Abstract
Objectives
Recurrent, severely painful episodes, known as vaso-occlusive crises (VOCs) are the hallmark of sickle cell disease (SCD) and the primary reason for hospitalization. Opioids have been the gold standard for VOC treatment without significant improvement pain outcomes. To aid analgesia and combat opioid related adverse effects (ORAEs), some SCD clinicians have trialed infusions of sub-anesthetic ketamine along with opioids to treat VOCs. In this retrospective analysis, we compared adult SCD patients who received early vs late adjunctive sub-anesthetic ketamine infusions for VOCs.
Methods
We identified adult SCD patients (age 18–50 years) who presented to Duke University with a VOC and received sub-anesthetic ketamine infusions from July 2015 to June 2019. We assessed both daily opioid consumption (measured as oral morphine milligram equivalents (MME)) and self-reported 0–10 numeric pain ratings (NPR) at 1, 2, and 3 days after infusion initiation, as well as 1 day after discontinuation.
Results
A total of 56 patients were identified with a median age of 30 years. Compared to late administration, early infusion of sub-anesthetic ketamine was associated with a 24.5% (P = .0003) and 25.9% (P = .0006) reduction, respectively, in median NPR at 1 day and 2 days after infusion initiation but did not persist at 3 days following initiation of the infusion. A statistically significant reduction in MME was not observed.
Conclusions
In a nonrandomized study of sickle cell patients with VOCs, early sub-anesthetic ketamine infusion led to greater reduction in subjective pain intensity than late initiation of the infusion. Randomized studies should further explore whether early vs late ketamine infusion improves management of acute SCD pain.
Keywords: Sickle Cell Disease, Acute Pain, Ketamine
Introduction
Sickle cell disease—an inherited hemoglobinopathy—is characterized by severe, recurrent painful episodes known as vaso-occlusive crises (VOCs). Frequent VOCs are associated with reduced life expectancy and decreased health-related quality of life [1]. The healthcare costs associated with VOCs are exceptionally high, although the majority of SCD patients manage their pain at home [2]. Emergency department visits and inpatient admissions for SCD are estimated to be ∼$2.4 billion annually. Approximately 70–80% of hospitalizations are due to VOCs, making management of VOCs the primary driver of SCD-related healthcare costs [3].
Yet despite this personal and economic burden associated with SCD, there has been slow progress in the development of new strategies for the treatment of pain secondary to VOCs for several reasons. First, the pathophysiology of pain secondary to VOCs is broad and complex [4]. Second, recent advancements in SCD research have led to the approval of three new SCD medications—voxelotor, crizanlizumab, and L-glutamine. These medications are important for the prevention and treatment of SCD-related complications; however, they cannot treat acute pain in hospitalized SCD patients [4]. Thus, effective analgesic medications for acute SCD pain are still required. Third, acute VOCs are no longer seen as brief, intermittent periods of pain with pain-free intervals in between. Instead, pain secondary to VOCs is intense and severe, and 30% of SCD patients experience daily pain and approximately 50% have chronic pain syndrome [2]. Thus, a SCD patient presenting with severe pain may be experiencing pain secondary to a VOC or an acute exacerbation of their chronic pain or both. Distinguishing these various types of severe SCD pain in the acute care setting is challenging both for providers and patients.
The complexity of pain secondary to VOCs should demand a multi-mechanistic approach to treatment. However, pain treatment regimens have remained palliative, and oral and parental opioids have remained the mainstay of therapy for decades without significant improvement in pain outcomes [5]. While short-term opioids can be effective treatment for severe acute SCD pain, there are notable harms and challenges associated with long-term and repeated opioid use, such as opioid tolerance in which increased doses of opioids are needed for relief of pain. The paradoxical phenomenon of opioid-induced hyperalgesia (OIH), whereby escalating doses of opioids leads to increased sensitivity to painful stimuli, can also occur [6]. Emerging evidence also suggests that opioids can also facilitate the development of chronic pain through central sensitization—nociceptive hyperexcitability of the central nervous system (CNS) [7]. Finally, inadequate relief of acute pain not only decreases opioid responsiveness but also increases the risk of developing chronic pain [8].
