Abstract
The human splicing factor 2, also called human alternative splicing factor (hASF), is the prototype of the highly conserved SR protein family involved in constitutive and regulated splicing of metazoan mRNA precursors. Here we report that the Drosophila homologue of hASF (dASF) lacks eight repeating arginine-serine dipeptides at its carboxyl-terminal region (RS domain), previously shown to be important for both localization and splicing activity of hASF. While this difference has no effect on dASF localization, it impedes its capacity to shuttle between the nucleus and cytoplasm and abolishes its phosphorylation by SR protein kinase 1 (SRPK1). dASF also has an altered splicing activity. While being competent for the regulation of 5′ alternative splice site choice and activation of specific splicing enhancers, dASF fails to complement S100-cytoplasmic splicing-deficient extracts. Moreover, targeted overexpression of dASF in transgenic flies leads to higher deleterious developmental defects than hASF overexpression, supporting the notion that the distinctive structural features at the RS domain between the two proteins are likely to be functionally relevant in vivo.
The accurate excision of intervening sequences (introns) from RNA polymerase II transcripts is crucial for the expression of most metazoan genes. This process occurs at the level of the spliceosome, a large multicomponent complex containing several small ribonucleoprotein particles (snRNPs) (for reviews, see references 25 and 52). In metazoans, the earliest detectable step triggering spliceosome formation involves the non-snRNP splicing factor U2AF (U2 snRNP auxiliary factor), U1 snRNP, and several other proteins (for a review, see reference 42). This step is a major control point for the initial recognition and pairing of splice sites and is therefore thought to be an important step in the regulation of alternative splicing (42). Among the proteins that contribute to this regulation, members of the SR protein family have been shown to influence splice site choice in a concentration-dependent manner (for reviews, see references 13 and 32). These proteins bind several classes of specific RNA motifs including purine-rich splicing enhancers known as exonic splicing elements, which have been demonstrated to play a key role in both alternative and constitutive splice site selection in several experimental systems (for reviews, see references 1 and 54).
SR proteins are characterized by the presence of one or two copies of an RNA recognition motif (RRM) and a carboxyl (C)-terminal domain rich in arginine and serine residues (13, 32). They are thus very closely related in domain structure, primary sequence, and functional properties. Functionally, many of the SR proteins are able to complement the splicing-deficient activity of postnuclear S100 extracts and can affect usage of alternative 5′ or 3′ splice sites in a concentration-dependent manner (13, 32). The RS domain is responsible for specific protein-protein interactions between RS domain-containing proteins (22, 58, 64). Such physical interactions indeed promote the binding of U1 snRNP to the 5′ splice site and constitute a bridge between 5′ and 3′ splice sites during splice site selection (58) at the earliest stages of spliceosome assembly (42). Although homophilic and heterophilic RS domain interactions are likely to be general mechanisms by which splice site selection is regulated, the rules governing these associations are still unknown. The specific phosphorylation of serine residues located within the RS domain may be one of the key determinants regulating splicing events. The findings that SR proteins are phosphorylated in vivo (47) and that the splicing activities of the U1 snRNP-specific protein (U1-70K) and splicing factor 2 (also called alternative splicing factor [ASF]) are influenced by the phosphorylation state of the RS domains support this hypothesis (56, 59; for a review, see reference 57).
Another level of regulation mediated by the RS domain can be attributed to cellular localization of SR proteins. Immunofluorescence analyses have shown that SR proteins are present in the nucleoplasm of interphase nuclei and exhibit a speckled pattern of staining (4, 34). The RS domain of some, but not all, SR proteins could serve as a targeting signal to the nuclear speckles (4, 19). Upon transcriptional activation of a gene, SR proteins are recruited from speckles to sites of transcription (35), and serine phosphorylation of the RS domain has been shown to be required for this recruitment (36). Given that some human SR proteins shuttle rapidly and continuously between the nucleus and the cytoplasm (5), it is possible that the phosphorylation levels of the RS domain can affect their shuttling properties. Consistent with this possibility, it has been shown that overexpression of the active form of Clk/Sty kinase (see below), but not its inactive form, results in cytoplasmic accumulation of human ASF (hASF), at the expense of the nuclear pool (5).
Information regarding the possible enzymatic activities involved in the phosphorylation of SR proteins has recently emerged. SR protein kinase 1 (SRPK1) can induce the disassembly of speckled intranuclear snRNP and SR protein-containing structures in interphase nuclei (17). Since SR proteins are reported to be hyperphosphorylated in metaphase cells (17), SRPK1 may be the kinase that causes dynamic changes in the phosphorylation state of these structures during the cell cycle. Consistent with this idea is the observation that the level of SRPK1 activity is highest during M phase (17). However, SRPK1 is not the only protein kinase that mediates SR protein phosphorylation and redistribution in the cell. ClK/Sty, a prototypical kinase with dual specificity which is able to phosphorylate tyrosines as well as serines and threonines, is also involved in SR protein phosphorylation, and as observed for SRPK1, overexpression of a catalytically active form of Clk/Sty causes a redistribution of SR proteins in the nucleoplasm of transformed cells (8). Recently, we have shown that DNA topoisomerase I (topo I), which is a constitutively expressed nuclear phosphoprotein that localizes to active transcription sites (57), is an atypical SR protein kinase (27, 45, 46). This kinase activity may allow topo I to participate in the coordination between transcription and splicing. Consistent with this observation, antitumor drugs targeting topo I specifically inhibit spliceosome assembly and splicing in vitro. Also, SR protein complete phosphorylation was inhibited following treatment of HeLa cells with DNA topo I blockers (45). Thus, the diversity of the kinases involved in the phosphorylation of these splicing factors is likely relevant to the function of SR proteins during cell differentiation and/or development. The relevance of the level of SR protein phosphorylation in mediating alternative or constitutive splicing in living cells is, however, unknown.
To understand the regulation of pre-mRNA splicing by SR proteins, it is essential to determine structural features of the RS domain that are tightly correlated with specific function and/or regulation by specific kinases. In this study, we have approached this problem by comparing the functional properties of hASF, a prototype of the SR protein family, to its Drosophila homologue (dASF). While the two proteins show strong overall sequence homology it is intriguing that dASF lacks a long region of repeating RS dipeptides at the beginning of its RS domain. Instead of eight repeating RS dipeptides, the C-terminal domain of dASF contains 14 glycine repeats. Starting from this observation, we have determined the functional incidence of such a difference on the phosphorylation, cellular distribution, and splicing properties of dASF. While being capable to regulate 5′ alternative splice site choice and to activate specific splicing enhancers, dASF differs in three respects from vertebrate SF2/ASF: it is not phosphorylated by SRPK1, it does not shuttle, and it fails to complement HeLa S100 extracts for splicing activation. The two proteins also show distinct abilities to induce developmental defects following overexpression in transgenic flies, implying that the specific structural features of dASF are key determinants for its function(s) during development.
MATERIALS AND METHODS
Sequencing and computer analysis.
BLAST searches in the Berkeley Drosophila Genome Project (BDGP) database for expressed sequence tags (49) that have homologies with human SR proteins revealed three cDNAs encoding dSC35 (LD32469), dASF (LD11844), and d9G8 (LD02483). These cDNAs are encoded by single-copy genes annotated in the GadFly genomic sequences of the BDGP database as CG5542 for the dSC35 gene, CG6987 for the dASF gene, and CG10203 for the d9G8 gene (37). The cytological positions of these genes are 33E5-7 on the left arm of chromosome II, 89B18-89C1 on the right arm of chromosome III, and 27C4-5 on the left arm of chromosome II, respectively. The ClustalW program from the GenBank database was used to determine pairwise sequence alignments between the human and Drosophila melanogaster SR proteins shown in Fig. 1. The DNA Strider program was used to analyze the structural features of the Drosophila SR proteins.
FIG. 1.
Amino acid sequence alignments of dASF (dmSF2/p28) with hASF (hsSF2/ASF), dSC35 (dmSC35) with human SC35 (hsSC35), and d9G8 (dm9G8) with human 9G8 (hs9G8) as obtained by the ClustalW program. Identical amino acid residues are on a black background, and conservative substitutions such as RKH, IVLM, ED, FY, and ST are marked in grey. The glycine-rich region between the RNA-binding domains and the RS domains are underlined. The positions of the conserved RNP-1 and RNP-2 submotifs are indicated. The amino acids belonging to the consensus C(X)2C(X)4H(X)4C forming the zinc knuckles of human and Drosophila 9G8 are represented in red.
Recombinant proteins; plasmids and purification.
Hexahistidine-tagged ASF proteins, wild type (ehASF) or truncated versions (ehASF ΔRS and RS domain of hASF), were produced and purified from Escherichia coli as described previously (27). To obtain a hexahistidine-tagged dASF protein (edASF), a 1.2-kb fragment corresponding to dASF cDNA was cloned in pTrcHis vector (Invitrogen) by PCR amplification of the coding region, using 5′ and 3′ oligonucleotides containing BamHI (Bam5′, cDNA positions 82 to 97) and EcoRI (EcoR3′, cDNA positions 824 to 849) restriction sites, and transformed into the E. coli strain BL21(DE3) (Novagen). The coding sequences of hASF and dASF were inserted in frame upstream of a histidine tag sequence into pVL1393 transfer vector (Invitrogen), and recombinant proteins were produced and purified from baculovirus-infected Sf9 cells as described previously (6, 14).
