Abstract
Agastache rugosa (popularly known as Korean mint) belongs to the Lamiaceae family and comprises 22 species of perennial aromatic medicinal species native to East Asian countries, such as Korea, Taiwan, Japan, and China. A. rugosa contains many phenolic compounds that exhibit pharmacological and physiological activities, including antioxidant, anticancer, antiviral, antifungal, and antibacterial activities. The highest concentrations of rosmarinic acid and its isomers have been reported in the roots of A. rugosa. In this in vitro study, hairy roots of A. rugosa were obtained and the carbohydrates (sorbitol, mannitol, glucose, maltose, galactose, mannose, and sucrose) were evaluated to determine those that were optimal for rosmarinic acid production and hairy root growth. Antioxidant and antibacterial activities of extracts of A. rugosa were also assessed. The best carbon source for A. rugosa hairy root cultures was sucrose, considering biomass productivity (0.460 ± 0.034 mg/30 mL), rosmarinic acid production (7.656 ± 0.407 mg/g dry weight), and total phenolic content (12.714 ± 0.202 mg/g gallic acid equivalent). Antioxidant and antimicrobial activities were displayed by A. rugosa hairy roots cultured in liquid medium supplemented with 100 mM sucrose. Twenty-five bacterial strains, including multidrug-resistant bacteria and one pathogenic yeast strain, were used for antimicrobial screening of A. rugosa hairy roots. The hairy root extracts displayed antibacterial activity against Micrococcus luteus (KCTC 3063) and Bacillus cereus (KCTC 3624). The inhibition of these bacteria was greater using A. rugosa hairy roots with the highest levels of phenolic compounds cultured in the presence of sucrose, compared to hairy roots with the lowest levels of phenolic compounds cultured in the presence of fructose. Considering hairy root biomass, phenolic compound production, and antibacterial activity, sucrose is the best carbon source for A. rugosa hairy root cultures.
Keywords: Agastache rugosa, hairy root cultures, rosmarinic acid, antibacterial effect
1. Introduction
Agastache rugosa, known as the Korean mint, belongs to the Lamiaceae family found in East Asia (China, Korea, and Japan). It is a traditional medicinal and ornamental plant [1]. In particular, A. rugosa has been a traditional herbal remedy for the treatment of miasma, cholera [2,3,4,5], anorexia, and vomiting [6]. A. rugosa can be used for physiological and pharmacological treatments, including antifungal [7], antibacterial [8], anticancer [9], and antiviral activities [10]. Furthermore, Park et al. [11] reported that different tissues (flower, stem, and leaf) contained phenolic compounds (catechin, chlorogenic acid, caffeic acid, ferulic acid, rutin, and kaempferol), and that methanolic extracts of the different plant tissues displayed antibacterial activity. Desta et al. [12] described the antioxidant activities of extracts of flower, root, stem, and leaf tissues of A. rugosa, which were rich in phenolic compounds. In particular, this study reported that A. rugosa roots contain high amounts of rosmarinic acid and its isomers compared with flower, leaf, and stem tissues [12].
Rosmarinic acid is a naturally occurring phenolic carboxylic acid that is commonly found in the Lamiaceae family. The family includes herbal plants such as Rosmarinus officinalis (rosemary), A. rugosa (Korean mint), Salvia officinalis (sage), Ocimum tenuiflorum (basil), Origanum vulgare (oregano), O. majorana (marjoram), and Melissa officinalis (lemon balm) [13]. Rosmarinic acid biosynthesis begins with formation of the 4-coumaroyl-4′-hydroxyphenyllactic acid precursor, which can be biosynthesized from two amino acids (phenylalanine and tyrosine). The precursor is subsequently converted to caffeoyl-4′-hydroxy phenyllactic acid, followed by conversion to rosmarinic acid [14]. Biological properties of rosmarinic acid include antioxidant [15] and antibacterial activities [16].
Agrobacterium rhizogenes-mediated transformation of a variety of hairy roots from many plant species has resulted in genetic stability, rapid root growth, and biosynthesis of bioactive compounds. Indeed, hairy roots have been obtained from many plant sources for the production of most groups of secondary metabolites, including phenolics, alkaloids, glucosinolates, terpenoids, and others. Many previous studies have confirmed that hairy roots can be a good source of plant-derived bioactive compounds, with flavonoid (anthocyanin) accumulation in hairy roots of Fagopyrum tataricum [17], flavone accumulation in hairy roots of Scultellaria baicalensis [18], ginsenoside production in hairy roots of Platycodon grandiflorum [19], glucosinolate production in hairy roots of Nasturtium officinale [20], resveratrol in hairy roots of Arachis hypogaea [21], tropane alkaloids in hairy roots of Hyoscyamus reticulatus [22], and tanshinone in hairy roots of Salvia miltiorrhiza [23].
A supply of carbohydrates is necessary for root cultures; these sugars are used as energy sources to survive in vitro and contribute to root growth and regeneration. Furthermore, the type of carbohydrate (monosaccharides and disaccharides) reportedly influences primary and secondary metabolism in root cultures. Park et al. [24] reported the enhanced biomass productivity and production of flavones (baicalin, baicalein, and wogonin) according to the sugar types (monosaccharides and disaccharides). Vinterhalter et al. [25] demonstrated optimized root growth and phenolic and xanthone production in Gentiana dinarica hairy roots using sucrose, fructose, and glucose, respectively, as the carbohydrate source at different concentrations. Additionally, the accumulation of withanolide A was reported to depend on the carbon source (sucrose, glucose, fructose, maltose, or a combination of sugars) in hairy roots of Withania somnifera [26].
