Abstract
The diversity of dynein's functions in mammalian cells is a manifestation of both the existence of multiple dynein heavy chain isoforms and an extensive set of associated protein subunits. In this study, we have identified and characterized a novel subunit of the mammalian cytoplasmic dynein 2 complex. The sequence similarity between this 33-kDa subunit and the light intermediate chains (LICs) of cytoplasmic dynein 1 suggests that this protein is a dynein 2 LIC (D2LIC). D2LIC contains a P-loop motif near its NH2 terminus, and it shares a short region of similarity to the yeast GTPases Spg1p and Tem1p. The D2LIC subunit interacts specifically with DHC2 (or cDhc1b) in both reciprocal immunoprecipitations and sedimentation assays. The expression of D2LIC also mirrors that of DHC2 in a variety of tissues. D2LIC colocalizes with DHC2 at the Golgi apparatus throughout the cell cycle. On brefeldin A-induced Golgi fragmentation, a fraction of D2LIC redistributes to the cytoplasm, leaving behind a subset of D2LIC that is localized around the centrosome. Our results suggest that D2LIC is a bona fide subunit of cytoplasmic dynein 2 that may play a role in maintaining Golgi organization by binding cytoplasmic dynein 2 to its Golgi-associated cargo.
INTRODUCTION
Dyneins are large, multisubunit motor proteins that are involved in a wide range of cellular processes. There are two classes of dyneins: axonemal and cytoplasmic. Axonemal dyneins drive and coordinate motility in cilia and flagella (reviewed in Gibbons, 1995; Porter, 1996), whereas cytoplasmic dyneins contribute to a variety of processes, including vesicle transport, formation and localization of the Golgi complex, mitotic spindle assembly and positioning, nuclear migration, and chromosome movements (reviewed in Holz-baur and Vallee, 1994; Hirokawa et al., 1998).
The cytoplasmic dynein family can be subdivided further. Three distinct isoforms of the dynein heavy chain (DHC) have been identified in HeLa cells (Vaisberg et al., 1996) and four in rat testis (Criswell and Asai, 1998). The most extensively studied member of this family is DHC1 (reviewed in Hirokawa, 1998) followed by DHC2, which has now been identified in a variety of cells and tissues (Gibbons et al., 1994; Tanaka et al., 1995; Criswell et al., 1996; Vaisberg et al., 1996; Criswell and Asai, 1998). DHC2 has been localized to the apical cytoplasm of ciliated epithelial cells (Criswell et al., 1996) and to the Golgi apparatus in nonciliated mammalian cells (Vaisberg et al., 1996). Microinjection of DHC2 antibodies resulted in the fragmentation and dispersal of the Golgi apparatus, indicating a role for dynein 2 in the formation and organization of the Golgi complex (Vaisberg et al., 1996). A homologous cytoplasmic dynein 2 heavy chain (cDhc1b) has been identified in Chlamydomonas flagella, where it is involved in the transport of flagellar assembly components (Pazour et al., 1999; Porter et al., 1999) and in Caenorhabditis elegans ciliated sensory neurons, where it is also implicated in intraflagellar transport (Wicks et al., 2000).
Dissection of the cytoplasmic dynein 1 complex has revealed that it is composed of two identical heavy chains (∼530 kDa), 2–3 distinct intermediate chains (∼70–74 kDa), several light intermediate chains (∼52–61 kDa), and numerous light chains (∼8–22 kDa). The DHCs are responsible for the ATPase and motor activities of the complex (reviewed in Vallee and Shpetner, 1990; Mazumdar et al., 1996), whereas the intermediate chains (ICs) interact with the p150Glued dynactin subunit and thereby link dynein to various organelles within the cell (reviewed in Karki and Holzbaur, 1999). Two-dimensional electrophoresis has identified several light intermediate chain (LIC) species, some of which are phosphorylated (Gill et al., 1994; Hughes et al., 1995). The light intermediate chains contain a P-loop consensus sequence of unknown function near their NH2 terminus. Dynein light chains (LCs) may contribute to the diversity in dynein function by mediating the attachment of specific cargoes to the dynein complex (King et al., 1996a,b; Tai et al., 1998; Bowman et al., 1999; Tai et al., 1999). Recent work has also examined the interaction of dynein LICs with specific cargos. One LIC, LIC1, binds to pericentrin, a structural component of the centrosome involved in microtubule organization and function (Doxsey et al., 1994; Purohit et al., 1999), suggesting that this subunit is involved in linking dynein to its cargo (Purohit et al., 1999; Tynan et al., 2000a).
To date, little has been learned about the polypeptide composition of other members of the cytoplasmic dynein family. In this study, we present information about a newly identified subunit of cytoplasmic dynein 2. We have found a polypeptide that coimmunoprecipitates specifically with DHC2 and have used microsequencing to identify a gene that encodes this novel dynein subunit. The sequence of its predicted product most closely resembles the light intermediate chains of cytoplasmic dynein 1. This cytoplasmic dynein 2 LIC (D2LIC) interacts with DHC2 in sedimentation assays and is expressed in a variety of tissues with a pattern similar to that of DHC2. D2LIC colocalizes with DHC2 at the Golgi apparatus throughout the cell cycle and may play a role in targeting and/or anchoring the dynein 2 complex to this organelle.
MATERIALS AND METHODS
Reagents and Antibodies
All biochemical reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise noted. Molecular biology reagents were purchased from Invitrogen (Carlsbad, CA) or Promega (Madison, WI). The monoclonal antibody (mAb) p58K-9 (Bloom and Brashear, 1989) was a gift from Dr. G. Bloom (University of Virginia, Charlottesville, VA). The 74.1 mAb to dynein 1 IC (IC74) (Dillman and Pfister, 1994) was provided by Dr. K. Pfister (University of Virginia School of Medicine, Charlottesville, VA). DHC2 and DHC1 antibodies were used as previously described (Vaisberg et al., 1996). The mAb to sea urchin β-tubulin was prepared by B. Neighbors (Neighbors et al., 1988), and the γ−tubulin mAb was purchased from Sigma-Aldrich. Alexa 488-conjugated goat anti-rabbit and Alexa 594-conjugated goat anti-rat antibodies from Molecular Probes (Eugene, OR) were used at a 1:400 dilution for secondary antibody staining.
Immunoprecipitation and Microsequencing Analysis
Rat testes were homogenized in PME buffer [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 5 mM MgSO4, 1 mM EGTA, 1 mM dithiothreitol (DTT), pH 6.9; supplemented with a protease inhibitor cocktail (1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μg/ml each of leupeptin, pepstatin A, aprotinin, and soybean trypsin inhibitor)], by using a Polytron homogenizer (model PT 10/35; Brinkmann Instruments, Westbury, NY). The homogenate was clarified by centrifugation at 30,000 × gav for 30 min, followed by a second spin at 100,000 × gav for 1 h. The supernatant was diluted 1:1 with NP-40 buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 1 mM DTT, and the protease inhibitor cocktail; pH 8.0), overlaid onto 5–20% sucrose gradients in the same buffer, and centrifuged at 150,000 × gav for 19 h. Immunoprecipitates were prepared from the 15 S peak of these sucrose gradients by using affinity-purified antibodies to DHC2 (Vaisberg et al., 1996). Immunoprecipitation was performed with protein A-Sepharose CL-4B using standard protocols (Harlow and Lane, 1988). After antibody binding, the beads were washed four times with 60 volumes of NP-40 buffer and the final bead pellet was resuspended in SDS-PAGE loading buffer. Polypeptides that were precipitated by DHC2 antibodies were separated on a 7–15% acrylamide gradient gel. A protein band of Mr = ∼33,000 Da was excised from the gel and microsequenced. Partial sequence analysis was performed at the Harvard Microchemistry Facility (Cambridge, MA) using tandem mass spectrometry on a Finnigan LCQ quadrupole ion trap mass spectrometer.