Phosphorylation of recombinant proteins in vitro and in yeast strains.
For protein kinase activity assays, reaction mixtures contained 100 ng of recombinant topo I or equivalent kinase activity (determined by titration using the RS domain of ASF as the standard substrate) of SRPK1 (a gift from T. Giannakouros) or glutathione S-transferase (GST)-Clk purified from E. coli as described previously (9), 300 ng of recombinant ehASF or edASF in buffer B (27), and 3 μCi of [γ-32P]ATP (3,000 Ci/mmol) in 15 μl (final volume). Following incubation at 30°C for 30 min, the samples were mixed with 5 μl of 3× Laemmli loading buffer and applied to a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel.
GFP fusion protein expression and heterokaryon assays.
Humanized green fluorescent protein (GFP) (pEGFP-C1 [Clontech]; GenBank accession number U55762) was fused in frame to the NH2 terminus of cDNA corresponding to hASF (BamHI-EcoRI fragment from plasmid pTrcHis-SF2/ASF [27]), dASF (BamHI-EcoRI fragment described above), or RBP1 (RBP1 coding sequences were obtained by PCR amplification from plasmid pGexRBP1 [28]). Each cDNA was inserted between BglII and EcoRI or ApaI sites of the pEGFP vector. To allow for expression of GFP fusions containing the RS domain of either hASF or dASF, cDNA fragments spanning codons 196 to 250 and 189 to 256, respectively, were generated by PCR from pTrcHis expression plasmids and cloned in pEGFP-C1 vector between BglII and EcoRI restriction sites. All open reading frames and in-frame fusions were entirely sequenced to verify their integrity. Sequences of the oligonucleotides used for all PCR amplifications are available upon request.
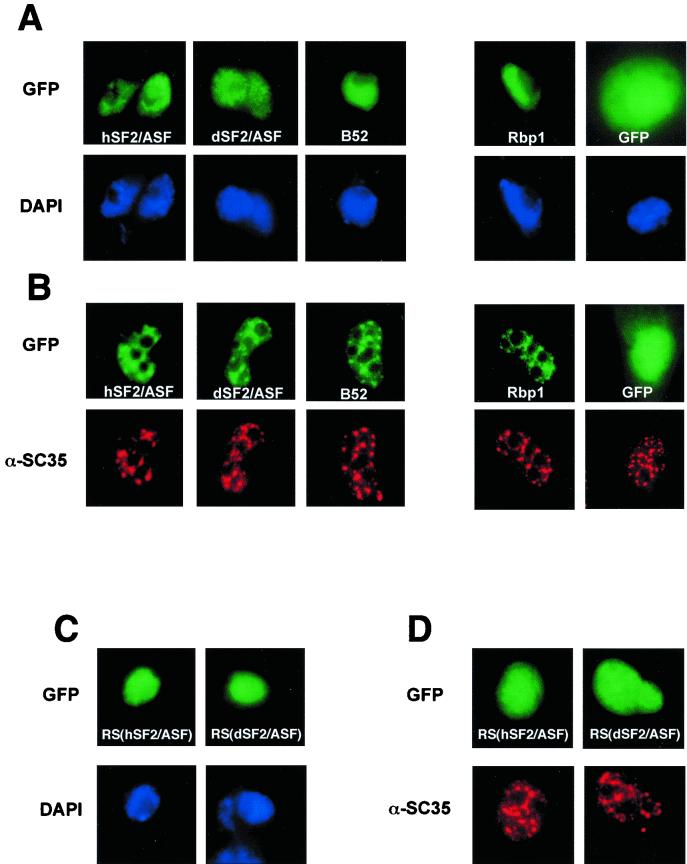
Monolayer Drosophila Schneider 2 (S2) or HeLa cells were grown in Schneider's Drosophila medium (Gibco BRL) or RPMI 1640 (Gibco BRL), respectively, supplemented with 10% fetal calf serum on 3-cm-diameter dishes (Nunc) to 70 to 80% confluence. They were transfected with 1 μg of the indicated plasmids and 19 mg of carrier DNA, using LipofectAMINE reagent (Gibco BRL) for HeLa cells or according to the manufacturer's procedure (DES kit; Invitrogen) for S2 cells. Twenty-four hours posttransfection, cells were washed with phosphate-buffered saline (PBS) and fixed for 15 min at room temperature with 3% paraformaldehyde. The fixed cells were then permeabilized with PBS–0.5% Triton X-100 for 5 min, washed two times with PBS, and then stained either with DAPI (4′,6′-diamidino-2-phenylindole) or with a monoclonal antibody (MAb) against SC35 (α-SC35) as previously described (12). Each experiment was reproduced in multiple independent transfections, and the cells shown in Fig. 3 are representative of the large effects observed under each set of conditions.
FIG. 3.
Cellular localization of GFP fusion proteins in Drosophila S2 (A) and HeLa (B) cells. Direct fluorescence of GFP (GFP), GFP-hASF (hSF2/ASF), GFP-dASF (dSF2/ASF), GFP-B52 (B52), and GFP-RBP1 (Rbp1) fusion proteins was analyzed 20 h after transfection. Expression of fusion proteins was confirmed by immunoblot analysis using anti-GFP antibody (data not shown). (C and D) Cellular localization of the GFP-RS domain of either hASF [RS(hSF2/ASF)] or dASF [RS(dSF2/ASF)] fusion proteins in S2 (C) and HeLa (D) cells. The position of nuclei was confirmed either by DAPI staining of S2 cells (DAPI) or by indirect immunofluorescent staining of HeLa cells with α-SC35, which showed the cellular localization of endogenous SR protein SC35. DAPI and α-SC35 staining were performed in the same cells transfected with GFP fusion proteins.
Shuttling experiments were performed essentially according to the method of Cáceres et al. (5). Briefly, HeLa cells were transfected with GFP constructs by electroporation and seeded on coverslips, followed by coincubation with an excess number of untransfected mouse NIH 3T3 cells for 3 h in the presence of 50 μg of cycloheximide/ml. The concentration of cycloheximide was then increased to 100 μg/ml, and the cells were incubated for an additional 30 min before fusion. Cell fusions were done as described previously (39), and the heterokaryons were incubated further for 2 h in medium containing 100 μg of cycloheximide/ml before fixation. Nuclei of cells were stained with 1 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/ml.
In vitro transcription, splicing assays, and U1 snRNP pre-mRNA binding experiments.
Radiolabeled RNAs were synthesized by in vitro transcription in the presence of 20 U of SP6 RNA polymerase (Boehringer), 1 μg of the suitable linearized plasmids, and 5 μM (α-32P]UTP (800 Ci/mmol) in 25-μl reactions according to the manufacturer's conditions. In vitro transcripts were purified on denaturing polyacrylamide-urea gels and quantitated by Cerenkov counting. Splicing reactions with HeLa nuclear extracts (NE) were done under standard conditions as described previously (6, 28). For S100 complementation experiments, Minx (62) or fushi tarazu (Ftz) (44) pre-mRNA substrates were added to splicing reactions containing 8 μl of HeLa S100 extract and 8 or 16 pmol of either baculovirus-produced hASF (bhASF) or baculovirus-produced dASF (bdASF) in a total volume of 25 μl; 2μl of HeLa NE was included in some reactions shown (e.g., in Fig. 5B). To compare the activities of individual SR proteins in the modulation of alternative splicing, we used various pre-mRNA substrates containing two competing 5′ splice sites, originating from either the adenovirus E1A gene [Sp4(13S−)], human β-globin gene (5′D16X), or simian virus 40 (SV40) early gene (pSVi66) (15, 40, 43). They were spliced for 100 to 120 min in a total volume of 25 μl containing 50 fmol of labeled pre-mRNA and 9 to 10 μl of HeLa NE in the presence of 3.2 mM MgCl2 and 60 mM KCl for Sp4(13S-) and 5′D16X pre-mRNAs or 2.2 mM MgCl2 and 48 mM KCl for SVi66 pre-mRNA. Splicing assays were supplemented with 16 pmol of 9G8 or 8 to 16 pmol of either bhASF or bdASF. To compare the activities of individual SR proteins in the activation of specific splicing enhancers, various transcripts containing Sp1-derived sequences and SR-specific splicing enhancers (7) were used. Splicing assays were performed in the presence of 7 μl of HeLa S100 extracts and 3 μl of 20 to 40% ammonium sulfate NE (NF20-40) in a total volume of 25 μl containing 3.2 mM MgCl2, 60 mM KCl, and ca. 16 pmol of individual purified SR proteins. Splicing products were analyzed by electrophoresis on denaturing polyacrylamide gels (PAGE) and revealed by autoradiography.
FIG. 5.
