Abstract
Mortality due to COVID-19 has been correlated with laboratory markers of inflammation, such as C-reactive protein (CRP). The lower mortality during Omicron variant infections could be explained by variant-specific immune responses or host factors, such as vaccination status. We hypothesized that infections due to Omicron variant cause less inflammation compared to Alpha and Delta, correlating with lower mortality. This was a retrospective cohort study of veterans hospitalized for COVID-19 at the Veterans Health Administration. We compared inflammatory markers among patients hospitalized during Omicron infection with those of Alpha and Delta. We reported the adjusted odds ratio (aOR) of the first laboratory results during hospitalization and in-hospital mortality, stratified by vaccination status. Of 2,075,564 Veterans tested for COVID-19, 29,075 Veterans met the criteria: Alpha (45.1%), Delta (23.9%), Omicron (31.0%). Odds of abnormal CRP in Delta (aOR = 1.85, 95% CI:1.64–2.09) and Alpha (aOR = 1.94, 95% CI:1.75–2.15) were significantly higher compared to Omicron. The same trend was observed for Ferritin, Alanine aminotransferase, Aspartate aminotransferase, Lactate dehydrogenase, and Albumin. The mortality in Delta (aOR = 1.92, 95% CI:1.73–2.12) and Alpha (aOR = 1.68, 95% CI:1.47–1.91) were higher than Omicron. The results remained significant after stratifying the outcomes based on vaccination status. Veterans infected with Omicron showed milder inflammatory responses and lower mortality than other variants.
Keywords: inflammatory markers, SARS-CoV-2 variants, alpha, delta, omicron
1. Introduction
In late 2021 a new variant of Severe Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) appeared and spread quickly throughout the world [1]. The new variant, named Omicron, was capable of escaping neutralization by antibodies generated by the previous infection and/or vaccination, raising concern for increasing hospitalization and mortality rates [2]. However, hospitalization and mortality rates were lower compared to previous variants [3,4]. Even though the Omicron variant was able to escape neutralizing antibodies to a large extent, pre-existing T and memory B cell immunity are believed to have an important role in limiting the severity of the coronavirus disease of 2019 (COVID-19). However, there is also some evidence to suggest that Omicron is less pathogenic by virtue of decreased fitness to replicate in human lung tissues [5,6]. This decreased pathogenicity may, in turn, result in less immunopathology and less severe COVID-19 disease.
In hospitalized patients with COVID-19 several clinical and laboratory factors have been shown to predict mortality and disease severity [7,8]. Specifically, laboratory markers of inflammation such as C-reactive protein (CRP) and ferritin have shown a strong correlation with disease severity and mortality. Recent data show mortality differences among the SARS-CoV-2 variants dominant in the USA, including Alpha, Delta, and Omicron, with lower mortality in Omicron-related infections. However, it has not been conclusively demonstrated whether the decline in mortality is correlated with the decrease in inflammatory markers or other factors such as vaccination. In this study, we explore if laboratory markers of inflammation differed among the three variants and the differences correlated with the mortality rates. We also were interested to see if such differences are primarily related to vaccination/immunity status or driven by SARS-CoV-2 variants.
2. Materials and Methods
We used the veteran health administration (VHA) Corporate Data Warehouse (CDW) [9], and VHA COVID-19 shared data resources [10]. The Research & Development Committee of the Michael E. DeBakey VA Medical Center and Baylor College of Medicine Institutional Review Board (IRB# H-47595) approved our study.
2.1. Cohort
This is a retrospective cohort study that includes all Veterans who tested positive for SARS-CoV-2 between 1 February 2020 and 7 July 2022 and were hospitalized related to COVID-19 infection. The inclusion criteria were the first positive test and hospital admission within 7 days after the SARS-CoV-2 test date or within 15 days prior to the test date [10,11,12]. The exclusion criteria were not being veterans and not being an active VHA healthcare user. We defined the active patients as those veterans that had a primary physician’s visit in the past two years within VHA health care systems.
2.2. Variables
2.2.1. Exposures
The three SARS-CoV-2 variants were determined based on the date of infection after consulting with the infectious disease experts (ST and AC): Alpha, 12 January 2020 to 6 January 2021, Delta, 9 January 2021 to 12 January 2021, and Omicron, 2 January 2022 to 7 July 2022. The dates based on phylogenetic trees were retrieved from Nextstrain [13]. The intervals with the highest entropy residues were determined for each variant (see Appendix A). The Nextstrain is an online SARS-CoV-2 database that maps the evolution and epidemiology of the virus [14,15]. Additionally, the VHA COVID-19 shared data resources provided a subsample of genome sequencing for the SARS-CoV-2 virus. We observed high accuracy (Alpha, 99.2%; Delta, 97.5%; Omicron, 98.1%) and precision (Alpha, 91.3%; Delta, 96.9%; Omicron, 100.0%) between the sequenced samples and date-based samples (see Appendix A).