Desta et al. [12] described the presence of high levels of phenolic compounds in leaves, flowers, stems, and roots of A. rugosa. Roots contained higher concentrations of rosmarinic acid and its isomers compared with the levels in the other tissues [12]. Several studies have reported that a supply of carbon sources is required for hairy root cultures of many plant species [24,25,26]. However, there are little studies on the effect of different carbohydrates on the growth and resveratrol production in hairy roots of A. rugosa. In the present study, A. rugosa hairy root induction was performed to evaluate the potential of sustainable medicinal bioresources for the production of rosmarinic acid. The optimal conditions for sustainable hairy root growth and rosmarinic acid production were evaluated using eight different carbon sources during culture. Additionally, the antioxidant and antibacterial properties of hairy root extracts were measured to gauge the potential therapeutic value of A. rugosa hairy roots.
2. Results
2.1. Establishment of A. rugosa Hairy Root Cultures
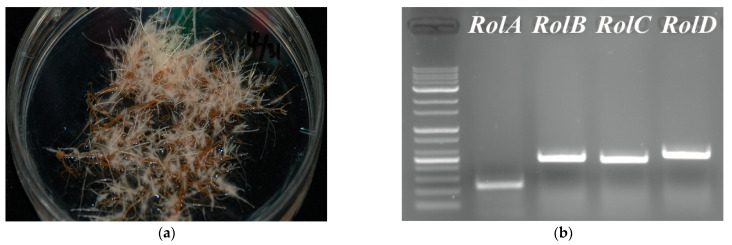
A. rhizogenes harbors a root-inducing plasmid (Ri-plasmid) with rol genes A, B, C, and D as transfer-DNA (T-DNA). Infection of host plants with A. rhizogenes introduced the T-DNA into the genome of the host plant, facilitating the induction of hairy roots from the plants. T-DNA introduction and transformation were confirmed by PCR analysis of rol genes, and transformation can be judged. The PCR analysis of rol genes and subsequent gel electrophoresis confirmed that A. rugosa hairy roots exhibited bands for the expected PCR product size, including rol A (233 bp), B (483 bp), C (464 bp), and D (525 bp) (Figure 1). The findings indicated that the induced roots were hairy roots originating from A. rugosa explants.
Figure 1.
(a) A. rhizogenes-mediated hairy root culture in A. rugosa; (b) PCR analysis of rol A (233 bp), B (483 bp), C (464 bp), and D (525 bp) genes in hairy roots of A. rugosa.
2.2. Growth Patterns of A. rugosa Hairy Root Cultures
A. rugosa hairy roots were incubated in half-strength MS broth with 100 mM of the selected carbohydrate (mannitol, sorbitol, glucose, fructose, galactose, mannose, maltose, or sucrose). The dry weight in A. rugosa hairy roots was evaluated after harvesting three biological replicates (Table 1). Maximum growth was obtained in the presence of galactose (0.462 ± 0.028 g dry weight (DW)/30 mL) and sucrose (0.460 ± 0.034 g DW/30 mL). The least growth occurred in the presence of mannitol (0.196 ± 0.015 g DW/30 mL), sorbitol (0.189 ± 0.023 g DW/30 mL), and mannose (0.193 ± 0.016 g DW/30 mL). These findings suggest that mannitol and sorbitol sugar alcohols are not suitable for the growth of A. rugosa hairy roots, while galactose, glucose, and sucrose are appropriate sugar sources for hairy root growth.
Table 1.
Effects of different carbohydrates on growth in hairy root cultures A. rugosa.
| Carbohydrates | Dry Weight (mg/30 mL) |
|---|---|
| Fructose | 0.402 ± 0.012 b 1 |
| Mannitol | 0.196 ± 0.015 d |
| Sorbitol | 0.189 ± 0.023 d |
| Glucose | 0.437 ± 0.032 ab |
| Maltose | 0.306 ± 0.021 c |
| Galactose | 0.462 ± 0.028 a |
| Mannose | 0.193 ± 0.016 d |
| Sucrose | 0.460 ± 0.034 a |
1 Means with the same letter are not significantly different at p < 0.05 using DMRT.
2.3. Rosmarinic Acid Production and Total Phenolic Contents Using Eight Different Sugar Sources
Table 2 shows the patterns of rosmarinic acid production by hairy roots in half-strength MS medium supplemented with 100 mM mannitol, sorbitol, glucose, fructose, galactose, mannose, maltose, and sucrose. Rosmarinic acid values ranged from 2.069 ± 0.081 (fructose) to 7.656 ± 0.407 mg/g DW (sucrose), a difference of 3.70 times. The highest value for sucrose was followed in order by high levels for galactose, mannose, and glucose; medium levels for maltose, mannitol, and sorbitol; and the low level for fructose. The addition of sucrose resulted in the highest level of rosmarinic acid which was 3.70 times higher than that of fructose, the lowest value. Additionally, the high levels of rosmarinic acid production were observed in hairy roots grown in galactose (6.061 ± 0.359 mg/g DW), mannose (5.448 ± 0.810 mg/g DW), and glucose (5.538 ± 1.234 mg/g DW), as well and medium the middle levels of rosmarinic acid accumulation were observed in hairy roots grown in maltose (4.682 ± 1.144 mg/g DW), mannitol (4.551 ± 0.529 mg/g DW), and sorbitol (4.474 ± 0.396 mg/g DW).The findings indicate that A. rugosa hairy root, as a sustainable bioresource, can be used for rosmarinic acid production.
Table 2.
