Abstract
Broad application of CuO nanoparticles (CuO-NP) for industrial and household purposes leads to a continuous increase in their discharge to, and, hence, ever-increasing environmental hazards for aquatic ecosystems. Microalgae-based technologies hold promise for bioremediation of diverse hazardous micropollutants (HMP), including NP, from wastewater. In this study, we tested the ability of the green microalga Desmodesmus sp. to accumulate CuO-NP or their components. We also assessed the tolerance of this microalga to the environmentally relevant concentrations of CuO-NP. Using scanning electron microscopy, we demonstrated that the average size of CuO-NP was 50–100 nm, and their purity was confirmed with elemental composition analysis. Tests of the colloidal suspensions of CuO-NP showed that the hydrodynamic diameter of CuO-NP and their aggregates was below 100 nm. Flow cytometry analysis showed that CuO-NP at a concentration of 100 µg L−1 slightly inhibited the viability of microalgae cells and led to an increase in their oxidative stress. The assessment of the condition of photosystem II showed that CuO-NP exert a multifaceted effect on the photosynthetic apparatus of Desmodesmus sp., depending on the concentration of and the exposure to the CuO-NP. Desmodesmus sp. turned to be relatively tolerant to CuO-NP. In addition, the ICP-MS method revealed increased bioaccumulation of copper by microalgae cells in the experimental groups. The outcomes of this study indicate that the Desmodesmus sp. has a significant potential for bioremoval of the copper-based nanostructured HMP from an aquatic environment.
Keywords: CuO-NP, microalgae, photosynthesis, oxidative stress, pigment composition, viability, bioaccumulation, bioremediation
1. Introduction
Since nanoparticles (NP) are extensively applied for commercial, environmental, and healthcare benefits, they are currently produced at an industrial scale [1,2]. CuO nanoparticles (CuO-NP) have become widespread, and their global production is forecasted to reach 1600 tons/year by 2025 [3]. Due to their unique chemical and physical properties, these NP have found broad applications in various commercial formulations, including agrochemicals, paints, semiconductor compounds, sensors, catalysts, and antimicrobial compounds [4]. In the process of production, transportation, storage, consumption, and disposal of the above-listed products, the CuO-NP are increasingly discharged into the environment; following diverse pathways, these NP end up in aquatic ecosystems [5,6].
In the aquatic environment, NP cause various impacts on its inhabitants. Thus, CuO-NP were reported to increase mortality, delay hatching, and distort heartbeat and embryo formation in Danio rerio [7]. Another study [8] showed that exposure to CuO-NP reduces the viability of sea urchin spermatozoa, disrupts mitochondrial activity, and increases the production of reactive oxygen species (ROS) and lipid peroxidation. CuO-NP inhibited growth, decreased carotenoid content, and deteriorated cellular metabolism of the microalga Chlamydomonas reinhardtii [9] and the cyanobacterium Lyngbya sp. [10].
CuO-NP are known to induce oxidative stress and to exert cytotoxicity, genotoxicity, and immunotoxicity effects [11]. The release of metal ions from NP is among the main causes of the NP toxicity for many organisms [12]. At the same time, it is necessary to take into account the whole set of the NP toxicity determinants, including their physico-chemical parameters and concentration [11].
It is also known that accumulating NP in living organisms can be transferred between trophic levels in food chains with concomitant build-up of their concentration in the organisms toward the higher trophic levels [13]. The upward transfer of NP across trophic levels was studied in a two-level system consisting of Artemia salina and Amatitlania nigrofasciata [14]. It was reported that CuO-NP accumulated by Chlorella vulgaris was detected in the Daphnia magna they grazed [15]. Therefore, the upward transfer of NP across trophic levels poses a great danger, both for natural ecosystems and for humans.
The deteriorative effects of the industrial CuO-NP on the environment call for the development of knowledge-based approaches to mitigation of these effects. Accordingly, the development of methods for bioremoval of NP from the environment gains increasing attention. Since mechanical cleaning systems are energy-intensive, the potential of microbial biotechnologies for the bioremoval of micropollutants comes to the foreground. Thus, a promising solution to the problem of wastewater treatment to remove CuO-NP is constituted by using microalgae, the organisms with a long story of application in the field of bioremediation [16]. The resulting biomass of microalgae can be utilized for the production of biofuels [17,18] or other industrially important compounds [19].
Indeed, microalgae are capable of accumulation of CuO-NP [9,15,20]. Still, high concentrations of these NP are toxic to many microalgal species [9,10].
Therefore, the foundation of the biotechnology for bioremediation of CuO-NP containing wastewater using microalgae requires the selection of species that would be (i) resilient to environmentally relevant concentrations of the NP (up to 1 mg L−1) and (ii) capable of bioaccumulating the NP or their components. Therefore, the aim of the present study was to assess the CuO-NP resilience and the bioaccumulation potential of the microalga Desmodesmus sp. IPPAS S-2014 [21,22], which showed the greatest (compared with the microalgae from the genera Lobosphaera sp. IPPAS C-2047, Micractinium IPPAS C-2056, and other representatives of the genus Desmodesmus) retention of photosynthetic capacity in a preliminary screening of CuO-NP resilience (for more information, see Figure S1 and [23]).
2. Materials and Methods
2.1. Nanoparticles
2.1.1. Nanoparticle Characterization
The CuO-NP from Sigma-Aldrich (St. Louis, MO, USA) were used in the work. The morphology and particle size were determined before the experiment by scanning electron microscopy (SEM) on a Tescan Vega 3 microscope (Czech Republic). The elemental analysis was carried out by the method of energy dispersive X-ray spectroscopy (EDX) using the EDX analyzer “10 mm2 SDD Detector-X-Act” (Oxford Instruments, Oxford, UK).