2.2.2. Laboratory Biomarkers
The primary outcomes were laboratory biomarkers. We curated the first laboratory drawn from the patients during the hospitalization intervals to measure the laboratory biomarkers of interest. The laboratory biomarkers of interest were recommended by our medical advisory team (AS, STT, AC). We collected the following laboratory biomarkers using the Logical Observation Identifiers Names and Codes (LOINC) codes: CRP (‘11039-5’, ‘14634-0’, ‘1988-5’, ‘30522-7’, ‘35648-5’, ‘48421-2’, ‘71426-1’, ‘76485-2’, ‘76486-0’), Ferritin (‘14723-1’, ‘14724-9’, ‘20567-4’, ‘2276-4’, ‘24373-3’, ‘35209-6’), alanine aminotransferase (‘1742-6’, ‘1743-4’, ‘1744-2’, ‘44785-4’, ‘76625-3’, ‘77144-4’, ‘16325-3’, ‘1916-6’, ‘1742-6’, ‘1743-4’, ‘1744-2’, ‘44785-4’, ‘76625-3’, ‘77144-4’, ‘48134-1’, ‘16325-3), aspartate aminotransferase (‘1920-8’, ‘27344-1’, ‘30239-8’, ‘44786-2’, ‘88112-8’, ‘48136-6’, ‘54500-4’, ‘1916-6’). Lactate dehydrogenase (‘2546-0’, ‘2545-2’, ‘2547-8’, ‘2549-4’, ‘2548-6’, ‘49279-3’, ‘5910-5’, ‘42929-0’), and Albumin (‘1751-7’, ‘18180-0’, ‘2862-1’, ‘43712-9’, ‘54347-0’, ‘61151-7’, ‘61152-5’, ‘62234-0’, ‘62235-7’, ‘76631-1’, ‘77148-5’). If any laboratories fall outside the normal range, we considered it as abnormal or one. The secondary outcome was in-hospital mortality. The in-hospital mortality documented by the COVID-19 shared data resource.
2.2.3. Other Variables
We used VHA Electronic Medical Record (EMR) to extract age (≥50 & <65, 65≥ & <75, ≥75 & <85, and ≥85 years), sex (male and female), BMI (categorized to <18.5, 18.5–30, and ≥30 kg/m2), race (White, Black, Others), Charlson Comorbidity Index (CCI), and frailty status. We used the validated VA frailty index (VA-FI) which is based on the accumulation of deficits framework and counts 31 age-related variables according to an established approach [16]. The VA-FI values were categorized as robust (≤0.1), prefrail (>0.1 and ≤0.2), and frail (>0.2). Any vaccination (any-Vax) referred to patients who received any dose of vaccines before the index date of hospitalization.
2.3. Statistical Analysis
Mean and standard deviation were calculated for continuous outcomes, and count and percentage were calculated for categorical outcomes. We used logistic regression models to calculate the odds ratio (OR) of abnormal laboratories. We adjusted the OR (aOR) with age, sex, BMI, race, ethnicity, CCI, VA-FI, and vaccine record. We reported the ORs of in-hospital mortality between variants and adjusted them with age, sex, BMI, race, ethnicity, CCI, and VA-FI.
We used the least absolute shrinkage and selection operator (LASSO) to identify the most important predictors of in-hospital mortality with 10-fold cross-validation. Then, we use multiple regression analysis to estimate the odds ratio. We forced the race variable to the multiple regression model. The regression was performed in those patients who had reported CRP laboratory biomarkers. The following variables were used as independent predictors: CRP, variants, age, gender, race, ethnicity, BMI, CCI, frailty status, and vaccination records. We reported odd ratios and 95 percent confidence intervals (95%CI) and Beta. The statistical significance was set at 2-sided p < 0.05. The statistical analyses were performed using IBM SPSS Statistics version 24 (IBM, Armonk, NY, USA) and R programming version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
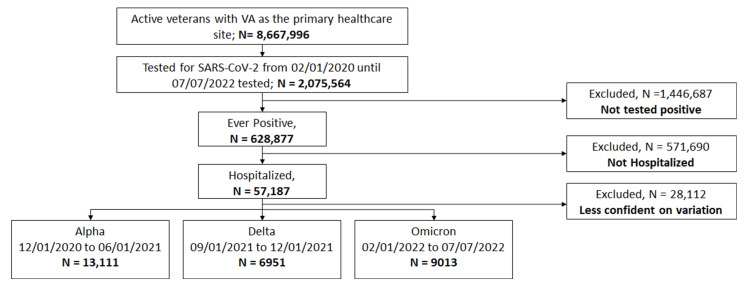
We classified 13,111 as Alpha, 6951 as Delta, and 9013 as Omicron. We excluded 28,112 as they tested positive outside the concurred intervals for each variant, Figure 1, and during these excluded periods, multiple variants may be circulating. The percentage of patients with age ≥ 65 years in the Omicron (72.8%) was higher than Alpha (67.6%) and Delta (65.4%). The percentage of patients with BMI ≥ 30 kg/m2 was lower in Omicron (32.3%) compared to Alpha (44.2%) and Delta (43.5%). The percentage of patients with frail status was lower in Omicron (29.7%) compared to Alpha (58.3%) and Delta (47.4%). We reported the number of laboratory results available for patients for each variant, along with the median and interquartile intervals, Table 1. The median of CRP in the Omicron (8.7 mg/L) was lower than Alpha (12.6 mg/L) and Delta (13.3 mg/L). The median of Ferritin in Omicron (233.0 ng/mL) was lower than Alpha (432.9 ng/mL) and Delta (460.5 ng/mL). The same trend was observed in alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase, Table 1.
Figure 1.
Strobe diagram of patient eligibility determinations for analysis.
Table 1.