Effects of dASF on splicing activation and alternative splicing in vitro. (A) Aliquots of 50 fmol of 32P-labeled SV40 derivative (left), E1A derivative (middle), and β-globin derivative (right) pre-mRNAs were incubated in HeLa cell NE under splicing conditions without complementation (lanes 1) or complemented with 8 or 16 pmol of bhASF (lanes 2 and 3), 8 or 16 pmol of bdASF (lanes 4 and 5), or 16 pmol of baculovirus-purified 9G8 (lanes 6). (B) Splicing-complementation activity of recombinant bdASF in S100-cytoplasmic splicing-deficient extracts. 32P-labeled Minx pre-mRNA (left) was incubated under splicing conditions (see Materials and Methods) either in HeLa S100 extracts without (lane 1) or with 4, 8, or 16 pmol of the indicated recombinant proteins (lanes 2 to 7) or in HeLa S100 extracts supplemented with 1/10 of HeLa NE in the absence (lane 9) or presence of added 8 or 16 pmol of the indicated recombinant proteins (lanes 10 to 13). Lane 8 represents a standard splicing reaction in HeLa NE. Right, splicing reactions performed as at the left, using an ftz pre-mRNA.
The ehASF- and edASF-U1 snRNP complex formation assays were performed as previously described (22) in 20 mM HEPES (pH 7.6)–5% glycerol–100 mM KCl–0.2 mM EDTA–1.5 mM MgCl2. Before loading half of the mixtures onto native gels, heparin and glycerol were added to final concentrations of 1 mg/ml and 15%, respectively.
Protein-protein interaction studies.
For far-Western analysis, purified recombinant proteins (around 1 μg of each) or purified U1 snRNP (3 μg) was separated by SDS-PAGE on 12% gels and transferred to nitrocellulose by electroblotting for 90 min in 10 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid; pH 11.0] transfer buffer containing 10% methanol. To renature the proteins, the filters were treated as previously described (22) and probed with 10 μg of labeled ASF in 10 ml of binding buffer. To label ehASF, 10 μg of the recombinant protein was incubated with 800 U of purified starfish cdc2 protein kinase (a generous gift from M. Dorée's laboratory) in the presence of 20 μCi of (γ-32P]ATP and 1 μM cold ATP in buffer B (27) for 1 h at 30°C. Unreacted nucleotides and cdc2 kinase were removed by binding labeled ASF to nickel-agarose beads and glutathione-Sepharose beads, respectively. After extensive washings of the beads with buffer B, labeled protein was eluted with 1 M imidazole.
Two-hybrid assays were performed according to the Clontech manual provided with the Matchmaker LexA system. Bait and prey plasmids were constructed by inserting hASF or dASF PCR-amplified coding sequences between EcoRI and XhoI restriction sites of pLexA or pB42AD vectors.
Drosophila stocks.
The UAS-hASF and UAS-dASF constructs were obtained by PCR amplification of hASF and dASF coding sequences and subcloning between NotI and XbaI restriction sites of the pUAST vector (2). These constructs were used to transform W1118 flies according to standard protocols (51) except that nondechorionated embryos were used for injections. Seven independent UAS-hASF or UAS-dASF transgenic lines (indicated by superscripts; e.g., UAS-hASF1 denotes line 1) were established. The transposon integration sites were mapped to individual chromosomes by standard crosses using balancer stocks. Three homozygous viable lines (3, 5, and 6) were used in this study. These transformed flies were crossed to homozygous (GMR [glass multimer reporter]-GAL4) GAL4-expressing lines and scored for phenotypes under a stereomicroscope. All files were reared at various temperatures between 18 and 28°C on standard medium. Homozygous double-insert lines were obtained by standard crosses using balancer stocks.
Nucleotide sequence accession numbers.
The complete sequences for cDNAs encoding dSC35 (LD32469), dASF (LD11844), and d9G8 (LD02483) have been submitted to the GenBank database under accession numbers AF232775, AF232773, and AF232774, respectively. Sequence data for the cDNA encoding the RS domain of dASF have been submitted to GenBank under accession number AF234157. Sequence data for the Drosophila SRPK1 (dSRPK1) gene have been assigned GenBank accession number AF301149.
RESULTS
Identification of three novel Drosophila SR protein orthologues of vertebrates.
Based on computational analysis of BDGP expressed sequence tag database resources, we identified three clones (LD11844, LD02483, and LD32469), derived from embryonic polyadenylated RNAs, whose products showed strong homology to members of the mammalian SR protein family. Sequence analysis revealed that these clones encode SR proteins, designated dASF, dSC35, and d9G8, with predicted molecular masses of 28.8, 27.8, and 21.4 kDa, respectively. Indeed, the overall homology and local regions of identity in the single N-terminal RRM-type motifs of dSC35 and d9G8 or the two RRMs of dASF, along with the size conservation, strongly suggest that these proteins are the true orthologues of human ASF, SC35, and 9G8, respectively (Fig. 1). Although we have not determined the exact transcriptional initiation sites of genes from which the clones are derived, the sizes of the inserts (1.39, 1.03, and 1.68 kb), which are about the same as those of major transcripts detected from Northern blot analysis (1.45, 1.2, and 1.7 kb, respectively [data not shown]), suggest that the 5′ ends of these cDNAs start close to the authentic transcription initiation sites.
However, most but not all of the sequence hallmarks of the mammalian SR proteins appear to be conserved in flies. In the case of d9G8, the regions of highest homology with human 9G8 include the N-terminal RRM and the zinc knuckle, but the C-terminal domain of d9G8 lacks many of RRSRSXSX repeats normally found in the RS domain (6). Another feature that distinguishes d9G8 from its human orthologue protein resides in a long tract of glycine residues that may form a flexible hinge between the N-terminal and C-terminal parts of d9G8. Drosophila SC35 and ASF display stronger identity with their human orthologues over the entire sequence (60%/69% and 69%/73% identity/similarity, respectively). However, important differences can be detected when functional domains are considered. First, the RS domain of dSC35 is shorter than that of human SC35 and lacks the most C-terminal 26 amino acids, which are highly conserved between birds and humans. Second, the single RRM domain and the RS domain are spaced by a series of additional glycine residues. Finally and most importantly, dASF has most of the signature of hASF, including an atypical second RRM and the invariant SWQDLKD sequence as well as a high conservation of the L1 and L3 loop sequences of both RRMs, which are known to be involved in defining the specificity of interaction with RNA sequences. In contrast, it lacks both a G-rich hinge region between the two RRMs and a perfect eight-RS-dipeptide repetition at the beginning of the RS domain. As already observed for d9G8 and dSC35, the RNA-binding domain and the RS domain are separated by a long G-rich stretch. Taken together, these analyses indicate that the C-terminal part of some Drosophila SR proteins might be subdivided into two separate structural domains: a portion with classical RS repeats and a preceding G-rich domain. In keeping with this suggestion, Drosophila RBP1 and B52, homologues of SRp20 and SRp55, respectively, also have an additional G-rich hinge region between the RNA-binding domain and the RS domain. Considering this new feature, dASF might represent an extreme case for which the G-rich region is more prominent than the RS repeats, and therefore its RS domain has diverged more during evolution than initially thought. Genomic sequencing and careful inspection of the obtained sequences did not reveal any open reading frame that could restore an RS domain with several RS repeats similar to those found in the vertebrate ASF. It is therefore very likely that the major protein encoded by the single dASF gene has the characteristics of the cDNA clone that we identified and lacks a long RS repeat at its RS domain.
dASF lacks at least SRPK1 phosphorylation sites.
At least eight members of the vertebrate SR family contain phosphopeptides that are recognized by MAb 104. However, immunoblotting of MAb 104 to NE from Drosophila Kc cells revealed that the SRp55 homologue B52 is the major immunoreactive polypeptide, whereas the other SR proteins are far less detectable (47). Thus, with the exception of B52, it was not clear if the Drosophila SR proteins exhibit a poor MAb 104 phosphoepitope due to weak phosphorylation or if their relative concentrations are lower in Drosophila Kc cells than in HeLa cells. Of several protein kinases that phosphorylate hASF within its RS domain in vitro, three have been firmly established to require the RS repeats for interaction and phosphorylation of the RS domain: SRPK, Clk/Sty (8), and topo I (27, 45). We therefore determined whether dASF can be used as a substrate for these kinases in vitro (Fig. 2). Using quantities of purified human recombinant SRPK1, Clk/Sty, and topo I normalized to give equivalent hASF RS domain phosphorylation levels, we found that purified bacterially expressed recombinant protein (edASF) is phosphorylated by both Clk/Sty (Fig. 2C, lane 2) and, to a lesser extent, topo I (Fig. 2D, lane 2) but not by SRPK1 (Fig. 2B, lane 2). However, quantitative analysis showed that under these conditions, the level of phosphorylation of edASF by Clk/Sty is four times lower than that of ehASF and can be correlated to the total number of serine residues in the RS domain. The results are consistent with previous observations showing that mutation of arginine residues to glycine in RS dipeptides results in loss of phosphorylation by SRPK1 (23) but has only a slight effect on the phosphorylation by Clk/Sty (9).
FIG. 2.
(A) Phosphorylation of purified E. coli-expressed recombinant ehASF and edASF proteins by dSRPK1 (A), human SRPK1 (B), Clk/Sty (C), and human topo I (D). Kinase assays were performed with equivalent activities of recombinant GST-Clk/Sty, SRPK1, and topo I to phosphorylate ehASF (lanes 1), edASF (lanes 2), or no substrate added other than the RS domain of hASF (lanes 3) as described in Materials and Methods. The RS domain of hASF was used as an internal control.
