Abstract
An important function of the RTG signaling pathway is maintenance of intracellular glutamate supplies in yeast cells with dysfunctional mitochondria. Herein, we report that MKS1 is a negative regulator of the RTG pathway, acting between Rtg2p, a proximal sensor of mitochondrial function, and the bHLH transcription factors Rtg1p and Rtg3p. In mks1Δ cells, RTG target gene expression is constitutive, bypassing the requirement for Rtg2p, and is no longer repressible by glutamate. We show further that Mks1p is a phosphoprotein whose phosphorylation pattern parallels that of Rtg3p in response to activation of the RTG pathway, and that Mks1p is in a complex with Rtg2p. MKS1 was previously implicated in the formation of [URE3], an inactive prion form of a negative regulator of the nitrogen catabolite repression pathway, Ure2p. rtgΔ mutations induce [URE3] and can do so independently of MKS1. We find that glutamate suppresses [URE3] formation, suggesting that the Mks1p effect on the formation of [URE3] can occur indirectly via regulation of the RTG pathway.
INTRODUCTION
Cells are able to monitor and respond to the functional state of their organelles. In animal cells, for example, compromises in mitochondrial function or certain external cues can lead to increased expression of genes encoding components of the mitochondrial oxidative phosphorylation apparatus and, in some instances, lead to a general increase in the biogenesis of mitochondria (Lunardi and Attardi, 1991; Scarpulla, 1997; Biswas et al., 1999; Heddi et al., 1999; Murdock et al., 1999; Wu et al., 1999; Amuthan et al., 2001). These events are controlled, in part, by transcriptional activators and coactivators whose targets include nuclear genes encoding mitochondrial proteins.
Yeast cells also respond to mitochondrial dysfunction by altering the expression of a subset of nuclear genes (Parikh et al., 1987, 1989). This response, called retrograde regulation, functions to better adapt cells to the mitochondrial defects. In derepressed, respiratory-deficient cells, such as those that have lost their mitochondrial DNA (ρο petites), the expression of genes involved in anaplerotic pathways, small molecule transport, peroxisomal activities, and stress responses is up-regulated (Liao et al., 1991; Liu and Butow, 1999; Hallstrom and Moye-Rowley, 2000; Traven et al., 2000; Epstein et al., 2001). In many cases, these changes in gene expression reflect activities that would compensate for the block in the tricarboxylic acid (TCA) cycle caused by the respiratory defect. Expression of a number of these retrograde responsive genes, such as CIT2, DLD3, and PDH1 encoding, respectively, a glyoxylate cycle isoform of citrate synthase, a cytosolic d-lactate dehydrogenase, and a protein involved in propionate metabolism, is controlled by RTG1, RTG2, and RTG3. Rtg1p and Rtg3p are basic helix-loop-helix transcription factors (Jia et al., 1997), and Rtg2p is a cytoplasmic protein with an N-terminal ATP-binding domain similar to that of the actin/sugar kinase/hsp70 superfamily (Bork et al., 1992). Rtg2p plays a pivotal role in the retrograde pathway, because it is both a sensor of the functional state of mitochondria and is required for the activation of RTG-dependent gene expression by promoting the cytoplasmic-to-nuclear translocation of Rtg1p and Rtg3p (Sekito et al., 2000). In addition, Rtg2p is required for the partial dephosphorylation of Rtg3p associated with its nuclear accumulation.
The retrograde pathway senses the functional state of mitochondria via the level of glutamate (Liu and Butow, 1999; Sekito et al., 2000; Epstein et al., 2001). Because glutamate is a potent repressor of RTG-dependent gene expression, the retrograde pathway is likely to be important for glutamate homeostasis. In cells with compromised or dysfunctional mitochondria, the expression of CIT1, ACO1, IDH1, and IDH2 (genes encoding the enzymes catalyzing the first three steps in the TCA cycle that lead to the production of α-ketoglutarate, the direct precursor of glutamate) is controlled by the RTG genes (Liu and Butow, 1999); in cells with robust mitochondrial function, expression of these genes is under control of the HAP transcription complex (Forsburg and Guarente, 1989; Rosenkrantz et al., 1994). A connection between the retrograde pathway and nitrogen metabolism has recently been uncovered by the finding that the target of rapamycin (TOR) kinase pathway in yeast also involves the RTG genes (Komeili et al., 2000; Shamji et al., 2000). The TOR kinase pathway promotes the formation of a complex between Gln3p, a transcription factor that controls the expression of genes required for the utilization of poor nitrogen sources (Mitchell and Magasanik, 1984; Courchesne and Magasanik, 1988; Coschigano and Magasanik, 1991; Blinder et al., 1996), and Ure2p, a negative regulator of Gln3p (Beck and Hall, 1999). When the TOR kinase pathway is inhibited by rapamycin, or when cells are grown on a poor nitrogen source such as urea, Rtg1p and Rtg3p translocate from the cytoplasm to the nucleus in an Rtg2p-dependent manner to activate target gene expression (Komeili et al., 2000).
Herein, we report the identification of a new regulatory gene in the RTG pathway, MKS1. Our data show that MKS1, originally described as a regulator of the Ras-cAMP pathway (Matsuura and Anraku, 1993), is a negative regulator of RTG-dependent gene expression. MKS1 was also known as LYS80, a negative regulator of lysine biosynthesis (Feller et al., 1997) and, more recently, proposed to regulate the nitrogen catabolite repression (NCR) pathway via its effects on the negative regulator of that pathway, Ure2p (Edskes et al., 1999). Mks1p was reported to be required for the generation of [URE3], the inactive prion form of Ure2p (Edskes and Wickner, 2000). We find that glutamate is a potent repressor of [URE3] formation, and that inactivation of the RTG pathway, which causes a decrease in the intracellular glutamate supply, induces [URE3].
MATERIALS AND METHODS
Media, Growth Conditions, Strains, and Constructs
Cells were grown at 30°C in YPR medium (YP + 2% raffinose), YNBR + cas medium (0.67% yeast nitrogen base containing 1% casamino acids and 2% raffinose), or YNBD (0.67% yeast nitrogen base and 2% dextrose). Glutamate was added at concentrations indicated in the text and figures. Where required, media were supplemented with 30 mg/l leucine, 30 mg/l lysine, 20 mg/l uracil as described (Rose et al., 1990). Ureidosuccinic acid was added at a final centration of 200 μg/ml. Rapamycin was dissolved in 10% Tween 20/90% ethanol and added at a final concentration of 0.2 μg/ml.
