Abstract
The Catharanthus roseus receptor-like kinase 1-like (CrRLK1L), which is a vital member of the plant receptor-like kinase family, plays versatile roles in plant growth, development, and stress response. Although the primary screening of tomato CrRLK1Ls has been reported previously, our knowledge of these proteins is still scarce. Using the latest genomic data annotations, a genome-wide re-identification and analysis of the CrRLK1Ls in tomatoes were conducted. In this study, 24 CrRLK1L members were identified in tomatoes and researched further. Subsequent gene structures, protein domains, Western blot analyses, and subcellular localization analyses all confirmed the accuracy of the newly identified SlCrRLK1L members. Phylogenetic analyses showed that the identified SlCrRLK1L proteins had homologs in Arabidopsis. Evolutionary analysis indicated that two pairs of the SlCrRLK1L genes had predicted segmental duplication events. Expression profiling analyses demonstrated that the SlCrRLK1L genes were expressed in various tissues, and most of them were up- or down-regulated by bacteria and PAMP treatments. Together, these results will lay the foundation for elaborating the biological roles of SlCrRLK1Ls in tomato growth, development, and stress response.
Keywords: CrRLK1L, Solanum lycopersicum, gene family analysis, genome-wide analysis
1. Introduction
As a crucial member of signal transduction, receptor-like kinases (RLKs) constitute the largest receptor family in plants and play a significant role in plant growth, development, stress, and pathogen response [1,2]. According to their diverse extracellular domains, plant RLKs can be mainly divided into the following: the S-domain, the wall-associated kinase domain, the legume lectin domain, the CRINKLY4 domain, the malectin-like (CrRLK1L) domain, the malectin-like leucine-rich repeat domain, the leucine-rich repeat malectin domain, the cysteine-rich repeat domain, the leucine-rich repeat (LRR) domain, the lysin motif domain, the pro-rich/extension domain, and the calcium-dependent lectin domain RLK family [3]. To the interest of many researchers, the plant-specific CrRLK1L protein kinases were firstly identified in Madagascar periwinkle and have since been found to exist in a variety of plant species [2,4,5]. Traditionally, CrRLK1Ls possess the following three conserved domains: the malectin-like domain, the transmembrane helix domain, and the kinase domain [5]. Some of the CrRLK1L members have been functionally identified, including FERONIA (FER), ANXUR1/2 (ANX1/2), THESEUS1 (THE1), BUDDHA’S PAPER SEAL1/2 (BUPS1/2), and HERCULES1 (HERK1).
The CrRLK1L family members are involved in a wide range of biological process regulations, including male–female gametophyte recognition, cell expansion, hormone signaling, energy production, stress tolerance, and host–pathogen interactions [2,6,7,8,9,10,11]. AtFER, which was originally identified from a pollen tube mutant, has become the most extensively investigated CrRLK1L protein in Arabidopsis [5,12,13]. AtFER takes part in different hormone signals that contain auxin, ethylene, brassinosteroid (BR), abscisic acid (ABA), and jasmonic acid (JA) [6,14,15,16,17]. Moreover, AtFER acts as a receptor for RALF1 (rapid alkalinization factor 1), RALF17, RALF23, RALF32, and RALF33 to regulate development and biotic/abiotic stress responses [18,19,20]. As for other Arabidopsis CrRLK1L members, AtTHE1 and AtHERK1 have been reported to regulate cell elongation [21,22], AtANX1/2 and AtBUPS1/2 participate in pollen tube growth regulation [11,23], and MEDOS1 (MDS1), MDS2, MDS3, and MDS4 have been reported to be involved in metal ion stress responses [24]. In addition, AtFER is involved in host–pathogen interactions, including Golovinomyces (syn. Erysiphe) orontii and Pseudomonas syringae pv. tomato DC3000 responses [17,25]. Several CrRLK1L members have also been functionally identified in other species. In rice, it has been reported that OsFLR1 (Oryza sativa FERONIA-like receptor1) and OsFLR2 (also named DRUS1 and DRUS2) are essential for maintaining architecture, reproduction, and seed yield [26,27]. Moreover, the ruptured pollen tube (RUPO) regulates the growth and integrity of pollen tubes [28]. Apple MdFERL1 (Malus domestica FERONIA-like1), MdFERL6, and tomato SlFERL (Solanum lycopersicum FERONIA-like) are involved in fruit ripening [29,30]. In soybean, GmLMM1 (Glycine max lesion mimic mutant1) regulates cell death and PTI (pattern-triggered immunity) processes, responding to bacterial and oomycete pathogen infections [31]. In pears (Pyrus bretchneideri), PbrCrRLK1L3 and PbrCrRLK1L26 take part in the pollen tube rupture process and growth [32]. Additionally, Chenopodium quinoa CqFER, soybean GmCrRLK1L20, and tobacco NtCrRLK1L47 have been reported to be involved in salt stress responses [33,34,35].
The tomato (Solanum lycopersicum), as an economically important fleshy fruit crop, is widely accepted as a model species for studying the developmental and postharvest biology of horticultural crops. The genome sequencing and annotations of tomatoes were completed for the first time in 2012 [36]. Based on this, a previous genome-wide receptor-like kinase (RLK) study found 23 CrRLK1L protein kinase subfamily members in the tomato genome, but it did not analyze the gene structure, protein motifs, phylogeny, subcellular localization, and gene expression of these proteins [37]. Over the past decade, with the increasing ability of technology, the annotation of the tomato genome has become more sophisticated and accurate. Therefore, it is necessary to re-identify and analyze the CrRLK1L protein kinases in tomatoes. In this study, taking advantage of the state-of-the-art and well-annotated tomato genome and protein database, genome-wide research of the CrRLK1L protein families was performed. As a result, 24 CrRLK1L protein kinase candidates were identified in the tomato genome. Further analysis showed the phylogenetic relationship, physicochemical properties, gene structure, subcellular localization, conserved protein domains, predicted motifs, and gene expression pattern of this family. It will give us new insight into the tomato CrRLK1L family and help us reveal the function of these proteins in the future.
2. Results
2.1. Identification of Tomato CrRLK1L Protein Kinases
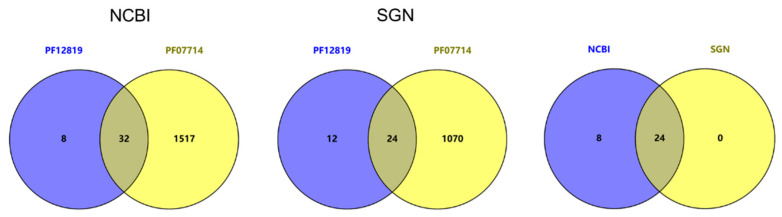
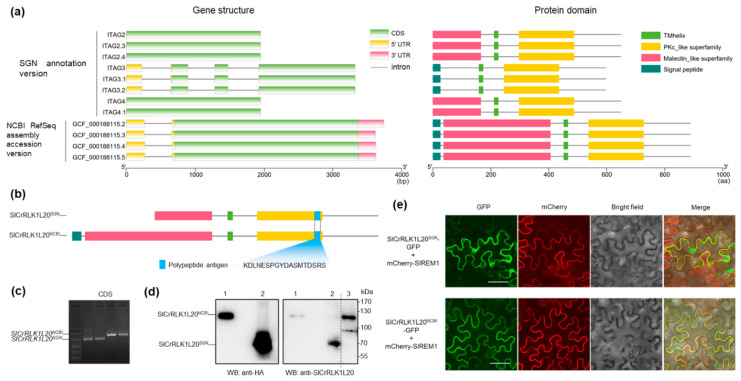
All CrRLK1L protein kinases consist of a malectin-like domain and a kinase domain. Arabidopsis CrRLK1L protein kinase sequences were submitted to the Pfam database, and two conserved domains (Pfam: PF12819 and PF07714) were acquired. Based on these criteria, the two conserved domains served as queries to screen the tomato protein databases in the National Center for Biotechnology Information (NCBI) and the Sol Genomics Network (SGN). As shown in Figure 1, 32 and 24 CrRLK1L protein kinase candidates were identified in NCBI and SGN, respectively. As a result, 24 tomato CrRLK1Ls were matched to both databases and named from SlCrRLK1L1 to SlCrRLK1L24 according to the location of the chromosomes (Table 1). Meanwhile, there were differences in the annotation of ten proteins in the NCBI and SGN databases (Figure S1; Table 1; Table S1). One of them was chosen to verify the accuracy. We analyzed the gene structure and protein domain of SlCrRLK1L20 from the SGN annotation versions (from ITAG2 to ITAG4.1) and the NCBI RefSeq assembly accession versions (from GCF_000188115.2 to GCF_000188155.5) and found that the NCBI RefSeq had complete UTR, CDS, intron, signal peptide, transmembrane helix, malectin-like, and protein kinase descriptions (Figure 2a). Moreover, the SlCrRLK1L20 gene sequences from SGN were all included in NCBI. Then, a unique polypeptide was used as an antigen to produce a SlCrRLK1L20 antibody, which could detect SlCrRLK1L20NCBI (NCBI GCF_000188115.5, the newest annotation protein) and SlCrRLK1L20SGN (SGN ITAG4.1, the newest annotation protein) simultaneously (Figure 2b). The SlCrRLK1L20NCBI and SlCrRLK1L20SGN CDSs were amplified by PCR (Figure 2c) and used to construct a plant expression vector. A Western blot assay showed that SlCrRLK1L20NCBI was detected by the anti-SlCrRLK1L20 antibody in the tomato but SlCrRLK1L20SGN was not (Figure 2d). At the same time, the subcellular localization of SlCrRLK1L20NCBI and SlCrRLK1L20SGN was conducted by confocal analysis. Solanum lycopersicum REMORIN1 (SlREM1) was identified as a plasma membrane-labeled protein in previous research [38]. As shown in Figure 2e, SlCrRLK1L20NCBI-GFP was co-localized with SlREM1 at the plasma membrane, while SlCrRLK1L20SGN was not. The above results revealed that the annotation of SlCrRLK1L20 from NCBI was more accurate than SGN. Therefore, the subsequent related research was mainly based on the NCBI database.
