Abstract
Steroidogenic factor-1 (SF-1, also termed Ad4BP; NR5A1 in the official nomenclature) is a nuclear receptor transcription factor that plays a crucial role in the regulation of adrenal and gonadal development, function and maintenance. In addition to its classical role in regulating the expression of P450 steroid hydroxylases and other steroidogenic genes, involvement in other key processes such as cell survival/proliferation and cytoskeleton dynamics have also been highlighted for SF-1. SF-1 has a restricted pattern of expression, being expressed along the hypothalamic-pituitary axis and in steroidogenic organs since the time of their establishment. Reduced SF-1 expression affects proper gonadal and adrenal organogenesis and function. On the other hand, SF-1 overexpression is found in adrenocortical carcinoma and represents a prognostic marker for patients’ survival. This review is focused on the current knowledge about SF-1 and the crucial importance of its dosage for adrenal gland development and function, from its involvement in adrenal cortex formation to tumorigenesis. Overall, data converge towards SF-1 being a key player in the complex network of transcriptional regulation within the adrenal gland in a dosage-dependent manner.
Keywords: steroidogenic factor 1, transcription factors, nuclear receptors, adrenal cortex, steroidogenesis, gene dosage, tumorigenesis, adrenocortical carcinoma
1. Introduction
Adrenal glands are small paired organs located above each kidney, which participate in the coordination of the mammalian stress response alongside the hypothalamus and the pituitary gland [1]. They have a concentric structure, with a central medullary zone and an external cortical zone surrounded by a connective tissue capsule. Within the adrenal cortex, the outer zona glomerulosa (zG) produces mineralocorticoids, the central zona fasciculata (zF), glucocorticoids, and the inner zona reticularis (zR), androgens under the control of corticotrophin (ACTH) released by the pituitary. Both in humans and in mice, the structure of the adrenal cortex undergoes substantial remodeling postnatally.
Nuclear receptors (NR) represent one of the largest families of transcription factors, with approximately fifty members in mammals. They regulate essential biological processes by binding DNA and regulating the expression of target genes. NR family members are grouped into three classes further divided into seven subfamilies, according to their structural similarities [2]. The NR5A subfamily includes steroidogenic factor-1 (SF-1, also known as Ad4BP or, according to the official nomenclature, NR5A1) and liver receptor homolog-1 (LRH-1, NR5A2), which have different and mostly non-overlapping functions [3].
SF-1 was initially identified as a positive regulator of steroid P450 hydroxylases in adrenals and gonads [4,5,6], but has a broader role in the establishment and function of endocrine glands. Its presence in all hypothalamic–pituitary–steroidogenic organs makes it an essential regulator of various biological processes [3,6]. In addition to its role in steroidogenesis, recent genomic studies have demonstrated the implication of SF-1 in the processes of angiogenesis, cell adhesion, cytoskeletal dynamics, proliferation, apoptosis, and transcriptional and post-transcriptional regulation of gene expression [7]. Various studies have introduced the notion of SF-1 functional dosage, demonstrating that an adequate dosage of this transcription factor is essential for proper adrenal and gonadal development and function (reviewed in [8]). Interestingly, the phenotypes associated with altered SF-1 dosage depend on the species involved. While gonads in patients carrying heterozygote NR5A1 mutations are most often affected by variable degrees of dysgenesis [9,10,11,12], haploinsufficiency of SF-1 causes adrenal hypoplasia and impaired function in mice [13,14]. In contrast, overexpression of SF-1 is associated with adrenocortical tumorigenesis in both species [8,15,16].
In this review, we have tried to provide a comprehensive overview of our current knowledge about the role of the transcription factor SF-1 in adrenal cortex physiopathology, with a special focus on its role in tumorigenesis.
2. SF-1 Structure
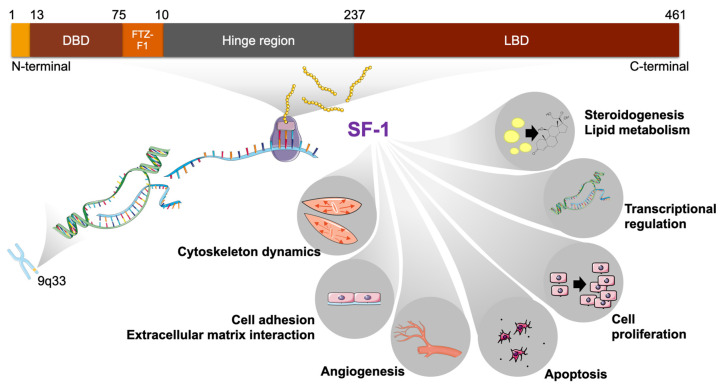
The SF-1 protein consists of four hundred and sixty-one, and four hundred sixty-two amino acids in humans and mice, respectively [6]. The gene (NR5A1; Nr5a1) encoding SF-1 is located on human chromosome 9 and mouse chromosome 2 [17], and consists of seven exons, the first being non-coding. SF-1 shares structural elements with other NRs (Figure 1).
Figure 1.
Structure of the human SF-1 protein and its target gene categories in tumor adrenocortical cells. The protein domains are shown and corresponding amino acid positions are indicated. SF-1 regulates several key cellular processes. DBD, DNA binding domain; FTZ-F1, FTZ-F1 box; LBD, ligand binding domain.
Notably, its structure includes a DNA-binding domain (DBD) composed of two zinc fingers harboring a proximal (P) and a distal (D) box conferring binding affinity to consensus 5′-YCAAGGYCR-3′ sequences [18]. Importantly, NR5A transcription factors contain a C-terminal extension (CTE) after the zinc fingers region of the DBD that contacts the 5′-YCA-3′ portion of the binding site, allowing the protein to bind DNA as a monomer, similarly to a restricted group of other nuclear receptors. In addition, unique to the NR5A subfamily is another highly conserved sequence referred to as the “FTZ-F1 box”, located immediately C-terminal to the DBD CTE, which is required for transcriptional activity and interaction with coactivators. It contains a bipartite nuclear localization signal (NLS) [19].
The SF-1 N-terminal region upstream of the DBD is very short and does not possess a classical AF-1 domain, as observed in other nuclear receptors. The C-terminal part of the SF-1 protein contains a ligand-binding domain (LBD or E-domain) that includes a well-conserved AF-2 transactivation motif, which is also present in other ligand-dependent NRs [6,20]. In the case of SF-1, the presence of the AF-2 domain alone does not allow it to be defined as a ligand-activated nuclear receptor since its activity is constitutive [21]. Structural studies have shown that phospholipids can bind the pocket inside the SF-1 LBD stabilizing its fold, but it is still unknown whether they represent true physiological ligands [22,23,24]. On the other hand, the presence of this AF-2 domain is of crucial importance for SF-1 transcriptional activity, allowing the recruitment of transcriptional cofactors [20]. Between the DBD and the LBD, a hinge region referred to as domain D harboring a proline-rich domain is the target of post-translational modifications that modulate SF-1 activity [21,25].
3. SF-1 Expression
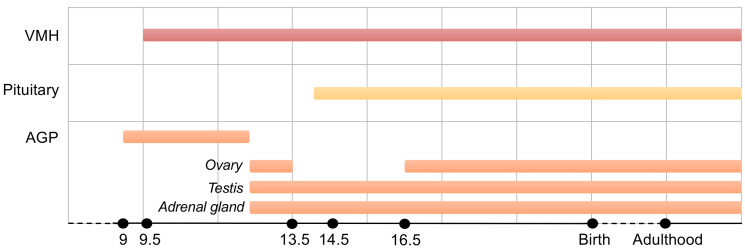
Spatial and temporal profiling of Nr5a1 transcripts in mice has revealed their presence as early as embryonic day (E) 9 in the adrenogonadal primordium (AGP), the common structure from which the adrenal gland and the gonads are derived [26,27,28]. In humans, NR5A1 transcripts are first detected in the urogenital ridge at 32 days post-ovulation (dpo)/Carnegie stage (CS) 14 [29], before adrenal and gonadal differentiation. In both species, SF-1 expression precedes that of the testis-determining SRY [6,29].
