Jiri Neuzil and colleagues review the processes and mechanisms that underlie horizontal mitochondrial transfer (HMT) and the metabolic consequences of HMT in cells.
Abstract
Mammalian genes were long thought to be constrained within somatic cells in most cell types. This concept was challenged recently when cellular organelles including mitochondria were shown to move between mammalian cells in culture via cytoplasmic bridges. Recent research in animals indicates transfer of mitochondria in cancer and during lung injury in vivo, with considerable functional consequences. Since these pioneering discoveries, many studies have confirmed horizontal mitochondrial transfer (HMT) in vivo, and its functional characteristics and consequences have been described. Additional support for this phenomenon has come from phylogenetic studies. Apparently, mitochondrial trafficking between cells occurs more frequently than previously thought and contributes to diverse processes including bioenergetic crosstalk and homeostasis, disease treatment and recovery, and development of resistance to cancer therapy. Here we highlight current knowledge of HMT between cells, focusing primarily on in vivo systems, and contend that this process is not only (patho)physiologically relevant, but also can be exploited for the design of novel therapeutic approaches.
Introduction
Mitochondria are essential organelles with multiple functions. As proteobacterial endosymbionts (Gray et al., 1999; Martin et al., 2019), mitochondria retained a part of the original bacterial genome. Mammalian mitochondrial DNA (mtDNA) of ∼15.6 kb codes for 13 mRNAs, whose products are subunits of mitochondrial respiratory complex I (CI), CIII, CIV, and CV, of which CI, CIII, and CIV form the respirasome crucial for CI-dependent respiration (Acin-Perez et al., 2008; Moreno-Lastres et al., 2012; Lapuente-Brun et al., 2013; Gu et al., 2016b). Mitochondrial DNA also encodes 22 tRNAs, 2 rRNAs, and the displacement loop (DLOOP; Falkenberg et al., 2017; Kukat and Larsson, 2013; Kukat et al., 2015; Gustafsson et al., 2016). For proper function, mitochondria need >1,500 proteins, the vast majority of which are encoded by nuclear DNA (nDNA; Liu and Butow, 2006; Neupert and Hermann, 2007; Ryan and Hoogenraad, 2007).
A prominent feature of mtDNA is its heteroplasmy (more than one mitochondrial genotype) mediated by tissue-specific non-random segregation (Burgstaller et al., 2014). Heteroplasmic variance modulates the number of pathological cells in a tissue (Aryaman et al., 2019b). Most eukaryotic cells carry multiple mtDNA copies, and the sequence of each mtDNA molecule can vary. This results in intracellular mitochondrial heterogeneity, which can lead to intercellular mitochondrial heterogeneity (Aryaman et al., 2019a). An intriguing paper proposed that exchange of mitochondria between cells helps maintain balanced heteroplasmy (Jayaprakash et al., 2015). This can be reconciled with the notion that for a particular phenotype, relevant heteroplasmy needs an elevated number of mtDNA copies with this genotype (Picard et al., 2014; Stewart and Chinnery, 2021).
Mitochondria have been increasingly linked to metabolic reprogramming and differentiation (Buck et al., 2016; Sykes et al., 2016; Seo et al., 2018). Mitochondrial function is associated with maintaining and dictating stem cell fate, and this plays a role in metabolic programming during quiescence, activation, self-renewal, proliferation, and differentiation (Bhattacharya and Scimè, 2020). It was found that mitochondrial Akt signaling modulates somatic cell reprogramming (Chen et al., 2019), and there is increasing understanding that mtDNA damage and loss of mitochondrial genome integrity play a critical role in the development of both severe early-onset maladies and chronic age-related diseases (Petros et al., 2005; Moreno-Loshuertos et al., 2006; Schon et al., 2012; Guha et al., 2014; Sharma and Sampath, 2019).
Although the role of mitochondria in cell biology and pathophysiology has been studied and is relatively well established, novel properties of mitochondria and their regulation keep emerging. For example, it has been shown that not all eukaryotes contain mitochondria, with metabolic function being at least in part maintained by complementary systems (Karnkowska et al., 2016). Mitochondria have been long considered autonomous, undergoing cytoplasmic inheritance (Birky, 2001). It is now apparent that there is a close relationship between mtDNA and the nucleus, including synchronized translation of nDNA- and mtDNA-encoded genes (Couvillion et al., 2016; Latorre-Pellicer et al., 2019). Regulation of mtDNA and mitochondrial proteins by epigenetic changes and post-translational modifications facilitate crosstalk between the nucleus and mitochondria that leads to maintenance of cellular homeostasis (Sharma et al., 2019). Consistent with this, mitochondria move to daughter cells during mitosis along microtubular structures (Kanfer et al., 2015).
