Abstract
Skin inflammation is a symptom of many skin diseases, such as eczema, psoriasis, and dermatitis, which cause rashes, redness, heat, or blistering. The use of natural products with anti-inflammatory properties has gained importance in treating these symptoms. Ursolic acid (UA), a promising natural compound that is used to treat skin diseases, exhibits low aqueous solubility, resulting in poor absorption and low bioavailability. Designing topical formulations focuses on providing adequate delivery via application to the skin surface. The aim of this study was to formulate and characterize lipid-surfactant-based systems for the delivery of UA. Microemulsions and liquid crystalline systems (LCs) were characterized by polarized light microscopy (PLM), rheology techniques, and textural and bioadhesive assays. PLM supported the self-assembly of these systems and elucidated their formation. Rheologic examination revealed pseudoplastic and thixotropic behavior appropriate, and assays confirmed the ability of these formulations to adhere to the skin. In vivo studies were performed, and inflammation induced by croton oil was assessed for response to microemulsions and LCs. UA anti-inflammatory activities of ~60% and 50% were demonstrated by two microemulsions and 40% and 35% by two LCs, respectively. These data support the continued development of colloidal systems to deliver UA to ameliorate skin inflammation.
Keywords: ursolic acid, skin, topical product, inflammation, bioadhesion, surfactant
1. Introduction
Ursolic acid (UA) is a type of natural pentacyclic triterpenoid carboxylic acid found mainly in the peels of fruits [1,2] and in spices such as rosemary [3,4]. UA has numerous pharmacological effects, including antioxidant, anti-inflammatory, antibacterial, and antifungal properties [5,6,7,8]. Due to its poor solubility in water, the incorporation of UA into alternate drug delivery systems has been explored and found to be highly efficient to improve its solubility [9], such as with polymeric nanoparticles [10], liposomes [11], polymeric micelles [12], and nanostructured lipid carriers [13].
Drug delivery systems have been widely studied for the improvement of drug administration to the skin [14]. Surfactant-based systems including microemulsions and liquid crystals comprise amphiphilic surfactants that dissolve in water and oil and self-assemble into various ordered structures [15]. Microemulsions are clear, thermodynamically stable isotropic liquids in which the dispersed phase takes the form of very small droplets [16,17,18]. A liquid crystal is a state of matter that exhibits both the ordered properties of solids and the flow characteristics of liquids [19,20,21,22,23,24,25]. Microemulsions, as well as lamellar, hexagonal, or cubic shaped mesophases, have attracted attention in the pharmaceutical field for their physicochemical properties, such as viscosity [26], bioadhesion, ability to control drug release [24,27], and loading of hydrophilic or lipophilic drugs [28,29,30].
Skin inflammation and rashes can cause redness, pain, itching, and dryness [31,32]. Common inflammatory skin conditions include dermatitis, psoriasis, poison ivy and poison oak, and drug rashes [32,33,34,35,36]. Unfortunately, the resilient barrier presented by epidermal layers of the skin, primarily the stratum corneum, severely limits the efficacy of dermal and transdermal drug delivery and inhibits the effective diffusion of drugs [37].
The aim of this study was to develop and characterize a surfactant-based system to improve topical anti-inflammatory drug delivery, and in addition to evaluate the biological performance of the drug delivery system.
2. Materials and Methods
2.1. Materials
Oleic acid (OA) was obtained from Synth (Diadema, Brazil), Procetyl® AWS (PPG-5-CETETH-20) was purchased from Croda (São Paulo, Brazil), and ursolic acid was obtained from Idealfarma (Anápolis, Brazil). Croton oil was purchased from Sigma-Aldrich (St. Louis, MO, USA). Purified water (W) was obtained by Milli-Q® Millipore™ Direct 8/16 system (Burlington, MA, USA). All other reagents were of analytical grade and were used as received without further purification.
2.2. Ternary Phase Diagram
The ternary phase diagrams were constructed by mixing the oil (O), OA, PPG-5-CETETH-20 surfactant (S), and purified W. Weighted proportions of each component were varied in 10% increments, bringing the ternary mixture to a total of 100%. All components were mixed and stirred by vigorous manual shaking. After 48 h, with systems at phase equilibria, they were classified by macroscopic appearance.
2.3. Selected Formulations and Incorporation of Ursolic Acid
Four formulations were selected based on ternary phase diagrams and designated A, B, C, and D. Component proportions (OA%/S%/W%) were 40/50/10, 30/50/20, 20/50/30, and 10/50/40 for formulation A, B, C, and D, respectively.
A solubility test of UA in OA was performed prior to incorporation into the formulations, prompting a target concentration of 5 mg/g. First, the UA was pulverized in a glass mortar with aid of a glass pestle in order to increase the contact surface of the particle and improve its solubility in the system. Next, OA was added, homogenizing until drug solubilization, then PPG-5-CETETH-20 was added and mixed.
The formulations were incubated for ten minutes in an Elmasonic E 120 H ultrasound bath (Singen, Germany) at 37 kHz and room temperature for total solubilization of UA. Finally, W was added to the system and mixed with vigorous manual agitation. Formulations omitting UA were prepared in the same manner, excluding the drug comminution and ultrasound steps.
2.4. Polarized Light Microscopy
PLM was performed using a Zeiss Axioskop 40 (Thornwood, NY, USA). A drop of each formulation was placed on a microscopy slide and covered with a cover slip. Images were acquired using a digital camera (Samsung DV-70, Suwon-si, Republic of Korea) at 40× magnification.
2.5. Flow Rheology
Flow curves were generated using a Discovery HR-1 Hybrid Rheometer (TA Instruments, New Castle, DE, USA) attached to a cone-plate geometry (∅ = 40 mm and θ = 2°) with a 52 μm gap. The sample was applied to base plate with minimal shear and fully filled the gap between plate and geometry. Experiments used a shear rate range of 0.1 to 100 (1/s) for the ascending curve and from 100 to 0.1 (1/s) for the descending curve, with each curve running for 120 s.
Quantitative analysis of flow behavior was calculated using the Herschel–Bulkley model with Equation (1) allowing determination of the consistency index (k) and the flow index (n):
| (1) |
where σ is shear stress (Pa), σo is yield shear stress (Pa), and γ is shear rate (1/s).
2.6. Oscillatory Rheology
Analysis of rheological behavior was performed using a Discovery HR-1 Hybrid Rheometer (TA Instruments, New Castle, DE, USA), using a cone-plate geometry (∅ = 40 mm and θ = 2°) with 52 μm gap. The sample was carefully applied to the base plate and the space between plate and cone was fully filled with samples. The linear viscosity range was determined at 1 Pa over a frequency range of 0.1 to 10 Hz. All analyses were performed at 32 °C in triplicate.
Quantitative data correlating the dependence of the storage modulus (G′) to the frequency were calculated by Equation (2):
| (2) |
where G′ is the storage modulus (Pa), ω is the frequency (Hz), S is the gel strength, and n is the viscoelastic exponent.
2.7. Texture Profile Analysis
Eight grams of each selected sample were placed in Falcon® tubes (50 mL) and centrifuged for 3 min at 2663× g to ensure a smooth surface and the elimination of air bubbles. The tubes were left to rest for 24 h and subsequently analyzed using a texture analyzer; TA.XTplus (Stable Micro Systems, Godalming, UK).