Demographics and clinical characteristics in the Alpha, Delta, and Omicron variation groups among veterans with COVID.
| Alpha | Delta | Omicron | |
|---|---|---|---|
| N | 13,111 (45.1) | 6951 (23.9) | 9013 (31.0) |
| Age: M (SD) | 68.5(13.9) | 67.6 (14.1) | 70.1 (14.3) |
| Age 19–50, N (%) | 1298 (9.9) | 807 (11.6) | 849 (9.4) |
| Age 50–65, N (%) | 2955 (22.5) | 1603 (23.1) | 1603 (17.8) |
| Age 65–75, N (%) | 4624 (35.3) | 2409 (34.7) | 2927 (32.5) |
| Age 75–85, N (%) | 2708 (20.7) | 1464 (21.1) | 2387 (26.5) |
| Age ≥ 85, N (%) | 1526 (11.6) | 668 (9.6) | 1247 (13.8) |
| Sex, Male, N (%) | 12,418 (94.7) | 6563 (94.4) | 8542 (94.8) |
| Race, N (%) | |||
| White | 8421 (64.2) | 5097 (73.3) | 6375 (70.7) |
| Black | 3461 (26.4) | 1252 (18.0) | 1825 (20.2) |
| Other | 1229 (9.4) | 602 (8.7) | 813 (9.0) |
| Ethnicity-Hispanic, N (%) | 1114 (8.5) | 469 (6.7) | 781 (8.7) |
| BMI, Kg/m2, M (SD) | 29.8 (7.1) | 29.5 (7.1) | 28.0 (6.8) |
| BMI < 18.5, N (%) | 372 (2.8) | 233 (3.4) | 414 (4.6) |
| BMI 18.5–30, N (%) | 6939 (52.9) | 3696 (53.2) | 5688 (63.1) |
| BMI ≥ 30, N (%) | 5800 (44.2) | 3022 (43.5) | 2911 (32.3) |
| Comorbid Conditions | |||
| CCI, M (SD) | 3.0 (2.7) | 2.7 (2.6) | 3.3 (2.9) |
| CCI ≥ 2, N (%) | 8378 (63.9) | 4069 (58.5) | 6043 (67.0) |
| Frailty, M (SD) | 0.3 (0.2) | 0.2 (0.2) | 0.1 (0.2) |
| Robust | 2309 (20.2) | 1462 (24.3) | 3992 (60.7) |
| Prefrail | 2469 (21.6) | 1334 (22.1) | 629 (9.6) |
| Frail | 6669 (58.3) | 3232 (53.6) | 1952 (29.7) |
| Any vaccine record | 1467 (11.2) | 3298 (47.4) | 6458 (71.7) |
| Laboratory Tests | |||
| C-reactive protein, N (N = 14,916) | 7570 (50.8) | 4011 (26.9) | 3335 (22.4) |
| C-reactive protein, mg/L, M(IQR) | 12.6 (4.5, 47.1) | 13.3 (5.1, 48.2) | 8.7 (2.5, 31.2) |
| Ferritin, N (N = 13,859) | 7073 (51.0) | 3411 (24.6) | 3375 (24.4) |
| Ferritin, ng/mL, M(IQR) | 432.9 (206.8, 811.0) | 460.5 (216.4, 926.4) | 233.0 (109.0, 494.6) |
| Alanine aminotransferase, N (N = 20,923) | 9897 (47.3) | 5187 (24.8) | 5839 (27.9) |
| Alanine aminotransferase, U/L, M(IQR) | 25.0 (17.0, 39.0) | 27.0 (18.0, 41.0) | 20.0 (14.0, 31.0) |
| Aspartate aminotransferase, N (N = 20,884) | 9918 (47.5) | 5187 (24.8) | 5779 (27.7) |
| Aspartate aminotransferase, U/L, M(IQR) | 32.0 (22.0, 47.0) | 34.0 (23.0, 51.0) | 24.0 (18.0, 37.0) |
| Lactate dehydrogenase, N (N = 11,127) | 5962 (53.6) | 2807 (25.2) | 2358 (21.2) |
| Lactate dehydrogenase, IU/L, M(IQR) | 267.0 (199.0, 369.0) | 284.0 (206.0, 406.0) | 203.0 (161.0, 281.0) |
| Albumin, N (N = 23,159) | 10,777 (46.5) | 5766 (24.9) | 6616 (28.6) |
| Albumin, g/dL, M(IQR) | 3.2 (2.8, 3.5) | 3.2 (2.8, 3.5) | 3.3 (2.9, 3.6) |
M (SD) = mean and standard deviation, M(IQR) = median and interquartile, CCI: Charlson Comorbidity Index.
The number of patients with abnormal laboratory findings are reported in Table 2. The percentage is calculated based on the total number of laboratories available in each variant group. The lowest percentage of abnormal CRP was observed in Omicron (80.0%) compared to Alpha (86.4%) and Delta (88.8%). The lowest percentage of abnormal ferritin was observed in Omicron (40.2%) compared to Alpha (61.4%) and Delta (63.6%). The same trend was observed in alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and albumin, Table 2. The adjusted odds of abnormal CRP among Alpha was 94% higher (aOR, 1.94, 95% CI: 1.75–2.15) compared to Omicron and among Delta 85% higher (aOR, 1.85, 95% CI: 1.64–2.09) compared to Omicron. The adjusted odds of abnormal ferritin among Alpha was 44% higher (aOR, 1.44, 95% CI: 1.30–1.60) compared to Omicron and among Delta 40% higher (aOR, 1.40, 95% CI: 1.24–1.58) compared to Omicron. The adjusted odds of abnormal alanine aminotransferase among Alpha were 41% higher (aOR, 1.41, 95% CI: 1.26–1.58) compared to Omicron and among Delta 25% higher (aOR, 1.25, 95% CI: 1.09–1.43) compared to Omicron. The adjusted odds of abnormal aspartate aminotransferase among Alpha were 53% higher (aOR, 1.53, 95% CI: 1.37–1.72) compared to Omicron and among Delta 36% higher (aOR, 1.36, 95% CI: 1.19–1.56) compared to Omicron. The adjusted odds of abnormal lactate dehydrogenase among Alpha were 69% higher (aOR, 1.69, 95% CI: 1.52–1.88) compared to Omicron and among Delta 79% higher (aOR, 1.79, 95% CI: 1.58–2.03) compared to Omicron. The adjusted odds of abnormal albumin among Alpha were 44% higher (aOR, 1.44, 95% CI: 1.26–1.63) compared to Omicron and among Delta 19% higher (aOR, 1.19, 95% CI: 1.02–1.38) compared to Omicron, Table 2.