A prominent place among recent intriguing findings is the identification of horizontal mitochondrial transfer (HMT) with their DNA payload between mammalian cells in vivo in a tumor setting (Tan et al., 2015; Berridge et al., 2015; Dong et al., 2017) and during lung injury (Islam et al., 2012; Ahmad et al., 2014), resulting in respiratory recovery. In the lung studies, mtDNA transfer was assumed but not directly demonstrated, while the cancer studies provide direct evidence for transfer of mtDNA based on distinct polymorphisms (Tan et al., 2015). The cancer studies used syngeneic tumors in transgenic mice with a mitochondria-associated fluorescence protein (su9DsRed2; Dong et al., 2017), while in mouse models of lung injury, the animals were injected with mesenchymal stem cells (MSCs) containing mitotracker-labeled mitochondria (Islam et al., 2012; Ahmad et al., 2014).
Prior to these studies, horizontal transfer of genes in eukaryotes had been considered rare (Keeling and Palmer, 2008), known only in lower eukaryotes (Gladyshev et al., 2008) and plants (Bergthorsson et al., 2003), promoting their biochemical diversification (Boschetti et al., 2012). In mammalian systems, there was evidence for intercellular trafficking of mitochondria in vitro (Rustom et al., 2004), but whether this occurred in vivo and had functional significance remained unclear (Rogers and Bhattacharya, 2013). The four cancer/lung studies discussed above made a strong case for HMT in vivo and for its relevance in pathological settings, and this was supported by phylogenetic evidence for intercellular transfer of mtDNA in canine transmissible venereal tumors (CTVT; Strakova et al., 2016; Strakova et al., 2020).
This Review highlights recent evidence of HMT between cells in vitro and, in particular, in vivo. The different possible modes of HMT, cellular and molecular mechanisms underlying each type of HMT, and metabolic consequences of HMT-related events in physiological and pathological settings linked to pervasive plasticity and therapeutic potential will be discussed.
Discovery of HMT
Intercellular communication is essential for maintaining homeostasis in multicellular organisms. The modes of cell-to-cell communication are diverse, including entry of various signaling “entities” across the cell membrane (Conner and Schmid, 2003), intercellular junctions (Dejana, 2004), or extracellular vesicles (exosomes, ectosomes, and microvesicles; Stoorvogel et al., 2002; Boelens et al., 2014; Cocucci and Meldolesi, 2015; Becker et al., 2016; Maas et al., 2017; Kumar et al., 2021). The role of such intercellular signaling has been shown in physiological and pathological context, and its therapeutic potential has been highlighted (EL Andaloussi et al., 2013). Interestingly, extracellular vesicles can carry across genetic material, i.e., microRNAs, that modulate the function of the recipient cells (Mittelbrunn and Sánchez-Madrid, 2012; Tomasetti et al., 2017).
A landmark paper defined the so-called “highways for intercellular organelle transport” (Rustom et al., 2004). This was based on the breakthrough discovery of a nanotubular network mediating long- and short-distance communication between cells via transient filamentous membrane protrusions that connect cytoplasm of neighboring or distant cells. These interconnections between cultured cells were shown to allow cell-to-cell movement of intracellular material. The authors coined the term “tunneling nanotubes” (TNTs) for this novel means of intercellular communication. They found that TNTs form between cells of different types. Transfer of cellular material via TNTs was inhibited by latrunculin B, pointing to the role of actin. TNTs were then studied in more detail and have been shown to carry across various cargo. This includes calcium ions (Watkins and Salter, 2005; Smith et al., 2011), bacteria (Onfelt et al., 2006), prions (Zhu et al., 2015; Gousset et al., 2009; Gousset and Zurzolo, 2009), or viruses (Rogers and Bhattacharya, 2013), as well as proteins (Reichert et al., 2016), as found in co-culture studies in vitro. The first report on viral transmission via TNTs involved the spread of human immunodeficiency virus from infected T cells to healthy cells (Sowinski et al., 2008). TNTs were also found to contribute to intercellular transfer of herpesvirus between live cells (Panasiuk et al., 2018) and to the spread of other types of viruses, including SARS-CoV-2 (Tiwari et al., 2021).