The recently completed sequence of the Drosophila genome revealed three genes encoding kinases of the SRPK type (CG8174, CG9085, and CG8565), among which the CG8174 gene showed the highest homology with human SRPK1 (37). To further confirm the above results, we decided to test whether the kinase encoded by this gene was able to phosphorylate dASF. For this purpose we isolated the cDNA clone of dSRPK1 and performed in vitro kinase assays with E. coli-purified recombinant dSRPK1. While ehASF and its RS domain were both efficiently phosphorylated by the recombinant enzyme (Fig. 2A, lanes 1 and 3), dASF, as expected, was not modified (lane 2). Furthermore, yeast SR-specific kinase Sky1, a conserved kinase which is structurally and functionally related to SRPK1 (50), was also unable to phosphorylate dASF (data not shown). Thus, these results clearly establish that dASF lacks key structural features required for its phosphorylation by SRPK-type kinases.
The dASF RS domain allows nuclear localization but not shuttling.
Utilization of ASF mutants that can be differentially phosphorylated by either SRPK1 or Clk kinases has led to the hypothesis that SRPK-mediated phosphorylation plays an important role in nuclear import, intranuclear localization, and nuclear export (17, 23, 30, 60). We therefore wished to test whether differences in the phosphorylation status between hASF and dASF would affect their cellular distribution. To this end, we fused GFP in frame to the amino terminus of hASF or dASF and transiently expressed the fusion proteins in either Drosophila S2 cells (Fig. 3A) or HeLa cells (Fig. 3B). Both GFP-hASF and GFP-dASF, as well as two other GFP-SR proteins from Drosophila, B52 and RBP1, are localized in the nucleus independently of the cell type in which they are expressed (Fig. 3A and B). In HeLa cells, the fusion proteins colocalized with both speckles and diffuse pools of splicing factors excluding the nucleoli (Fig. 3B), while in S2 cells only a diffuse pattern was seen (Fig. 3A). Deletion of the RS domain from either hASF or dASF did not interfere with their localization in speckles from HeLa cells (data not shown), a result consistent with previous data showing that subnuclear targeting to speckles can be mediated by the two RRMs of hASF (4). Moreover, this result indicates that the two RRMs of dASF are equivalent to those of hASF in targeting the fusion proteins to speckles. We also tested the capacity of the RS domain of dASF to behave as a nuclear localization signal in the absence of the two RRMs. As shown in Fig. 3C and D, the GFP fusion protein that harbors the RS domain of dASF displays the same nuclear distribution as the one with the RS domain of hASF, demonstrating that both types of RS domains act as nuclear localization signals. None of them, however, has the ability to direct the GFP reporter to nuclear speckles.
The RS domain of hASF is also involved in shuttling of the protein between the nucleus and the cytoplasm, and a recent study suggested that the stable interactions between hASF and SRPKs may be a key determinant of this subcellular distribution (23). Therefore, dASF constitutes an ideal candidate to directly test this suggestion, since it is not a substrate for SRPK1. To compare the shuttling properties of hASF and dASF, GFP fusion proteins were transiently expressed in HeLa cells, which were then fused to mouse NIH 3T3 cells to form heterokaryons. Before fusion, the cells were treated with cycloheximide to avoid further protein synthesis in the heterokaryons. Detection of GFP within the mouse nuclei in the heterokaryons, which are easily distinguishable by DAPI staining, is indicative of shuttling. In agreement with results of a previous study in which epitope-tagged hASF was used (5), GFP-hASF was detected in the mouse nuclei (Fig. 4, GFP column, hSF2/ASF), indicating that the GFP reporter does not interfere with the shuttling properties of the GFP-hASF fusion. In sharp contrast, GFP-dASF, while expressed at high levels in HeLa nuclei, was not found in the mouse nuclei (Fig. 4, GFP column, dSF2/ASF), demonstrating that dASF is not a shuttling protein. The possibility that shuttling behavior is restricted to SR proteins from vertebrates can be ruled out because RBP1, like its human homologue SRp20 (5), does shuttle (Fig. 4, GFP column, RBP1). Interestingly, deletion of the perfect RS repeats (residues 197 to 216) at the RS domain of hASF prevents shuttling (Fig. 4, GFP column, hSF2/ASF Δ197–216). Given that these RS repeats are also required for efficient phosphorylation by SRPK1, it is likely that this kinase contributes to the cellular distribution of hASF and, perhaps, of other shuttling proteins as well.
FIG. 4.
Analysis of nucleocytoplasmic shuttling of GFP-dASF, GFP-RBP1, GFP-hASF, and GFP-hASF Δ197–216 (a mutant of hASF lacking the RS repeats at the RS domain) fusion proteins by transient expression in interspecies heterokaryons (see Materials and Methods). Phase-contrast images of the heterokaryons are shown (left column). Localization of the expressed proteins was determined by direct fluorescence of GFP (middle column). The cells were simultaneously incubated with DAPI for differential staining of human and mouse nuclei within heterokaryons (right column). Arrows indicate the mouse nuclei within human-mouse heterokaryons.
dASF has a switching activity but does not complement S100 extracts.
hASF was originally identified in mammalian cells by two different assays. In one assay, hASF was shown to switch utilization of the downstream small t-antigen 5′ splice site at the expense of the upstream large T-antigen 5′ splice site in an SV40 early pre-mRNA and was therefore called alternative switch factor (15). In the second assay, hASF was shown to be a constitutive splicing factor able to complement splicing activity in postnuclear S100 extracts (24). The RS repeats at the RS domain of hASF are critical for its function in constitutive splicing (3, 63). Thus, the finding that the long RS dipeptide repetition is missing from dASF was intriguing and prompted us to test whether the structural differences in the RS domain between dASF and hASF are critical for either of the demonstrable activities of hASF.
To assess the switching activity of dASF, we used three model pre-mRNA substrates with two competing 5′ splice sites. The substrates were chosen to contain either two identical 5′ splice sites (5′D16X, a β-globin pre-mRNA derivative with duplicated 5′ splice site from the first intron [43]), an authentic 5′ splice site competing with a cryptic 5′ splice site [Sp4(13S−), an adenovirus derivative from the E1A transcription unit in which the 13S 5′ splice site is mutated [40]), or two different 5′ splice sites (SVi66, an SV40 derivative containing an upstream and a downstream 5′ splice site to generate large T and small t mRNAs [15]). Figure 5A (left) clearly shows that bdASF, when added to HeLa NE, could stimulate use of the proximal 5′ splice sites of all substrates in a dose-dependent manner (lanes 4 and 5). The amount of bdASF required to achieve this stimulation was almost identical to that of bhASF (compare lanes 2 and 3 with lanes 4 and 5), indicating that these two factors have similar switching activities. These results confirm previous work showing that deletion of the entire human RS domain does not affect hASF activity in this assay (3). Furthermore, dASF is more likely to meet hASF criteria for splice site choice than other SR proteins. Indeed, a recombinant baculovirus-produced 9G8 protein is able to promote relatively efficient switching of large T to small t from the SVi66 transcript (left, lane 6), but it fails to switch the 12S 5′ splice site for the proximal cryptic site within the Sp4(13S−) transcript (middle, lane 6) and the proximal site of β-globin within the 5′D16X pre-mRNA (right, lane 6).
Attempts to further characterize recombinant bdASF biochemically with HeLa cell S100 extracts in a complementation assay were unsuccessful using either the Minx synthetic mRNA precursor, a model pre-mRNA derived from the adenovirus major late transcription unit (62) (Fig. 5B, left, lanes 5 to 7), or the Drosophila ftz pre-mRNA gene (44) (Fig. 5B, right, lanes 4 and 5). Both substrates were, however, efficiently spliced in S100 extracts complemented with recombinant bhASF (Fig. 5B, left, lanes 2 to 4 and 10 to 11; right, lanes 2, 3, 8, and 9), showing that failure of bdASF to activate splicing of these substrates was not due to differences in the composition of insect and human intron sequence elements. We also tested other model substrates, like β-globin and adenovirus E1A derivatives, and observed S100 rescue with bhASF but not with bdASF (data not shown). The trivial possibility that bdASF was inactive because a heterologous system was used for complementation can also be discounted, since bhASF but not bdASF allowed splicing in S100 extracts from Drosophila Kc cells (data not shown). Given that previous work had shown that deletion of the RS repeats (residues 197 to 224) abolishes the activity of hASF in this assay (63), differences at the RS domain between dASF and hASF could be a more likely explanation to account for the inability of bdASF to carry out essential splicing functions executed by vertebrate SR proteins.
Since RBP1 has also been shown to be unable to complement S100 extracts (21), we used more refined conditions to seek for factors in HeLa NE that could reveal a complementing activity of bdASF in S100 extracts. Therefore, we designed an assay in which S100 extracts were supplemented with limiting amounts of NE promoting very little if any splicing of either Minx (Fig. 5B, left, lane 9) or Ftz (right, lane 7) pre-mRNA. Compared to the previous assays, addition of NE had only a moderate effect on the splicing activation of these substrates in S100 extracts by recombinant bhASF (Fig. 5B, left, lanes 10 and 11; right, lanes 8 and 9) but significantly stimulated the splicing activation mediated by recombinant bdASF (left, lanes 12 and 13; right, lanes 10 and 11). Notably, the concentration of recombinant bdASF was critical for splicing activation, since concentrations twice that required to stimulate splicing were inhibitory (Fig. 5B, left, lane 13; right, lane 11). Previous experiments have also shown that very high concentrations of ASF itself can also block splicing (15). However, the dASF-induced inhibition is distinct from this, as it occurred when the concentration of the protein is below that required for hASF self-inhibition. Moreover, S100 extracts containing moderate amounts of recombinant bhASF, which do not allow splicing of either Ftz or Minx, became active when supplemented with the same moderate amounts of recombinant bdASF (data not shown). These results suggest that stoichiometric amounts of bdASF and bhASF or other SR proteins from NE can function in constitutive splicing, presumably through formation of homo or heteromeric complexes in which at least one partner has an RS dipeptide cluster that allows efficient interactions with a component of the basic splicing machinery. This fits well with data presented below showing a direct interaction of dASF with itself and with hASF.
dASF interacts with itself and with hASF and allows efficient interaction of U1 snRNP with 5′ splice site.