2.1.2. Obtaining of the NP Suspension
To introduce the NP into the algae cultivation medium, aqueous dispersions of the NP were prepared. The NP powder samples (0.5 mg) were weighed using ViBRA HT analytical scales (Shinko Denshi, Tokyo, Japan), placed into cylindrical tubes with screw caps with 10 mL of sterile distilled water (pH 7.1 ± 0.2). The suspensions were mixed and sonicated (VBS-41H, Vilitek, Moscow, Russia) for 10 min (the ultrasonic power—180 W, the bath volume—4 L) to obtain a 500 µg 10 mL−1 NP stock. The stock suspension was diluted to obtain the working suspensions of 50 µg 10 mL−1 and 5 µg 10 mL−1. To assess the stability of the obtained dispersed systems, the zeta potential of the NP in the suspensions was analyzed using the ZetasizerNanoZS analyzer (Malvern Panalytical, Malvern, UK) [24,25]. The size and the aggregates of the particles in the obtained colloidal solutions were determined by the method of dynamic light scattering (on the same ZetasizerNanoZS device) [24,26].
2.2. Testing of the Effect of the NP on Microalgae
A stress-resilient unicellular green alga Desmodesmus sp., strain IPPAS S-2014 (referred to herein as Desmodesmus sp.) was selected as the object of the study. This strain of microalgae was previously isolated from fragments of the invertebrate animal hydroid Dynamena pumila [21], but its tolerance to nanopollution has never been tested before.
The Desmodesmus sp. cells were cultivated in BG-11 medium [27] in glass conical flasks with a capacity of 250 mL containing 150 mL of the culture under constant illumination with white LED lamps (480 µM PAR quanta/m2/s) at a temperature of 27 °C and pH 7.0–7.2.
2.2.1. Experimental Design
The experiment was started by the addition of NP suspension to the test culture of the microalga. Specifically, 6-mL aliquots of the microalgal culture (OD = 0.6 at 560 nm) were introduced into each of well of the 6-well plate (Eppendorf, Hamburg, Germany) together with either 120 µL of the corresponding NP suspension or 120 µL of distilled water (the control) and gently mixed. The final NP concentration was 10, 100, or 1000 µg mL−1, which is in the range of environmentally relevant NP concentrations [28,29,30].
2.2.2. Cell Viability Tests
The changes in the viability of the NP-treated culture (expressed as % of that in the untreated control culture, see Figure S2) was considered the primary indicator of the microalgal culture condition and, correspondingly, the effect of the NP. This cell viability was assessed using the cell analyzer Muse Cell Analyzer, (Merck Millipore, Darmstadt, Germany) using the reagent Muse Count & Viability from the same manufacturer. Since the working cell suspensions were of high density, the sampled suspension was diluted (1:20) with fresh BG-11. After that, the cell cultures were further diluted with the Muse Count & Viability reagent (1:20) and incubated for 5 min before the measurement. The cell density of the culture samples was additionally determined in the NP-treated and the untreated control [31].
2.2.3. Oxidative Stress Assessment
The degree of oxidative stress in the algal culture samples was gauged using the Muse Cell Analyzer and Muse Oxidative Stress reagent kit from the same manufacturer. The stock solution of the Muse Oxidative Stress reagent was diluted (1:100) with the 1× Assay buffer from the kit. The working solution was prepared by further diluting the stock solution with the 1× Assay buffer. The algal culture samples were also diluted (1:20) with the 1× Assay buffer. After that, 10 µL of the diluted cell suspension was added to 190 µL of the working solution. The obtained samples were incubated for 30 min at 37 °C before performing the measurements [32].
2.2.4. Photosynthetic Pigment Assay
The pigments in the microalgal samples were quantified spectrophotometrically [33] in dimethyl sulfoxide (DMSO) extracts. Briefly, 1 mL of the cell suspension was centrifuged (5 min, 5000× g) on a MiniSpin centrifuge (Eppendorf, Hamburg, Germany). The supernatant was discarded, and the cell pellet was incubated in DMSO at 70 °C for 10 min with intensive stirring and the cells’ debris was then removed by centrifugation.
The concentrations of chlorophylls a and b, as well as the total carotenoids in the extract was carried out spectrophotometrically in 96-well plates on a Multiskan Sky spectrophotometer (Thermo Scientific, Waltham, MA, USA) using the equations reported in [33]:
| CChl a (mg/L) = 13.44 D666 − 4.85 D650; | (1) |
| CChl b (mg/L) = 24.58 D650 − 6.65 D666; | (2) |
| CCar(mg/L) = (1000 D480 − 1.29 CChl a − 53.76 CChl b)/220 | (3) |
where CChl a, CChl b, and CCar are the concentrations of chlorophylls a and b and carotenoids, respectively; and Dλ is the optical density of the extract at the wavelength λ (nm).
2.2.5. Measurement of Photosynthetic Activity of the Microalgae
The photosynthetic activity of the microalgae was assessed as a potential maximal photochemical quantum yield of photosystem 2, PS II Qy = (Fm − Fo)/Fm = Fo)/Fm, where Fo is the minimum and Fm is the maximum intensity of Chl, a fluorescence excited with a weak measuring light and a strong saturating pulse, respectively, in the microalgal cells dark-adapted for 15 min. The PS II Qy was measured with a PAM-fluorometer FluorPen FP S100 (Photon Systems Instruments, Drasov, Czech Republic) according to the manufacturer’s protocol. The PS II Qy parameter is frequently used to monitor direct and PSII-mediated stress effects on photosynthetic organisms. Generally, any damage to the cells directly or indirectly causing inhibition of the photosystem II or damage to its reaction centers leads to an observable decline on Fv/Fm [34].
2.3. Assessment of the NP Absorption and/or Internalization by the Microalgal Cells
The content of the NP or their components in the microalgal cells and the residual NP content of particles in the medium were determined by inductively coupled plasma mass spectrometry (ICP-MS) on an ICP-MS spectrometer (Bruker, San Jose, CA, USA). The samples for the analysis were prepared by filtering 2 mL of cell suspension through a membrane filter (Millipore, Burlington, MA, USA) with a pore size of 0.45 microns. The microalgal culture incubated with the maximum concentration (1000 µg mL−1) of the NP was used for this analysis. Since large aggregates of NP could be detained by the filter in addition to the NP accumulated by/adsorbed on the microalgal cells, a reference analysis was conducted by filtering the nutrient medium containing the same amount of the NP, but the lacking microalgal cells culture was additionally filtered. The obtained data were taken into account when calculating the NP bioaccumulation efficiency. Furthermore, to avoid an error in the determination of the Cu content due to handling the cells deposited on the filter, we re-measured the cell density of the cell suspension washed from the filter (it was actually used for the ICP assay) and corrected the final result accordingly.