The ρ+ and ρο derivative of strain PSY142 (MATα leu2 lys2 ura3) and their rtg2Δ and rtg3Δ derivatives have been previously described, as well as the derivatives of these strains containing a single chromosomal copy of a CIT2-lacZ reporter gene (Liu and Butow, 1999; Sekito et al., 2000). Similar derivatives of strain MLY42 (MATα ura3 leu2), a Σ1278b derivative, and CC34 (MATα trp1 ade2 leu2 his3 ura2::HIS3) were constructed as done for strain PSY142. The LEU2+ derivatives of MLY42 were obtained by transforming cells with YDp-LEU2 digested with BamHI. mks1Δ derivatives of these strains were constructed by replacing the MKS1 locus (from −66 to +1595) with URA3 or LEU2. The various ura2Δ derivatives of these strains used to detect ureidosuccinic acid (USA)+ colonies (see below) were obtained by replacing the URA2 locus (from +45 to +6594) with the kanMX4 cassette (Wach et al., 1994). Strain RBY453 (MATa ura2 met) was used as a tester in crosses for the genetic properties of USA+ cells. The original mks1 mutants were obtained by screening ethyl methanesulfonate-mutagenized PSY142 rtg2Δ ρ+ cells containing an integrated CIT2-lacZ reporter gene for the appearance of dark blue colonies on solid YNBR + cas medium containing X-Gal.
pRS416Mks1-GFP was constructed by polymerase chain reaction (PCR) amplification of the MKS1 coding and 5′-flanking regions from −1007 to +1752 by using the oligonucleotides 5′-TAGGAGCTCGGGGATGCCCAAGTTTAT-3′ and 5′-TTACTCGAGCTATTCGCCCTCCATTACTC-3′ (restriction sites used for cloning are underlined). The oligonucleotides 5′-CGAGGTACCTTACTAAGTTAAATAAATCAGATA-3′ and 5′-TTACTCGAGCTATTCGCCCTCCATTACTC-3′ were used to PCR amplify 550 base pairs of the 3′-untranslated region of MKS1. The PCR products were cleaved with the appropriate restriction enzymes, and a 727-base pair XhoI-KpnI fragment containing the coding region of a bright green version of green fluorescent protein (GFP) was cloned into the XbaI-BamHI site of the yeast centromere plasmid pRS416. Transplacement of Mks1-GFP into the genomic MKS1 locus was conducted as described by Sekito et al. (2000). Transplacement of Rtg3-GFP into the genomic RTG3 locus was described previously (Sekito et al., 2000).
Assays
β-Galactosidase assays were carried out as described (Liu and Butow, 1999). Assays were conducted in triplicate and independent experiments were carried out two or three times. Trichloroacetic acid precipitation of total yeast cell proteins, treatment with calf intestinal phosphatase, SDS-PAGE, and immunodetection of proteins by Western blotting were carried out as described (Sekito et al., 2000). Rtg3p was detected with anti-Rtg3p antiserum (Sekito et al., 2000). Mks1p was detected as a C-terminal, triple hemagglutinin (HA)-tagged derivative, Mks1p(HA)3, in cells from a construct cloned into the plasmid pRS416 by using a monoclonal anti-HA antibody (12CA5). Dephosphorylation of whole-cell extracts was performed by incubation of neutralized TCA precipitates at 30°C for 30 min, with 5 U of calf intestinal phosphatase or 80 U of λPPase. For Northern blot analysis, cells were grown to OD 0.8 in YNBR + cas medium. RNA extraction and Northern blot analysis were performed as described (Liu and Butow, 1999). A 3.3-kb MKS1 DNA probe was labeled using a Random primed DNA labeling kit (Roche Applied Science, Indianapolis, IN). The Rtg1p, Mks1p, and Rtg3p-GFP fusion proteins were expressed from constructs transplaced into the respective RTG1 and RTG3 chromosomal loci and their visualization in cells by immunofluorescence microscopy was carried out as described (Sekito et al., 2000). To assay for the USA+ phenotype, the various strains noted in the text containing a ura2Δ mutation were individually cultured to saturation in YPD (1% yeast extract, 2% bacto peptone, and 2% dextrose). Between 106 and 107 cells were plated on YNBD medium supplemented with appropriate amino acids, and 200 μg/ml USA. The number of USA+ colonies per plate was determined after 5 d of incubation at 30°C. Genetic analyses of the USA+ phenotype were carried out by standard procedures. Guanidine curing of USA+ cells was carried out by plating cells from USA+ colonies on YPD plates containing 5 mM guanidine HCl. After 3 d growth at 30°C, the cells were replated on YPD-5 mM guanidine HCl plates, grown again for 3 d, and then scored for USA+ phenotype as described above.
Coimmunoprecipitation Experiments
Preparations of cell extract for coimmunoprecipitation were done as previously described (Sekito et al., 2000) except that solution A containing phosphatase inhibitors (0.1 mM Na3VO4, 50 mM NaF, and 5 mM sodium pyrophosphate). Five hundred-microliter cell extracts (300 ng/μl protein) were incubated at 4°C for 2 h with monoclonal anti-myc antibody (9E10), after which 100 μl of a 50% slurry of protein G-Sepharose (Roche Applied Science) was added for each reaction then further incubated at 4°C for 2 h. The immune complexes bound to the Sepharose beads were released by boiling in SDS-PAGE sample buffer after washing five times with solution A. The released immune complexes were analyzed by Western blotting with a monoclonal anti-myc antibody or monoclonal anti-HA antibody. A construct encoding Mks1p(myc)3 was transplaced into the chromosomal MKS1 locus. Rtg2p(HA)3 and Rtg3p(HA)3 were expressed in cells from constructs cloned into the plasmid pRS416. A similar protocol was used in the reciprocal coimmunoprecipitation experiments with Mks1(HA)3 and myc-tagged Rtg2p and Rtg3p derivatives.
RESULTS
MKS1 Mutations Bypass the Requirement for Rtg2p in Rtg1p-Rtg3p Target Gene Expression
To identify additional regulatory genes in the retrograde pathway, we performed a suppressor screen for mutations that would allow expression of an integrated CIT2-lacZ reporter gene in rtg2Δ cells. This screen yielded 14 recessive, single-gene mutations that fell into two complementation groups. Subsequent screening of the mutants transformed with a wild-type yeast centromeric-based plasmid library revealed that the high level of CIT2-lacZ expression in the mutagenized rtg2Δ cells of one of the complementation groups could be restored to a low level of expression by MKS1. Details of the other complementation group will be reported elsewhere.
