Abstract
Breast cancer (BC) is one of the most widely diagnosed cancers and a leading cause of cancer death among women worldwide. Globally, BC is the second most frequent cancer and first most frequent gynecological one, affecting women with a relatively low case-mortality rate. Surgery, radiotherapy, and chemotherapy are the main treatments for BC, even though the latter are often not aways successful because of the common side effects and the damage caused to healthy tissues and organs. Aggressive and metastatic BCs are difficult to treat, thus new studies are needed in order to find new therapies and strategies for managing these diseases. In this review, we intend to give an overview of studies in this field, presenting the data from the literature concerning the classification of BCs and the drugs used in therapy for the treatment of BCs, along with drugs in clinical studies.
Keywords: breast cancer, chemotherapy, endocrine therapy, therapeutic targets
1. Introduction
BC is considered to be the second most common cancer subtype investigated worldwide [1] and it is estimated that 2.3 million new cases are diagnosed each year worldwide [2]. As per the 2021 WHO statistics, BC has become the most prevalent cancer in the world, and it is predicted that it could constitute more than 30% of all cancers diagnosed in women [3]. Its patterns and trends vary in distinct countries, being the second driving cause of cancer death among women overall—after lung cancer—and the head cause of cancer death in Africa and America among Black and Hispanic women [4,5]. About 5–10% of all BC patients are genetically predisposed to cancers [6,7] and almost 80% of patients with BC are individuals aged >50. Nevertheless, BC is the most common cancer diagnosed and the second most common cause of cancer death in women aged less than 40 years [8]. The number of risk factors of BC is significant and includes modifiable and non-modifiable factors, such as excessive body weight, physical inactivity, and alcohol intake [9]. A recent study suggested that negative emotions also significantly increase the incidence risk for BC [10]. Prevention through mammography screening is fundamental for allowing early therapy and a substantial reduction in BC mortality [11]. BC treatment is multidisciplinary; indeed, breast-conserving surgery with radiotherapy or mastectomy is generally used in early-stage BCs and sentinel node biopsy is used for axillary staging. In most cases, endocrine therapy and/or chemotherapy are needed together with adjuvant and neo-adjuvant systemic therapies that are often used in the majority of women based on proven survival outcomes [12]. The survival rate from BC has improved in recent years; for instance, the 5-year survival rate for non-invasive BC is 99%, while the 5-year survival rate is 85% for invasive BC diffused to the regional lymph nodes. However, the survival rate reduces to 27% if distant parts of the body are also affected [13]. Approximately 70% of BC metastases occur in the bone [14] and metastatic breast cancer (MBC) is currently considered incurable. Lei et al. [15] recently assessed that the incidence and mortality rates of BC increased rapidly in China and South Korea but decreased in the USA. In 2020, the diagnosis and treatment of cancer was negatively affected by the coronavirus disease 2019 (COVID-19) pandemic [16]. Indeed, the reduced access to care due to the closure of healthcare settings along with the fear of COVID-19 exposure have led to delays in diagnosis and treatment bringing to a short-term drop in cancer incidence, followed by an increase in advanced-stage disease and, ultimately, in mortality [17]. Obviously, patients suffering from BC and contemporaneously infected with SARS-CoV-2 have a high probability of mortality [18].
2. Classification of BCs
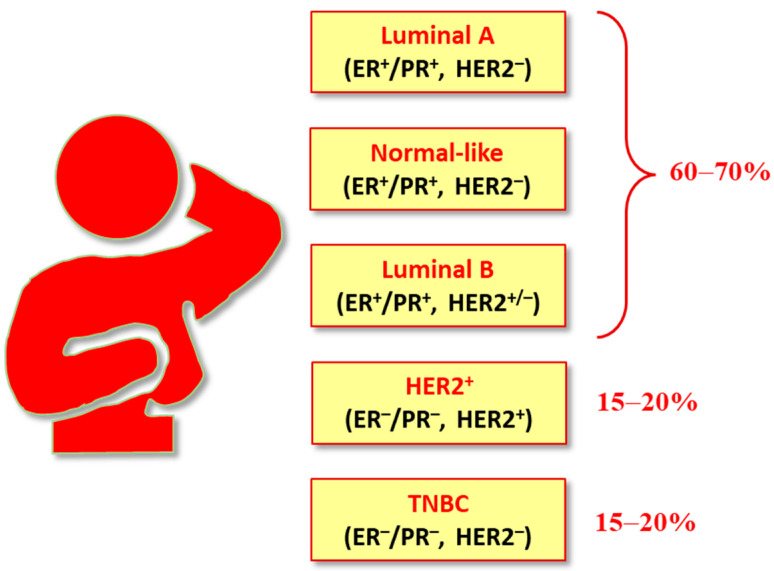
Different classifications have been proposed for BC (Figure 1). It is generally categorized into five major subtypes based on the presence or absence of receptors expressed by tumor cells: luminal A (LumA), luminal B (LumB), HER2-overexpressing (or HER2-enriched or HER2+), triple negative breast cancer (TNBC), and basal-like and normal-like (or normal breast tissue-like) [19]. The luminal A, B, and HER2+ subtypes are positive for hormone receptors (HRs), i.e., estrogen receptor (ER) and/or progesterone receptor (PR). In luminal B and HER2+ subtypes, HER2 overexpression is also noticed. The majority (60–70%) of breast tumors are represented by luminal A and B breast cancers subtypes. The HER2+ breast cancer subtype that constitutes 10–15% of invasive breast cancers is characterized by the amplification/activation of the HER2 gene, which results in the HER2 receptor overexpression on the surface of breast cancer cells. TNBC was so named (“triple negative”) because it lacks the expression of the three molecular markers (ER, PR, and HER2), and is the most aggressive subtype, poorly prognosed, often observed in young women, representing the 15–20% of all BCs.
Figure 1.
Molecular subtypes of BCs.
Another hormone receptor (HR) that is not generally classified in HRs is the prolactin (PRL) receptor (PRLR) that is overexpressed in most of the ER+ breast tumors. The role of PRLR in the etiology and proliferation of breast carcinoma induced by PRL has been well established [20]. Moreover, another subtype of BC has been described, namely the “claudin-low”, defined by gene expression characteristics—most prominently a low expression of cell–cell adhesion genes, high expression of epithelial–mesenchymal transition (EMT) genes, and stem cell-like/less differentiated gene expression patterns [21,22]. However, a vast degree of heterogeneity has been observed in this subtype of BC, suggesting a need for more investigations into this form of cancer [23]. Recently, HER2 has been named ERBB2 or ErbB2, being part of the ErbB family of receptor tyrosine kinases (also known as epidermal growth factor receptor (EGFR)-family) and consisting of four closely related receptors which includes EGFR (ErbB1/HER1), ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4) [24]. A more recent classification of BCs is based on the presence or absence of molecular markers for ERs or PRs and human epidermal growth factor 2 (ERBB2; formerly HER2): hormone receptor positive/ERBB2 negative (70% of patients), ERBB2 positive (15–20%), and triple-negative (tumors lacking all three standard molecular markers; 15%) [25]. All the subtypes have distinct risk profiles and treatment strategies, depending on tumor subtype, anatomic cancer stage, and patient preferences. BC is one of the first malignancies for which targeted therapies have been used successfully [26] and, in the period from 2010 to 2020, 30 drugs have been approved by the FDA for the treatment of BC [27]. However, side effects, often related to the lack of treatment adherence, are still reported [28]. Despite the efforts made in treating cancers during the past decades, resistance to known chemotherapeutic agents and/or new targeted drugs continues to be a great dilemma in cancer therapy [29]. Drug resistance may be intrinsic—i.e., existing before treatment—or acquired, generally generated after the therapy, but both of them are responsible for most relapses of cancer, one of the main causes of death from BC [30]. Thus, finding new strategies for curbing BC is nowadays a field of great interest. For instance, nanoparticles (NPs) and engineered NPs have been proposed as a next-generation platform with preventive and therapeutic effects against BC [31]. In this review, we highlight the most common therapeutic strategies used to restrain BC and most recent studies for novel compounds designed as potential anticancer drugs for fighting BC in the future.
3. Genetic Mutations in BCs
Genetic mutations in specific genes enhance the probability of acquiring breast cancer and may influences its severity. It is known that mutations in the p53, BRCA1, and PTEN genes account for about 10% of familial breast and ovarian cancer cases overall [32]. BReast CAncer genes (BRCA) 1 and 2 encode DNA repair enzymes and took their name just because of their association with BC [33,34]. BRCA mutations are highly associated with TNBC, as well as the loss of BRCA1 or BRCA2 activity [35]. Phosphatase and Tensin homolog deleted on chromosome 10 (PTEN) is a tumor suppressor gene located in the 10q23 region of chromosome 10 that has been found to be mutated in many types of cancers, including BC, and encodes for a dual lipid and protein phosphatase [36]. PTEN inactivity is associated with an aggressive triple-negative phenotype cancer, as in TNBC [37].
4. Breast Cancer Therapies
The approach to BC therapy is multidisciplinary and the current major treatments for are represented by surgery, cytotoxic chemotherapy, targeted therapy, radiotherapy, endocrine therapy, and immuno-therapy [38,39]. The plurality of women with early-stage BC is subjected to breast-conserving surgery with radiotherapy or, alternatively, mastectomy and the sentinel node biopsy is utilized for axillary staging, minimizing the demand for axillary dissection in sentinel node-positive women. Neoadjuvant and adjuvant therapies are often used to treat BC and may include chemo- and radiotherapy, hormone and/or immune-therapy and targeted therapy. The major difference is that neoadjuvant (or preoperative) therapies are delivered before the cancerous tumor is surgically removed, helping to reduce the size of the tumor or kill cancer cells that may have spread, whereas the adjuvant therapy is usually used after a cancerous tumor has been surgically removed in order to destroy the remaining cancer cells (Table 1) [12].
Table 1.
Characteristics of adjuvant and neoadjuvant therapies.
| Characteristics | Adjuvant | Neoadjuvant |
|---|---|---|
| Definition | Adjuvant therapy is the treatment after tumor removal | Neoadjuvant therapy is the treatment before tumor removal |
| Advantages | Can help prevent cancer recurrence and kills the remaining cancerous cells | Helps shrink the size of tumors making them easier to cut out |
| Disadvantages | The treatments can further weaken the organism due to unpleasant side effects | Delay in surgical removal of the tumor could mean the cancer spread |
| Cancer caused by genetic mutations | Most often recommended because the risk of cancer recurrence is high where mutations are inherited | Not often recommended if cancer is not due to inherited mutations but is likely due to environmental factors |
| Early insight into treatment plan | Does not provide early insight into the effects of chemotherapy and radiation until after surgery | Useful in indicating how a patient does with certain chemotherapy or radiation treatments before surgery, allowing for changes to be made after surgery |
Moreover, new methods—such as targeted and immuno-therapy—have been developed to improve patient survival and prognosis. The era of targeted therapies has offered a new avenue in the search for potentially more efficacious strategies. Recently, cancer stem cell (CSC)-targeted therapies, including those inducing CSC apoptosis and inhibiting the self-renewal and division, have been under study [40]. Recently, sirtuins (SIRT1–7) have demonstrated a great potential as biomarkers and/or targets for the treatment of BCs and the dual role of SIRT1–7 as tumor promoters or suppressors in BCs have been widely discussed [41]. Common therapies will be discussed below.
4.1. Endocrine Therapy
Endocrine therapy can be used for all patients affected by BC that express ERs and PRs—specifically, LumA, LumB and normal-like BC and as adjuvant and neoadjuvant in several types of BCs [42,43]. However, due to the well-known limits of this type of therapy, the search for new ER inhibitors is still ongoing [44]. The majority (80%) of BC patients are hormone-dependent or ER+ [45]. Amongst ER receptors, ERα subtype is the major responsible for estrogen effect in the breast, whereas the activity of ERβ prevents tumor formation in response to estrogens. Recent studies have focused on ESR1, which is the gene spanning q24–q27 of chromosome 6 and encodes for ERα, the nuclear transcription factor most commonly implicated in BC [46]. However, when BRCA1 or BRCA2 mutations are present, patients have poor survival outcomes and hence screening for BRCA mutations might help in strategizing the treatment and improving the survival [47]. For MBC patients with ER+ disease, endocrine therapy—with or without the addition of targeted agents—is recommended as first line systemic anti-cancer therapy (SACT) [48,49]. The most commonly used endocrine therapies are based on the use of selective estrogen-receptor modulators (SERMs), selective estrogen receptor downregulators or degraders (SERDs), aromatase inhibitors (AIs), gonadotropin-releasing hormone (GnRH) agonists. However, mutations on the ESR1 gene may produce resistance to endocrine therapies and patients with ESR1 mutant–positive metastases are resistant to standard-of-care endocrine therapy and evidence a worst overall survival [50]. However, resistance to endocrine treatment often occurs in BC patients, as assessed by REVERT clinical study. Recently, the combined use of the drug eribulin has been suggested, as it may sensitize the tumor to the hormonal treatment due to the switch of cancer cell phenotype [51].
4.1.1. Selective Estrogen-Receptor Modulators (SERMs)
SERMs specifically control the ERs and limit the progression of breast malignancy regulating particularly ERα, which is mainly responsible for the initiation and progression of BC. They act as antagonists of the transcription process in the BC cell and as agonists in other tissues (bone and endometrium); however, the long-term use of traditionally marketed SERMs is associated with several side effects, such as the development of endometrial cancer and other disorders [52]. SERMs include tamoxifen, raloxifene, bazedoxifene, and lasofoxifene. For over 30 years, tamoxifen—a triphenylethylene SERM (Table 2)—has been the drug of choice solely to treat ER+ BC patients. It is a partial nonsteroidal estrogen agonist, which represents the prototype of SERMs, acting as a type II competitive inhibitor of estradiol used for early and metastatic BC. Tamoxifen is metabolized to the more active metabolites 4-OH-tamoxifen and endoxifen by the CYP2D6 and CYP3A4/5 enzymes; thus, may be considered a prodrug [53]. Raloxifene hydrochloride (Evista®), a benzothiophene derivative acting as estrogen antagonist was the first SERM approved by the US FDA as a protective and therapeutic agent for postmenopausal osteoporosis. Later, raloxifene was also approved for reducing breast cancer risk, and it has been more recently investigated for breast cancer management [54]. Unfortunately, raloxifene has a reduced bioavailability (not higher than 2%), being subjected to dramatic first pass metabolism due to its poor water solubility; thus, more recently, raloxifene-loaded semisolid self-nanoemulsifying system (SSNES) have been proposed, with minimized globule size in order to improve drug solubility, tumor penetration, and antitumor activity [55]. Lasofoxifene (Fablyn®), a SERM with benefits on bone health and potential for breast cancer prevention, has been investigated in mouse models of endocrine therapy-resistant BC with ERα mutations, Y537S and D538G, which have low sensitivity to fulvestrant inhibition, demonstrating its potential as an effective therapy for women with advanced or metastatic ER+ BC expressing the most common constitutively active ERα mutations [56]. Lasofoxifene demonstrated interesting activity in the clinical trial ELAINE 1 in which it was compared with fulvestrant in ESR1-mutated MBC patients with progression on CDK4/6i, and all clinical outcomes were in favored lasofoxifene. The association of lasofoxifene with abemaciclib, in phase II trial ELAINE 2, showed efficacy in heavily pretreated patients with ESR1-mutated MBC post-CDK4/6i (ASCO 2022) [57]. Bazedoxifene (Duavive®) is a synthetic SERM, which received approval by the United States Food and Drug Administration for the treatment of osteoporosis in postmenopausal women. It is now being studied as a repositioned drug for its anticancer activity in different types of cancers including BC [58]. Recently, the combination of BZA and palbociclib showed clinical efficacy and an acceptable safety profile in a heavily pretreated patient population with advanced HR+/HER2− BC [59]. However, complicacies due to SERMs remain because of the onset of many dangerous adverse effects as endometrial carcinoma, hot flashes, and VTE (venous thromboembolism), thus novel candidates with no or lower adverse effects for ER+ BC prevention are needed [60].
Table 2.
Endocrine drugs in therapy and/or in clinical study for BC.
