Abstract
It is proven that the blood concentration of antioxidants can impress the severity of viral infections, including COVID-19. However, the lack of a comprehensive study accumulating existing data regarding COVID-19 can be perceived. Therefore, this systematic review is aimed to report the association between the blood concentration of several antioxidants and the overall health condition of COVID-19 patients. We summarized the available data surrounding the serum antioxidant level in COVID-19 patients and COVID-19 outcomes. A systematic search was performed in PubMed, Scopus, Web of Science, and Cochrane, and studies that evaluated the association between antioxidants and COVID-19 outcomes were included. Of 4101 articles that were viewed in the database search, 38 articles were included after the title, abstract, and full-text review. Twenty-nine studies indicated that lower serum antioxidants are associated with worse outcomes, and one study reported no association between serum zinc (Zn) level and COVID-19 outcomes. In most cases, antioxidant deficiency was associated with high inflammatory factors, high mortality, acute kidney injury, thrombosis, intensive care unit (ICU) admission, acute respiratory distress syndrome, cardiac injury, and the need for mechanical ventilation (MV), and there was no significant association between serum antioxidants level and ICU or hospital length of stay (LOS). It seems that higher levels of antioxidants in COVID-19 patients may be beneficial to prevent disease progression. However, clinical trials are needed to confirm this conclusion.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12011-023-03588-1.
Keywords: COVID-19, Coronavirus, Antioxidants, Systematic review
Introduction
COVID-19 first appeared by causing pneumonia symptoms in affected people in China [1, 2]. The World Health Organization (WHO) designated coronavirus (COVID-19) to be a pandemic disease in March 2020 as a result of the rapid spread of this progressive disease [3]. Person-to-person transmission of this virus via droplets and airborne contamination is the most typical method of transmission [4, 5]. Since this virus has infected over ten million cases worldwide, discovering a prevention, treatment, or management strategy draws much attention to itself [6]. Despite that several medications are exhibiting in vitro activity against COVID-19 (such as corticosteroids, antivirals, and anticoagulants), there is a lack of clinical data to promote a medication as a COVID-19 treatment agent [5, 7]. Hence, most coping strategies rely on symptomatic treatment and support [2, 5]. One of these supportive strategies that medical institutions have considered is using antioxidant agents in conjunction with other treatments to reduce oxidative stress [8, 9]. It is nearly accepted by all related studies that COVID-19 infection and replication causes oxidative stress by inducing excessive production of free radicals and cytokines (e.g., tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and IL-10) [10, 11]. Therefore, it is assumed that regulating free radicals in infected patients will help with the management of hyper-inflammation and protection of tissues against oxidative injury as well as decreasing replication of the virus [12]. With that being said, consuming antioxidants in COVID-19 patients becomes a promising strategy as a supportive treatment [13]. As a result of having a high diversity of antioxidants in addition to their possible beneficial effect on COVID-19 patients, investigating the role of different antioxidants in the prevention and improvement of COVID-19 has attracted more attention towards itself [14]. Therefore, several studies have been conducted concerning this issue; however, most of these researches have been carried out individually. Hence, a lack of a comprehensive study comparing the specific potencies of each antioxidant substance can be perceived. Furthermore, an accumulation of remarkable observations eases comparing antioxidants’ benefits in COVID-19 patients and helps to reveal the most effective antioxidants for future patients [15]. According to the proposed efficacy, safety, effects on the onset of disease, and their low cost, antioxidants (vitamins A, C, E, selenium (Se), and Zn) represent beneficial agents for clinicians to be used for the COVID-19 pandemic. The present systematic review reports the association of vitamins A, C, E, Se, and Zn as exogenous and endogenous antioxidants with the overall health condition of COVID-19 patients and further proposes the most beneficial ones to be considered for future applications.
Methods
The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) were used to conduct this systematic review [16]. The PRISMA checklist can be found in Supplementary Table 1. Prior to beginning the study, a thorough research protocol was created and registered in the international prospective register of systematic reviews (PROSPERO) with CRD42023394386 ID and then followed throughout the process. The main question of this study is as follows: in COVID-19 patients, what is the association between different serum levels of antioxidants and the outcomes and severity of the disease?
Search Strategy
A thorough search was carried out for articles published before April 2022 on PubMed, Web of Science, Scopus, and Cochrane. The complete syntax set on the bases of following search terms including Medical Subject Heading (MeSH) and non-MeSH keywords was as follows: (zinc[tiab] OR zinc[MeSH] OR zn[tiab] OR zink[tiab] OR “vitamin c”[tiab] OR “ascorbic acid”[tiab] OR “ascorbic acid”[MeSH] OR ascorbic[tiab] OR ascorbate[tiab] OR selenium[tiab] OR selenium[MeSH] OR Se[tiab] OR selenoprotein[tiab] OR selenoproteins[MeSH] OR “selenoprotein P”[tiab] OR “selenoprotein P”[MeSH] OR “glutathione peroxidase”[tiab] OR “glutathione peroxidase”[MeSH] OR “selenoglutathione peroxidase”[tiab] OR “vitamin A”[tiab] OR “vitamin A”[MeSH] OR carotene[tiab] OR carotenoids[MeSH] OR “beta carotene”[tiab] OR “beta carotene”[MeSH] OR “ß-carotene”[tiab] OR carotenoid[tiab] OR retinol[tiab] OR retinoid[tiab] OR retinoids[MeSH] OR “retinoic acid”[tiab] OR tretinoin[tiab] OR tretinoin[MeSH] OR isotretinoin[tiab] OR isotretinoin[MeSH] OR zeaxanthin[tiab] OR zeaxanthins[MeSH] OR lutein[tiab] OR lutein[MeSH] OR lycopene[tiab] OR lycopene[MeSH] OR cryptoxanthin[tiab] OR cryptoxanthins[MeSH] OR “vitamin E”[tiab] OR “vitamin E”[MeSH] OR tocopherol[tiab] OR tocopherols[MeSH]) AND (covid-19[tiab] OR covid-19[MeSH] covid[tiab] OR “covid 19”[tiab] OR covid19[tiab] OR SARS-COV-2[tiab] OR SARS-COV-2[MeSH]). Regarding the publication date, there were no limitations imposed. All publications were gathered into an EndNote library, and duplicates were eliminated.
Inclusion Criteria
After excluding duplicated studies, two independent researchers (A.H. and R.A.) conducted the first step of screening by reading titles and abstracts. Through the second screening step, all the observational studies (case controls, cohorts, and cross-sectionals) investigating the association between the serum level of antioxidants and COVID-19 outcomes were considered eligible to be included. Desired outcomes were defined to be hospital length of stay (LOS), intensive care units (ICU) LOS, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), TNF-α level, IL-6 level, mortality rate, need to mechanical ventilation (MV), cardiac injury, acute kidney injury (AKI), ICU admission, thrombosis, and acute respiratory distress syndrome (ARDS). Disagreements regarding article selection were discussed with the principal investigator.
Exclusion Criteria
These criteria were used to exclude studies: (1) non-human participants; (2) gray literature, including book chapters, correspondence, conference abstracts, and comments; (3) molecular research; (4) environmental studies; (5) review papers; (6) full-text publications in languages other than English; (7) desired data not reported; and (8) studies that focused on pregnant women.
Data Extraction
Data from papers were individually gathered by two reviewers and entered into Microsoft Excel.
The data comprised the following:
Details about the publication, such as the title, journal, publication date, and stated goals
Study characteristics: study location, design, sample size, mean age, gender, and health condition of participants
Critical data: the substance studied, data on outcomes as much detailed as possible
Quality Assessment
The Newcastle–Ottawa Scale (NOS) was used to rate the quality of the included articles [17]. NOS is based on a star scoring system, in which a maximum of nine (for cohort and case–control studies) and ten stars (for cross-sectional studies) can be awarded to each study. The quality assessment was checked independently by two authors, and any disagreements were discussed with the principal investigator.