Effects of different carbohydrates on the accumulation of rosmarinic acid in hairy root cultures of A. rugosa.
| Carbohydrates | Rosmarinic Acid (mg/g Dry Weight) |
|---|---|
| Fructose | 2.069 ± 0.081 d 1 |
| Mannitol | 4.551 ± 0.529 c |
| Sorbitol | 4.474 ± 0.396 c |
| Glucose | 5.538 ± 1.234b c |
| Maltose | 4.682 ± 1.144 c |
| Galactose | 6.061 ± 0.359 b |
| Mannose | 5.448 ± 0.810 bc |
| Sucrose | 7.656 ± 0.407 a |
1 Means with the same letter are not significantly different at p < 0.05 using DMRT.
The total phenolic content of hairy roots cultured in the different carbon sources, expressed as mg/g gallic acid equivalent (GAE), decreased in the order of sucrose, fructose, mannitol, sorbitol, glucose, maltose, galactose, and mannose (Table 3). These findings are similar to those of rosmarinic acid. The highest level of total phenolics was recorded in hairy roots cultured in sucrose, followed by galactose, glucose, mannitol, mannose, maltose, sorbitol, and fructose. The findings in Table 2 and Table 3 demonstrate that, of the tested carbohydrates, sucrose is the best for production of rosmarinic acid and total phenolics.
Table 3.
Effects of different carbohydrates on the total phenolic contents in hairy root cultures of A. rugosa.
| Carbohydrates | Total Phenolic Contents (mg/g GAE) |
|---|---|
| Fructose | 4.286 ± 0.404 e 1 |
| Mannitol | 8.738 ± 0.168 c |
| Sorbitol | 6.619 ± 0.539 d |
| Glucose | 8.810 ± 0.471 c |
| Maltose | 7.643 ± 1.717 cd |
| Galactose | 10.095 ± 0.539 b |
| Mannose | 8.238 ± 0.606 c |
| Sucrose | 12.714 ± 0.202 a |
1 Means with the same letter are not significantly different at p < 0.05 using DMRT.
2.4. In Vitro Antibacterial Properties of A. rugosa Hairy Root Cultures
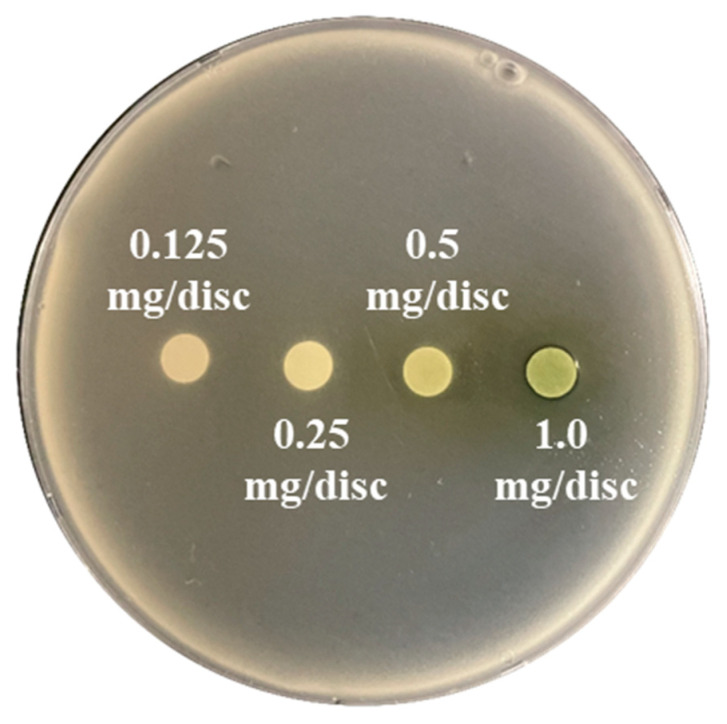
Zones of growth inhibition surrounding the paper discs containing A. rugosa hairy root extracts cultured with different concentrations of sucrose (Figure 2 displays representative results) or fructose were determined to assess in vitro antimicrobial activities (Table 4). Growth was inhibited by the methanol extracts from A. rugosa hairy roots on the agar plates containing M. luteus (KCTC 3063) and B. cereus (KCTC 3624). Growth was not inhibited on the plates containing the select multidrug-resistant bacteria, bacterial pathogens, and one opportunistic yeast (Table 4).
Figure 2.
Representative image showing antibacterial activity of A. rugosa hairy roots cultured with sucrose against B. cereus.
Table 4.
Antibacterial activity of methanol extracts of A. rugosa hairy root cultured with sucrose and fructose.
| Group | Bacterial Strains | Zone of Inhibition (mm) | |
|---|---|---|---|
| Extracts from Hairy Roots Cultured with Sucrose | Extracts from Hairy Roots Cultured with Fructose | ||
| Multidrug-resistant bacteria | P. aeruginosa (KBN-10-p01827) | – 1 | – |
| P. aeruginosa (KBN-10-p01828) | – | – | |
| P. aeruginosa (0225) | – | – | |
| P. aeruginosa (0254) | – | – | |
| P. aeruginosa (0826) | – | – | |
| P. aeruginosa (1113) | – | – | |
| P. aeruginosa (1378) | – | – | |
| P. aeruginosa (1731) | – | – | |
| Pathogens | P. aeruginosa (KCCM 11803) | – | – |
| Escherichia coli (KCTC 1682) | – | – | |
| Staphylococcus aureus (KCTC 3881) | – | – | |
| Salmonella paratyphi C (KCCM 41577) | – | – | |
| Shigella flexneri (KCTC 2517) | – | – | |
| Chryseobacterium gleum (KCTC 2094) | – | – | |
| Staphylococcus epidermidis (KCTC 3958) | – | – | |
| Streptococcus mutans (KCTC 3065) | – | – | |
| Proteus vulgaris (KCTC 2512) | – | – | |
| Proteus mirabilis (KCTC 2510) | – | – | |
| Enterococcus avium (ATCC 14025) | – | – | |
| Vibrio parahaemolyticus (KCTC 2471) | – | – | |
| Corynebacterium xerosis (KCTC 3435) | – | – | |
| Klebsiella pneumoniae subsp. pneumonia (KCTC 2690) | – | – | |
| Sphingomonas paucimobilis (KCTC 2834) | – | – | |
| Micrococcus luteus (KCTC 3063) | 10.7 ± 0.52 **,2 | 6.1 ± 0.34 | |
| Bacillus cereus (KCTC 3624) | 7.1 ± 0.21 * | 6.5 ± 0.14 | |
| Pathogenic yeast | Candida albicans (ATCC 28367) | – | – |
1 –, negative; 2 asterisks indicate significant differences (t-test, * p < 0.05, ** p < 0.01).