Table 2.
Comparing the abnormality after testing positive for COVID-19 among 3 variants groups: Alpha, Delta, and Omicron.
| Odds Ratio (95%Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|
| Abnormality Status | Variation Status, N (%) | Alpha vs. Omicron | Delta vs. Omicron | ||||
| Alpha | Delta | Omicron | Unadjusted | Adjusted † | Unadjusted | Adjusted † | |
| Inflammatory Markers | |||||||
| C-reactive protein | 6540 (86.4) | 3562 (88.8) | 2669 (80.0) |
2.29 * (2.14, 2.45) |
1.94 * (1.75, 2.15) |
2.30 * (2.18, 2.44) |
1.85 * (1.64, 2.09) |
| Ferritin | 4344 (61.4) | 2168 (63.6) | 1357 (40.2) |
1.54 * (1.45, 1.65) |
1.44 * (1.30, 1.60) |
1.91 * (1.81, 2.02) |
1.40 * (1.24, 1.58) |
| Liver Inflammation Markers | |||||||
| Alanine aminotransferase | 1701 (17.2) | 996 (19.2) |
853 (14.6) |
1.49 * (1.38, 1.60) |
1.41 * (1.26, 1.58) |
1.60 * (1.50, 1.70) |
1.25 * (1.09, 1.43) |
| Aspartate aminotransferase | 3607 (36.4) | 2212 (42.6) | 1490 (25.8) |
1.55 * (1.44, 1.67) |
1.53 * (1.37, 1.72) |
1.69 * (1.59, 1.80) |
1.36 * (1.19, 1.56) |
| Lactate dehydrogenase | 3796 (63.7) | 1833 (65.3) | 992 (42.1) |
1.85 * (1.73, 1.98) |
1.69 * (1.52, 1.88) |
2.31 * (2.18, 2.45) |
1.79 * (1.58, 2.03) |
| Liver Metabolic Function | |||||||
| Albumin | 6857 (63.6) | 3685 (63.9) | 3741 (56.5) |
1.66 * (1.52, 1.80) |
1.44 * (1.26, 1.63) |
1.61 * (1.50, 1.73) |
1.19 * (1.02, 1.38) |
† Results were adjusted by age, sex, body mass index, race, ethnicity, Charlson Comorbidity Index (CCI), VA frailty index, and vaccine record status. * The p-value < 0.05.
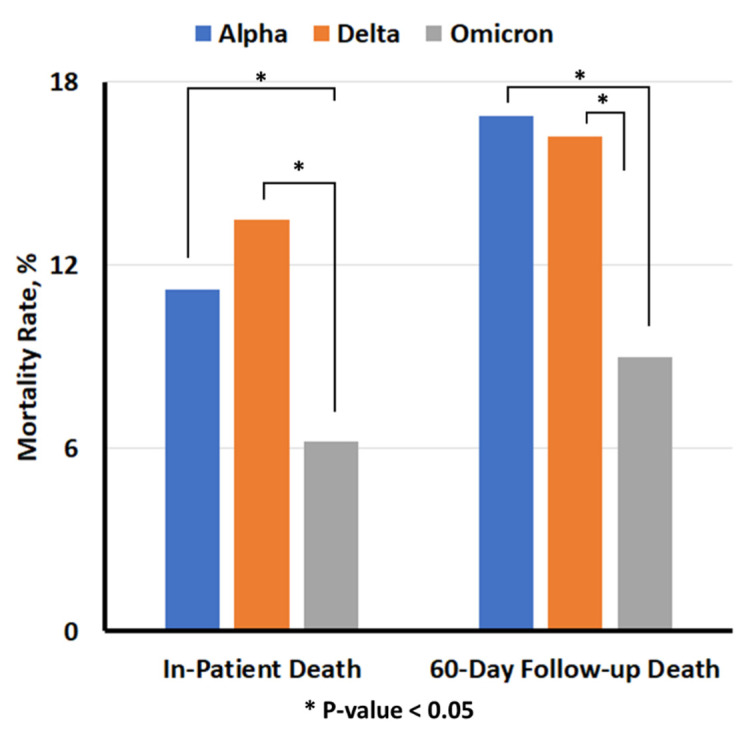
The lowest in-hospital mortality was observed in the Omicron (6.2%) compared to Alpha (11.2%) and Delta (13.5%) in Table 3 and Appendix B. The adjusted odds of in-hospital mortality were 1.68-times higher in Alpha (aOR, 1.68, 95% CI: 1.47, 1.91) compared to Omicron, and it was 1.92-time higher in Delta compared to Omicron (aOR, 1.92, 95% CI: 1.73, 2.12). The adjusted odds of 60-day follow-up mortality were 81% higher in Alpha (aOR, 1.81, 95% CI: 1.62, 2.02) compared to Omicron, and it was 2.04-time higher in Delta compared to Omicron (aOR, 2.04, 95% CI: 1.88, 2.23). The sensitivity analysis showed the same significant trend when we stratified the patients based on vaccination status, Table 3.
