolone (1 g/d). The NCCN guidelines state that peripheral neuropathies include life-threatening autonomic dysfunction and that the treatments are the same. Supportive treatments, i.e., antiemetics, pain medication, bowel regimen, antipyretics, antihypertensives, and bedrest, are recommended [84,158].
9.4. Myasthenia gravis
9.4.1. Description and pathophysiology
MG is a disease in which nicotinic acetylcholine receptor autoantibodies block transmission at the neuromuscular junction, causing muscle fatigue and weakness. As a result of immune enhancement, ICIs can exacerbate pre-existing MG [159] or cause de novo MG [160]; 75%–85% MG irAEs are new onset [67,161]. Myasthenia and myositis irAE overlap is well described [70,162,163]. NirAE myasthenia shows T cell infiltration in the skeletal muscle, as well as acetylcholine receptor autoantibodies [164].
9.4.2. Epidemiology
The overall incidence of NirAE MG is 0.12% [53]. MG, however, was the most frequent high-grade NirAE (35%) in a 2022 systemic review; PD-1 inhibitors were most often involved [46]. MG has been most frequently documented with PD-1 therapy and appears earlier than other neurotoxicities [147]. About 2/3 of MG cases overlap with myositis, and 40% overlap with myocarditis [163].
9.4.3. Symptoms
The typical symptoms of MG include ptosis, dyspnea, limb weakness, dysphagia, and diplopia, in descending order of frequency [67]. Unlike GBS, autonomic symptoms do not occur [165]. Bulbar and respiratory symptoms are the most specific. The neuromuscular junction disorder can be difficult to distinguish from ICI myopathy because ocular and bulbar muscles are frequently involved in the irAE version [166]. MG is often associated with other irAEs, including myositis (30%) and myocarditis (25%). Myositis, myocarditis, and MG overlap syndrome has a poor prognosis; therefore, the presence of one should prompt investigation for others [53,70]. Because ICI therapy may precipitate myasthenic crisis in patients with subclinical MG, some have advocated screening patients with myasthenia antibodies before starting immunotherapy [164], but this practice is controversial and best left to consultants.
9.4.4. Testing
The diagnostic work-up for MG should include creatine phosphokinase, acetylcholine receptor antibodies, and anti-striational antibodies. Acetylcholine receptor antibodies are unique to MG, and although they are anti-muscle-specific kinase antibodies, anti-striational antibodies are specific to myositis associated with MG [167]. However, acetylcholine receptor antibodies are found with less frequency and when they are detected the titers are much lower when they are associated with ICI therapy as compared to titers seen in ICI naïve patients [147]. The ICE test, edrophonium (Tensilon), or pyridostigmine challenge may confirm MG. Alternatively, the antibodies may be present without clinical symptoms [46]. The presence of anti-muscle-specific kinase antibodies and anti-striated muscle antibodies portend a fulminant and often fatal course [167]. Frequently, there is co-existent CK elevation but not necessarily myositis [53,168]. Aldolase may be elevated in myositis even if CK is normal [169]. Screening for concurrent myocarditis should include EKG and cardiac enzymes. Diagnostic imaging to rule out other diagnoses should be directed by symptoms. Repetitive nerve simulation or electromyography may localize post-synaptic neuromuscular junction disorder [161]. Once the diagnosis is established, vital capacity and negative inspiratory force should be monitored [[17], [18], [19]]. Diaphragm ultrasound has been used to assess respiratory effort in MG patients [170].
9.4.5. Grading and treatment
The Myasthenia Gravis Foundation of America (MGFA) system classifies MG as class I to V. Class I is only ocular muscle weakness, class II is mild non-ocular (limb or bulbar) muscle weakness, class III is moderate weakness, class IV is severe weakness, and class V is weakness requiring intubation [165,171]. ASCO refers to these grades in their guidelines; otherwise, grading is similar to that for other NirAEs. There is no G1. G2 corresponds to MGFA class I or II (isolated ocular or mild non-ocular weakness). Treatment includes pyridostigmine (30 mg 3 × daily) and prednisone (0.5 mg/kg daily), both by mouth. Hospital admission is recommended. G3/G4 corresponds to MGFA class III-V. Treatment is similar to that for G2 plus IVIG or plasmapheresis. Rituximab can also be added for refractory cases. PFTs and ICU care should also be considered [17].
Early neurology consultation before starting steroids is essential since steroids may transiently exacerbate symptoms in MG leading to life threatening decline. Some experts recommend starting IVIG or plasmapheresis before steroids in severe cases with bulbar or respiratory involvement [[17], [18], [19],172]. A multi-disciplinary team of rheumatologists, cardiologists, and critical care specialists is recommended in severe cases or potential overlap syndromes [[17], [18], [19]].
