Key Points
Question
Does once-daily oral verapamil preserve pancreatic beta cell function in children and adolescents with newly diagnosed type 1 diabetes?
Findings
In a randomized clinical trial including 88 children and adolescents with newly diagnosed type 1 diabetes, C-peptide levels (a measure of pancreatic beta cell function) measured during a mixed-meal tolerance test 52 weeks after diagnosis were 30% higher with verapamil compared with placebo. The percentage of participants with a 52-week peak C-peptide level of 0.2 pmol/mL or greater was 95% in the verapamil group vs 71% in the placebo group. Verapamil had few adverse events.
Meaning
Verapamil partially preserved stimulated C-peptide secretion at 52 weeks compared with placebo and was well tolerated in children and adolescents with newly diagnosed type 1 diabetes.
Abstract
Importance
In preclinical studies, thioredoxin-interacting protein overexpression induces pancreatic beta cell apoptosis and is involved in glucotoxicity-induced beta cell death. Calcium channel blockers reduce these effects and may be beneficial to beta cell preservation in type 1 diabetes.
Objective
To determine the effect of verapamil on pancreatic beta cell function in children and adolescents with newly diagnosed type 1 diabetes.
Design, Setting, and Participants
This double-blind, randomized clinical trial including children and adolescents aged 7 to 17 years with newly diagnosed type 1 diabetes who weighed 30 kg or greater was conducted at 6 centers in the US (randomized participants between July 20, 2020, and October 13, 2021) and follow-up was completed on September 15, 2022.
Interventions
Participants were randomly assigned 1:1 to once-daily oral verapamil (n = 47) or placebo (n = 41) as part of a factorial design in which participants also were assigned to receive either intensive diabetes management or standard diabetes care.
Main Outcomes and Measures
The primary outcome was area under the curve values for C-peptide level (a measure of pancreatic beta cell function) stimulated by a mixed-meal tolerance test at 52 weeks from diagnosis of type 1 diabetes.
Results
Among 88 participants (mean age, 12.7 [SD, 2.4] years; 36 were female [41%]; and the mean time from diagnosis to randomization was 24 [SD, 4] days), 83 (94%) completed the trial. In the verapamil group, the mean C-peptide area under the curve was 0.66 pmol/mL at baseline and 0.65 pmol/mL at 52 weeks compared with 0.60 pmol/mL at baseline and 0.44 pmol/mL at 52 weeks in the placebo group (adjusted between-group difference, 0.14 pmol/mL [95% CI, 0.01 to 0.27 pmol/mL]; P = .04). This equates to a 30% higher C-peptide level at 52 weeks with verapamil. The percentage of participants with a 52-week peak C-peptide level of 0.2 pmol/mL or greater was 95% (41 of 43 participants) in the verapamil group vs 71% (27 of 38 participants) in the placebo group. At 52 weeks, hemoglobin A1c was 6.6% in the verapamil group vs 6.9% in the placebo group (adjusted between-group difference, −0.3% [95% CI, −1.0% to 0.4%]). Eight participants (17%) in the verapamil group and 8 participants (20%) in the placebo group had a nonserious adverse event considered to be related to treatment.
Conclusions and Relevance
In children and adolescents with newly diagnosed type 1 diabetes, verapamil partially preserved stimulated C-peptide secretion at 52 weeks from diagnosis compared with placebo. Further studies are needed to determine the longitudinal durability of C-peptide improvement and the optimal length of therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT04233034
This randomized clinical trial compares the effect of verapamil on pancreatic beta cell function vs placebo in children and adolescents with newly diagnosed type 1 diabetes.
Introduction
Type 1 diabetes, an autoimmune condition resulting in the destruction of insulin-producing pancreatic beta cells, affects more than 1 million people in the US.1 Maintenance of even modest residual beta cell function, which is assessed by stimulated C-peptide secretion, is a desirable goal and is associated with lower risk of diabetes-related vascular complications and hypoglycemia.2,3
Over the past 3 decades, a variety of approaches aimed at preserving C-peptide secretion after the diagnosis of type 1 diabetes have been tested. A small number of medications have shown efficacy in preserving C-peptide secretion in newly diagnosed patients with type 1 diabetes when tested in sufficiently powered randomized clinical trials.4,5 Despite beneficial effects on beta cell function and insulin secretion, only 1 medication has advanced to approval by the US Food and Drug Administration, and thus far none has achieved widespread clinical implementation. Teplizumab was recently approved for the treatment of stage 2 type 1 diabetes (positive diabetes-related autoantibodies with evidence of dysglycemia), but not for the treatment of stage 3 type 1 diabetes (new-onset, clinically evident). To date, the majority of disease modification strategies for type 1 diabetes have focused on targeting immune responses.4
In recent years, a growing number of pathways intrinsic to the beta cell have been linked with type 1 diabetes pathophysiology. Thioredoxin-interacting protein overexpression has been shown to induce pancreatic beta cell apoptosis and be involved in glucotoxicity-induced beta cell death in culture and mouse models.6 Calcium channel blockers, such as verapamil, reduce thioredoxin-interacting protein expression and beta cell apoptosis7,8,9 and may be beneficial in beta cell preservation after a type 1 diabetes diagnosis. In 2018, a placebo-controlled, pilot and feasibility randomized clinical trial was published10 that found a 35% relative increase in stimulated C-peptide levels after 12 months in 11 adults with newly diagnosed type 1 diabetes treated with oral verapamil compared with 13 adults taking placebo.
We conducted a double-blind, placebo-controlled randomized clinical trial of children and adolescents aged 7 years to 17 years with newly diagnosed stage 3 (clinically apparent) type 1 diabetes to assess the safety and efficacy of verapamil in preserving beta cell function 12 months after diagnosis.