Table 3.
Comparing in-hospital mortality among three variation groups: Alpha, Delta, and Omicron after the COVID-19 test.
| Variation Status, N (%) | Odds Ratio (95%Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|
| Alpha | Delta | Omicron | Alpha vs. Omicron | Delta vs. Omicron | |||
| Unadjusted | Adjusted † | Unadjusted | Adjusted † | ||||
| In-hospital Mortality | 1471 (11.2) | 939 (13.5) |
557 (6.2) |
2.17 * (1.89, 2.49) |
1.68 * (1.47, 1.91) |
2.37 * (2.12, 2.65) |
1.92 * (1.73, 2.12) |
| 60-day follow-up Mortality | 2214 (16.9) | 1127 (16.2) | 815 (9.0) |
1.85 * (1.63, 2.08) |
1.81 * (1.62, 2.02) |
1.95 * (1.77, 2.14) |
2.04 * (1.88, 2.23) |
| Stratifying Analysis based on vaccination records | |||||||
| Mortality in any-Vax | 2 (0.1) |
348 (10.6) |
374 (5.8) |
NA | NA | 1.92 * (1.65, 2.24) |
1.49 * (1.21, 1.83) |
| 60-day follow-up Mortality | 5 (0.3) |
432 (13.1) |
559 (8.7) |
NA | NA | 1.59 * (1.39, 1.82) |
1.27 * (1.06, 1.51) |
† Results were adjusted by age, sex, body mass index, race, ethnicity, Charlson Comorbidity Index (CCI), and VA frailty index. * The p-value < 0.05.
The LASSO algorithm excluded race as the most important variable. We forced-fed the race variable to report the ORs. We observed that age ≥ 85 years (Beta, 2.16, OR, 8.64, 95% CI: 6.06, 12.32), age 75–85 (Beta, 1.74, OR, 5.73, 95% CI: 4.04, 8.12), age 65–75 (Beta, 1.40, OR, 4.06, 95% CI: 2.88, 5.74), Delta variant (Beta, 0.74, OR, 2.10, 95% CI: 1.83, 2.41), BMI < 18.5 (Beta, 0.58, OR, 1.78, 95% CI: 1.47, 2.15), Frailty Status-frail (Beta, 0.51, OR, 1.67, 95% CI: 1.44, 1.93) are the top predictors of in-hospital mortality, Table 4. The sensitivity analysis showed the same significant trend when we stratified the patients based on vaccination status, Table 4.
Table 4.
Results of multivariate logistic regression for 14,916 patients with reported C-Reactive Protein laboratory results.
| All | Vaccinated | |||
|---|---|---|---|---|
| OR (95%CI) | Beta | OR (95%CI) | Beta | |
| Abnormal CRP | 1.24 (1.14, 1.36) * | 0.22 | 1.26 (1.06, 1.50) * | 0.23 |
| Omicron | Reference | Reference | Reference | Reference |
| Delta | 2.10 (1.83, 2.41) * | 0.74 | 1.45 (1.18, 1.78) * | 0.37 |
| Alpha | 1.61 (1.41, 1.84) * | 0.48 | NA | NA |
| Age < 50 | Reference | Reference | Reference | Reference |
| age 50–65 | 1.99 (1.40, 2.85) * | 0.69 | 0.94 (0.40, 2.18) | −0.07 |
| age 65–75 | 4.06 (2.88, 5.74) * | 1.40 | 1.81 (0.82, 4.00) | 0.60 |
| age 75–85 | 5.73 (4.04, 8.12) * | 1.74 | 2.85 (1.30, 6.28) * | 1.05 |
| age ≥ 85 | 8.64 (6.06, 12.32) * | 2.16 | 4.08 (1.84, 9.03) * | 1.41 |
| Sex-Male | 1.52 (1.15, 2.00) * | 0.42 | 2.57 (1.13, 5.86) * | 0.94 |
| Race-White | Reference | Reference | Reference | Reference |
| Race-Black | 0.90 (0.80, 1.00) | −0.11 | 0.83 (0.65, 1.07) | −0.18 |
| Race-Other | 1.06 (0.91, 1.24) | 0.06 | 0.86 (0.60, 1.21) | −0.15 |
| Ethnicity | ||||
| BMI 18.5–30 | Reference | Reference | Reference | Reference |
| BMI < 18.5 | 1.78 (1.47, 2.15) * | 0.58 | 2.50 (1.82, 3.44) * | 0.92 |
| BMI ≥ 30 | 0.98 (0.90, 1.08) | −0.02 | 0.83 (0.68, 1.02) | −0.18 |
| CCI ≥ 2 | 1.11 (1.00, 1.23) * | 0.10 | 1.56 (1.21, 2.01) * | 0.45 |
| Frailty Status -Robust | Reference | Reference | Reference | Reference |
| Frailty Status -prefrail | 1.61 (1.37, 1.89) * | 0.48 | 1.21 (0.85, 1.74) | 0.19 |
| Frailty Status -frail | 1.67 (1.44, 1.93) * | 0.51 | 1.83 (1.44, 2.33) * | 0.60 |
OR (95%CI) = odds ratio and 95 percent confidence intervals, CRP = reported C-Reactive Protein laboratory, BMI = body Mass Index, CCI = Charlson Comorbidity Index. * The p-value < 0.05.