For the analysis, the biomass samples were mineralized with a mixture of saturated HCl and HNO3 (3:1, vol./vol.). The efficiency of the bioaccumulation/adsorption of the NP by the microalgae cells was calculated via the following equation [35,36]:
| Bioaccumulation/adsorption (%) = (Ci/Cf)·100% | (4) |
where Ci is the NP concentration in the microalgal cell sample and Cf is the initial NP concentration in the medium.
2.4. Statistical Treatment of the Data
All the experiments were carried out in three biological repetitions with three technical samplings for each biological replica. The statistical significance of the difference of the main indicators of the culture condition (chlorophyll and carotenoid contents, cell density, cell viability, and the oxidative stress degree) was evaluated using the Student’s t-criterion (at the 0.05 significance level). For the Fv/Fm parameter, a nonparametric Mann–Whitney U-test was used (at the 0.05 significance level) since the chlorophyll fluorescence parameters generally do not feature the normal distribution [37]. The average values and their standard deviations are shown in the figures and tables. Statistical treatment of the data has been carried out with Excel spreadsheet software (Microsoft, Redmond, WA, USA).
3. Results
3.1. Characterization of the CuO-NP
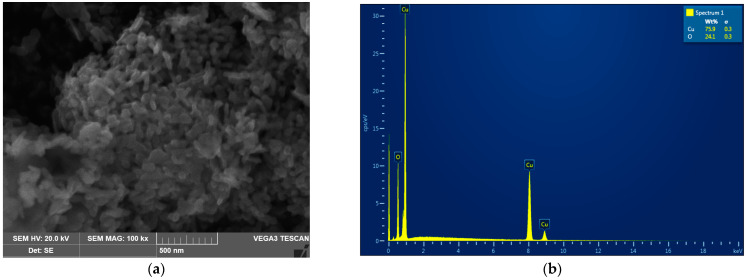
The analysis of the CuO-NP revealed that the NP used in this work were rod-shaped with rounded ends, assembled into aggregates (Figure 1a).
Figure 1.
The analysis of the CuO-NP used in this work. (a) Scanning electron micrograph. (b) Element composition (EDX spectrum).
As can be seen from the presented microphotographs, the individual NP were, on average, 50 nm in diameter and 70–100 nm long. Energy dispersive X-ray spectroscopy showed that the analyzed NP powder is composed of copper oxide without any impurities (Figure 1b). Collectively, both analyses confirmed that the studied NP samples were indeed represented by nano-sized (50–100 nm) particles of pure CuO.
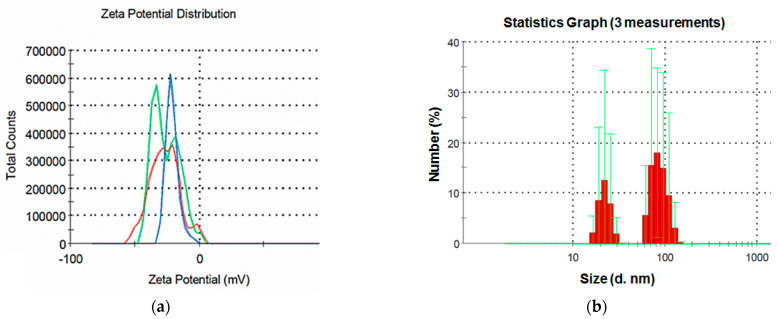
3.2. Properties of the Colloidal Systems of the NP
The studied NP suspension featured a zeta potential of 21.4 mV (Figure 2a), which indicates a relatively low stability of the dispersed system.
Figure 2.
The CuO-NP suspension (a) zeta potential and (b) particle size distribution.
The results of the analysis of the CuO particle size in the colloidal solutions demonstrate a bimodal particle size distribution (Figure 2b). The first peak is in the range of 20–30 nm, the second is 70–100 nm. The results are consistent with the SEM data obtained in this study, and the presence of two peaks can be explained by the elongated shape of the particles, as well as their aggregation in the aqueous environment.
In general, the results of the analysis of the dispersed composition of colloidal suspensions of the NP show that, despite the use of sonication, the NP aggregated in the aqueous medium, but the size of the aggregates was not large.
3.3. CuO-NP Effects on the Microalgal Cultures
3.3.1. Cell Viability
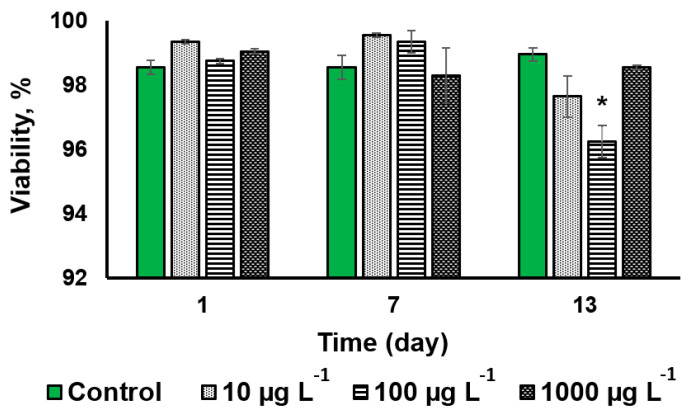
The CuO-NP treatment at a concentration of 100 µg L−1 induced a statistically significant effect (2.7% decline of the cell viability) only on the 13th day of exposure (Figure 3 and Figure S2).
Figure 3.
The analysis of the viability of Desmodesmus sp. cells incubated with CuO-NP as compared to the untreated cells (Values significantly differing from the control are marked with an asterisk, *).