Methods
Trial Conduct and Oversight
The trial was conducted at 6 pediatric diabetes centers in the US. The trial protocol was approved by the Jaeb Center for Health Research institutional review board (the trial protocol and statistical analysis plan appear in Supplement 1). Written informed consent was obtained from a parent or legal guardian and assent was obtained from the participants (however, if they turned 18 years of age during the trial, the participants signed the informed consent form). An independent data and safety monitoring board provided trial oversight. The trial protocol included a factorial design and also evaluated the effect of intensive diabetes management using an automated insulin delivery system vs standard diabetes management on beta cell function, which is reported separately.11
Trial Design and Participants
Eligible participants were aged 7 years to 17 years with type 1 diabetes diagnosed within 31 days of randomization, had at least 1 positive islet autoantibody, weighed 30 kg or greater, and had no contraindication to use of verapamil (complete inclusion and exclusion criteria appear in eTable 1 in Supplement 2 and an overview of the study procedures at the screening, randomization, and follow-up visits appears in eTable 2 in Supplement 2). The lower weight limit was due to verapamil dosing constraints.
Using a balanced factorial design (1:1:1:1), each participant was randomly assigned to the verapamil group or the placebo group and also to receive either intensive diabetes management with an automated insulin delivery system (from either Tandem Diabetes Care or Medtronic) or standard care diabetes management (Figure 1). Standard care diabetes management, which included use of a continuous glucose monitor (Dexcom G6), was determined by the participant and their health care provider. The computer-generated randomization schedule was stratified by site and used a permuted-block design.
Figure 1. Recruitment, Randomization, and Follow-up of Participants in a Trial of Verapamil vs Placebo for the Preservation of Beta Cell Function in Children and Adolescents With Newly Diagnosed Type 1 Diabetes.
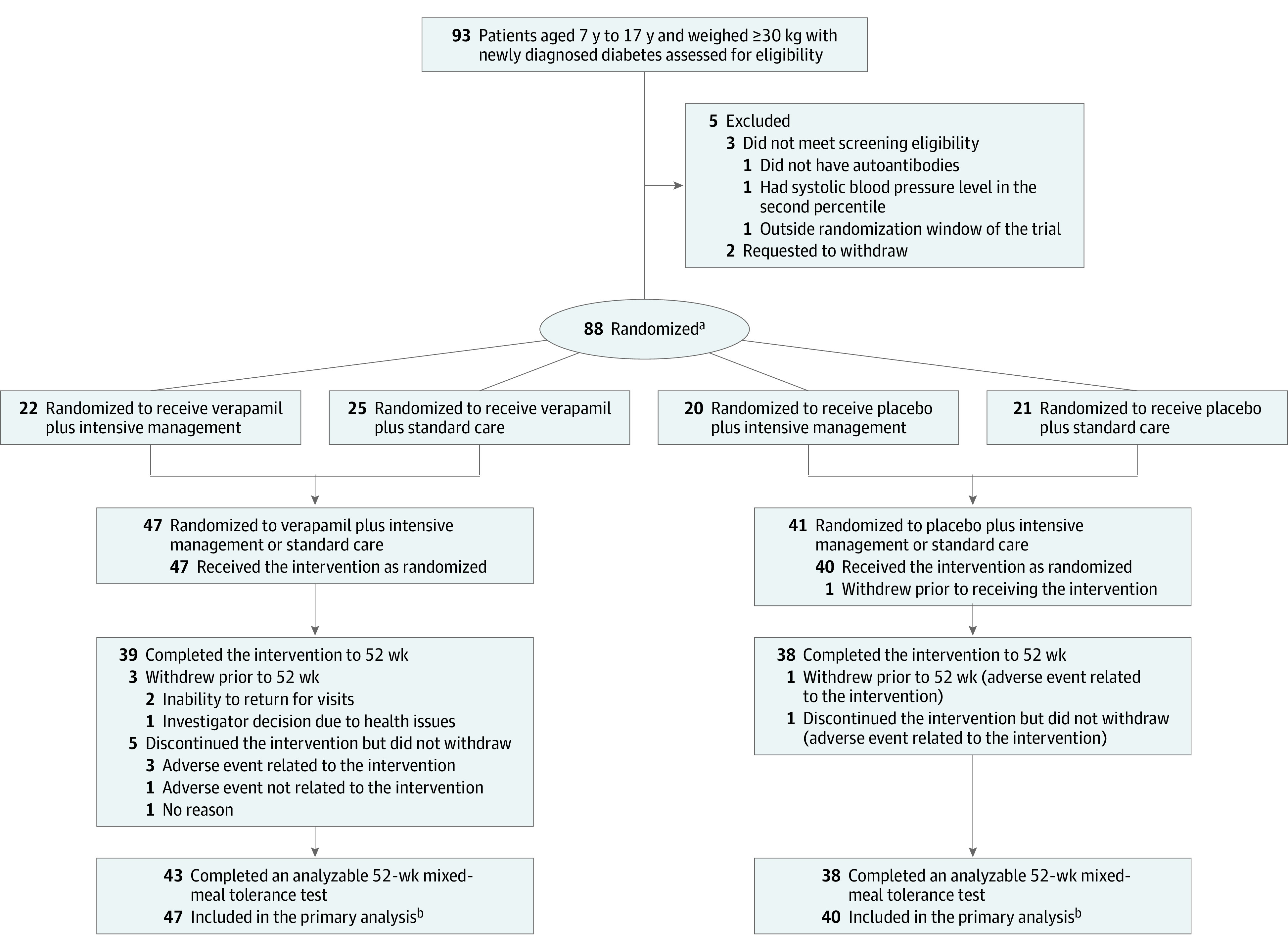
aRandomization was stratified by site with a permuted block design. Intensive diabetes management included an automated insulin delivery system.
bHad at least 1 analyzable area under the curve value for C-peptide level (a measure of pancreatic beta cell function) stimulated by a mixed-meal tolerance test. One participant in the placebo group who dropped out immediately after randomization before completing the baseline mixed-meal tolerance test was not included in the primary analysis.
An extended-release formulation of verapamil was used. The drug dose was dependent on each participant’s weight and was started with 60 mg/d or 120 mg/d. The dose was escalated at 2- to 4-week intervals to a maximum of dose of 360 mg/d for participants weighing more than 50 kg. The dose was held, decreased, or discontinued if adverse events occurred. Safety visits occurred approximately 1 week after initiation of the study drug and after each dose increase to measure blood pressure and pulse. Levels of liver enzymes (alanine transaminase and aspartate aminotransferase) were measured and an electrocardiogram was performed at screening and at the 6-week, 26-week, and 52-week visits. Adherence was assessed with pill counts based on the bottles dispensed and returned.