| Structure | Name | Class | Phase Study |
|---|---|---|---|
|
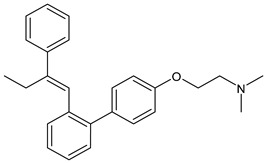
Tamoxifen (Nolvadex®, Kessar®, Nomafen®, Tamoxene®) | SERM | FDA approved |
|
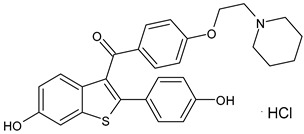
Raloxifene Hydrochloride (Evista®) |
SERM | FDA approved |
|
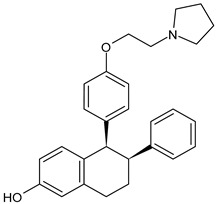
Lasofoxifene (Fablyn®) | SERM | FDA approved |
|
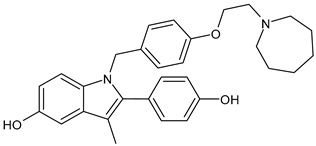
Bazedoxifene (Duavive®) | SERM | FDA approved |
|
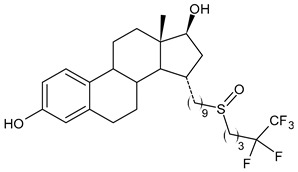
Fulvestrant (Faslodex®) | SERD | FDA approved |
|
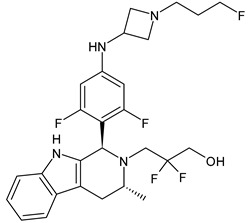
Giredestrant (GDC-9545) |
SERD | Phase III |
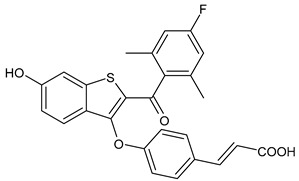
|
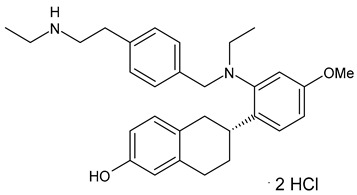
Rintodestrant (GIT48) |
SERD | Phase I |
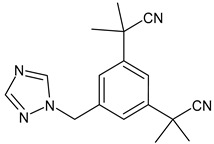
|
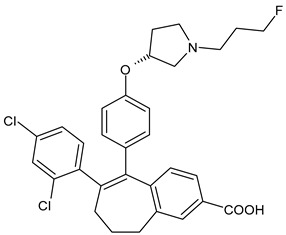
Amcenestrant (SAR439859) | SERD | Phase III |
|
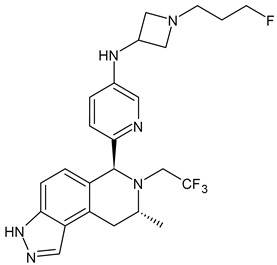
Camizestrant (AZD9833) | SERD | Phase III |
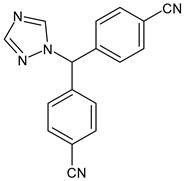
|
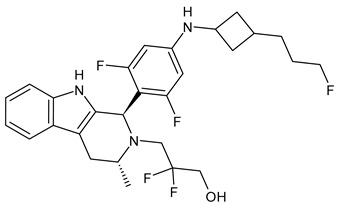
Elacestrant (RAD1901) |
SERD | Phase III |
|
Imlunestrant (LY3484356) | SERD | Phase I |
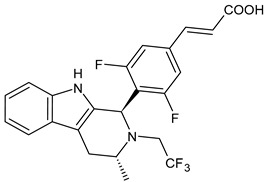
|
AZD9496 | SERD | Phase I |
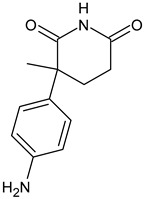
|
Aminoglutethimide (Orimeten) | AI First generation type II (non-steroidal) |
FDA approved |
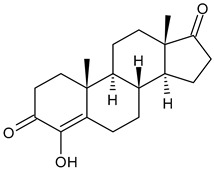
|
Formestane (Lentaron) | AI Second generation type I (steroidal) |
FDA approved |
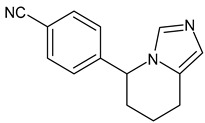
|
Fadrazole (Afema®) | AI Second generation type II (non-steroidal) |
FDA approved |
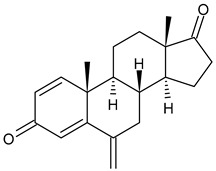
|
Exemestane (Aromasin®) | AI Third generation type I (steroidal) |
FDA approved |
|
Anastrozole (Arimidex®) | AI Third generation type II (non-steroidal) |
FDA approved |
|
Letrozole (Femara®) | AI Third generation type II (non-steroidal) |
FDA approved |
|
Goserelin | GnRH agonist | FDA approved |
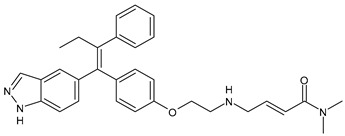
|
H3B-5942 | SERCA | Precursor of SERCA H3B-6545 |
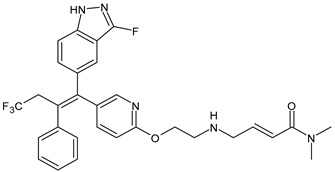
|
H3B-6545 | SERCA | Phase II |
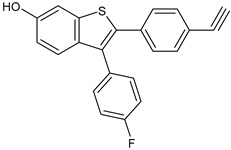
|
BMI-135 | ShERPA | Phase I |
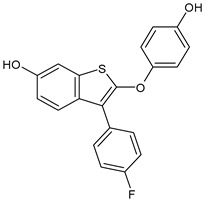
|
TTC-352 | ShERPA | Phase I |
4.1.2. Selective Estrogen Receptor Downregulators or Degraders (SERDs)
SERDs include fulvestrant and investigational SERDs (AZD9496, AZD9833, LY3484356, GDC-0810, GDC-0927, GDC-9545, and SAR439859), which inhibit ER-mediated cellular proliferation through ERα degradation [61]. Fulvestrant (Faslodex®) is a first-generation SERD approved by the FDA in 2007 for the treatment of metastatic luminal BC in postmenopausal patients following progression on prior endocrine therapy with aromatase inhibitors or tamoxifen [62]. It has also shown interesting activity in patients with ESR1 mutations in the second line treatment setting. It is the unique SERD approved for use as a second-line endocrine therapy effective in treating endocrine therapy-resistant tumors [63]. It can degrade and completely antagonize ERα in all settings tested and has proven to be superior to the aromatase inhibitor anastrozole in clinical trials [64]; it is also used in MBC. Nevertheless, its effectiveness in metastatic disease has been validated, the challenges of drug resistance remain even for this drug [65] and it has the limit of being an injectable with considerable pharmaceutical liabilities [66]. Thus, several SERDs available as oral formulations are under study as monotherapy or in combination with other drugs [67,68,69,70]. GDC-9545 (giredestrant) was identified as an oral SERD with an exceptional preclinical profile [71]. It is now in phase III clinical trials as monotheraphy [72,73], and in association with palcociclib [74,75,76,77] and its use was suggested in order to overcome acquired resistance, since it was observed that BCs with mutant ERα remain sensitive to giredestrant and reversed progesterone hypersensitivity driven by this mutation [78]. Rintodestrant (G1T48) is an orally bioavailable, nonsteroidal SERD that was developed through structure-guided investigations driven by activity in BC cell lines. It was obtained by a structural modification of raloxifene, with the introduction of an acrylic acid side chain [79] and is currently under clinical development for the treatment of patients with HR+ breast cancer [80,81,82]. Amcenestrant (SAR439859) is a nonsteroidal, orally bioavailable, SERD studied in clinical trials (AMEERA) in postmenopausal women with HR+/HER2− advanced breast cancer [83] and in association with CDK4/6 inhibitors, such as palbociclib for previously untreated ER+/HER2− advanced breast cancer [84]. AZD9833 (camizestrant) is an oral tricyclic indazole that has demonstrated high potency in preclinical cancer models as SERD and pure ER antagonist [85]. Two phase III trials are ongoing to evaluate the effectiveness of camizestrant in combination with palbociclib: SERENA-4 (NCT04711252) is a randomized, multicenter, double-blind, trial for evaluating the safety and efficacy for patients with ER+/HER2− advanced breast cancer who have not received systemic treatment in the advanced disease setting [86]; SERENA-6 is a study to assess the efficacy and safety of camizestrant in combination with palbociclib or abemaciclib in patients with ER+/HER2− MBC with detectable ESR1 mutations who have not experienced disease progression on first-line therapy, compared with aromatase inhibitors [87]. Elacestrant (RAD1901) is the first oral selective ER degrader demonstrating a significant progression-free survival (PFS) improvement compared to the standard-of-care (SOC) endocrine monotherapy both in the overall population and in patients with ESR1 mutations, with manageable safety in a phase III trial (EMERALD) for patients with ER+/HER2− advanced BC or MBC [88]. Imlunestrant (LY3484356) is a novel orally bioavailable SERD with pure antagonistic properties that showed a favorable profile as monotherapy in the study EMBER (NCT04188548) in ER+, HER2− advanced breast cancer [89]. AZD9496, an oral nonsteroidal small molecule inhibitor of ERα, is a potent and selective antagonist and downregulator of ERα in vitro and in vivo in ER+ models of breast cancer [90,91]. It is well tolerated with an acceptable safety profile, showing evidence of prolonged disease stabilization in heavily pretreated patients with ER+/HER2− advanced breast cancer [92]. Other oral investigational drugs for BC have been summarized by Chen et al. (2022) [93].
4.1.3. Aromatase Inhibitors (AIs)
AIs—such as anastrozole, letrozole, and exemestane—are effective targeted therapy in patients with ER+ BC, used in early and metastatic BC and in pre- and post-menopausal BC patients. They act as inhibitors of the CYP19 aromatase enzyme that catalyzes essential steps in estrogen biosynthesis, blocking the production of estrogens and, thereby, the downstream ER signaling. Since AIs deplete systemic estrogen levels in post-menopausal patients by blocking the conversion of androgens to estrogens, they are more effective than SERMs because they block both the genomic and nongenomic activities of ER. To date, first-generation (e.g., aminoglutethimide), second-generation (e.g., formestane and fadrazole), and third-generation (e.g., anastrozole, letrozole, and exemestane) AIs have been approved by the FDA and the third-generation is currently used in the standard treatment for postmenopausal breast cancer [94]. AIs can be categorized into Type I (steroidal) and Type II (non-steroidal) inhibitors, based upon their structure. Type I inhibitors, known as aromatase inactivators, have a steroidal structure similar to androgens and inactivate the enzyme irreversibly by blocking the substrate-binding site; whereas type II inhibitors are nonsteroidal and their action is reversible [95]. Most of the type II inhibitors are heterocyclic compounds—such as azoles, chromene, coumarin, xanthone, triphenylethylene, indole, sulfonamide, pyrimidines, pyridine, quinolone, and thiourea [96]. The treatment with AIs in early BC patients determined improvements of clinical psychological features, and health related quality of life was observed in BC survivors [97]. However, the therapy with AIs is associated to several side effects related to estrogen depletion in the body, which include menopausal symptoms—such as hot flashes, insomnia, accelerated bone loss—leading to higher osteoporosis risk, and most significantly, arthralgias [98]. Moreover, the potential role of AIs in the onset of autoimmune disorders—such as rheumatoid arthritis [99], anti-synthetase syndrome [100], and anti-phospholipid syndrome [101]—has been described. Finally, postmenopausal women with BC treated with AIs have an increased risk of cardiovascular events in comparison with tamoxifen [102].
4.1.4. Gonadotropin-Releasing Hormone (GnRH) Agonists
GnRH (also known as luteinizing hormone-releasing hormone (LHRH), gonadoliberin, luliberin, gonadorelin) is produced by the hypothalamus in the form of decapeptide. It binds specifically the GnRH receptor (GnRH-R) on the surface of gonadotropic cells in the anterior pituitary gland and promotes the production and the release of gonadotropins in both adult men and women. GnRH-R is a G-protein coupled receptor, which activates the mitogen-activated protein kinase (MAPK) cascade leading to growth and proliferation of the cells. GnRH-R is overexpressed in cancers that are dependent on gonadal steroids such as breast cancer, representing the 50% of the cases [103]. GnRH agonists bind GnRH-R in the pituitary gland, resulting in the secretion and initial surge of FSH and LH, thus stimulating the production of serum testosterone or estrogen. Thereafter, the negative feedback at the pituitary gland causes the downregulation of GnRH receptors suppressing the downstream effects of follicle-stimulating hormone and luteinizing hormone, ultimately leading to a decreased estrogen production in premenopausal ovaries [104]. GnRH agonists cause ovarian suppression that can aid fertility preservation useful for the successive fertility treatments and pregnancies in women with a previous diagnosis of BC [105]. The most common GnRH agonists are goserelin, triptorelin, buserelin, and leuprolide [106,107], used only in premenopausal women with BC. GnRH agonists are often administered in combination with cyclofosfamide [108], tamoxifen [109], or cyclin-dependent kinase 4/6 inhibitor [110,111] in the adjuvant or metastatic settings in patients with premenopausal or perimenopause endocrine positive BC. One advantage of the use of GnRH agonists is the protection of ovarian reserve that was observed with the use of goserelin during chemotherapy, even though the exact mechanism has not been fully elucidated yet [112]. The impact of GnRH agonists on ischemic heart disease has not been established, but some authors report an increased risk of cardiovascular toxicity after treatment with GnRH agonists, especially in men with prostate cancer [113,114], whereas it has not been demonstrated in women with BC. A recent study found that female patients with breast cancer receiving GnRH agonists had a lower risk of developing ischemic heart disease than patients not receiving them [115].
4.1.5. Complete Estrogen Receptor Antagonists (CERANs)
CERANs are small molecules that degrade ER as a SERD, but also hamper ER function, particularly blocking the activity of both the AF1 and AF2 transcriptional activation functions, inhibit ER-driven breast cancer cell growth, and induce ER degradation [116]. OP-1250 is an orally bioavailable CERAN currently in phase I/II open-label, multi-center, dose-escalation and expansion clinical trial (NCT04505826) for the treatment of ER+ advanced BC and/or MBC [117,118]. OP-1250 potently competes with the endogenous activating estrogenic ligand 17-beta estradiol for binding the ligand binding pocket [119].
4.1.6. Selective Estrogen Receptor Covalent Antagonists (SERCAs)
Several years ago, covalent targeting of ERα had been accomplished using estrogenic (ketonoestrol aziridine) and antiestrogenic (tamoxifen aziridine) affinity labels as tools to map the hormone-binding domain [120]. SERCAs are addressed specifically to a positionally non-conserved cysteine 530 (C530) in the ligand-binding pocket of ERα that could be covalently modified [121,122]. H3B-5942 is the first-in-class experimental oral compound showing exquisite selectivity for C530 and antitumor activity superior to fulvestrant therapy in BC xenograft models with ESR1 WT or ESR1 Y37S mutation [123]. It is known for being the precursor of compound H3B-6545, which was suggested for the potential treatment of endocrine therapy-resistant ERα+ BC harboring wild-type or mutant ESR1 (clinical trials: NCT03250676, NCT04568902, NCT04288089) [124]. It is noteworthy that the name SERCA must not be confused with sarcoplasmic reticulum (ER/SR) Ca2+-ATPase, which possesses the same acronym and is also involved in studies regarding BC [125,126,127].
4.1.7. Selective Human Estrogen Receptor Partial Agonist (ShERPA)
ShERPAs target and bind ER into the nucleus, causing ER translocation to extra-nuclear sites, which cause ER+ tumor cell growth inhibition [128]. They are based on the benzothiophene scaffold of raloxifene and mimics the effects of estradiol [129]. Two ShERPAs, namely BMI-135 and TTC-352, have been studied [130] and TTC-352 has completed successfully the phase I clinical trial for treatment of hormone-refractory HR+ BC demonstrating a favorable safety profile and early clinical evidence of antitumor activity [131].
4.2. Chemotherapy
Historical chemotherapic cancer treatments include direct DNA-damaging chemotherapy, such as platinum salts (cisplatin), alkylating agents (cyclophosphamide), antimetabolites (methotrexate, pemetrexed, gemcitabine, capecitabine, 5-fluorouracil), and topoisomerase I/II inhibitors. Chemotherapy represents the first line treatment option for BC with no hormone receptors [132]. For HR+/HER2− MBC, international guidelines recommend endocrine therapy as first-line treatment, except in case of ‘visceral crisis’, for which the cytotoxic chemotherapy is preferable [133]. Moreover, as neoadjuvant and adjuvant therapy, chemotherapy can be used also in other types of BC, even though its role is still controversial, especially in certain cases [134,135]. Unfortunately, patients frequently develop resistance. Chemotherapy is often used in association with immune checkpoint inhibitors to treat BC.
4.2.1. Targeted Therapy
In 1971, Judah Folkman first proposed that tumor angiogenesis could serve as a potential target for anticancer therapy [136]. Since then, targeted therapies against vulnerabilities in the key cell signaling pathways within the cancer cell began to be developed in the 1990s. Important advances have been obtained with targeted therapies in all the forms of cancer. Specifically, targeted therapies are represented by drugs that target particular enzymes, growth factor receptors, and signal transducers, thus interfering with various oncogenic cellular processes [137]. HER2, encoded by the oncogene ErbB2, is the transmembrane protein in human cells [138] that is involved in regulating cell proliferation, differentiation, and apoptosis through the activation of signal transduction by homo- or hetero-dimerization [139]. It was among the first to be discovered [140] and now represents one of the most promising targets in oncology research, especially in BC; numerous therapeutic agents are addressed to this target [141,142].
Topoisomerase (TOP) Inhibitors
DNA topoisomerases (TOPs) are crucial enzymes that modulate the double helix of DNA, by regulating DNA under- and overwinding and the removal of tangles and knots from the genome. TOP (or TOPO) inhibitors cause DNA double-strand breaks during DNA replication: they have been widely used as anti-cancer drugs during the past 20 years [143]. TOPO1 (TOP1 or TOPOI) inhibitors selectively trap TOPO1 cleavage complexes resulting in DNA double-strand breaks during replication, which are repaired by homologous recombination. Camptothecin (Table 3) is a natural alkaloid from the Chinese tree Camptotheca acuminata acting as TOPO1 inhibitor. Due to its extremely low solubility, the original natural product is not used as a drug but the semisynthetic derivatives topotecan and irinotecan were employed and clinically approved as TOPO1 inhibitors [144]. Camptothecin and its derivatives are currently used as second- or third-line treatment for patients with endocrine-resistant BCs [145,146]. Etirinotecan pegol (NKTR-102) is a long-acting topoisomerase-I inhibitor used for patients with MBC and brain metastases. However, in the phase III ATTAIN randomized clinical trial, no statistically significant difference in survival outcomes was observed in patients treated with etirinotecan pegol and patients treated with chemotherapy of physician’s choice [147]. These findings are questionable [148] and further studies are needed.
Table 3.
Chemotherapics in therapy and/or in clinical study for BC.
| Structure | Name | Activity |
|---|---|---|
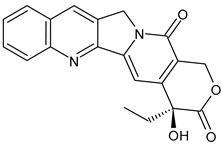
|
Camptothecin | TOPOI inhibitor |
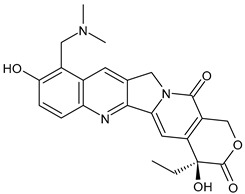
|
Topotecan | TOPOI inhibitor |
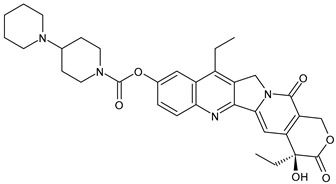
|
Irinotecan | TOPOI inhibitor |
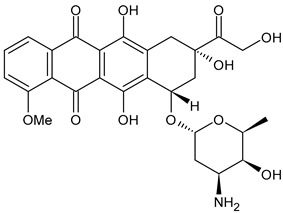
|
Adriamycin or doxorubicin | TOPOII inhibitor |
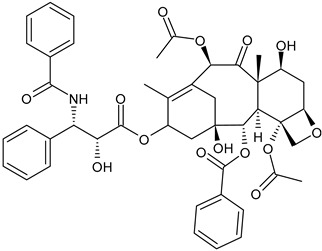
|
Paclitaxel | Tubulin stabilizer |
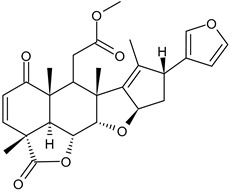
|
Nimbolide | Actin regulator |
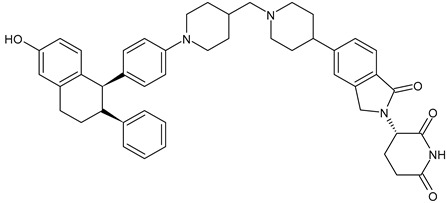
|
ARV-471 | PROTACs |
Type II topoisomerases (TOP2s, TOPO2s, or TOPOIIs) alter DNA topology through the generation of a transient double-stranded break in one segment of DNA, thus allowing another segment to pass through the DNA gate. Adriamycin, also known as doxorubicin, is a DNA TOPOII inhibitor belonging to the family of anthracycline anticancer drugs isolated from Streptomyces peucetius [149]. It is currently one of the most effective chemotherapeutic drugs for BC patients. However, several studies showed that adriamycin chemotherapy can cause adverse cardiac reactions in BC patients [150], and even heart failure [151]. Moreover, growing chemotherapeutic resistance to adriamycin has emerged as one of the essential reasons for treatment failure and poor outcome in BC [152,153]. Finally, it has been recently suggested that adriamycin therapy may accelerate BC metastasis in a SIRT7/TEK (TIE2) dependent manner [154]. Several mechanisms have been proposed to explain adriamycin resistance [155], including circular RNA (circRNA) in TNBC [156], RBM38 that may be negatively regulated by miR-320b, accelerating drug resistance in BC [157] and several strategies are addressed to overcome adriamycin resistance. Placenta-specific 8 (also known as PLAC8 or Onzin) is a highly conserved protein functioning as an oncogene or tumor suppressor in various tumors: it was suggested as a therapeutic target, through the participation of p62, in BC treatment and for its potential clinical application in overcoming adriamycin resistance [158]. Other promising strategies for adriamycin-resistant BCs include targeting exosomes or exosomal miR-222 [159]. The most common therapies for BC in association are: adriamycin/cyclophosphamide (AC), adriamycin/cyclophosphamide/paclitaxel (AC-T), cyclophosphamide/methotrexate/5-fluorouracil (CMF), and docetaxel/cyclophosphamide (TC) (Waks 2019). Neoadjuvant chemotherapy aids in debulking the tumor before surgery and is recommended for locally advanced BC patients [160]. Adjuvant chemotherapy aids in eradication of remaining BC tumor cells as well as undiscovered micrometastases and was demonstrated to reduce mortality by one third [161].
