Abstract
Recent studies suggest estradiol (E2)/natural progesterone (P) confers less breast cancer risk compared with conjugated equine estrogens (CEE)/synthetic progestogens. We investigate if differences in the regulation of breast cancer-related gene expression could provide some explanation. This study is a subset of a monocentric, 2-way, open observer-blinded, phase 4 randomized controlled trial on healthy postmenopausal women with climacteric symptoms (ClinicalTrials.gov; EUCTR-2005/001016-51). Study medication was two 28-day cycles of sequential hormone treatment with oral 0.625 mg CEE and 5 mg of oral medroxyprogesterone acetate (MPA) or 1.5 mg E2 as percutaneous gel/day with the addition of 200 mg oral micronized P. MPA and P were added days 15–28/cycle. Material from two core-needle breast biopsies in 15 women in each group was subject to quantitative PCR (Q-PCR). The primary endpoint was a change in breast carcinoma development gene expression. In the first eight consecutive women, RNA was extracted at baseline and after two months of treatment and subjected to microarray for 28856 genes and Ingenuity Pathways Analysis (IPA) to identify risk factor genes. Microarray analysis showed 3272 genes regulated with a fold-change of >±1.4. IPA showed 225 genes belonging to mammary-tumor development function: 198 for CEE/MPA vs. 34 for E2/P. Sixteen genes involved in mammary tumor inclination were subject to Q-PCR, inclining the CEE/MPA group towards an increased risk for breast carcinoma compared to the E2/P group at a very high significance level (p = 3.1 × 10−8, z-score 1.94). The combination of E2/P affected breast cancer-related genes much less than CEE/MPA.
Keywords: breast cancer gene expression, estradiol/micronized progesterone, conjugated equine estrogens/medroxyprogesterone acetate, healthy postmenopausal women, core needle biopsies, menopausal hormone treatment and breast cancer risk
1. Introduction
Menopausal hormone treatment (MHT) is used for the alleviation of climacteric symptoms but has been associated with an increased risk of breast cancer after long-term treatment [1,2,3,4,5,6]. Since breast cancer is so common, even a small increase in the odds ratio (OR) will have a great impact on the absolute number of breast cancer cases. The increased risk of developing breast cancer in women using CEE in combination with the synthetic progestogen MPA was one of the reasons for the premature termination of the CEE-MPA arm in the WHI study [1]. Since then, many women have discontinued their MHT despite sometimes severe climacteric symptoms.
Sex hormones in different combinations, doses, regimens, and durations may have various effects on the breast. Our group and others have shown that CEE in combination with the synthetic progestogen MPA induces a different genetic and proliferative response in breast cells in vivo and in vitro than when given together with natural micronized progesterone [6,7,8]. In a previous study by our group at the Karolinska Institutet, mitogenic activity was found to increase when oral estradiol was combined with noretisterone acetate as well as with dienogest [9]. The use of natural and topical MHT seems to have less impact on breast cancer risk. In the French E3N cohort, women on estrogen in combination with different synthetic progestogens conferred an increased risk of breast cancer compared to women taking estrogen and natural progesterone formulations [10,11,12]. We used a 2.0 mm core needle biopsy (CNB) to increase the cell amount [13]. All samples were retrieved before and at the end of two months of treatment, making every subject under their own control. The stimulation of proliferation in breast cells can already be seen after two months of treatment [8,14,15,16].
The purpose of this study was to evaluate any differences in the change in expression of several genes relevant to mammary tumor development in the treatment groups.
2. Results
Fifteen women receiving CEE/MPA and fifteen women receiving(E2)/P were the subsets for gene expression analysis. Patient demographics at screening are illustrated in Table 1.
Table 1.
Patient demographics.
| CEE/MPA n = 15 | E2/P n = 15 | ||
|---|---|---|---|
| Age | Mean | 55.8 | 58.0 |
| Median | 56.0 | 58.0 | |
| IQR | 54.0–60.0 | 56.0–60.0 | |
| BMI | Mean | 25.9 | 24.9 |
| Median | 26.0 | 24.6 | |
| IQR | 24.0–28.0 | 23.3–26.9 | |
| Parity | Mean | 1.9 | 2.1 |
| Median | 2.0 | 2.0 | |
| IQR | 1.0–3.0 | 1.0–2.8 | |
| YSMP | Mean | 6.7 | 7.0 |
| Median | 6.0 | 5.0 | |
| IQR | 3.5–10.0 | 3.2–10.0 |
There were no significant differences in any of these parameters at baseline.