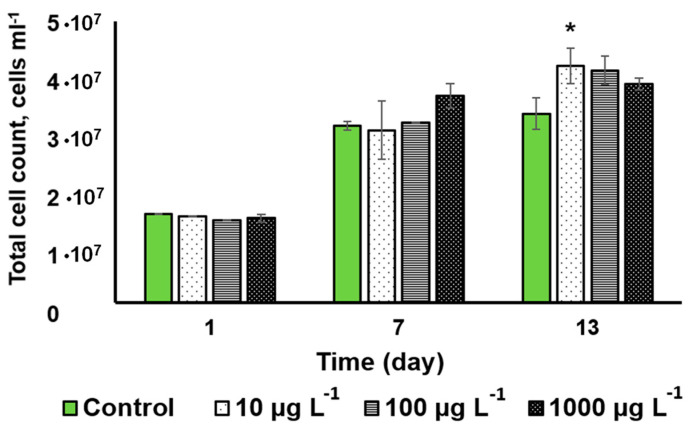
Figure 4 shows the dynamics of the changes in the cell density of the cultures incubated with the CuO-NP and the untreated control. The CuO-NP exerted no significant effect on microalgal cell density except for a slight increase observed on the 13th day in the culture incubated with 10 µg L−1 CuO-NP.
Figure 4.
The changes in Desmodesmus sp. cell density in the cultures as a function of the added CuO-NP concentration (Values significantly differing from the control are marked with an asterisk, *).
Judging from the cell viability and cell density, the tested microalga Desmodesmus sp. turned out to be relatively resilient to the effects of CuO-NP.
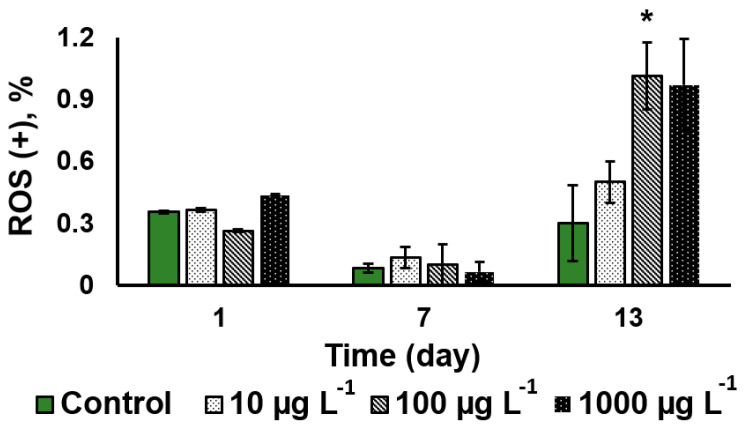
3.3.2. Oxidative Stress Severity
The assessment of the relative severity of oxidative stress to the algal cells showed that CuO-NP did not induce a sizeable an increase in the oxidative stress intensity during the first seven days of the NP exposure regardless of the CuO-NP concentration. It was only on the 13th day of the exposure to the 100 µg L−1 CuO-NP that a three-fold increase in the severity of oxidative stress was observed, as well as in the cultures treated with 1000 µg L−1 CuO-NP (Figure 5).
Figure 5.
Comparative analysis of the oxidative stress severity in the Desmodesmus sp. cells as a function of the added CuO-NP concentration (Values significantly differing from the control are marked with an asterisk, *).
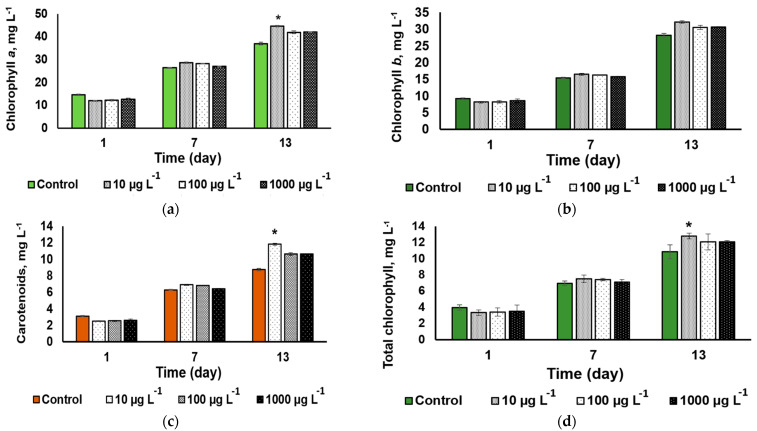
3.3.3. Cell Pigment Composition
A statistically significant CuO-NP effect on the microalgal cell pigment composition (a slight increase) was revealed only on 13th day of exposure to the NP concentration of 100 µg L−1 (Figure 6a,c). The same trend was revealed in the total chlorophylls (a + b) concentration of chlorophyll (Figure 6d). Otherwise, the cultures exhibited a steady accumulation of both pigment groups regardless of the added CuO-NP concentration, showing no sign of impairment of the pigment apparatus of the cell.
Figure 6.
The dynamics of (a) chlorophyll a, (b) chlorophyll b, (c) total carotenoid, and (d) chlorophyll a + b contents in the cultures of Desmodesmus sp. incubated in the presence of different concentrations of CuO-NP (Values significantly differing from the control are marked with an asterisk, *).
The calculation of per cell pigment content revealed no significant changes in the carotenid or chlorophyll content (Figure S3), therefore the increase in the volumetric pigment content in the variant “10 µg L−1” is caused by the increase in the cell number (Figure 4).
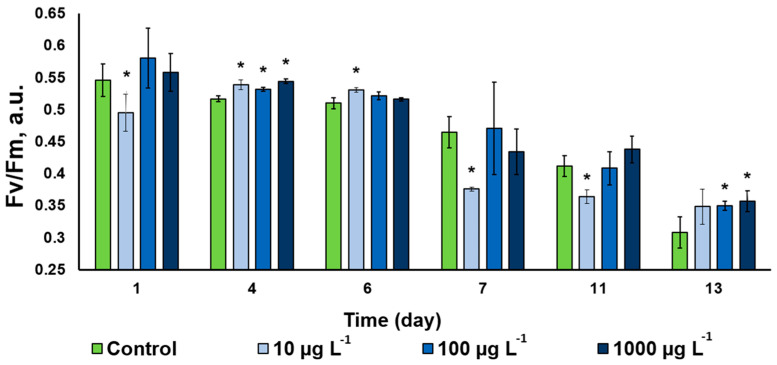
3.3.4. Photosystem II Functional Condition
The photochemical activity of the Desmodesmus sp. cells assessed as potential maximal quantum yield of photosystem II displayed a steady decline throughout the observation period (Figure 7). This trend was evident in the untreated control, likely due to slow-down of the cell division in the studied culture manifesting the onset of the stationary phase of the culture growth. The CuO-NP-treated cultures followed approximately the same trend. Interestingly, the NP in the lowest concentration used triggered a small decline of the PS II Qy, while the cultures treated with higher NP concentrations frequently possessed a higher photochemical activity than that in the control (Figure 7). There was no clearly expressed pattern in the CuO-NP effect on the functioning of the photosynthetic apparatus of the studied microalgal cultures. Intermittent inhibition and stimulation effects have been observed in our experiments on the background of the overall decline in the photosynthetic activity of the cultures taking place, regardless of the presence and/or concentration of the NP.