Figure 1.
Identification of tomato CrRLK1Ls in the NCBI and SGN databases. The predicted numbers of tomato CrRLK1Ls in the NCBI and SGN databases are shown. PF12819: Malectin-like; PF07714: PK-Tyr-Ser-Thr.
Table 1.
List of the predicted CrRLK1L proteins in tomatoes.
| Protein Name | Protein ID in NCBI |
Protein Length (aa) |
Molecular Weight (Da) |
Theoretical pI |
GRAVY | Matched SGN Locus |
Annotation Difference in NCBI and SGN |
|---|---|---|---|---|---|---|---|
| SlCrRLK1L1 | XP_025886195.1 | 854 | 95,039.67 | 6.86 | −0.268 | Solyc01g059910 | Y |
| SlCrRLK1L2 | XP_004230878.1 | 876 | 96,273.18 | 5.82 | −0.212 | Solyc01g109950 | N |
| SlCrRLK1L3 | XP_019067666.1 | 1152 | 129,597.94 | 6.93 | −0.174 | Solyc02g014030 | N |
| SlCrRLK1L4 | XP_004233885.1 | 868 | 96,438.64 | 5.49 | −0.28 | Solyc02g069970 | Y |
| SlCrRLK1L5 | XP_004233025.1 | 1002 | 112,230.67 | 6.36 | −0.312 | Solyc02g071860 | Y |
| SlCrRLK1L6 | XP_010316862.1 | 995 | 111,071.97 | 5.79 | −0.108 | Solyc02g071880 | N |
| SlCrRLK1L7 | XP_004232151.1 | 869 | 97,152.91 | 6.14 | −0.234 | Solyc02g089090 | Y |
| SlCrRLK1L8 | NP_001234869.1 | 903 | 101,461.32 | 8.92 | −0.197 | Solyc02g091590 | N |
| SlCrRLK1L9 | XP_004234657.2 | 817 | 91,323.59 | 5.9 | −0.168 | Solyc03g044160 | Y |
| SlCrRLK1L10 | XP_010318169.1 | 865 | 97,835 | 5.41 | −0.201 | Solyc03g093380 | N |
| SlCrRLK1L11 | XP_025886103.1 | 1340 | 150,300.63 | 6.39 | −0.226 | Solyc03g115710 | Y |
| SlCrRLK1L12 | XP_010318523.1 | 894 | 99,669.44 | 5.49 | −0.16 | Solyc03g121230 | N |
| SlCrRLK1L13 | XP_004239170.1 | 926 | 103,366.23 | 5.88 | −0.267 | Solyc05g014240 | N |
| SlCrRLK1L14 | XP_004240198.2 | 840 | 92,846.45 | 6.26 | 0.021 | Solyc05g054680 | N |
| SlCrRLK1L15 | XP_004239762.1 | 811 | 90,461.22 | 5.66 | −0.079 | Solyc05g054860 | N |
| SlCrRLK1L16 | XP_004240568.1 | 887 | 97,458.64 | 5.73 | −0.264 | Solyc06g009540 | N |
| SlCrRLK1L17 | XP_004240569.1 | 880 | 97,075.96 | 6.33 | −0.246 | Solyc06g009550 | Y |
| SlCrRLK1L18 | XP_004243035.1 | 854 | 94,945.25 | 6.36 | −0.259 | Solyc07g008400 | N |
| SlCrRLK1L19 | XP_004246699.1 | 928 | 102,646.36 | 5.82 | −0.212 | Solyc09g007280 | N |
| SlCrRLK1L20 | XP_004246282.1 | 889 | 97,348.77 | 5.78 | −0.22 | Solyc09g015830 | Y |
| SlCrRLK1L21 | XP_004247083.1 | 904 | 101,257.01 | 5.48 | −0.107 | Solyc09g060110 | N |
| SlCrRLK1L22 | XP_010327142.1 | 840 | 91,947.64 | 5.26 | 0.032 | Solyc10g006870 | N |
| SlCrRLK1L23 | XP_004248695.3 | 868 | 96,419.44 | 6.09 | −0.221 | Solyc10g054050 | Y |
| SlCrRLK1L24 | XP_004251295.1 | 836 | 91,944.54 | 5.84 | −0.1 | Solyc11g072910 | Y |
The putative tomato CrRLK1Ls are listed in Table 1. The protein length, molecular weight (MW), theoretical isoelectric point (pI), and grand average of hydropathicity (GRAVY) were analyzed. As shown in Table 1, the protein length ranged from 811 to 1340 aa, the MW ranged from 90,461.22 to 150,300.63 Da, the theoretical pI ranged from 5.26 to 8.92, and the GRAVY ranged from −0.312 to 0.032.
Figure 2.
The accuracy of the tomato CrRLK1L annotations in NCBI was higher than that in SGN. (a) The different versions of the gene structure and protein domain of SlCrRLK1L20 in the NCBI and SGN databases. The data were extracted from NCBI and SGN and then analyzed for visualization; detailed information can be found in Table S2. (b) Polypeptide antigen location in SlCrRLK1L20NCBI and SlCrRLK1L20SGN. (c) Amplification of the SlCrRLK1L20NCBI and SlCrRLK1L20SGN CDSs. (d) Western blot analysis of SlCrRLK1L20NCBI and SlCrRLK1L20SGN. 1: N. benthamiana leaves transiently expressing CaMV35S::SlCrRLK1L20NCBI-HA; 2: N. benthamiana leaves transiently expressing CaMV35S::SlCrRLK1L20SGN-HA; 3: S. lycopersicum fruit. The uncropped Western blot gel image can be found in Figure S2. (e) Confocal analysis of SlCrRLK1L20NCBI and SlCrRLK1L20SGN subcellular localization. Bars = 50 μm.