Participants completed a follow-up visit 6 weeks after randomization and then at 13, 26, 39, and 52 weeks from the time of type 1 diabetes diagnosis. At randomization and at most follow-up visits (except at 6 weeks), blood was drawn for central laboratory measurement of hemoglobin A1c using the Tosoh G8 HPLC Analyzer and a 2-hour mixed-meal tolerance test was performed for central laboratory measurements of C-peptide levels using the Roche Cobas e801 unit.
Race and ethnicity information were collected to characterize the cohort. Race information was solicited from the participant in response to this question: “Which of the following racial designations best describes you?” The participant could select race from fixed categories, which allowed for the reporting of more than 1 race. Hispanic ethnicity information was solicited from the participant in response to this question: “Do you consider yourself to be Hispanic/Latino or not Hispanic/Latino?”
Outcomes
The primary outcome was area under the curve (AUC) values for C-peptide level (a measure of pancreatic beta cell function) stimulated by a mixed-meal tolerance test at 52 weeks from diagnosis of type 1 diabetes. The secondary outcomes included (1) peak C-peptide level and the proportion with a peak C-peptide level of 0.2 pmol/mL or greater; (2) hemoglobin A1c levels; and (3) the following glucose metrics measured with continuous glucose monitoring12 during the 28 days prior to each visit: mean glucose concentration and percentage of time with glucose concentration in the range of 70 to 180 mg/dL or 70 to 140 mg/dL and percentage of time with a glucose concentration greater than 180 mg/dL, greater than 250 mg/dL, less than 70 mg/dL, or less than 54 mg/dL. The safety outcomes included severe hypoglycemia and diabetic ketoacidosis.
Sample Size and Power Calculation
The sample size was calculated to be 98 participants to provide 90% power with a 2-sided type I error rate of 5% to detect a 52-week treatment difference in C-peptide AUC between the verapamil group and the placebo group, assuming the true relative between-group difference in the target population was 50% and a dropout rate of no more than 10% (additional information appears in the eStatistical Methods in Supplement 2). Recruitment was affected by the COVID-19 pandemic and was stopped after 88 participants were randomized, providing 88% statistical power with the lower than expected dropout rate of 5.7%. This recruitment decision was related to funding considerations and was made without knowledge of the treatment effect.
Statistical Analysis
Participants were analyzed in the group to which they were assigned by randomization regardless of the actual treatment received. All participants were included in the primary and secondary analyses unless otherwise indicated. Treatment group comparisons for the primary C-peptide outcome included all randomized participants with at least 1 analyzable C-peptide AUC value (at baseline or follow-up) and were made with a constrained longitudinal analysis by fitting the log(AUC + 1) for each mixed-meal tolerance test as the outcome and testing for the drug use indicator (verapamil or placebo), controlling for age, time from diagnosis to randomization, and assignment to intensive diabetes management or standard diabetes management. The backtransformation of the mean value, exp(y) − 1, was reported as the geometriclike mean. The primary outcome was tested for a possible device × drug interaction using the same model described above with the inclusion of a drug × type of diabetes management interaction term.
Missing data were handled using the direct likelihood method, which maximizes the likelihood function integrated over the possible values of the missing data. Additional sensitivity analyses handled missing data by using the multiple imputation method of Rubin,13 using multiple imputation with a pattern mixture model, and using available cases only. Another sensitivity analysis was performed that included Tanner stage and body mass index percentile as covariates in the model assessing the primary outcome because of imbalances between the treatment groups at randomization. A per-protocol analysis was performed using the same method as the primary analysis in which the analysis cohort was restricted to participants in the verapamil group and in the placebo group who took at least 80% of the prescribed study drug (based on pill counts) and who did not have data missing for 52-week C-peptide level.
Except for the primary analysis, the P values were adjusted to control the false discovery rate using the 2-stage Benjamini-Hochberg procedure. A 2-tailed P = .05 was the threshold for statistical significance for all analyses. Additional details appear in the eStatistical Methods in Supplement 2. The analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Participants and Follow-up
Between July 20, 2020, and October 13, 2021, 88 participants were randomly assigned to the verapamil group (n = 47) or the placebo group (n = 41) (Figure 1). The ages of the participants ranged from 7.5 years to 17.9 years (mean age, 12.7 years [SD, 2.4 years]); 36 were female (47%). Of the 88 participants, 3 (3%) identified as Black or African American, 79 (90%) identified as White, and 4 (5%) identified as having multiple races. Fifteen participants (17%) identified as being of Hispanic ethnicity (Table 1). The mean time from type 1 diabetes diagnosis to randomization was 24 days (SD, 4 days).
Table 1. Baseline Characteristics.
| Verapamil (n = 47) | Placebo (n = 41) | |
|---|---|---|
| Age, y | ||
| Mean (SD) | 13.1 (2.6) | 12.3 (2.1) |
| Median (IQR) | 12.7 (10.9-15.5) | 12.2 (10.9-13.5) |
| Sex, No. (%) | ||
| Female | 20 (43) | 16 (39) |
| Male | 27 (57) | 25 (61) |
| Race, No. (%)a | ||
| American Indian or Alaska Native | 1 (2) | 0 |
| Asian | 0 | 1 (2) |
| Black or African American | 2 (4) | 1 (2) |
| White | 44 (94) | 35 (85) |
| Multiple | 0 | 4 (10)b |
| Hispanic ethnicity, No. (%)c | 8 (17) | 7 (17) |
| Annual household income, No. (%) | (n = 46) | (n = 34) |
| <$50 000 | 10 (22) | 5 (15) |
| $50 000-<$100 000 | 9 (20) | 9 (26) |
| ≥$100 000 | 27 (59) | 20 (59) |
| Highest parental education, No. (%) | ||
| <Bachelor’s degree | 12 (26) | 13 (32) |
| Bachelor’s degree | 21 (45) | 16 (39) |
| Graduate or professional degree | 14 (30) | 12 (29) |
| Health insurance, No. (%) | ||
| Private | 38 (81) | 37 (90) |
| Government-sponsored | 7 (15) | 4 (10) |
| None | 2 (4) | 0 |
| Body mass index percentile, median (IQR)d | 73 (33-90) | 57 (32-84) [n = 40] |
| Time from diagnosis of type 1 diabetes to randomization, d | ||
| Mean (SD) | 24 (5) | 25 (4) |
| Median (IQR) | 25 (21-27) | 26 (23-27) |
| Hemoglobin A1c at randomization, mean (SD), % | 10.3 (1.7) | 10.2 (1.2) [n = 39] |
| Diabetic ketoacidosis at diagnosis, No./total (%)e | 28/46 (61) | 23/41 (56) |
| Tanner stage, No. (%)f | ||
| 1g | 9 (19) | 12 (29) |
| 2-5h | 38 (81) | 29 (71) |
Solicited from the participant in response to this question: “Which of the following racial designations best describes you?” The fixed categories allowed for the reporting of more than 1 race.
