Abstract
Infectious diarrhoea contributes to high morbidity and mortality in young children from sub-Saharan Africa. The aim of this study was to assess the prevalence of single and multiple diarrhoeal-causing pathogen combinations in children suffering from diarrhoea from rural and peri-urban communities in South Africa. A total of 275 diarrhoea stool specimens were collected between 2014 and 2016 from Hospitals and Primary Health Care clinics. The BioFire® FilmArray® Gastrointestinal panel was used to simultaneously detect 22 diarrhoea pathogens (viruses, bacteria, parasites) known to cause diarrhoea. A total of 82% (226/275) enteric pathogens were detected in the stool specimens. The two most detected bacterial, viral and parasitic pathogens each included: EAEC (42%), EPEC (32%), Adenovirus F40/41 (19%), Norovirus (15%), Giardia (8%) and Cryptosporidium (6%), respectively. Single enteric pathogen infections were recorded in 24% (65/275) specimens with EAEC, and Norovirus was found in 26% (17/65) and 14% (9/65) of the specimens, respectively. Multiple enteric pathogen combinations were recorded in 59% (161/275) of the stool specimens with 53% (85/161) containing two pathogens, 22% (35/161) containing three pathogens and 25% (41/161) containing four or more pathogens. The results from this study demonstrated the complex nature of pathogen co-infections in diarrhoeal episodes which could have an impact on treatment effectiveness.
Keywords: diarrhoea, infectious, pathogens, paediatric patients, South Africa, stool specimens
1. Introduction
Diarrhoea is defined as passage of three or more unusually loose stool or watery stool of any frequency within 24 h [1]. The World Health Organisation (WHO) reports that almost 1.7 billion cases of childhood diarrhoeal diseases are recorded annually, with 525,000 children under the age of 5 years dying [2]. Sub-Saharan Africa and South Asia have the highest mortality rates, ranging from 50 to 150 per 100,000 people [3]. In Africa, 800,000 children die each year from diarrhoea and dehydration, which account for 25% to 75% of all childhood diseases, respectively [4,5]. In South Africa, the 2010 General Household Survey (GHS) showed that there were over 60,000 cases of childhood diarrhoea per month recorded in health care facilities, and approximately 9000 child diarrhoeal deaths in the same year [6].
The primary health care in South Africa is based on a system in which Primary Health Care clinics (PHC clinics) serve the rural and peri-urban communities, and nurses determine on the severity of the diarrhoea and whether it is necessary to refer the patient to the hospital where a medical doctor takes over the treatment [7,8]. Generally, children with diarrhoea in rural and peri-urban areas are treated with oral rehydration and/or antibiotic regimes without identifying the pathogen(s) responsible for infection. Thus, broad-spectrum antibiotics are overused, and bacterial resistance develops [9].
To improve clinical care and public health surveillance, it is important to correctly diagnose the aetiology of infectious diarrhoea [10]. A systematic literature review done in 2013 [11] on deaths due to diarrhoeal diseases, estimated that 70% deaths are attributable to 13 pathogens, of which Rotavirus, Enteropathogenic Escherichia coli (EPEC), Enterotoxigenic Escherichia coli (ETEC), Calicivirus and Shigella were the most important pathogens. The Global Enteric Multicenter Study (GEMS) agrees with this, but also includes Cryptosporidium spp. as an important pathogen [12]. The MAL-ED study [13,14] included the collection of 5399 stool specimens for surveillance purposes. Of these, 5160 (95.6%) were available for testing. However, only 3458 (64%) were included in the analysis of aetiologies of diarrhoeal episodes. Several geographical regions were included in the analyses, of which the Vhembe district of South Africa was one. Re-analysis of stool samples using probe-based qualitative PCR assays compartmentalised in TaqMan® Array cards (Thermo Fisher, Waltham, MA, USA) for the detection of 29 enteric pathogens showed that for the Vhenda region in South Africa, the ten pathogens with the highest attributable incidence (from highest to lowest) were: Shigella spp., norovirus, sapovirus, Campylobacter spp., astrovirus, rotavirus, adenovirus, ETEC, tEPEC and Cryptosporidium spp. [13,14].
For public health interventions to be successful, it is important to determine the relative burden of pathogen-specific diarrhoea to compare the results to other studies with similar settings; to determine if the principal aetiologies of diarrhoea in the community are similar to those for more severe disease; and to understand the importance of mixed infection [15]. Although some diarrhoea cases have a single defined pathogen, most diarrhoea cases are thought to be caused by multiple pathogens [2]. Shrivastavha et al. [16] hypothesized that in cases with multiple infections, pathogens may lead to severe diarrhoea. The laboratories at referral hospitals in rural and peri-urban areas are not always able to perform standard tests on a regular basis [17]. Recent advances of molecular diagnostics tests that use multiplex assays able to target several different pathogens (bacteria, viruses and parasites) within a single reaction have made it possible to characterize the clinical aetiology and epidemiology of infectious diarrhoea [18,19,20,21,22]. In this study, the BioFire Filmarray Gastrointestinal (GI) panel, which detects 22 diarrhoeal pathogens, was used to determine the prevalence of single and multiple viral, bacterial and parasitic pathogens causing diarrhoea in children younger than five years of age from rural and peri-urban communities in South Africa to understand the aetiology of diarrhoea. Using this panel, the complex nature of co-infections could be assessed in treating diarrhoeal episodes.
2. Materials and Methods
2.1. Patient Enrolment
Only children who have lived in the Vhembe district for at least 7 days or more and experienced more than two watery/loose stools per day and have not taken any antibiotics during the week prior to providing a stool specimen were included in this study.
2.2. Study Population and Stool Collection
The study focused on the Vhembe District Municipality in the northern part of the Limpopo province in South Africa, which comprises of four Local Municipalities, Musina, Mutale, Thulamela and Makhado. Two district hospitals (Louis Trichardt Memorial Hospital and Donald Fraser Hospital) and one regional hospital (Tshilidzini Hospital) were included in the study. Each of these hospitals have several Primary Health Care (PHC) clinics that serve under them and were included in this study for stool sample collection. Only severe cases of diarrhoea were referred by the nurses at the PHC clinics to the hospitals for further treatment. Both the Donald Fraser and Tshilidzini hospitals have clinics responsible for outpatient treatment.
Stool specimens were collected between 2014 and 2016 from rural and peri-urban PHC facilities and for five months during 2016 at the study hospitals from children under the age of five years suffering from diarrhoea as part of a larger study regarding the cost of treating severe diarrhoea. Nurses assisted in the collection of stool specimens, which were kept at 4 °C until collected and delivered to the laboratory where they were processed.
2.3. Pathogen Detection
The commercially available BioFire® Film Array® GI Panel system was used per the manufacturer’s instructions (BioFire Diagnostics, Salt Lake City, UT, USA) to analyse all stool specimens. Briefly: only one specimen at a time was processed within a closed pouch where 200 μL of the stool specimen is added together with the supplied hydration solution. The system performs a nucleic acid extraction, multiplex PCR and melting analysis in 60 min and provides a printout of the results. The test simultaneously detects and identifies nucleic acids from the following 22 diarrhoea pathogens:
Bacteria: Campylobacter [C. jejuni; C. coli; C. upsaliansis], Clostridium difficile [toxin A/B], Plesiomonas shigelloides, Salmonella, V. cholerae; Vibrio spp [V. cholerae; V. parahaemolyticus; V. vulnificus], Yersinia enterocolitica, Enteroaggregative E. coli (EAEC), Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC [lt/st]), Shiga-like-toxin-producing E. coli [STEC stx1/stx2], E. coli O157, Shigella-EIEC.
Viruses: Adenovirus F40/41, Sapovirus [I; II; IV; V], Astrovirus [HAstV 1-8], Norovirus [GI; GII], Rotavirus A.
Parasites: Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia [G. intestinalis; G. duodenalis].
2.4. Statistical Analysis
All data were imported to a Microsoft Excel spreadsheet and analysed using the Strata 14 statistical package. The hospital samples were collected for only five months during 2016 and therefore descriptive data were used. Statistical analysis was reported using the Fischer’s exact test.
