Abstract
Stenotrophomonas maltophilia is a clinically relevant bacterial pathogen, particularly in cystic fibrosis (CF) patients. Despite the well-known ability to form biofilms inherently resistant to antibiotics and host immunity, many aspects involved in S. maltophilia biofilm formation are yet to be elucidated. In the present study, a proteomic approach was used to elucidate the differential protein expression patterns observed during the planktonic-to-biofilm transition of S. maltophilia Sm126, a strong biofilm producer causing chronic infection in a CF patient, to identify determinants potentially associated with S. maltophilia biofilm formation. In all, 57 proteins were differentially (3-fold; p < 0.01) expressed in biofilm cells compared with planktonic counterparts: 38 were overexpressed, and 19 were down-expressed. It is worth noting that 34 proteins were exclusively found in biofilm, mainly associated with quorum sensing-mediated intercellular communication, augmented glycolysis, amino acid metabolism, biosynthesis of secondary metabolites, phosphate signaling, response to nutrient starvation, and general stress. Further work is warranted to evaluate if these proteins can be suitable targets for developing anti-biofilm strategies effective against S. maltophilia.
Keywords: Stenotrophomonas maltophilia, biofilm formation, proteomic analysis, cystic fibrosis
1. Introduction
Stenotrophomonas maltophilia is an environmental microorganism associated with plant rhizosphere and involved in the nitrogen and sulfur cycles [1]. However, as a human opportunist, it represents an emerging pathogen of significant concern to susceptible patient populations, especially the elderly and immunocompromised persons [2]. Indeed, this microorganism can cause many opportunistic infections, including those of the respiratory tract, bloodstream and heart, nervous system, gastrointestinal tract, urinary tract, bone, and soft tissue [3].
In addition to these nosocomial and community-acquired infections, the chronic colonization of the airways of patients with cystic fibrosis (CF) is also increasingly reported. With about a 10% colonization rate in these patients, S. maltophilia represents an independent risk factor for pulmonary exacerbation associated with impaired lung function, lung transplantation need, and death [4].
Even though it is considered a low-grade pathogen, the increased occurrence of S. maltophilia nosocomial infections is primarily the result of alarmingly high drug resistance rates, further complicated by biofilm formation, a relevant virulence feature [3,5]. S. maltophilia can adhere to several surfaces and grow as a biofilm, a microbial consortium embedded in a self-produced polymeric matrix and inherently resistant to antibiotic therapy and the host immune response [6]. Several studies have recently indicated that the ability to form biofilm is highly conserved in clinically relevant S. maltophilia isolates [7]. Biofilm formation can occur on inert surfaces (e.g., respiratory tubes and nebulizers, intravenous cannulae, prosthetic devices, and dental unit waterlines), causing medical implant-associated infections [3]. Regarding the biotic surfaces, S. maltophilia adheres to HEp-2 cells, mucin, and tight junctions of human epithelial respiratory cells [4]. Interestingly, it colonizes bronchial CF-derived cells as biofilms [8], highlighting the significance of biofilm formation in the persistence of S. maltophilia in the airways of CF patients because of a selective adaptation to CF airways.
For these reasons, S. maltophilia biofilm research has recently accelerated using genomics profiling to understand better the mechanisms underlying biofilm formation. The genetic regulation of biofilm formation in S. maltophilia is complex, as suggested by the significant number of genes involved [6]. Several studies indicated that the ability of S. maltophilia to form biofilm is mainly influenced by bacterial virulence factors (e.g., flagella, pili, LPS/exopolysaccharide biosynthesis, motility) [7], cell surface traits (e.g., outer membrane proteins, hydrophobicity) [9,10], and quorum-sensing communication [11].
However, as already observed for other bacterial pathogens, S. maltophilia biofilm cells show gene expression patterns significantly different from those of their planktonic counterparts [12]. The findings from bacterial transcriptomic analysis do not necessarily correlate with the detected proteins and their functionality, particularly in the case of CF strains in which the low genotype-phenotype correlation is probably the “biological cost” bacteria must pay to adapt to a highly stressful environment such as CF lung [13]. In this respect, the proteomics approach can provide a glimpse into the presence of functional molecules.
Despite the wealth of information derived from genetic studies on biofilm formation [6], studies have yet to compare the protein expression profiles between biofilm and planktonic S. maltophilia cells. To gain insights into the underlying mechanisms of S. maltophilia biofilm formation, in the present work, we used a proteomic approach to assess the changes in protein expression of a CF S. maltophilia strain when growing in biofilm versus planktonic lifestyles. The findings from the present study will help deliver comprehensive knowledge about the cellular processes and metabolic pathways involved in S. maltophilia biofilm to come up with potential targets useful to biofilm-inhibiting strategies. In this regard, the comparative analysis of the proteomes showed distinct differences between the protein profiles and related metabolic pathways.
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
The S. maltophilia Sm126 strain, isolated from the airways of a CF patient with chronic infection, was used throughout this study. This strain was previously characterized as multidrug-resistant and with a propensity to produce a relevant biofilm [14].
Planktonic and biofilm cells for proteomic analysis were prepared from a standardized inoculum. Briefly, some colonies grew overnight on Muller-Hinton agar (Oxoid SpA; Garbagnate M.se, Milan, Italy) were suspended in 40 mL Trypticase Soy broth (TSB; Oxoid SpA) and incubated overnight at 37 °C, under agitation (130 rpm). Then, the broth culture was diluted with TSB to an OD550 of 1.5 and added to 560 mL TSB. The resulting 600 mL suspension was incubated at 37 °C under agitation (200 rpm) for 4 h to achieve a final OD550 of 0.6 (corresponding to 0.5–1 × 109 CFU/mL).
2.2. Collection of Planktonic Cells
A volume of 150 mL of the standardized inoculum was incubated at 37 °C for 6 h, under agitation (200 rpm), when an exponential growth phase was achieved, corresponding to an OD550 of 0.6 (0.5−1 × 109 CFU/mL). Planktonic cells were then harvested by centrifugation (10,000× g, 10 min, 4 °C), and the obtained pellet was washed thrice with Phosphate Buffer Solution (PBS) pH 7.3 (Merk Life Science Srl; Milan, Italy) before being subjected to lysis.
2.3. Collection of Biofilm Cells and Kinetics of Biofilm Formation
Sixty milliliters of the standardized inoculum were dispensed in each of five polystyrene, 150 mm diameter, tissue culture (TC)-treated Petri dishes (Iwaki). The plates were statically incubated at 37 °C for 24 h, then the biofilm samples were washed (3 times with PBS pH 7.3) and scraped to collect biofilm cells in a polypropylene vial (Nalgene; Thermo Scientific Italia) containing 150 mL of PBS. This suspension was centrifuged (10,000× g, 10 min, 4 °C), and the obtained pellet was washed three times with PBS and finally subjected to lysis.
The kinetics of biofilm formation in 96-well polystyrene microplates was spectrophotometrically monitored over 72 h using a crystal violet-based colorimetric assay [9].
2.4. Bacterial Lysis and Protein Extraction
Lysis was carried out using a Q Proteome Bacterial Protein Prep kit (Qiagen srl; Milan, Italy) containing 100 mg/mL lysozyme. Benzonase 0.25 U/µL (Sigma-Aldrich srl; Milan, Italy) was then added to degrade nucleic acids. Samples were incubated at room temperature on ice, then centrifuged (14,000× g, 30 min, 4 °C); the supernatant was collected in polypropylene vials (Nalgene) and newly centrifuged (50,000× g, 30 min). Aliquots of the supernatant underwent to SpeedVac (mod. SC110-SAVANT) and were finally stored at −80 °C until protein purification. Before purification, the three samples obtained by independent extractions were pooled for planktonic and biofilm samples [15]. Samples were purified using a Ready Prep 2D Cleanup kit (Bio-Rad; Milan, Italy), and the protein precipitates were treated with 50 µL solubilization buffer for two-dimensional gel electrophoresis (2-DE) (2 M thiourea, 7 M urea, 50 mM DTT, 4% CHAPS, 0.2% Bio-Lyte 3/10 ampholyte, and 0.002% bromophenol blue). After protein resuspension, each sample was centrifuged at 12,000× g, and the clear phase was transferred to a new vial where the buffer was added to yield a final volume of 450 µL. Protein concentration was determined, according to Lowry, by RC-DC Protein Assay (Bio-Rad). Concentrations of 8 and 16 µg/µL were obtained for planktonic and biofilm samples, respectively. Samples were finally stored at −20 °C until needed.
2.5. 2-D Electrophoresis
Immobilized pH gradient precast Ready Strips with a non-linear gradient (pH 3 to 10) (Bio-Rad) were rehydrated for 12 h with 80 Wg protein in 8 M urea, 2 mM tributyl phosphine, 2% ampholytes pH 3–10, 2% CHAPS and traces of bromophenol blue. The first dimension was carried out using the Protean IEF Cell (Bio-Rad) for 63.7 kVh. After isoelectric focusing, the strips were first equilibrated for 15 min in equilibration buffer (6 M urea, 0.375 M Tris pH 8.8, 2% SDS, and 20% glycerol). The same solution with 2.5% iodoacetamide was used for further 15 min equilibration period. The second-dimensional separation was a vertical SDS-PAGE with 12.5% acrylamide resolving gels in Protean II XL (Bio-Rad). Separation was performed at 20°C under constant amperage (40 mA). Gels were stained with 0.15% colloidal Coomassie blue G-250 (Sigma-Aldrich srl; Milan, Italy) for 48 h, then scanned using a GS-800 Imaging densitometer (Bio-Rad).