4. Discussion
We conducted a retrospective study using VHA administrative databases among veterans who tested positive for SARS-CoV-2 for the first time and were hospitalized in the VHA healthcare systems. We categorized patients into three groups, Alpha, Delta, and Omicron, based on the defined time intervals when each was the dominant circulating strain. We observed that Omicron patients showed significantly lower inflammatory profiles compared to Alpha and Delta variants of SARS-CoV-2. Further, mortality rates differed significantly among the three variants’ times, with the lowest mortality associated with the Omicron period. The findings persisted after stratifying for vaccination status. Among risk factors of in-hospital mortality, high chronicle age (≥65 years) had a similar expected change in log odds of mortality per unit change. Non-Omicron variants (i.e., Delta), BMI < 18.5, and being frail were the other risk factors for mortality with the same expected changes. Additionally, the CRP inflammatory biomarker was selected as one of the most important significant predictors of in-hospital mortality along with other factors.
COVID-19 results in two inflammatory phases, including direct virus-mediated tissue damage, followed by the second phase of local and systemic inflammation related to the host’s immune response [17]. The resulting inflammation may vary depending on the virus and the host and correlate with the spectrum and severity of the clinical presentation of the disease and related mortality [18]. Hui and colleagues showed that the Omicron variant replicates faster than other variants in the bronchi but less efficiently in the lung parenchyma [5]. Suzuki and colleagues showed that compared to Alpha and Delta, Omicron resulted in fewer pathological findings in the lungs of hamsters [19]. Data indicates that Omicron transmissibility, including breakthrough infections, was higher compared to other variants, and this correlated with deletions and mutations in its genetic structure [20,21]. Our data, consistent with the literature, show that patients admitted with COVID-19 during the Omicron dominant period had a lower mortality rate compared to those infected during the Alpha and Delta dominant periods [3,20,21,22,23,24,25]. Thus, clinical as well as in vivo and in vitro data suggest a decoupling between clinical presentation and transmissibility of Omicron when compared to Alpha and Delta variants. Our data clearly shows that systemic markers of inflammation are lower in Omicron compared to Alpha and Delta. Interestingly, even after adjustment for various factors, including the study variants, CRP significantly predicted higher mortality. This finding is novel and may stem from a combined effect of the inherent ability of a virus causing inflammation and the genetic composition of the host to mount inflammation.
Madhi and colleagues postulated that the cell-mediated immunity of prior COVID-19 partly explained the decoupling. Bhattacharyya and colleagues suggested that part of the reduced clinical severity of Omicron compared to Delta may stem from the stronger immune-evasion capability of Omicron compared to prior variants [26]. This strong immune evasion in turn allows Omicron to infect those with prior history of COVID-19 infection. A novel aspect of our study is that we enrolled individuals with first-time documented severe infection with the virus. Although prior infection and or vaccination offers some degree of protection against Omicron, even in first-time infected individuals, Omicron results in more favorable clinical outcomes than Delta and Alpha.
Studies show that COVID-19 vaccines significantly lower the incidence of clinically severe disease. Data from Altarawneh and colleagues showed that previous infection and or vaccination with BNT162b2 strongly reduced the rate of severe, critical, or fatal COVID-19 infection [27]. Our data is novel as it showed that the variances in mortality rates among the studied groups remained significant even in vaccinated patients. The data show that the adjusted ORs of in-hospital mortality is significantly higher than vaccinated patients. Thus, prior vaccination only to a mild degree explains the differences in the study outcomes between the Delta and Omicron groups.
The majority of the studies showing favorable clinical outcomes in Omicron versus prior variants are in population with age less than 65 [28]. Bhattacharyya also suggested that immunosenescence related to aging may explain worse clinical outcomes in Delta compared to Omicron [26]. Auvigne and colleagues reported that the lower severity of clinical illness in Omicron compared to Delta is less prominent in the elderly [29]. In our cohort, the average age in the three groups was similar, which makes the immunosenescence of less importance in our cohort. Krutikov and colleagues studied nursing home residents with a median age of 84.5 years and reported favorable outcomes, including hospitalization and mortality in Omicron compared to prior variants [30]. In our study as well as Krutikov and colleagues, the more favorable clinical outcomes in Omicron versus Alpha and Delta remained significant after adjusting for age, sex, prior infection, and vaccination status. To differentiate the differential mortality rates of the three studied variants, we used a validated index of frailty. Favorable mortality rates in Omicron compared to the others continued after adjusting for frailty status.
Our team, as well as others, showed that mortality and poor clinical outcomes in COVID-19 are linked to the presence of comorbid conditions as assessed by Charlson Comorbidity Index [11,31]. Our data shows that prior to or after adjustment for comorbid conditions using CCI; mortality remains significantly lower in Omicron compared to the two other study variants.
