Abstract
Background: Escherichia coli (E. coli) is one of the main etiological agents responsible for bovine mastitis (BM), neonatal calf diarrhea (NCD), and avian colibacillosis (AC). This study aimed to assess resistance and virulence genes content, biofilm-forming ability, phylogenetic groups, and genetic relatedness in E. coli isolates recovered from clinical cases of BM, NCD, and AC. Materials/Methods: A total of 120 samples including samples of milk (n = 70) and feces (n = 50) from cows with BM and calves with NCD, respectively, were collected from different farms in Northern Tunisia. Bacterial isolation and identification were performed. Then, E. coli isolates were examined by disk diffusion and broth microdilution method for their antimicrobial susceptibility and biofilm-forming ability. PCR was used to detect antimicrobial resistance genes (ARGs), virulence genes (VGs), phylogenetic groups, and Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) for their clonal relationship. Results: Among the 120 samples, 67 E. coli isolates (25 from BM, 22 from AC, and 20 from NCD) were collected. Overall, 83.6% of isolates were multidrug resistant. Thirty-six (53.73%) isolates were phenotypically colistin-resistant (CREC), 28.3% (19/67) were ESBL producers (ESBL-EC), and forty-nine (73.1%) formed biofilm. The blaTEM gene was found in 73.7% (14/19) of isolates from the three diseases, whilst the blaCTXM-g-1 gene was detected in 47.3% (9/19) of isolates, all from AC. The most common VG was the fimA gene (26/36, 72.2%), followed by aer (12/36, 33.3%), cnf1 (6/36, 16.6%), papC (4/36, 11.1%), and stx1 and stx2 genes (2/36; 5.5% for each). Phylogenetic analysis showed that isolates belonged to three groups: A (20/36; 55.5%), B2 (7/36; 19.4%), and D (6/36; 16.6%). Molecular typing by ERIC-PCR showed high genetic diversity of CREC and ESBL E. coli isolates from the three animal diseases and gave evidence of their clonal dissemination within farms in Tunisia. Conclusion: The present study sheds new light on the biofilm-forming ability and clonality within CREC and ESBL-EC isolated from three different animal diseases in Tunisian farm animals.
Keywords: E. coli, colistin resistance, biofilm-forming ability, animal diseases, genetic relatedness
1. Introduction
Escherichia coli (E. coli) is a highly diverse group of Gram-negative bacteria with the ability to colonize and persist in humans, warm-blooded animals, and abiotic environments [1,2]. However, some pathovars of E. coli are responsible for severe gastrointestinal diseases and a range of extra-intestinal infections in both humans and animals [3,4]. In addition, E. coli is one of the main etiological agents responsible for bovine mastitis (BM), neonatal calf diarrhea (NCD), and avian colibacillosis (AC), causing important economic losses [5,6,7].
There are two major groups of pathogenic E. coli: intestinal pathogenic E. coli (InPEC) and extraintestinal pathogenic E. coli (ExPEC). InPECs are divided into five main pathovars according to the clinical manifestation of the disease, the site of infection, and the virulence factors (VFs) repertoire. These pathovars include enterotoxigenic (ETEC), enteropathogenic (EPEC), enterohemorrhagic (EHEC), enteroinvasive (EIEC), and enteroaggregative (EAEC) [8]. Interestingly, ETEC and EPEC are also the most common pathovars associated with NCD [4,9]. On the other hand, ExPEC strains are classified into four pathovars, neonatal meningitis E. coli (NMEC), sepsis-associated E. coli (SEPEC), uropathogenic E. coli (UPEC), and avian pathogenic E. coli (APEC), based on the type of disorders they cause and their mode of interaction with the host [10]. APEC strains are the leading cause of avian colibacillosis responsible for diverse local and systemic infections in poultry, including chickens, turkeys, ducks, and many other avian species [7]. Although some bovine mastitis-associated E. coli (MAEC) strains carry genes associated with ExPEC virulence, most published data have not identified them as ExPEC due to their variable virulence factors (VFs) content. Indeed, the absence of a core set of VFs associated with bovine mastitis-associated E. coli has led to the proposal of MAEC as a distinct pathovar group [11]. Pathogenic E. coli are reservoirs of a wide range of VFs, including adhesins, invasins, toxins, and several uptake systems for various nutrients [12,13]. Additionally, it has been demonstrated that biofilms have important implications in the early stages of bacterial infection [4]. Indeed, the ability of pathogenic E. coli to adhere to epithelial cells, cause diseases, and enhance its antimicrobial resistance capacity is increased by biofilm formation [14].
Previous studies have revealed that APEC and ExPEC strains causing infections in humans are quite closely phylogenetically related and share some of the same virulence genes [15]. Moreover, EHEC strains have been involved in life-threatening gastrointestinal tract infections in humans, with bovines being their natural reservoir [16].
Apart from β-lactams, aminoglycosides, fluoroquinolones, and tetracyclines antibiotic families commonly used in animals [17]; colistin is considered one of the most critically important antimicrobials and has been increasingly used in animal husbandry [18]. Consequently, selection pressure exerted by inadequate use of antibiotics has led to the emergence of multidrug-resistant (MDR) E. coli (MDR), colistin-resistant E. coli (CREC), and Extended-spectrum β-lactamases E. coli (ESBL-EC).
The earlier reports so far from Tunisia have reported the occurrence of CREC and/or ESBL-EC from diseased [19,20,21] and healthy livestock [22,23]. However, it remains unclear if CREC and ESBL-EC isolates recovered from different animal diseases are genetically related and capable of biofilm formation. Therefore, this study aimed to (i) investigate the occurrence of CREC and ESBL-EC in cows with mastitis, diarrheic calves, and chickens with colibacillosis in Tunisia; (ii) assess their biofilm-forming ability and the molecular determinants of their resistance and virulence; and (iii) to determine their phylogenetic groups and their genetic relatedness.
2. Materials and Methods
2.1. Sampling and Sample Collection
In the period from February to April 2016, a total of 120 samples were collected from cows with mastitis and diarrheic calves in three adjacent farms (FIV, FV, and FVI) located in Bizerte and Ariana governorates in Northern Tunisia. Animals showed repetitive episodes of illness without death and were subjected to clinical examination by veterinarians. Following oral consent from animals’ owners, samples including mastitis milk and feces were collected from cows (n = 70) and calves (n = 50), respectively. Farms included in the present survey were characterized by a number of animals ranging from 15 to 32 and were not under control by official veterinary services. According to animals’ owners, the most commonly used antibiotics in treating diseased animals in these farms included β-lactams, aminoglycosides, fluoroquinolones, and tetracyclines. Cows and calves included in the present investigation shared the grazing environment, food, and water sources. In addition, cow’s milk was used to feed young calves. All samples were transported to the laboratory within a few hours of collection in refrigerated boxes and processed immediately. Twenty-two E. coli isolates previously identified from chickens who died of colibacillosis in three different farms (I, II and III) located in Nabeul, Ben Arous, and Zaghouane governorates in Northeast Tunisia were included in the present study for further analysis [20].
2.2. Isolation and Bacteria Identification
One hundred microliters from each sample was placed in brain heart infusion broth (Oxoid Ltd., Basingstoke, UK) and incubated aerobically at 37 °C for 24 h. Then, 10 µL of culture suspensions were seeded onto MacConkey agar (Merck, Darmstadt, Germany) plates and incubated overnight at 37 °C. Isolates with typical E. coli morphology were selected and seeded onto Endo agar (Merck) and incubated overnight at 37 °C. One presumptive colony per sample was selected and identified by conventional methods including Gram staining and biochemical tests (oxidase, catalase, urea-Indole, lactose, and glucose fermentation gas production ability in Kigler-Hajna agar) and by an API 20E system (BioMerieux, Marcy l’Etoile, France). Bacterial DNA for polymerase chain reaction (PCR) analysis was prepared by boiling a loopful of bacteria in 400 µL of TE buffer (10 mMTris–2 mM EDTA) for 10 min, followed by centrifugation for 15 min at 10,000× g. Subsequently, isolates were confirmed as E. coli using species-specific PCR targeting the uidA gene encoding for β-glucuronidase structural protein [24] (Table 1).
2.3. Antimicrobial Susceptibility Testing and Screening for ESBL Production
Antimicrobial susceptibility of all E. coli isolates was determined using the disc diffusion method and interpreted according to the Clinical and Laboratory Standards Institute [25] and the European Committee on Antimicrobial Susceptibility Testing guidelines [26]. The following antibiotics (Oxoid) were used (µg/disk): Ampicillin (AMP, 10 µg), nalidixic acid (NA, 30 µg), tetracycline (TET, 30 µg), trimethoprim–sulfamethoxazole (SXT, 1.25/23.75 µg), streptomycin (STR, 10 µg), cefotaxime (CTX, 30 µg), cefoxitin (FOX, 30 µg), ceftiofur (XNL, 30 µg), cefsulodine (CFZ, 30 µg), gentamicin (GN, 30 µg), enrofloxacin (ENR, 5 µg), imipenem (IMP, 30 µg), meropenem (MEM, 30 µg), ertapenem (ETP, 30 µg), chloramphenicol (CHL, 30 µg), and colistin (CST, 50 µg). The double-disk synergy test (DDST) with cefotaxime (CTX, 30 µg), ceftazidime (CAZ, 30 µg), aztreonam (ATM, 30 µg), and cefepime (FEP, 30 µg) in the proximity of amoxicillin-clavulanic acid (AMC, 20/10 µg) was used for the screening of ESBL production. E. coli ATCC25922 and Klebsiella pneumonia ATCC700603 were used as ESBL-negative and positive control strains, respectively. The isolates were defined as multidrug-resistant (MDR) if they exhibited resistance to at least one agent belonging to three or more antimicrobial families [27].
2.4. Colistin Susceptibility Testing and Screening of Colistin Resistance Genes
The minimum inhibitory concentration (MIC) of colistin was determined using the broth microdilution method (BMD) according to the CLSI guidelines [28]. Dilution methods were performed using colistin sulfate (Sigma-Aldrich, Merck, Darmstadt, Germany) tested over a range from 0.25 to 128 µg/mL. All experiments were performed in triplicate. E. coli ATCC 25922 was used as a quality control strain. The mcr-1, mcr-2, mcr-3, and mcr-4 genes encoding for colistin resistance were investigated by PCR in all isolates with MIC ≥2 µg/mL as described elsewhere [18,29,30,31].
2.5. Detection of Resistance Genes in CREC Isolates
The 36 CREC isolates (12 from avian colibacillosis, 18 from mastitis, and 6 from diarrhea) were selected for further molecular characterization. CREC isolates were screened by PCR for the presence of antimicrobial resistance genes conferring resistance to streptomycin (aadA, strA, strB), phenicols (cmlA, floR), tetracyclines (tetA, tetB), trimethoprim (dfrAI, dfrVII), and sulfonamides (sul1, sul2) as previously described [32,33] (Table 1).