2.6. Analysis of Protein Patterns
Six gels resulting from three independent protein extractions were comparatively analyzed for each condition (planktonic, biofilm). Only reproducible spots present in four to six gels were considered in the analysis. Gels were analyzed with PD-Quest software (Bio-Rad; ver. 7.0) for qualitative and quantitative analysis of protein spots. Statistical analysis was performed by GraphPad (GraphPad Software Inc.; Boston, MA, USA): Student’s t-test was carried out to ensure significant (p < 0.05) changes in the value of protein spots. Calibration of gels with an isoelectric point (pI) and molecular masses was carried out using internal 2-D SDS-PAGE protein standards (Bio-Rad).
2.7. Protein Identification by MALDI-TOF-MS and N-Terminal Sequencing
The 2-D gel protein spots, stained with Coomassie blue, were digested with 0.5 µg/µL trypsin. Peptides were mixed with a 10 mg l31 solution of K-cyano-4-hydroxycinnamic acid, placed on the sample plate to air-dry, then analyzed by MALDI-TOF-MS (Reflex IV, Bruker Daltonics, Brehme, Germany). Monoisotopic peptide masses obtained from mass spectra were acquired, and proteins were identified by matching the sequences derived from peptide MS/MS spectra with sequences in the S. maltophilia protein sequence database (https://www.ncbi.nlm.nih.gov/protein; accessed on 1 February 2015) using Mascot Daemon database searching software (MatrixScience; ver. 2.7). The following parameters were used in the searches: protein molecular mass range from 1000 to 100,000 Da, trypsin digest with one missing cleavage, fragment ion mass tolerance of T 50 ppm, and possible methionine oxidation. N-terminal amino acid sequences were obtained by transferring the selected proteins to polyvinylidene difluoride membranes (Sequi-Blot PVDF Membrane, Bio-Rad), and then micro-sequenced using an automatic Beckman/Porton LF3000 protein sequencer. Sequence homology was evaluated with the BLAST program for MALDI-TOF-MS and N-terminal sequencing identification.
2.8. Scanning Electron Microscopy
Biofilm samples were allowed to form onto 35-mm TC-treated polystyrene dishes (Iwaki), air-dried overnight, and then fixed for 16 h in 2% (v/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.15 M sodium cacodylate buffer pH 7.4, added with the cationic dye 0.1% alcian blue (Polysciences Europe, Germany). Samples were rinsed three times in 0.2 M cacodylate buffer for 10 min and post-fixed for 1 h in 1% OsO4 (v/v). Samples were rinsed in 0.15 M cacodylate buffer and then dehydrated in a graded ethanol series (50, 70, 80, 95, and 100%) before critical point drying. Samples were mounted on aluminum stubs, coated with 15-nm Au film, and then observed with a Philips XL30CP SEM.
3. Results and Discussion
3.1. Growth Kinetics of Planktonic and Biofilm S. maltophilia Cells
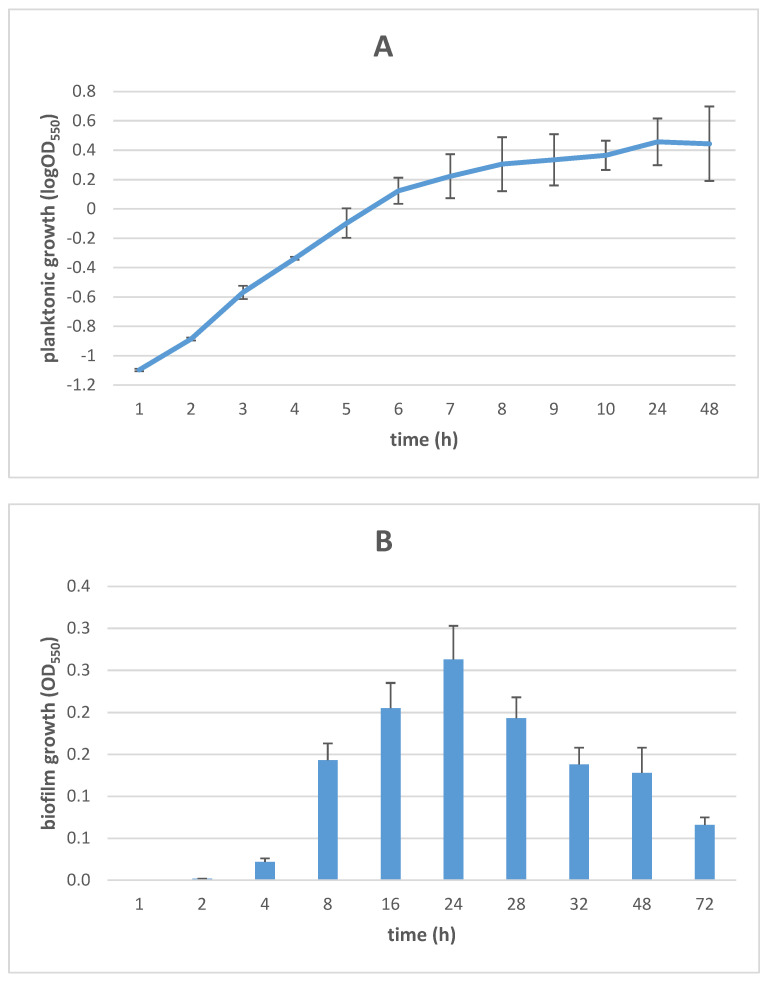
To standardize the preparation of samples for proteomics analysis, growth curves of planktonic and biofilm cells were obtained under the experimental conditions used, as shown in Figure 1. The growth of planktonic cells was spectrophotometrically monitored over 48 h, as previously described [13]. The growth curve analysis revealed an exponential phase entry after 4 h-incubation (OD550: 0.46), lasting up to 8 h (OD550: 2.02) (Figure 1A). Therefore, planktonic cells for proteomic analysis were collected following 6 h-incubation when an OD550 value of ~1.33 (corresponding to ~1 × 109 CFU/mL) is reached as indicative of the mid-exponential phase.
Figure 1.
Growth kinetics of planktonic and biofilm S. maltophilia Sm126 cells. (A) Planktonic kinetics was measured in Trypticase Soy broth, under dynamic conditions, and results are shown as the log of mean OD550 ± SD values (n = 6). (B) Biofilm formation kinetics was measured in a 96-well polystyrene microplate using a crystal violet-based colorimetric assay, and results are shown as mean OD550 + SD values (n = 6).
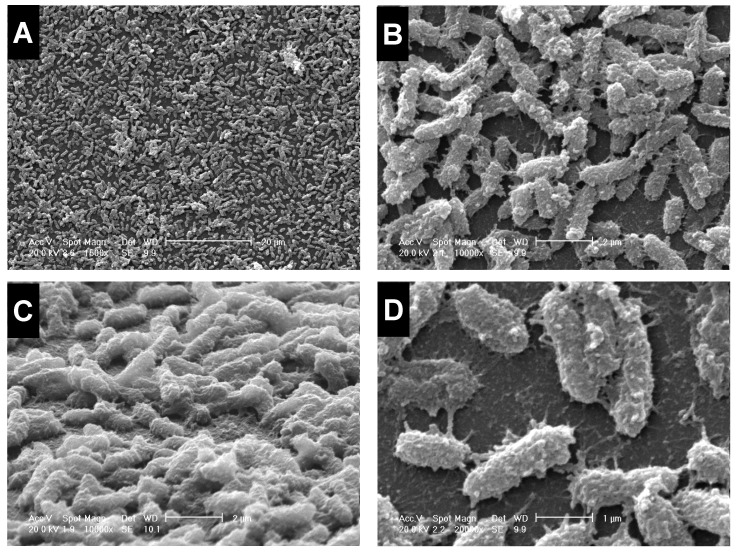
The kinetics of biofilm formation on polystyrene is shown in Figure 1B. The biofilm biomass increased over time until 24 h-incubation when a plateau was reached (OD550: 0.263), then gradually decreasing towards 72 h. As a result, the biofilm cells for proteomic analysis were collected after 24 h-incubation when the biofilm differentiated into a “mature” structure consisting of a multilayer architecture embedded in large amounts of extracellular matrix appearing as fine polymeric fibrils bridging the cells, as shown by scanning electron microscopy analysis (Figure 2).
Figure 2.
Scanning electron microscopy observation of S. maltophilia mature biofilm. The Sm126 strain was allowed to form biofilm onto 35-mm TC-treated polystyrene dishes for 24 h at 37 °C under static conditions. Magnifications: ×1.500 (A); ×10.000 (B); ×10.000 (C) (60°-inclined); ×20.000 (D).
3.2. Proteomic Analysis: Biofilm versus Planktonic Lifestyles
To identify the differentially expressed proteins in S. maltophilia during the transition from planktonic to sessile lifestyles, the proteomic profiles expressed under these conditions were compared using 2-DE, and peptide sequences were identified by MALDI-TOF. The identification of proteins of interest was then carried out by research in the sequenced genome of S. maltophilia and by analysis of sequence homology with known proteins of other microorganisms. Although the genome of S. maltophilia has been sequenced, the functions of most genes have yet to be defined [16].