9.4.6. Prognosis
MG is the most potentially fatal of all neurotoxicities, usually presenting at MGFA grade III-IV, with a reported MG-specific mortality rate of >30%, particularly if there is respiratory involvement [53,160,173]. Of patients who survive, only half experience complete resolution of symptoms [53]. More than 1/3 of NirAE MG patients develop cardiac complications, with high mortality despite appropriate management [166]. In the largest case series to date (63 patients), nearly half of the patients experienced respiratory failure that required mechanical ventilation or NIPPV. Of those who died, 3/4 had concurrent myositis or myocarditis [67].
10. Conclusion
NirAEs have the potential to cause great harm to individuals; therefore, it is imperative that health care providers recognize and treat these patients as quickly as possible. The early recognition of low-grade presentations with the potential for rapid progression to death (myasthenia, Guillain-Barre like syndromes) needs to be the uppermost consideration when evaluating NirAE patients. Other high-mortality NirAEs include encephalitis and enteric neuropathy with pseudo-obstruction. NirAEs are increasing in incidence. We have presented an approach that was designed for acute care clinicians to help diagnose and stratify these patients. Randomized trials are needed to further advance our understanding of ICI related neurotoxicities. For instance, there is a need for research into predictors of NirAEs, as well as confirmatory serum and CSF tests. The role of adjunctive cancer treatments on incidence of NirAEs is unclear. Treatment guidelines need to be improved through trials comparing IVIG, Plasmapheresis, and immunosuppressants for conditions where choice is left to the practitioner. Undoubtedly these will happen as ICI use and NirAE incidence increases. Until then, consensus treatment guidelines should be used to guide treatment. Table 1 provides a summary combining the guidelines of the three major oncologic organizations.
As a narrative review this work was intended to educate practitioners. A systematic review looking at studies that addresses the various questions above might improve on our conclusions by eliminating studies with methodological issues.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare the following conflict of interests: S.-C. Yeung reports grants from Bausch Health Companies, Inc., Assertio Therapeutics, Inc. (previously Depomed, Inc.), and Bristol-Myer Squibb, and expert panel member at Celgene, Inc. outside the submitted work. DN Lipe is employed by IQVIA Biotech. No potential conflicts of interest were disclosed by the other authors.
Acknowledgement
The authors thank Ann M. Sutton from the Research Medical Library at The University of Texas MD Anderson Cancer Center for editing this manuscript, and Kate J. Krause, Senior Librarian, for the literature search.
References
- 1.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 2.Twomey J.D., Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin-Acevedo J.A., Kimbrough E.O., Lou Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021;14(1):45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA approves anti-LAG3 checkpoint. Nat. Biotechnol. 2022;40:625. doi: 10.1038/s41587-022-01331-0. [DOI] [PubMed] [Google Scholar]
- 5.Tawbi H.A., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 2022;386(1):24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi F.S., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer R.J., et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 2015;33(13):1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T., et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 11.Ansell S.M., et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 2021;16(1):223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 13.Schmid P., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 14.Horn L., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo A., et al. Impact of clinicopathological features on survival in patients treated with first-line immune checkpoint inhibitors plus tyrosine kinase inhibitors for renal cell carcinoma: a meta-analysis of randomized clinical trials. Eur. Urol. Focus. 2022;8(2):514–521. doi: 10.1016/j.euf.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo A., et al. Is there a role for immunotherapy in prostate cancer? Cells. 2020;9(9):2051. doi: 10.3390/cells9092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 18.National N.C.C.N. 2022. Comprehensive cancer Network guidelines in oncology. Management of immunotherapy-related toxicities. Version 1. 2022 february 28.https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf Accessed April 22, 2022] April 22, 2022]; Available from: (sign in required) [DOI] [PubMed] [Google Scholar]
- 19.Brahmer J.R., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer. 2021;9(6):e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goleva E., et al. Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann. Allergy Asthma Immunol. 2021;126(6):630–638. doi: 10.1016/j.anai.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dermani F.K., et al. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J. Cell. Physiol. 2019;234(2):1313–1325. doi: 10.1002/jcp.27172. [DOI] [PubMed] [Google Scholar]