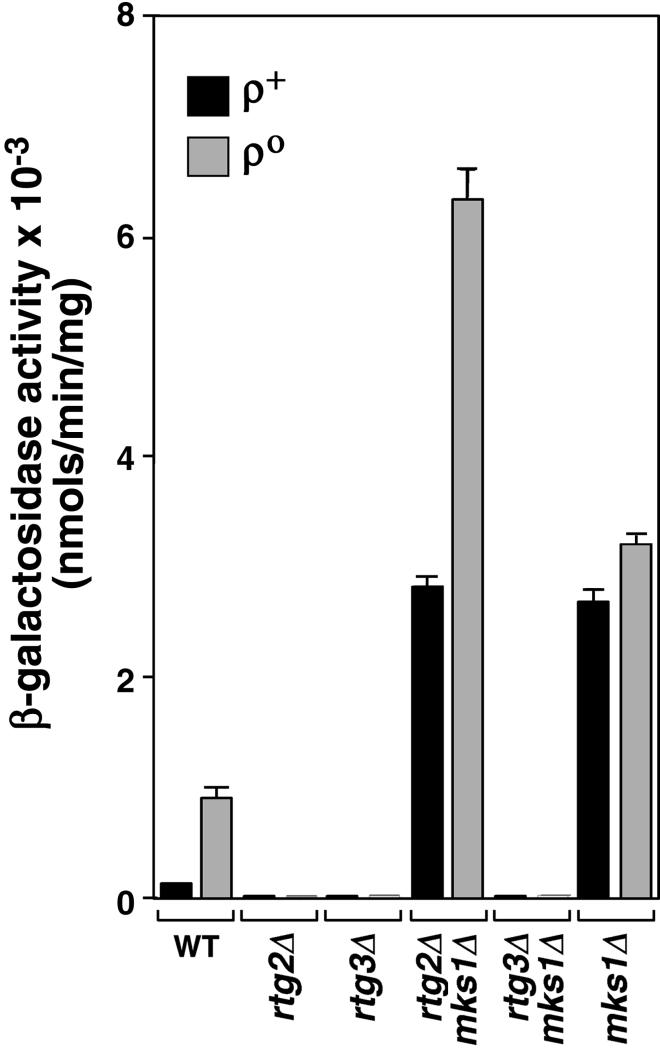
Sequencing of two of the mks1 mutant alleles showed that each contained a chain termination codon (our unpublished data). To verify the mutant phenotype and to characterize more generally the effects of MKS1 on RTG-dependent gene expression, we examined the effects of an mks1Δ mutation on the expression of an integrated CIT2-lacZ reporter gene in wild-type and rtg2Δ or rtg3Δ derivatives of ρ+ and ρο cells of strain PSY142 (Figure 1). Typical of the retrograde response, reporter gene expression was greater in respiratory-deficient ρο cells, and expression in both ρ+ and ρο cells was completely dependent on RTG2 and RTG3. However, the block in reporter gene expression in rtg2Δ cells was not only suppressed by the mks1Δ mutation but also in both the ρ+ and ρο rtg2Δ mks1Δ double mutants, expression levels were significantly greater than observed in otherwise wild-type ρο cells. In contrast, the mks1Δ mutation was unable to suppress the block in reporter gene expression in rtg3Δ cells, suggesting that suppression of the rtg2Δ mutation by mks1Δ does not occur by activation of some alternative pathway. Finally, the mks1Δ mutation alone resulted in high levels of CIT2-lacZ expression, exceeding even that observed in ρο cells.
Figure 1.
Inactivation of MKS1 reverses the block in CIT2 expression in rtg2Δ cells. β-Galactosidase activities were determined in triplicate in whole-cell extracts of the indicated strains grown to midlog phase in YNBR + cas medium. Each strain contained a CIT2-lacZ reporter gene integrated at the CIT2 locus.
MKS1 Negatively Regulates RTG-dependent Gene Expression Upstream of Rtg1p-Rtg3p
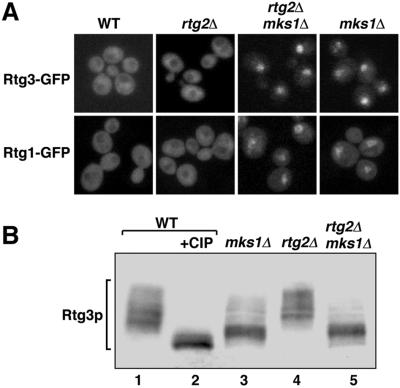
To investigate the cellular and molecular events associated with MKS1 regulation of the RTG pathway, we first examined the intracellular localization of functional derivatives of Rtg3p and Rtg1p, each tagged at their C terminus with GFP and transplaced into the respective RTG3 and RTG1 chromosomal loci. In agreement with our previous findings (Sekito et al., 2000), these transcription factors were predominantly cytoplasmic in wild-type ρ+ cells in which CIT2 expression is low (Figure 2A). In mks1Δ and mks1Δ rtg2Δ double mutant ρ+ cells, however, they were predominantly nuclear, consistent with the high level of CIT2-lacZ expression in those mutants. Nuclear localization of Rtg3p was confirmed by colocalization of nuclear DNA stained with 4′,6′-diamino-2-phenylindole (our unpublished data).
Figure 2.
Effects of inactivation of MKS1 in wild-type and rtg2Δ cells on Rtg1p and Rtg3p. (A) Subcellular localization of C-terminal GFP derivatives of Rtg1p and Rtg3p in ρ+ wild-type (WT) and mutant derivatives of strain PSY142. Constructs encoding the GFP derivatives were transplaced into the respective RTG1 and RTG3 loci. (B) Rtg3p is dephosphorylated in mks1Δ cells grown in YNBD + cas medium. Rtg3p was detected by Western blotting with a polyclonal anti-Rtg3p antibody raised against recombinant Rtg3p (Sekito et al., 2000). Lane 2 shows that the mobility of Rtg3p collapses to a faster migrating species when an extract of wild-type cells was pretreated with 5 U of calf intestinal phosphatase (CIP).
Previous work established that in wild-type ρ+ cells, the cytoplasmic form of Rtg3p is phosphorylated at multiple sites (Sekito et al., 2000), and that activation of RTG-dependent gene expression in respiratory-deficient ρo cells leads to the partial dephosphosphorylation of Rtg3p accompanying its nuclear accumulation (together with nuclear accumulation of Rtg1p). Furthermore, in rtg2Δ mutant cells in which CIT2 expression is undetectable, not only is the nuclear accumulation of Rtg3p and Rtg1p blocked but also Rtg3p becomes hyperphosphorylated relative to its phosphorylation state in wild-type ρ+ cells. We therefore determined whether the inactivation of MKS1 in ρ+ wild-type and rtg2Δ cells also affected the phosphorylation state of Rtg3p. Using polyclonal antiserum raised against recombinant Rtg3p to detect Rtg3p by Western blotting, we found that in wild-type and rtg2Δ cells, introduction of the mks1Δ mutation resulted in an extensive dephosphorylation of Rtg3p (Figure 2B).
RTG-dependent gene expression is also regulated by glutamate via a negative feedback loop, whereby low levels of glutamate activate the RTG pathway, and high levels of glutamate inhibit it (Liu and Butow, 1999; Sekito et al., 2000). This regulation reflects one of the primary functions of the RTG pathway: to ensure that adequate supplies of glutamate are available for biosynthetic reactions. When the RTG pathway is debilitated, for instance, in rtg2Δ or rtg3Δ strains with compromised or dysfunctional mitochondria, these strains are glutamate auxotrophs (Liao and Butow, 1993; Jia et al., 1997; Liu and Butow, 1999). We therefore wished to determine whether Mks1p also functions in glutamate regulation of the RTG pathway.