Human ASF was previously shown to cooperate with the U1 snRNP particle in forming a stable complex at the 5′ splice site (22). We therefore tested whether dASF itself was defective in constitutive splicing because it could not stabilize the binding of U1 snRNP to the 5′ splice site. Different combinations of recombinant ehASF, purified U1 snRNP, and edASF were incubated with either 32P-labeled PIP7.A or PIP75′AU, a mutant version in which the invariant GU dinucleotide at the 5′ splice site is changed to AU (22) (Fig. 6A). The mixes were then analyzed by native gel electrophoresis to separate the U1-containing complexes from the free probe. No complex was detected with the mutated substrate (Fig. 6A, lanes 3, 8, and 13), indicating that an intact, functional 5′ splice site is required for formation of U1 snRNP–hASF–pre-mRNA complex. In agreement with previous findings (22), U1 snRNP alone gave rise to low levels of U1 snRNP–pre-mRNA complex formation (lane 2), whereas when both U1 snRNP and ehASF were incubated with the pre-mRNA, a stable complex was detected (lanes 5 to 7). Similar efficient ternary complex formation was observed with edASF, implying that the failure to activate S100 extracts is not due to the inability of dASF to form a complex with the U1 snRNP particle (lanes 10 to 12).
FIG. 6.
(A) Effect of dASF on U1 snRNP binding to the 5′ splice site. U1 snRNP–ASF–pre-mRNA complex formation assays were performed as previously described (22). Reactions in 10 ml contained 1.7 pmol of U1 snRNP (lanes 2, 3, 5 to 8, and 10 to 13), 40 (lanes 4, 5, and 8), 20 (lane 6), and 10 (lane 7) pmol of bhASF, 40 (lanes 9, 10, and 13), 20 (lane 11), and 10 (lane 12) pmol of bdASF, and 1.5 fmol of either 32P-labeled PIP7.A (lanes 1, 2, 4 to 7, and 9 to 12) or PIP75′AU (lanes 3, 8, and 13) pre-mRNA. (B) Physical interaction between the hASF and dASF by far-Western analysis (22). The indicated proteins (lanes 1 to 3), purified U1 snRNP (lane 4), and purified SR proteins treated with calf alkaline phosphatase (lane 5) were separated by SDS-PAGE on a 12% gel, transferred to nitrocellulose, renatured, and probed with 32P-labeled ehASF. SR proteins were purified as described by Zahler et al. (61). (C) dASF interacts with both hASF and itself, as revealed by yeast two-hybrid system.
The RS domain of hASF is required not only for its direct interaction with the U1-70K protein but also for its self-association and association with other members of the SR protein family. To test for a physical interaction between dASF and hASF, we used far-Western blotting, which has been successfully used to show specific interactions between members of the SR protein family and other splicing factors (28). After SDS-PAGE analysis, purified proteins were transferred to filters, renatured, and probed with 32P-labeled ehASF (Fig. 6B). The specificity of binding of modified ehASF was confirmed by its ability to bind to itself (lane 1) but not to a truncated version with a deletion of the RS domain (lane 2). The probe cross-reacted with immobilized SR proteins (lane 5) and edASF (lane 3), as well as with U1-70K (lane 4), providing direct evidence that the structural difference at the RS domain between hASF and dASF does not detectably affect their association.
To independently confirm the specificity of these protein-protein interactions and to establish whether dASF can form homodimers and heterodimers or multimers with hASF, we used the yeast two-hybrid interaction assay as follows. We introduced the cDNA encoding dASF or hASF into both the bait and prey expression plasmids of the two-hybrid system and analyzed β-galactosidase activity (Fig. 6C). Although the levels of β-galactosidase activity are not accurate quantitative measures of the strength of interactions between proteins, we observed slighly higher levels of β-galactosidase activity in the dASF or hASF homophilic interactions than in the hASF-dASF heterophilic interactions, implying that dASF, like other SR proteins, is capable of forming both homodimers and heterodimers or higher oligomers.
dASF and hASF have the same pre-mRNA substrate specificities.
In addition to their general functions in the basic splicing reactions, SR proteins play a major role in exon-dependent splicing. High-affinity RNA-binding sites for several SR proteins identified by iterative in vitro binding selection (SELEX) can function as splicing enhancers when placed downstream of a weak 3′ splice site (54). To determine whether bdASF could activate splicing of substrates containing hASF high-affinity RNA targets, we devised a complementation assay in which recombinant proteins were added to S100 extracts supplemented with NF20–40 (53). As splicing substrates, we used E1A derivative specific pre-mRNAs whose second exon contains a single copy of high-affinity targets for either 9G8, SRp20, or hASF that are known to be responsive to corresponding SR proteins in vitro (7, 54). As shown in Fig. 7, bdASF is active in this assay if the substrate contains hASF high-affinity sequences (lane 4) but not when it contains those of 9G8 (lane 10) or SRp20 (lane 15). While the splicing activation exhibited by bdASF was lower than that of bhASF (compare lanes 3 and 4), 9G8 (lane 5) could not activate splicing from the same substrate. As previously shown (7), however, both 9G8 (lane 9) and SRp20 (lane 13) were able to activate efficient splicing of substrates containing the respective cognate sequences. These results are in keeping with the high degree of conservation of the RNA-binding domain between Drosophila and human ASF and are consistent with the view that dASF could act in splicing through the same cognate sequences as hASF. However, in agreement with previous work (7), hASF, but not dASF, activates splicing of the substrate with an SRp20-target sequence (lane 14), implying that hASF has broader substrate specificity than dASF. The additional RS repeats at the RS domain of hASF may allow interactions with factors present in the NF20-40 which mediate interaction with SRp20-target sequence and/or increase the splicing efficiency. In agreement with this idea, duplicating the RS domain, which by definition increases the the RS content and doubles the number of interacting regions, leads to a proportional increase in the rate of splicing (16).
FIG. 7.
dASF specifically activates enhancer-dependent splicing. 32P-labeled Sp1 transcripts containing hASF (left), 9G8 (middle), and SRp20 (right) high-affinity binding sites were incubated in a mixture of S100 cytoplasmic extracts and NF20–40 (7). Assays were supplemented with no SR protein (lanes 2, 7, and 12) or with 16 pmol of bhASF (lanes 3, 8, and 14), 16 pmol of bdASF (lanes 4, 10, and 15), 16 pmol of baculovirus-purified 9G8 (lanes 5 and 9), or 16 pmol of SRp20 (lane 13). Lanes 1, 6, and 11 represent control splicing assays using NE. ESE, exonic splicing element.
Targeted overexpression of hASF and dASF in living flies.
Since structural differences exist between the RS domains of hASF and dASF, we considered the possibility that this difference would contribute to the specific functions of the two proteins in vivo. Our biochemical data provided evidence that the levels of hASF and dASF can modulate splicing of specific pre-mRNAs in vitro; we therefore assessed whether ectopic overexpression of either protein in a living animal would affect Drosophila development and/or survival, as previously observed for SRp55 (B52) (26, 28). The GAL4-upstream activation sequence (UAS) binary expression system (2) was used to drive prolonged high expression levels of either hASF or dASF in a tissue-specific fashion. To this end, wild-type hASF or dASF cDNA was placed within a P-element transposon under transcriptional control of the yeast GAL4-responding upstream activating sequence (to yield UAS-hASF and UAS-dASF elements), and seven independent transgenic lines for either construct were established. Transgenic flies carrying a single UAS-hASF or UAS-dASF element were mated to flies which express the yeast transcriptional activator GAL4 in a cell- or tissue-specific fashion to drive robust expression of hASF or dASF. To examine the tissue-specific expression of GAL4 in each cross, lines carrying GAL4 elements were crossed to a transgenic line carrying the E. coli β-galactosidase-encoding gene under the control of the GAL4 activator (UAS-lacZ [2]).