2.1. Serum Hormones
Serum hormone levels at baseline and after two months of treatment were assessed on the same day as the CNBs. Estradiol (E2) (p < 0.01) and sex hormone binding globulin (SHBG) (p < 0.05) increased significantly during treatment in both treatment groups. Free testosterone (fT) decreased significantly only for women on CEE/MPA (p = 0.002). Insulin growth factor 1 (IGF-1) (p < 0.01) and insulin growth factor binding protein 3 (IGFBP-3) (p = 0.01) decreased significantly in both groups.
2.2. Microarray
For the eight patients (CEE/MPA n = 4 and (E2)/P n = 4), with biopsies before and on one of the days 54–56 of treatment, the expression values of 28,856 genes were further analyzed.
During treatment, 3272 [2735 unique for CEE/MPA; 340 for (E2)/P; and 197 common] genes were changed with a fold change of <−1.4 or >1.4 and subject to further analysis with IPA.
Among the 3272 genes, IPA classified 225 genes as “affecting mammary tumor development”, 198 genes for CEE/MPA, and 34 for (E2)/P. For 18/225 genes, it was indicated in the IPA database whether up- or down-regulation would increase mammary tumor inclination: Fourteen of these eighteen genes were concluded to increase mammary tumor development more for CEE/MPA than for (E2)/P. The corresponding figure for tumor inclination was 4/18 genes more for (E2)/P than CEE/MPA. For 11 of the 18 genes, microarray data indicated enough mRNA content in the normal breast tissue to be further assessed with Q-PCR. In addition, we chose to study another five genes of interest according to the literature: the MKi-67, Bcl-2, PGR (B), IGF-1, and cERB-B3 genes (Table 2).
Table 2.
The 16 target genes for PCR.
| Affymetrix ID | Gene Abbreviation |
Full Gene Name | Taqman Assay ID |
|---|---|---|---|
| 8084710 | ADIPOQ | Adiponectin, C1Q and collagen domain containing | Hs 00605917_m1 |
| 8155849 | ANXA1 | Annexin A1 | Hs 00167549_m1 |
| 8056909 | ATF2 | Activating transcription factor 2 | Hs 01095345_m1 |
| 8023646 | BCl-2 | Apoptosis regulator Bcl 2, B-cell lymphoma 2 | Hs 00608023_m1 |
| 8135594 | CAV1 | Caveolin 1, coding caveolae protein 22 kDa | Hs 00971716_m1 |
| 8089771 | CD80 | Cluster of Differentiation 80 | Hs 00175478_m1 |
| 7956120 | ERBB3 | Receptor tyrosine-kinase erbB-3, HER3 (human epidermal growth factor) | Hs 00176538_m1 |
| 8122843 | ESR1 | Estrogen receptor 1 | Hs 00174860_m1 |
| 7980908 | FBLN5 | Fibulin 5 | Hs 00197064_m1 |
| 8105111 | FBXO4 | F box protein 4 | Hs 00254777_m1 |
| 7902227 | GADD45A | Growth arrest and DNA damage inducible α | Hs 00169255_m1 |
| 7965873 | IGF1 | Insulin-like growth factor 1 | Hs 01547656_m1 |
| 7937020 | MKI67 | Monoclonal antibody Ki 67 | Hs 01032443_m1 |
| 7951165 | PGR | Progesterone receptor | Hs 01556702_m1 |
| 8124185 | PRL | Prolactin | Hs 00168730_m1 |
| 7912145 | TNFRSF9 | Tumor necrosis factor receptor superfamily, member 9 | Hs 00155512_m1 |
2.3. Q-PCR
The change in Q-PCR expression of the 16 genes (11 + 5), with sufficient mRNA in both specimens, for the 30 patients (15 in each treatment group), is given in Table 3.
Table 3.
Effects of the 2 MHTs on fold changes of the 16 genes assessed by Q-PCR. * p < 0.05 within group.