Sequence and Molecular Analysis of D2LIC
Two peptide sequences provided by the Harvard Microchemistry Facility, ELIDPFPIPLVIIGSK and LQAHSPMELWK, were used to search the National Center for Biotechnology Information database of expressed sequence tags (dbEST) by using the Advanced BLAST program with the filter off and an expect value of 10,000 (http://www.ncbi.nlm.nih.gov/). Multiple human clones were identified, obtained from American Type Culture Collection (Manassas, VA), and sequenced, using an ABI PRISM Big Dye Terminator cycle sequencer (PE Biosystems, Foster City, CA). Sequence alignments were performed using the BESTFIT program (GCG Wisconsin Package, version 10.1; Genetics Computer Group, Madison, WI). The ProtParam tool at http://www.expasy.ch/cgi-bin/protparam was used to calculate the predicted molecular weight and theoretical pI of D2LIC. The ClustalW Multiple Sequence Alignment program (Thompson et al., 1994), courtesy of BCM search launcher (http://searchlauncher.bcm.tmc.edu/seq-search/protein-search-genomes.html), was used for phylogenetic sequence analysis and the MOTIFS program (Genetics Computer Group Wisconsin Package) was used to identify conserved structural motifs. The coils program available at http://www.ch.embnet.org/software/COILS_form.html (Lupas et al., 1991) was used for coiled coil predictions and protein motif fingerprints were identified using the PRINTS BLAST database at http://www.biochem.ucl.ac.uk/bsm/dbbrowser/PRINTS/PRINTS.html.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed using the SuperScript One-Step RT-PCR system (Invitrogen). Total HeLa RNA was used as the template with four different gene-specific primer combinations (primer set 1: 5′-TGTGACGTTTGCGGCAGCCAG-3′ and 5′-GGAGATCCTATTTGACCGAA-3′; primer set 2: 5′-TGTGACGTTTGCGGCAGCCAG-3′ and 5′-CTTCCAATTATGACCAGAGG-3′; primer set 3: 5′-TTCTCGTTCTGGATCTTTCAA-3′ and 5′-GGAGATCCTATTTGACCGAA-3′; and primer set 4: 5′-TTCGGTCAAATAGGATCTCC-3′ and 5′-GATATACAGGGAGATGCACT-3′). Northern blotting was performed using total RNA, isolated with RNA Isolator (Genosys Biotechnologies, The Woodlands, TX), and poly(A+) RNA, prepared with PolyATtract System 1000 (Promega). Twenty micrograms of total RNA and 1 μg of poly(A+) RNA were separated on a 6% formaldehyde-denaturing agarose gel, transferred to Hybond-N+ nylon membrane (Amersham Biosciences, Piscataway, NJ), and probed as described in Vaisberg et al. (1993).
D2LIC Fusion Protein and Antibody Preparation
A 6xHis-D2LIC construct was generated by fusing an 855-base pair fragment corresponding to the COOH-terminal region of D2LIC into the pQE-32 expression vector (QIAGEN, Valencia, CA). The D2LIC clone (accession no. AA312584) was digested with NdeI, the overhanging ends were filled in with the Klenow fragment of DNA polymerase, and then subsequently digested with PstI. This fragment was cloned into the pQE32 bacterial expression vector, which had been previously digested with SmaI and PstI. The resultant plasmid was transformed into Escherichia coli strain M15[pREP4]. Inclusion bodies were purified from cells expressing 6xHis-D2LIC-1 fusion proteins (Lin and Cheng, 1991) and then subjected to PAGE. The 6xHis-D2LIC-1 protein bands were excised from the gel, electroeluted using an Elutrap electro-separation chamber (Schleicher & Schuell, Keene, NH), and then dialyzed against phosphate-buffered saline (PBS). The resulting purified 6xHis-D2LIC-1 fusion protein was sent to Strategic BioSolutions (Ramona, CA) for the generation of antisera in rats.
Purification of Antibodies and Immunoblot Analysis
We generated a second, slightly larger fusion protein by digesting the full-length D2LIC clone with BamHI/SalI and fusing this fragment into the pGEX-KG vector (Guan and Dixon, 1991). This GST-D2LIC fusion protein was gel purified as described above and coupled to a CNBr-activated Sepharose 4B column for antibody purification. This GST full-length D2LIC fusion protein was chosen as the ligand for affinity-column preparation to eliminate the purification of antibodies cross-reacting to the 6xHis sequence while maximizing the recovery of D2LIC antibodies. The immune serum was passed through this affinity column; after extensive washing with PBS, antibodies were eluted, first with 0.1 M glycine, pH 2.1, and then with 0.1 M triethylamine, pH 11.5. Eluted fractions were immediately neutralized and then dialyzed versus Tris-buffered saline (10 mM Tris, 150 mM NaCl; pH 8.0). The affinity-purified anti-D2LIC antibodies were used at a concentration of 0.05–0.2 μg/ml. Immunoblots were blocked with 4% dry milk in Tris-buffered saline + 0.05% Tween 20 and developed using SuperSignal West Dura Extended Duration Substrate (Pierce Chemical, Rockford, IL).
Preparation of COS-7 Cell Extracts and Sucrose Gradient Fractionation
COS-7 cells were collected by scraping into ice-cold PBS, washed twice with PBS, once with Tris buffer (50 mM Tris, 150 mM NaCl; pH 8.0), resuspended in NP-40 buffer, and then lysed by passing through a 26-gauge needle on ice. The cell lysate was clarified by centrifugation at 1000 × gav for 10 min, followed by additional centrifugation at 100,000 × gav for 30 min. The clarified supernatant was overlaid onto a 5–20% sucrose gradient in NP-40 buffer and centrifuged at 150,000 × gav for 19 h. Fractions were collected from the bottom of the tube and analyzed by immunoblotting.
Immunoprecipitation assays were also performed using the clarified COS-7 homogenate prepared as described above. Protein A or protein G-Sepharose beads were used to precipitate the appropriate antibody–antigen complexes, employing methods previously described (Harlow and Lane, 1988).
Mouse Tissue Blot
Samples were prepared by homogenizing various mouse tissues in PHEM [60 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 25 mM HEPES, 5 mM MgSO4, 1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail; pH 6.9) by using a Polytron homogenizer. Homogenates were centrifuged at 24,000 × gav for 35 min and the supernatants collected. SDS was added to the clarified supernatants to a final concentration of 0.2% and the samples were centrifuged for an additional 30 min at 60,000 × gav. These tissue homogenate samples were then separated by SDS-PAGE on a 7–15% acrylamide gradient gel (35 μg of total protein per lane), transferred to nitrocellulose, and probed with the antibodies indicated.
Cell Culture and Immunofluorescence Microscopy
African green monkey kidney (COS-7) cells and human cervical carcinoma (HeLa) cells were grown in DMEM and Eagle's minimal essential medium, respectively, supplemented with 10% fetal calf serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate (Invitrogen). All cell cultures were maintained in 5% CO2 at 37°C. Cells were plated on glass coverslips, allowed to grow for 24–48 h, fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) in PBS for 10 min, permeabilized with 0.1% Triton X-100 in PBS for 2 min, and then processed for immunofluorescence as previously described (Vaisberg et al., 1996). D2LIC and DHC2 antibodies were used at a concentration of 5–10 μg/ml for immunostaining. DNA was visualized in all fixed cells by using the DNA-specific stain 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescence microscopy was performed on a Zeiss Axiophot 2 with a plan neofluor 40× objective lens. Images were acquired with a Cooke SensiCam charge-coupled devise camera and quantitated using the SlideBook software package (Intelligent Imaging Innovations, Denver, CO).