3. Results
3.1. Gender and Age Demographics of Study Group
A total of 275 children suffering from diarrhoea were part of the study and included 140 (52%) males and 128 (48%) females under the age of 5 years old. No statistical difference was seen between the male and female patients visiting the PHC clinics and the Hospitals (p = 0.303). No information on gender was recorded for seven of the patients during stool specimen collections (Table 1).
Table 1.
Gender distribution per recruitment site.
| Gender | Health Facility | Frequency (%) | * Total (%) |
|---|---|---|---|
| Male | Hospitals | 52 (57%) | 140 (52%) |
| Primary Health Care clinics | 88 (50%) | ||
| Female | Hospitals | 39 (43%) | 128 (48%) |
| Primary Health Care clinics | 89 (50%) |
* 7 stool samples had no information on gender.
The majority (80%; 221/275) of stool samples were collected from children aged between birth and 24 months. Only 265 stool specimens (96%) had data on the age of patient. The overall median age distribution for the study participants was 10 months [IQR 6 to 18 months], whereas the hospital cohort had the median age of 12 months [IQR 9 to 17 months] and the PHC clinic cohort had the median age of 9 months [IQR 4 to 18 months]. No statistical difference was observed between the age distribution of patients from the hospitals and PHC clinics (p = 0.293). Table 2 provides a breakdown of age distribution and frequency of stools received by age group and health facility.
Table 2.
Age distribution of patients by recruitment site.
| Age (Months) |
Healthcare Facility | |
|---|---|---|
| Hospitals [Frequency (%)] |
Primary Health Care facilities [Frequency (%)] | |
| Birth—11 | 55 | 60 |
| 12–23 | 30 | 22 |
| 24–36 | 4 | 6 |
| 37–48 | 10 | 6 |
| 49–60 | 1 | 5 |
3.2. Symptoms Reported in Study Cohort
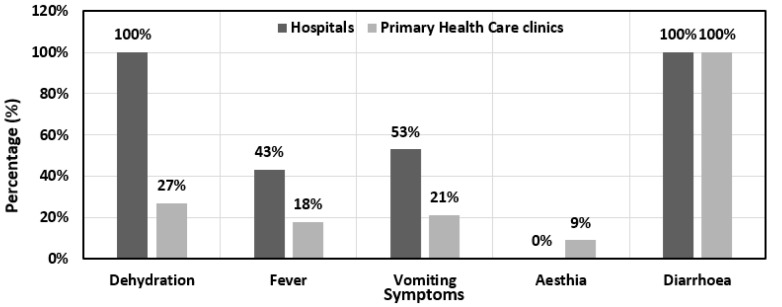
In the study cohort, 33% (90/275) of the patients recorded only one symptom, while 67% (185/275) of patients experienced multiple symptoms. All children (100%; 91/91) from the hospital group had multiple symptoms, whereas 49% (90/184) and 51% (95/184) of the children in the PHC clinic group had single and multiple symptoms, respectively. This was statistically significant (p = 0.00). The symptoms recorded for each patient on the questionnaire included dehydration, fever, vomiting, diarrhoea, and aesthia/listlessness (Figure 1).
Figure 1.
Distribution by recruitment site of clinical symptoms displayed by patients.
3.3. Pathogen Prevalence in Stool Samples
From June 2014 to June 2016, 184 stool samples were collected from PHC clinics serving rural and peri-urban communities, with 78% (144/184) being positive for one or more pathogens. Viruses were found in 27% of the samples, parasites in 2% of the samples and bacteria in 69% of the samples. Between August and December 2016, 91 stool samples were collected from the three study hospitals, with 90% (82/91) of the samples being positive for one or more pathogens. Viruses were found in 59% of the samples, parasites in 5% of the samples and bacteria in 37% of the samples. Overall, a total of 82% (226/275) of the samples were positive for at least one pathogen in the GI panel; viruses were found in 11% (25/226), bacteria were detected in 17% (38/226) and parasites were detected in 1% (2/226) of the samples.
3.4. Single vs. Multiple Infections
In total, 24% (65/275) of the stool specimens were positive for only one pathogen (Table 3). Overall, single infections were detected in 23% (43/184) of the clinic patient specimens and in 24% (22/91) of the hospital patient specimens. The most prevalent pathogens responsible for single infections were EAEC in 26% (17/65), Norovirus GI/GII in 14% (9/65), Shigella-EIEC in 12% (8/65), ETEC in 11% (7/65) and Adenovirus F40/41 in 11% (7/65) of the stools. A total of 59% (161/275) of the study cohort were infected with multiple pathogens (Table 3). Multiple or co-infections were detected in 55% (101/184) of the stool specimens from the clinic patients and 66% (60/91) were detected in the stool specimens from the hospital patients.
Table 3.
Single infections detected in the stool specimens (n = 65).
| Pathogen Detected |
Hospital Patients (n) |
PHC Clinic Patients (n) |
Total Patients (n) |
|---|---|---|---|
| Bacteria infection | 8 | 30 | 38 |
| Campylobacter [jejuni, coli, upsaliansis] | - | 1 | 1 |
| EAEC | 4 | 13 | 17 |
| EPEC | 2 | 5 | 7 |
| ETEC [lt/st] | - | 4 | 4 |
| STEC [stx1, stx2] | - | 1 | 1 |
| Shigella/EIEC | 2 | 6 | 8 |
| Virus infection | 13 | 12 | 25 |
| Adenovirus F40/41 | 2 | 5 | 7 |
| Astrovirus | - | 3 | 3 |
| Norovirus [GI/GII] | 8 | 1 | 9 |
| Rotavirus A | 2 | 3 | 5 |
| Sapovirus [I, II, IV, V] | 1 | - | 1 |
| Parasite infection | 1 | 1 | 2 |
| Giardia lamblia [intestinalis, duodenalis] |
1 | - | 1 |
| Cryptosporidium | - | 1 | 1 |
| Total: | 22 (24%) | 43 (23%) | 65 (24%) |
Table 4 indicates that bacteria/bacteria combinations were detected in 29% (46/161) of stool specimens; bacteria/parasite combinations were detected in 14% (23/161) of stool specimens; bacteria/virus/parasite combinations were detected in 6% (9/161) of stool specimens; virus/virus combinations were detected in 3% (5/161) of stool specimens; bacteria/virus combinations were detected in 47% (76/161) of stool specimens; virus/parasite and parasite/parasite combinations were detected in 1% (1/161) of stool specimens. Two pathogens were detected in 53% (85/161) of stool specimens; three pathogens were detected in 22% (35/161) of stool specimens; and four or more pathogens were detected in 25% (41/161) of stool specimens.
Table 4.
Multiple infections of diarrhoea-causing pathogens detected in stool samples (n = 161).
