Abstract
Following the COVID-19 virus epidemic, extensive, coordinated international research has led to the rapid development of effective vaccines. Although vaccines are now considered the best way to achieve collective safety and control mortality, due to the critical situation, these vaccines have been issued the emergency use licenses and some of their potential subsequence side effects have been overlooked. At the same time, there are many reports of side effects after getting a COVID-19 vaccine. According to these reports, vaccination can have an adverse event, especially on nervous system. The most important and common complications are cerebrovascular disorders including cerebral venous sinus thrombosis, transient ischemic attack, intracerebral hemorrhage, ischemic stroke, and demyelinating disorders including transverse myelitis, first manifestation of MS, and neuromyelitis optica. These effects are often acute and transient, but they can be severe and even fatal in a few cases. Herein, we have provided a comprehensive review of documents reporting neurological side effects of COVID-19 vaccines in international databases from 2020 to 2022 and discussed neurological disorders possibly caused by vaccination.
Keywords: SARS Covid-2, COVID-19, Vaccine, Neurological side effects, Thrombosis, Myelitis
Background
In December 2019, the SARS Covid-2 virus was introduced to the world. A virus that was much more contagious than SARS Covid-1 and spread to different parts of the world in a short time. Following that situation in 2020, the World Health Organization had to declare a global health emergency. This virus is known to cause widespread lung infection and hypoxia [1]. As of November 2022, 630.3 million people have been diagnosed with COVID-19 and 6.58 million deaths worldwide, according to WHO figures [2].
In early 2021, the first vaccines were introduced to stop the pandemic. Also, approximately 68.2% of the world's population has been fully vaccinated against this disease. There are four major strategies for producing COVID-19 vaccines, including nucleic acid-based vaccine (DNA–mRNA), viral vector (replication–non-replication), live inactivated (or attenuated) virus, and protein (spike protein or its subunits). In nucleic acid and adenovirus-based vaccines, fragments of the virus mRNA or genome enter human cells and induce the production of viral proteins [3]. These viral proteins are eventually identified as antigens and stimulate antibody production. In vaccines containing inactive or protein viruses, virus particles and proteins, as antigens, trigger the immune system [4]. As of November 2021, 11 candidate vaccines for COVID-19 have been approved by the World Health Organization for mass vaccination after leaving phase 3 of clinical studies. However, in order to prove the effectiveness of the vaccine in terms of safety and side effects, the implementation of phase 4 of clinical studies is necessary. Because the results of the phase 4 studies are the proper criteria for how the vaccine works in the real world [5].
Vaccines have always been known to be the most effective and safest drugs; however, different side effects have been identified for them, for example, the link between influenza, hepatitis, and HPV vaccines with demyelinating syndromes has been discovered, and the injection of influenza vaccine is a reason for the incidence of narcolepsy in young people [6].
Because COVID-19 vaccines are urgently approved, meaning they do not complete the standard clinical trials, the adverse effects of each vaccine should be closely monitored. It is necessary to pay attention to the fact that in mass vaccination, due to different races, disease history, age, lifestyle, and other effective factors, the incidence of adverse effects of vaccination is higher. According to data from the CDC, VAERS, and EMA databases, the short-term outcome of COVID-19 vaccination is promising, but in the medium and long term, especially with some vaccines, side effects have been reported that are worrisome. VST is the most severe disorder that should be diagnosed and controlled immediately. Therefore, physicians and personnel of medical centers related to these patients should recognize these complications and intervene as soon as possible.