The sample was placed beneath a cylindrical analytical probe (∅ = 10 mm) and compression was initiated at a velocity of 0.5 mm/s. When the probe reached 10 mm of depth the probe was raised out of the sample at 0.1 mm/s. After 5 s, the probe ran a second cycle at the same parameters. All analyses were performed at 25 °C and each sample was analyzed in triplicate.
Graphs of force by time were acquired and peaks of hardness and areas under curve (AUC) of compressibility and adhesiveness were achieved using Exponent® (Stable Micro Systems Data Analysis Godalming, UK). Cohesion was calculated as the difference of compressibility area between the second and first cycle.
2.8. Bioadhesion Studies
Pig ears were obtained from a local slaughterhouse and pre-treated according to Dick and Scott [38] for subsequent analyses. Ears in good condition were sanitized with water at room temperature and the skin was separated from the cartilage with the aid of a scalpel. Ears that presented some type of injury such as wounds or minor lesions were discarded.
After sorting and pre-treatment, a layer of 400 μm was separated from the adipose layer using the Nouvag® TCM 300 dermatometer (Goldach, Switzerland), retaining only the stratum corneum and epidermis. Any existing hairs were cut, and the specimens were kept in 0.9% sodium chloride solution for 30 min.
An 8 g sample of each selected formulation was placed in a Falcon® tube (50 mL) and centrifuged for 3 min at 2663× g. The tubes were left to rest for 24 h and analyzed using texture analyzer TA.XTplus (Stable Micro Systems). On the day of the experiment, the tubes containing the samples were kept in a thermostatic water bath at 32 °C.
Using a cylindrical analytical probe (∅ = 10 mm), an ear skin specimen was attached to the bottom of the probe and secured with a rubber band. The probe was set to descend at a constant velocity of 1 mm/s and the sample was maintained in contact with the skin for a period of 60 s. During the contact stage, the probe was maintained at a constant depth and at end the probe was set to rise at 0.5 mm/s. Skin contact was interrupted and the work and force required to separate was calculated using Exponent® Stable Micro Systems Data Analysis.
2.9. Croton-Oil-Induced Ear Edema
2.9.1. Animals
Male Swiss mice (Mus musculus), 25–30 g, were housed at an average temperature of 22 °C (±2 °C) and kept in a 12 h light/dark cycle with feed and water ad libitum. The experimental protocol was approved by the Ethics Committee of the Use of Animals at the School of Pharmaceutical Sciences (CEUA-FCF) under protocol number 46/2018 in accordance with the guidelines, procedures, and bioethics of animal procedures from the Brazilian Council for Control of Animal Experimentation (CONCEA) standards.
2.9.2. Treatments
Mice were randomized to 9 groups (n = 6) and all treatments were administered to the right ear. Group 1 were naïve mice that received no induction nor any treatment. Group 2 received no treatment (negative control); group 3 received formulation A without UA. Groups 4–7 were administered formulations A to D loaded with AU (5 mg/g), respectively; group 8 received UA solubilized in OA (5 mg/g); and group 9 was administered dexamethasone cream (DEX) (0.1 mg/g).
2.9.3. Edema Induced by Croton Oil
The ear edema was induced on the external right ear by topical application of 20 µL of croton oil in acetone (50 µg/mL, 1 µg/ear). At 30 min prior to the induction, 20 mg of drug formulation was applied topically to the inner right ear of the animals according to the groups outlined above. Ear edema was measured both before and 6 h after induction of inflammation. Animals were euthanized, the ears hole-punched, and the isolated specimens were weighed. Ear edema was quantified as the difference in weight (in mg) of the section removed from the right ear (treated with phlogistic agent, formulations, and positive control) and the weight (in mg) of the section removed from the left ear (naïve group).
The average percent inhibition of edema (%) was calculated using the following Equation (3):
| (3) |
where NC is negative control group; NV is naïve group; and T is tested samples.
2.10. Statistical Analysis
When applicable, significant differences between the means of the values obtained were submitted to analysis of variance (ANOVA) followed by multiple comparisons applying the Tukey method (p < 0.05).
3. Results and Discussion
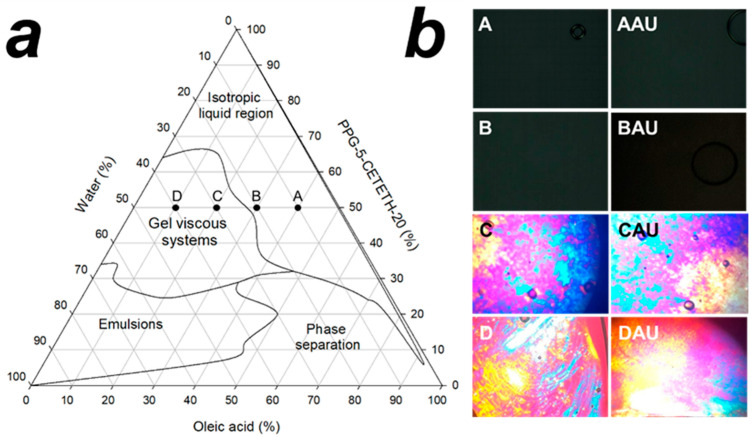
A ternary phase diagram was obtained for oil, water, and surfactant mixtures and is shown in Figure 1a. Higher proportions of surfactant lead to the formation of isotropic liquid systems, such as micelles and microemulsions, observable at the upper vertex of the diagram. However, when the water content increased, it undermined the clear gelling systems and led to characteristic hexagonal or cubic mesophases. The ternary phase diagram shows these vertices in two regions: the left side, characterized by opaque emulsion-like systems, and a phase separation region on the right side.
Figure 1.
Phase ternary diagram of PPG-5-CETETH-20, oleic acid, and water mixture and their macroscopic visual characterization regions: inner symbols denoted the chosen formulations (a); polarized light microscopy obtained at 40× of magnification (b). A = formulation with OA%/S%/W% ratio of 40/50/10; B = formulation with OA%/S%/W% ratio of 30/50/20; C = formulation with OA%/S%/W% ratio of 20/50/30; D = formulation with OA%/S%/W% ratio of 10/50/40; AAU = formulation A loaded with AU; BAU = formulation B loaded with AU; CAU = formulation C loaded with AU; DAU = formulation Dloaded with AU.
Surfactants are known to self-assemble to several types of colloidal systems, microemulsions, macroemulsions, and lyotropic liquid crystals [17,18,39,40], and these colloidal systems have been examined for topical drug delivery to skin [41,42,43,44,45,46,47,48,49]. The formation of microemulsions and lyotropic liquid crystals is dependent on several factors, including temperature, salt concentrations, hydrophilic–lipophilic balance (HLB) of surfactant, water solvation, and supramolecular interactions between the components [50]. Recently, the critical packing parameter (CPP) of surfactants has been used to explain these self-assemblies and the behavior of mixtures undergoing the phase change to lyotropic liquid crystals [51,52].