Figure 7.
Changes of the Fv/Fm parameter in the of Desmodesmus sp. incubated with different CuO-NP concentrations (Values significantly differing from the control are marked with an asterisk, *).
3.3.5. Uptake and adsorption of the CuO-NP
The copper content assay showed that the volumetric amount of copper taken up and adsorbed by Desmodesmus sp. exposed to the 1000 µg mL−1 NP for 13 days was 330 µg L−1; the cell density of the culture increased ca. 2.5 times by that time (Figure 4). This amount is constituted by the copper adsorbed to the cell surface, as well as the copper internalized by the cells both as NP and in ionic form. These figures should be interpreted while taking into account the constitutive copper content integral to the enzyme molecules in the cell, as well as the copper taken up from the growth medium. The cell density of the culture in the beginning of the experiment was 1.36·107 cells mL–1, which yield the “background” level of ca. 4.23 fg Cu per cell. The results of the copper assay in the different sample types including the filtrates of the medium are shown in Table 1.
Table 1.
Copper content in different sample types.
| Sample | Copper, µg L−1 | Copper, fg/Cell |
|---|---|---|
| Desmodesmus sp. (the control) | 57.6 | 4.23 |
| Desmodesmus sp. after the CuO-NP exposure | 367.2 * | 9.18 |
| The filtrate of BG-11 medium | 632.8 | - |
| The filtrate of the algal cell-free BG-11 medium with CuO-NP | 962.8 | - |
* Including 37.2 µg L−1 of copper adsorbed on the filter (see Methods).
Table 1 shows that the microalgal culture unexposed to CuO-NP contained 57.6 µg L−1 copper, whereas the CuO-NP-exposed culture Cu contained six times more copper. The efficiency of bioaccumulation and adsorption of CuO-NP (or copper contained therein) by Desmodesmus sp. cells was 33%, meaning that the concentration of CuO-NP in the growth medium was reduced by ca. 33%. Notably, the ICP-MS analyses cannot distinguish between the copper in the NP and that within the microalgal enzymes. There are also reports on the ability of microalgae to absorb trace elements, including Cu, from the environment [38,39]. Therefore, some of the copper discovered in the Desmodesmus sp. cells under our experiment conditions can stem from the micro-salts of the BG-11 nutrient medium, but contribution of the medium cannot be significant. Thus, there was no increase in the copper content of the control culture cells. Collectively, our findings suggest that the NP or their components were the major source of the Cu discovered in the NP-treated cells.
4. Discussion
Taken together, the results of the biochemical and functional tests suggest that the microalga selected as the test object for our study turned out to be quite resilient to the CuO-NP in the medium, even at concentrations as high as 1000 µg L−1. Moreover, under the conditions studied, the cells of the microalgae exhibited a pronounced ability to take up copper from the nutrient medium. The latter observation is very important in the context of the development of biotechnology for bioremediation on nanopolluted wastewater, as well as water with a high concentration of ionic copper forms.
At the same time, CuO-NP at a concentration of 100 µg L−1 were able to reduce the viability of the microalgae culture, likely via enhancing ROS production exacerbating the oxidative stress [40], which was also observed in these experimental variants.
The ‘nonlinear’ toxic effect of the studied NP is consistent with the results obtained in our previous work [41] and other studies [42,43]. Such a toxicity pattern may be associated with stepwise kinetics of the acclimation of the microalga to the NP-induced stress, the cross-talk of the NP-induced effects, and the cellular signaling [44]. Studies on the patterns of the slightly expressed toxicity are very important for understanding the momentary and accumulated effects of hazardous micropollutants, as well as for control of undesirable microflora development with chemicals acting in low doses.
Interestingly, all the cultures studied displayed a steady accumulation of the photosynthetic pigments, regardless of the presence of the CuO-NP or their concentration. Most likely, this finding reflects a steady increase in cell density [10], although direct counting of the cells could not be performed under our conditions due to the cell aggregate formation. An indirect indication on the culture growth and the onset of the stationary phase by the 13th day of cultivation is the decline in PS Qy taking place due to over-reduction of the electron transport chain components in the chloroplast membranes of the cell. Although many studies indicate that copper is deteriorative for the photosynthetic apparatus [45,46,47,48], in our study, the CuO-NP and the ionic forms of Cu potentially present in the medium did not exert a profound inhibitory effect of PS II. The intermittent decline in photosynthetic activity recorded may be associated with Cu2+ ions likely released from the NP surface during sonication of the NP suspensions [40]. Another plausible source of the ‘nonlinear’ pattern of the NP toxicity can be the diversity of the NP size and shape [43].
The specific mechanisms of CuO-NP- and Cu2+-mediated inhibition of photosynthesis are related to the disruption of the oxygen-evolving complex of photosystem II [49]. Copper ions also affect photosynthesis via interaction with the electron transport chain and interfering with the uptake of Fe ions essential for the biosynthesis of porphyrins, especially cytochromes [50]. Overall, the main mechanism of CuO-NP toxicity is the release of Cu ions [20,51,52]. In addition to this, CuO-NP and their aggregates are able to attach to the surface of microalgae cells, leading to direct or indirect toxicity through physical damage, internalization, or light shading [53]. We also found that the CuO-NP used in our experiment tended to aggregate in the aqueous medium, possibly causing some shading of the cells.
