Key Points
Question
Can the outcomes of pivotal trials conducted among patients with extensive-stage small cell lung cancer (ES-SCLC) be applied in a clinical practice setting?
Findings
In this cohort study of 207 patients with ES-SCLC who received chemoimmunotherapy as the first-line treatment, the overall treatment outcome was comparable to that reported in pivotal clinical trials. However, treatment outcomes after chemoimmunotherapy might differ between trial-eligible and trial-ineligible patients.
Meaning
These findings suggest that it may be useful to consider trial-eligibility criteria for clinical decision-making in a clinical practice setting; further studies using high-quality clinical practice data are required to elucidate the association of eligibility criteria with clinical outcomes.
Abstract
Importance
Chemoimmunotherapy is the standard first-line therapy for patients with extensive-stage small cell lung cancer (ES-SCLC). However, whether findings from pivotal trials can be extrapolated to the clinical practice setting remains unclear.
Objective
To compare treatment outcome gaps following first-line chemoimmunotherapy for patients with ES-SCLC between those who met and did not meet the eligibility criteria used in previous clinical trials.
Design, Setting, and Participants
A prospective cohort study was conducted from September 1, 2019, to September 30, 2020, at 32 hospitals in Japan, with at least 12 months of follow-up. Participants included consecutive patients with ES-SCLC who received carboplatin and etoposide with atezolizumab as first-line therapy.
Exposures
Patients who met eligibility criteria for pivotal phase 3 clinical trials were considered trial-eligible.
Main Outcomes and Measures
The primary outcome was 6-month progression-free survival. The secondary outcomes were differences in progression-free survival, overall survival, and safety according to whether key clinical trial eligibility criteria were met.
Results
A total of 207 patients were analyzed (median age, 72 years; range, 46-87 years; 170 [82%] were male). Sixty-four patients (31%) were older adults (age ≥75 years), and most (184 [89%]) had an Eastern Cooperative Oncology Group performance status of 0 or 1. There were 132 (64%) trial-eligible patients. The 6-month progression-free survival rate for all patients was 38.8% (95% CI, 32.4%-45.7%). The median progression-free survival was 5.1 months in trial-eligible patients and 4.7 months in trial-ineligible patients (hazard ratio, 0.72; 95% CI, 0.53-0.97; P = .03). The proportion of patients who achieved disease control was 93% (118 of 127) in trial-eligible patients and 77% (55 of 71) in trial-ineligible patients (P = .002). The median overall survival was 15.8 months in trial-eligible patients and 13.1 months in trial-ineligible patients (hazard ratio, 0.73; 95% CI, 0.51-1.07; P = .10). The rate of severe adverse events was numerically higher among trial-ineligible patients than among trial-eligible patients (39% vs 27%; P = .07).
Conclusions and Relevance
In this cohort study, the overall treatment outcome was comparable to that reported in pivotal clinical trials. However, treatment outcomes after chemoimmunotherapy might differ between trial-eligible and trial-ineligible patients. These findings suggest that trial-eligibility criteria may be useful in clinical practice, and further studies using data from clinical practice settings are required to inform regulatory approval and clinical decision-making.
This cohort study compares the outcomes of patients with extensive-stage small cell lung cancer who were included in clinical trials with outcomes of patients who were ineligible for those trials.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide.1 Small cell lung cancer (SCLC) accounts for 15% to 20% of all lung cancer cases, and extensive-stage SCLC (ES-SCLC) accounts for approximately two-thirds of all SCLC cases.1,2 The development of programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) checkpoint inhibitors has markedly changed the treatment strategy for ES-SCLC. In a pivotal phase 3 trial, the addition of atezolizumab to chemotherapy resulted in longer progression-free survival (PFS) (5.2 vs 4.3 months; P = .02; hazard ratio [HR], 0.77; 95% CI, 0.62-0.96) and overall survival (12.3 vs 10.3 months; P = .007; HR, 0.70; 95% CI, 0.54-0.91) than chemotherapy.3 Consequently, chemoimmunotherapy, ie, the addition of PD-L1 inhibitors to the combination of a platinum agent and etoposide, has become a standard first-line treatment option for ES-SCLC.3,4,5,6
Because of the safety and efficacy data of clinical trials for ES-SCLC, chemoimmunotherapy has been used for patients in clinical practice. However, many patients in this setting have been excluded from previous clinical trials due to the strict eligibility criteria, which mainly included patients in good medical condition.7,8,9 Thus, the outcomes of these clinical trials are not entirely representative of those of patients in clinical practice settings. There has been increased interest in using clinical practice data to address clinical and policy-relevant questions that cannot be answered with clinical trial data.10,11,12,13 One important use of data from clinical practice is to evaluate the outcomes of medical treatments for underrepresented populations, including clinical trial–ineligible patients.14
A recent important retrospective cohort study15 did not show a survival difference between immune checkpoint inhibitors (ICIs) and non-ICI therapies in trial-ineligible patients compared with that in trial-eligible patients. However, there were many missing values in the eligibility criteria for this study, and only a few common exclusion criteria were used owing to the retrospective nature of the research. In addition, the study included patients with 4 carcinomas other than ES-SCLC who received a variety of regimens, few of which were chemoimmunotherapy. However, many cancers, including ES-SCLC, are increasingly being treated with chemoimmunotherapy as first-line treatment.16,17,18,19 Therefore, to examine the external validity of previous findings of pivotal clinical trials, a prospective study of first-line chemoimmunotherapy that includes trial-ineligible populations is critical. In this prospective cohort study, we aimed to investigate the clinical outcomes of chemoimmunotherapy as a first-line treatment in patients with ES-SCLC and focused on the association between eligibility criteria and clinical outcomes.