Search method
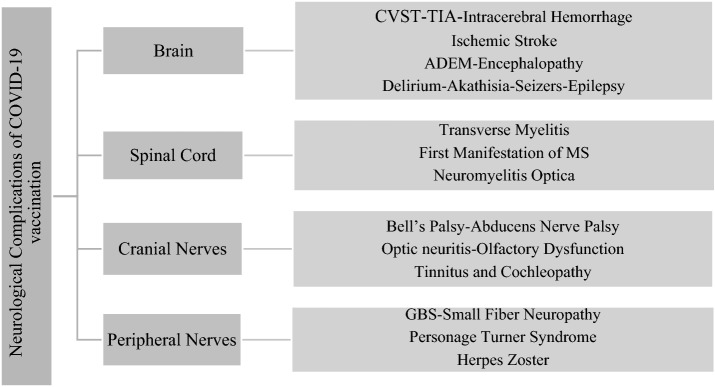
Research, Review, and Case Report articles related to adverse effects of COVID-19 vaccination from 2020 to February 2022 were searched and reviewed in Google Scholar, PubMed, and NCBI databases. Many Case Report articles were not considered due to the lack of a convincing link between the complication and vaccination. Keywords used for this search included COVID-19, SARS-CoV-2, vaccination, side effects, complications, vascular thrombosis, thrombocytopenia, myelitis, demyelination, and all kind of mRNA vaccines, Adenovirus vaccine, Pfizer, AstraZeneca, Johnson & Johnson, Moderna, Sinovac, Sinopharm, Sputnik, and Covaxin. For ease of understanding the various side effects of COVID-19 vaccination, the main categories are shown in Fig. 1.
Fig. 1.
Classification of neurological complications observed after COVID-19 vaccination
Neurological complications following COVID-19 vaccination
According to reports published in the VAERS database, COVID-19 vaccines have several local and systemic neurological complications that occur in different people, from mild to severe, depending on age, sex, history of the disease, and pre-existing immunity [7]. Complications usually appear within one day to 1 month after injection and are usually acute, transient, and self-limiting, but in severe cases lead to hospitalization and intensive care [8]. On the other hand, women have the highest incidence of neurological complications because they induce a stronger immune response against foreign antigens, which can lead to the targeting of self-antigens and lead to autoimmune disorders [9]. Adverse reactions after the second dose of the vaccine are reported more than in the first dose [5].
Mild neurological effects of the COVID-19 vaccine include weakness, numbness, headache, dizziness, imbalance, fatigue, muscle spasms, joint pain, and restless leg syndrome are more common, while tremors, tinnitus, and herpes zoster are less common. On the other hand, severe neurological complications included Bell's palsy, Guillain–Barre syndrome (GBS), stroke, seizures, anaphylaxis, and demyelinating syndromes such as transverse myelitis and acute encephalomyelitis [10]. Among these, the most dangerous neurological complication caused by COVID-19 vaccines, especially adenovirus-based, is cerebral venous sinus thrombosis in women of childbearing age [8].
According to the WHO, in the case of side effects of inactivated virus-based vaccines, especially Sinopharm, the most common local and systemic adverse reactions are injection site reactions, fatigue, fever, headache, and allergic dermatitis, which are self-limiting, and the person does not need to be hospitalized [11, 12]. It is noteworthy that rare and scattered reports have been published on the side effects of Sinopharm and other inactivated virus-based vaccines (Table 1). Vaccine reactivity has been linked to a temporary increase in inflammatory cytokines that act on blood vessels, muscles, and other tissues. In other words, we will observe the flu-like syndrome for several consecutive days after vaccination [13]. According to a recent report on the Sputnik vaccine, side effects are included headache, joint pain, fever, and flu-like symptoms [14]. According to published information on the side effects of other adenovirus vaccines, it is essential to properly evaluate the efficacy of the Sputnik vaccine and publish relevant data to decide on its side effects. COVID-19 vaccination can sometimes have severe side effects on nervous system, including the brain, spinal cord, cranial nerves, and peripheral nerves, and has been shown to have adverse vascular, metabolic, inflammatory, and functional effects on the brain [1].
Table 1.
Reported neurological complications for inactivated virus-based vaccines
| Vaccine name | Complications | References |
|---|---|---|
| Inactivated virus | ||
| Sinovac | Headache | [11, 12] |
| Transverse myelitis | [15–18] | |
| Bell’s palsy | [19] | |
| Acute disseminated encephalomyelitis | [15, 20] | |
| neuromyelitis optica | [21] | |
| Sinopharm | Multiple sclerosis relapse | [9, 22] |
| Neuromyelitis optica | [21] | |
| Covaxin | Bell’s palsy | [19, 23–25] |
The two main mechanisms, ectopic immune reactions, and molecular mimicry, have been proposed for the pathogenicity of vaccines and how these complications occur.