At E12.5 (mouse) or 44 dpo/CS18 (humans), gonadal differentiation begins with the formation of testicular cords and in mice is accompanied by sexual dimorphism of Nr5a1 expression. In fact, in this species, Nr5a1 transcripts are detected throughout the development of the testis, whereas in the ovary it is not detected between E13.5 and E16.5 [6,26,27]. Conversely, in humans, NR5A1 transcripts are detectable in the ovary throughout its development [12,29]. In mice, variations in Nr5a1 expression have also been observed during adrenal development. After the formation of the gonadal (AG) and adrenal primordia (AP) from the AGP between E10.5 and E11, Nr5a1 continues to be expressed in adrenal progenitor cells [30]. However, after separation of the adrenal cortex and medulla, between E18.5 and postnatal day (PND) 6, its expression transiently decreases. At this time and until adulthood, SF-1 expression is restricted to the cortex [31]. In adult mice, SF-1 has restricted and specific expression patterns in the hypothalamic–pituitary–steroidogenic axis [26,32]. In female mice, it is expressed in granulosa and theca cells starting from the beginning of ovarian folliculogenesis [32,33] and, later on, in the corpus luteum [4]. Its presence has also been detected in ovarian interstitial cells [34,35]. In male mice, it is found both in Leydig and in Sertoli cells [26,36]. In mouse tissues, other than in the gonads and steroidogenic cells of the adrenal cortex, SF-1 is expressed in ventromedial hypothalamus [26,37,38] and gonadotropes of the pituitary gland [39,40,41] (Figure 2). In addition, it is expressed in a subset of hippocampal neurons [42], the spleen and its veinous sinuses [43].
Figure 2.
Timing of SF-1 expression in the mouse embryo along the hypothalamic–pituitary–adrenogonadal axis. SF-1 is expressed in those organs starting from the early stages of development, in particular in the AGP. The developmental stages before birth are indicated in days post-conception.
Given its function as transcription factor, SF-1 is localized in the cell nucleus [3]. It has also been shown to colocalize with the centrosome marker γ-tubulin in mouse MA-10 Leydig and Y1 adrenocortical cells. Deletion analysis identified a centrosome localization signal at aa 348-367, which may be of importance during cell division to prevent centrosome overduplication and genomic instability [44,45].
4. A Key Role for SF-1 in Adrenal Gland Organogenesis
Adrenal gland formation is a complex process and even if differences exist between humans and mice in its organogenesis, several genes involved are common to both species [46,47]. Briefly, once the AP is formed from the AGP, the adrenocortical lineage forms morphologically and functionally distinct areas: the fetal inner area adjacent to the medulla (termed X-zone in mice) and the definitive outer area [48]. The fetal cortex is gradually replaced by the definitive cortex and the encapsulation of the fetal cortex marks the beginning of the transition. At that time, the different zones of the cortex are not established yet. This event, which involves cell differentiation, occurs during early postnatal life. The maintenance of those zones is then ensured by a constant renewal process throughout life [49]. During embryogenesis in humans and in some primate species, the adrenal cortex is composed in the largest extent of large cells producing adrenal androgens [mainly dehydroepiandrosterone sulfate (DHEAS)] (fetal zone: FZ), separated by a transitional zone (TZ) from an outer layer of smaller cells (definitive zone: DZ), which contains the adrenal precursor cells. After birth, the FZ undergoes rapid involution, while the DZ expands and differentiates to form the characteristic zones of the adult cortex zG, zF, and later on, zR [50]. Many of the processes occurring during adrenal formation involve SF-1, from organogenesis to maintenance in the adult [49]. Different mouse models as well as high throughput genomic analyses have expanded our knowledge about SF-1 function, which far exceeds its classic function in the regulation of genes involved in steroidogenesis [7,31]. An essential role for SF-1 in adrenal gland development was demonstrated using knock-out mice models for Nr5a1 that lack adrenals and gonads at birth [36,51]. In those animals, adrenal glands and gonads initiated a normal developmental program, however since E12, the AP and AG regressed due to apoptosis and pups died because of adrenal insufficiency [36]. As mentioned above, the first SF-1 expression during adrenal development is found at E9 in mice and at 30 dpc in humans [26,27,29]. These stages of embryonic development coincide with the establishment of the adrenal gland and gonadal precursor cells. The understanding of the mechanisms underlying adrenal development in humans is still far from exhaustive [52]. While both the adrenal cortex and gonads derive from the AGP in rodents, that originates from the thickening of the coelomic epithelium at E9 [53], it has recently been shown that in humans and cynomolgus monkeys the adrenal and gonadal precursors arise from opposite regions of the coelomic epithelium and at distinct developmental stages, respectively anterior at 30 dpc and posterior at 33 dpc [47]. Despite the differences in the emergence of adrenal precursor cells in these two species, a high expression of SF-1 is a common element. Current data suggest that SF-1 in these adrenal precursor cells is important to initiate the steroidogenic cell differentiation program. Additionally, exogenous SF-1 expression in mouse embryonic stem cells has been shown to trigger the expression of Cyp11a1, a key enzyme involved in the process of steroidogenesis, as well as progesterone synthesis in response to cAMP [54]. Further works on the differentiation of mesenchymal [55], murine [56,57] or human [58] bone marrow, adipose tissue [59] or umbilical cord [60] stem cells, have demonstrated a key role for SF-1 in establishing a differentiation program into steroidogenic cells. Similarly, human induced steroidogenic cells (hiSCs) were generated from fibroblasts, blood-, and urine-derived cells through forced expression of SF-1 and activation of the PKA pathway [61].
In mice, SF-1 expression is induced shortly after that of Gata4 and Wt1, which have an important role in the formation of the AGP [28,30]. Subsequently, through the action of homeobox proteins and positive feedback involving the fetal adrenal enhancer (FadE) located in the fourth intron of the Nr5a1 gene, SF-1 expression is specifically increased in the AP [62]. An in vivo model using the FadE to induce overexpression of Ad4BP/SF-1 during mouse development supported the idea that not only SF-1 affects steroidogenic cell fate determination, but also that its precise regulation is essential for proper adrenal development. In this model of SF-1 overexpression, ectopic adrenal tissue formation in the thorax was reported, as well as an increase in adrenal size without an impact on cell proliferation [63]. The presence of such a regulatory element in the human gene sequence has not yet been demonstrated, although the FadE locus shares a high degree of homology in both species [62]. The interaction between SF-1 and DAX-1, a negative regulator of SF-1 activity [64], in synergy with the SUMOylation of SF-1 (see below), is required to extinguish FadE activation in the postnatal fetal cortex, which then undergoes regression [65,66,67]. This is consistent with data showing that the X zone is maintained in adult male Dax1-/y mice [68].
In addition to its role in cell fate determination, SF-1 is required for adrenocortical cell proliferation. SF-1 overexpression leads to increased cell proliferation in the mouse definitive adrenal cortex and in the H295R adrenocortical tumor cell line [8,15]. Conversely, Nr5a1+/− mouse embryos exhibit hypoplastic adrenals resulting in adrenal insufficiency and, therefore, altered stress response [13]. In the Nr5a1 knock-out mouse model, exogenous expression of Ad4BP/SF-1 rescued the gonadal but not the adrenal defects [69], indicating that an altered SF-1 dosage severely affects adrenal development in mice. Interestingly, when the alteration of SF-1 expression is a consequence of the deletion of genes such as Cited2, Wt1 [28], M33 [70], or Fgfr2b [71], adrenal development is impaired (Table 1). This emphasizes the major role of SF-1 in the development of the adrenal gland and indicates that not only the presence of SF-1 but also its adequate levels are required for proper adrenal development.
Table 1.