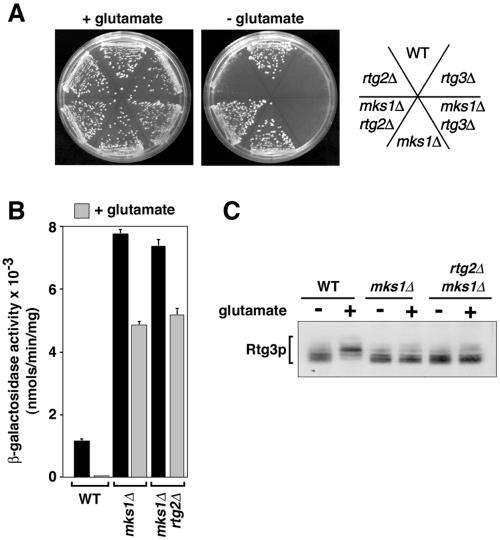
We first tested the effect of the mks1Δ mutation on the glutamate auxotrophy of rtg2Δ and rtg3Δ cells. As shown in Figure 3A, the inability of rtg2Δ cells to grow in medium lacking glutamate was reversed by introduction of the mks1Δ mutation; rtg3Δ cells, however, remained glutamate auxotrophs when MKS1 was inactivated. These observations are consistent with the restoration of CIT2 expression by the mks1Δ mutation in rtg2Δ, but not in rtg3Δ cells. In the converse experiment (Figure 3B), glutamate repression of CIT2-lacZ reporter gene expression in ρ+ cells grown in minimal dextrose medium was not only largely attenuated in mks1Δ and mks1Δ rtg2Δ cells but also the mks1Δ mutation by itself resulted in an even greater level of reporter gene expression than was observed in wild-type cells grown in medium lacking glutamate. Accompanying the strong glutamate repression of CIT2-lacZ expression was an increased phosphorylation of Rtg3p, an effect that was also suppressed by the mks1Δ mutation alone or in the presence of an rtg2Δ mutation (Figure 3C). Collectively, these data strongly support the conclusion that MKS1 is a negative regulator of the RTG pathway and suggest that Mks1p acts downstream of Rtg2p, but upstream of Rtg1p-Rtg3p.
Figure 3.
Inactivation of MKS1 alters glutamate regulation of the RTG pathway. (A) Glutamate auxotrophy of rtg2Δ but not of rtg3Δ cells is reversed by the mks1Δ mutation. Derivatives of PSY142 cells were plated on YNBD medium without or with 0.01% glutamate. (B) CIT2-lacZ reporter gene expression is not significantly repressed by glutamate in mks1Δ cells. β-Galactosidase assays were carried out on whole-cell extracts of the indicated strains derived from PSY142 grown in YNBD medium with or without supplementation with 0.2% glutamate. (C) Glutamate-dependent phosphorylation of Rtg3p is reversed by the mks1Δ mutation. The increase in phosphorylation of Rtg3p is observed when wild-type PSY142 ρ+ cells are grown in YNBD medium containing 0.2% glutamate but not in mks1Δ or mks1Δ rtg2Δ derivatives.
Mks1p Is a Phosphoprotein
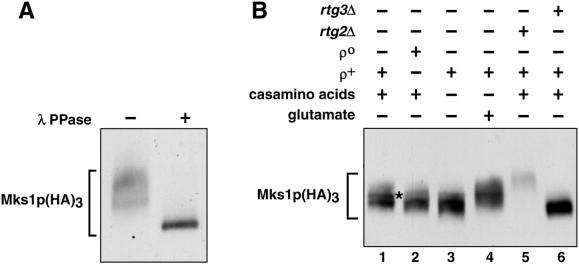
To investigate further the relation between MKS1 and RTG-dependent gene expression, we constructed a chimeric gene encoding a triple HA3 tag fused to the C terminus of Mks1p. This HA-tagged version of Mks1p [Mks1p(HA)3], which can complement the mks1Δ mutation, was used to follow the fate of Mks1p in a variety of cell types and growth conditions. Immunoblot analysis of Mks1p(HA)3 in extracts of ρ+ cells grown in medium containing 0.2% glutamate showed multiple electrophoretic forms that collapsed to a single, faster migrating species upon pretreatment of the extract with λ phosphatase, indicating that Mks1p is a phosphoprotein (Figure 4A). A comparison of ρ+ versus ρο cells grown in rich medium shows that Mks1p(HA)3 is more phosphorylated in ρ+ than in ρο cells (Figure 4B, lanes 1 and 2). Similarly, the addition of 0.2% glutamate to the growth medium resulted in an increase in the phosphorylation of Mks1p(HA)3 (compare Figure 4B, lanes 3 and 4). In rtg2Δ cells, Mks1p(HA)3 was much less abundant, but that which could be detected was hyperphosphorylated (Figure 4B, lane 5). In contrast, Mks1p(HA)3 was abundant and dephosphorylated in rtg3Δ cells (Figure 4B, lane 6). These results show that Mks1p is a phosphoprotein whose phosphorylation pattern changes in response to signals that affect RTG-dependent gene expression.
Figure 4.
Mks1p is a phosphoprotein. (A) Mks1(HA)3 was expressed in ρ+ mks1Δ cells grown in YNBD medium containing 0.2% glutamate. Mks1p(HA)3 was detected by Western blotting with a monoclonal anti-HA antibody either with or without pretreatment with λ phosphatase (λ PPase). (B) Western blot analysis of Mks1p(HA)3 in extracts of ρ+ or ρο cells grown in YNBD medium with or without the addition of 1% casamino acids or 0.2% glutamate as indicated. All strains were derived from PSY142. The asterisk indicates the position of a more phosphorylated species.
Rapamycin Treatment Results in Dephosphorylation of Mks1p and Rtg3p
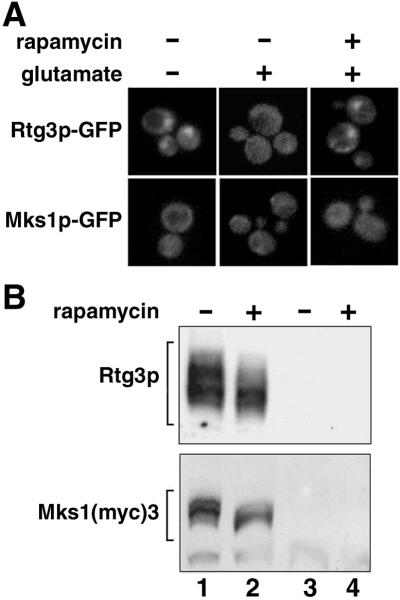
In addition to changes in the functional state of mitochondria, the intracellular localization of Rtg1p and Rtg3p has been shown to be affected by the quality of the nitrogen source in the medium (Komeili et al., 2000). Thus, in cells grown in medium containing poor nitrogen sources, CIT2 expression is activated and Rtg1p and Rtg3p accumulate in the nucleus. Activation of CIT2 expression and nuclear accumulation of these transcription factors was also shown to occur when cells were treated with rapamycin, an inhibitor of the TOR kinase pathway. We first confirmed that rapamycin addition to PSY142 cells grown in medium containing glutamate resulted in the nuclear localization of an Rtg3p-GFP derivative (Figure 5A). Under these conditions of treatment of cells with rapamycin there was a clear, partial dephosphorylation of Rtg3p (Figure 5B), consistent with our previous observations (Sekito et al., 2000) that activation of CIT2 expression and nuclear localization of Rtg3p (and Rtg1p) is associated with a partial dephosphorylation of Rtg3p. Mks1p also was partially dephosphorylated when cells were treated with rapamycin, but in contrast to Rtg3p, an Mks1-GFP derivative remained cytoplasmic in the presence of rapamycin or by the exclusion of glutamate from the growth medium. The similarity in the phosphorylation behavior of Mks1p and Rtg3p (Sekito et al., 2000) to conditions that affect RTG-dependent gene expression suggests that these proteins may be modified by the same kinase/phosphatase pathway.