Due to this great need for opioid-sparing adjuncts for VOC treatment, sickle cell clinicians have looked to ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist with demonstrated efficacy as an opioid sparing analgesic in other painful disease states. Activation of the NMDA receptor, a glutamate receptor and ion channel found in nerve cells, plays a key role in central sensitization, OIH, and opioid tolerance—factors that, as discussed above, can make the treatment of VOCs challenging [9]. The evidence supporting the use of ketamine in SCD is limited [10–13]. As a result, initiation of a ketamine infusion for a VOC often occurs late in the hospital course of a patient when his/her pain is refractory to standard opioid therapy. In this study, we conducted a retrospective analysis of a cohort of adult SCD patients who received ketamine infusions early vs late during a hospitalization for a VOC at a single academic center. We hypothesized that early ketamine administration (within three days of hospitalization) would be associated with a greater reduction in opioid consumption and pain intensity than late ketamine administration (after 3 days of hospitalization).
Methods
Study Design and Data Collection
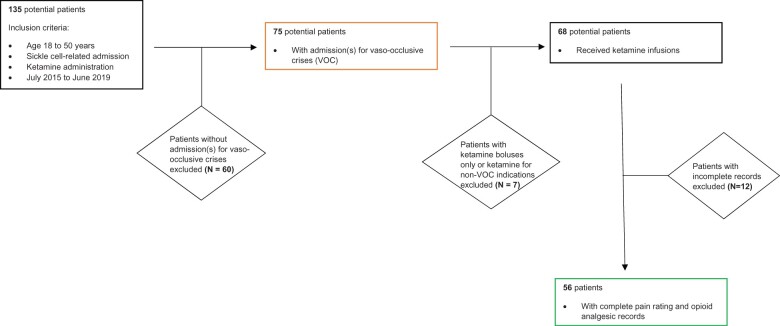
We included adult patients (age 18–50 years) who presented to Duke University Medical Center from July 2015 to June 2019 with a primary admitting diagnosis of VOC. We identified 135 potential subjects using a web-based institutional clinical research query tool to search for International Classification of Diseases, Revision 10 (ICD-10) for SCD admissions (D57.20, D57.21, D57.211, D57.212, D57.211, D57.212, D57.219, D57.411, D57.412, D57.419, D57.8, D57.811, D57.812, D57.819) and pharmacy orders for ketamine. We then manually reviewed the query to select for subjects with VOC admissions which excluded 60 patients. Seven patients who received only ketamine boluses without infusions and/or received ketamine for other indications (e.g., post-surgical pain) were excluded. Twelve patients with incomplete pain scores and/or opioid analgesic records were also excluded (Figure 1). For patients with more than one qualifying admission, the most recent admission was selected for data collection. Due to the retrospective nature of this study, all treatment decisions and disposition plans were made at the discretion of the clinical team.
Figure 1.
Flowchart depicting process for patient selection for the study.
Patient characteristics, such as sex, age, genotype, VOC frequency, and ketamine infusion characteristics were collected via manual review of the electronic medical record (EMR) by a single reviewer. Baseline pain scores are defined as the average pain level experienced by an individual patient outside of a VOC. Pain scores (baseline and during admission) were reported by patients to nursing or physician providers using 0–10 numeric pain rating (NPR) scale. NPR scores and daily opioid consumption were collected via a data scrapper built in Python 3.9.1 (Python Software Foundation; Beaverton, Oregon, USA). Data collected by the data scrapper tool were then sent through a de-identification script written in Python 3.9.2. Pain scores and daily opioid use were also manually reviewed in the EMR and compared to the corresponding data retrieved from the data scrapper for 15% of the patients to ensure for accuracy. All data were stored securely in the Research Electronic Data Capture (REDCap) platform [14] hosted at University of North Carolina Chapel Hill (UNCCH). This study was approved by UNCCH and Duke University’s Institutional Review Boards.