The CPP is a geometric parameter of a surfactant defined as volume of the tail chain (vs) divided by the product of the area of the head group at the head–tail interface (a0) and critical length of the tail chain (lc) [51], as shown in Equation (4). Thus, the aggregation shape of amphiphilic molecules can be determined according to the value of CPP. For example, from 0 < CPP ≤ 1/3 only spherical micelles exist in solution [51]. However, when 1/3 < CPP ≤ 1/2, aggregations with a cylindrical micelle or worm-like micelle are most likely. Finally, for 1/2 < CPP ≤ 1, there is a balance between sizes of the head group and tail, which causes the surfactant molecule to form planer aggregates with a bi-layer structure [51].
| (4) |
Water content is an influencing factor of phase transition, and with the decreasing water content, different structures are derived, such as the normal micelle (oil in water), the normal cubic phase, the normal hexagonal phase, the lamellar phase, the reversed cubic phase (water in oil), and the reversed hexagonal phase [50]. In our case, dilution of water in the ternary phase system shows a transition from the isotropic region to a viscous isotropic region, which characterizes lyotropic liquid crystalline systems. In this instance, water molecules solvate the head group of surfactants, changing the negative curvature and leading to the generation of lyotropic liquid crystals.
The selected formulations chosen all represented 50% (w/w) of the surfactant and oil/water ratios with variations shown to correspond with the inner area of the ternary phase diagram (Figure 1a). Four formulations were carefully chosen, with two selected due to their liquid macroscopic appearance and two selected due to gel-like viscosity. Formulations were analyzed by PLM: the liquid formulations (named A and B) displayed isotropic-like properties and a dark field was seen under PLM. Alternatively, formulations C and D showed anisotropic properties under PLM and were characterized as liquid crystalline systems (Figure 1b). PLM is a characterization technique and must be used to accurately categorize LCs [53]; isotropic mixtures show a dark field distinctive for microemulsions [43,54] and cubic mesophases [55]. These two systems are then distinguishable by viscosity, with microemulsions presenting viscosity behavior similar to water [17], while cubic mesophases are categorized as a firm clear gel. Anisotropy is characteristically used for classification of lyotropic LCs [53], while lamellar mesophases display Maltese’s crosses [44,47] and hexagonal mesophases present as stretch marks under PLM [46]. Formulations A and B were characterized as microemulsions, while C and D proved to be hexagonal mesophases. The addition of AU did not change the polarizing appearance of samples, as shown in Figure 1b.
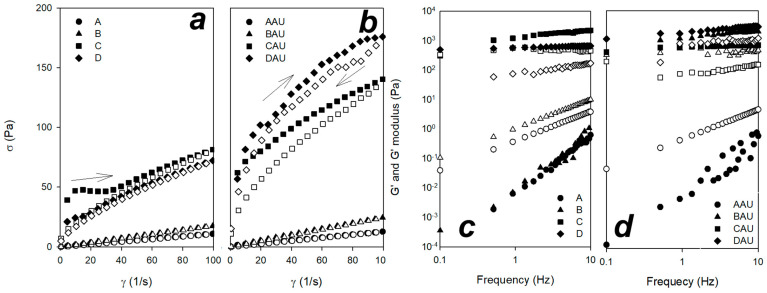
Formulations A to D were analyzed by flow and oscillatory rheology (Figure 2). Flow rheology of all formulations showed a shear-thinning behavior, revealing fluids whose viscosity decreases under shear strain, as well as a hysteresis region revealing thixotropic fluid behavior. For loaded and unloaded formulations A and B, a low viscosity was exhibited, while C and D formulations demonstrated high viscosity values. For n < 1, the fluid is shear-thinning, whereas for n > 1 the fluid is shear-thickening. If n = 1 and the yield stress rate is 0, this model reduces to the Newtonian fluid. The Herschel–Bulkley model characterizes A and B formulations with n values close to 1 and no yield stress rate values, classifying them as Newtonian fluids, with the exception of sample AAU which presented a yield stress rate and was classified as ideal Bingham plastic (Table 1). All other samples presented n < 1 (values between 0.8 and 0.3) and were classified as pseudoplastic or shear-thinning fluids. Only samples C and D, characterized as hexagonal liquid crystals, were able to exhibit that a minimum stress is required to break down, to some extent, the internal ‘structure’ of the fluid before any movement (yield stress rates exhibited in Table 1). Samples characterized as microemulsions (A and B) do not show this behavior, explained by their ‘weak’ structure.
Figure 2.
Rheograms obtained from unloaded and loaded formulations ((a,b), respectively). Ascending curves are denoted by close symbols and descending curves are denoted by open symbols; oscillatory sweep obtained from unloaded and loaded formulations ((c,d), respectively). G′ and G″ moduli are shown as closed and open symbols, respectively. A = formulation with OA%/S%/W% ratio of 40/50/10; B = formulation with OA%/S%/W% ratio of 30/50/20; C = formulation with OA%/S%/W% ratio of 20/50/30; D = formulation with OA%/S%/W% ratio of 10/50/40; AAU = formulation A loaded with AU; BAU = formulation B loaded with AU; CAU = formulation C loaded with AU; DAU = formulation Dloaded with AU.
Table 1.
Values of constants obtained by Herschel–Bulkley model and Power Law model from rheology assays and bioadhesive measurement. AAU = A with A; BAU = B with A; CAU = C with A; DAU = D with A.
| Sample | Herschel–Bulkley Model | Power Law Model | Bioadhesive Measurement | |||||
|---|---|---|---|---|---|---|---|---|
| σo (Pa) | k (Pa.sn) | n | R2 Adjusted | S | n | R2 Adjusted | Force of Detachment (mN) | |
| A | - | 0.1469 | 0.9360 | 0.9999 | 0.0075 | 1.8735 | 0.9621 | 3.0 ± 1.0 |
| AAU | 1.0245 | 0.0890 | 1.0628 | 0.9991 | 0.0023 | 2.4691 | 0.7578 | 1.0 ± 1.5 |
| B | - | 0.2219 | 0.9501 | 1.0000 | 0.0010 | 3.0376 | 0.8452 | 4.0 ± 4.5 |
| BAU | - | 0.3777 | 0.9077 | 1.0000 | 1182.0945 | 0.2531 | 0.9348 | 3.0 ± 1.8 |
| C | 41.8661 | 0.0494 | 0.8606 | 0.9805 | 1239.1706 | 0.2676 | 0.9457 | 31.0 ± 6.4 |
| CAU | 52.0744 | 3.6331 | 0.6951 | 0.9991 | 581.3953 | 0.0808 | 0.8715 | 65.0 ± 18.5 |
| D | 15.3213 | 1.1716 | 0.8473 | 0.9971 | 575.5286 | 0.0625 | 0.9885 | 78.0 ± 7.7 |
| DAU | 6.5332 | 29.3480 | 0.3870 | 0.9928 | 1851.0950 | 0.2184 | 0.9704 | 33.0 ± 16.6 |
Upon application of shear force, the sample undergoes disorganization of molecules, resulting in low viscosity. For the application of topical products to skin, this is analogous to thinning of the product at the high shear rates typical of application [56]. Thixotropy provides an indication of the degree to which the product flows into the skin surface. Quick recuperation of viscosity in a topical product leads the formulation to remain on the skin, giving a greasy feel. Shear thinning and moderate thixotropic characteristics in a topical product represent very favorable rheological behaviors for providing uniform coverage of the skin surface [56]. To this end, skin care products and cosmetics have been formulated to exbibit pseudoplastic behavior, and among these products, we can refer to examples of emulsions and lotions [57,58,59], microemulsions [60,61], hydrogels [61,62,63], and lyotropic liquid crystals [45,64,65]. Previous studies performed by our group demonstrated that lyotropic liquid crystals designed with other surfactants and natural oils are ideal for formulating Non-Newtonian fluids, showing pseudoplastic behavior and thixotropic characteristics [46,47,66].