Pharmacological Treatments
Nocodazole (2.5 mg/ml in DMSO) and brefeldin A (2.5 mg/ml in ethanol) were added to the cell culture medium to a final concentration of 33 μM and 10 μg/ml, respectively. Cells were treated at 37°C for the times indicated in each figure legend, placed on ice, and then washed with ice-cold PBS before fixation. Cells were fixed and processed for immunofluorescence as described above.
RESULTS
Identification and Sequence Analysis of D2LIC
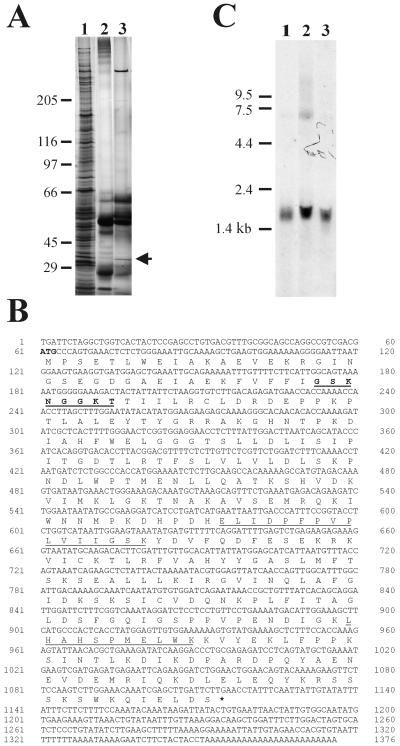
In a previous study, we identified a second isoform of cytoplasmic dynein, showed that it sedimented at 15 S, and prepared affinity-purified antibodies specific to its heavy chain (DHC2) (Vaisberg et al., 1996). Here, we have performed immunoprecipitations with these antibodies on the material sedimenting at 15 S during sucrose gradient fractionation of the soluble proteins from either rat testes or COS-7 cells. DHC2 affinity-purified antibodies immunoprecipitated the heavy chain, as expected, but also a polypeptide of Mr = ∼33,000 Da (Figure 1A, lane 3). The corresponding preimmune antibody did not immunoprecipitate either of these polypeptides (Figure 1A, lane 2). Densitometry scans of silver-stained gels, although not quantitative, indicated that the 33-kDa component coimmunoprecipitated with DHC2 in a stoichiometric ratio that was maintained whether the precipitation was performed from whole homogenates of rat testes or from 15 S sucrose gradient fractions of testes homogenate (our unpublished data).
Figure 1.
Identification and molecular analysis of human D2LIC. (A) Silver-stained gel of sucrose gradient fractions and immunoprecipitated complexes. COS-7 cell homogenate was fractionated on sucrose gradients and 15 S peak fractions pooled (lane 1). Immunoprecipitations from the 15 S fractions were performed using preimmune serum (lane 2) and DHC2 affinity-purified antibodies (lane 3). An ∼33-kDa polypeptide (arrow) that coimmunoprecipitated with DHC2 was excised from the gel and microsequenced. (B) DNA and predicted protein sequence for the human D2LIC. Two peptide fragments generated from microsequencing (underlined) were used to identify a clone (D2LIC) from the human dbEST database. The P-loop consensus motif is underlined and in bold. (C) Northern blot analysis. COS-7 poly(A+) RNA (lane 1), total COS-7 RNA (lane 2), and total HeLa RNA (lane 3) were size fractionated on a gel, transferred to nylon, and probed with the full-length D2LIC clone (shown in B). A predominant 1.6-kb message is evident.
The 33-kDa gel band was excised and microsequenced by tandem mass spectrometry, and two peptide sequences were obtained (Figure 1B). Blast searches for these sequences in the National Center for Biotechnology Information Human dbEST database identified three clones (accession no AA312584, AA476487, and AA224466). We determined by standard sequencing techniques that the largest clone, AA312584, was 1376 base pairs in length and contained a single open reading frame of 351 residues with a predicted mass of 39,624 Da (Figure 1B) and a calculated pI of 7.1. Both peptide sequences identified by microsequencing were found in clone AA312584. The clone also included a 60-base pair 5′ region upstream of the first methionine and a 261-base pair region 3′ of the first stop codon, which contained a polyadenylation signal (base pairs 1329–1334) that preceded a poly(A+) tail. Sequence analysis of clones AA476487 and AA224466 indicated that they are contained entirely within clone AA312584.
This predicted peptide sequence was analyzed for conserved motifs and for secondary structure. The MOTIFS program revealed a P-loop sequence near the NH2 terminus, running from amino acids 38–45, suggesting a possible ATP/GTP-binding site (Figure 1B). The COILS program identified a possible coiled coil of 21 amino acids (aa 315 to 336) in the COOH terminus, by using both the MTK and MTIDK matrices. The identification of our novel protein sequence by coimmunoprecipitation with DHC2 and sequence similarities described in detail below, led us to call the 33-kDa band, D2LIC, denoting the light intermediate chain of cytoplasmic dynein 2.
To confirm the integrity of the cDNA clones and to investigate the possibility of alternative splicing, we performed a series of RT-PCR reactions, as well as a Northern blot analysis. RT-PCR on total HeLa RNA, by using four different gene-specific primer combinations (see MATERIALS AND METHODS), resulted in products of the size predicted from clone AA312584. In addition, a single message of ∼1.6 kb in both COS-7 and HeLa cells was detected by Northern blot analysis (Figure 1C). These results suggested that D2LIC is not alternatively spliced in these cell types and that the cDNA correctly represents D2LIC mRNA. Furthermore, examination of the National Center for Biotechnology Information Human Genome database revealed that this gene maps to the left arm of Homo sapiens chromosome 2 at LOC51626: CGI-60 protein (Lai et al., 2000; Pruitt and Maglott, 2001).
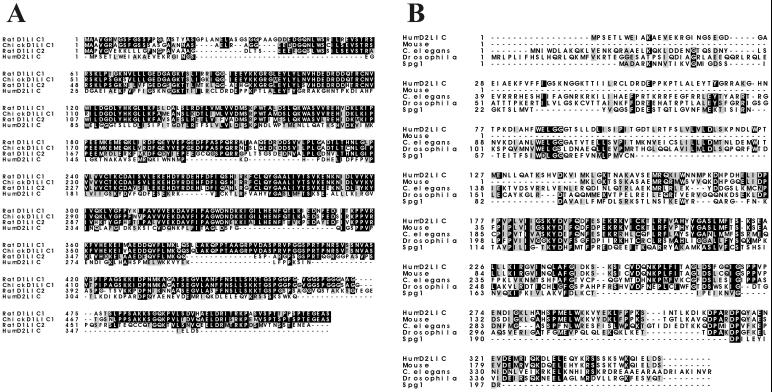
Searches of National Center for Biotechnology Information GenBank with the predicted D2LIC protein sequence revealed similarities to a family of dynein LICs previously identified as part of the conventional cytoplasmic dynein 1 complex (Figure 2A). Sequence analysis using the BESTFIT program indicated that D2LIC is 27% identical (37% similar) to rat dynein LIC2 from aa 4 to 263 (Hughes et al., 1995), 25% identical (36% similar) to rat dynein LIC1 from aa 32 to 268 (Purohit et al., 1999; Tynan et al., 2000a), and 24% identical (36% similar) to chicken dynein LIC1, DLC-A, from aa 32 to 268 (Gill et al., 1994). The similarity of D2LIC to all known dynein 1 light intermediate chains (D1LICs) drops significantly toward the COOH terminus of the polypeptides (following amino acid 268 of the D2LIC sequence), whereas D2LIC and all D1LICs contain P-loop motifs near the NH2 terminus. No sequence homology was observed between D2LIC and other components of dynein 1, including known dynein ICs or LCs.