3. Discussion
A. rugosa hairy roots were successfully generated from in vitro cultured plants by infection with A. rhizogenes strain R1000 (wild type), as confirmed by PCR analysis of rol genes A–D (Figure 1). The hairy roots were able to produce rosmarinic acid; concentrations ranged from 2.069 ± 0.081 to 7.656 ± 0.407 mg/g DW according to the carbon source. Similarly, Kim, Oh, and Lee [27] reported that all hairy roots generated by five different strains (13333, 15834, R1000, R1200, and R1601) of A. rhizogenes produced rosmarinic acid, ranged from 14.3 ± 1.3 to 22.6 ± 2.1 mg/g DW, with the highest levels produced by strains R1000 and R1601. Furthermore, Lee et al., 2008 reported that the rosmarinic acid content of A. rugosa hairy roots reached its maximum after 14 days of culture with 116 mg/g DW [4]. This might be due to different root culture conditions, such as gaseous atmosphere for oxygen supply and carbon dioxide exchange, nutrients, minerals, temperature, and pH [28]. Additionally, previous studies reported that electrical conductivity [29], root zone temperature [30], and indole-3-acetic acid (IAA) [31] treatment affected the accumulation of rosmarinic acid in the intact plants of A. rugosa and its roots contained higher levels of rosmarinic acid compared with those of the other organs. In particular, the intact roots produced rosmarinic acid contents ranging from 10.163 to 21.141 mg/g DW according to the electrical conductivity conditions [29], ranging from 21.083 to 37.915 mg/g DW according to the root zone temperature [30], and ranging from 10.348 to 29.925 mg/g DW according to the IAA concentrations [31], respectively. Considering these studies, hairy roots of A. rugosa can be a good source for the production of resveratrol due to its high levels of rosmarinic acid comparable to those of roots of intact plants.
The supply of carbohydrates as an energy source is necessary for in vitro root cultures, including seedling, adventitious, and hairy roots. Among them, sucrose has been used for plant cell and tissue cultures because most plant species contain this disaccharide in their phloem sap and because sucrose is readily available and cost-effective [24,32,33,34]. In this study, supplementation with eight carbohydrates affected root growth and rosmarinic acid production in A. rugosa hairy roots. Sucrose contributed to the highest productivity of hairy root biomass and rosmarinic acid accumulation. These findings are consistent with prior observations of enhanced natural product accumulation in root cultures supplemented with different carbohydrates. Previously, sucrose was the best source for root growth and production of baicalin, baicalein, and wogonin in Scutellaria baicalensis hairy roots [24], and for biomass productivity and accumulation of betacyanin and betaxanthin in Beta vulgaris hairy roots [35]. Praveena and Murthy [26] reported that the supply of sucrose resulted in higher root biomass and production of withanolide A in Withania somnifera hairy roots compared with the other carbohydrate sources. Verma et al. [36] reported that Picrorhiza kurroa hairy root cultured with sucrose displayed higher growth rate and productions of kutkoside and picroside I than the other nine carbohydrate sources examined. Furthermore, galactose showed the highest productivity of hairy root biomass comparable with that of sucrose and the second highest production of rosmarinic acid and total phenolics in A. rugosa hairy roots. Similarly, Park et al. [24] reported that galactose at a concentration of 100 mM was most beneficial to root growth and production of baicalin, baicalein, and wogonin in S. baicalensis hairy roots, and Guo et al. [37] reported that supplementation with galactose yielded the highest growth values and production of rosmarinic acid in Salvia castanea hairy roots. Thus, this study also suggested that galactose can be a good carbon source for the production of rosmarinic acid in A. rugosa hairy roots.
In the present study, methanol extracts from A. rugosa hairy roots cultured with sucrose and fructose, respectively, were used for antimicrobial screening. The extracts displayed antibacterial activities against two pathogens (M. luteus and B. cereus). M. luteus causes infections that include pneumonia, bacteremia, endocarditis, and peritonitis in immunosuppressed patients [38]. B. cereus is responsible for emetic and diarrheal syndrome food poisoning [39]. These extracts did not display antimicrobial activities against multidrug-resistant bacteria and a pathogenic yeast. Similar to these results, essential oils from A. rugosa leaves reportedly had antibacterial effects against B. cereus [8]. However, until the current study, the antimicrobial properties of extracts of A. rugosa hairy roots against B. cereus and M. luteus have been unclear. Additionally, antibacterial activities were higher in hairy roots cultured with sucrose than in those cultured with fructose. This might be due to the higher levels of rosmarinic acid and total phenolics in hairy roots cultured with sucrose. Previous studies have reported that rosmarinic acid possesses antibacterial properties against B. cereus and M. luteus [40,41,42] and antimicrobial activity of plant phenolic compounds [43]. In this study, the enhanced production of rosmarinic acid and total phenolic compounds in A. rugosa hairy roots cultured with sucrose and greater antibacterial activity of the extracts are supported by previous studies reporting that A. rugosa flowers contain higher levels of phenolic compounds and have more pronounced antibacterial properties than those of leaves and stems [11]. White flowers of Angelica gigas, containing higher levels of phenolic compounds, showed higher antibacterial activities than those of pink and violet flowers [44]. However, we observed that the growth of only two bacterial pathogens (M. luteus and B. cereus) was inhibited by A. rugosa hairy root extract. This might be because only 1 mg of extract was loaded onto the discs. In further studies, antibacterial properties against the pathogens tested in this study should be confirmed using higher concentrations of A. rugosa hairy root extract. Furthermore, essential oils derived from plants (arborvitae, cassia, clove, lemongrass, oregano, tea tree, and thyme) displayed antimicrobial activities against multidrug-resistant pathogens (Enterobacter cloaceae, Proteus mirabilis, Morganella morganii, P. aeruginosa, and E. coli) [45]. Thus, antibacterial activities against multidrug-resistant pathogens should be performed with essential oils derived from A. rugosa in further studies.