The consistency index (k), shown in Table 1, reveals higher values when a drug is added to the systems. In addition, when internal water is increased in the system, k values are also high, suggesting internal supramolecular self-assembled formulations. K values have been studied to characterize polymers and polymer solutions, with increasing polymer concentration in solution or cross-linking of polymeric chains generally leading to higher k values [67,68]. This parameter is also directly related to the viscosity of the material [69].
In oscillatory rheology, the storage modulus, G′, represents the elastic portion of viscoelastic behavior, which describes the solid-state behavior of the sample. Meanwhile, the loss modulus, G″, characterizes the viscous portion of viscoelastic behavior, or the liquid-state behavior of the sample [70]. Viscoelastic solids have a higher storage modulus than loss modulus (G′ > G″) due to links inside the material, for example chemical bonds or physical–chemical interactions [71]. On the other hand, viscoelastic liquids with a higher loss modulus than storage modulus (G″ > G′) [72,73] generally have no such strong bonds between the individual molecules. The addition of drugs changed this behavior in formulation B (BAU), where this sample showed a more structured system. Alternately, higher values of S have been related to density structures, molecular interconnections, or the packing of molecules [74]. The use of the Power Law has been studied to predict the viscoelastic behavior of hydrogels [75,76,77]. The use of this technique has also been applied to the study of colloidal systems, such as microemulsions and lyotropic liquid crystals [78,79]. For a quantitative analysis, a G′ modulus value is fitted by the Power Law model to give the S constant (relating to gel strength) and n as the viscoelastic exponent. These values are shown in Table 1. Note the value of n decreases with increasing cross-linking density due to microstructure.
For samples characterized as microemulsions, these moduli correspond to G″ > G′, and they show a ‘weak’ microstructure of material. When water was added to these systems, it resulted in a self-assembly to lyotropic liquid crystals and a switch to G′ > G″. Graphs (Figure 2c,d) show frequency-dependent sweeps, that is, with increasing frequency there is a deformation or breakdown of the material, and it is possible to weaken bonds and the interactions of microemulsions. However, when the material is structured, no changes are exhibited and even with increasing frequency the stored energy is constant. These behaviors have been described for micelles, microemulsions, or lamellar mesophases with a G″ > G′ [42,78] and hexagonal and cubic mesophases denoted by a G′ > G″ [64,65].
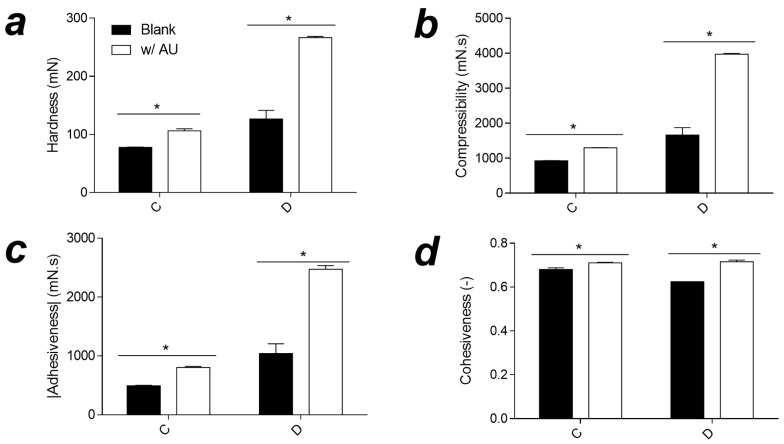
TPA is a technique for evaluating the textural properties of semisolid products, and this technique allows measurement of hardness, adhesiveness, compressibility, and cohesiveness (Figure 3) [80]. Hardness is related to the maximum force required to rupture the matrix, adhesiveness is the sample’s force under the probe surface, compressibility is the force to deform the semisolid product under first compression, and cohesiveness is related to the ratio of areas under the curve of the second and first cycles of compression [81].
Figure 3.
Hardness (a), adhesiveness (b), compressibility (c), and cohesiveness (d) obtained from C and D loaded and unloaded with ursolic acid samples. * p < 0.05 between same group. A = formulation with OA%/S%/W% ratio of 40/50/10; B = formulation with OA%/S%/W% ratio of 30/50/20; C = formulation with OA%/S%/W% ratio of 20/50/30; D = formulation with OA%/S%/W% ratio of 10/50/40.
Due to the unsuitability of liquid samples in this analysis, liquid samples were not analyzed. Only semisolid samples were analyzed and show increasing values for hardness, adhesiveness, and compressibility. The loading of UA leads to an increase in values, corroborating the flow rheology measurements, since the hardness and compressibility were due to the attendant increased viscosities of these formulations [81,82]. Adhesiveness is more of a surface characteristic and depends on the combined effect of adhesive and cohesive forces [83]. It is also related to bioadhesive or mucoadhesive properties [84,85]. To achieve adequate efficacy and user acceptance of topical products, spreadability is an important property, related primarily to hardness and compressibility [86,87].
Bioadhesion can be defined as the state in which two materials, at least one of which is biological in nature, are maintained together for a prolonged time period by means of interfacial forces [88]. Prolonged efficacy via dermal administration of creams, solutions, and lotions is unexpected, since such preparations can be easily removed from the skin by moisture, temperature, and physical movements [89]. Therefore, the design of novel dosage formulations with bioadhesive properties is desirable for establishing long residence times at the application site and reducing product administration frequency [90]. Table 1 shows force of detachment values and establishes that liquid crystalline systems have more ability to adhere to the skin’s surface. This phenomenon can be explained by the viscosity of lyotropic liquid crystals and has already been reported by other authors for cubic and hexagonal mesophases [91,92].
UA has been studied for its anti-inflammatory activities [93] and has been shown to suppress the activation of nuclear factor kappa B (NF-κB), nuclear factor of activated T-cells (NF-AT), and activator protein 1 (AP-1) in lymphocytes of mice [94]. Treatment with ursolic acid leads to reduction in the pro-inflammatory cytokines interleukin 6 (IL-6) and interferon gamma (IFN-γ) [90], and in vivo anti-nociceptive activity has been demonstrated [95,96,97].
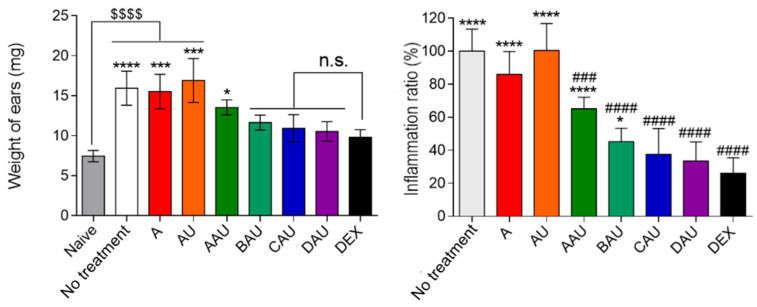
Figure 4 shows the weight of ear samples after treatment and the potential of topical ursolic acid administration. Formulations compared to dexamethasone showed no significant difference in the mean weight, with the exception of AAU. Free UA, vehicle (A), and untreated groups were significantly changed compared to the dexamethasone (p < 0.05) and naïve (p < 0.001) groups. The index of edema was measured as close to 100% for untreated, vehicle (A), and free UA, and these values show significant differences compared to dexamethasone. For the formulations, all demonstrated differences compared to the untreated group, but when compared to dexamethasone only BAU and AAU showed differences. The treatment showed an inhibition of ~60%, 50%, 40%, 35%, and 25% to AAU, BAU, CAU, DAU, and dexamethasone as the positive control, respectively.