We used a large national cohort of patients from a healthcare system with an approximate distribution of race and ethnicity of the US population. Additionally, our cohort benefited from near-complete demographics and characteristics with up-to-date information retrievals. Importantly, our analysis is among the first and largest to compare the inflammatory laboratory biomarkers between three major SARS-CoV-2 variants in the US. Our study outcomes were limited to in-hospital mortality due to delays in reporting the out-of-hospital death reports. We also limited the number of reported laboratory biomarkers due to the fact that some patients may not have all the laboratory biomarkers of interest. Our analysis was limited to sets of variables available in the dataset, and we were not able to report on social determinants of health. Due to the large scale of this study, manual chart review was not feasible, and we are unable to determine the indications for SARS-CoV-2 testing and which symptoms, if any, the patients experienced.
5. Conclusions
In summary, our data established a close correlation among variants of SAR-CoV-2 variants, mortality, and severity of inflammation. Our clinical data support the notion that the lower immunopathogenesis of Omicron and, thus, lower produced inflammation is a major reason for a more favorable clinical outcome compared to Alpha and Delta variants.
Acknowledgments
We would like to express our appreciation to Peter Hotez, who helped us to enrich our results and findings by providing scientific feedbacks. We are grateful to the VA Informatics and Computing Infrastructure (VINCI) and VA COVID-19 Shared Data Resource.
Appendix A
We determined the SARS-CoV-2 variants based on the time intervals that our team of clinicians provided us. The time intervals determined based on the based on phylogenetic trees were retrieved from Nextstrain [13]. To ensure confidence in the variant, we used the COVID-19 shared data resource (CSDR) in the VHA, where they reported the results of genetic sequence for the subsample of the SARS-CoV-2 virus. According to the CSDR, there were 2670 samples. Table A1 shows the distribution of samples based on one date versus the genetic sequences.
Table A1.
Reporting the number of cases grouped into three SARS-CoV-2 variants based on the genetic sequencing versus the date.
| Variant Determined Based on the Genetic Sequencing | |||||
|---|---|---|---|---|---|
| Alpha | Delta | Omicron | Total | ||
| Variant determined based on the date | Alpha | 42 | 2 | 2 | 46 |
| Delta | 18 | 2031 | 48 | 2097 | |
| Omicron | 0 | 0 | 527 | 527 | |
| Total | 60 | 2033 | 577 | 2670 | |
According to Table A1, we reported the precision, negative predictive value (NPV), sensitivity, specificity, and accuracy for each variant based on the one-versus-rest approach (see Table A2) [32].
Table A2.
Reporting the performance of the interval-based method for identifying SARS-CoV-2 variants versus the genetic sequencing on subsample 2670 cases.
| Alpha | Delta | Omicron | |
|---|---|---|---|
| Precision | 0.913 | 0.969 | 1.000 |
| Negative Predictive Value | 0.993 | 0.997 | 0.977 |
| Sensitivity | 0.700 | 0.999 | 0.913 |
| Specificity | 0.998 | 0.896 | 1.000 |
| Accuracy | 0.992 | 0.975 | 0.981 |
Appendix B
Figure A1.
In-hospital mortality among three variation groups: Alpha, Delta, and Omicron after the COVID-19 test.
Author Contributions
Study design, data analysis, interpreting results, and writing—original draft preparation, C.P.; study design and review and revising the original draft, S.T.-T.; study design, data analysis, and writing—original draft preparation, A.S.; study design and review and revising the original draft, A.C.; Study design, data analysis, and writing—original draft preparation, J.R.; Review and revising the original draft, B.J.S., B.H. and C.I.A.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Baylor College of Medicine (protocol code: H-47595; date of approval: 9 April 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available behind the VHA firewall, and they cannot leave the VHA electronic health records. Any request for data access requires official approval process.
Conflicts of Interest
The funding sources had no role in study design, methods, data collection and analysis, interpretation, and submission of the results. All authors declare no competing interests.
Funding Statement
The analysis was supported by seed funding from Baylor College of Medicine, Houston, Texas, United States, the Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, TX, United states and a national institute of health (NIH), National Heart, Lung, and Blood Institute (NHLBI) K25 funding (#:1K25HL152006-01), VA Clinical Science Research & Development (IK2 CX001981), and Artificial Intelligence/Machine Learning Consortium to Advance Health Equity and Researcher Diversity (AIM-AHEAD) funding (OD032581-01S1).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rubin E.J., Baden L.R., Morrissey S. Audio Interview: Understanding the Omicron Variant of SARS-CoV-2. N. Engl. J. Med. 2022;386:e27. doi: 10.1056/NEJMe2202699. [DOI] [PubMed] [Google Scholar]
- 2.Hachmann N.P., Miller J., Collier A.Y., Ventura J.D., Yu J., Rowe M., Bondzie E.A., Powers O., Surve N., Hall K., et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M., Amoako D.G., Everatt J., Bhiman J.N., Scheepers C., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/s0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulloa A.C., Buchan S.A., Daneman N., Brown K.A. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA. 2022;327:1286–1288. doi: 10.1001/jama.2022.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., Kam T.T., Gu H., Sit K.Y., Hsin M.K.Y., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 6.Peacock T.P., Brown J.C., Zhou J., Thakur N., Sukhova K., Newman J., Kugathasan R., Yan A.W.C., Furnon W., De Lorenzo G., et al. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. bioRxiv. 2022 doi: 10.1101/2021.12.31.474653. [DOI] [Google Scholar]