Figure 2.
Dynein 2 light intermediate chain is a member of a family. (A) ClustalW Multiple Sequence Alignment of D2LIC and cytoplasmic dynein 1 LICs: rat LIC1, chicken DLC-A (D1LIC1), and rat LIC2. (B) ClustalW Multiple Sequence Alignment of dynein 2 light intermediate chains from mouse, C. elegans, D. melanogaster, and the yeast GTPase Spg1p. BOXSHADE was used to illustrate amino acid identity (darkly shaded residues) and similarity (lightly shaded residues). Accession numbers are rat D1LIC1, AF181992 (Purohit et al., 1999; Tynan et al., 2000a); chicken DLC-A (D1LIC1), Q90828 (Gill et al., 1994); rat D1LIC2, Q62698 (Hughes et al., 1995); mouse locus AK008822, BAB25915 (M. musculus Genome Sequencing Consortium); C. elegans hypothetical protein F02D8.3, T20505 (C. elegans Genome Sequencing Consortium); D. melanogaster CG3769 gene product, AAF52775.1 (Adams et al., 2000); and Spg1p, T45541 (Schmidt et al., 1997).
Sequence analysis of D2LIC also revealed a 44 aa segment, distinct from the P-loop, that shows significant similarity to the yeast GTPases Spg1p (Schmidt et al., 1997) and Tem1p (Goffeau et al., 1996). D2LIC is 43% identical (49% similar) to Spg1p from aa 182 to 226 and 29% identical (42% similar) to Tem1p in this same region (Figure 2B). Examination of the D2LIC sequence by using the PRINTS database also identified the same 44 aa segment of homology to Spg1p and Tem1p as a RAS motif signature common to RAS-related transforming proteins.
D2LIC-like proteins have been identified in genome sequencing projects of Mus musculus, C. elegans, and Drosophila melanogaster. A ClustalW Multiple Sequence Alignment of the D2LIC family illustrates several highly conserved regions (Figure 2B), including the block shared with Spg1p and Tem1p (D2LIC aa 182–226). In addition, partial sequence analysis has identified a D2LIC homolog in Chlamydomonas (Dr. Mary Porter, personal communication).
D2LIC Is Associated with Cytoplasmic Dynein 2 Heavy Chain
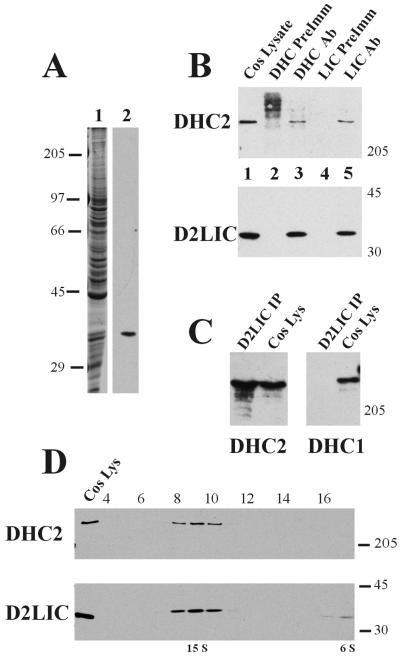
To determine whether the D2LIC protein is associated with its corresponding heavy chain in cells, as would be expected of a component of the cytoplasmic dynein 2 complex, we generated antisera specific to D2LIC. A fragment of D2LIC was used to generate an antigen that lacked the first 66 aa at the NH2 terminus of the D2LIC protein. This fragment was chosen because it avoids the P-loop motif and thus the possibility of generating antibodies to a family of ATP/GTPases. The affinity-purified antibodies produced (see MATERIALS AND METHODS) were highly specific and recognized a single band of the expected molecular mass of 39 kDa on Western blots of COS-7 cell homogenate (Figure 3A).
Figure 3.
Immunoprecipitation and sedimentation analysis of the heavy chain and light intermediate chain of dynein 2. (A) D2LIC antibody specificity. Affinity-purified antibodies to D2LIC were prepared and analyzed on Western blots. COS-7 cell homogenate was fractionated by SDS-PAGE (Coomassie-stained gel; lane 1), transferred to nitrocellulose, and probed with affinity-purified antibodies to D2LIC (lane 2). (B) Immunoprecipitation analysis. Immunoprecipitates were prepared from COS-7 cell lysate (lane 1) by using rabbit preimmune serum for DHC2 (lane 2), DHC2 affinity-purified antibodies (lane 3), rat preimmune serum for D2LIC (lane 4), and D2LIC affinity-purified antibodies (lane 5), and then analyzed by Western blotting. The top panel was probed with antibodies to DHC2, and the bottom panel was probed with antibodies to D2LIC. (C) Specificity of immunoprecipitated complexes. Western blots of D2LIC immunoprecipitates (Figure 3B, lane 5) and COS-7 lysate (20 μg/lane) (Figure 3B, lane 1) were probed with affinity-purified antibodies to DHC2 (left) and to DHC1 (right). (D) Sedimentation analysis. Fractionation of COS-7 cell homogenate on a sucrose gradient was performed as described in MATERIALS AND METHODS. COS-7 cell lysate and gradient fractions were analyzed by Western blot with affinity-purified antibodies to DHC2 (top) and D2LIC (bottom).
To determine whether the D2LIC protein is specifically associated with DHC2 in vitro, we performed a series of immunoprecipitation reactions using DHC2 and D2LIC affinity-purified antibodies. COS-7 cell homogenates were mixed with antibodies to DHC2, or D2LIC, or with the corresponding preimmune sera for each antibody. Immunological analysis of the resulting precipitates revealed that antibodies to DHC2 precipitated D2LIC as well as the heavy chain, whereas the preimmune control serum did not (Figure 3B). Similarly, antibodies to D2LIC immunoprecipitated the heavy chain as well as the LIC, whereas preimmune control serum did not (Figure 3B). We tested the specificity of these interactions by probing the same immunoprecipitated complexes with antibodies to conventional cytoplasmic DHC1 and to DHC2. Immunoprecipitates from COS-7 cell homogenate by using affinity-purified D2LIC antibodies were analyzed on Western blots. D2LIC antibodies immunoprecipitated the heavy chain of dynein 2 (DHC2) but not the heavy chain of dynein 1 (DHC1) (Figure 3C). The specificity of the interaction between D2LIC and DHC2 suggests that these two polypeptides are associated in a single complex.
We have also examined immunoprecipitates, prepared using DHC2 antibodies, for the presence of various dynein 1 subunits, including the IC, IC74, and several light chains: Tctex-1, RP3, and the 8-kDa Chlamydomonas light chain (antibodies generously provided by Dr. K. Pfister, University of Virginia School of Medicine, Charlottesville, VA; and Dr. S. King, University of Connecticut Health Center, Farmington, CT). To date, we have not detected any of these dynein 1 subunits in our preparations (our unpublished data).