- 22.Robert C. LAG-3 and PD-1 blockade raises the bar for melanoma. Nat. Cancer. 2021;2(12):1251–1253. doi: 10.1038/s43018-021-00276-8. [DOI] [PubMed] [Google Scholar]
- 23.Hryniewicki A.T., et al. Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J. Emerg. Med. 2018;55(4):489–502. doi: 10.1016/j.jemermed.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Baroudjian B., et al. Management of immune-related adverse events resulting from immune checkpoint blockade. Expert Rev. Anticancer Ther. 2019;19(3):209–222. doi: 10.1080/14737140.2019.1562342. [DOI] [PubMed] [Google Scholar]
- 25.Olsen T.A., et al. Advances in knowledge and management of immune-related adverse events in cancer immunotherapy. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.779915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluim D., et al. Enzyme linked immunosorbent assay for the quantification of nivolumab and pembrolizumab in human serum and cerebrospinal fluid. J. Pharm. Biomed. Anal. 2019;164:128–134. doi: 10.1016/j.jpba.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Lorger M., et al. Immune checkpoint blockade - how does it work in brain metastases? Front. Mol. Neurosci. 2019;12:282. doi: 10.3389/fnmol.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parakh S., et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br. J. Cancer. 2017;116(12):1558–1563. doi: 10.1038/bjc.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Giorgi U., et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int. 2019;123(1):98–105. doi: 10.1111/bju.14461. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg S.B., et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(5):655–663. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schagen S.B., et al. Cognitive adverse effects of chemotherapy and immunotherapy: are interventions within reach? Nat. Rev. Neurol. 2022;18(3):173–185. doi: 10.1038/s41582-021-00617-2. [DOI] [PubMed] [Google Scholar]
- 32.Yshii L.M., Hohlfeld R., Liblau R.S. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat. Rev. Neurol. 2017;13(12):755–763. doi: 10.1038/nrneurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 33.Duong S.L., Pruss H. Paraneoplastic autoimmune neurological syndromes and the role of immune checkpoint inhibitors. Neurotherapeutics. 2022;19(3):848–863. doi: 10.1007/s13311-022-01184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogrig A., et al. Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420932797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graus F., Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019;16(9):535–548. doi: 10.1038/s41571-019-0194-4. [DOI] [PubMed] [Google Scholar]
- 36.Binks S., et al. Paraneoplastic neurological syndromes: a practical approach to diagnosis and management. Practical Neurol. 2022;22(1):19–31. doi: 10.1136/practneurol-2021-003073. [DOI] [PubMed] [Google Scholar]
- 37.Vogrig A., et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2019;6(6) doi: 10.1212/NXI.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogrig A., et al. Central nervous system complications associated with immune checkpoint inhibitors. J. Neurol. Neurosurg. Psychiatry. 2020;91(7):772–778. doi: 10.1136/jnnp-2020-323055. [DOI] [PubMed] [Google Scholar]
- 39.Graus F., et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(4):e1014. doi: 10.1212/NXI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin J., et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22(6):709–718. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K., et al. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J. Neuro Oncol. 2019;145(1):1–9. doi: 10.1007/s11060-019-03273-1. [DOI] [PubMed] [Google Scholar]
- 42.Cuzzubbo S., et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer. 2017;73:1–8. doi: 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Farooq M.Z., et al. Association of immune checkpoint inhibitors with neurologic adverse events: a systematic review and meta-analysis. JAMA Netw. Open. 2022;5(4):e227722. doi: 10.1001/jamanetworkopen.2022.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohn N., et al. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy-review of the literature and future outlook. J. Clin. Med. 2019;8(11) doi: 10.3390/jcm8111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marini A., et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology. 2021;96(16):754–766. doi: 10.1212/WNL.0000000000011795. [DOI] [PubMed] [Google Scholar]
- 46.Khan E., et al. CNS and PNS manifestation in immune checkpoint inhibitors: a systematic review. J. Neurol. Sci. 2022;432 doi: 10.1016/j.jns.2021.120089. [DOI] [PubMed] [Google Scholar]
- 47.Dubey D., et al. Severe neurological toxicity of immune checkpoint inhibitors: growing spectrum. Ann. Neurol. 2020;87(5):659–669. doi: 10.1002/ana.25708. [DOI] [PubMed] [Google Scholar]
- 48.NCI. National Cancer Institute . 2017. Common Terminology criteria for adverse events (CTCAE) version 5.0.https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 Accessed April 22, 2022]; Available from: [Google Scholar]