|
Bacteria—Bacteria combinations (n = 46): E. coli 0157—STEC—EAEC (n = 1) E. coli 0157—STEC (n = 1) EAEC—Campylobacter (n = 1) EAEC—Clostridium difficile toxin A/B (n = 1) EAEC—EPEC—Campylobacter (n = 3) EAEC—Salmonella (n = 1) EPEC—Campylobacter (n = 2) EPEC—Clostridium difficile toxin A/B (n = 1) EPEC—EAEC (n = 8) ETEC—EAEC (n = 7) ETEC—EPEC—Campylobacter (n = 1) ETEC—EPEC—EAEC (n = 1) ETEC—EPEC (n = 4) Shigella/EIEC—Campylobacter (n = 1) Shigella/EIEC—EPEC—EAEC (n = 1) Shigella/EIEC—EPEC (n = 2) Shigella/EIEC—ETEC—EAEC—Campylobacter (n = 1) Shigella/EIEC—ETEC—EAEC (n = 2) Shigella/EIEC—ETEC—EPEC—Clostridium difficile toxin A/B—Campylobacter (n = 1) Shigella/EIEC—ETEC—EPEC—EAEC (n = 1) Shigella/EIEC—ETEC (n = 3) STEC—Campylobacter (n = 1) STEC—ETEC—EAEC—Plesiomonas shigelloides (n = 1) Bacteria—Parasite combinations (n = 23): EAEC—Cryptosporidium parvum (n = 4) EAEC—Giardia lamblia (n = 1) EPEC—Cryptosporidium parvum (n = 1) EPEC—EAEC—Giardia lamblia (n = 3) EPEC—EAEC—Salmonella—Shigella/EIEC—Campylobacter—Clostridium difficile toxin A/B—Cryptosporidium parvum (n = 1) EPEC—Giardia lamblia (n = 3) EPEC—Shigella/EIEC—Giardia lamblia (n = 1) ETEC—EAEC—Campylobacter—Cryptosporidium parvum (n = 1) ETEC—EAEC—Cryptosporidium parvum (n = 1) ETEC—EPEC—EAEC—Cryptosporidium parvum (n = 1) ETEC—EPEC—EAEC—Giardia lamblia (n = 1) ETEC—EPEC—Shigella/EIEC—Cryptosporidium parvum (n = 1) ETEC—Giardia lamblia (n = 1) ETEC—Shigella/EIEC—Giardia lamblia (n = 1) Shigella/EIEC—Giardia lamblia (n = 2) Bacteria—Virus—Parasite combinations (n = 9): EAEC—Rotavirus A—Giardia lamblia (n = 1) EPEC—Rotavirus A—Giardia lamblia (n = 1) EPEC—Adenovirus F40/41—Cryptosporidium parvum (n = 1) ETEC—Norovirus GI/GII—Campylobacter—Cryptosporidium parvum (n = 1) Shigella/EIEC—Norovirus GI/GII—Giardia lamblia (n = 1) Shigella/EIEC—Adenovirus F40/41—Giardia lamblia (n = 1) Shigella/EIEC—Rotavirus A—Giardia lamblia (n = 1) Shigella/EIEC—EPEC—Sapovirus—Cryptosporidium parvum (n = 1) Shigella/EIEC—EPEC—EAEC—Adenovirus F40/41—Giardia lamblia (n = 1) Virus—Virus combinations (n = 5): Rotavirus A—Adenovirus F40/41 (n = 3) Norovirus—Adenovirus F40/41 (n = 1) Rotavirus A—Norovirus—Adenovirus F40/41 (n = 1) Parasite—Parasite combinations (n = 1): Giardia lamblia—Cryptosporidium parvum (n = 1) |
Bacteria—Virus combinations (n = 76): EAEC—Adenovirus F40/41—Rotavirus A (n = 1) EAEC—Adenovirus F40/41 (n = 4) EAEC—Campylobacter—Norovirus GI/GII (n = 1) EAEC—Norovirus GI/GII (n = 3) EAEC—Rotavirus A (n = 4) EAEC—Sapovirus (n = 1) EAEC—Shigella/EIEC—Adenovirus F40/41 (n = 1) EAEC—Shigella/EIEC—Sapovirus (n = 1) EAEC—STEC—Adenovirus F40/41 (n = 1) EAEC—STEC—Shigella/EIEC—Salmonella—Adenovirus F40/41 (n = 1) EPEC—Clostridium difficile toxin A/B—Norovirus GI/GII (n = 2) EPEC—EAEC—Adenovirus F40/41 (n = 1) EPEC—EAEC—Astrovirus (n = 1) EPEC—EAEC—Clostridium difficile toxin A/B—Norovirus GI/GII—Adenovirus F40/41 (n = 1) EPEC—EAEC—Norovirus GI/GII (n = 6) EPEC—EAEC—Rotavirus A—Norovirus GI/GII (n = 1) EPEC—EAEC—Shigella/EIEC—Adenovirus F40/41 (n = 2) EPEC—EAEC—Shigella/EIEC—Campylobacter—Adenovirus F40/41 (n = 1) EPEC—EAEC—Shigella/EIEC—Norovirus GI/GII—Adenovirus F40/41 (n = 1) EPEC—EAEC—Shigella/EIEC—Norovirus GI/GII—Adenovirus F40/41—Rotavirus A (n = 1) EPEC—EAEC—Shigella/EIEC—Norovirus GI/GII (n = 1) EPEC—EAEC—Shigella/EIEC—Rotavirus A (n = 1) EPEC—ETEC—EAEC—Adenovirus F40/41(n = 1) EPEC—ETEC—EAEC—Adenovirus F40/41—Rotavirus A (n = 1) EPEC—ETEC—EAEC—Norovirus GI/GII (n = 1) EPEC—ETEC—EAEC—Sapovirus (n = 1) EPEC—Norovirus GI/GII (n = 2) EPEC—Rotavirus A (n = 3) EPEC—Salmonella—Rotavirus A (n = 1) EPEC—Shigella/EIEC—Astrovirus (n = 1) ETEC—Adenovirus F40/41 (n = 1) ETEC—EAEC—Rotavirus A—Adenovirus F40/41 (n = 1) ETEC—EAEC—Rotavirus A (n = 1) ETEC—EAEC—Sapovirus—Adenovirus F40/41 (n = 1) ETEC—EAEC—Shigella/EIEC—Norovirus GI/GII—Rotavirus A (n = 1) ETEC—EAEC—Shigella/EIEC—Norovirus GI/GII (n = 1) ETEC—EAEC—Shigella/EIEC—Plesiomonas shigelloides—Norovirus GI/GII—Adenovirus F40/41—Rotavirus A (n = 1) ETEC—EAEC—STEC—Campylobacter—Adenovirus F40/41 (n = 1) ETEC—EAEC—STEC—Shigella/EIEC—Adenovirus F40/41 (n = 1) ETEC—EPEC—EAEC—Campylobacter—Shigella/EIEC—Adenovirus F40/41 (n = 1) ETEC—EPEC—EAEC—Campylobacter—Shigella/EIEC—Norovirus GI/GII (n = 1) ETEC—EPEC—EAEC—Campylobacter -Norovirus GI/GII (n = 1) ETEC—EPEC—EAEC—Shigella/EIEC—Adenovirus F40/41—Norovirus GI/GII (n = 1) ETEC—EPEC—EAEC—Shigella/EIEC—Adenovirus F40/41—Rotavirus A (n = 1) ETEC—EPEC—EAEC—Shigella/EIEC—Adenovirus F40/41 (n = 2) ETEC—EPEC—Rotavirus A (n = 1) ETEC—EPEC—Shigella/EIEC—Adenovirus F40/41 (n = 1) ETEC—Norovirus GI/GII (n = 3) Adenovirus F40/41—Campylobacter (n = 3) Salmonella—Sapovirus (n = 1) Shigella/EIEC—Adenovirus F40/41—Rotavirus A (n = 1) Shigella/EIEC—Adenovirus F40/41 (n = 1) Shigella/EIEC—Campylobacter—Adenovirus F40/41 (n = 1) Virus—Parasite combinations (n = 1): Sapovirus—Adenovirus F40/41—Giardia lamblia (n = 1) |
EPEC/EAEC combination was the highest mixed infection, detected in 5% (8/161) of the specimens. ETEC/EAEC combination was detected in 4.4% (7/161) of the specimens. EPEC/EAEC/Noro [GI/GII] combination was detected in 3.7% (6/161) of the specimens. Combinations of either ETEC/EPEC or EAEC/Rotavirus A or EAEC/Adenovirus F 40/41 or EAEC/Cryptosporidium were detected in 2.5% (4/161) of the stool specimens, respectively.
4. Discussion
The objective of this study was to show the prevalence of single and multiple diarrhoeal pathogen combinations in stool specimens collected from children under the age of five from rural and peri-urban communities. Several studies have reported that children in rural and peri-urban communities are exposed to enteric pathogens through a variety of transmission pathways including contaminated food, water, exposure to soil contaminated with animal faeces and through person-to-person contact [23,24,25,26].
The results in this study have shown that most stool specimens positive for diarrhoeagenic pathogens were collected from children aged between 0–24 months (Table 3), which correlates with other previously reported studies in the Limpopo province of South Africa [27,28].
Diarrhoeagenic E. coli strains have been frequently identified as a predominant cause of diarrhoea in children under the age of five years. In Mozambique, a study reported diarrhoeagenic E. coli as the cause of 42% of diarrhoea cases [29]. In Tanzania, a study showed that diarrhoeagenic E. coli was responsible for 70% of all diarrhoea cases [30]. In the present study, diarrhoeagenic E. coli strains were highly detected in single or mixed infections with other bacterial strains, viruses or parasites (Table 3, Table 4 and Table 5).
Table 5.