One participant identified as Black, Native American, and White; 1 participant identified as White and Asian; 1 participant identified as White and Native American; and 1 participant identified as White and Black.
Solicited from the participant in response to this question: “Do you consider yourself to be Hispanic/Latino or not Hispanic/Latino?”
Based on age, sex, body weight, and height using growth charts developed by the US Centers for Disease Control and Prevention.
Defined as hyperglycemia associated with a level of serum ketones greater than 1.5 mmol/L or large or moderate urine ketones; arterial blood pH less than 7.30, venous pH less than 7.24, or serum bicarbonate less than 15; and treatment provided in a health care facility.
Based on pubic hair growth for males and females plus breast development for females.
Stage reflects no pubic hair at all and no breast glandular tissue (typically age of ≤10 years).
Stages reflect gradually greater amount of pubic hair and breast development.
Follow-up was completed on September 15, 2022. The trial was completed by 44 of the 47 participants (94%) in the verapamil group and 39 of the 41 participants (95%) in the placebo group. The reasons for trial discontinuation appear in eTable 3 in Supplement 2. Among the trial completers, the visit completion rate for the 5 follow-up visits was 96% in each group. The C-peptide results were missing for 1 participant in each group at baseline and for 4 participants in the verapamil group and 3 participants in the placebo group at 12 months. Intensive diabetes management with an automated insulin delivery system was provided to 22 participants (47%) in the verapamil group and 20 participants (49%) in the placebo group and standard diabetes care was provided to 25 (53%) and 21 (51%), respectively.
Drug Dose and Drug Adherence
The mean starting drug dose was 1.7 mg/kg/d (SD, 0.3 mg/kg/d) in the verapamil group and 1.7 mg/kg/d (SD, 0.3 mg/kg/d) in the placebo group and the mean ending doses were 5.8 mg/kg/d (SD, 1.3 mg/kg/d) and 6.3 mg/kg/d (SD, 1.4 mg/kg/d), respectively. At randomization, 25 of 46 participants (54%) in the verapamil group and 26 of 40 participants (65%) in the placebo group were prescribed 60 mg/d, whereas 21 (46%) and 14 (35%), respectively, were prescribed 120 mg/d. At 52 weeks, 4 of 39 participants (10%) in the verapamil group were taking 120 mg/d, 13 (33%) were taking 240 mg/d, and 21 (54%) were taking 360 mg/d. At 52 weeks, 3 of 38 participants (8%) in the placebo group were taking 120 mg/d, 14 (37%) were taking 240 mg/d, and 20 (53%) were taking 360 mg/d.
In addition to the 5 participants who dropped out of the trial, the study drug was discontinued due to presumed adverse drug events by 3 participants in the verapamil group and by 1 participant in the placebo group and was discontinued for other reasons by 2 participants in the verapamil group (eTable 4 in Supplement 2). A permanent or temporary dose reduction due to a presumed adverse drug event was made for 3 participants in the verapamil group and 3 participants in the placebo group (eTable 4 in Supplement 2).
The median drug adherence from pill counts was 94% (IQR, 85%-98%) in the verapamil group and was 93% (IQR, 84%-99%) in the placebo group; 84% and 79% of participants, respectively, had a drug adherence level of 80% or greater.
Primary Outcome
In the verapamil group, the mean C-peptide AUC was 0.66 pmol/mL at baseline and 0.65 pmol/mL at 52 weeks from diagnosis compared with 0.60 pmol/mL at baseline and 0.44 pmol/mL at 52 weeks in the placebo group. The adjusted between-group treatment difference at 52 weeks was 0.14 pmol/mL (95% CI, 0.01 to 0.27 pmol/mL; P = .04), representing a 30% higher C-peptide level with verapamil than with placebo (Table 2 and Figure 2A). After increasing from baseline to 13 weeks in both groups, the mean C-peptide AUC declined in the placebo group, whereas the mean was stable in the verapamil group through 26 weeks and then declined (Table 2 and Figure 2B).
Table 2. Primary and Secondary Outcomes.
| Verapamil (n = 43)a | Placebo (n = 38)a | Adjusted between-group difference (95% CI) |
P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Area under the curve values for C-peptide level at 52 wk from diagnosis of type 1 diabetes, geometriclike mean (SD), pmol/mLb | 0.65 (0.31) | 0.44 (0.30) | 0.14 (0.01 to 0.27)c | .04c |
| Secondary outcomes | ||||
| Hemoglobin A1c, mean (SD), % | 6.6 (1.0) [n = 43] | 6.9 (1.2) [n = 39] | −0.3 (−1.0 to 0.4)d | .65d |
| Continuous glucose monitoring outcomese | (n = 44) | (n = 37) | ||
| Time spent with glucose concentration in range, mean (SD), % | ||||
| 70-180 mg/dL | 74 (18) | 70 (21) | 2 (−9 to 13)d | .74d |
| 70-140 mg/dL | 51 (19) | 49 (21) | 0 (−12 to 11)d | .95d |
| Glucose concentration, mean (SD), mg/dL | 151 (27) | 159 (41) | −4 (−25 to 16)d | .74d |
| Time spent with glucose concentration, mean (SD), % | ||||
| >180 mg/dL | 25 (18) | 28 (21) | −2 (−13 to 9)d | .74d |
| >250 mg/dLf | 5 (6) | 8 (10) | −1 (−6 to 4) | .74 |
| <54 mg/dLf | 0.20 (0.24) | 0.24 (0.26) | −0.02 (−0.19 to 0.15) | .79 |
| <70 mg/dLf | 1.3 (1.2) | 1.6 (1.3) | −0.2 (−1.0 to 0.6) | .74 |
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Had analyzable area under the curve C-peptide measurements at 52 weeks. Four participants in the verapamil group were missing data at 52 weeks (3 dropped out prior to 52 weeks and 1 did not complete a mixed-meal tolerance test due to a medical issue). Two participants in the placebo group were missing data at 52 weeks (1 dropped out prior to 52 weeks and 1 had an area under the curve C-peptide measurement that was unanalyzable).