Headache
The first and most common systemic side effect of COVID-19 vaccines is headache, which is mild to severe and is felt in the frontal area of the head. Post-vaccination headaches can be caused by stress, vascular spasm, and intracerebral or subarachnoid hemorrhage. Vaccines based on mRNA and adenovirus have been reported to be most likely to cause headaches [26].
Vascular complications in the brain
Due to the activity of the immune system, after the injection of COVID-19 vaccines, especially adenovirus-based type, thrombocytopenia, cerebral venous sinus thrombosis, ischemic stroke and intracerebral hemorrhage, have also been reported [27]. The proposed mechanism for thrombocytopenia is the synthesis of IgG antibodies against platelet factor 4 (PF4), which activates platelets and blood clots in large venous arteries [28]. Adenovirus-based vaccines are at the forefront of causing this complication due to the transfer of the nucleic acids encoding the viral spike (S) protein. Due to the leakage of these genetic materials and their binding to factor 4 platelet, autoimmunity develops [29]. Venous sinus thrombosis is associated with excessive coagulation. Vaccine viral antigens activate platelets or indirectly cause blood to clot by activating complement pathways and increasing thrombin production. Venous sinus thrombosis and cerebral hemorrhage are more common in women between the ages of 30 and 50 than in men (Table 2) [8].
Table 2.
Reported neurological complications for adenovirus-based vaccines
| Vaccine name | Complications | References |
|---|---|---|
| Adenovirus-based vaccine | ||
| AstraZeneca | Headache | [8] |
| Cerebral venous sinus thrombosis | [30–38] | |
| Transverse myelitis | [16–18, 39–44] | |
| GBS | [45–48] | |
| Bell’s palsy | [19, 23–25] | |
| Acute disseminated encephalomyelitis | [49] | |
| Intracerebral hemorrhage | [10, 50] | |
| Ischemic stroke | [19] | |
| Encephalopathy | [51] | |
| Parsonage–Turner syndrome | [52] | |
| Herpes zoster | [53] | |
| Tinnitus and cochleopathy | [54] | |
| Seizures | [10] | |
| Unilateral and bilateral optic neuritis | [10] | |
| Johnson & Johnson | Cerebral venous sinus thrombosis | [31, 34, 38] |
| Transverse myelitis | [16–18, 39] | |
| GBS | [55–58] | |
| Transient ischemic attack | [59] | |
| Sputnik | Headache | [14] |
| Multiple sclerosis | [60] | |
Acute neurological disorders
These disorders include, transverse myelitis, acute diffuse encephalomyelitis (ADEM), Bell’s palsy, GBS, encephalopathy and seizures. Each type of vaccine can play a different role in increasing the risk of manifestation of these disorders (Tables 2, 3). The COVID-19 vaccine-related convulsions can be attributed to the synthesis and release of spike proteins, which cause severe inflammation and hyperthermia. Hyperthermia, in turn, increases glial cell activity and increases blood–brain barrier permeability. Following these events, as expected, peripheral blood cells and albumin enter the brain and disrupt the osmotic balance [10]. In connection with brain disorders, the possible mechanism is the entry of inflammatory mediators secreted by peripheral blood cells into the brain and the destruction of myelin and axonal degeneration. The presence of SARS-CoV-2 spike domain S1 antibodies in CSF may explain neurological complications after vaccination, such as encephalopathy and seizures [61].
Table 3.