To further assess the interaction between D2LIC and DHC2, we examined the fractionation of these two polypeptides by sucrose gradient centrifugation. COS-7 cell homogenate was fractionated on sucrose gradients as described in MATERIALS AND METHODS. Immunological analysis of the fractions from these gradients confirmed that DHC2 sedimented at ∼15 S, as reported previously (Figure 3D) (Vaisberg et al., 1996). Examination of these same fractions with D2LIC antibodies revealed that the majority of D2LIC cosedimented with DHC2 at ∼15 S in fractions 8–10 (Figure 3D). There was, however, a small amount of D2LIC that sedimented near the top of the gradient at ∼6 S (fractions 16 and 17). This 6 S fraction of D2LIC may represent a pool of D2LIC that is not bound to the heavy chain, a result that is consistent with previous observations that both the intermediate and light chains of dynein 1 sediment at low S after removal from the heavy chain (Steffen et al., 1996). The reciprocal coimmunoprecipitation and the cosedimentation of D2LIC and DHC2 indicate that these polypeptides are stably associated with one another in the same 15 S complex.
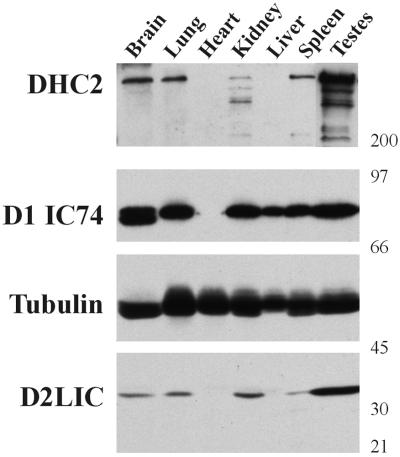
Previously, we demonstrated that DHC2 is expressed in a variety of cells and tissues (Vaisberg et al., 1996). We therefore examined the expression of D2LIC relative to DHC2 and compared their levels of expression with that of cytoplasmic dynein 1. Mouse tissue samples were analyzed immunologically with antibodies to cytoplasmic dynein 1 IC (D1 IC74), DHC2, and D2LIC, using β-tubulin as a sample loading control (Figure 4). D1IC74 was expressed at its highest level in testes and brain, at slightly lower levels in lung and kidney, and at even lower levels in spleen and liver. These results are consistent with data previously reported (King et al., 1998). DHC2 was expressed at high levels in testes and at lower levels in brain, lung, spleen, and kidney. Expression of DHC2 was not detectible in either liver or heart. This pattern of expression was mirrored by that of D2LIC. The lower molecular weight bands, recognized by DHC2 antibodies in the kidney sample, are probably due to proteolysis of the dynein 2 heavy chain in this preparation; therefore comparisons to the quantity of D2LIC expression for this sample may be misleading. The relatively equal expression of β-tubulin in all tissues was used as a control for gel loading and sample preparation. These data show that the levels of DHC2 and D2LIC expression are similar in a variety of tissues, as would be expected for two polypeptides that form a complex in vivo.
Figure 4.
Tissue-specific distribution of D2LIC and DHC2. An immunoblot of multiple tissue samples from mouse was prepared and cut at the molecular weight markers indicated to the right. The panels were probed with antibodies to either DHC2, the 74.1k IC of dynein 1 (D1 IC74), tubulin, or D2LIC.
Colocalization of D2LIC and DHC2 to the Golgi Apparatus
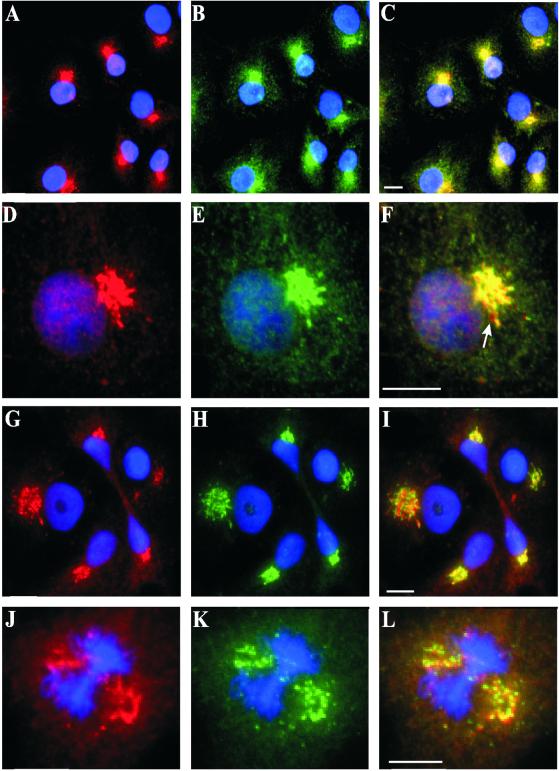
To further explore the association of D2LIC with DHC2, we examined the subcellular localization of D2LIC in COS-7 cells by immunocytochemistry (see MATERIALS AND METHODS). D2LIC colocalized with DHC2, clearly labeling a juxtanuclear structure that resembled the Golgi apparatus (Figure 5, A–C). As previously observed, DHC2 antibodies stained some cytoplasmic vesicles, but D2LIC antibodies stained fewer of these vesicles, and those only faintly (Figure 5, D–F). At higher magnification, it became evident that D2LIC and DHC2 did not colocalize perfectly; there were some small regions where D2LIC appeared to stain vesicular compartments adjacent to or independent of DHC2-stained structures (Figure 5, D–F, arrow). We were concerned that these discrepancies in colocalization might result from background noise or misregistration between the fluorescent channels used to collect the images. However, we measured pixel intensity of the fluorescent signals and determined that the subset of vesicles uniquely stained by D2LIC antibodies was clearly above background noise (see MATERIALS AND METHODS). Misregistration was not the explanation because the uniquely stained vesicles were positioned in many orientations relative to their neighbors in a single micrograph. Therefore, immunolocalization suggests that some vesicles bind DHC2 but not D2LIC and vice versa.
Figure 5.
Localization of D2LIC and DHC2 to the Golgi apparatus in COS-7 Cells. COS-7 cells were costained with D2LIC affinity-purified antibodies (shown in red) (A and D) and DHC2 affinity-purified antibodies (shown in green) (B and E). The merged images are shown in C and F. The DNA in all cells is visualized with DAPI (shown in blue). Cells were also double labeled with D2LIC antibodies (shown in red) (G and J) and antibodies to the Golgi maker p58 (shown in green) (H and K). The corresponding merged images are shown in I and L. Bars, 10 μm.
We have previously demonstrated that the DHC2 is localized predominantly to the Golgi apparatus in a variety of cultured mammalian cells (Vaisberg et al., 1996). To test the Golgi localization of D2LIC, we double labeled COS-7 cells with anti-D2LIC antibodies and anti-p58 mAb (Bloom and Brashear, 1989), a marker for the pre-Golgi intermediate compartment and cis-Golgi elements (Saraste and Svensson, 1991). In COS-7 cells, D2LIC and p58 had nearly identical staining patterns (Figure 5, G–I). Moreover, D2LIC colocalized with p58 at the Golgi not only during interphase but also during the initial break down of the Golgi apparatus in the early stages of mitosis; it remained associated with fragmented Golgi vesicles during prometaphase/metaphase (Figure 5, J–L). Previous studies on the reorganization of the Golgi complex during the final stages of cell division have shown that the Golgi complex goes through a synchronized change in location after karyokinesis and before completion of cytokinesis (reviewed in Thyberg and Moskalewski, 1999). During the final stages of division, D2LIC remained associated with the Golgi as it relocalized from the region adjacent to the intercellular bridge to the other side of the nucleus at the pole (Figure 5, G–I).