Figure 4.
Anti-inflammatory activity of the colloidal systems with ursolic acid. The vehicle is the A formulation, and the control is an ointment containing dexamethasone 0.5% (w/w). Left graph shows weight of ears and right graph shows the inflammatory index (%) obtained. The data represent the mean ± S.D. of 5 mice. The statistical significances of ear thickness and inflammatory index were analyzed using variance analysis following Tukey’s multiple comparison test; * p < 0.05, *** p < 0.001, **** p ≤ 0.0001, and n.s. p > 0.05 compared to dexamethasone group; $$$$ is p ≤ 0.0001 compared to naïve group; ### p < 0.001 and #### p ≤ 0.0001 compared to no treatment group. A = formulation with OA%/S%/W% ratio of 40/50/10; B = formulation with OA%/S%/W% ratio of 30/50/20; C = formulation with OA%/S%/W% ratio of 20/50/30; D = formulation with OA%/S%/W% ratio of 10/50/40; AAU = formulation A loaded with AU; BAU = formulation B loaded with AU; CAU = formulation C loaded with AU; DAU = formulation D loaded with AU; DEX = dexamethasone.
Our previous study showed that curcumin loaded into lamellar and hexagonal mesophases exhibited an ability to reduce paw edema due to increased contact time with the skin surface, aiding the absorption of curcumin [46]. Another study examined the incorporation of trans-resveratrol into the lamellar/hexagonal mesophase; the maximal inhibition of inflammation was found to be between 63.4–27.4%, and these formulations improved bioadhesive properties [47]. Lamellar structures have the ability to deliver trans-resveratrol and reduce symptoms of UVB-irradiation-induced skin damage by inhibiting edema, neutrophil recruitment, lipid hydroperoxidation, and oxidative stress [48]. Cubic and hexagonal mesophases were also investigated in an aerosol-induced rat paw edema model, and the results showed reduced inflammation with celecoxib [98]. These data support the use of colloidal systems for delivery of drugs to ameliorate skin inflammation.
4. Conclusions
Colloidal systems containing OA, PPG-5-CETETH-20, and purified water were prepared for the incorporation of UA, a hydrophobic drug. These systems have the potential to meet some of the challenges involved in hydrophobic drug nanoformulations for topical cutaneous application. The rheological properties of microemulsions and liquid crystals showed pseudoplastic behavior and thixotropic properties desirable for effective topical use. In addition, oscillatory studies were performed and indicated that the microstructure self-assembled. TPA showed a viscosity dependence, and these systems showed bioadhesive ability, allowing for prolonged exposure upon topical application to skin and offering long-lasting activity. The pharmacological study demonstrated up to 65% inhibition of inflammation on mouse ears, with improved inhibition for those formulations that presented liquid crystal mesophases, suggesting these colloidal formulations hold great potential in the delivery of UA to the skin.
Acknowledgments
The authors thank the following Brazilian funding agencies for scholarships support: National Research Council of Brazil (CNPq/Brazil), Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil), and São Paulo Research Foundation.
Author Contributions
B.F.-S.: supervision, data curation, writing—original draft preparation; G.A.A.: methodology; P.S.F.: methodology; F.D.V.: methodology; A.M.P.: investigation, methodology; V.H.S.A.: investigation, methodology; S.G.C.: writing—review and editing; M.C.: funding acquisition, writing—review and editing, contextualization. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The experimental protocol was approved by the Ethics Committee of the Use of Animals at the School of Pharmaceutical Sciences (CEUA-FCF) under protocol number 46/2018 in accordance with the guidelines, procedures, and bioethics of animal procedures from the Brazilian Council for Control of Animal Experimentation (CONCEA) standards.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Research Council of Brazil (CNPq/Brazil), Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil), and São Paulo Research Foundation (grant numbers 15/05394-2;16/22544-0; 17/10016-2; 17/22928-6; 17/23357-2; and 19/10761-5).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Frighetto R.T., Welendorf R.M., Nigro E.N., Frighetto N., Siani A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008;106:767–771. doi: 10.1016/j.foodchem.2007.06.003. [DOI] [Google Scholar]
- 2.Butkevičiūtė A., Liaudanskas M., Kviklys D., Zymonė K., Raudonis R., Viškelis J., Uselis N., Janulis V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018;21:1716–1727. doi: 10.1080/10942912.2018.1506478. [DOI] [Google Scholar]
- 3.Zou S., Chen W. Determination of oleanolic and ursolic acids in different parts of Perilla frutescens by high-performance liquid chromatography. J. Braz. Chem. Soc. 2008;19:1429–1432. doi: 10.1590/S0103-50532008000700030. [DOI] [Google Scholar]
- 4.Huang M.T., Ho C.T., Wang Z.Y., Ferraro T., Lou Y.R., Stauber K., Ma W., Georgiadis C., Laskin J.D., Conney A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 5.Lee S.Y., Kim Y.J., Chung S.O., Park S.U. Recent studies on ursolic acid and its biological and pharmacological activity. EXCLI J. 2016;15:221–228. doi: 10.17179/excli2016-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules. 2019;24:2751. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam M., Ali S., Ahmed S., Elasbali A.M., Adnan M., Islam A., Hassan I., Yadav D.K. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 2021;22:12162. doi: 10.3390/ijms222212162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samsonowicz M., Kalinowska M., Gryko K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials. 2021;14:264. doi: 10.3390/ma14020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pironi A.M., De Araújo P.R., Fernandes M.A., Salgado H.R.N., Chorilli M. Characteristics, Biological Properties and Analytical Methods of Ursolic Acid: A Review. Crit. Rev. Anal. Chem. 2018;48:86–93. doi: 10.1080/10408347.2017.1390425. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Li X., Ding J., Xu H., Dai X., Hou Z., Zhang K., Sun K., Sun W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2) Int. J. Pharm. 2013;441:261–268. doi: 10.1016/j.ijpharm.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Zhao T., Liu Y., Wang Q., Xing S., Li L., Wang L., Liu L., Gao D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C. 2017;71:1231–1240. doi: 10.1016/j.msec.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M., Yi Y., Liu L., Lin Y., Li J., Ruan J., Zhong Z. Polymeric micelles loading with ursolic acid enhancing anti-tumor effect on hepatocellular carcinoma. J. Cancer. 2019;10:5820–5831. doi: 10.7150/jca.30865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S., Ghosh S., De A.K., Bera T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017;102:996–1008. doi: 10.1016/j.ijbiomac.2017.04.098. [DOI] [PubMed] [Google Scholar]
