Abstract
Periodontitis is the sixth most prevalent chronic disease globally and places significant burdens on societies and economies worldwide. Behavioral modification, risk factor control, coupled with cause-related therapy have been the “gold standard” treatment for managing periodontitis. Given that host inflammatory and immunological responses play critical roles in the pathogenesis of periodontitis and impact treatment responses, several adjunctive strategies aimed at modulating host responses and improving the results of periodontal therapy and maintenance have been proposed. Of the many pharmacological host modulators, we focused on non-steroidal anti-inflammatory drugs (NSAIDs), due to their long history and extensive use in relieving inflammation and pain and reducing platelet aggregation. NSAIDs have been routinely indicated for treating rheumatic fever and osteoarthritis and utilized for the prevention of cardiovascular events. Although several efforts have been made to incorporate NSAIDs into the treatment of periodontitis, their effects on periodontal health remain poorly characterized, and concerns over the risk–benefit ratio were also raised. Moreover, there is emerging evidence highlighting the potential of NSAIDs, especially aspirin, for use in periodontal regeneration. This review summarizes and discusses the use of NSAIDs in various aspects of periodontal therapy and regeneration, demonstrating that the benefits of NSAIDs as adjuncts to conventional periodontal therapy remain controversial. More recent evidence suggests a promising role for NSAIDs in periodontal tissue engineering and regeneration.
Keywords: Non-steroidal anti-inflammatory drug, Aspirin, Periodontitis, Periodontal regeneration
Introduction
Periodontal diseases are notably the most common and prevalent chronic diseases in the world [1]. They include a spectrum of inflammatory conditions encompassing gingivitis and periodontitis; the latter is a non-communicable, multifactorial chronic inflammatory infectious disease characterized by clinical attachment loss and alveolar bone destruction, which can result in tooth migration, drifting, tooth hypermobility, tooth loss, and ultimately masticatory dysfunction [2]. Periodontitis has been the major cause of tooth loss in the adult population worldwide that negatively impacts quality of life and accounts for the huge socio-economic impact and healthcare costs globally [3]. The causes of periodontitis have been extensively investigated, and its initiation and progression has been attributed to the uncontrolled accumulation of dental biofilm, which interacts with the individual susceptibility profile [4]. Genetics, tobacco and alcohol use, diabetes, obesity, poor nutrition, physical inactivity and impaired host responses have all been associated with an increased risk of periodontitis [5]. Periodontal therapy varies based on a proper diagnosis of disease severity, extent, rate of progression, risk factors, complexity of management, and interrelationship with general health [6]. According to the European Federation of Periodontology (EFP) S3 level clinical practice guideline for periodontitis [7], the fundamental cornerstones of periodontal therapy involves 4 sequential steps: Step 1—Behavior change and risk factor control; Step 2—Cause-related therapy; Step 3—Surgical interventions and Step 4—Supportive care; which are delivered in a stepwise incremental approach depending on the disease stage. The essence of the first step of therapy is both preventive and therapeutic which aims to control systemic and local risk factors. The second step of cause-related therapy targets the control of subgingival biofilm and calculus via subgingival mechanical instrumentation, namely non-surgical periodontal therapy (NSPT), and may also include the use of adjunctive physical or chemical agents, local or systemic adjunctive host-modulating agents or antimicrobials. For areas that are not responding favorably to cause-related therapy, surgical interventions may be indicated to facilitate access to root surface debridement, to regenerate or resect intra-bony or furcation periodontal defects [8, 9]. The recent advances in biological materials, therapeutic agents, and surgical techniques for regenerative periodontal therapy has enabled its success in restoring lost periodontal tissue and changing tooth prognosis [10]. While standard NSPT and supportive periodontal maintenance by means of scaling and root surface debridement remain the “gold-standard” treatment for Stage I-III periodontitis [7], there still exist patients or sites with poor response to NSPT and long-term supportive maintenance efforts that may be attributed to sustained dysbiosis, periodontopathic bacteria tissue invasion, or a non-resolving chronic inflammatory response [11]. Hence, there has been a constant pursuit for adjunctive therapies to boost the outcomes of periodontal therapy.
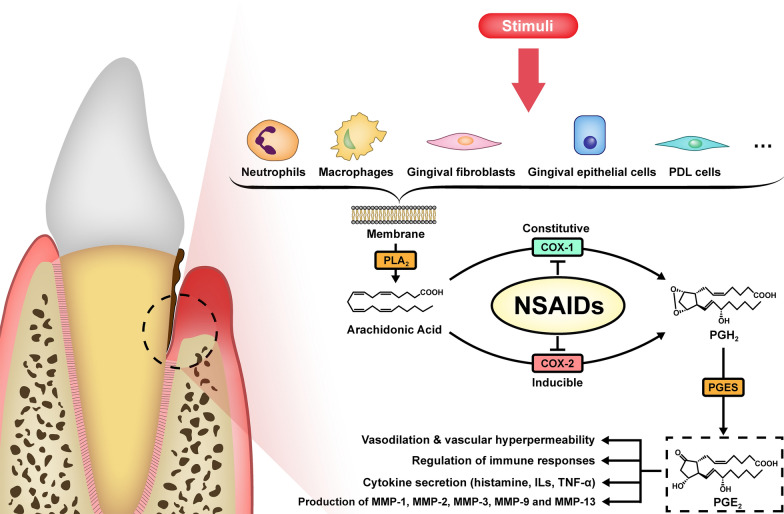
Even though biofilms are initiators of periodontal disease, the unique individual susceptibility profile reflected in the host’s immunoinflammatory response plays an important role in periodontal tissue destruction through the local synthesis and secretion of immunological factors, and will impact the treatment responses to all four steps of periodontal therapy [12, 13]. Therefore, various host modulators have been proposed as adjuncts to conventional periodontal treatment. Among these host modulators, non-steroidal anti-inflammatory drugs (NSAIDs) are the most well-known and broadly prescribed [14]. Their pharmacological function is based on the blocking of cyclooxygenase (COX), which was discovered in the late 1980s and exists as two isoforms [15] (Fig. 1). COX-1 is the constitutive isoform and produces basal levels of prostaglandins (PGs), which control platelet activation and protect the lining of the gastrointestinal tract; COX-2 is the inducible isoform and is released in response to inflammatory stimuli such as hypoxia, interleukin (IL)-1, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, leading to swelling and pain at the inflammation site [16]. Both COX isoforms can transform arachidonic acid in the plasma membrane into PGG2, which is reduced to PGH2 by a subsequent peroxidase reaction. PGH2 is then converted to biologically active primary PGs: PGD2, PGE2, PGF2 , PGI2, and thromboxane A2 (TxA2) [15].
Fig. 1.
The synthesis and effects of PGE2 in periodontitis. COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; ILs, interleukins; MMPs, matrix metalloproteinases; NSAIDs, nonsteroidal anti-inflammatory drugs; PGE synthase; PGE2, prostaglandin E2; PGES, PGH2, prostaglandin H2; PLA2, Phospholipase A2
NSAIDs are classified into four categories based on their affinity and/or selectivity for COX isoenzymes (Table 1). The first category comprises compounds with potential to produce complete inhibition of both COX-1 and COX-2 and is exemplified by aspirin, indomethacin, diclofenac, naproxen, and ibuprofen. The second category of compounds consists of preferential COX-2 inhibitors (5- to 50-fold selectivity) and includes meloxicam, celecoxib, nimesulide, and etodolac. The third category comprises compounds with strong selectivity (> 50-fold) for COX-2, such as diisopropyl fluorophosphate, L-745337, rofecoxib, and NS398. The fourth category of compounds has low affinities for both COX-1 and COX-2 and comprises compounds such as sulfasalazine, sodium salicylate, and nabumetone [17, 18]. Notably, aspirin is unique in that it is an irreversible inhibitor of COXs, whereas all of the other NSAIDs exhibit some degree of reversible inhibition [19].
Table 1.
Classification of NSAIDs according to their selectivity for COX isoenzymes
| Category | Properties | Examples |
|---|---|---|
| 1 | Inhibitors of COX-1 and COX-2 | indomethacin, aspirin, diclofenac, naproxen, ibuprofen |
| 2 | 5- to 50-fold COX-2 selective | etodolac, meloxicam, celecoxib, nimesulide |
| 3 | > 50-fold COX-2 selective | diisopropyl fluorophosphate, L-745337, rofecoxib, NS398 |
| 4 | Weak inhibitors of COX-1 and COX-2 | 5-Aminosalicylic acid, nabumetone, sodium salicylate, sulfasalazine |
NSAIDs can inhibit PG synthesis and consequently exert analgesic, anti-pyretic, and anti-inflammatory effects. Therefore, they are indicated to relieve pain and fever and treat inflammation in rheumatic, osteoarthritic, and other diseases. In addition, they can be used to prevent cardiovascular events, premature labor, and perturb the microenvironments of tumors [20, 21]. However, some nonspecific cytotoxic effects of NSAIDs can be detrimental to the human body; such complications should not be ignored [22].
In this review, we summarize laboratory and clinical evidence for the utility of NSAIDs in the conventional treatment of periodontitis, which includes both surgical and non-surgical periodontal treatment, but without regenerative approaches, and in periodontal tissue engineering and regeneration. In the first part of the review, we discuss the role of PGs in periodontitis and the administration of NSAIDs during cause-related non-surgical or surgical therapies. In the second part of the review, we describe the combination of NSAIDs with current periodontal regeneration techniques.