Mouse models with altered SF-1 expression.
| Mouse Model | Adrenal Phenotype | Observed in the Model | Reference |
|---|---|---|---|
| Cited2 − /− | Absence of adrenal glands | Lack of Sf-1 expression | [28] |
| Cited2+/−; Wt1+/− | Marked decrease in adrenal size at E11.5 |
Decrease in Sf-1 expression due to activation of Nr5a1 promoter by Cited2 et Wt1 | [28] |
| Six1−/−; Six4−/− | Smaller adrenal glands than wild type counterparts |
Reduced Nr5a1 expression due to the ability of Six1 and Six4 to bind to the Nr5a1 promoter |
[72] |
| M33 − /− | Underdeveloped adrenal glands | Decreased Nr5a1 expression through indirect binding to the Nr5a1 locus | [70] |
| Insr−/−; Igf1r−/− | Adrenal agenesis for the majority of mutant animals; drastic reduction in adrenal size for a small number of mutants | Altered expression of the core AGP program including a reduction in Nr5a1 transcript levels | [73] |
| Pbx1 − /− | Absence of adrenal glands | Decrease in SF-1 expression in Pbx1-/- embryos correlated with the absence of the adrenocortical cell population | [74] |
| Fgfr2b − / − | Adrenal hypoplasia | Lack of FGFR2b splice variant which causes reduced Sf-1 mRNA levels and reduction in inner cortical cell proliferation | [71] |
| Sf12KR /2KR | Smaller adrenal glands and delayed postnatal regression of adrenal X-zone |
SUMOylation-deficiency leading to persistent FadE activity in X-zone |
[66,67] |
| Dax1 −/y | Delayed postnatal regression of adrenal X-zone |
Dax1-deficiency leading to persistent FadE activity in X-zone due to its repressor role towards Sf-1 | [67] |
5. Regulation of SF-1 Expression and Activity in Adrenal Glands
Regulation of both SF-1 expression and activity appears to be crucial as altered functional dosage results in disturbed adrenal and gonadal development and function, causing different pathologies.
The Nr5a1 gene is regulated by multiple tissue-specific enhancers [75]. As mentioned above, overexpression of SF-1 using the FadE results in a forced commitment to a steroidogenic fate [63]. Regarding other organs, a VMH-specific enhancer [76] and a pituitary enhancer [77], both localized in the sixth intron of the mouse Nr5a1 gene locus, have been identified that allow localization of SF-1 expression to the respective tissues. Further, tissue-specific regulation of SF-1 may be achieved by interacting proteins which positively or negatively regulate its activity, and are themselves expressed in a tissue-specific manner. A relevant example is DAX-1 [78], which was introduced above. Its expression pattern closely follows that of SF-1, and phenotypes resulting from an impaired DAX-1 expression resemble those induced by an altered dosage of SF-1, as reviewed in [79]. DAX-1 inhibits SF-1-dependent gene expression, either through direct binding to SF-1 [64], or by binding to the promoter region of its target genes [80]. In addition to tissue specificity, the timing of expression seems to control SF-1 activity and, therefore, adrenal development. In an attempt to rescue adrenal development in SF-1 null mice, BAC transgenesis was used. Adrenal glands failed to develop, while the spleen and the gonad developed normally [69]. Early SF-1 expression in the adrenogonadal primordium is under the control of Wt1 and Cited2 [28]; Wt1, in turn, being regulated by Odd1 [81] and Sall1 [82]. MicroRNAs, which are small non-coding RNAs controlling gene expression, might also regulate SF-1 at the post-translational level. This type of regulation remains poorly explored, however several elements suggest that they might limit SF-1 expression. The NR5A1 3′UTR of different vertebrate species harbors a predicted miR125b binding site. This miRNA is significantly downregulated in pediatric adrenocortical tumors (ACT) [7,83] and may be implicated in the mechanism of SF-1 upregulation in those tumors, especially in cases lacking NR5A1 copy number gain (see below).
At the post-translational level, SF-1 activity is regulated by SUMOylation. This transcription factor is constitutively targeted to the nucleus, however, because of this post-translational modification, its nuclear distribution is modified. The main SUMOylation site of SF-1 is Lys194. This modification is associated with repression of its activity by recruitment of negative regulators, such as the DEAD-box protein DP103. The localization of SUMOylated SF-1 is restricted to nuclear speckles, thus, preventing its DNA-binding activity [65,84]. Conversely, the localization of SF-1 in active foci within the nucleus, which allows for transcription of target genes, has been reported to be under the control of acetylation at the KQQKK motif in the FTZ-F1 box and of the cAMP pathway [85]. The acetylation state of SF-1 has a positive impact on its half-life and activity [86]. In addition, in Y1 mouse adrenocortical cells, cAMP stimulated the expression of the histone acetyltransferase p300, which associated with SF-1, increases its acetylation and DNA binding [85]. Thereby, a cAMP-dependent mechanism has been thought to promote interactions between SF-1 and cofactors [85,87,88] and to induce the synthesis of activating ligands for SF-1 [89,90]. Other post-translational modifications such as phosphorylation at Ser203 [25] or ubiquitination [91] do not affect SF-1 subcellular localization or its binding to DNA, however Ser203 phosphorylation is necessary to achieve SF-1 maximal activity [25]. Two kinases are known to phosphorylate SF-1, ERK2 and CDK7 [25,92,93]. CDK7-induced phosphorylation of SF-1 can be inhibited by its SUMOylation [94,95].
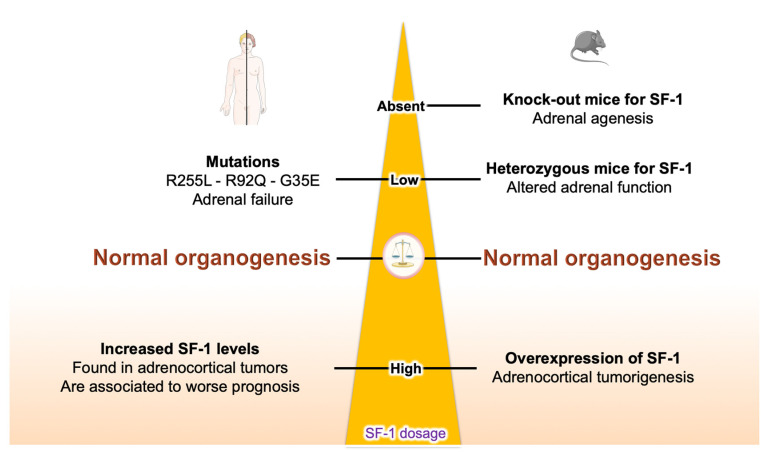
A crucial factor regulating SF-1 expression and biological activity is represented by its dosage. The significance of SF-1 gene dosage has already been introduced above with regard to the adrenal defects in mice resulting from altered SF-1 expression. In general, any altered SF-1 dosage leads to non-physiological conditions both in mice and humans. In the case of heterozygous mutations of NR5A1, a wide range of phenotypes is observed in patients: both gonadal and, more uncommonly, adrenal development and function may be affected, which leads to different degrees of gonadal dysgenesis, male infertility, primary ovarian and adrenal insufficiency [96]. Reported cases of NR5A1 mutations causing adrenal failure are the G35E (heterozygote), R92Q (homozygote) and R255L (heterozygote) missense mutations affecting DNA binding. Among those cases, only the R255L mutation selectively affected adrenal function without producing a gonadal phenotype [9,96,97].
6. SF-1 Dosage Is not Only Critical for Adrenal Development but also for Tumorigenesis
Analysis of both human and murine adrenal tissues has linked SF-1 overexpression to adrenocortical tumorigenesis, providing insights into the underlying mechanisms of tumor formation (reviewed in [8]). An intriguing aspect comes from the study of a group of pediatric ACT from southern Brazil, where the incidence of this malignancy is remarkably higher than in the rest of the world because of the high prevalence of a specific TP53 germline mutation (p.R337H) in the population [98]. In this cohort, frequent amplification of the 9q33–q34 region, in which the NR5A1 gene is located, was found [99]. Later on, increases in NR5A1 copy number and SF-1 protein overexpression were demonstrated in most cases of pediatric ACT [100,101,102]. High levels of SF-1 were also reported in adrenocortical carcinoma (ACC) in adults and negatively correlated to patients’ overall survival (OS) [16].