Using the GMR-GAL4 [11] line, we directed expression and activity of dASF to differentiating photoreceptor cells of the developing eye. Adult progeny consistently showed retinal defects, the severity of which depended upon the UAS-dASF transgenic line used (Fig. 8B, lower row; Table 1). Relatively weak defects of retinal development were observed with UAS-dASF line 7 (Fig. 8B, image e) compared to lines 1 (image f) and 2 (image g), which gave rise to adults with rough eyes. On elevating the level of overexpression of dASF by raising the offspring at higher temperatures (22, 25, or 28°C), the same phenotypes were aggravated (Table 1) but most emerging adults were females (all males died as late pupae), indicating that a proper level of dASF protein is critical for male viability and normal eye development. Interestingly, overexpression of hASF under the same genetic background used for dASF overproduction led to a less pronounced eye phenotype (Fig. 8B, upper row). Even when progeny were raised at 22 or 25°C, all adults eclosed and there was no sexual bias in the eclosion rate between males and females (Table 1). However, among viable progeny raised at higher temperature (28°C), several adults exhibited a fully penetrant eye phenotype very similar to those observed among the offspring derived from crosses with the various UAS-dASF lines raised at low temperature (Table 1; Fig. 8B, compare image d to images e and f). Given that increasing temperature appears to enhance the transcriptional activation by GAL4 in Drosophila (2), it can be assumed that expression of the target gene is similarly increased. Quantitative reverse transcription-PCR confirmed this assumption (data not shown); thus, high levels of hASF expression in flies give rise to the same phenotype as low levels of expression of dASF. The same result was obtained when expression of the proteins was directed to precursor cells of all sensory organs owing to an imaginal disc-specific enhancer of the scabrous gene. Whereas progeny overexpressing dASF have thin bristles, only moderate or no phenotypes were observed with overexpression of hASF (data not shown). Since a major structural difference between the two proteins resides in the RS domain, these data suggest that the latter is likely involved in a fine-tuning control of the availability of active protein in vivo.
FIG. 8.
High levels of dASF overexpression in differentiating photoreceptor cells lead to more severe adult eye defects than hASF overexpression. (A) Virgin female flies carrying a P-element insert in which the GAL4 coding sequence was placed under the control of five glass-binding sites (GMR enhancer) were mated to male flies carrying a single UAS-hASF line 5, 1, or 6 or UAS-dASF line 7, 1, or 2 element. (B) Stereomicroscope views of adult compound eyes. Genotypes: a, GMR-GAL4/+; UAS-lacZ/+; b, GMR-GAL4/UAS-ASF5; c, GMR-GAL4/+; UAS-ASF1/+; d, GMR-GAL4/+; UAS-ASF6/+; e, GMR-GAL4/UAS-dASF7; f, GMR-GAL4/+; UAS-dASF1/+; g, GMR-GAL4/+; UAS-dASF1/+. All progeny were raised at 25°C. Overexpression of dASF in the developing retina results in necrosis of ommatidia that can be moderate (e) or severe (f and g), depending on the site of chromosomal integration of the transgene.
TABLE 1.
Phenotypes of progeny from crosses between UAS-dSF2/ASF or UAS-hSF2/ASF transgenic lines and GMR-GAL4 line reared at different temperaturesa
| Genotype of transgenic flies overexpressing: | Temp (°C) | Observed phenotype (relative strength of retina defects) |
|---|---|---|
| dSF2/ASF | ||
| W1118; UAS-dASF1/GMR-GAL4 | 22 | Adults of both sexes with retina defects (++) |
| 25 | No adult males; adult females with retina defects (+++) | |
| 28 | Late pupal lethality | |
| W1118; UAS-dASF22/GMR-GAL4 | 22 | Adults of both sexes with retina defects (+++) |
| 25 | No adult males; adult females with retina defects (+++) | |
| 28 | Late pupal lethality | |
| W1118; GMR-GAL4/+; UAS-dASF23/+ | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | Late pupal lethality | |
| W1118; GMR-GAL4/+; UAS-dASF24/+ | 22 | Adults of both sexes with retina defects (++) |
| 25 | Adults of both sexes with retina defects (+++) | |
| 28 | Late pupal lethality | |
| W1118;GMR-GAL4/+; UAS-dASF25/+ | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | Late pupal lethality | |
| W1118; GMR-GAL4/+; UAS-dASF26/+ | 22 | Adults without apparent abnormalities |
| 25 | Adults of both sexes with retina defects (+) | |
| 28 | Adults of both sexes with retina defects (++) | |
| W1118; UAS-dASF27/GMR-GAL4 | 22 | Adults of both sexes with retina defects (++) |
| 25 | Rare adult males; all adults with retina defects (+++) | |
| 28 | Late pupal lethality | |
| hSF2/ASF | ||
| W1118; UAS-hASF21/GMR-GAL4 | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | No males; adult females with retina defects (+++) | |
| W1118; UAS-hASF22/GMR-GAL4 | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | Late pupal lethality | |
| W1118;GMR-GAL4/+; UAS-hASF23/+ | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | No males; adult females with retina defects (+++) | |
| W1118;GMR-GAL4/+; UAS-hASF24/+ | 22 | Adults without apparent abnormalities |
| 25 | Adults of both sexes with retina defects (+) | |
| 28 | Adults of both sexes with retina defects (+++) | |
| W1118; UAS-hASF25/ GMR-GAL4 | 22 | Adults without apparent abnormalities |
| 25 | Adults of both sexes with retina defects (+) | |
| 28 | No males; Adult females with retina defects (+++) | |
| W1118; GMR-GAL4/+; UAS-hASF26/+ | 22 | Adults of both sexes with retina defects (+) |
| 25 | Adults of both sexes with retina defects (++) | |
| 28 | No males; adult females with retina defects (+++) | |
| W1118; UAS-hASF27/ GMR-GAL4 | 22 | Adults without apparent abnormalities |
| 25 | Adults of both sexes with retina defects (+) | |
| 28 | No males; adult females with retina defects (+++) |
More than 100 progeny were examined.
DISCUSSION
We have taken advantage of the availability of cDNAs corresponding to over 40% of the genes in the fruit fly D. melanogaster (49) to analyze several cDNAs representing three members of the SR family which are still uncharacterized in Drosophila. Sequence alignments (Fig. 1 and reference 37) indicate that these newly identified members are the closest relatives of vertebrates ASF, 9G8, and SC35. Initially, partial characterization of SR proteins from different sources (48, 61) suggested that their apparent sizes and amino acid sequences are conserved in the animal kingdom. Only two SR proteins from Drosophila, RBP1 and B52, homologues of SRp20 and SRp55, respectively, allowed this comparison, and it was not certain whether other SR proteins from Drosophila would follow the same rules. The present study shows that Drosophila and human ASF have similar sizes and that the corresponding unphosphorylated recombinant proteins have the same electrophoretic mobility. It is therefore very likely that the 30-kDa band faintly detected by MAb 104 corresponds to dASF (48). The possibility that the 30-kDa band contains in addition dSC35 and/or d9G8 can be ruled out because when cDNAs corresponding to these SR proteins were expressed in yeast or S2 cells, they showed electrophoretic mobility different from that of dASF expressed under the same conditions (E. Allemand and J. Tazi, unpublished results).
Whereas RS domains of individual vertebrate SR proteins have little more in common than the overall composition and the presence of many consecutive RS or SR dipeptides, several Drosophila SR proteins have a glycine hinge region between the RNA-binding domain (with one or two RRMs) and the RS domain. This region has been shown to be required for the function of RBP1 in the regulation of doublesex (dsx) pre-mRNA splicing and to be involved in protein-protein interactions (20). Whether the glycine region is similarly important for function of dASF warrants further investigation. However, the lack of RS repeats at the beginning of the RS domain and the weak detection with MAb 104 make a clear difference between Drosophila and vertebrate ASF. In this study, we took advantage of this difference to elucidate features of the RS domain of dASF which are important for its function in splicing and cellular localization. Biochemical analysis and cellular localization studies revealed three features that distinguish dASF from hASF: (i) dASF is hypophosphorylated and lacks SRPK phosphorylation sites, (ii) it does not shuttle, and (iii) it does not activate splicing from S100-cytoplasmic splicing-deficient extracts. Similarities between the two factors consisted of their capacities to switch usage of competing 5′ splice sites and to activate splicing through the same exonic splicing enhancer sequences. These findings argue in favor of the fact that vertebrate ASF has acquired long RS repeats at the RS domain to regulate its cellular compartmentalization and splicing activation potency but not splicing specificity. Moreover, the distinctive features between the two factors are likely to be relevant for their in vivo functions, since overexpression of hASF in transgenic flies has moderate effects on development as compared to dASF overexpression.
RS domain phosphorylation and splicing activity of dASF.