2.2. Phylogenetic Analysis of the Tomato CrRLK1L Protein Kinases
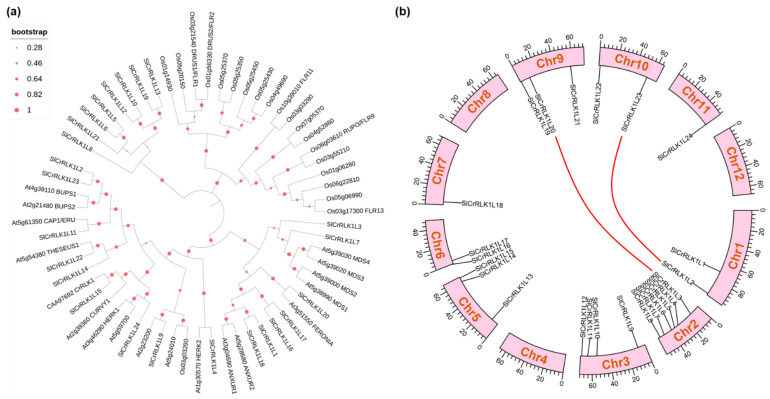
In order to explore the relationship between Arabidopsis, rice, Madagascar periwinkle, and tomato CrRLK1L protein kinases, a phylogenetic analysis using the whole CrRLK1L amino acid from the above species was conducted (Figure 3a). The results revealed that most of the tomato SlCrRLK1Ls had several homologous Arabidopsis members but only one homologous member in rice, indicating that the evolution in different species is independent. Arabidopsis and tomato had a much closer evolutionary relationship than rice (Figure 3a). Several CrRLK1L proteins, including AtFERONIA, AtCAP1/ERU, AtCURVY1, AtTHESEUS1, AtANXUR1/2, AtBUPS1/2, and AtHERK1, have been well characterized in Arabidopsis. In tomato, one copy of SlFERONIA (SlCrRLK1L20), SlCAP1/ERU (SlCrRLK1L11), and SlCURVY1 (SlRLK1L15) and two copies of SlTHESEUS1 (SlCrRLK1L22/SlCrRLK1L14), SlANXUR1/2 (SlCrRLK1L1/SlCrRLK1L18), SlBUPS1/2 (SlCrRLK1L2/SlCrRLK1L23), and SlHERK1/2 (SlCrRLK1L24/SlCrRLK1L4), were identified.
Figure 3.
Evolutionary analysis of SlCrRLK1Ls. (a) Phylogenetic analysis of CrRLK1Ls in tomato, Arabidopsis, rice, and Madagascar periwinkle. (b) Chromosome position and collinearity of SlCrRLK1Ls. The collinearity relationship is marked by red lines. Unit: Mb.
2.3. Tomato CrRLK1L Gene Locations and Duplication on Tomato Chromosome
To better understand the relationship between tomato CrRLK1L genes, the chromosomal distribution and collinearity of these genes were analyzed by TBtools. The results were as follows: The tomato CrRLK1L genes were distributed on chromosomes 1 to 3, 5 to 7, and 9 to 11, and not distributed on chromosomes 4, 8, and 12 (Figure 3b). Chromosome 2 had the largest number of SlCrRLK1L genes, and chromosomes 7 and 11 had only one SlCrRLK1L gene (Figure 3b). Segmental duplication played an important role in the gene family expansion. During this study, one-step MCScanX was used to reveal the collinearity of the SlCrRLK1L genes. As shown in Figure 3b, there was a collinearity relationship between SlCrRLK1L2, SlCrRLK1L23, SlCrRLK1L3, and SlCrRLK1L20, which showed duplication events of these genes.
2.4. Tomato CrRLK1L Protein Domain and Gene Structure
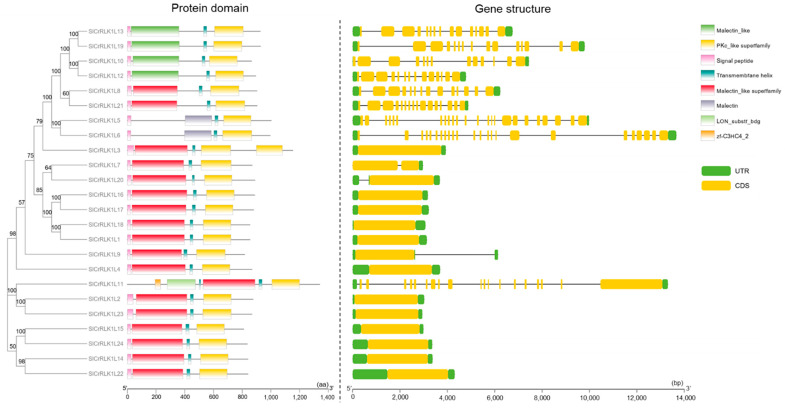
In order to further confirm the SlCrRLK1L proteins, conserved domain detection was carried out. All of the sequences were submitted to the NCBI Batch CD-Search to search for common domains. As a result, the malectin domain, the malectin-like domain, and the PKc-like domain were verified (Figure 4). At the same time, DeepTMHMM (https://dtu.biolib.com/DeepTMHMM) (accessed on 3 September 2022) was used to detect the signal peptide and transmembrane helix of SlCrRLK1Ls. As shown in Figure 4, Tables S3 and S4, all of the SlCrRLK1L proteins held one signal peptide and one transmembrane helix, except for SlCrRLK1L11. SlCrRLK1L11 had two transmembrane helices and no signal peptides.
Figure 4.
Protein domains and gene structures of SlCrRLK1Ls. The signal peptides, malectin-like domains, transmembrane helices, kinase domains, UTRs, and CDSs are marked in different colors.
As for the gene structure, 13 out of the 24 SlCrRLK1L genes possessed continuous CDSs, and 11 SlCrRLK1L genes had no introns (Figure 4). A total of 4 of the SlCrRLK1L members (SlCrRLK1L1, 7, 9, and 20) had only one intron, while the other 9 members (SlCrRLK1L5, 6, 8, 10, 11, 12, 13, 19, and 21) had multiple introns.
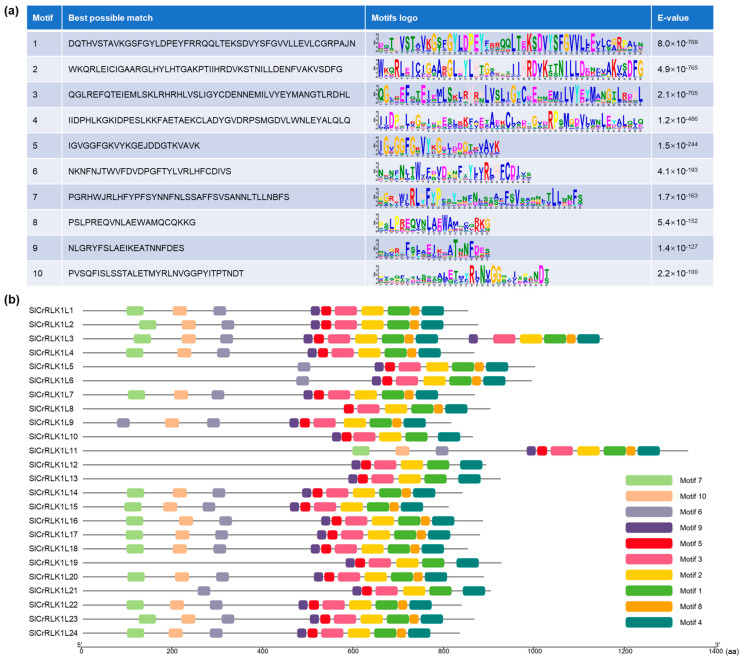
2.5. Prediction of SlCrRLK1L Conserved Protein Motifs
The SlCrRLK1L conserved protein motifs were analyzed by MEME (https://meme-suite.org/meme/tools/meme) (accessed on 1 September 2022). In total, ten conserved motifs were acquired (Figure 5, motif one to ten); the amino acid numbers ranged from 21 to 50. Among them, motifs one to five could be found in all of the 24 members, while motif seven could only be found in 14 members (Figure 5). The similarities in the characteristic motifs between the SlCrRLK1L proteins may reflect functional similarities.
Figure 5.
Predicted conserved motifs of SlCrRLK1Ls. (a) The best possible match sequences and motif logos are listed here. (b) Motif positions on the SlCrRLK1Ls.