NSAIDs as adjuncts to conventional periodontal treatment
The role of PGs in periodontitis
PGs in the pathogenesis of periodontitis
Arachidonic acid metabolites have been implicated in a variety of inflammatory conditions such as rheumatoid arthritis, psoriasis, diabetes mellitus, obesity, and periodontitis [13, 23–25]. Among the subfamilies of arachidonic acid metabolites, PGs are the most critical inflammatory mediators of periodontitis. They regulate local inflammation in both autocrine and paracrine manners, with impacts on vasodilation, vascular permeability, edema, and pain [26]. PGE2, one of the most abundant PGs in periodontal tissue, plays a prominent role in pathogenesis. In periodontal tissue, PGE2 has been shown to be produced by neutrophils, macrophages, periodontal ligament cells, gingival fibroblast cells, gingival epithelial cells, osteoblasts, and cementoblasts [27–32]. PGE2 is mainly known for its pro-inflammatory effects. It causes vasodilation and vascular hyperpermeability, facilitating the leakage of immune cells and plasma components into extracellular space and leading to local inflammation [33].
Recently, lymphocyte responses have been recognized as being involved in the onset of periodontal diseases; PGE2 has been shown to directly stimulate T cell activation and the production of various cytokines. Yao et al. reported that PGE2 promotes Th1 cell differentiation and Th17 cell expansion both in vitro and in vivo [34, 35]. Th1 cells secrete IFN-γ and other cytokines, activating immune cells such as macrophages, dendritic cells, and B cells, which play a critical role in defending the body against infectious diseases such as periodontitis [36]. In addition, PGE2 stimulates the production of IL-17A by Th17 cells, and that IL-17A can in turn increase the expression of PGE2 in osteoblasts [37, 38], indicating a magnification of the effects of PGE2 at the site of periodontitis.
A growing body of clinical evidence also suggests the relationship between PGE2 and periodontitis. Goodson et al. first reported a tenfold increase in PGE2 concentrations in periodontally compromised tissues compared with healthy tissues [39]. Varying concentrations of PGE2 have been observed in gingival crevicular fluid, which were later shown to be correlated with the severity of periodontal inflammation [40, 41]. High concentrations of PGE2 observed in the saliva of patients with severe periodontitis and high PPDs [42].
PGE2 and periodontal tissue destruction
The destruction of both soft and hard tissues in periodontitis is largely attributable to the inflammatory host responses to pathogens [43]. Of the various cytokines and growth factors secreted at the sites of periodontitis, matrix metalloproteinases (MMPs) is one of the most vital contributors to soft tissue destruction by degrading collagen within the gingiva and periodontal ligament [44]. Yen et al. [45] and Khan et al. [46] demonstrated that PGE2 directly promotes the expression of MMP-9 in dendritic cells and macrophages; other MMPs such as MMP-1, MMP-2, MMP-3, and MMP-13 have also been shown to be regulated by PGE2 [32, 47–49]. Furthermore, PGE2 may also indirectly activate MMPs by inducing the production of pro-inflammatory cytokines that regulate MMPs, such as IL-1β, IL-6, and IL-17, during the progression of periodontitis [50, 51].
Regarding hard tissue destruction, early in vitro and in vivo studies have shown the effects of PGE1 and PGE2 on bone resorption [52, 53]. Studies have demonstrated the direct role of PGE2 in bone resorption by showing that PGE2 can bind to its receptors (EPs) on osteoclasts, thereby stimulating osteoclastic activity [54, 55]. Another mechanism by which PGE2 induces osteoclastogenesis is cellular communication between osteoclasts and osteoblastic cells through the receptor-activator of nuclear factor-κB ligand (RANKL)/receptor-activator of nuclear factor-κB (RANK)/osteoprotegerin (OPG) pathway. It has been shown that PGE2 upregulates the expression of RANKL, which binds to its receptor RANK on osteoclastic precursors to facilitate differentiation and downregulate the expression of OPG in mesenchymal lineage cells [56]. In periodontal tissue, PGE2 exposure has been reported to inhibit OPG expression in both periodontal ligament cells and osteoblasts while selectively promoting RANKL expression [57]. Collectively, this overwhelming body of evidence has confirmed the participation of PGE2 in the pathogenesis of periodontal diseases and its destructive impact on periodontal tissue.
Application of NSAIDs in non-clinical studies
Given the critical role of PGs, especially PGE2, in the progression of periodontal diseases, therapeutic strategies that use NSAIDs in periodontitis have been explored. The first investigation in this field was conducted 2 years after the mechanisms of NSAIDs were elucidated in 1971 [58]. In an in vitro study, Goldhaber et al. [59] reported that blocking PG synthesis by indomethacin significantly alleviated alveolar bone resorption caused by gingival cytokines in a dose-dependent manner, indicating that it acted on local inflammatory responses. To further examine the effects of indomethacin in vivo, Nyman et al. [60] established a ligature-induced periodontitis model in beagle dogs. The dogs were administered either no drug or indomethacin at 1 mg/kg orally for 29 days. Periodontal tissue was then biopsied and analyzed for the progression of inflammatory reactions and alveolar bone resorption. The results demonstrated that indomethacin delayed the initiation and reduced the severity of ligature-induced periodontitis and suppressed bone resorption. The findings were further verified by another study in squirrel monkeys [61].
Flurbiprofen is another NSAID that has been frequently explored in the context of therapies for periodontitis. Williams et al. [62] explored the effects of flurbiprofen on periodontitis in beagles by administering it to the dogs for 12 months. The bone loss rates of the treated groups largely decreased after drug administration compared with the baseline, indicating This that flurbiprofen could be used as an adjunct to periodontal surgical treatment. A subsequent study with the same animal model was conducted to explore whether the therapeutic effects of flurbiprofen could be sustained after terminating drug administration [63]. The rate of bone loss decreased over the course of pharmaceutical treatment, but returned to baseline at the sixth month after discontinuing treatment. Interestingly, despite the profound reduction in alveolar bone loss by flurbiprofen, gingival inflammation was not affected during the whole process.
Williams et al. [64] further investigated the effects of ibuprofen, an analogue of flurbiprofen, on periodontitis in beagle dogs. They employed a polymer matrix for the sustained release of ibuprofen. Although both normal and sustained release of the drug inhibited alveolar bone loss, sustained release was more effective than normal administration. Furthermore, the dogs in the sustained release group lost fewer teeth during the 7-month treatment period than those in other groups. These results highlight a more potent way of continuously releasing and obtaining a consistently high blood concentration of the drug. The authors also demonstrated the preventive effect of another NSAID, naproxen, against alveolar bone loss in beagle dogs, although this effect was attenuated after long-term use [65].
Application of NSAIDs in clinical studies
Significant efforts have been made to evaluate the application of NSAIDs in the progression of periodontal diseases in humans. Some earlier studies have shown that NSAIDs may have a therapeutic role in the process. Jeffcoat et al. [66] recruited 15 patients with active moderate-to-severe periodontitis and administered 50 mg b.i.d. flurbiprofen or placebo for 2 months. Radiography showed significantly less bone loss during the 2-month study period in the flurbiprofen-treated patients compared with the placebo-treated patients. More tooth sites with bone loss were also detected in the placebo group than in the flurbiprofen-treated group. In a 44-patient study conducted by Williams et al. [67], periodontitis patients were prescribed 50 mg b.i.d. flurbiprofen or placebo alongside NSPT for 2 years. Compared with the control group, the flurbiprofen-treated patients reported a lower rate of bone loss and less bleeding at probing spots after 1 year of therapy.
However, more recent clinical studies have revealed conflicting results regarding the treatment of periodontitis with NSAIDs. Haffajee et al. [68] examined the effects of three adjunct medications in periodontal treatment. The patients were given ibuprofen (400 mg t.i.d.), antibiotics (Augmentin™ [amoxycillin with clavulanic acid] or tetracycline), or a placebo, during a 1-month period of active periodontal treatment. Ibuprofen and placebo treatment exhibited similar decreases in PPD and gains or losses of clinical attachment level (CAL), which were inferior to the effects of treatment with either of the two antibiotics. A similar study on the adjunctive benefits of flurbiprofen administered at 200 mg q.d. during 10 days of periodontal therapy was conducted on periodontitis patients with different smoking statuses [69]. After periodontal therapy, a reduction in the plaque and gingival index was observed in all groups, while the PPD and CAL remained unchanged. However, NSAID treatment had no impact on either the clinical or biological parameters. Notably, the medicine was administered only for a short period of periodontal treatment in these studies. This was different from previous studies, wherein the effects of NSAIDs became apparent only after months of application [67].