| Genes | Fold Change CEE/MPA | Fold Change E2/P |
|---|---|---|
| MKi- | 14.16 * | 2.67 |
| IGF-1 | 1.83 * | 1.07 |
| PRL | −1.13 | −14.88 * |
| BCl-2 | −1.03 | −1.64 * |
| ESR 1 | −1.80 | −2.86 |
| PGR B | 2.47 | 2.02 |
| TNFSR9 | 1.03 | −2.43 |
| ANXA-1 | −1.52 | −1.22 |
| CD 80 | 2.22 | −1.56 |
| ATF 2 | 1.01 | −1.11 |
| ADIPOQ | −1.01 | −0.52 |
| GADD 45A | 1.03 | −0.89 |
| FBX 04 | −0.18 | 1.09 |
| CAV 1 | −1.16 | −1.77 |
| Fibulin 5 | 3.02 | −1.29 |
| cERB B3 | −1.70 | 1.02 |
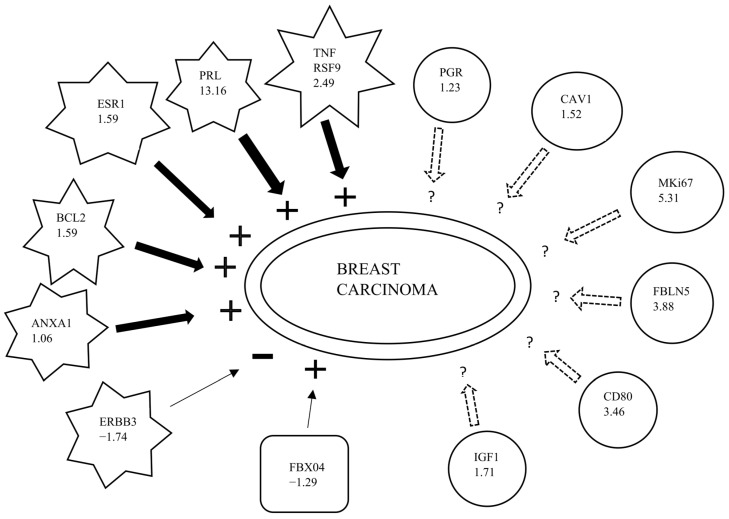
Between treatments, FC ratios from Q-PCR of the 16 genes as specified in Table 1 were re-uploaded to IPA and compared. The biological function “breast carcinoma” was augmented more for CEE/MPA than for (E2)/P at a very high significance level (p = 3.08 × 10−8, z-score = 1.94). Moreover, 13 out of the 16 genes were involved in this biological function, where 6/13 genes were shown to augment the IPA- function “breast carcinoma” more for CEE/MPA than (E2)/P vs. 1/13 genes to augment this function more for (E2)/P than CEE/MPA (Figure 1 and Table 4).
Figure 1.
Fold change ratios (no’: s within symbols) for CEE/MPA vs. (E2)/P for thirteen genes affecting breast carcinoma. Star-shaped symbols represent genes increasing breast carcinoma when activated. Plus: +—sign represents net effect of increasing breast carcinoma inclination. Minus: −sign represents net effect decreasing breast carcinoma inclination. Rectangle represents gene with protective effect against breast carcinoma. Thick black arrows represent positive FC ratios. Thin black arrows represent negative FC ratios. Dotted arrows indicate findings inconsistent with state of downstream molecule. The biological function “breast carcinoma” was augmented more for CEE/MPA than for (E2)/P at a very high significance level (p = 3.1 × 10−8, z-score = 1.94). (Adapted information from IPA database, Ingenuity Systems).
Table 4.
Between treatment effects [a] (CEE/MPA vs. E2/P FC ratios) from Q-PCR of the thirteen genes affecting the IPA function “breast carcinoma”. [b]: Literature findings concerning increased expression of the respective gene on “breast carcinoma”.
| Affymetrix ID | Genes | Predicted Effect [a] | Fold Change Ratio | Findings [b] |
|---|---|---|---|---|
| 7937020 | PRL | Increased | 13.60 | Increases |
| 7980908 | MKi-67 | Affected | 5.307 | Affects |
| 8089771 | FBLN5 | Affected | 3.884 | Affects |
| 7912145 | CD80 | Affected | 3.456 | Affects |
| 7965873 | TNFRSF9 | Increased | 2.491 | Increases |
| 8122843 | IGF1 | Affected | 1.708 | Affects |
| 8023646 | ESR1 | Increased | 1.591 | Increases |
| 8135594 | BCL-2 | Increased | 1.587 | Increases |
| 7951165 | CAV1 | Affected | 1.518 | Affects |
| 8155849 | PGR | Affected | 1.226 | Affects |
| 8105111 | ANXA1 | Increased | 1.062 | Increases |
| 7956120 | FBXO4 | Increased | −1.290 | Decreases |
| 7956120 | ERBB3 | Decreased | −1.741 | Increases |
Adapted from IPA database, Ingenuity Systems.
A significant increase in MKi-67 and IGF-1 gene expression (p < 0.05) was found in the CEE/MPA group only. In the (E2)/P group, the prolactin and Bcl-2 genes were down-regulated (p < 0.05), (Table 3).
For the eight subjects who were analyzed by both microarray and Q-PCR, we found a high correlation between the methods for the mRNA expression fold changes of the sixteen genes given in Table 1 and Table 3 (Rs = 0.5; p = 0.005).