Methods
Study Design and Participants
This was a multicenter, prospective, hospital-based cohort study of consecutive patients with ES-SCLC who received carboplatin and etoposide with atezolizumab as the first-line treatment at any of the 32 participating hospitals in Japan between September 1, 2019, and September 30, 2020. The patients received atezolizumab until the occurrence of unacceptable toxic effects or disease progression. The study was approved by the ethical/institutional review board of each participating institution and was conducted following the provisions of the Declaration of Helsinki.20 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. All patients provided written informed consent before study entry. Patients did not receive financial compensation. The study was registered with the University Medical Information Network Clinical Trials Registry.
Clinical End Points
The primary outcome of this study was 6-month PFS. Secondary outcomes comprised differences in PFS, overall survival (OS), and safety, according to whether key eligibility criteria of previous trials were met, OS, PFS, objective response rate, and safety.
Definitions and Assessments
Our study defined patients who met the eligibility criteria for pivotal phase 3 clinical trials as trial-eligible patients. The key eligibility criteria of previous pivotal trials are described in eTable 1 in Supplement 1. Because 2 pivotal trials used different criteria for patients with brain metastases, our study established 2 criteria: in criteria 1, patients with untreated asymptomatic brain metastases were considered trial-eligible; in criteria 2, these patients were considered trial-ineligible.3,4
Older patients were defined as those aged 75 years or older, and poor performance status (PS) was defined as an Eastern Cooperative Oncology Group (ECOG) PS score of 2 or higher. Smoking status was categorized as never (never smoked), current (smoked within 1 year of diagnosis), or former (other smoking status). Clinical staging was performed according to the TNM staging system (eighth edition). Extensive stage was diagnosed based on the Veterans Administration Lung Study Group staging system.21 Antitumor responses were assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Progression-free survival was calculated as the interval between the initiation date of combination therapy and the date of disease progression or death from any cause. Overall survival was calculated as the date of death from any cause.
The attending physician evaluated adverse events (AEs) according to the Common Terminology Criteria for Adverse Events (version 5.0). Safety was investigated using AE data related to combination therapy, including grade 3 or higher nonhematologic AEs and grade 4 or higher hematologic AEs. Severe AEs were defined as grade 3 or higher nonhematologic AEs.
Clinically important factors (age, sex, ECOG PS, key trial eligibility criteria, and brain metastases) were used to determine the associations between patient characteristics and treatment outcomes, considering the results of previous studies.3,4,14 Chest-abdominal computed tomography and brain imaging studies were recommended every 6 to 9 weeks to evaluate treatment efficacy. The data cutoff date for the current analysis was October 1, 2021.
Statistical Analysis
The purpose of the primary analysis was to test the hypothesis that carboplatin and etoposide with atezolizumab, considered effective in pivotal phase 3 clinical trials, show similar outcomes in a clinical practice population. The 6-month PFS rate in patients with ES-SCLC who received standard carboplatin and etoposide chemotherapy was 22%, and the rate in those who received carboplatin and etoposide with atezolizumab was 31% in a pivotal phase 3 study.3 In this study, the 6 month PFS rate threshold and expected 6 months PFS rate were set as 22% and 31%, respectively. Under this assumption, the required sample size to test the difference was 188 (α = 0.05, 2-sided, and 1 − β = 0.80). Allowing for protocol deviation in 5% of patients, we planned the total number of patients as 200.
Age was compared using the t test. Categorical variables are presented as numbers and percentages and were compared using the χ2 test or Fisher exact test. The Kaplan-Meier method was used to estimate survival outcomes, and groups were compared using the log-rank test and Cox proportional hazards regression model. Results are expressed as HRs with 95% CIs. A 2-sided P value <.05 was considered statistically significant. Statistical analysis was performed using JMP pro, version 16 software (SAS Institute Inc).