2.6. Detection of β-Lactamase-Encoding Genes
All ESBL-EC isolates were screened for the presence of five β-lactamase-encoding genes (blaTEM, blaSHV, blaCTX-M-g-1, blaCTX-M-g-8, and blaCTX-M-g-9) using PCR conditions as previously described [24,32] (Table 1).
2.7. Biofilm Formation Assay
The biofilm formation ability of the 67 E. coli isolates was performed in 96-well microtiter plates [34]. Briefly, an overnight culture was diluted (1:100) in TSB containing 1% glucose and inoculated onto microtiter plates at 37 °C for 18 h without aeration. The free-floating planktonic bacteria were removed and washed, dried for 60 min at 60 °C, and stained with 0.06% crystal violet. The biofilm was quantified in duplicate, after adding 200 μL of 95% ethanol using a microtiter plate reader by an enzyme-linked immunosorbent assay plate reader at 570 nm (BioRad). Each strain was tested in triplicate and each assay was performed in duplicate. E. coli ATCC25922 and S. epidermidis strain ATCC12228 were used as positive and negative controls, respectively. The isolates were classified as strong biofilm producer: 4 × ODC< OD; moderate biofilm producer: 2 × ODc < OD ≤ 4 × ODc; weak biofilm producer: ODc < OD < 2 × ODc; and no biofilm producer: OD ≤ ODc [35]. The cut-off value (ODc) is defined as three standard deviations (SD) above the mean OD of the negative control (TSB plus 1% glucose, without bacterial cells) [35].
2.8. Detection of Virulence and Biofilm Encoding Genes
Biofilm and virulence-associated genes (fimA, papC, hly, aer, cnf1, stx1 and stx2) were investigated in CREC isolates by PCR using sets of primers as described in previous studies [36,37] (Table 1).
2.9. E. coli Phylogenetic Typing
Phylogenetic groups (A, B1, B2, or D) and sub-groups (A0, A1, B1, B22, B23, D1, and D2) of all E. coli isolates were determined using a triplex PCR targeting the chuA, yjaA genes, and the DNA fragment tspE4.C2 as described by Clermont et al., 2000 [38] and Escobar-Paramo et al. (2006) [39] (Table 1).
Table 1.
Primers, amplicon size and annealing temperature used for the detection of resistance genes, integrons, virulence genes, phylogenetic groups, and genotyping of E. coli isolates.
| Primer Name | Oligonucleotide Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temp. °C | Specificity | Reference |
|---|---|---|---|---|---|
| E. coli Identification | |||||
| UidA | F: ATCACCGTGGTGACGCATGTCGC | 486 | 51 | β-glucuronidase enzyme | [24] |
| R: CACCACGATGCCATGTTCATCTGC | |||||
| Resistance Genes | |||||
| mcr1 | F: CGGTCAGTCCGTTTGTTC | 309 | 58 | Colistin | [18] |
| R: CTTGGTCGGTCTGTAGGG | |||||
| mcr2 | F:TGTTGCTTGTGCCGATTGGA | 567 | 58 | [29] | |
| R: AGATGGTATTGTTGGTTGCTG | |||||
| mcr-3 | F: TTGGCACTGTATTTTGCATTT | 542 | 50 | [30] | |
| R: TTAACGAAATTGGCTGGAACA | |||||
| mcr-4 | F: ATTGGGATAGTCGCCTTTTT | 487 | 56 | [31] | |
| R: TTACAGCCAGAATCATTATCA | |||||
| bla TEM | F: ATTCTTGAAGACGAAAGGGC | 1150 | 60 | Bêtalactamases | [32] |
| R: ACGCTCAGTGGAACGAAAAC | |||||
| tet(A) | F:AATTCTGAGCACTGTCGC | 937 | 62 | Tetracyclines | |
| R: CTGCCTGGACAACATTGCTT | |||||
| tet(B) | F: CTCAGTATTCCAAGCCTTTG | 416 | 57 | ||
| R: CTAAGCACTTGTCTCCTGTT | |||||
| strA | F: ATTCTGACTGGTTGCCTGTC | 1562 | 55 | Streptomycin | |
| R: CGCAGATAGAAGGCAAGG | |||||
| strB | F: TTCTCATTGCGGACAACCT | 1562 | 55 | ||
| R: TAGATCGCGTTGCTCCTCTT | |||||
| DfrAI | F: GTGAAACTATCACTAATGG | 474 | 55 | Trimethoprim | |
| R: TTAACCCTTTTGCCAGATTT | |||||
| DfrVII | F: TTGAAAATTTCATTGATT | 474 | 55 | ||
| R: TTAGCCTTTTTTCCAAATCT | |||||
| sul1 | F:TGGTGACGGTGTTCGGCATTC | 789 | 63 | Sulfamides | |
| R: GCGAGGGTTTCCGAGAAGGTG | |||||
| sul2 | F: CGGCATCGTCAACATAACC | 722 | 50 | ||
| R: GTGTGCGGATGAAGTCAG | |||||
| aadA | F: GCAGCGCAATGACATTCTTG | 282 | 60 | Streptomycin | [33] |
| R: ATCCTTCGGCGCGATTTTG | |||||
| floR | F: CACGTTGAGCCTCTATAT | 868 | 55 | Florfenicol | [32] |
| R: ATGCAGAAGTAGAACGCG | |||||
| cmlA | F: TGTCATTTACGGCATACTCG | 455 | 55 | Chloramphenicol | |
| R: ATCAGGCATCCCATTCCCAT | |||||
| bla SHV | F: CACTCAAGGATGTATTGTG | 885 | 52 | β-lactamases | [24] |
| R: TTAGCGTTGCCAGTGCTCG | |||||
| bla CTX-M-g-1 | F: GTTACAATGTGTGAGAAGCAG | 1041 | 50 | ||
| R: CCGTTTCCGCTATTACAAAC | |||||
| bla CTX-M-g-8 | F: TGATGAGACATCGCGTTAAG | 666 | 52 | ||
| R: TAACCGTCGGTGACGATTTT | |||||
| bla CTX-M-g-9 | F: GTGACAAAGAGAGTGCAACGG | 856 | 62 | ||
| R: ATGATTCTCGCCGCTGAAGCC | |||||
| Virulence Genes | |||||
| fimA | F: GTTGTTCTGTCGGCTCTGTC | 447 | 55 | Type 1 Fimbriae | [36] |
| R: ATGGTGTTGGTTCCGTTATTC | |||||
| aer | F: TACCGGATTGTCATATGCAGACCGT | 602 | 55 | Aerobactin iron uptake system | |
| R: AATATCTTCCTCCAGTCCGGAGAAG | |||||
| stx1 | F: CTGGATTTAATGTCGCATAGTG | 150 | 55 | Type 1 Shiga-toxin | [37] |
| R: AGAACGCCCACTGAGATCATC | |||||
| stx2 | F: GGCACTGTCTGAAACTGCTCC | 255 | 55 | Type 2 Shiga-toxin | |
| R: TCGCCAGTTATCTGACATTCTG | |||||
| hlyA | F: AACAAGGATAAGCACTGTTCTGGCT | 1177 | 55 | Alpha-hemolysin | [36] |
| R: ACCATATAAGCGGTCATTCCCGTCA | |||||
| cnf1 | F: AAGATGGAGTTTCCTATGCAGGAG | 498 | 55 | Cytotoxic necrotizing factor 1 | |
| R: CATTCAGAGTCCTGCCCTCATTATT | |||||
| papC | F:GACGGCTGTACTGCAGGGTGTGGCG | 328 | 55 | P Fimbriae | |
| R: ATATCCTTTCTGCAGGGATGCAATA | |||||
| Phylogenetic Groups | |||||
| chuA | F: GACGAACCAACGGTCAGGAT | 279 | 55 | Phylogenetic groups | [38,39] |
| R: TGCCGCCAGTACCAAAGACA | |||||
| yjaA | F: TGAAGTGTCAGGAGACGCTG | 211 | 55 | ||
| R: ATGGAGAATGCGTTCCTCAAC | |||||
| tspE4.C2 | F: GAGTAATGTCGGGGCATTCA | 152 | 55 | ||
| R: CGCGCCAACAAAGTATTACG | |||||
| Genotyping | |||||
| ERIC | F: ATGTAAGCTCCTGGGGATTCAC | * | 52 | Enterobacterial Repetitive Intergenic Consensus | [40] |
| R: AAGTAAGTGACTGGGGTGAGCG | |||||
* Bands profile.
2.10. E.coli Molecular Typing by ERIC-PCR
CREC isolates were fingerprinted by ERIC-PCR as described by Bilung et al. (2018) [40] (Table 1) and different ERIC-PCR profiles were analyzed visually and numerically according to Tenover et al. (1998) [41]. Then, the phylogenetic tree was established using MVSP 3.2 software. The comparison between ERIC-PCR profiles was conducted using the Jaccard coefficient, and a dendrogram was constructed using the unweighted pair group method with arithmetic mean (UPGMA).
2.11. Statistical Analysis
Statistical analysis was performed in IBM SPSS 22.0. A chi-squared test χ² using the Pearson Chi-square test was employed to estimate differences between colistin resistance, virulence genes, biofilm formation, and ESBL production rates in E. coli from the three animal diseases, whereby a probability of less than 0.05 was considered statistically significant.
3. Results
3.1. Collected E. coli Isolates
A total of 45 (37.5%) out of 120 bovine samples displayed a positive culture for E. coli (25 isolates from BM and 20 from NCD). In addition, 22 E. coli isolates recovered from chickens that died of AC, which were previously identified, were added to the collection for further analysis. Overall, 67 E. coli isolates were included in the present investigation.
3.2. Antimicrobial Susceptibility Testing and Screening for ESBL Production
The highest rates of antibiotic resistance in the 67 E. coli isolates were found for cefsulodine (67/67; 100%), followed by ceftazidime (56/67; 83.6%), streptomycin (55/67; 82.1%), cefotaxime (49/67; 73.1%), tetracycline (44/67; 65.7%), colistin (36/67; 53.7%), trimethoprim-sulfamethoxazole (29/67; 43.3%), nalidixic acid (27/67; 40.3%), ampicillin (24/67; 35.8%), enrofloxacin (20/67; 29.8%), chloramphenicol (19/67; 28.3%), cefoxitin (18/67; 26.8%), and meropenem and amoxicillin-clavulanic acid (17/67; 25.4%) for each. However, E. coli isolates exhibited lower frequencies of resistance to aztreonam (12/67; 17.9%), ceftiofur (8/67; 11.9%), gentamicin (5/67; 7.4%), ertapenem and imipenem (2/67; 3%) for each, and cefepime (1/67; 1.5%) (Table 2).
Table 2.