Reported neurological complications for mRNA-based vaccines
| Vaccine name | Complications | References |
|---|---|---|
| mRNA-based vaccine | ||
| Pfizer | Headache | [73, 74] |
| First manifestation of multiple sclerosis | [75–77] | |
| Transverse myelitis | [78, 79] | |
| GBS | [55, 56, 80–83] | |
| Bell’s palsy | [19, 23–25, 84–86] | |
| Acute disseminated encephalomyelitis | [87] | |
| Neuromyelitis optica | [21, 76] | |
| Small fiber neuropathy | [64, 77] | |
| Encephalopathy | [10] | |
| Olfactory dysfunction and phantosmia | [66, 88] | |
| Tinnitus and cochleopathy | [89] | |
| abducens nerve palsy | [90] | |
| Parsonage–Turner syndrome | [1] | |
| Seizers | [10] | |
| Herpes zoster | [91, 92] | |
| Akathisia | [93] | |
| Delirium | [1] | |
| Intracerebral hemorrhage | [94] | |
| Ischemic stroke | [10, 95] | |
| Transient ischemic attack | [10] | |
| Moderna | Transverse myelitis | [15, 17, 96] |
| First manifestation of multiple sclerosis | [76, 94] | |
| Bell’s palsy | [19, 97] | |
| Encephalopathy | [98] | |
| Small fiber neuropathy | [77] | |
| Herpes zoster | [91, 99] | |
| Epilepsy | [100, 101] | |
| Intracerebral hemorrhage | [102] | |
Transverse myelitis is an inflammation of a part of the spinal cord that usually occurs after infection and is associated with impaired sensory, motor, and autonomic function (bladder and intestines) in areas below the area of inflammation in the spinal cord. The mechanism of induction of this disorder is the development of autoimmunity by molecular mimicry. In fact, the viral antigens of the vaccine stimulate an immunological response in the spinal cord [62]. Transverse myelitis has been observed after injection of mRNA and adenovirus-based vaccines, and it is noteworthy that mRNA-based vaccines can cause exacerbation or early manifestation of MS and neuromyelitis optica. More generally, the majority of demyelinating syndromes are related to mRNA-based vaccines, followed by adenovirus-based vaccines. According to reports, these complications are more common in men and women between the ages of 20 and 60 [9].
COVID-19 vaccination also affects the cranial and peripheral nerves and causes side effects such as Bell's palsy (facial nerve palsy—7 cranial nerve), abducens nerve palsy (lateral rectus ocular muscle nerve palsy—6 cranial nerve), impaired vision, olfactory, hearing, Guillain–Barre syndrome (GBS), small fiber neuropathy, Parsonage–Turner syndrome, and also herpes zoster. In this case, too, the known mechanism is the induction of autoimmunity by molecular mimicry. Bell's palsy and small fiber neuropathy are more commonly observed in mRNA-based vaccines [63, 64]. GBS is also a peripheral nerves and nerve roots injury that presents with severe motor weakness and paralysis of the legs or four limbs and is more common in the elderly after vaccination with adenovirus-based vaccines [65]. There have been many reports of the Pfizer vaccine being associated with olfactory [66], visual [67], auditory [68, 69], and sometimes abducens nerve palsy. Olfactory dysfunction ranges from a lack of sense of smell to an olfactory hallucination (phantosmia) that results from a bilateral disturbance or enhancement of the olfactory pathway and the olfactory bulb. Hearing disorders can vary from hearing loss to tinnitus and dizziness. Also, there is ample evidence that the Pfizer and AstraZeneca vaccines are associated with optic nerve inflammation and vision disorders and are more common in middle-aged people [70].
Herpes zoster is a disease that occurs as a result of the reactivation of the varicella-zoster virus (VZV) after receiving the COVID-19 vaccine. The process that causes the disorder is probably explained by the fact that the varicella-zoster virus CD8+ killer cells, after vaccination, are temporarily unable to control VZV due to the extensive change of simple CD8+ cells to the COVID-19 virus CD8+ killer cells. Therefore, vaccination is like a shock to the recurrence of VZV and subsequent herpes zoster [71]. mRNA-based vaccines can increase the risk of herpes zoster [72]. There was a recent report of Ramsey Hunt Syndrome (RHS after the Pfizer vaccination. RHS leads to facial nerve palsy, vestibulocochlear neuropathy, and glossopharyngeal nerve neuropathy, so it causes numbness of the face, tongue, and hearing loss. In addition, skin blisters have been observed in the ear area, leading us to hypothesize that reactivation of VZV could be a cause for RHS as well as Bell's palsy [71].