- 49.Katsumoto T.R., et al. Plasma exchange for severe immune-related adverse events from checkpoint inhibitors: an early window of opportunity? Immunother. Adv. 2022;2(1):ltac012. doi: 10.1093/immadv/ltac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D.Y., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan S., et al. Neurological immune-related adverse events associated with immune checkpoint inhibitors: a review of the literature. Asia Pac. J. Clin. Oncol. 2020;16(6):291–298. doi: 10.1111/ajco.13375. [DOI] [PubMed] [Google Scholar]
- 52.Zimmer L., et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S., et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89(11):1127–1134. doi: 10.1212/WNL.0000000000004359. [DOI] [PubMed] [Google Scholar]
- 54.Velasco R., et al. Encephalitis induced by immune checkpoint inhibitors: a systematic review. JAMA Neurol. 2021;78(7):864–873. doi: 10.1001/jamaneurol.2021.0249. [DOI] [PubMed] [Google Scholar]
- 55.Guidon A.C., et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J. Immunother. Cancer. 2021;9(7):e002890. doi: 10.1136/jitc-2021-002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villagran-Garcia M., Velasco R. Neurotoxicity and safety of the rechallenge of immune checkpoint inhibitors: a growing issue in neuro-oncology practice. Neurol. Sci. 2022;43(4):2339–2361. doi: 10.1007/s10072-022-05920-4. [DOI] [PubMed] [Google Scholar]
- 57.Solinas C., et al. Cancer immunotherapy-associated hypophysitis. Semin. Oncol. 2018;45(3):181–186. doi: 10.1053/j.seminoncol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Wick W., Hertenstein A., Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17(12):e529–e541. doi: 10.1016/S1470-2045(16)30571-X. [DOI] [PubMed] [Google Scholar]
- 59.Li Y., Zhang X., Zhao C. Guillain-barre syndrome-like polyneuropathy associated with immune checkpoint inhibitors: a systematic review of 33 cases. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/9800488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssen J.B.E., et al. Immune checkpoint inhibitor-related Guillain-Barre syndrome: a case series and review of the literature. J. Immunother. 2021;44(7):276–282. doi: 10.1097/CJI.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 61.Brzezinska B.N., Higgins R.V., Rungruang B. Guillain-Barre Syndrome in a patient with uterine adenocarcinoma undergoing treatment with immune-checkpoint inhibitor therapy: a case report and review of the literature. Gynecol. Oncol. Rep. 2021;36 doi: 10.1016/j.gore.2021.100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Appelbaum J., et al. Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report. J. Immunother. Cancer. 2018;6(1):82. doi: 10.1186/s40425-018-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dubey D., et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology. 2019;93(11):e1093–e1103. doi: 10.1212/WNL.0000000000008091. [DOI] [PubMed] [Google Scholar]
- 64.Gao C.A., et al. Seronegative autoimmune autonomic ganglionopathy from dual immune checkpoint inhibition in a patient with metastatic melanoma. J. Immunother. Cancer. 2019;7(1):262. doi: 10.1186/s40425-019-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trontzas I.P., et al. Enteric plexus neuropathy associated with PD-L1 blockade in a patient with small-cell lung cancer. Immunotherapy. 2021;13(13):1085–1092. doi: 10.2217/imt-2020-0350. [DOI] [PubMed] [Google Scholar]
- 66.Mohn N., et al. Diagnosis and differential diagnosis of neurological adverse events during immune checkpoint inhibitor therapy. JAMA Oncol. 2020;2020 doi: 10.1155/2020/8865054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safa H., et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J. Immunother. Cancer. 2019;7(1):319. doi: 10.1186/s40425-019-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spain L., et al. How we treat neurological toxicity from immune checkpoint inhibitors. ESMO Open. 2019;4(Suppl 4):e000540. doi: 10.1136/esmoopen-2019-000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Racca F., et al. Practical approach to respiratory emergencies in neurological diseases. Neurol. Sci. 2020;41(3):497–508. doi: 10.1007/s10072-019-04163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipe D.N., et al. Immune checkpoint inhibitor-associated myasthenia gravis, myositis, and myocarditis overlap syndrome. Am. J. Emerg. Med. 2021;46:51–55. doi: 10.1016/j.ajem.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Chen J.H., et al. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: a case report and literature review. Medicine. 2017;96(50):e9262. doi: 10.1097/MD.0000000000009262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohn N., et al. Acute progressive neuropathy-myositis-myasthenia-like syndrome associated with immune-checkpoint inhibitor therapy in patients with metastatic melanoma. Melanoma Res. 2019;29(4):435–440. doi: 10.1097/CMR.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 73.Charabi S., et al. Case report: longitudinal extensive transverse myelitis with novel autoantibodies following two rounds of pembrolizumab. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.655283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abolhassani A.R., et al. C-reactive protein as an early marker of immune-related adverse events. J. Cancer Res. Clin. Oncol. 2019;145(10):2625–2631. doi: 10.1007/s00432-019-03002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung V.T.F., et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br. J. Cancer. 2020;123(2):207–215. doi: 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isik B., et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int. Rep. 2021;6(4):1022–1031. doi: 10.1016/j.ekir.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Husain B., et al. Inflammatory markers in autoimmunity induced by checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2021;147(6):1623–1630. doi: 10.1007/s00432-021-03550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lauwyck J., et al. C-reactive protein as a biomarker for immune-related adverse events in melanoma patients treated with immune checkpoint inhibitors in the adjuvant setting. Melanoma Res. 2021;31(4):371–377. doi: 10.1097/CMR.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 79.Dimitriou F., et al. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur. J. Cancer. 2021;157:214–224. doi: 10.1016/j.ejca.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 80.Zivelonghi C., Zekeridou A. Neurological complications of immune checkpoint inhibitor cancer immunotherapy. J. Neurol. Sci. 2021;424 doi: 10.1016/j.jns.2021.117424. [DOI] [PubMed] [Google Scholar]
- 81.Gardin T., Longbrake E.E. The role of immune checkpoint therapy in propagating neurologic immune-related adverse events: inducing or "unmasking" autoimmunity? Neurology. 2021;96(16):733–734. doi: 10.1212/WNL.0000000000011812. [DOI] [PubMed] [Google Scholar]
- 82.Thouvenin L., et al. Immune checkpoint inhibitor-induced aseptic meningitis and encephalitis: a case-series and narrative review. Ther. Adv. Drug Saf. 2021;12 doi: 10.1177/20420986211004745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson D.B., et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer. 2019;7(1):134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haanen J., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 85.Touat M., et al. Neurological toxicities associated with immune-checkpoint inhibitors. Curr. Opin. Neurol. 2017;30(6):659–668. doi: 10.1097/WCO.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki S. Encephalitis as an immune-related adverse event. J. Neurol. Neurosurg. Psychiatry. 2020;91(7):680. doi: 10.1136/jnnp-2020-323212. [DOI] [PubMed] [Google Scholar]
- 87.Seki M., Kitano S., Suzuki S. Neurological disorders associated with immune checkpoint inhibitors: an association with autoantibodies. Cancer Immunol. Immunother. 2022;71(4):769–775. doi: 10.1007/s00262-021-03053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nannini S., et al. Immune-related aseptic meningitis and strategies to manage immune checkpoint inhibitor therapy: a systematic review. J. Neuro Oncol. 2022;157(3):533–550. doi: 10.1007/s11060-022-03997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blackmon J.T., Viator T.M., Conry R.M. Central nervous system toxicities of anti-cancer immune checkpoint blockade. J. Neurol. Neuromed. 2016;1(4):39–45. [Google Scholar]
- 90.de Ribaupierre S., et al. The role of posterior fossa decompression in acute cerebellitis. Childs Nerv. Syst. 2005;21(11):970–974. doi: 10.1007/s00381-005-1176-7. [DOI] [PubMed] [Google Scholar]
- 91.Mitoma H., Manto M., Hadjivassiliou M. Immune-Mediated cerebellar ataxias: clinical diagnosis and treatment based on immunological and physiological mechanisms. J. Mov. Disord. 2021;14(1):10–28. doi: 10.14802/jmd.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vitt J.R., et al. Autoimmune pancerebellitis associated with pembrolizumab therapy. Neurology. 2018;91(2):91–93. doi: 10.1212/WNL.0000000000005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zurko J., Mehta A. Association of immune-mediated cerebellitis with immune checkpoint inhibitor therapy. Mayo Clin. Proc. Innov. Qual. Outcomes. 2018;2(1):74–77. doi: 10.1016/j.mayocpiqo.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saikawa H., et al. Acute cerebellar ataxia due to Epstein-Barr virus under administration of an immune checkpoint inhibitor. BMJ Case Rep. 2019;12(12):e231520. doi: 10.1136/bcr-2019-231520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardwick M., et al. CD8 T-cell-mediated cerebellitis directed against Purkinje cell antigen after ipilimumab for small cell lung cancer. Neuropathol. Appl. Neurobiol. 2022;48(2) doi: 10.1111/nan.12755. [DOI] [PubMed] [Google Scholar]
- 96.Bartynski W.S. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am. J. Neuroradiol. 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaughn C., Zhang L., Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr. Oncol. Rep. 2008;10(1):86–91. doi: 10.1007/s11912-008-0013-z. [DOI] [PubMed] [Google Scholar]