Results
Patient Characteristics
Between September 1, 2019, and September 30, 2020, 208 patients were enrolled. A flow diagram summarizing the patient selection procedure is presented in eFigure 1 in Supplement 1. We analyzed 207 patients (170 were male [82%] and 37 [18%] were female) with ES-SCLC who received carboplatin and etoposide with atezolizumab as the first-line treatment. The median age was 72 years (range, 46-87 years), and 64 (31%) patients were aged 75 years or older. Most patients (184 [89%]) had PS of 0 or 1. The number of trial-eligible patients based on criteria 1 was 132 (64%) and criteria 2 was 116 (56%). The baseline patient characteristics and reasons for ineligibility are described in Table 1. The median follow-up period was 12.7 (IQR, 6.7-16.1) months.
Table 1. Baseline Patient Characteristics.
| Characteristics | Overall, No. (%) |
|---|---|
| No. | 207 |
| Age, y | |
| Median (range) | 72 (46-87) |
| ≥75 | 64 (31) |
| Sex | |
| Male | 170 (82) |
| Female | 37 (18) |
| Smoking status | |
| Current | 100 (48) |
| Former | 101 (49) |
| Never | 6 (3) |
| ECOG performance status | |
| 0 | 60 (29) |
| 1 | 124 (60) |
| 2 | 17 (8) |
| 3 | 6 (3) |
| Stage | |
| 3 | 9 (4) |
| 4 | 161 (78) |
| Recurrent | 37 (18) |
| Metastatic sites | |
| Brain metastases | 54 (26) |
| Eligibility using criteria 1a | |
| Eligible | 132 (64) |
| Ineligible | 75 (36) |
| Eligibility using criteria 2a | |
| Eligible | 116 (56) |
| Ineligible | 91 (44) |
| Reason for ineligibility | |
| ECOG performance status 2-3 | 23 (11) |
| Active metastases | |
| Symptomatic CNS metastases | 12 (6) |
| Untreated asymptomatic CNS metastases | 25 (12) |
| Uncontrolled pleural effusion, pericardial effusion, or ascites requiring recurrent drainage procedures | 3 (1) |
| Abnormal laboratory findings | |
| Inadequate hematologic function | 5 (2) |
| Increased AST and ALT | 8 (4) |
| Serum bilirubin >1.25 × ULN | 3 (1) |
| Serum creatinine >1.5 × ULN | 5 (2) |
| Comorbidity | |
| History of autoimmune diseases | 2 (1) |
| Preexisting interstitial lung disease | 8 (4) |
| Treatment with systemic immunosuppressive medications | 9 (4) |
| Cancers other than SCLC within 5 y | 25 (12) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; SCLC, small cell lung cancer; ULN, the upper limit of normal.
In criteria 1, those with untreated asymptomatic brain metastases were considered trial-eligible, while in criteria 2, they were considered trial-ineligible.
Outcomes in the Overall Population
Of the 198 patients who had target lesions at baseline, the total objective response rate was 71%; specifically, 2.5% of patients achieved a complete response and 68.7% achieved a partial response. Overall, 16% of patients had stable disease, 6% had progressive disease (PD), and 7% were not evaluated. One hundred fifty-four patients (74%) received 4 cycles of the induction triple-drug combination. There were 186 PFS events (90%) and 117 OS events (57%) during follow-up. The median PFS of the combination therapy group was 4.9 months (95% CI, 4.6-5.4 months) and the median OS was 14.7 months (95% CI, 12.6-16.2 months) (Figure 1). The 6-month PFS probability was 38.8% (95% CI, 32.4%-45.7%). The 12-month OS probability was 60.8% (95% CI, 53.9%–67.3%) and the 18-month OS probability was 40.4% (95% CI, 33.1%-48.2%). Among patients who discontinued therapy (n = 183), 128 patients (70%) received subsequent anticancer therapies.
Figure 1. Survival Curves in the Overall Population of Patients With Extensive-Stage Small Cell Lung Cancer.