A longer treatment duration with NSAIDs, in conjunction with conventional periodontal therapy, has been shown to improve periodontal outcomes. A 6-week administration of naproxen improved clinical parameters such as the gingival index, plaque index, and PPD, but no difference was observed in immunological parameters compared with placebo-treated patients [70]. Yen et al. [71] investigated a more extended application of celecoxib, a COX-2 inhibitor, for 12 months. They systematically prescribed 131 patients with chronic periodontitis either celecoxib or placebo combined with SRP. Compared with patients in the placebo group, patients administered celecoxib showed more prominent PPD reduction and CAL gains, especially in moderate-to-severe pockets. Furthermore, celecoxib-treated patients showed more sites with attachment gains and fewer sites with attachment loss than those in the placebo group. Bleeding on probing also improved in celecoxib-treated patients. Oduncuoglu et al.[72] employed a cyclic regimen with 50 mg diclofenac potassium for 2 months followed by 2 months of washout and another 2 months of prescription, and their group reported significant reduction in PPD at 6 months. However, regarding the topical application of NSAIDs, the current findings are inconclusive. The use of a 1% flurbiprofen toothpaste twice a day for 12 months did not improve PPD reduction compared to a placebo toothpaste [73]. In another randomized controlled trial, supragingival daily irrigation with 300 ml of water immediately followed by 200 ml of buffered 0.3% acetylsalicylic acid did not improve PPD at 6 months compared to irrigation with water alone [74]. Due to the conflicting results so far, future studies are still needed to elucidate the efficacy of the adjunctive use of NSAIDs to subgingival instrumentation in different conditions.
A focus on aspirin
Aspirin is an ancient analgesic and anti-pyretic drug. The history of using salicylic acid, the major metabolite of aspirin, can be traced back to 3,500 years ago, when willow bark was utilized as a traditional medicine by ancient Sumerians and Egyptians [75]. In recent years, researchers have explored whether aspirin can also be used to in the treatment of periodontitis. Coimbra et al. [76] investigated the effects of aspirin on ligature-induced periodontitis in rats. The animals in the experimental group were administered aspirin intragastrically at 30 mg/kg body weight for 15 days. Aspirin significantly reduced the level of inflammation when assessed by histometric analysis and in terms of inflammatory factors such as TxA2, TNF-α, and IL-6.
In addition to its anti-inflammatory effect at high doses via the inhibition of COX-1/2, aspirin may also contribute to inflammation relief through other mechanisms. Lipoxin (LX) is an arachidonic acid metabolite synthesized by lipoxygenases (LOs). Aspirin acetylates COX-2 to generate the LX epimers 15-epi-LXA4 and 15-epi-LXB4 in the presence of 5-LO. These epimers, termed aspirin-triggered lipoxins (ATLs), can better alleviate inflammation than native LXs and have been used to treat diseases such as asthma, peritonitis, sepsis, and cardiovascular diseases [77, 78]. Serhan et al. [77] showed that the topical application of ATLs drastically inhibits inflammatory infiltration and alveolar bone loss in a rabbit periodontitis model. Other studies have documented that aspirin resulted in the synthesis of aspirin-triggered resolvins and aspirin-triggered protectins, which are anti-inflammatory molecules, along with omega-3 polyunsaturated fatty acids (PUFAs) [79, 80]. El-Sharkaway et al. [81] reported significant PPD improvements and reduced number of sites requiring additional therapy with the intervention of 3 g omega-3 PUFA with 81 mg aspirin daily for 6 months. A recent study by Castro Dos Santos et al. [82] showed that aspirin, administered at a low dose of 100 mg/day in conjunction with 3 g of fish oil for 2 months, improved both clinical and immunological parameters in patients with periodontitis and type 2 diabetes. Specifically, in patients taking medication after conventional periodontal treatment, aspirin in combination with omega-3 PUFA significantly improved clinical attachment level gains, reduced IL-6, IL-8, and IFN-γ in gingival crevicular fluid, and decreased HbA1c concentrations in peripheral blood.
In clinical settings, aspirin has long been employed for the treatment of various diseases and conditions such as headache, osteoarthritis, rheumatic diseases, cardiovascular diseases, and even cancers because of its versatile pharmacologic activities of pain relief, anti-inflammation, and anti-platelet function [83–85]. Therefore, several observational studies have been carried out to investigate its effects in subjects with a history of aspirin intake in terms of periodontal parameters. Feldman et al. [86] demonstrated that arthritic patients with daily consumption of aspirin and indomethacin presented significantly fewer sites with ≥ 10% bone loss than healthy individuals, highlighting the possible protective effect of aspirin on periodontal tissue. A similar finding was reported in a later study, suggesting that low-dose aspirin may reduce the risk of clinical attachment loss [87]. However, a nationally representative report from the United States involving 2,335 subjects demonstrated that aspirin users and non-users did not differ significantly in terms of PPD and clinical attachment loss, suggesting that low-dose aspirin use is not associated with the reduced prevalence of periodontitis [88]. It is also critical to understand that daily aspirin exposure has been shown to increase the appearance of bleeding on probing that could impact on diagnosis and treatment planning for periodontitis [89, 90].
Smokers are prone to periodontal disease and tend to have inferior responses to non-surgical and surgical periodontal treatment compared to non-smokers [91–93]. A cross-sectional study of non-smokers and ex-smokers who took low-dose aspirin (≤ 300 mg/day) for at least 2 years showed that aspirin takers had less attachment loss (by 0.3 mm) than non-aspirin takers, regardless of smoking status [94]. Another recent randomized controlled pilot study [95] explored the effects of long-term aspirin use as an adjunct to periodontal therapy in smokers. In conjunction with SRP, patients administered 325 mg/day of aspirin for 12 months showed better improvements in clinical parameters such as PPD and CAL than those in the placebo group, with no adverse effects.
NSAIDs in periodontal tissue engineering and regeneration
Non-surgical and access flap periodontal surgeries in anatomically challenging areas are effective procedures for mechanically removing biofilms and thereby relieve local inflammation in patients with periodontal diseases. These techniques aim to remove infectious substances, halt disease progression, and restore periodontal health; however, healing is mainly by repair and not regeneration of lost periodontal tissues [96]. To regenerate new supportive apparatus for teeth, strategies such as, minimally invasive surgical techniques in periodontal regeneration [97, 98] in combination with biologics, guided tissue regeneration (GTR), bone replacement grafts, and periodontal tissue engineering have been developed [99]. GTR involves placing an absorbable or non-absorbable membrane above the area to be restored. This prevents the undesired migration of epithelial cells and gingival fibroblasts and promotes the growth of bone progenitor cells [100]. The benefits of GTR have been adequately discussed in previous reviews, such as decreased PPD, increased gains in attainment level, and the formation of new bone [101, 102].
Currently, bone replacement grafts, biologics such as amelogenins and various biomaterials can be utilized either by themselves or as scaffolds in conjunction with different surgical techniques. Bone replacement grafts use osteoinductive and osteoconductive bone graft materials to fill the bone defect areas, with the aim of regenerating the most important component of supportive tissue, the alveolar bone [103]. Common graft materials are autologous bone, bovine xenograft, and demineralized freeze-dried bone allograft (DFDBA) [104–106]. Several efforts have been dedicated to exploring novel biomaterials in recent decades [107]. Biomaterials are normally used as scaffolds in tissue engineering to facilitate cell attachment, tissue formation, and mechanical support. Given the complexity of the periodontium, materials such as hydroxyapatite (HA), biphasic calcium phosphate, and tricalcium phosphate (TCP) have been used to reconstruct bone tissue [108]. Some innovative biomaterials that mimic the structure and characteristics of the cementum, periodontal ligament, and alveolar bone have also been used to restore naturally structured periodontal tissues [109].
Anti-inflammatory effects of NSAIDs on periodontal regeneration
Despite the added benefits of periodontal regeneration procedures as compared with access flap procedure in terms of gains in clinical attachment, there is a significant inter-center variability in the outcomes [110] due to the implanted biomaterials-triggered inflammatory responses [111] or systemic inflammatory conditions such as diabetes mellitus. Therefore, anti-inflammatory biomaterials design strategies, such as modification of surface chemistry, topography, and biomolecules, have been developed to reduce the adverse immunological responses in periodontal regeneration [112].
The incorporation of NSAIDs in biomaterial synthesis has been extensively studied (summarized in the Table 2). Veronese et al. [113] manufactured biodegradable polyphosphazene membranes and microspheres for periodontal regeneration. These materials were loaded with naproxen and had a release rate that yielded therapeutically adequate blood concentrations. However, the biological effects of this biomaterial were not investigated. The incorporation of salicylic acid into poly(anhydride-ester) was shown to relieve inflammation around implants [114], and promote bone regeneration more effectively than the bone graft alone in a critical-size bone defect model in rats [115]. The effects of ibuprofen were also explored on a polycaprolactone (PCL) membrane. An in vivo study using a mouse periodontitis model demonstrated that the combination of ibuprofen and PCL membrane prevented clinical attachment loss and inhibited osteoclastogenesis, thereby improving periodontal regeneration [116].
Table 2.
