Abstract
In a previous study, we found that the SHIP2 protein became tyrosine phosphorylated and associated with the Shc adapter protein in response to the treatment of cells with growth factors and insulin (T. Habib, J. A. Hejna, R. E. Moses, and S. J. Decker, J. Biol. Chem. 273:18605–18609, 1998). We describe here a novel interaction between SHIP2 and the p130Cas adapter protein, a mediator of actin cytoskeleton organization. SHIP2 and p130Cas association was detected in anti-SHIP2 immunoprecipitates from several cell types. Reattachment of trypsinized cells stimulated tyrosine phosphorylation of SHIP2 and increased the formation of a complex containing SHIP2 and a faster-migrating tyrosine-phosphorylated form of p130Cas. The faster-migrating form of p130Cas was no longer recognized by antibodies to the amino terminus of p130Cas and appeared to be generated through proteolysis. Interaction of the SHIP2 protein with the various forms of p130Cas was mediated primarily through the SH2 domain of SHIP2. Immunofluorescence studies indicated that SHIP2 localized to focal contacts and to lamellipodia. Increased adhesion was observed in HeLa cells transiently expressing exogenous WT-SHIP2. These effects were not seen with SHIP2 possessing a mutation in the SH2 domain (R47G). Transfection of a catalytic domain deletion mutant of SHIP2 (ΔRV) inhibited cell spreading. Taken together, our studies suggest an important role for SHIP2 in adhesion and spreading.
Products of phosphatidylinositol (PI) metabolism are important second messengers in cellular signaling pathways (1, 11, 70, 76). Activation of PI 3′-kinase, which phosphorylates the 3′ position of the inositol ring of PI, is a critical event in growth factor, insulin, and G protein-mediated signal transduction (14, 25, 49). In addition, PI 3′-kinase plays an important role in the regulation of adhesion and migration (68). PI 3′-kinase activation localizes to cell-cell and cell-matrix adhesion sites in epithelial cells, as well as to membrane ruffles in fibroblasts (78). Inhibition of PI 3′-kinase attenuated integrin-mediated adhesion and migration in several cell types, while expression of the catalytic p110 subunit of PI 3′-kinase enhanced cell adhesion (18, 22, 29, 31, 33, 34, 52, 80). Moreover, p85, the regulatory subunit of PI 3′-kinase, interacted with proteins regulating adhesion and migration such as focal adhesion kinase (FAK) and p130Crk-associated substrate (p130Cas) (3, 8, 43).
In vivo, the major substrate for PI 3′-kinase is phosphatidylinositol-4,5-bisphosphate [PI-(4,5)-P2] leading to the formation of phosphatidylinositol-3,4,5-trisphosphate [PI-(3,4,5)-P3] (63). Significant pools of phosphatidylinositol-3,4-bisphosphate [PI-(3,4)-P2] are also generated following PI 3′-kinase activation primarily through dephosphorylation of PI-(3,4,5)-P3 by 5′ inositol phosphatases (27). PI-(3,4,5)-P3 and PI-(3,4)-P2 specifically interact with pleckstrin homology (PH) domains of proteins, regulating activity or intracellular localization of cellular enzymes such as Akt/PKB and its upstream kinase phosphoinositide-dependent kinase 1 (PDK1) (75). In addition to Akt and PDK1, phospholipid products of PI 3′-kinase regulate the activity of a number of other cellular proteins containing PH domains. These include the Btk family tyrosine kinases, as well as guanine nucleotide exchange factors such as Vav, Dbl, and general receptor for phosphoinositides 1 (Grp1) (54). Vav, Dbl, and Grp1 are important regulators of cytoskeletal organization, adhesion, invasion, vesicle budding, membrane trafficking, and cell spreading (7, 39, 77).
Inositol phosphatases are important in regulating the cellular levels of lipid second messengers. Inactivation of the tumor suppressor gene PTEN/MMAC1, which hydrolyzes the 3′-phosphate of PI-(3,4,5)-P3, is frequently observed in tumor cells (2), leading to increased basal levels of PI-(3,4,5)-P3 and activation of downstream targets of PI 3′-kinase (23). PTEN also regulates integrin-mediated activation of extracellular-signal-regulated kinase (ERK), interacts with FAK, and inhibits adhesion, migration, and invasion processes (19, 44, 71, 72). Thus, PTEN has been implicated in the regulation of adhesion and/or integrin-mediated survival signaling and detachment-induced cell death or “anoikis” (5, 69). Several inositol phosphatases that dephosphorylate the 5′ position of PI-(3,4,5)-P3 have been cloned (79). Among the known 5′ inositol phosphatases, SH2-containing inositol 5′-phosphatases 1 and 2 (SHIP1 and SHIP2) are specific for PI-(3,4,5)-P3 and inositol-(1,3,4,5)-tetrakiphosphate (12). SHIP1 is expressed primarily in hematopoietic tissues, while SHIP2 expression appears to be more ubiquitous (15, 24, 48, 59, 67). Studies using SHIP1 knockout mice revealed a negative regulatory role for SHIP1 in myeloid cell proliferation and immune system function (46, 47). Negative regulation of growth factor and antigen receptor-mediated signaling by SHIP1 is well documented (28, 45). On the other hand, the role of SHIP2 in cellular functions remains largely unknown, although some studies suggest a negative role for SHIP2 in insulin and FcγRIIB receptor signaling (30, 53). Besides an amino-terminal SH2 domain, both SHIP1 and SHIP2 possess a proline-rich region and NPXY motifs that could possibly provide interaction sites with proteins. Indeed, SHIP1 forms complexes with adapter proteins, Shc and Grb2, as well as tyrosine phosphatase SHP-2 (6, 26, 42, 61). We have previously reported that SHIP2 was tyrosine phosphorylated and associated with adapter protein Shc in response to growth factors and insulin (24). In this study, we report a novel function for SHIP2 in cell adhesion and spreading. We have identified p130Cas, an important regulator of adhesion and migration processes, as an SHIP2-interacting protein. In HeLa cells, SHIP2 localized to focal contacts during attachment and to leading edges of membranes, lamellipodia, in spreading cells. Wild-type SHIP2 promoted adhesion, while catalytically inactive SHIP2 inhibited the spreading of HeLa cells.
MATERIALS AND METHODS
Materials.
A monoclonal antibody directed against an epitope (amino acids [aa] 644 to 819) at the C terminus of p130Cas (C-Cas) was from Transduction Laboratories. Another monoclonal antibody raised against full-length p130Cas (CAS-14) was obtained from Neomarkers. Rabbit polyclonal anti-p130Cas (N-17) raised against an N-terminus epitope and rabbit polyclonal anti-GST (Z-5) were purchased from Santa Cruz Biotech. Anti-FLAG (M2) antibody, rat tail collagen I, and calpain inhibitor E-64d were from Sigma Chemicals. Antiphosphotyrosine (clone 4G10) was from Upstate Biotech. Anti-mouse immunoglobulin G (IgG)-Oregon Green, anti-rabbit IgG-Oregon Green, and anti-mouse IgG-Texas Red were from Molecular Probes. We obtained horseradish peroxidase (HRP)-conjugated anti-mouse IgG from BioRad, and anti-rabbit IgG-HRP and purified Abl kinase from NEB. Rabbit polyclonal anti-SHIP2 antiserum was raised as described earlier (24). The protease inhibitors calpeptin, MG132, MG115, ALLN, and lactacystin were purchased from Alexis Biochemicals. The caspase-I inhibitor, Z-VAD-fmk, was from Calbiochem. Mammalian expression construct for the glutathione S-transferase (GST)-p130Cas (pEB6-Cas) fusion protein was a kind gift from Bruce Mayer, Harvard University Medical School, Cambridge, Mass. (50).
Cell culture.
HeLa and 293T cells were routinely cultured in Dulbecco modified Eagle medium (with high glucose, pyridoxine hydrochloride, and l-glutamine and without sodium pyruvate) containing 10% heat-inactivated fetal bovine serum (FBS). Transient transfection of HeLa or 293T cells was carried out using Lipofectamine-Plus reagent (Gibco-BRL).
Construction of expression vectors encoding epitope-tagged SHIP2 and GST fusion proteins.
cDNAs encoding full-length SHIP2 without (untagged SHIP2) or with a FLAG epitope at the carboxy terminus (SHIP2-FLAG) were cloned into the pcDNA3 mammalian expression vector. Deletion in the catalytic domain of SHIP2 was achieved by digestion with EcoRV enzyme with sites flanking aa 616 to 812, followed by religation. Site-directed mutagenesis was used to replace the arginine at codon 47 with glycine (R47G). This mutation disrupted the FLVR motif in the SH2 domain (aa 20 to 108). These constructs were tagged with the FLAG epitope at the carboxy terminus. The FLAG epitope was attached to the full-length rat p130Cas at the amino terminus by PCR using pEB6-Cas as a template and cloned into pcDNA3. SHIP2 cDNA fragments encoding aa 20 to 118 encompassing the SH2 domain and aa 890 to 1258 encompassing the C-terminus proline-rich region and the sterile alpha motif domain were generated by PCR and cloned into pGEX-KG (21) in frame with the N-terminus coding region for GST. Fragments of cDNA encoding the SH2 domain of the Shc adapter protein (aa 487 to 583) (51) and the full-length EWS/FLI1-activated transcript2 (EAT2) (73) were also cloned similarly into the pGEX-KG vector.
Preparation of GST fusion proteins and GST pulldown assays.