Summary of diarrhoeal pathogens detected in stool samples ordered from most to least prevalent.
| Diarrhoea Pathogen |
Study Cohort % Positive (n) [95% Confidence Interval] |
Pathogen Distribution in Stool Samples per Health Care Facility | Pathogen Distribution in Stool Samples per Age Group [Only Age Information for 265 Stool Samples] |
|||||
|---|---|---|---|---|---|---|---|---|
| Hospital Cohort % Positive (n) |
PHC Clinic Cohort % Positive (n) |
≤12 Months |
13–24 Months | 25–36 Months | 37–48 Months | 49–60 Months | ||
| EAEC | 42 (115/275) [35.92–47.89] |
49 (45/91) | 38 (70/184) | 69 | 24 | 5 | 12 | 1 |
| EPEC | 32 (88/275) [26.52–36.87] |
37 (34/91) | 29 (54/184) | 48 | 26 | 2 | 8 | 2 |
| ETEC [lt/st] | 21 (59/275) [16.75–26.78] |
24 (22/91) | 20 (37/184) | 26 | 16 | 5 | 10 | - |
| Shigella-EIEC | 20 (55/275) [15.43–25.22] |
19 (17/91) | 21 (38/184) | 25 | 15 | 5 | 7 | 2 |
| Adenovirus F40/41 | 19 (51/275) [14.13–23.65] |
14 (13/91) | 21 (38/184) | 36 | 9 | 2 | 4 | - |
| Norovirus [GI/GII] | 15 (42/275) [11.23–20.08] |
32 (29/91) | 7 (13/184) | 24 | 13 | 1 | 2 | - |
| Rotavirus A | 13 (36/275) [9.34–17.66] |
10 (9/91) | 15 (27/184) | 16 | 11 | - | 5 | 2 |
| Campylobacter [jejuni, coli, upsaliansis] |
9 (25/275) [5.96–13.12] |
13 (12/91) | 7 (13/184) | 19 | 4 | 1 | 1 | - |
| Giardia lamblia [intestinalis, duodenalis] | 8 (21/275) [4.79–11.44] |
8 (7/91) | 8 (14/184) | 7 | 6 | 1 | 2 | 3 |
| Cryptosporidium | 6 (16/275) [3.36–9.27] |
11 (10/91) | 3 (6/184) | 7 | 8 | 1 | - | - |
| Sapovirus [I, II, IV, V] | 3 (9/275) [1.40–6.12] |
2 (2/91) | 4 (7/184) | 4 | 2 | 2 | 1 | - |
| STEC [stx1, stex2] | 3 (9/275) [1.50–6.12] |
2 (2/91) | 4 (7/184) | 4 | 2 | - | 2 | - |
|
Clostridium difficile
toxin A/B |
3 (7/275) [1.03–5.17] |
3 (3/91) | 2 (4/184) | 6 | - | 1 | - | - |
| Astrovirus | 2 (5/275) [0.59–4.19] |
1 (1/91) | 2 (4/184) | 3 | 2 | - | - | - |
| Salmonella | 2 (5/275) [0.59–4.19] |
3 (3/91) | 1 (2/184) | 5 | - | - | - | - |
| Plesiomonas shigelloides | 1 (2/275) [0.09–2.60] |
2 (2/91) | 0 (0/184) | 1 | - | - | 1 | - |
| E. coli 0157 | 1 (2/275) [0.09–2.60] |
0 (0/91) | 1 (2/184) | - | 1 | - | - | - |
| Single infections (see Table 3) |
24 (65/275) | 24 (22/91) | 23 (43/184) | 39 | 14 | 4 | 5 | 3 |
| Multiple infections (see Table 4) | 59 (161/275) | 66 (60/91) | 55 (101/184) | 86 | 43 | 9 | 14 | 3 |
However, several studies in Africa have also indicated a high carriage level of diarrhoeagenic E. coli detection in asymptomatic cases [31,32]. In this study, EPEC was detected in 32% of the participants. EPEC are important in paediatric endemic diarrhoea and diarrhoea outbreaks [33]; atypical EPEC has been reported in association with prolonged diarrhoea [34] and has also been demonstrated to be more prevalent than typical EPEC in both developed and developing countries [33]. Nguyen et al. [35] have reported that diarrhoea caused by atypical EPEC is usually mild and generally not associated with dehydration; its importance lies in its association with prolonged diarrhoea, which is a major contributor to childhood illness in developing countries. In this study, ETEC was found in 22% of the participants. ETEC is a multivalent pathogen that causes recurrent infections that may adversely affect the nutritional status of children younger than two years of age and the susceptibility of infants and young children due to poor public health and hygiene conditions [36,37]. In this study, EAEC was the highest detected pathogen, in 42% of the participants. EAEC has been linked with persistent diarrhoea in children living in areas where EAEC is endemic [38]. Contaminated food appears to be the main source of EAEC infection and has been implicated in several foodborne outbreaks of diarrhoea [39,40]. The MAL-ED study [41] has shown the importance of subclinical EAEC infection and co-infections in children younger than two years of age and emphasized the importance of adequate maternal and child care, hygiene, sanitation and socio-economic factors.
High proportions of co-infections have been reported in low-income countries, where children may be exposed simultaneously to multiple enteric pathogens due to poor sanitary conditions [25,42,43]. Co-infections are a risk in persistent diarrhoea lasting longer than 14 days, which leads to death in young children [44]. Mixed or co-infections in Africa are common and have been reported by several studies [28,32,45]. From the results (Table 4), we did see co-infections of virus–bacteria–parasites combinations in 9 stool samples; co-infections of virus–parasite combinations in 1 stool sample; co-infections of different bacterial strain combinations in 46 stool samples; co-infections of bacteria–parasite combinations in 23 stool samples; co-infections of bacteria–virus combinations in 76 stool samples; and only 1 stool sample with different parasite (Giardia and Cryptosporidium) combinations. However, it may be difficult to indicate the exact role of each pathogen and to interpret if mechanisms of synergic enhancement of disease severity may be enacted.
Nyholm et al. [46] reported that Diarrhoeagenic E. coli can acquire virulence genes from other pathogroups via horizontal gene transfer, resulting in the development of what is termed intermediate pathogroups [47], or also referred to as hybrid [48] or blended virulence profiles and virulence combination [49]. The combinations of EHEC/EAEC from Germany [50] and STEC/ETEC from Finland are E. coli hybrid strains that have been reported to cause severe diarrhoea in humans [46]. Other E. coli combinations that have also been reported include EPEC/EAEC [51] and the E. coli O104:H4 strain in Germany, which possesses EAEC- and STEC-associated virulence genes [48]. This explains both the possibility of the emergence of extremely pathogenic strains [52] as well as the limitations of molecular techniques in distinguishing these strains from others in mixed cultures. More researchers are looking at the complex mechanisms of host–microbe and microbe–microbe interactions in the gastrointestinal tract, and details regarding the complex mechanisms of action and interaction are emerging [53,54].
Viruses such as Adenovirus F40/41, Sapovirus [I; II; IV; V], Astrovirus [HAstV 1-8], Norovirus [GI; GII] and Rotavirus A were all detected in the stool samples as either single or co-infections (Table 3, Table 4 and Table 5). Kotlofff et al. [12] conducted a multi-centre case-control study in Africa and South Asia and found that Rotavirus and, to a lesser extent, Adenovirus 40/41 (<5%) were the most common viruses causing moderate-to-severe diarrhoea in children. In the present study, Norovirus and Adenoviruses were the most frequent viruses isolated from the stool specimens, whereas Rotaviruses, Sapoviruses and Astroviruses were detected in low percentages (Table 4 and Table 5). Positive results from patients for adenovirus (17.8%) were higher compared to other studies conducted in other African countries: 10.4% in Tunisia [55], 8.0% in Senegal [56], 2.9% in Mozambique [57], 5.7% in the Central African Republic [58] and 3.8% in Angola [59]. Similarly, more patients tested positive for norovirus (14.7%) in the current study compared to: 4.2% in Mozambique [57] and 9.9% in the Central African Republic [58]. Detection rates of astrovirus in the present study (2.5%) were like those in studies conducted by Nhampossa et al. [57] and Gasparinho et al. [59] in Mozambique (1.7%) and Angola (2.6%), respectively. According to GEMS, Sapovirus prevalence, along with other enteric pathogens, has been linked with moderate to severe diarrhoea in developing countries [12]. Sapovirus infections were found in 36.6% (15/41) of African studies, with the GI and GII subtypes being more prevalent [60]. Detection rates of Sapovirus ranged between 0.8% to 18% in Burkina Faso, with studies in South Africa reporting prevalence of 4.1% and 7.7%, respectively [60].