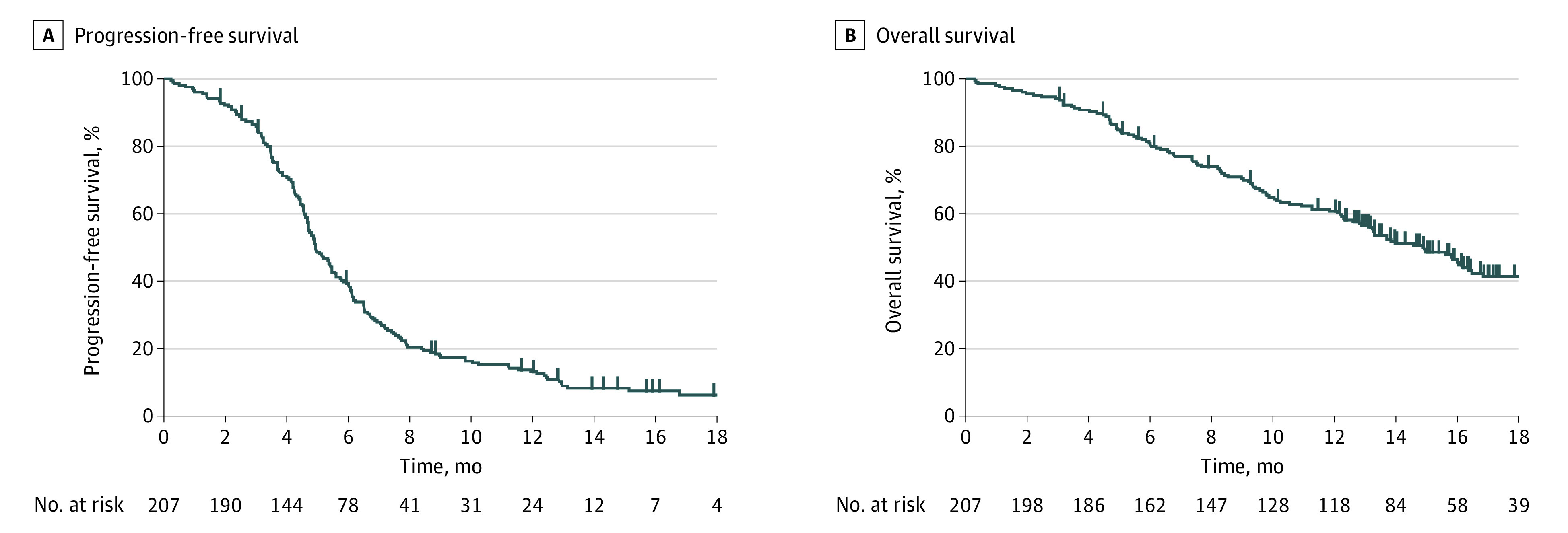
Progression-free survival (A) and overall survival (B).
Outcomes According to the Eligibility Criteria and Patient Characteristics
The objective response rate was 72% (92 of 127) in trial-eligible patients and 69% (49 of 71) in trial-ineligible patients using eligibility criteria 1 (P = .61) and 73% (83 of 113) in trial-eligible patients and 68% (58 of 85) in trial-ineligible patients using eligibility criteria 2 (P = .42). The disease control rate was 93% (118 of 127) in trial-eligible patients and 77% (55 of 71) in trial-ineligible patients using eligibility criteria 1 (P = .002) and 93% (105 of 113) in trial-eligible patients and 80% (68 of 85) of trial-ineligible patients using eligibility criteria 2 (P = .007). Kaplan-Meier curves for PFS and OS based on eligibility criteria 1 and 2 are shown in Figure 2 and eFigure 2 in Supplement 1. Based on criteria 1, the median PFS was 5.1 months in trial-eligible patients and 4.7 months in trial-ineligible patients (HR, 0.72; 95% CI, 0.53-0.97; P = .03), and the 6-month PFS probability was 41.9% in trial-eligible patients and 32.0% in trial-eligible patients. The median OS was 15.8 months in trial-eligible patients and 13.1 months in trial-ineligible patients (HR, 0.73; 95% CI, 0.51-1.07; P = .10). Based on criteria 2, a similar difference was observed for PFS (5.1 vs 4.7 months; HR, 0.82; 95% CI, 0.62-1.10; P = .19), 6-month PFS probability (41.7% vs 34.1%), and OS (15.8 vs 13.1 months; HR, 0.82; 95% CI, 0.57-1.19; P = .30). In the survival analysis according to each eligibility criterion and important patient characteristics, a PS of 0 to 1 (HR, 0.60; 95% CI, 0.39-0.97; P = .03) was associated with significantly better PFS, and a PS of 0 to 1 (HR, 0.51; 95% CI, 0.31-0.89; P = .01) and younger age (<75 years) (HR, 0.66; 95% CI, 0.45-0.97; P = .03) were associated with significantly better OS (Table 2). The Kaplan-Meier curves for PFS and OS stratified according to PS and age are shown in eFigure 3 and eFigure 4 in Supplement 1.
Figure 2. Kaplan-Meier Survival Curves According to Eligibility Criteria in Patients With Extensive-Stage Small Cell Lung Cancer.