3. Discussion
This is the first prospective randomized study to describe the effects on genetic expression in mammary tissue from healthy postmenopausal women during treatment with sequential MHT with either natural (E2)/P or synthetic CEE/MPA. The effects of (E2), (E2)/NETA, and tibolone have previously been evaluated [17]. Repeated CNBs allowed the women to act as their own controls and made it possible to measure the actual change incurred by treatment for every investigated gene. The use of a housekeeping gene in each sample and the same primers for all genes assessed by microarray and PCR improved assessment accuracy, as evidenced by the high correlation between methods. Microarray data showed five times more genes to be affected by CEE/MPA as compared to (E2)/P treatment.
IPA Upstream Regulator Analysis (URA) and Downstream Effects Analysis (DEA) are powerful tools to assess the activity of a transcriptional regulator as well as biological functions and diseases that are downstream of genes with altered expression during treatment. The information in IPA is collected from numerous experimental systems into a continuously updated knowledge base [18].
We found a remarkable difference between the two alternatives for sequential MHT in healthy postmenopausal women. Between-treatment FCs from Q-PCR for the 16 genes as specified in Table 1 were re-uploaded to IPA and compared. The biological function “breast carcinoma” was augmented more for CEE/MPA than for (E2)/P at a very high significance level (p = 3.08 × 10−8), indicating a striking difference between the two MHTs for this important adverse effect.
The CEE/MPA preparation conferred an augmented breast cancer risk in the WHI study. This study may contribute to some explanation for this risk, based on our results concerning breast cancer gene expression. The French E3N-cohort data indicating MHT with(E2)/P as less detrimental is also in concordance with our current findings [6,7,8,19,20,21,22]. Although these data seem very favorable for (E2)//P, in this study there was also a marked variation in response between individual women [8,9,15]. A few women, tentatively more sensitive to hormones than the majority, had a proliferative response also during E2/P treatment and a concomitant MKi-67 gene activation.
An anti-proliferative drug in the normal breast found previously was the anti-progesterone mifepristone, where a significant down-regulation of Ki-67 protein in the breast was seen in premenopausal women treated for leiomyoma. In that study, material from an FNA biopsy was not sufficient for gene expression studies [23].
The present gene data from the current subset of 15 + 15 patients were compared to previously reported findings on proliferation and apoptosis using the Ki-67 and Bcl-2 antibodies in the same clinical material [8]. In the total subset material, there was a highly significant correlation (Rs = 0.67; p = 0.026) between the difference in expression of the MKi-67 gene and the increase in the percentage of Ki-67- MIB1-positive cells during treatment. A positive correlation between the change in IGF-1 gene expression and the Ki-67 MIB1 protein was found in the CEE/MPA group but not in the E2/P group. This correlation, apparent only for CEE/MPA, is interesting. It indicates the IGF-1 gene as one of the candidate genes for possible mechanisms behind the observed proliferative effects induced by this treatment. Previously, a significant correlation between IGF-1 mRNA and Ki-67 protein was seen in women during hormonal contraception with ethinylestradiol/levonorgestrel. High IGF-1 levels were found to be a risk factor for breast cancer in epidemiologic studies as well as a mitogen for many breast cancer cell lines [24,25,26].
We also found a marked down-regulation of the proliferative prolactin gene in the E2/P group, which was not apparent during CEE/MPA treatment. The drop in endogenous estradiol and progesterone at parturition induces prolactin gene activation, stimulating lactation [27]. Obviously, in postmenopausal women with low endogenous E2 and P levels, natural E2/P treatment induces the logical opposite effect of down-regulating the prolactin gene. Here we find that CEE/MPA treatment is devoid of this physiologic capacity [28,29].
The anti-apoptotic Bcl-2 gene was down-regulated by E2/P, which was not evident for CEE/MPA, where the MKi-67 gene increased during treatment [30].
The significant positive correlation between the MKi-67 gene and Ki-67 protein expression for the total material supports the opinion that proliferative responses on both MHTs coincide with increased Ki-67 protein production and not with reduced protein degradation [31].
4. Materials and Methods
4.1. Study Design and Patients
We performed a monocentric, 2-way, open (observer-blinded), phase 4 randomzsed controlled trial (investigator-sponsored study) at the Clinical Research Unit of the Department of Obstetrics and Gynecology at the Karolinska University Hospital/Institutet, and in the Unilabs Mammography Department, Capio St Göran’s Hospital, in Stockholm, Sweden.
Postmenopausal, apparently healthy women, non-smokers, aged 44 to 66 years without known breast pathology, with normal mammograms and a body mass index (BMI) of 18–30 kg/m2 were recruited for the study. They were post-menopausal for at least 12 months before entering the study, as confirmed at the screening by follicle-stimulating hormone (FSH) levels >25 IU/L and E2 levels < 90 pmol/L according to the reference values at the Karolinska Hospital accredited laboratory. The washout period for previous MHT users was three months. The study was approved by the independent ethics committee and the Swedish Medical Products Agency: IRB-2005/762-31, IRB-2013/963-32, and EUCTR-2005/001016-51, respectively. All women gave their written informed consent before inclusion.