NSAID application in periodontal tissue engineering and regeneration
| Year | Drug | Material | Study type | In vivo design | Outcome | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| 2007 | SA/diflunisal | PAE | in vivo | Rat calvarial bone defect model | Relieved inflammation around implants | Anti-inflammation | [114] |
| 2013 | SA | PAE | in vivo | Rat mandibular bone defect model | Enhanced bone regeneration | Anti-inflammation | [115] |
| 2018 | Ibuprofen | PCL | in vitro & in vivo | Mouse periodontitis model | Improved CAL and inhibited bone destruction | Anti-inflammation; inhibited migration of ECs and FBs | [116] |
| 2018 | Diclofenac | Alginate/CS hydrogel fibers | in vitro | – | Enhanced bone osteogenesis and relieved inflammation | Anti-inflammation | [120] |
| 2011 | Aspirin + omega-3 polyunsaturated fatty acids | DFDBA | clinical | Randomized controlled trial (a total of 40 patients) | Improved CAL, probing depth, gingival index, and gingival bleeding index at 6-month follow-up | Anti-inflammation | [117] |
| 2011 | Aspirin | Heprasil/Gelin-S hydrogel | in vivo | Rat calvarial bone defect model | Enhanced bone regeneration | Modulation of T cells | [126] |
| 2017 | Aspirin | HA/TCP | in vitro & in vivo | Rat mandibular bone defect model | Enhanced bone regeneration | Inhibited M1 polarization of macrophages | [121] |
| 2018 | Aspirin | Bio-Oss® | in vitro & in vivo | Rat calvarial bone defect model | Enhanced bone regeneration | Improved osteogenic differentiation of DPSCs | [129] |
| 2019 | Aspirin | Liposomes/PCL | in vitro & in vivo | Subcutaneous implantation in nude mice | Enhanced bone regeneration | Anti-inflammation; improved osteogenic differentiation of MSCs | [135] |
| 2019 | Aspirin + EPO | CS/b-GP/gelatin hydrogel | in vivo | Rat periodontitis model | Improved CAL and inhibited bone destruction | Anti-inflammation | [137] |
| 2019 | Aspirin | HA/TCP & hydrogel | in vitro & in vivo | Rat mandibular bone defect model | Enhanced bone regeneration | Inhibited osteoclastogenic differentiation of DCs | [139] |
| 2020 | Aspirin | Sr-α-CSH/n-HA | in vitro & in vivo | Rat tibial bone defect model | Enhanced bone regeneration | Improved osteogenic differentiation, proliferation, and migration of BMSCs | [134] |
| 2022 | Aspirin | Tetra-PEG-CS composite gel | in vitro & in vivo | Rat calvarial bone defect model | Enhanced bone regeneration | Enhanced M2 polarization of macrophages; improved osteogenic differentiation and proliferation of PDLSCs | [136] |
CAL, clinical attachment level; CS, chitosan; DCs, dendric cells; DFDBA, demineralized freeze-dried bone allograft; DPSCs, dental pulp stem cells; ECs, epithelial cells; FBs, fibroblasts; HA, hydroxyapatite; MSCs, mesenchymal stem cells; PAE, poly(anhydride-ester); PCL, polycaprolactone; PDLSCs, periodontal ligament stem cells; PEG, poly(ethylene glycol); SA, salicylic acid; Sr-α-CSH/n-HA, Strontium (Sr)-containing α-hemihydrate calcium sulfate/nano-hydroxyapatite; TCP, tricalcium phosphate
The combination of omega-3 PUFA and low-dose aspirin was found to be effective as an adjunct to regenerative periodontal surgery. Elkhouli et al. [117] demonstrated that DFDBA implantation in conjunction with omega-3 PUFA and low-dose aspirin administration for 6 months resulted in greater PPD reduction and increased gains in CAL compared with DFDBA alone. IL-1β and IL-10 concentrations in gingival crevicular fluid were also downregulated by the administration of omega-3 PUFA and low-dose aspirin.
Of the various cell types involved in bone remodeling, macrophages are of great importance as they can switch to either the pro-inflammatory M1 or anti-inflammatory M2 phenotype and thus secrete different cytokines to regulate bone formation [118]. The typical cytokines produced by M1 macrophages are inducible nitric oxide synthase (iNOS), IL-1β, and TNF-α, which are associated with inflammatory responses. M2 macrophages secrete cytokines such as IL-4, IL-10, and TGF-β and promote cell proliferation and tissue regeneration [119]. As excessive inflammation hinders bone regeneration, some strategies have been developed to regulate the biology of macrophages. Lin et al. [120] established a natural polymer system for the sustained release of diclofenac, an NSAID. They embedded diclofenac in a porous alginate hydrogel scaffold and coated it with a layer of chitosan hydrogel to slow the release of diclofenac. When co-cultured with LPS-stimulated macrophages, this biomaterial significantly reduced iNOS, IL-6, and TNF-α secretion from the cells, thus suppressing inflammation. Furthermore, the direct treatment of macrophages with aspirin was reported to inhibit their LPS-stimulated phenotypes [121]. Mechanistically, aspirin was shown to downregulate the expression of iNOS and TNF-α via the COX2/PGE2/EP2/nuclear factor (NF)-κB and the inhibitor of NF-κB kinase (IκK) /inhibitor of NF-κB (IκB)/NF-κB signaling pathways. Further in vivo studies confirmed that aspirin inhibits the infiltration of LPS-induced iNOS+ macrophages and thereby promotes the regeneration of periodontal alveolar bone [121].
However, in terms of bone fracture healing, NSAIDs have typically been associated with delayed healing [122]. The inhibition of cyclooxygenase is documented to induce fracture non-unions and incomplete unions [123]. One possible explanation for their different effects on bone fracture healing and periodontal bone regeneration might be due to their different biological microenvironments. During periodontal regeneration, residual bacteria, newly formed biofilm, and the foreign body response to implanted biomaterials may intensify inflammation to a degree greater than that in closed fracture healing where COX-2 inhibitors can act to dampen the inflammation to a level more compatible for regeneration to occur, as opposed to closed bone fracture healing where the physiological level of naturally occurring inflammation and cyclooxygenase level might be more optimal for angiogenesis, osteogenesis and healing to occur [124]. Since it has been established that excessive inflammation impairs tissue regeneration [125], the involvement of inflammatory inhibitors may benefit periodontal regeneration. One study showed that a BMSC-based scaffold failed to regenerate tissue due to the elevated secretion of the inflammatory factors IFN-γ and TNF-α by T cells; relieving this inflammation by aspirin improved the regenerative progress [126].
Effects of aspirin on periodontal regeneration
The distinct effect of aspirin on bone metabolism, supplementary to its anti-inflammatory effect, has increasingly attracted the attention of researchers. Inspired by an epidemiological study that indicated some beneficial effects of aspirin on bone mineral density [127], Yamaza et al. [128] investigated the association between aspirin and bone homeostasis. In an ovariectomy-induced osteoporosis model in mice, the systemic delivery of aspirin significantly improved trabecular and cortical bone density. They established that aspirin blocked Fas ligand-induced BMSC apoptosis and promoted the osteogenesis of BMSCs by increasing telomerase activity. As BMSCs are the most important cellular reservoirs for bone formation and the most commonly used seed cells in bone tissue engineering, the effects of aspirin on BMSCs may impact bone regeneration. Further studies demonstrated that aspirin could improve the osteogenic differentiation of dental pulp and periodontal ligament stem cells [129, 130]. The adipogenesis of BMSCs was also inhibited by aspirin via histone acetylation [131]. Adipogenesis and osteogenesis are generally two sides of cell fate determination, with factors inducing adipogenesis often inhibiting osteogenesis and vice versa [132]. Furthermore, aspirin has been reported to enhance cell migration and stem cell homing [133]. Together, these studies suggest a pro-osteogenic effect of aspirin that can potentially benefit bone formation during periodontal regeneration. Figure 2 shows a representative utilization of aspirin in periodontal regeneration.
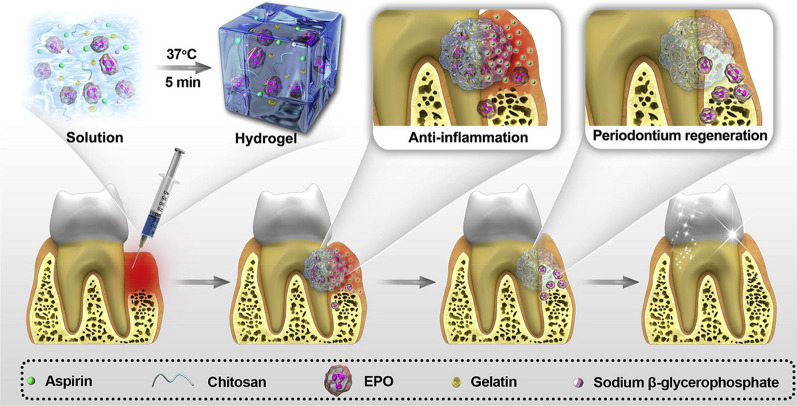
Fig. 2.
A representative application of aspirin-loaded biomaterials in periodontal regeneration.
Reproduced with permission from reference [137]. Copyright 2019 Elsevier
Strontium (Sr)-containing α-hemihydrate calcium sulfate/nano-hydroxyapatite (Sr-α-CSH/n-HA) is a biocompatible, osteoinductive, and osteoconductive biomaterial with weak compressive strength. Jiang et al. [134] added aspirin to balance the concentration of Sr and develop a material suitable for bone regeneration. In a critical-sized tibial bone defect model in rats, the increasing amount of aspirin in the material enhanced the number of cells undergoing osteogenic differentiation and inhibited osteoclastogenesis. Micro-computed tomography images revealed that the overall bone formation was significantly improved by aspirin, which also inhibited osteoclastogenesis around the defect. In vitro experiments showed that BMSCs co-cultured with the aspirin-loaded Sr-α-CSH/n-HA scaffold exhibited better osteogenic ability, proliferation, and migration ability than the scaffold without aspirin.