- 98.Fugate J.E., Rabinstein A.A. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 99.Fischer M., Schmutzhard E. Posterior reversible encephalopathy syndrome. J. Neurol. 2017;264(8):1608–1616. doi: 10.1007/s00415-016-8377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mergen S., Long B., Matlock A. Posterior reversible encephalopathy syndrome: a narrative review for emergency clinicians. J. Emerg. Med. 2021;61(6):666–673. doi: 10.1016/j.jemermed.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Fugate J.E., et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin. Proc. 2010;85(5):427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grewal J., Grewal H.K., Forman A.D. Seizures and epilepsy in cancer: etiologies, evaluation, and management. Curr. Oncol. Rep. 2008;10(1):63–71. doi: 10.1007/s11912-008-0010-2. [DOI] [PubMed] [Google Scholar]
- 103.Singer S., et al. Posterior reversible encephalopathy syndrome in patients with cancer. Oncol. 2015;20(7):806–811. doi: 10.1634/theoncologist.2014-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maur M., et al. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J. Clin. Oncol. 2012;30(6):e76–e78. doi: 10.1200/JCO.2011.38.7886. [DOI] [PubMed] [Google Scholar]
- 105.Hussein H.M., Dornfeld B., Schneider D.J. Nivolumab-induced posterior reversible encephalopathy syndrome. Neurol. Clin. Pract. 2017;7(5):455–456. doi: 10.1212/CPJ.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.LaPorte J., Solh M., Ouanounou S. Posterior reversible encephalopathy syndrome following pembrolizumab therapy for relapsed Hodgkin's lymphoma. J. Oncol. Pharm. Pract. 2017;23(1):71–74. doi: 10.1177/1078155215620922. [DOI] [PubMed] [Google Scholar]
- 107.Mancone S., et al. Severe neurologic complications of immune checkpoint inhibitors: a single-center review. J. Neurol. 2018;265(7):1636–1642. doi: 10.1007/s00415-018-8890-z. [DOI] [PubMed] [Google Scholar]
- 108.Kim D. Posterior reversible encephalopathy syndrome induced by nivolumab immunotherapy for non-small-cell lung cancer. Clin. Case Rep. 2019;7(5):935–938. doi: 10.1002/ccr3.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barber F.D. Identification and management of posterior reversible encephalopathy syndrome in a patient enrolled in an immunotherapy combination phase I clinical trial: a case study. Asia Pac. J. Oncol. Nurs. 2021;8(1):103–105. doi: 10.4103/apjon.apjon_49_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sabile J.M., et al. Posterior reversible encephalopathy syndrome (PRES) and drug-induced hypersensitivity syndrome (DIHS) following immunotherapy and BRAF/MEK inhibition with continued response in metastatic melanoma. Case Rep. Oncol. Med. 2021;2021 doi: 10.1155/2021/8845063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foulser P.F.G., et al. Posterior reversible encephalopathy syndrome associated with use of Atezolizumab for the treatment of relapsed triple negative breast cancer. Cancer Treat. Res. Commun. 2022;31 doi: 10.1016/j.ctarc.2022.100548. [DOI] [PubMed] [Google Scholar]
- 112.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple sclerosis. N. Engl. J. Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGinley M.P., Goldschmidt C.H., Rae-Grant A.D. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–779. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 114.Collongues N., et al. A narrative review on axonal neuroprotection in multiple sclerosis. Neurol. Ther. 2022;11(3):981–1042. doi: 10.1007/s40120-022-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia C.R., et al. Multiple sclerosis outcomes after cancer immunotherapy. Clin. Transl. Oncol. 2019;21(10):1336–1342. doi: 10.1007/s12094-019-02060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan P.C., Haggiagi A. Neurologic immune-related adverse events associated with immune checkpoint inhibition. Curr. Oncol. Rep. 2019;21(12):108. doi: 10.1007/s11912-019-0859-2. [DOI] [PubMed] [Google Scholar]
- 117.Oliveira M.C.B., de Brito M.H., Simabukuro M.M. Central nervous system demyelination associated with immune checkpoint inhibitors: review of the literature. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.538695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Albarran V., et al. Neurologic toxicity of immune checkpoint inhibitors: a review of literature. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.774170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caputo F., et al. Durvalumab and multiple sclerosis: a causal link or simple unmasking? Eur. J. Clin. Pharmacol. 2020;76(12):1773–1774. doi: 10.1007/s00228-020-02959-0. [DOI] [PubMed] [Google Scholar]