2.6. Subcellular Localization
Previous studies have found that most CrRLK1L proteins are localized in the plasma membrane. In this study, three assays were used to predict the subcellular localization of the SlCrRLK1L proteins. As shown in Table S3, almost all of the SlCrRLK1L proteins were predicted to localize in the plasma membrane, which was consistent with our SlCrRLK1L20 subcellular localization results. Meanwhile, the results obtained by different prediction methods were also different. The CELLO and MultiLoc2 shared most of their results, while the Plant-mPLoc Computation did not, owing to their various predicted algorithms. In addition, the signal peptide and transmembrane helix predictions of the SlCrRLK1L proteins further demonstrated the membrane localization of these proteins.
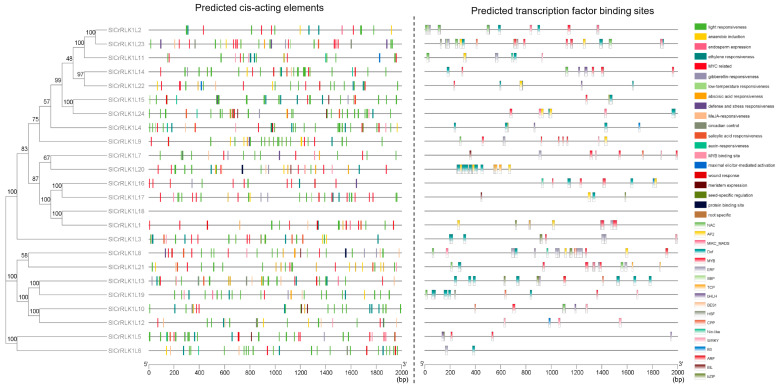
2.7. SlCrRLK1L Gene Promoter Analysis
To better explore the putative functions in tomatoes, the SlCrRLK1L promoters were analyzed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 3 September 2022) and PlantTFDB (http://planttfdb.gao-lab.org/index.php) (accessed on 5 September 2022). The PlantCARE tool was used to detect the predicted cis-acting elements. As a result, 709 cis-acting elements were predicted in the SlCrRLK1L promoters, which were divided into 20 featured categories (Figure 6; Table S5). The predicted cis-acting elements were mainly related to light, low temperature, ethylene, gibberellin, abscisic acid (ABA), methyl jasmonate (MeJA), salicylic acid (SA), auxin, and wound responsiveness, suggesting that SlCrRLK1L may participate in hormone, stress, and defense responses. In addition, PlantTFDB was selected to predict transcription factor binding sites. As shown in Figure 6 and Table S6, 712 binding sites were identified in the SlCrRLK1L promoters; the represented sites were visualized and belonged to various types of transcription factors. Among them, NAC, AP2, MIKC-MADS, Dof, and MYB were the most abundant. However, there was no available data on the SlCrRLK1L18 promoter because of incomplete sequencing or annotation of the genome.
Figure 6.
Predicted cis-acting elements and transcription factor binding sites of SlCrRLK1L promotors. The regions are represented by colored rectangles.
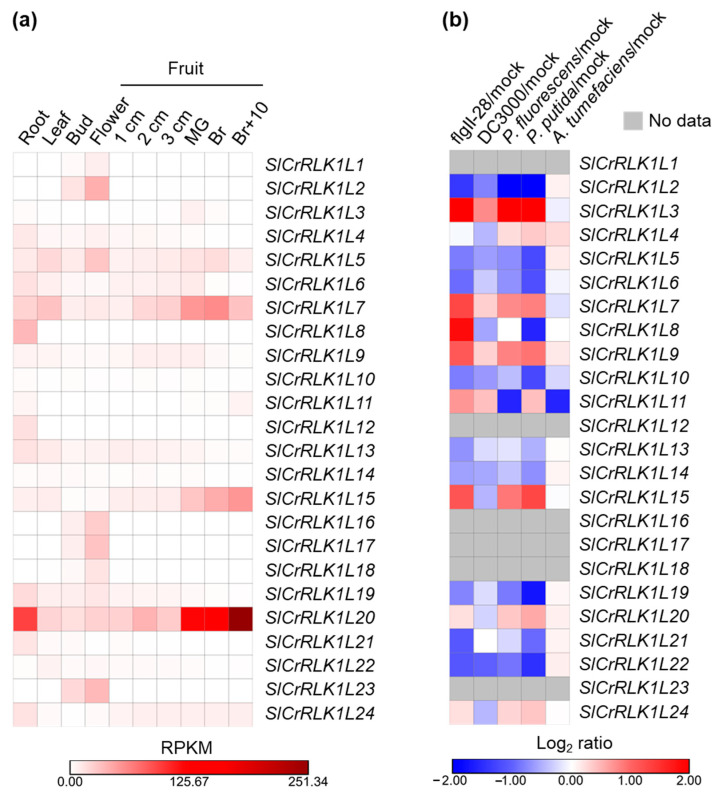
2.8. SlCrRLK1L Gene Expression Pattern Analysis
It has been experimentally demonstrated that the CrRLK1L genes have tissue-specific expression patterns in Arabidopsis, tobacco, and apple [34,39,40]. To study the tissue-specific expression in tomato CrRLK1L genes, the expression profiles of all of the 24 SlCrRLK1L genes were examined in ten samples (root, leaf, bud, flower, from 1 cm to 3 cm of fruit, mature green fruit, breaker fruit, and breaker plus a 10-day fruit). The original RNA-seq data was extracted from the SGN tomato functional genomic database (SGN-TFGD, http://ted.bti.cornell.edu/cgi-bin/TFGD/digital/home.cgi) (accessed on 4 September 2022) [36,41]. As shown in Figure 7a, SlCrRLK1L20 was dominantly expressed in all ten samples, especially in the roots and fruits. SlCrRLK1L2, SlCrRLK1L5, SlCrRLK1L16, SlCrRLK1L17, and SlCrRLK1L23 were mainly expressed in the flowers. SlCrRLK1L7 and SlCrRLK1L15 had relatively high expression levels in the fruits, and SlCrRLK1L7 held the highest expression level in the leaves as compared to the other genes. Compared with other tissues, SlCrRLK1L8 and SlCrRLK1L12 had relatively high expression levels in the roots. The other SlCrRLK1L genes held relatively low expression levels in all of the examined samples.
Figure 7.
Expression pattern analysis of SlCrRLK1Ls. (a) Heatmap of SlCrRLK1L expressions in a variety of tissues. RPKM: reads per kilobase per million mapped reads. (b) Heatmap of SlCrRLK1L expressions in various bacteria and PAMP. No available data are represented by a gray color. All of the data were acquired from the SGN RNA-seq database.
The promoter analysis of the SlCrRLK1L genes indicated that SlCrRLK1L might not only be involved in plant growth but also in defense responses. To explore this query, we calculated and compared the expression ratios of SlCrRLK1Ls treated with different bacteria and PAMP using the RNA-seq data from SGN-TFGD. The expression levels of the SlCrRLK1L genes changed with the different treatments, yet some of them possessed no available data (Figure 7b). When treated with flgII-28, a pathogen-associated molecular pattern (PAMP) founded in Pseudomonas syringae pv. tomato T1, SlCrRLK1L3, 7, 8, 9, and 15 were up-regulated and SlCrRLK1L2, 21, and 22 were down-regulated. Only SlCrRLK1L22 and SlCrRLK1L11 were significantly down-regulated by Pseudomonas syringae pv. tomato DC3000 or Agrobacterium tumefaciens infections. As for the Pseudomonas fluorescens and Pseudomonas putida treatments, SlCrRLK1L2 was significantly down-regulated by P. fluorescens and P. putida, while SlCrRLK1L3 was significantly up-regulated by these two bacteria.
3. Discussion
As an important member of the plant RLK family, CrRLK1Ls have been found in many species, including angiosperms (for example, Arabidopsis, rice, and apple), gymnosperms (Picea abies), and early diverging lineages (for example, the Closterium peracerosum-strigosumlittorale complex, Marchantia polymorpha, and Physcomitrella patens) (Table 2). However, the structural characteristics and functions of the tomato CrRLK1L gene family remain unclear. Based on this, we comprehensively analyzed the physicochemical properties, structural characteristics, and expression patterns of tomato CrRLK1Ls.