The GST fusion proteins, except GST-p130Cas, were purified from Escherichia coli. Bacterial cells expressing fusion proteins were suspended in lysis buffer (20 mM HEPES, pH 7.4; 0.5 mM EDTA; 0.1% Triton X-100; 1 mM phenylmethylsulfonyl fluoride [PMSF]) and lysed by sonication. GST fusion proteins were purified from sonicates using glutathione-Sepharose (Pharmacia) beads. GST-p130Cas fusion protein was expressed and purified from 293T cells. pEB6.GST-Cas (10 μg/100-mm dish) was transiently transfected into 293T cells using Lipofectamine-Plus (20 μl of Plus reagent and 30 μl of Lipofectamine reagent per 100-mm dish) reagent. At 48 h posttransfection, cells were lysed in HNTG buffer (50 mM HEPES, pH 7.4; 150 mM NaCl; 1% Triton X-100; 10% glycerol; 1 mM EGTA; 1 mM EDTA; 10 mM sodium pyrophosphate; 100 mM sodium fluoride; 0.2 mM sodium orthovanadate; 1 mM PMSF; plus protease inhibitor cocktail [Boehringer Mannheim]) and precleared by spinning at 14,000 × g at 4°C for 10 min. GST-Cas was purified from the precleared lysate with glutathione-Sepharose beads.
For the pulldown assays, HeLa cells, cultured as indicated, were lysed in HNTG buffer. Lysates were centrifuged at 14,000 × g at 4°C for 10 min, followed by incubation for 90 min at 4°C with fusion proteins bound to glutathione-Sepharose beads as indicated. Beads were then washed four times with HNTG buffer and resuspended in sodium dodecyl sulfate (SDS) sample buffer. Bound proteins were analyzed on SDS-gels, transferred to nitrocellulose, and probed with antiphosphotyrosine or anti-p130Cas antibodies. Blots were developed with chemiluminescence reagents.
For GST-Cas pulldown experiments, GST-Cas bound to glutathione beads was washed two times with Abl kinase buffer (NEB) and incubated in Abl kinase buffer supplemented with bovine serum albumin (BSA; 0.1 mg/ml), 200 μM ATP, and with or without 100 U of purified Abl kinase (NEB). In vitro kinase reaction was carried out at 30°C for 30 min, followed by two washes with TNTG buffer (10 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1% Triton X-100; 10% glycerol; 1 mM PMSF; 0.2 mM sodium orthovanadate) (50). Equal amounts of GST-Cas, bound to glutathione beads pretreated with or without Abl as described above, were incubated for 60 min at 4°C with lysates from 293T cells that were transiently transfected with pcDNA3 (vector), pcDNA3-SHIP2-FLAG, pcDNA3-ΔRV-FLAG, or pcDNA3-R47G-FLAG (10 μg of DNA/100-mm dish). Beads were washed four times with HNTG buffer and resuspended in SDS sample buffer. Bound proteins were resolved on SDS-polyacrylamide gel electrophoresis (PAGE) gels and blotted with anti-FLAG (M2) antibody. Lipofectamine-Plus reagent was used for 293T cell transfection. 293T cell lysates were prepared 30 h posttransfection in HNTG buffer, and the amounts of FLAG-tagged proteins in the lysates were determined by an anti-FLAG (M2) Western blot.
Far-Western analyses.
GST-Cas or Abl phosphorylated GST-Cas (GST-Cas-PY), prepared as described above, was run on SDS-PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked for 5 h at 4°C in blocking buffer (2% BSA; 20 mM Tris-HCl, pH 7.5; 100 mM NaCl; 0.1 mM EDTA; 0.1% Tween 20; 1 mM dithiothreitol). SHIP2-FLAG was expressed in 293T cells, purified on anti-FLAG (M2)-agarose beads, and eluted with FLAG peptide (100 μg/ml). Approximately 2.5 μg of purified SHIP2-FLAG per ml in the blocking buffer was incubated with the membranes for 12 h at 4°C. Membranes were washed three times for 5 min each time with washing buffer (essentially same as the blocking buffer but without BSA), followed by sequential probing with anti-FLAG (M2) antibody and anti-mouse IgG-HRP in the blocking buffer. Duplicate membranes were incubated with anti-FLAG and anti-mouse IgG-HRP alone. Purified SHIP2-FLAG protein was run alongside as a positive control for anti-FLAG probing. Reverse analyses were carried out by running anti-FLAG immunoprecipitates from 293T cells transfected with pcDNA3 (V) or SHIP2-FLAG on the gel. Proteins were transferred to the nitrocellulose membranes, followed by blocking as described above. Abl-phosphorylated GST-Cas (GST-Cas-PY) was eluted off the glutathione-Sepharose beads with 20 mM glutathione and incubated with the membranes (2.5 μg/ml) in the blocking buffer for 12 h at 4°C. Following three 5-min washes, the membranes were sequentially probed with anti-GST and anti-rabbit IgG-HRP in the blocking buffer. Duplicate membrane was incubated with anti-GST and anti-rabbit IgG-HRP alone. All antibody incubations were done for 1 h at room temperature. Membranes were washed three times for 5 min each time with washing buffer at room temperature at the end of each probing. Blots were developed using chemiluminescence reagents.
Immunoprecipitation and Western blot analyses.
HeLa cells cultured under various conditions as indicated were lysed in HNTG buffer. For adherent samples used in the experiments, confluent cells in 100-mm tissue dishes were washed once with cold phosphate-buffered saline (PBS) and scraped into lysis buffer. Lysates from trypsinized cells were prepared after treatment with 1× trypsin-EDTA (Gibco-BRL; 0.25% trypsin, 1 mM EDTA) for 3 min. Trypsin was inactivated by using complete medium containing FBS. Cells were then centrifuged for 3 min at 50 × g in a tabletop centrifuge and washed once with PBS prior to lysis. For samples from reattaching cells, trypsinized cells were replated for indicated intervals on 100-mm tissue culture dishes with no additional coating or on bacterial petri dishes coated with collagen I (6 μg/cm2 in PBS for 1 h), poly-l-lysine (Sigma) (0.1% solution in PBS) or PBS alone. After the indicated intervals, adherent cells were gently washed once with cold PBS and scraped into lysis buffer. The medium and the PBS wash containing nonadherent cells from each sample were centrifuged, and the resulting cell pellet was combined with the respective lysate. Immunoprecipitations and Western blots were done as described earlier (62). Briefly, samples were centrifuged at 14,000 × g for 10 min at 4°C, precleared with protein A/G-agarose beads for 30 min at 4°C, and immunoprecipitated with the specified antibodies and protein A/G-agarose beads. The beads were washed three times with lysis buffer and resuspended in SDS sample buffer. Whole-cell lysates were prepared in 1× SDS sample buffer containing 2% SDS, 10% sucrose, 25 mM Tris-HCl (pH 7.4), and 2.5 mM EDTA. Samples were boiled in the presence of 5% 2-mercaptoethanol prior to SDS-PAGE analyses. Proteins were resolved on SDS-gels, transferred to nitrocellulose, and probed with the appropriate antibodies. Blots were developed with chemiluminescence reagents. p130Cas immunoprecipitations and Western blot analyses were carried out using C-Cas antibody (Transduction Laboratories), and HNTG buffer was used to prepare the cell lysates unless stated otherwise. The anti-p130Cas immunoblots described in Fig. 6 were carried out with N-17 (Santa Cruz Biotech) and CAS-14 (Neomarkers) antibodies as indicated.
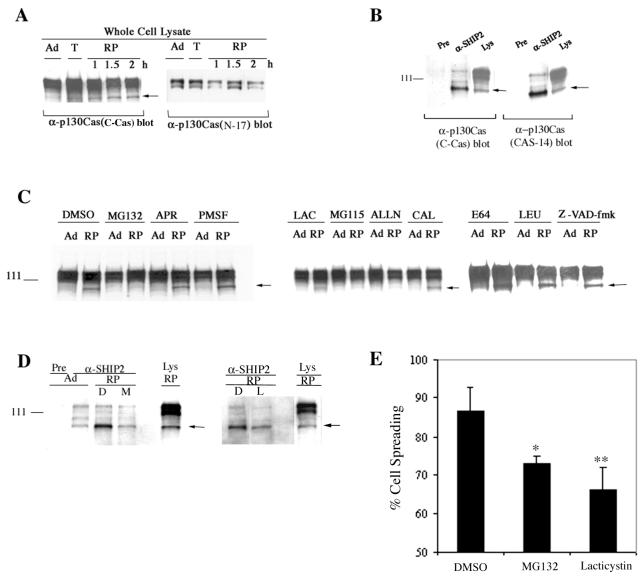
FIG. 6.
Adhesion-dependent processing of p130Cas. (A) Anti-p130Cas blots of whole-cell lysates prepared from HeLa cells that were adherent (Ad), detached by trypsinization (T) or replated (RP) for 1 or 2 h. The arrow indicates the p105 form of p130Cas. Antibody raised against the carboxy-terminal region (C-Cas) or the amino-terminal region (N-17) of p130Cas was used. (B) Anti-SHIP2 or preimmune serum (Pre) immunoprecipitates or whole-cell lysates (Lys) from HeLa cells replated for 2 h were blotted with C-Cas (Transduction Laboratories) or with CAS-14 (monoclonal antibody raised against full-length p130Cas; Neomarkers). The arrow indicates the p105 form of p130Cas. The amount of immunoprecipitates blotted were half of that shown in Fig. 5A. (C) Whole-cell lysates from adherent cells (Ad) or from those replated for 2 h (RP) were blotted with anti-p130Cas (C-Cas). Cells were pretreated with vehicle (DMSO) or various protease inhibitors for 30 min prior to trypsin treatment and were replated for 2 h along with vehicle or inhibitors, respectively. Adherent cells were treated for 2.5 h with vehicle or protease inhibitors. The protease inhibitors MG132 (25 μM), MG115 (20 μM), ALLN (100 μM), lactacystin (10 μM), E64 (100 μM), calpeptin (20 μM), caspase-I inhibitor Z-VAD-fmk (100 μM), aprotinin (2 μg/ml), PMSF (1 mM), and leupeptin (100 μM) were used. The arrow indicates the p105 form of p130Cas. (D) Anti-p130Cas blots of preimmune serum (Pre) or anti-SHIP2 immunoprecipitates from HeLa cells that were adherent (Ad) or replated for 2 h (RP) in the presence of DMSO, MG132 (25 μM), or lactacystin (10 μM) as described above. The amount of immunoprecipitates blotted were half of that shown in Fig. 5A. Whole-cell lysates (Lys) from untreated HeLa cells replated for 2 h were run as a control. The arrow indicates the p105 form of p130Cas. (E) HeLa cells treated with DMSO, MG132 (25 μM), or lactacystin (10 μM) were plated on collagen I-coated chamber slides for 1 h. Cells were then fixed and scored for spreading. At least 300 cells were counted for each sample, and the percentage of cell spreading (average of two experiments done in duplicate) is shown. ∗, P < 0.01; ∗∗, P < 0.001 (as determined by paired Student's t test).