4.2. Analytical Methods
Circulating sex steroid levels and hormone-binding globulins were quantified by routine hospital methods. Serum concentrations of E2 and Sex Hormone Binding Globulin (SHBG) were determined by direct chemiluminescence enzyme immunoassay and total testosterone (T) by direct RIA with a commercial kit (Coat-a-Count Testosterone) (Siemens Healthcare Corporation, Deerfield, IL, USA). Concentrations of free testosterone (fT) were calculated from values for T, SHBG, and a fixed albumin concentration of 40 g/L. IGF-1 was determined by chemiluminescence enzyme immunoassay using a commercial kit (Advantages) obtained from Nichols Products Corporation, San Juan Capistrano, CA, USA. IGF-BP3 was analyzed by ELIZA using a commercial kit obtained from Diagnostic Systems Laboratories Inc., (Webster, TX, USA). The detection limits and within- and between-assay coefficients of variation were for T: 0.1 nmol/L, 6%, and 12%; SHBG: 0.2 nmol/L, 6.5%, and 8.7%; E2 (Spectria): 5 pmol/L, 7.4%, and 10.3% (Orion Diagnostica Oy, Espoo, Finland); E2 (Immulite): 55 pmol/L, 9.3%, and 10.6% (Diagnostic Products Corporation, Los Angeles, CA, USA), and for P: 0.6 nmol/L, 8.2%, and 9.3%; PRL: 0.04 µg/L, 1.9%, and 3.2%; IGF1: 6 µg/L, 4.8%, and 6.7%; and IGF-BP3: 0.04 µg/L, 9%, and 10%.
4.3. Randomisation and Masking
Eligible patients were randomized (1:1) into two groups: MHT with oral CEE/MPA or percutaneous E2/oral P. Randomization was done from a list created by a random number generator external to the Department of Obstetrics and Gynecology, and the randomization sequence was kept concealed. The investigators but not the patients were masked to group assignment. The evaluation of IHC, microarray, and PCR data was conducted blinded to treatment.
4.4. Study Medication
Seventy-seven healthy women were randomized to sequential hormone therapy with two 28-day cycles of either oral 0.625 mg conjugated equine estrogens (CEE) or 2.5 g 0.06% (1.5 mg E2) percutaneous E2-gel daily, with the addition of 5 mg of oral medroxyprogesterone acetate (MPA) or 200 mg of oral micronized P, daily, 14/28 days per cycle.
4.5. Biopsies
Three CNB specimens from each patient were prepared at baseline and on days 54–56 of treatment. We directed the biopsies stereo-tactically towards areas of the highest mammographic density of the upper outer quadrant of the left breast under local anesthesia on a prone table (LORAD) using a 14G needle and normal breast tissue was procured [32]. Detailed IHC data for Ki-67 and Bcl-2 have been published from the clinical trial [8].
One specimen stored in RNA-Later® was used for this current study, namely, gene expression analyses with microarray and Q-PCR according to the manufacturer’s instructions (Life Technologies Ltd., Paisley, UK).
4.6. RNA Extraction, cDNA Synthesis and Microarray
In eight consecutive patients from the clinical trial, four from the CEE/MPA group and four from the E2/P group, RNA was extracted in the samples before and at the end of treatment, subject to reverse transcription and amplification, and a cDNA microarray was performed for expression using 28,856 genes derived after background noise reduction on a platform at the Karolinska Bioinformatics and Expression Analysis (BEA) center. Samples were homogenized using a Retsch® tissue mill (Retsch KG, Hahn, Germany) and maintained in liquid nitrogen for 2 min using a shaking frequency of 30/s. Total RNA was first extracted in Trizol® reagent (Life Technologies; Invitrogen Corp. & Applied Biosystems, Inc., Carlsbad, CA, USA) and then stored in a −80 °C freezer until reverse transcription into cDNA was made. Total RNA was purified with the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany), including treatment with DNase, all steps in accordance with the manufacturer’s instructions.
Total RNA from each sample was used in the standard protocol from NuGen (San Carlos, CA, USA) to label targets. The RNA was reverse transcribed by Affymetrix (Santa Clara, CA, USA) in vitro into single-stranded sense target cDNA, and 1.6 µg per sample was hybridized to Gene Chip® Human Gene 1.0 ST Array Gene Chips according to their expression analysis manual. All samples were of high quality, with an OD 260/280 ratio > 1.8. Genes with a fold change of ≤−1.4 or ≥1.4 were analyzed using Ingenuity Pathways Analysis (IPA) software (Ingenuity© Systems, Inc., www.ingenuity.com May 2018).