Result
Study Selection
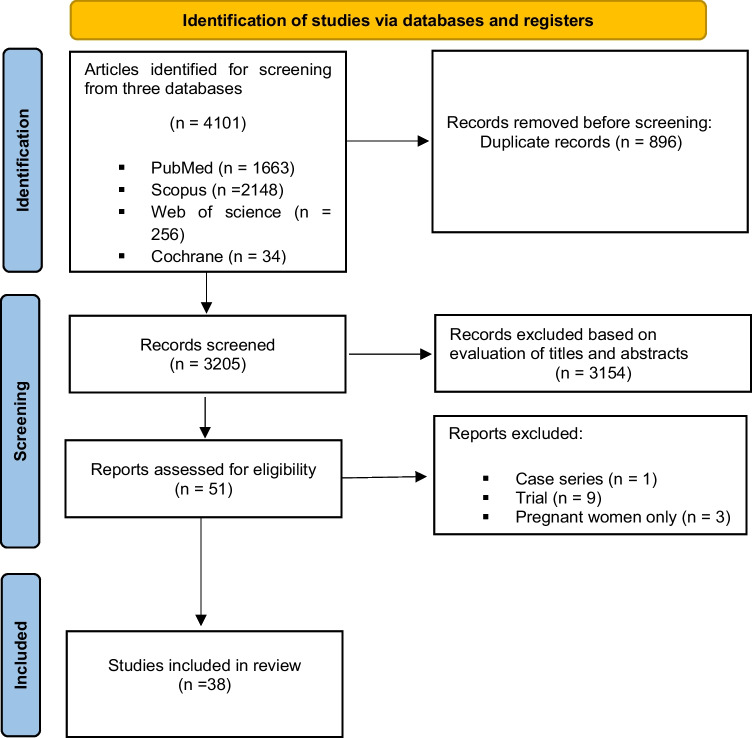
Detailed information on the study selection procedure can be found in Fig. 1. Four thousand one hundred one articles were initially acquired up until April 2022, of which 1663 were obtained from PubMed, 2148 from Scopus, 256 from Web of Science, and 34 from Cochrane. After removing 896 duplicates and 3154 irrelevant articles during the first screening stage, 51 articles were retained. After performing stricter criteria in the second screening stage, 13 articles were excluded, which 9 of them were trial studies, 3 of them focused on pregnant women, and one of them was a case series study. At last, 38 eligible studies were included in this systematic review.
Fig. 1.
Flow diagram of the study selection for the systematic review
Basic Characteristics of the Selected Studies
Table 1 provides an overview of the included studies’ characteristics. The 38 included articles were published between 2019 and 2022. All of them used an observational design. Out of 38 studies, 22 of them were cohort, 5 of them used case–control design, and 11 were cross-sectional. Investigations were conducted in different countries: 9 studies were conducted in Iran [3, 4, 6, 9, 15, 18–21], while the remaining were performed in Turkey [22–26], China [7, 10, 27, 28], Spain [14, 29, 30], Saudi Arabia [1, 5, 31], France [12, 32], Belgium [33], Korea [34], Nigeria [8], India [35, 36] Brazil [2], and Japan [37]. Twenty-one studies just analyzed the association between Zn and COVID-19 outcomes [2, 4, 5, 9, 12, 13, 15, 18–20, 22, 26, 29, 30, 32, 33, 35–38], three studies analyzed the association between Se and COVID-19 outcomes [8, 39], one analyzed the association between vitamin A and COVID-19 outcomes [40], eight studies analyzed the association between vitamin C and COVID-19 outcomes [1, 7, 10, 23–25, 27, 28], and eight studies investigated the association between mixed antioxidant vitamins, Se, and Zn with COVID-19 [3, 8, 9, 14, 21, 31, 34, 41].
Table 1.
Baseline characteristics of studies that reported the association between antioxidants and covid-19 outcomes
| Study characteristics | Sample characteristics | NOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year |
Country | Design | Antioxidant | Measurements | OR, RR, HR (%95 CI)/ ß/ mean value | Male/female | (Treatment or survivors/Control or Non-survivors) | BMI (kg/m2) | Mean age | Adjustments | ||
| 1 | Yasui et al. 2020 | Japan | Cohort | Zinc | Serum level | 62.4 ± 19.2 (μg/dl) | 34/ 28 | - | 26 ± 5.4 | 62.7 ± 15.3 | COVID-19 PCR | 5 |
| 2 | Al Sulaiman et al. 2020 |
Saudi Arabia |
Cohort | Vitamin C |
Mortality MV ICU LOS AKI Thrombosis Hospital LOS |
OR (95% CI): 0.77 (0.47, 1.23) OR (95% CI): 1.05 (0.51, 2.14) OR (95% CI): 0.50 (0.330, 0.759) OR (95% CI): 1.34 (0.837, 2.150) OR (95% CI): 0.42 (0.184, 0.937) ß: 0.73 (0.51, 0.95) |
296 Both |
Treatment/Control 148/148 |
NR | 60.6 ± 15.5 | COVID-19 PCR, Age | 7 |
| 3 | Gonçalves et al. 2020 | Brazil | Cohort | Zinc | ARDS | OR (95% CI): 15.4 (6.5–36.3) | 138/131 | - | 30[24.7-32] | 74 [66–81] | COVID-19 PCR, Age, CT scan | 7 |
| 4 | Notz et al. 2020 | Germany | Cohort | Zinc | CRP | 24[19–32] vs. 15[8-21] (mg/ml) | 14/ 8 |
Admission/10-14 day 22/19 |
NR | 60.5 [50-69] | COVID-19 PCR, Age | 8 |
| Selenium |
IL-6 TNF-α |
501[168–1211] vs.110[54-306] (pg/ml) 0.8[0.1–1.9] vs. 0.6[0.1-1.2] (pg/ml) |
||||||||||
| 5 | Kumar et al. 2021 | India | Cross-sectional | Zinc | Serum level | 56.6[40-73] vs. 60.5[48-74] (μg/dl) | 124/ 76 | Patients/Control150/50 | NR | NR | COVID-19 PCR, Age | 7 |
| 6 | Hosseini et al. 2020 | Iran | Cross-sectional | Zinc | Serum level | 72.1±18.18 vs. 82.1±17.9 (μg/dl) | 31/ 25 |
Patients/Control 56/44 |
25.88 ± 1.77 | 54.1 ± 20.7 | COVID-19 PCR, Age, CT scan | 9 |
| 7 | Jothimani et al. 2020 [36] | India | Case-control | Zinc |
Serum level CRP D-dimer ARDS IL-6 Hospital LOS ICU admission Mortality |
74.5[53-94] vs. 105.8[95-120] (mg/dl) 11[3.5–48.5] (mg/ml) 499[237–603] (ng/ml) %18.5% vs. 0% 33.3% vs. 15% OR (95% CI): 3.39 (0.99–11.57) OR (95% CI): 3.15 (0.58–17.67) OR (95% CI): 5.84 ( 0.61–49.35) |
92 Both |
Patients/Control 47/45 |
NR | 18 to 77 | COVID-19 PCR, Age | 8 |
| 8 | Younesian et al. 2021 [6] | Iran | Cross-sectional | Selenium | Serum level | 77. 8±13.9 vs. 91.7±16.7 (μg/l) | 31/ 19 |
Patients/Control 50/50 |
NR | 42 to 77 | COVID-19 PCR , Age | 8 |
| 9 | Muhammad et al. 2020 | Nigeria | cross-sectional | Zinc | Serum level | 58 ± 7 vs. 64.9 ± 6.2 (μg/dl) | 46/ 25 |
Patients/Control 50/21 |
21.9 ± 2.2 | 43.8 ± 13.8 | COVID-19 PCR, Age | 7 |
| Selenium | Serum level | 25.3 ± 2.4 vs. 29.1 ± 1.9 (ng/dl) | ||||||||||
| vitamin A | Serum level | 26.5 ± 2.3 vs. 28 ± 1.1 (μg/dl) | ||||||||||
| vitamin C | Serum level | 0.3 ± 0.4 vs. 0.4 ± 0.3 (mg/dl) | ||||||||||
| vitamin E | Serum level | 0.6 ± 0.05 vs. 0.8 ± 0.06 (mg/dl) | ||||||||||
| 10 | Al-Saleh et al. 2020 |
Saudi Arabia |
Cohort | Zinc | Serum level | 1.30 ± 1.81 (μg/mL) | 77/ 78 | - | 29.4 ± 6.4 | 49.8 ± 16.2 | COVID-19 PCR, Age, Medial history | 7 |
| Selenium | Serum level | 76.6 ± 23.54 (μg/L) | ||||||||||
| vitamin A | Serum level | 0.422 ± 0.275 (mg/L) | ||||||||||
| vitamin E | Serum level | 13.92 ± 6.2 (mg/L) | ||||||||||
| 11 | Shakeri et al. 2020 | Iran | cross-sectional | Zinc | Serum level | 118.8 ± 34.4 vs. 94.1 ± 25.9 (μg/dl) | 146/147 |
Survivors/Non-survivors 251/42 |
NR | 53[38-65] | COVID-19 PCR, CT scan | 8 |
| 12 | Al Sulaiman et al. 2021 [1] |
Saudi Arabia |
Cohort | Zinc |
AKI Liver injury Thrombosis MV CRP Mortality ICU LOS |
OR (95% CI): 0.46 (0.19, 1.06) OR (95% CI): 0.24 (0.05, 1.26) OR (95% CI): 0.46 (0.11, 1.98) OR (95% CI): 0.98 (0.31, 3.14) ß (95% CI): -0.05 (-0.36, 0.27) HR (95% CI): 0.64 (0.37, 1.10) HR (95% CI): 0.10 (-0.16, 0.36) |
164 Both |
Treatment/Control 82/82 |
NR | ≥18 | COVID-19 PCR, Age | 8 |
| 13 | Ivanova et al. 2021 | Bulgaria | Cohort | Zinc |
Serum level CRP |
12 ± 3.71vs. 12.8 ± 3.71(μmol/l) 50[15.2–86] vs. 2.42 (mg/l) |
75/ 90 |
Patients/Control 97/68 |
NR | 53.7 ± 12.8 | CT scan | 7 |
| 14 | Maares et al. 2022 [38] | Germany | Cross-sectional | Zinc | Serum level | 0.4 ± 0.2 vs. 0.2 ± 0.1 (nM/l) | 14/ 19 |
Survivors/Non-survivors 27/6 |
NR | 65 to 89 | COVID-19 PCR | 6 |