Astroviruses in this study were found in three stool samples, as well as in combination with either EPEC-EAEC and EPEC-Shigella/EIEC, respectively (Table 4 and Table 5). In South Africa, Astroviruses have been frequently associated with Rotavirus infections in children [61]. Finkbeiner et al. [62] have shown a diversity of Astroviruses present in stool specimens from diarrhoea patients and have further speculated that there might be more unrecognized Astroviruses responsible for diarrhoea cases. Although no further classification of the Norovirus isolates in this study was performed, a previous study on Norovirus strains circulating in the Vhembe district in 2017 found that the GII subtype is more prevalent than the GI subtype in children under five years of age [63]. The presence of Rotavirus A in some stool samples in the present study might have been due to recombinant vaccine strains, which have been shown to shed in stool samples for up to eight weeks following inoculation [64]. Madhi et al. [65] and Groome et al. [66] have shown that the administration of Rotavirus vaccination treatment decreased the incidence of diarrhoea caused by the virus in South Africa. Other studies have also reported that there is an increase in the frequency of Norovirus as a cause of acute gastroenteritis in countries where the rotavirus vaccine has been introduced [67,68,69,70].
A recent study by Ikeda et al. [71] examining seasonal factors in relation to hospital-admitted diarrhoea cases in South Africa reported that higher cases of diarrhoea were observed during the warmer season than in the usual conditions in the dry winter season when temperatures range between 5 to 10 °C, and that dry conditions were more associated with diarrhoea in children younger than five years of age. The dry conditions in rural areas can lead to increased water storage which then increases the risk of water contamination, as well as less water accessible for personal hygiene, which increases the risk of potential diseases [2,26]. Virus detection may have been influenced by seasonality; Sapovirus and Rotavirus are more prevalent in cold months [72,73], while Astrovirus infections are more likely to occur in spring and summer [74]. In the current study, Norovirus was more prevalent in specimens collected from patients presenting with diarrhoea during the rainy season, and this is consistent with previous studies [75,76]. Seasonal and other environmental variations appear to have no influence on the prevalence of Adenovirus infection [77,78].
Only Cryptosporidium (13%) and Giardia lamblia (8%) parasites were detected in the stool samples. According to Omoladi et al. [79], the overall prevalence of Cryptosporidium spp infection in Southern African countries was 16.8%, which is mainly due to the high number of immunocompromised individuals in the region suffering from HIV/Aids. In this study, no samples tested positive for Entamoema histolytica or Cyclospora cayetanensis. However, Samie et al. [80] have previously reported on E. histolytica infections in the Vhembe region to be associated with diarrhoea in both healthy and immunocompromised patients. Also, in the Venda region, relatively low percentage of study participants testing positive for C. cayetanensis have been observed [81].
In a study conducted by Walker et al. [82], paired stool specimens were collected from 117 children under the age of five with acute, non-bloody, community-acquired diarrhoea who were admitted to one of four hospitals in Botswana. This study also used the BioFire® Film Array® (BioFire Diagnostics, Salt Lake City, UT, USA), and when the Walker et al. [82] study results were compared to the present study, similar rates of detection were noted for EAEC (42.7%), EPEC (41.0%), ETEC (17.9%), G. lamblia (9.4%), sapovirus (6.8%), Cryptosporidium spp. (6.0%), astrovirus (1.7%), Salmonella spp. (0.9%), C. cayetanensis (0.0%), E. histolytica (0.0%), Y. enterocolitica (0.0%) [82]. Detection rates were higher in participants in the Walker study for Campylobacter spp. (17.1%), C. difficile (7.7%), V. cholerae (2.6%) and rotavirus, which was detected in over half (52.1%) of the study participants, compared with only 12.9% in the present study [82]. Compared to the present study, detection rates were lower in participants in the Walker study for P. shigelloides, STEC, Shigella/EIEC (13.7%), adenovirus (10.3%) and norovirus (9.4%) [82].
Limitations of the study included: (1) PHC clinic specimens were collected over a period of 24 months from various clinics and then stored at −20 degrees; (2) Hospital specimens were the only specimens collected and tested within a short period of time; (3) Hospital specimens were only collected for 5 months in the study; (4) Participation in the study was voluntary, and so not all children presenting with diarrhoea at the respective health care facilities included in the study were recruited; (5) If the PHC clinic nurses experienced high patient volumes, the required time for specimen collection was not available and thus not all patients under the age of five presenting with diarrhoea at the clinic could be included in the study; (6) no asymptomatic stools were collected or tested; (7) the BioFire® Film Array® GI Panel (BioFire Diagnostics, Salt Lake City, UT, USA) does not distinguish between active infection and asymptomatic colonisation of the patient by potential pathogens [83]. Non-viable DNA and/or RNA may also be detected during analysis and could be misinterpreted as a true positive [83]. Further complicating the interpretation of results obtained by utilisation of the BioFire® Film Array® GI Panel (BioFire Diagnostics, Salt Lake City, UT, USA) is that several enteric pathogens are shed over a prolonged period by patients who have already recovered from an episode of diarrhoea and are asymptomatic at the time of testing [19]. Diagnoses should therefore be made by clinicians by interpreting laboratory results in conjunction with the clinical presentations of diarrhoea cases and the symptoms reported by patients. The rates of detection of pathogens in frozen versus fresh stool specimens using the BioFire® (BioFire Diagnostics, Salt Lake City, UT, USA) system has not yet been published and thus it is unclear if the use of frozen specimens influenced the results obtained in the present study. The diversity of diarrhoeal pathogens detected by the BioFire® Film Array® panel in this study allows for the collection of useful data on the prevalence of diarrhoeal pathogens and co-infections.
5. Conclusions
The simultaneous detection of a wide variety of pathogens in individual patients presenting with diarrhoea is becoming increasingly important as co-infection with multiple microbes has been shown to increase both morbidity and mortality even though the exact interactions of these organisms during pathogenesis has yet to be fully elucidated. Results of the current study reveal that diarrhoeagenic E. coli (specifically EAEC) are the predominant cause of diarrhoea in the population group tested in the Vhembe district, South Africa. The detection of such a high proportion of multiple enteric pathogens in mixed infections in the current study raises public health concerns. The results from this study also demonstrates the need for continuous epidemiological monitoring and surveillance of antimicrobial resistance to ensure correct treatment of acute and persistent diarrhoea. The data will guide the Department of Health in the patient treatment and management of diarrhoea, especially in outbreak situations or in critically ill patients.
Acknowledgments
The authors acknowledge all the nurses for their assistance in the study and all the caregivers who provided the information and specimen required to perform the study.
Author Contributions
N.P. was project leader and designed the study protocol; J.P.K.N., T.Z., L.S.M. and S.E.L. carried out the field work; L.H. carried out the BioFire® Film Array® analysis; N.P., A.N.T., L.S.M. and T.G.B. supervised the post graduate students between 2014 and 2017; N.P., L.H., A.N.T., L.S.M., J.P.K.N., S.E.L. and T.G.B. assisted in the analysis and interpretation of the data; P.B. assisted in the statistical analysis of the data. All authors have contributed equally to the conceptualization, validation, writing, original draft preparation, review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.for studies involving humans. The Higher Degrees Ethics Committee of the University of Venda (SMNS/16/MBY/07/2904) and the Higher Degrees and Research Ethics Committees of the University of Johannesburg (HDC-01-100-2015 and REC-241112-035) approved this study. Permission to undertake the study was approved by the Limpopo Provincial Department of Health in the Vhembe District and the Chief Executive Officers from each of the hospitals in this study.