Conclusion
According to the vaccine study literature, adverse effects have always been part of the mass vaccination strategy, but ultimately the desired effects of the vaccination are more significant. Side effects of COVID-19 vaccination have been reported more frequently in people with a history of immune-related diseases or who are more sensitive to age and physiological conditions. The most important and most common complications are cerebral venous sinus thrombosis (more about AstraZeneca), transverse myelitis (more about Pfizer, Moderna, AstraZeneca, and Johnson & Johnson), Bell's palsy (more about Pfizer, Moderna, AstraZeneca), GBS (more about Pfizer, AstraZeneca, and Johnson & Johnson), and the first manifestation of MS (more about Pfizer). Finally, discovering whether these disorders are accidental or whether the vaccine is the main cause of them requires future studies, ongoing efforts to gather evidence, and long-term monitoring.
Acknowledgements
Not applicable.
Author contributions
RH: carried out the searched publications, classified the documents, and wrote the manuscript draft. NA: supervised the study and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2021 doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.who.int. https://covid19.who.int/mapFilter=deaths. Accessed 13 Novr 2022
- 3.Nagy A, Alhatlani B. An overview of current COVID-19 vaccine platforms. Comput Struct Biotechnol J. 2021;19:2508–2517. doi: 10.1016/j.csbj.2021.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham G, Bhalala OG, de Bakker PI, Ripatti S, Inouye M. Towards a molecular systems model of coronary artery disease. Curr Cardiol Rep. 2014;16(6):1–10. doi: 10.1007/s11886-014-0488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, Shaban D, Al Khames Aga LA, Agha MY, Traqchi M. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588–6594. doi: 10.1002/jmv.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S, Kaur R, Charan J, Bhardwaj P, Ambwani SR, Babu S, Goyal JP, Haque M. Analysis of neurological adverse events reported in VigiBase from COVID-19 vaccines. Cureus. 2022 doi: 10.7759/cureus.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand. 2022;145(1):5–9. doi: 10.1111/ane.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362:577765. doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assiri SA, Althaqafi RM, Alswat K, Alghamdi AA, Alomairi NE, Nemenqani DM, Ibrahim ZS, Elkady A. Post COVID-19 vaccination-associated neurological complications. Neuropsychiatr Dis Treat. 2022;18:137. doi: 10.2147/NDT.S343438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiasi N, Valizadeh R, Arabsorkhi M, Hoseyni TS, Esfandiari K, Sadighpour T, Jahantigh HR. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathologia Persa. 2021;7(2):31. doi: 10.34172/ipp.2021.31. [DOI] [Google Scholar]
- 12.Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis. 2021;111:219–226. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadyrova I, Yegorov S, Negmetzhanov B, Kolesnikova Y, Kolesnichenko S, Korshukov I, Baiken Y, Matkarimov B, Miller MS, Hortelano GH. Sputnik-V reactogenicity and immunogenicity in the blood and mucosa: a prospective cohort study. medRxiv. 2022 doi: 10.1038/s41598-022-17514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021;122(3):793–795. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdem NŞ, Demirci S, Özel T, Mamadova K, Karaali K, Çelik HT, Uslu FI, Özkaynak SS. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogyaszati Szemle. 2021;74(7–08):273–276. doi: 10.18071/isz.74.0273. [DOI] [PubMed] [Google Scholar]
- 17.Khan E, Shrestha AK, Colantonio MA, Liberio RN, Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol. 2021;269(3):1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra HS, Gupta P, Prabhu V, Garg RK, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM: An Int J Med. 2021 doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrêa DG, Cañete LAQ, Dos Santos GAC, de Oliveira RV, Brandão CO, da Cruz Jr LCH. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: Cause or coincidence? Clin Imaging. 2021;80:348–352. doi: 10.1016/j.clinimag.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozgen Kenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089–1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Fan X-R, He S, Zhang J-W, Li S-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci. 2021;42(9):3537–3539. doi: 10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyed Ahadi M, Ghadiri F, Naser Moghadasi A. Acute attack in a patient with multiple sclerosis 2 days after COVID vaccination: a case report. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Reports CP. 2021;14(7):e243829. doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ercoli T, Lutzoni L, Orofino G, Muroni A, Defazio G. Functional neurological disorder after COVID-19 vaccination. Neurol Sci. 2021;42(10):3989–3990. doi: 10.1007/s10072-021-05504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ish S, Ish P. Facial nerve palsy after COVID-19 vaccination–A rare association or a coincidence. Indian J Ophthalmol. 2021;69(9):2550. doi: 10.4103/ijo.IJO_1658_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekizoglu E, Gezegen H, Yalınay Dikmen P, Orhan EK, Ertaş M, Baykan B. The characteristics of COVID-19 vaccine-related headache: Clues gathered from the healthcare personnel in the pandemic. Cephalalgia. 2021;42(4–5):366–375. doi: 10.1177/03331024211042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C, Petzold GC, Piccininni M, Poli S, Röhrig R. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):627–639. doi: 10.1002/ana.26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2022;50(1):e80. doi: 10.1097/CCM.0000000000005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. 2021;121:102662. doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM, Al Maqrashi ZA, Shroff A, Lessard M-C, Blais N. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. New Engl J Med. 2021;385(8):720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RT, Johnson L, Billotti J, Foulds G, Ketels T, Heard K, Hynes EC. Early outcomes of bivalirudin therapy for thrombotic thrombocytopenia and cerebral venous sinus thrombosis after Ad26. COV2.S vaccination. Ann Emergency Med. 2021;78(4):511–514. doi: 10.1016/j.annemergmed.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, Sirabella G, Marano I, Granata V, Grassi R. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Personal Med. 2021;11(4):285. doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franchini M, Testa S, Pezzo M, Glingani C, Caruso B, Terenziani I, Pognani C, Bellometti SA, Castelli G. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb Res. 2021;202:182–183. doi: 10.1016/j.thromres.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Azorín D, Do TP, Gantenbein AR, Hansen JM, Souza MNP, Obermann M, Pohl H, Schankin CJ, Schytz HW, Sinclair A. Delayed headache after COVID-19 vaccination: a red flag for vaccine induced cerebral venous thrombosis. J Headache Pain. 2021;22(1):1–5. doi: 10.1186/s10194-021-01324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George G, Friedman KD, Curtis BR, Lind SE. Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26. COV2. S vaccination. Am J Hematol. 2021;96(8):E301–E303. doi: 10.1002/ajh.26237. [DOI] [PubMed] [Google Scholar]
- 36.Ramdeny S, Lang A, Al-Izzi S, Hung A, Anwar I, Kumar P. Management of a patient with a rare congenital limb malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and thrombocytopenia (VITT) Br J Haematol. 2021 doi: 10.1111/bjh.17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. New Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607. doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J-J, Tseng H-P, Lin C-L, Shiu J-S, Lee M-H, Liu C-H. Acute transverse myelitis following COVID-19 vaccination. Vaccines. 2021;9(9):1008. doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notghi AA, Atley J, Silva M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin Med (Northfield Il) 2021;21(5):e535. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tahir N, Koorapati G, Prasad S, Jeelani HM, Sherchan R, Shrestha J, Shayuk M. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021 doi: 10.7759/cureus.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vegezzi E, Ravaglia S, Buongarzone G, Bini P, Diamanti L, Gastaldi M, Prunetti P, Rognone E, Marchioni E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J Neuroimmunol. 2021;359:577686. doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, Evans JR. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 46.Bonifacio GB, Patel D, Cook S, Purcaru E, Couzins M, Domjan J, Ryan S, Alareed A, Tuohy O, Slaght S. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 47.Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Reports CP. 2021;14(6):e243629. doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohli S, Varshney M, Mangla S, Jaiswal B, Chhabra PH. Guillain-Barré syndrome after COVID-19 vaccine: should we assume a causal Link? Int J Med Pharm Case Rep: 20-24. 2021 doi: 10.9734/ijmpcr/2021/v14i130124. [DOI] [Google Scholar]