4. Materials and Methods
4.1. Hairy Root Induction and Cultures
Hairy root induction began with the infection of A. rugosa explants with Agrobacteriun rhizogenes R1000 (optical density at 600 nm, OD600 = 0.6), followed by co-cultivation for 48 h. After removing A. rhizogenes from the surface of the explants by washing ten times with sterilized water, the plant parts were placed on half-strength Murashige and Skoog (MS) medium containing 500 mg/L cefotaxime and incubated at 25 °C in the dark. Within 2 weeks, hairy roots emerged from the injured parts of the explants. The roots were allowed to grow to at least 10 cm in length before being aseptically cut from the mother plant explants. The cut portions were transferred to basal medium containing 500 mg/L cefotaxime. After a 1-month culture, the hairy roots were transferred to basal medium containing 250 mg/L cefotaxime. After a further 1-month incubation, the hairy roots were incubated on basal medium without cefotaxime to confirm elimination of Agrobacterium. To obtain sufficient biomass to assess the effect of different carbon sources, hairy roots were cultured in half-strength MS liquid medium. Thereafter, hairy roots [4 g (fresh weight)] were cultured in the dark in 30 mL of half-strength MS containing 100 mM different carbohydrates (sorbitol, mannitol, glucose, maltose, galactose, mannose, or sucrose) at 25 °C on a gyratory shaker operating at 100 rpm. Each carbohydrate was tested using triplicate cultures. After 5 weeks, A. rugosa hairy roots were harvested and freeze-dried to measure dry weight and perform further analyses.
4.2. High-Performance Liquid Chromatography and Determination of Total Phenolic Content
Rosmarinic acid was analyzed as previously described [46,47]. Dried powder (100 mg) of A. rugosa hairy roots cultured in different carbohydrates was extracted with 2 mL of 80% methanol in a sonicator for 1 h and then centrifuged at 3000× g for 15 min. The supernatant was then syringe-filtered into a vial and analyzed by NS-4000 HPLC system (Futecs Co., Daejeon, Republic of Korea) as previously described [29,30]. Rosmarinic acid in A. rugosa hairy roots was compared with the retention time of the standard (100 ppm rosmarinic acid) and spike test. Quantification of rosmarinic acid was performed using a calibration curve established using dilutions of the standard. Extracts were also used for total phenolic content analysis using the Folin–Ciocalteu method. Briefly, 0.1 mL of each extract from hairy root cultured in the different carbon sources was added to a tube containing 3 mL of HPLC grade water, followed by 0.5 mL of 2 N Folin and Ciocalteu phenol reagent. Each mixture was incubated for 3 min, followed by the addition of 2 mL of sodium carbonate (20%, w/v). After incubation for 3 min, the absorbance of each sample was measured at 760 nm using a spectrophotometer. Calibration curve equivalent was determined using the following gallic acid standard curve equation: y = 0.002x − 0.0226 (r2 = 0.9997).
4.3. PCR Analysis
Genomic DNA of A. rugosa hairy roots cultured in different carbohydrates was isolated using a previously described one-step DNA extraction protocol [48]. The rol genes (A, B, C, and D) were amplified using the primers listed in Table S1. PCR amplification was performed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s, extension at 72 °C for 1 min, followed by a final elongation step at 72 °C for 10 min. One percent agarose gel electrophoresis was used to verify the expected lengths of the rol A (233 bp), B (483 bp), C (464 bp), and D (525 bp).
4.4. Screening for Antibacterial Activity
Antimicrobial screening of A. rugosa hairy roots cultured with sucrose and fructose was performed using an established disk diffusion method [49]. A. rugosa hairy root powder (300 mg) was soaked in 30 mL of methanol, and the mixture was sonicated for 24 h. Afterwards, the crude extracts were filtered through filter paper. The solvent was evaporated using a rotary vacuum evaporator. The final extracts were stored at 4 °C until required for analysis. Pseudomonas aeruginosa (KBN-10-p01827), P. aeruginosa (KBN-10-p01828), P. aeruginosa (0225), P. aeruginosa (0254), P. aeruginosa (0826), P. aeruginosa (1113), P. aeruginosa (1378), P. aeruginosa (1731), P. aeruginosa (KCCM 11803), Escherichia coli (KCTC 1682), Staphylococcus aureus (KCTC 3881), Salmonella paratyphi C (KCCM 41577), Proteus mirabilis (KCTC 2510), Klebsiella pneumoniae subsp. pneumoniae (KCTC 2690), Shigella flexneri (KCTC 2517), Chryseobacterium gleum (KCTC 2094), and Bacillus cereus (KCTC 3624) were incubated at 30 °C to OD600 = 1.0 in nutrient broth (NB). S. epidermidis (KCTC 3958), P. vulgaris (KCTC 2512), and Enterococcus avium (ATCC 14025) were incubated at 100 rpm at 37 °C to OD600 = 1.0, in Trypticase Soy broth. Additionally, Corynebacterium xerosis (KCTC 3435), Micrococcus luteus (KCTC 3063), Vibrio parahaemolyticus (KCTC 2471), and Streptococcus mutans (KCTC 3065) were incubated at 100 rpm and 30 °C to OD600 = 1.0, in no. 2 enriched NB, marine agar, and Brain Heart Infusion broth, respectively. Sphingomonas paucimobilis (KCTC 2834) was cultured at 100 rpm at 30 °C to OD600 = 1.0 in Reasoner’s 2A broth (R2A). Candida albicans (ATCC 28367) was cultured at 100 rpm at 25 °C to OD600 = 1.0 in a Yeast Malt broth. Subsequently, the warm agar medium containing one hundred microliter aliquots of each culture at an OD600 of 1.0 was poured into a plastic plates. Four sterilized paper discs were placed on the agar plates. Each was saturated with 20 μL of A. rugosa hairy roots incubated with 0.125, 0.25, 0.5, or 1 mg/mL of sucrose or fructose. Each plate was incubated at the appropriate temperature for the particular bacterium for 24 h. Diameters of the resulting zones of growth inhibition were measured.