Data on C-peptide level were obtained during a mixed-meal tolerance test from which the area under the curve was computed. The baseline values for the geometriclike means were 0.66 (SD, 0.20) pmol/mL in the verapamil group and 0.60 (SD, 0.22) pmol/mL in the placebo group. In individuals with type 1 diabetes, the level can decline to less than 0.05 pmol/mL, which represents the limit of assay detection.
All randomized participants with at least 1 analyzable C-peptide measurement were included in the primary outcome analysis. In the verapamil group, all 47 participants were included in the primary outcome analysis. In the placebo group, 40 of 41 participants were included in the primary outcome analysis because there was 1 participant without C-peptide measurements at baseline or follow-up. Data were estimated from a constrained, longitudinal, mixed-effects model including age, time from type 1 diabetes diagnosis to randomization, and intensity of care (intensive diabetes management or standard diabetes care) as fixed effects and clinical site as a random effect. Values of log(area under the curve + 1) at randomization, at 13 weeks, at 26 weeks, at 39 weeks, and at 52 weeks were included in the response.
Estimated from a longitudinal, mixed-effects model including age, time from type 1 diabetes diagnosis to randomization, and intensity of care (intensive diabetes management or standard diabetes care) as fixed effects and clinical site as a random effect. The 95% CIs and P values have been adjusted for multiplicity using the 2-step Benjamini-Hochberg procedure.
Computed from up to 288 glucose values per day over approximately 28 days at 12 months. The median number of glucose values per participant was 7751 (IQR, 7513-7918) in the verapamil group and 7836 (IQR, 7537-7926) in the placebo group.
Robust means and SDs are reported for these outcomes with a skewed distribution. Estimates and 95% CIs were calculated using robust regression with M estimation. The models adjusted for age, days from type 1 diabetes diagnosis to randomization, and intensity of care (intensive diabetes management or standard diabetes care) as covariates.
Figure 2. Area Under the Curve Values for 52-Week C-Peptide Level, Area Under the Curve Values for C-Peptide Level at Each Time Point, and Peak C-Peptide Levels at Each Time Point.
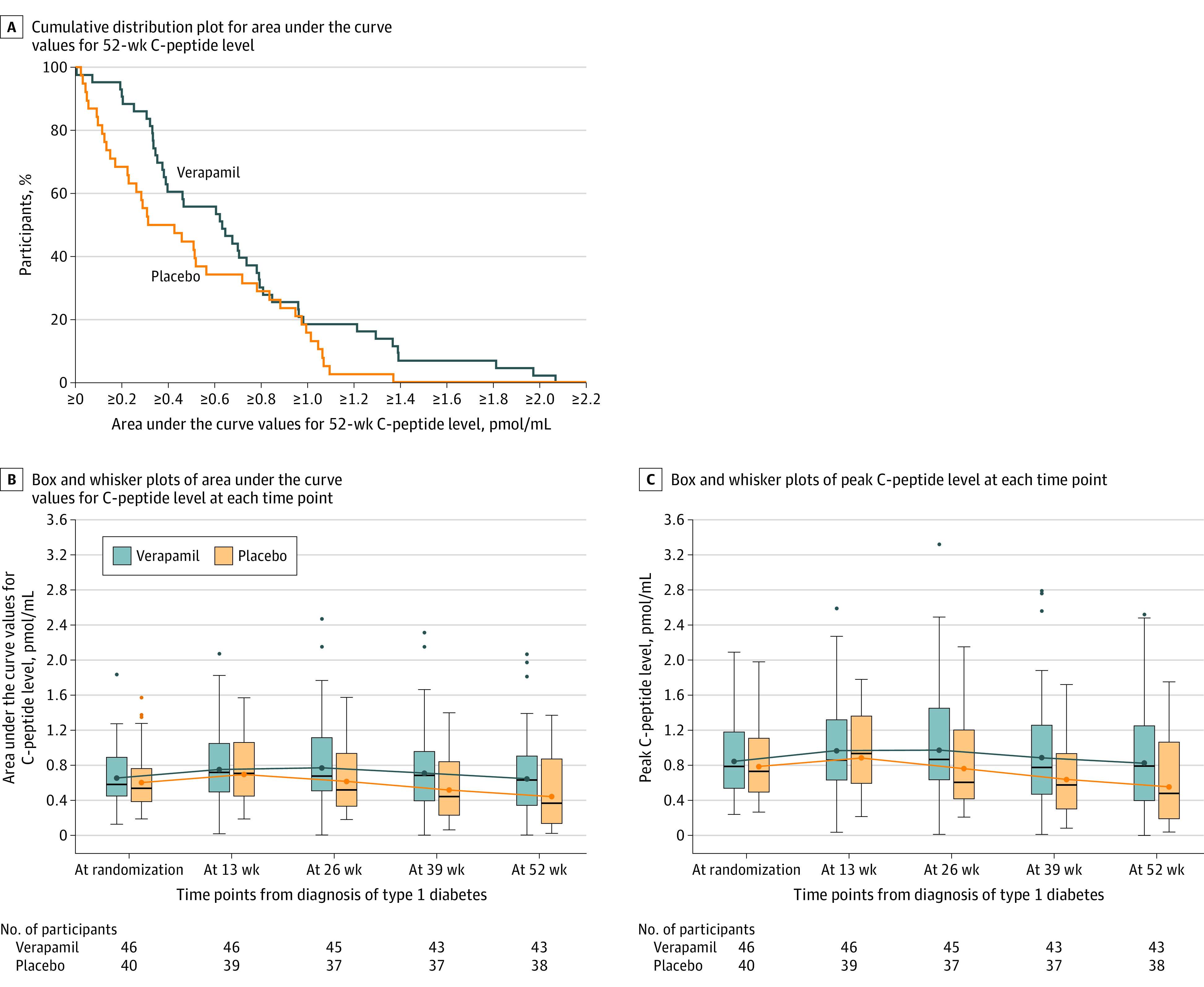
In A and B, the area under the curve values for the C-peptide levels were obtained from a mixed-meal tolerance test and computed using the trapezoidal rule as a weighted sum of the measurements for C-peptide level at time 0 and after 15, 30, 60, 90, and 120 minutes. In A, for any given C-peptide area under the curve level, the percentage of participants in each treatment group with a value at that level or higher can be determined from the Figure. In B and C, for each box and whisker plot, the blue or orange dot inside the box represents the geometric mean, the horizontal black line represents the median, the ends of the box represent the 25th and 75th percentiles, and the whiskers represent the highest or lowest values within 1.5 × the IQR. The numbers beneath the x-axis reflect the number of participants in each group completing a mixed-meal tolerance test at each time point.