- 14.Barry B.W. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002;54:S31–S40. doi: 10.1016/S0169-409X(02)00113-8. [DOI] [PubMed] [Google Scholar]
- 15.Drummond C.J., Fong C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr. Opin. Colloid Interface Sci. 1999;4:449–456. doi: 10.1016/S1359-0294(00)00020-0. [DOI] [Google Scholar]
- 16.Hamed R., Abu Kwiak A.D., Al-Adhami Y., Hammad A.M., Obaidat R., Abusara O.H., Abu Huwaij R. Microemulsions as Lipid Nanosystems Loaded into Thermoresponsive In Situ Microgels for Local Ocular Delivery of Prednisolone. Pharmaceutics. 2022;14:1975. doi: 10.3390/pharmaceutics14091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClements D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- 18.Solans C., García-Celma M.J. Surfactants for microemulsions. Curr. Opin. Colloid Interface Sci. 1997;2:464–471. doi: 10.1016/S1359-0294(97)80093-3. [DOI] [Google Scholar]
- 19.Garti N., Libster D., Aserin A. Lipid polymorphism in lyotropic liquid crystals for triggered release of bioactives. Food Funct. 2012;3:700–713. doi: 10.1039/c2fo00005a. [DOI] [PubMed] [Google Scholar]
- 20.Martiel I., Baumann N., Vallooran J.J., Bergfreund J., Sagalowicz L., Mezzenga R. Oil and drug control the release rate from lyotropic liquid crystals. J. Control. Release. 2015;204:78–84. doi: 10.1016/j.jconrel.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Milak S., Zimmer A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015;478:569–587. doi: 10.1016/j.ijpharm.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 22.de Souza I.F.F., dos Santos T.Q., Placido R.V., Mangerona B.A., Carvalho F.C., Boralli V.B., Ruela A.L.M., Pereira G.R. The liquid crystalline phase behaviour of a nasal formulation modifies the brain disposition of donepezil in rats in the treatment of Alzheimer’s disease. Colloids Surf. B Biointerfaces. 2021;203:111721. doi: 10.1016/j.colsurfb.2021.111721. [DOI] [PubMed] [Google Scholar]
- 23.Aida K.L., Kreling P.F., Caiaffa K.S., Calixto G.M.F., Chorilli M., Spolidorio D.M., Santos-Filho N.A., Cilli E.M., Duque C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018;13:3081–3091. doi: 10.2147/IJN.S155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos M.A.D.S., Calixto G.M.F., de Toledo L.G., Bonifácio B.V., dos Santos L.C., de Almeida M.T.G., Chorilli M., Bauab T.M. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int. J. Nanomed. 2015;10:7455–7466. doi: 10.2147/IJN.S92638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonçalez M.L., Corrêa M.A., Chorilli M. Skin Delivery of Kojic Acid-Loaded Nanotechnology-Based Drug Delivery Systems for the Treatment of Skin Aging. BioMed Res. Int. 2013;2013:271276. doi: 10.1155/2013/271276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo C., Wang J., Cao F., Lee R.J., Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today. 2010;15:1032–1040. doi: 10.1016/j.drudis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Zhang Y., Gui S., Huang J., Cao J., Li Z., Li Q., Chu X. Characterization of Lipid-Based Lyotropic Liquid Crystal and Effects of Guest Molecules on Its Microstructure: A Systematic Review. AAPS PharmSciTech. 2018;19:2023–2040. doi: 10.1208/s12249-018-1069-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y., Ma P., Gui S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. BioMed Res. Int. 2014;2014:815981. doi: 10.1155/2014/815981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajabalaya R., Musa M.N., Kifli N., David S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Dev. Ther. 2017;11:393–406. doi: 10.2147/DDDT.S103505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah J.C. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001;47:229–250. doi: 10.1016/S0169-409X(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 31.Segre J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halder R.M., Nootheti P.K. Ethnic skin disorders overview. J. Am. Acad. Dermatol. 2003;48:S143–S148. doi: 10.1067/mjd.2003.274. [DOI] [PubMed] [Google Scholar]
- 33.Gladman A.C. Toxicodendron Dermatitis: Poison Ivy, Oak, and Sumac. Wilderness Environ. Med. 2006;17:120–128. doi: 10.1580/PR31-05.1. [DOI] [PubMed] [Google Scholar]
- 34.Howell M.D., Kim B.E., Gao P., Grant A.V., Boguniewicz M., DeBenedetto A., Schneider L., Beck L.A., Barnes K.C., Leung D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009;124:R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Luger T., Amagai M., Dreno B., Dagnelie M.-A., Liao W., Kabashima K., Schikowski T., Proksch E., Elias P.M., Simon M., et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021;102:142–157. doi: 10.1016/j.jdermsci.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Pfisterer K., Shaw L.E., Symmank D., Weninger W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021;9:682414. doi: 10.3389/fcell.2021.682414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huzil J.T., Sivaloganathan S., Kohandel M., Foldvari M. Drug delivery through the skin: Molecular simulations of barrier lipids to design more effective noninvasive dermal and transdermal delivery systems for small molecules, biologics, and cosmetics. WIREs Nanomed. Nanobiotechnol. 2011;3:449–462. doi: 10.1002/wnan.147. [DOI] [PubMed] [Google Scholar]
- 38.Dick I.P., Scott R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992;44:640–645. doi: 10.1111/j.2042-7158.1992.tb05485.x. [DOI] [PubMed] [Google Scholar]
- 39.Santos A.D.J., Macêdo N.A., Cavalcanti S.C.D.H., Sarmento V.H.V., Lira A.A.M., dos Santos C.P., Santos R.L.C., Nunes R.D.S. Larvicidal formulation containing N-tosylindole: A viable alternative to chemical control of Aedes aegypti. Colloids Surf. B Biointerfaces. 2022;213:112380. doi: 10.1016/j.colsurfb.2022.112380. [DOI] [PubMed] [Google Scholar]
- 40.Kralova I., Sjöblom J. Surfactants Used in Food Industry: A Review. J. Dispers. Sci. Technol. 2009;30:1363–1383. doi: 10.1080/01932690902735561. [DOI] [Google Scholar]
- 41.Salikolimi K., Sudhakar A.A., Ishida Y. Functional Ionic Liquid Crystals. Langmuir. 2020;36:11702–11731. doi: 10.1021/acs.langmuir.0c01935. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca-Santos B., Bonifácio B.V., Baub T.M., Gremião M.P.D., Chorilli M. In-Situ Gelling Liquid Crystal Mucoadhesive Vehicle for Curcumin Buccal Administration and Its Potential Application in the Treatment of Oral Candidiasis. J. Biomed. Nanotechnol. 2019;15:1334–1344. doi: 10.1166/jbn.2019.2758. [DOI] [PubMed] [Google Scholar]