For anti-p130Cas immunoprecipitations described in Fig. 2A and B, cell lysates were prepared either in HNTG buffer or in NP-40 buffer (1% NP-40, 50 mM Tris-HCl [pH 7.4], 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 0.2 mM sodium orthovanadate, 4 μg of leupeptin per ml, 1 μg of aprotinin per ml, 1 μg of pepstatin per ml, 1 mM PMSF), as indicated. Lysates were precleared at 14,000 × g for 10 min at 4°C, followed by incubation with a combination of two monoclonal antibodies, C-Cas (Transduction Laboratories) and CAS-14 (Neomarkers), for 1 h at 4°C. Immunoprecipitates were then collected on protein A/G-agarose beads, washed three times with the respective lysis buffer, and resuspended in the SDS sample buffer. SDS-PAGE and Western blot analyses were done essentially as described above.
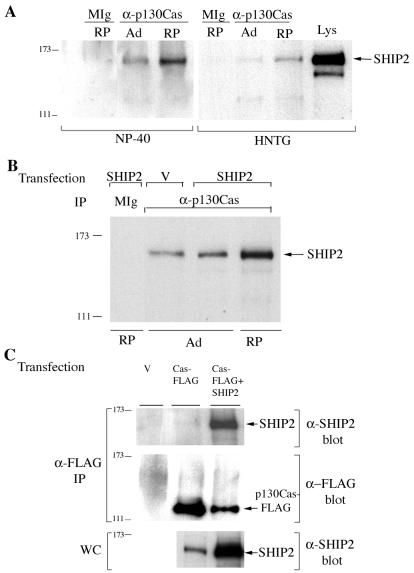
FIG. 2.
SHIP2 coprecipitates with anti-p130Cas. (A) Anti-p130Cas (a combination of C-Cas and CAS-14 antibodies) or mouse IgG (MIg) immunoprecipitates from HeLa cells that were adherent (Ad) or replated for 2 h (RP) were blotted with anti-SHIP2. Lysis conditions are indicated at the bottom of the figure. HeLa cell lysate from adherent cells (Lys) was run as a control. (B) HeLa cells transfected with pcDNA3 vector alone (V) or with pcDNA3-SHIP2-FLAG (SHIP2) were lysed with NP-40 buffer. Anti-p130Cas (a combination of C-Cas and CAS-14 antibodies) immunoprecipitates were blotted with anti-SHIP2. Mouse IgG (MIg) was used as a control. (C) 293T cells, transfected with pcDNA3 (V), pcDNA3-FLAG-tagged p130Cas (Cas-FLAG), or Cas-FLAG plus untagged SHIP2, were lysed in HNTG and immunoprecipitated with anti-FLAG. Anti-SHIP2 and anti-FLAG blots of anti-FLAG immunoprecipitates, as well as an anti-SHIP2 blot of whole-cell lysate, are shown.
Immunofluorescence staining.
HeLa cells, cultured in 35-mm dishes, were transiently transfected with expression constructs of FLAG-tagged wild-type SHIP2, ΔRV-FLAG-SHIP2, or R47G-FLAG-SHIP2 mutants cloned into pcDNA3 vector. A green fluorescent protein (GFP) expression construct (pEGFP-C1; Clontech), with the same cytomegalovirus promoter as that of SHIP2 constructs, was used as control. At 48 h posttransfection, the cells were trypsinized and replated for 1 h on collagen I-coated chamber slides (6 μg/cm2 for 1 h), followed by anti-FLAG (M2) immunofluorescence staining. Duplicate dishes of transfected cells were stained directly on 35-mm dishes with anti-FLAG (M2) antibody. After a gentle PBS wash, cells were fixed with 4% paraformaldehyde in PBS for 15 min and permeabilized with 0.2% Triton X-100 in PBS for an additional 15 min. Blocking was performed in PBS containing 2% BSA (PBS-BSA) and 1:1,000-diluted normal goat serum for 1 h. Cells were then treated with 1.5 μg of anti-FLAG antibody (M2) per ml in PBS-BSA for 1 h. After five washes with PBS, the cells were further incubated with 0.75 μg of Oregon green-conjugated anti-mouse IgG per ml for 45 min. Final washes were done in PBS five times prior to confocal microscopy. Staining for endogenous SHIP2 was done on HeLa cells that were adherent or replated for 1 h on collagen I-coated chamber slides. Double staining of HeLa cells, plated for 1 h on collagen I-coated chamber slides, was done with a combination of anti-paxillin (monoclonal antibody, 1 μg/ml) and anti-SHIP2 (1:250). After being washed, the cells were stained for 45 min with Texas red-conjugated anti-mouse IgG (0.5 μg/ml) and Oregon green-conjugated anti-rabbit IgG (0.75 μg/ml). Cells were washed five times with PBS prior to confocal microscopy. All incubations were done at room temperature in humidified chambers. Control GFP-transfected cells were fixed in 4% paraformaldehyde and processed for microscopy after several PBS washes.
Adhesion and spreading assays.
Anti-FLAG staining of HeLa cells was carried out as described above after 1 h of replating on collagen I-coated chamber slides. Positively stained (green) cells that were adherent were counted from five random fields (magnification, ×60). The total number of adherent cells in five fields (magnification, ×60) was ca. 300 to 350 both in untransfected and transfected cells. Among the positive cells that were adherent, the numbers of spread or nonspread and/or round ones were determined. The average numbers of positive (green) cells that were adherent are presented in histogram form after the values were normalized for transfection efficiency. The numbers of positively stained cells (at least 300 per sample) on a duplicate 35-mm dish were counted in three random fields (magnification, ×20) to calculate relative transfection and/or expression efficiency that was employed to normalize the above values. The percentage of spreading among adherent green cells was calculated from three separate experiments. The statistical significance was calculated by paired Student's t test. The effect of proteasome inhibition on cell spreading was evaluated as follows. HeLa cells were pretreated with dimethyl sulfoxide (DMSO), MG132 (25 μM), or lactacystin (10 μM) for 30 min prior to trypsinization. Cells were then replated for 1 h in the presence of DMSO, MG132, or lactacystin on collagen I-coated chamber slides. The cells were fixed and scored for spreading. The percentage of cells that were spread was calculated by scoring at least 300 cells under a 60× objective.
RESULTS
Interaction of SHIP2 with p130Cas.
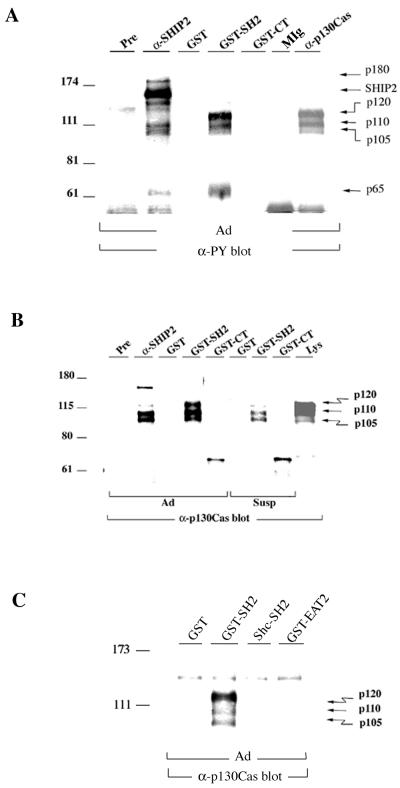
Several tyrosine phosphorylated proteins were found to coprecipitate with SHIP2 protein from HeLa cell lysates (Fig. 1A). In SHIP2 immunoprecipitates from untreated cells, a basal level of tyrosine phosphorylation of SHIP2 protein was found along with coprecipitating tyrosine phosphorylated proteins with Mrs of approximately 65,000, 105,000, 110,000, and 180,000. The 105,000- and 110,000-Mr SHIP2-associated proteins comigrated with immunoprecipitated forms of the p130Cas adapter protein in anti-phosphotyrosine immunoblots. They were also detected in anti-p130Cas immunoblots and comigrated with the faster-migrating forms of p130Cas (Fig. 1B). A slower-migrating 120,000-Mr form of p130Cas was also weakly detectable in anti-SHIP2 immunoprecipitates. In whole-cell lysates of HeLa cells, p130Cas forms appeared as a doublet with Mrs of 110,000 and 120,000, with a low-abundance third form with an Mr of 105,000 as previously observed by others (13, 58). SHIP2 and tyrosine phosphorylated p130Cas could directly associate through the SH2 domain of SHIP2 or through interaction of proline-rich regions in the carboxyl terminus of SHIP2 with the SH3 domain of p130Cas. To examine these possibilities, HeLa cell lysates were incubated with the GST-SHIP2 SH2 domain or GST-SHIP2 carboxyl-terminus fusion proteins bound to glutathione-Sepharose beads. As shown in Fig. 1A, tyrosine-phosphorylated proteins migrating with p130Cas specifically bound the GST-SHIP2 SH2 domain, with little binding to the GST-SHIP2 carboxyl terminus or to GST alone. Additional tyrosine-phosphorylated bands with Mrs of 180,000 and 60,000 to 70,000 were present in GST-SHIP2 SH2 pulldowns (Fig. 1A and see also Fig. 5B). Anti-p130Cas immunoblots confirmed that the 105,000- to 120,000-Mr SHIP2 SH2 domain-associated proteins were indeed three forms of p130Cas (Fig. 1B). As controls, we tested GST-Shc SH2 domain (51) and GST-EWS/FLI1-activated transcript 2 (EAT2) fusion proteins (73) for interaction with p130Cas. EAT2 is a small protein comprising mostly a SH2 domain and is closely related to the SH2 domain of SHIP2. Neither GST-Shc SH2 nor GST-EAT2 interacted with p130Cas (Fig. 1C). To confirm the in vivo association between SHIP2 and p130Cas, reciprocal immunoprecipitation experiments were performed. In these experiments SHIP2 was found in anti-p130Cas immunoprecipitates (Fig. 2A). The association was enhanced in cells that were allowed to reattach after trypsinization. Overexpression of SHIP2 further increased the coprecipitating amounts of SHIP2 in HeLa cells upon replating (Fig. 2B). In addition, SHIP2 was detected in anti-FLAG immunoprecipitates when FLAG-tagged p130Cas and untagged SHIP2 were coexpressed in 293T cells (Fig. 2C).