Antibiotic resistance rates in 67 E. coli isolates recovered from bovine mastitis, calves’ diarrhea, and avian colibacillosis.
| Antibiotic | Class | Mastitis (n = 25) n (%) |
Colibacillosis (n = 22) n (%) |
Diarrhea (n = 20) n (%) |
Total (n = 67) n (%) |
|---|---|---|---|---|---|
| Ampicillin | β-lactams | 8 (32) | 10 (45.4) | 6 (30) | 24 (35.8) |
| Amoxicillin-clavulanic acid | 2 (8) | 10 (45.4) | 5 (25) | 17 (25.4) | |
| Aztreonam | 2 (8) | 10 (45.4) | 0 (0) | 12 (17.9) | |
| Cefotaxime | 21 (84) | 11 (50) | 17 (85) | 49 (73.1) | |
| Cefoxitin | 2 (8) | 1 (4.5) | 15 (75) | 18 (26.8) | |
| Ceftiofur | 2 (8) | 6 (27.3) | 0 (0) | 8 (11.9) | |
| Cefsulodine | 25 (100) | 22 (100) | 20 (100) | 67 (100) | |
| Ceftazidime | 25 (100) | 11 (50) | 20 (100) | 56 (83.6) | |
| Cefepime | 0 (0) | 1 (4.5) | 0 (0) | 1 (1.5) | |
| Imipenem | Carbapenems | 1 (4) | 1 (4.5) | 0 (0) | 2 (3) |
| Meropenem | 4 (16) | 12 (54.5) | 1 (5) | 17 (25.4) | |
| Ertapenem | 1 (4) | 1 (4.5) | 0 (0) | 2 (3) | |
| Tetracyclines | Tetracyclines | 10 (40) | 19 (86.3) | 15 (75) | 44 (65.7) |
| Enrofloxacin | Fluoroquinolones | 1 (4) | 16 (72.7) | 3 (15) | 20 (29.8) |
| Nalidixic acid | Quinolones | 3 (12) | 21 (95.4) | 3 (15) | 27 (40.3) |
| Gentamycin | Aminoglycosides | 1 (4) | 1 (4.5) | 3 (15) | 5 (7.4) |
| Streptomycin | 20 (80) | 21 (95.4) | 14 (70) | 55 (82.1) | |
| Trimethoprim-sulfamethoxazole | Dihdrofolatreductase/Sulfonamide | 6 (24) | 15 (68.2) | 8 (40) | 29 (43.3) |
| Chloramphenicol | Phenicols | 2 (8) | 15 (68.2) | 2 (10) | 19 (28.3) |
| Colistin | Polymyxins | 18 (72) | 12 (54.5) | 6 (30) | 36 (53.7) |
Avian isolates showed the most important antibiotic resistance rates for the majority of antibiotics tested except for cefotaxime, ceftazidime, and colistin, for which mastitis isolates displayed the highest resistance rates as shown in Table 2. Although diarrheal isolates showed the lowest resistance rates to the majority of antibiotics, they revealed higher resistance frequencies for ceftazidime, cefotaxime, cefoxitin, and gentamicin than those in avian isolates.
Of the 67 E. coli isolates, only 19 (28.3%) were ESBL producers. Among them, nine (47.3%) originated from chickens with colibacillosis, seven (36.8%) from cows with mastitis, and three (15.8%) from calves with diarrhea (Table 3).
Table 3.
Phenotypic resistance patterns, minimal inhibitory concentrations of colistin, ESBL production, and biofilm-forming ability in 67 E. coli isolates recovered from bovine mastitis, calves’ diarrhea, and avian colibacillosis in farms in Tunisia.
| Farm | Strain ID | Origin | Phenotypic Resistance Profile | Colistin MIC (µg/mL) | Biofilm Formation |
|---|---|---|---|---|---|
| FI | E2 a | AC | AMP/CFZ/CHL/ENR/MEM/NA/STR/SXT/TET/CST b | 8 | SBF |
| E3 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/MEM/STR/XNL/NA/SXT | 1 | SBF | |
| E9 a | AC | AMP/CFZ/CHL/ENR/MEM/NA/STR/SXT/TET/XNL/CST b | 1 | NBF | |
| E12 a | AC | AMP/CFZ/NA/STR/SXT/TET/CST b | 8 | SBF | |
| E13 a | AC | AMP/CAZ/CFZ/CTX/ENR/CST b | 8 | SBF | |
| E15 a | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/ETP/MEM/NA/STR/SXT/TET/CST b | 4 | SBF | |
| E17 a | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/GN/NA/STR/TET/XNL/CST b | 4 | MBF | |
| E19 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/NA/STR | 0.25 | SBF | |
| E22 | AC | CFZ/ENR/NA/STR/SXT | 0.5 | SBF | |
| E28 a | AC | AMP/CFZ/CHL/STR/TET/XNL/CST b | 4 | NBF | |
| E35 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/IMP/MEM/NA/STR/SXT/TET/XNL | 0.5 | SBF | |
| E42 | AC | CFZ/CHL/ENR/MEM/STR/NA/SXT/TET | 1 | SBF | |
| E46 | AC | AMP/CFZ/CHL/ENR/NA/STR/SXT/TET/CST b | 8 | SBF | |
| E22 | AC | CFZ/ENR/NA/STR/SXT | 0.5 | SBF | |
| E28 a | AC | AMP/CFZ/CHL/STR/TET/XNL/CST b | 4 | NBF | |
| E35 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/IMP/MEM/NA/STR/SXT/TET/XNL | 0.5 | SBF | |
| E42 | AC | CFZ/CHL/ENR/MEM/STR/NA/SXT/TET | 1 | SBF | |
| E46 | AC | AMP/CFZ/CHL/ENR/NA/STR/SXT/TET/CST b | 8 | SBF | |
| E47 | AC | CFZ/MEM/NA/STR/TET | 1 | NBF | |
| E48 | AC | ATM/AMC/CAZ/CFZ/CTX/NA/STR/TET/CST b | 4 | SBF | |
| E50 a | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/ENR/MEM/NA/STR/SXT/TET/CST b | 4 | NBF | |
| FII | E18 | AC | CFZ/CHL/ENR/NA/STR/SXT/TET | 0.5 | SBF |
| E38 | AC | CFZ/MEM/STR/CHL/NA/SXT/TET | 0.5 | NBF | |
| E31 a | AC | CFZ/CHL/MEM/NA/STR/SXT/TET/CST b | 8 | SBF | |
| FIII | E20 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/MEM/NA/STR/TET | 0.5 | SBF |
| E27 | AC | ATM/AMC/CAZ/CFZ/CTX/CHL/ENR/MEM/NA/STR/SXT/TET/XNL | 0.25 | SBF | |
| E41 | AC | ATM/AMC/CAZ/CFZ/CTX/ENR/NA/SXT/TET/CST b | 4 | SBF | |
| FIV | L1 | BM | CAZ/CFZ/STR/CST b | 32 | SBF |
| L3 a | BM | AMP/CAZ/CFZ/CTX/FOX/STR/NA/CST b | 64 | SBF | |
| L7 a | BM | AMP/CAZ/CFZ/CTX/STR/CST b | 32 | SBF | |
| FIV | L9 a | BM | AMP/CAZ/CFZ/STR/CST b | 64 | SBF |
| L11 | BM | CAZ/CFZ/CTX/STR/CST b | 64 | SBF | |
| L12 a | BM | AMP/CAZ/CFZ/CTX/STR/CST b | 32 | SBF | |
| L13 | BM | CAZ/CFZ/STR/CST b | 128 | SBF | |
| L19 | BM | CAZ/CFZ/CTX | 0.5 | SBF | |
| L23 | BM | AMC/CAZ/CFZ/CTX/STR/SXT/TET/CST b | 128 | NBF | |
| L25 | BM | CAZ/CFZ/CTX/STR/TET/CST b | 64 | SBF | |
| L26 a | BM | CAZ/CFZ/CTX | 0.5 | SBF | |
| D3 | DC | CAZ/CFZ/FOX/TET | 1 | SBF | |
| D7 | DC | CAZ/CFZ/CTX//FOX/MEM/TET | 1 | NBF | |
| D9 | DC | CAZ/CFZ/CTX/TET | 0.5 | SBF | |
| D12 | DC | CAZ/CFZ/CTX/STR/TET/ENR/CHL/GN/SXT | 0.5 | MBF | |
| D13 | DC | AMC/CAZ/CFZ/CTX/FOX/NA/STR/SXT/TET | 0.5 | SBF | |
| D14 | DC | AMC/CAZ/CFZ/ENR/FOX/NA/STR/SXT/TET | 0.5 | MBF | |
| D18 | DC | AMC/CAZ/CFZ/FOX/STR/SXT/TET | 1 | NBF | |
| D22 a | DC | AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/GN/STR/SXT/TET/CST b | 32 | NBF | |
| FV | L31 | BM | CAZ/CFZ/CTX/CST b | 64 | SBF |
| L37 | BM | CAZ/CFZ/CTX/STR/TET/CST b | 32 | NBF | |
| L39 a | BM | CAZ/CFZ/CST b | 64 | SBF | |
| L49 | BM | CAZ/CFZ/CTX/GN/CST b | 32 | SBF | |
| D24 | DC | CAZ/CFZ/CTX/FOX/GN/STR/TET | 1 | SBF | |
| D29 a | DC | AMP/CAZ/CFZ/CTX/TET | 0.5 | SBF | |
| D34 | DC | CAZ/CFZ/CTX/FOX/SXT/TET | 0.5 | NBF | |
| D35 | DC | AMP/CAZ/CFZ/CTX/FOX/STR/CST b | 32 | SBF | |
| D38 | DC | CAZ/CFZ/CTX/STR/SXT/TET/CST b | 32 | NBF | |
| D41 | DC | CAZ/CFZ/CTX/FOX/TET | 0.25 | MBF | |
| L58 | BM | CAZ/CFZ/CTX/STR | 1 | NBF | |
| L61 | BM | AMP/CAZ/CFZ/CTX/CHL/ENR/MEM/STR/SXT/TET/XNL/CST b | 128 | SBF | |
| L62 | BM | CAZ/CFZ/CTX//CHL/IMP/MEM/STR/SXT/TET | 1 | NBF | |
| L64 | BM | AMP/CAZ/CFZ/CTX/MEM/STR/SXT/TET/CST b | 64 | SBF | |
| L78 | BM | ATM/AMP/AMC/CAZ/CFZ/CTX/ETP/MEM/STR/XNL/CST b | 128 | SBF | |
| L79 | BM | ATM/CAZ/CFZ/CTX/STR/SXT/TET | 1 | SBF | |
| L82 a | BM | AMP/CAZ/CFZ/CTX/STR | 0.25 | SBF | |
| L94 | BM | CAZ/CFZ/CTX/STR/TET | 0.25 | NBF | |
| L129 | BM | CAZ/CFZ/CTX/STR/TET/CST b | 64 | SBF | |
| D43 | DC | AMC/CAZ/CFZ/CTX/SXT/STR/TET/FOX/CST b | 32 | NBF | |
| D45 | DC | AMP/CAZ/CFZ/CTX/FOX/STR/CST b | 64 | SBF | |
| D46 | DC | CAZ/CFZ/CTX/STR/CST b | 32 | NBF | |
| D50 | DC | CAZ/CFZ/CTX/FOX/STR/TET | 1 | SBF | |
| D52 a | DC | AMP/CAZ/CFZ/CTX/FOX/STR | 0.5 | MBF | |
| D53 | DC | CAZ/CFZ/CTX/CST b | 0.5 | NBF |
AMC, amoxicillin–clavulanic acid; AMP, Ampicillin; ATM, aztreonam; CAZ, ceftazidime; CFZ, cefsulodine; CHL, chloramphenicol; CTX, cefotaxime; ENR, Enrofloxacin; ETP, ertapenem; FOX, cefoxitin; GN, gentamycin; MEM, meropenem; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracyclines; XNL, ceftiofur; CST, colistin; AC, Avian colibacillosis; BM, bovine mastitis; DC, Diarrheic calves; ESBL: Extended-spectrum β-lactamase-producing strains. a ESBL producer, b Colistin-resistant strains (MIC ranging from 4 to 128 ug/mL), SBF: strong biofilm-forming, MBF: moderate biofilm-forming, NBF: non-biofilm-forming.