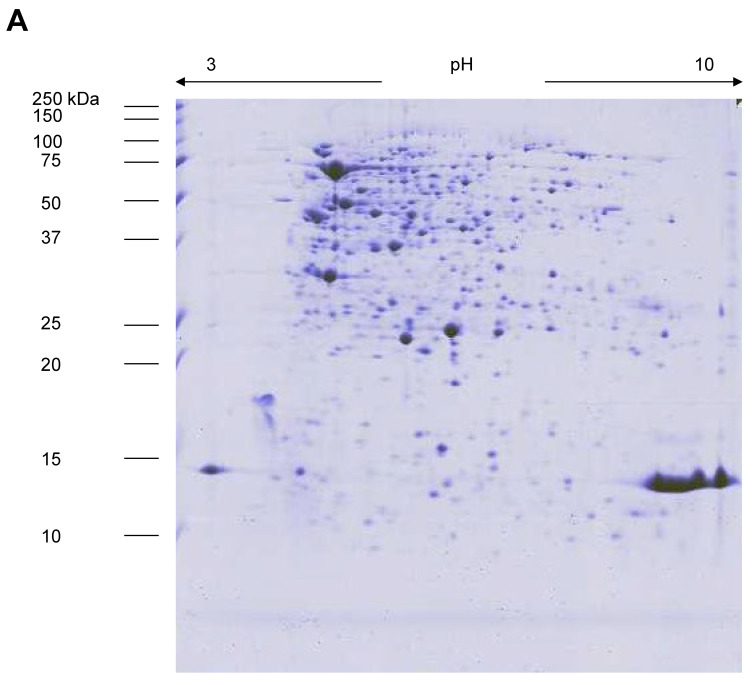
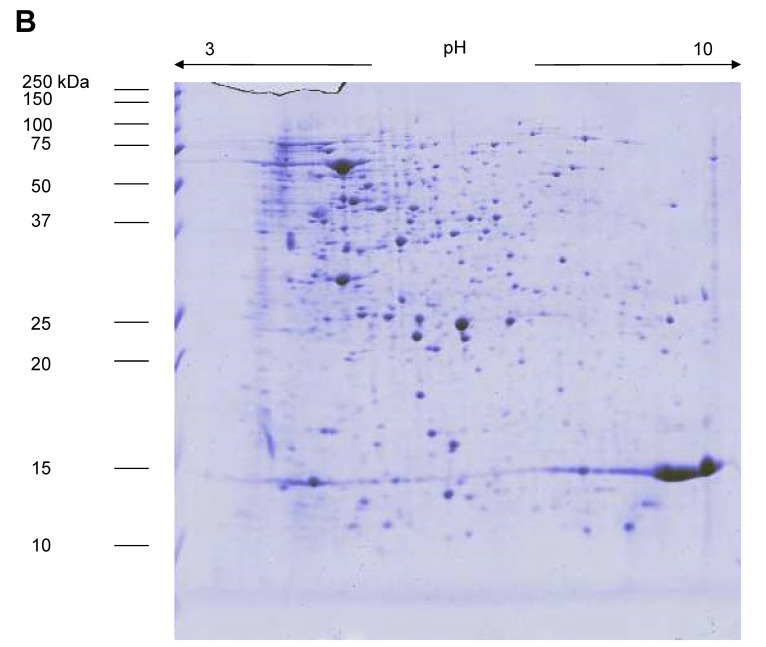
The image analysis showed approximately 350 spots/gels after staining with colloidal blue Coomassie G-250, irrespective of the sample (planktonic or biofilm cells) considered (Figure 3). Most proteins migrated with pI between pH 4 and 8. High-reproducible gels with a correlation coefficient of at least 80% could be achieved. Among these 350 spots, the comparison of 2-DE proteomic maps in the considered pH range (3–10 non-linear) revealed 198 protein spots not differentially expressed between biofilm and planktonic cells. On the contrary, 149 spots displayed statistically significant differences (at least 1.5-fold; p < 0.05, t-test) in their level of protein expression between biofilm and planktonic growth. Particularly, 76 in biofilm cells (42 upregulated and 34 exclusive proteins) (Supplementary Materials, Tables S1 and S2), and 73 in planktonic cells (56 upregulated and 17 exclusive proteins) (Supplementary Materials, Tables S3 and S4).
Figure 3.
The 2-DE gel electrophoresis of S. maltophilia Sm126 proteins from biofilm (A) and planktonic (B) cells.
To strengthen the comparison between the biofilm and planktonic growth states among the 149 differently expressed spots, only those displaying a signal >1000 ppm, and with a difference of ≥ 3-fold between biofilm and planktonic growth (p < 0.01, t-test) in the signal intensity were selected and underwent identification by MALDI-TOF-MS and N-terminal sequencing. Specifically, 34, 19, and 38 spots were respectively exclusive, upregulated, or downregulated in biofilm cells. Not all the selected spots could be identified due to technical reasons (i.e., no sequence found due to the incomplete genomic database or the lacking sequence for the strain under study; lack of a sufficient amount of available protein). In addition, some proteins occurred as isoforms, and most were characterized by small mass changes but clearly different pI values.
3.3. Proteins and Related Pathways Expressed Only in Biofilm Cells
Sixteen proteins, among those present only in biofilm cells, were identified by sequence homology with those deposited in databases (software Mascot, ver. 2.7; Matrix Science): fourteen in the S. maltophilia database, while two were matched with those in the database of other bacteria (i.e., B1305 from Streptomyces sviceus, B3102 from Geobacter spp.) (Table 1).
Table 1.
Proteins (n = 16; signal >1000 ppm; p < 0.01, t-test) expressed only in biofilm-grown S. maltophilia Sm126 cells.
| Spot No. | Protein | Biological Process | Molecular Function | Peptides Matched a | Sequence Coverage b | pI c | MW d | GI e |
|---|---|---|---|---|---|---|---|---|
| B1305 | regulatory protein | Not known | Not known | 6 | 33 | 4.83/5.96 | 36,096/25,543 | 197784663 |
| B5613 | putative amidohydrolase | Nitrogen compound metabolic process | Hydrolase activity | 11 | 31 | 5.65/6.38 | 49,616/52,720 | 190575191 |
| B6708 | putative electron transfer flavoprotein-ubiquinone oxidoreductase | Energy production and conversion |
Electron-transferring-flavoprotein dehydrogenase activity; Metal ion binding | 11 | 29 | 5.89/6.60 | 61,233/72,075 | 190572277 |
| B8509 | putative 3-ketoacyl-CoA thiolase | Fatty acid beta-oxidation |
Acetyl-CoA C-acyltransferase |
14 | 43 | 7.15/8.66 | 46,154/45,239 | 190572243 |
| B7007 | hypothetical protein Smal | Not known | Not known | 8 | 48 | 6.09/7.19 | 16,432/11,577 | 194367327 |
| B4409 | putative isocitrate/isopropylmalate dehydrogenase | Leucine biosynthesis | Oxidoreductase activity; Mg and NAD binding | 16 | 57 | 5.61/6.17 | 35,841/39,353 | 190573015 |
| B3102 | chorismate synthase | Aromatic amino acid family biosynthetic process |
Lyase chorismate synthase activity |
13 | 45 | 5.41/5.56 | 42,701/20,525 | 191161246 |
| B3712 | putative acetyl-coenzyme A synthetase | Acetyl-CoA biosynthesis from acetate | Metal ion binding; AMP/ATP binding; Acetate-CoA ligase activity |
17 | 28 | 5.55/5.61 | 72,107/67,921 | 190576408 |
| B6109 | putative heat shock protein | Not known | Not known | 10 | 74 | 5.74/6.61 | 19,550/17,758 | 190575907 |
| B8812 | conserved hypothetical exported protein | Not known | Catalytic activity | 28 | 43 | 8.76/8.73 | 77,975/83,683 | 190576412 |
| B3808 | putative polyribonucleotide phosphorylase | RNA processing; mRNA catabolic processing; 3′-5-exoribonuclease activity; RNA binding; Polyribonucleotide nucleotidyl-transferase |
Nucleotidyl-transferase | 20 | 34 | 5.42/5.63 | 75,448/79,698 | 190575258 |
| B7203 | phosphoglycerate mutase 1 family | Gluconeogenesis; Glycolysis |
Isomerase | 9 | 54 | 6.51/7.24 | 28,036/26,920 | 194364976 |
| B5617 | putative 4-aminobutyrate-2-oxoglutarate aminotransferase | Alanine, glutamate, and glutamine metabolism | Pyridoxal phosphate binding; 4-amino-butyrate:2-oxoglutarate transaminase activity | 9 | 26 | 5.82/6.58 | 52,261/54,309 | 190573417 |
| B8206 | 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase | Lysine and diamonipimelate biosynthesis | 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase activity |
8 | 18 | 9.71/8.76 | 37,654/29,196 | 190573512 |
| B9202 | putative dihydrolipoamide succinyltransferase, E2 component | Tricarboxylic acid cycle | dihydrolipoyllysine-residue succinyltransferase activity | 9 | 18 | 5.71/9.39 | 41,992/26,817 | 190575085 |
| B6314 | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | Gluconeogenesis; Glycolysis |
2,3-bisphosphoglycerate-dependent phosphoglycerate mutase activity | 11 | 61 | 6.32/6.93 | 28,062/27,123 | 190573432 |
a Number of tryptic peptides matched to protein sequence based on MS/MS spectra. b Percentage of amino acid coverage (peptides observed/theoretical number from sequence data given in S. maltophilia database). c Theoretical and practical pI of the protein. d Theoretical and practical mass, expressed in Da, of the protein. e GI accession (GenInfo identifier; sequence identification).