Pain Management and Ketamine Administration
At Duke University, analgesic management for VOCs, including opioid patient-controlled analgesia (PCA) dosing strategy, is usually dictated by a patient’s individualized care plan (ICP). The ICPs provide guidance for the details of patients’ opioid therapy—that is specific opioid type, dose, frequency, mode of delivery—based on patients’ prior history and admissions. The plans are embedded in the EMR and are accessible to the treating providers. Weight-based PCA or intermittent opioid dosing is used for patients who do not have an ICP due to a lack of prior admissions to Duke.
Duke University uses a standard institutional protocol that provides guidance for ketamine administration. Any provider may order or initiate ketamine with the approval and recommendation of the inpatient pain service (IPS). A consult with the IPS must be obtained within 24 hours of initiation of ketamine. A standard infusion concentration of 2 mg/mL (500 mg/250 mL) is used, and infusions are administered via locked infusion pumps. For adults, the typical starting dose is 0.25 mg/kg/hour with a dosing range of 0.1–0.35 mg/kg/hour. Infusion rates are titrated based on clinical response and can be increased every 8–12 hours with a physician order. Patient monitoring includes respiratory rate, oxygenation saturation, heart rate, and pain and sedation scores. Infusion orders typically expire after 5 days, and an IPS provider must authorize infusions of longer duration. Weaning of infusions is at the discretion of IPS providers with consideration for the indication for the infusion, duration, and patient’s readiness for discharge.
Overall disposition plans for SCD patients are at the discretion of the treating inpatient SCD provider. However, in general, providers consider the following factors in assessing readiness for hospital discharge: (1) improvement in overall pain, (2) baseline pain prior to admission, (3) ability for timely outpatient follow-up, and (4) overall clinical improvement.
Outcomes
The primary outcome was change in daily opioid consumption (measured as oral morphine milligram equivalents (MME) per day) from baseline (the day immediately prior to ketamine initiation) to four different time points: 1, 2, and 3 days after initiation of ketamine infusion and the day after discontinuation of the infusion. The total daily opioid consumed was converted to MME using an online opioid conversion calculator (https://globalrph.com/medcalcs). The secondary outcome was change in self-reported numeric pain rating (NPR) from baseline to the four different time points, as defined previously.
Statistical Analysis
Based on sample size and non-normal distributions of data, nonparametric analyses were determined as the most appropriate for this study. A Wilcoxon Signed-Rank test was used to determine significant differences in median MME or numeric pain ratings at the four defined time points (1, 2, and 3 days after initiation of ketamine infusion and the day after discontinuation of the infusion), each time point compared to baseline. Differences in both baseline MME and numeric pain scores at these time points were calculated (baseline vs 1, 2, and 3 days after initiation of ketamine infusion and the day after discontinuation of the infusion) and used as endpoints in subsequent analyses. To determine associations with categorical patient and infusion characteristics with these endpoints, two-sample Wilcoxon Rank-Sum tests were used with Hodges-Lehmann estimators for the shift parameter and 95% confidence interval. A Spearman Rank Correlation test was performed between age (a continuous variable) and MME or pain score differences, with 95% Fisher transformation confidence interval for the Spearman correlation coefficient. Due to the number of statistical tests being performed, statistical significance was assessed at P < .0125 after a Bonferroni correction to account for k = 4 significance tests per endpoint and a subgroup variable. Scatter plots were created to visualize distributions of MME and pain score differences across patient and infusion characteristics. SAS OnDemand for Academics (SAS Institute Inc.; Cary, North Carolina, USA) was utilized for all statistical analyses, while Python 3.7.1.2 with seaborn data visualization library was used to create scatter plots for the study.
Results
Patient Characteristics
We identified a total of 56 patients admitted for VOCs who received sub-anesthetic ketamine infusions during their hospital course. Forty-five (80%) had sickle cell anemia (HbSS), and slightly more than half (54%) were males. The median age of the patients was 30 years. A majority (94%) were on opioid therapy prior to admission, with a baseline median NPR of 4 out of 10 and a median of four sickle-cell related hospitalizations within the year of the hospitalization of interest. Median length of stay for the hospitalization of interest was 12 days (Table 1).
Table 1.