Figure 5.
Effects of rapamycin on Rtg3p and Mks1p. (A) Effects of rapamycin and glutamate on the subcellular localization of Rtg3p-GFP and Mks1p-GFP. PSY142 ρ+ cells expressing Rtg3p-GFP or Mks1p-GFP from constructs transplaced into the respective chromosomal RTG3 or MKS1 loci treated for 30 min with 200 ng/ml rapamycin (+) or vehicle alone (−) were grown in YNBD with or without the addition of 0.2% glutamate. (B) Rapamycin induces dephosphorylation of Rtg3p and Mks1p. Extracts from PSY142 ρ+ cells grown in YNBD supplemented with 0.01% glutamate and treated with rapamycin or vehicle as described in A were analyzed by Western blotting with Rtg3p polyclonal anti-Rtg3p antiserum or monoclonal anti-myc antibody. Lanes 3 and 4 are extracts prepared from rtg3Δ cells (top) or from cells not containing the construct encoding Mks1(myc)3 (bottom).
Mks1p Is in a Complex with Rtg2p
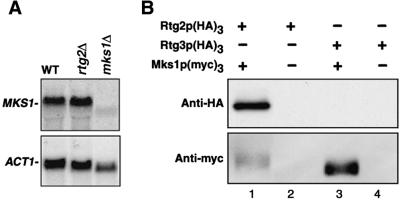
The dramatic decrease in the abundance of Mks1p(HA)3 observed in rtg2Δ cells was intriguing. Northern blot analysis of MKS1 mRNA showed that there was no difference in transcript levels between wild-type and rtg2Δ cells (Figure 6A). This raised the possibility that Mks1p might be present in a complex with Rtg2p and that in rtg2Δ cells, Mks1p would be destabilized. We therefore determined whether Mks1p and Rtg2p could be coprecipitated from whole-cell extracts of ρ+ cells grown in rich raffinose medium. Extracts of cells coexpressing a C-terminal, triple myc tag of Mks1p [Mks1p(myc)3] and an N-terminal triple HA-tagged derivative of Rtg2p [Rtg2p(HA)3] were immunoprecipitated with anti-myc antibody and analyzed by Western blotting. These experiments show that the anti-myc antibody coprecipitated Rtg2p(HA)3, and did so only in cells expressing Mks1p(myc)3 (Figure 6B, lanes 1 and 2); no detectable HA reactivity was observed in the immunoprecipitates of extracts from cells expressing a different HA-tagged protein, Rtg3p(HA)3 (Figure 6B, lanes 3 and 4). In a reciprocal experiment in which extracts from cells expressing Rtg2p(myc)3 and Mks1p(HA)3 or Rtg3p(HA)3 derivatives were immunoprecipitated with anti-myc antibody to pull-down Rtg2p, Mks1p(HA)3, but not Rtg3p(HA)3, was coprecipitated (our unpublished data). These data strongly suggest that Rtg2p and Mks1p are present in a complex that is not likely to include Rtg3p.
Figure 6.
Coimmunoprecipitation of Mks1p and Rtg2p. (A) Northern blot analysis of MKS1 transcripts were determined on total RNA prepared from PSY142 cells (B) Coimmunoprecipitation of (myc)3- and (HA)3-tagged derivatives of Mks1p and Rtg2p, respectively.
Inactivation of RTG Pathway Induces [URE3]
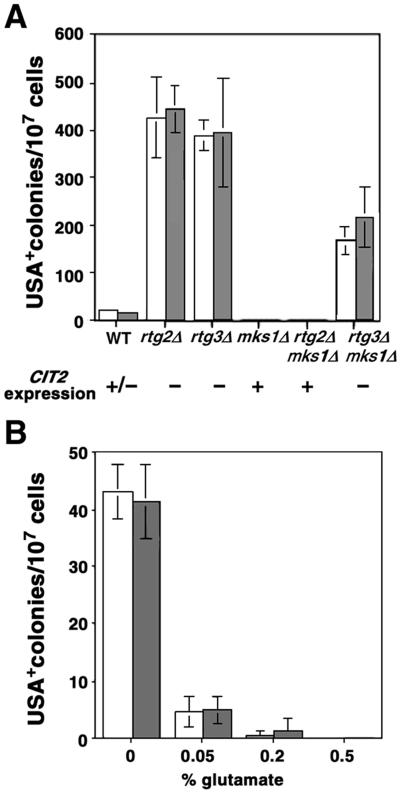
Recent studies have shown that Mks1p is required for the formation of [URE3], the inactive prion form of the negative regulator of Gln3, Ure2p (Edskes and Wickner, 2000). [URE3] was originally identified as a dominant, non-Mendelian determinant (Lacroute, 1971; Aigle and Lacroute, 1975), and first proposed by Wickner (1994) to be a yeast version of a prion. The infectious [URE3] prion is inherited as a stable, cytoplasmic element and shows altered structural properties based on the appearance of aggregates in vivo, protease resistance, and the ability to form amyloid in vitro (Taylor et al., 1999; Wickner et al., 2000). In the presence of high-quality nitrogen sources, the NCR pathway is inhibited by interaction between Ure2p and Gln3. Inactivation of Ure2p by its conversion to [URE3] releases Gln3p, allowing the expression of genes, such as DAL5 encoding an allantoate permease, thus enabling cells to take up USA. Given the apparent dual role of Mks1p in the RTG pathway and in [URE3] formation, we asked whether the RTG pathway itself influences [URE3] formation. To this end, we determined the effect of rtg2Δ and rtg3Δ mutations on the appearance of USA+ cells in two separate cultures of two randomly selected colonies of a derivative of strain MLY42 (Figure 7A). In the rtg2Δ and rtg3Δ strains, the frequency of USA+ colonies increased dramatically 20–30-fold over the spontaneous level of USA+ colonies in wild-type cells. Similar results were obtained with strain CC34 (our unpublished data). We verified that the USA+ cells that arose in cultures of the rtg2Δ and rtg3Δ strains were [URE3]: first, when mated to a USA− tester strain, the resultant diploids were USA+. Analysis of tetrads obtained after sporulation of the USA+ diploids showed a non-Mendelian segregation pattern for the USA+ phenotype, e.g., 4:0 and 0:4, in contrast to the strict 2:2 segregation of nuclear markers. Second, the USA+ phenotype could be cured to USA− by growth of cells on YPD medium containing 5 mM guanidine HCl, consistent with a previous report on the curing of the [URE3] prion (Wickner, 1994). Finally, to exclude the possibility that the rtg2 mutations result in some metabolic changes or inhibition of Ure2p function in some fraction of the cells that allow those cells to take up ureidosuccinic acid, and which is independent of [URE3] formation, we transformed a functional RTG2 gene on a centromeric plasmid into rtg2Δ USA+ cells. Despite restoration of the RTG pathway, all of the transformants remained USA+ (our unpublished data). These findings, together with the properties of dominance, non-Mendelian meiotic segregation, and curing, confirm that the USA+ colonies were [URE3].
Figure 7.