- 49.Permezel F, Borojevic B, Lau S, de Boer HH. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol. 2021 doi: 10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjørnstad-Tuveng TH, Rudjord A, Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskrift for Den norske legeforening. 2021 doi: 10.4045/tidsskr.21.0312. [DOI] [PubMed] [Google Scholar]
- 51.Baldelli L, Amore G, Montini A, Panzera I, Rossi S, Cortelli P, Guarino M, Rinaldi R, D'Angelo R. Hyperacute reversible encephalopathy related to cytokine storm following COVID-19 vaccine. J Neuroimmunol. 2021;358:577661. doi: 10.1016/j.jneuroim.2021.577661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonio Crespo Burillo J, Martínez CL, Arguedas CG, Pueyo FJM. Amyotrophic neuralgia secondary to Vaxzevria (AstraZeneca) COVID-19 vaccine. Neurologia (Barc, Ed impr) 2021 doi: 10.1016/j.nrleng.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alpalhão M, Filipe P. Herpes Zoster following SARS-CoV-2 vaccination–a series of four cases. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tseng P-T, Chen T-Y, Sun Y-S, Chen Y-W, Chen J-J. The reversible tinnitus and cochleopathy followed first-dose AstraZeneca COVID-19 vaccination. QJM An Int J Med. 2021 doi: 10.1093/qjmed/hcab210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finsterer J, Scorza FA, Scorza CA. Post SARS-CoV-2 vaccination Guillain-Barre syndrome in 19 patients. Clinics. 2021 doi: 10.6061/clinics/2021/e3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Introna A, Caputo F, Santoro C, Guerra T, Ucci M, Mezzapesa DM, Trojano M. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: a causal or casual association? Clin Neurol Neurosurg. 2021;208:106887. doi: 10.1016/j.clineuro.2021.106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain E, Pandav K, Regmi P, Michel G, Altshuler I. Facial diplegia: a rare, atypical variant of Guillain-Barré syndrome and Ad26. COV2. S Vaccine. Cureus. 2021 doi: 10.7759/cureus.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loza AMM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021;96(22):1052–1054. doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 59.Malik B, Kalantary A, Rikabi K, Kunadi A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Reports CP. 2021;14(7):e243975. doi: 10.1136/bcr-2021-243975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etemadifar M, Sigari AA, Sedaghat N, Salari M, Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother. 2021;17(10):3481–3483. doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan H-T, Lin Y-Y, Chiang W-F, Lin C-Y, Chen M-H, Wu K-A, Chan J-S, Kao Y-H, Shyu H-Y, Hsiao P-J. COVID-19 vaccine-induced encephalitis and status epilepticus. QJM: An Int J Med. 2022 doi: 10.1093/qjmed/hcab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM): clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front Immunol. 2021;12:879. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dyer O. Covid-19: Regulators warn that rare Guillain-Barré cases may link to J&J and AstraZeneca vaccines. Br Med J Publ Gr. 2021 doi: 10.1136/bmj.n1786. [DOI] [PubMed] [Google Scholar]
- 66.Keir G, Maria NI, Kirsch CF. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133–137. doi: 10.1097/RMR.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 67.Santovito LS, Pinna G. Acute reduction of visual acuity and visual field after Pfizer-BioNTech COVID-19 vaccine 2nd dose: a case report. Inflammation Res. 2021;70(9):931–933. doi: 10.1007/s00011-021-01476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol. 2021 doi: 10.1080/14992027.2021.1931969. [DOI] [PubMed] [Google Scholar]
- 69.Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID-19 vaccination: the house ear clinic experience. Otol Neurotol. 2021;42(9):e1213. doi: 10.1097/MAO.0000000000003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Žorić L, Rajović-Mrkić I, Čolak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. 2021;14:349. doi: 10.2147/IMCRJ.S315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woo CJ, Chou OHI, Cheung BMY. Ramsay Hunt syndrome following COVID-19 vaccination. Postgrad Med J. 2022 doi: 10.1136/postgradmedj-2021-141022. [DOI] [PubMed] [Google Scholar]