3.3.1. Quorum Sensing-Mediated Intercellular Communication
Gram-negative bacteria modulate and synchronize gene expression involved in pathogenicity through a mechanism based on the population density called “quorum sensing” (QS) [17]. It acts by the production and perception by the bacterial population of one or more diffusible “signal” molecules whose concentration reflects cell density. The signal reaching a threshold concentration is perceived by a sensor system that activates the QS [17]. The most frequently produced signals from Proteobacteria have been grouped in the class of N-acyl homoserine lactones (N-AHSLs), almost always synthesized through a LuxI-like synthase and perceived through a sensor-regulator system LuxR-like [18]. In S. maltophilia, the main QS system relies on the Diffusible Signal Factor (DSF) cis-11-methyl-2-dodecenoic acid, which modulates motility, biofilm formation, antibiotic resistance, and virulence [19]. DSF produced by S. maltophilia influences several virulence traits of P. aeruginosa—e.g., biofilm formation and antibiotic resistance—and its persistence in CF lungs [20]. On the other hand, although S. maltophilia cannot produce AHLs, it also responds to AHL signal molecules produced by P. aeruginosa [21].
The existence of QS makes it plausible that both the host and the bacteria could have developed “quorum quenching” (QQ) strategies to interfere with this intercellular communication system. Confirming this, N-AHSLs can be rapidly degraded in nature [22] by environmental pH and temperature or by bacteria, plants, and animals through the production of antagonists [23] or degrading enzymes such as lactonases [24] and starch hydrolases [25]. In this frame, we demonstrated for the first time that S. maltophilia specifically produces starch hydrolase (B5613 protein; S. maltophilia K279a) in the biofilm phenotype, suggesting the presence of a QQ system. Indeed, the cleavage of N-AHSLs by this enzyme might generate homoserine and an acyl chain that cannot spontaneously regenerate a functional QS signal.
This ability might have relevant implications in CF interspecies communication. S. maltophilia might indeed attenuate P. aeruginosa pathogenicity by a QQ-based mechanism, degrading N-AHL signal molecules that modulate several virulence traits of P. aeruginosa—e.g., biofilm formation and antibiotic resistance—and its persistence in CF lungs [20,26]. Further studies aimed at understanding the behavior of bacterial populations during CF infection and testing the therapeutic QQ antivirulence potential of S. maltophilia biofilms are warranted.
3.3.2. General Stress Response and Nutrient Starvation
Our findings showed that some proteins exclusively expressed in S. maltophilia Sm126 biofilm-grown cells are involved in nutrient limitation and general stress response, including a putative heat shock protein (B6109 protein), the acetyl-CoA synthetase (B3712 protein), and chorismate-synthetase (B3102 protein).
Putative Heat Shock Protein (B6109 Protein)
Universal stress proteins are expressed in nearly all bacteria, playing a role in adaptation to oxidative stress, low pH, high temperature, and hypoxia [27]. Heat shock proteins (HSPs) are implicated in the bacterial response to environmental stresses and the pathogenesis of infection. For example, in P. aeruginosa the HSP DNA has a significant effect on pathogenicity, promoting bacterial adhesion and biofilm formation [28]. This effect is mainly due to a decreased extracellular DNA—one of the most critical components in biofilms—secondary to the DNA-mediated reduction of the QS-autoinducer 2-heptyl-3-hydroxy-4(1H) -quinolone [28].
Acetyl-CoA Synthetase (B3712 Protein)
To survive, many cells must be able to modulate their physiology, growing rapidly in the presence of abundant nutrients and then slowdown in nutrient deficiency. During this transition, the bacteria pass from a program of rapid growth, which produces and frees the acetate (dissimilation) in the environment, to a program of slow growth facilitated by the uptake and utilization (assimilation) of the acetate previously excreted. This phenomenon, called the “acetate switch”, occurs when cellular metabolism reduces the environmental content in acetate-producing sources (acetogenins), such as D-glucose or L-serine, and they begin to depend on their ability to recover environmental acetate [29]. The key molecule in the “acetate switch” is acetyl-coenzyme A (acetyl-CoA), a high-energy intermediate of the central metabolism. Recently there has been a renewed interest in this phenomenon resulting from the theory that acetyl-phosphate, a high-energy intermediate of the dissimilation pathway, can work similarly to a global signal [29]. In particular, considerable evidence supports the regulatory role of acetyl-phosphate in numerous cellular processes. Acetyl-phosphate is associated with a reduction in gene expression involved in flagellar synthesis [30]. On the other hand, it causes increased gene expression related to the assembly of pilus type I, the synthesis of colonic acid (extracellular polysaccharide or capsule), and the response to multiple stresses. It has also been suggested that acetyl-phosphate coordinates the expression of surface structures and cellular processes involved in biofilm formation [31]. It is fascinating, in this regard, the recent observation that the formation and disintegration of structures such as biofilm, formed intracellularly by Escherichia coli uropathogens, depend on the same cellular structures regulated by acetyl-phosphate, such as flagella, pili-type 1 and capsule [32]. The induction of acetyl-CoA synthetase in the biofilm of S. maltophilia could, therefore, be the consequence of the “acetate switch” during the cellular transition from a fast-growing physiological program (planktonic forms) to one characterized by cellular quiescence (sessile forms formed biofilm).
Chorismate-Synthetase (B3102 Protein) and Polyribonucleotide phosphorylase (B3808 Protein)
It has been suggested that under low levels of folic acid, some enzymes of the shikimate pathway, aimed at the biosynthesis of folates and aromatic amino acids, may be considered excellent targets for antibiotic therapy since, essential in bacteria, fungi, and other plants, such enzymes are not present in animal cells. The chorismate-synthetase is the seventh enzyme of this pathway where it catalyzes the NADH- and FMN-dependent synthesis of chorismate, a precursor of aromatic amino acids (phenylalanine, tyrosine, and tryptophan), naphthoquinones (plant pigments with antibiotic and immunomodulatory activity), and menaquinones (vitamins K). It has recently been seen that cyanobacteria are provided with QS and release the mediator C8-AHL (N-octanoyl homoserine lactone). In response to C8-AHL, during the self-induction phenomenon, cyanobacteria have been found to increase their levels of chorizo-synthase [33] significantly. The chorismate-synthase observed exclusively in the biofilm phenotype of S. maltophilia again supports the hypothesis that this microorganism is also equipped with a QS N-AHSL.
Polyribonucleotide phosphorylase (PNPase) is a conserved enzyme in bacteria, plants, and mammals where it affects gene expression [34]. It is involved in two reactions [35]: (i) the processive phosphorolytic degradation of mRNA from the 3′-end, and (ii) a polymerase activity catalyzing reaction using nucleoside diphosphates as substrates to add NMPs to the 3′-end of the RNA chain. In addition, PNPase can be directly or indirectly involved in several functions, such as virulence, stress responses, biofilm formation, and motility [35]. In Salmonella enterica Serovar Typhimurium, PNPase negatively controls biofilm formation by affecting CsgD expression [36]. At the same time, in E. coli it represses poly-N-acetylglucosamine production [37] and the expression of protein 43, an outer membrane protein promoting cell aggregation and affecting motility [38]. Considering that PNPase negatively modulates biofilm formation, the exclusive production we observed in S. maltophilia biofilms warrants further investigation.
3.3.3. Carbohydrate Metabolism and Energy Production
Other proteins exclusive to S. maltophilia biofilm are involved in sugar metabolism and energy generation
The induced expression of 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase and phosphoglycerate mutase (B6314 and B7203 proteins) in biofilm samples confirmed earlier findings indicating that this glycolytic enzyme (GpmA) modulates biofilm formation in S. maltophilia on airway epithelial cells and abiotic surfaces [39]. It has been hypothesized that amino acid carbons are shuttled into the central metabolism through GpmA and toward pentose phosphate metabolism, producing an unknown compound able to stimulate growth and biofilm [40]. A greater understanding of these unique metabolic pathways is needed to define novel methods for inhibiting S. maltophilia growth and biofilm. This enzyme is also involved in the fermentative pathway. It is well known that oxygen tension is significantly reduced in the deeper layers of the biofilm [41] and that E. coli gene expression is altered due to reduced oxygen availability [42]. For this reason, the expression of phosphoglycerate-mutase could be a consequence of the poor oxygenation in the biofilm formed by S. maltophilia. On the other hand, glycolytic enzymes have been shown to have additional properties when located at the cell surface. For example, streptococcal α-enolase can bind plasminogen highly when associated with cell surface [43]. Similarly, in Staphylococcus aureus, a superficial protein, the glycolytic glyceraldehyde-3-phosphate dehydrogenase enzyme, with high transferrin affinity, has been identified. Therefore, the glycolytic pathway is one of the essential factors among biofilm survival mechanisms in S. maltophilia. As in other microorganisms, the expression of glycolytic enzymes in S. maltophilia biofilm can be regulated, not only by oxygen but also by other stimuli, thus indicating they play a role in a complex microbial population.
The second protein exclusively expressed in biofilm cells was identified as isocitrate dehydrogenase (B4409 protein) (IDH), a key enzyme in the tricarboxylic acid cycle, which is involved in biofilm formation by Bacillus cereus and S. aureus. In both species, the complete loss of IDH reduces biofilm yield and changes its morphology, while the growth seems not to be affected [44,45]. Specifically, in S. aureus, the phosphorylation of IDH resulted in the complete loss of activity and was restored upon dephosphorylation [45]. It has been suggested that IDH, in B. cereus and S. aureus, may regulate biofilm formation acting on intracellular redox homeostasis [44,45]. No studies have been published in this regard on S. maltophilia. However, the induced expression of the hypothetical oxidoreductase protein SACOL1851 we observed might indicate a similar mechanism also in S. maltophilia.