Effects of rtg mutations and glutamate on [URE3] formation. Two independent colonies of an MLY42 derivative (MATα ura2) were analyzed in parallel as indicated by the unshaded and shaded bars. (A) Effects of inactivation of RTG and MKS1 on [URE3] formation. Wild-type (derived from MLY42, a Σ1278b background strain) and isogenic rtg2Δ, rtg3Δ, mks1Δ, rtg2Δ mks1Δ, and mks1Δ rtg3Δ mutant derivatives (all contain an ura2Δ::kanMX4 mutation) were grown in YPD medium for 2 d to saturation. Cells were pelleted and washed twice with sterile water. For each strain, 106 or 107 cells were plated on each of seven YNBD plates supplemented with 200 μg/ml ureidosuccinic acid and 0.01% glutamate. The number of USA+ colonies per plate was determined after 5 d of incubation at 30°C. Two independent colonies of each strain were analyzed as indicated by the shaded and unshaded bars. Error bars indicate SD of colony numbers from seven plates. CIT2 expression refers to the qualitative level of CIT2 expression in the various strains grown in YPD medium. (B) Glutamate inhibits [URE3] formation. Cells were cultured and plated in the same way as in A except that YNBD plates supplemented with 200 μg/ml ureidosuccinic acid and the indicated amounts of glutamate were used. Error bars indicate SD of colony numbers from seven plates.
In agreement with the findings from Wickner's laboratory (Edskes et al., 1999; Edskes and Wickner, 2000), inactivation of MKS1 blocked the appearance of spontaneous USA+ colonies (Figure 7A). In a similar manner, the mks1Δ mutation blocked the appearance of USA+ colonies in the rtg2Δ mutants. In striking contrast, however, the rtg3Δ mutation reversed the block in USA+ colony formation observed in mks1Δ cells, giving a level of USA+ colonies about one-half of that observed in rtg3Δ cells alone. It is important to note, as is indicated in Figure 7A, that these effects of the mks1Δ mutation correlate directly with the presence or absence of CIT2 expression in those mutant cells (Figure 1). These results suggest that the control of [URE3] formation by MKS1 is effected at least in part through its control of RTG gene function. It has been reported that overproduction of Ure2p can lead to an increase in [URE3] formation (Wickner, 1994). Thus, one formal explanation for the increase in [URE3] formation when the retrograde pathway is inactivated is that it leads to an increased amount of Ure2p. However, using a functional GFP-tagged version of Ure2p and GFP-specific antiserum, we found that in rtg2 mutant cells, there was no detectable increase in the amount of Ure2p in cells in which the RTG pathway was inactivated (our unpublished data).
Glutamate Represses [URE3] Formation
How does the RTG pathway regulate [URE3] formation? Because a primary function of RTG target gene expression is to maintain intracellular supplies of glutamate, we reasoned that glutamate itself might regulate [URE3] formation. To test this, we determined the frequency of USA+ colonies that arose spontaneously in cultures of wild-type cells grown on minimal ammonia medium containing increasing concentrations of glutamate (Figure 7B). The results of this experiment show that the number of USA+ colonies decreased with increasing concentrations of glutamate in the medium. At 0.5% glutamate, USA+ colony formation was essentially completely inhibited. In control experiments, we have determined that glutamate has no effect on the survival of USA+ cells, does not convert USA+ to USA− cells, i.e., does not cure the [URE3] prion, and that the USA+ [URE3] cells can grow on USA medium containing 0.2% glutamate (our unpublished data). Similar observations for growth of [URE3] cells were noted by Aigle and Lacroute (1975). Together, these data indicate that glutamate is a negative regulator of [URE3] formation.
DISCUSSION
The findings presented herein show that MKS1 is a negative regulator of the RTG pathway. We identified MKS1 in a screen for mutations that would suppress the block in RTG-dependent gene expression in rtg2Δ cells. When MKS1 is inactivated, RTG-dependent gene expression is constitutive and no longer requires Rtg2p. Previous studies have established that Rtg2p is a proximal sensor of mitochondrial dysfunction, acting upstream of the transcription factors Rtg1p and Rtg3p (Rothermel et al., 1997; Liu and Butow, 1999; Sekito et al., 2000). Because the suppression of the block in gene expression in rtg2Δ cells by an mks1 null mutation still requires Rtg3p (as well as Rtg1p), the suppression is not likely to involve the activation of some alternative pathway of gene expression, for example, by the recruitment of surrogate transcription factors to the CIT2 promoter.
A number of well-defined physiological and molecular signatures are associated with regulation of the RTG pathway (Liao and Butow, 1993; Liu and Butow, 1999; Sekito et al., 2000). These include 1) glutamate auxotrophy of rtg mutant cells with compromised or reduced mitochondrial function, 2) translocation of Rtg1p and Rtg3p from the cytoplasm to the nucleus when RTG target gene expression is activated, 3) partial dephosphorylation of Rtg3p that accompanies its nuclear accumulation, and 4) hyperphosphorylation and cytoplasmic retention of Rtg3p in rtg2Δ cells. Inactivation of MKS1 in wild-type ρ+ and in rtg2Δ cells affects all of the above-mentioned processes in a manner fully consistent with the high-level of CIT2 expression observed in those mutants. Together, these findings suggest that Mks1p acts between Rtg2p and the Rtg1p-Rtg3p transcription complex in its negative regulation of RTG-dependent gene expression (Figure 8). A recent report has also suggested that Mks1p regulates Rtg1p-Rtg3p activity (Shamji et al., 2000). However, in contrast to the conclusions drawn from our findings, Mks1p was proposed to be a positive rather than a negative regulator of the RTG pathway. That conclusion was based on the observation that RTG target gene expression is not responsive to rapamycin in mks1Δ cells. In the experiments of Shamji et al. (2000), it is likely that the level of expression of the RTG target genes examined was already at or close to maximum because of the mks1Δ mutation, as we have described herein, and thus expression of those genes would have appeared to have been unresponsive to rapamycin.
Figure 8.
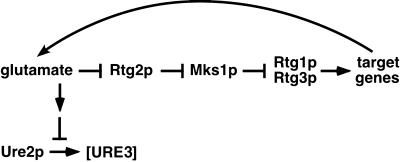
Model linking the negative feedback control of glutamate levels by the RTG pathway and [URE3] formation. Glutamate (or glutamine) could inhibit [URE3] formation directly or through an intermediary sensing mechanism.
Our studies have revealed for the first time that Mks1p is a phosphoprotein located in the cytoplasm whose phosphorylation state is regulated. It is intriguing that the phosphorylation state of Mks1p responds to the same signals and in the same way as Rtg3p phosphorylation. One obvious implication of these findings is that the phosphorylation state of Mks1p affects its regulation of the RTG pathway. Although we found that Mks1p is dephosphorylated in rtg3Δ cells in which the RTG pathway is inactive, that result can be easily reconciled with the notion that dephosphorylation of Mks1p relieves its negative regulation of RTG-dependent gene expression: we previously demonstrated that there is a feedback response in cells lacking a functional Rtg3p, as if these cells were attempting to activate the RTG pathway (Sekito et al., 2000). In those experiments, cells containing a cytoplasmic derivative of Rtg3p (ΔNLS) that was unable to translocate into the nucleus because it lacked its nuclear localization sequence, Rtg3p was dephosphorylated. When those cells were also transformed with a construct expressing a functional copy of Rtg3p, the ΔNLS Rtg3p derivative, still cytoplasmic, became phosphorylated. Ongoing studies directed at a mutational analysis of potential phosphorylation sites in Mks1p should clarify the relation between phosphorylation of the protein and its regulation of the RTG pathway.