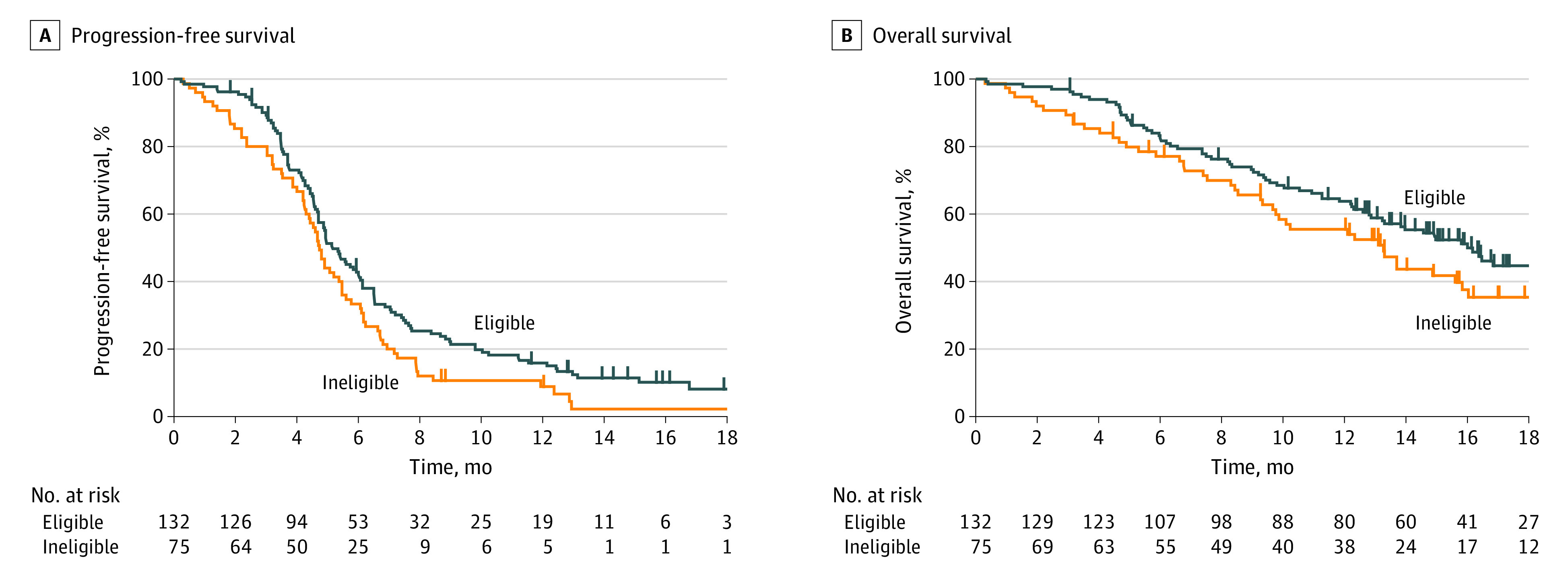
Progression-free survival (A) and overall survival (B) stratified by eligibility criteria 1.
Table 2. Analyses of PFS and OS According to Patient Characteristics.
| Characteristic | Patients, No. (%) | PFS | OS | ||||
|---|---|---|---|---|---|---|---|
| Median PFS, mo | HR (95% CI) | P value | Median OS, mo | HR (95% CI) | P value | ||
| Age, y | |||||||
| <75 | 143 (69) | 5.0 | 0.77 (0.57-1.05) | .09 | 16 | 0.66 (0.45-0.97) | .03 |
| >75 | 64 (31) | 4.7 | 1 [Reference] | 12 | 1 [Reference] | ||
| Sex | |||||||
| Male | 170 (82) | 5.0 | 0.84 (0.58-1.24) | .37 | 14.4 | 0.92 (0.60-1.49) | .74 |
| Female | 37 (18) | 4.3 | 1 [Reference] | 15.6 | 1 [Reference] | ||
| Brain metastases | |||||||
| Yes | 54 (26) | 5.3 | 0.80 (0.57-1.12) | .21 | 16.6 | 0.68 (0.42-1.04) | .08 |
| No | 153 (74) | 4.8 | 1 [Reference] | 13.5 | 1 [Reference] | ||
| Eligibility criteria | |||||||
| ECOG performance status | .03 | .01 | |||||
| 0-1 | 184 (89) | 5.1 | 0.60 (0.39-0.97) | 15.6 | 0.51 (0.31-0.89) | ||
| 2-3 | 23 (11) | 4.1 | 1 [Reference] | 7.4 | 1 [Reference] | ||
| Active metastases (criteria 1a) | |||||||
| Yes | 15 (7) | 5.1 | 1.23 (0.68-2.04) | .45 | 15.6 | 0.75 (0.32-1.50) | .47 |
| No | 192 (93) | 4.9 | 1 [Reference] | 14.7 | 1 [Reference] | ||
| Active metastases (criteria 2a) | |||||||
| Yes | 39 (19) | 4.9 | 1.01 (0.70-1.48) | .97 | 14.4 | 1.18 (0.74-1.99) | .49 |
| No | 168 (81) | 4.9 | 1 [Reference] | 15.6 | 1 [Reference] | ||
| Abnormal laboratory findings | |||||||
| Yes | 18 (9) | 4.3 | 1.27 (0.74-2.03) | .34 | 10.1 | 1.26 (0.64-2.23) | .47 |
| No | 189 (91) | 5.0 | 1 [Reference] | 15.4 | 1 [Reference] | ||
| Comorbidity | |||||||
| Yes | 40 (19) | 4.8 | 1.16 (0.80-1.65) | .41 | 13.5 | 1.27 (0.80-1.94) | .30 |
| No | 167 (81) | 4.9 | 1 [Reference] | 14.7 | 1 [Reference] | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
In criteria 1, those with untreated asymptomatic brain metastases were considered trial-eligible, while in criteria 2, they were considered trial-ineligible.