Nocodazole and BFA Disperse the Golgi Localization of D2LIC
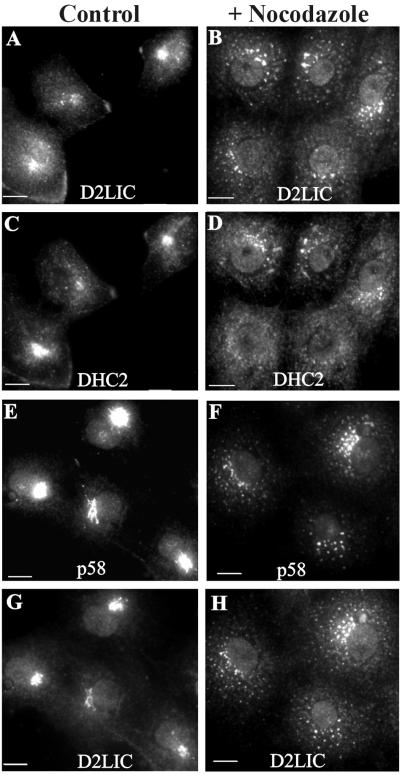
We further examined the relationship between D2LIC and Golgi elements by treating cells with pharmacological agents known to disrupt the structural integrity of the Golgi apparatus. When cells are treated with nocodazole, microtubules are depolymerized and Golgi membranes lose their association with the centrosomal region, resulting in the disruption and dispersal of cisternal Golgi stacks throughout the cytoplasm (Thyberg and Moskalewski, 1985; Kreis, 1990). On treatment of COS-7 cells with nocodazole, D2LIC and DHC2 remained associated with these Golgi fragments, brightly staining the structures that were now scattered throughout the cytoplasm (Figure 6, B and D). This pattern is consistent with the Golgi staining of nocodazole-treated cells seen with a variety of Golgi markers, including p58 (Figure 6F) (Rogalski et al., 1984; Turner and Tartakoff, 1989). Furthermore, D2LIC colocalized to the dispersed Golgi fragments stained with p58 antibodies (Figure 6H). The localization of D2LIC, DHC2, and p58 was not affected by control treatments of COS-7 cells with DMSO (Figure 6, A, C, E, and G). We conclude that the interaction of D2LIC and DHC2 with the Golgi apparatus is microtubule independent.
Figure 6.
Effect of nocodazole treatment on the localization of D2LIC and DHC2 to the Golgi. COS-7 cells were treated with 0.3% DMSO (A, C, E, and G) or 33 μM nocodazole dissolved in DMSO (B, D, F, and H) for 1 h at 37°C. Cells were then fixed as described in MATERIALS AND METHODS and double labeled with anti-D2LIC (A and B) and anti-DHC2 (C and D). Treated cells were also double labeled with anti-D2LIC (G and H) and with antibodies to the Golgi marker p58 (E and F). Bars, 10 μm.
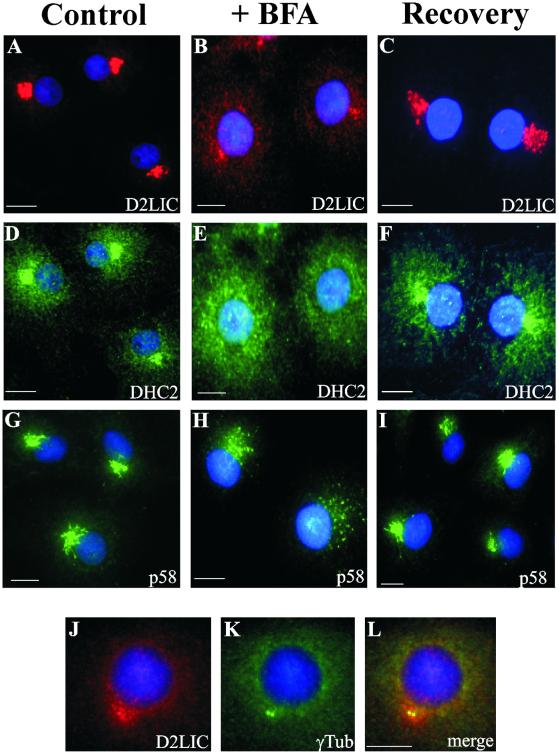
Brefeldin A (BFA) is a fungal metabolite that causes the reversible disassembly of the Golgi complex into a tubular network that is absorbed into the ER (Fujiwara et al., 1988; Doms et al., 1989; Lippincott-Schwartz et al., 1989, 1990; Orci et al., 1991; Klausner et al., 1992). This compound, whose mechanism of action is distinct from nocodazole, was used to perturb Golgi structure and follow D2LIC localization in cells. After treatment of COS-7 cells with BFA, some D2LIC and most DHC2 disappeared from the Golgi apparatus, resulting in a cytoplasmic staining (Figure 7, B and E) that does not colocalize with p58-stained structures. Additionally, D2LIC antibodies stained a bright pericentrosomal region. This residual D2LIC is clearly localized to the area around the centrosome, as indicated by its colocalization with γ-tubulin (Figure 7, J–L), a protein associated with the pericentriolar material (Stearns et al., 1991; Zheng et al., 1991). The D2LIC staining pattern seen here (Figure 7, B and J) is different from what has been observed with proteins intrinsic to the Golgi complex, such as p58 (Figure 7H) (Lippincott-Schwartz et al., 1990). Control treatments of COS-7 cells with ethanol had no effect on the localization of D2LIC, DHC2, and p58 (Figure 7, A, D, and G).
Figure 7.
Effect of brefeldin A on the localization of D2LIC and DHC2 to the Golgi. COS-7 cells were treated at 37°C with 0.4% ethanol (A, D, and G), 10 μg/ml BFA dissolved in ethanol (B, E, H, and J–L) for 30 min, or 10 μg/ml BFA for 30 min followed by recovery in the absence of BFA for 90 min (C, F, and I). After these treatments, cells were fixed and double labeled with anti-D2LIC (A–C) and anti-DHC2 (D–F) or stained with anti-p58 (G–I). DNA in all cells is visualized with DAPI (shown in blue). BFA-treated cells were also double labeled with anti-D2LIC (J) and anti-γ-tubulin (K). The corresponding merged image is shown in L. Bars, 10 μm.
BFA-induced Golgi disruption is reversible (Doms et al., 1989; Lippincott-Schwartz et al., 1989), so we asked whether the BFA-induced D2LIC and DHC2 localization patterns were likewise reversible. After BFA removal and recovery, the Golgi apparatus reforms, as evidenced by the localization of p58 (Figure 7I). Similarly, both the D2LIC and DHC2 staining patterns returned to the pericentrosomal region characteristic of the Golgi apparatus (Figure 7, C and F). The differences in p58 and D2LIC staining patterns in BFA-treated cells suggest that the dynein 2 complex and more specifically, D2LIC, may be involved in targeting the Golgi complex to the centrosome and/or the establishment of the Golgi apparatus organization in cells.
To test this hypothesis, we conducted a series of experiments to examine the function of D2LIC in vivo. Previously, we have shown that injection of anti-DHC2 antibodies into NRK cells leads to the dispersal of the Golgi apparatus, suggesting that cytoplasmic dynein 2 is involved in establishing proper Golgi organization (Vaisberg et al., 1996). We therefore injected rat anti-D2LIC affinity-purified antibodies into COS-7 cells at a concentration of 1 mg/ml. At 5 and 24 h postinjection, cells were fixed and stained with goat anti-rat antibody and anti-p58 mAb to visualize Golgi membranes. We saw staining of a normal Golgi apparatus with both antibodies, including cells that were obviously sisters, and thus had dispersed and reformed their Golgi complex during division (our unpublished data). These observations suggested that the D2LIC antibodies we have generated do not block detectable manifestations of the protein's function.