Mks1p has been proposed to act within a number of different pathways in the cell, including the Ras-cAMP pathway (Matsuura and Anraku, 1993), lysine biosynthesis (Feller et al., 1997), the TOR kinase pathway (Shamji et al., 2000), and as a regulator of Ure2p (Edskes et al., 1999; Edskes and Wickner, 2000). Our finding that Mks1p is in a complex with Rtg2p implies an interplay between these two proteins in their regulation of RTG-dependent gene expression. The biochemical function of neither of these proteins is known, so that it is not possible to conclude at this time how their colocalization in a complex might regulate RTG-dependent gene expression. However, if we assume that the phosphorylation state of Mks1p is important for its regulation of the retrograde pathway, phosphorylation is not likely to be involved in the entry or release of either of these components into a common complex, because Mks1p and Rtg2p can be coimmunoprecipitated independently of the observed changes in Mks1p phosphorylation (Sekito, unpublished observations).
One possible clue to a function for Mks1p is that there are two regions of the protein, which together, share ∼50% sequence similarity to a 234-amino acid stretch in the N-terminal region of the Ppz1p phosphatase of yeast. The N-terminal domain of Ppz1p has been proposed to have a regulatory function for its catalytic phosphatase domain located in the C-terminal part of protein (Hughes et al., 1993). This observation, together with the finding that Mks1p is present in a complex with Rtg2p, may offer some clues as to how Mks1p might regulate the RTG pathway. As noted above, Rtg2p functions as a proximal sensor of mitochondrial dysfunction, translating mitochondrial signals to the Rtg1p–Rtg3p complex. Rtg2p thus acts as a switch to keep Rtg1p and Rtg3p tethered in the cytoplasm when the retrograde pathway is off, and to enable their release for nuclear entry when the pathway is turned on. This Rtg2p switch could be effected by modulating, via Mks1p, a phosphatase activity acting upon Rtg3p, so as to initiate Rtg3p's translocation to the nucleus. In the absence of Mks1p, the putative phosphatase would be constitutively active, resulting in RTG target gene expression that is no longer dependent on Rtg2p.
Recent genome-wide transcription studies and analyses of changes in gene expression resulting from dysfunctional mitochondria, treatment of cells with rapamycin, or growth of cells on different nitrogen sources show that both carbon and nitrogen metabolism are connected via the RTG pathway (Komeili et al., 2000; Shamji et al., 2000; Epstein et al., 2001). Our experiments are in agreement with previous studies (Komeili et al., 2000; Shamji et al., 2000) showing that treatment of cells with rapamycin activates RTG-dependent gene expression. We have found, however, that rapamycin treatment, as well as all other regimes we have tested that activate RTG-dependent gene expression, results in a dephosphorylation of Rtg3p, rather than an increase in the phosphorylation of the protein as reported by Komeili et al. (2000). Presently, we have no explanation for this discrepancy. It is worth noting that mutation of a number of potential phosphorylation sites in Rtg3p results in its constitutive nuclear localization (Sekito, unpublished observations), consistent with the view that dephosphorylation of Rtg3p is associated with its nuclear accumulation.
A striking connection between the RTG pathway and nitrogen metabolism was revealed by the finding that inactivation of the RTG-pathway by the rtg2Δ or rtg3Δ mutations results in a dramatic increase in the frequency of [URE3] cells. This increase in [URE3] formation can occur independently of MKS1 and is clearly related to a loss of RTG target gene expression. Thus, in rtg2Δ mks1Δ cells, in which CIT2 expression is high, [URE3] formation is at background levels, whereas in an rtg3Δ mks1Δ mutant, in which CIT2 expression is blocked, [URE3] formation is significantly higher than in wild-type cells. Our result showing that glutamate is a negative regulator of [URE3] formation explains how the rtg2Δ or rtg3Δ mutations would increase [URE3] formation and how the mks1Δ mutation alone decreases it (Edskes and Wickner, 2000): inactivation of the RTG pathway starves cells for glutamate (indeed, such cells are glutamate auxotrophs) (Liao and Butow, 1993; Jia et al., 1997). The low levels of glutamate would result in an increase in frequency of [URE3] formation; in contrast, high levels of RTG gene activity due to the inactivation of MKS1 would lead to increased glutamate levels and a suppression of [URE3] formation. As summarized in the model of Figure 8, the targets of the RTG genes that are directly involved in glutamate synthesis, together with the negative feedback control of the RTG pathway by glutamate, would influence the formation of the [URE3] prion. Our results do not rule out the possibility, however, that Mks1p might also regulate [URE3] formation by direct interaction with Ure2p, as suggested by Edskes and Wickner (2000), especially given the result shown in Figure 7A that the dramatic increase in [URE3] formation in rtg3Δ cells is somewhat suppressed by the mks1Δ mutation.
What might be the logic behind the link between the RTG pathway and [URE3] formation? As noted above, when cells are faced with poor nitrogen sources, both the NCR pathway and RTG-dependent gene expression are activated (Komeili et al., 2000; Shamji et al., 2000). The NCR pathway is activated because Ure2p has been released from a complex with Gln3p, allowing Gln3p to translocate to the nucleus (Beck and Hall, 1999). Because Ure2p needs to remain in an “active” state, poised to reassociate with and inactivate Gln3p when a high-quality nitrogen source becomes available, it would be a clear advantage to the cell to suppress conversion of Ure2p to the inactive [URE3] prion form, so as allow cells to switch rapidly between states suitable for the utilization of different quality nitrogen sources. As we have shown herein, such suppression occurs when RTG-dependent gene activity is high.
Finally, our results also shed light on how MKS1 could behave as a negative regulator of lysine biosynthesis (Feller et al., 1997). α-Ketoglutarate is a precursor to lysine, so that down-regulation of α-ketoglutarate synthesis would also down-regulate the lysine biosynthetic pathway. Because MKS1 negatively regulates RTG gene function, which is required for the production of α-ketoglutarate (Liu and Butow, 1999), a strong down-regulation of the RTG pathway by MKS1 would also be manifest as a down-regulation of lysine biosynthesis. Altogether, these findings underscore the central role played by the RTG pathway in connecting nitrogen and carbon metabolism to the functional state of mitochondria.
ACKNOWLEDGMENTS
We thank J. Heitman, A. Chelstowska, and C. Cullin for yeast strains. We also thank C. Cullin for advice on [URE3], A. Tizenor for graphics, as well as members of the Butow laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health and The Robert A. Welch Foundation (to R.A.B.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–09–0473. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–09–0473.