Drugs Targeting Tubulin
Taxanes, commonly used to treat different types of cancer, including BC, inhibit mytosis by binding the β-tubulin subunit of microtubules and induce apoptosis by preventing depolymerization of microtubules. Although they are effective against many cancer types, usual adverse effects are arrhyhtmias, hypersensitivity reactions, myelosuppression, nausea, vomiting, and neuropathy [162]. The broad-spectrum anticancer drug paclitaxel is currently one of the most used taxanes, besides other formulations such as docetaxel, cabazitaxel, and nab-paclitaxel. Paclitaxel is a diterpene pseudoalkaloid with a taxane ring as nucleus (C47H51NO14), first extracted and isolated from the bark of the Pacific yew tree Taxus brevifolia Nutt. Then, it was obtained with semi-synthetical procedures, starting from a paclitaxel precursor from the European yew tree’s needles. Its interaction with microtubules impairs the abnormal proliferation and mitosis rate of tumor cells. Specifically, paclitaxel enters in the microtubule lattice through small openings and binds β-tubulin, interfering with the dynamic process [163], leading to a strengthening between the tubulin subunit’s lateral contacts. Therefore, the depolymerization is suppressed and the microtubules are stabilized [164]. The application of paclitaxel is difficult because of its hydrophobic properties; thus, fat-soluble solvents such as cremophor are applied for injection purposes in order to improve its poor solubility. However, solvent-based paclitaxel can cause side reactions, including bone marrow suppression, allergic reactions, peripheral neuropathy [165], and cosolvent-induced toxicity. In order to improve the water solubility and safety of paclitaxel, nab-paclitaxel (nanoparticle albumin-bound paclitaxel)—a 130 nm particle solvent-free formulation, comprising albumin nanoparticles and paclitaxel with non-covalent bonds—has been successfully used for microtubule depolymerization inhibition [166]. Food and Drug Administration (FDA) lists nab-paclitaxel as a vital drug for the treatment of non-small cell lung cancer, pancreatic, and breast cancers, and it has recently suggested that nab-paclitaxel may promote the cancer-immunity cycle, acting as a potential immunomodulator [167]. Docetaxel, a semi-synthetic and side-chain analog of taxol, produced from 10-deacetylbaccatin III [168] has been used to treat MBC in combination with capecitabine after failure of first-line anthracycline-based treatment or in combination with HER2-targeted therapy in HER2+ MBC, or as monotherapy in second or later palliative lines [169]. Adverse effects are represented by of neutropenia, febrile neutropenia, neuropathy, onycholysis, epiphora, and toxicity, often leading to treatment discontinuation. Moreover, docetaxel is a highly lipophilic drug with very poor water solubility and instability [170]. Cabazitaxel is a recent taxoid agent showing in vitro and in vivo activity against cell lines and tumors resistant to docetaxel and paclitaxel [171]. Promising results were shown for the use of cabazitaxel in MBC patients previously treated with taxanes, and specifically after the development of taxane resistance [172].
Drugs Targeting Actin
Metastasis is a multi-step process driven by the dynamic reorganization of the actin-myosin cytoskeleton [173]. About five decades ago, it was found that actin filaments were disrupted in the malignant transformed cells. Indeed, migration and establishment of metastatic colonies require dynamic cytoskeletal modifications, characterized by polymerization and depolymerization of actin. Thus, actin became a possible and useful therapeutic target for chemotherapy and also an indicator for the efficacy of chemotherapeutic drugs [174] and several drugs hampering actin polymerization have been studied. Actin cytoskeletal modifications are initiated and regulated mainly by extracellular matrix (ECM) factors through integrin-focal adhesion kinase (ITG-FAK) signaling. Nimbolide, a terpenoid lactone found in leaves and flowers of Azadirachta indica, is widely known as ‘neem’ and in a recent study by Arumugam [175] it was demonstrated to inhibit TNBC growth, by altering AKT/mTOR and MAPK signaling, thereby inducing apoptosis. Nimbolide also inhibits ITG-FAK and Rac1/Cdc42-signaling pathways leading to a subsequent induction of actin depolymerization that has been suggested as a promising approach to reduce metastasis.
Trophoblast Cell Surface Antigen 2 (TROP2) Inhibitors
TROP2 is a type I transmembrane glycoprotein and calcium signal transducer first identified as a cell surface marker for trophoblast cells in the placenta [176]. Successively, a large body of work has implicated TROP2 as a major tumorigenic factor, being overexpressed in a number of malignant tumors, including BC and especially TNBC [177], thus representing a valid drug target [178,179]. Moreover, the use of specific monoclonal antibody targeting TROP2, such as TrMab-6 and TrMab-29, has been suggested to evaluate TROP2 expression in BCs [180,181].
4.2.2. HR+ Targeted Therapies
mTOR Inhibitors
The mTOR pathway was studied in the late 1970s, after the discovery of the antifungal metabolite rapamycin produced by Streptomyces hygroscopicus. TOR took its name from rapamycin: “target-of-rapamycin” [182]. mTOR is a Ser/Thr kinase belonging to the phosphoinositide 3-kinase related kinase (PIKK) family and exists as two distinct complexes, mTORC1 and mTORC2. mTOR signaling is often overactive in multiple cancer types including breast cancer [183] and mTOR antagonist are employed in clinical studies for BC, especially in association with cytotoxic chemotherapy. Rapamycin (sirolimus, HY-10219, Rapamune) possesses immunosuppressive and antiproliferative properties and is under study in HR+/HER2− BCs, generally in association with tamoxifen. Rapamycin regulates cell growth and survival through the mediation of the EGFR signaling pathway [184]. The other mTOR inhibitors are often mentioned as “mammalian target-of-rapamycin” inhibitors, that may cause confusion, since rapamycin is already an inhibitor. Everolimus (Afinitor, HY-10218) is a derivative of sirolimus that binds with high affinity its intracellular receptor, 12-kD FK506-binding protein (FKBP12), a protein belonging to the immunophilin family, forming a complex that is an allosteric inhibitor of mTORC1. Everolimus is approved for the treatment of ER+, HER2− MBCs in combination with hormonal therapies [185] and, considering the common resistance to CDK4/6 inhibitors, it is also used in recurrent BCs treated with CDK4/6 inhibitors in clinical settings [186]. Temsirolimus (CCI-779, HY-50910, Torisel) is an ester, analog of rapamycin, with improved aqueous solubility and pharmacokinetic properties that binds the FKBP12 forming a complex that is studied in combination with neratinib in ER+/HER2+ MBC [187]. Sapanisertib (TAK-228, MLN0128) is an oral, potent, and highly selective inhibitor of mTOR kinase exhibiting activity against both mTORC1 and mTORC2. It is studied in combination with exemestane or fulvestrant in postmenopausal women with ER+/HER2− advanced BC [188] and is currently under study in combination with another mTOR inhibitor, serabelisib (TAK-117), and paclitaxel for gynecological cancers, including BC [189].
Phosphatidylinositol 3-Kinase (PI3K) Inhibitors
PI3Ks are lipid kinases that regulate various cellular processes such as proliferation, adhesion, survival, and motility. PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) at the 3′ position on its inositol ring, converting PIP2 to phosphatidylinositol-3,4,5-trisphosphate (PIP3) [190]. Dysregulated PI3K pathway signaling occurs in one-third of human tumors [191]. PI3Ks are classified into three classes (I, II, and III) based on their primary conformations and binding substrates. Class I PI3K is grouped into class IA (PI3K isoforms α, β, and δ) and class IB (PI3K isoform γ) [192]. PI3Kα is the most commonly associated with solid tumors via gene amplification or mutations of the PIK3CA gene, which encodes the p110α catalytic subunit of PI3Kα. Approximately 30% of BCs and about 40% of HR+/HER2− BC bear aberrant PI3Kα signaling [193]. Several PI3K inhibitors are used as monotherapy or in combination therapies for various cancer, including BC [194]. Alpelisib (NVP-BYL719, Piqray, Table 4) is an oral PI3Kα selective inhibitor that was approved by FDA in 2019 for PIK3CA-mutated, HR+/HER2− advanced BC in combination with fulvestrant [195]. Other PI3K inhibitors are in clinical study, such as Inavolisib (GDC-0077), a PI3Kα-specific inhibitor that also promotes degradation of mutant p110α [196]. It has demonstrated encouraging preliminary results in a phase I/Ib clinical study in patients with PIK3CA-mutated HR+/HER− MBC as a monotherapy, and in combination with palbociclib and/or endocrine therapy. A phase III study of inavolisib + palbociclib + fulvestrant is undergoing (NCT03006172) [197]. Taselisib (Genentech), a dual PI3Kα/PI3Kδ inhibitor, entered phase III studies in BC [198]. However, the modest clinical benefit and considerable side effects (probably related to PI3Kδ inhibition) have led to its discontinuation [199]. Buparlisib (BKM120) and Pictilisib (GDC-0941) are pan-PI3K inhibitors and the second is one of the very first pan-class I selective PI3K inhibitors evaluated in patients with advanced cancer [200], being currently in phase Ib clinical study in combination with paclitaxel for advanced BC [201]. Buparlisib is an oral 2,6-dimorpholino pyrimidine derivative that potently inhibits the PI3K downstream signaling, including the downregulation of p-Akt and p-S6R and apoptosis of cancer cells [202,203]. Recent clinical studies are ongoing for the combined use of buparlisib (or alpelisib) and endocrine therapies [204,205].
Table 4.
Targeted therapies for BC.
| Structure | Name | Class | Reference Number of Clinical Trials |
|---|---|---|---|
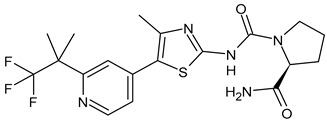
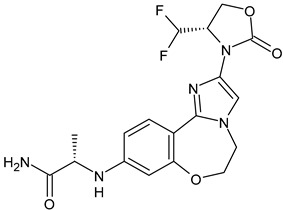
|
Alpelisib | PI3K inhibitor (selective PI3Kα) |
NCT02437318 |
|
Inavolisib | PI3K inhibitor (selective PI3Kα) |
NCT03006172 |
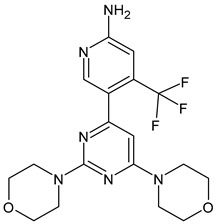
|
Buparlisib | PI3K inhibitor (pan-PI3K) |
NCT01790932, NCT01629615 |
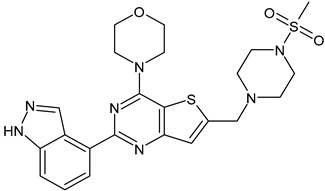
|
Pictilisib (GDC-0941) |
PI3K inhibitor (pan-PI3K) |
NCT01740336 |
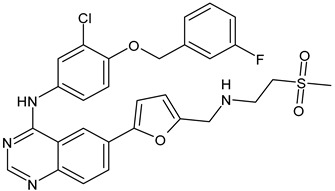
|
Lapatinib (GW-572016, Tyverb) |
TKI | NCT03894410 |
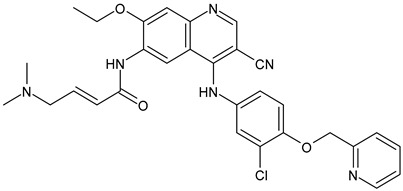
|
Neratinib (HKI-272) (Nerlynx™) |
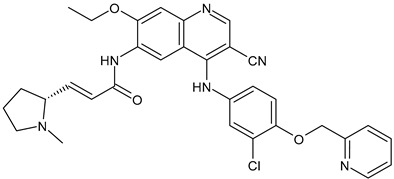
TKI | NCT01670877 |
|
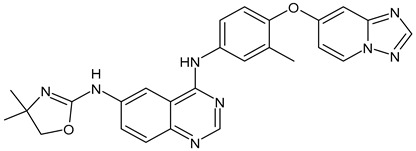
Pyrotinib | TKI | NCT03863223 |
|
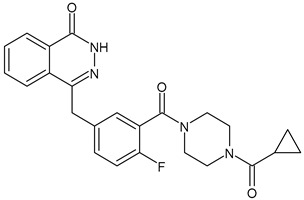
Tucatinib (Tukysa®) | TKI | NCT02614794 |
|
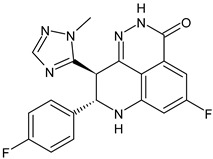
Olaparib (AZD-2281, Lynparza) |
PARPi | NCT01034033 |
|
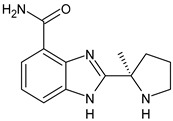
Talazoparib (BMN-673, Talzenna) |
PARPi | NCT04039230 |
|
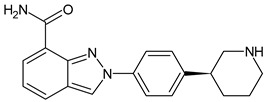
Veliparib (ABT-888) |
PARPi | NCT01149083 |
|
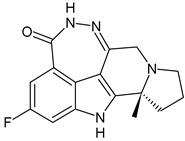
Niraparib (MK-4827) |
PARPi | NCT04837209 |
|
Pamiparib (BGB-290) |
PARPi | NCT03575065 |
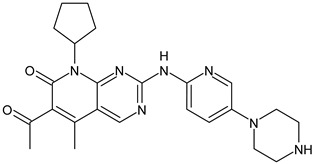
|
Palbociclib (Ibrance) |
CDK4/6 inhibitor | NCT04711252 |
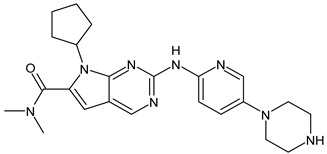
|
Ribociclib (Kisqali) |
CDK4/6 inhibitor | NCT03577197 |
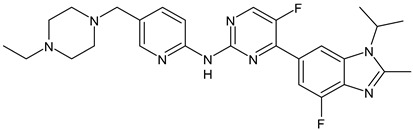
|
Abemaciclib (Verzenios) |
CDK4/6 inhibitor | NCT02246621 |
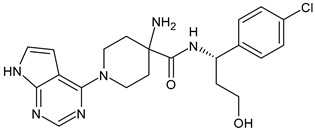
|
Capivasertib | Akt inhibitor | NCT03742102 |
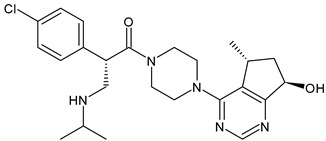
|
Ipatasertib | Akt inhibitor | NCT03337724, NCT04177108 |
Protein Kinase B (Akt) Inhibitors
Akt (or PKB) is a Ser/Thr kinase that is phosphorylated after phosphatidylinositol-3,4,5-triphosphate formation, leading to the downstream activation of the mTORC1 and mTORC2 complexes. The Akt kinase family comprises three isoforms: AKT1, AKT2, and AKT3 that are encoded by different genes with high sequence homology and display a conserved protein structure [206]. AKT activity is controlled in an Akt-dependent manner via phosphorylation and dephosphorylation. In cancer cells, AKT1 is implicated in proliferation and growth, promoting tumor initiation and suppressing apoptosis, while AKT2 regulates cytoskeleton dynamics, favoring invasiveness and metastatization. The function of AKT3 hyperactivation is still controversial. However, a potential stimulation of cell proliferation has been hypothesized [207]. According to the current knowledge about isoform-specific effects in BC, the inhibition of a specific isoform alone is not recommended [208]. Akt inhibitors have been classified into three categories depending on their mechanism of action: ATP-competitive inhibitors (capivasertib, ipatasertib, uprosertib, and GSK690693) that reduce the phosphorylation of Akt by competing with ATP; allosteric inhibitors (MK-2206), which prevent Akt from interacting with its substrate by causing conformational transitions in enzymic structure and finally irreversible inhibitors, which are less common. Capivasertib and ipatasertib (GDC-0068) are pan-AKT inhibitors and have been widely tested in phase I and II clinical trials as monotherapy or in association with chemotherapy or endocrine therapy. Then, phase III trials are ongoing in HR+ and TNBC [207].
PI3K/Akt/mTOR (PAM) Inhibitors
The three protein components—PI3K, Akt, and mTOR complexes—are primary regulators of the PAM pathway, which is associated with cell growth, survival, and proliferation. Targeting this pathway has been largely studied in cancer treatment for endocrine-resistant BCs, given the increasing resistance to endocrine therapy with upregulation of the PAM pathway. Most recent studies were widely summarized in the literature [209]. However, to increase the therapeutic benefit and overcome the resistance to PAM inhibitors, drug combinations are needed to obtain the highest efficacy and lowest toxicity rate [210,211].
4.2.3. HER2-Positive (HER2+) Targeted Therapies
HER2 is a membrane tyrosine kinase which gene is overexpressed or amplified in about 20% of BCs, playing a critical role in cellular transformation and carcinogenesis. Before the occurrence of targeted therapy, patients with HER2+ BC had an increased risk of recurrence and death, but since then their outcomes have substantially improved and the situation has changed considerably [212]. Blocking the HER2 pathway is considered a good therapy for BC and at present, several HER2-targeted agents are available to treat HER2-overexpressing BC [213].
Tyrosine Kinase Inhibitors (TKIs)
Lapatinib is a dual inhibitor of HER1 and HER2 receptors that competitively and reversibly binds their intracellular ATP-binding domains and slows tumor growth. It received its first FDA approval in 2007 and has been used in combination with capecitabine for previously treated MBC overexpressing HER-2 [214]. Since 2010, lapatinib has been approved in association with letrozole in the treatment of postmenopausal women with advanced HER2− and HR+ BC [215]. Recently, the association of lapatinib with trastuzumab as neoadjuvant therapy for HER2+ early BC has shown a better outcome over monotherapy with trastuzumab (CHER-Lob trial) [216].
Neratinib (HKI-272, Nerlynx™) is an oral, irreversible inhibitor of HER1, HER2, and HER4 receptors used in the treatment of BC [217]. In 2017, it received its first approval for HER2+ early-stage BC in the US [218]. Pyrotinib is an oral, irreversible pan-ErbB (or pan-HER) kinase inhibitor with activity against HER1, HER2, and HER4 [219]. The combination of PYR and ADM has shown synergistic effects both in vitro and in vivo. It has been demonstrated that PYR suppresses the proliferation, migration, and invasion of breast cancers through the downregulation of the Akt/p65/FOXC1 pathway [220]. The most recent TKI studied is tucatinib (Tukysa) that received its first approval by the FDA in 2020 to treat HER2+ MBC [221]. Compared to the other TKIs, tucatinib is highly selective and is more than 1000-fold specific for HER2 than EGFR. Moreover, it is better able to penetrate central nervous system (CNS) than lapatinib and neratinib, thus it can a very interesting potential treatment for MBC with CNS metastases [222].
Monoclonal Antibodies
Monoclonal antibodies are an effective therapeutic strategy for HER2+ BC and have been around for more than 20 years. Trastuzumab, a recombinant antibody targeting HER2, was the first biological drug approved by the FDA for the treatment of HER2+ BC in 1998. Trastuzumab is a first-line treatment option for early and advanced HER2+ BC, in mono- or combined therapy, due to its established safety and profile of efficacy. It is used in intravenous and subcutaneous formulations which have similar efficacy and safety. However, the main challenge of trastuzumab-based treatment is represented by the onset of resistance phenomena.