Metal NP are also able to interfere with the enzymes of the antioxidant system, triggering the processes mimicking the response of the plant’s low-molecular antioxidant system to stress [47,54]. It is possible that the increase in carotenoids in the microalgae cells, which was observed in our study, reflects an antioxidant response that was quite efficient, judging from the low percentage of the cells exhibiting the symptoms of oxidative stress.
Desmodesmus sp. was able to uptake and/or absorb CuO-NP or copper emitted from the NP from the aqueous medium. The exact pathways of NP uptake by the microalgae were not considered in this study, although it is possible that the internalization of NP proceeded via energy-dependent clathrin-mediated endocytosis, as reported in [53]. To be internalized by the algal cells, NP need to penetrate two barriers—the cell wall and the cytoplasmic membranes. In the case of microalgae, relatively thick and rigid cell walls are usually considered as the first and the toughest barrier preventing internalization of NP. At the same time, the cell walls of algae are semi-permeable and usually porous. The pore diameter is normally in the range of 5–20 nm, thereby allowing smaller NP to readily pass through the cell wall [55]. In our study, the average diameter of the individual CuO particles was about 50 nm, and their length was 70–100 nm, therefore, they would hardly be able to pass the cell wall for subsequent internalization by the microalgae cells. Accordingly, adsorption of NP on the cell surface but not uptake can be suggested as the main mechanism of the CuO-NP bioremoval from the medium. Still, NP can alter the cell wall and membrane properties, facilitating their penetration into the cells [50], therefore this issue requires further studies.
To conclude, the efficiency of bioremoval of the CuO-NP or the copper they harbor was around 33%, which makes the Desmodesmus sp. a potential vehicle for bioremediation of wastewater contaminated with Cu-containing NP. The efficiency reached in this work can be improved using physico-chemical and biotechnological approaches facilitating the adsorption of the NP on the surface of, as well as uptake of the NP by the cells. One of the ways of accomplishing this is the selection of microalgal strains for increasing their NP (ad)sorption capacity, isolating or constructing the microbial consortia with synergistically increased NP bioremoval efficiency. Another promising approach is the chemical and/or physical stimulation of the ability of microalgae to the biotransformation of NP [50] to better mitigate the negative consequences of nanopollution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13040737/s1, Figure S1: Preliminary screening of CuO-NP effects on different microalgal strains via PS II Qy (Fv/Fm): (a) Desmodesmus sp. IPPAS C-2014; (b) Lobosphaera sp. IPPAS C-2047; (c) Desmodesmus sp. 1 Dp66E-1; (d) Micractinium simplicissimum IPPAS C-2053; Figure S2: Analysis of the viability of Desmodesmus sp. cells incubated with CuO-NP as compared to the untreated cells; Figure S3: The dynamics of (a) chlorophyll a, (b) chlorophyll b, (c) total carotenoid, and (d) chlorophyll a + b content per cells of Desmodesmus sp. Incubated in the presence of different concentrations of CuO-NP.
Author Contributions
Conceptualization, O.V.Z. and S.P.C.; methodology, O.V.Z., S.P.C., A.A.G., P.A.B., A.E.S. and E.V.S.; software, E.A.K.; validation, A.E.S.; formal analysis, S.P.C., O.V.Z., P.A.B., M.A.A. and A.S.Y.; investigation, S.P.C., O.V.Z., A.A.G., P.A.B., A.S.Y. and E.S.L.; resources, A.E.S., O.V.Z., A.A.G. and E.V.S.; data curation, O.V.Z. and A.E.S.; writing—original draft preparation, S.P.C.; writing—review and editing S.P.C., M.A.A., J.X., M.A.A. and O.V.Z.; visualization, S.P.C., O.V.Z., A.A.G. and E.A.K.; supervision, O.V.Z.; project administration, O.V.Z. and A.E.S.; funding acquisition, O.V.Z., A.A.G. and A.E.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Microalgal cultivation was supported by an international joint cooperation project between the Russian Science Foundation and the National Science Foundation of China, grant #23-44-00006 (RSF)/22261132515 (NSFC). All other works were funded by the Russian Science Foundation, grant number 21-74-20004.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pérez-Hernández H., Pérez-Moreno A., Sarabia-Castillo C.R., García-Mayagoitia S., Medina-Pérez G., López-Valdez F., Campos-Montiel R.G., Jayanta-Kumar P., Fernández-Luqueño F. Ecological drawbacks of nanomaterials produced on an industrial scale: Collateral Effect on Human and Environmental Health. Water Air Soil Pollut. 2021;232:435. doi: 10.1007/s11270-021-05370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charitidis C.A., Georgiou P., Koklioti M.A., Trompeta A.-F., Markakis V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014;1:11. doi: 10.1051/mfreview/2014009. [DOI] [Google Scholar]
- 3.Khan R., Inam M.A., Park D.R., Khan S., Akram M., Yeom I.T. The Removal of CuO nanoparticles from water by conventional treatment C/F/S: The Effect of pH and Natural Organic Matter. Molecules. 2019;24:914. doi: 10.3390/molecules24050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonin M., Cantarel A.A.M., Crouzet A., Gervaix J., Martins J.M.F., Richaume A. Negative effects of copper oxide nanoparticles on carbon and nitrogen cycle microbial activities in contrasting agricultural soils and in presence of plants. Front. Microbiol. 2018;9:3102. doi: 10.3389/fmicb.2018.03102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray A., Gautam A., Das S., Pal K., Das S., Karmakar P., Ray M., Ray S. Effects of copper oxide nanoparticle on gill filtration rate, respiration rate, hemocyte associated immune parameters and oxidative status of an Indian freshwater mussel. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020;237:108855. doi: 10.1016/j.cbpc.2020.108855. [DOI] [PubMed] [Google Scholar]