Patient and infusion characteristics
| Characteristic | N (%) |
|---|---|
| Sex | |
| Female | 26 (46%) |
| Male | 30 (54%) |
| Age (years), median (25th–75th) | 30 (26–36) |
| Genotype | |
| HbSS | 45 (80%) |
| HbSC | 6 (11%) |
| HbS/β0 thalassemia | 3 (5%) |
| HbS/β+ thalassemia | 0 |
| Other | 2 (4%) |
| Pre-admission analgesics | |
| Opioids | 49 (94%) |
| NSAIDs | 2 (4%) |
| Acetaminophen | 0 (0%) |
| Antidepressants | 8 (15%) |
| Anticonvulsants (Gabapentin, Pregabalin) | 17 (32%) |
| Benzodiazepine | 1 (2%) |
| Clonidine | 2 (4%) |
| Hydroxyurea therapy | |
| Yes | 53 (95%) |
| No | 3 (5%) |
| Hospitalization year prior to admission median (25th–75th) | 4 (2–7) |
| Preadmission (baseline) pain NPR* | 4 (3–7) |
| Hospitalization duration | 12 (7–15) |
| Ketamine infusion | |
| Early initiation (3 days or less) | 23 (41%) |
| Late initiation (more than 3 days) | 33 (59%) |
| Duration (days), median (25th–75th) | 3 (2–5) |
Baseline characteristics of SCD patients who received ketamine infusion during their VOC hospitalization.
NPR = numeric pain rating.
Infusion Characteristics
Of the 56 patients, 23 (41%) were treated with ketamine infusions within 3 days of their hospitalization. The median NPR immediately prior to the initiation of the infusion was 9 out of 10. Median duration of the infusion was 3 days. There were no records of significant complications, such as respiratory depression or hemodynamic changes requiring intervention.
Outcomes
Our primary and secondary outcomes were changes in opioid consumption and numeric pain rating respectively at four different time points: 1, 2, and 3 days after initiation of ketamine infusion and the day after discontinuation of the infusion. However, not all 56 patients were hospitalized long enough to provide assessment at each of these defined time points (1-day N = 56, 2-day N = 43, 3-day N = 36, 1-day after discontinuation N = 33). A significant reduction in pain rating was noted for 1 and 2 days after initiation of the ketamine infusion (P = .0013 and P = .0121 respectively, Table 2) but did not persist for 3 days after initiation of the infusion. However, ketamine infusions were not associated with a significant reduction in opioid consumption, as measured by MME, at the observed time points (Table 2).
Table 2.
Change in opioid consumption and pain ratings from baseline: median difference (P values)
| Outcomes | +1 Day | +2 Days | +3 Days | +1 Day After Discontinuation |
|---|---|---|---|---|
| MME† | −1.4 (0.5560) | −12.0 (0.6432) | 9.7 (0.7545) | −12.0 (0.3655) |
| Numeric pain rating | −0.54 (0.0013)* | −0.34 (0.0121)* | −0.58 (0.0157) | −0.56 (0.0071)* |
Median differences in opioid consumption (as measured by oral morphine milligram equivalents (MME) per day) scores and self-reported numeric pain rating (NPR) for each day compared to baseline (1 day before infusion start). Negative values indicate reduction, while positive values indicate increase. P values from Wilcoxon Signed-Rank Test. Median and confidence intervals (CI) are Hodges-Lehmann estimates for each day compared to baseline (one day before infusion start). With Bonferroni correction, P values are significant at P < .0125.
Statistically significant values (P < .0125).
MME = morphine milligram equivalents.