Liposomes, as a novel delivery system, can directly deliver active molecules to the site of action, reduce systematic toxicity, and achieve sustained release. Aspirin-laden liposomes on PCL-based composite scaffolds have been shown to significantly promote mineralization and osteogenic gene expression and inhibit osteoclastogenesis and the expression of inflammatory factors such as TNF-α and IFN-γ [135]. The subcutaneous implantation of scaffolds containing aspirin in nude mice revealed its elevated potential for bone regeneration.
Hydrogel, due to its excellent biocompatibility and biodegradability, can also be used as a scaffold for aspirin delivery. Tetra-armed poly(ethylene glycol)-chitosan hydrogels encapsulating aspirin were shown to possess favorable physicochemical properties and suitable pore structure for periodontal regeneration [136]. Aspirin was released from these scaffolds in a sustained manner and promoted osteogenesis in vitro. In a critical-size defect model in mice, hydrogels loaded with aspirin resulted in a significantly higher volume of newly formed bone than hydrogels without aspirin. The underlying mechanism was the facilitation of M2 polarization by aspirin. Chitosan β-sodium glycerophosphate/gelatin injectable hydrogels combined with aspirin and erythropoietin (EPO) were established as a controlled-release system to regenerate periodontal tissues [137]. When injected subcutaneously into nude mice, these hydrogels in conjunction with aspirin showed strong anti-inflammatory effects coupled with a reduction in COX-2 and MMP-9 expression. In a periodontitis model, the use of aspirin-loaded hydrogels also yielded better gains in the CAL and greater bone regeneration than the use of hydrogels alone, with the combination of aspirin and EPO showing the most robust effects.
Aspirin may also enhance bone formation by modifying osteoclastogenesis. Zeng et al. [138] assessed the effects of aspirin on osteoclastogenic differentiation in the mouse macrophage cell line RAW264.7. Aspirin significantly reduced the number of differentiated osteoclasts and suppressed the mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways in RANKL-treated cells. Another study showed that aspirin could inhibit the osteoclastogenesis of dendritic cells [139]. In this study, loading aspirin onto HA/TCP hydrogels also improved bone regeneration in a rat mandibular defect model.
Conclusions and future perspectives
Although several studies have investigated the effects of NSAIDs on the treatment of periodontitis, either alone or as adjuncts to non-surgical or surgical periodontal treatments, it remains challenging to draw a definitive conclusion on their efficacy. This is exacerbated by the fact that conventional periodontal therapies have proven safe and efficacious in resolving inflammation and halting tissue destruction [140, 141], making it unnecessary to use NSAIDs as adjunctive therapy for most conditions [7]. Furthermore, NSAIDs have long-term adverse effects that cannot be ignored; non-selective NSAIDs can cause gastrointestinal bleeding, renal effects, hypertension, hepatic injury, and headache, and COX-2 inhibitors have similar adverse cardiovascular and renal effects [142, 143]. Moreover, aspirin exerts anti-platelet effects even at low doses, as it inhibits thromboxane synthesis, which increases the risk of bleeding [144]. This may increase periodontal bleeding in patients with or without periodontal diseases [89, 145, 146]. Therefore, considering the costs and benefits, systemic or local use of NSAIDs as an adjunct to conventional periodontal treatment is not recommended by the EFP [7].
Notwithstanding the limitations, recent preclinical and clinical research on host modulation utilizing NSAIDs, especially aspirin, in combination with omega-3 PUFA for NSPT and alone in periodontal regeneration has yielded promising outcomes. These promising preliminary results signal their potential use as adjunctive treatment in selective patient groups with high susceptibility risk profile or poor response. Studies on the mechanism of action of aspirin have shown that, in addition to inhibiting PG synthesis, it activates various signaling pathways [147–149]. They have also documented its role in promoting osteogenesis, inhibiting adipogenesis, attenuating osteoclastogenesis, modulating macrophage polarization, and inducing cell migration and stem cell homing, all of which support periodontal regeneration. However, some of the results must be interpreted with caution as many of these preclinical studies have used critical-size defect models of the tibia and cranium in animals. Given the position of defects, local infection, and inflammation level, it remains to be determined whether the results from these models can precisely reflect the chronic inflammation condition in the periodontium. Well-designed, high quality randomized controlled trials investigating the adjunctive use of NSAIDs in periodontal therapy alone or in combination are also needed to further substantiate their additional benefits, determine dosing regimen, and establish safety.
The timing of administering NSAIDs also warrants consideration. Tissue healing comprises two inflammatory phases, the initiation and resolution of acute inflammation, characterized by immune cell infiltration, cellular debris clearance, and the production of cytokines and growth factors that facilitate tissue healing [122]. Disturbing either of these phases may delay healing [150]. Moreover, it has been shown that pro-inflammatory signals, when delivered at the early inflammatory stage, can aid tissue repair [151]. Therefore, the controlled release of anti-inflammatory drugs in the resolution phase, rather than the initiation phase, may boost tissue regeneration [115]. Smart biomaterials that can sense, respond, and adapt to stimuli have attracted increasing attention [152]. It is thus possible that a combination of anti-inflammatory agents and smart biomaterials will generate a precisely controlled microenvironment that can maximize the benefits and potential for periodontal regeneration in the future.
Acknowledgements
Not applicable.
Abbreviations
- ATLs
Aspirin-triggered lipoxins
- BMSCs
Bone marrow stem cells
- CAL
Clinical attachment level
- COX
Cyclooxygenase
- CS
Chitosan
- DCs
Dendric cells
- DFDBA
Demineralized freeze-dried bone allograft
- DPSCs
Dental pulp stem cells
- ECs
Epithelial cells
- EFP
European Federation of Periodontology
- EPO
Erythropoietin
- EPs
PGE2 receptors
- FBs
Fibroblasts
- GCF
Gingival crevicular fluid
- GTR
Guided tissue regeneration
- HA
Hydroxyapatite
- IFN
Interferon
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LOs
Lipoxygenases
- LPS
Lipopolysaccharide
- LX
Lipoxin
- MAPK
Mitogen-activated protein kinase
- MMPs
Matrix metalloproteinases
- MSCs
Mesenchymal stem cells
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NSPT
Non-surgical periodontal therapy
- OPG
Osteoprotegerin
- PAE
Poly(anhydride-ester)
- PCL
Polycaprolactone
- PDLSCs
Periodontal ligament stem cells
- PEG
Poly(ethylene glycol)
- PGs
Prostaglandins
- PPD
Probing pocket depth
- PUFAs
Polyunsaturated fatty acids
- RANK
Receptor-activator of nuclear factor-κB
- RANKL
Receptor-activator of nuclear factor-κB ligand
- SA
Salicylic acid
- Sr-α-CSH
Strontium (Sr)-containing α-hemihydrate calcium sulfate
- TCP
Tricalcium phosphate
- TNF
Tumor necrosis factor
- TxA2
Thromboxane A2
Author contributions
RJ, conceived the idea and draft manuscript; MF, YZ, and BH revised the manuscript; YL conceived the idea and critically revised manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Health and Medical Research Fund Project of Hong Kong (No. 19201421) and the National Natural Science Foundation of China (51972005).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish this manuscript.
Competing interests
All the authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bing Han, Email: kqbinghan@bjmu.edu.cn.
Yifan Lin, Email: yflin@hku.hk.