3.3.4. Other Pathways
Regarding the hypothetical protein Smal (B7007 protein), although the structure of this protein has not been determined previously, it is probably a phosphate-binding protein from S. maltophilia (UniProt ID B4SL31; gene ID Smal_2208). Because biofilm formation is commonly controlled by phosphate signaling, SmeI might play a role in biofilm formation by mediating the cellular uptake of phosphate ions [46]. In P. aeruginosa, a functionally similar protein, PstS, has been shown to contribute to the structural integrity of biofilm, even though phosphate binding per se is not required for PstS biofilm activity [47]. Future studies are needed to characterize how SmaI contributes to biofilm formation in S. maltophilia, and whether it may represent a novel target for antibiofilm strategies.
No studies were published on the involvement of other biofilm-related proteins—putative electron transfer flavoprotein-ubiquinone oxidoreductase (B6708), putative 3-ketoacyl-CoA thiolase (B8509), conserved hypothetical exported protein (B8812), putative 4-aminobutyrate-2-oxoglutarate aminotransferase (B5617), 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase (B8206), and putative dihydrolipoamide succinyltransferase E2 component (B9202)—in bacterial biofilm formation. Therefore, the function of these proteins in S. maltophilia biofilm production remains unclear, thus warranting further studies.
3.4. Proteins Significantly Upregulated in Biofilm Cells
Nine protein spots upregulated in the biofilm cells were identified, all by searching the S. maltophilia database (Table 2).
Table 2.
Proteins (n = 9; signal > 1000 ppm; ≥ 3-fold vs. planktonic growth; p < 0.01, t-test) significantly upregulated in biofilm-grown S. maltophilia Sm126 cells.
| Spot No. | Protein | Biological Process | Molecular Function |
Fold Up-Regulation a | Peptides Matched b | Sequence Coverage c | pI d | MW e | GI f |
|---|---|---|---|---|---|---|---|---|---|
| MB4713 | Putative urocanate hydratase | Aminoacid degradation |
Conversion of urocanate to 4-imidazolone-5-propionate | 7.8 | 13 | 30 | 5.58/6.06 | 60,368/69,065 | 190575003 |
| MB4807 | Putative zinc metallopeptidase |
Cell division; Protein catabolysis; Proteolysis |
Metallopeptidase activity; Zinc ion binding |
10.9 | 15 | 25 | 5.73/6.05 | 76,512/82,630 | 190572608 |
| MB1806 | Putative 30S ribosomal protein | Protein synthesis | Protein synthesis | 3.0 | 4.44/4.79 | 66,234/72,144 | 1702195087 | ||
| MB6403 | Putative aminotransferase |
Protein metabolism; Gluconeogenesis | Transamination | 4.7 | 7 | 31 | 6.07/6.91 | 42,450/43,319 | 190575152 |
| MB6405 | Putative NADP-dependent alcohol dehydrogenase | Response to reactive oxygen species | Metal ion binding; Butanol dehydrogenase activity | 4.3 | 4 | 17 | 5.71/6.93 | 38,658/41,493 | 190575829 |
| MB7303 | Putative threonine 3-dehydrogenase | Threonine catabolism |
L-threonine 3-dehydrogenase activity | 3.5 | 20 | 47 | 6.21/7.26 | 37,502/35,401 | 190572994 |
| MB7502 | Putative serine hydroxymethyltransferase | Glycine biosynthesis from serine |
Glycine hydroxymethyltransferase activity | 10.1 | 9 | 27 | 6.12/7.44 | 45,122/49,476 | 190572766 |
| MB8406 | Conserved hypothetical protein |
Not known | Not known | 7.8 | 22 | 59 | 7.15/5.99 | 43,882/85,923 | 190576469 |
| MB8602 | Putative biotin carboxylase |
Fatty acid biosynthesis |
ATP binding; Metal ion binding |
5.2 | 13 | 34 | 6.55/7.97 | 49,609/60,821 | 190576068 |
a Ratio of mean percentage integrated optical density for each protein from biofilm-grown and planktonic S. maltophilia cells (n = 6). b Number of tryptic peptides matched to protein sequence based on MS/MS spectra. c The percentage of amino acid coverage (peptides observed/theoretical number from sequence data given in S. maltophilia database). d Theoretical and practical pI of the protein. e Theoretical and practical mass, expressed in Da, of the protein. f GI accession (GenInfo identifier; sequence identification).
3.4.1. Putative Urocanate Hydratase
The first three enzymatic steps by which organisms degrade L-histidine to L-glutamate eventually lead to purine/pyrimidine biosynthesis via carbamoyl-phosphate and carbamoyl-aspartate, which are universally conserved in many species from all kingdoms of life. A histidine ammonia-lyase catalyzes 1,2-elimination of the α-amino group from l-histidine; a urocanate hydratase converts urocanate to 4-imidazolone-5-propionate, and this intermediate is hydrolyzed to N-formimino-l-glutamate by an imidazolone-propionase [48]. In S. aureus, the endogenous production of glutamate—a major amino acid in central metabolism—enhances biofilm formation in conjunction with the TCA and urea cycles [49]. In other work, Hassanov et al. [50] showed that the development of Bacillus subtilis, Enterococcus faecalis, and P. aeruginosa biofilms depends specifically on the use of glutamine or glutamate as a nitrogen source, contrarily to planktonic cells. Specifically, in B. subtilis biofilms, glutamine is necessary only for the dividing cells at the edges, while the inner cells use lactic acid, thus indicating a differential metabolic requirement within the biofilm. Our results confirmed that glutamine is an essential trigger for biofilm development also in S. maltophilia; targeting the metabolism might lead to novel anti-biofilm therapies.
3.4.2. NADP-Dependent Alcohol Dehydrogenase
ADH is one of the dehydrogenase enzymes occurring in many organisms to catalyze ethanol metabolism. An increasing number of evidence demonstrated that ADH is also involved in the bacterial QS system playing a crucial role in bacteria biofilm formation. Both in clinical and reference, Acinetobacter baumannii, S. aureus, and P. aeruginosa strains ADH leads to dose-dependent increases in bacterial growth, motility, and biofilm development, along with an increased expression of QS-related genes [51]. In another work, Becker et al. [52] reported upregulated in S. aureus biofilms by ADH1. Contrarily, the disruption of ADH1 significantly enhanced the ability of Candida albicans to form biofilm. The differential role of this enzyme in different microbial biofilms may have implications for the interactions in a mixed-species milieu. It is likely that bacteria overexpress ADH not only to enhance their ability to form biofilm, but also to inhibit Candida biofilm formation. These findings suggest that ADH may represent a new target to control biofilm-associated S. maltophilia infections.
3.4.3. Serine Hydroxymethyltransferase
Serine hydroxymethyltransferase (SHMT) is a pyridoxal 5′-phosphate (PLP)-dependent enzyme. In P. aeruginosa, the reversible conversion of the amino acids glycine to serine by hydroxymethyltransferase (SHMT) impacts the pathogen’s sessile lifestyle and virulence. The inactivation of SMHT leads to the rugose small-colony variants phenotype of P. aeruginosa and increases biofilm formation and exopolysaccharide synthesis while decreasing swarming motility [53]. SHMT controls biofilm formation by regulating the second messenger cyclic diguanylate (c-di-GMP). Whole transcriptome analysis also revealed that SHMT is associated with the cell’s redox state [53]. The upregulation of SHMT expression we found in biofilm cells suggests a differential role of the enzyme in S. maltophilia, although we do not know the underlying mechanisms.
3.4.4. Acetyl-CoA Carboxylase Biotin Carboxylase Subunit
Acetyl-CoA carboxylase (ACC) plays a critical role in fatty acid metabolism, catalyzing the first committed and rate-limiting step in fatty acid biosynthesis. In E. coli, the ACC reaction consists of two partial reactions catalyzed by different components of a multi-subunit complex. In the first reaction, the accC-encoded biotin carboxylase subunit allows the carboxylation of biotin by bicarbonate in an ATP-dependent reaction to form carboxybiotin [54]. The increased AccC expression we observed in biofilm cells might be QS-related. Indeed, in S. maltophilia the QS system is based on the fatty acid signal DSF (cis-11-methyl-2-dodecenoic acid) [55] that positively regulates biofilm formation and the induction of L1 and L2 β-lactamase production [56]. In addition, the presence of DSF signals in CF sputum was associated with the patient’s colonization exclusively by S. maltophilia and B. cenocepacia, thus, indicating the potential for interspecies signaling involving DSF family signals within the CF lung [57]. These findings indicate that acetyl-CoA carboxylase might represent a valuable antibiotic and antivirulence therapeutic target.
3.4.5. Aminotransferase
Weiland-Bräuer et al. [58] recently identified nine QQ proteins that significantly inhibit biofilm formation of E. coli K12 MG1655, P. aeruginosa, B. subtilis, and S. aureus likely due to the interference with AI-2 and AHL-based QS. The sequence-based prediction analysis revealed aminotransferase among these novel QQ proteins.