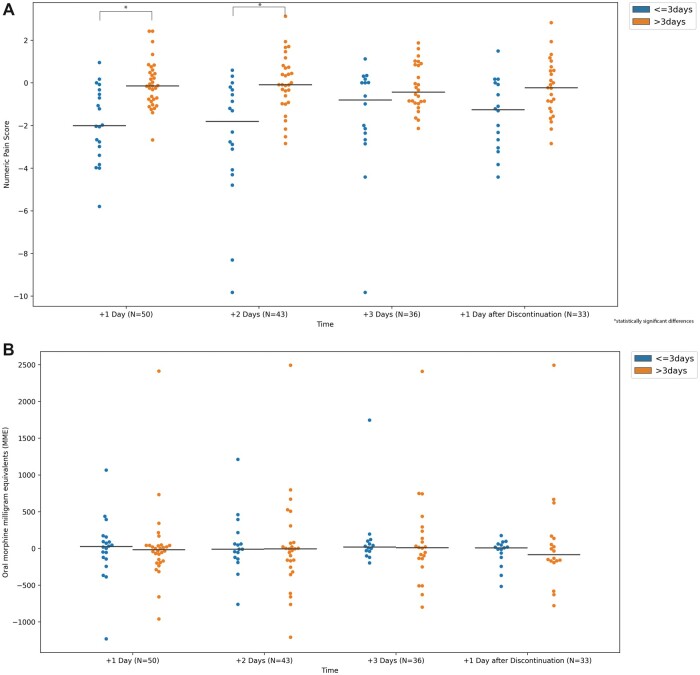
We examined the association of various patient and infusion characteristics with reductions in opioid consumption and pain ratings. No associations were noted with respect to patient age, sex, or healthcare utilization (Tables 3A and 3B). With respect to ketamine infusion characteristics, we did not observe a significant reduction in median NPR or MME in association with the duration of infusion. However, greater median reductions in NPR were observed for early (≤3 days of hospitalization) vs late (>3 days of hospitalization) initiation of ketamine infusion at the first and second days after the initiation of the infusion (P = .0003 and P = .0006, Table 3B). This corresponded to a percent reduction in median NPR of 24.5% and 25.9% for these two time points, respectively, for early ketamine initiation compared to 2% and 1.2% reduction in median NPR for late ketamine initiation. This statistically significant reduction in median NPR did not persist for 3 days after infusion initiation or following the discontinuation of the infusion (Tables 3B), although the overall trend was toward a reduction in NPR (Figure 2). A statistically significant association, however, was not noted with opioid consumption (Table 3A).
Table 3A.
Changes in opioid consumption (MME) scores based on patient and infusion characteristics: median difference (confidence intervals)
| +1 Day (N = 50) | +2 Days (N = 43) | +3 Days (N = 36) | +1 Day After Discontinuation (N = 33) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age† | 0.04 (−0.24, 0.32) | 0.25 (−0.05, 0.51) | 0.02 (−0.31, 0.35) | 0.20 (−0.15, 0.51) |
| Sex (females vs males) | 3.55 (−127.20, 123.80) | −99.95 (−357.20, 76.00) | 79.50 (-84.00, 288.20) | −27.95 (-199.50, 131.60) |
| Health care utilization within 1 year (3+ vs <3 admissions) | 71.35 (−80.0, 264.28) | −0.32 (−78.92, 503.83) | 58.69 (-142.00, 431.20) | 158.00 (-9.20, 431.40) |
| Infusion characteristics | ||||
| Early vs late (≤3 of hospitalization or >3 of hospitalization) | 46.84 (−90.00, 167.00) | 40.00 (−136.50, 301.00) | 33.46 (−134.80, 212.40) | 36.10 (−179.20, 186.00) |
| Infusion duration (<24 hr vs >24 hr) | −121.10 (−242.00, 47.00) | −18.80 (−270.29, 211.20) | 85.00 (−129.80, 394.06) | −102.05 (−247.20, 100.20) |
Changes in opioid consumption (as measured by oral morphine milligram equivalents (MME) per day) scores by subgroup. Negative values indicate reduction, while positive values indicate increase. P values from Wilcoxon Rank-Sum Test. Median and confidence intervals (CI) are Hodges-Lehmann estimates for each day compared to baseline (one day before infusion start). †Estimate for age is Spearman rho correlation and 95% Fisher transformation confidence interval. With Bonferroni correction, P values are significant at P < .0125.
† Spearman correlation coefficients with 95% Fisher's Transformation Confidence Intervals.
No significant associations were found.
Table 3B.