Pertuzumab is a recombinant, humanized monoclonal antibody that targets the extracellular dimerization domain of HER2 (domain II), distinct from the binding site of trastuzumab (domain IV) [223]. It is approved by FDA for use in association with trastuzumab and docetaxel in MBC, and in combination with trastuzumab and chemotherapy as neoadjuvant or adjuvant therapy in non-metastatic disease. The combination of pertuzumab and trastuzumab has shown low cardiac toxicity and favorable efficacy outcomes as demonstrated by the BERENICE study [224].
Margetuximab (MGAH22) is a second-generation monoclonal antibody for the treatment of HER2+ breast cancer and other cancers [225] that binds the same epitope on HER2 as trastuzumab. Margetuximab-cmkb (MARGENZA) received approval by the FDA on 16 December 2020, for its usage in combination with chemotherapy for the treatment of for advanced or HER2+ MBC [226]. Recently, the combination of margetuximab or T-DXd with small molecule inhibitors such as TKIs has been suggested to further improve the efficacy of monoclonal antibodies in the treatment of HER2+ MBC patients [225].
Antibody-Drug Conjugates (ADCs)
ADCs derive from the conjugation of traditional chemotherapy agents with monoclonal antibodies. Recent studies are addressed to ADC formed by monoclonal antibodies and inhibitors of TOPOI or TROP2, which are overexpressed in BC. Trastuzumab emtansine (Kadcyla, T-DM1) was the first ADC approved by the FDA on 22 February 2013 for HER2+ BC [227]. T-DM1 is composed of a trastuzumab backbone linked via a thioether linker to mertansine (also known as DM1), which is a tubulin polymerization inhibitor. On 20 December 2019, the FDA granted accelerated approval for the treatment of unresectable or metastatic HER2+ BC to Fam-trastuzumab deruxtecan-Nxki (T-DxD, Enhertu®), composed of trastuzumab and topoisomerase I inhibitor (DXd) [228]. Current studies compare the efficacy of T-DXd and T-DM1 [229]: the randomized phase III trial DESTINY-Breast03 showed a superior progression-free survival and a manageable safety profile for T-Dxd [230]. Moreover, trastuzumab deruxtecan has demonstrated a high intracranial response rate in patients with active brain metastases from HER2+ BC in the TUXEDO-1 trial (NCT04752059, EudraCT 2020-000981-41) [231]. Datopotamab deruxtecan (Dato-DXd, DS-1062) is an ADC composed of a humanized anti-TROP2 IgG1 monoclonal antibody attached to a highly potent topoisomerase I inhibitor payload (an exatecan derivative DXd) via a stable cleavable linker. It is now in phase III clinical trial for the treatment of metastatic TNBC (TROPION-Breast02, NCT05374512) [232].
4.2.4. HER2-Negative (HER2−) Targeted Therapies
Poly(ADP-Ribose) Polymerase Inhibitors (PARPi)
PARPs represent a family of 17 proteins that are activated by DNA damage. BRCA1 and BRCA2 are tumor-suppressor genes that encode proteins involved in the repair of DNA double-strand breaks. Cells that lack functional BRCA1 or BRCA2 are sensitive to PARP inhibition; thus, PARPi are useful agents in the treatment of BC patients with germline mutations in DNA repair genes, particularly those with deleterious BRCA1 and BRCA2 mutations, which constitutes 3–4% of all women with breast cancer and includes 10–20% of those with TNBC [233]. Olaparib (AZD-2281, Lynparza™) is an oral, small molecule, it is a PARP1/2 inhibitor first approved in 2014 for BRCA mutation-positive ovarian cancer [234]. On 8 January 2018, the US FDA granted approval of olaparib for patients with germline BRCA mutations (gBRCAm) HER2− MBC [235] and on October 2018, the PARPi talazoparib (BMN-673, Talzenna) received US FDA approval [236]. Veliparib (ABT-888) is generally used as neoadjuvant therapy in TNBC, even though its effectiveness is still controversial [237]. It has demonstrated interesting results in the BROCADE3 trial as a maintenance monotherapy following chemotherapy of veliparib in combination with carboplatin/paclitaxel for HER2− advanced germline gBRCAm BC [238,239]. The phase III Bravo trial investigating the role of the PARPi niraparib (MK-4827) versus chemotherapy in BRCA-mutated BC, was prematurely closed because of a severe discontinuation rate in the control arm [240]. The phase III clinical trial ZEST (NCT04915755) is ongoing to assess the efficacy of niraparib in patients with wild-type or mutated tumor BRCA HER2− TNBC who have detectable circulating tumor DNA (ctDNA) after completion of definitive therapy [241]. Pamiparib (BGB-290) has been approved in China for the treatment of ovarian cancer, fallopian tube cancer and peritoneal cancer [242] and phase II studies are evaluating its efficacy as treatment for HER2− BC with BRCA mutation (NCT03575065) [243].
Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors
CDK4/6 inhibitors belong to a new class of drugs that interrupt proliferation of malignant cells by inhibiting the progression through the cell cycle. Up to now, three different generations of CDKs inhibitors have been developed [244]. The third generation CDKs selectively target CDK4/6. Specifically, palbociclib, ribociclib, and abemaciclib were recently approved in combination with anti-estrogen therapy for the treatment of advanced and/or metastatic HR+/HER2− BC patients [245].
Antibody-Drug Conjugates (ADCs)
Sacituzumab govitecan (IMMU-132) is a third-generation ADC, composed of anti-human trophoblast cell-surface antigen 2 (Trop-2) monoclonal antibody, a hydrolyzable CL2A linker, and a cytotoxin (SN38), which inhibits topoisomerase 1, which is useful against TNBC cells. Trop-2 is present in BC cells, therefore, the anti-Trop-2 antibody allows IMMU-132 to specifically deliver SN-38 to the BC cells and the surrounding tumor via the cleavable linker [246]. Sacituzumab govitecan (Trodelvy™) was approved by FDA in 2020 as a third-line treatment for metastatic TNBC, in patients with a history of two prior metastatic treatments [247]. Other ADCs under study are widely described by Liu et al. (2022) [248].
4.2.5. Targeted Protein Degradation (TPD) Technologies
Unlike interfering with protein-protein interaction or other classical approaches, TPD triggers the protein degradation pathway. TPD technologies used for BCs include PROteolysis Targeting Chimeras (PROTACs) and LYsosome Targeting Chimeras (LYTACs), which determine ER degradation taking advantages of protein destruction mechanism in cells and compensates the shortcomings of traditional small molecular inhibitors. Ribonuclease-targeting chimeras (RIBOTAC) and small interfering RNA (siRNA) may inhibit the production of ER protein and are the subject of several recent medicinal chemistry studies [249,250].
Proteolysis-Targeting Chimeras (PROTACs)
The technology termed PROTAC, proteolysis targeting chimera, has been recently developed for inducing protein degradation by a targeting molecule [251,252]. PROTACs has been labeled with the mantra of being able to “drug the undruggable” [253,254,255]. A PROTAC is a heterobifunctional small molecule with three chemical elements: a ligand, binding a target protein (the protein of interest, POI), a ligand binding E3 ubiquitin ligase, and a linker that conjugates these two ligands. PROTAC is a chemical knockdown strategy that degrades the target protein through the ubiquitin-proteasome system. Unlike the competitive- and occupancy-driven process of traditional inhibitors, PROTACs have a catalytic mode of action, thus they have the potential to degrade the target pathogenic proteins and regulate the related signaling pathways, which cannot be achieved by traditional therapy (inhibitor/activator) [256]. The resulting PROTAC molecule then specifically recruits the E3 ligase ubiquitin machinery to the target protein of interest (POI) and exploits the cellular ubiquitin proteasome system (UPS) or lysosomal degradation pathways to degrade the protein [257]. The first PROTAC, reported by the groups of Craig M. Crews and Raymond J. Deshaies in 2001, showed the degradation induction of methionine aminopeptidase-2 (MetAp-2) [258]. Then, PROTACs were demonstrated to induce degradation of the androgen receptor and ER, hence expanding the target scope [259]. In the literature other names are also used for these compounds, such as specific and non-genetic IAP-dependent protein erasers (SNIPER); degrader; degronimids; PROteolysis TArgeting Peptide (PROTAP); Protein Degradation Probe (PDP). ARV-471 is a novel, potent, orally bioavailable PROTAC that selectively targets the ER and is currently in phase I/II clinical trial in association with palcociclib (NCT04072952) and as monotherapy in the VERITAC phase II for the treatment of ER+/HER2− locally advanced or metastatic BC [260,261].
Lysosome Targeting Chimeras (LYTACs)
The lysosomes represent another protein degradation system within the cell. LYTACs overcome the limitations of PROTACs and other degrader systems, which cannot degrade extracellular proteins and may have greater potential, since they are bifunctional molecules that bind both extracellular proteins and membrane-bound proteins, which are the products of approximately 40% of all protein-encoding genes [262]. PROTAC and LYTAC in combination could reduce the extracellular membrane and intracellular EGFR protein levels in BC cells: this association has been suggested as a potential strategy for treating EGFR antagonist resistance [263].
Metronomic Chemotherapy (MCT)
MCT is a suitable treatment option for selected MBC patients, first introduced to the clinic in international guidelines in 2017 for the treatment of advanced breast cancer. It consists in the continuous administration of low-dose chemotherapeutic agents with no or short regular treatment-free intervals [264]. MCT exerts its effects via immunomodulation, antiangiogenesis, and direct cytotoxic effects. Oral administration of MCT is safe, easy to handle, and allows for flexible drug dosing [265]. However, randomized controlled trials comparing MCT with conventional chemotherapic therapy are needed. The combination of MCT with other therapeutic interventions in BC is also currently under study [266].
5. Androgen Receptor Targeted Therapies
The AR, which belongs to the hormonal nuclear receptor superfamily, has been emerging as an interesting feature widely expressed in human BCs, both as a prognostic marker and for AR-targeting approaches [267,268]. However, its role in ER+ BC is controversial and has not yet been clearly established, thus constraining implementation of AR-directed therapies. Some authors report that AR suppression exerts potent antitumor activity in cancer [269], whereas other authors suggest that the activation of this receptor is responsible for the antitumor activity in ER+ BCs, including endocrine resistance [270]. AR has been considered a useful biomarker in BC, depending on the context of breast cancer subtypes [271]. It has also been shown to be predictive for the potential response to adjuvant hormonal therapy in ER+ BCs and neoadjuvant chemotherapy in TNBC and clinical trials using AR antagonists in BC are ongoing. Targeting AR, alone or combined with other therapeutic agents, provides alternatives to the existing therapies for BC, for instance enzalutamide and bicalutamide are two AR antagonists, which are in clinical trials to determine their efficacy alone to treat ER+ BC (NCT01889238 and NCT00468715, respectively). Moreover, a phase II trial (NCT02091960) that combined enzalutamide with the anti-HER2 antibody trastuzumab, in advanced AR+/HER2+/ER− BC groups, indicated that about a quarter of the participants exhibit partial response or stable disease during the 24-week treatment period [272]. An ongoing phase II trial (NCT02750358) shows promising results in indicating AR+ patients’ tolerance to enzalutamide [267]. The combination of bicalutamide with the CDK4/6 selective inhibitors palbociclib and ribociclib is also under study in advanced AR+ TNBC (NCT02605486 and NCT03090165, respectively) [273]. On the other hand, AR agonists combined with standard-of-care agents enhanced therapeutic responses [270].
6. Immune Checkpoint Inhibitors (ICIs)
ICIs—which target the programmed cell death receptor 1 (PD-1) and programmed death ligand 1 (PD-L1)—have been widely explored in the field of BCs, including both early and advanced disease. The development of immunotherapy in BC is relatively slow as it is traditionally considered to be poorly immunogenic [274]. However, chemotherapy and ICIs act in a synergistic manner when administered together: chemotherapy triggers the release of cancer antigens by killing tumor cells, thus enhancing cancer antigens presentation by antigen-presenting cells [275]. In the ongoing NCT03036488 study, carried out on early TNBC patients, the percentage of a pathological complete response was considerably higher among patients who received pembrolizumab plus neoadjuvant chemotherapy than among those who received placebo plus neoadjuvant chemotherapy [276]. Recently, atezolizumab targeting PD-L1 in combination with paclitaxel was approved by the FDA for the treatment of TNBC [277].
7. Treatment of Breast Cancer Stem Cells
Cancer stem cells, or cancer initiating cells or tumor-initiating cells, are smaller cells within tumor that can repopulate cancer cells over a long period of time and maintain their ability to regenerate. Thus, they act as normal stem cells but in a diseased manner. The activation of 5′-adenosine monophosphate-activated protein kinase (AMPK) phosphorylates and targets several cellular pathways involved in cell growth and proliferation and is emerging as a target of choice in the treatment of cancer cells and cancer stem cells. For instance, paclitaxel involves the activation of AMPK during its therapeutic pathway [278,279]. Metformin, a drug commonly used to treat type 2 diabetes, has been proposed as a repositioned drug due to its ability to activate AMPK. Moreover, vanadium compounds are well-known PTP inhibitors and AMPK activators [280].
8. Natural Products
Plant-derived natural compounds are developing as a prospective therapeutic tool in cancer biology research due to their easy accessibility and cost-effectiveness [281]. The vinca alkaloids—vinblastine and vincristine—isolated from the Madagascar periwinkle plant Catharanthus roseus G. Don. (Apocynaceae), were the first natural products with potent antitumor properties for use in clinical cancer therapy. They interfere with the mitotic spindle apparatus, thus triggering cellular arrest in metaphase during mitosis leading to tumor cell death by apoptosis. These compounds are usually used in BC patients in combination chemotherapy or as a therapeutic option if other chemotherapy agents fail [282]. Vinorelbine and vinflunine are analogs of vinca alkaloids and act as antimitotics that bind tubulin, inhibit microtubule function and arrest mitosis [283]. Various natural agents—including curcumin, indol-3-carbinol, resveratrol, kaempferol, epigallocatechin gallate (EGCG), and genistein—are used in preclinical or clinical settings for the management of cancer [284]. Moreover, epidemiological data suggest that high nutritional intake of fruits and vegetables lower the risk of cancer [285].
9. Nanomedicines and Nanopharmaceuticals
Numerous papers evaluating a great variety of nanoformulations have been published, ranging from inorganic to organic nanoparticles, with high versatility, controllable size, and shape. The use of active targeted nanomedicine is an explored strategy to obtain the selective delivery of antineoplastics to cancer cells, thus reducing their adverse effects and increasing their efficacy. However, the toxicological effects of nanomaterials must be taken into account, even though some toxic effects are not yet completely known. It is mandatory to implement guidelines to standardize preclinical nanomedicine research [286]. In recent decades, several active targeted nanoformulations have been approved or reached clinical investigation with very good results. Besides ADC, which are probably the most promising nanomedicines, other active targeted nanosystems have reached the clinic for the treatment of BC including Abraxane® and Nab-rapamicyn (albumin nanoparticles entrapping rapamycin) and various liposomes (MM-302, C225-ILS-Dox, and MM-310) loaded with doxorubicin or docetaxel and coated with ligands targeting HER-2 and other receptors, such as Ephrin 2. Abraxane consists of albumin nanoparticles of around 130 nm entrapping paclitaxel and received approval by the FDA and EMA in the 2000s for the treatment of different cancers, including BC. Nowadays, it is used in MBC after failure of combination chemotherapy and when standard treatment including an anthracycline is inadequate. Recently, it has been approved in combination with atezolizumab in PD-L1 overexpressing unresectable locally advanced tumors or metastatic TNBC. Several clinical studies are ongoing to evaluate the efficacy of combination therapy with abraxane [287].
10. Conclusions
BC is the most commonly diagnosed cancer in women worldwide with more than 2 million new cases in 2020. The way to view BC is widely and constantly changing, mostly after the further characterization of its molecular hallmarks that includes, for instance, the immunohistochemical markers ER, PR, HER2; the genomic markers BRCA1, BRCA2, and PIK3CA; and immunomarkers such as PD-L1. The neoadjuvant combination therapy represents a standard strategy for treating HER2+ and triple negative BCs, radiotherapy is still a valid cornerstone for BCs therapy, endocrine therapy and chemotherapy have been the treatment of choice, mostly in the last 10 years, for ER+ tumors, whereas targeted therapies using CDK4/6, PI3K, PARP inhibitors, and anti-PD-L1 immunotherapy, have been adopted for MBC. It is clear that the availability of wide treatment options reflects the complexity of BC today. It must also be considered that an optimal therapy should take into account the tumor subtype, cancer stage, and patients’ compliance and preferences. Thus, the future research in BC needs to be focused not only on new drugs discovery and repurposing, but mostly on the personalization of the therapy and the “precision medicine”. As an example, different approved agents (namely, PARP or PI3K inhibitors, amongst many others) work selectively against tumors exhibiting certain biomarkers or mutations. Some targeted therapy drugs, as monoclonal antibodies, control cancer cells and boost the immune system as well; thus, they can be considered as immunotherapy, reaching different parts of the body and are useful against MBC. Moreover, the use of antibody–drug conjugates for the treatment of TNBC as sacituzumab govitecan, targeting TROP2 successfully improved the progression-free survival and overall. New endocrine agents, as selective ER downregulators, have been developed to prevent or overcome the endocrine resistance, which is due, for instance, to ESR1 mutations. Finally, it is important to highlight the concept of finding the appropriate combination of treatments for each type of BC, because cancer cells could become resistant to targeted therapies over time. In this review, we presented the classification of BCs and listed the most commonly adopted therapies, shedding light on the complexity and the constant evolution of classic and emerging strategies and ongoing clinical trials.