We also looked for perturbation of dynein 2 function by overexpressing either wild-type or mutant forms of D2LIC. COS-7 cells were transfected with either a NH2-tagged myc-D2LIC construct, a COOH-tagged myc-D2LIC construct, or a COOH-tagged myc-D2LIC P-loop mutant (Gly43 to Glu or Thr45 to Asn). At times ranging from 4 to 48 h post transfection, the cells were fixed and stained with anti-myc antibodies. All of the myc-tagged constructs localized to both the Golgi apparatus and the interphase nucleus, but there was no detectable disruption in Golgi morphology or organization.
DISCUSSION
Identification of a Novel Cytoplasmic Dynein 2 Subunit
Cytoplasmic dyneins contribute to diverse cellular processes (reviewed in Holzbaur and Vallee, 1994; Hirokawa et al., 1998). Unlike the various tasks accomplished by members of the kinesin superfamily, this array of functions appears to be due to only a few isoforms of cytoplasmic dynein. The functional diversification of cytoplasmic dyneins may instead be a result of the extensive subunit complexity of these large polypeptide clusters. It is therefore important to identify and characterize the light and intermediate molecular weight components of each dynein complex.
In this study, we have used biochemical and immunological techniques to identify a novel polypeptide subunit of the cytoplasmic dynein 2 complex. The reciprocal immunoprecipitations of this subunit (D2LIC) with the heavy chain (DHC2), their cosedimentation on sucrose gradients, their colocalization by immunofluorescence, and their similar expression in a variety of tissues indicate that these two polypeptides are present in cells in the same 15 S complex. We have also demonstrated through immunoprecipitation and sedimentation analysis that D2LIC specifically interacts with DHC2, and not DHC1. Similarly, the differential expression of D2LIC relative to D1IC74 suggests that cytoplasmic dyneins 1 and 2 do not share this LIC subunit.
We have used peptide microsequencing to clone the coding sequence for this D2LIC gene. It shares sequence similarity with members of the D1LIC subunit family. The amino-terminal P-loop sequence that it shares with all members of the D1LIC family suggests a possible ATPase activity, but to date, this activity has not been detected in any of the dynein light intermediate chains. Moreover, mutations in the P-loop of D1LIC1 do not appear to perturb the binding of D1LIC1 to the heavy chain of dynein 1 or to another binding partner, pericentrin (Tynan et al., 2000a,b). D2LIC is most similar to D1LIC2, but the homology to all D1LICs decreases significantly in the carboxy-terminal region of the polypeptide. Perhaps a further investigation of this region will elucidate some functional differences among the LICs of dynein 1 and dynein 2.
Sequence analysis has identified D2LIC homologs from several organisms, including C. elegans, D. melanogaster, M. musculus, and Chlamydomonas (Dr. Mary Porter, personal communication). We have also identified a region of sequence similarity with the yeast GTPases Spg1p and Tem1p. The significance of this similarity is unknown but Spg1p and Tem1p may represent distant homologs of D2LIC in yeast. Alternatively, the 44 amino acid block of shared homology between D2LIC and Spg1p and Tem1p, which includes a RAS signature motif common to all RAS-related transforming proteins, may reflect a previously undetected mode of regulation for cytoplasmic dynein function in cells. It is interesting to note that this RAS motif is present in all members of the D2LIC family as well as the D1LIC family. Further work will be required to understand the significance of this motif and its implication in dynein regulation.
D2LIC Subunit Function within the Cell
Several reports have suggested that cytoplasmic dynein 1 plays a role in the organization and function of the Golgi apparatus. Dynein 1 is thought to mediate centrosomal localization of the Golgi apparatus (Corthesy-Theulaz et al., 1992), ER-Golgi transport (Presley et al., 1997), and the partitioning of the Golgi apparatus between daughter cells (Lucocq and Warren, 1987; Lucocq et al., 1989). Previous studies of mice lacking DHC1 revealed that cultured DHC1−/− blastocysts had a disrupted Golgi apparatus that was distributed throughout the cytoplasm (Harada et al., 1998).
In a previous study, we demonstrated that the injection of affinity-purified DHC2 antibodies resulted in the dispersal of the Golgi complex (Vaisberg et al., 1996), suggesting that DHC2 also plays a role in Golgi organization. Function-blocking antibodies to DHC1 had no such effect (Vaisberg et al., 1993), suggesting that in cultured cells, the dynein 2 complex is more important than the dynein 1 complex for Golgi organization. Our observations on the persistent colocalization of the D2LIC subunit with DHC2 at the Golgi apparatus, throughout the cell cycle, supports the role of cytoplasmic dynein 2 complex in Golgi organization. Moreover, the colocalization of D2LIC and DHC2 with Golgi fragments induced by the depolymerization of microtubules with nocodazole provides further evidence about the relationship between dynein 2 and the Golgi apparatus and suggests that the dynein 2 complex interacts with the Golgi apparatus in a microtubule-independent manner.
Additional information about the possible role of dynein 2 in Golgi organization is provided by our observations following BFA-induced Golgi fragmentation. After BFA treatment, some D2LIC loses its association with the Golgi and remains localized near the centrosome. It is unclear whether BFA treatment unmasks D2LIC that is normally localized to the centrosomal region, or whether this staining pattern is due to the dissociation of D2LIC from the Golgi complex. Recent work has shown that D1LIC1 binds to the centrosomal protein pericentrin (Purohit et al., 1999; Tynan et al., 2000a). We have used immunoprecipitation to look for associations between D2LIC and two obvious centrosomal candidates, pericentrin and γ-tubulin, but have seen no evidence that such interactions exist (our unpublished data). These findings are nevertheless intriguing and suggest that dynein 2, and more specifically the D2LIC subunit, may be responsible for targeting of the Golgi apparatus to the centrosome and/or establishing proper Golgi organization.
Our attempts to disrupt D2LIC function in vivo have thus far yielded no information. The lack of function-perturbing tools and the technical difficulties associated with these experiments suggest a genetic approach. The generation of mutants that lack a functional D2LIC subunit in either mammals or other organisms may be required to elucidate D2LIC function in vivo.
Dynein 2 Regulation
An important unresolved aspect of cytoplasmic dynein function is its spatial and temporal regulation. These control mechanisms may include posttranslation modifications such as phosphorylation/dephosphorylation, G proteins involved with signal transduction cascades, the diversification of dynein subunit composition, and the regulation of subunit chain binding to the dynein complex. Previous studies have presented evidence that D1LIC1 is phosphorylated (Gill et al., 1994; Hughes et al., 1995) in a cell cycle-dependent manner, resulting in a reduction in the rate of retrograde transport by decreasing the levels of membrane-associated dynein 1 (Niclas et al., 1996). Whether D2LIC is phosphorylated in a similar manner remains to be determined. However, the sequence of RAS-like homology between D2LIC and Spg1p/Tem1p proposes an interesting question regarding the regulatory significance of this polypeptide.
In this study, we report the first identification of a dynein 2 subunit. Yet, based on our knowledge of dynein 1 holoenzyme, there are probably multiple subunits comprising the dynein 2 motor, including possible intermediate and light chains. In addition to subunit composition, dyneins may also be regulated by the association/disassociation of these various subunit chains. A previous study on Tctex-1, a dynein 1 light chain, has shown that there is a population of Tctex-1 that is not associated with the IC at steady state (Tai et al., 1998). Additionally, Tctex-1 competes with a related protein, RP3, for binding to cytoplasmic dynein 1, resulting in the missorting of apical proteins and suggesting that dynein cargo specificity is regulated by subunit composition (Tai et al., 2001). Our observations also indicate that there is a small population of D2LIC that is not associated with its heavy chain; immunofluorescence reveals a subset of organelles stained with D2LIC but not DHC2 antibodies. Similarly, sedimentation analysis of the D2LIC indicates a subpopulation of the polypeptide that sediments at 6 S on sucrose gradients. This fraction may represent a pool that is not bound to the dynein 2 complex. It will be interesting to determine whether there are distinct dynein 2 populations and how they contribute to functional diversity within the cell.