Table 2.
The identified CrRLK1L numbers in various species.
| Species | Number of CrRLK1Ls | Reference |
|---|---|---|
| Arabidopsis thaliana | 17 | [42] |
| Oryza sativa (rice) | 16 | [43] |
| Malus domestica (apple) | 74 | [40] |
| Fragaria vesca (strawberry) | 62 | [44] |
| Citrus sinensis ‘Valencia’ (Citrus) | 47 | [45] |
| Glycine max L. (soybean) | 38 | [33] |
| Nicotiana tabacum L. (tobacco) | 48 | [34] |
| Nicotiana benthamiana | 31 | [46] |
| Chenopodium quinoa | 26 | [35] |
| Pyrus bretchneideri (pear) | 26 | [32] |
| Populus trichocarpa (black cottonwood) | 42 | [47] |
| Gossypium raimondii, G. arboreum, and G. hirsutum TM-1 (cotton) | 44 | [48] |
| Boea hygrometrica | 18 | [49] |
| Solanum tuberosum (potato) | 17 | [50] |
| Solanum lycopersicum (tomato) | 24 | This study |
| Amborella trichopoda | 9 | [51] |
| Marchantia polymorpha | 1 | [52] |
| Physcomitrella patens | 6 | [53] |
| Selaginella moellendorffii | 2 | [54] |
| Picea abies | 7 | [55] |
| Closterium peracerosum-strigosumlittorale complex | 1 | [56] |
A previous study found that there were 23 CrRLK1L subfamily members in the tomato genome [37]. In this study, after sequence analysis, we used a new method to search the state-of-the-art and well-annotated tomato protein databases, and 24 SlCrRLK1Ls were re-identified. A comparison between the tomato CrRLK1L proteins in “this study” and a “previous study [37]” was also carried out. As shown in Figure S1, eight proteins were identified here for the first time. These proteins have not been identified previously, maybe due to the different analytical methods and genome annotations used. In addition, the annotations in SGN and NCBI had some differences. In short, some of the SlCrRLK1L gene structures from the SGN database lacked well-annotated UTRs and CDSs (Figure S1). To ensure the accuracy of the results, SlCrRLK1L20 was selected for further analysis. The results showed that NCBI had better annotations than SGN at this point (Figure 2). This situation is also present in other species. After the first identification, subsequent re-studies found that the number of CrRLK1Ls was different from that in previous studies of Arabidopsis and rice [27,57].
Homologous proteins often have similar functions. The phylogenetic analysis revealed that tomato CrRLK1Ls were closely related to Arabidopsis. Our homology search showed that 11 out of the 24 SlCrRLK1Ls had Arabidopsis homologs with known functions. It is speculated that these homologous genes may have evolved from a common ancestor, implying that they may have similar functions in some signaling pathways.
In Arabidopsis, rice, apple, strawberry, and soybean, CrRLK1Ls have been proven to be involved in development, fertility, environmental responses, and immunity [2]. Our cis-activating elements and transcription factor binding site analysis indicated that SlCrRLK1Ls may be involved in plant development, hormones, and environmental responses, such as auxin, ethylene, abscisic acid, wounds, light, and temperature (Figure 6), some of which were confirmed in Arabidopsis homologs, as illustrated above. Gene expressions were closely linked to their functions. During fruit ripening, SlCrRLK1L20 showed a very high abundance of expression (Figure 7), which was consistent with its function in regulating fruit ripening [30]. Moreover, the expression pattern analysis suggested that SlCrRLK1Ls may participate in the response to bacterial infections. Upon treatment, SlCrRLK1L2, 3, 8, 11, 15, 19, and 22 displayed relatively strong responses (Figure 7), indicating that these genes may be involved in plant–pathogen interactions. The functions of these SlCrRLK1L members need further exploration in the future.
In conclusion, we identified and analyzed the CrRLK1L family in tomato by bioinformatic, biochemical, and cell biology assays and provided a theoretical basis and guidance for further functional studies of these proteins.
4. Materials and Methods
4.1. Protein Identification and Phylogenetic Analysis
The genome sequence and annotations of tomatoes were downloaded from the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) (Bethesda, MD, USA, accessed on 31 August 2022) and the Sol Genomics Network (SGN, https://solgenomics.net/) (Ithaca, NY, USA, accessed on 30 August 2022) [36,58]. The Arabidopsis, rice, and Catharanthus roseus CrRLK1L protein sequences were downloaded from TAIR (https://www.arabidopsis.org/) (Newark, CA, USA, accessed on 20 April 2020) [59], EnsemblPlants (http://plants.ensembl.org/index.html) (Hinxton, United Kingdom, accessed on 20 April 2020) [60], and NCBI, according to their accession numbers. Firstly, the Arabidopsis CrRLK1L protein sequences were set as queries to Pfam (http://pfam.xfam.org/) (Hinxton, United Kingdom, accessed on 6 September 2022) [61] to identify their conserved domains. As a result, malectin-like (PF12819) and PK-Tyr-Ser-Thr (PF07714) HMM profiles were obtained and subjected to Simple HMM Search tools from TBtools v1.108 (Guangzhou, China) [62] to screen the tomato CrRLK1L protein candidates (sequence and domain scores, E-value < 0.05). The Venn image was illustrated by VENNY, version 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) (Madrid, Spain, accessed on 19 September 2022). The predicted molecular weight (MW), theoretical isoelectric point (pI), and grand average of hydropathicity (GRAVY) of the SlCrRLK1Ls were determined by ExPASy-ProtParam (https://www.expasy.org/resources/protparam) (Lausanne, Switzerland, accessed on 19 September 2022) [63].
The obtained tomato, Arabidopsis, rice, and Catharanthus roseus CrRLK1L whole protein sequences were aligned by ClustalW and submitted to MEGA, version 11 (State College, PA, USA) [64], to construct a neighbor-joining phylogenetic tree with 1000 bootstrap replicates, a pairwise deletion, and a Poisson model. Then, the tree file was optimized by iTOL, version 6 (https://itol.embl.de/) (Heidelberg, Germany, accessed on 23 September 2022) [65].
4.2. Antibody Preparation
The specific polypeptide (KDLNESPGYDASMTDSRS) was synthesized and used as an antigen for immunizing rabbits in order to prepare the anti-SlCrRLK1L20 polyclonal antibody by the Abmart (Shanghai) company (Shanghai, China).
4.3. Western Blot Assay
The total proteins were extracted as described previously [30] and separated using a 10% SDS-PAGE gel. After the electrophoresis, the proteins were transferred to a PVDF membrane. The PVDF membrane was blocked in 5% skim milk for 1 h and then incubated with anti-HA (Abmart; 1:5000) and anti-SlCrRLK1L20 (this study; 1:1000) antibodies for 1 h, respectively. The images were captured by a chemiluminescent imaging system (Tanon). SlCrRLK1L20-HA and SlCrRLK1L20 were detected with the anti-HA and anti-SlCrRLK1L20 antibodies, respectively.
4.4. Gene Location and Collinearity Analysis
The location information of the SlCrRLK1L genes on the tomato chromosomes was obtained from the NCBI database and was illustrated by Advanced Circos (TBtools v1.108) [62]. The collinearity analysis of the SlCrRLK1L genes was conducted using the one-step MCScanX from TBtools with the default parameters [62].
4.5. Subcellular Localization Analysis
The SlCrRLK1L protein sequences were submitted to the Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) (Shanghai, China, accessed on 4 September 2022), CELLO, version 2.5, (http://cello.life.nctu.edu.tw/) (accessed on 4 September 2022) and MultiLoc2 (https://abi-services.informatik.uni-tuebingen.de/multiloc2/webloc.cgi) (Tübingen, Germany, accessed on 4 September 2022) webtools to predict their possible subcellular localization using the default parameters [66,67,68]. As for the subcellular localization of SlCrRLK1L20, a confocal assay was used. The SlCrRLK1L20 CDS was amplified by PCR and then inserted into pCAMBIA2300-GFP vectors. The recombinant plasmids were transferred into the Agrobacterium tumefaciens strain GV3101 and then infiltrated into the epidermal cells of Nicotiana benthamiana. The leaves were observed at 48 h post-infiltration by a laser scanning confocal microscope.