Abbreviations
ADC, antibody-drug conjugate; Akt, protein kinase B; AI, aromatase inhibitor; BC, breast cancer; BRCA, breast cancer genes; CDK, cyclin-dependent kinase; CERAN, complete estrogen receptor antagonist; COVID-19, coronavirus disease 2019; ER, estrogen receptor; ErbB, human epidermal growth factor receptor; EGFR, epidermal growth factor receptor; EMT, epithelial–mesenchymal transition; ERK1/2, extracellular signal-regulated kinase 1/2; FDA, Food and Drug Administration; GnRH, gonadotropin-releasing hormone; HER2− = HER2-negative; HER2+ = HER2-enriched or HER2-positive; HR, hormone receptor; MBC, metastatic breast cancer; PAM, PI3K/Akt/mTOR; PARP, Poly (ADP-ribose) polymerase; PI3K, Phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol-4, 5-bisphosphate; PIP3 phosphatidylinositol-3,4,5-trisphosphate; PR, progesterone receptor; PRL, prolactin; PTEN, phosphatase and tensin homolog, deleted on chromosome TEN; SERCA, selective estrogen receptor covalent antagonist; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator; ShERPA, selective human estrogen receptor partial agonist; TKI, tyrosine kinase inhibitor; TNBC, triple-negative breast cancer; TOPOI or TOPO1, topoisomerase I; TOPOII or TOPO2, topoisomerase II; TROP2, trophoblast cell surface antigen 2, WHO, World Health Organization
Author Contributions
Conceptualization, N.B. and M.S.S.; Writing—original draft preparation, D.I., J.C., and N.B.; Methodology and data curation, D.I. and J.C.; Writing—review and editing, A.C.; Supervision, M.S.S. and A.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Łukasiewicz S., Czeczelewski M., Forma A., Baj J., Sitarz R., Stanisławek A. Breast cancer–epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers. 2021;13:4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breast Cancer. [(accessed on 7 December 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
- 4.Giaquinto A.N., Miller K.D., Tossas K.Y., Winn R.A., Jemal A., Siegel R.L. Cancer statistics for African American/Black people 2022. CA Cancer J. Clin. 2022;72:202–229. doi: 10.3322/caac.21718. [DOI] [PubMed] [Google Scholar]
- 5.Miller K.D., Ortiz A.P., Pinheiro P.S., Bandi P., Minihan A., Fuchs H.E., Tyson D.M., Tortolero-Luna G., Fedewa S.A., Jemal A.M., et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J. Clin. 2021;71:466–487. doi: 10.3322/caac.21695. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2021;28:1167–1180. doi: 10.1007/s12282-020-01148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey-Jones E.J., Lord C.J., Tutt A.N. Systemic therapy for hereditary breast cancers. Hematol. Oncol. Clin. 2021;37:203–224. doi: 10.1016/j.hoc.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Daly A.A., Rolph R., Cutress R.I., Copson E.R. A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer Targets Ther. 2021;13:241. doi: 10.2147/BCTT.S268401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islami F., Goding Sauer A., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 10.Xu C., Ganesan K., Liu X., Ye Q., Cheung Y., Liu D., Zhong S., Chen J. Prognostic value of negative emotions on the incidence of breast cancer: A systematic review and meta-analysis of 129,621 patients with breast cancer. Cancers. 2022;14:475. doi: 10.3390/cancers14030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhushan A., Gonsalves A., Menon J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics. 2021;13:723. doi: 10.3390/pharmaceutics13050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moo T.A., Sanford R., Dang C., Morrow M. Overview of breast cancer therapy. PET Clin. 2018;13:339–354. doi: 10.1016/j.cpet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arya N., Saha S. Multi-modal advanced deep learning architectures for breast cancer survival prediction. Knowl. Based Syst. 2021;221:106965. doi: 10.1016/j.knosys.2021.106965. [DOI] [Google Scholar]
- 14.Ihle C.L., Wright-Hobart S.J., Owens P. Therapeutics targeting the metastatic breast cancer bone microenvironment. Pharmacol. Ther. 2022;239:108280. doi: 10.1016/j.pharmthera.2022.108280. [DOI] [PubMed] [Google Scholar]
- 15.Lei S., Zheng R., Zhang S., Wang S., Chen R., Sun K., Zeng H., Zhou J., Wei W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021;41:1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iacopetta D., Ceramella J., Catalano A., Saturnino C., Pellegrino M., Mariconda A., Longo P., Sinicropi M.S., Aquaro S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses. 2022;14:573. doi: 10.3390/v14030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:E180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliamento M., Agostinetto E., Bruzzone M., Ceppi M., Saini K.S., de Azambuja E., Punie K., Westphalen C.B., Morgan G., Pronzato P., et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021;163:103365. doi: 10.1016/j.critrevonc.2021.103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yersal O., Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014;5:412–424. doi: 10.5306/wjco.v5.i3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavarthapu R., Anbazhagan R., Dufau M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers. 2021;13:4685. doi: 10.3390/cancers13184685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal S.K., Childs B.H., Pegram M. Triple negative breast cancer: Unmet medical needs. Breast Cancer Res. Treat. 2011;125:627–636. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prat A., Parker J.S., Karginova O., Fan C., Livasy C., Herschkowitz J.I., He X., Perou C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fougner C., Bergholtz H., Norum J.H., Sørlie T. Re-definition of claudin-low as a breast cancer phenotype. Nat. Commun. 2020;11:1787. doi: 10.1038/s41467-020-15574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohanapure N.S., Khandeparkar S.G.S., Saragade P.B., Gogate B.P., Joshi A.R., Mehta S.R. Immunohistochemical study of epidermal growth factor receptor, human epidermal growth factor receptor 2/neu, p53, and Ki67 in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2022;26:127. doi: 10.4103/jomfp.jomfp_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waks A.G., Winer E.P. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 26.Meisel J.L., Venur V.A., Gnant M., Carey L. Evolution of targeted therapy in breast cancer: Where precision medicine began. Am. Soc. Clin. Oncol. Educat. Book. 2018;38:78–86. doi: 10.1200/EDBK_201037. [DOI] [PubMed] [Google Scholar]
- 27.Arora S., Narayan P., Osgood C.L., Wedam S., Prowell T.M., Gao J.J., Shah M., Krol D., Wahby S., Royce M., et al. U.S. FDA drug approvals for breast cancer: A decade in review. Clin. Cancer Res. 2022;28:1072–1086. doi: 10.1158/1078-0432.CCR-21-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabieva N., Fasching P.A. Endocrine Treatment for Breast Cancer Patients Revisited—History, Standard of Care, and Possibilities of Improvement. Cancers. 2021;13:5643. doi: 10.3390/cancers13225643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalano A., Iacopetta D., Ceramella J., Scumaci D., Giuzio F., Saturnino C., Aquaro S., Rosano C., Sinicropi M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules. 2022;27:616. doi: 10.3390/molecules27030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Zhang H., Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganesan K., Wang Y., Gao F., Liu Q., Zhang C., Li P., Zhang J., Chen J. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics. 2021;13:1829. doi: 10.3390/pharmaceutics13111829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muggia F., Safra T., Dubeau L. BRCA genes: Lessons learned from experimental and clinical cancer. Ann. Oncol. 2011;22:i7–i10. doi: 10.1093/annonc/mdq659. [DOI] [PubMed] [Google Scholar]
- 33.De Sabando A.R., Lafuente E.U., García-Amigot F., Alonso Sánchez A., Morales Garofalo L., Moreno S., Ardanaz E., Ramos-Arroyo M.A. Genetic and clinical characterization of BRCA-associated hereditary breast and ovarian cancer in Navarra (Spain) BMC Cancer. 2019;19:1145. doi: 10.1186/s12885-019-6458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Breast Cancer Foundation BRCA: The Breast Cancer Gene. [(accessed on 22 December 2022)]. Available online: https://www.nationalbreastcancer.org/what-is-brca.
- 35.Menghi F., Banda K., Kumar P., Straub R., Dobrolecki L., Rodriguez I.V., Yost S.E., Chandok H., Radke M.R., Somlo G., et al. Genomic and Epigenomic BRCA alterations predict adaptive resistance and response to platinum-based therapy in aptients with triple-negative breast and ovarian carcinomas. Sci. Trans. Med. 2022;14:eabn1926. doi: 10.1126/scitranslmed.abn1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumura N., Yoshida H., Kitagishi Y., Nishimura Y., Matsuda S. Alternative splicings on p53, BRCA1 and PTEN genes involved in breast cancer. Biochem. Biophys. Res. Comm. 2011;413:395–399. doi: 10.1016/j.bbrc.2011.08.098. [DOI] [PubMed] [Google Scholar]
- 37.Chai C., Wu H.H., Abuetabh Y., Sergi C., Leng R. Regulation of the tumor suppressor PTEN in triple-negative breast cancer. Cancer Lett. 2021;527:41–48. doi: 10.1016/j.canlet.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Lau K.H., Tan A.M., Shi Y. New and emerging targeted therapies for advanced breast cancer. Int. J. Mol. Sci. 2022;23:2288. doi: 10.3390/ijms23042288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burguin A., Diorio C., Durocher F. Breast cancer treatments: Updates and new challenges. J. Pers. Med. 2021;11:808. doi: 10.3390/jpm11080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng X., Liu C., Yao J., Wan H., Wan G., Li Y., Chen N. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol. Res. 2021;163:105320. doi: 10.1016/j.phrs.2020.105320. [DOI] [PubMed] [Google Scholar]
- 41.Onyiba C.I., Scarlett C.J., Weidenhofer J. The mechanistic roles of sirtuins in breast and prostate cancer. Cancers. 2022;14:5118. doi: 10.3390/cancers14205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss A., King T.A. What is the role of neoadjuvant endocrine therapy for breast cancer? Adv. Surg. 2022;56:275–286. doi: 10.1016/j.yasu.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Cucciniello L., Gerratana L., del Mastro L., Puglisi F. Tailoring adjuvant endocrine therapy in early breast cancer: When, how, and how long? Cancer Treat. Rev. 2022;110:102445. doi: 10.1016/j.ctrv.2022.102445. [DOI] [PubMed] [Google Scholar]
- 44.Ferraro E., Walsh E.M., Tao J.J., Chandarlapaty S., Jhaveri K. Accelerating drug development in breast cancer: New frontiers for ER inhibition. Cancer Treat. Rev. 2022;109:102432. doi: 10.1016/j.ctrv.2022.102432. [DOI] [PubMed] [Google Scholar]
- 45.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer (Primer) Nat. Rev. Dis. Prim. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 46.Grinshpun A., Chen V., Sandusky Z.M., Fanning S.W., Jeselsohn R. ESR1 activating mutations: From structure to clinical application. Biochim. Biophys. Acta BBA Rev. Cancer. 2023;1878:188830. doi: 10.1016/j.bbcan.2022.188830. [DOI] [PubMed] [Google Scholar]
- 47.Liu M., Xie F., Liu M., Zhang Y., Wang S. Association between BRCA mutational status and survival in patients with breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2021;186:591–605. doi: 10.1007/s10549-021-06104-y. [DOI] [PubMed] [Google Scholar]
- 48.Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., Andre F., Harbeck N., Aguilar Lopez B., Barrios C.H., Bergh J., et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) dagger. Ann. Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NICE Pathway Managing Advanced Breast Cancer. 2020. [(accessed on 13 December 2022)]. Available online: https://breastcancernow.org/sites/default/files/advanced-breast-cancer-managing-advanced-breast-cancer.pdf.
- 50.Dustin D., Gu G., Fuqua S.A. ESR1 mutations in breast cancer. Cancer. 2019;125:3714–3728. doi: 10.1002/cncr.32345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.López González A., del Barco Berrón S., Grau I., Galan M., Castelo Fernández B., Cortés A., Sánchez Rovira P., Martinez-Bueno A., Gonzalez X., García A., et al. Challenging endocrine sensitivity of hormone receptor-positive/HER2-negative advanced breast cancer with the combination of eribulin and endocrine therapy: The REVERT study. Cancers. 2022;14:5880. doi: 10.3390/cancers14235880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das S., Kulkarni S., Singh Y., Kumar P., Thareja S. Selective Estrogen Receptor Modulators (SERMs) for the treatment of ER+ breast cancer: An overview. J. Mol. Struct. 2022;1270:133853. doi: 10.1016/j.molstruc.2022.133853. [DOI] [Google Scholar]
- 53.Abel M.K., Morgan T., Angeles A., Goldman M. Gynecological management of the breast cancer survivor. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;82:69–80. doi: 10.1016/j.bpobgyn.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Murthy A., Rao Ravi P., Kathuria H., Malekar S. Oral Bioavailability Enhancement of Raloxifene with Nanostructured Lipid Carriers. Nanomaterials. 2020;10:1085. doi: 10.3390/nano10061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badr-Eldin S.M., Aldawsari H.M., Ahmed O.A., Alhakamy N.A., Neamatallah T., Okbazghi S.Z., Fahmy U.A. Optimized semisolid self-nanoemulsifying system based on glyceryl behenate: A potential nanoplatform for enhancing antitumor activity of raloxifene hydrochloride in MCF-7 human breast cancer cells. Int. J. Pharm. 2021;600:120493. doi: 10.1016/j.ijpharm.2021.120493. [DOI] [PubMed] [Google Scholar]
- 56.Lainé M., Fanning S.W., Chang Y.-F., Green B., Greene M.E., Komm B., Kurleto J.D., Phung L., Greene G.L. Lasofoxifene as a Potential Treatment for Therapy-Resistant ER-Positive Metastatic Breast Cancer. Breast Cancer Res. 2021;23:54. doi: 10.1186/s13058-021-01431-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goetz M.P., Plourde P., Stover D.G., Bagegni N., Vidal G.A., Brufsky A., Rugo H.S., Portman D.J., Gal-Yam E. LBA20 Open-label, randomized study of lasofoxifene (LAS) vs. fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2-breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. Ann. Oncol. 2022;33:S1387–S1388. [Google Scholar]
- 58.Zafar E., Maqbool M.F., Iqbal A., Maryam A., Shakir H.A., Irfan M., Khan M., Ma T. A comprehensive review on anticancer mechanism of bazedoxifene. Biotechnol. Appl. Biochem. 2022;69:767–782. doi: 10.1002/bab.2150. [DOI] [PubMed] [Google Scholar]
- 59.Tsuji J., Li T., Grinshpun A., Coorens T., Russo D., Anderson L., Rees R., Nardone A., Patterson C., Lennon N.J., et al. Clinical efficacy and whole-exome sequencing of liquid biopsies in a phase IB/II study of bazedoxifene and palbociclib in advanced hormone receptor—positive breast cancer. Clin. Cancer Res. 2022;28:5066–5078. doi: 10.1158/1078-0432.CCR-22-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das A., Lavanya K.J., Kaur K., Jaitak V. Effectiveness of selective estrogen receptor modulators in breast cancer therapy: An update. Curr. Med. Chem. :2022. doi: 10.2174/0929867329666221006110528. in press . [DOI] [PubMed] [Google Scholar]
- 61.Lu Y., Liu W. Selective estrogen receptor degraders (SERDs): A promising strategy for estrogen receptor positive endocrine-resistant breast cancer. J. Med. Chem. 2020;63:15094–15114. doi: 10.1021/acs.jmedchem.0c00913. [DOI] [PubMed] [Google Scholar]