4.7. Quantitative PCR (Q-PCR)
This study is a subset of the clinical trial. Fifteen consecutive women in each group receiving CEE/MPA and E2/P, respectively, were subjected to gene expression analysis with Q-PCR. After reverse-transcription of equal amounts of total RNA, cDNA was formed after pre-treatment with DNAse using Superscript II Reverse Transcriptase (Invitrogen, CA, USA) and Ribonuclease Inhibitor (Promega Corp., Masden, WI, USA).
Gene expression levels were quantified by Q-PCR using Taqman Gene Expression Assays and Taqman Gene Expression Master Mix (Life Technologies) in multiplex reactions. mRNA levels were normalized to the level of endogenous control 18S. Sixteen probes for target genes, eleven genes identified as increasing mammary tumors by the IPA database, and an additional five genes with specific relevance for hormonal effects/risk of cancer in mammary tissue were assessed (Table 1).
We ran the reactions in a Step One 7300 Real-Time multiplex PCR system (Life Technologies). All the reactions contained 10 ng of cDNA in a 25 µL reaction volume. The PCR efficiency with all amplicons was 90–100%, and we performed all determinations in triplicates and included duplicate negative (no-template) and positive controls (Human Placenta Total RNA, Lot No. 030302520J, Ambion Life Technologies, Austin, TX, USA).
Q-PCR reactions were performed on 30 consecutive patients (15 from each treatment group) both at baseline and at the end of the treatment in 96-well optical PCR plates. Target gene TaqMan probes were FAM™ dye-labeled, and 18S cDNA probes were VIC™ dye-labeled; all products, including oligonucleotide primers, were purchased from Applied Biosystems. All plates included 18S rRNA amplification of each sample as an endogenous control for data normalization. The cycling conditions were: 50 °C for 2 min, followed by 95 °C for 10 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. For all sixteen genes, we used the same PCR primers as for the previous microarray.
We analyzed the Q-PCR data using the comparative Ct method, where Ct is the cycle number when the fluorescence first exceeds the threshold, and calculated ΔCt by subtracting the Ct value of the endogenous control from the Ct value of the target gene. This quantification gave us the RQ value [33].
4.8. Statistical Analysis
All IPA data were analyzed by Fischer’s exact test within the IPA core analysis program. Power calculations were performed according to earlier studies on Ki-67 protein from FNA biopsies since mRNA studies on normal breast tissue were never previously performed when the study was designed.
Non-IPA data are presented as the arithmetic mean, median, and 25th–75th percentile. Comparisons within the same group of women were carried out by Wilcoxon’s signed-rank test for paired observations and between the two groups by Mann–Whitney U-test. Correlations were performed by Spearman’s rank correlation test. Non-parametric methods were chosen due to the skewed distribution of data. The significance level was set at p < 0.05.
5. Conclusions
In summary, hormone therapy with CEE/MPA induced a more adverse regulation of genes involved in breast carcinoma inclination compared with E2/P in healthy postmenopausal women with moderate climacteric symptoms.
Limitations
This study was carried out some time ago using microarray, and the results were validated by real-time PCR. However, using the latest technology such as RNA-seq may help us understand the differential gene expression in greater depth.
Acknowledgments
Skilful technical assistance was provided by Berit Legerstam, Lotta Blomberg, and Eva Andersson.
Author Contributions
Conceptualization, G.S. and D.M.; methodology, G.S., P.G.L.L., B.B. and D.U.; software, G.S., P.G.L.L. and B.B.; validation, G.S., P.G.L.L., B.B. and D.U.; formal analysis, B.B., D.M. and D.U.; investigation, G.S., P.G.L.L., B.B., D.M. and D.U.; resources, P.G.L.L., B.B., E.T., E.L. and G.S.; data curation, G.S., P.G.L.L., B.B., D.U. and D.M.; writing—original draft preparation, E.L., D.M. and G.S.; writing—review and editing, G.S., E.L. and P.G.L.L.; visualization, P.G.L.L., E.L. and G.S.; supervision, G.S.; project administration, G.S., P.G.L.L. and B.B.; funding acquisition, G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Karolinska Institutet independent ethics committee and the Swedish Medical Products Agency: IRB-2005/762-31, IRB-2013/963-32, and EUCTR-2005/001016-51, respectively.
Informed Consent Statement
All women gave their written informed consent before inclusion.