| 15 | Xia et al. 2020 | China | Cohort | Vitamin C |
CRP IL-6 level TNF-α level |
ß: 0.153 (0.028 0.628) ß: 0.188 (0.097-0.533) ß: 0.185 (0.028 -0.252) |
106/130 |
Treatment/Control 85/151 |
NR | 57 to 76 | COVID-19 PCR, Age, Medical history | 8 |
| 16 | Zhao et al. 2020 | China | Cohort | Vitamin C |
CRP ESR |
1.2[0.5-7.6] vs. 0.5 [0.5, 7.3] (mg/l) 33[10-76] vs. 40.5[21-74.3] (ml/h) |
68/ 42 |
Treatment/Control 55/55 |
NR | 36 [31-47] | COVID-19 PCR, Age | 8 |
| 17 | Xia et al. 2020 | China | Cohort | Vitamin C | Improvement of cardiac injury | OR (95% CI):2.42(1.022-5.729) | 52/ 61 |
Treatment/Control 51/62 |
NR | 59 to 77 | COVID-19 PCR, Age, Myocardial damage | 7 |
| 18 | L. Hess et al. 2020 | USA | Cohort | Vitamin C |
ICU admission ICU LOS MV Cardiac arrest Mortality CRP D-dimer |
OR (95% CI): 0.3 (0.1, 1.3) OR (95% CI): 4.0 (-7.4, 9.3) OR (95% CI): 0.3 (0.1, 1.0) OR (95% CI): 0.2 (0.1, 1.0) HR (95% CI): 0.8 (0.4, 1.6) 126.0± 76.3 vs. 165± 98 (mg-l) 1968 ± 3186 vs. 2553 ± 2720 (ng/ml) |
55/ 45 |
Treatment/Control 25/75 |
35.9 ± 9.7 | 58.3 ± 14.2 | COVID-19 PCR, Age | 8 |
| 19 | Fromonot et al. 2020 | France | Cohort | Zinc | Serum level | 27.6% vs. 11.4% |
240 Both |
Patients/control 152/88 |
NR | 26 to 58 | Age | 6 |
| 20 | Dubourg et al. 2020 | France | Cohort | Zinc | Serum level | 841.1± 198.8 (mg/L) |
275 Both |
- | NR | 53.4 ± 17 | COVID-19 PCR, Age | 6 |
| 21 | Irriguible et al. 2020 | Spain | cross-sectional | Vitamin A | ICU admission | OR (95% CI): 5.26 (1.68–16.46) | 77/ 43 | - | 29.7 ± 12 | 58.7 ± 13.9 | COVID-19 PCR, Age | 7 |
| Zinc |
MV ICU admission Serum level |
OR (95% CI): 6.66 (2.10–21.15) OR (95% CI): 3.84 (1.27–11.65) 63.5 ± 13.5 (μg/dl) |
||||||||||
| 22 | Ghanei et al. 2021 | Iran | Case-control | Zinc | Serum level | 56[123] vs. 110[27] (ng/ml) | 68/ 117 |
Patients/Control 90/95 |
NR | 52 ± 16 | COVID-19 PCR, Age | 7 |
| 23 | Vogel-González et al. 2020 | Spain | Cohort | Zinc |
CRP IL-6 D-dimer |
14.6 [5–24] (mg/dl) 77 [39–145] (pg/mL) 935 [540– 1700] (UI/l) |
127/122 | - | NR | 65[54-75] | COVID-19 PCR, Medical history, Age | 6 |
| 24 | Hyoung Im et al. 2020 | Korea | Cohort | Zinc | Serum level | 87.2[81.9–96.7] (mg/dl) | 29/ 21 |
Patients/Control 50/150 |
NR | 52.2 ± 20.7 | COVID-19 PCR, Age | 7 |
| Selenium | Serum level | 98.3[90.3–107.6] (ng/ml) | ||||||||||
| 25 | Verschelden et al. 2020 | Belgium | Cohort | Zinc |
Serum level MV Mortality |
57[45-67] vs. 74[64-84] (μg/dl) OR (95% CI): 0.98 (0.95–1.00) OR (95% CI): 0.97 (0.94–1.00) |
91/ 48 |
Patients/Control 139/1513 |
27[23–48] | 65[54-77] | COVID-19 PCR, Age | 6 |
| 26 | Moghaddam et al. 2020 | Germany | Cross-sectional | Selenium | Serum level | 53.3 ± 16.2 vs. 40.8 ± 8.1 (μg/L) | NR |
Survivors/ Non-survivors 132/34 |
NR | 38 to 94 | COVID-19 PCR, Age | 8 |
| 27 | Arvinte et al. 2020 [24] | USA | Cohort | Vitamin C | Serum level | 29.1 vs. 15.4 ± 7.6 (μmol/L) | 15/ 6 |
Survivor/ Non-survivor 11/10 |
31.6 ± 7.3 | 60.2 ± 17.4 | Age | 7 |
| 28 | Bagher Pour et al. 2020 | Iran | Cohort | Zinc | Serum level | 67.87 ± 1.12 (μg/dl) | 114/112 | - | NR | 56.3 ± 18.5 | COVID-19 PCR, Age | 8 |
| Selenium | Serum level | 126.6 ± 2 (μg/L) | ||||||||||
| 29 | Shahvali et al. 2020 | Iran | Case-control | Zinc | Serum level | 67.6 ± 15.1vs. 86.6±11.7 (μg/dl) | 41/ 52 |
Case-control 93/186 |
NR | 51[40-61] | COVID-19 PCR, Age | 8 |
| 30 | Arrieta et al. 2021 [30] | Spain | Cohort | zinc |
CRP IL-6 D-dimer |
172 ± 122 vs. 92 ± 66 (mg/L) 47± 922 vs. 1029 ± 2374 (pg/mL) 5183±11270 vs. 7810±14534 (μg/mL) |
30/ 5 | Before and after treatment | 30.3±8.4 | 65 ± 10 | COVID-19 PCR, | 8 |
| 31 | Ekemen Keleş et al. 2020 | Turkey | Cohort | Zinc | Serum level | 88[77–100] vs. 98[84-111] (ng/ml) | 44/ 56 |
Patients/control 100/269 |
NR | 13.3[8–15.4] | COVID-19 PCR | 8 |
| 32 | Beigmohammadi et al. 2020 | Iran | Cross-sectional | Vitamin A | Serum level | 0.2 ± 0.39(μmol/l) | 39/ 21 | - | 25.9±2.7 | 53.5[12.75] | COVID-19 PCR, Medical history | 8 |
| Vitamin C | Serum level | 0.25 ± 0.27 (mg/dl) | ||||||||||
| Vitamin E | Serum level | 7.3 ± 6 (μg/ml) | ||||||||||
| Zinc | Serum level | 50.5± 18 (μg/dl) | ||||||||||
| 33 | Golabi et al. 2021 [15] | Iran | Cross-sectional | Zinc | Serum level | 101±18 vs. 114±13 (μg/dl) | 36/ 17 | Patient/Control 53/53 | 27±5 | 41±13 | COVID-19 PCR, Age, Medical history | 8 |
| 34 | Razeghi et al. 2020 | Iran | Cohort | Selenium | Serum level | 3.33 ± 0.33(μg/ml) | 47/ 37 | - | NR | 81 ± 7 | COVID-19 PCR , CT scan, Age | 7 |
| Zinc | Serum level | 3.95 ± 0.29 (μg/ml) | ||||||||||
| 35 | Gao et al. 2020 | China | Cohort | Vitamin C | Mortality | HR (95% CI): 0.14 (0.03-0.72) | 35/ 41 |
Treatment/Control 46/30 |
NR | 61[52-71] | COVID-19 PCR | 7 |
| 36 | Li et al. 2020 | USA | Cohort | Vitamin C |
ICU LOS Mortality |
18 ±13 vs. 16±14 88% vs 79% |
12/ 20 |
Treatment/Control 8/24 |
NR | 64.1 ± 8.3 | COVID-19 PCR | 8 |
| 37 | Tepasse et al. 2020 | Germany | Cross-sectional | Vitamin A |
ARDS Mortality IL-6 CRP |
OR (95%CI): 5.54 (1.01–30.26) OR (95%CI): 5.21 (1.06–25.5) 88[37–199] vs. 33[16-95] (pg-ml) 14[8.5–27 ] vs. 6[2-13.7] (mg/dl) |
40 Both |
Patients/Control 40/47 |
23 to 28 | 30 to 82 | COVID-19 PCR | 8 |
| 38 | M. Carlucci et al. 2020 [26] | USA | Case-control | Zinc |
ICU admission MV D-dimer CRP ICU LOS |
OR (95%CI): 0.733(0.471–1.14) OR (95%CI): 0.804(0.487–1.33) 341[214–565] vs. 334[215-587] (ng/ml) 104.9[51–158] vs. 108[53-157] (mg/l) 4.8[1.9–7.9] vs. 5.5[2.6-9.3] |
584/348 |
Treatment/Control 411/521 |
29.1 [25.8-33.4] | 63.1 ± 15.1 | COVID-19 PCR | 8 |
Abbreviation, NOS Newcastle–Ottawa Scale; OR odds ratio; RR relative risk; HR: hazard ratio; CI confidence interval; BMI body mass index; MV mechanical ventilation; ICU intensive care unit; LOS: length of stay; AKI acute kidney injury; ARDS acute respiratory distress syndrome; CRP C-reactive protein; ESR erythrocyte sedimentation rate; IL interleukin; TNF tumor necrosis factor; HR Hazard ratio; hs-cTnI high-sensitivity troponin I; M male; F female; NR not reported
Quality Assessment of the Included Studies
A complete report of the quality assessment procedure for the included studies is outlined in Supplementary Table 2. According to the NOS scores, we classified 6 studies as fair quality and thirty-five as high quality.