- 62.Robertson J.F., Lindemann J., Garnett S., Anderson E., Nicholson R.I., Kuter I., Gee J.M. A Good drug made better: The fulvestrant dose-response story. Clin. Breast Cancer. 2014;14:381–389. doi: 10.1016/j.clbc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Boer K. Fulvestrant in advanced breast cancer: Evidence to date and place in therapy. Adv Med. Oncol. 2017;9:465–479. doi: 10.1177/1758834017711097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson J.F.R., Bondarenko I.M., Trishkina E., Dvorkin M., Panasci L., Manikhas A., Shparyk Y., Cardona-Huerta S., Cheung K.-L., Philco-Salas M.J., et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 65.Tsuboi K., Kaneko Y., Nagatomo T., Fujii R., Hanamura T., Gohno T., Yamaguchi Y., Niwa T., Hayashi S.I. Different epigenetic mechanisms of ERalpha implicated in the fate of fulvestrant-resistant breast cancer. J. Steroid. Biochem. Mol. Biol. 2017;167:115–125. doi: 10.1016/j.jsbmb.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 66.McDonnell D.P., Wardell S.E., Chang C.Y., Norris J.D. Next-generation endocrine therapies for breast cancer. J. Clin Oncol. 2021;39:1383. doi: 10.1200/JCO.20.03565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Downton T., Zhou F., Segara D., Jeselsohn R., Lim E. Oral Selective Estrogen Receptor Degraders (SERDs) in Breast Cancer: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2022;16:2933–2948. doi: 10.2147/DDDT.S380925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Fructuoso I., Gomez-Bravo R., Schettini F. Integrating new oral selective oestrogen receptor degraders in the breast cancer treatment. Curr. Opin. Oncol. 2022;34:635–642. doi: 10.1097/CCO.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 69.Lloyd M.R., Wander S.A., Hamilton E., Razavi P., Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: Current and emerging role. Ther. Adv. Med. Oncol. :2022. doi: 10.1177/17588359221113694. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hernando C., Ortega-Morillo B., Tapia M., Moragón S., Martínez M.T., Eroles P., Garrido-Cano I., Adam-Artigues A., Lluch A., Bermejo B., et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: Present and future from a clinical perspective. Int. J. Mol. Sci. 2021;22:7812. doi: 10.3390/ijms22157812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang J., Zbieg J.R., Blake R.A., Chang J.H., Daly S., DiPasquale A.G., Friedman L.S., Gelzleichter T., Gill M., Giltnane J.M., et al. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J. Med. Chem. 2021;64:11841–11856. doi: 10.1021/acs.jmedchem.1c00847. [DOI] [PubMed] [Google Scholar]
- 72.Jimenez M.M., Lim E., Mac Gregor M.C., Bardia A., Wu J., Zhang Q., Nowecki Z., Cruz F., Safin R., Kim S.B., et al. 211MO Giredestrant (GDC-9545) vs. physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2–locally advanced/metastatic breast cancer (LA/mBC): Primary analysis of the phase II, randomised, open-label acelERA BC study. Ann. Oncol. 2022;33:S633–S634. doi: 10.1016/j.annonc.2022.07.250. [DOI] [Google Scholar]
- 73.Bardia A., Schmid P., Harbeck N., Rimawi M.F., Hurvitz S.A., Loi S., Saji S., Jung K.H., Werutsky G., Stroyakovskii D., et al. Abstract OT2-11-09: Lidera breast cancer: A phase III adjuvant study of giredestrant (GDC-9545) vs. physician’s choice of endocrine therapy (ET) in patients (pts) with estrogen receptor-positive, HER2-negative early breast cancer (ER+/HER2-EBC) Cancer Res. 2022;82:OT2-11-09. doi: 10.1158/1538-7445.SABCS21-OT2-11-09. [DOI] [Google Scholar]
- 74.Hurvitz S.A., Quiroga V., Park Y.H., Bardia A., López-Valverde V., Steinseifer J., Fernando T.M., Spera G., Xue C., Fasching P.A., et al. Abstract PD13-06: Neoadjuvant giredestrant (GDC-9545)+ palbociclib versus anastrozole+ palbociclib in postmenopausal women with estrogen receptor-positive, HER2-negative, untreated early breast cancer: Primary analysis of the randomized, open-label, phase II coopERA breast cancer study. Cancer Res. 2022;82:PD13-06. [Google Scholar]
- 75.Bardia A., Fernando T.M., Fasching P.A., Garcia V.Q., Park Y.H., Giltnane J.M., Xue C., Lopez Valverde V., Steinzeifer-Szabo J., Pérez-Moreno P.D., et al. 144P–Neoadjuvant giredestrant (GDC-9545) + palbociclib (P) vs. anastrozole (A)+ P in postmenopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2–eBC): Biomarker subgroup analysis of the randomised, phase II coopERA BC study. Ann. Oncol. 2022;33:S605–S606. [Google Scholar]
- 76.Fasching P.A., Bardia A., Quiroga V., Park Y.H., Blancas I., Alonso J.L., Vasilyev A., Adamchuk H., Salgado M.R.T., Yardley D.A., et al. Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2− eBC): Final analysis of the randomized, open-label, international phase 2 coopERA BC study. J. Clin. Oncol. 2022;40:589. [Google Scholar]
- 77.Neilan T.G., Villanueva-Vázquez R., Bellet M., López-Miranda E., García-Estévez L., Kabos P., Bond J., Gates M.R., Chang C.W., Boni V., et al. Abstract P5-18-07: Heart rate changes, cardiac safety, and exercise tolerance from a phase Ia/b study of giredestrant (GDC-9545) ± palbociclib in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Cancer Res. 2022;82:P5–P18. doi: 10.1158/1538-7445.SABCS21-P5-18-07. [DOI] [Google Scholar]
- 78.Liang J., Ingalla E.R., Yao X., Wang B.E., Tai L., Giltnane J., Liang Y., Daemen A., Moore H.M., Aimi J., et al. Giredestrant reverses progesterone hypersensitivity driven by estrogen receptor mutations in breast cancer. Sci. Transl. Med. 2022;14:eabo5959. doi: 10.1126/scitranslmed.abo5959. [DOI] [PubMed] [Google Scholar]
- 79.Andreano K.J., Wardell S.E., Baker J.G., Desautels T.K., Baldi R., Chao C.A. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res. Treat. 2020;180:635–646. doi: 10.1007/s10549-020-05575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dees E.C., Aftimos P.G., van Oordt H., de Vries E.G.E., Neven P., Pegram M.D., Iqbal R., Boers J., Xiao J., Sipes C., et al. Dose-escalation study of G1T48, an oral selective estrogen receptor degrader (SERD), in postmenopausal women with ER+/HER2-locally advanced or metastatic breast cancer (ABC) Ann. Oncol. 2019;30:v121–v122. doi: 10.1093/annonc/mdz242.035. [DOI] [Google Scholar]
- 81.Aftimos P., Neven P., Pegram M., van Menke-van der Houven Oordt C.W., Jager A., Bulat I., Chap L., Maglakelidze M., Dees E.C., Schorder C., et al. Abstract PS12-04: Rintodestrant (G1T48), an oral selective estrogen receptor degrader in ER+/HER2-locally advanced or metastatic breast cancer: Updated phase 1 results and dose selection. Cancer Res. 2021;81:PS12-04. doi: 10.1158/1538-7445.SABCS20-PS12-04. [DOI] [Google Scholar]
- 82.Beelen A.P., Li C., Jarugula P., Gopalakrishnan M., Sorrentino J.A., Wolfgang C.D., Jain S., Tao W. Abstract PS17-08: Population pharmacokinetic and exposure-response modeling of the oral selective estrogen receptor degrader, rintodestrant (G1T48), in patients with ER+/HER2-advanced breast cancer. Cancer Res. 2021;81:PS17-08. doi: 10.1158/1538-7445.SABCS20-PS17-08. [DOI] [Google Scholar]
- 83.Bardia A., Chandarlapaty S., Linden H.M., Ulaner G.A., Gosselin A., Cartot-Cotton S., Cohen P., Doroumian S., Paux G., Celanovic M., et al. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat. Commun. 2022;13:4116. doi: 10.1038/s41467-022-31668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bardia A., Cortes J., Hurvitz S.A., Delaloge S., Iwata H., Shao Z.M., Kanavagel D., Cohen P., Liu Q., Cartot-Cotton S., et al. AMEERA-5: A randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2–advanced breast cancer. Ther. Adv. Med. Oncol. 2022;14:1–12. doi: 10.1177/17588359221083956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott J.S., Moss T.A., Balazs A., Barlaam B., Breed J., Carbajo R.J., Chiarparin E., Davey P.R.J., Delpuech O., Fawell S., et al. Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J. Med. Chem. 2020;63:14530–14559. doi: 10.1021/acs.jmedchem.0c01163. [DOI] [PubMed] [Google Scholar]
- 86.André F., Im S.A., Neven P., Baird R.D., Ettl J., Goetz M.P., Hamilton E., Iwata H., Jiang Z., Joy A.A., et al. Abstract OT2-11-06: SERENA-4: A Phase III comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive/HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. Cancer Res. 2022;82:OT2-11. [Google Scholar]
- 87.Bidard F.C., Kalinsky K., Cristofanilli M., Bianchini G., Chia S.K., Janni W., Ma C.X., Mayer E.L., Park Y.H., Fox S., et al. Abstract OT2-11-05: SERENA-6: A Phase III study to assess the efficacy and safety of AZD9833 (camizestrant) compared with aromatase inhibitors when given in combination with palbociclib or abemaciclib in patients with HR+/HER2-metastatic breast cancer with detectable ESR1 m who have not experienced disease progression on first-line therapy. Cancer Res. 2022;82:OT2-11. [Google Scholar]
- 88.Bardia A., Aftimos P., Bihani T., Anderson-Villaluz A.T., Jung J., Conlan M.G., Kaklamani V.G. EMERALD: Phase III trial of elacestrant (RAD1901) vs. endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019;15:3209–3218. doi: 10.2217/fon-2019-0370. [DOI] [PubMed] [Google Scholar]
- 89.Jhaveri K.L., Jeselsohn R., Lim E., Hamilton E.P., Yonemori K., Beck J.T., Kaufman P.A., Sammons S., Bhave M.A., Saura C., et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Monotherapy results from EMBER. J. Clin. Oncol. 2022;40:1021. doi: 10.1200/JCO.2022.40.16_suppl.1021. [DOI] [Google Scholar]
- 90.Weir H.M., Bradbury R.H., Lawson M., Rabow A.A., Buttar D., Callis R.J., Curwen J.O., de Almeida C., Ballard P., Hulse M., et al. AZD9496: An oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models pharmacology of SERD inhibitor in breast cancer models. Cancer Res. 2016;76:3307–3318. doi: 10.1158/0008-5472.CAN-15-2357. [DOI] [PubMed] [Google Scholar]
- 91.Paoletti C., Schiavon G., Dolce E.M., Darga E.P., Carr T.H., Geradts J., Hoch M., Klinowska T., Lindemann J., Marshall G., et al. Circulating biomarkers and resistance to endocrine therapy in metastatic breast cancers: Correlative results from AZD9496 oral SERD phase I trial liquid biopsies, metastatic breast cancer, oral SERD AZD9496. Clin. Cancer Res. 2018;24:5860–5872. doi: 10.1158/1078-0432.CCR-18-1569. [DOI] [PubMed] [Google Scholar]
- 92.Hamilton E.P., Patel M.R., Armstrong A.C., Baird R.D., Jhaveri K., Hoch M., Klinowska T., Lindemann J.P.O., Morgan S.R., Schiavon G., et al. A first-in-human study of the new oral selective estrogen receptor degrader AZD9496 for ER+/HER2− advanced breast cancer. Clin. Cancer Res. 2018;24:3510–3518. doi: 10.1158/1078-0432.CCR-17-3102. [DOI] [PubMed] [Google Scholar]
- 93.Chen Y.C., Yu J., Metcalfe C., de Bruyn T., Gelzleichter T., Malhi V., Perez-Moreno P.D., Wang X. Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Exp. Opin. Investig. Drugs. 2022;31:515–529. doi: 10.1080/13543784.2021.1983542. [DOI] [PubMed] [Google Scholar]
- 94.Ratre P., Mishra K., Dubey A., Vyas A., Jain A., Thareja S. Aromatase inhibitors for the treatment of breast cancer: A journey from the scratch. Anticancer Agents Med. Chem. 2020;20:1994–2004. doi: 10.2174/1871520620666200627204105. [DOI] [PubMed] [Google Scholar]
- 95.Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int. J. Women’s Health. 2015;7:493–499. doi: 10.2147/IJWH.S69907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rani S., Raheja K., Luxami V., Paul K. A review on diverse heterocyclic compounds as the privileged scaffolds in non-steroidal aromatase inhibitors. Bioorg. Chem. 2021;113:105017. doi: 10.1016/j.bioorg.2021.105017. [DOI] [PubMed] [Google Scholar]
- 97.Martino G., Catalano A., Agostino R.M., Bellone F., Morabito N., Lasco C.G., Vicario C.M., Schwarz P., Feldt-Rasmussen U. Quality of life and psychological functioning in postmenopausal women undergoing aromatase inhibitor treatment for early breast cancer. PLoS ONE. 2020;15:e0230681. doi: 10.1371/journal.pone.0230681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tenti S., Correale P., Cheleschi S., Fioravanti A., Pirtoli L. Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020;21:5625. doi: 10.3390/ijms21165625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caprioli M., Carrara G., Sakellariou G., Silvagni E., Scirè C.A. Influence of aromatase inhibitors therapy on the occurrence of rheumatoid arthritis in women with breast cancer: Results from a large population-based study of the Italian Society for Rheumatology. RMD Open. 2017;3:e000523. doi: 10.1136/rmdopen-2017-000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mascella F., Gianni L., Affatato A., Fantini M. Aromatase inhibitors and antisynthetase syndrome. Int. J. Immunopathol. Pharmacol. 2016;29:494–497. doi: 10.1177/0394632016651086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tenti S., Giordano N., Cutolo M., Giannini F., Fioravanti A. Primary antiphospholipid syndrome during aromatase inhibitors therapy: A case report and review of the literature. Medicine. 2019;98:e15052. doi: 10.1097/MD.0000000000015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boszkiewicz K., Piwowar A., Petryszyn P. Aromatase inhibitors and risk of metabolic and cardiovascular adverse effects in breast cancer patients—A systematic review and meta-analysis. J. Clin. Med. 2022;11:3133. doi: 10.3390/jcm11113133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghaly H.S.A., Varamini P. New drug delivery strategies targeting GnRH receptor in breast and other cancers. Endocr. Relat. Cancer. 2021;28:R251–R269. doi: 10.1530/ERC-20-0442. [DOI] [PubMed] [Google Scholar]
- 104.Robertson J., Blamey R. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur. J. Cancer. 2003;39:861–869. doi: 10.1016/S0959-8049(02)00810-9. [DOI] [PubMed] [Google Scholar]
- 105.Ulrich N.D., Raja N.S., Moravek M.B. A review of fertility preservation in patients with breast cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;82:60–68. doi: 10.1016/j.bpobgyn.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Huerta-Reyes M., Maya-Núñez G., Pérez-Solis M.A., López-Muñoz E., Guillén N., Olivo-Marin J.C., Aguilar-Rojas A. Treatment of breast cancer with gonadotropin-releasing hormone analogs. Front. Oncol. 2019;9:943. doi: 10.3389/fonc.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Lauro L., Pizzuti L., Barba M., Sergi D., Sperduti I., Mottolese M., Amoreo C.A., Belli F., Vici P., Speirs V., et al. Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: Results from a pooled analysis. J. Hematol. Oncol. 2015;8:53. doi: 10.1186/s13045-015-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karimi-Zarchi M., Forat-Yazdi M., Vafaeenasab M.R., Nakhaie-Moghadam M., Miratashi-Yazdi A., Teimoori S., Dehghani-Tafti A. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur. J. Gynaecol. Oncol. 2014;35:59–61. [PubMed] [Google Scholar]
- 109.Jonat W., Kaufmann M., Sauerbrei W., Blamey R., Cuzick J., Namer M., de Haes F.J.C., de Matteis A., Stewart A., Eiermann W., et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The zoladex early breast cancer research association study. J. Clin. Oncol. 2002;20:4628–4635. doi: 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 110.Tripathy D., Im S.A., Colleoni M., Franke F., Bardia A., Harbeck N., Hurvitz S.A., Chow L., Sohn J., Lee K.S., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 111.Im S.A., Lu Y.S., Bardia A., Harbeck N., Colleoni M., Franke F., Chow L., Sohn J., Lee K.S., Campos-Gomez S., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 112.Wang S., Pei L., Hu T., Jia M., Wang S. Protective effect of goserelin on ovarian reserve during (neo) adjuvant chemotherapy in young breast cancer patients: A prospective cohort study in China. Hum. Reproduct. 2021;36:976–986. doi: 10.1093/humrep/deaa349. [DOI] [PubMed] [Google Scholar]
- 113.Kakarla M., Gambo M.A., Salama M.Y., Ismail N.H., Tavalla P., Uppal P., Mohammed S.A., Rajashekar S., Ravindran S.G., Hamid P., et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer patients: A systematic review. Cureus. 2022;14:e26209. doi: 10.7759/cureus.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boland J., Choi W., Lee M., Lin J. Cardiovascular toxicity of androgen deprivation therapy. Curr. Cardiol. Rep. 2021;23:109. doi: 10.1007/s11886-021-01561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chou Y.S., Wang C.C., Hsu L.F., Chuang P.H., Cheng C.F., Li N.H., Chen C.C., Chen C.L., Lai Y.J., Yen Y.F., et al. Gonadotropin—Releasing hormone agonist treatment and ischemic heart disease among female patients with breast cancer: A cohort study. Cancer Med. :2022. doi: 10.1002/cam4.5390. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alemany C., Patel M., Mitri Z., Sparano J., Borges V., Makower D., Klein P., Lawrence J., Le T., Zujewski J.A., et al. Abstract P037: A phase 1/2 dose escalation and expansion study of OP-1250 in adults with advanced and/or metastatic hormone receptor-positive (HR+), HER2-negative (HER2−) breast cancer. Mol. Cancer Ther. 2021;20:P037. doi: 10.1158/1535-7163.TARG-21-P037. [DOI] [Google Scholar]
- 117.Parisian A.D., Miller C., Ortega F., Hodges-Gallagher L., Barratt S., Azofeifa J., Kushner P.J., Kulp D., Harmon C.L. Precision run-on sequencing (PRO-seq) analysis of a treatment time course in ER+ breast cancer cell lines reveals the transcriptional changes underlying response to complete estrogen receptor antagonist (CERAN) OP-1250. Cancer Res. 2022;82:5375. doi: 10.1158/1538-7445.AM2022-5375. [DOI] [Google Scholar]
- 118.Patel M., Alemany C., Mitri Z., Makower D., Borges V., Sparano J., Le T., Klein P., Lawrence J., Khushner P.J., et al. Preliminary data from a phase 1/2, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in patients with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer. Cancer Res. 2022;82:P1-17-12. [Google Scholar]
- 119. [(accessed on 22 December 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04505826.