No published studies reported the role of threonine 3-dehydrogenase, involved in the catabolism of threonine and the synthesis of glycine in E. coli [59], in biofilm formation. Finally, we observed a conserved hypothetical protein (NMB1475) upregulated in biofilm cells.
3.5. Proteins Significantly Downregulated in Biofilm Cells
Eight proteins, among those down expressed in biofilm cells, were identified by sequence homology with those deposited in databases (software Mascot, ver. 2.7; Matrix Science): 7 in the S. maltophilia database, and one (MP8009) in the database of Campylobacter rectus RM3267 (Table 3).
Table 3.
Proteins (n = 8; signal > 1000 ppm; ≥ 3-fold vs. biofilm growth; p < 0.01, t-test) significantly downregulated in biofilm-grown S. maltophilia Sm126 cells.
| Spot No. | Protein | Biological Process | Molecular Function |
Fold Up-Regulation a | Peptides Matched b | Sequence Coverage c | pI d | MW e | GI f |
|---|---|---|---|---|---|---|---|---|---|
| MP0304 | OmpA/MotB domain-containing protein |
Ion transport | Porin activity | 19.0 | 11 | 43 | 4.80/4.36 | 39,148/37,464 | 194364578 |
| MP4106 | Hypothetical protein Smal_0271 | Not known | Not known | 13.5 | 9 | 44 | 5.80/5.97 | 21,071/16,406 | 194364049 |
| MP8009 | Thioester dehydrase family protein | Fatty acid biosynthetic process |
Dehydrase activity | 3.6 | 8 | 43 | 4.90/8.21 | 17,497/7237 | 223039807 |
| MP0011 | Ribosomal protein L7/L12 | Cytoplasmatic translation |
Structural constituent of ribosome | 4.0 | 4 | 50 | 4.79/4.51 | 12,589/10,677 | 254524344 |
| MP0201 | Thioredoxin | Removal of superoxide radicals | Detoxification | 4.4 | 3 | 14 | 4.57/4.34 | 30,871/31,825 | 194366686 |
| MP1006 | 50S ribosomal protein L9 |
Cytoplasmatic translation |
Large ribosomal subunit rRNA binding | 10.3 | 6 | 36 | 5.24/4.9 | 15,719/8238 | 190575039 |
| MP8002 | Putative sigma-54 modulation protein | Translation regulation |
Translation regulation |
5.5 | 2 | 26 | 6.64/8.17 | 11,739/10,127 | 190573137 |
| MP8004 | Cold shock protein | Response to stress | Nucleic acid binding | 9.8 | 3 | 40 | 8.15/8.80 | 7573/7464 | 190573988 |
a Ratio of mean percentage integrated optical density for each protein from planktonic and biofilm-grown S. maltophilia cells (n = 6). b Number of tryptic peptides matched to protein sequence based on MS/MS spectra. c The percentage of amino acid coverage (peptides observed/theoretical number from sequence data given in S. maltophilia database). d Theoretical and practical pI of the protein. e Theoretical and practical mass, expressed in Da, of the protein. f GI accession (GenInfo identifier; sequence identification).
3.5.1. OmpA/MotB Proteins
The proteins of the OmpA/MotB domains act as porin-like integral membrane proteins in most Gram-negative bacteria, which are required for cell morphology, membrane stability, and motility [60]. OmpA is the most abundant outer membrane protein in S. maltophilia, providing the structural integrity needed for flagellar assembly and swimming motility [61]. In P. aeruginosa, the major flagellar motor proteins MotA and MotB form a complex that acts as the stator and generates the torque that drives flagellar rotation. Therefore, the decrease in the expression of proteins of the OmpA/MotB domain observed in biofilm cells is consistent with our previous findings confirming that in S. maltophilia flagella are critical only during initial stages of attachment, while being turned off in mature biofilms [8,62].
3.5.2. Thioester Dehydrase
Transcriptomic studies showed that in acute infection, planktonic P. aeruginosa cells rely on amino acids as a carbon source, while in biofilm infection sessile cells may utilize fatty acids for carbon and energy [63]. A similar pathway might be hypothesized in S. maltophilia based on the down expression of the thioester dehydrase family protein involved in the biosynthesis of fatty acids. In addition, the degradation of the fatty acids would improve acetyl-CoA production, as also suggested by the unique expression of acetyl-CoA synthetase we observed in sessile cells, potentially feeding the expression of surface structures, such as flagella, pili, and exo-polysaccharides, involved in the initial stages of biofilm development [43].
3.5.3. Thioredoxin
The redox protein thioredoxin has been shown to modulate various virulence factors in Gram-negative pathogens. Previous research has shown that it is critical for the regulation of motility and gene transcription in Listeria monocytogenes [64], reduction of mucin to improve Helicobacter pylori adhesion to the epithelial surface [65], and contributes to organ burdens, host response, and increased survival in a model of sepsis caused by A. baumannii [66]. The decreased expression of thioredoxin we observed during the planktonic-to-biofilm transition might, therefore, indicate that bacteria lower their virulence by forming biofilm so that it can achieve persistent infection in vivo. Consistent with this hypothesis, previous studies reported the downregulation of genes encoding for virulence factors in biofilm cells relative to planktonic cells of several bacterial species [67,68,69]. Specific to CF, P. aeruginosa adaptation in CF airways selects patho-adaptive variants with a lower ability to cause acute infection [70]. Similarly, we previously showed that the virulence potential of S. maltophilia plays little if any role in its ability to persist in CF airways [71]; confirming this, the S. maltophilia Sm126 strain used in the present study was responsible for chronic infection in a CF patient.
3.5.4. Cold Shock Protein
The cold shock protein (Csp) superfamily consists of homologous proteins highly conserved in many bacteria [72]. Although most research has focused on their role in bacterial adaption and survival at suboptimal temperatures, recent studies indicate that some Csps’ also contribute to general stress response and virulence. Specifically, numerous pieces of evidence indicated that Csp homologs positively regulate the motility and attachment to surfaces, as observed in Xylella fastidiosa [73], L. monocytogenes [74], and Salmonella Typhimurium [75]. These findings might explain the downregulation of Csp we observed in cells collected from a 24-h-old biofilm. Indeed, both motility and adhesion are needed in the early phase of biofilm formation, although being quenched in mature biofilm.
4. Conclusions
This work reports the first proteomic analysis of an S. maltophilia biofilm, highlighting that biofilm development results in significant changes in the whole-cell protein profiles of S. maltophilia growing planktonically and as a biofilm on polystyrene.
The proteins identified are implicated at various levels of cellular physiology, indicating that the biofilm phenotype results in complex patterns of gene regulation. In particular, the proteins we observed being exclusively associated with the biofilm phenotype—i.e., starch hydrolases (QS-mediated intercellular communication); a putative heat shock protein, acetyl-CoA synthetase, and chorismate-synthetase (response to stress and nutrient starvation); polyribonucleotide phosphorylase (phosphate signaling); 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase, phosphoglycerate mutase, and isocitrate dehydrogenase (exalted carbohydrate metabolism)—may play an important role in the regulation of the biofilm development in S. maltophilia and, therefore, representing interesting targets to develop suitable anti-biofilm agents. Further functional and gene mutation studies are warranted to prove the exact role of the identified proteins in S. maltophilia biofilm development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11020442/s1.