- 120.Filippi M., et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain. 2019;142(7):1858–1875. doi: 10.1093/brain/awz144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ziemssen T., Akgun K., Bruck W. Molecular biomarkers in multiple sclerosis. J. Neuroinflammation. 2019;16(1):272. doi: 10.1186/s12974-019-1674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodriguez Y., et al. Guillain-Barre syndrome, transverse myelitis and infectious diseases. Cell. Mol. Immunol. 2018;15(6):547–562. doi: 10.1038/cmi.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lopez Chiriboga S., Flanagan E.P. Myelitis and other autoimmune Myelopathies. Continuum. 2021;27(1):62–92. doi: 10.1212/CON.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 124.Liao B., et al. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16(4):589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang V.A., et al. Infliximab for treatment-refractory transverse myelitis following immune therapy and radiation. J. Immunother. Cancer. 2018;6(1):153. doi: 10.1186/s40425-018-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson R., et al. Seronegative antibody-mediated neurology after immune checkpoint inhibitors. Ann. Clin. Transl. Neurol. 2018;5(5):640–645. doi: 10.1002/acn3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nowosielski M., et al. Encephalomyeloneuritis and arthritis after treatment with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(4) doi: 10.1212/NXI.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bolz S., et al. Detect it so you can treat it: a case series and proposed checklist to detect neurotoxicity in checkpoint therapy. eNeurologicalSci. 2021;22 doi: 10.1016/j.ensci.2021.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moodie T., Alshaqi O., Alchaki A. Longitudinal extensive transverse myelitis after chemoradiation therapy with durvalumab, a rare complication: case report. BMC Neurol. 2022;22(1):107. doi: 10.1186/s12883-022-02576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Picca A., et al. Longitudinally extensive myelitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(3) doi: 10.1212/NXI.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Picca A., et al. Anti-Interleukin-6 and Janus kinase inhibitors for severe neurologic toxicity of checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(6) doi: 10.1212/NXI.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Francis J.H., et al. Immune checkpoint inhibitor-associated optic neuritis. Ophthalmology. 2020;127(11):1585–1589. doi: 10.1016/j.ophtha.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jarius S., Wildemann B., Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 2014;176(2):149–164. doi: 10.1111/cei.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nasralla S., Abboud H. Is neuromyelitis optica without AQP4-IgG a T-cell mediated disease? insights from checkpoint inhibitor immune-related adverse events. Multi Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102451. [DOI] [PubMed] [Google Scholar]
- 135.Khimani K., et al. Case report: neuromyelitis optica after treatment of uveal melanoma with nivolumab and ipilimumab. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.806501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weiss D., et al. Lethal form of a late-onset aquaporin-4 antibody-positive NMOSD related to the immune checkpoint inhibitor nivolumab. J. Neurol. 2022;269(5):2778–2780. doi: 10.1007/s00415-021-10913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pohl D., et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87(9 Suppl 2):S38–S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 138.Mader I., et al. Acute haemorrhagic encephalomyelitis (AHEM): MRI findings. Neuropediatrics. 2004;35(2):143–146. doi: 10.1055/s-2004-817906. [DOI] [PubMed] [Google Scholar]
- 139.Matsuoka H., et al. Nivolumab-induced limbic encephalitis with anti-hu antibody in a patient with advanced pleomorphic carcinoma of the lung. Clin. Lung Cancer. 2018;19(5):e597–e599. doi: 10.1016/j.cllc.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 140.Zafar Z., et al. Nivolumab-associated acute demyelinating encephalitis: a case report and literature review. Clin. Med. Res. 2019;17(1–2):29–33. doi: 10.3121/cmr.2019.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bjursten S., et al. Early rise in brain damage markers and high ICOS expression in CD4+ and CD8+ T cells during checkpoint inhibitor-induced encephalomyelitis. J. Immunother. Cancer. 2021;9(7):e002732. doi: 10.1136/jitc-2021-002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fan Q., et al. Guillain-Barre syndrome in patients treated with immune checkpoint inhibitors. J. Neurol. 2021;268(6):2169–2174. doi: 10.1007/s00415-021-10404-0. [DOI] [PubMed] [Google Scholar]
- 143.Shahrizaila N., Lehmann H.C., Kuwabara S. Guillain-Barre syndrome. Lancet. 2021;397(10280):1214–1228. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- 144.McNeill C.J., et al. A rare case of Miller Fisher variant of Guillain-Barre Syndrome (GBS) induced by a checkpoint inhibitor. BMJ Case Rep. 2019;12(8) doi: 10.1136/bcr-2019-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yildirim N., et al. Fatal acute motor axonal neuropathy induced by nivolumab: a case report and literature review. Clin. Genitourin. Cancer. 2019;17(6):e1104–e1107. doi: 10.1016/j.clgc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 146.Okada K., et al. Polyradiculoneuropathy induced by immune checkpoint inhibitors: a case series and review of the literature. J. Neurol. 2021;268(2):680–688. doi: 10.1007/s00415-020-10213-x. [DOI] [PubMed] [Google Scholar]