- 120.Harlow K.W., Smith D.N., Katzenellenbogen J.A., Greene G.L., Katzenellenbogen B.S. Identification of cysteine 530 as the covalent attachment site of an affinity-labeling estrogen (ketononestrol aziridine) and antiestrogen (tamoxifen aziridine) in the human estrogen receptor. J. Biol. Chem. 1989;264:17476–17485. doi: 10.1016/S0021-9258(18)71519-6. [DOI] [PubMed] [Google Scholar]
- 121.Furman C., Hao M.H., Prajapati S., Reynolds D., Rimkunas V., Zheng G.Z., Zhu P., Korpal M. Estrogen receptor covalent antagonists: The best is yet to come. Cancer Res. 2019;79:1740–1745. doi: 10.1158/0008-5472.CAN-18-3634. [DOI] [PubMed] [Google Scholar]
- 122.Scott J.S., Barlaam B. Selective estrogen receptor degraders (SERDs) and covalent antagonists (SERCAs): A patent review (2015-present) Exp. Opin. Ther. Pat. 2022;32:131–151. doi: 10.1080/13543776.2022.2006185. [DOI] [PubMed] [Google Scholar]
- 123.Puyang X., Furman C., Zheng G.Z., Wu Z.J., Banka D., Aithal K., Agoulnik S., Bolduc D.M., Buonamici S., Caleb B., et al. Discovery of selective estrogen receptor covalent antagonists for the treatment of ERalpha (WT) and ERalpha (MUT) breast cancer. Cancer Discov. 2018;8:1176–1193. doi: 10.1158/2159-8290.CD-17-1229. [DOI] [PubMed] [Google Scholar]
- 124.Furman C., Puyang X., Zhang Z., Wu Z.J., Banka D., Aithal K.B., Albacker L.A., Hao M.H., Irwin S., Kim A., et al. Covalent ERα antagonist H3B-6545 demonstrates encouraging preclinical activity in therapy-resistant breast cancer. Mol. Cancer Ther. 2022;21:890–902. doi: 10.1158/1535-7163.MCT-21-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Colomer-Saucedo J.B., Loulousis M.M., Copello V.A., Krager S.L., Tischkau S.L., Copello J.A. Pharmacological targeting of SERCA in breast cancer. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.03404. [DOI] [Google Scholar]
- 126.So C.L., Saunus J.M., Roberts-Thomson S.J., Monteith G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2019;94:74–83. doi: 10.1016/j.semcdb.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Christodoulou P., Yiallouris A., Michail A., Christodoulou M.I., Politis P.K., Patrikios I. Altered SERCA expression in breast cancer. Medicina. 2021;57:1074. doi: 10.3390/medicina57101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiong R., Patel H.K., Gutgesell L.M., Zhao J., Delgado-Rivera L., Pham T.N., Zhao H., Carlson K., Martin T., Katzenellenbogen J.A., et al. Selective human estrogen receptor partial agonists (ShERPAs) for tamoxifen-resistant breast cancer. J. Med. Chem. 2016;59:219–237. doi: 10.1021/acs.jmedchem.5b01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pagliuca M., Donato M., D’Amato A.L., Rosanova M., Russo A.O.M., Scafetta R., de Angelis C., Trivedi M.V., André F., Arpino G., et al. New steps on an old path: Novel estrogen receptor inhibitors in breast cancer. Crit. Rev. Oncol. Hematol. 2022;180:103861. doi: 10.1016/j.critrevonc.2022.103861. [DOI] [PubMed] [Google Scholar]
- 130.Abderrahman B., Maximov P.Y., Curpan R.F., Hanspal J.S., Fan P., Xiong R., Tonetti D.A., Thatcher G.R., Jordan V.C. Pharmacology and molecular mechanisms of clinically relevant estrogen estetrol and estrogen mimic BMI-135 for the treatment of endocrine-resistant breast cancer. Mol. Pharmacol. 2020;98:364–381. doi: 10.1124/molpharm.120.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dudek A.Z., Liu L.C., Fischer J.H., Wiley E.L., Sachdev J.C., Bleeker J., Hurley R.W., Tonetti D.A., Thatcher G.R.J., Venuti R.P., et al. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res. Treat. 2020;183:617–627. doi: 10.1007/s10549-020-05787-z. [DOI] [PubMed] [Google Scholar]
- 132.Moy B., Rumble R.B., Come S.E., Davidson N.E., di Leo A., Gralow J.R., Hortobagyi G.N., Yee D., Smith I.E., Chavez-MacGregor M., et al. Chemotherapy and targeted therapy for patients with human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline update. J. Clin. Oncol. 2021;39:3938–3958. doi: 10.1200/JCO.21.01374. [DOI] [PubMed] [Google Scholar]
- 133.Jacquet E., Lardy-Cléaud A., Pistilli B., Franck S., Cottu P., Delaloge S., Debled M., Vanlemmens L., Leheurteur M., Guizard A., et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor–positive HER2-negative metastatic breast cancer patients. Eur. J. Cancer. 2018;95:93–101. doi: 10.1016/j.ejca.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 134.Trapani D., Gandini S., Corti C., Crimini E., Bellerba F., Minchella I., Criscitiello C., Tarantino P., Curigliano G. Benefit of adjuvant chemotherapy in patients with lobular breast cancer: A systematic review of the literature and metanalysis. Cancer Treat. Rev. 2021;97:102205. doi: 10.1016/j.ctrv.2021.102205. [DOI] [PubMed] [Google Scholar]
- 135.Rossi P.G., Lebeau A., Canelo-Aybar C., Saz-Parkinson Z., Quinn C., Langendam M., McGarrigle H., Warman S., Rigau D., Alonso-Coello P., et al. Recommendations from the European Commission Initiative on Breast Cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, HER-2 negative. Br. J. Cancer. 2021;124:1503–1512. doi: 10.1038/s41416-020-01247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 137.Tsimberidou A.M. Targeted therapy in cancer. Cancer Chemother. Pharmacol. 2015;76:1113–1132. doi: 10.1007/s00280-015-2861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitri Z., Constantine T., O’Regan R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 140.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 141.Simmons C., Rayson D., Joy A.A., Henning J.W., Lemieux J., McArthur H., Card P.B., Dent R., Bresden-Masley C. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther. Adv. Med. Oncol. :2022. doi: 10.1177/17588359211066677. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oh D.Y., Bang Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 143.Buzun K., Bielawska A., Bielawski K., Gornowicz A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2020;35:1781–1799. doi: 10.1080/14756366.2020.1821676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomas A., Pommier Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. 2019;25:6581–6589. doi: 10.1158/1078-0432.CCR-19-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tesauro C., Simonsen A.K., Andersen M.B., Petersen K.W., Kristoffersen E.L., Algreen L., Hansen N.Y., Andersen A.B., Jakobsen A.K., Stougaard M., et al. Topoisomerase I activity and sensitivity to camptothecin in breast cancer-derived cells: A comparative study. BMC Cancer. 2019;19:1158. doi: 10.1186/s12885-019-6371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kümler I., Brünner N., Stenvang J., Balslev E., Nielsen D.L. A systematic review on topoisomerase 1 inhibition in the treatment of metastatic breast cancer. Breast Cancer Res. Treat. 2013;138:347–358. doi: 10.1007/s10549-013-2476-3. [DOI] [PubMed] [Google Scholar]
- 147.Tripathy D., Tolaney S.M., Seidman A.D., Anders C.K., Ibrahim N., Rugo H.S., Twelves C., Diéras V., Müller V., Du Y., et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases. JAMA Oncol. 2022;8:1047–1052. doi: 10.1001/jamaoncol.2022.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang B., Zhang S. Etirinotecan Pegol treatment for patients with metastatic breast cancer and brain metastases. JAMA Oncol. 2022;8:1700–1701. doi: 10.1001/jamaoncol.2022.4343. [DOI] [PubMed] [Google Scholar]
- 149.Khallaf S.M., Roshdy J., Ibrahim A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J. Egypt. Natl. Cancer Inst. 2020;32:20. doi: 10.1186/s43046-020-00034-4. [DOI] [PubMed] [Google Scholar]
- 150.Hu G., Wang D. Analysis of cardiac adverse reactions caused by different doses of adriamycin chemotherapy in patients with breast cancer. J. Healthc. Eng. 2022;2022:1642244. doi: 10.1155/2022/1642244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Swain S.M., Ewer M.S., Viale G., Delaloge S., Ferrero J.-M., Verrill M., Colomer R., Vieira C., Werner T.L., Douthwaite H., et al. Pertuzumab, trastuzumab, and standard anthracycline-and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018;29:646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Elshimali Y.I., Wu Y., Khaddour H., Wu Y., Gradinaru D., Sukhija H., Chung S.S., Vadgama J.V. Optimization of cancer treatment through overcoming drug resistance. J. Cancer Res. Oncobiol. 2018;1:107. doi: 10.31021/jcro.20181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang M., Li H., Li Y., Ruan Y., Quan C. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol. Med. Rep. 2018;17:6211–6226. doi: 10.3892/mmr.2018.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang F., Hu Y., Shao L., Zhuang J., Huo Q., He S., Chen S., Wang J., Xie N. SIRT7 interacts with TEK (TIE2) to promote adriamycin induced metastasis in breast cancer. Cell Oncol. 2021;44:1405–1424. doi: 10.1007/s13402-021-00649-2. [DOI] [PubMed] [Google Scholar]
- 155.Liu Z., Gao J., Gu R., Shi Y., Hu H., Liu J., Huang J., Zhong C., Zhou W., Yang Y., et al. Comprehensive analysis of transcriptomics and genetic alterations identifies potential mechanisms underlying anthracycline therapy resistance in breast cancer. Biomolecules. 2022;12:1834. doi: 10.3390/biom12121834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen J., Shi P., Zhang J., Li Y., Ma J., Zhang Y., Liu H. CircRNA_0044556 diminishes the sensitivity of triple-negative breast cancer cells to adriamycin by sponging miR-145 and regulating NRAS. Mol. Med. Rep. 2022;25:1–12. doi: 10.3892/mmr.2021.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ke J., Ni K., Xue H., Li J. RBM38 is negatively regulated by miR-320b and enhances Adriamycin resistance in breast cancer cells. Oncol. Lett. 2022;23:1–12. doi: 10.3892/ol.2021.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen Y., Jia Y., Mao M., Gu Y., Xu C., Yang J., Hu W., Shen J., Hu D., Chen C., et al. PLAC8 promotes adriamycin resistance via blocking autophagy in breast cancer. J. Cell. Mol. Med. 2021;25:6948–6962. doi: 10.1111/jcmm.16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen W.-X., Wang D.-D., Zhu B., Zhu Y.-Z., Zheng L., Feng Z.-Q., Qin X.-H. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging. 2021;13:10415–10430. doi: 10.18632/aging.202802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Masood S. Neoadjuvant chemotherapy in breast cancers. Women’s Health. 2016;12:480–491. doi: 10.1177/1745505716677139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R., Davies C., Godwin J., Gray R., Pan H.C., Clarke M., Cutter D., Darby S., McGale P., et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pourkarim F., Rahimpour E., Alvani S., Jouyban A. Solubilization methods for paclitaxel and docetaxel: An overview. Pharm. Sci. 2021;28:208–223. doi: 10.34172/PS.2021.30. [DOI] [Google Scholar]
- 163.Risinger A.L., Riffle S.M., Lopus M., Jordan M.A., Wilson L., Mooberry S.L. The taccalonolides and paclitaxel cause distinct effects on microtubule dynamics and aster formation. Mol. Cancer. 2014;13:41. doi: 10.1186/1476-4598-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Prota A.E., Bargsten K., Zurwerra D., Field J.J., Díaz J.F., Altmann K.-H., Steinmetz M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587–590. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 165.Klein I., Lehmann H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics. 2021;9:229. doi: 10.3390/toxics9100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tang W., Yang J., Yuan Y., Zhao Z., Lian Z., Liang G. Paclitaxel nanoparticle awakens immune system to fight against cancer. Nanoscale. 2017;9:6529–6536. doi: 10.1039/C6NR09895A. [DOI] [PubMed] [Google Scholar]
- 167.Chen Y., Liu R., Li C., Song Y., Liu G., Huang Q., Yu L., Zhu D., Lu C., Lu A., et al. Nab-paclitaxel promotes the cancer-immunity cycle as a potential immunomodulator. Am. J. Cancer Res. 2021;11:3445. [PMC free article] [PubMed] [Google Scholar]
- 168.Denis J.N., Greene A.E., Guenard D., Gueritte-Voegelein F., Mangatal L., Potier P. Highly efficient, practical approach to natural taxol. J. Am. Chem. Soc. 1988;110:5917–5919. doi: 10.1021/ja00225a063. [DOI] [Google Scholar]
- 169.Swain S.M., Miles D., Kim S.-B., Im Y.-H., Im S.-A., Semiglazov V., Ciruelos E., Schneeweiss A., Loi S., Monturus E., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 170.Van Eijk M., Vermunt M.A., van Werkhoven E., Wilthagen E.A., Huitema A.D., Beijnen J.H. The influence of docetaxel schedule on treatment tolerability and efficacy in patients with metastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2022;22:104. doi: 10.1186/s12885-022-09196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vrignaud P., Semiond D., Benning V., Beys E., Bouchard H., Gupta S. Preclinical profile of cabazitaxel. Drug Des. Dev. Ther. 2014;8:1851–1867. doi: 10.2147/DDDT.S64940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koutras A., Zagouri F., Koliou G., Psoma E., Chryssogonidis I., Lazaridis G., Tryfonopoulos D., Kotsakis A., Res E., Kentepozidis N., et al. Phase 2 study of cabazitaxel as second-line treatment in patients with HER2-negative metastatic breast cancer previously treated with taxanes-a Hellenic Cooperative Oncology Group (HeCOG) trial. Br. J. Cancer. 2020;123:355–361. doi: 10.1038/s41416-020-0909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Olson M.F., Sahai E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis. 2009;26:273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 174.Suresh R., Diaz R.J. The remodelling of actin composition as a hallmark of cancer. Translat. Oncol. 2021;14:101051. doi: 10.1016/j.tranon.2021.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arumugam A., Subramani R., Lakshmanaswamy R. Involvement of actin cytoskeletal modifications in the inhibition of triple-negative breast cancer growth and metastasis by nimbolide. Mol. Ther. Oncolytics. 2021;20:596–606. doi: 10.1016/j.omto.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lipinski M., Parks D.R., Rouse R.V., Herzenberg L.A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc. Natl. Acad. Sci. USA. 1981;78:5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McDougall A.R., Tolcos M., Hooper S.B., Cole T.J., Wallace M.J. Trop2: From development to disease. Dev. Dyn. 2015;244:99–109. doi: 10.1002/dvdy.24242. [DOI] [PubMed] [Google Scholar]
- 178.Cortesi M., Zanoni M., Maltoni R., Ravaioli S., Tumedei M.M., Pirini F., Bravaccini S. TROP2 (trophoblast cell-surface antigen 2): A drug target for breast cancer. Exp. Opin. Ther. Targets. 2022;26:593–602. doi: 10.1080/14728222.2022.2113513. [DOI] [PubMed] [Google Scholar]
- 179.Alberti S., Trerotola M., Guerra E. The Hu2G10 tumor-selective anti-Trop-2 monoclonal antibody targets the cleaved-activated Trop-2 and shows therapeutic efficacy against multiple human cancers. Cancer Res. 2022;82:340. doi: 10.1158/1538-7445.AM2022-340. [DOI] [Google Scholar]
- 180.Sayama Y., Kaneko M.K., Kato Y. Development and characterization of TrMab-6, a novel anti-TROP2 monoclonal antibody for antigen detection in breast cancer. Mol. Med. Rep. 2021;23:92. doi: 10.3892/mmr.2020.11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sayama Y., Kaneko M.K., Takei J., Hosono H., Sano M., Asano T., Kato Y. Establishment of a novel anti-TROP2 monoclonal antibody TrMab-29 for immunohistochemical analysis. Biochem. Biophys. Rep. 2021;25:100902. doi: 10.1016/j.bbrep.2020.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kauffman H.M., Cherikh W.S., Cheng Y., Hanto D.W., Kahan B.D. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.TP.0000184006.43152.8D. [DOI] [PubMed] [Google Scholar]
- 183.Hare S.H., Harvey A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017;7:383. [PMC free article] [PubMed] [Google Scholar]
- 184.Chaturvedi D., Gao X., Cohen M.S., Taunton J., Patel T.B. Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene. 2009;28:1187–1196. doi: 10.1038/onc.2008.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Voutsadakis I.A. Biomarkers of everolimus efficacy in breast cancer therapy. J. Oncol. Pharm. Pract. 2022;28:945–959. doi: 10.1177/10781552211073673. [DOI] [PubMed] [Google Scholar]
- 186.Kitano S., Honda A., Itoi N., Lee T. Everolimus for treating hormone receptor-positive metastatic breast cancer previously treated with cyclin-dependent kinase 4/6 inhibitors. Anticancer Res. 2022;42:3913–3919. doi: 10.21873/anticanres.15885. [DOI] [PubMed] [Google Scholar]
- 187.Gandhi L., Bahleda R., Tolaney S.M., Kwak E.L., Cleary J.M., Pandya S.S., Hollebecque A., Abbas R., Ananthakrishnan R., Berkenblit A., et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 2014;32:68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 188.Diamond J., David P., Salkeni M., Silverman P., Haddad T., Forget F., Awada A., Canon J.L., Danso M., Lortholary A., et al. Phase 2 safety and efficacy results of TAK-228 in combination with exemestane or fulvestrant in postmenopausal women with ER-positive/HER2-negative metastatic breast cancer previously treated with everolimus. Cancer Res. 2019;79:PD1-09. doi: 10.1158/1538-7445.SABCS18-PD1-09. [DOI] [Google Scholar]
- 189.Starks D., Rojas-Espaillat L., Meissner T., Williams C. Patient reported outcomes and final results of phase 1 evaluation of the safety and clinical activity of sapanisertib in combination with serabelisib and paclitaxel in patients with advanced ovarian, endometrial, or breast cancer (044) Gynecol. Oncol. 2022;166:S30–S31. doi: 10.1016/S0090-8258(22)01263-X. [DOI] [PubMed] [Google Scholar]
- 190.Lien E.C., Dibble C.C., Toker A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Akinleye A., Avvaru P., Furqan M., Song Y., Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sabbah D.A., Hajjo R., Bardaweel S.K., Zhong H.A. Phosphatidylinositol 3-kinase (PI3K) inhibitors: A recent update on inhibitor design and clinical trials (2016–2020) Expert Opin. Ther. Pat. 2021;31:877–892. doi: 10.1080/13543776.2021.1924150. [DOI] [PubMed] [Google Scholar]
- 193.Vanhaesebroeck B., Perry M.W.D., Brown J.R., André F., Okkenhaug K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021;20:741–769. doi: 10.1038/s41573-021-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mishra R., Patel H., Alanazi S., Kilroy M.K., Garrett J.T. PI3K inhibitors in cancer: Clinical implications and adverse effects. Int. J. Mol. Sci. 2021;22:3464. doi: 10.3390/ijms22073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Narayan P., Prowell T.M., Gao J.J., Fernandes L.L., Li E., Jiang X., Qiu J., Fan J., Song P., Yu J., et al. FDA approval summary: Alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin. Cancer Res. 2021;27:1842–1849. doi: 10.1158/1078-0432.CCR-20-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hanan E.J., Braun M.G., Heald R.A., MacLeod C., Chan C., Clausen S., Hedgar K.A., Eigenbrot C., Elliott R., Endres N., et al. Discovery of GDC-0077 (inavolisib), a highly selective inhibitor and degrader of mutant PI3Kα. J. Med. Chem. :2022. doi: 10.1021/acs.jmedchem.2c01422. in press . [DOI] [PubMed] [Google Scholar]
- 197.Bedard P.L., Accordino M.K., Cervantes A., Gambardella V., Hamilton E.P., Italiano A., Juric D., Kalinsky K., Krop I.E., Oliveira M., et al. Long-term safety of inavolisib (GDC-0077) in an ongoing phase 1/1b study evaluating monotherapy and in combination (combo) with palbociclib and/or endocrine therapy in patients (pts) with PIK3CA-mutated, hormone receptor-positive/HER2-negative (HR+/HER2−) metastatic breast cancer (BC) J. Clin. Oncol. 2022;40:1052. [Google Scholar]
- 198.Moore H.M., Savage H.M., O’Brien C., Zhou W., Sokol E.S., Goldberg M.E., Metcalfe C., Friedman L.S., Leckner M.R., Wilson T.R., et al. Predictive and pharmacodynamic biomarkers of response to the phosphatidylinositol 3-kinase inhibitor Taselisib in breast cancer preclinical models. Mol. Cancer Ther. 2019;19:292–303. doi: 10.1158/1535-7163.MCT-19-0284. [DOI] [PubMed] [Google Scholar]
- 199.Dent S., Cortés J., Im Y.H., Diéras V., Harbeck N., Krop I.E., Wilson T.R., Cui N., Schimmoller F., Hsu J.Y., et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021;32:197–207. doi: 10.1016/j.annonc.2020.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sarker D., Ang J.E., Baird R., Kristeleit R., Shah K., Moreno V., Clarke P.A., Raynaud F.I., Levy G., Ware J.A., et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schoffski P., Cresta S., Mayer I.A., Wildiers H., Damian S., Gendreau S., Rooney I., Morrissey K.M., Spoerke J.M., Ng V.W., et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018;20:109. doi: 10.1186/s13058-018-1015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sirohi B., Rastogi S., Dawood S. Buparlisib in Breast Cancer. Future Oncol. 2015;11:1463–1470. doi: 10.2217/fon.15.56. [DOI] [PubMed] [Google Scholar]
- 203.Luo Q., Lu H., Zhou X., Wang Y. The efficacy and safety of neoadjuvant buparlisib for breast cancer: A meta-analysis of randomized controlled studies. Medicine. 2019;98:e17614. doi: 10.1097/MD.0000000000017614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu Y.S., Lee K.S., Chao T.Y., Tseng L.M., Chitapanarux I., Chen S.C., Liu C.T., Sohn J., Kim J.H., Chang Y.C., et al. A Phase Ib study of alpelisib or buparlisib combined with tamoxifen plus goserelin in premenopausal women with HR-positive HER2-negative advanced breast cancer. Clin. Cancer Res. 2021;27:408–417. doi: 10.1158/1078-0432.CCR-20-1008. [DOI] [PubMed] [Google Scholar]
- 205.Tolaney S.M., Im Y.H., Calvo E., Lu Y.S., Hamilton E., Forero-Torres A., Bachelot T., Maur M., Fasolo A., Tiedt R., et al. Phase Ib study of ribociclib plus fulvestrant and ribociclib plus fulvestrant plus PI3K inhibitor (alpelisib or buparlisib) for HR(+) advanced breast cancer. Clin. Cancer Res. 2021;27:418–428. doi: 10.1158/1078-0432.CCR-20-0645. [DOI] [PubMed] [Google Scholar]
- 206.Song M., Bode A.M., Dong Z., Lee M.H. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 207.Martorana F., Motta G., Pavone G., Motta L., Stella S., Vitale S.R., Manzella L., Vigneri P. AKT inhibitors: New weapons in the fight against breast cancer? Front. Pharmacol. 2021;12:662232. doi: 10.3389/fphar.2021.662232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hinz N., Jücker M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell. Commun. Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhu K., Wu Y., He P., Fan Y., Zhong X., Zheng H., Luo T. PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells. 2022;11:2508. doi: 10.3390/cells11162508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li H., Prever L., Hirsch E., Gulluni F. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers. 2021;13:3517. doi: 10.3390/cancers13143517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peng Y., Wang Y., Zhou C., Mei W., Zeng C. PI3K/Akt/mtor pathway and its role in cancer therapeutics: Are we making headway? Front. Oncol. 2022;12:819128. doi: 10.3389/fonc.2022.819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jelovac D., Wolff A.C. The adjuvant treatment of HER2-positive breast cancer. Curr. Treat. Options Oncol. 2012;13:230–239. doi: 10.1007/s11864-012-0186-4. [DOI] [PubMed] [Google Scholar]
- 213.Schlam I., Swain S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer. 2021;7:56. doi: 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ryan Q., Ibrahim A., Cohen M.H., Johnson J., Ko C.W., Sridhara R., Justice R., Pazdur R. FDA drug approval summary: Lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13:1114–1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- 215.Voigtlaender M., Schneider-Merck T., Trepel M. Lapatinib. Recent Results Cancer Res. 2018;211:19–44. doi: 10.1007/978-3-319-91442-8_2. [DOI] [PubMed] [Google Scholar]
- 216.Guarneri V., Dieci M.V., Griguolo G., Miglietta F., Girardi F., Bisagni G., Generali D.G., Cagossi K., Sarti S., Frassoldati A., et al. Trastuzumab-lapatinib as neoadjuvant therapy for HER2-positive early breast cancer: Survival analyses of the CHER-Lob trial. Eur. J. Cancer. 2021;153:133–141. doi: 10.1016/j.ejca.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 217.Roskoski R., Jr. Orally effective FDA-approved protein kinase targeted covalent inhibitors (TCIs) Pharmacol. Res. 2021;165:105422. doi: 10.1016/j.phrs.2021.105422. [DOI] [PubMed] [Google Scholar]
- 218.Deeks E.D. Neratinib: First Global Approval. Drugs. 2017;77:1695–1704. doi: 10.1007/s40265-017-0811-4. [DOI] [PubMed] [Google Scholar]
- 219.Ma F., Li Q., Chen S., Zhu W., Fan Y., Wang J., Luo Y., Xing P., Lan B., Li M., et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J. Clin. Oncol. 2017;35:3105–3112. doi: 10.1200/JCO.2016.69.6179. [DOI] [PubMed] [Google Scholar]
- 220.Wang C., Deng S., Chen J., Xu X., Hu X., Kong D., Liang G., Yuan X., Li Y., Wang X., et al. The synergistic effects of pyrotinib combined with adriamycin on HER2-positive breast cancer. Front. Oncol. 2021;11:1603. doi: 10.3389/fonc.2021.616443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee A. Tucatinib: First approval. Drugs. 2020;80:1033–1038. doi: 10.1007/s40265-020-01340-w. [DOI] [PubMed] [Google Scholar]
- 222.Ulrich L., Okines A.F.C. Treating advanced unresectable or metastatic HER2-positive breast cancer: A spotlight on tucatinib. Breast Cancer. 2021;13:361–381. doi: 10.2147/BCTT.S268451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Capelan M., Pugliano L., de Azambuja E., Bozovic I., Saini K.S., Sotiriou C., Loi S., Piccart-Gebhart M.J. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann. Oncol. 2013;24:273–282. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 224.Dang C., Ewer M.S., Delaloge S., Ferrero J.-M., Colomer R., de la Cruz-Merino L., Werner T.L., Dadswell K., Verrill M., Eiger D., et al. BERENICE final analysis: Cardiac safety study of neoadjuvant pertuzumab, trastuzumab, and chemotherapy followed by adjuvant pertuzumab and trastuzumab in HER2-positive early breast cancer. Cancers. 2022;14:2596. doi: 10.3390/cancers14112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Husna S.M.N., Wong K.K. Margetuximab and trastuzumab deruxtecan: New generation of anti-HER2 immunotherapeutic agents for breast cancer. Mol. Immunol. 2022;152:45–54. doi: 10.1016/j.molimm.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 226.Royce M., Osgood C.L., Amatya A.K., Fiero M.H., Chang C.G., Ricks T.K., Shetty K.A., Kraft J., Qiu J., Song P., et al. FDA approval summary: Margetuximab plus chemotherapy for advanced or metastatic HER2-positive breast cancer. Clin. Cancer Res. 2022;28:1487–1492. doi: 10.1158/1078-0432.CCR-21-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Amiri-Kordestani L., Blumenthal G.M., Xu Q.C., Zhang L., Tang S.W., Ha L., Weinberg W.C., Chi B., Candau-Chacon R., Hughes P., et al. FDA approval: Ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin. Cancer Res. 2014;20:4436–4441. doi: 10.1158/1078-0432.CCR-14-0012. [DOI] [PubMed] [Google Scholar]
- 228.Narayan P., Osgood C.L., Singh H., Chiu H.J., Ricks T.K., Chiu Yuen Chow E., Qui J., Song P., Yu J., Namuswe F., et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast CancerFDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki. Clin. Cancer Res. 2021;27:4478–4485. doi: 10.1158/1078-0432.CCR-20-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cortés J., Kim S.-B., Chung W.-P., Im S.-A., Park Y.H., Hegg R., Kim M.H., Tseng L.-M., Petry V., Chung C.-F., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 230.Hurvitz S., Kim S.B., Chung W.P., Im S.A., Park Y.H., Hegg R., Kim M.H., Tseng L.M., Petry V., Chung C.F., et al. GS3-01. Trastuzumab Deruxtecan (T-DXd; DS-8201a) vs. Trastuzumab Emtansine (T-DM1) in Patients (pts) with HER2+ Metastatic Breast Cancer (mBC): Subgroup Analyses from the Randomized Phase 3 Study DESTINY-Breast03. 2021. [(accessed on 16 December 2022)]. Available online: https://www.abstractsonline.com/pp8/#!/10462/presentation/649.