- 7.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringer D., Braude P., Myint P.K., Evans L., Collins J.T., Verduri A., Quinn T.J., Vilches-Moraga A., Stechman M.J., Pearce L., et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021;50:420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhargava A., Kim T., Quine D.B., Hauser R.G. A 20-year evaluation of LOINC in the United States’ largest integrated health system. Arch. Pathol. Lab. Med. 2020;144:478–484. doi: 10.5858/arpa.2019-0055-OA. [DOI] [PubMed] [Google Scholar]
- 10.Park C., Razjouyan J., Hanania N.A., Helmer D.A., Naik A.D., Lynch K.E., Amos C.I., Sharafkhaneh A. Elevated Risk of Chronic Respiratory Conditions within 60 Days of COVID-19 Hospitalization in Veterans. Healthcare. 2022;10:300. doi: 10.3390/healthcare10020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razjouyan J., Helmer D.A., Li A., Naik A.D., Amos C.I., Bandi V., Sharafkhaneh A. Differences in COVID-19-related testing and healthcare utilization by race and ethnicity in the veterans health administration. J. Racial. Ethn. Health Disparities. 2022;9:519–526. doi: 10.1007/s40615-021-00982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razjouyan J., Helmer D.A., Lynch K.E., Hanania N.A., Klotman P.E., Sharafkhaneh A., Amos C.I. Smoking status and factors associated with COVID-19 in-hospital mortality among US veterans. Nicotine Tob. Res. 2022;24:785–793. doi: 10.1093/ntr/ntab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., Sagulenko P., Bedford T., Neher R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty C., Sharma A.R., Bhattacharya M., Mallik B., Nandi S.S., Lee S.-S. Comparative genomics, evolutionary epidemiology, and RBD-hACE2 receptor binding pattern in B.1.1.7 (Alpha) and B.1.617.2 (Delta) related to their pandemic response in UK and India. Infect. Genet. Evol. 2022;101:105282. doi: 10.1016/j.meegid.2022.105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paudel S., Dahal A., Bhattarai H.K. Temporal Analysis of SARS-CoV-2 Variants during the COVID-19 Pandemic in Nepal. COVID. 2021;1:423–434. [Google Scholar]
- 16.Orkaby A.R., Nussbaum L., Ho Y.L., Gagnon D., Quach L., Ward R., Quaden R., Yaksic E., Harrington K., Paik J.M., et al. The Burden of Frailty Among U.S. Veterans and Its Association With Mortality, 2002–2012. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1257–1264. doi: 10.1093/gerona/gly232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 18.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki R., Yamasoba D., Kimura I., Wang L., Kishimoto M., Ito J., Morioka Y., Nao N., Nasser H., Uriu K., et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jassat W., Abdool Karim S.S., Mudara C., Welch R., Ozougwu L., Groome M.J., Govender N., von Gottberg A., Wolter N., Wolmarans M., et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: A retrospective observational study. Lancet Glob. Health. 2022;10:e961–e969. doi: 10.1016/S2214-109X(22)00114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fall A., Eldesouki R.E., Sachithanandham J., Morris C.P., Norton J.M., Gaston D.C., Forman M., Abdullah O., Gallagher N., Li M., et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: An investigation of hospital admissions and upper respiratory viral loads. EBioMedicine. 2022;79:104008. doi: 10.1016/j.ebiom.2022.104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhi S.A., Kwatra G., Myers J.E., Jassat W., Dhar N., Mukendi C.K., Nana A.J., Blumberg L., Welch R., Ngorima-Mabhena N., et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022;386:1314–1326. doi: 10.1056/NEJMoa2119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledford H. How severe are Omicron infections? Nature. 2021;600:577–578. doi: 10.1038/d41586-021-03794-8. [DOI] [PubMed] [Google Scholar]
- 24.Skarbinski J., Wood M.S., Chervo T.C., Schapiro J.M., Elkin E.P., Valice E., Amsden L.B., Hsiao C., Quesenberry C., Corley D.A., et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: A retrospective cohort study. Lancet Reg. Health Am. 2022;12:100297. doi: 10.1016/j.lana.2022.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., Louca P., May A., Figueiredo J.C., Hu C., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharyya R.P., Hanage W.P. Challenges in Inferring Intrinsic Severity of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022;386:e14. doi: 10.1056/NEJMp2119682. [DOI] [PubMed] [Google Scholar]
- 27.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., Al-Khatib H.A., Smatti M.K., Coyle P., Al-Kanaani Z., et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz M.J., Jump R.L.P. Omicron infection milder in nursing home residents. Lancet Healthy Longev. 2022;3:e314–e315. doi: 10.1016/S2666-7568(22)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auvigne V., Vaux S., Strat Y.L., Schaeffer J., Fournier L., Tamandjou C., Montagnat C., Coignard B., Levy-Bruhl D., Parent du Chatelet I. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: A retrospective, population-based, matched cohort study. EClinicalMedicine. 2022;48:101455. doi: 10.1016/j.eclinm.2022.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krutikov M., Stirrup O., Nacer-Laidi H., Azmi B., Fuller C., Tut G., Palmer T., Shrotri M., Irwin-Singer A., Baynton V., et al. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): A prospective, cohort study. Lancet Healthy Longev. 2022;3:e347–e355. doi: 10.1016/S2666-7568(22)00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., Huang I., Lukito A.A., Suastika K., Kuswardhani R.A.T., et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2021;93:104324. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownlee J. One-vs-Rest and One-vs-One for Multi-Class Classification. Machine Learning Mastery. 2020. [(accessed on 6 January 2023)]. Available online: https://machinelearningmastery.com/one-vs-rest-and-one-vs-one-for-multi-class-classification/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available behind the VHA firewall, and they cannot leave the VHA electronic health records. Any request for data access requires official approval process.