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study. Study information was provided to the caregiver prior to signing the consent form, which included permission to access hospital records of the child, completion of a questionnaire and allowing the collection of a stool sample. A copy of the study information and the signed consent form was given to the caregiver after signing.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to protection of patients.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was funded by the Water Research Commission (Project K3/7150; Pretoria, South Africa) and the Directorate of Publications and Research at University of Venda, South Africa (Project I395).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. 4th ed. WHO; Geneva, Switserland: 2005. [Google Scholar]
- 2.WHO Scope of Diarrhoeal Disease. [(accessed on 10 October 2021)];2017 Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
- 3.Dadonaite B., Ritchie H., Roser M. “Diarrheal diseases”. 2018. [(accessed on 10 October 2021)]. Published online at OurWorldInData.org. Available online: https://ourworldindata.org/diarrheal-diseases.
- 4.Hamer D.H., Simon J., Thea D., Keusch G.T., Hernandez-Avila M., Lazcano-Ponce E. Childhood Diarrhea in Sub-Saharan Africa. Child Health Res. Proj. Spec. Rep. 1998;2:32. [Google Scholar]
- 5.Ugboko H.U., Ninyi O.C., Oranusi S.U., Oyewale J.O. Childhood diarrhoeal diseases in developing countries. Heliyon. 2020;6:e03690. doi: 10.1016/j.heliyon.2020.e03690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chola L., Micha L.J., Tugendhaft A., Hoffman K. Reducing diarrhoea deaths in South Africa: Costs and effects of scaling up essential interventions to prevent and treat diarrhoea in under-five children. BMC Public Health. 2015;15:1. doi: 10.1186/s12889-015-1689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Primary Health Care in South Arica. 1983. [(accessed on 10 October 2021)]. Available online: https://disa.ukzn.ac.za/sites/default/files/pdf_files/ChMay83.1024.8196.000.009.May1983.10.pdf.
- 8.PRIMASYS [Primary Health Care Systems]: Case Study from South Africa. World Health Organization; Geneva, Switzerland: 2017. Abridged Version. [Google Scholar]
- 9.Chiyangi Y., Muma J.B., Malama S., Manyahi J., Abade A., Kwenda G., Matee M.I. Identification and antimicrobial resistance patterns of bacterial enteropathoens from children aged 0-59 months at the University teaching hospital, Lusaka, Zambia: A prospective cross sectional study. BMC Infect. Dis. 2017;17:117. doi: 10.1186/s12879-017-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrant R.L., Van Gilder T., Steiner T.S., Thielman N.M., Slutsker L., Tauxe R.V., Hennessy T., Griffen P.M., DuPont R.B., Tarr P., et al. Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 2001;32:31–50. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 11.Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E., Child Health Epidemiology Reference Group of the World Health Organization and UNICEF Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE. 2013;8:e72799. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 13.Platts-Mills J.A., Babji S., Bodhidatta L., Gratz J., Haque R., Havt A., McCormick B.J., McGrath M., Olortegui M.P., Samie A., et al. Pathogen-Specific Burdens of Community Diarrhoea on Developing Countries: A Multisite Birth Cohort Study (MAL-ED) Lancet Glob. Health. 2015;3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platts-Mills J.A., Liu J., Rogawski E.T., Kabir F., Lertsethtakarn P., Siguas M., Khan S.S., Praharaj I., Murei A., Nshama R., et al. Use of Quantitative Molecular Diagnostic Methods to Assess the Aetiology, Burden, and Clinical Characteristics of Diarrhoea in Children in Low-Resource Settings: A Reanalysis of the MAL-ED Cohort Study. Lancet Glob. Health. 2018;6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platts-Mills J.A., McCormick B.J.J., Kosek M., Pan W.K., Checkley W., Houpt E.R., the MAL-ED Investigators Methods of analysis of enteropathogen infection in the MAL-ED cohort study. Clin. Infect. Dis. 2014;59((Suppl. 4)):S233–S238. doi: 10.1093/cid/ciu408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrivastavha A.K., Kumar S., Mohakud N.K., Suar M., Sahu P.S. Multiple etiologies of infectious diarrhoea and concurrent infections in a pediatric outpatient-based screening study in Odisha, India. Gut Pathog. 2017;9:16. doi: 10.1186/s13099-017-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eibach S., Krumkamp R., Hahn A., Sarpong N., Adu-Sarkodie Y., Leva A., Käsmaier J., Panning M., May J., Tannich E. Application of a multiplex PCR assay for the detection of gastrointestinal pathogens in a rural African steeing. BMC Infect. Dis. 2016;16:150. doi: 10.1186/s12879-016-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khare R., Espy M.J., Cebelinkski E., Boxrud D., Sloan L.M., Cunningham S.A., Pritt B.S., Patel R., Binnicker M.J. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J. Clin. Microbiol. 2014;52:3667–3673. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buss S.N., Leber A., Chapin K., Fey P.D., Bankowski M.J., Jones M.K., Rogatcheva M., Kanack K.J., Bourzac K.M. Multicenter Evaluation of the BioFire FilmArray Gastrointestinal Panel for Etiologic Diagnosis of Infectious Gastroenteritis. J. Clin. Microbiol. 2015;53:915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang R.S.P., Johnson C.L., Pritchard L., Hepler R., Ton T.T., Dunn J.J. Performance of the Verigene® enteric pathogens test, Biofire FilmArrayTM gastrointestinal panel and Luminex xTAG® gastrointestinal pathogen panel for detection of common enteric pathogens. Diagn. Microbiol. Infect. Dis. 2016;86:336–339. doi: 10.1016/j.diagmicrobio.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Piralla A., Lunghi G., Ardissino G., Girello A., Premoli M., Bava E., Arghittu M., Colombo M.R., Cognetto A., Bono P., et al. FilmArray™ GI Panel Performance for the Diagnosis of Acute Gastroenteritis or Hemorragic Diarrhea. BMC Microbiol. 2017;17:111. doi: 10.1186/s12866-017-1018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo I.H., Kang H.M., Suh W., Cho H., Yoo I.Y., Jo S.J., Park Y.J., Jeong D.C. Quality Improvements in Management of Children with Acute Diarrhea Using a Multiplex-PCR-Based Gastrointestinal Pathogen Panel. Diagnostics. 2021;11:1175. doi: 10.3390/diagnostics11071175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MAL-ED Investigators The MAL-ED study: A multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource poor environments. Clin. Infect. Dis. 2014;59((Suppl. 4)):S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 24.Wang S., Xiao S.Z., Gu F.F., Tang J., Guo X.K., Ni Y.X., Qu J.M., Han L.Z. Antimicrobial Susceptibility and Molecular Epidemiology of Clinical Enterobacter cloacae Bloodstream Isolates in Shanghai, China. PLoS ONE. 2017;12:e0189713. doi: 10.1371/journal.pone.0189713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medgyesi D.N., Brogan J.M., Sewell D.K., Creve-Coeur J.P., Kwong L.H., Baker K.K. Where children plau: Young child exposure to environmental hazards during play in public areas in a trasitioning internally displaced persons in Haiti. Int. J. Environ. Res. Public Health. 2018;15:1646. doi: 10.3390/ijerph15081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troeger C., Blacker B.F., Khalil I.A., Roa P.C., Cao S., Zimsen S.R., Albertson S.B., Deshpande A., Fara T., Abebe Z., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabue J.P., Maeder E., Hunter P.R., Potgieter N. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J. Clin. Virol. 2016;84:12–18. doi: 10.1016/j.jcv.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledwaba S.E., Kabue J.P., Barnard T.G., Potgieter N. Enteric pathogen co-infections in the paediatric population from rural communities in the Vhembe District, South Africa. South Afr. J. Child Health. 2018;12:170–174. doi: 10.7196/SAJCH.2018.v12i4.1550. [DOI] [Google Scholar]