The RS domain of hASF is required for constitutive splicing in vitro (3, 63), a redundant function shared by all vertebrate and some Drosophila SR proteins (13, 32, 61). In particular, hASF mutant protein lacking residues 198 to 224 including the eight consecutive RS dipeptides fails to restore splicing to cytoplasmic S100 extracts, while deletion of the last carboxyl terminal 24 residues has no adverse effect (63). Significantly, dASF, which naturally lacks the RS repeats and has instead a G-rich region, is also inactive in this assay, implying that this G-rich region cannot substitute for the RS repeats to mediate splicing activation. This observation is also consistent with data showing that substitutions of arginines with glycines at the RS domain of ASF inactivate its capacity to act as a constitutive factor (3). However, dASF enables U1 snRNP to bind efficiently to the 5′ splice site, suggesting that its RS domain performs well known crucial protein-protein interactions with U1-70K (22). Moreover, dASF interacts with itself and with other members of the SR protein family such as ASF, SC35, B52 (SRp55), and dSC35 (E.A. and J.T., unpublished), implying that the overall structural organization of dASF is not incompatible with a function as a constitutive splicing factor. One possibility accounting for the poor capacity of dASF to activate constitutive splicing is that the phosphorylated RS repeats at the beginning of the RS domain of hASF mediate interactions with splicing components other than U1 snRNP, i.e., Drosophila U2AF, to perform initial steps of the spliceosome assembly. Although some of the interactions of SR proteins with splicing factors have been defined in recent years, the roles of these specific interactions and their regulation by phosphorylation need further investigation. A final consideration is that hyperphosphorylation of the RS domain may compete with RNA-binding proteins known as hnRNPs (heterogeneous ribonucleoproteins) which may block splice site recognition (55). The positively charged arginines might enable SR proteins to accumulate around RNA while phosphorylation of alternating serine residues might reduce the repelling forces between neighboring positive charges. This could be another plausible explanation why dASF, which contains 14 neutral charges (glycines) instead of RS repeats, does not activate S100 extracts unless added in combination with hASF or other factors in HeLa NE or NF20-40, which contain more positively charged residues. Given that the most C-terminal part of dASF contain several RS repeats and that other SR proteins with fewer RS repeats than hASF, like 9G8, are active in constitutive splicing (6, 31), alternative explanations are still possible. For example, the local environment of the RS clusters might influence their phosphorylation by specific kinases and/or their ability to participate in protein-protein interactions. It is therefore not surprising that RS domains from different SR proteins display distinct splicing activities (16).
dASF substrate specificity and regulation of alternative splicing.
The result shown in Fig. 7 confirms that the specific association of individual SR proteins with constitutive and regulated splicing enhancers could be connected to their ability to promote splicing in vitro (54). Indeed, dASF, which shares extensive homology over its two RRMs with hASF but little with other members of the SR family, was expected to bind similar RNA recognition sequences as hASF and activate splicing through the same sequences. Although additional work is needed to establish whether dASF and hASF can recognize the same enhancer sequences naturally occurring in pre-mRNAs, it is striking that a similar set of SR proteins from HeLa and Kc extracts bind the Drosophila dsx splicing enhancer (31), a cis-acting regulatory sequence required for dsx pre-mRNA splicing regulation in the cascade of splicing events leading to female sexual differentiation. In particular, a protein designated dSRp30 with the same molecular mass as dASF was detected in Kc extracts and found to bind to the purine-rich element with a specificity similar to that of hASF from HeLa cells (31). Since d9G8 and dSC35 differ in apparent molecular mass from dASF, it is likely that dSRp30 indeed corresponds to dASF. In addition to SR proteins, the regulation of dsx pre-mRNA splicing requires Tra (transformer) and Tra2. Specific interaction of each of these splicing factors with RNA is highly dependent on the presence of the other proteins (31), implying that specific RNA sequence recognition is likely to be a combinatorial mechanism involving weak RNA-protein as well as protein-protein interactions. Consistently, dASF interacts physically with other SR proteins (E.A. and J.T., unpublished) and allows cooperative binding of U1 snRNP at the 5′ splice site (Fig. 5A).
The biochemical effects of dASF on alternative 5′ splice site selection do not seem to involve the divergent RS domain. Indeed, lack of the RS repeats at the beginning of the RS domain can be assimilated to a deletion of the RS domain which was shown previously to have no significant effect on splice site switching activity (3, 63). It is striking that the second RRM is highly conserved between human and Drosophila ASF, arguing for a highly specific role. In agreement with this, it was found that both RRMs of hASF are required for optimal activity (3, 63) and that the second RRM has a dominant role in substrate specificity (33). The situation in vivo is likely to be more complex because of the possibility of multiple protein-protein interactions through the RS domain of dASF. It is therefore not surprising that targeted overexpression of dASF in Drosophila tissues has more deleterious effects on development than targeted overexpression of hASF (Fig. 8).
Phosphorylation and cellular localization of dASF.
Previous studies showed that kinases that phosphorylate the RS domain of SR proteins may contribute to their spatial and temporal regulation (8, 17, 36) and modulate their activity (56, 59). Considering that SR proteins can affect splice site selection in a concentration-dependent manner, the regulation of this nuclear traffic of splicing factors may also play an important role in the regulation of alternative splicing. In this context, it is significant that dASF could be phosphorylated in vitro by SR protein-specific kinases (Fig. 2). In particular, the RS domain of dASF was specifically phosphorylated by topo I, a kinase that may participate in the coordination between transcription and splicing (27, 45, 46, 57), and by Clk, a kinase that can directly modulate SR protein splicing activity and cellular distribution (8, 41). In contrast, dASF was not phosphorylated at all in vitro by Drosophila or human SRPK1, making it unlikely that this enzyme is involved in the cellular localization and/or splicing function of dASF. Consistent with this, the RS domain of dASF is as efficient as the RS domain of hASF to trigger GFP fusions to the nucleus (Fig. 3 C and D). Interestingly, though, dASF, unlike its human homologue, does not shuttle between the nucleus and the cytoplasm, suggesting that part of the shuttling properties could be mediated by the RS repeats at the RS domain and that this cellular event might be subjected to regulation by phosphorylation from SRPK1. In keeping with this suggestion, deletion of the RS repeats abolished both shuttling (Fig. 4) and phosphorylation by SRPK1 (data not shown). Even more interestingly, shuttling of DNA and RNA-binding proteins seems to be important during spermatogenesis in testis (18), a tissue where SRPK1 is highly expressed (38). Furthermore, Npl3, a yeast protein which is a major substrate for Sky and SRPK1 (50), is also a shuttling protein (10). It is therefore possible that SRPK1 regulates shuttling properties of some SR proteins but also of other proteins such as protamines (38), supporting the notion that SRPK, originally thought to be an enzyme involved only in pre-mRNA splicing, plays a broader role in cellular regulation. Thus, the diversity of the kinases involved in the phosphorylation of SR proteins is likely relevant to their function(s) during cell differentiation and/or development.
Ongoing experiments, including genetic approaches, should unravel how the activities of SR proteins are regulated in response to developmental cues. Since SR proteins are key determinants of splicing regulation during differentiation and development of multicellular organisms, a full understanding of their functions will require both biochemical and genetic approaches. The high degree of conservation between SR proteins from vertebrates and Drosophila (37) makes the latter organism ideal to study these proteins. The availability of the complete sequence of Drosophila genome and Drosophila mutants generated by random P-element insertions should facilitate a better understanding of SR protein functions in vivo.
ACKNOWLEDGMENTS
We are grateful to R. Bordonné for helpful discussions and for setting up the yeast system in the lab. We thank M. Vervood and the Bloomington Stock Center for fly strains, R. Lührmann for purified U1 snRNP and PIP7 constructs, M. Dorée for purified cdc2 kinase, J. Manley for ASF cDNA and GST-Clk1 expression plasmid, and T. Giannakouros for purified SRPK1. Special thanks go to G. Hildwein for excellent technical assistance. H.-M.B. thanks L. Joulia for his generous help for the transgenic experiments and D. Cribbs for laboratory support.
This work was supported by a grant from the ARC, GEFLUC, and CNRS-INSERM. E.A. was supported by graduate fellowships from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT), and benefited from a graduate training fellowship from the ARC.