The Association Between the Serum Level of Different Antioxidants in COVID-19 Patients (Substance-Based)
A summary of the findings on the correlation between serum levels of several antioxidants and COVID-19 results can be found in Table 1.
Selenium
Starting with cross-sectional studies, the first study was conducted by Younesian et al. in Iran[6] and revealed that no significant differences existed between the groups of survivors (mean serum Se level 77.9 ± 14.3 µg/l) and non-survivors (77.2 ± 12.3 µg/l), despite the fact that when compared to healthy control subjects (91.7 ± 16.7 µg/l), the serum Se level in COVID-19 patients was significantly lower (77.8 ± 13.9 µg/l). The second cross-sectional study regarding Se [8] in Nigeria revealed that the plasma concentration of Se in COVID-19 patients was considerably lower than in controls (25.3 ± 2.4 vs. 29.1 ± 1.9 ng/dl, P < 0.001). In Germany, a cross-sectional study [39] showed that the samples from COVID-19 survivors had considerably greater Se serum status (53.3 ± 16.2 µg/l) than samples from non-survivors (40.8 ± 8.1 µg/l). Moreover, a cohort study in Saudi Arabia [31] showed that 30% of COVID-19 patients were deficient in serum Se level (< 70.08 µg/l). Another cohort study in Korea [34] found that 42% of patients were Se deficient. In the same way, the next cohort study in Iran [3] demonstrated that serum Se levels in ICU patients were lower than the non-ICU ward patients; however, the difference was not significant (123.06 ± 2.58 vs. 130.19 ± 3.19, P = 0.084). Finally, the next cohort study [9] which was conducted in Iran reported that the mean serum Se levels were 47.07 ± 20.82 ng/ml in the mild group, 47.36 ± 25.6 ng/ml in the moderate group, and 29.86 ± 11.48 ng/ml in the severe group. A substantial inverse relationship between serum Se level and COVID-19 severity was found (standardized coefficient = − 0.28, P value = 0.01).
Zinc
A comparative cross-sectional study by Kumar et al. [35] revealed that the Zn level was remarkably lower in COVID-19 cases compared to healthy individuals (P < 0.0001). Zn levels were found to be reduced with the increasing severity of COVID-19 (mild, 56.7 µg/dl; moderate, 50.5 µg/dl; and severe, 42.89 µg/dl). Another cross-sectional study [4] in Iran reported the average serum Zn levels in severe cases, non-severe cases, and healthy volunteers as follows: 72.10 ± 18.18 µg/dl, 78.72 ± 22.58 µg/dl, and 82.10 ± 17.96 µg/dl (P < 0.05), respectively. The findings also showed that individuals with pulmonary involvement had significantly lower Zn levels than healthy volunteers (74.29 ± 20.94 µg/dl vs. 82.10 ± 17.95 µg/dl, P = 0.02). Another cross-sectional [8] study in Nigeria showed that the plasma concentrations of Zn were significantly lower in COVID-19 patients (58.1 ± 7.0 µg/dl) compared to controls (64.9 ± 6.2 µg/dl, P = 0.039). Likewise, a cross-sectional study [18] carried out in Iran indicated that the serum level of Zn was lower in patients who expired (94.17 ± 25.95 µg/dl) than in those who were in ICU (98.83 ± 30.49 µg/dl) or non-ICU-admitted (118.8 ± 34.40 µg/dl) (P = 0.002). According to the authors, clinical outcomes in COVID-19 patients can be impacted by the serum Zn level at the time of admission. In line with this, a cross-sectional study [38] conducted in Germany showed that surviving patients (0.4 ± 0.2 nM) significantly had higher levels of Zn than non-survivors (0.2 ± 0.1 nM; P = 0.0004). Another cross-sectional study in Spain [14] realized that 74% of patients had low levels of Zn (63.5 ± 13.5 µg/dl) with respect to the normal range (> 84 µg/dl). In a cross-sectional study [15], infected individuals had lower serum Zn concentrations (101 ± 18 µg/dl) than non-infected participants (114 ± 13 µg/dl; P = 0.01). Another cross-sectional [21] study was conducted on ICU-admitted patients in Iran. The results indicated that the serum levels of Zn were remarkably lower in the group with an Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) score of more than 25 (50.50 ± 18 µg/dl) in compassion to the group with an APACHE score < 25 (80 ± 32.75 µg/dl) (P < 0.001). In India, a case–control study [36] found that Zn levels were considerably lower in COVID-19 patients than in healthy individuals (median; IQR 74.5 mg/dl; 53.4–94.6 vs. 105.8 mg/dl; 95.65–120.90; P < 0.001). Another case–control study in Iran [19] indicated that the median of Zn levels in the cases was lower than the controls (median; IQR 56 ng/ml; 23 vs. 110 ng/ml; 27; respectively) (P < 0.01). Another case–control study in Turkey [22] found that serum Zn level was significantly lower among COVID-19 patients (median serum Zn level, 88.5; IQR, 77.2–100 µg/d) compared to the control group (median serum Zn level, 98; IQR, 84–111 µg/dl). Another case–control study in Iran [20] found that 52% of patients had Zn deficiency, and serum Zn levels of patients were lower than the healthy individuals (67.61 ± 15.10 µg/dl vs. 86.66 ± 11.76 µg/dl; P < 0.001). In the same way, a cohort study [37] showed that hypozincemia (Zn < 70 mg/dl) was a risk factor for a severe case of COVID-19. Another cohort study in Saudi Arabia [31] showed that 25% were deficient in serum Zn level (< 0.693 µg/ml). Another cohort study [13] was carried out on 97 acute and non-acute COVID-19 patients in Bulgaria. Results showed that acute patients did not vary from healthy controls (P = 0.999) but had remarkably lower Zn levels in comparison to non-acute patients (P = 0.023). Similar to the previous study, a cohort study in France [12] found that hypozincemia was more common in patients than in healthy individuals (27.6% vs. 11.4%; P = 0.003). Additionally, a French cohort research [32] discovered that the median blood Zn level was considerably lower in patients with poor clinical outcomes (N = 75) compared to individuals with favorable clinical outcomes (840 mg/l vs. 970 mg/l; P < 0.0001). Likewise, a cohort study in Korea[34] found that no patients were Zn-deficient. In Belgium, another cohort study [33] demonstrated that the most of COVID-19 patients (96%) were Zn-deficient. Similarly, a cohort study in Iran[3] indicated that serum Zn levels were lower in ICU patients (67.3 ± 1.79 µg/dl) compared to non-ICU ward patients (68.42 ± 1.35 µg/dl), and the difference was not significant (P = 0.619). However, low Zn levels were found to be associated with death among COVID-19 patients (recovered 69.66 ± 1.34 µg/dl vs. deceased 62.43 ± 1.81 µg/dl; P = 0.005). Also another cohort study in Iran[9] revealed a substantial inverse relationship between serum Zn levels and COVID-19 severity (standardized coefficient = − 0.26, P value = 0.02).