Author Contributions
Conceptualization, G.D.B. and A.P.; methodology, G.D.B., C.P., V.L., and A.P.; validation, G.D.B., and A.P.; formal analysis, G.D.B., and A.P.; investigation, G.D.B., C.P., V.L., and A.P.; resources, G.D.B., and A.P.; data curation, G.D.B., and A.P.; writing-original draft preparation, G.D.B., and A.P.; writing-review and editing, G.D.B., C.P., V.L., and A.P.; supervision, A.P.; project administration, G.D.B., and A.P.; funding acquisition, G.D.B., and A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by the University of Chieti-Pescara, grant number “FAR, ex-60%-2020”, and the Italian Cystic Fibrosis Research Foundation, Verona (Italy), grant number “FFC 7/2007”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.An S.Q., Berg G. Stenotrophomonas maltophilia. Trends Microbiol. 2018;26:637–638. doi: 10.1016/j.tim.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Insuwanno W., Kiratisin P., Jitmuang A. Stenotrophomonas maltophilia infections: Clinical characteristics and factors associated with mortality of hospitalized patients. Infect. Drug. Resist. 2020;13:1559–1566. doi: 10.2147/IDR.S253949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke J.S. Advances in the microbiology of Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 2021;34:e0003019. doi: 10.1128/CMR.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis F., Enaud R., Soret P., Lussac-Sorton F., Avalos-Fernandez M., MucoFong Investigation Group. Bui S., Fayon M., Thiébaut R., Delhaes L. New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project. J. Clin. Med. 2021;10:3725. doi: 10.3390/jcm10163725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trifonova A., Strateva T. Stenotrophomonas maltophilia—A low-grade pathogen with numerous virulence factors. Infect. Dis. 2019;51:168–178. doi: 10.1080/23744235.2018.1531145. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Treviño S., Bocanegra-Ibarias P., Camacho-Ortiz A., Morfín-Otero R., Salazar-Sesatty H.A., Garza-González E. Stenotrophomonas maltophilia biofilm: Its role in infectious diseases. Expert. Rev. Anti. Infect. Ther. 2019;17:877–893. doi: 10.1080/14787210.2019.1685875. [DOI] [PubMed] [Google Scholar]
- 7.Pompilio A., Ranalli M., Piccirilli A., Perilli M., Vukovic D., Savic B., Krutova M., Drevinek P., Jonas D., Fiscarelli E.V., et al. Biofilm Formation among Stenotrophomonas maltophilia Isolates Has Clinical Relevance: The ANSELM Prospective Multicenter Study. Microorganisms. 2020;9:49. doi: 10.3390/microorganisms9010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pompilio A., Crocetta V., Confalone P., Nicoletti M., Petrucca A., Guarnieri S., Fiscarelli E., Savini V., Piccolomini R., Di Bonaventura G. Adhesion to and biofilm formation on IB3-1 bronchial cells by Stenotrophomonas maltophilia isolates from cystic fibrosis patients. BMC Microbiol. 2010;10:102. doi: 10.1186/1471-2180-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pompilio A., Piccolomini R., Picciani C., D’Antonio D., Savini V., Di Bonaventura G. Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: The role of cell surface hydrophobicity and motility. FEMS Microbiol. Lett. 2008;287:41–47. doi: 10.1111/j.1574-6968.2008.01292.x. [DOI] [PubMed] [Google Scholar]
- 10.An S.Q., Tang J.I. The Ax21 protein influences virulence and biofilm formation in Stenotrophomonas maltophilia. Arch Microbiol. 2018;200:183–187. doi: 10.1007/s00203-017-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An S., Tang J. Diffusible signal factor signaling regulates multiple functions in the opportunistic pathogen Stenotrophomonas maltophilia. BMC Res. Notes. 2018;11:569. doi: 10.1186/s13104-018-3690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alio I., Gudzuhn M., Pérez García P., Danso D., Schoelmerich M.C., Mamat U., Schaible U.E., Steinmann J., Yero D., Gibert I., et al. Phenotypic and Transcriptomic Analyses of Seven Clinical Stenotrophomonas maltophilia Isolates Identify a Small Set of Shared and Commonly Regulated Genes Involved in the Biofilm Lifestyle. Appl. Environ. Microbiol. 2020;86:e02038-20. doi: 10.1128/AEM.02038-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito A., Pompilio A., Bettua C., Crocetta V., Giacobazzi E., Fiscarelli E., Jousson O., Di Bonaventura G. Evolution of Stenotrophomonas maltophilia in Cystic Fibrosis Lung over Chronic Infection: A Genomic and Phenotypic Population Study. Front. Microbiol. 2017;8:1590. doi: 10.3389/fmicb.2017.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pompilio A., Crocetta V., Scocchi M., Pomponio S., Di Vincenzo V., Mardirossian M., Gherardi G., Fiscarelli E., Dicuonzo G., Gennaro R., et al. Potential novel therapeutic strategies in cystic fibrosis: Antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012;12:145. doi: 10.1186/1471-2180-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karp N.A., Lilley K.S. Investigating sample pooling strategies for DIGE experiments to address biological variability. Proteomics. 2009;9:388–397. doi: 10.1002/pmic.200800485. [DOI] [PubMed] [Google Scholar]
- 16.Crossman L.C., Gould V.C., Dow J.M., Vernikos G.S., Okazaki A., Sebaihia M., Saunders D., Arrowsmith C., Carver T., Peters N., et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008;9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Bian Z., Wang Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022;106:6365–6381. doi: 10.1007/s00253-022-12150-3. [DOI] [PubMed] [Google Scholar]
- 18.Simanek K.A., Paczkowski J.E. Resistance Is Not Futile: The Role of Quorum Sensing Plasticity in Pseudomonas aeruginosa Infections and Its Link to Intrinsic Mechanisms of Antibiotic Resistance. Microorganisms. 2022;10:1247. doi: 10.3390/microorganisms10061247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huedo P., Yero D., Martinez-Servat S., Ruyra À., Roher N., Daura X., Gibert I. Decoding the genetic and functional diversity of the DSF quorum-sensing system in Stenotrophomonas maltophilia. Front. Microbiol. 2015;6:761. doi: 10.3389/fmicb.2015.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pompilio A., Crocetta V., De Nicola S., Verginelli F., Fiscarelli E., Di Bonaventura G. Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 2015;6:951. doi: 10.3389/fmicb.2015.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez P., Huedo P., Martinez-Servat S., Planell R., Ferrer-Navarro M., Daura X., Yero D., Gilbert I. Stenotrophomonas maltophilia responds to exogenous AHL signals through the LuxR solo SmoR (Smlt1839) Front. Cell. Infect. Microbiol. 2015;5:41. doi: 10.3389/fcimb.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Givskov M., Rasmussen B.T. Quorum sensing inhibitors: A bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 23.Givskov M.R., De Nys M., Manefield L., Gram R., Maximilien L., Eberl S., Molin P., Steinberg D., Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uroz S., Oger P.M., Chapelle E., Adeline M.T., Faure D., Dessaux Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol. 2008;74:1357–1366. doi: 10.1128/AEM.02014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S.Y., Kang H.O., Jang H.S., Lee J.K., Koo B.T., Yum D.Y. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp and its application to quorum quenching. Appl. Environ. Microbiol. 2005;71:2632–2641. doi: 10.1128/AEM.71.5.2632-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb K., Cámara M., Zain N.M.M., Halliday N., Bruce K.D., Nash E.F., Whitehouse J.L., Knox A., Forrester D., Smyth A.R., et al. Novel detection of specific bacterial quorum sensing molecules in saliva: Potential non-invasive biomarkers for pulmonary Pseudomonas aeruginosa in cystic fibrosis. J. Cyst. Fibros. 2022;21:626–629. doi: 10.1016/j.jcf.2021.08.030. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor A., McClean S. The role of universal stress proteins in bacterial infections. Curr. Med. Chem. 2017;24:3970–3976. doi: 10.2174/0929867324666170124145543. [DOI] [PubMed] [Google Scholar]
- 28.Zeng B., Wang C., Zhang P., Guo Z., Chen L., Duan K. Heat Shock Protein DnaJ in Pseudomonas aeruginosa Affects Biofilm Formation via Pyocyanin Production. Microorganisms. 2020;8:395. doi: 10.3390/microorganisms8030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe A.J. The acetate switch. Microbiol. Mol. Biol. Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pru¨ß B.M., Wolfe A.J. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe A.J., Chang D.E., Walker J.D., Seitz-Partridge J.E., Vidaurri M.D., Lange C.F., Pru¨ß B.M., Henk M.C., Larkin J.C., Conway T. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 2003;48:977–988. doi: 10.1046/j.1365-2958.2003.03457.x. [DOI] [PubMed] [Google Scholar]
- 32.Anderson G.G., Palermo J.J., Schilling J.D., Roth R., Heuser J., Hultgren S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 33.Sharif D.I., Gallon J., Smith C.J., Dudley E. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium. Gloeothece PCCISME J. 2008;2:1171–1182. doi: 10.1038/ismej.2008.68. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar D., Fisher P.B. Polynucleotide phosphorylase: An evolutionary conserved gene with an expanding repertoire of functions. Pharmacol. Ther. 2006;112:243–263. doi: 10.1016/j.pharmthera.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Ladjouzi R., Duban M., Lucau-Danila A., Drider D. The absence of PNPase activity in Enterococcus faecalis results in alterations of the bacterial cell-wall but induces high proteolytic and adhesion activities. Gene. 2022;833:146610. doi: 10.1016/j.gene.2022.146610. [DOI] [PubMed] [Google Scholar]