- 43.Souto D.E., Fonseca A.M., Barragan J.T., Luz R.D.C., Andrade H.M., Damos F.S., Kubota L.T. SPR analysis of the interaction between a recombinant protein of unknown function in Leishmania infantum immobilised on dendrimers and antibodies of the visceral leishmaniasis: A potential use in immunodiagnosis. Biosens. Bioelectron. 2015;70:275–281. doi: 10.1016/j.bios.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Benigni M., Pescina S., Grimaudo M.A., Padula C., Santi P., Nicoli S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018;545:197–205. doi: 10.1016/j.ijpharm.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 45.Chorilli M., Prestes P.S., Rigon R.B., Leonardi G.R., Chiavacci L.A., Sarmento V.H.V., Oliveira A.G., Scarpa M.V. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf. B Biointerfaces. 2011;85:182–188. doi: 10.1016/j.colsurfb.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca-Santos B., dos Santos A.M., Rodero C.F., Gremião M.P.D., Chorilli M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int. J. Nanomed. 2016;11:4553–4562. doi: 10.2147/IJN.S108675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonseca-Santos B., Satake C.Y., Calixto G.M.F., dos Santos A.M., Chorilli M. Trans-resveratrol-loaded nonionic lamellar liquid-crystalline systems: Structural, rheological, mechanical, textural, and bioadhesive characterization and evaluation of in vivo anti-inflammatory activity. Int. J. Nanomed. 2017;12:6883–6893. doi: 10.2147/IJN.S138629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimura A.T., Martinez R.M., Pinho-Ribeiro F.A., da Silva A.M.L.D., Baracat M.M., Georgetti S.R., Verri W.A., Chorilli M., Casagrande R. Resveratrol-Loaded Liquid-Crystalline System Inhibits UVB-Induced Skin Inflammation and Oxidative Stress in Mice. J. Nat. Prod. 2016;79:1329–1338. doi: 10.1021/acs.jnatprod.5b01132. [DOI] [PubMed] [Google Scholar]
- 49.Naoui W., Bolzinger M.-A., Fenet B., Pelletier J., Valour J.-P., Kalfat R., Chevalier Y. Microemulsion Microstructure Influences the Skin Delivery of an Hydrophilic Drug. Pharm. Res. 2011;28:1683–1695. doi: 10.1007/s11095-011-0404-y. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y., Gui S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018;8:6978–6987. doi: 10.1039/C7RA12008G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Israelachvili J.N., Mitchell D.J., Ninham B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976;72:1525–1568. doi: 10.1039/f29767201525. [DOI] [Google Scholar]
- 52.Fong C., Le T., Drummond C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012;41:1297–1322. doi: 10.1039/C1CS15148G. [DOI] [PubMed] [Google Scholar]
- 53.Manaia E.B., Abuçafy M.P., Chiari-Andréo B.G., Silva B.L., Oshiro-Júnior J.A., Chiavacci L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017;12:4991–5011. doi: 10.2147/IJN.S133832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Badawi A.A., Nour S.A., Sakran W.S., El-Mancy S.M.S. Preparation and Evaluation of Microemulsion Systems Containing Salicylic Acid. AAPS PharmSciTech. 2009;10:1081–1084. doi: 10.1208/s12249-009-9301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szlezak M., Nieciecka D., Joniec A., Pękała M., Gorecka E., Emo M., Stébé M.J., Krysiński P., Bilewicz R. Monoolein Cubic Phase Gels and Cubosomes Doped with Magnetic Nanoparticles–Hybrid Materials for Controlled Drug Release. ACS Appl. Mater. Interfaces. 2017;9:2796–2805. doi: 10.1021/acsami.6b12889. [DOI] [PubMed] [Google Scholar]
- 56.Ryklin I., Byers B. Delivery System Handbook for Personal Care and Cosmetic Products. Elsevier; Amsterdam, The Netherlands: 2005. Shear-Thinning Lamellar Gel Network Emulsions as Delivery Systems; pp. 547–568. [Google Scholar]
- 57.Kwak M.-S., Ahn H.-J., Song K.-W. Rheological investigation of body cream and body lotion in actual application conditions. Korea Aust. Rheol. J. 2015;27:241–251. doi: 10.1007/s13367-015-0024-x. [DOI] [Google Scholar]
- 58.Gulão E.D.S., De Souza C.J.F., Da Costa A.R., Da Rocha-Leão M.H.M., Garcia-Rojas E.E. Stability and rheological behavior of coconut oil-in-water emulsions formed by biopolymers. Polímeros. 2018;28:413–421. doi: 10.1590/0104-1428.08017. [DOI] [Google Scholar]
- 59.Otsubo Y., Prud’Homme R.K. Flow behavior of oil-in-water emulsions. J. Rheol. 1993;37:561. doi: 10.1122/1.550423. [DOI] [Google Scholar]
- 60.Resende K.X., Corrêa M.A., De Oliveira A.G., Scarpa M.V. Effect of cosurfactant on the supramolecular structure and physicochemical properties of non-ionic biocompatible microemulsions. Rev. Bras. Ciências Farm. 2008;44:35–42. doi: 10.1590/S1516-93322008000100005. [DOI] [Google Scholar]
- 61.Oliveira M.B., Calixto G., Graminha M., Cerecetto H., González M., Chorilli M. Development, Characterization, andIn VitroBiological Performance of Fluconazole-Loaded Microemulsions for the Topical Treatment of Cutaneous Leishmaniasis. BioMed Res. Int. 2015;2015:396894. doi: 10.1155/2015/396894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carvalho F.C., Calixto G., Hatakeyama I.N., Luz G.M., Gremião M.P.D., Chorilli M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013;39:1750–1757. doi: 10.3109/03639045.2012.734510. [DOI] [PubMed] [Google Scholar]
- 63.Rigon C., Marchiori M.C.L., Jardim F.D.S., Pegoraro N.S., Chaves P.D.S., Velho M.C., Beck R.C.R., Ourique A., Sari M.H.M., de Oliveira S.M., et al. Hydrogel containing silibinin nanocapsules presents effective anti-inflammatory action in a model of irritant contact dermatitis in mice. Eur. J. Pharm. Sci. 2019;137:104969. doi: 10.1016/j.ejps.2019.104969. [DOI] [PubMed] [Google Scholar]
- 64.De Araújo P.R., Calixto G.M.F., Silva I., Zago L.H.D.P., Junior J.A.O., Pavan F., Ribeiro A.O., Fontana C.R., Chorilli M. Mucoadhesive In Situ Gelling Liquid Crystalline Precursor System to Improve the Vaginal Administration of Drugs. AAPS PharmSciTech. 2019;20:225. doi: 10.1208/s12249-019-1439-3. [DOI] [PubMed] [Google Scholar]
- 65.Rodero C.F., Calixto G.M.F., Dos Santos K.C., Sato M., Ramos M.A.D.S., Miró M.S., Rodríguez E., Vigezzi C., Bauab T.M., Sotomayor C.E., et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018;15:4491–4504. doi: 10.1021/acs.molpharmaceut.8b00507. [DOI] [PubMed] [Google Scholar]
- 66.Fonseca-Santos B., Pacheco C.D.N., Pinto M.C., Chorilli M. An effective mosquito-repellent topical product from liquid crystal-based tea tree oil. Ind. Crops Prod. 2019;128:488–495. doi: 10.1016/j.indcrop.2018.11.020. [DOI] [Google Scholar]
- 67.Hao Z.-Q., Chen Z.-J., Chang M.-C., Meng J.-L., Liu J.-Y., Feng C.-P. Rheological properties and gel characteristics of polysaccharides from fruit-bodies of Sparassis crispa. Int. J. Food Prop. 2018;21:2283–2295. doi: 10.1080/10942912.2018.1510838. [DOI] [Google Scholar]
- 68.Hassan M.A., Pathak M., Khan M.K. Thermorheological Characterization of Elastoviscoplastic Carbopol Ultrez 20 Gel. J. Eng. Mater. Technol. 2015;137:031002. doi: 10.1115/1.4030004. [DOI] [Google Scholar]