Vitamin C
A cross-sectional investigation by Muhammad et al. [8] demonstrated that COVID-19 patients’ plasma concentrations of vitamin C were considerably lower than those of controls (0.33 ± 0.43 mg/dl vs. 0.44 ± 0.32 mg/dl; P < 0.001). Similarly, a cross-sectional study in Spain [14] found that all patients had low levels of vitamin C with a mean value of 0.14 ± 0.05 mg/dl (normal range > 0.4 mg/dl). In Iran, 60 COVID-19 patients admitted to the ICU underwent another cross-sectional research [21]. The findings revealed that the group with an APACHE score > 25 had lower serum levels of vitamin C than the group with an APACHE score < 25 (0.25 ± 0.27 µg/dl vs. 0.40 ± 0.50 µg/dl, respectively, P = 0.063). Moreover, a cohort study [24] was conducted on 21 COVID-19 patients who were admitted to ICU in the USA. The results demonstrated that most of the patients who were severely ill had low serum levels of vitamin C, and serum levels of vitamin C among non-survivors were lower than survivors (15.4 ± 7.6 μmol/l 29.1 ± 23.3 μmol/l; P = 0.106).
Vitamin A
Involving 71 COVID-19 patients in Nigeria, Muhammad et al. [8] carried out a cross-sectional study. The findings demonstrated that individuals with COVID-19 had considerably lower plasma concentrations of vitamin A than did controls (26.5 ± 2.3 µg/dl vs. 28 ± 1.1 µg/dl, P < 0.001). Similar findings were made by a cross-sectional study [14] in Spain. It was discovered that 71% of the patients had low levels of vitamin A (0.17 ± 0.06 mg/l, normal range > 0.3 mg/l). Similar to this, a cross-sectional study [21] on 60 COVID-19 patients admitted to an Iranian ICU was carried out. The results revealed that the serum level of vitamin A was lower in the group with APACHE score > 25 (0.20 ± 0.39 µmol/l) when compared to the group with APACHE score < 25 (0.30 ± 0.37 µmol/l) (P = 0.841). 40 COVID-19 patients were the subject of another cross-sectional study [40] in Germany. The results demonstrated that Vitamin A level was significantly lower in hospitalized patients (moderate, severe, and critical status) compared to convalescent persons (median; IQR 0.48 mg/l; 0.29–0.56, 0.32 mg/l; 0.21–0.42, 0.25 mg/l 0.16–0.38, and 0.60 mg/l; 0.51–0.69, respectively; P < 0.001). Similarly, a cohort study [31] in Saudi Arabia indicated that 36.5% of patients had vitamin A levels below the lower reference limits of 0.343 mg/l.
Vitamin E
A cross-sectional investigation in Nigeria was carried out by Muhammad et al. [8]. The findings demonstrated that COVID-19 patients’ plasma concentrations of vitamin E were considerably lower than those of controls (0.63 ± 0.05 mg/dl vs. 0.87 ± 0.06 mg/dl; P < 0.001). One more cross-sectional study [21] was conducted on 60 COVID-19 patients admitted to ICU in Iran. The findings showed that the group with an APACHE score > 25 had lower serum levels of vitamin E than the group with an APACHE score < 25 (7.30 ± 6 µg/ml vs. 7.75 ± 7.22 µg/ml; P = 0.406, respectively). In a similar way, a cohort study [31] in Saudi Arabia showed that 10.2% of patients had vitamin E levels lower than the reference limits of 5.5 mg/l.
The Association Between the Serum Level of Different Antioxidants in COVID-19 Patients (Outcome-Based)
Mechanical Ventilation in COVID-19 Patients
Shaken et al. [18] conducted a cross-sectional study in Iran. The results showed that there is no noticeable difference in serum Zn level between patients who needed intubation and did not. The necessity for intubation was directly correlated with low levels of vitamin A (vitamins deficient 92.3% vs. non-deficient 7.7%; P = 0.001) and zinc (87.5% vs. 12.5%; P = 0.002), according to a second cross-sectional study [14] done in Spain. In the same way, in the USA, a case–control study [26] was carried out on 411 patients receiving Zn sulfate and 521 patients who did not. According to the findings, the addition of Zn sulfate was linked to a reduced need for MV (OR, 0.562; 95% CI, 0.354–0.891). In a similar way, a cohort study [1] in Saudi Arabia was conducted on 296 patients who were admitted to ICU. According to the findings, patients who received the ascorbic acid had a statistically insignificant increased rate of liver damage and respiratory failure necessitating MV. Likewise, a cohort study [5] in Saudi Arabia demonstrated that patients who received Zinc sulfate had a longer median of ventilator-free days than patients who did not (beta coefficient, 0.33; 95%CI, 0.21, 0.87; P = 0.22). Similar to this, a cohort research [23] in the USA showed that patients who received high-dose intravenous vitamin C (HDIVC) had a noticeably decreased MV rate (52.93% vs. 73.14%; OR = 0.27; P = 0.049). Another cohort study [33] in Belgium showed that the median plasma Zn level was systematically lower in MV-needed patients compared to non-MV COVID-19 cases (P < 0.001).
CRP, ESR, TNF-α, and IL-6 Levels in COVID-19 Patients
Tepasse et al. [40] conducted a cross-sectional study in Germany. The findings demonstrated a strong correlation between elevated CRP levels and decreased plasma levels of vitamin A (r = − 0.54, P < 0.001). In a similar way, in the USA, a case–control study [26] was carried out on 411 patients receiving zinc sulfate and 521 patients who did not. The findings demonstrated that there was no variation in CRP between groups (median; IQR 104.95 mg/l; 51.1–158.69 vs. 108.13 mg/l; 53–157.11; P = 0.958). Also, another case–control study [22] was conducted in Turkey. The findings demonstrated that serum Zn levels and CRP did not correlate (median; IQR, 2.6 mg/l; 0.80–8.80 vs. 2.2 mg/l; 0.80–5.50; P = 1.000). Another case–control study [36] in India showed that IL-6 levels were raised in more patients in the Zn-deficient group than in those with normal Zn levels (33.3% vs. 15%, P = 0.110). In the same way, a cohort study [41] conducted on 22 patients who were admitted to ICU showed that sufficient Se and Zn homeostasis was furthermore associated with reduced parameters of inflammation. Se and Zn were inversely correlated with CRP and positively associated with the number of natural killers (NK) cells. Another cohort study [5] in Saudi Arabia showed that patients who received Zn in the ICU had a lower CRP (beta coefficient, − 0.05; 95%CI, − 0.36, 0.27; P = 0.92). Similar to this, a cohort study [13] conducted in Bulgaria on individuals with COVID-19 revealed that acute patients had higher CRP levels at hospital admission. Likewise, a cohort study [28] conducted in China found that the percentages of reduction in inflammatory marker levels were higher in patients receiving HDIVC compared with those in patients treated without HDIVC. Similarly, a cohort study [7] conducted in China found that from day 0 (on admission) to day 7, the HDIVC group exhibited increased CD4 + T cells (P = 0.04) and decreased CRP levels (P = 0.005). In the USA, another cohort study [23] (HDIVC group, 25; control group, 75) revealed that on the fifth day of treatment, CRP levels in the HDIVC patients were non-significantly reduced (mean ± SD, 126.0 ± 7 6.3 mg/l vs. 165.3 ± 98.5 mg/l; P = 0.130). Similarly, a cohort study [29] was performed in Spain. The results indicated that individuals with serum Zn levels < 50 µg/dl had significantly higher CRP (Zn-deficient individuals 14.6 mg/dl vs. non-Zn-deficient individuals 7 mg/dl; P = 0.03) and especially IL-6 (77 pg/ml vs. 32 pg/ml; P < 0.001).
Mortality in COVID-19 Patients
Tepasse et al. [40] conducted a cross-sectional study in Germany. The findings demonstrated a strong association between plasma vitamin A levels below 0.2 mg/l and death (OR, 5.21; 95%CI, 1.06–25.5; P = 0.042). Similar to this, a case–control study [26] was carried out in the USA on 411 patients using zinc sulfate and 521 patients who did not. The results showed that the consumption of zinc sulfate was associated with decreased mortality (OR, 0.511; 95% CI, 0.359–0.726). Another case–control study [36] conducted in India demonstrated that when compared to patients with normal Zn levels, documented deaths were greater in the Zn-deficient group 18.5% vs. 0% (P = 0.06). Similar to this, a cohort study [1] on 296 Saudi Arabian patients who were brought to the ICU was carried out. According to the findings, individuals who took ascorbic acid had much lower hospital mortality rates (33.6%) than those who did not (49.3%) (P = 0.0006). Likewise, a cohort study [5] in Saudi Arabia showed that the 30-day mortality was lower in patients who received 220 mg zinc sulfate/day (HR, 0.52; 95%CI, 0.29, 0.92; P = 0.03). Similarly, a cohort study [23] was performed in the USA. According to the findings, HDIVC patients had a considerably longer mean time to expiry (22.9 days on average vs. 13.7 days for control patients; P = 0.013). Another cohort study [33] in Belgium indicated that the plasma Zn concentration was not significantly associated with the risk of mortality or morbidity (OR, 0.97; 95% CI = 0.94–1.00; P = 0.065). Another cohort study [27] in China showed that compared to the COVID-19 group’s conventional therapy, the high-dose vitamin C treatment lowered the chance of 28-day mortality (HR, 0.14; 95% CI, 0.03–0.72). Similarly, a cohort study [25] was performed in the USA. Eight patients who received daily IV vitamin C were matched to 24 patients who did not. The results showed that higher rates of hospital mortality were observed in the IV vitamin C group of patients (N = 7 (88%) vs. N = 19 (79%), P = 0.049).