References
- 1.Jin L, Lamster I, Greenspan J, Pitts N, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22:609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 2.Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J. 2009;54:S11–26. doi: 10.1111/j.1834-7819.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 4.Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 5.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 6.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47(Suppl 22):4–60. doi: 10.1111/jcpe.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:508–524.e5. doi: 10.1016/j.adaj.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 9.John MT, Michalowicz BS, Kotsakis GA, Chu H. Network meta-analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. J Clin Periodontol. 2017;44:603–611. doi: 10.1111/jcpe.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortellini P, Stalpers G, Mollo A, Tonetti MS. Periodontal regeneration versus extraction and dental implant or prosthetic replacement of teeth severely compromised by attachment loss to the apex: a randomized controlled clinical trial reporting 10-year outcomes, survival analysis and mean cumulative cost of recurrence. J Clin Periodontol. 2020;47:768–776. doi: 10.1111/jcpe.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas AN, Furlaneto F, Gaio EJ, Gomes SC, Palioto DB, Castilho RM, et al. New tendencies in non-surgical periodontal therapy. Braz Oral Res. 2021;35:e095. doi: 10.1590/1807-3107bor-2021.vol35.0095. [DOI] [PubMed] [Google Scholar]
- 12.Salvi G, Lang N. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. CPD. 2005;11:1757–1769. doi: 10.2174/1381612053764878. [DOI] [PubMed] [Google Scholar]
- 13.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE 2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 14.Meek IL, van de Laar MAFJ, Vonkeman HE. Non-steroidal anti-inflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals. 2010;3:2146–2162. doi: 10.3390/ph3072146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11:52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- 16.Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 1998;47:78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 17.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunimov N, Laneuville O. Cyclooxygenase inhibitors: instrumental drugs to understand cardiovascular homeostasis and arterial thrombosis. Cardiovasc Hematol Disord Drug Targets. 2008;8:268–277. doi: 10.2174/187152908786786250. [DOI] [PubMed] [Google Scholar]
- 20.Moore N, Duong M, Gulmez SE, Blin P, Droz C. Pharmacoepidemiology of non-steroidal anti-inflammatory drugs. Therapies. 2019;74:271–277. doi: 10.1016/j.therap.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, et al. Anti-inflammatory drugs as anticancer agents. Int J Mol Sci. 2020;21:2605. doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AG, Quinn DI, Day RO. Non-steroidal anti-inflammatory drugs. Med J Aust. 1995;163:155–158. doi: 10.5694/j.1326-5377.1995.tb127972.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Wang X, Xu L, Li H, Wang R. LOX inhibitor HOEC interfered arachidonic acid metabolic flux in collagen-induced arthritis rats. Am J Transl Res. 2018;10:2542–2554. [PMC free article] [PubMed] [Google Scholar]
- 24.Sorokin AV, Domenichiello AF, Dey AK, Yuan Z-X, Goyal A, Rose SM, et al. Bioactive lipid mediator profiles in human psoriasis skin and blood. J Invest Dermatol. 2018;138:1518–1528. doi: 10.1016/j.jid.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mašek T, Filipović N, Hamzić LF, Puljak L, Starčević K. Long-term streptozotocin diabetes impairs arachidonic and docosahexaenoic acid metabolism and ∆5 desaturation indices in aged rats. Exp Gerontol. 2014;60:140–146. doi: 10.1016/j.exger.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Yucel-Lindberg T, Båge T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
- 27.Okamura H, Yamaguchi M, Abiko Y. Enhancement of lipopolysaccharide-stimulated PGE2 and IL-1β production in gingival fibroblast cells from old rats. Exp Gerontol. 1999;34:379–392. doi: 10.1016/S0531-5565(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 29.Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol. 2006;177:1879–1885. doi: 10.4049/jimmunol.177.3.1879. [DOI] [PubMed] [Google Scholar]
- 30.Inaba H, Tagashira M, Honma D, Kanda T, Kou Y, Ohtake Y, et al. Identification of hop polyphenolic components which inhibit prostaglandin E2 production by gingival epithelial cells stimulated with periodontal pathogen. Biol Pharm Bull. 2008;31:527–530. doi: 10.1248/bpb.31.527. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y-G, Nam J-H, Kim K-H, Lee K-S. FAK Pathway regulates PGE 2 production in compressed periodontal ligament cells. J Dent Res. 2010;89:1444–1449. doi: 10.1177/0022034510378521. [DOI] [PubMed] [Google Scholar]
- 32.Sanchavanakit N, Saengtong W, Manokawinchoke J, Pavasant P. TNF-α stimulates MMP-3 production via PGE2 signalling through the NF-kB and p38 MAPK pathway in a murine cementoblast cell line. Arch Oral Biol. 2015;60:1066–1074. doi: 10.1016/j.archoralbio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Omori K, Kida T, Hori M, Ozaki H, Murata T. Multiple roles of the PGE2-EP receptor signal in vascular permeability. Br J Pharmacol. 2014;171:4879–4889. doi: 10.1111/bph.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2–EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 36.Figueredo CM, Lira-Junior R, Love RM. T and B cells in periodontal disease: new functions in a complex scenario. Int J Mol Sci. 2019;20:3949. doi: 10.3390/ijms20163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Aoki T, Thumkeo D, Siriwach R, Yao C, Narumiya S. T cell-intrinsic prostaglandin E2-EP2/EP4 signaling is critical in pathogenic TH17 cell-driven inflammation. J Allergy Clin Immunol. 2019;143:631–643. doi: 10.1016/j.jaci.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodson JM, Dewhirst FE, Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974;6:81–85. doi: 10.1016/S0090-6980(74)80043-2. [DOI] [PubMed] [Google Scholar]
- 40.Offenbacher S, Farr DH, Goodson JM. Measurement of prostaglandin E in crevicular fluid. J Clin Periodontol. 1981;8:359–367. doi: 10.1111/j.1600-051X.1981.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 41.Offenbacher S, Odle BM, Gray RC, Van Dyke TE. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J Periodontal Res. 1984;19:1–13. doi: 10.1111/j.1600-0765.1984.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez GA, Miozza VA, Delgado A, Busch L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J Clin Periodontol. 2013;40:1112–1117. doi: 10.1111/jcpe.12164. [DOI] [PubMed] [Google Scholar]
- 43.Bascones A, Noronha S, Gómez M, Mota P, Gónzalez Moles MA, Villarroel DM. Tissue destruction in periodontitis: bacteria or cytokines fault? Quintessence Int. 2005;36:299–306. [PubMed] [Google Scholar]
- 44.Checchi V, Maravic T, Bellini P, Generali L, Consolo U, Breschi L, et al. The role of matrix metalloproteinases in periodontal disease. Int J Environ Res Public Health. 2020;17:4923. doi: 10.3390/ijerph17144923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen J-H, Kocieda VP, Jing H, Ganea D. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286:38913–38923. doi: 10.1074/jbc.M111.252932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan KMF, Kothari P, Du B, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-dependent microsomal prostaglandin E synthase (mPGES)-1 expression in macrophages: role of TNF-α and the EP4 prostanoid receptor1. J Immunol. 2012;188:1970–1980. doi: 10.4049/jimmunol.1102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim C-H, Park Y-G, Noh S-H, Kim Y-K. PGE2 induces the gene expression of bone matrix metalloproteinase-1 in mouse osteoblasts by cAMP-PKA signaling pathway. Int J Biochem Cell Biol. 2005;37:375–385. doi: 10.1016/j.biocel.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Shankavaram UT, Lai WC, Netzel-Arnett S, Mangan PR, Ardans JA, Caterina N, et al. Monocyte membrane type 1-matrix metalloproteinase. Prostaglandin-dependent regulation and role in metalloproteinase-2 activation. J Biol Chem. 2001;276:19027–19032. doi: 10.1074/jbc.M009562200. [DOI] [PubMed] [Google Scholar]
- 49.Oka H, Miyauchi M, Sakamoto K, Kitagawa M, Noguchi K, Somerman MJ, et al. Prostaglandin E2 inhibits mineralization and enhances matrix metalloproteinase-13 in mature cementoblasts mainly via the EP4 pathway. Arch Oral Biol. 2008;53:243–249. doi: 10.1016/j.archoralbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol. 2000;2007(43):85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 51.Beklen A, Ainola M, Hukkanen M, Gürgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 52.Goodson JM, McClatchy K, Revell C. Prostaglandin-induced resorption of the adult rat calvarium. J Dent Res. 1974;53:670–677. doi: 10.1177/00220345740530032601. [DOI] [Google Scholar]
- 53.Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 54.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 55.Jiang W, Jin Y, Zhang S, Ding Y, Huo K, Yang J, et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022;10:27. doi: 10.1038/s41413-022-00201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayahara K, Yamaguchi A, Takenouchi H, Kariya T, Taguchi H, Shimizu N. Osteoblasts stimulate osteoclastogenesis via RANKL expression more strongly than periodontal ligament cells do in response to PGE(2) Arch Oral Biol. 2012;57:1377–1384. doi: 10.1016/j.archoralbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 59.Goldhaber P, Rabadjija L, Beyer WR, Kornhauser A. Bone resorption in tissue culture and its relevance to periodontal disease. J Am Dent Assoc. 1973;87:1027–1033. doi: 10.14219/jada.archive.1973.0010. [DOI] [PubMed] [Google Scholar]
- 60.Nyman S, Schroeder HE, Lindhe J. Suppression of inflammation and bone resorption by indomethacin during experimental periodontitis in dogs. J Periodontol. 1979;50:450–461. doi: 10.1902/jop.1979.50.9.450. [DOI] [PubMed] [Google Scholar]