In an animal model, transgenic mice harboring YAC clones containing several copies of the rat Nr5a1 gene developed adrenocortical neoplastic lesions (both dysplastic and nodular) with a frequency increasing with age [15,103]. In these animals, tumors arose in the adrenal subcapsular region and expressed the gonadal markers Gata4 and AMH, suggesting that tumor cells are derived from undifferentiated adrenogonadal precursors [15]. The critical role of SF-1 in adrenocortical tumorigenesis is reinforced by data from a human adrenocortical cell line (H295R) where SF-1 can be overexpressed in a doxycycline-inducible manner. SF-1 overexpression in H295R cells is able to sharpen their malignant phenotype, increasing their proliferation and invasive capacity in vitro and decreasing apoptosis [15,104]. This effect is dependent on SF-1 transcriptional activity, since overexpression of an AF-2 SF-1 mutant has no effect on cell proliferation. In a concordant fashion, in that cell model, changes in steroid hormone biosynthesis induced by SF-1 overexpression recapitulated those present in pediatric ACT, which produce high DHEAS and lower aldosterone and cortisol levels [15]. Furthermore, an SF-1 synthetic inverse agonist prevents the increase in adrenocortical cell proliferation and the changes in hormone production induced by SF-1 overexpression in H295R cells, indicating that this transcription factor is a druggable target in ACC [105].
7. SF-1 Dosage-Dependent Target Genes: Their Roles in ACC and in Adrenal Gland Development
It is remarkable that SF-1 overexpression in the H295R cell model was sufficient to induce modifications of gene expression which are consistent with patterns found in pediatric ACT, modulating the expression of genes related to cell cycle, apoptosis, cell adhesion and steroidogenesis [15,106]. ChIP-seq studies coupled to gene expression profiling have shown that SF-1 regulates distinct, mostly non-overlapping sets of target genes in ACC cells according to its dosage [7,107]. Functional studies about the molecular and cellular roles of some among those SF-1 dosage-dependent target genes have elucidated their important roles in adrenocortical tumorigenesis:
-
-
NOV/CCN3 encodes a secreted protein with pro-apoptotic properties which is a marker of the DZ in the human fetal adrenal cortex. Its expression is reduced in ACC. SF-1 overexpression in H295R cells significantly reduces NOV expression [108];
-
-
FATE1 encodes a protein enriched in mitochondria-associated membranes (MAM) which has an important role in modulating calcium transfer between the endoplasmic reticulum and mitochondria in ACC cells, being involved in the resistance to chemotherapeutic drugs [109,110]. In addition, FATE1 is a prognostic factor in ACC and a cancer-testis antigen against which an immune response is present in patients with ACC [111], making it a potential target for immunotherapy;
-
-
VAV2 encodes a guanine nucleotide exchange factor (GEF) which activates Rho family GTPases, important regulators of cell cytoskeleton, motility and invasion. VAV2 is a prognostic factor in ACC and its knockdown significantly inhibits H295R cell invasion in Matrigel activated by SF-1 overexpression [104].
Altogether, these findings support a ‘Goldilocks’ model of transcriptional regulation by SF-1, in which the levels of active transcription factor protein must be ‘just right’ to direct transcriptional regulation of its physiological target genes [112]. Decrease or increase in SF-1 activity/levels below or in excess of a critical threshold leads to defects in adrenal gland development or is associated with adrenal tumorigenesis, respectively (Figure 3).
Figure 3.
Adrenal phenotypes associated with an altered SF-1 dosage in mice and humans.
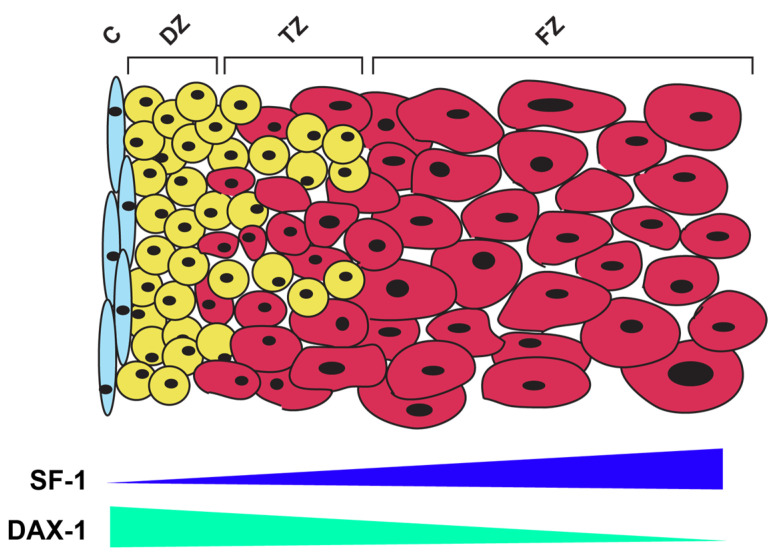
These properties of SF-1 may also have an important role during human adrenal development: it has been reported, in fact, that higher levels of SF-1 are expressed in the FZ compared with the DZ [47], which correlates to the suppression of genes involved in the production of cortisol [15] and to the reduced expression of DZ marker genes (NOV/CCN3) induced by an increased dosage of SF-1 in the H295R cell model [108]. It is then tempting to speculate that changes in SF-1 expression and/or activity have an important role in the modulation of adrenocortical cell differentiation towards a FZ vs. DZ phenotype. Interestingly, the expression pattern of the DAX-1 repressor in the fetal adrenal is the opposite of SF-1, with higher expression and more pronounced nuclear localization in the DZ compared with the FZ [113]. In this model, lower SF-1 activity in the DZ would allow for progenitor cell maintenance and subsequent differentiation into cells of the definitive cortex, while higher SF-1 activity in the FZ is associated with their androgenic steroid secretion pattern (Figure 4).
Figure 4.
A model for the role of SF-1 in the control of adrenocortical cell differentiation in the human fetal adrenal. A gradient of SF-1 expression exists, with higher levels in the FZ compared with the DZ. Additionally, the DAX-1 repressor is expressed at higher levels and shows more pronounced nuclear localization in the DZ compared with the FZ. This leads to presumed higher transcriptional activity of SF-1 in the FZ compared with the DZ and differential regulation of the expression of its dosage-dependent target genes. C, adrenal capsule; DZ, definitive zone; TZ, transitional zone; FZ, fetal zone.
The identification of upstream factors and signaling pathways finely tuning the differential expression of SF-1 in the different cell layers of the human fetal adrenal gland is then of utmost importance for future studies. This may be made easier by the recent development of an in vitro system to produce human fetal adrenal cells from induced pluripotent stem cells (iPS) [114].
8. SF-1 and the Tumor Microenvironment (TME) in ACC
Homogeneous culture conditions obtained from patient-derived cell lines are a simple and low-cost tool to study druggable targets and mechanisms. They are widely used in pre-clinical studies, where many authors have identified drugs that can potentially be used in clinical trials [115]. However, gene expression profiles may present differences in tumor cell lines compared with tumor samples. Data from RNA expression profiling in tumors represent a genetic mosaic, displaying heterogeneous gene expression patterns for each cell type, containing reads from blood, stroma and immune infiltrate, beyond tumor cells themselves. When bulk RNA-seq data from tumors are considered, network analysis can exploit this mixture of gene expression profiles from different cell types. Therefore, the concept of regulatory networks based on transcription factors (TF), as described elsewhere [116,117], takes into consideration target genes either directly or indirectly stimulated or inhibited by the TF.