4.5. Statistical Analysis
Duncan’s multiple range test and t-test were performed for data from dry weight evaluation, rosmarinic acid analysis, total phenolic content analysis, and screening for antibacterial activity using SAS software (version 9.4, 2013; SAS Institute, Inc., Cary, NC, USA).
5. Conclusions
This study aimed to optimize carbon sources for biomass productivity and rosmarinic acid production in hairy root cultures of A. rugosa infected with A. rhizogenes R1000. Furthermore, the antimicrobial activities of A. rugosa hairy root extracts were investigated to provide insights into the synergistic antimicrobial activities of phenolic compounds derived from its extracts. According to Table 1, Table 2 and Table 3, monosaccharides (galactose and glucose) and disaccharides (sucrose) are appropriate sugar sources for hairy root growth. Sucrose is considered the best carbohydrate for phenolic compounds. Furthermore, A. rugosa hairy roots had significant antibacterial properties against B. cereus and M. luteus. In particular, A. rugosa hairy roots cultured in the presence of sucrose contained the highest levels of rosmarinic acid and total phenolics, and displayed superior antibacterial activities compared to those of A. rugosa hairy roots cultured in the presence of fructose, which contained the lowest levels of phenolic compounds. These results suggest that A. rugosa hairy root can be a good biomaterial based on its rosmarinic acid production and antibacterial effect, and that the best carbohydrate source is sucrose for A. rugosa hairy root cultures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12040797/s1, Table S1: Gene-specific primers used to confirm the establishment of transformation.
Author Contributions
C.H.P. and S.U.P. conceived the study; H.J.Y., C.H.P., M.J.K., S.Y.H., J.C.J. and C.Y.K. performed the experiments and analyzed the data; H.J.Y. and M.J.K. writing original draft; H.J.Y., M.J.K., S.Y.H., C.H.P. and S.U.P.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2020R1A6A3A01097231).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kang M.J., Sundan S., Lee G.A., Ko H.C., Chung J.W., Huh Y.C., Gwag J.G., Oh S.J., Kim Y.G., Cho G.T. Genetic Diversity and Population Structure of Korean Mint Agastache rugosa (Fisch & Meyer) Kuntze (Lamiaceae) Using ISSR Markers. Korean J. Plant Resour. 2013;26:362–369. [Google Scholar]
- 2.Kim M.H., Chung W.T., Kim Y.K., Lee J.H., Lee H.Y., Hwang B., Park Y.S., Hwang S.J., Kim J.H. The effect of the oil of Agastache rugosa O. Kuntze and three of its components on human cancer cell lines. J. Essent. Oil Res. 2001;13:214–218. doi: 10.1080/10412905.2001.9699669. [DOI] [Google Scholar]
- 3.Ohk H.-C., Song J.-S., Chae Y.-A. Variability of the Volatile Composition of Agastache rugosa in South Korea. Korean J. Breed. 2002;34:100–104. [Google Scholar]
- 4.Lee S.Y., Xu H., Kim Y.K., Park S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbiol. Biotechnol. 2008;24:969–972. doi: 10.1007/s11274-007-9560-y. [DOI] [Google Scholar]
- 5.Li H.Q., Liu Q.Z., Liu Z.L., Du S.S., Deng Z.W. Chemical composition and nematicidal activity of essential oil of Agastache rugosa against Meloidogyne incognita. Molecules. 2013;18:4170–4180. doi: 10.3390/molecules18044170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J. Phytotoxic and antimicrobial activities and chemical analysis of leaf essential oil from Agastache rugosa. J. Plant Biol. 2008;51:276–283. doi: 10.1007/BF03036127. [DOI] [Google Scholar]
- 7.Shin S., Kang C.A. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Lett. Appl. Microbiol. 2003;36:111–115. doi: 10.1046/j.1472-765X.2003.01271.x. [DOI] [PubMed] [Google Scholar]
- 8.Song J.-H., Kim M.-J., Kwon H.-D., Park I.-H. Antimicrobial activity and components of extracts from Agastache rugosa during growth period. Prev. Nutr. Food Sci. 2001;6:10–15. [Google Scholar]
- 9.Hong J.-J., Choi J.-H., Oh S.-R., Lee H.-K., Park J.-H., Lee K.-Y., Kim J.-J., Jeong T.-S., Oh G.T. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001;495:142–147. doi: 10.1016/S0014-5793(01)02379-1. [DOI] [PubMed] [Google Scholar]
- 10.Min B.S., Hattori M., Lee H.K., Kim Y.H. Inhibitory constituents against HIV-1 protease from Agastache rugosa. Arch. Pharmacal Res. 1999;22:75–77. doi: 10.1007/BF02976440. [DOI] [PubMed] [Google Scholar]
- 11.Park C.H., Yeo H.J., Baskar T.B., Park Y.E., Park J.S., Lee S.Y., Park S.U. In vitro antioxidant and antimicrobial properties of flower, leaf, and stem extracts of Korean mint. Antioxidants. 2019;8:75. doi: 10.3390/antiox8030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desta K.T., Kim G.S., Kim Y.H., Lee W.S., Lee S.J., Jin J.S., Abd El-Aty A., Shin H.C., Shim J.H., Shin S.C. The polyphenolic profiles and antioxidant effects of Agastache rugosa Kuntze (Banga) flower, leaf, stem and root. Biomed. Chromatogr. 2016;30:225–231. doi: 10.1002/bmc.3539. [DOI] [PubMed] [Google Scholar]