- 72.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, Desai SR, French LE, Lim HW, Thiers BH. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Göbel CH, Heinze A, Karstedt S, Morscheck M, Tashiro L, Cirkel A, Hamid Q, Halwani R, Temsah M-H, Ziemann M. Clinical characteristics of headache after vaccination against COVID-19 (coronavirus SARS-CoV-2) with the BNT162b2 mRNA vaccine: a multicentre observational cohort study. Brain Commun. 2021;3(3):169. doi: 10.1093/braincomms/fcab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269(1):55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khayat-Khoei M, Bhattacharyya S, Katz J, Harrison D, Tauhid S, Bruso P, Houtchens MK, Edwards KR, Bakshi R. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2021 doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alshararni A. Acute transverse myelitis associated with COVID-19 vaccine: a case report. Int J Res Pharma Sci. 2021;12:2083–2087. doi: 10.26452/ijrps.v12i3.4818. [DOI] [Google Scholar]
- 79.McLean P, Trefts L. Transverse myelitis 48 hours after the administration of an mRNA COVID 19 vaccine. Neuroimmunology Reports. 2021;1:100019. doi: 10.1016/j.nerep.2021.100019. [DOI] [Google Scholar]
- 80.Finsterer J. Exacerbating Guillain-Barré Syndrome Eight Days after vector-based COVID-19 vaccination. Case Rep Infect Dis. 2021 doi: 10.1155/2021/3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: a temporal occurrence, not a causal association. IDCases. 2021;24:e01143. doi: 10.1016/j.idcr.2021.e01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann Med Surg. 2021;67:102540. doi: 10.1016/j.amsu.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021 doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Terreros Caro GG, Díaz SG, Alé MP, Gimeno MM. Bell’s palsy following COVID-19 vaccination: a case report. Neurologia (Barcelona, Spain) 2021 doi: 10.1016/j.nrleng.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42(11):4397–4399. doi: 10.1007/s10072-021-05496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogrig A, Janes F, Gigli GL, Curcio F, Del Negro I, D’Agostini S, Fabris M, Valente M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed SH, Waseem S, Shaikh TG, Qadir NA, Siddiqui SA, Ullah I, Waris A, Yousaf Z. SARS-CoV-2 vaccine-associated-tinnitus: a review. Ann Med Surg. 2022;75:103293. doi: 10.1016/j.amsu.2022.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J Am Assoc Pediatr Ophthalmol Strabismus. 2021;25(5):302–303. doi: 10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiu H-H, Wei K-C, Chen A, Wang W-H. Herpes zoster following COVID-19 vaccine: a report of three cases. QJM: An Int J Med. 2021;114(7):531–532. doi: 10.1093/qjmed/hcab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodríguez-Jiménez P, Chicharro P, Cabrera L-M, Seguí M, Morales-Caballero Á, Llamas-Velasco M, Sánchez-Pérez J. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salinas MR, Dieppa M. Transient akathisia after the SARS-Cov-2 vaccine. Clin Park Relat Disord. 2021;4:100098. doi: 10.1016/j.prdoa.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finsterer J, Redzic Z. Symptomatic peduncular, cavernous bleeding following SARS-CoV-2 vaccination induced immune thrombocytopenia. Brain Hemorrhages. 2021;2(4):169–171. doi: 10.1016/j.hest.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitzsimmons W, Nance CS. Sudden onset of myelitis after COVID-19 vaccination: an under-recognized severe rare adverse event. SSRN. 2021 doi: 10.2139/ssrn.3841558. [DOI] [Google Scholar]
- 97.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno M, de la Nogal-Fernandez B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269(1):47–48. doi: 10.1007/s00415-021-10617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Mashdali AF, Ata YM, Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: a case report. Ann Med Surg. 2021;69:102803. doi: 10.1016/j.amsu.2021.102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Channa L, Torre K, Rothe M. Herpes zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 vaccination. JAAD Case Rep. 2021;15:60–61. doi: 10.1016/j.jdcr.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu BD, Ugolini C, Jha P. Two cases of post-Moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021 doi: 10.7759/cureus.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Šín R, Štruncová D. Status epilepticus as a complication after COVID-19 mRNA-1273 vaccine: a case report. World J Clin Cases. 2021;9(24):7218. doi: 10.12998/wjcc.v9.i24.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Athyros VG, Doumas M. A possible case of hypertensive crisis with intracranial haemorrhage after an mRNA anti-COVID-19 vaccine. Angiology. 2022;73(1):87–87. doi: 10.1177/00033197211018323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.