TNTs have been reported to translocate various organelles, including endosomes (Rustom et al., 2004; Bukoreshtliev et al., 2009; Wang et al., 2011), ER (Kadiu and Gendelman, 2011a; Kadiu and Gendelman, 2011b), Golgi/ER (Wang et al., 2011), lysosomes (Rustom et al., 2004; Onfelt et al., 2006; Abounit et al., 2016), melanosomes (Gerdes et al., 2007), and mitochondria (Tavi et al., 2010; Koyanagi et al., 2005; Spees et al., 2006; Acquistapace et al., 2011). While other means of HMT between cells have also been identified, TNTs remain a major transport route. The types of cells that “donate” and “import” mitochondria are numerous. Transfer may be homotypic or heterotypic, i.e., donor and acceptor cells are of the same type or of different types, respectively.
Donor cells are often MSCs (Islam et al., 2012; Ahmad et al., 2014; Spees et al., 2006; Otsu et al., 2009; Plotnikov et al., 2008; Boukelmoune et al., 2018). Other types of donor cells, i.e., fibroblasts (Marlein et al., 2017; Ippolito et al., 2019), astrocytes (Sun et al., 2019), hematopoietic cells (Golan et al., 2020), cardiomyocytes (CMs; Nicolas-Avila et al., 2020), or adipocytes (Brestoff et al., 2021; Crewe et al., 2021), have also been reported. Acceptor cells are also of various types, including epithelial cells (Konari et al., 2019), endothelial cells (ECs; D’Souza et al., 2021), CMs (Marti Gutierrez et al., 2022), neuronal cells (Babenko et al., 2018; Li et al., 2019), neural stem cells (Boukelmoune et al., 2018), T lymphocytes (Hough et al., 2018), cancer cells (Spees et al., 2006; Marlein et al., 2017; Moschoi et al., 2016), and macrophages (Brestoff et al., 2021; Jackson et al., 2016a; Jackson et al., 2016b). Intercellular HMT has been reported under both pathological and physiological situations (Table 1).
Table 1.
Overview of donor/acceptor cells (types of cells) involved in mitochondrial transfer
| Donor cells | Acceptor cells | Type of transfer | References |
|---|---|---|---|
| Rat neuroendocrine pheochromocytoma cells | Rat neuroendocrine pheochromocytoma cells | TNTs | Rustom et al., 2004 |
| Human embryonic kidney cells | Human embryonic kidney cells | TNTs | Rustom et al., 2004 |
| Normal rat kidney cells | Normal rat kidney cells | TNTs | Rustom et al., 2004 |
| Rat neonatal CMs | Human endothelial progenitor cells | Nanotubes | Koyanagi et al., 2005 |
| Human macrophage | Human macrophages | TNTs | Onfelt et al., 2006 |
| Human MSCs | Human alveolar adenocarcinoma cells | TNTs | Spees et al., 2006 |
| Human skin fibroblast | Human alveolar adenocarcinoma cells | TNTs | Spees et al., 2006 |
| Human MSCs | Rat CM | Nanotubes | Plotnikov et al., 2008 |
| Rat MSCs | Rat lung ECs | Cx43-based intercellular gap junctional communication | Otsu et al., 2009 |
| MSCs | Rat cardiomyoblasts | Nanotubes | Cselenyak et al., 2010 |
| Rat kidney renal tubular cells | Human MSCs | TNTs and gap junctions | Plotnikov et al., 2010 |
| Human MSCs | Rat kidney renal tubular cells | TNTs and gap junctions | Plotnikov et al., 2010 |
| Mouse endothelial progenitor cells | Human umbilical vein ECs | TNTs | Yasuda et al., 2010 |
| Human adipose-derived stem cells | Mouse CMs | Partial cell fusion | Acquistapace et al., 2011 |
| Human proximal tubular epithelial cells | Human proximal tubular epithelial cells | TNT-like structures | Domhan et al., 2011 |
| Rat ventricular CMs | Rat fibroblasts | Nanotubes | He et al., 2011 |
| Rat ventricular CMs | Rat ventricular CMs | Nanotubes | He et al., 2011 |
| Rat fibroblasts | Rat fibroblasts | Nanotubes | He et al., 2011 |
| Rat fibroblasts | Rat ventricular CMs | Nanotubes | He et al., 2011 |