FIG. 1.
SHIP2 associates with p130Cas. (A) Lysates of adherent (Ad) HeLa cells were immunoprecipitated with preimmune serum (Pre), anti-SHIP2, control mouse IgG (MIg), or anti-p130Cas. Pulldown assays were carried out by incubating adherent HeLa cell lysates with GST, GST-SHIP2 SH2 fusion protein (GST-SH2), or GST-SHIP2 carboxyl-terminus fusion protein (GST-CT). Precipitates were blotted with antiphosphotyrosine antibody (α-PY; 4G10). The arrows point to coprecipitating tyrosine-phosphorylated proteins. (B) Preimmune serum (Pre) or anti-SHIP2 immunoprecipitates from adherent (Ad) HeLa cells, GST pulldown samples from adherent (Ad) cells, or those held in suspension for 30 min (Susp) were immunoblotted with anti-p130Cas. A pulldown assay was carried out by incubating lysate with GST, GST-SHIP2 SH2 fusion protein (GST-SH2), or GST-SHIP2 carboxyl-terminus fusion protein (GST-CT). HeLa cell lysate from adherent cells (Lys) was run as a control. The arrows indicate different forms of p130Cas. (C) Anti-p130Cas immunoblot of GST pulldown samples from adherent (Ad) HeLa cells carried out using GST-SHIP2 SH2 fusion protein (GST-SH2), GST-Shc SH2 fusion protein (Shc-SH2), or GST-EAT2 fusion protein immobilized on glutathione-Sepharose beads. The arrows indicate different forms of p130Cas.
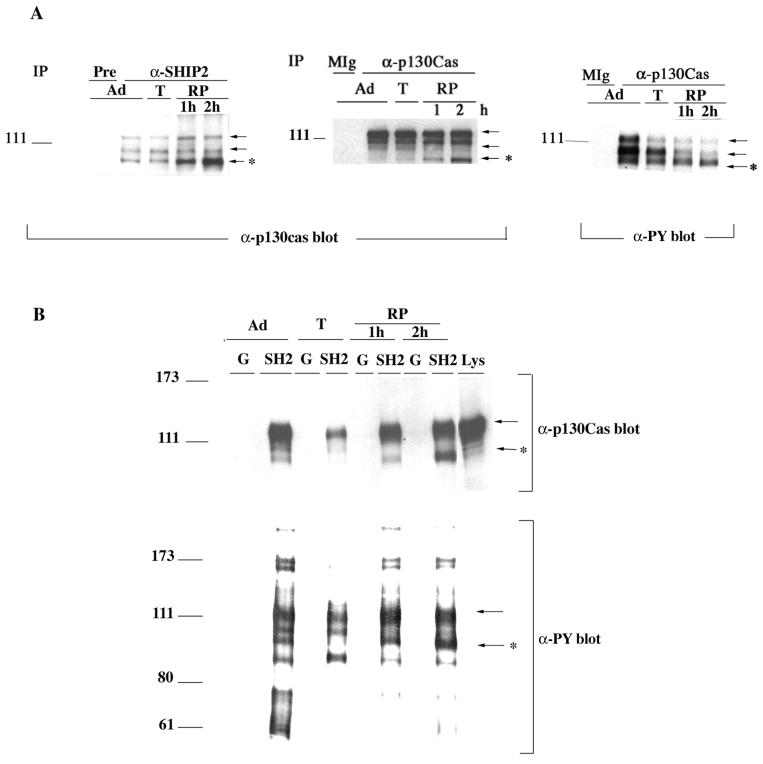
FIG. 5.
Adhesion-dependent changes in the interaction of SHIP2 with p130Cas. (A) Anti-p130Cas blots of anti-SHIP2 immunoprecipitates or anti-p130Cas immunoprecipitates and anti-phosphotyrosine antibody (α-PY) blot of anti-p130Cas immunoprecipitates from HeLa cells that were cultured as indicated: adherent (Ad), detached by trypsinization (T), or replated (RP) for 1 or 2 h. The control lanes are preimmune serum (Pre) and mouse IgG (MIg). (B) GST (G) or GST-SHIP2-SH2 domain (SH2) beads were incubated with lysates prepared from HeLa cells that were adherent (Ad), detached by trypsinization (T), or replated (RP) for 1 or for 2 h. Pulldown samples were blotted with anti-p130Cas or anti-phosphotyrosine (α-PY) antibodies as indicated. The arrows point to the coprecipitating p130Cas forms, and the asterisk highlights the changes in the p105 form of p130Cas.
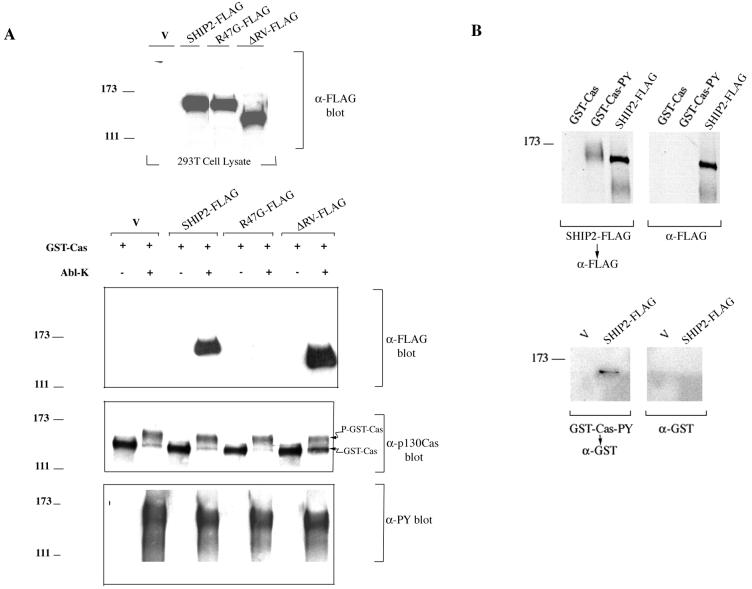
To further characterize the SHIP2-p130Cas interaction, pulldown assays were carried out using purified GST-Cas from 293T cells. Tyrosine phosphorylation of GST-Cas purified from 293T cells was not detectable by antiphosphotyrosine blotting, but incubation with purified Abl (Abl-K; NEB) and ATP in vitro resulted in the appearance of a slower-migrating form of Cas (P-GST-Cas) which was strongly reactive with antiphosphotyrosine antibody (Fig. 3A). Lysates from 293T cells transiently transfected with FLAG-tagged SHIP2, with a SH2-defective mutant (in which the critical arginine of FLVR motif in the SH2 domain was changed to glycine; R47G-FLAG), or with a catalytic region deletion mutant (ΔRV-FLAG) were incubated with GST-Cas bound to glutathione beads that were pretreated with or without Abl kinase. GST-Cas coprecipitated SHIP2-FLAG and ΔRV-FLAG only when Cas was prephosphorylated in vitro by purified Abl kinase. The SH2 domain-defective mutant, R47G-FLAG, on the other hand, failed to associate with GST-Cas even when Cas was tyrosine phosphorylated (Fig. 3A). Far-Western analyses provided further evidence for direct interaction between p130Cas and SHIP2 (Fig. 3B). SHIP2-FLAG purified from 293T cells interacted specifically with nitrocellulose membrane-bound tyrosine-phosphorylated GST-Cas and purified GST-Cas-PY (GST-Cas tyrosine phosphorylated by Abl) bound to SHIP2-FLAG that was immobilized to the membrane (Fig. 3B).
FIG. 3.
The SH2 domain of SHIP2 mediates interaction with tyrosine-phosphorylated p130Cas. (A) Lysates from 293T cells transfected with vector pcDNA3 (V), FLAG-tagged WT-SHIP2, ΔRV-SHIP2, or R47G-SHIP2 were blotted with anti-FLAG antibody to determine levels of expression. In the bottom panel, 293T lysates containing equal amounts of FLAG-tagged proteins were incubated with purified GST-p130Cas beads that were pretreated in vitro with or without Abl kinase as described in Materials and Methods. Proteins bound to GST-p130Cas beads were blotted with anti-FLAG. Vector-transfected cell lysate (V) was included as a control. GST-p130Cas pulldown samples were also blotted with anti-p130Cas and antiphosphotyrosine antibodies. (B) In far-Western experiments, GST-Cas or GST-Cas-PY (GST-Cas tyrosine phosphorylated by Abl as described above) were transferred to nitrocellulose and incubated with purified SHIP2-FLAG, followed by detection with anti-FLAG and goat anti-mouse IgG-HRP. A duplicate membrane was probed only with anti-FLAG and goat anti-mouse IgG-HRP as a control. As a positive control for anti-FLAG reactivity, purified SHIP2-FLAG was run alongside. GST-Cas-PY migrated at approximately Mr 150,000 to 160,000. In the bottom panel, anti-FLAG immunoprecipitates from 293T cells transfected with pcDNA3 (V) or SHIP2-FLAG were transferred to nitrocellulose, followed by incubation with purified GST-Cas-PY. The interaction was detected with anti-GST (rabbit polyclonal antibody) and goat anti-rabbit IgG-HRP. A duplicate membrane was incubated with only anti-GST and goat anti-rabbit IgG-HRP as a control.