Secondary Outcomes
During the 52-week mixed-meal tolerance test, the mean peak C-peptide level was 0.83 pmol/mL (SD, 0.37 pmol/mL) in the verapamil group compared with 0.55 pmol/mL (SD, 0.34 pmol/mL) in the placebo group (Figure 2C). The 52-week peak C-peptide level was 0.2 pmol/mL or greater in 41 of 43 participants (95%) in the verapamil group compared with 27 of 38 participants (71%) in the placebo group (eTable 5 in Supplement 2).
In the per-protocol and sensitivity analyses, which included adjustment for baseline imbalances between groups in body mass index percentile and prepubertal status (Tanner stage 1), the results were consistent with the primary analysis (eTable 6 in Supplement 2). The results also appeared consistent among the subgroups (eFigure in Supplement 2). Assignment to intensive diabetes management vs standard diabetes management did not influence the primary outcome results. In the verapamil group, the mean C-peptide AUC was 0.64 pmol/mL (SD, 0.32 pmol/mL) in the participants who received intensive diabetes management compared with 0.65 pmol/mL (SD, 0.30 pmol/mL) in those who received standard diabetes management, and in the placebo group, the mean C-peptide AUC was 0.45 pmol/mL (SD, 0.31 pmol/mL) in the participants who received intensive diabetes management compared with 0.44 pmol/mL (SD, 0.29 pmol/mL) in those who received standard diabetes management (P = .19 for interaction).
Hemoglobin A1c decreased in the verapamil group from 10.3% at baseline to 6.6% at 52 weeks and in the placebo group from 10.2% at baseline to 6.9% at 52 weeks (mean between-group difference, −0.3% [95% CI, −1.0% to 0.4%]; Table 2 and eTable 7 in Supplement 2). The mean percentage of time with glucose concentration (measured with continuous glucose monitoring) in the range of 70 mg/dL to 180 mg/dL at 52 weeks was 74% in the verapamil group compared with 70% in the placebo group (mean between-group difference, 2% [95% CI, −9% to 13%]; Table 2 and eTables 8 and 9 in Supplement 2). In the verapamil group, the total insulin dose was 0.74 units/kg/d at baseline and 0.65 units/kg/d at 52 weeks compared with 0.64 units/kg/d and 0.74 units/kg/d, respectively, in the placebo group (mean between-group difference at 52 weeks, −0.12 units/kg/d [95% CI −0.30 to 0.05 units/kg/d]).
Adverse Events
Thirty-nine participants (83%) in the verapamil group experienced 134 adverse events and 30 participants (73%) in the placebo group experienced 91 adverse events (Table 3 and eTable 10 in Supplement 2). One event of severe hypoglycemia occurred in each group and 1 event of diabetic ketoacidosis occurred in the placebo group. Three participants in each group experienced other serious adverse events that were not considered related to treatment.
Table 3. Adverse Events During the Trial.
| Verapamil (n = 47) | Placebo (n = 41) | |
|---|---|---|
| Adverse eventsa | ||
| No. of events | 134 | 91 |
| Participants with ≥1 event, No. (%) | 39 (83) | 30 (73) |
| Incidence per 100 person-yearsb | 307.5 | 245.9 |
| Severe hypoglycemiac | ||
| No. of events | 1 | 1 |
| Participants with ≥1 event, No. (%) | 1 (2) | 1 (2) |
| Incidence per 100 person-yearsb | 2.3 | 2.7 |
| Diabetic ketoacidosisd | ||
| No. of events | 0 | 1 |
| Participants with ≥1 event, No. (%) | 0 | 1 (2) |
| Incidence per 100 person-yearsb | 0 | 2.7 |
| Other serious adverse eventse | ||
| No. of events | 7 | 3 |
| Participants with ≥1 event, No. (%) | 3 (6) | 3 (7) |
| Incidence per 100 person-yearsb | 16.0 | 8.1 |
| Specific serious adverse events among participants with ≥1 event, No. (%) | ||
| Depression or suicidal ideation | 3 (6) | 1 (2) |
| Asthma | 0 | 1 (2) |
| Dehydration and ketosis | 0 | 1 (2) |
| Nonserious adverse events of special interest among participants with ≥1 event, No. (%) | ||
| Nausea or vomiting | 5 (11) | 0 |
| Headache | 4 (9) | 7 (17) |
| Constipation | 3 (6) | 1 (2) |
| Electrocardiogram abnormalityf | 3 (6) | 0 |
| Elevated level of liver enzymesg | 2 (4) | 2 (5) |
| Hypotensionh | 1 (2) | 0 |
| Bradycardiah | 0 | 1 (2) |
| Dizziness | 0 | 0 |
Eight participants in each group experienced an adverse event considered to be related to treatment. In the verapamil group, there were 10 events in 8 participants (2 with constipation, 2 with hypotension, 1 with headache, 1 with fatigue, 1 with both a prolonged PR interval on electrocardiogram and elevated levels of liver enzymes, 1 with elevated levels of liver enzymes [alanine transaminase and aspartate aminotransferase], 1 with second-degree heart block and a prolonged PR interval, and 1 with first-degree heart block). In the placebo group, there were 8 events in 8 participants (4 with headache, 2 with elevated levels of liver enzymes, 1 with constipation, and 1 with fatigue).