REFERENCES
- Aigle M, Lacroute F. Genetical aspects of [URE3], a non mitochondrial, cytoplasmically inherited mutation in yeast. Mol Gen Genet. 1975;136:327–335. doi: 10.1007/BF00341717. [DOI] [PubMed] [Google Scholar]
- Amuthan G, Biswas G, Zhang SY, Klein-Szanto A, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signaling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinder D, Coschigano PW, Magasanik B. Interaction of the GATA factor Gln3p with the nitrogen regulator Ure2p in Saccharomyces cerevisiae. J Bacteriol. 1996;178:4734–4746. doi: 10.1128/jb.178.15.4734-4736.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin and hsp70 heat shock proteins. Proc Natl Acad Sci USA. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano PW, Magasanik B. The URE2 gene product of Saccharomyces cerevisiae plays an important role in the cellular response to the nitrogen source and has homology to glutathione s-transferases. Mol Cell Biol. 1991;11:822–832. doi: 10.1128/mcb.11.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne WE, Magasanik B. Regulation of nitrogen assimilation in Saccharomyces cerevisiae: roles of the URE2 and GLN3 genes. J Bacteriol. 1988;170:708–713. doi: 10.1128/jb.170.2.708-713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes HK, Hanover JA, Wickner RB. Mks1p is a regulator of nitrogen catabolism upstream of Ure2p in Saccharomyces cerevisiae. Genetics. 1999;153:585–594. doi: 10.1093/genetics/153.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes HK, Wickner RB. A protein required for prion generation: [URE3] induction requires the Ras-regulated Mks1 protein. Proc Natl Acad Sci USA. 2000;97:6625–6629. doi: 10.1073/pnas.120168697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CB, Waddle JA, Hale IV W, Davé V, Thornton J, Macatee TL, Garner HR, Butow HR. Genome-wide responses to mitochondrial dysfunctions. Mol Biol Cell. 2001;12:297–308. doi: 10.1091/mbc.12.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A, Ramos F, Pierard A, Dubois E. Lys80p of Saccharomyces cerevisiae, previously proposed as a specific repressor of LYS genes, is a pleiotropic regulatory factor identical to Mks1p. Yeast. 1997;13:1337–1346. doi: 10.1002/(SICI)1097-0061(199711)13:14<1337::AID-YEA186>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Forsburg SL, Guarente L. Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol. 1989;5:153–180. doi: 10.1146/annurev.cb.05.110189.001101. [DOI] [PubMed] [Google Scholar]
- Hallstrom TC, Moye-Rowley S. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J Biol Chem. 2000;275:34347–37356. doi: 10.1074/jbc.M007338200. [DOI] [PubMed] [Google Scholar]
- Heddi A, Stepien G, Benke PJ, Wallace DC. Coordinate induction of energy gene expression in tissues of mitochondrial disease patients. J Biol Chem. 1999;274:22968–22976. doi: 10.1074/jbc.274.33.22968. [DOI] [PubMed] [Google Scholar]
- Hughes V, Mulle A, Stark MJR, Cohen PTW. Both isoforms of protein phosphatase Z are essential for the maintenance of cell size and integrity in Saccharomyces cerevisiae in response to osmotic stress. Eur J Biochem. 1993;216:269–279. doi: 10.1111/j.1432-1033.1993.tb18142.x. [DOI] [PubMed] [Google Scholar]
- Jia Y, Rothermel B, Thornton J, Butow RA. A basic helix-loop-helix zipper transcription complex functions in a signaling pathway from mitochondria to the nucleus. Mol Cell Biol. 1997;17:1110–1117. doi: 10.1128/mcb.17.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili A, Wedaman KP, O'Shea EK, Powers T. Mechanism of metabolic control: target of rapamycin signaling links nitrogen quality to the activity of the Rtg1 and Rtg3 transcription factors. J Cell Biol. 2000;151:863–878. doi: 10.1083/jcb.151.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971;106:519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Butow RA. RTG1 and RTG2: Two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell. 1993;72:61–71. doi: 10.1016/0092-8674(93)90050-z. [DOI] [PubMed] [Google Scholar]
- Liao XS, Small WC, Srere PA, Butow RA. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Butow RA. A transcriptional switch in the expression of yeast tricarboxylic acid cycle genes in response to a reduction or loss of respiratory function. Mol Cell Biol. 1999;19:6720–6728. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardi J, Attardi G. Differential regulation of expression of the multiple ADP/ATP translocase genes in human cells. J Biol Chem. 1991;266:16534–16540. [PubMed] [Google Scholar]
- Matsuura A, Anraku Y. Characterization of the MKS1 gene, a new negative regulator of the Ras-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Gen Genet. 1993;238:6–16. doi: 10.1007/BF00279524. [DOI] [PubMed] [Google Scholar]
- Mitchell AP, Magasanik B. Regulation of glutamine-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:2758–2766. doi: 10.1128/mcb.4.12.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock DG, Boone BE, Esposito LA, Wallace DC. Up-regulation of nuclear and mitochondrial genes in the skeletal muscle of mice lacking the heart/muscle isoform of the adenine nucleotide translocator. J Biol Chem. 1999;274:14429–14433. doi: 10.1074/jbc.274.20.14429. [DOI] [PubMed] [Google Scholar]
- Parikh VS, Conrad-Webb H, Docherty R, Butow RA. Interaction between the yeast mitochondrial and nuclear genomes influences the abundance of novel transcripts derived from the spacer region of the nuclear ribosomal DNA repeat. Mol Cell Biol. 1989;9:1897–1907. doi: 10.1128/mcb.9.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh VS, Morgan MM, Scott R, Clements LS, Butow RA. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987;235:576–580. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Heiter P. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- Rosenkrantz M, Kell CS, Pennell EA, Devenish LJ. The HAP2,3,4 transcriptional activator is required for derepression of the yeast citrate synthase gene, CIT1. Mol Microbiol. 1994;13:119–131. doi: 10.1111/j.1365-2958.1994.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Rothermel B, Thornton J, Butow RA. Rtg3p, a basic helix-loop-helix/leucine zipper protein functions in mitochondrial-induced changes in gene expression, contains independent activation domains. J Biol Chem. 1997;272:19801–19807. doi: 10.1074/jbc.272.32.19801. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression in mammalian cells. J Bioenerg Biomembr. 1997;29:109–119. doi: 10.1023/a:1022681828846. [DOI] [PubMed] [Google Scholar]
- Sekito T, Thornton J, Butow RA. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol Biol Cell. 2000;11:2103–2115. doi: 10.1091/mbc.11.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamji AF, Kuruvilla FG, Schreiber SL. Partitioning the transcriptional program induced by rapamycin among the effectors of the TOR proteins. Curr Biol. 2000;10:1574–1581. doi: 10.1016/s0960-9822(00)00866-6. [DOI] [PubMed] [Google Scholar]
- Taylor KL, Cheng N, Williams RW, Steven AC, Wickner RB. Prion domain initiation of amyloid formation in vitro from native Ure2p. Science. 1999;283:1339–1343. doi: 10.1126/science.283.5406.1339. [DOI] [PubMed] [Google Scholar]
- Traven A, Wong JM, Xu D, Sopta M, Ingles CJ. Inter-organellar communication: altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J Biol Chem. 2000;27:27. doi: 10.1074/jbc.M006807200. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Taylor KL, Edskes HK, Maddelein ML, Moriyama H, Roberts BT. Prions of yeast as heritable amyloidoses. J Struct Biol. 2000;130:310–322. doi: 10.1006/jsbi.2000.4250. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]