Adhesion-dependent tyrosine phosphorylation of SHIP2 and interactions between SHIP2 and p130Cas.
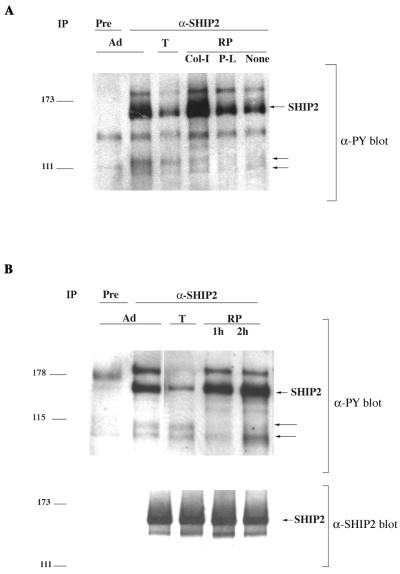
The interaction between SHIP2 and p130Cas protein suggested a possible role for SHIP2 in regulation of cellular adhesion and migration processes. In HeLa cells, SHIP2 is constitutively tyrosine phosphorylated and the tyrosine phosphorylation observed in unstimulated cells could be decreased by detachment from the substratum through trypsinization (Fig. 4A). HeLa cells replated on bacterial petri dishes coated with collagen I (integrin-dependent adhesion) displayed increased SHIP2 tyrosine phosphorylation compared to those plated on poly-l-lysine (integrin-independent adhesion) or PBS-coated petri dishes (Fig. 4A). Replating of cells on regular tissue culture dishes restored SHIP2 tyrosine phosphorylation as well (Fig. 4B).
FIG. 4.
Effects of adhesion on tyrosine phosphorylation of SHIP2. (A) Immunoprecipitates were prepared from cells that were adherent (Ad), detached with trypsin (T), or replated (RP) for 1 h on a bacterial petri dish coated with collagen I (Col-I), poly-l-lysine (P-L), or PBS (None). Preimmune serum (Pre) or anti-SHIP2 immunoprecipitates of lysates of cells from these treatments were blotted with anti-phosphotyrosine antibody (α-PY). (B) Anti-phosphotyrosine antibody (α-PY) and anti-SHIP2 blots of preimmune serum (Pre) or anti-SHIP2 immunoprecipitates from HeLa cells that were cultured as indicated: adherent (Ad), detached by trypsinization (T), or replated on tissue culture dishes (RP) for 1 h or 2 h. Arrows in both panels point to the coprecipitating tyrosine-phosphorylated doublet of Mr 110,000 and Mr 105,000 corresponding to the p130Cas forms.
Trypsin treatment appeared to have little effect on the association between SHIP2 and p130Cas or on the tyrosine phosphorylation of p130Cas (Fig. 4B and 5A). However reattachment preferentially induced association of SHIP2 with the tyrosine-phosphorylated smaller Mr 105,000 form p130Cas. Anti-p130Cas antibody immunoblots of anti-p130Cas immunoprecipitates and of whole-cell lysates indicated that levels of the Mr 105,000 form increased during adhesion, while the levels of the Mr 110,000 and 120,000 forms were relatively unchanged (Fig. 5A and 6A). The stoichiometry of tyrosine phosphorylation of the all p130Cas forms appeared to decrease during adhesion. Overall tyrosine phosphorylation of the Mr 110,000 and 120,000 forms of p130Cas was greatly reduced during adhesion, while tyrosine phosphorylation of the proportion of these proteins bound to SHIP2 was not changed. Both the Mr 105,000 and 120,000 proteins were present in GST-SHIP2 SH2 domain pulldown reattachment experiments with a time-dependent increase in the amount of bound Mr 105,000 protein (Fig. 5B).
Origin of the Mr 105,000 form of p130Cas.
Reactivity of p130Cas forms with antibodies recognizing the carboxy-terminal or the amino-terminal region of p130Cas were compared using whole-cell lysates of HeLa cells (Fig. 6A). The amino-terminal-specific anti-p130Cas (N-17) antibody failed to recognize the p105 form of p130Cas, which was readily apparent with the carboxy-terminal-specific antibody (Fig. 6A). A second monoclonal anti-p130Cas antibody (raised against full-length p130Cas; CAS-14) also detected the p105 form of p130Cas in whole-cell lysates, as well as in anti-SHIP2 immunoprecipitates (Fig. 6B). This suggested that the p105 form of p130Cas in HeLa cells might be an N-terminally truncated form generated due to proteolytic processing. The addition of the proteasome inhibitors MG132, MG115, lactacystin, and ALLN reduced the adhesion-dependent appearance of the p105 form of p130Cas (Fig. 6C). The calpain inhibitors E64d and calpeptin, the inhibitors of trypsin-like proteases aprotinin, PMSF, and leupeptin, and the caspase-I inhibitor Z-VAD-fmk did not appear to affect processing to the Mr 105,000 form. In addition, treatment with MG132 and lactacystin decreased the association between SHIP2 and the p105 form of p130Cas (Fig. 6D), and proteasome inhibition with MG132 and lactacystin significantly delayed the spreading of cells plated on collagen-coated surface (Fig. 6E).
SHIP2 localizes to focal contacts and lamellipodia.
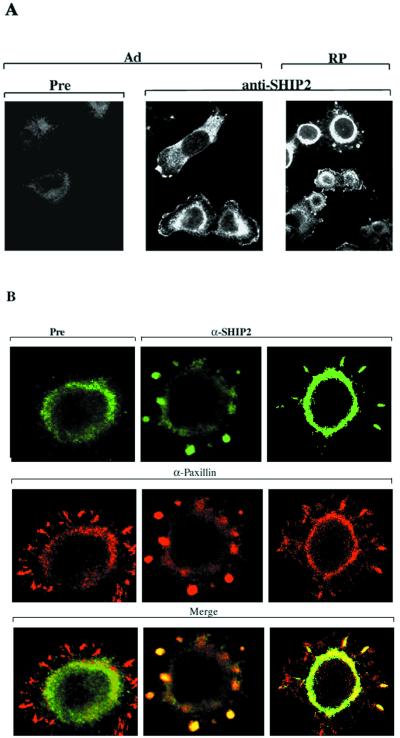
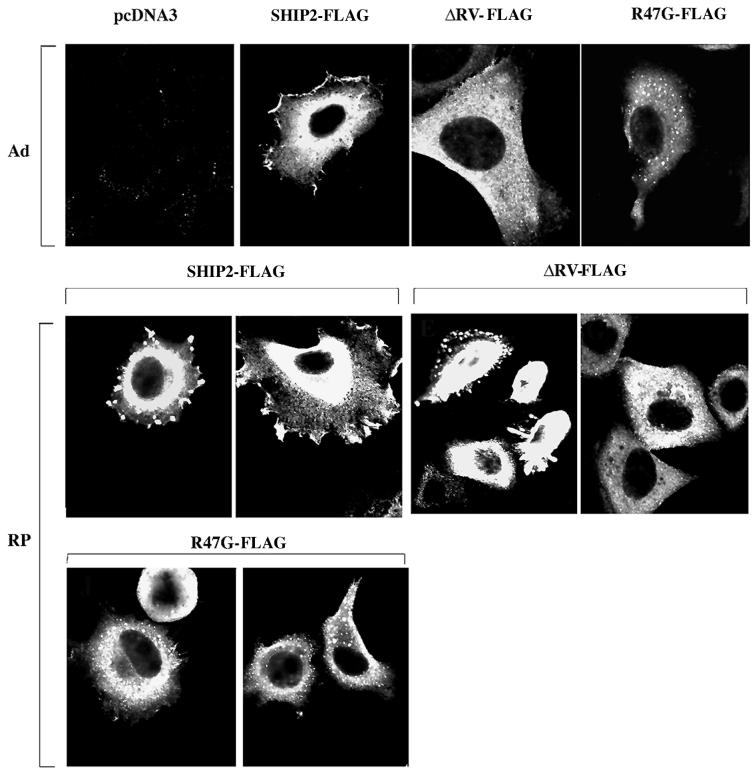
Adhesion-dependent phosphorylation of SHIP2 and its interaction with p130Cas suggested that SHIP2 might function in the organization of the actin cytoskeleton. To examine this possibility, we studied the cellular localization of SHIP2 in HeLa cells. Immunofluorescence staining with anti-SHIP2 revealed that SHIP2 localized largely to peripheral membrane protrusions and lamellipodia in adherent cells (Fig. 7A). In addition, diffuse perinuclear cytoplasmic staining, somewhat stronger than that seen with preimmune serum alone, was apparent. When cells were detached by trypsinization and allowed to reattach on collagen-coated slides, SHIP2 localized primarily to focal contacts or focal complexes that formed in the initial stages of spreading and to membrane protrusions of lamellipodia type structures in later stages of spreading. In Fig. 7B, cells were double stained with anti-SHIP2 and a monoclonal antibody to a focal contact component protein, paxillin. Colocalization of SHIP2 (in green) and paxillin (in red) to focal contacts was observed in adhering cells. In order to test the roles of the SH2 domain and catalytic domain in SHIP2 localization, anti-FLAG immunofluorescence staining was done on HeLa cells transiently transfected with FLAG-tagged versions of WT-SHIP2, ΔRV, and R47G mutants (Fig. 8). In adherent cells WT-SHIP2-FLAG localized to peripheral membranes, whereas the ΔRV and R47G mutants were generally cytoplasmic, displaying a punctate pattern of variable intensity lacking significant peripheral membrane staining. Upon replating, localization of WT-SHIP2 and ΔRV-FLAG appeared similar, concentrating in the focal contacts (Fig. 8). ΔRV-FLAG- and R47G-FLAG-SHIP2 was largely absent from membrane periphery and appeared punctate in spread cells. The SH2-defective mutant, R47G-FLAG, was largely absent from the peripheral membranes, showing a more prominent punctate pattern of cytoplasmic staining (Fig. 8).