Data Availability Statement
Confidential disclosure agreement for the clinical trial from where this subset study is derived prevents public access.
Conflicts of Interest
P.G.L.L. Lalitkumar has received consulting fees from IVF access, India. No disclosures were reported by the other authors.
Funding Statement
Swedish Research Council (Project. No. 5982); ALF funding from Karolinska Institutet/Stockholm County-council; Unrestricted grant to the project from Besins International, Brussels, Belgium. Gunnar Söderqvist was project leader.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A.A., Howard B.V., Johnsin K.C., et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Santen R.J., Heitjan D.F., Gompel A., Lumsden M.A., Pinkerton J.A.V., Davies S.R., Stuenkel C.A. Underlying Breast Cancer Risk and Menopausal Hormone Therapy. J. Clin. Endocrinol. Metab. 2020;105:2299–2307. doi: 10.1210/clinem/dgaa073. [DOI] [PubMed] [Google Scholar]
- 3.Weiss L.K., Burkman R.T., Cushing-Haugen K.L., Voigt L.F., Simon M.S., Daling J.R. Hormone Replacement Therapy Regimens and Breast Cancer Risk. Obstet. Gynecol. 2002;100:1148–1158. doi: 10.1016/s0029-7844(02)02502-4. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski R.T., Hendrix S.L., Langer R.D., Stefanik M.L., Gass M., Lane D., Rodabough R.J., Gilligan M.A., Cyr M.G., Thomson C.A., et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. The Women’s Initiative Randomized Trial. JAMA. 2003;289:3243–3353. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 5.Manson J.E., Chlebowski R.T., Stefanick M.L., Aragaki A.K., Rossouw J.E., Prentice R.L., Anderson G., Howard B.V., Thomson C.A., LaCroix A.Z., et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlebowski R.T., Anderson G.L., Aaron K., Aragaki A.K., Manson J.A.E., Stefanick M.L., Pan K., Barrington W., Kuller L.H., Simon M.S., et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA. 2020;324:369–380. doi: 10.1001/jama.2020.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood C.E., Bransetter D., Jacob A.P., Cline M.J., Register T.C., Rohrbach K., Huang L.-Y., Borgerink H., Dougall W.C. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013;15:R62. doi: 10.1186/bcr3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murkes D., Conner P., Leifland K., Tani E., Beliard A., Lundström E., Söderqvist G. Effects of percutaneous estradiol-oral progesterone versus oral conjugated equine estrogen-medroxyprogesterone acetate on breast cell proliferation and bcl-2 protein in healthy women. Fertil. Steril. 2011;95:1188–1191. doi: 10.1016/j.fertnstert.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 9.Conner P., Söderqvist G., Skoog L., Gräser T., Walter F., Tani E., Carlström K., von Shoultz B. Breast cell proliferation in postmenopausal women during HRT evaluated through fine needle aspiration cytology. Breast Cancer Res. Treat. 2003;78:159–165. doi: 10.1023/A:1022987618445. [DOI] [PubMed] [Google Scholar]
- 10.Fournier A., Berrino F., Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008;108:103–111. doi: 10.1007/s10549-007-9604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadeau C., Fournier A., Mesrine S., Clavel-Chapelon F., Fagherazzi G., Boutron-Ruault M.C. Postmenopausal breast cancer risk and interactions between body mass index, menopausal hormone therapy use, and vitamin D supplementation: Evidence from the E3N cohort. Int. J. Cancer. 2016;139:2193–2200. doi: 10.1002/ijc.30282. [DOI] [PubMed] [Google Scholar]
- 12.Trabert B., Sherman M.E., Kannan N., Stanczyk F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020;41:320–344. doi: 10.1210/endrev/bnz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leifland K., Lundquist H., Lagerstedt U. Comparison of stereotactic fine needle aspiration cytology and core needle biopsy in 522 non-palpable breast lesions. Acta Radiol. 2003;44:387–391. doi: 10.1080/j.1600-0455.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 14.Conner P., Register T., Skoog L., Tani E., von Schoultz B., Cline M. Expression of p53 and markers for apoptosis in breast tissue during long-term hormone therapy in cynomolgus monkeys. Am. J. Gynecol. 2005;193:58–63. doi: 10.1016/j.ajog.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 15.Isaksson E., von Schoultz E., Odlind V., Söderqvist G., Csemiczky G., Carlström K., Skoog L., von Schoultz B. Effects of oral contraceptives on breast proliferation. Breast Cancer Res. Treat. 2001;65:163–169. doi: 10.1023/A:1006482418082. [DOI] [PubMed] [Google Scholar]