REFERENCES
- 1.Biencowe B J. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. 2000;25:106–110. doi: 10.1016/s0968-0004(00)01549-8. [DOI] [PubMed] [Google Scholar]
- 2.Brand A H, Manoukian A S, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 3.Cáceres J F, Krainer A R. Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 1993;12:4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cáceres J F, Misteli T, Screaton G R, Spector D L, Krainer A R. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cáceres J F, Screaton G R, Krainer A R. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavaloc Y, Popielarz M, Fuchs J P, Gattoni R, Stevenin J. Characterization and cloning of the human splicing factor 9G8: a novel 35 kDa factor of the serine/arginine protein family. EMBO J. 1994;13:2639–2649. doi: 10.1002/j.1460-2075.1994.tb06554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaloc Y, Bourgeois C F, Kister L, Stevenin J. The splicing factors 9G8 and SRp20 transactivate splicing through different and specific enhancers. RNA. 1999;5:468–483. doi: 10.1017/s1355838299981967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwill K, Pawson T, Andrews B, Prasad J, Manley J L, Bell J C, Duncan P I. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- 9.Colwill K, Feng L L, Yeakley J M, Gish G D, Cáceres J F, Pawson T, Fu X D. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J Biol Chem. 1996;271:24569–24575. doi: 10.1074/jbc.271.40.24569. [DOI] [PubMed] [Google Scholar]
- 10.Flach J, Bossie M, Vogel J, Corbett A, Jinks T, Willins D A, Silver P A. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol Cell Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 12.Fu X D, Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992;256:535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- 13.Fu X D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego M E, Gattoni R, Stevenin J, Marie J, Expert-Bezancon A. The SR splicing factors ASF/SF2 and SC35 have antagonistic effects on intronic enhancer-dependent splicing of the beta-tropomyosin alternative exon 6A. EMBO J. 1997;16:1772–1784. doi: 10.1093/emboj/16.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge H, Zuo P, Manley J L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- 16.Graveley B R, Hertel K J, Maniatis T. A systematic analysis of the factors that determine the strength of pre-mRNA splicing enhancers. EMBO J. 1998;17:6747–6756. doi: 10.1093/emboj/17.22.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui J F, Lane W S, Fu X D. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 18.Hecht N B. Intracellular and intercellular transport of many germ cell mRNAs is mediated by the DNA- and RNA-binding protein, testis-brain-RNA-binding protein (TB-RBP) Mol Reprod Dev. 2000;56:252–253. doi: 10.1002/(SICI)1098-2795(200006)56:2+<252::AID-MRD8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Hedley M L, Amrein H, Maniatis T. An amino acid sequence motif sufficient for subnuclear localization of an arginine/serine-rich splicing factor. Proc Natl Acad Sci USA. 1995;92:11524–11528. doi: 10.1073/pnas.92.25.11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrichs V, Baker B S. In vivo analysis of the functional domains of the Drosophila splicing regulator RBP1. Proc Natl Acad Sci USA. 1997;94:115–120. doi: 10.1073/pnas.94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y J, Zuo P, Manley J L, Baker B S. The Drosophila RNA-binding protein RBP1 is localized to transcriptionally active sites of chromosomes and shows a functional similarity to human splicing factor ASF/SF2. Genes Dev. 1992;6:2569–2579. doi: 10.1101/gad.6.12b.2569. [DOI] [PubMed] [Google Scholar]
- 22.Kohtz J D, Jamison S F, Will C L, Zuo P, Luhrmann R, Garcia-Blanco M A, Manley J L. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi J, Okamoto Y, Onogi H, Mayeda A, Krainer A R, Hagiwara M. The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs) J Biol Chem. 1999;274:11125–11131. doi: 10.1074/jbc.274.16.11125. [DOI] [PubMed] [Google Scholar]
- 24.Krainer A R, Mayeda A, Kozak D, Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991;66:383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- 25.Krämer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 26.Kraus M E, Lis J T. The concentration of B52, an essential splicing factor and regulator of splice site choice in vitro, is critical for Drosophila development. Mol Cell Biol. 1994;14:5360–5370. doi: 10.1128/mcb.14.8.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labourier E, Rossi F, Gallouzi I E, Allemand E, Divita G, Tazi J. Interaction between the N-terminal domain of human DNA topoisomerase I and the arginine-serine domain of its substrate determines phosphorylation of SF2/ASF splicing factor. Nucleic Acids Res. 1998;26:2955–2962. doi: 10.1093/nar/26.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labourier E, Bourbon H M, Gallouzi I, Fostier M, Allemand E, Tazi J. Antagonism between RSF1 and SR proteins for both splice site recognition in vitro and Drosophila development. Genes Dev. 1999;13:740–753. doi: 10.1101/gad.13.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labourier E, Riou J F, Prudhomme M, Carrasco C, Bailly C, Tazi J. Poisoning of topoisomerase I by an antitumor indolocarbazole drug: stabilization of topoisomerase I-DNA covalent complexes and specific inhibition of the protein kinase activity. Cancer Res. 1999;59:52–55. [PubMed] [Google Scholar]
- 30.Lai M C, Lin R I, Huang S Y, Tsai C W, Tarn W Y. A human importin-beta family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J Biol Chem. 2000;275:7950–7957. doi: 10.1074/jbc.275.11.7950. [DOI] [PubMed] [Google Scholar]
- 31.Lynch K W, Maniatis T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev. 1996;10:2089–2101. doi: 10.1101/gad.10.16.2089. [DOI] [PubMed] [Google Scholar]
- 32.Manley J L, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 33.Mayeda A, Screaton G R, Chandler S D, Fu X D, Krainer A R. Substrate specificities of SR proteins in constitutive splicing are determined by their RNA recognition motifs and composite pre-mRNA exonic elements. Mol Cell Biol. 1999;19:1853–1863. doi: 10.1128/mcb.19.3.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintz P J, Spector D L. Compartmentalization of RNA processing factors within nuclear speckles. J Struct Biol. 2000;129:241–251. doi: 10.1006/jsbi.2000.4213. [DOI] [PubMed] [Google Scholar]
- 35.Misteli T, Cáceres J F, Spector D L. The dynamics of a pre-mRNA splicing factor in living cells. Nature. 1997;387:523–527. doi: 10.1038/387523a0. [DOI] [PubMed] [Google Scholar]
- 36.Misteli T, Cáceres J F, Clement J Q, Krainer A R, Wilkinson M F, Spector D L. Serine phosphorylation of SR proteins is required for their recruitment to sites of transcription in vivo. J Cell Biol. 1998;143:297–307. doi: 10.1083/jcb.143.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mount S M, Salz H K. Pre-messenger RNA processing factors in the Drosophila genome. J Cell Biol. 2000;150:F37–F44. doi: 10.1083/jcb.150.2.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papoutsopoulou S, Nikolakaki E, Chalepakis G, Kruft V, Chevaillier P, Giannakouros T. SR protein-specific kinase 1 is highly expressed in testis and phosphorylates protamine 1. Nucleic Acids Res. 1999;27:2972–2980. doi: 10.1093/nar/27.14.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 40.Popielarz M, Gattoni R, Stevenin J. Contrasted cis-acting effects of downstream 5′ splice sites on the splicing of a retained intron: the adenoviral E1A pre-mRNA model. Nucleic Acids Res. 1993;21:5144–5151. doi: 10.1093/nar/21.22.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasad J, Colwill K, Pawson T, Manley J L. The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing. Mol Cell Biol. 1999;19:6991–7000. doi: 10.1128/mcb.19.10.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed R. Mechanisms of fidelity in pre-mRNA splicing. Curr Opin Cell Biol. 2000;12:340–345. doi: 10.1016/s0955-0674(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 43.Reed R, Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986;46:681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- 44.Rio D C. Accurate and efficient pre-mRNA splicing in Drosophila cell-free extracts. Proc Natl Acad Sci USA. 1988;85:2904–2908. doi: 10.1073/pnas.85.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou J F, Antoine E, Cathala G, Brunel C, Tazi J. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 1996;381:80–82. doi: 10.1038/381080a0. [DOI] [PubMed] [Google Scholar]
- 46.Rossi F, Labourier E, Gallouzi I E, Derancourt J, Allemand E, Divita G, Tazi J. The C-terminal domain but not the tyrosine 723 of human DNA topoisomerase I active site contributes to kinase activity. Nucleic Acids Res. 1998;26:2963–2970. doi: 10.1093/nar/26.12.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth M B, Murphy C, Gall J G. A monoclonal antibody that recognizes a phosphorylated epitope stains lambrush chromosome loops and small granules in the amphibian germinal vesicle. J Cell Biol. 1990;111:2217–2223. doi: 10.1083/jcb.111.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth M B, Zahler A M, Stolk J A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin G M, Hong L, Brokstein P, Evans-Holm M, Frise E, Stapleton M, Harvey D A. A Drosophila complementary DNA resource. Science. 2000;287:2222–2224. doi: 10.1126/science.287.5461.2222. [DOI] [PubMed] [Google Scholar]
- 50.Siebel C W, Feng L, Guthrie C, Fu X D. Conservation in budding yeast of a kinase specific for SR splicing factors. Proc Natl Acad Sci USA. 1999;96:5440–5445. doi: 10.1073/pnas.96.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spradling A C, Rubin G M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 52.Staley J P, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 53.Tacke R, Manley J L. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities. EMBO J. 1995;14:3540–3551. doi: 10.1002/j.1460-2075.1995.tb07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tacke R, Manley J L. Determinants of SR protein specificity. Curr Opin Cell Biol. 1999;11:358–362. doi: 10.1016/S0955-0674(99)80050-7. [DOI] [PubMed] [Google Scholar]
- 55.Tazi J, Temsamani J, Alibert C, Rhead W, Khellil S, Cathala G, Brunel C, Jeanteur P. Purified U5 small nuclear ribonucleoprotein can relieve the inhibition of spliceosome assembly and splicing by snRNP-free nuclear proteins. Nucleic Acids Res. 1989;17:5223–5243. doi: 10.1093/nar/17.13.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tazi J, Kornstadt U, Rossi F, Jeanteur P, Cathala G, Brunel C, Lührmann R. Thiophosphorylation of U1–70K protein inhibits pre-mRNA splicing. Nature. 1993;363:283–286. doi: 10.1038/363283a0. [DOI] [PubMed] [Google Scholar]
- 57.Tazi J, Rossi F, Labourier E, Gallouzi I, Brunel C, Antoine E. DNA topoisomerase I: customs officer at the border between DNA and RNA worlds? J Mol Med. 1997;75:786–800. doi: 10.1007/s001090050168. [DOI] [PubMed] [Google Scholar]
- 58.Wu J Y, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 59.Xiao S H, Manley J L. Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J. 1998;17:6359–6367. doi: 10.1093/emboj/17.21.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeakley J M, Tronchere H, Olesen J, Dyck J A, Wang H Y, Fu X D. Phosphorylation regulates in vivo interaction and molecular targeting of serine/arginine-rich pre-mRNA splicing factors. J Cell Biol. 1999;145:447–455. doi: 10.1083/jcb.145.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zahler A M, Lane W S, Stolk J A, Roth M B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 62.Zillmann M, Zapp M L, Berget S M. Gel electrophoretic isolation of splicing complexes containing U1 small nuclear ribonucleoprotein particles. Mol Cell Biol. 1988;8:814–821. doi: 10.1128/mcb.8.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo P, Manley J L. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993;12:4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]