- 61.Weaks-Dybvig M, Sanavi F, Zander H, Rifkin BR. The effect of indomethacin on alveolar bone loss in experimental periodontitis. J Periodontal Res. 1982;17:90–100. doi: 10.1111/j.1600-0765.1982.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 62.Williams RC, Jeffcoat MK, Kaplan ML, Goldhaber P, Johnson HG, Wechter WJ. Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science. 1985;227:640–642. doi: 10.1126/science.3969553. [DOI] [PubMed] [Google Scholar]
- 63.Jeffcot MK, Williams RC, Wechter WJ, Johnson HG, Kaplan ML, Gandrup JS, et al. Flurbiprofen treatment of periodontal disease in beagles. J Periodontal Res. 1986;21:624–633. doi: 10.1111/j.1600-0765.1986.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 64.Williams RC, Jeffcoat MK, Howell TH, Reddy MS, Johnson HG, Hall CM, et al. Ibuprofen: an inhibitor of alveolar bone resorption in beagles. J Periodontal Res. 1988;23:225–229. doi: 10.1111/j.1600-0765.1988.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 65.Howell TH, Jeffcoat MK, Goldhaber P, Reddy MS, Kaplan ML, Johnson HG, et al. Inhibition of alveolar bone loss in beagles with the NSAID naproxen. J Periodontal Res. 1991;26:498–501. doi: 10.1111/j.1600-0765.1991.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 66.Jeffcoat MK, Williams RC, Reddy MS, English R, Goldhaber P. Flurbiprofen treatment of human periodontitis: effect on alveolar bone height and metabolism. J Periodontal Res. 1988;23:381–385. doi: 10.1111/j.1600-0765.1988.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 67.Williams RC, Jeffcoat MK, Howard Howell T, Rolla A, Stubbs D, Teoh KW, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. 1989;60:485–490. doi: 10.1902/jop.1989.60.9.485. [DOI] [PubMed] [Google Scholar]
- 68.Haffajee AD, Dibart S, Kent RL, Socransky SS. Clinical and microbiological changes associated with the use of 4 adjunctive systemically administered agents in the treatment of periodontal infections. J Clin Periodontol. 1995;22:618–627. doi: 10.1111/j.1600-051X.1995.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 69.Kurtiş B, Tüter G, Serdar M, Pınar S, Demirel İ, Toyman U. Gingival crevicular fluid prostaglandin E2 and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen. J Periodontol. 2007;78:104–111. doi: 10.1902/jop.2007.060217. [DOI] [PubMed] [Google Scholar]
- 70.Aras H, Çağlayan F, Güncü GN, Berberoğlu A, Kılınç K. Effect of systemically administered naproxen sodium on clinical parameters and myeloperoxidase and elastase-like activity levels in gingival crevicular fluid. J Periodontol. 2007;78:868–873. doi: 10.1902/jop.2007.060412. [DOI] [PubMed] [Google Scholar]
- 71.Yen CA, Damoulis PD, Stark PC, Hibberd PL, Singh M, Papas AS. The effect of a selective cyclooxygenase-2 inhibitor (celecoxib) on chronic periodontitis. J Periodontol. 2008;79:104–113. doi: 10.1902/jop.2008.070271. [DOI] [PubMed] [Google Scholar]
- 72.Oduncuoglu BF, Kayar NA, Haliloglu S, Serpek B, Ataoglu T, Alptekin NO. Effects of a cyclic NSAID regimen on levels of gingival crevicular fluid prostaglandin E2 and interleukin-1β: A 6-month randomized controlled clinical trial. Niger J Clin Pract. 2018;21:658–666. doi: 10.4103/njcp.njcp_221_17. [DOI] [PubMed] [Google Scholar]
- 73.Heasman PA, Benn DK, Kelly PJ, Seymour RA, Aitken D. The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease. J Clin Periodontol. 1993;20:457–464. doi: 10.1111/j.1600-051X.1993.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 74.Flemmig TF, Epp B, Funkenhauser Z, Newman MG, Kornman KS, Haubitz I, et al. Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy. J Clin Periodontol. 1995;22:427–433. doi: 10.1111/j.1600-051X.1995.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 75.Desborough MJR, Keeling DM. The aspirin story – from willow to wonder drug. Br J Haematol. 2017;177:674–683. doi: 10.1111/bjh.14520. [DOI] [PubMed] [Google Scholar]
- 76.Coimbra LS, Rossa C, Guimarães MR, Gerlach RF, Muscará MN, Spolidorio DMP, et al. Influence of antiplatelet drugs in the pathogenesis of experimental periodontitis and periodontal repair in rats. J Periodontol. 2011;82:767–777. doi: 10.1902/jop.2010.100555. [DOI] [PubMed] [Google Scholar]
- 77.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Romano M, Cianci E, Simiele F, Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 79.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, et al. Novel pro-resolving aspirin-triggered DHA pathway. Chem Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 fatty acids and low-dose aspirin. J Periodontol. 2010;81:1635–1643. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- 82.Castro Dos Santos NC, Andere NMRB, Araujo CF, de Marco AC, Kantarci A, Van Dyke TE, et al. Omega-3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus: randomized clinical trial. J Periodontol. 2020;91:1318–1327. doi: 10.1002/JPER.19-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/S0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 84.Patrono C. Low-dose aspirin in primary prevention: cardioprotection, chemoprevention, both, or neither? Eur Heart J. 2013;34:3403–3411. doi: 10.1093/eurheartj/eht058. [DOI] [PubMed] [Google Scholar]
- 85.Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16:675–686. doi: 10.1038/s41569-019-0225-y. [DOI] [PubMed] [Google Scholar]
- 86.Feldman RS, Szeto B, Chauncey HH, Goldhaber P. Non-steroidal anti-inflammatory drugs in the reduction of human alveolar bone loss. J Clin Periodontol. 1983;10:131–136. doi: 10.1111/j.1600-051X.1983.tb02201.x. [DOI] [PubMed] [Google Scholar]
- 87.Faizuddin M, Tarannum F, Korla N, Swamy S. Association between long-term aspirin use and periodontal attachment level in humans: a cross-sectional investigation. Aust Dent J. 2012;57:45–50. doi: 10.1111/j.1834-7819.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 88.Kotsakis GA, Thai A, Ioannou AL, Demmer RT, Michalowicz BS. Association between low-dose aspirin and periodontal disease: results from the continuous national health and nutrition examination survey (NHANES) 2011–2012. J Clin Periodontol. 2015;42:333–341. doi: 10.1111/jcpe.12380. [DOI] [PubMed] [Google Scholar]
- 89.Schrodi J, Recio L, Fiorellini J, Howell H, Goodson M, Karimbux N. The effect of aspirin on the periodontal parameter bleeding on probing. J Periodontol. 2002;73:871–876. doi: 10.1902/jop.2002.73.8.871. [DOI] [PubMed] [Google Scholar]
- 90.Royzman D, Recio L, Badovinac RL, Fiorellini J, Goodson M, Howell H, et al. The effect of aspirin intake on bleeding on probing in patients with gingivitis. J Periodontol. 2004;75:679–684. doi: 10.1902/jop.2004.75.5.679. [DOI] [PubMed] [Google Scholar]
- 91.Tonetti MS, Pini-Prato G, Cortellini P. Effect of cigarette smoking on periodontal healing following GTR in infrabony defects. A preliminary retrospective study. J Clin Periodontol. 1995;22:229–234. doi: 10.1111/j.1600-051X.1995.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 92.Tomasi C, Leyland AH, Wennström JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol. 2007;34:682–690. doi: 10.1111/j.1600-051X.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 93.Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol. 2000;2007(44):178–194. doi: 10.1111/j.1600-0757.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 94.Drouganis A, Hirsch R. Low-dose aspirin therapy and periodontal attachment loss in ex- and non-smokers. J Clin Periodontol. 2001;28:38–45. doi: 10.1034/j.1600-051x.2001.280106.x. [DOI] [PubMed] [Google Scholar]
- 95.Shiloah J, Bland PS, Scarbecz M, Patters MR, Stein SH, Tipton DA. The effect of long-term aspirin intake on the outcome of non-surgical periodontal therapy in smokers: a double-blind, randomized pilot study. J Periodont Res. 2014;49:102–109. doi: 10.1111/jre.12085. [DOI] [PubMed] [Google Scholar]
- 96.Woo HN, Cho YJ, Tarafder S, Lee CH. The recent advances in scaffolds for integrated periodontal regeneration. Bioactive Materials. 2021;6:3328–3342. doi: 10.1016/j.bioactmat.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cortellini P, Tonetti MS. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: a novel approach to limit morbidity. J Clin Periodontol. 2007;34:87–93. doi: 10.1111/j.1600-051X.2006.01020.x. [DOI] [PubMed] [Google Scholar]
- 98.Trombelli L, Farina R, Franceschetti G, Calura G. Single-flap approach with buccal access in periodontal reconstructive procedures. J Periodontol. 2009;80:353–360. doi: 10.1902/jop.2009.080420. [DOI] [PubMed] [Google Scholar]
- 99.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple ILC, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol. 2000;2015(68):182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 100.Nyman S. Bone regeneration using the principle of guided tissue regeneration. J Clin Periodontol. 1991;18:494–498. doi: 10.1111/j.1600-051X.1991.tb02322.x. [DOI] [PubMed] [Google Scholar]
- 101.Kao RT, Nares S, Reynolds MA. Periodontal regeneration—intrabony defects: a systematic review from the AAP regeneration workshop. J Periodontol. 2015;86:S77–104. doi: 10.1902/jop.2015.130685. [DOI] [PubMed] [Google Scholar]
- 102.Wu Y-C, Lin L-K, Song C-J, Su Y-X, Tu Y-K. Comparisons of periodontal regenerative therapies: a meta-analysis on the long-term efficacy. J Clin Periodontol. 2017;44:511–519. doi: 10.1111/jcpe.12715. [DOI] [PubMed] [Google Scholar]
- 103.Chiapasco M, Casentini P. Horizontal bone-augmentation procedures in implant dentistry: prosthetically guided regeneration. Periodontol. 2000;2018(77):213–240. doi: 10.1111/prd.12219. [DOI] [PubMed] [Google Scholar]
- 104.Rezende ML, Consolaro A, Sant’Ana AC, Damante CA, Greghi SL, Passanezi E. Demineralization of the contacting surfaces in autologous onlay bone grafts improves bone formation and bone consolidation. J Periodontol. 2014;85:e121–129. doi: 10.1902/jop.2013.130298. [DOI] [PubMed] [Google Scholar]