Hospital LOS in COVID-19 Patients
In the USA, M. Carlussi [26] conducted a case–control study on 411 patients receiving zinc sulfate and 521 patients who did not. The results showed that the hospital LOS did not shorten when zinc sulfate was added(median; IQR Zn, 6; 4–9 days vs. no-Zn: 6; 3–9 days P = 0.646). In the same way, a cohort study [1] was conducted on 296 COVID-19 patients who were admitted to ICU in Saudi Arabia. 21.3% of the patients who were included got ascorbic acid, compared to 78.7% who did not. They noticed that the hospital LOS was prolonged for severe patients who received an additional dosage of ascorbic acid as supplementary therapy (beta coefficient, 0.50; 95% CI, 0.29, 0.71; P < 0.0001). Another case–control study [36] in India demonstrated that compared to individuals with normal Zn levels, more COVID-19 patients with Zn deficiency had lengthy hospital admissions (> 7 days) (59.2% vs. 30.0%, P = 0.047). Likewise, a case–control study [22] in Turkey revealed that 11.2% and 45.5% of patients needed hospitalization in normal and low serum Zn levels, and there was not any significant difference in the hospital LOS (4 days vs. 5.4 days; P = 0.360). Likewise, a cohort study [30] on 35 COVID-19 patients who needed parenteral nutrition with Zn found that serum Zn level was inversely correlated with a total hospital stay in the first week of parenteral nutrition and at the end of it (r = 0.413, P = 0.014, and r = 0.386, P = 0.022, respectively).
ICU LOS in COVID-19 Patients
In the USA, M. Carlussi [26] conducted a case–control study on 411 patients receiving zinc sulfate and 521 patients who did not. The results showed that a shorter LOS in the ICU was not correlated with the addition of zinc (median; IQR 4.85 days; 1.97–7.94 vs. 5.54 days; 2.65–9.32, respectively; P = 0.504). In the same way, a cohort study [1] was conducted on 296 COVID-19 patients who were admitted to ICU in Saudi Arabia. They noticed that severe patients who got additional ascorbic acid as supplementary therapy stayed in the ICU for a longer period of time (beta coefficient, 0.47; 95% CI, 0.26, 0.68; P < 0.0001). Likewise, a cohort study [5] was carried out in Saudi Arabia. Zinc sulfate 220 mg (50 mg of elemental Zn) enteral tablet once daily was newly initiated in the ICU to 90 patients. The result demonstrated that there was no noticeable difference in ICU LOS between the two groups (beta coefficient, 0.10; 95% CI, − 0.16, 0.36; P = 0.46). Similarly, a cohort study [23] in the USA (HDIVC group, 25, and control group, 75) revealed that individuals with COVID-19 who received HDIVC had considerably longer average ICU stays than those who did not (median; IQR 11.8 days; 7.9–15.8 vs. 7.9 days; 5.1–10.7) (OR, 4.0; 95%CI, − 7.4, 9.3; P = 0.141). Another cohort study [25] in the USA demonstrated that there was no difference in ICU LOS between patients who received 6gr IV vitamin C daily and patients that not received IV vitamin C (18 ± 13 days vs. 16 ± 14 days, respectively) (P = 0.71).
Thrombosis in COVID-19 Patients
In the USA, M. Carlussi [26] conducted a case–control study on 411 patients receiving zinc sulfate and 521 patients who did not. The results demonstrated that there was no difference between the D-dimer of groups (median; IQR 341 ng/ml; 214–565 vs. 334 ng/ml; 215–587, respectively; P = 0.753). Similarly, a cohort study [1] was conducted on 296 COVID-19 patients who were admitted to ICU in Saudi Arabia. It was revealed that utilizing the ascorbic acid was related to a lower risk of thrombosis (6.1% vs. 13%, respectively) (OR, 0.42; 95% CI, 0.184, 0.937; P = 0.03). Likewise, a cohort study [5] was carried out on 164 COVID-19 patients in Saudi Arabia. Zinc sulfate 220 mg (50 mg of elemental Zn) enteral tablet once daily was newly initiated in the ICU for 90 patients. The results showed that no significant difference was observed in thrombosis/infraction (OR, 0.46; 95%CI, 0.11, 1.98; P = 0.29). In the same way, a cohort study [23] in the USA (HDIVC group, 25, and control group, 75) found that on the seventh day of treatment, the HDIVC patients’ mean D-dimer level was likewise significantly lower (1968.3 ± 3186 ng/ml vs. 2553.3 ± 2720 ng/ml; P = 0.016).
AKI in COVID-19 Patients
Al Sulaiman et al. [1] performed a cohort study on 296 COVID-19 patients who were hospitalized in the ICU in Saudi Arabia. The findings indicated that the ascorbic acid group had a statistically insignificant increased rate of AKI. Also, another cohort study [5] in Saudi Arabia showed that Zinc sulfate was associated with lower odds of AKI development during ICU stay (OR, 0.46; 95% CI, 0.19–1.06; P = 0.07).
ARDS in COVID-19 Patients
Tepasse et al. [40] conducted a cross-sectional study in Germany. The results showed that plasma vitamin A concentrations below 0.2 mg/l were strongly linked to the onset of ARDS (OR = 5.54; 95%CI, 1.01–30.26; P = 0.048). In the same way, a case–control study [36] in India found that patients with ARDS were more likely to be in the Zn-deficient group than those with normal Zn levels (18.5% vs. 0%, P = 0.063). In the same way, a cohort study [20] was performed on 269 ICU patients and showed that there was an association between severe ARDS and low Zn levels (OR, 14.4; 95% CI, 6.2–33.5; P < 0.001).
Cardiac Injury
Xia et al. [10] conducted a cohort study in China. The findings revealed that HDIVC administration can ameliorate cardiac injury by alleviating hyper-inflammation in severe COVID-19 patients. Another cohort study [23] in the USA (HDIVC group, 25, and control group, 75) showed that patients who received HDIVC had a remarkably lower rate of cardiac arrest (2.46% vs. 9.06%; OR = 0.2 (0.1–1.0); P = 0.043).
ICU Admission in COVID-19 Patients
In a cross-sectional investigation in Spain, Irriguible et al. [14] discovered that low levels of vitamin A and zinc were linked to the requirement for ICU admission (62.1% vs. 20.7%; P = 0.048 and 61.8% vs. 29%; P = 0.002, respectively). In the same way, a case–control study [36] in India showed that the requirement of ICU was higher in the Zn-deficient group when compared to patients with normal Zn levels (25.9% vs. 10%, P = 0.266). Another case–control study [26] was carried out in the USA on 411 individuals receiving zinc sulfate and 521 who did not. The findings indicated that the consumption of zinc sulfate was linked to a reduced requirement for ICU care (OR, 0.545; 95% CI, 0.362–0.821).
Discussion
In the present study, we systematically reviewed the association between different serum levels of antioxidants such as Se; Zn; vitamins A, C, and E; and COVID-19 outcomes. There were different stratifications in various articles, such as stratification based on survived and non-survived patients, and stratification based on the severity of COVID-19, in which most articles were divided into mild, moderate, and severe. We observed that low levels of antioxidants may increase the severity of COVID-19 outcomes. Although most studies focused on the same outcomes including the need for MV, mortality, rise in the inflammatory factors, ICU admission, ICU LOS, hospital LOS, AKI, thrombosis, ARDS, and cardiac injury, there are disagreements concerning their findings. Therefore, no clear recommendation can be drawn. In most studies, it seems that the serum level of antioxidants decreases when going from mild to severe COVID-19. Moreover, the serum levels of antioxidants were higher in the survivors than in the expired patients. Also, patients who had an appreciative level of antioxidants had low levels of mortality, inflammatory factors, cardiac injury, ARDS, ICU admission, low risk for thrombosis, and the requirement to MV. But there were no significant difference in ICU LOS and hospital LOS. In accordance with the main findings of the present study, previous studies have also demonstrated that the lack of antioxidants may result in the severity of COVID-19. A recent systematic review by Jovis et al. [42] which examined the association of vitamins in facing COVID-19 indicated that vitamins A, C, D, and E are potentially advantageous by acting as antioxidants, immunomodulators, and natural barriers. Another study confirmed the association between antioxidants and COVID-19 [42] suggested that high doses of vitamin C may lower mortality and thrombosis rates, as well as improve oxygenation. Another systematic review [43] indicated that vitamin C supplementation in the range of 1–2 g per day enhanced endothelium function and reduced CRP. Zn supplementation in the range of nearly 50 mg per day significantly decreased CRP. Another systematic review [44] demonstrated that Se levels were lower in COVID-19 patients than in healthy people, and Se deficit was often linked to worse outcomes. They came to the conclusion that Se supplementation in COVID-19 patients may help to stop the progression of the disease. Inconsistently, Kwak et al. [45] showed that the length of hospitalization did not differ significantly between the HDIVC and control groups. But their meta-analysis revealed that HDIVC lowered the in-hospital mortality rate in patients with severe COVID-19. On the other hand, another systematic review and meta-analysis [46] indicated that compared to the placebo group, short-term intravenous vitamin C treatment did not lessen the severity or expiry in COVID-19 patients. Moreover, a systematic review and meta-analysis [47] demonstrated that antioxidants, especially Zn, and Se, vitamins C and D, improve COVID-19 clinical outcomes and reduce the severity. Another systematic review and meta-analysis [48] revealed that vitamin C supplementation reduces hospital mortality in COVID-19 patients, but ICU LOS and hospital LOS were longer in the patients who were treated with vitamin C.