Changes in numeric pain rating based on patient and infusion characteristics: median difference (confidence intervals)
| +1 Day (N = 50) | +2 Days (N = 43) | +3 Days (N = 36) | +1 Day After Discontinuation (N = 33) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age† | −0.06 (−0.33, 0.21) | 0.03 (−0.26, 0.31) | 0.09 (−0.22, 0.39) | 0.06 (−0.25, 0.36) |
| Sex (females vs males) | −0.55 (−1.38, 0.23) | 1.07 (0.19, 2.15) | −0.35 (−1.30, 0.50) | −0.42 (−1.42, 0.67) |
| Health care utilization within one year (3+ vs <3 admissions) | 0.13 (−0.74, 1.02) | −0.07 (−1.12, 1.02) | 1.11 (0.22, 1.94) | 1.10 (0.08, 2.13) |
| Infusion characteristics | ||||
| Early vs late (≤3 of hospitalization or >3 of hospitalization) | −1.81 (−2.74, −0.84)* | −1.96 (−3.29, −0.78)** | −0.94 (−1.88, 0.21) | −1.19 (−2.35, −0.20) |
| Infusion duration (<24 hr vs >24 hr.) | −0.04 (−1.08, 1.00) | 0.65 (−0.73, 1.98) | 0.69 (−0.60, 1.98) | 0.04 (−1.22, 1.22) |
Changes in self-reported numeric pain rating (NPR) by subgroup. Negative values indicate reduction, while positive values indicate increase. P values from Wilcoxon Rank-Sum Test. Median and confidence intervals (CI) are Hodges-Lehmann estimates for each day compared to baseline (1 day before infusion start).
Estimate for age is Spearman rho correlation and 95% Fisher transformation confidence interval. With Bonferroni correction, P values are significant at P < 0.125.
Statistically significant values (P = .0003).
Statistically significant values (P = .0006).
† Spearman correlation coefficients with 95% Fisher's Transformation Confidence Intervals.
Figure 2.
Changes in median opioid consumption (as measured by oral morphine milligram equivalents (MME) per day) and median numeric pain rating (NPR) based on timing of ketamine infusion (≤ 3 days of hospitalization or > 3 days of hospitalization). Negative values indicate reduction, while positive values indicate increase. Horizontal bar indicates median change in numeric pain score.
We also examined the data distribution based on the previously mentioned patient and infusion characteristics (Figure 2). Excluding a few outliers, the data appeared to be evenly distributed for NPRs and MMEs when patient characteristics—age, sex, and healthcare utilization—and ketamine infusion duration were assessed. However, the data for patients who were initiated on ketamine early (within 3 days of their hospitalization) showed a strong trend toward overall reduction in NPR (Figure 2A).
Discussion
In recent years, there has been heighted interest in non-opioid alternatives and adjuncts for treatment of acute sickle cell pain. Guidelines by the American Society for Hematology include ketamine as a late adjunct for treatment of VOC pain judged by the provider to be refractory to first-line agents. However, the evidence for ketamine usage in SCD remains of low certainty [15]. There are unanswered questions regarding the timing of ketamine administration during the VOC course and the characteristics of SCD patients who would most benefit from low-dose ketamine infusions. Our study, to our knowledge, is the largest descriptive analysis of adult SCD patients treated with sub-anesthetic ketamine infusions for a VOC. We aimed to assess changes in opioid consumption and pain intensity for SCD patients started on ketamine early vs late in their hospital course.
In this study, we found that early initiation of ketamine was associated with a greater reduction in self-reported NPRs, specifically, a nearly 2-point or 25% reduction in the median pain ratings on first and second day after the initiation of the infusion. Two studies suggest that a clinically significant difference of 13 mm on the 100 mm Visual Analog Scale constitutes a clinically significant pain intensity difference among SCD patients in the emergency department with a VOC [16, 17]. Translating those reductions to clinically meaningful reductions as measured by NPRs in SCD has not been done. However, consensus guidelines from the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT) state that a 1-point or 15–20% reduction is “minimally important” and greater than a 2-point or 30% reduction to is a “meaningful” decrease in pain intensity [18]. Although these recommendations are for chronic pain clinical trials, they can be applied to SCD, given that it is a chronic painful disease in which a significant proportion of patients experience daily chronic pain. So we conclude our observed reduction in median pain ratings as valid and clinically meaningful.