- 16.Lundström E., Söderqvist G., Svane G., Azavedo E., Olovsson M., Skoog L., von Shoultz E., von Schoultz B. Digitized assessment of mammographic breast density in patients who received low-dose intrauterine levonorgestrel in continuous combination with oral estradiol valerate: A pilot study. Fertil. Steril. 2006;85:989–995. doi: 10.1016/j.fertnstert.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Sieuwerts A., De Napoli G., van Galen A., Kloosterboer H., de Weerd V., Zhang H., Martens J., Foekens J., De Geyter G. Hormone replacement therapy dependent changes in breast cancer-related gene expression in breast tissue of healthy postmenopausal women. Mol. Oncol. 2011;5:504–516. doi: 10.1016/j.molonc.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krämer A. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16:1644–1653. doi: 10.3109/13697137.2013.808563. [DOI] [PubMed] [Google Scholar]
- 20.Dartois L., Fagherazzi G., Baglietto L., Boutron-Ruault M.-C., Delaloge S., Mesrine S., Clavel-Chapelon F. Proportion of premenopausal and postmenopausal breast cancers attributable to known risk factors: Estimates from the E3N-EPIC cohort. Int. J. Cancer. 2016;138:2415–2427. doi: 10.1002/ijc.29987. [DOI] [PubMed] [Google Scholar]
- 21.Lindén-Hirschberg A., Tani E., Brismar K., Lundström E. Effects of drospirenone and norethisterone acetate combined with estradiol on mammographic density and proliferation of breast epithelial cells—A prospective randomized trial. Maturitas. 2019;126:18–24. doi: 10.1016/j.maturitas.2019.04.205. [DOI] [PubMed] [Google Scholar]
- 22.Lundström E., Ivana Virijevic I., Söderqvist G. Differences in breast cell proliferation between oral estradiol/norethisterone acetate, sequential conjugated equine estrogen/medroxyprogesterone acetate and oral estradiol-valerate/low-dose levo-norgestrel intrauterine system, in healthy postmenopausal women. Horm. Mol. Biol. Clin. Investig. 2020;41:20190051. doi: 10.1515/HMBCI-2019-0051. [DOI] [PubMed] [Google Scholar]
- 23.Engman M., Skoog L., Söderqvist G., Gemzell-Danielsson K. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum. Reprod. 2008;23:2072–2079. doi: 10.1093/humrep/den228. [DOI] [PubMed] [Google Scholar]
- 24.Isaksson E., Sahlin L., Söderqvist G., von Schoultz E., Masironi B., Wickman M., Wilking N., von Schoultz B., Skoog L. Expression of sex steroid receptors and IGF-1 mRNA in breast tissue—Effects of hormonal treatment. J. Steroid Biochem. Mol. Biol. 1999;70:257–262. doi: 10.1016/S0960-0760(99)00115-6. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson S.E., Willett W.C., Colditz G.A., Hunter D.J., Michaud D.S., Deroo B., Rosner B., Speizer F.E., Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1293–1296. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 26.Key T.J., Appleby P.N., Reeves G.K., Roddam A.W. The Breast Cancer Collaborative Group. Endogenous hormones and breast cancer: Insulin-like growth factor 1 (IGF-1) IGF-binding protein 3 (IGFBP-3) and breast cancer risk. Pooled individual analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris J.R., Lippman M.E., Morrow M., Hellman S. Diseases of the Breast. Lippincott-Raven; Philadelphia, PA, USA: 1996. [Google Scholar]
- 28.Wang M., Wu X., Chai F., Zhang Y., Jiang J. Plasma prolactin and breast cancer risk: A meta-analysis. Sci. Rep. 2016;6:25998. doi: 10.1038/srep25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carver K.C., Arendt L.M., Schuler L.A. Complex prolactin cross-talk in breast cancer: New therapeutical implications. Mol. Cell Endocrinol. 2009;307:1–7. doi: 10.1016/j.mce.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huh S.J., Oh H., Peterson M.A., Almendro V., Hu R., Bowden M., Lis R.L., Cotter M.B., Loda M., Barry W.T., et al. The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women. Cancer Res. 2016;76:1926–1934. doi: 10.1158/0008-5472.CAN-15-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston-Martin S., Pike M.C., Ross R.K., Jones P.A., Henderson B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- 32.Leifland K., Lundquist H., Lagerstedt U., Svane G. Comparison of pre-operative simultaneous stereotactic fine needle aspiration biopsy and stereotactic core needle biopsy in ductal carcinoma in situ of the breast. Acta Radiol. 2003;44:213–217. doi: 10.1034/j.1600-0455.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen T.D., Livak K.J. Analyzing real-time PCR-data by the comparative method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Confidential disclosure agreement for the clinical trial from where this subset study is derived prevents public access.