A great deal of effort has been focused on defining the role of TFs associated with the pathogenesis and/or prognosis of ACC. Those studies have been made possible by accessing publicly available transcriptome databases to evaluate the intratumoral microenvironment. Using the RNA-seq and clinical data from 78 samples from the TCGA ACC cohort [118], Muzzi et al. identified 369 regulatory units composed of a TF and its targets, named regulons, associated with OS in multivariate Cox analysis [119]. Of these 369 units, 346 were also significantly correlated to OS in the European Network for the Study of Adrenal Tumors (ENSAT) cohort with 44 ACC cases [120]. The NR5A1 regulon represented one of the largest regulons in this analysis with 248 targets [119]. Overexpression of this TF correlated with low OS in the multivariate Cox and Kaplan–Meier analyses in the TCGA ACC cohort as well as in the ENSAT ACC cohort. From the regulatory network analysis, the NR5A1 regulon activity was found to be related to worse outcomes in OS and progression-free interval both in the TCGA-ACC cohort, as well as in the ENSAT cohort. Interestingly, of the 369 prognostic regulons, the NR5A1 regulon presented the highest negative correlation with the TGF beta response, and a negative correlation with IFN gamma response, lymphocyte infiltration signature, and leukocyte fraction. On the other hand, it was positively correlated with proliferation and wound healing signatures [119]. These results indicate an association of NR5A1 with immune suppression observed in most ACC cases [121,122] and may be related to the stimulation of steroidogenesis by this TF. However, a full understanding of the role of NR5A1 in the immune response in ACC still has to be obtained. Interestingly, the regulatory network analysis revealed genes that may be indirect targets of SF-1, not just in tumor cells but also in other cell types in the ACC TME.
9. Conclusions and Future Perspectives
Since its discovery, the SF-1 transcription factor has generated wide interest, which has led to its designation as a “master regulator” of major endocrine organ development and physiology. A crucial role in the cellular processes allowing the establishment of the gonads and adrenals, their development and function has been widely demonstrated for SF-1. In particular, adrenal gland formation, maintenance, steroidogenesis and tumorigenesis are dependent on the tight regulation of SF-1 dosage in a time- and tissue-specific manner. Further studies are needed to improve our understanding of the mechanisms regulating both the expression and the biological activity of SF-1, which would allow us to better understand and counteract the pathological consequences of the alterations of its functional dosage.
Author Contributions
Writing—original draft preparation, L.R.; writing—review and editing, L.R., M.D.-B., C.R., J.C.D.M., B.C.F. and E.L.; supervision, E.L.; funding acquisition, E.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was funded by the CNRS EXPOGEN-CANCER IRP and Agence Nationale de la Recherche (ANR), grant number ANR20-CE14-0007 (Goldilocks) to E.L.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Berger I., Werdermann M., Bornstein S.R., Steenblock C. The adrenal gland in stress—Adaptation on a cellular level. J. Steroid Biochem. Mol. Biol. 2019;190:198–206. doi: 10.1016/j.jsbmb.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z., Burch P.E., Cooney A.J., Lanz R.B., Pereira F.A., Wu J., Gibbs R.A., Weinstock G., Wheeler D.A. Genomic analysis of the nuclear receptor family: New insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004;14:580–590. doi: 10.1101/gr.2160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meinsohn M.C., Smith O.E., Bertolin K., Murphy B.D. The orphan nuclear receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, regulation, and essential roles in mammalian reproduction. Physiol. Rev. 2019;99:1249–1279. doi: 10.1152/physrev.00019.2018. [DOI] [PubMed] [Google Scholar]
- 4.Lala D.S., Rice D.A., Parker K.L. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 1992;6:1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- 5.Morohashi K., Honda S., Inomata Y., Handa H., Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J. Biol. Chem. 1992;267:17913–17919. doi: 10.1016/S0021-9258(19)37129-7. [DOI] [PubMed] [Google Scholar]
- 6.Parker K.L., Schimmer B.P. Steroidogenic factor 1: A key determinant of endocrine development and function. Endocr. Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- 7.Lalli E., Doghman M., Latre de Late P., El Wakil A., Mus-Veteau I. Beyond steroidogenesis: Novel target genes for SF-1 discovered by genomics. Mol. Cell. Endocrinol. 2013;371:154–159. doi: 10.1016/j.mce.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Doghman M., Lalli E. A matter of dosage: SF-1 in adrenocortical development and cancer. Ann. Endocrinol. 2009;70:148–152. doi: 10.1016/j.ando.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Achermann J.C., Ito M., Ito M., Hindmarsh P.C., Jameson J.L. A mutation in the gene encoding Steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 1998;22:125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 10.Lin L., Philibert P., Ferraz-de-Souza B., Kelberman D., Homfray T., Albanese A., Molini V., Sebire N.J., Einaudi S., Conway G.S., et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J. Clin. Endocrinol. Metab. 2007;92:991–999. doi: 10.1210/jc.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L., Achermann J.C. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2:200–209. doi: 10.1159/000152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashamboo A., McElreavey K. NR5A1/SF-1 and development and function of the ovary. Ann. Endocrinol. 2010;71:177–182. doi: 10.1016/j.ando.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Bland M.L., Jamieson C.A., Akana S.F., Bornstein S.R., Eisenhofer G., Dallman M.F., Ingraham H.A. Haploinsufficiency of Steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc. Natl. Acad. Sci. USA. 2000;97:14488–14493. doi: 10.1073/pnas.97.26.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beuschlein F., Mutch C., Bavers D.L., Ulrich-Lai Y.M., Engeland W.C., Keegan C., Hammer G.D. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143:3122–3135. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- 15.Doghman M., Karpova T., Rodrigues G.A., Arhatte M., De Moura J., Cavalli L.R., Virolle V., Barbry P., Zambetti G.P., Figueiredo B.C., et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 16.Sbiera S., Schmull S., Assie G., Voelker H.U., Kraus L., Beyer M., Ragazzon B., Beuschlein F., Willenberg H.S., Hahner S., et al. High diagnostic and prognostic value of Steroidogenic factor-1 expression in adrenal tumors. J. Clin. Endocrinol. Metab. 2010;95:E161–E171. doi: 10.1210/jc.2010-0653. [DOI] [PubMed] [Google Scholar]
- 17.Taketo M., Parker K.L., Howard T.A., Tsukiyama T., Wong M., Niwa O., Morton C.C., Miron P.M., Seldin M.F. Homologs of Drosophila Fushi-Tarazu factor 1 map to mouse chromosome 2 and human chromosome 9q33. Genomics. 1995;25:565–567. doi: 10.1016/0888-7543(95)80059-U. [DOI] [PubMed] [Google Scholar]
- 18.Evans R.M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L.A., Chiang E.F., Chen J.C., Hsu N.C., Chen Y.J., Chung B.-C. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol. Endocrinol. 1999;13:1588–1598. doi: 10.1210/mend.13.9.0349. [DOI] [PubMed] [Google Scholar]