3.3. MIC of Colistin and Detection of Resistance Genes
A total of 36 colistin-resistant E. coli isolates were found (36/67; 53.7%) using the microdilution test with MICs of colistin ranging from 4 to 128 µg/µL (Table 3). Mastitis-associated isolates showed the greatest colistin resistance rate (18/25; 72%), followed by colibacillosis-related isolates (12/22; 54.5%), and finally calves’ isolates (6/20; 30%). The mcr-1 gene was detected only in 10 E. coli isolates, all collected from chickens with colibacillosis (10/36; 27.78%). However, the remaining mcr genes (2, 3, and 4) were not detected (Table 4). A statistically significant relationship was found between colistin resistance, carriage of the mcr-1 gene and the origin of isolates (p < 0.05) (Table 5).
Table 4.
Phenotypic and genotypic antibiotic resistance profiles, virulence genes, and biofilm-forming ability of 36 colistin-resistant E. coli isolates recovered from bovine mastitis, calves’ diarrhea, and avian colibacillosis in Tunisia.
| Strain ID | Farm | Animal Pathology |
Resistance Phenotypic Profile | Resistance Genes | Virulence Genes | Biofilm Formation |
Phylo-Group | ERIC Profile |
|---|---|---|---|---|---|---|---|---|
| E12 a | FI | AC | AMP/CFZ/NA/STR/SXT/TET/CST b | mcr-1, blaTEM, blaCTX-M-g-1, aadA, tetA, dfrAI | fimA, stx1, aer | SBF | A1 | J |
| E13 a | FI | AC | AMP/CAZ/CFZ/CTX/ENR/CST b | mcr-1, blaCTX-M-g-1, floR | fimA, stx2 | SBF | B23 | F |
| E15 a | FI | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/ETP/MEM/NA/STR/SXT/TET/CST b | mcr-1, blaCTX-M-g-1, tetA, aadA, floR, strA, dfrAI | fimA | SBF | D2 | C |
| E17 a | FI | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/GN/NA/STR/TET/XNL/CST b | mcr-1, tetA, blaCTX-M-g-1 | --- | MBF | A1 | F |
| E2 a | FI | AC | AMP/CFZ/CHL/ENR/MEM/NA/STR/SXT/TET/CST b | mcr-1, blaTEM, blaCTX-M-1, cmlA | fimA, stx1, stx2, aer, papC | SBF | B23 | G |
| E28 a | FI | AC | AMP/CFZ/CHL/STR/TET/XNL/CST b | mcr-1, blaTEM, blaCTX-M-g-1, aadA, tetA, strA | fimA, aer | NBF | B23 | G |
| E46 a | FI | AC | AMP/CFZ/CHL/ENR/NA/STR/SXT/TET/CST b | mcr-1, blaCTX-M-1 | fimA, aer | SBF | B23 | E |
| E48 | FI | AC | ATM/AMC/CAZ/CFZ/CTX/NA/STR/TET/CST b | dfrAI, aadA | aer | SBF | B23 | F |
| E50 a | FI | AC | ATM/AMP/AMC/CAZ/CFZ/CTX/ENR/MEM/NA/STR/SXT/TET/CST b | mcr-1, blaCTX-M-g-1, floR, strA, dfrAI | fimA, aer | NBF | B23 | G |
| E31 a | FII | AC | CFZ/CHL/MEM/NA/STR/SXT/TET/CST b | mcr-1 | fimA, aer | SBF | B22 | F |
| E41 | FIII | AC | ATM/AMC/CAZ/CFZ/CTX/ENR/NA/SXT/TET/CST b | tetA | --- | SBF | D1 | C |
| E9 a | FIII | AC | AMP/CFZ/CHL/ENR/MEM/NA/STR/SXT/TET/XNL/CST b | mcr-1, blaTEM, blaSHV, blaCTX-M-1, aadA, floR, strB, dfrAI | fimA | NBF | B22 | G |
| D22 a | FIV | DC | AMP/AMC/CAZ/CFZ/CTX/CHL/ENR/GN/STR/SXT/TET/CST b | blaTEM, aadA, tetA, sul1, dfrAI | --- | NBF | A1 | K |
| L1 | FIV | BM | CAZ/CFZ/STR/CST b | aadA | fimA, cnf1, papC | SBF | A1 | A |
| L7 a | FIV | BM | AMP/CAZ/CFZ/CTX/STR/CST b | blaTEM, aadA | fimA, cnf1, papC | SBF | A1 | A |
| L9 a | FIV | BM | AMP/CAZ/CFZ/STR/CST b | blaTEM, aadA | fimA, cnf1, papC | SBF | A1 | A |
| L12 a | FIV | BM | AMP/CAZ/CFZ/CTX/STR/CST b | blaTEM, aadA | fimA | SBF | A1 | B |
| L25 | FIV | BM | CAZ/CFZ/CTX/STR/TET/CST b | aadA | fimA | SBF | D2 | C |
| L11 | FIV | BM | CAZ/CFZ/CTX/STR/CST b | aadA, tetA | fimA, aer | SBF | A1 | D |
| L13 | FIV | BM | CAZ/CFZ/STR/CST b | aadA | fimA, aer | SBF | D1 | M |
| L23 | FIV | BM | AMC/CAZ/CFZ/CTX/STR/SXT/TET/CST b | aadA, tetA, sul1, dfrAI | fimA | NBF | B23 | E |
| L3 a | FIV | BM | AMP/CAZ/CFZ/CTX/FOX/STR/NA/CST b | blaTEM, aadA | fimA | SBF | D1 | L |
| D35 | FV | DC | AMP/CAZ/CFZ/CTX/FOX/STR/CST b | blaTEM, aadA | --- | SBF | A1 | B |
| D38 | FV | DC | CAZ/CFZ/CTX/STR/SXT/TET/CST b | aadA, tetA, sul1, dfrAI | --- | NBF | A1 | B |
| L31 | FV | BM | CAZ/CFZ/CTX/CST b | --- | fimA, cnf1 | SBF | A1 | A |
| L37 | FV | BM | CAZ/CFZ/CTX/STR/TET/CST b | aadA, tetA | -- | NBF | A1 | A |
| L49 | FV | BM | CAZ/CFZ/CTX/GN/CST b | --- | fimA, cnf1 | SBF | A1 | A |
| L39 a | FV | BM | CAZ/CFZ/CST b | --- | fimA | SBF | A1 | I |
| D45 | FVI | DC | AMP/CAZ/CFZ/CTX/FOX/STR/CST b | blaTEM, aadA | --- | SBF | A1 | B |
| D43 | FVI | DC | AMC/CAZ/CFZ/CTX/SXT/STR/TET/FOX/CST b | aadA, tetA, sul1, dfrAI | --- | NBF | D1 | N |
| D46 | FVI | DC | CAZ/CFZ/CTX/STR/CST b | aadA | --- | NBF | A1 | H |
| L78 | FVI | BM | ATM/AMP/AMC/CAZ/CFZ/CTX/ETP/MEM/STR/XNL/CST b | blaTEM, aadA | fimA, cnf1 | SBF | A1 | A |
| L129 | FVI | BM | CAZ/CFZ/CTX/STR/TET/CST b | aadA, tetA | fimA | SBF | A1 | B |
| L51 | FVI | BM | CAZ/CFZ/CTX/STR/SXT/TET/CST b | strA, tetA, sul1, dfrAI | fimA, aer | SBF | A1 | D |
| L61 | FVI | BM | AMP/CAZ/CFZ/CTX/CHL/ENR/MEM/STR/SXT/TET/XNL/CST b | blaTEM, strA, floR, tetA, sul1, dfrAI | fimA, aer | SBF | A1 | D |
| L64 | FVI | BM | AMP/CAZ/CFZ/CTX/MEM/STR/SXT/TET/CST b | blaTEM, strA, tetA, sul1 | fimA, aer | SBF | B23 | E |
AMC, amoxicillin–clavulanic acid; AMP, Ampicillin; ATM, aztreonam; CAZ, ceftazidime; CFZ, cefsulodine; CHL, chloramphenicol; CTX, cefotaxime; ENR, Enrofloxacin; ETP, ertapenem; FOX, cefoxitin; GN, gentamycin; MEM, meropenem; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracyclines; XNL ceftiofur; CST, colistin; AC, Avian colibacillosis; BM, bovine mastitis; DC, Diarrheic calves; a Extended-spectrum β-lactamase-producing strains. b Colistin-resistant strains (MIC ranging from 4 to 128 ug/mL).
Table 5.
Relationship between animal diseases and colistin resistance, mcr-1 gene carriage, ESBL production, and biofilm-forming ability of 67 E. coli isolates recovered from bovine mastitis, calves’ diarrhea, and avian colibacillosis in Tunisia.
| Mastitis (n = 25) n (%) | Colibacillosis (n = 22) n (%) | Diarrhea (n = 20) n (%) | Total (n = 67) n (%) |
p-Value | |
|---|---|---|---|---|---|
| Colistin resistance | 18 (72) | 12 (54.5) | 6 (30) | 36 (53.7) | 0.000 * |
| mcr-1 gene | 0 (0) | 10 (27.7) | 0 (0) | 10 (14.9) | 0.000 * |
| ESBL production | 7 (28) | 9 (40) | 3 (15) | 19 (28.3) | 0.004 * |
| Biofilm formation | 20 (80) | 17 (77.3) | 12 (60) | 49 (73.1) | 0.049 * |
* Statistically significant relationship between animal diseases and colistin resistance, mcr-1 gene carriage, ESBL production, and biofilm-forming ability of E. coli isolates.