4.6. Protein Domain and Gene Structure Analyses
For the protein domain analyses, the SlCrRLK1L protein sequences were submitted to the NCBI Batch CD-Search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi?) (Bethesda, MD, USA, accessed on 3 September 2022) [69] and processed using the default parameters, and the results (E-value < 1 × 10−10) were then obtained. The signal peptide and transmembrane helix regions were predicted by DeepTMHMM (https://dtu.biolib.com/DeepTMHMM) (Copenhagen, Denmark, accessed on 3 September 2022) [70] using the default parameters. The detailed protein domain data are listed in Table S4. For the gene structure analyses, the SlCrRLK1L gene annotation files were obtained from NCBI and subjected to the Visualize Gene Structure tools from TBtools for visualization.
4.7. Conserved Protein Motif Analysis
The SlCrRLK1L protein sequences were submitted to the MEME suite 5.5.0 webtool (https://meme-suite.org/meme/tools/meme) (San Diego, CA, USA, accessed on 1 September 2022) [71] and processed using the default parameters, and the result file was visualized using the Visualize MEME/MAST Motif Pattern (TBtools v1.108).
4.8. Promoter Analysis
The 2000 bp region upstream of the SlCrRLK1L CDS start sites was obtained from the tomato genome using GXF Sequence Extract (TBtools) and then submitted to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Gent, Belgium, accessed on 3 September 2022) [72] and PlantTFDB, version 5.0 (http://planttfdb.gao-lab.org/index.php) (Beijing, China, accessed on 5 September 2022) [73], to identify the cis-acting elements and transcription factor binding sites using the default parameters. The results are listed in Tables S5 and S6 and visualized using the Simple BioSequence Viewer (TBtools v1.108).
4.9. Gene Expression Pattern Analysis
The SlCrRLK1L gene expression pattern analysis used RNA-seq data from the SGN tomato functional genomic database (SGN-TFGD, http://ted.bti.cornell.edu/cgi-bin/TFGD/digital/home.cgi) (Ithaca, NY, USA, accessed on 4 September 2022) [36,41]. For the expression pattern of the bacteria and the PAMP treatment, the expression data ratio was calculated and transformed with log2 to normalize. The data are listed in Tables S7 and S8. Morpheus (https://software.broadinstitute.org/morpheus/) (Cambridge, MA, USA, accessed on 4 September 2022) was adopted to illustrate the heatmap.
4.10. Accession Number
The detailed accession number can be found in Table S9.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24043142/s1.
Author Contributions
Conceptualization, D.J.; formal analysis, W.M. and D.J.; investigation, D.J. and W.M.; writing—original draft preparation, D.J. and W.M.; writing—review and editing, W.M. and D.J.; visualization, D.J. and W.M.; supervision, X.L., K.C. and X.Y.; project administration, D.J.; funding acquisition, D.J. and W.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Shandong University of Technology Doctoral Research Startup Fund, grant number 4041/421073, 4041/422015, and the Shandong Provincial Natural Science Foundation, China, grant number ZR2022QC256.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ji D., Chen T., Zhang Z., Li B., Tian S. Versatile Roles of the Receptor-like Kinase Feronia in Plant Growth, Development and Host-Pathogen Interaction. Int. J. Mol. Sci. 2020;21:7881. doi: 10.3390/ijms21217881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu S., Fu Q., Xu F., Zheng H., Yu F. New Paradigms in Cell Adaptation: Decades of Discoveries on the CrRLK1L Receptor Kinase Signalling Network. New Phytol. 2021;232:1168–1183. doi: 10.1111/nph.17683. [DOI] [PubMed] [Google Scholar]
- 3.Dievart A., Gottin C., Périn C., Ranwez V., Chantret N. Origin and Diversity of Plant Receptor-like Kinases. Annu. Rev. Plant Biol. 2020;71:131–156. doi: 10.1146/annurev-arplant-073019-025927. [DOI] [PubMed] [Google Scholar]
- 4.Schulze-Muth P., Irmler S., Schröder G., Schröder J. Novel Type of Receptor-like Protein Kinase from a Higher Plant (Catharanthus Roseus) J. Biol. Chem. 1996;271:26684–26689. doi: 10.1074/jbc.271.43.26684. [DOI] [PubMed] [Google Scholar]
- 5.Franck C.M., Westermann J., Boisson-Dernier A. Plant Malectin-like Receptor Kinases: From Cell Wall Integrity to Immunity and Beyond. Annu. Rev. Plant. Biol. 2018;69:301–328. doi: 10.1146/annurev-arplant-042817-040557. [DOI] [PubMed] [Google Scholar]
- 6.Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. FERONIA Receptor-like Kinase Regulates RHO GTPase Signaling of Root Hair Development. Proc. Natl. Acad. Sci. USA. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T., Wang L., Li C., Liu Y., Zhu S., Qi Y., Liu X., Lin Q., Luan S., Yu F. Receptor Protein Kinase FERONIA Controls Leaf Starch Accumulation by Interacting with Glyceraldehyde-3-Phosphate Dehydrogenase. Biochem. Biophys. Res. Commun. 2015;465:77–82. doi: 10.1016/j.bbrc.2015.07.132. [DOI] [PubMed] [Google Scholar]
- 9.Li C., Wu H.M., Cheung A.Y. FERONIA and Her Pals: Functions and Mechanisms. Plant Physiol. 2016;171:2379–2392. doi: 10.1104/pp.16.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C. The Receptor Kinase FER Is a RALF-Regulated Scaffold Controlling Plant Immune Signaling. Science. 2017;355:287–289. doi: 10.1126/science.aal2541. [DOI] [PubMed] [Google Scholar]
- 11.Ge Z., Bergonci T., Zhao Y., Zou Y., Du S., Liu M., Luo X., Ruan H., García-Valencia L.E., Zhong S., et al. Arabidopsis Pollen Tube Integrity and Sperm Release Are Regulated by RALF-Mediated Signaling. Science. 2017;358:1596–1600. doi: 10.1126/science.aao3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huck N., Moore J.M., Federer M., Grossniklaus U. The Arabidopsis Mutant Feronia Disrupts the Female Gametophytic Control of Pollen Tube Reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Yang Z., Wu D., Yu F. RALF–FERONIA Signaling: Linking Plant Immune Response with Cell Growth. Plant Commun. 2020;1:100084. doi: 10.1016/j.xplc.2020.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deslauriers S.D., Larsen P.B. FERONIA Is a Key Modulator of Brassinosteroid and Ethylene Responsiveness in Arabidopsis Hypocotyls. Mol. Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 15.Mao D., Yu F., Li J., Van de Poel B., Tan D., Li J., Liu Y., Li X., Dong M., Chen L., et al. FERONIA Receptor Kinase Interacts with S-Adenosylmethionine Synthetase and Suppresses S-Adenosylmethionine Production and Ethylene Biosynthesis in Arabidopsis. Plant Cell Environ. 2015;38:2566–2574. doi: 10.1111/pce.12570. [DOI] [PubMed] [Google Scholar]
- 16.Chen J., Yu F., Liu Y., Du C., Li X., Zhu S., Wang X., Lan W., Rodriguez P.L., Liu X., et al. FERONIA Interacts with ABI2-Type Phosphatases to Facilitate Signaling Cross-Talk between Abscisic Acid and RALF Peptide in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2016;113:E5519–E5527. doi: 10.1073/pnas.1608449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H., Nolan T.M., Song G., Liu S., Xie Z., Chen J., Schnable P.S., Walley J.W., Yin Y. FERONIA Receptor Kinase Contributes to Plant Immunity by Suppressing Jasmonic Acid Signaling in Arabidopsis thaliana. Curr. Biol. 2018;28:3316–3324. doi: 10.1016/j.cub.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y., Chakravorty D., Assmann S.M. The G Protein β-Subunit, AGB1, Interacts with FERONIA in RALF1-Regulated Stomatal Movement. Plant Physiol. 2018;176:2426–2440. doi: 10.1104/pp.17.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Z., Dresselhaus T., Qu L.J. How CrRLK1L Receptor Complexes Perceive RALF Signals. Trends Plant Sci. 2019;24:978–981. doi: 10.1016/j.tplants.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Y., Stegmann M., Han Z., DeFalco T.A., Parys K., Xu L., Belkhadir Y., Zipfel C., Chai J. Mechanisms of RALF Peptide Perception by a Heterotypic Receptor Complex. Nature. 2019;572:270–274. doi: 10.1038/s41586-019-1409-7. [DOI] [PubMed] [Google Scholar]