- 20.Pawlak M., Lefebvre P., Staels B. General molecular biology and architecture of nuclear receptors. Curr. Top. Med. Chem. 2012;12:486–504. doi: 10.2174/156802612799436641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desclozeaux M., Krylova I.N., Horn F., Fletterick R.J., Ingraham H.A. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor Steroidogenic factor 1. Mol. Cell. Biol. 2002;22:7193–7203. doi: 10.1128/MCB.22.20.7193-7203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krylova I.N., Sablin E.P., Moore J., Xu R.X., Waitt G.M., MacKay J.A., Juzumiene D., Bynum J.M., Madauss K., Montana V., et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Choi M., Cavey G., Daugherty J., Suino K., Kovach A., Bingham N.C., Kliewer S.A., Xu H.E. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor Steroidogenic factor-1. Mol. Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Zhang C., Marimuthu A., Krupka H.I., Tabrizizad M., Shelloe R., Mehra U., Eng K., Nguyen H., Settachatgul C., et al. The crystal structures of human Steroidogenic factor-1 and Liver receptor homologue-1. Proc. Natl. Acad. Sci. USA. 2005;102:7505–7510. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer G.D., Krylova I., Zhang Y., Darimont B.D., Simpson K., Weigel N.L., Ingraham H.A. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: Integration of hormone signaling in reproduction and stress. Mol. Cell. 1999;3:521–526. doi: 10.1016/S1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y., Shen W.H., Ingraham H.A., Parker K.L. Developmental expression of mouse Steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 27.Hatano O., Takakusu A., Nomura M., Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 28.Val P., Martinez-Barbera J.P., Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- 29.Hanley N.A., Ball S.G., Clement-Jones M., Hagan D.M., Strachan T., Lindsay S., Robson S., Ostrer H., Parker K.L., Wilson D.I. Expression of Steroidogenic factor 1 and Wilms’ tumour 1 during early human gonadal development and sex determination. Mech. Dev. 1999;87:175–180. doi: 10.1016/S0925-4773(99)00123-9. [DOI] [PubMed] [Google Scholar]
- 30.Abou Nader N., Boyer A. Adrenal cortex development and maintenance: Knowledge acquired from mouse models. Endocrinology. 2021;162:bqab187. doi: 10.1210/endocr/bqab187. [DOI] [PubMed] [Google Scholar]
- 31.Lalli E. Adrenocortical development and cancer: Focus on SF-1. J. Mol. Endocrinol. 2010;44:301–307. doi: 10.1677/JME-09-0143. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda Y., Lala D.S., Luo X., Kim E., Moisan M.P., Parker K.L. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol. Endocrinol. 1993;7:852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- 33.Morohashi K., Iida H., Nomura M., Hatano O., Honda S., Tsukiyama T., Niwa O., Hara T., Takakusu A., Shibata Y., et al. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol. Endocrinol. 1994;8:643–653. doi: 10.1210/mend.8.5.8058072. [DOI] [PubMed] [Google Scholar]
- 34.Falender A.E., Lanz R., Malenfant D., Belanger L., Richards J. Differential expression of Steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144:3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- 35.Park M., Shin E., Won M., Kim J.H., Go H., Kim H.L., Ko J.J., Lee K., Bae J. FOXL2 interacts with steroidogenic factor-1 (SF-1) and represses SF-1-induced CYP17 transcription in granulosa cells. Mol. Endocrinol. 2010;24:1024–1036. doi: 10.1210/me.2009-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo X., Ikeda Y., Parker K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 37.Shinoda K., Lei H., Yoshii H., Nomura M., Nagano M., Shiba H., Sasaki H., Osawa Y., Ninomiya Y., Niwa O., et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- 38.Kurrasch D.M., Cheung C.C., Lee F.Y., Tran P.V., Hata K., Ingraham H.A. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J. Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingraham H.A., Lala D.S., Ikeda Y., Luo X., Shen W.H., Nachtigal M.W., Abbud R., Nilson J.H., Parker K.L. The nuclear receptor Steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 40.Barnhart K.M., Mellon P.L. The orphan nuclear receptor, Steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol. Endocrinol. 1994;8:878–885. doi: 10.1210/mend.8.7.7527122. [DOI] [PubMed] [Google Scholar]
- 41.Ngan E.S., Cheng P.K., Leung P.C., Chow B.K. Steroidogenic factor-1 interacts with a gonadotrope-specific element within the first exon of the human gonadotropin-releasing hormone receptor gene to mediate gonadotrope-specific expression. Endocrinology. 1999;140:2452–2462. doi: 10.1210/endo.140.6.6759. [DOI] [PubMed] [Google Scholar]
- 42.Wehrenberg U., Prange-Kiel J., Rune G.M. Steroidogenic factor-1 expression in marmoset and rat hippocampus: Co-localization with StAR and aromatase. J. Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 43.Morohashi K., Tsuboi-Asai H., Matsushita S., Suda M., Nakashima M., Sasano H., Hataba Y., Li C.L., Fukata J., Irie J., et al. Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood. 1999;93:1586–1594. [PubMed] [Google Scholar]
- 44.Lai P.Y., Wang C.Y., Chen W.Y., Kao Y.H., Tsai H.M., Tachibana T., Chang W.C., Chung B.C. Steroidogenic Factor 1 (NR5A1) resides in centrosomes and maintains genomic stability by controlling centrosome homeostasis. Cell Death Differ. 2011;18:1836–1844. doi: 10.1038/cdd.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C.Y., Kao Y.H., Lai P.Y., Chen W.Y., Chung B.C. Steroidogenic factor 1 (NR5A1) maintains centrosome homeostasis in steroidogenic cells by restricting centrosomal DNA-dependent protein kinase activation. Mol. Cell. Biol. 2013;33:476–484. doi: 10.1128/MCB.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keegan C.E., Hammer G.D. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol. Metab. 2002;13:200–208. doi: 10.1016/S1043-2760(02)00602-1. [DOI] [PubMed] [Google Scholar]
- 47.Cheng K., Seita Y., Moriwaki T., Noshiro K., Sakata Y., Hwang Y.S., Torigoe T., Saitou M., Tsuchiya H., Iwatani C., et al. The developmental origin and the specification of the adrenal cortex in humans and cynomolgus monkeys. Sci. Adv. 2022;8:eabn8485. doi: 10.1126/sciadv.abn8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zubair M., Parker K.L., Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol. Cell. Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman B.D., Kempna P.B., Carlone D.L., Shah M., Guagliardo N.A., Barrett P.Q., Gomez-Sanchez C.E., Majzoub J.A., Breault D.T. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesiano S., Jaffe R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 51.Sadovsky Y., Crawford P.A., Woodson K.G., Polish J.A., Clements M.A., Tourtellotte L.M., Simburger K., Milbrandt J. Mice deficient in the orphan receptor Steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc. Natl. Acad. Sci. USA. 1995;92:10939–10945. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pignatti E., du Toit T., Flück C.E. Development and function of the fetal adrenal. Rev. Endocr. Metab. Disord. 2023;24:5–21. doi: 10.1007/s11154-022-09756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki K., Oguchi A., Cheng K., Murakawa Y., Okamoto I., Ohta H., Yabuta Y., Iwatani C., Tsuchiya H., Yamamoto T., et al. The embryonic ontogeny of the gonadal somatic cells in mice and monkeys. Cell Rep. 2021;35:109075. doi: 10.1016/j.celrep.2021.109075. [DOI] [PubMed] [Google Scholar]
- 54.Crawford P.A., Sadovsky Y., Milbrandt J. Nuclear receptor Steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol. Cell. Biol. 1997;17:3997–4006. doi: 10.1128/MCB.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazawa T., Imamichi Y., Miyamoto K., Khan M.R., Uwada J., Umezawa A., Taniguchi T. Regulation of steroidogenesis, development, and cell differentiation by Steroidogenic Factor-1 and Liver Receptor Homolog-1. Zoolog. Sci. 2015;32:323–330. doi: 10.2108/zs140237. [DOI] [PubMed] [Google Scholar]
- 56.Gondo S., Yanase T., Okabe T., Tanaka T., Morinaga H., Nomura M., Goto K., Nawata H. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells. 2004;9:1239–1247. doi: 10.1111/j.1365-2443.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 57.Yazawa T., Mizutani T., Yamada K., Kawata H., Sekiguchi T., Yoshino M., Kajitani T., Shou Z., Umezawa A., Miyamoto K. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka T., Gondo S., Okabe T., Ohe K., Shirohzu H., Morinaga H., Nomura M., Tani K., Takayanagi R., Nawata H., et al. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J. Mol. Endocrinol. 2007;39:343–350. doi: 10.1677/JME-07-0076. [DOI] [PubMed] [Google Scholar]