This observed inconsistency among included studies may be due to the following causes: (a) antioxidants were used as the primary treatment in certain investigations, while in others, they served as a co-adjuvant to formal experimental therapy. (b) The hospital setting (inpatient vs. outpatient) and ethnicity varied between studies. (c) There was no consistency among studies regarding their administration protocol; a high degree of heterogeneity was observed in the dosage, administration, and treatment duration. (d) Finally, some studies gave vitamins to individuals with varying risk factors, levels of COVID-19 infection, and times (before, during, or after the infection).
Regarding the underlying mechanisms, one possible explanation is that COVID-19 outcomes may be due to oxidative stress [49–51] caused by binding of the virus to the ACE2. ACE2’s bioavailability is decreased as a result of SARS-CoV-2 bindings to it, which also allows the virus to enter cells. When angiotensin II binds to angiotensin II receptor type 1(AT1R), it modulates nicotinamide adenine dinucleotide phosphate (NADPH) activation. Because ACE2 is now less bioavailable due to SARS-CoV-2 binding, angiotensin II can link with AT1R and send signals that activate NADPH oxidase, cause oxidative stress, and trigger inflammatory reactions, all of which increase the severity of COVID-19. Another relevant mechanism [49] explains the interaction of ACE2 with oxidative stress in the etiology of COVID-19, including endothelial dysfunction brought on by NADPH oxidase’s production of ROS, which lowers nitric oxide bioavailability, which in turn causes vasospasm, inflammation, redox imbalance, and endothelial dysfunction. As a result, the traditional renin–angiotensin–aldosterone system (RAAS) transforms into a strong pro-oxidant system in vessels when ACE2 is dysfunctional or its levels are decreased as a result of SARS-CoV-2. A higher generation and release of pro-inflammatory cytokines are one way that coronaviruses, specifically COVID-19, are capable to trigger the cytokine storm. This verifies the high levels of inflammatory factors seen in COVID-19 patients. It is interesting that one of the factors discovered is the nonspecific CRP, a popular biomarker for the identification of sepsis. Furthermore, COVID-19 severity and mortality have been linked to increased levels of inflammatory cytokines and chemokines. Regarding inflammatory mediators, COVID-19 has been reported to have elevated plasma concentrations of TNF and IL such as IL-6 and interferon (IFN) among others. This gave rise to the theory that sepsis and septic shock in these patients could be brought on by the dysregulated activation of a broad variety of hyper-inflammatory factors linked to COVID-19. It is obvious that oxidative stress, particularly when combined with pulmonary dysfunction, the cytokine storm, or viral sepsis caused by COVID-19 infection, is a crucial element that worsens COVID-19 in some patients. Antioxidant therapy has been suggested as an adjuvant therapy for sepsis. The therapeutic potential of these substances in COVID-19 may therefore be hypothesized using the knowledge gained from other studies about antioxidants in sepsis.
Our systematic review has several strengths. All of the information used in this study was taken from original studies that had high NOS system quality ratings. Antioxidants may be administered to patients with infectious disorders given their high level of safety, low price, and potential for convenience production.
However, the following limitations of this study are noted: there is currently no evidence-based advice for use of antioxidants in COVID-19 patients, and more thorough randomized controlled trials (RCTs) are needed to assess and optimize the timing, dosage, and duration of antioxidants treatment. The majority of current investigations have been observational, and some have used studies with inadequate sample sizes that could cause bias.
Conclusions
According to the primary findings of the current study, it appears that the majority of studies have shown that antioxidants can improve COVID-19 results. To draw a reliable conclusion, additional clinical trials with adequate sample sizes should be carried out.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
ED and ShZ designed the article. AH and RA wrote syntaxes for primary and advanced searchings from all databases, performed first and second screenings for exclusion and inclusion, and extracted the data from all articles. AH, RA, and ED evaluated the quality of all the included studies. AH and RA wrote the body of the article. ED finalized the grammatical changes to the manuscript. All authors read the article and approved the submitted version.
Data Availability
The original contributions presented in this study are included in the article.
Declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
This study was approved by the ethics committee of the Alborz University of Medical Sciences, Karaj, Iran (82–5026).
Conflict of Interest
The authors declare no competing interests.
Footnotes
Elnaz Daneshzad and Shokoofeh Zamani are equally co-corresponding author
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al Sulaiman K, Aljuhani O, Saleh K Bin, Badreldin HA, Al Harthi A, Alenazi M, et al (2021) Author Correction: Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study. Sci Rep 11:19433 [DOI] [PMC free article] [PubMed]
- 2.Gonçalves TJM, Gonçalves SEAB, Guarnieri A, Risegato RC, Guimarães MP, de Freitas DC, et al. Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus SARS-CoV-2. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2021;36(1):186–191. doi: 10.1002/ncp.10612. [DOI] [PubMed] [Google Scholar]
- 3.Pour OB, Yahyavi Y, Karimi A, Khamaneh AM, Milani M, Khalili M, Sharifi A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int J Infect Dis. 2021;111:164–168. doi: 10.1016/j.ijid.2021.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosseini SJ, Moradi B, Marhemati M, Firouzian AA, Ildarabadi E, Abedi A, et al. Comparing serum levels of vitamin D and zinc in novel coronavirus-infected patients and healthy individuals in northeastern Iran, 2020. Infect Dis Clin Pract (Baltim Md) 2021;29(6):e390–e394. doi: 10.1097/IPC.0000000000001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulaiman A, Care C, Sulaiman K Al, Aljuhani O, Shaya AI Al, Kharbosh A, et al (2021) Evaluation of zinc sulfate as an adjunctive therapy in COVID‑19 critically ill patients: a two center propensity ‑ score matched study. Crit Care [Internet]. 1–8. Available from: 10.1186/s13054-021-03785-1 [DOI] [PMC free article] [PubMed]
- 6.Younesian O, Khodabakhshi B, Abdolahi N, Norouzi A, Behnampour N. (2021) Decreased serum selenium levels of COVID‑19 patients in comparison with healthy individuals. Biol Trace Elem Res [Internet]. (0123456789). Available from: 10.1007/s12011-021-02797-w [DOI] [PMC free article] [PubMed]
- 7.Zhao B, Liu M, Liu P, Peng Y, Huang J, Li M, et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol. 2021;12:638556. doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhammad Y, Kani YA, Iliya S, Muhammad JB, Binji A, El-Fulaty Ahmad A, et al. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa. Northwestern Nigeria SAGE open Med. 2021;9:2050312121991246. doi: 10.1177/2050312121991246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RazeghiJahromi S, Moradi Tabriz H, Togha M, Ariyanfar S, Ghorbani Z, Naeeni S, et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect Dis. 2021;21(1):899. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia G, Qin B, Ma C, Zhu Y, Zheng Q. High-dose vitamin C ameliorates cardiac injury in COVID-19 pandemic: a retrospective cohort study. Aging (Albany NY) 2021;13(17):20906–20914. doi: 10.18632/aging.203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erol SA, Polat N, Akdas S, AribalAyral P, Anuk AT, OzdenTokalioglu E, et al. Maternal selenium status plays a crucial role on clinical outcomes of pregnant women with COVID-19 infection. J Med Virol. 2021;93(9):5438–5445. doi: 10.1002/jmv.27064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromonot J, Gette M, Lassoued AB, Guéant JL, Guéant-Rodriguez RM, Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin Nutr. 2022;41(12):3115–3119. doi: 10.1016/j.clnu.2021.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanova ID, Pal A, Simonelli I, Atanasova B, Ventriglia M, Rongioletti M, Squitti R (2022) Evaluation of zinc, copper, and Cu: Zn ratio in serum, and their implications in the course of COVID-19. J Trace Elem Med Biol 71:126944 [DOI] [PMC free article] [PubMed]