- 21.Hématy K., Sado P.-E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.-P., Höfte H. A Receptor-like Kinase Mediates the Response of Arabidopsis Cells to the Inhibition of Cellulose Synthesis. Curr. Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Guo H., Li L., Ye H., Yu X., Algreen A., Yin Y. Three Related Receptor-like Kinases Are Required for Optimal Cell Elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki S., Murata T., Sakurai-Ozato N., Kubo M., Demura T., Fukuda H., Hasebe M. ANXUR1 and 2, Sister Genes to FERONIA/SIRENE, Are Male Factors for Coordinated Fertilization. Curr. Biol. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Richter J., Watson J.M., Stasnik P., Borowska M., Neuhold J., Berger M., Stolt-Bergner P., Schoft V., Hauser M.T. Multiplex Mutagenesis of Four Clustered CrRLK1L with CRISPR/Cas9 Exposes Their Growth Regulatory Roles in Response to Metal Ions. Sci. Rep. 2018;8:12182. doi: 10.1038/s41598-018-30711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler S.A., Shimosato-Asano H., Keinath N.F., Wuest S.E., Ingram G., Panstruga R., Grossniklaus U. Conserved Molecular Components for Pollen Tube Reception and Fungal Invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 26.Li C., Wang L., Cui Y., He L., Qi Y., Zhang J., Lin J., Liao H., Lin Q., Yang T., et al. Two FERONIA-like Receptor (FLR) Genes Are Required to Maintain Architecture, Fertility, and Seed Yield in Rice. Mol. Breeding. 2016;36:151. doi: 10.1007/s11032-016-0580-x. [DOI] [Google Scholar]
- 27.Pu C.X., Han Y.F., Zhu S., Song F.Y., Zhao Y., Wang C., Zhang Y., Yang Q., Wang J., Bu S.-L., et al. The Rice Receptor-like Kinases DWARF AND RUNTISH SPIKELET1 and 2 Repress Cell Death and Affect Sugar Utilization during Reproductive Development. Plant Cell. 2017;29:70–89. doi: 10.1105/tpc.16.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L., Zheng C., Kuang B., Wei L., Yan L., Wang T. Receptor-like Kinase RUPO Interacts with Potassium Transporters to Regulate Pollen Tube Growth and Integrity in Rice. PLoS Genet. 2016;12:e1006085. doi: 10.1371/journal.pgen.1006085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia M., Du P., Ding N., Zhang Q., Xing S., Wei L., Zhao Y., Mao W., Li J., Li B., et al. Two FERONIA-like Receptor Kinases Regulate Apple Fruit Ripening by Modulating Ethylene Production. Front. Plant Sci. 2017;8:1406. doi: 10.3389/fpls.2017.01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji D., Cui X., Qin G., Chen T., Tian S. SlFERL Interacts with S-Adenosylmethionine Synthetase to Regulate Fruit Ripening. Plant Physiol. 2020;184:2168–2181. doi: 10.1104/pp.20.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Liang X., Bao Y., Yang S., Zhang X., Yu H., Zhang Q., Xu G., Feng X., Dou D. A Malectin-like Receptor Kinase Regulates Cell Death and Pattern-triggered Immunity in Soybean. EMBO Rep. 2020;21:e50442. doi: 10.15252/embr.202050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kou X., Qi K., Qiao X., Yin H., Liu X., Zhang S., Wu J. Evolution, Expression Analysis, and Functional Verification of Catharanthus Roseus RLK1-like Kinase (CrRLK1L) Family Proteins in Pear (Pyrus Bretchneideri) Genomics. 2017;109:290–301. doi: 10.1016/j.ygeno.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z.-Q., Yu T.-F., Sun G.-Z., Zheng J.-C., Chen J., Zhou Y.-B., Chen M., Ma Y.-Z., Wei W.-L., Xu Z.-S. Genome-Wide Analysis of the Catharanthus Roseus RLK1-Like in Soybean and GmCrRLK1L20 Responds to Drought and Salt Stresses. Front. Plant Sci. 2021;12:614909. doi: 10.3389/fpls.2021.614909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Guo C., Wang Q., Li Z., Cai J., Wu D., Li Y., Yang A., Guo Y., Gao J., et al. Systematic Analysis of Tobacco CrRLK1L Family Genes and Functional Identification of NtCrRLK1L47 in Environmental Stresses. Front. Plant Sci. 2022;13:838857. doi: 10.3389/fpls.2022.838857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang W., Li C., Li L., Li Y., Wang Z., Yu F., Yi F., Zhang J., Zhu J.-K., Zhang H., et al. Genome-Wide Analysis of CqCrRLK1L and CqRALF Gene Families in Chenopodium Quinoa and Their Roles in Salt Stress Response. Front. Plant Sci. 2022;13:918594. doi: 10.3389/fpls.2022.918594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomato Genome Consortium The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature. 2012;485:635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakamoto T., Deguchi M., Brustolini O.J.B., Santos A.A., Silva F.F., Fontes E.P.B. The Tomato RLK Superfamily: Phylogeny and Functional Predictions about the Role of the LRRII-RLK Subfamily in Antiviral Defense. BMC Plant Biol. 2012;12:229. doi: 10.1186/1471-2229-12-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J., Qin G., Chen T., Tian S. The Mode of Action of Remorin1 in Regulating Fruit Ripening at Transcriptional and Post-Transcriptional Levels. New Phytol. 2018;219:1406–1420. doi: 10.1111/nph.15264. [DOI] [PubMed] [Google Scholar]
- 39.Boisson-Dernier A., Roy S., Kritsas K., Grobei M.A., Jaciubek M., Schroeder J.I., Grossniklaus U. Disruption of the Pollen-Expressed FERONIA Homologs ANXUR1 and ANXUR2 Triggers Pollen Tube Discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo C., Zhang W., Ma Z., Chu M., Mao J., An Z., Chen B. Genome-Wide Identification and Expression Analysis of the CrRLK1L Gene Family in Apple (Malus Domestica) Plant Mol. Biol. Rep. 2018;36:844–857. doi: 10.1007/s11105-018-1125-8. [DOI] [Google Scholar]
- 41.Rosli H.G., Zheng Y., Pombo M.A., Zhong S., Bombarely A., Fei Z., Collmer A., Martin G.B. Transcriptomics-Based Screen for Genes Induced by Flagellin and Repressed by Pathogen Effectors Identifies a Cell Wall-Associated Kinase Involved in Plant Immunity. Genome Biol. 2013;14:R139. doi: 10.1186/gb-2013-14-12-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindner H., Müller L.M., Boisson-Dernier A., Grossniklaus U. CrRLK1L Receptor-like Kinases: Not Just Another Brick in the Wall. Curr. Opin. Plant Biol. 2012;15:659–669. doi: 10.1016/j.pbi.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen Q.-N., Lee Y.-S., Cho L.-H., Jeong H.-J., An G., Jung K.-H. Genome-Wide Identification and Analysis of Catharanthus Roseus RLK1-like Kinases in Rice. Planta. 2015;241:603–613. doi: 10.1007/s00425-014-2203-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q., Jia M., Xing Y., Qin L., Li B., Jia W. Genome-Wide Identification and Expression Analysis of MRLK Family Genes Associated with Strawberry (Fragaria Vesca) Fruit Ripening and Abiotic Stress Responses. PLoS ONE. 2016;11:e0163647. doi: 10.1371/journal.pone.0163647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo C., Sun Q., Zhang F., Zhang D., Liu C., Wu Q., Shu B. Genome-Wide Identification and Expression Analysis of the Citrus Malectin Domain-Containing Receptor-like Kinases in Response to Arbuscular Mycorrhizal Fungi Colonization and Drought. Hortic. Environ. Biotechnol. 2020;61:891–901. doi: 10.1007/s13580-020-00273-3. [DOI] [Google Scholar]