| Rat hippocampal astrocytes | Rat hippocampal astrocytes | TNTs | Wang et al., 2011 |
| Human MSCs | Human osteosarcoma cells | Partial cell fusion | Cho et al., 2012 |
| Mouse BM-derived stromal cells | Mouse BM-derived stromal cells | Cx43-containing gap junctional channels, nanotubes, MVs | Islam et al., 2012 |
| Human BM-derived stromal cells | Human BM-derived stromal cells | Cx43-containing gap junctional channels, nanotubes, MVs | Islam et al., 2012 |
| Mouse BM-derived stromal cells* | Mouse alveolar epithelial cell* | Cx43-containing gap junctional channels, nanotubes, MVs | Islam et al., 2012 |
| Human BM-derived stromal cells* | Mouse alveolar epithelial cell* | Cx43-containing gap junctional channels, nanotubes, MVs | Islam et al., 2012 |
| Human pleural mesothelioma cells | Human pleural mesothelioma cells | TNTs | Lou et al., 2012 |
| Human MSCs | Human vascular smooth muscle cells | TNTs | Vallabhaneni et al., 2012 |
| Human vascular smooth muscle cells | Human MSCs | TNTs | Vallabhaneni et al., 2012 |
| Human retinal pigment epithelial cells | Human retinal pigment epithelial cells | TNTs | Witting et al., 2012) |
| Human ECs | Human ovarian cancer cells | TNTs | Pasquier et al., 2013 |
| Human ECs | Human breast cancer cells | TNTs | Pasquier et al., 2013 |
| MSC* | Bronchial epithelial cells* | TNTs | Ahmad et al., 2014 |
| Platelets | Neutrophils | ECVs | Boudreau et al., 2014 |
| Murine neuronal cells* | Murine glial cells* | Protrusions | Davis et al., 2014 |
| MSCs* | Lung epithelial cells* | TNTs | Li et al., 2014 |
| MSCs | Human umbilical vein ECs | TNTs | Li et al., 2014 |
| Human multipotent MSCs | Rat neuronal cells | Intercellular contacts | Babenko et al., 2015 |
| Human multipotent MSCs | Rat glial cells | Intercellular contacts | Babenko et al., 2015 |
| Breast cancer cells | Breast cancer cells | n.s. | Jayaprakash et al., 2015 |
| Normal fibroblast cells | Normal fibroblast cells | n.s. | Jayaprakash et al., 2015 |
| Human MSCs | Macrophages | MVs | Phinney et al., 2015 |
| Human MSCs* | Macrophages* | MVs | Phinney et al., 2015 |
| Pheochromocytoma cells | Pheochromocytoma cells | TNTs | Okafo et al., 2017 |
| MSCs | Rat cardiomyoblasts | TNTs | Han et al., 2016 |
| Astrocytes* | Neuronal cells* | Extracellular mitochondrial particles | Hayakawa et al., 2016 |
| MSCs* | Lung alveolar macrophages* | TNTs | Jackson et al., 2016a, b |
| MSCs | Corneal epithelial cells | TNTs | Jiang et al., 2016 |
| Mouse sister germ cells | Mouse oocytes | Microtubes | Lei and Spradling, 2016 |
| BM stromal cells | Acute myeloid leukemic cells | Endocytosis | Moschoi et al., 2016 |
| BM stromal cells* | Acute myeloid leukemic cells* | Endocytosis | Moschoi et al., 2016 |
| MSCs* | Murine CMs* | TNTs | Zhang et al., 2016 |
| MSCs | Murine CMs | TNTs | Zhang et al., 2016 |
| MSCs | MERRF cybrid cells | n.s. | Chuang et al., 2017 |
| Malignant urothelial carcinoma cells | Non-malignant urinary papillary urothelial tumor cells | TNTs | Lu et al., 2017 |
| Human MSCs | Human CMs | n.s. | Mahrouf-Yorgov et al., 2017 |
| Human MSCs | Human ECs | n.s. | Mahrouf-Yorgov et al., 2017 |
| Human CMs | Human MSCs | n.s. | Mahrouf-Yorgov et al., 2017 |
| Human ECs | Human MSCs | n.s. | Mahrouf-Yorgov et al., 2017 |
| BM stromal cells* | Acute myeloid leukemia cells* | TNTs | Marlein et al., 2017 |
| Mesenchymal stromal cells* | Macrophages* | EVs | Morrison et al., 2017 |
| Human healthy astrocytes | Human stressed astrocytes | TNTs | Rostami et al., 2017 |
| Human multipotent MSCs | Rat astrocytes | TNTs | Babenko et al., 2018 |