Safety in the Overall Population
Treatment-related AEs are described in eTable 2 in Supplement 1. Overall, 64 patients (31%) experienced grade 3 or higher nonhematologic AEs, and 73 patients (35%) experienced grade 4 or higher hematologic AEs. Among the patients who developed grade 3 or higher nonhematologic AEs, the most frequent AE was febrile neutropenia (13%). In total, 25 patients (12%) discontinued all treatment components due to AEs (including refusal to continue treatment due to AEs). Of these AEs, the main ones were pneumonitis (6 [24%]) and infection (5 [20%]). Four patients (2%) died from treatment-related AEs attributed to combination therapy. The AEs included pneumonitis (n = 2), lung infection (n = 1), and hepatic failure (n = 1).
Safety According to Eligibility Criteria and Patient Characteristics
A comparison between patients with and without severe AEs is reported in Table 3. Older age was associated with severe AEs. Severe AEs were numerically more frequent in the following age categories: younger than 65 years (24%), 65 to 74 years (30%), and 75 years or older (36%). Based on both criteria 1 and 2, the rate of severe AEs was numerically higher in trial-ineligible patients vs trial-eligible patients (criteria 1, 39% vs 27%, P = .07; criteria 2, 35% vs 28%; P = .24), although this difference was not statistically significant.
Table 3. Comparisons Between Patients With and Without Severe Adverse Events.
| Variable | No. (%) | P value | |
|---|---|---|---|
| With severe AEs | Without severe AEs | ||
| No. | 64 | 143 | |
| Age, mean (SD), y | 72.1 (7.2) | 69.8 (7.6) | .049 |
| Sex | |||
| Male | 51 (80) | 119 (83) | .54 |
| Female | 13 (20) | 24 (17) | |
| Eligibility using criteria 1 | .07 | ||
| Eligible | 35 (55) | 97 (68) | |
| Ineligible | 29 (45) | 46 (32) | |
| Eligibility using criteria 2 | |||
| Eligible | 32 (50) | 84 (59) | .24 |
| Ineligible | 32 (50) | 59 (41) | |
| Reason for ineligibility | |||
| ECOG performance status 2-3 | 11 (17) | 12 (8) | .07 |
| Active metastase | |||
| Criteria 1 | 13 (20) | 26 (18) | .72 |
| Criteria 2 | 5 (8) | 10 (7) | .83 |
| Abnormal laboratory findings | 9 (14) | 9 (6) | .07 |
| Comorbidity | 14 (22) | 26 (18) | .54 |
Abbreviations: AEs, adverse events; ECOG, Eastern Cooperative Oncology Group.
Discussion
Herein, we investigated clinical practice setting treatment outcomes in patients with ES-SCLC who received chemoimmunotherapy, using what we believe to be one of the largest prospective cohorts to date. The PFS and OS among overall patients in this study were comparable to those in pivotal trials of chemoimmunotherapy for ES-SCLC.3,4,5,6 However, the rates of treatment-related severe AEs and treatment discontinuation due to toxic effects were slightly higher than those reported in previous clinical trials.3,4,5,6 Several previous reports have shown an efficacy-effectiveness gap in cancer treatment.22,23,24 These reports indicate that clinical practice setting effectiveness is inferior to the efficacy in pivotal clinical trials, but this gap varies widely among the evaluated clinical trials.24 In addition, the overall quality of previous studies assessing the efficacy-effectiveness gap was very low.24 Actually, similar outcomes to those in the present study have been observed in recent retrospective observational studies of chemoimmunotherapy for lung cancer.25,26,27 Collectively, these results suggest comparable outcomes to those in clinical trials, while safety is somewhat inferior in clinical practice settings in which patients with lung cancer receive chemoimmunotherapy. However, the external validity of previous clinical trial results could not be fully investigated in these previous retrospective studies, as patients who met the eligibility criteria of clinical trials were expected to have similar outcomes to those in pivotal clinical trials, even in clinical practice settings. Thus, it may be useful to investigate the gap in treatment outcomes between trial-eligible and trial-ineligible patients in a high-quality cohort, such as that in the present study.