- 46.Rao S., Wu X., Zheng H., Lu Y., Peng J., Wu G., Chen J., Yan F. Genome-Wide Identification and Analysis of Catharanthus Roseus RLK1-like Kinases in Nicotiana benthamiana. BMC Plant Biol. 2021;21:425. doi: 10.1186/s12870-021-03208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V., Donev E.N., Barbut F.R., Kushwah S., Mannapperuma C., Urbancsok J., Mellerowicz E.J. Genome-Wide Identification of Populus Malectin/Malectin-like Domain-Containing Proteins and Expression Analyses Reveal Novel Candidates for Signaling and Regulation of Wood Development. Front. Plant Sci. 2020;11:588846. doi: 10.3389/fpls.2020.588846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niu E., Cai C., Zheng Y., Shang X., Fang L., Guo W. Genome-Wide Analysis of CrRLK1L Gene Family in Gossypium and Identification of Candidate CrRLK1L Genes Related to Fiber Development. Mol. Genet. Genom. 2016;291:1137–1154. doi: 10.1007/s00438-016-1169-0. [DOI] [PubMed] [Google Scholar]
- 49.Tang L., Wang Y., Wang W., Deng X., Wang X. Genome-Wide Identification of CrRLK1L Gene Family and Desiccation-Induced Expression Profiles in Boea hygrometrica. Curr. Plant Biol. 2022;31:100256. doi: 10.1016/j.cpb.2022.100256. [DOI] [Google Scholar]
- 50.Yu H.-F., Zhang W.-N., Kang Y.-C., Fan Y.-L., Yang X.-Y., Shi M.-F., Zhang R.-Y., Zhang J.-L., Qin S.-H. Genome-Wide Identification and Expression Patterns in Response to Signals from Phytophthora Infestans of CrRLK1Ls Gene Family in Potato. Acta Agronom. Sin. 2021;48:249–258. doi: 10.3724/SP.J.1006.2022.14019. [DOI] [Google Scholar]
- 51.Amborella Genome Project. Albert V.A., Barbazuk W.B., de Pamphilis C.W., Der J.P., Leebens-Mack J., Ma H., Palmer J.D., Rounsley S., Sankoff D., et al. The Amborella Genome and the Evolution of Flowering Plants. Science. 2013;342:1241089. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki G., Katoh K., Hirose N., Suga H., Kuma K., Miyata T., Su Z.-H. Multiple Receptor-like Kinase CDNAs from Liverwort Marchantia Polymorpha and Two Charophycean Green Algae, Closterium Ehrenbergii and Nitella Axillaris: Extensive Gene Duplications and Gene Shufflings in the Early Evolution of Streptophytes. Gene. 2007;401:135–144. doi: 10.1016/j.gene.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Rensing S.A., Lang D., Zimmer A.D., Terry A., Salamov A., Shapiro H., Nishiyama T., Perroud P.-F., Lindquist E.A., Kamisugi Y., et al. The Physcomitrella Genome Reveals Evolutionary Insights into the Conquest of Land by Plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 54.Banks J.A., Nishiyama T., Hasebe M., Bowman J.L., Gribskov M., de Pamphilis C., Albert V.A., Aono N., Aoyama T., Ambrose B.A., et al. The Selaginella Genome Identifies Genetic Changes Associated with the Evolution of Vascular Plants. Science. 2011;332:960–963. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nystedt B., Street N.R., Wetterbom A., Zuccolo A., Lin Y.-C., Scofield D.G., Vezzi F., Delhomme N., Giacomello S., Alexeyenko A., et al. The Norway Spruce Genome Sequence and Conifer Genome Evolution. Nature. 2013;497:579–584. doi: 10.1038/nature12211. [DOI] [PubMed] [Google Scholar]
- 56.Hirano N., Marukawa Y., Abe J., Hashiba S., Ichikawa M., Tanabe Y., Ito M., Nishii I., Tsuchikane Y., Sekimoto H. A Receptor-like Kinase, Related to Cell Wall Sensor of Higher Plants, Is Required for Sexual Reproduction in the Unicellular Charophycean Alga, Closterium Peracerosum–Strigosum–Littorale Complex. Plant Cell Physiol. 2015;56:1456–1462. doi: 10.1093/pcp/pcv065. [DOI] [PubMed] [Google Scholar]
- 57.Jing X.-Q., Shalmani A., Zhou M.-R., Shi P.-T., Muhammad I., Shi Y., Sharif R., Li W.-Q., Liu W.-T., Chen K.-M. Genome-Wide Identification of Malectin/Malectin-like Domain Containing Protein Family Genes in Rice and Their Expression Regulation under Various Hormones, Abiotic Stresses, and Heavy Metal Treatments. J. Plant Growth Regul. 2020;39:492–506. doi: 10.1007/s00344-019-09997-8. [DOI] [Google Scholar]
- 58.Fernandez-Pozo N., Menda N., Edwards J.D., Saha S., Tecle I.Y., Strickler S.R., Bombarely A., Fisher-York T., Pujar A., Foerster H., et al. The Sol Genomics Network (SGN)—From Genotype to Phenotype to Breeding. Nucleic Acids Res. 2015;43:D1036–D1041. doi: 10.1093/nar/gku1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berardini T.Z., Reiser L., Li D., Mezheritsky Y., Muller R., Strait E., Huala E. The Arabidopsis Information Resource: Making and Mining the “Gold Standard” Annotated Reference Plant Genome: Tair: Making and Mining the “Gold Standard” Plant Genome. Genesis. 2015;53:474–485. doi: 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yates A.D., Allen J., Amode R.M., Azov A.G., Barba M., Becerra A., Bhai J., Campbell L.I., Carbajo Martinez M., Chakiachvili M., et al. Ensembl Genomes 2022: An Expanding Genome Resource for Non-Vertebrates. Nucleic Acids Res. 2022;50:D996–D1003. doi: 10.1093/nar/gkab1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J., et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Wilkins M.R., Gasteiger E., Bairoch A., Sanchez J.-C., Williams K.L., Appel R.D., Hochstrasser D.F. 2-D Proteome Analysis Protocols. Volume 112. Humana Press; Totowa, NJ, USA: 1998. Protein Identification and Analysis Tools in the ExPASy Server; pp. 531–552. [DOI] [PubMed] [Google Scholar]
- 64.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Letunic I., Bork P. Interactive Tree of Life (ITOL): An Online Tool for Phylogenetic Tree Display and Annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 66.Yu C.-S., Chen Y.-C., Lu C.-H., Hwang J.-K. Prediction of Protein Subcellular Localization. Proteins. 2006;64:643–651. doi: 10.1002/prot.21018. [DOI] [PubMed] [Google Scholar]
- 67.Blum T., Briesemeister S., Kohlbacher O. MultiLoc2: Integrating Phylogeny and Gene Ontology Terms Improves Subcellular Protein Localization Prediction. BMC Bioinform. 2009;10:274. doi: 10.1186/1471-2105-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chou K.-C., Shen H.-B. Plant-MPLoc: A Top-down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE. 2010;5:e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S., et al. CDD/SPARCLE: The Conserved Domain Database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hallgren J., Tsirigos K.D., Pedersen M.D., Almagro Armenteros J.J., Marcatili P., Nielsen H., Krogh A., Winther O. DeepTMHMM Predicts Alpha and Beta Transmembrane Proteins Using Deep Neural Networks. bioRxiv. 2022 doi: 10.1101/2022.04.08.487609. [DOI] [Google Scholar]
- 71.Bailey T.L., Elkan C. Fitting a Mixture Model by Expectation Maximization to Discover Motifs in Biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994;2:28–36. [PubMed] [Google Scholar]
- 72.Lescot M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian F., Yang D.-C., Meng Y.-Q., Jin J., Gao G. PlantRegMap: Charting Functional Regulatory Maps in Plants. Nucleic Acids Res. 2019;48:gkz1020. doi: 10.1093/nar/gkz1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.