- 6.Roubeau Dumont E., Elger A., Azéma C., Castillo Michel H., Surble S., Larue C. Cutting-edge spectroscopy techniques highlight toxicity mechanisms of copper oxide nanoparticles in the aquatic plant Myriophyllum spicatum. Sci. Total Environ. 2022;803:150001. doi: 10.1016/j.scitotenv.2021.150001. [DOI] [PubMed] [Google Scholar]
- 7.Chao S.-J., Huang C.P., Lam C.-C., Hua L.-C., Chang S.-H., Huang C. Transformation of copper oxide nanoparticles as affected by ionic strength and its effects on the toxicity and bioaccumulation of copper in zebrafish embryo. Ecotoxicol. Environ. Saf. 2021;225:112759. doi: 10.1016/j.ecoenv.2021.112759. [DOI] [PubMed] [Google Scholar]
- 8.Gallo A., Manfra L., Boni R., Rotini A., Migliore L., Tosti E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018;118:325–333. doi: 10.1016/j.envint.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Melegari S.P., Perreault F., Costa R.H., Popovic R., Matias W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013;142–143:431–440. doi: 10.1016/j.aquatox.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Janani B., Al Farraj D.A., Raju L.L., Elshikh M.S., Alkubaisi N.A., Thomas A.M., Das A., Sudheer Khan S. Cytotoxicological evaluation of copper oxide nanoparticles on green algae, bacteria and crustacean systems. J. Environ. Health Sci. Eng. 2020;18:1465–1472. doi: 10.1007/s40201-020-00561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naz S., Gul A., Zia M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020;14:1–13. doi: 10.1049/iet-nbt.2019.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Li N., Zhao J., White J.C., Qu P., Xing B. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem. Res. Toxicol. 2012;25:1512–1521. doi: 10.1021/tx3002093. [DOI] [PubMed] [Google Scholar]
- 13.Peng C., Zhang W., Gao H., Li Y., Tong X., Li K., Zhu X., Wang Y., Chen Y. Behavior and Potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials. 2017;7:21. doi: 10.3390/nano7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemati T., Sarkheil M., Johari S.A. Trophic transfer of CuO nanoparticles from brine shrimp (Artemia salina) nauplii to convict cichlid (Amatitlania nigrofasciata) larvae: Uptake, accumulation and elimination. Environ. Sci. Pollut. Res. 2019;26:9610–9618. doi: 10.1007/s11356-019-04263-6. [DOI] [PubMed] [Google Scholar]
- 15.Wu F., Bortvedt A., Harper B.J., Crandon L.E., Harper S.L. Uptake and toxicity of CuO nanoparticles to Daphnia magna varies between indirect dietary and direct waterborne exposures. Aquat. Toxicol. 2017;190:78–86. doi: 10.1016/j.aquatox.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Rempel A., Gutkoski J.P., Nazari M.T., Biolchi G.N., Cavanhi V.A.F., Treichel H., Colla L.M. Current advances in microalgae-based bioremediation and other technologies for emerging contaminants treatment. Sci. Total Environ. 2021;772:144918. doi: 10.1016/j.scitotenv.2020.144918. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Horsman M., Wu N., Lan C.Q., Dubois-Calero N. Biofuels from microalgae. Biotechnol. Prog. 2008;24:815–820. doi: 10.1021/bp070371k. [DOI] [PubMed] [Google Scholar]
- 18.Hossain N., Mahlia T.M.I., Saidur R. Latest development in microalgae-biofuel production with nano-additives. Biotechnol. Biofuels. 2019;12:125. doi: 10.1186/s13068-019-1465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miazek K., Iwanek W., Remacle C., Richel A., Goffin D. Effect of Metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A Review. Int. J. Mol. Sci. 2015;16:23929–23969. doi: 10.3390/ijms161023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Cao X., Liu X., Wang Z., Zhang C., White J.C., Xing B. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity. Nanotoxicology. 2016;10:1297–1305. doi: 10.1080/17435390.2016.1206149. [DOI] [PubMed] [Google Scholar]
- 21.Solovchenko A., Gorelova O., Selyakh I., Semenova L., Chivkunova O., Baulina O., Lobakova E. Desmodesmus sp. 3Dp86E-1-a novel symbiotic chlorophyte capable of growth on pure CO2. Mar. Biotechnol. 2014;16:495–501. doi: 10.1007/s10126-014-9572-1. [DOI] [PubMed] [Google Scholar]
- 22.Solovchenko A., Gorelova O., Selyakh I., Pogosyan S., Baulina O., Semenova L., Chivkunova O., Voronova E., Konyukhov I., Scherbakov P., et al. A novel CO2-tolerant symbiotic Desmodesmus (Chlorophyceae, Desmodesmaceae): Acclimation to and performance at a high carbon dioxide level. Algal Res. 2015;11:399–410. doi: 10.1016/j.algal.2015.04.011. [DOI] [Google Scholar]
- 23.Solovchenko A.E., Vasilieva S.G., Zaitsev P., Lukyanov A.A., Skripnikova E.V., Antal T.K. Approaches to rapid screening of pharmaceutical xenobiotic effects on microalgae via monitoring of photosynthetic apparatus condition. J. Appl. Phycol. 2022;34:353–361. doi: 10.1007/s10811-021-02660-4. [DOI] [Google Scholar]
- 24.Shnoudeh A.J., Hamad I., Abdo R.W., Qadumii L., Jaber A.Y., Surchi H.S., Alkelany S.Z. Chapter 15-Synthesis, Characterization, and Applications of Metal Nanoparticles. In: Tekade R.K., editor. Biomaterials and Bionanotechnology. Academic Press; London, UK: 2019. pp. 527–612. [Google Scholar]
- 25.Clogston J.D., Patri A.K. Zeta Potential Measurement. In: McNeil S.E., editor. Characterization of Nanoparticles Intended for Drug Delivery. Humana Press; Totowa, NJ, USA: 2011. pp. 63–70. [Google Scholar]
- 26.Carvalho P.M., Felício M.R., Santos N.C., Gonçalves S., Domingues M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018;6:237. doi: 10.3389/fchem.2018.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanier R., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Microbiol. Mol. Biol. Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Marchi L., Coppola F., Soares A.M.V.M., Pretti C., Monserrat J.M., Torre C.d., Freitas R. Engineered nanomaterials: From their properties and applications, to their toxicity towards marine bivalves in a changing environment. Environ. Res. 2019;178:108683. doi: 10.1016/j.envres.2019.108683. [DOI] [PubMed] [Google Scholar]