To our knowledge, this is the first prospective study to investigate outcomes based on patient eligibility criteria in pivotal clinical trials for ES-SCLC. In this study, trial ineligibility was common, despite the use of chemoimmunotherapy, which is more toxic than conventional therapy. In addition, trial-ineligible patients might have poor treatment outcomes and higher rates of severe AEs. Notably, long-term PFS was limited among these patients. Thus, accumulating evidence suggests the possibility of disparities between clinical practice data and clinical trial data, depending on the proportion of trial-eligible and -ineligible patients.
Strengths and Limitations
The strength of our study is that it was conducted prospectively. A recent important retrospective cohort study involving patients with advanced cancers who preferentially received first-line ICI therapy also focused on the impact of eligibility criteria.15 The study did not report significant differences in outcomes between ICI and non-ICI therapies among trial-ineligible patients. However, in this previous study, ECOG PS data were missing for nearly half of the cohort, and only kidney and liver function were examined as eligibility criteria owing to the retrospective nature of the study. Furthermore, safety data were not available and thus could not be examined. It seems difficult to properly assess trial eligibility and the safety profile without a prospective approach, such as that in the present study. Therefore, prospective studies are needed to assess whether more toxic treatments should be considered the new standard, especially for patients considered ineligible for clinical trials.
There is growing interest by regulatory agencies in using clinical practice data to evaluate the safety and effectiveness of medical treatments. The 21st Century Cures Act passed by the US Congress directed the US Food and Drug Administration to evaluate the use of real-world data to support its regulatory processes.28,29,30 As a result, there has been increased interest in designing and implementing clinical trials that leverage external data sets.31,32,33 These efforts are also very important in lung cancer–related research, as attempts to subdivide patients by biomarkers are increasingly popular in lung cancer treatment, even in the case of SCLC.34,35 Despite the enthusiasm for the regulatory use of clinical practice data, more attention should be paid to the heterogeneity of the data collected, including both trial-eligible and trial-ineligible patients. Consequently, the outcome of a clinical study that uses clinical practice data will depend on the proportion of eligible and ineligible patients if there is a gap in clinical outcomes between them, as suggested in our study. Therefore, it is important to investigate trial eligibility criteria using various clinical practice data, and our findings may be useful for consideration in future clinical practice data–based studies.
This study has some limitations. First, we examined only patients with ES-SCLC who could receive chemoimmunotherapy as the first-line therapy; patients unable to receive this combination therapy were not represented in our study. It is possible that in selecting chemoimmunotherapy, which is a more toxic treatment than conventional therapy, patients in good condition were selected into the study cohort. Second, the data were underpowered for subgroup analyses, especially for each trial ineligibility criterion, precluding any firm conclusions. Future studies are needed to investigate various trial ineligibility criteria. Third, most of the patients in the cohort were Japanese; therefore, our results may not be generalizable to people of other ethnicities.
Conclusions
In this cohort study, the overall treatment outcome in patients with ES-SCLC who received chemoimmunotherapy as the first-line treatment in clinical practice settings was comparable to that reported in pivotal clinical trials. However, treatment outcomes after chemoimmunotherapy might differ between trial-eligible and trial-ineligible patients. Our findings suggest that the positive results among trial-eligible patients may not apply to patients with ES-SCLC who are ineligible for such trials. Trial-eligibility criteria should be considered in clinical practice, and further studies using clinical practice data are required to inform regulatory approval and clinical decision-making.
eTable 1. Key Eligibility Criteria of Previous Pivotal Trials
eTable 2. Treatment-Related Adverse Events (AEs)
eFigure 1. Study Profile
eFigure 2. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Eligibility Criteria 2
eFigure 3. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Performance Status
eFigure 4. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Age
Data Sharing Statement
References
- 1.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7(1):1339. doi: 10.1038/s41598-017-01571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664-672. doi: 10.1002/cncr.29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn L, Mansfield AS, Szczęsna A, et al. ; IMpower133 Study Group . First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L, Dvorkin M, Chen Y, et al. ; CASPIAN Investigators . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 5.Goldman JW, Dvorkin M, Chen Y, et al. ; CASPIAN Investigators . Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51-65. doi: 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 6.Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619-630. doi: 10.1200/JCO.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to enrollment in non–small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6(1):98-102. doi: 10.1097/JTO.0b013e3181fb50d8 [DOI] [PubMed] [Google Scholar]