3.4. Biofilm Formation Assay
A total of 49 of the 67 isolates (73.13%) formed biofilm (OD > 4 × 10−3), while 18 (26.87%) did not (OD ≤ 4 ×10−3). Among biofilm-forming isolates, 44 (65.67%) were classified as strong biofilm-forming isolates (OD > 16 × 10−3), while 5 strains (7.46%) were moderately biofilm-forming isolates (8 × 10−3 < DO ≤ 16 × 10−3). In this study, no strains were classified as weak biofilm formers (4 × 10− 3 < DO ≤ 8 × 10−3). The highest rate of biofilm-forming ability was observed in mastitis-associated isolates (20/25; 80%), followed by avian colibacillosis-associated isolates (17/22; 77.3%), and calve diarrheic isolates (12/20; 60%) (Table 3). Biofilm-forming ability in mastitis isolates was significantly higher than that in colibacillosis and diarrheic isolates (p < 0.05) (Table 5). Interestingly, the majority of colistin-resistant isolates were rather strong biofilm-forming (SBF) (26/36; 72.2%) or moderate biofilm-forming isolates (MBF) (2/36; 5.5%), whilst only nine colistin-resistant isolates were not biofilm producers (9/36; 25%) (Table 4). A statistically significant correlation was observed between colistin resistance and biofilm-forming ability (p < 0.05) (Table 5).
3.5. Detection Genes Encoding ESBL Enzymes and Other Resistance Markers
The blaTEM gene encoding for ESBL production was found in 73.7% (14/19) of isolates (seven from cows, four from chickens, and three from calves). However, the blaCTXM-g-1 gene was only detected in nine (9/19; 47.3%) phenotypically ESBL-EC strains. All blaCTXM-g-1-carrying isolates were from chickens with colibacillosis, whilst none of the mastitis and diarrhea isolates carried this gene. The blaSHV gene was detected in a single isolate of avian origin (1/19; 5.2%), whilst all isolates were free of the blaCTX-M-g-8 and blaCTX-M-g-9 genes (Table 4). A statistically significant relationship was found between ESBL production and the type of animal diseases (p < 0.05) (Table 5).
Among the 55 streptomycin-resistant isolates, aadA, strA, and strB genes were found in 23 (41.8%), 6 (10.9%), and 1 (1.8%) isolate, respectively. Of the 44 phenotypically tetracycline-resistant isolates, 15 (34.1%) were positive for the tetA gene (7 from cows, 5 from chickens, and 3 from calves). The dfrAI gene was detected in 11 out of the 29 (37.9%) trimethoprim-resistant isolates, whereas the sul1 gene was found in 7 isolates (7/29; 24.1%). Among the 19 chloramphenicol-resistant isolates, the floR and cmlA genes were detected in 26.3% (5/19) and 5.2% (1/19) of isolates, respectively. All isolates were negative for tetB, sul2, and dfrIIV genes (Table 4).
3.6. Detection of Virulence and Biofilm Encoding Genes in CREC Isolates
The fimA gene was detected in 27 of the 36 CREC isolates (75%). Among these strains, 17 (63%) were from cows with mastitis and 9 (33.3%) from chickens with colibacillosis. In chickens, the stx1 and stx2 genes were observed in two isolates (9% for each), whilst isolates from mastitis and diarrhea were free of these genes. The papC gene was observed in three and one isolates from mastitis and chickens, respectively. The aer gene encoding for aerobactin was detected in 7 out of the 22 chicken isolates (31.8%) and 5 out of the 25 mastitis isolates (20%). In addition, the cnfl gene was found in three isolates from both chickens and mastitis (13.6% and 12%, respectively). Mastitis isolates harboring VGs contained either the fimA gene alone or in combination with aer, cnf1, and papC genes. However, none of the diarrheal isolates was positive for the analyzed genes.
3.7. E. coli Phylogenetic Typing in CREC Isolates
Phylogroup analysis of the 36 CREC isolates showed that they belonged to three phylogroups: A (20/36; 55.5%), B2 (10/36; 27.7%), and D (6/36; 16.6%). Regarding subgroups, isolates were allotted as follows: A1 (20/36; 55.5%); D1 (4/36; 11.1%); B23 (8/36; 22.2%); and B22 and D2(2/36; 5.5% for each). While E coli isolates recovered from chickens were placed in five different subgroups (A1, D1, D2, B22, and B23), those originating from bovine mastitis and calves with diarrhea were allotted to four (A1, B23, D1, and D2) and two subgroups (A1 and D1), respectively (Table 4). Interestingly, a statistically significant relationship between phylogroups and the type of animal diseases, biofilm-forming ability, and ESBL production was found (p < 0.05).
3.8. CREC Molecular Typing by ERIC-PCR
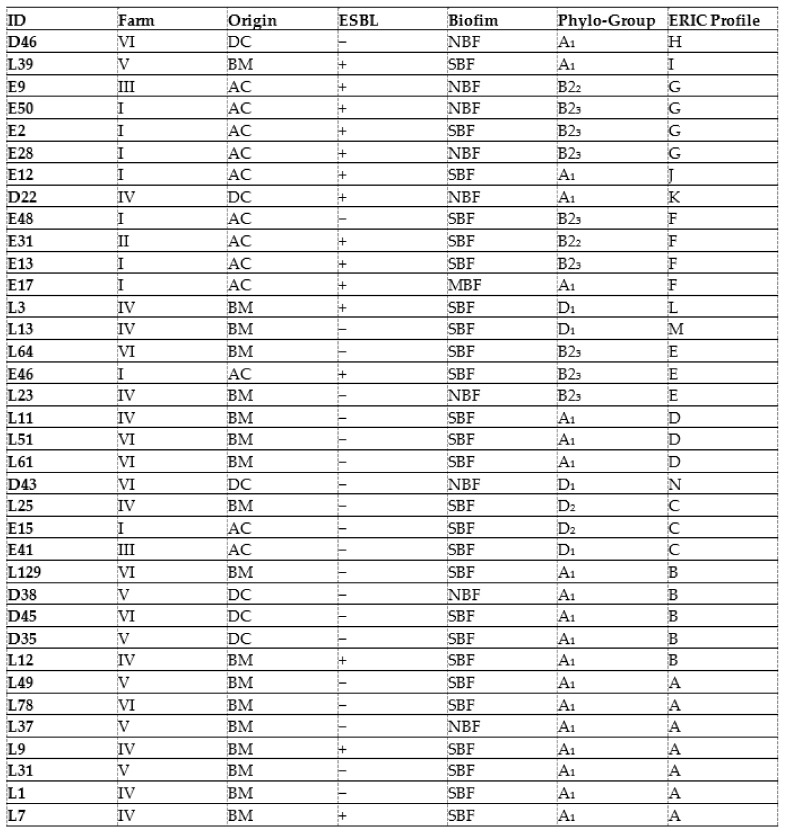
Genetic relationship analysis for the 36 CREC isolates using ERIC-PCR showed 14 different ERIC types (ETs). Identical ETs were allocated letters from A to G, while unique ETs were assigned letters from H to N (Figure 1). The UPGMA method indicated seven different clones (A, B, C, D, E, F, G). The most prevalent ET was the type A (7/36; 19.4%) identified in the mastitis isolates, followed by the type B (5; 13.8%) found in isolates from diarrhea and mastitis, then the types F and G (4/36; 11.1%, for each) detected in avian isolates. The ETs C, D, and E (3/36; 8.3%, for each) were found in either mastitis or avian isolates. While ETs C and E were observed in mastitis and avian isolates, the type D was only detected in mastitis isolates (Table 4, Figure 1).
Figure 1.
ERIC dendrogram representing the genetic relatedness and characteristics of 36 CREC isolates recovered from cows with mastitis, diarrheic calves, and chickens with colibacillosis in farms from Tunisia, using UPGMA (Unweighted Pair Group Method with Arithmetic mean using Jaccard’s coefficient). AC, avian colibacillosis; BM, bovine mastitis; DC, diarrheic calves; ESBL, extended-spectrum β-lactamases; A, B, C, D, F, and G, ERIC groups; NBF, non-biofilm forming; MBF, moderate biofilm-forming; SBF, strong biofilm forming.
The dendrogram analysis indicates that the highest genetic diversity was observed in the eighteen CREC isolates collected from bovine mastitis with the presence of eight different ETs (A, B, C, D, E, M, L, and E). Among these ETs, the types A and D detected in mastitis isolates were found in different farms (FIV, FV, and FVI; FIV). Likewise, the ETs C and E including mastitis and avian isolates were found in three different farms, (FI, FIII, FIV) and (FI, FIV, FVI), respectively. Type B was detected in isolates collected from mastitis, diarrhea, and mastitis belonging to FIV, FV, and FVI. However, the ETs F and G included only avian isolates from three different farms (FI, FII, FII) (Table 4, Figure 1).
4. Discussion
In the present study, 67 E. coli isolates were recovered from bovine mastitis, calves’ diarrhea, and avian colibacillosis from different farms in Tunisia. Overall, isolates showed a high rate of multidrug resistance (83.6%). In cows, the highest frequencies of resistance were recorded for cefsulodine and ceftazidime (100% for each), cefotaxime (84%), streptomycin (80%), colistin (53.7%), and tetracyclines (40%). These frequencies were higher than those obtained in the study of Yu et al. (2020) [42], who recorded lower frequencies of resistance for cefotaxime, streptomycin, and tetracyclines in E. coli isolated from bovine mastitis (18.1%, 13.3%, and 12%, respectively). The resistance rate to enrofloxacin (4%) in our study was close to that reported in the study of Yu et al. (2020) [42] (4.8%). Contrarily, resistance rates to gentamicin (4%) in mastitis isolates were lower than those recorded in that study (12%) [42].
In calves, the same trend in resistance rates was observed regarding cefsulodine, ceftazidime (100% for each), and cefotaxime (85%). The lowest resistance rate was found for streptomycin (70%) and cefoxitin (75% for each), whilst the highest resistance rate to tetracyclines (75%) was recorded compared to mastitis isolates. These resistance rates are higher than those found by Srivani et al. (2017) [43].
In chickens, the highest rate of antibiotic resistance was found regarding cefsulodine (100%), streptomycin and nalidixic acid (95.4% for each), and tetracyclines (86.3%). The resistance rates for streptomycin (95.4%) and tetracyclines (86.3%) were higher than those found by Wang et al., 2021 [44] in chickens with colibacillosis in China. However, the resistance rate for gentamicin (4.5%) was lower compared to what was recorded in that study [44].
A total of 36 phenotypically colistin-resistant E. coli strains were found (36/67; 53.73%). The highest colistin resistance rate was detected in avian isolates (17/22, 77%) followed by bovine mastitis isolates (18/25, 72%) and diarrheal isolates (6/20, 30%). Although the microdilution test showed the highest rate of colistin resistance in mastitis isolates (18/36; 50%), the mcr-1 gene was detected in ten E. coli isolates all from chickens with colibacillosis (10/36; 27.78%). The colistin resistance rate found in avian isolates (27.78%) is close to that obtained in the study of Johar et al. (2021) [45] (28.5%) in chickens with colibacillosis from Qatar. In cows, the mcr-1 gene frequency was lower than that reported by Liu et al. (2020) [46], who detected 2% of colistin-resistant mcr-1 positive E. coli isolates collected from cows with mastitis. Conversely, the mcr-1 was not detected in any E. coli isolated from calves with diarrhea corroborating the results found by Umpiérrez et al., 2017 [47].