- 59.Gondo S., Okabe T., Tanaka T., Morinaga H., Nomura M., Takayanagi R., Nawata H., Yanase T. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of Steroidogenic factor 1. Endocrinology. 2008;149:4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- 60.Wei X., Peng G., Zheng S., Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101–110. doi: 10.1111/j.1365-2184.2012.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz-Babot G., Balyura M., Hadjidemetriou I., Ajodha S.J., Taylor D.R., Ghataore L., Taylor N.F., Schubert U., Ziegler C.G., Storr H.L., et al. Modeling congenital adrenal hyperplasia and testing interventions for adrenal insufficiency using donor-specific reprogrammed cells. Cell Rep. 2018;22:1236–1249. doi: 10.1016/j.celrep.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zubair M., Ishihara S., Oka S., Okumura K., Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: Initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zubair M., Oka S., Parker K.L., Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol. Endocrinol. 2009;23:1657–1667. doi: 10.1210/me.2009-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito M., Yu R., Jameson J.L. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol. Cell. Biol. 1997;17:1476–1483. doi: 10.1128/MCB.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M.B., Lebedeva L.A., Suzawa M., Wadekar S.A., Desclozeaux M., Ingraham H.A. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee F.Y., Faivre E.J., Suzawa M., Lontok E., Ebert D., Cai F., Belsham D.D., Ingraham H.A. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev. Cell. 2011;21:315–327. doi: 10.1016/j.devcel.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing Y., Morohashi K.I., Ingraham H.A., Hammer G.D. Timing of adrenal regression controlled by synergistic interaction between Sf1 SUMOylation and Dax1. Development. 2017;144:3798–3807. doi: 10.1242/dev.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu R.N., Ito M., Saunders T.L., Camper S.A., Jameson J.L. Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- 69.Fatchiyah, Zubair M., Shima Y., Oka S., Ishihara S., Fukui-Katoh Y., Morohashi K. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem. Biophys. Res. Commun. 2006;341:1036–1045. doi: 10.1016/j.bbrc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 70.Katoh-Fukui Y., Owaki A., Toyama Y., Kusaka M., Shinohara Y., Maekawa M., Toshimori K., Morohashi K. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood. 2005;106:1612–1620. doi: 10.1182/blood-2004-08-3367. [DOI] [PubMed] [Google Scholar]
- 71.Guasti L., Candy Sze W.C., McKay T., Grose R., King P.J. FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol. Cell. Endocrinol. 2013;371:182–188. doi: 10.1016/j.mce.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujimoto Y., Tanaka S.S., Yamaguchi Y.L., Kobayashi H., Kuroki S., Tachibana M., Shinomura M., Kanai Y., Morohashi K., Kawakami K., et al. Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev. Cell. 2013;26:416–430. doi: 10.1016/j.devcel.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Pitetti J.L., Calvel P., Romero Y., Conne B., Truong V., Papaioannou M.D., Schaad O., Docquier M., Herrera P.L., Wilhelm D., et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013;9:e1003160. doi: 10.1371/journal.pgen.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schnabel C.A., Selleri L., Cleary M.L. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis. 2003;37:123–130. doi: 10.1002/gene.10235. [DOI] [PubMed] [Google Scholar]
- 75.Morohashi K., Zubair M. The fetal and adult adrenal cortex. Mol. Cell. Endocrinol. 2011;336:193–197. doi: 10.1016/j.mce.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 76.Shima Y., Zubair M., Ishihara S., Shinohara Y., Oka S., Kimura S., Okamoto S., Minokoshi Y., Suita S., Morohashi K. Ventromedial hypothalamic nucleus-specific enhancer of Ad4BP/SF-1 gene. Mol. Endocrinol. 2005;19:2812–2823. doi: 10.1210/me.2004-0431. [DOI] [PubMed] [Google Scholar]
- 77.Shima Y., Zubair M., Komatsu T., Oka S., Yokoyama C., Tachibana T., Hjalt T.A., Drouin J., Morohashi K. Pituitary homeobox 2 regulates adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol. Endocrinol. 2008;22:1633–1646. doi: 10.1210/me.2007-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikeda Y., Swain A., Weber T.J., Hentges K.E., Zanaria E., Lalli E., Tamai K.T., Sassone-Corsi P., Lovell-Badge R., Camerino G., et al. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: Potential links in endocrine development. Mol. Endocrinol. 1996;10:1261–1272. doi: 10.1210/mend.10.10.9121493. [DOI] [PubMed] [Google Scholar]
- 79.Lalli E. Role of orphan nuclear receptor DAX-1/NR0B1 in development, physiology and disease. Adv. Biol. 2014;2014:582749. doi: 10.1155/2014/582749. [DOI] [Google Scholar]
- 80.Zazopoulos E., Lalli E., Stocco D.M., Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390:311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q., Lan Y., Cho E.S., Maltby K.M., Jiang R. Odd-skipped related 1 (Odd1) is an essential regulator of heart and urogenital development. Dev. Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishinakamura R., Matsumoto Y., Nakao K., Nakamura K., Sato A., Copeland N.G., Gilbert D.J., Jenkins N.A., Scully S., Lacey D.L., et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- 83.Doghman M., El Wakil A., Cardinaud B., Thomas E., Wang J., Zhao W., Peralta-Del Valle M.H., Figueiredo B.C., Zambetti G.P., Lalli E. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70:4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W.Y., Lee W.C., Hsu N.C., Huang F., Chung B.C. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (Steroidogenic factor-1) J. Biol. Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- 85.Chen W.Y., Juan L.J., Chung B.C. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol. Cell. Biol. 2005;25:10442–10453. doi: 10.1128/MCB.25.23.10442-10453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacob A.L., Lund J., Martinez P., Hedin L. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J. Biol. Chem. 2001;276:37659–37664. doi: 10.1074/jbc.M104427200. [DOI] [PubMed] [Google Scholar]
- 87.Fan W., Yanase T., Wu Y., Kawate H., Saitoh M., Oba K., Nomura M., Okabe T., Goto K., Yanagisawa J., et al. Protein kinase A potentiates adrenal 4 binding protein/steroidogenic factor 1 transactivation by reintegrating the subcellular dynamic interactions of the nuclear receptor with its cofactors, general control nonderepressed-5/transformation/transcription domain-associated protein, and suppressor, dosage-sensitive sex reversal-1: A laser confocal imaging study in living KGN cells. Mol. Endocrinol. 2004;18:127–141. doi: 10.1210/me.2003-0110. [DOI] [PubMed] [Google Scholar]
- 88.Winnay J.N., Hammer G.D. Adrenocorticotropic hormone-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor 1-dependent transcriptional activation. Mol. Endocrinol. 2006;20:147–166. doi: 10.1210/me.2005-0215. [DOI] [PubMed] [Google Scholar]
- 89.Urs A.N., Dammer E., Sewer M.B. Sphingosine regulates the transcription of CYP17 by binding to Steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- 90.Li D., Urs A.N., Allegood J., Leon A., Merrill A.H., Jr., Sewer M.B. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol. Cell. Biol. 2007;27:6669–6685. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen W.Y., Weng J.H., Huang C.C., Chung B.C. Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1) Mol. Cell. Biol. 2007;27:7284–7290. doi: 10.1128/MCB.00476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fowkes R.C., Desclozeaux M., Patel M.V., Aylwin S.J., King P., Ingraham H.A., Burrin J.M. Steroidogenic factor-1 and the gonadotrope-specific element enhance basal and pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein hormone alpha-subunit gene in gonadotropes. Mol. Endocrinol. 2003;17:2177–2188. doi: 10.1210/me.2002-0393. [DOI] [PubMed] [Google Scholar]