- 8.Kawachi H, Fujimoto D, Morimoto T, et al. Clinical characteristics and prognosis of patients with advanced non–small-cell lung cancer who are ineligible for clinical trials. Clin Lung Cancer. 2018;19(5):e721-e734. doi: 10.1016/j.cllc.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Horn L, Keedy VL, Campbell N, et al. Identifying barriers associated with enrollment of patients with lung cancer into clinical trials. Clin Lung Cancer. 2013;14(1):14-18. doi: 10.1016/j.cllc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 10.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293-2297. doi: 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 11.Booth CM, Karim S, Mackillop WJ. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16(5):312-325. doi: 10.1038/s41571-019-0167-7 [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Garcia E, Haibe-Kains B, Siu LL. Using real-word data to evaluate the effects of broadening eligibility criteria in oncology trials. Cancer Cell. 2021;39(6):750-752. doi: 10.1016/j.ccell.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Pasello G, Pavan A, Attili I, et al. Real world data in the era of immune checkpoint inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87:102031. doi: 10.1016/j.ctrv.2020.102031 [DOI] [PubMed] [Google Scholar]
- 14.Rzeniewicz K, Larkin J, Menzies AM, Turajlic S. Immunotherapy use outside clinical trial populations: never say never? Ann Oncol. 2021;32(7):866-880. doi: 10.1016/j.annonc.2021.03.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh RB, Min EJ, Wileyto EP, et al. Uptake and survival outcomes following immune checkpoint inhibitor therapy among trial-ineligible patients with advanced solid cancers. JAMA Oncol. 2021;7(12):1843-1850. doi: 10.1001/jamaoncol.2021.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JM, Shen L, Shah MA, et al. ; KEYNOTE-590 Investigators . Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759-771. doi: 10.1016/S0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 18.Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators . Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 19.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37(3):271-276. doi: 10.1016/S0169-5002(02)00072-7 [DOI] [PubMed] [Google Scholar]
- 22.Templeton AJ, Booth CM, Tannock IF. Informing patients about expected outcomes: the efficacy-effectiveness gap. J Clin Oncol. 2020;38(15):1651-1654. doi: 10.1200/JCO.19.02035 [DOI] [PubMed] [Google Scholar]
- 23.Phillips CM, Parmar A, Guo H, et al. Assessing the efficacy-effectiveness gap for cancer therapies: a comparison of overall survival and toxicity between clinical trial and population-based, real-world data for contemporary parenteral cancer therapeutics. Cancer. 2020;126(8):1717-1726. doi: 10.1002/cncr.32697 [DOI] [PubMed] [Google Scholar]
- 24.Boyle JM, Hegarty G, Frampton C, et al. Real-world outcomes associated with new cancer medicines approved by the Food and Drug Administration and European Medicines Agency: a retrospective cohort study. Eur J Cancer. 2021;155:136-144. doi: 10.1016/j.ejca.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elegbede AA, Gibson AJ, Fung AS, et al. A real-world evaluation of atezolizumab plus platinum-etoposide chemotherapy in patients with extensive-stage SCLC in Canada. JTO Clin Res Rep. 2021;2(12):100249. doi: 10.1016/j.jtocrr.2021.100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto D, Miura S, Yoshimura K, et al. A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin Res Rep. 2021;3(2):100265. doi: 10.1016/j.jtocrr.2021.100265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita R, Okishio K, Shimizu J, et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: a multicenter retrospective observational study in Japan. Lung Cancer. 2020;140:8-18. doi: 10.1016/j.lungcan.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration . Real-world evidence. December 12, 2022. Accessed April 1, 2022. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- 29.ElZarrad MK, Corrigan-Curay J. The US Food and Drug Administration’s real-world evidence framework: a commitment for engagement and transparency on real-world evidence. Clin Pharmacol Ther. 2019;106(1):33-35. doi: 10.1002/cpt.1389 [DOI] [PubMed] [Google Scholar]
- 30.Raphael MJ, Gyawali B, Booth CM. Real-world evidence and regulatory drug approval. Nat Rev Clin Oncol. 2020;17(5):271-272. doi: 10.1038/s41571-020-0345-7 [DOI] [PubMed] [Google Scholar]
- 31.Mishra-Kalyani PS, Amiri Kordestani L, Rivera DR, et al. External control arms in oncology: current use and future directions. Ann Oncol. 2022;33(4):376-383. doi: 10.1016/j.annonc.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 32.Rahman R, Ventz S, McDunn J, et al. Leveraging external data in the design and analysis of clinical trials in neuro-oncology. Lancet Oncol. 2021;22(10):e456-e465. doi: 10.1016/S1470-2045(21)00488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ventz S, Lai A, Cloughesy TF, Wen PY, Trippa L, Alexander BM. Design and evaluation of an external control arm using prior clinical trials and real-world data. Clin Cancer Res. 2019;25(16):4993-5001. doi: 10.1158/1078-0432.CCR-19-0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289-297. doi: 10.1038/s41568-019-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baine MK, Hsieh MS, Lai WV, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15(12):1823-1835. doi: 10.1016/j.jtho.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Key Eligibility Criteria of Previous Pivotal Trials
eTable 2. Treatment-Related Adverse Events (AEs)
eFigure 1. Study Profile
eFigure 2. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Eligibility Criteria 2
eFigure 3. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Performance Status
eFigure 4. Kaplan-Meier Survival Curves: Progression-Free Survival and Overall Survival Stratified by Age
Data Sharing Statement