FIG. 7.
Subcellular localization of SHIP2. (A) Immunofluorescence staining of adherent HeLa (Ad) or HeLa cells replated for 1 h on collagen I-coated surface (RP) using preimmune serum (Pre) or anti-SHIP2. (B) HeLa cells replated for 1 h on collagen I-coated surface were stained with anti-paxillin antibody and preimmune serum (Pre) or anti-SHIP2 antibody. Control (Pre) and anti-SHIP2 antibody (green color) were detected by Oregon green-conjugated anti-rabbit IgG, and anti-paxillin antibody was detected by Texas red-conjugated anti-mouse IgG.
FIG. 8.
Role for SH2 and catalytic domains in SHIP2 subcellular localization. Anti-FLAG staining of HeLa cells transiently transfected with FLAG-tagged WT-SHIP2, ΔRV-SHIP2-FLAG, or R47G-SHIP2-FLAG mutants. Staining was performed on adherent cells (Ad) or on cells that were replated for 1 h on a collagen I-coated surface (RP).
Effects of WT-SHIP2 and SHIP2 mutants on cellular adhesion and spreading.
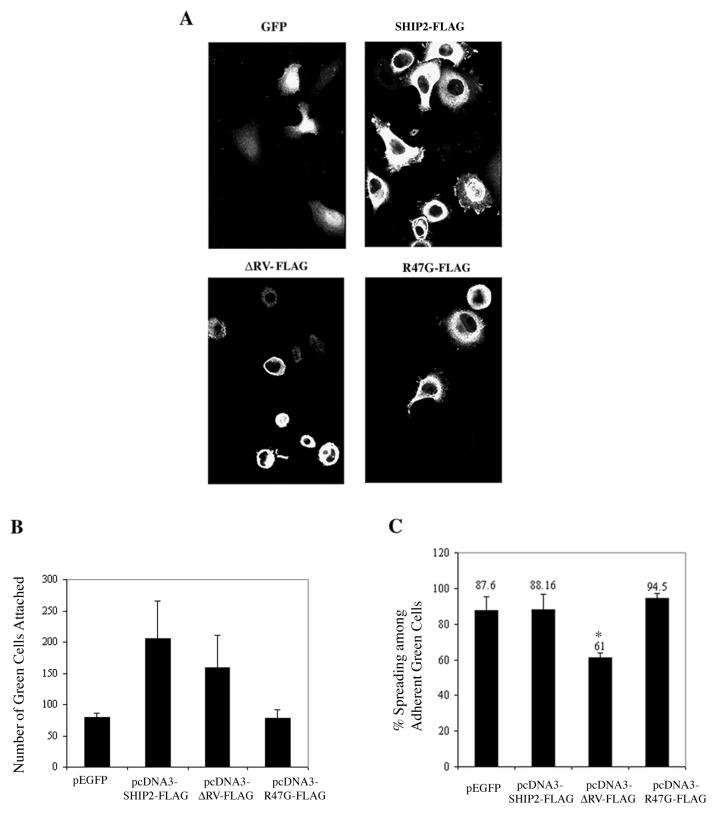
We next examined the effect of transient expression of exogenous WT-SHIP2 or SHIP2 mutants on adhesion and spreading of HeLa cells (Fig. 9). As illustrated in the representative photomicrographs (Fig. 9A) and in the histogram form (Fig. 9B), the overexpression of WT-SHIP2 resulted in an approximately two- to three-fold increase in the number of adherent cells compared to the control GFP group. The proadhesion effect of WT-SHIP2 was not seen with R47G-SHIP2, indicating the importance of SH2 domain interactions in this effect. ΔRV-FLAG promoted adhesion to a lesser degree than the wild-type SHIP2, and the R47G mutant was inactive. The effects of WT-SHIP2 and the SHIP2 mutants on cell spreading were also examined (Fig. 9C). At 1 h postplating, only ca. 60% of RV-FLAG-positive adherent cells were spread compared to ∼90% of wild-type SHIP2 or GFP-positive cells. The transfection of R47G-FLAG had no effect on spreading.
FIG. 9.
Role for SHIP2 in adhesion and spreading. (A) Representative fields of anti-FLAG staining of HeLa cells that were transiently transfected with FLAG-tagged SHIP2 or the SHIP2 mutants ΔRV-FLAG and R47G-FLAG. Cells were trypsinized and replated on collagen I-coated plates for 1 h, followed by immunofluorescence staining. Cells transfected with GFP were used as a control. (B) Histogram showing the number of green cells (GFP or FLAG-positive) that were adherent after 1 h of plating. Average data from two experiments are shown in which five random fields (magnification, ×60) were counted and normalized for transfection efficiency as described under Materials and Methods. (C) Percentage of spreading among the adherent green (GFP or FLAG-positive) cells. The data are from an average of three experiments in which five random fields (magnification, ×60) were counted. ∗, P < 0.01 (compared with SHIP2-FLAG as determined by paired Student's t test).
DISCUSSION
This study describes a novel interaction between SHIP2 and p130Cas mediated primarily through the SH2 domain of SHIP2. SHIP2 was tyrosine phosphorylated in an adhesion-dependent manner and was localized to focal contacts as well as to lamellipodia. Transient expression of exogenous SHIP2 in HeLa cells increased adhesion, and this effect was abolished by mutation of the SH2 domain (R47G). Cell spreading was severely reduced by a catalytic domain deleted version of SHIP2 (ΔRV). Our data clearly indicated an important role for SHIP2 in cellular adhesion and spreading.
SHIP2 was tyrosine phosphorylated in unstimulated HeLa cells and was rapidly tyrosine phosphorylation decreased upon detachment. Similar results were observed in two other cell lines, MDCK and SH-SY5Y, as well (unpublished observation). It is unclear how tyrosine phosphorylation of SHIP2 might affect its activity. Although in vitro phosphorylation of SHIP1 has been reported to decrease enzymatic activity, this effect has not been confirmed in vivo (57). Growth factors, cytokines, and other activation signals induce tyrosine phosphorylation of SHIP1 and SHIP2, but no significant alteration in the inositol phosphatase activity of either enzyme was observed under these conditions (24, 28, 38). A recent study by Phee et al. (60) shows that membrane localization may be the critical mechanism that regulates the activity of SHIP1. Signal-dependent tyrosine phosphorylation of SHIP2 may cause recruitment of other signaling molecules with SH2 and phosphotyrosine-binding (PTB) domains, as has been shown for Shc (24).
The SH2 domain of SHIP2 may be important in initiating interaction with tyrosine-phosphorylated signaling molecules. Accordingly, SHIP2 was shown to associate with immunoreceptor tyrosine-based inhibition motif (ITIM) of FcγRII in B cells. Mutations in the ITIM sequence of FcγRII abrogated SHIP2 binding and subsequent tyrosine phosphorylation (53). Tyrosine phosphorylation of p130Cas upon integrin activation may provide binding sites for the SH2 domain of SHIP2 (55). Our data clearly indicate that tyrosine phosphorylation of p130Cas is important for the interaction with SHIP2. It is conceivable that the interaction may more likely occur through the carboxyl terminus substrate domains of p130Cas that contains several tyrosine phosphorylation sites. p130Cas has been shown to interact with tyrosine kinases, FAK and c-Src, as well as p85 subunit of PI 3′-kinase, in an adhesion-dependent manner (20, 66). p130Cas may bring SHIP2 to the proximity of the above enzymes facilitating tyrosine phosphorylation of SHIP2 as well as hydrolysis of PI-(3,4,5)-P3 generated at the sites of cell-matrix interaction. In agreement, PI 3′-kinase-dependent PI-(3,4)-P2 generation has been reported upon integrin-mediated aggregation in platelets (74) and during adhesion in fibroblasts (34). p130Cas is an important regulator of adhesion and migration processes (35, 56, 72), and recruitment of SHIP2 to the sites of focal contacts and lamellipodia may modulate PI 3′-kinase signaling events at these sites by means of the generation of PI-(3,4)-P2.
Several distinct forms of p130Cas have been previously observed (13, 55, 58). No functional distinction between these forms is currently known. We found here the adhesion-dependent generation of an Mr 105,000 form of p130Cas which preferentially binds SHIP2 during adhesion. Low levels of the Mr 105,000 form were detectable in adherent cells which greatly increased during reattachment. Generation of the Mr 105,000 form appeared to result from proteolysis at the amino-terminal end of a larger form of p130Cas. Surprisingly, several proteasome inhibitors decreased the processing to the Mr 105,000 form during adhesion and significantly delayed the spreading process. Proteolytic processing of p130Cas of a related protein, human enhancer of filamentation 1 (HEF1), has been observed during programmed cell death (36, 37, 40, 41). While caspase family proteases play a major role in the degradation of p130Cas and HEF1 (36, 40), proteasome-dependent processing also appears to occur (40). A proapoptotic role has been attributed to an Mr 28,000 form of HEF1 (40) and during mitosis HEF1 was shown to be cleaved by caspases to generate a Mr 55,000 form that localized to the mitotic spindle, suggesting a role of HEF1 proteolysis in remodeling of actin cytoskeleton (41). In addition, an as-yet-unidentified proteasome-dependent event is shown to be necessary for adhesion process in HL60 cells (32). In HeLa cells the p105 form of p130Cas was the major tyrosine-phosphorylated species of p130Cas seen during reattachment. The p105 form of p130Cas may participate in the dynamic process of assembly and disassembly of adhesion complexes, which occurs during remodeling of the actin cytoskeleton.