| Human multipotent MSCs | Neuron-like PC12 pheochromocytoma ρ0 cells | TNTs | Babenko et al., 2018 |
| MSCs | Neural stem cells | Actin-based intercellular structures | Boukelmoune et al., 2018 |
| Monkey kidney cells | Monkey kidney cells | TNTs | Guo et al., 2018 |
| Porcine alveolar macrophages | Porcine alveolar macrophages | TNTs | Guo et al., 2018 |
| Porcine umbilical cord MSCs | Porcine alveolar macrophages | TNTs | Guo et al., 2018 |
| Endothelial progenitor cells | Brain ECs | Endothelial progenitor cell–derived mitochondrial particles | Hayakawa et al., 2018 |
| Myeloid-derived regulatory cells | T Cells | ECVs | Hough et al., 2018 |
| Cardiac myofibroblasts | Cardiomyocytes | Nanotubes | Shen et al., 2018 |
| T cell acute lymphoblastic leukemia cells | MSCs | TNTs | Wang e al., 2018 |
| MSCs | T Cell acute lymphoblastic leukemia cells | TNTs | Wang et al., 2018 |
| Human induced pluripotent stem cell–derived MSCs | Human bronchial epithelium cells | TNTs (Cx43 mediated) | Yao et al., 2018 |
| Human induced pluripotent stem cell–derived MSCs* | Murine epithelial cells* | TNTs (Cx43 mediated) | Yao et al., 2018 |
| Scattered tubular-like cells | Tubular epithelial cells | ECVs | Zou et al., 2018 |
| MSCs | Acute lymphoblastic leukemia cells | TNTs | Burt et al., 2019 |
| BM MSCs | Human ECs | TNTs | Feng et al., 2019 |
| Human astrocytes | Human astrocytes | n.s. | Gao et al., 2019 |
| Human neuronal cells | Human astrocytes | n.s. | Gao et al., 2019 |
| Cancer-associated fibroblasts | Prostate cancer cells | Cellular bridges | Ippolito et al., 2019 |
| Cancer-associated fibroblasts* | Prostate cancer cells* | Cellular bridges | Ippolito et al., 2019 |
| BM-derived MSCs | Proximal tubular epithelial cells | n.s. | Konari et al., 2019 |
| BM-derived MSCs* | Proximal tubular epithelial cells* | n.s. | Konari et al., 2019 |
| Rat MSCs | Rat neurons | Gap junction intercellular communication | Li et al., 2019 |
| Rat MSCs* | Rat neurons* | Gap junction intercellular communication | Li et al., 2019 |
| Astrocytes | Primary rat neuronal cells | n.s. | Lippert and Borlongan, 2019 |
| BM stromal cells | MM cells | TNTs | Marlein et al., 2019 |
| BM stromal cells | Hematopoietic stem cells | Gap junction | Mistry et al., 2019 |
| BM stromal cells* | Hematopoietic stem cells* | Gap junction | Mistry et al., 2019 |
| Astrocytes | Neurons | n.s. | English et al., 2020 |
| Murine hematopoietic stem and progenitor cells* | Murine mesenchymal stromal cells* | Cell-contact dependent, Cx43-mediated | Golan et al., 2020 |
| Murine CMs* | Murine macrophages* | Cardiomyocyte-derived exophers (subcellular particles) | Nocolas-Avila et al., 2020 |
| Mesenchymal stromal cells | Islet β cells | TNTs | Rackham et al., 2020 |
| Murine white adipocytes* | Murine macrophages* | n.s. | Brestoff et al., 2021 |
| Human MSCs | Injured alveolar epithelial cells | Cx43-containing gap junctional channels | Huang et al., 2021 |
| Platelets | MSCs | Dynamin-dependent clathrin-mediated endocytosis | Levoux et al., 2021 |
| Platelets* | MSCs* | Dynamin-dependent clathrin-mediated endocytosis | Levoux et al., 2021 |
| BM stromal cells | Myeloma cells | TNTs and partial cell fusion | Matula et al., 2021 |
| Myeloma cells | BM stromal cells | TNTs and partial cell fusion | Matula et al., 2021 |
| Murine ovarian follicles | Murine ovarian follicles | Gap junction internalization | Norris, 2021 |
| Glioblastoma stem cells | Glioblastoma stem cells | TNTs | Pinto et al., 2021 |
| 3D-glioblastoma organoids | 3D-glioblastoma organoids | TNTs | Pinto et al., 2021 |
| Human breast epithelial cancer cells | Human breast epithelial cancer cells | ECVs | Rabas et al., 2021 |