Interestingly, all mcr-1-positive E. coli isolates were multidrug-resistant, exhibiting resistance to common antimicrobials. This finding is in agreement with those found by Liu et al. (2020) [46].
In our study, E. coli isolates from the three different origins were free of the mcr-2, mcr-3, or mcr-4 genes. This result may be explained by the possession of other mcr gene variants or chromosomal mutation(s) [48]. Subsequently, further molecular investigations are needed to identify genes involved in colistin resistance in these isolates.
In the present investigation, a statistically significant association between colistin resistance, mcr-1 carriage, and biofilm formation ability was found supporting previous studies [49].
Strains isolated from chickens showed an important ESBL production rate (9/22, 40.90%) that was lower than that found by Parvin et al. (2020) (86%) [50] and higher than that observed in the study of Johar et al., 2021(3.8%) [45] in E. coli isolated from chickens. In cows with mastitis, 28% (7/25) of the E. coli isolates were phenotypically ESBL producers. This frequency is close to that obtained by Liu et al. (2020) [46], who recorded only 20% of ESBL-producing E. coli among 249 strains isolated from milk from cows with mastitis. The lowest rate of ESBL-producing isolates (3/20, 15%) was recorded in isolates from calves’ diarrhea.
The blaCTXM-g-1 was detected only in ESBL-producing avian isolates, whilst the blaTEM gene was found in isolates from the three animal pathologies. This result is consistent with previous studies in which the blaTEM gene was the most predominant ESBL encoding gene in E. coli isolated from avian colibacillosis, diarrheic calves, and bovine mastitis [20,51,52].
A total of 33 out of the 36 colistin-resistant E. coli isolates contained at least one of the following genes: tetA, sul1, mcr-1, aadA, floR, strB, dfrAI cmlA, strA, and strB, demonstrating the important antibiotic resistance pool in CREC isolates recovered from the three animal pathologies. Previous studies have demonstrated the detection of the same aforementioned genes in E. coli from cows with mastitis [42], avian colibacillosis [44], and diarrheic calves [51]. In the present study, antibiotics were widely used in farm animals either for treating mastitis, colibacillosis, and diarrhea or even to enhance their productivity. This practice promotes the dissemination of ARB that could reach food products, causing serious public health issues [53,54].
A total of 49 of the 67 isolates (49/67; 73.1%) formed biofilm. In cows, the percentage of biofilm-forming E. coli isolates (20/25, 80%) was lower than that found by [55], who reported 100% of biofilm-forming E. coli isolates from acute clinical environmental bovine mastitis in Brazil. In chickens, 77.3% (17/22) of isolates showed biofilm-forming ability. This frequency was markedly higher than that reported in the study of [49], in which only 45% of the APEC strains showed biofilm formation ability. The lowest rate (12/20, 60%) of biofilm formation was observed in calves when compared to the other origins. These results are higher than those reported in the study of [47], in which 45% of E. coli isolated from calves with diarrhea in Uruguay formed biofilm. Based on the observations of the biofilm formation assay, the study suggested that mastitis isolates were more biofilm producers than those of avian and calf origins. Our findings show that bovine mastitis, avian colibacillosis, and neonatal calf diarrhea may be biofilm-related diseases as biofilm-forming bacteria can be resilient to the immune system, antibiotics, and other treatments [17,49,56]. In addition, biofilm plays a key role in horizontal gene transfer (HGT) facilitated by highly dense cells nearby [57] which smooth the movement of RGs and virulence factors, especially under the selective pressure of antibiotics [58,59]. In the biofilm formation process, the key event is the attachment to the surface leading to subsequent aggregation and mature biofilm formation. This increases the stability of bacteria to cause diseases and enhances their drug resistance capacity [14].
In the present investigation, the most common virulence determinant found in E. coli isolates was the fimA gene (26/36, 72.2%) followed by aer (12/36, 33.3%), supporting anterior investigations [60]. The highest frequency of the fimA gene was found in mastitis isolates (17/25, 68%), corroborating previous findings by Jouini et al., (2021) [21] (66.67%). The fimA gene was found in 33.33% (9/27) of chicken isolates but was absent in diarrheal isolates. Genes encoding for Shiga toxins (stx1 and stx2) were detected only in avian isolates with lower rates (2/36; 5.5% for each) than those reported in the study of Elmonir et al. (2021) [61] (20% and 17.1% of isolates, respectively).
The co-occurrence of various VGs encoding for Shiga toxins known as diarrhea genic (stx1/2), aerobactin synthesis (aer), fimbria type I (fimA), and P-fimbriae involved in septicemia (papC) emphasizes the fact that these avian isolates might be incriminated in the morbidity of chickens. Diagnosing APEC based on virulence genes is difficult since there is no specific set of virulence genes systemically associated with APEC [7]. However, based on the presence of specific genes, three isolates could be categorized as Shiga toxin-producing E. coli (STEC), containing stx1, stx2, or stx1 and stx2 genes [62]. STEC represents a public health threat if transmitted to humans as they can adhere to host epithelial cells and cause damage [63]. Furthermore, previous studies have provided evidence of potential zoonotic transmission of STEC isolates recovered from diarrheic cattle and their food products to humans, representing an emerging public health threat [61].
Mastitis isolates harbored fimA, aer, cnf1, and papC. However, the presence of these genes could not determine whether these isolates are ExPEC or not. Other virulence genes associated with ExPEC such as traT, fyuA, and iutA genes were found in E. coli from bovine mastitis in previous studies [10]. The cnf1 genes were detected in 24%of mastitis isolates, which is contradictory to the study of Suojala et al., 2011 [12] from Finland, in which all E. coli isolates from bovine mastitis were free of this gene. However, the stx1, stx2, and hlyA genes were not detected in bovine isolates in our study following a previous study [12].
Although diarrheal isolates were free of the analyzed VGs, they might be reservoirs for other VGs such as fimH and csgA [44]. Animals and humans in contact with calves may become infected through their feces serving as reservoirs for antibiotic and virulence genes.
The results of the assessment of VGs showed high genetic heterogenicity among isolates as shown in other studies [47]. This heterogeneity might be the result of the acquisition and/or the deletion of genetic elements and localization of many virulence-associated genes on bacteriophages, plasmids, transposons, and pathogenicity islands contributing to either the gain or loss of these pathogenic attributes [64]. Although many VGs were not detected in E. coli isolates, the biofilm-forming ability might be due to the presence of pathogenicity islands and the expression of other virulence determinants.
It is important to take into account that given the small number of isolates, particularly those originating from calves, it is difficult to draw a clear conclusion about their virulence patterns. Thus, further molecular characterization of VRs in all isolates would be of great relevance to better elucidate the virulence background of E. coli incriminated in the three animal pathologies. Phylogroup distribution showed that most of the CREC isolates (20/36; 55.5%) were allotted to phylogroup A. In contrast to colibacillosis isolates that belonged mostly to the B2 phylogroup (8/12), mastitis and diarrheal isolates were mostly of the A phylogroup (13/18 and 5/6, respectively). This finding might be due to the difference in the origin of samples and the health status of animals. Indeed, avian E. coli isolates were recovered from dead chickens, whilst mastitis and diarrhea were isolated from diseased animals. The phylogroup results found in this study demonstrate the high pathogenicity of avian isolates compared to those from cows and calves. In previous studies, the virulent B2 group was frequently detected in ExPEC incriminated in severe human infections [12], which demonstrates the high zoonotic potential of avian isolates. Mastitis isolates were of phylogroups A1, D1, or B2. In contrast to phylogenetic type A, phylogroups D and B2 were considered virulent by Clermont et al. 2000 [38].
In this study, most of the mastitis isolates (72.2%) belonged to phylogroup A, a finding that agrees with similar studies that revealed the predominance of A and D phylogroups in E. coli isolates from cows with mastitis [65]. Contrarily, lower amounts of isolates were allotted to the D and B2 phylogroups, corroborating previous studies [65].
In the present investigation, the ERIC-PCR-based genotyping analysis of the 36 CREC isolates recovered from BM, NCD, and AC showed an important level of genetic diversity (14 ERIC profiles). The ERIC band patterns ranged from 1 to 10 bands with a size range from 100 to 2000 bp, comparable with reports from Egypt [61]. The twenty-two mastitis isolates showed the most identical ERIC profiles. Among these isolates, seven displayed the same ET (A), and three belonged to the ET (D), demonstrating clonal dissemination of E. coli among cows with mastitis. This result is consistent with that of Nüesch-Inderbinen et al., 2019 [10], who found high genetic diversity in E. coli isolates collected from cases of bovine mastitis. The six strains isolated from calves’ diarrhea showed four different profiles (groups B, H, K, and N); among them, ET (B) included three isolates. This result is in agreement with the study conducted by Gharieb et al. (2019) [66], who observed seven clusters in E. coli isolates recovered from diarrheic calves.
It is worth noting that the study of genetic relatedness using ERIC-PCR in avian isolates showed concordance with phylogenetic analysis results found in a previous study using the technique of pulsed-field electrophoresis (PFGE), considered as a reference technique for the molecular typing of bacteria [20]. In the present investigation, ERIC-PCR genotyping showed that some mastitis and colibacillosis isolates belonged to the same ET (groups C and E). This result is different from that of Grami et al. (2014) [67], who found no clonal relationship between strains from colibacillosis and bovine mastitis in Tunisia. In addition, ET (B) included strains from mastitis (n = 2) and calf diarrhea (n = 3), whilst no identical ETs were observed in chickens and calves’ isolates. ERIC-PCR analysis showed not only identical profiles but also unrelated patterns among CREC recovered from the three animal pathologies, which may reflect the diversity of CREC clones incriminated in these animal diseases in Tunisia.
The ETs (A) and (D) detected in mastitis isolates were found in different farms (FIV, FV, and FVI and FIV and FVI, respectively). This result indicates the dissemination of two different CREC clones in farms included in this study. This could be explained by the movement of animals between farms and their sharing of grazing and water sources. In addition, the (C) and (E) ETs including mastitis and avian isolates were found in three different farms (FI, FIII, and FIV and FI, FIV, and FVI, respectively). This finding demonstrates the involvement of the same CREC clones in avian colibacillosis and bovine mastitis in Tunisia. The ET (B) was detected in isolates collected from diarrhea and mastitis belonging to FIV, FV, and FVI, indicating that this clone disseminated among diseased calves and cows from different farms in Tunisia. However, the ETs (F) and (G) found in CREC of avian origin were circulating in three different farms (FI, FII, FII) but were absent in mastitis and calves’ isolates. There were no identical CREC clones between diarrheal and colibacillosis isolates. However, some diarrheal isolates (D22 and D43) showed close ETs with avian isolates clustered in the ETs (F) and (C), respectively.