- 13.Alfieri A., Mann G.E. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. Elsevier; Amsterdam, The Netherlands: 2015. Bioactive nutraceuticals and stroke: Activation of endogenous antioxidant pathways and molecular mechanisms underlying neurovascular protection; pp. 365–379. [Google Scholar]
- 14.Coiai S., Campanella B., Paulert R., Cicogna F., Bramanti E., Lazzeri A., Pistelli L., Coltelli M.-B. Rosmarinic acid and Ulvan from terrestrial and marine sources in anti-microbial bionanosystems and biomaterials. Appl. Sci. 2021;11:9249. doi: 10.3390/app11199249. [DOI] [Google Scholar]
- 15.Cao H., Cheng W.-X., Li C., Pan X.-L., Xie X.-G., Li T.-H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. THEOCHEM. 2005;719:177–183. doi: 10.1016/j.theochem.2005.01.029. [DOI] [Google Scholar]
- 16.Petersen M., Simmonds M.S. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/S0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 17.Park C.H., Park Y.E., Yeo H.J., Park N.I., Park S.U. Effect of light and dark on the phenolic compound accumulation in Tartary buckwheat hairy roots overexpressing ZmLC. Int. J. Mol. Sci. 2021;22:4702. doi: 10.3390/ijms22094702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park C.H., Xu H., Yeo H.J., Park Y.E., Hwang G.-S., Park N.I., Park S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021;64:64–73. doi: 10.1016/j.ymben.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.-K., Sathasivam R., Kim Y.B., Kim J.K., Park S.U. Transcriptomic Analysis, Cloning, Characterization, and Expression Analysis of Triterpene Biosynthetic Genes and Triterpene Accumulation in the Hairy Roots of Platycodon grandiflorum Exposed to Methyl Jasmonate. ACS Omega. 2021;6:12820–12830. doi: 10.1021/acsomega.1c01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuong D.M., Park C.H., Bong S.J., Kim N.S., Kim J.K., Park S.U. Enhancement of glucosinolate production in watercress (Nasturtium officinale) hairy roots by overexpressing cabbage transcription factors. J. Agric. Food Chem. 2019;67:4860–4867. doi: 10.1021/acs.jafc.9b00440. [DOI] [PubMed] [Google Scholar]
- 21.Park Y.-E., Park C.-H., Yeo H.-J., Chung Y.-S., Park S.-U. Resveratrol biosynthesis in hairy root cultures of tan and purple seed coat peanuts. Agronomy. 2021;11:975. doi: 10.3390/agronomy11050975. [DOI] [Google Scholar]
- 22.Shameh S., Hosseini B., Palazon J. Genetic engineering of tropane alkaloid biosynthesis of hyoscyamus reticulatus L. hairy roots by pmt gene overexpression and feeding with putrescine. Ind. Crops Prod. 2021;170:113716. doi: 10.1016/j.indcrop.2021.113716. [DOI] [Google Scholar]
- 23.Shi M., Zhu R., Zhang Y., Zhang S., Liu T., Li K., Liu S., Wang L., Wang Y., Zhou W. A novel WRKY34-bZIP3 module regulates phenolic acid and tanshinone biosynthesis in Salvia miltiorrhiza. Metab. Eng. 2022;73:182–191. doi: 10.1016/j.ymben.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Park C.H., Kim Y.S., Li X., Kim H.H., Arasu M.V., Al-Dhabi N.A., Lee S.Y., Park S.U. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. 2016;11:1934578X1601100625. doi: 10.1177/1934578X1601100625. [DOI] [PubMed] [Google Scholar]
- 25.Vinterhalter B., Krstić-Milošević D., Janković T., Pljevljakušić D., Ninković S., Smigocki A., Vinterhalter D. Gentiana dinarica Beck. hairy root cultures and evaluation of factors affecting growth and xanthone production. Plant Cell Tissue Organ Cult. (PCTOC) 2015;121:667–679. doi: 10.1007/s11240-015-0737-z. [DOI] [Google Scholar]
- 26.Praveen N., Murthy H. Synthesis of withanolide A depends on carbon source and medium pH in hairy root cultures of Withania somnifera. Ind. Crops Prod. 2012;35:241–243. doi: 10.1016/j.indcrop.2011.07.009. [DOI] [Google Scholar]
- 27.Kim J.-S., Oh E.-J., Lee S.-Y. Influence of Different Strains of Agrobacterium rhizogenes on Hairy Root Induction and Rosmarinic Acid Production in Agastache rugosa Kuntze. Korean J. Plant Resour. 2010;23:14–18. [Google Scholar]
- 28.Yancheva S., Georgieva L., Badjakov I., Dincheva I., Georgieva M., Georgiev V., Kondakova V. Application of bioreactor technology in plant propagation and secondary metabolite production. J. Cent. Eur. Agric. 2019;20:321–340. doi: 10.5513/JCEA01/20.1.2224. [DOI] [Google Scholar]
- 29.Lam V.P., Kim S.J., Park J.S. Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy. 2020;10:76. doi: 10.3390/agronomy10010076. [DOI] [Google Scholar]