- 29.Gottschalk F., Sun T., Nowack B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Kiser M.A., Westerhoff P., Benn T., Wang Y., Pérez-Rivera J., Hristovski K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009;43:6757–6763. doi: 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- 31.Zedler J.A., Gangl D., Guerra T., Santos E., Verdelho V.V., Robinson C. Pilot-scale cultivation of wall-deficient transgenic Chlamydomonas reinhardtii strains expressing recombinant proteins in the chloroplast. Appl. Microbiol. Biotechnol. 2016;100:7061–7070. doi: 10.1007/s00253-016-7430-y. [DOI] [PubMed] [Google Scholar]
- 32.Hazeem L.J., Yesilay G., Bououdina M., Perna S., Cetin D., Suludere Z., Barras A., Boukherroub R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020;156:111278. doi: 10.1016/j.marpolbul.2020.111278. [DOI] [PubMed] [Google Scholar]
- 33.Solovchenko A., Merzlyak M.N., Khozin-Goldberg I., Cohen Z., Boussiba S. coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010;46:763–772. doi: 10.1111/j.1529-8817.2010.00849.x. [DOI] [Google Scholar]
- 34.Murchie E.H., Lawson T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013;64:3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- 35.Sarı A., Tuzen M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008;152:302–308. doi: 10.1016/j.jhazmat.2007.06.097. [DOI] [PubMed] [Google Scholar]
- 36.Afkar E., Ababna H., Fathi A.A. Toxicological response of the green alga Chlorella vulgaris, to Some heavy metals. Am. J. Environ. Sci. 2010;6:230. doi: 10.3844/ajessp.2010.230.237. [DOI] [Google Scholar]
- 37.Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct. Plant Biol. FPB. 2006;33:9–30. doi: 10.1071/FP05095. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima A., Horikoshi T., Sakaguchi T. Uptake of copper ion by green microalgae. Agric. Biol. Chem. 1979;43:1455–1460. doi: 10.1080/00021369.1979.10863659. [DOI] [Google Scholar]
- 39.Éva M., Molnár S., Attila K., Virág D., Pénzes-Kónya E. Study of microelement accumulating characteristic of microalgae. Acta Bot. Hung. 2011;53:167. doi: 10.1556/ABot.53.2011.1-2.15. [DOI] [Google Scholar]
- 40.Che X., Ding R., Li Y., Zhang Z., Gao H., Wang W. Mechanism of long-term toxicity of CuO NPs to microalgae. Nanotoxicology. 2018;12:923–939. doi: 10.1080/17435390.2018.1498928. [DOI] [PubMed] [Google Scholar]
- 41.Zakharova O.V., Godymchuk A.Y., Gusev A.A., Gulchenko S.I., Vasyukova I.A., Kuznetsov D.V. Considerable Variation of antibacterial activity of cu nanoparticles suspensions depending on the storage time, dispersive medium, and particle sizes. BioMed Res. Int. 2015;2015:412530. doi: 10.1155/2015/412530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayade G., Rong Wu M., Massicotte R., Gennad’evich Deryabin D., Yahia L.H. Biocidal properties of copper nanoparticles. Eng. Biomater. 2021;24:2–17. doi: 10.34821/eng.biomat.159.2021.2-17. [DOI] [Google Scholar]
- 43.Bell I.R., Ives J.A., Jonas W.B. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Response. 2013;12:202–232. doi: 10.2203/dose-response.13-025.Bell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stovbun S.V., Kiselev A.V., Zanin A.M., Kalinina T.S., Voronina T.A., Mikhailov A.I., Berlin A.A. Effects of physicochemical forms of phenazepam and Panavir on their action at ultra-low doses. Bull. Exp. Biol. Med. 2012;153:455–458. doi: 10.1007/s10517-012-1739-z. [DOI] [PubMed] [Google Scholar]
- 45.Purbonegoro T., Suratno, Puspitasari R., Husna N.A. Toxicity of copper on the growth of marine microalgae Pavlova sp. and its chlorophyll-a. IOP Conf. Ser. Earth Environ. Sci. 2018;118:012060. doi: 10.1088/1755-1315/118/1/012060. [DOI] [Google Scholar]
- 46.Cid A., Herrero C., Torres E., Abalde J. Copper toxicity on the marine microalga Phaeodactylum tricornutum: Effects on photosynthesis and related parameters. Aquat. Toxicol. 1995;31:165–174. doi: 10.1016/0166-445X(94)00071-W. [DOI] [Google Scholar]
- 47.Shakya K., Chettri M.K., Sawidis T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008;54:412–421. doi: 10.1007/s00244-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 48.Mazumder P. Effect of copper on growth and chlorophyll content in tea plants (Camellia sinensis (L.) O. Kuntze) Impact Int. J. Res. Appl. Nat. Soc. Sci. (IMPACT IJRANSS) 2014;2:223–230. [Google Scholar]
- 49.Joonas E., Aruoja V., Olli K., Kahru A. Environmental safety data on CuO and TiO2 nanoparticles for multiple algal species in natural water: Filling the data gaps for risk assessment. Sci. Total Environ. 2019;647:973–980. doi: 10.1016/j.scitotenv.2018.07.446. [DOI] [PubMed] [Google Scholar]
- 50.Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aruoja V., Dubourguier H.-C., Kasemets K., Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 52.Mueller E., Behra R., Sigg L. Toxicity of engineered copper (Cu0) nanoparticles to the green alga Chlamydomonas reinhardtii. Environ. Chem. 2015;13:457–463. doi: 10.1071/EN15132. [DOI] [Google Scholar]
- 53.Kamat J.P., Devasagayam T.P., Priyadarsini K.I., Mohan H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 2000;155:55–61. doi: 10.1016/S0300-483X(00)00277-8. [DOI] [PubMed] [Google Scholar]
- 54.Venzhik Y.V., Moshkov I.E., Dykman L.A. Influence of nanoparticles of metals and their oxides on the photosynthetic apparatus of plants. Biol. Bull. 2021;48:140–155. doi: 10.1134/S106235902102014X. [DOI] [Google Scholar]
- 55.Wang F., Guan W., Xu L., Ding Z., Ma H., Ma A., Terry N. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl. Sci. 2019;9:1534. doi: 10.3390/app9081534. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author on reasonable request.