ESBL and non-ESBL-producing CREC isolates from the three animal pathologies in the different farms were found to be related by ERIC genotyping. Moreover, ERIC-PCR has revealed a clonal relationship between E. coli biofilm-producer isolates from cows with BM following previous investigations in Brazil [68].
In the present study, the combinations of identical ETs, biofilm-forming ability, phylogenetic groups, virulence, and resistance profiles among some of the CREC isolates from the same or different animal pathology highlights the potential intra-species cross-transmission of these isolates and/or their genes in the study region.
On combining data, the majority of strong biofilm-producing CREC isolates were of either mastitis or colibacillosis origin and were allotted to the A, B2, and D phylogroups. These isolates displayed seven ETs that were circulating between cows, calves, and chickens, suggesting clonal dissemination of strong biofilm-producing CREC isolates with clinically relevant phylogroups in farms from Tunisia.
5. Conclusions
The present study showed a high prevalence of MDR E. coli (83.6%) isolated from BM, NCD, and AC. CREC and ESBL-EC isolates were shown from the three different origins. E. coli isolates harbored a combination of resistance, virulence, and β-lactamase-encoding genes and were assigned to the A, B, and D phylogroups. This is the first report of the biofilm formation ability in E. coli isolated from clinical cases of bovine mastitis, avian colibacillosis, and neonatal calves’ diarrhea in Tunisia. Our study revealed a high propensity of E. coli isolates recovered from diseased animals to produce biofilm, suggesting the importance of biofilm-forming ability in the pathogenesis process. Further, this paper sheds new light on the diversity and the clonality observed within CREC and ESBL-EC isolates from three different animal diseases in farms in Northern Tunisia.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at University of Bisha for funding this research through the general research project under grant number (UB-GRP-66-1444).
Author Contributions
Conceptualization, R.B.E. and S.D.; methodology, S.D., S.C. (Salsabil Chedli). and A.R.; formal analysis, S.D., W.B. and S.C. (Salsabil Chedli).; data curation, S.D., L.S. and S.C. (Salsabil Chedli); writing—original draft preparation, S.D., M.S.A. and R.B.E.; writing—review and editing, W.M. and A.C.; supervision, W.M., A.C. and R.B.E.; project administration, R.B.E., S.C. (Soufiene Chaari). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was subjected to ethical review and given approval by the ethics committee in animal experimentation (CEEA, ENMV) at the National School of Veterinary Medicine of Sidi Thabet, Ariana, Tunisia, ref: 01. 2019/ISBST.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from Dr Ramzi Boubaker Elandoulsi.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was carried out within the framework of the MOBIDOC device, financed by the program of support to Education, MOtility, Research and Innovation (EMORI) subject of the financing agreement between the Government of the Republic of Tunisia and the European Union dated 31 March 2017 and having the accounting number ENI/2016/39506; ENI/2016/39771; ENI/2016/39772, and the subsidy contract under established between the ANPR and the European Union having the accounting number ENI/2017/387-249.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wirth T., Falush D., Lan R., Colles F., Mensa P., Wieler L.H., Karch H., Reeves P.R., Maiden M.C., Ochman H., et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenaillon O., Skurnik D., Picard B., Denamur E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 3.Johnson J.R., Russo T.A. Extraintestinal pathogenic Escherichia coli: “the other bad E. coli”. J. Lab. Clin. Med. 2002;139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 4.Kaper J.B., Nataro J.P., Mobley H.L. Pathogenic Escherichia coli. Nature reviews. Microbiology. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 5.Hogeveen H., Huijps K., Lam T. Economic aspects of mastitis: New developments. N. Z. Vet. J. 2011;59:16–23. doi: 10.1080/00480169.2011.547165. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz I., Fagan J., More S.J. Calf health from birth to weaning. II. Management of diarrhoea in pre-weaned calves. Ir. Vet. J. 2011;64:9. doi: 10.1186/2046-0481-64-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guabiraba R., Schouler C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015;362:fnv118. doi: 10.1093/femsle/fnv118. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva G.J., Mendonça N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence. 2012;3:18–28. doi: 10.4161/viru.3.1.18382. [DOI] [PubMed] [Google Scholar]
- 9.Mainil J.G., Fairbrother J. Pathogenic Escherichia coli in domestic mammals and birds. In: Morabito S., editor. Pathogenic Escherichia coli. Molecular and Cellular Microbiology. 1st ed. Caister Academic Press; Norfolk, UK: 2014. pp. 19–44. [Google Scholar]
- 10.Nüesch-Inderbinen M., Käppeli N., Morach M., Eicher C., Corti S., Stephan R. Molecular types, virulence profiles and antimicrobial resistance of Escherichia coli causing bovine mastitis. Vet. Rec. Open. 2019;6:e000369. doi: 10.1136/vetreco-2019-000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suojala L., Pohjanvirta T., Simojoki H., Myllyniemi A.L., Pitkälä A., Pelkonen S., Pyörälä S. Phylogeny, virulence factors and antimicrobial susceptibility of Escherichia coli isolated in clinical bovine mastitis. Vet. Microbiol. 2011;147:383–388. doi: 10.1016/j.vetmic.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Johnson T.J., Wannemuehler Y., Johnson S.J., Stell A.L., Doetkott C., Johnson J.R., Kim K.S., Spanjaard L., Nolan L.K. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl. Environ. Microbiol. 2008;74:7043–7050. doi: 10.1128/AEM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terlizzi M.E., Gribaudo G., Maffei M.E. UroPathogenic Escherichia coli (UPEC) infections: Cirulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vysakh A., Midhun S.J., Jayesh K., Jyothis M., Latha M.S. Studies on biofilm formation and virulence factors associated with uropathogenic Escherichia coli isolated from patient with acute pyelonephritis. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2018;25:381–387. doi: 10.1016/j.pathophys.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Manges A.R., Harel J., Masson L., Edens T.J., Portt A., Reid-Smith R.J., Zhanel G.G., Kropinski A.M., Boerlin P. Multilocus sequence typing and virulence gene profiles associated with Escherichia coli from human and animal sources. Foodborne Pathog. Dis. 2015;12:302–310. doi: 10.1089/fpd.2014.1860. [DOI] [PubMed] [Google Scholar]
- 16.Menge C., Wieler L.H., Schlapp T., Baljer G. Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect. Immun. 1999;67:2209–2217. doi: 10.1128/IAI.67.5.2209-2217.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constable P.D. Antimicrobial use in the treatment of calf diarrhea. J. Vet. Intern. Med. 2004;18:8–17. doi: 10.1111/j.1939-1676.2004.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.Saidani M., Messadi L., Soudani A., Daaloul-Jedidi M., Châtre P., Ben Chehida F., Mamlouk A., Mahjoub W., Madec J.Y., Haenni M. Epidemiology, Antimicrobial Resistance, and Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Clinical Bovine Mastitis in Tunisia. Microb. Drug Resist. 2018;24:1242–1248. doi: 10.1089/mdr.2018.0049. [DOI] [PubMed] [Google Scholar]
- 20.Dhaouadi S., Soufi L., Hamza A., Fedida D., Zied C., Awadhi E., Mtibaa M., Hassen B., Cherif A., Torres C., et al. Co-occurrence of mcr-1 mediated colistin resistance and β-lactamase-encoding genes in multidrug-resistant Escherichia coli from broiler chickens with colibacillosis in Tunisia. J. Glob. Antimicrob. Resist. 2020;22:538–545. doi: 10.1016/j.jgar.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Jouini A., Klibi A., Elarbi I., Chaabene M.B., Hamrouni S., Souiai O., Hanachi M., Ghram A., Maaroufi A. First Detection of Human ST131-CTX-M-15-O25-B2 Clone and High-Risk Clonal Lineages of ESBL/pAmpC-Producing E. coli Isolates from Diarrheic Poultry in Tunisia. Antibiotics. 2021;10:670. doi: 10.3390/antibiotics10060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassen B., Saloua B., Abbassi M.S., Ruiz-Ripa L., Mama O.M., Hassen A., Hammami S., Torres C. mcr-1 encoding colistin resistance in CTX-M-1/CTX-M-15- producing Escherichia coli isolates of bovine and caprine origins in Tunisia. First report of CTX-M-15-ST394/D E. coli from goats. Comp. Immunol. Microbiol. Infect. Dis. 2019;67:101366. doi: 10.1016/j.cimid.2019.101366. [DOI] [PubMed] [Google Scholar]
- 23.Hassen B., Abbassi M.S., Ruiz-Ripa L., Mama O.M., Hassen A., Torres C., Hammami S. High prevalence of mcr-1 encoding colistin resistance and first identification of bla(CTX-M-55) in ESBL/CMY-2-producing Escherichia coli isolated from chicken faeces and retail meat in Tunisia. Int. J. Food Microbiol. 2020;318:108478. doi: 10.1016/j.ijfoodmicro.2019.108478. [DOI] [PubMed] [Google Scholar]
- 24.Jouini A., Vinué L., Slama K.B., Sáenz Y., Klibi N., Hammami S., Boudabous A., Torres C. Characterization of CTX-M and SHV extended-spectrum beta-lactamases and associated resistance genes in Escherichia coli strains of food samples in Tunisia. J. Antimicrob. Chemother. 2007;60:1137–1141. doi: 10.1093/jac/dkm316. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Disk Susceptibility Tests. 12th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. [Google Scholar]
- 26.The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Breakpoint Tables for Interpretation of MICs and Zone Diameters, v7.1. 2017. [(accessed on 16 October 2021)]. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf.