- 30.Lam V.P., Kim S.J., Bok G.J., Lee J.W., Park J.S. The effects of root temperature on growth, physiology, and accumulation of bioactive compounds of Agastache rugosa. Agriculture. 2020;10:162. doi: 10.3390/agriculture10050162. [DOI] [Google Scholar]
- 31.Lam V.P., Lee M.H., Park J.S. Optimization of indole-3-acetic acid concentration in a nutrient solution for increasing bioactive compound accumulation and production of Agastache rugosa in a plant factory. Agriculture. 2020;10:343. doi: 10.3390/agriculture10080343. [DOI] [Google Scholar]
- 32.Thompson M.R., Thorpe T.A. Cell and Tissue Culture in Forestry. Springer; Berlin/Heidelberg, Germany: 1987. Metabolic and non-metabolic roles of carbohydrates; pp. 89–112. [Google Scholar]
- 33.Ahmad T., Abbasi N.A., Hafiz I.A., Ali A. Comparison of sucrose and sorbitol as main carbon energy sources in microprogation of peach rootstock GF-677. Pak. J. Bot. 2007;39:1269. [Google Scholar]
- 34.Fuentes S.R., Calheiros M.B., Manetti-Filho J., Vieira L.G. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult. 2000;60:5–13. doi: 10.1023/A:1006474324652. [DOI] [Google Scholar]
- 35.Bhagyalakshmi N., Thimmaraju R., Narayan M. Various hexoses and di-hexoses differently influence growth, morphology and pigment synthesis in transformed root cultures of red beet (Beta vulgaris) Plant Cell Tissue Organ Cult. 2004;78:183–195. doi: 10.1023/B:TICU.0000022557.84867.db. [DOI] [Google Scholar]
- 36.Verma P.C., Singh H., Negi A.S., Saxena G., Rahman L.-u., Banerjee S. Yield enhancement strategies for the production of picroliv from hairy root culture of Picrorhiza kurroa Royle ex Benth. Plant Signal. Behav. 2015;10:e1023976. doi: 10.1080/15592324.2015.1023976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y.-H., Wang F.-Y., You H.-Q., Wei Y.-K., Yang Z.-Q., Liang Z.-S., Yang D.-F. Effects of different carbon sources on growth and active component contents in Salvia miltiorrhiza and S. castanea f. tomentosa hairy roots. China J. Chin. Mater. Med. 2020;45:2509–2514. doi: 10.19540/j.cnki.cjcmm.20200329.112. [DOI] [PubMed] [Google Scholar]
- 38.Erbasan F. Brain abscess caused by Micrococcus luteus in a patient with systemic lupus erythematosus: Case-based review. Rheumatol. Int. 2018;38:2323–2328. doi: 10.1007/s00296-018-4182-2. [DOI] [PubMed] [Google Scholar]
- 39.Bhunia A.K. Bacillus cereus and Bacillus anthracis. Foodborne Microb. Pathog. Mech. Pathog. 2008;66:135–148. [Google Scholar]
- 40.Slobodníková L., Fialová S., Hupková H., Grančai D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013;8:1934578X1300801223. doi: 10.1177/1934578X1300801223. [DOI] [PubMed] [Google Scholar]
- 41.Klančnik A., Piskernik S., Jeršek B., Možina S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods. 2010;81:121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Kuhnt M., Pröbstle A., Rimpler H., Bauer R., Heinrich M. Biological and pharmacological activities and further constituents of Hyptis verticillata. Planta Med. 1995;61:227–232. doi: 10.1055/s-2006-958061. [DOI] [PubMed] [Google Scholar]
- 43.Zacchino S.A., Butassi E., Di Liberto M., Raimondi M., Postigo A., Sortino M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine. 2017;37:27–48. doi: 10.1016/j.phymed.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Park C.H., Park H.W., Yeo H.J., Jung D.H., Jeon K.S., Kim T.J., Kim J.K., Park S.U. Combined transcriptome and metabolome analysis and evaluation of antioxidant and antibacterial activities in white, pink, and violet flowers of Angelica gigas. Ind. Crops Prod. 2022;188:115605. doi: 10.1016/j.indcrop.2022.115605. [DOI] [Google Scholar]
- 45.Bučková M., Puškárová A., Kalászová V., Kisová Z., Pangallo D. Essential oils against multidrug resistant gram-negative bacteria. Biologia. 2018;73:803–808. doi: 10.2478/s11756-018-0090-x. [DOI] [Google Scholar]
- 46.Yeo H.J., Park C.H., Park Y.E., Hyeon H., Kim J.K., Lee S.Y., Park S.U. Metabolic profiling and antioxidant activity during flower development in Agastache rugosa. Physiol. Mol. Biol. Plants. 2021;27:445–455. doi: 10.1007/s12298-021-00945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park C.H., Yeo H.J., Park C., Chung Y.S., Park S.U. The Effect of Different Drying Methods on Primary and Secondary Metabolites in Korean Mint Flower. Agronomy. 2021;11:698. doi: 10.3390/agronomy11040698. [DOI] [Google Scholar]
- 48.Edwards K., Johnstone C., Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park C.H., Sathasivam R., Kim T.J., Park B.B., Kim J.K., Park S.U. Metabolic profiling and secondary metabolite accumulation during fruit development of Cornus officinalis Sieb. et Zucc. Ind. Crops Prod. 2022;189:115779. doi: 10.1016/j.indcrop.2022.115779. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.