| Murine high-metastatic lung carcinoma cells | Murine low-metastatic lung carcinoma cells | ECVs | Takenaga et al., 2021 |
| Murine low-metastatic lung carcinoma cells | Murine high-metastatic lung carcinoma cells | ECVs | Takenaga et al., 2021 |
| Murine high-metastatic lung carcinoma cells* | Murine low-metastatic lung carcinoma cells and cancer-associated fibroblasts* | ECVs | Takenaga et al., 2021 |
| MSCs | Neurons | Cell-to-cell contact | Tseng et al., 2021 |
| Human glioblastoma cells | Human primary astrocytes | TNTs | Valdebenito et al., 2021 |
| Human neurons | Human astrocytes | TNTs | Lampinen et al., 2022 |
| Murine neurons | Murine astrocytes | TNTs | Lampinen et al., 2022 |
| Murine brown adipocytes* | Murine adipocytes* | ECVs | Rosina et al., 2022 |
| Effector immune cells | Breast cancer cells | TNTs | Saha et al., 2022 |
| T cells* | Lung carcinoma cells* | TNTs | Saha et al., 2022 |
| T cells* | Melanoma cells* | TNTs | Saha et al., 2022 |
in vivo work
Note: TNTs, nanotubes, protrusions, microtubes, actin-based intercellular structures, cellular bridges—types of filamentous tubes that function as intercellular bridges. n.s., not specified in original paper.
In vivo relevance of HMT has been established by several key publications. HMT from MSCs grafted into lungs of mice challenged with lipopolysaccharide (LPS) was documented using mitochondria-competent MSCs, which alleviated the injury (Islam et al., 2012). Using a similar system, a subsequent paper reported on a link of the therapeutic effect of grafted MSCs with the Miro-1 protein (Ahmad et al., 2014), indicating involvement of the kinesin mobility system (MacAskill and Kittler, 2010). Tan and colleagues demonstrated that horizontal transfer of mitochondria into respiration-deficient cancer cells restored their tumorigenic potential and allowed tumor formation (Tan et al., 2015). To date, several other studies demonstrate that HMT occurs in vivo from MSCs to recipient cells to protect the latter or to repair their mitochondrial injury (Ippolito et al., 2019; Crewe et al., 2021; Moschoi et al., 2016; Jackson et al., 2016a; Jackson et al., 2016b), as well as in a variety of other systems with functional consequences. This is consistent with respiratory competent mitochondria being essential for cancer (Wallace, 2012).
Pathophysiological and phylogenic evidence of HMT and its role in cancer
Mitochondrial dysfunction is associated with many types of human disease (Paliwal et al., 2018; Liu et al., 2014; Berridge et al., 2020). Transfer of healthy mitochondria appears to be an effective rejuvenation process in several types of damaged cells, such as cancer cells, epithelial cells, ECs, and CMs (Paliwal et al., 2018), in the context of multiple diseases. These include ischemia-reperfusion models (Liu et al., 2014), amelioration of acute renal ischemia/reperfusion injury (Gu et al., 2016a), recovery of mitochondrial function in rat CMs after ischemia/reperfusion injury (Han et al., 2016), attenuation of alveolar destruction and altered severity of fibrosis in models of cigarette smoke–induced lung damage (Li et al., 2014), neuroprotective effects, and decline of infarct volume in the brain (Babenko et al., 2015). Moschoi and colleagues found that acute myeloid leukemia cells take up functional mitochondria from murine or human bone marrow (BM) stromal cells following chemotherapy (Moschoi et al., 2016). Concerning physiology, Lei and Spradling showed that germ cells in primordial germ cysts donate organelles, including mitochondria, to facilitate differentiation of mature mouse oocytes (Lei and Spradling, 2016).