In current clinical practice, ketamine is usually only initiated once acute sickle cell pain is refractory to standard opioid therapy [15]. This means that SCD patients can experience poorly controlled, severe pain and receive high dose opioids for several, if not many, days, before an adjunct such as ketamine is trialed. Waiting for a prolonged period can make obtaining optimal pain management challenging, even with the addition of non-opioid adjuncts. Our results provide some of the earliest evidence that it is reasonable for SCD clinicians to consider a trial of ketamine infusion early in a patient’s hospitalization, rather than wait for refractory pain.
Another retrospective study assessing pediatric SCD patients noted that younger patients and males had a greater reduction in pain scores than older patients and females when placed on sub-anesthetic ketamine infusions for VOCs [12]. Our study did not find differences in opioid consumption and NPR based on patient characteristics, such as sex, age, or healthcare utilization. Unlike our study, this pediatric study had a larger sample size and included more than one hospitalization per patient.
We did not observe an association of early ketamine administration with a reduction in opioid consumption. In general, because daily opioid consumption was obtained directly from data automatically recorded in patient-controlled analgesia (PCA) devices or medication administration records in the EMR, our data is reliable. However, our patients in this study received large boluses of opioids from their PCAs or intermittent intravenous doses. Thus, small changes in opioid usage may not be detected, especially given our small sample size.
The results of our study should be interpreted within the context of some limitations. First, data were collected retrospectively from a single medical institution; therefore, conclusions regarding the effectiveness of sub-anesthetic or low-dose ketamine infusions for VOCs cannot be made. Second, treatment decisions, including timing for the initiation of ketamine and the duration of the infusion, were at the discretion of the attending and consulting physicians. Third, because the data was collected retrospectively, we cannot account for the potential confounders, such as effect of other analgesic adjuncts. Fourth, our study is limited by a relatively small sample size. Lastly, pain is a multidimensional experience that impacts physical function, affect/mood, and sleep. While self-reported pain scores are the gold standard for assessing analgesic responsiveness in acute SCD pain management, comprehensive assessment of these other domains are also needed. As this was a retrospective study, other measures of improvement in pain, such as improvement in physical function, affect/mood, and sleep could not be reliably assessed.
While subject to these limitations, our study provides informative data regarding the use of low-dose or subanesthetic ketamine infusions in SCD patients. To our knowledge, this is the largest descriptive analysis of adult sickle cell patients who were treated with sub-anesthetic ketamine infusions as an adjunctive analgesic for VOCs. And, although there are no published randomized controlled trials (RCT) of low-dose ketamine infusions for VOC treatment, there are two RCTs which report that single boluses of ketamine for an acute VOC, when administered during a SCD patient’s presentation to the emergency department, can lead to reductions in pain ratings and opioid consumption [10, 11]. These studies along with ours imply that early initiation of sub-anesthetic ketamine infusions may led to greater SCD pain reduction. However, it is important to highlight that ketamine infusions, rather than single ketamine bolus, have greater analgesic and opioid sparing properties [9]. Multicenter prospective RCTs are required to further investigate the analgesic and opioid-sparing benefits of sub-anesthetic ketamine infusions for VOCs. Future studies may include prospective responder analyses that investigate the proportion of subjects who have met a clinically significant reduction in NRS and/or MME. If proven effective in the future, early administration of sub-anesthetic ketamine infusions, that is at the initial phase of a VOC when a patient presents to a sickle cell infusion center or day hospital, could decrease both the economic burden of SCD and reduce patient suffering from poorly controlled pain and opioid related harms. As we wait for multicenter RCTs, it is important for sickle cell clinicians to consider trialing ketamine early during a patient’s hospitalization.
Contributor Information
Martha O Kenney, Division of Pediatric Anesthesiology, Department of Anesthesiology, The University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA; Department of Pediatrics, The University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
Benjamin Becerra, Center for Health Equity, Department of Information & Decision Sciences, California State University, San Bernardino, California, USA.