SHIP2 in the membrane localized largely to broad extensions of the lamellipodia type. In cells that were freshly plated, SHIP2 was predominantly found in focal contacts formed early in spreading cells and in membrane protrusions of the lamellipodia type in spread cells. Interestingly, the SH2-defective SHIP2 mutant (R47G-FLAG) did not localize to focal contacts and was largely absent from the membrane periphery in spread cells. This suggests that interactions through the SHIP2 SH2 domain are critical to SHIP2 localization. On the other hand, ΔRV-FLAG, which still carries an intact SH2 domain, localized to focal contacts in the early stages but not to lamellipodia of spread cells. Catalytic activity of SHIP2 may aid in the localization of SHIP2 to lamellipodia. The product of SHIP2 activity, PI-(3,4)-P2, may indirectly stabilize SHIP2 membrane localization through interaction with putative PH-domain-containing proteins that could also interact with phosphorylated tyrosines in SHIP2. Such dual-adapter proteins with SH2 and PH domains or PTB and PH domains have been cloned (9, 10). It may also be possible that deletion of catalytic domain might have inadvertently deleted other putative sites of protein interaction that stabilize the membrane localization. Alternatively, a direct effect of phosphoinositides on SHIP2 localization might also be plausible through SH2 interaction, since SH2 domains from p85 subunit of PI 3′-kinase, pp60c-Src, and phospholipase C-γ have been reported to interact with phosphoinositol lipids (4, 64). The catalytic domain of SHIP2 appears to be dispensable for increased adhesion but was clearly essential for cell spreading, since a large number of ΔRV-expressing cells remained round. The R47G-SHIP2 mutant did not affect adhesion or the spreading of cells compared to the GFP control and failed to enhance adhesion, unlike wild-type SHIP2. The R47G-SHIP2 mutant did not behave as a dominant negative, but our results suggest that a functional SH2 domain was necessary for the proadhesive function of SHIP2. Combining our observations that the SH2 domain mediated interaction of SHIP2 with p130Cas and that the R47G mutation in the SHIP2 SH2 domain inhibited membrane localization, as well as the proadhesive effect of SHIP2, we suggest that interaction with p130Cas may mediate membrane localization and proadhesive function of SHIP2.
Although this is the first report demonstrating a biological role for SHIP2 in cell adhesion and migration, earlier studies with the other PI phosphatases PTEN and SHIP1 provide some precedence. Expression of PTEN, a 3′ PI phosphatase, inhibited the growth and invasion of mammary epithelial and glioblastoma cells (72). PTEN associated with FAK and caused dephosphorylation of FAK (71). p130Cas reversed the effect of PTEN on invasion but not on growth. SHIP1 has been shown to promote LFA-1-dependent adhesion to intracellular adhesion molecule-1 (65), and SHIP1 tyrosine phosphorylation and membrane localization were induced by the integrin-mediated aggregation of platelets (16, 17).
Our observations that SHIP2 localizes to focal contacts as well as lamellipodia, that SHIP2 promotes adhesion, and that catalytic domain-deleted SHIP2 prevented cell spreading suggest a significant role for SHIP2 in cell adhesion and spreading. PI-(3,4)-P2 generation during integrin-dependent signaling may be due to the action of SHIP2. A dynamic process involving turnover of PI-(3,4,5)-P3 and PI-(3,4)-P2 may be critical for sequential cell attachment and release events needed for cell motility.
ACKNOWLEDGMENTS
We thank Bruce Mayer for GST-Cas expression construct and Alan Saltiel and Roman Herrera for helpful discussions.
REFERENCES
- 1.Alessi D R, Downes C P. The role of PI 3-kinase in insulin action. Biochim Biophys Acta. 1998;1436:151–164. doi: 10.1016/s0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 2.Ali I U, Schriml L M, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 3.Bachelot C, Rameh L, Parsons T, Cantley L C. Association of phosphatidylinositol 3-kinase, via the SH2 domains of p85, with focal adhesion kinase in polyoma middle t-transformed fibroblasts. Biochim Biophys Acta. 1996;1311:45–52. doi: 10.1016/0167-4889(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 4.Bae Y S, Cantley L G, Chen C S, Kim S R, Kwon K S, Rhee S G. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 5.Besson A, Robbins S M, Yong V W. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–611. doi: 10.1046/j.1432-1327.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruyns C, Pesesse X, Moreau C, Blero D, Erneux C. The two SH2-domain-containing inositol 5-phosphatases SHIP1 and SHIP2 are coexpressed in human T lymphocytes. Biol Chem. 1999;380:969–974. doi: 10.1515/BC.1999.120. [DOI] [PubMed] [Google Scholar]
- 7.Bustelo X R. The VAV family of signal transduction molecules. Crit Rev Oncog. 1996;7:65–88. doi: 10.1615/critrevoncog.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 8.Chen H C, Guan J L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong F, Yuan B, Goff S P. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol Cell Biol. 1999;19:8314–8325. doi: 10.1128/mcb.19.12.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowler S, Currie R A, Downes C P, Alessi D R. DAPP1: a dual adaptor for phosphotyrosine and 3-phosphoinositides. Biochem J. 1999;342:7–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Downes C P, Carter A N. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3:501–513. doi: 10.1016/0898-6568(91)90027-r. [DOI] [PubMed] [Google Scholar]
- 12.Erneux C, Govaerts C, Communi D, Pesesse X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim Biophys Acta. 1998;1436:185–199. doi: 10.1016/s0005-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- 13.Fagerstrom S, Pahlman S, Nanberg E. Protein kinase C-dependent tyrosine phosphorylation of p130cas in differentiating neuroblastoma cells. J Biol Chem. 1998;273:2336–2343. doi: 10.1074/jbc.273.4.2336. [DOI] [PubMed] [Google Scholar]
- 14.Fukui Y, Ihara S, Nagata S. Downstream of phosphatidylinositol-3 kinase, a multifunctional signaling molecule, and its regulation in cell responses. J Biochem. 1998;124:1–7. doi: 10.1093/oxfordjournals.jbchem.a022067. [DOI] [PubMed] [Google Scholar]
- 15.Geier S J, Algate P A, Carlberg K, Flowers D, Friedman C, Trask B, Rohrschneider L R. The human SHIP gene is differentially expressed in cell lineages of the bone marrow and blood. Blood. 1997;89:1876–1885. [PubMed] [Google Scholar]
- 16.Giuriato S, Bodin S, Erneux C, Woscholski R, Plantavid M, Chap H, Payrastre B. pp60c-src associates with the SH2-containing inositol-5-phosphatase SHIP1 and is involved in its tyrosine phosphorylation downstream of alphaIIbbeta3 integrin in human platelets. Biochem J. 2000;348(Pt. 1):107–112. [PMC free article] [PubMed] [Google Scholar]
- 17.Giuriato S, Payrastre B, Drayer A L, Plantavid M, Woscholski R, Parker P, Erneux C, Chap H. Tyrosine phosphorylation and relocation of SHIP are integrin-mediated in thrombin-stimulated human blood platelets. J Biol Chem. 1997;272:26857–26863. doi: 10.1074/jbc.272.43.26857. [DOI] [PubMed] [Google Scholar]
- 18.Gratacap M P, Payrastre B, Viala C, Mauco G, Plantavid M, Chap H. Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-gamma2 is an early key event in FcgammaRIIA-mediated activation of human platelets. J Biol Chem. 1998;273:24314–24321. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, Tamura M, Yamada K M. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan J L. Focal adhesion kinase in integrin signaling. Matrix Biol. 1997;16:195–200. doi: 10.1016/s0945-053x(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 21.Guan K L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 22.Guilherme A, Torres K, Czech M P. Cross-talk between insulin receptor and integrin alpha5 beta1 signaling pathways. J Biol Chem. 1998;273:22899–22903. doi: 10.1074/jbc.273.36.22899. [DOI] [PubMed] [Google Scholar]
- 23.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 24.Habib T, Hejna J A, Moses R E, Decker S J. Growth factors and insulin stimulate tyrosine phosphorylation of the 51C/SHIP2 protein. J Biol Chem. 1998;273:18605–18609. doi: 10.1074/jbc.273.29.18605. [DOI] [PubMed] [Google Scholar]
- 25.Hall R K, Granner D K. Insulin regulates expression of metabolic genes through divergent signaling pathways. J Basic Clin Physiol Pharmacol. 1999;10:119–133. doi: 10.1515/jbcpp.1999.10.2.119. [DOI] [PubMed] [Google Scholar]
- 26.Harmer S L, DeFranco A L. The src homology domain 2-containing inositol phosphatase SHIP forms a ternary complex with Shc and Grb2 in antigen receptor-stimulated B lymphocytes. J Biol Chem. 1999;274:12183–12191. doi: 10.1074/jbc.274.17.12183. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins P T, Jackson T R, Stephens L R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 28.Huber M, Helgason C D, Damen J E, Scheid M, Duronio V, Liu L, Ware M D, Humphries R K, Krystal G. The role of SHIP in growth factor induced signalling. Prog Biophys Mol Biol. 1999;71:423–434. doi: 10.1016/s0079-6107(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 29.Imai Y, Clemmons D R. Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyriboncleic acid synthesis by insulin-like growth factor-I. Endocrinology. 1999;140:4228–4235. doi: 10.1210/endo.140.9.6980. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara H, Sasaoka T, Hori H, Wada T, Hirai H, Haruta T, Langlois W J, Kobayashi M. Molecular cloning of rat SH2-containing inositol phosphatase 2 (SHIP2) and its role in the regulation of insulin signaling. Biochem Biophys Res Commun. 1999;260:265–272. doi: 10.1006/bbrc.1999.0888. [DOI] [PubMed] [Google Scholar]