- 231.Bartsch R., Berghoff A.S., Furtner J., Marhold M., Bergen E.S., Roider-Schur S., Starzer A.M., Forstner H., Rottenmanner B., Dieckmann K., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022;28:1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen N., Michaels E., Howard F., Nanda R. The evolving therapeutic landscape of antibody-drug conjugates in breast cancer. Exp. Rev. Anticancer Ther. 2022;12:1235–1331. doi: 10.1080/14737140.2022.2147510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sharma P., Klemp J.R., Kimler B.F., Mahnken J.D., Geier L.J., Khan Q.J., Elia M., Connor C.S., McGinness M.K., Mammen J.M., et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res. Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deeks E.D. Olaparib: First global approval. Drugs. 2015;75:231–240. doi: 10.1007/s40265-015-0345-6. [DOI] [PubMed] [Google Scholar]
- 235.U.S Food and Drug Administration FDA Approves Olaparib for Germline BRCA-Mutated Metastatic Breast Cancer. [(accessed on 16 January 2023)];2018 Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm592357.htm.
- 236.McCann K.E. Advances in the use of PARP inhibitors for BRCA1/2-associated breast cancer: Talazoparib. Future Oncol. 2019;15:1707–1715. doi: 10.2217/fon-2018-0751. [DOI] [PubMed] [Google Scholar]
- 237.Geyer C.E., Sikov W.M., Huober J., Rugo H.S., Wolmark N., O’Shaughnessy J., Maag D., Untch M., Golshan M., Ponce Lorenzo J., et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 2022;33:384–394. doi: 10.1016/j.annonc.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 238.Han H.S., Arun B.K., Kaufman B., Wildiers H., Friedlander M., Ayoub J.P., Puhalla S.L., Bach B.A., Kundu M.G., Khandelwal N., et al. Veliparib monotherapy following carboplatin/paclitaxel plus veliparib combination therapy in patients with germline BRCA-associated advanced breast cancer: Results of exploratory analyses from the phase III BROCADE3 trial. Ann. Oncol. 2022;33:299–309. doi: 10.1016/j.annonc.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 239.Stodtmann S., Eckert D., Joshi R., Nuthalapati S., Ratajczak C.K., Menon R., Mensing S., Xiong H. Exposure-response model with time-varying predictors to estimate the effects of veliparib in combination with carboplatin/paclitaxel and as monotherapy: Veliparib phase 3 study in BRCA-mutated advanced breast cancer (BROCADE3) trial. J. Clin. Pharmacol. :2022. doi: 10.1002/jcph.2061. in press . [DOI] [PubMed] [Google Scholar]
- 240.Turner N.C., Balmaña J., Poncet C., Goulioti T., Tryfonidis K., Honkoop A.H., Zoppoli G., Razis E., Johannsson O.T., Colleoni M., et al. Niraparib for Advanced Breast Cancer with Germline BRCA1 and BRCA2 Mutations: The EORTC 1307-BCG/BIG5-13/TESARO PR-30-50-10-C BRAVO Study. Clin. Cancer Res. 2021;27:5482–5491. doi: 10.1158/1078-0432.CCR-21-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Turner N.C., Cescon D.W., Loibl S., Janni W., Rugo H., Balmaña J., Crowley C., Chung J., Fucli G., Hofstatter E., et al. Abstract OT2-24-02: ZEST: Randomized phase III study evaluating efficacy and safety of niraparib in patients with HER2-negative BRCA-mutated or triple-negative breast cancer with detectable circulating tumor DNA after definitive therapy. Cancer Res. 2022;82:OT2-24. doi: 10.1158/1538-7445.SABCS21-OT2-24-02. [DOI] [Google Scholar]
- 242.Markham A. Pamiparib: First approval. Drugs. 2021;81:1343–1348. doi: 10.1007/s40265-021-01552-8. [DOI] [PubMed] [Google Scholar]
- 243.Xu B., Sun T., Shi Y., Cui J., Yin Y., Ouyang Q., Liu Q., Zhang Q., Chen Y., Wang S., et al. Pamiparib in patients with locally advanced or metastatic HER2-negative breast cancer with germline BRCA mutations: A phase II study. Breast Cancer Res. Treat. :2022. doi: 10.1007/s10549-022-06785-z. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Poratti M., Marzaro G. Third-generation CDK inhibitors: A review on the synthesis and binding modes of palbociclib, ribociclib and abemaciclib. Eur. J. Med. Chem. 2019;172:143–153. doi: 10.1016/j.ejmech.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 245.Braal C.L., Jongbloed E.M., Wilting S.M., Mathijssen R.H., Koolen S.L., Jager A. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: Similarities and differences. Drugs. 2021;81:317–331. doi: 10.1007/s40265-020-01461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bardia A., Mayer I.A., Vahdat L.T., Tolaney S.M., Isakoff S.J., Diamond J.R., O’Shaughnessy J., Moroose R.L., Santin A.D., Abramson V.G., et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 247.Syed Y.Y. Sacituzumab govitecan: First approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu X., Deng J., Yuan Y., Chen W., Sun W., Wang Y., Huang H., Liang B., Ming T., Wen J., et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022;239:108296. doi: 10.1016/j.pharmthera.2022.108296. [DOI] [PubMed] [Google Scholar]
- 249.Meyer S.M., Tanaka T., Zanon P.R., Baisden J.T., Abegg D., Yang X., Akahori Y., Alshakarchi Z., Cameron M.D., Adibekian A., et al. DNA-encoded library screening to inform design of a Ribonuclease Targeting Chimera (RiboTAC) J. Am. Chem. Soc. 2022;144:21096–21102. doi: 10.1021/jacs.2c07217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang W., He S., Dong G., Sheng C. Nucleic-acid-based targeted degradation in drug discovery. J. Med. Chem. 2022;65:10217–10232. doi: 10.1021/acs.jmedchem.2c00875. [DOI] [PubMed] [Google Scholar]
- 251.Pettersson M., Crews C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Salerno A., Seghetti F., Caciolla J., Uliassi E., Testi E., Guardigni M., Roberti M., Milelli A., Bolognesi M.L. Enriching proteolysis targeting chimeras with a second modality: When two are better than one. J. Med. Chem. 2022;65:9507–9530. doi: 10.1021/acs.jmedchem.2c00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kessler D., Gmachl M., Mantoulidis A., Martin L.J., Zoephel A., Mayer M., Gollner A., Covini D., Fischer S., Gerstberger T., et al. Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. USA. 2019;116:15823–15829. doi: 10.1073/pnas.1904529116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rudolph J., Settleman J., Malek S. Emerging trends in cancer drug discovery-from drugging the “undruggable” to overcoming resistance. Cancer Discov. 2021;11:815–821. doi: 10.1158/2159-8290.CD-21-0260. [DOI] [PubMed] [Google Scholar]
- 255.Grimster N.P. Covalent PROTACs: The best of both worlds? RSC Med. Chem. 2021;12:1452–1458. doi: 10.1039/D1MD00191D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gao H., Sun X., Rao Y. PROTAC technology: Opportunities and challenges. ACS Med. Chem. Lett. 2020;11:237–240. doi: 10.1021/acsmedchemlett.9b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.LaPlante G., Zhang W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule inhibitors. Cancers. 2021;13:3079. doi: 10.3390/cancers13123079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sakamoto K.M., Kim K.B., Kumagai A., Mercurio F., Crews C.M., Deshaies R.J. PROTACs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sakamoto K.M., Kim K.B., Verma R., Ransick A., Stein B., Crews C.M., Deshaies R. Development of Protacs to Target Cancer-promoting Proteins for Ubiquitination and Degradation. Mol. Cell. Proteom. 2003;2:1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 260.Hamilton E., Vahdat L., Han H.S., Ranciato J., Gedrich R., Keung C.F., Chirnomas D., Hurvitz S. Abstract PD13-08: First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2-locally advanced or metastatic breast cancer. Cancer Res. 2022;82:PD13-08. doi: 10.1158/1538-7445.SABCS21-PD13-08. [DOI] [Google Scholar]
- 261.Hamilton E.P., Schott A.F., Nanda R., Lu H., Keung C.F., Gedrich R., Parameswaran J., Han H.S., Hurvitz S.A. ARV-471, an estrogen receptor (ER) PROTAC degrader, combined with palbociclib in advanced ER+/human epidermal growth factor receptor 2–negative (HER2-) breast cancer: Phase 1b cohort (part C) of a phase 1/2 study. J. Clin. Oncol. 2022;40:TPS1120. doi: 10.1200/JCO.2022.40.16_suppl.TPS1120. [DOI] [Google Scholar]
- 262.Banik S.M., Pedram K., Wisnovsky S., Ahn G., Riley N.M., Bertozzi C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584:291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li X., Zhao L., Chen C., Nie J., Jiao B. Can EGFR be a therapeutic target in breast cancer? Biochim. Biophys. Acta BBA Rev. Cancer. 2022;1877:188789. doi: 10.1016/j.bbcan.2022.188789. [DOI] [PubMed] [Google Scholar]
- 264.Cazzaniga M.E., Capici S., Cordani N., Cogliati V., Pepe F.F., Riva F., Cerrito M.G. Metronomic chemotherapy for metastatic breast cancer treatment: Clinical and preclinical data between lights and shadows. J. Clin. Med. 2022;11:4710. doi: 10.3390/jcm11164710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Krajnak S., Battista M.J., Hasenburg A., Schmidt M. Metronomic Chemotherapy for Metastatic Breast Cancer. Oncol. Res. Treat. 2022;45:12–17. doi: 10.1159/000520236. [DOI] [PubMed] [Google Scholar]
- 266.Liu J., He M., Wang Z., Li Q., Xu B. Current research status of metronomic chemotherapy in combination treatment of breast cancer. Oncol. Res. Treat. 2022;45:681–692. doi: 10.1159/000526481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Michmerhuizen A.R., Spratt D.E., Pierce L.J., Speers C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer. 2020;6:47. doi: 10.1038/s41523-020-00190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.You C.P., Leung M.H., Tsang W.C., Khoo U.S., Tsoi H. Androgen receptor as an emerging feasible biomarker for breast cancer. Biomolecules. 2022;12:72. doi: 10.3390/biom12010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Forooshani M.K., Scarpitta R., Fanelli G.N., Miccoli M., Naccarato A.G., Scatena C. Is it time to consider the androgen receptor as a therapeutic target in breast cancer? Anti-Cancer Agents Med. Chem. 2021;22:775–786. doi: 10.2174/1871520621666211201150818. [DOI] [PubMed] [Google Scholar]
- 270.Hickey T.E., Selth L.A., Chia K.M., Laven-Law G., Milioli H.H., Roden D., Jindal S., Hui M., Finlay-Schultz J., Ebrahimie E., et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021;27:310. doi: 10.1038/s41591-020-01168-7. [DOI] [PubMed] [Google Scholar]
- 271.Kolyvas E.A., Caldas C., Kelly K., Ahmad S.S. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. 2022;24:79. doi: 10.1186/s13058-022-01574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wardley A., Cortes J., Provencher L., Miller K., Chien A.J., Rugo H.S., Steinberg J., Sugg J., Tudor I.C., Huizing M., et al. The efficacy and safety of enzalutamide with trastuzumab in patients with HER2+ and androgen receptor-positive metastatic or locally advanced breast cancer. Breast Cancer Res. Treat. 2021;187:155–165. doi: 10.1007/s10549-021-06109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li Y., Zhang H., Merkher Y., Chen L., Liu N., Leonov S., Chen Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022;15:121. doi: 10.1186/s13045-022-01341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen F., Chen N., Gao Y., Jia L., Lyu Z., Cui J. Clinical progress of PD-1/L1 inhibitors in breast cancer immunotherapy. Front. Oncol. 2022;11:724424. doi: 10.3389/fonc.2021.724424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mezni E., Behi K., Gonçalves A. Immunotherapy and breast cancer: An overview. Curr. Opin. Oncol. 2022;34:587–594. doi: 10.1097/CCO.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 276.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 277.Jabbarzadeh Kaboli P., Salimian F., Aghapour S., Xiang S., Zhao Q., Li M., Wu X., Du F., Zhao Y., Shen J., et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020;156:104806. doi: 10.1016/j.phrs.2020.104806. [DOI] [PubMed] [Google Scholar]
- 278.Kim J.H., Lee J.O., Kim N., Lee H.J., Lee Y.W., Kim H.I., Kim S.J., Park S.H., Kim H.S. Paclitaxel suppresses the viability of breast tumor MCF7 cells through the regulation of EF1á and FOXO3a by AMPK signaling. Int. J. Oncol. 2015;47:1874–1880. doi: 10.3892/ijo.2015.3153. [DOI] [PubMed] [Google Scholar]
- 279.D’Antona L., Dattilo V., Catalogna G., Scumaci D., Fiumara C.V., Musumeci F., Perrotti G., Schenone S., Tallerico R., Spoleti C.B., et al. In Preclinical Model of Ovarian Cancer, the SGK1 Inhibitor SI113 Counteracts the Development of Paclitaxel Resistance and Restores Drug Sensitivity. Transl. Oncol. 2019;12:1045–1055. doi: 10.1016/j.tranon.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uprety B., Abrahamse H. Targeting breast cancer and their stem cell population through AMPK activation: Novel insights. Cells. 2022;11:576. doi: 10.3390/cells11030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Muhammad N., Usmani D., Tarique M., Naz H., Ashraf M., Raliya R., Tabrez S., Zughaibi T.A., Alsaieedi A., Hakeem I.J., et al. The role of natural products and their multitargeted approach to treat solid cancer. Cells. 2022;11:2209. doi: 10.3390/cells11142209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.González-Burgos E., Gómez-Serranillos M.P. Discovery and Development of Anti-Breast Cancer Agents from Natural Products. Elsevier Inc.; Amsterdam, The Netherlands: 2021. Vinca alkaloids as chemotherapeutic agents against breast cancer; pp. 69–101. [Google Scholar]
- 283.Roskoski R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacolog. Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 284.Tuli H.S., Tuorkey M.J., Thakral F., Sak K., Kumar M., Sharma A.K., Sharma U., Jain A., Aggarwal V., Bishayee A., et al. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019;10:1336. doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Goh Y.X., Jalil J., Lam K.W., Husain K., Premakumar C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022;13:820969. doi: 10.3389/fphar.2022.820969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rodríguez F., Caruana P., de la Fuente N., Español P., Gámez M., Balart J., Llurba E., Rovira R., Ruiz R., Martín-Lorente C., et al. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules. 2022;12:784. doi: 10.3390/biom12060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fraguas-Sánchez A.I., Lozza I., Torres-Suárez A.I. Actively targeted nanomedicines in breast cancer: From pre-clinical investigation to clinic. Cancers. 2022;14:1198. doi: 10.3390/cancers14051198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.