Arvind Mallikarjunan, Department of Pediatric Hematology/Oncology, Duke University Medical Center, Durham, North Carolina, USA.
Nirmish Shah, Department of Pediatric Hematology/Oncology, Duke University Medical Center, Durham, North Carolina, USA.
Wally R Smith, Division of General Internal Medicine, Department of Internal Medicine, Virginia Commonwealth University School of Medicine, Richmond, Virginia, USA.
Data Availability Statement
The data and supporting materials are available from corresponding author upon request.
Ethics of Approval Statement
This study was approved by Duke University’s Institutional Review Board.
Funding sources: M.O.K. was supported by funding from the National Heart, Lung, and Blood Institute’s Programs to Increase Diversity Among Individuals engaged in Health-Related Research (PRIDE): R25HL106365 and funding from University of North Carolina, Chapel Hill’s Simmons Scholars Program and Children’s Hospital Foundation.
Conflicts of interest: Dr. Kenney has current research funding from Global Blood Therapeutics. Dr. Shah reports receiving fees from Novartis, Global Blood Therapeutics, Forma, and Alexion. Dr. Smith has funding from the National Heart, Lung, and Blood Institute (NHLBI), Health Resources and Services Administration (HRSA), Patient-Centered Outcomes Research Institute, Pfizer, Novartis, Emmaus Life Sciences, Imara Inc., and Shire Pharmaceuticals. He serves as a consultant for Novartis, Pfizer, Global Blood Therapeutics and Emmaus Life Sciences. No other authors report potential conflicts of interest.
References
- 1. Elmariah H, Garrett ME, De Castro LM, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol 2014;89(5):530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148(2):94–101. [DOI] [PubMed] [Google Scholar]
- 3. Lanzkron S, Carroll CP, Haywood C Jr.. The burden of emergency department use for sickle-cell disease: An analysis of the national emergency department sample database. Am J Hematol 2010;85(10):797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darbari DS, Sheehan VA, Ballas SK.. The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management. Eur J Haematol 2020;105(3):237–46. [DOI] [PubMed] [Google Scholar]
- 5. Carroll CP. Opioid treatment for acute and chronic pain in patients with sickle cell disease. Neurosci Lett 2020;714:134534. [DOI] [PubMed] [Google Scholar]
- 6. Machelska H, Celik M.. Advances in achieving opioid analgesia without side effects. Front Pharmacol 2018;9:1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rivat C, Ballantyne J.. The dark side of opioids in pain management: Basic science explains clinical observation. Pain Rep 2016;1(2):e570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pak DJ, Yong RJ, Kaye AD, Urman RD.. Chronification of pain: Mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep 2018;22(2):9. [DOI] [PubMed] [Google Scholar]
- 9. Schwenk ES, Viscusi ER, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018;43:456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alshahrani MS, AlSulaibikh AH, ElTahan MR, et al. Ketamine administration for acute painful sickle cell crisis: A randomized controlled trial. Acad Emerg Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lubega FA, DeSilva MS, Munube D, et al. Low dose ketamine versus morphine for acute severe vaso occlusive pain in children: A randomized controlled trial. Scand J Pain 2018;18(1):19–27. [DOI] [PubMed] [Google Scholar]
- 12. Nobrega R, Sheehy KA, Lippold C, Rice AL, Finkel JC, Quezado ZMN.. Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res 2018;83(2):445–54. [DOI] [PubMed] [Google Scholar]
- 13. Sheehy KA, Lippold C, Rice AL, Nobrega R, Finkel JC, Quezado ZM.. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: A single-center cohort study. J Pain Res 2017; 10:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: Management of acute and chronic pain. Blood Adv 2020;4(12):2656–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Todd KH, Funk KG, Funk JP, Bonacci R.. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27(4):485–9. [DOI] [PubMed] [Google Scholar]
- 17. Lopez BL, Flenders P, Davis-Moon L, Corbin T, Ballas SK.. Clinically significant differences in the visual analog pain scale in acute vasoocclusive sickle cell crisis. Hemoglobin 2007;31(4):427–32. [DOI] [PubMed] [Google Scholar]
- 18. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and supporting materials are available from corresponding author upon request.