- 93.Lewis A.E., Rusten M., Hoivik E.A., Vikse E.L., Hansson M.L., Wallberg A.E., Bakke M. Phosphorylation of Steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol. Endocrinol. 2008;22:91–104. doi: 10.1210/me.2006-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell L.A., Faivre E.J., Show M.D., Ingraham J.G., Flinders J., Gross J.D., Ingraham H.A. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1) Mol. Cell. Biol. 2008;28:7476–7486. doi: 10.1128/MCB.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang W.H., Heaton J.H., Brevig H., Mukherjee S., Iñiguez-Lluhí J.A., Hammer G.D. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol. Cell. Biol. 2009;29:613–625. doi: 10.1128/MCB.00295-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suntharalingham J.P., Buonocore F., Duncan A.J., Achermann J.C. DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:607–619. doi: 10.1016/j.beem.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biason-Lauber A., Schoenle E.J. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am. J. Hum. Genet. 2000;67:1563–1568. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Custódio G., Parise G.A., Kiesel Filho N., Komechen H., Sabbaga C.C., Rosati R., Grisa L., Parise I.Z., Pianovski M.A., Fiori C.M., et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 2013;31:2619–2626. doi: 10.1200/JCO.2012.46.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Figueiredo B.C., Stratakis C.A., Sandrini R., DeLacerda L., Pianovski M.A., Giatzakis C., Young H.M., Haddad B.R. Comparative genomic hybridization analysis of adrenocortical tumors of childhood. J. Clin. Endocrinol. Metab. 1999;84:1116–1121. doi: 10.1210/jc.84.3.1116. [DOI] [PubMed] [Google Scholar]
- 100.Figueiredo B.C., Cavalli L.R., Pianovski M.A., Lalli E., Sandrini R., Ribeiro R.C., Zambetti G., DeLacerda L., Rodrigues G.A., Haddad B.R. Amplification of the Steroidogenic factor 1 gene in childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- 101.Pianovski M.A., Cavalli L.R., Figueiredo B.C., Santos S.C., Doghman M., Ribeiro R.C., Oliveira A.G., Michalkiewicz E., Rodrigues G.A., Zambetti G., et al. SF-1 overexpression in childhood adrenocortical tumours. Eur. J. Cancer. 2006;42:1040–1043. doi: 10.1016/j.ejca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 102.Almeida M.Q., Soares I.C., Ribeiro T.C., Fragoso M.C., Marins L.V., Wakamatsu A., Ressio R.A., Nishi M.Y., Jorge A.A., Lerario A.M., et al. Steroidogenic factor 1 overexpression and gene amplification are more frequent in adrenocortical tumors from children than from adults. J. Clin. Endocrinol. Metab. 2010;95:1458–1462. doi: 10.1210/jc.2009-2040. [DOI] [PubMed] [Google Scholar]
- 103.Latre de Late P., El Wakil A., Jarjat M., de Krijger R.R., Heckert L.L., Naquet P., Lalli E. Vanin-1 inactivation antagonizes the development of adrenocortical neoplasia in Sf-1 transgenic mice. Endocrinology. 2014;155:2349–2354. doi: 10.1210/en.2014-1088. [DOI] [PubMed] [Google Scholar]
- 104.Ruggiero C., Doghman-Bouguerra M., Sbiera S., Sbiera I., Parsons M., Ragazzon B., Morin A., Robidel E., Favier J., Bertherat J., et al. Dosage-dependent regulation of VAV2 expression by Steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci. Signal. 2017;10:eaal2464. doi: 10.1126/scisignal.aal2464. [DOI] [PubMed] [Google Scholar]
- 105.Doghman M., Cazareth J., Douguet D., Madoux F., Hodder P., Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J. Clin. Endocrinol. Metab. 2009;94:178–183. doi: 10.1210/jc.2008-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.West A.N., Neale G.A., Pounds S., Figueiredo B.C., Rodriguez Galindo C., Pianovski M.A., Oliveira Filho A.G., Malkin D., Lalli E., Ribeiro R., et al. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 107.Doghman M., Figueiredo B.C., Volante M., Papotti M., Lalli E. Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation. Nucleic Acids Res. 2013;41:8896–8907. doi: 10.1093/nar/gkt658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doghman M., Arhatte M., Thibout H., Rodrigues G., De Moura J., Grosso S., West A.N., Laurent M., Mas J.C., Bongain A., et al. Nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic factor, is down-regulated in childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 2007;92:3253–3260. doi: 10.1210/jc.2007-0342. [DOI] [PubMed] [Google Scholar]
- 109.Doghman-Bouguerra M., Granatiero V., Sbiera S., Sbiera I., Lacas-Gervais S., Brau F., Fassnacht M., Rizzuto R., Lalli E. FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep. 2016;7:1264–1280. doi: 10.15252/embr.201541504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Doghman-Bouguerra M., Lalli E. ER-mitochondria interactions: Both strength and weakness within cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:650–662. doi: 10.1016/j.bbamcr.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 111.Doghman-Bouguerra M., Finetti P., Durand N., Parise I.Z.S., Sbiera S., Cantini G., Canu L., Hescot S., Figueiredo M.M.O., Komechen H., et al. Cancer-testis antigen FATE1 expression in adrenocortical tumors is associated with a pervasive autoimmune response and is a marker of malignancy in adult, but not children, ACC. Cancers. 2020;12:689. doi: 10.3390/cancers12030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ruggiero C., Doghman M., Lalli E. How genomic studies have improved our understanding of the mechanisms of transcriptional regulation by NR5A nuclear receptors. Mol. Cell. Endocrinol. 2015;408:138–144. doi: 10.1016/j.mce.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 113.Battista M.C., Otis M., Côté M., Laforest A., Peter M., Lalli E., Gallo-Payet N. Extracellular matrix and hormones modulate DAX-1 localization in the human fetal adrenal gland. J. Clin. Endocrinol. Metab. 2005;90:5426–5431. doi: 10.1210/jc.2005-0666. [DOI] [PubMed] [Google Scholar]
- 114.Sakata Y., Cheng K., Mayama M., Seita Y., Detlefsen A.J., Mesaros C.A., Penning T.M., Shishikura K., Yang W., Auchus R.J., et al. Reconstitution of human adrenocortical specification and steroidogenesis using induced pluripotent stem cells. Dev. Cell. 2022;57:2566–2583.e8. doi: 10.1016/j.devcel.2022.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilding J.L., Bodmer W.F. Cancer cell lines for drug discovery and development. Cancer Res. 2014;74:2377–2384. doi: 10.1158/0008-5472.CAN-13-2971. [DOI] [PubMed] [Google Scholar]
- 116.Fletcher M.N., Castro M.A., Wang X., de Santiago I., O’Reilly M., Chin S.F., Rueda O.M., Caldas C., Ponder B.A., Markowetz F., et al. Master regulators of FGFR2 signalling and breast cancer risk. Nat. Commun. 2013;4:2464. doi: 10.1038/ncomms3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castro M.A., de Santiago I., Campbell T.M., Vaughn C., Hickey T.E., Ross E., Tilley W.D., Markowetz F., Ponder B.A., Meyer K.B. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat. Genet. 2016;48:12–21. doi: 10.1038/ng.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng S., Cherniack A.D., Dewal N., Moffitt R.A., Danilova L., Murray B.A., Lerario A.M., Else T., Knijnenburg T.A., Ciriello G., et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29:723–736. doi: 10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muzzi J.C.D., Magno J.M., Souza J.S., Alvarenga L.M., de Moura J.F., Figueiredo B.C., Castro M.A.A. Comprehensive characterization of the regulatory landscape of adrenocortical carcinoma: Novel transcription factors and targets associated with prognosis. Cancers. 2022;14:5279. doi: 10.3390/cancers14215279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Assié G., Letouzé E., Fassnacht M., Jouinot A., Luscap W., Barreau O., Omeiri H., Rodriguez S., Perlemoine K., René-Corail F., et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014;46:607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 121.Landwehr L.S., Altieri B., Schreiner J., Sbiera I., Weigand I., Kroiss M., Fassnacht M., Sbiera S. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J. Immunother. Cancer. 2020;8:e000469. doi: 10.1136/jitc-2019-000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muzzi J.C.D., Magno J.M., Cardoso M.A., de Moura J., Castro M.A.A., Figueiredo B.C. Adrenocortical carcinoma steroid profiles: In silico pan-cancer analysis of TCGA data uncovers immunotherapy targets for potential improved outcomes. Front. Endocrinol. 2021;12:672319. doi: 10.3389/fendo.2021.672319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.