Our results imply that there are multiple cytoplasmic dyneins, each with its own associated subunits, that are responsible for the diverse functions attributed to dynein motors in cells. It remains an interesting challenge to identify and characterize additional subunits of the dynein 2 complex, to differentiate specific cargo proteins that bind to them, and to determine their roles in cellular organization and motility.
ACKNOWLEDGMENTS
We thank Drs. Heidi Browning, Robert West, and Katya Grishchuk for assistance and advice throughout this project, and Drs. Mary Porter and John Ohlsson for critical reading of the manuscript. We also thank Dr. Mike Klymkowsky for performing the antibody injection experiments described in this study and Yuming Han (University of Colorado Sequencing Facility) for the D2LIC sequence. This work was supported by the National Institutes of Health grant GM-R01-36663 to J.R.M., who is a Research Professor of the American Cancer Society.
Abbreviations used:
- BFA
brefeldin A
- DAPI
4,6-diamidino-2-phenylindole
- DHC
cytoplasmic dynein heavy chain
- DHC1
cytoplasmic dynein 1 heavy chain
- DHC2/cDhc1b
cytoplasmic dynein 2 heavy chain
- ER
endoplasmic reticulum
- IC
intermediate chain
- LC
light chain
- LIC
light intermediate chain
- mAb
monoclonal antibody
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0402. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0402.
REFERENCES
- Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LSB, King SM. Drosophila roadblock and ChlamydomonasLC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J Cell Biol. 1999;146:165–179. [PMC free article] [PubMed] [Google Scholar]
- Corthesy-Theulaz I, Pauloin A, Rfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell PS, Asai DJ. Evidence for four cytoplasmic dynein heavy chain isoforms in rat testis. Mol Biol Cell. 1998;9:237–247. doi: 10.1091/mbc.9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell PS, Ostrowski LE, Asai DJ. A novel cytoplasmic dynein heavy chain: expression of DHC1b in mammalian ciliated epithelial cells. J Cell Sci. 1996;109:1891–1898. doi: 10.1242/jcs.109.7.1891. [DOI] [PubMed] [Google Scholar]
- Dillman JF, III, Pfister KK. Differential phosphorylation in vivoof cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;125 127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Gibbons BH, Asai DJ, Tang WJY, Hays TS, Gibbons IR. Phylogeny and expression of axonemal and cytoplasmic dynein genes in sea urchins. Mol Biol Cell. 1994;5:57–70. doi: 10.1091/mbc.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons IR. Dynein family of motor proteins: present status and future questions. Cell Motil Cytoskeleton. 1995;32:136–144. doi: 10.1002/cm.970320214. [DOI] [PubMed] [Google Scholar]
- Gill SR, Cleveland DW, Schroer TA. Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol Biol Cell. 1994;5:645–654. doi: 10.1091/mbc.5.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A, et al. Life with 6000 genes. Science. 1996;274:546–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. pp. 464–470. [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10:60–73. doi: 10.1016/s0955-0674(98)80087-2. [DOI] [PubMed] [Google Scholar]
- Holzbaur ELF, Vallee RB. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Vaughan KT, Herskovits JS, Vallee RB. Molecular analysis of a cytoplasmic dynein light intermediate chain reveals homology to a family of ATPases. J Cell Sci. 1995;108:17–24. doi: 10.1242/jcs.108.1.17. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- King SM, Barbarese E, Dillman JF, III, Benashski SE, Do KT, Patel-King RS, Pfister KK. Cytoplasmic dynein contains a family of differentially expressed light chains. Biochemistry. 1998;37:15033–15041. doi: 10.1021/bi9810813. [DOI] [PubMed] [Google Scholar]
- King SM, Barbarese E, Dillman JF, III, Patel-King RS, Carson JH, Pfister KK. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996a;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- King SM, Dillman JF, III, Benashski SE, Lye RJ, Patel-King RS, Pfister KK. The mouse t-complex-encoded protein Tctex-1 is a light chain of brain cytoplasmic dynein. J Biol Chem. 1996b;271:32281–32287. doi: 10.1074/jbc.271.50.32281. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE. Role of microtubules in the organization of the Golgi apparatus. Cell Motil Cytoskeleton. 1990;15:67–70. doi: 10.1002/cm.970150202. [DOI] [PubMed] [Google Scholar]
- Lai CH, Chou CY, Ch'ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegansby comparative proteomics. Genome Res. 2000;10:703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Cheng SY. An efficient method to purify active eukaryotic proteins from the inclusion bodies in Escherichia coli. Biotechniques. 1991;11:748–753. [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Berger EG, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq JM, Warren G. Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Mikami A, Gee MA, Vallee RB. In vitromotility from recombinant dynein heavy chain. Proc Natl Acad Sci USA. 1996;93:6552–6556. doi: 10.1073/pnas.93.13.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors BW, Williams RC, McIntosh JR. Localization of kinesin in cultured cells. J Cell Biol. 1988;106:1193–1204. doi: 10.1083/jcb.106.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Tagaya M, Amherdt M, Perrelet A, Donaldson JG, Lippincott-Schwartz J, Klausner RD, Rothman JE. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoforms of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME. Axonemal dyneins: assembly, organization, and regulation. Curr Opin Cell Biol. 1996;8:10–17. doi: 10.1016/s0955-0674(96)80042-1. [DOI] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Pruitt KD, Maglott DR. RefSeq, and LocusLink. NCBI gene-centered resources. Nucleic Acids Res. 2001;29:137–140. doi: 10.1093/nar/29.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A, Tynan SH, Vallee R, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol. 1999;147:481–491. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski AA, Bergmann JE, Singer SJ. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J Cell Biol. 1984;99:1101–1109. doi: 10.1083/jcb.99.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Steffen W, Hodgkinson JL, Wiche G. Immunogold localization of the intermediate chain within the protein complex of cytoplasmic dynein. J Struct Biol. 1996;117:227–235. doi: 10.1006/jsbi.1996.0087. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Bode C, Wolfrum U, Sung C-H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Sung C-H. Localization of Tctex-1, a cytoplasmic dynein light chain, to the Golgi apparatus and evidence for dynein complex heterogeneity. J Biol Chem. 1998;273:19639–19649. doi: 10.1074/jbc.273.31.19639. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang J-Z, Sung C-H. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J Cell Biol. 2001;153:1499–1509. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Zhang Z, Hirokawa N. Identification and molecular evolution of new dynein-like protein sequences in rat brain. J Cell Sci. 1995;108:1883–1893. doi: 10.1242/jcs.108.5.1883. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985;159:1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Turner JR, Tartakoff AM. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan SH, Gee MA, Vallee RB. Distinct but overlapping sites within the cytoplasmic dynein heavy chain for dimerization and for intermediate chain and light intermediate chain binding. J Biol Chem. 2000b;275:32769–32774. doi: 10.1074/jbc.M001537200. [DOI] [PubMed] [Google Scholar]
- Tynan SH, Purohit A, Doxsey SJ, Vallee RB. Light intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. J Biol Chem. 2000a;275:32763–32768. doi: 10.1074/jbc.M001536200. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Shpetner HS. Motor proteins of cytoplasmic microtubules. Annu Rev Biochem. 1990;59:909–932. doi: 10.1146/annurev.bi.59.070190.004401. [DOI] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HGAM, Plasterk RHA. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR. Gamma-tubulin is present in Drosophila melanogaster and Homo sapiensand is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]