- 69.Ghica M.V., Hîrjău M., Lupuleasa D., Dinu-Pîrvu C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules. 2016;21:786. doi: 10.3390/molecules21060786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu T., Malcolm K., Woolfson D., Jones D.S., Andrews G.P. Vaginal gel drug delivery systems: Understanding rheological characteristics and performance. Expert Opin. Drug Deliv. 2011;8:1309–1322. doi: 10.1517/17425247.2011.600119. [DOI] [PubMed] [Google Scholar]
- 71.Torres L.G., Iturbe R., Snowden M.J., Chowdhry B.Z., Leharne S.A. Preparation of o/w emulsions stabilized by solid particles and their characterization by oscillatory rheology. Colloids Surf. A Physicochem. Eng. Asp. 2007;302:439–448. doi: 10.1016/j.colsurfa.2007.03.009. [DOI] [Google Scholar]
- 72.Mezger T. The Rheology Handbook: For Users of Rotational and Oscillator Y Rheometers. 5th ed. Vincentz Network; Hannover, Germany: 2020. [Google Scholar]
- 73.Tabilo-Munizaga G., Barbosa-Cánovas G.V. Rheology for the food industry. J. Food Eng. 2005;67:147–156. doi: 10.1016/j.jfoodeng.2004.05.062. [DOI] [Google Scholar]
- 74.Saxena A., Kaloti M., Bohidar H. Rheological properties of binary and ternary protein–polysaccharide co-hydrogels and comparative release kinetics of salbutamol sulphate from their matrices. Int. J. Biol. Macromol. 2011;48:263–270. doi: 10.1016/j.ijbiomac.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Maurer S., Junghans A., Vilgis T.A. Impact of xanthan gum, sucrose and fructose on the viscoelastic properties of agarose hydrogels. Food Hydrocoll. 2012;29:298–307. doi: 10.1016/j.foodhyd.2012.03.002. [DOI] [Google Scholar]
- 76.Sharma A., Rawat K., Solanki P.R., Aswal V., Kohlbrecher J., Bohidar H. Internal structure and thermo-viscoelastic properties of agar ionogels. Carbohydr. Polym. 2015;134:617–626. doi: 10.1016/j.carbpol.2015.07.094. [DOI] [PubMed] [Google Scholar]
- 77.Singh S.S., Aswal V.K., Bohidar H.B. Structural evolution of aging agar-gelatin co-hydrogels. Polymer. 2009;50:5589–5597. doi: 10.1016/j.polymer.2009.09.032. [DOI] [Google Scholar]
- 78.Carvalho F.C., Campos M.L., Peccinini R.G., Gremião M.P.D. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur. J. Pharm. Biopharm. 2013;84:219–227. doi: 10.1016/j.ejpb.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 79.Carvalho F.C., Barbi M.S., Sarmento V.H.V., Chiavacci L.A., Netto F.M., Gremião M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010;62:430–439. doi: 10.1211/jpp.62.04.0004. [DOI] [PubMed] [Google Scholar]
- 80.Jones D.S., Woolfson A.D., Djokic J. Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterization and investigation of interactions between formulation components. J. Appl. Polym. Sci. 1996;61:2229–2234. doi: 10.1002/(SICI)1097-4628(19960919)61:12<2229::AID-APP24>3.0.CO;2-0. [DOI] [Google Scholar]
- 81.Jones D.S., Woolfson A.D., Brown A.F. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm. Res. 1997;14:450–457. doi: 10.1023/A:1012091231023. [DOI] [PubMed] [Google Scholar]
- 82.Cardoso V.M.d.O., Cury B.S.F., Evangelista R.C., Gremião M.P.D. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J. Mech. Behav. Biomed. Mater. 2017;65:317–333. doi: 10.1016/j.jmbbm.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Huang M., Kennedy J., Li B., Xu X., Xie B. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr. Polym. 2007;69:411–418. doi: 10.1016/j.carbpol.2006.12.025. [DOI] [Google Scholar]
- 84.Bassi Da Silva J., Ferreira S.B.D.S., Reis A.V., Cook M.T., Bruschi M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers. 2018;10:254. doi: 10.3390/polym10030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cevher E., Sensoy D., Taha M.A.M., Araman A. Effect of Thiolated Polymers to Textural and Mucoadhesive Properties of Vaginal Gel Formulations Prepared with Polycarbophil and Chitosan. AAPS PharmSciTech. 2008;9:953–965. doi: 10.1208/s12249-008-9132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foegeding E.A. Rheology and sensory texture of biopolymer gels. Curr. Opin. Colloid Interface Sci. 2007;12:242–250. doi: 10.1016/j.cocis.2007.07.001. [DOI] [Google Scholar]
- 87.Estanqueiro M., Conceição J., Amaral M.H., Santos D., Silva J.B., Lobo J.M.S. Characterization and stability studies of emulsion systems containing pumice. Braz. J. Pharm. Sci. 2014;50:361–369. doi: 10.1590/S1984-82502014000200016. [DOI] [Google Scholar]
- 88.Smart J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Palacio M.L.B., Bhushan B. Bioadhesion: A review of concepts and applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012;370:2321–2347. doi: 10.1098/rsta.2011.0483. [DOI] [PubMed] [Google Scholar]
- 90.Parente M.E., Andrade A.O., Ares G., Russo F., Jiménez-Kairuz Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015;37:511–518. doi: 10.1111/ics.12227. [DOI] [PubMed] [Google Scholar]
- 91.Da Silva P.B., Calixto G.M.F., Júnior J.A.O., Bombardelli R.L., Fonseca-Santos B., Rodero C.F., Chorilli M. Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers. 2017;9:30. doi: 10.3390/polym9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oyafuso M.H., Carvalho F.C., Takeshita T.M., De Souza A.L.R., Araújo D.R., Merino V., Gremião M.P.D., Chorilli M. Development and In Vitro Evaluation of Lyotropic Liquid Crystals for the Controlled Release of Dexamethasone. Polymers. 2017;9:330. doi: 10.3390/polym9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ikeda Y., Murakami A., Ohigashi H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008;52:26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- 94.Checker R., Sandur S.K., Sharma D., Patwardhan R.S., Jayakumar S., Kohli V., Sethi G., Aggarwal B.B., Sainis K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE. 2012;7:e31318. doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martínez A.L., González-Trujano M.E., Chávez M., Pellicer F. Antinociceptive effectiveness of triterpenes from rosemary in visceral nociception. J. Ethnopharmacol. 2012;142:28–34. doi: 10.1016/j.jep.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 96.Tapondjou L.A., Lontsi D., Luc S.B., JongWon C., Kyung-Tae L., Hyun-Ju J., Hee-Juhn P. In Vivo anti-nociceptive and anti-inflammatory effect of the two triterpenes, ursolic acid and 23-hydroxyursolic acid, fromcussonia bancoensis. Arch. Pharmacal Res. 2003;26:143–146. doi: 10.1007/BF02976660. [DOI] [PubMed] [Google Scholar]
- 97.Verano J., González-Trujano M.E., Déciga-Campos M., Ventura-Martínez R., Pellicer F. Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol. Biochem. Behav. 2013;110:255–264. doi: 10.1016/j.pbb.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 98.Dante M.D.C.L., Borgheti-Cardoso L.N., Fantini M.C.D.A., Praça F.S.G., Medina W.S.G., Pierre M.B.R., Lara M.G. Liquid Crystalline Systems Based on Glyceryl Monooleate and Penetration Enhancers for Skin Delivery of Celecoxib: Characterization, In Vitro Drug Release, and In Vivo Studies. J. Pharm. Sci. 2018;107:870–878. doi: 10.1016/j.xphs.2017.10.039. [DOI] [PubMed] [Google Scholar]