- 147.Haugh A.M., Probasco J.C., Johnson D.B. Neurologic complications of immune checkpoint inhibitors. Expet Opin. Drug Saf. 2020;19(4):479–488. doi: 10.1080/14740338.2020.1738382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu M., et al. Risk of neurological toxicities following the use of different immune checkpoint inhibitor regimens in solid tumors: a systematic review and meta-analysis. Neurologist. 2019;24(3):75–83. doi: 10.1097/NRL.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 149.Kao J.C., Brickshawana A., Liewluck T. Neuromuscular complications of programmed cell death-1 (PD-1) inhibitors. Curr. Neurol. Neurosci. Rep. 2018;18(10):63. doi: 10.1007/s11910-018-0878-7. [DOI] [PubMed] [Google Scholar]
- 150.Watson J.C., Dyck P.J. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin. Proc. 2015;90(7):940–951. doi: 10.1016/j.mayocp.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Hammi C., Yeung B. StatPearls. StatPearls Publishing LLC; Treasure Island (FL): 2022. Neuropathy. StatPearls Publishing Copyright © 2022. [Google Scholar]
- 152.Hottinger A.F. Neurologic complications of immune checkpoint inhibitors. Curr. Opin. Neurol. 2016;29(6):806–812. doi: 10.1097/WCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 153.Astaras C., et al. Neurological adverse events associated with immune checkpoint inhibitors: diagnosis and management. Curr. Neurol. Neurosci. Rep. 2018;18(1):3. doi: 10.1007/s11910-018-0810-1. [DOI] [PubMed] [Google Scholar]
- 154.Man J., et al. Treatment-related toxicities of immune checkpoint inhibitors in advanced cancers: a meta-analysis. Asia Pac. J. Clin. Oncol. 2018;14(3):141–152. doi: 10.1111/ajco.12838. [DOI] [PubMed] [Google Scholar]
- 155.Chen X., et al. Electrophysiological findings in immune checkpoint inhibitor-related peripheral neuropathy. Clin. Neurophysiol. 2019;130(8):1440–1445. doi: 10.1016/j.clinph.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Archibald W.J., et al. Brief communication: immune checkpoint inhibitor-induced diaphragmatic dysfunction: a case series. J. Immunother. 2020;43(3):104–106. doi: 10.1097/CJI.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 157.Srinivasan M., et al. Acute bilateral phrenic nerve neuropathy causing hypercapnic respiratory associated with checkpoint inhibitor immunotherapy. Respir. Med. Case Rep. 2021;34 doi: 10.1016/j.rmcr.2021.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brahmer J.R., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abdel-Wahab N., et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann. Intern. Med. 2018;168(2):121–130. doi: 10.7326/M17-2073. [DOI] [PubMed] [Google Scholar]
- 160.Cooper D.S., et al. Severe exacerbation of myasthenia gravis associated with checkpoint inhibitor immunotherapy. J. Neuromuscul. Dis. 2017;4(2):169–173. doi: 10.3233/JND-170219. [DOI] [PubMed] [Google Scholar]
- 161.Makarious D., Horwood K., Coward J.I.G. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur. J. Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 162.Puwanant A., et al. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul. Disord. 2019;29(2):127–133. doi: 10.1016/j.nmd.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 163.Shi J., et al. Association between clinical factors and result of immune checkpoint inhibitor related myasthenia gravis: a single center experience and systematic review. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.858628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kimura T., et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016;107(7):1055–1058. doi: 10.1111/cas.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beloor Suresh A., Asuncion R.M.D. StatPearls. StatPearls Publishing LLC; Treasure Island (FL): 2022. Myasthenia gravis. StatPearls Publishing Copyright © 2022. [PubMed] [Google Scholar]
- 166.Johansen A., et al. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology. 2019;92(14):663–674. doi: 10.1212/WNL.0000000000007235. [DOI] [PubMed] [Google Scholar]
- 167.Takamatsu K., et al. Immune checkpoint inhibitors in the onset of myasthenia gravis with hyperCKemia. Ann. Clin. Transl. Neurol. 2018;5(11):1421–1427. doi: 10.1002/acn3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gonzalez N.L., et al. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul. Disord. 2017;27(3):266–268. doi: 10.1016/j.nmd.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 169.Pathak R., et al. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist. 2021;26(12):1052–1061. doi: 10.1002/onco.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saishu Y., et al. Nivolumab-related myasthenia gravis with myositis requiring prolonged mechanical ventilation: a case report. J. Med. Case Rep. 2022;16(1):61. doi: 10.1186/s13256-022-03286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jaretzki A., 3rd, et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology. 2000;55(1):16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 172.Reynolds K.L., Guidon A.C. Diagnosis and management of immune checkpoint inhibitor-associated neurologic toxicity: illustrative case and review of the literature. Oncologist. 2019;24(4):435–443. doi: 10.1634/theoncologist.2018-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Becquart O., et al. Myasthenia gravis induced by immune checkpoint inhibitors. J. Immunother. 2019;42(8):309–312. doi: 10.1097/CJI.0000000000000278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.