- 36.Rouf S.F., Ahmad I., Anwar N., Vodnala S.K., Kader A., Römling U., Rhen M. Opposing contributions of polynucleotide phosphorylase and the membrane protein NlpI to biofilm formation by Salmonella enterica serovar Typhimurium. J. Bacteriol. 2011;193:580–582. doi: 10.1128/JB.00905-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carzaniga T., Antoniani D., Dehò G., Briani F., Landini P. The RNA processing enzyme polynucleotide phosphorylase negatively controls biofilm formation by repressing poly-N-acetylglucosamine (PNAG) production in Escherichia coli C. BMC Microbiol. 2012;12:270. doi: 10.1186/1471-2180-12-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulett G.C., Webb R.I., Schembri M.A. Antigen-43-mediated autoaggregation impairs motility in Escherichia coli. Microbiology. 2006;152:2101–2110. doi: 10.1099/mic.0.28607-0. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Hegazy L., Chakravarty S., Anderson G.G. Phosphoglycerate mutase affects Stenotrophomonas maltophilia attachment to biotic and abiotic surfaces. Microbes Infect. 2020;22:60–64. doi: 10.1016/j.micinf.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isom C.M., Fort B., Anderson G.G. Evaluating Metabolic Pathways and Biofilm Formation in Stenotrophomonas maltophilia. J. Bacteriol. 2022;204:e0039821. doi: 10.1128/JB.00398-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu K.D., Stewart P.S., Xia F., Huang C.T., McFeters G.A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 1998;64:4035–4039. doi: 10.1128/AEM.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prigent-Combaret C., Vidal O., Dorel C., Lejeune P. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 1999;181:5993–6002. doi: 10.1128/JB.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pancholi V., Fischetti A.V. a-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 1998;273:14503–14515. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L., Liu Q., Huang Q., Liu F., Liu H., Wang G. Isocitrate dehydrogenase of Bacillus cereus is involved in biofilm formation. World J. Microbiol. Biotechnol. 2021;37:207. doi: 10.1007/s11274-021-03175-3. [DOI] [PubMed] [Google Scholar]
- 45.Prasad U.V., Vasu D., Yeswanth S., Swarupa V., Sunitha M.M., Choudhary A., Sarma P.V. Phosphorylation controls the functioning of Staphylococcus aureus isocitrate dehydrogenase--favours biofilm formation. J. Enzyme Inhib. Med. Chem. 2015;30:655–661. doi: 10.3109/14756366.2014.959945. [DOI] [PubMed] [Google Scholar]
- 46.Keegan R., Waterman D.G., Hopper D.J., Coates L., Taylor G., Guo J., Coker A.R., Erskine P.T., Wood S.P., Cooper J.B. The 1.1 Å resolution structure of a periplasmic phosphate-binding protein from Stenotrophomonas maltophilia: A crystallization contaminant identified by molecular replacement using the entire Protein Data Bank. Acta Crystallogr. D. Struct. Biol. 2016;72:933–943. doi: 10.1107/S2059798316010433. [DOI] [PubMed] [Google Scholar]
- 47.Neznansky A., Blus-Kadosh I., Yerushalmi G., Banin E., Opatowsky Y. The Pseudomonas aeruginosa phosphate transport protein PstS plays a phosphate-independent role in biofilm formation. FASEB J. 2014;28:5223–5233. doi: 10.1096/fj.14-258293. [DOI] [PubMed] [Google Scholar]
- 48.Beliaeva M.A., Atac R., Seebeck F.P. Bacterial Degradation of Nτ-Methylhistidine. ACS Chem. Biol. 2022;17:1989–1995. doi: 10.1021/acschembio.2c00437. [DOI] [PubMed] [Google Scholar]
- 49.Shibamura-Fujiogi M., Wang X., Maisat W., Koutsogiannaki S., Li Y., Chen Y., Lee J.C., Yuki K. GltS regulates biofilm formation in methicillin-resistant Staphylococcus aureus. Commun. Biol. 2022;5:1284. doi: 10.1038/s42003-022-04239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassanov T., Karunker I., Steinberg N., Erez A., Kolodkin-Gal I. Novel antibiofilm chemotherapies target nitrogen from glutamate and glutamine. Sci. Rep. 2018;8:7097. doi: 10.1038/s41598-018-25401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K., Yang X., Yang J., Qiao X., Li F., Liu X., Wei J., Wang L. Alcohol dehydrogenase modulates quorum sensing in biofilm formations of Acinetobacter baumannii. Microb. Pathog. 2020;148:104451. doi: 10.1016/j.micpath.2020.104451. [DOI] [PubMed] [Google Scholar]
- 52.Becker P., Hufnagle W., Peters G., Herrmann M. Detection of differential gene expression in biofilm-forming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Appl. Environ. Microbiol. 2001;67:2958–2965. doi: 10.1128/AEM.67.7.2958-2965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pu M., Sheng L., Song S., Gong T., Wood T.K. Serine Hydroxymethyltransferase ShrA (PA2444) Controls Rugose Small-Colony Variant Formation in Pseudomonas aeruginosa. Front. Microbiol. 2018;9:315. doi: 10.3389/fmicb.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi-Rhee E., Cronan J.E. The biotin carboxylase-biotin carboxyl carrier protein complex of Escherichia coli acetyl-CoA carboxylase. J. Biol. Chem. 2003;278:30806–30812. doi: 10.1074/jbc.M302507200. [DOI] [PubMed] [Google Scholar]
- 55.Huedo P., Coves X., Daura X., Gibert I., Yero D. Quorum Sensing Signaling and Quenching in the Multidrug-Resistant Pathogen Stenotrophomonas maltophilia. Front. Cell. Infect. Microbiol. 2018;8:122. doi: 10.3389/fcimb.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alcaraz E., García C., Friedman L., de Rossi B.P. The rpf/DSF signalling system of Stenotrophomonas maltophilia positively regulates biofilm formation, production of virulence-associated factors and β-lactamase induction. FEMS Microbiol. Lett. 2019;366:fnz069. doi: 10.1093/femsle/fnz069. [DOI] [PubMed] [Google Scholar]
- 57.Twomey K.B., O’Connell O.J., McCarthy Y., Dow J.M., O’Toole G.A., Plant B.J., Ryan R.P. Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. ISME J. 2012;6:939–950. doi: 10.1038/ismej.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiland-Bräuer N., Kisch M.J., Pinnow N., Liese A., Schmitz R.A. Highly Effective Inhibition of Biofilm Formation by the First Metagenome-Derived AI-2 Quenching Enzyme. Front. Microbiol. 2016;7:1098. doi: 10.3389/fmicb.2016.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma F., Wang T., Ma X., Wang P. Identification and characterization of protein encoded by orf382 as L-threonine dehydrogenase. J. Microbiol. Biotechnol. 2014;24:748–755. doi: 10.4014/jmb.1312.12030. [DOI] [PubMed] [Google Scholar]
- 60.Mougous J.D., Cuff M.E., Raunser S., Shen A., Zhou M., Gifford C.A., Goodman A.L., Joachimiak G., Ordoñez C.L., Lory S., et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao C.H., Chang C.L., Huang H.H., Lin Y.T., Li L.H., Yang T.C. Interplay between OmpA and RpoN Regulates Flagellar Synthesis in Stenotrophomonas maltophilia. Microorganisms. 2021;9:1216. doi: 10.3390/microorganisms9061216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Bonaventura G., Prosseda G., Del Chierico F., Cannavacciuolo S., Cipriani P., Petrucca A., Superti F., Ammendolia M.G., Concato C., Fiscarelli E., et al. Molecular characterization of virulence determinants of Stenotrophomonas maltophilia strains isolated from patients affected by cystic fibrosis. Int. J. Immunopathol. Pharmacol. 2007;20:529–537. doi: 10.1177/039463200702000311. [DOI] [PubMed] [Google Scholar]
- 63.D’Arpa P., Karna S.L.R., Chen T., Leung K.P. Pseudomonas aeruginosa transcriptome adaptations from colonization to biofilm infection of skin wounds. Sci. Rep. 2021;11:20632. doi: 10.1038/s41598-021-00073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng C., Dong Z., Han X., Wang H., Jiang L., Sun J., Yang Y., Ma T., Shao C., Wang X., et al. Thioredoxin A is essential for motility and contributes to host infection of Listeria monocytogenes via redox interactions. Front. Cell. Infect. Microbiol. 2017;7:287. doi: 10.3389/fcimb.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Windle H.J., Fox A., Ni Eidhin D., Kelleher D. The thioredoxin system of Helicobacter pylori. J. Biol. Chem. 2000;275:5081–5089. doi: 10.1074/jbc.275.7.5081. [DOI] [PubMed] [Google Scholar]
- 66.May H.C., Yu J.J., Zhang H., Wang Y., Cap A.P., Chambers J.P., Guentzel M.N., Arulanandam B.P. Thioredoxin-A is a virulence factor and mediator of the type IV pilus system in Acinetobacter baumannii. PLoS ONE. 2019;14:e0218505. doi: 10.1371/journal.pone.0218505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilmore K.S., Srinivas P., Akins D.R., Hatter K.L., Gilmore M.S. Growth, development, and gene expression in a persistent Streptococcus gordonii biofilm. Infect. Immun. 2003;71:4759–4766. doi: 10.1128/IAI.71.8.4759-4766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Petrova O.E., Su S., Lau G.W., Panmanee W., Na R., Hassett D.J., Davies D.G., Sauer K. BdlA, DipA and induced dispersion contribute to acute virulence and chronic persistence of Pseudomonas aeruginosa. PLoS Pathog. 2014;10:e1004168. doi: 10.1371/journal.ppat.1004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamilton S., Bongaerts R.J., Mulholland F., Cochrane B., Porter J., Lucchini S., Lappin-Scott H.M., Hinton J.C. The transcriptional program of Salmonella enterica serovar Typhimurium reveals a key role for tryptophan metabolism in biofilms. BMC Genomics. 2009;10:599. doi: 10.1186/1471-2164-10-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkesson A., Jelsbak L., Yang L., Johansen H.K., Ciofu O., Høiby N., Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012;10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 71.Pompilio A., Crocetta V., Ghosh D., Chakrabarti M., Gherardi G., Vitali L.A., Fiscarelli E., Di Bonaventura G. Stenotrophomonas maltophilia Phenotypic and Genotypic Diversity during a 10-year Colonization in the Lungs of a Cystic Fibrosis Patient. Front. Microbiol. 2016;7:1551. doi: 10.3389/fmicb.2016.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu T., Keto-Timonen R., Jiang X., Virtanen J.P., Korkeala H. Insights into the phylogeny and evolution of cold shock proteins: From enteropathogenic Yersinia and Escherichia coli to Eubacteria. Int. J. Mol. Sci. 2019;20:4059. doi: 10.3390/ijms20164059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei W., Sawyer T., Burbank L. Csp1, a Cold Shock Protein Homolog in Xylella fastidiosa Influences Cell Attachment, Pili Formation, and Gene Expression. Microbiol. Spectr. 2021;9:e0159121. doi: 10.1128/Spectrum.01591-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muchaamba F., Stephan R., Tasara T. Listeria monocytogenes Cold Shock Proteins: Small Proteins with A Huge Impact. Microorganisms. 2021;9:1061. doi: 10.3390/microorganisms9051061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray S., Da Costa R., Thakur S., Nandi D. Salmonella Typhimurium encoded cold shock protein E is essential for motility and biofilm formation. Microbiology. 2020;166:460–473. doi: 10.1099/mic.0.000900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.