- 105.Nooh N, Ramalingam S, Al-Kindi M, Al-Rasheed A, Al-Hamdan KS, Al-Hezaimi K. Real-time assessment of guided bone regeneration in standardized calvarial defects in rats using bio-oss with and without collagen membrane: an in vivo microcomputed tomographic and histologic experiment. Int J Periodontics Restorative Dent. 2016;36(Suppl):s139–149. doi: 10.11607/prd.2354. [DOI] [PubMed] [Google Scholar]
- 106.Pajnigara NG, Kolte AP, Kolte RA, Pajnigara NG. Volumetric assessment of regenerative efficacy of demineralized freeze-dried bone allograft with or without amnion membrane in grade II furcation defects: a cone beam computed tomography study. Int J Periodontics Restorative Dent. 2017;37:255–262. doi: 10.11607/prd.2901. [DOI] [PubMed] [Google Scholar]
- 107.Shen P, Chen Y, Luo S, Fan Z, Wang J, Chang J, et al. Applications of biomaterials for immunosuppression in tissue repair and regeneration. Acta Biomater. 2021;126:31–44. doi: 10.1016/j.actbio.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 108.Liang Y, Luan X, Liu X. Recent advances in periodontal regeneration: a biomaterial perspective. Bioact Mater. 2020;5:297–308. doi: 10.1016/j.bioactmat.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20:1342–1351. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tonetti MS, Cortellini P, Suvan JE, Adriaens P, Baldi C, Dubravec D, et al. Generalizability of the added benefits of guided tissue regeneration in the treatment of deep intrabony defects. Evaluation in a multi-center randomized controlled clinical trial. J Periodontol. 1998;69:1183–1192. doi: 10.1902/jop.1998.69.11.1183. [DOI] [PubMed] [Google Scholar]
- 111.Chu C, Zhao X, Rung S, Xiao W, Liu L, Qu Y, et al. Application of biomaterials in periodontal tissue repair and reconstruction in the presence of inflammation under periodontitis through the foreign body response: recent progress and perspectives. J Biomed Mater Res. 2022;110:7–17. doi: 10.1002/jbm.b.34891. [DOI] [PubMed] [Google Scholar]
- 112.Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthcare Mater. 2019;8:1801106. doi: 10.1002/adhm.201801106. [DOI] [PubMed] [Google Scholar]
- 113.Veronese FM, Marsilio F, Lora S, Caliceti P, Passi P, Orsolini P. Polyphosphazene membranes and microspheres in periodontal diseases and implant surgery. Biomaterials. 1999;20:91–98. doi: 10.1016/S0142-9612(97)00104-X. [DOI] [PubMed] [Google Scholar]
- 114.Reynolds MA, Prudencio A, Aichelmann-Reidy ME, Woodward K, Uhrich KE. Non-Steroidal Anti-inflammatory Drug (NSAID)-derived poly(anhydrideesters) in bone and periodontal regeneration. CDD. 2007;4:233–239. doi: 10.2174/156720107781023866. [DOI] [PubMed] [Google Scholar]
- 115.Wada K, Yu W, Elazizi M, Barakat S, Ouimet MA, Rosario-Meléndez R, et al. Locally delivered salicylic acid from a poly(anhydride-ester): impact on diabetic bone regeneration. J Control Release. 2013;171:33–37. doi: 10.1016/j.jconrel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Batool F, Morand D-N, Thomas L, Bugueno IM, Aragon J, Irusta S, et al. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: an in vitro and in vivo study. Materials. 2018;11:580. doi: 10.3390/ma11040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elkhouli AM. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study) J Periodontal Res. 2011;46:261–268. doi: 10.1111/j.1600-0765.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 118.Sadowska JM, Ginebra M-P. Inflammation and biomaterials: role of the immune response in bone regeneration by inorganic scaffolds. J Mater Chem B. 2020;8:9404–9427. doi: 10.1039/D0TB01379J. [DOI] [PubMed] [Google Scholar]
- 119.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin H-Y, Chang T-W, Peng T-K. Three-dimensional plotted alginate fibers embedded with diclofenac and bone cells coated with chitosan for bone regeneration during inflammation. J Biomed Mater Res A. 2018;106:1511–1521. doi: 10.1002/jbm.a.36357. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Fang S, Li X, Feng J, Du J, Guo L, et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci Rep. 2017;7:11549. doi: 10.1038/s41598-017-10720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Newman H, Shih YV, Varghese S. Resolution of inflammation in bone regeneration: from understandings to therapeutic applications. Biomaterials. 2021;277:121114. doi: 10.1016/j.biomaterials.2021.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 124.Kellinsalmi M, Parikka V, Risteli J, Hentunen T, Leskelä H-V, Lehtonen S, et al. Inhibition of cyclooxygenase-2 down-regulates osteoclast and osteoblast differentiation and favours adipocyte formation in vitro. Eur J Pharmacol. 2007;572:102–110. doi: 10.1016/j.ejphar.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 125.Gao F, Lv T-R, Zhou J-C, Qin X-D. Effects of obesity on the healing of bone fracture in mice. J Orthop Surg Res. 2018;13:145. doi: 10.1186/s13018-018-0837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, et al. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18:1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 128.Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan M, Zhan Y, Hu W, Li Y, Xie X, Miao N, et al. Aspirin promotes osteogenic differentiation of human dental pulp stem cells. Int J Mol Med. 2018;42:1967–1976. doi: 10.3892/ijmm.2018.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abd Rahman F, Mohd Ali J, Abdullah M, Abu Kasim NH, Musa S. Aspirin enhances osteogenic Potential of Periodontal Ligament Stem Cells (PDLSCs) and modulates the expression profile of growth factor-associated genes in PDLSCs. J Periodontol. 2016;87:837–847. doi: 10.1902/jop.2016.150610. [DOI] [PubMed] [Google Scholar]
- 131.Zhan Y, He Z, Liu X, Miao N, Lin F, Xu W, et al. Aspirin-induced attenuation of adipogenic differentiation of bone marrow mesenchymal stem cells is accompanied by the disturbed epigenetic modification. Int J Biochem Cell Biol. 2018;98:29–42. doi: 10.1016/j.biocel.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 132.James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16:2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu H, Li W, Liu Y, Zhang X, Zhou Y. Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin. Stem Cell Res Ther. 2015;6:200. doi: 10.1186/s13287-015-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang Y, Qin H, Wan H, Yang J, Yu Q, Jiang M, et al. Asprin-loaded strontium-containing α-calcium sulphate hemihydrate/nano-hydroxyapatite composite promotes regeneration of critical bone defects. J Cell Mol Med. 2020;24:13690–13702. doi: 10.1111/jcmm.15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y, Bai Y, Pan J, Wang H, Li H, Xu X, et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J Mater Chem B. 2019;7:619–629. doi: 10.1039/C8TB02756K. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y, Dou X, Zhang L, Wang H, Zhang T, Bai R, et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact Mater. 2022;11:130–139. doi: 10.1016/j.bioactmat.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu X, Gu Z, Chen X, Shi C, Liu C, Liu M, et al. An injectable and thermosensitive hydrogel: promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019;86:235–246. doi: 10.1016/j.actbio.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 138.Zeng Y-P, Yang C, Li Y, Fan Y, Yang H-J, Liu B, et al. Aspirin inhibits osteoclastogenesis by suppressing the activation of NF-κB and MAPKs in RANKL-induced RAW264.7 cells. Mol Med Rep. 2016;14:1957–1962. doi: 10.3892/mmr.2016.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu L, Luo Z, Liu Y, Jia L, Jiang Y, Du J, et al. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-κB and NFATc1 activation. Stem Cell Res Ther. 2019;10:375. doi: 10.1186/s13287-019-1500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heitz-Mayfield LJA, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):92–102. doi: 10.1034/j.1600-051X.29.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 141.Mailoa J, Lin G-H, Khoshkam V, MacEachern M, Chan H-L, Wang H-L. Long-term effect of four surgical periodontal therapies and one non-surgical therapy: a systematic review and meta-analysis. J Periodontol. 2015;86:1150–1158. doi: 10.1902/jop.2015.150159. [DOI] [PubMed] [Google Scholar]
- 142.Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15:S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma JN, Jawad NM. Adverse effects of COX-2 inhibitors. ScientificWorldJournal. 2005;5:629–645. doi: 10.1100/tsw.2005.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sostres C, Lanas A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol. 2011;8:385–394. doi: 10.1038/nrgastro.2011.97. [DOI] [PubMed] [Google Scholar]
- 145.Kim DM, Koszeghy KL, Badovinac RL, Kawai T, Hosokawa I, Howell TH, et al. The effect of aspirin on gingival crevicular fluid levels of inflammatory and anti-inflammatory mediators in patients with gingivitis. J Periodontol. 2007;78:1620–1626. doi: 10.1902/jop.2007.070011. [DOI] [PubMed] [Google Scholar]
- 146.Elad S, Chackartchi T, Shapira L, Findler M. A critically severe gingival bleeding following non-surgical periodontal treatment in patients medicated with anti-platelet. J Clin Periodontol. 2008;35:342–345. doi: 10.1111/j.1600-051X.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 147.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 148.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 149.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hozain S, Cottrell J. CDllb+ targeted depletion of macrophages negatively affects bone fracture healing. Bone. 2020;138:115479. doi: 10.1016/j.bone.2020.115479. [DOI] [PubMed] [Google Scholar]
- 151.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Montoya C, Du Y, Gianforcaro AL, Orrego S, Yang M, Lelkes PI. On the road to smart biomaterials for bone research: definitions, concepts, advances, and outlook. Bone Res. 2021;9:12. doi: 10.1038/s41413-020-00131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.