- 31.Ji P, Haimovich B. Integrin alpha IIb beta 3-mediated pp125FAK phosphorylation and platelet spreading on fibrinogen are regulated by PI 3-kinase. Biochim Biophys Acta. 1999;1448:543–552. doi: 10.1016/s0167-4889(98)00160-8. [DOI] [PubMed] [Google Scholar]
- 32.Katagiri K, Yokosawa H, Kinashi T, Kawashima S, Irie S, Tanaka K, Katagiri T. Ubiquitin-proteasome system is involved in induction of LFA-1/ICAM-1-dependent adhesion of HL-60 cells. J Leukoc Biol. 1999;65:778–785. doi: 10.1002/jlb.65.6.778. [DOI] [PubMed] [Google Scholar]
- 33.Kinashi T, Asaoka T, Setoguchi R, Takatsu K. Affinity modulation of very late antigen-5 through phosphatidylinositol 3-kinase in mast cells. J Immunol. 1999;162:2850–2857. [PubMed] [Google Scholar]
- 34.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kook S, Shim S R, Choi S J, Ahnn J, Kim J I, Eom S H, Jung Y K, Paik S G, Song W K. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol Biol Cell. 2000;11:929–939. doi: 10.1091/mbc.11.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kook S, Shim S R, Kim J I, Ahnn J H, Jung Y K, Paik S G, Song W K. Degradation of focal adhesion proteins during nocodazole-induced apoptosis in rat-1 cells. Cell Biochem Funct. 2000;18:1–7. doi: 10.1002/(SICI)1099-0844(200001/03)18:1<1::AID-CBF840>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 38.Krystal G, Damen J E, Helgason C D, Huber M, Hughes M R, Kalesnikoff J, Lam V, Rosten P, Ware M D, Yew S, Humphries R K. SHIPs ahoy. Int J Biochem Cell Biol. 1999;31:1007–1010. doi: 10.1016/s1357-2725(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 39.Langille S E, Patki V, Klarlund J K, Buxton J M, Holik J J, Chawla A, Corvera S, Czech M P. ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1. J Biol Chem. 1999;274:27099–27104. doi: 10.1074/jbc.274.38.27099. [DOI] [PubMed] [Google Scholar]
- 40.Law S F, O'Neill G M, Fashena S J, Einarson M B, Golemis E A. The docking protein HEF1 is an apoptotic mediator at focal adhesion sites. Mol Cell Biol. 2000;20:5184–5195. doi: 10.1128/mcb.20.14.5184-5195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law S F, Zhang Y Z, Klein Szanto A J P, Golemis E A. Cell cycle-regulated processing of HEF1 to multiple protein forms differentially targeted to multiple subcellular compartments. Mol Cell Biol. 1998;18:3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecoq-Lafon C, Verdier F, Fichelson S, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP, and phosphatidylinositol 3-kinase. Blood. 1999;93:2578–2585. [PubMed] [Google Scholar]
- 43.Li E, Stupack D G, Brown S L, Klemke R, Schlaepfer D D, Nemerow G R. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000;275:14729–14735. doi: 10.1074/jbc.275.19.14729. [DOI] [PubMed] [Google Scholar]
- 44.Liliental J, Moon S Y, Lesche R, Mamillapalli R, Li D, Zheng Y, Sun H, Wu H. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Damen J E, Ware M, Hughes M, Krystal G. SHIP, a new player in cytokine-induced signalling. Leukemia. 1997;11:181–184. doi: 10.1038/sj.leu.2400559. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Oliveira-Dos-Santos A J, Mariathasan S, Bouchard D, Jones J, Sarao R, Kozieradzki I, Ohashi P S, Penninger J M, Dumont D J. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J Exp Med. 1998;188:1333–1342. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont D J, Penninger J M. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Q, Shalaby F, Jones J, Bouchard D, Dumont D J. The SH2-containing inositol polyphosphate 5-phosphatase, ship, is expressed during hematopoiesis and spermatogenesis. Blood. 1998;91:2753–2759. [PubMed] [Google Scholar]
- 49.Maruta H, He H, Tikoo A, Vuong T, Nur E K M. G proteins, phosphoinositides, and actin-cytoskeleton in the control of cancer growth. Microsc Res Tech. 1999;47:61–66. doi: 10.1002/(SICI)1097-0029(19991001)47:1<61::AID-JEMT6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 50.Mayer B J, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- 51.Migliaccio E, Mele S, Salcini A E, Pelicci G, Lai K M, Superti-Furga G, Pawson T, Di Fiore P P, Lanfrancone L, Pelicci P G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mine S, Tanaka Y, Suematu M, Aso M, Fujisaki T, Yamada S, Eto S. Hepatocyte growth factor is a potent trigger of neutrophil adhesion through rapid activation of lymphocyte function-associated antigen-1. Lab Investig. 1998;78:1395–1404. [PubMed] [Google Scholar]
- 53.Muraille E, Bruhns P, Pesesse X, Daeron M, Erneux C. The SH2 domain containing inositol 5-phosphatase SHIP2 associates to the immunoreceptor tyrosine-based inhibition motif of Fc gammaRIIB in B cells under negative signaling. Immunol Lett. 2000;72:7–15. doi: 10.1016/s0165-2478(00)00162-0. [DOI] [PubMed] [Google Scholar]
- 54.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 55.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 56.O'Neill G M, Fashena S J, Golemis E A. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 57.Osborne M A, Zenner G, Lubinus M, Zhang X, Songyang Z, Cantley L C, Majerus P, Burn P, Kochan J P. The inositol 5′-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high-affinity IgE receptor aggregation. J Biol Chem. 1996;271:29271–29278. doi: 10.1074/jbc.271.46.29271. [DOI] [PubMed] [Google Scholar]
- 58.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pesesse X, Deleu S, De Smedt F, Drayer L, Erneux C. Identification of a second SH2-domain-containing protein closely related to the phosphatidylinositol polyphosphate 5-phosphatase SHIP. Biochem Biophys Res Commun. 1997;239:697–700. doi: 10.1006/bbrc.1997.7538. [DOI] [PubMed] [Google Scholar]
- 60.Phee H, Jacob A, Coggeshall K M. Enzymatic activity of the src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem. 2000;275:19090–19097. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- 61.Pradhan M, Coggeshall K M. Activation-induced bi-dentate interaction of SHIP and Shc in B lymphocytes. J Cell Biochem. 1997;67:32–42. doi: 10.1002/(sici)1097-4644(19971001)67:1<32::aid-jcb4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 62.Prasad N, Topping R S, Zhou D, Decker S J. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1) Biochemistry. 2000;39:6929–6935. doi: 10.1021/bi000387i. [DOI] [PubMed] [Google Scholar]
- 63.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 64.Rameh L E, Chen C S, Cantley L C. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 65.Rey-Ladino J A, Huber M, Liu L, Damen J E, Krystal G, Takei F. The SH2-containing inositol-5′-phosphatase enhances LFA-1-mediated cell adhesion and defines two signaling pathways for LFA-1 activation. J Immunol. 1999;162:5792–5799. [PubMed] [Google Scholar]
- 66.Schlaepfer D D, Hauck C R, Sieg D J. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 67.Schurmans S, Carrio R, Behrends J, Pouillon V, Merino J, Clement S. The mouse SHIP2 (Inppl1) gene: complementary DNA, genomic structure, promoter analysis, and gene expression in the embryo and adult mouse. Genomics. 1999;62:260–271. doi: 10.1006/geno.1999.5995. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu Y, Hunt S W., III Regulating integrin-mediated adhesion: one more function for PI 3-kinase? Immunol Today. 1996;17:565–573. doi: 10.1016/s0167-5699(96)10061-x. [DOI] [PubMed] [Google Scholar]
- 69.Stambolic V, Mak T W, Woodgett J R. Modulation of cellular apoptotic potential: contributions to oncogenesis. Oncogene. 1999;18:6094–6103. doi: 10.1038/sj.onc.1203126. [DOI] [PubMed] [Google Scholar]
- 70.Stenmark H, Aasland R. FYVE-finger proteins—effectors of an inositol lipid. J Cell Sci. 1999;112:4175–4183. doi: 10.1242/jcs.112.23.4175. [DOI] [PubMed] [Google Scholar]
- 71.Tamura M, Gu J, Danen E H, Takino T, Miyamoto S, Yamada K M. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 72.Tamura M, Gu J, Takino T, Yamada K M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- 73.Thompson A D, Braun B S, Arvand A, Stewart S D, May W A, Chen E, Korenberg J, Denny C. EAT-2 is a novel SH2 domain containing protein that is up regulated by Ewing's sarcoma EWS/FLI1 fusion gene. Oncogene. 1996;13:2649–2658. [PubMed] [Google Scholar]
- 74.Torti M, Bertoni A, Sinigaglia F, Balduini C, Payrastre B, Plantavid M, Chap H, Mauco G. The platelet cytoskeleton regulates the aggregation-dependent synthesis of phosphatidylinositol 3,4-bisphosphate induced by thrombin. FEBS Lett. 2000;466:355–358. doi: 10.1016/s0014-5793(00)01100-5. [DOI] [PubMed] [Google Scholar]
- 75.Vanhaesebroeck B, Alessi D R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 76.Vanhaesebroeck B, Waterfield M D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 77.Venkateswarlu K, Cullen P J. Signalling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem J. 2000;345(Pt. 3):719–724. [PMC free article] [PubMed] [Google Scholar]
- 78.Watton S J, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9:433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- 79.Woscholski R, Parker P J. Inositol lipid 5-phosphatases—traffic signals and signal traffic. Trends Biochem Sci. 1997;22:427–431. doi: 10.1016/s0968-0004(97)01120-1. [DOI] [PubMed] [Google Scholar]
- 80.Zheng D Q, Woodard A S, Tallini G, Languino L R. Substrate specificity of αvβ3 integrin-mediated cell migration and PI 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]