- 29.Rapelli P., Folgosa E., Solinas M.L., DaCosta J.L., Pisanu C., Sidat M., Melo J., Cappuccinelli P., Columbo M.M. Pathogenic enteric Escherichia coli in children with and without diarrhea in Maputo, Mozambique. FEMS Immunilogy Med. Microbiol. 2005;43:67–72. doi: 10.1016/j.femsim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Vargas M., Gascon J., Casals C., Schellenberg D., Urassa H., Kahigwa E., Ruiz J., Vila J. Etiology of diarrhea in children less than five years of age in ifakao, Tanzania. Am. J. Trop. Med. Hyg. 2014;70:536–539. doi: 10.4269/ajtmh.2004.70.536. [DOI] [PubMed] [Google Scholar]
- 31.Okeke I.N., Lamikanra A., Czeczulin J., Dubovsky F., Kaper J.B., Nataro P. Heterogeneous virulence of enteroagrregative Escherichia coli strains isolated from children in southwest Nigeria. J. Infect. Dis. 2000;181:252–260. doi: 10.1086/315204. [DOI] [PubMed] [Google Scholar]
- 32.Campellone K.G., Giese A., Tipper D.J., Leong J.M. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Molecular Microbiology. 2003;43:1227–1241. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa T.J., Contreras C.A. Enteropathogenic E. coli (EPEC) infection in children. Curr. Opin. Infect. Dis. 2011;24:478–483. doi: 10.1097/QCO.0b013e32834a8b8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afset J.E., Bevanger L., Romundstad P., Bergh K. Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhoea. J. Med. Microbiol. 2004;53:1137–1144. doi: 10.1099/jmm.0.45719-0. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen R.N., Taylor L.S., Tauschek M., Robins-Browne R.M. Atypical Enteropathogenic Escherichia coli Infection and Prolonged Diarrhea in Children. Emerg. Infect. Dis. 2006;12:597–603. doi: 10.3201/eid1204.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri F., Svennerholm A.M., Faruque A.S., Sack R.B. Enterotoxigenic Escherichia coli in Developing Countries: Epidemiology, Microbiology, Clinical Features, Treatment, and Prevention. Clin. Microbiol. Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qadri F., Wenneras C., Ahmed F., Asaduzzman M., Saha D., Albert M.J., Sack R.B., Svennerholm A. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine. 2000;18:2704–2712. doi: 10.1016/S0264-410X(00)00056-6. [DOI] [PubMed] [Google Scholar]
- 38.Wanke C.A., Schorling J.B., Barlett L.J., DeSouza M.A., Guerrant R.L. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr. Infect. Dis. J. 1991;10:746–751. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Hedberg C., Savarino S., Besser J., Paulus C., Thelen V., Myers L.J., Cameron D.N., Barrett T.J., Kaper J.B., Osterholm M.T. An outbreak of foodborne illness caused by Echerichia coli O39:NM, an agent not fitting into the existing scheme for classifying diarrhoeanic E. Coli. J. Infect. Dis. 1997;176:1625–1628. doi: 10.1086/517342. [DOI] [PubMed] [Google Scholar]
- 40.Itoh Y., Nagano I., Kunishima M., Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable: H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 1997;35:2546–2550. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima A.A.M., Soares A.M., Filho J.Q.S., Havt A., Lima I.F.N., Lima Noélia L., Abreu Cláudia B., Junior Francisco S., Mota Rosa M.S., Pan William K.-Y., et al. Enteroaggregative Escherichia coli subclinical infection and coinfection and impaired child growth in the MAL-ED cohort study. J. Pediatr. Gastroenterol. Nutr. 2018;66:325–333. doi: 10.1097/MPG.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 42.Le Guyader F.S., Le Saux J.-C., Ambert-Balay K., Krol J., Serais O., Parnaudeau S., Giraudon H., Delmas G., Pommepuy M., Pothier P., et al. Aichi virus, norovirus, astrovirus, enterovirus and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 2008;46:4011–4017. doi: 10.1128/JCM.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Zeng J., Liu W., Zhao F., Hu Z., Zhao C., Wang Q., Wang X., Chen H., Li H., et al. Emergence of a Hypervirulent Carbapenem-Resistant Klebsiella pneumoniae Isolate from Clinical Infections in China. J. Infect. 2015;71:553–560. doi: 10.1016/j.jinf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 44.McAuliffe J.F., Shields D.S., Auxiliadora de Sousa M., Sakell J., Schorling J., Guerrant R.L. Prolonged and recurring diarrhea in the northeast of Brazil: Examination of cases from a community-based study. J. Pediatr. Gastroenterol. Nutr. 1986;5:902–906. doi: 10.1097/00005176-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Chukwu M.O., Abia AL K., Ubomba-Jaswa E., Dewar J.B., Obi C.L. Mixed Aetiology of Diarrhoea in Infants Attending Clinics in the North-West Province of South Africa: Potential for Sub-Optimal Treatment. Pathogens. 2020;9:198. doi: 10.3390/pathogens9030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyholm O., Heinikainen S., Pelkonen S., Hallanvuo S., Haukka K., Siitonen A. Hybrids of Shigatoxigenic and Enterotoxigenic Escherichia coli (STEC/ETEC) Among Human and Animal Isolates in Finland. Zoonoses Public Health. 2015;62:518–524. doi: 10.1111/zph.12177. [DOI] [PubMed] [Google Scholar]
- 47.Müller D., Greune L., Heusipp G., Karch H., Fruth A., Tschäpe H., Schmidt M.A. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 2007;73:3380–3390. doi: 10.1128/AEM.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellmann A., Harmsen D., Cummings C.A., Zentz E.B., Leopold S.R., Rico A., Prior K., Szczepanowski R., Ji Y., Zhang W., et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE. 2011;6:e22751. doi: 10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bielaszewska M., Mellmann A., Zhang W., Köck R., Fruth A., Bauwens A., Peters G., Karch H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 50.Prager R., Lang C., Aurass P., Fruth A., Tietze E., Flieger A. Two Novel EHEC/EAEC Hybrid Strains Isolated from Human Infections. PLoS ONE. 2014;9:e95379. doi: 10.1371/journal.pone.0095379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liebchen A., Benz I., Mellmann A., Karch H., Gomes T.A., Yamamoto D., Hernandes R.T., Sampaio J., Sampaio S.C.F., Fruth A., et al. Characterization of Escherichia coli strains isolated from patients with diarrhea in Sao Paulo, Brazil: Identifcation of intermediate virulence factor profles by multiplex PCR. J. Clin. Microbiol. 2011;49:2274–2278. doi: 10.1128/JCM.00386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.EFSA . John Wiley & Sons, Ltd., European Food Safety Authority (EFSA); Parma, Italy: 2011. 2011 Annual Report Annual report the EFSA Scientific Network for Risk Assessment of GMOs. EFSA Supporting PublicationsVolume 8, Issue 4, Apr 2011, European Food Safety Authority. [Google Scholar]
- 53.Almand E.A., Moore M.D., Jaykus L.A. Virus-Bacteria Interactions: An Emerging Topic in Human Infection. Viruses. 2017;9:58. doi: 10.3390/v9030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger A.K., Mainou B.A. Interactions between Enteric Bacteria and Eukaryotic Viruses Impact the Outcome of Infection. Viruses. 2018;10:19. doi: 10.3390/v10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Gallas N., Bahri O., Bouratbeen A., Ben Haasen A., Ben Aissa R. Etiology of Acute Diarrhea in Children and Adults in Tunis, Tunisia, with Emphasis on Diarrheagenic Escherichia coli: Prevalence, Phenotyping, and Molecular Epidemiology. Am. J. Trop. Med. Hyg. 2007;77:571–582. doi: 10.4269/ajtmh.2007.77.571. [DOI] [PubMed] [Google Scholar]
- 56.Sire J.M., Garin B., Chartier L., Fall N.K., Tall A., Seck A., Weill F.X., Breurec S., Vray M. Community-Acquired Infectious Diarrhoea in Children Under 5 Years of Age in Dakar, Senegal. Paediatr. Int. Child Health. 2013;33:139–144. doi: 10.1179/2046905512Y.0000000046. [DOI] [PubMed] [Google Scholar]