- 14.Tomasa-Irriguible TM, Bielsa-Berrocal L, Bordejé-Laguna L, Tural-Llàcher C, Barallat J, Manresa-Domínguez JM, et al. Low levels of few micronutrients may impact COVID-19 disease progression: an observational study on the first wave. Metabolites. 2021;11(9):1–13. doi: 10.3390/metabo11090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golabi S, Adelipour M, Mobarak S, Piri M, Seyedtabib M, Bagheri R, Suzuki K, Ashtary-Larky D, Maghsoudi F, Naghashpour M. The association between vitamin D and zinc status and the progression of clinical symptoms among outpatients infected with SARS-CoV-2 and potentially non-infected participants: a cross-sectional study. Nutrients. 2021;13(10):3368. doi: 10.3390/nu13103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ [Internet] 2009;339(7716):332–6. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Shakeri H, Azimian A, Ghasemzadeh-Moghaddam H, Safdari M, Haresabadi M, Daneshmand T, Namdar Ahmadabad H. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J Med Virol. 2022;94(1):141–146. doi: 10.1002/jmv.27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanei E, Baghani M, Moravvej H, Talebi A, Bahmanjahromi A, Abdollahimajd F. Low serum levels of zinc and 25-hydroxyvitmain D as potential risk factors for COVID-19 susceptibility: a pilot case-control study. Eur J Clin Nutr. 2022;76(9):1297–1302. doi: 10.1038/s41430-022-01095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elham AS, Azam K, Azam J, Mostafa L, Nasrin B, Marzieh N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin Nutr ESPEN. 2021;43:276–282. doi: 10.1016/j.clnesp.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigmohammadi MT, Bitarafan S, Abdollahi A, Amoozadeh L, Salahshour F, Soltani D, Motallebnejad ZA (2021) The association between serum levels of micronutrients and the severity of disease in patients with COVID-19. Nutrition 91:111400 [DOI] [PMC free article] [PubMed]
- 22.EkemenKeleş Y, Yılmaz Çiftdoğan D, Çolak A, Kara Aksay A, Üstündag G, Şahin A, et al. Serum zinc levels in pediatric patients with COVID-19. Eur J Pediatr. 2022;181(4):1575–1584. doi: 10.1007/s00431-021-04348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess AL, Halalau A, Dokter JJ, Paydawy TS, Karabon P, Bastani A, Baker RE, Balla AK, Galens SA. High-dose intravenous vitamin C decreases rates of mechanical ventilation and cardiac arrest in severe COVID-19. Intern Emerg Med. 2022;17(6):1759–1768. doi: 10.1007/s11739-022-02954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvinte C, Singh M, Marik PE (2020) Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American community hospital intensive care unit in May 2020: a pilot study. Medicine in Drug Discovery 8:100064 [DOI] [PMC free article] [PubMed]
- 25.Li M, Ching TH, Hipple C, Lopez R, Sahibzada A, Rahman H. Use of intravenous vitamin C in critically ill patients with COVID-19 infection. J Pharm Pract. 2023;36(1):60–66. doi: 10.1177/08971900211015052. [DOI] [PubMed] [Google Scholar]
- 26.Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D, Xu M, Wang G, Lv J, Ma X, Guo Y, et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging (Albany NY) 2021;13(5):7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia G, Fan D, He Y, Zhu Y, Zheng Q (2021) High-dose intravenous vitamin C attenuates hyperinflammation in severe coronavirus disease 2019. Nutrition 91:111405 [DOI] [PMC free article] [PubMed]
- 29.Vogel-González M, Talló-Parra M, Herrera-Fernández V, Pérez-Vilaró G, Chillón M, Nogués X, et al. Low zinc levels at admission associates with poor clinical outcomes in sars-cov-2 infection. Nutrients. 2021;13(2):1–13. doi: 10.3390/nu13020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrieta F, Martinez-Vaello V, Bengoa N, Jiménez-Mendiguchia L, Rosillo M, de Pablo A, et al. Serum zinc and copper in people with COVID-19 and zinc supplementation in parenteral nutrition. Nutrition. 2021;91–92:111467. doi: 10.1016/j.nut.2021.111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nujud IA, Hissah A, Rola A. Essential metals, vitamins and antioxidant enzyme activities in COVID-19 patients and their potential associations with the disease severity. BioMetals [Internet] 2022;35(1):125–45. doi: 10.1007/s10534-021-00355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubourg G, Lagier J-C, Brouqui P, Casalta J-P, Jacomo V, La Scola B, et al. Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: is there a need to supplement with zinc COVID-19 patients? J Microbiol Immunol Infect. 2021;54(5):997–1000. doi: 10.1016/j.jmii.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verschelden G, Noeparast M, Noparast M, Goossens MC, Lauwers M, Cotton F, Michel C, Goyvaerts C, Hites M (2021) Plasma zinc status and hyperinflammatory syndrome in hospitalized COVID-19 patients: an observational study. Int Immunopharmacol 100:108163 [DOI] [PMC free article] [PubMed]
- 34.Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pvsn KK, Tomo S, Purohit P, Sankanagoudar S, Charan J, Purohit A, et al (2022) Comparative analysis of serum zinc, copper and magnesium level and their relations in association with severity and mortality in SARS-CoV-2 patients. Biol Trace Elem Res [Internet]. (0123456789). Available from: 10.1007/s12011-022-03124-7 [DOI] [PMC free article] [PubMed]
- 36.Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, Manoharan S, Ramani V, Narasimhan G, Kaliamoorthy I, Rela M. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasui Y, Yasui H, Suzuki K, Saitou T, Yamamoto Y, Ishizaka T, et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment - relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maares M, Hackler J, Haupt A, Heller RA, Bachmann M, Diegmann J, Moghaddam A, Schomburg L, Haase H. Free zinc as a predictive marker for COVID-19 mortality risk. Nutrients. 2022;14(7):1407. doi: 10.3390/nu14071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tepasse PR, Vollenberg R, Fobker M, Kabar I, Schmidt H, Meier JA, Nowacki T, Hüsing-Kabar A. Vitamin A plasma levels in COVID-19 patients: a prospective multicenter study and hypothesis. Nutrients. 2021;13(7):2173. doi: 10.3390/nu13072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Notz Q, Herrmann J, Schlesinger T, Helmer P, Sudowe S, Sun Q, Hackler J, Roeder D, Lotz C, Meybohm P, Kranke P. Clinical significance of micronutrient supplementation in critically ill COVID-19 patients with severe ARDS. Nutrients. 2021;13(6):2113. doi: 10.3390/nu13062113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9):2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrao S, Mallaci Bocchio R, Lo Monaco M, Natoli G, Cavezzi A, Troiani E, Argano C. Does evidence exist to blunt inflammatory response by nutraceutical supplementation during covid-19 pandemic? An overview of systematic reviews of vitamin D, vitamin C, melatonin, and zinc. Nutrients. 2021;13(4):1261. doi: 10.3390/nu13041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, Karimi F, Mozafari AM. COVID-19 and selenium deficiency: a systematic review. Biol Trace Elem Res. 2022;200(9):3945–3956. doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwak SG, Choo YJ, Chang MC (2022) The effectiveness of high-dose intravenous vitamin C for patients with coronavirus disease 2019: a systematic review and meta-analysis. Complement Ther Med 64:102797 [DOI] [PMC free article] [PubMed]
- 46.Ao G, Li J, Yuan Y, Wang Y, Nasr B, Bao M, Gao M, Qi X. Intravenous vitamin C use and risk of severity and mortality in COVID-19: a systematic review and meta-analysis. Nutr Clin Pract. 2022;37(2):274–281. doi: 10.1002/ncp.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foshati S, Mirjalili F, Rezazadegan M, Fakoorziba F, Amani R. Antioxidants and clinical outcomes of patients with coronavirus disease 2019: a systematic review of observational and interventional studies. Food Sci Nutr. 2022;10(12):4112–4125. doi: 10.1002/fsn3.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olczak-Pruc M, Swieczkowski D, Ladny JR, Pruc M, Juarez-Vela R, Rafique Z, Peacock FW, Szarpak L. Vitamin C supplementation for the treatment of COVID-19: a systematic review and meta-analysis. Nutrients. 2022;14(19):4217. doi: 10.3390/nu14194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, et al. Oxidative stress and inflammation in covid-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9(10):1–20. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cárdenas-Rodríguez N, Bandala C, Vanoye-Carlo A, Ignacio-Mejia I, Gómez-Manzo S, Hernandez-Cruz EY, Pedraza-Chaverri J, Carmona-Aparicio L, Hernandez-Ochoa B. Use of antioxidants for the neuro-therapeutic management of COVID-19. Antioxidants. 2021;10(6):971. doi: 10.3390/antiox10060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto ME, Guarner-Lans V, Soria-Castro E, Pech LM, Pérez-Torres I. Is antioxidant therapy a useful complementary measure for covid-19 treatment? An algorithm for its application Med. 2020;56(8):1–29. doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article.