Calculated as the number of events in the numerator and the total amount of study time across all participants in the group in years in the denominator multiplied by 100.
Defined as hypoglycemia that required the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions due to the participant being impaired cognitively to the point that they were unable to treat themselves, were unable to verbalize their needs, were incoherent, disoriented, combative, or a combination of these, or experienced seizure or loss of consciousness.
Defined as hyperglycemia associated with serum level of ketones greater than 1.5 mmol/L or large or moderate urine ketones; arterial blood pH less than 7.30, venous pH less than 7.24, or serum bicarbonate less than 15; and treatment provided in a health care facility.
Defined as any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent disability or incapacity, is a congenital abnormality or birth defect, or requires a medical or surgical intervention to prevent permanent impairment. In the verapamil group, 1 participant had 2 hospitalizations reported for suicidal ideation and depression, 1 participant had 1 hospitalization for suicidal ideation, and 1 participant had 1 hospitalization for depression and 3 hospitalizations for suicidal ideation. In the placebo group, 3 participants each had 1 hospitalization for suicidal ideation, exacerbation of asthma, and dehydration with ketosis.
Included prolonged PR interval in 1 participant, second-degree heart block and prolonged PR interval in 1 participant, and first-degree heart block in 1 participant.
Reported as an adverse event when the laboratory normal range was exceeded for alanine transaminase and aspartate aminotransferase.
Pulse was considered abnormal if less than second percentile for age and sex. Blood pressure (either systolic or diastolic) was considered to be abnormal if less than the fifth percentile for age, sex, and height.
Eight participants (17%) in the verapamil group and 8 participants (20%) in the placebo group experienced a nonserious adverse event considered to be related to treatment. In the verapamil group, 3 participants experienced electrocardiogram abnormalities (prolonged PR interval in 1 participant, second-degree heart block and prolonged PR interval in 1 participant, and first-degree heart block in 1 participant) and 1 participant experienced hypotension. In the placebo group, 1 participant experienced bradycardia (Table 3). Mean blood pressure and mean heart rate collected at each visit did not show appreciable differences between groups (eTable 11 in Supplement 2).
Discussion
Once-daily oral verapamil started within 31 days after diagnosis of type 1 diabetes slowed beta cell decline over 52 weeks in children and adolescents aged 7 years to 17 years. The natural history of type 1 diabetes involves a slight improvement in C-peptide secretion immediately after diagnosis and initiation of insulin therapy, followed by progressive C-peptide decline related to autoimmune beta cell destruction.14,15 An initial improvement in stimulated C-peptide secretion was observed in both groups, but the verapamil group experienced a longer period of stability compared with the placebo group before C-peptide levels began to decline. In view of the favorable safety profile compared with immunosuppressive agents, once-daily oral administration, and low cost, initiation of verapamil therapy could be considered for patients with newly diagnosed type 1 diabetes.
The current randomized clinical trial confirms the findings of the pilot trial by Ovalle et al10 in adults with newly diagnosed type 1 diabetes. That study found a relative difference in mean C-peptide AUC of 35% after 12 months comparing 11 participants treated with verapamil vs 14 participants treated with placebo, similar to the finding of a 30% relative difference in the current pediatric and adolescent population. Further follow-up of the adult participants in the pilot study suggested a possible sustained benefit of verapamil at 2 years, although treatment during year 2 was not controlled.16
The 30% 1-year improvement in C-peptide secretion with verapamil is in the midrange of improvement reported for immunosuppressive agents that have been evaluated for newly diagnosed type 1 diabetes in randomized clinical trials.4,17,18 However, it is difficult to compare treatment effects across studies due to lack of precision in the point estimates and differences in the study designs and characteristics of the enrolled cohorts. These immunosuppressive therapies are most effective shortly after the diagnosis of type 1 diabetes, with differences in C-peptide outcomes between treated and control participants becoming established within 6 months followed by a subsequent decline in C-peptide secretion similar to what was observed in the current trial with verapamil. Two years of treatment with abatacept,19,20 1 year with golimumab,5,21 2 annual courses with teplizumab,18 and single-course treatment with rituximab22,23 and antithymocyte globulin4,17 all demonstrated this pattern. The C-peptide benefits that persisted, sometimes for years, were due to early gains. There was minimal evidence that longer therapy added benefit. Whether the same is true for verapamil, an agent proposed to act via mechanisms that are not immunomediating, is unknown.
Significant differences in C-peptide secretion were not accompanied by meaningful treatment group differences in hemoglobin A1c levels, continuous glucose monitoring parameters, or insulin dose. Glycemic control in the placebo group was very good, which may be reflective of the use of either an automated insulin delivery system or continuous glucose monitoring for diabetes management plus the presence of the honeymoon phase during the first year after type 1 diabetes diagnosis in which most patients have some residual beta cell function present. This presumption is supported by the finding that 71% of the placebo group had a peak C-peptide level of 0.2 pmol/mL or greater 52 weeks after diagnosis even though this percentage was less than the 95% of the verapamil group with a peak C-peptide level of 0.2 pmol/mL or greater. There was no effect of the factorial design randomization on either the intensive diabetes management groups (plus verapamil or placebo) or the standard diabetes management groups on the treatment group comparison of C-peptide levels.
We observed few treatment-related adverse events, none of which were serious, which is consistent with the known safety profile of verapamil.24,25 It should be noted that drug therapy was approached cautiously because of our lack of experience in using verapamil in children with gradual weight- and symptom-based dose escalation over 2 months and close monitoring for bradycardia, hypotension, electrocardiogram changes, and liver function.