- 57.Nhampossa T., Mandomando I., Acacio S., Quintó L., Vubil D., Ruiz J., Nhalungo D., Sacoor C., Nhabanga A., Nhacolo A., et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0-59 Months Seeking Care at Health Facilities. PLoS ONE. 2015;14:e0119824. doi: 10.1371/journal.pone.0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breurec S., Vanel N., Bata P., Chartier L., Farra A., Favennec L., Franck T., Giles-Vernick T., Gody J.C., Luong Nguyen L.B., et al. Etiology and Epidemiology of Diarrhea in Hospitalized Children from Low Income Country: A Matched Case-Control Study in Central African Republic. PLoS Negl. Trop. Dis. 2016;10:e0004283. doi: 10.1371/journal.pntd.0004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gasparinho C., Mirante M.C., Centeno-Lima S., Istrate C., Mayer A.C., Tavira L., Nery S.V., Brito M. Etiology of Diarrhea in Children Younger Than 5 Years Attending the Bengo General Hospital in Angola. Pediatr. Infect. Dis. J. 2016;35:e28–e34. doi: 10.1097/INF.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 60.Magwalivha M., Kabue J.P., Traore A.N., Potgieter N. Prevalence of human sapovirus in low and middle income countries. Adv. Virol. 2018;2018:5986549. doi: 10.1155/2018/5986549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadan S., Taylor M.B., Groome M.J., Cohen C., Madhi S.A., Page N.A. Epidemiology of human astroviruses among children younger than 5 years: Prospective hospital-based sentinel surveillance in South Africa, 2009-2014. J. Med. Virol. 2019;91:225–234. doi: 10.1002/jmv.25308. [DOI] [PubMed] [Google Scholar]
- 62.Finkbeiner S.R., Holtz L.R., Jiang Y., Rajendran P., Franz C.J., Zhao G., Kang G., Wang D. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol. J. 2009;6:161. doi: 10.1186/1743-422X-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabue J.P., Maeder E., Hunter P.R., Potgieter N. Genetic characterisation of Norovirus strains in outpatient children from rural communities of Vhembe district, South Africa, 2014-2015. J. Clin. Virol. 2017;94:100–106. doi: 10.1016/j.jcv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Ye S., Whiley D.M., Ware R.S., Kirkwood C.D., Lambert S.B., Grimwood K. Multivalent Rotavirus Vaccine and Wild-type Rotavirus Strain Shedding in Australian Infants: A Birth Cohort Study. Clin. Infect. Dis. 2018;66:1411–1418. doi: 10.1093/cid/cix1022. [DOI] [PubMed] [Google Scholar]
- 65.Madhi S.A., Cunliffe N.A., Steele D., Witte D., Kirsten M., Louw C., Ngwira B., Victor J.C., Gillard P.H., Cheuvart B.B., et al. Effect of Human Rotavirus Vaccine on Severe Diarrhea in African Infants. New Engl. J. Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 66.Groome M.J., Zell E.R., Solomon F., Nzenze S., Parashar U.D., Izu A., Madhi S.A. Temporal Association of Rotavirus Vaccine Introduction and Reduction in All-Cause Childhood Diarrheal Hospitalizations in South Africa. Clinical Infectious Diseases. 2016;62((Suppl. 2)):188–195. doi: 10.1093/cid/civ1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riddle M., Walker R. Status of vaccine research and development for norovirus. Vaccine. 2016;34:2895–2899. doi: 10.1016/j.vaccine.2016.03.077. [DOI] [PubMed] [Google Scholar]
- 68.Makhaole K., Moyo S., Lechile K., Goldfarb D.M., Kebaabetswe L.P. Genetic and epidemiological analysis of norovirus from children with gastroenteritis in Botswana, 2013-2015. BMC Infect. Dis. 2019;18:246. doi: 10.1186/s12879-018-3157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olivares A.I.O., Leitȃo G.A.A., Pimenta Y.C., Cantelli C.P., Fumian T.M., Fialho A.M., da Silva e Mouta Junior S., Delgado I.F., Nordgre J., Svensson L., et al. Epidemiology of enteric virus infections in children living in the Amazon region. Int. J. Infect. Dis. 2021;108:494–502. doi: 10.1016/j.ijid.2021.05.060. [DOI] [PubMed] [Google Scholar]
- 70.Farahmand M., Moghoofei M., Dorost A., Shoja Z., Ghorbani S., Kiani S.J., Khales P., Esteghamati A., Sayyahfar S., Jafarzadeh M., et al. Global prevalence and genotype distribution of norovirus infection in children with gastroenteritis: A meta-analysis on 6 years of research from 2015-2020. Rev. Med. Virol. 2022;32:e2237. doi: 10.1002/rmv.2237. [DOI] [PubMed] [Google Scholar]
- 71.Ikeda T., Kapwata T., Behera S.K., Minakawa N., Hashizume M., Sweijd N., Mathee A., Wright C.J. Climatic factors in relation to diarrhoea hospital admissions in rural Limpopo, South Africa. Atmosphere. 2019;10:522. doi: 10.3390/atmos10090522. [DOI] [Google Scholar]
- 72.Varela M.F., Ouardani I., Kato T., Kadoya S., Aouni M., Sano D., Romalde J.L. Sapovirus in Wastewater Treatment Plants in Tunisia: Prevalence, Removal, and Genetic Characterization. Appl. Environ. Microbiol. 2018;84:e02093-17. doi: 10.1128/AEM.02093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lestari F.B., Vongpunsawad S., Wanlapakorn N., Poovorawan Y. Rotavirus Infection in Children in Southeast Asia 2008–2018: Disease Burden, Genotype Distribution, Seasonality, and Vaccination. J. Biomed. Sci. 2020;27:66. doi: 10.1186/s12929-020-00649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El Sayed Zaki M., Mashaly G.E., Alsayed MAL annd Nomir M.M. Molecular study of human astrovirus in Egyptian children with acute gastroenteritis. Germs. 2020;10:167–173. doi: 10.18683/germs.2020.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mans J., Armah G.E., Steele A.D., Taylor M.B. Norovirus Epidemiology in Africa: A Review. PLoS ONE. 2016;11:e0146280. doi: 10.1371/journal.pone.0146280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shamkhali Chenar S., Deng Z. Environmental indicators for human norovirus outbreaks. Int. J. Environ. Health Res. 2017;27:40–51. doi: 10.1080/09603123.2016.1257705. [DOI] [PubMed] [Google Scholar]
- 77.Basu G., Rossouw J., Sebunya T.K., Gashe B.A., de Beer M., Dewar J.B., Steele A.D. Prevalence of rotavirus, adenovirus and astrovirus infection in young children with gastroenteritis in Gaborone, Botswana. East Afr. Med. J. 2003;80:652–655. doi: 10.4314/eamj.v80i12.8783. [DOI] [PubMed] [Google Scholar]
- 78.Celik C., Gozel M.G., Turkay H., Bakici M.Z., Güven A.S., Elaldi N. Rotavirus and adenovirus gastroenteritis: Time series analysis. Pediatr. Int. 2015;57:590–596. doi: 10.1111/ped.12592. [DOI] [PubMed] [Google Scholar]
- 79.Omoladi K.F., Odeniran P.O., Soliman M.E. A meta-analysis of Cryptosporidium species in humans from Southern Africa (2000–2020) J. Parasit. Dis. 2021;46:304–316. doi: 10.1007/s12639-021-01436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samie A., Obi L.C., Bessong P.O., Stroup S., Houpt E., Guerrant R.L. Prevalence and species distribution of E. histolytica and E. dispar in the Venda region, Limpopo, South Africa. Am. J. Trop. Med. Hyg. 2006;75:565–571. doi: 10.4269/ajtmh.2006.75.565. [DOI] [PubMed] [Google Scholar]
- 81.Samie A., Guerrant R.L., Barrett L., Bessong P.O., Igumbor E.O., Obi C.L. Prevalence of intestinal parasitic and bacterial pathogens in diarrhoeal and non-diarroeal human stools from Vhembe district, South Africa. J. Health Popul. Nutr. 2009;27:739–745. doi: 10.3329/jhpn.v27i6.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker C.R., Lechiile K., Mokomane M., Steenhoff A.P., Arscott-Mills T., Penica J.M., Goldfarb D.M. Evaluation of Anatomically Designed Flocked Rectal Swabs for Use with the BioFire FilmArray Gastrointestinal Panel for Detection of Enteric Pathogens in Children Admitted to Hospital with Severe Gastroenteritis. Journal of Clinical Microbiology. 2019;57:e00962-19. doi: 10.1128/JCM.00962-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Axelrad J.E., Freedberg D.E., Whittier S., Greendyke W., Lebwohl B., Green D.A. Impact of Gastrointestinal Panel Implementation on Health Care Utilization and Outcomes. J. Clin. Microbiol. 2019;57:e01775-18. doi: 10.1128/JCM.01775-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to protection of patients.