- 27.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. CLSI; Philadelphia, PA, USA: 2013. Twenty-Third Information Supplement CLSI Document M100-S23. [Google Scholar]
- 29.Xavier B.B., Lammens C., Ruhal R., Kumar-Singh S., Butaye P., Goossens H., Malhotra-Kumar S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance. 2016;21:30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 30.Yin W., Li H., Shen Y., Liu Z., Wang S., Shen Z., Zhang R., Walsh T.R., Shen J., Wang Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio. 2017;8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carattoli A., Villa L., Feudi C., Curcio L., Orsini S., Luppi A., Pezzotti G., Magistrali C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance. 2017;22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sáenz Y., Briñas L., Domínguez E., Ruiz J., Zarazaga M., Vila J., Torres C. Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob. Agents Chemother. 2004;48:3996–4001. doi: 10.1128/AAC.48.10.3996-4001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bean D.C., Livermore D.M., Hall L.M. Plasmids imparting sulfonamide resistance in Escherichia coli: Implications for persistence. Antimicrob. Agents Chemother. 2009;53:1088–1093. doi: 10.1128/AAC.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwasny S.M., Opperman T.J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010;50:13A.8.1–13A.8.23. doi: 10.1002/0471141755.ph13a08s50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepanović S., Vuković D., Hola V., Di Bonaventura G., Djukić S., Cirković I., Ruzicka F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS Acta Pathol. Microbiol. Et Immunol. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz J., Simon K., Horcajada J.P., Velasco M., Barranco M., Roig G., Moreno-Martínez A., Martínez J.A., Jiménez de Anta T., Mensa J., et al. Differences in virulence factors among clinical isolates of Escherichia coli causing cystitis and pyelonephritis in women and prostatitis in men. J. Clin. Microbiol. 2002;40:4445–4449. doi: 10.1128/JCM.40.12.4445-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contreras C.A., Ochoa T.J., Ruiz J., Lacher D.W., Rivera F.P., Saenz Y., Chea-Woo E., Zavaleta N., Gil A.I., Lanata C.F., et al. Phylogenetic relationships of Shiga toxin-producing Escherichia coli isolated from Peruvian children. J. Med. Microbiol. 2011;60:639–646. doi: 10.1099/jmm.0.026666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clermont O., Bonacorsi S., Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000;66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escobar-Páramo P., Le Menac’h A., Le Gall T., Amorin C., Gouriou S., Picard B., Skurnik D., Denamur E. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. 2006;8:1975–1984. doi: 10.1111/j.1462-2920.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 40.Bilung L.M., Pui C.F., Su’ut L., Apun K. Evaluation of BOX-PCR and ERIC-PCR as Molecular Typing Tools for Pathogenic Leptospira. Dis. Mrk. 2018;2018:1351634. doi: 10.1155/2018/1351634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenover F.C., Arbeit R.D., Goering R.V., Mickelsen P.A., Murray B.E., Persing D.H., Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z.N., Wang J., Ho H., Wang Y.T., Huang S.N., Han R.W. Prevalence and antimicrobial-resistance phenotypes and genotypes of Escherichia coli isolated from raw milk samples from mastitis cases in four regions of China. J. Glob. Antimicrob. Resist. 2020;22:94–101. doi: 10.1016/j.jgar.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Srivani M., Reddy Y.N., Subramanyam K.V., Reddy M.R., Rao T.S. Prevalence and antimicrobial resistance pattern of Shiga toxigenic Escherichia coli in diarrheic buffalo calves. Vet. World. 2017;10:774–778. doi: 10.14202/vetworld.2017.774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M., Jiang M., Wang Z., Chen R., Zhuge X., Dai J. Characterization of antimicrobial resistance in chicken-source phylogroup F Escherichia coli: Similar populations and resistance spectrums between E. coli recovered from chicken colibacillosis tissues and retail raw meats in Eastern China. Poult. Sci. 2021;100:101370. doi: 10.1016/j.psj.2021.101370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johar A., Al-Thani N., Al-Hadidi S.H., Dlissi E., Mahmoud M.H., Eltai N.O. Antibiotic Resistance and Virulence Gene Patterns Associated with Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Qatar. Antibiotics. 2021;10:564. doi: 10.3390/antibiotics10050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G., Ali T., Gao J., Ur Rahman S., Yu D., Barkema H.W., Huo W., Xu S., Shi Y., Kastelic J.P., et al. Co-Occurrence of Plasmid-Mediated Colistin Resistance (mcr-1) and Extended-Spectrum β-Lactamase Encoding Genes in Escherichia coli from Bovine Mastitic Milk in China. Microb. Drug Resist. 2020;26:685–696. doi: 10.1089/mdr.2019.0333. [DOI] [PubMed] [Google Scholar]
- 47.Hassen B., Hammami S., Hassen A., Abbassi M.S. Molecular mechanisms and clonal lineages of colistin-resistant bacteria across the African continent: A scoping review. Lett. Appl. Microbiol. 2022;75:1390–1422. doi: 10.1111/lam.13818. [DOI] [PubMed] [Google Scholar]
- 48.Umpiérrez A., Bado I., Oliver M., Acquistapace S., Etcheverría A., Padola N.L., Vignoli R., Zunino P. Zoonotic Potential and Antibiotic Resistance of Escherichia coli in Neonatal Calves in Uruguay. Microbes Environ. 2017;32:275–282. doi: 10.1264/jsme2.ME17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvin M.S., Talukder S., Ali M.Y., Chowdhury E.H., Rahman M.T., Islam M.T. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Frozen Chicken Meat in Bangladesh. Pathogens. 2020;9:420. doi: 10.3390/pathogens9060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yue S., Zhang Z., Liu Y., Zhou Y., Wu C., Huang W., Chen N., Zhu Z. Phenotypic and molecular characterizations of multidrug-resistant diarrheagenic E. coli of calf origin. Anim. Dis. 2021;1:14. doi: 10.1186/s44149-021-00019-3. [DOI] [Google Scholar]
- 51.Tahar S., Nabil M.M., Safia T., Ngaiganam E.P., Omar A., Hafidha C., Hanane Z., Rolain J.M., Diene S.M. Molecular Characterization of Multidrug-Resistant Escherichia coli Isolated from Milk of Dairy Cows with Clinical Mastitis in Algeria. J. Food Prot. 2020;83:2173–2178. doi: 10.4315/JFP-20-198. [DOI] [PubMed] [Google Scholar]
- 52.Ramchandani M., Manges A.R., DebRoy C., Smith S.P., Johnson J.R., Riley L.W. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005;40:251–257. doi: 10.1086/426819. [DOI] [PubMed] [Google Scholar]
- 53.Garofalo C., Vignaroli C., Zandri G., Aquilanti L., Bordoni D., Osimani A., Clementi F., Biavasco F. Direct detection of antibiotic resistance genes in specimens of chicken and pork meat. Int. J. Food Microbiol. 2007;113:75–83. doi: 10.1016/j.ijfoodmicro.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes J.B., Zanardo L.G., Galvão N.N., Carvalho I.A., Nero L.A., Moreira M.A. Escherichia coli from clinical mastitis: Serotypes and virulence factors. J. Vet. Diagn. Investig. 2011;23:1146–1152. doi: 10.1177/1040638711425581. [DOI] [PubMed] [Google Scholar]
- 55.Goudarztalejerdi A., Mohammadzadeh A., Niazi K., Mohammad Mirzaei M. High Prevalence of Multidrug Resistance and Biofilm-Formation Ability Among Avian Escherichia coli Isolated from Broilers in Iran. Microb. Drug Resist. 2021;28:244–254. doi: 10.1089/mdr.2021.0091. [DOI] [PubMed] [Google Scholar]
- 56.González M.J., Robino L., Iribarnegaray V., Zunino P., Scavone P. Effect of different antibiotics on biofilm produced by uropathogenic Escherichia coli isolated from children with urinary tract infection. Pathog. Dis. 2017;75:ftx053. doi: 10.1093/femspd/ftx053. [DOI] [PubMed] [Google Scholar]
- 57.Bajpai T., Varma M., Bhatambare G., Pandey M. Escherichia coli biofilms: Accepting the therapeutic challenges. Int. J. Health Allied Sci. 2016;5:204–209. doi: 10.4103/2278-344X.194081. [DOI] [Google Scholar]
- 58.Tajbakhsh E., Ahmadi P., Abedpour-Dehkordi E., Arbab-Soleimani N., Khamesipour F. Biofilm formation, antimicrobial susceptibility, serogroups and virulence genes of uropathogenic E. coli isolated from clinical samples in Iran. Antimicrob. Resist. Infect. Control. 2016;5:11. doi: 10.1186/s13756-016-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruber C.J., Lang S., Rajendra V.K.H., Nuk M., Raffl S., Schildbach J.F., Zechner E.L. Conjugative DNA Transfer Is Enhanced by Plasmid R1 Partitioning Proteins. Front. Mol. Biosci. 2016;3:32. doi: 10.3389/fmolb.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soufi L., Abbassi M.S., Sáenz Y., Vinué L., Somalo S., Zarazaga M., Abbas A., Dbaya R., Khanfir L., Ben Hassen A., et al. Prevalence and diversity of integrons and associated resistance genes in Escherichia coli isolates from poultry meat in Tunisia. Foodborne Pathog. Dis. 2009;6:1067–1073. doi: 10.1089/fpd.2009.0284. [DOI] [PubMed] [Google Scholar]
- 61.Elmonir W., Shalaan S., Tahoun A., Mahmoud S.F., Remela E.M.A., Eissa R., El-Sharkawy H., Shukry M., Zahran R.N. Prevalence, antimicrobial resistance, and genotyping of Shiga toxin-producing Escherichia coli in foods of cattle origin, diarrheic cattle, and diarrheic humans in Egypt. Gut Pathog. 2021;13:8. doi: 10.1186/s13099-021-00402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coura F.M., de Araújo Diniz S., Mussi J.M.S., Silva M.X., Lage A.P., Heinemann M.B. Characterization of virulence factors and phylogenetic group determination of Escherichia coli isolated from diarrheic and non-diarrheic calves from Brazil. Folia Microbiol. 2017;62:139–144. doi: 10.1007/s12223-016-0480-9. [DOI] [PubMed] [Google Scholar]
- 63.Tanabe R.H.S., Vieira M.A., Mariano N.A.B., Dias R.C.B., da Silva R.V., Castro C.M., Dos Santos L.F., Camargo C.H., Yamatogi R.S., Rall V.L.M., et al. Identification and characterization of atypical enteropathogenic and Shiga toxin-producing Escherichia coli isolated from ground beef and poultry breast purchased in Botucatu, Brazil. Braz. J. Microbiol. 2019;50:1099–1103. doi: 10.1007/s42770-019-00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jafari A., Aslani M.M., Bouzari S. Enteroaggregative Escherichia coli, a heterogenous, underestimated and under-diagnosed E. coli pathotype in Iran. Gastroenterol. Hepatol. Bed Bench. 2013;6:71–79. [PMC free article] [PubMed] [Google Scholar]
- 65.Aslam N., Khan S.-U.-H., Usman T., Ali T. Phylogenetic genotyping, virulence genes and antimicrobial susceptibility of Escherichia coli isolates from cases of bovine mastitis. J. Dairy Res. 2021;88:78–79. doi: 10.1017/S002202992100011X. [DOI] [PubMed] [Google Scholar]
- 66.Gharieb R., Fawzi E., Elsohaby I. Antibiogram, virulotyping and genetic diversity of Escherichia coli and Salmonella serovars isolated from diarrheic calves and calf handlers. Comp. Immunol. Microbiol. Infect. Dis. 2019;67:101367. doi: 10.1016/j.cimid.2019.101367. [DOI] [PubMed] [Google Scholar]
- 67.Grami R., Mansour W., Mehri W., Bouallègue O., Boujaâfar N., Madec J.Y., Haenni M. Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Eurosurveillance. 2016;21:30144. doi: 10.2807/1560-7917.ES.2016.21.8.30144. [DOI] [PubMed] [Google Scholar]
- 68.Oliveira S.V., Freitas E.I., Scatamburlo M.M.A. Clonal relationship of Escherichia coli biofilm producer isolates obtained from mastitic milk. Can. J. Microbiol. 2013;59:291–293. doi: 10.1139/cjm-2013-0053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from Dr Ramzi Boubaker Elandoulsi.