The strengths of this trial include the multicenter, double-blind, randomized design; the investigation of a drug with a mechanism of action distinct from immunotherapies; use of a drug with a prior safety profile in other populations; inclusion of a cohort confined to children and adolescents; the ethnic diversity of the participants; and the initiation of therapy within 31 days of diagnosis with type 1 diabetes.
Limitations
This trial had limitations. First, the trial only included children who weighed 30 kg or greater due to the commercially available dosing options for extended-release verapamil. Second, due to the challenges surrounding the enrollment of participants during the COVID-19 pandemic in early 2020, we did not reach our original enrollment target. Third, the sample size was small for assessing adverse events of treatment.
Conclusions
In children and adolescents with newly diagnosed type 1 diabetes, verapamil partially preserved stimulated C-peptide secretion at 52 weeks from diagnosis compared with placebo. Further studies are needed to determine the longitudinal durability of C-peptide improvement and the optimal length of therapy.
Trial protocol and statistical analysis plan
The CLVer Study Group
Data and Safety Monitoring Board
eStatistical Methods
eTable 1. Eligibility Criteria
eTable 2. Study Procedures and Visits
eTable 3. Listing of Randomized Participants Who Did Not Complete the Trial
eTable 4. Drug Discontinuation and Dose Reduction
eTable 5. C-Peptide Results
eTable 6. Per-Protocol and Sensitivity Analyses
eTable 7. HbA1c Metrics
eTable 8. CGM Metrics
eTable 9. Time in Range 70-180 mg/dL by Drug and HCL Use
eTable 10. All Reported Adverse Events
eTable 11. Blood Pressure and Heart Rate
eFigure. Treatment Effect within Baseline Subgroups
Nonauthor collaborators
Data sharing statement
References
- 1.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. doi: 10.15585/mmwr.mm6906a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonfanti R, Bazzigaluppi E, Calori G, et al. Parameters associated with residual insulin secretion during the first year of disease in children and adolescents with type 1 diabetes mellitus. Diabet Med. 1998;15(10):844-850. doi: [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832-836. doi: 10.2337/diacare.26.3.832 [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen LM, Bundy BN, Greco MN, et al. Comparing beta cell preservation across clinical trials in recent-onset type 1 diabetes. Diabetes Technol Ther. 2020;22(12):948-953. doi: 10.1089/dia.2020.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quattrin T, Haller MJ, Steck AK, et al. ; T1GER Study Investigators . Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383(21):2007-2017. doi: 10.1056/NEJMoa2006136 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57(4):938-944. doi: 10.2337/db07-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296(5):E1133-E1139. doi: 10.1152/ajpendo.90944.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848-856. doi: 10.2337/db11-0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borowiec AM, Właszczuk A, Olakowska E, Lewin-Kowalik J. TXNIP inhibition in the treatment of diabetes: verapamil as a novel therapeutic modality in diabetic patients. Med Pharm Rep. 2022;95(3):243-250. doi: 10.15386/mpr-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24(8):1108-1112. doi: 10.1038/s41591-018-0089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McVean J, Forlenza GP, Beck RW, et al. ; CLVer Study Group . Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized trial. JAMA. Published online February 24, 2023. doi: 10.1001/jama.2023.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin DB. Inference and missing data. Biometrika. 1976;63:581-592. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 14.Buckingham B, Beck RW, Ruedy KJ, et al. ; Diabetes Research in Children Network (DirecNet) Study Group; Type 1 Diabetes TrialNet Study Group . Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care. 2013;36(12):4030-4035. doi: 10.2337/dc13-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum CJ, Beam CA, Boulware D, et al. ; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes. 2012;61(8):2066-2073. doi: 10.2337/db11-1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu G, Grimes TD, Grayson TB, et al. Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat Commun. 2022;13(1):1159. doi: 10.1038/s41467-022-28826-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller MJ, Schatz DA, Skyler JS, et al. ; Type 1 Diabetes TrialNet ATG-GCSF Study Group . Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care. 2018;41(9):1917-1925. doi: 10.2337/dc18-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold KC, Gitelman SE, Ehlers MR, et al. ; AbATE Study Team . Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766-3774. doi: 10.2337/db13-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orban T, Bundy B, Becker DJ, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group . Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069-1075. doi: 10.2337/dc13-0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orban T, Bundy B, Becker DJ, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group . Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412-419. doi: 10.1016/S0140-6736(11)60886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigby MR, Hayes B, Li Y, Vercruysse F, Hedrick JA, Quattrin T. Two-year follow-up from the T1GER study: continued off-therapy metabolic improvements in children and young adults with new-onset T1D treated with golimumab and characterization of responders. Diabetes Care. Published online December 28, 2022. doi: 10.2337/dc22-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143-2152. doi: 10.1056/NEJMoa0904452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pescovitz MD, Greenbaum CJ, Bundy B, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group . B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37(2):453-459. doi: 10.2337/dc13-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn JT, Pasko DA. Calcium channel blockers: pharmacology and place in therapy of pediatric hypertension. Pediatr Nephrol. 2000;15(3-4):302-316. doi: 10.1007/s004670000480 [DOI] [PubMed] [Google Scholar]
- 25.Porter CJ, Garson A Jr, Gillette PC. Verapamil: an effective calcium blocking agent for pediatric patients. Pediatrics. 1983;71(5):748-755. doi: 10.1542/peds.71.5.748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
The CLVer Study Group
Data and Safety Monitoring Board
eStatistical Methods
eTable 1. Eligibility Criteria
eTable 2. Study Procedures and Visits
eTable 3. Listing of Randomized Participants Who Did Not Complete the Trial
eTable 4. Drug Discontinuation and Dose Reduction
eTable 5. C-Peptide Results
eTable 6. Per-Protocol and Sensitivity Analyses
eTable 7. HbA1c Metrics
eTable 8. CGM Metrics
eTable 9. Time in Range 70-180 mg/dL by Drug and HCL Use
eTable 10. All Reported Adverse Events
eTable 11. Blood Pressure and Heart Rate
eFigure. Treatment Effect within Baseline Subgroups
Nonauthor collaborators
Data sharing statement