Random accumulation of mtDNA deletions and the subsequent mosaic of respiratory chain deficiencies accelerate the aging process (Baris et al., 2015; Krishnan et al., 2008), and the main purpose of HMT is to restore the respiratory activity of acceptor cells with aberrant mitochondrial function (Patananan et al., 2016). Initial studies that demonstrated intercellular HMT were performed in vitro. Human lung carcinoma cells were depleted of mtDNA, with the resulting ρ0 cells devoid of respiratory function. Co-culture with MSCs or skin fibroblasts rescued ρ0 cells by import of functional mitochondria (Spees et al., 2006; Katrangi et al., 2007). Human osteosarcoma 143B ρ0 cells featured collapse of their mitochondrial electron transfer chain (ETC) function and thymidine kinase activity, with impaired de novo synthesis of pyrimidines (King and Attardi, 1989; Cho et al., 2012). When co-cultured with MSCs, they acquired functional mitochondria. The authors reasoned that HMT likely occurs under situations of highly compromised mitochondrial respiratory function (Cho et al., 2012).
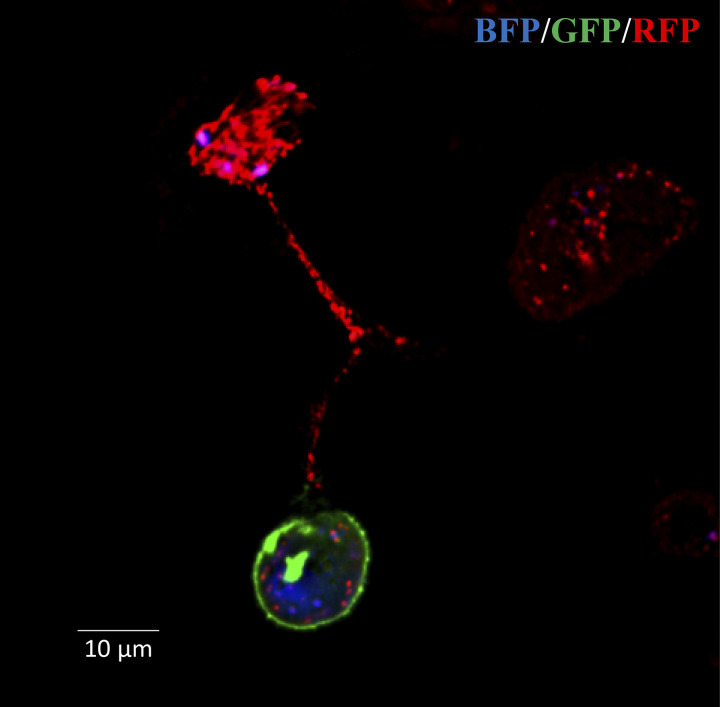
We used a similar system to investigate HMT in vivo and found that ρ0 tumor cells lacking mitochondrial respiratory function showed delayed tumor growth. Acquisition of mtDNA from host cells in the tumor stroma by ρ0 tumor cells re-established respiration as well as tumor-initiating and metastatic potential (Tan et al., 2015). We reported that whole mitochondria were transferred from stromal to ρ0 tumor cells with their mtDNA payload prior to recovery of tumorigenic capacity (Dong et al., 2017; Bajzikova et al., 2019; Fig. 1).
Figure 1.
Movement of mitochondria between MSCs and B16 ρ0 melanoma cells. MSCs isolated from a transgenic Su9DsRed mouse expressing RFP in their mitochondria were co-cultured with B16 ρ0 cells labeled with BFP targeted to nuclei and GFP targeted to the plasma membrane. Confocal microscopy shows the presence of RFP mitochondria in a TNT connecting an MSC and a B16 ρ0 cell (Dong et al., 2017).
While complete mtDNA removal is an extreme case that informs on mechanism but is not likely to occur in cancer, HMT was also reported in a more “natural” cancer settings. BM stromal cells donate mitochondria to acute myeloid leukemic cells in xenotransplants in vivo, conferring resistance to chemotherapy and survival advantage (Moschoi et al., 2016) to support their high energy demand and protection from metabolic stress (Marlein et al., 2017; Wang et al., 2018), as also shown in a mouse model of radiation-exposed glioblastoma multiformae (GBM; Osswald et al., 2015; Saha et al., 2022).
HMT has an intriguing role in transmissible cancer in feral dogs and likely in the Tasmanian devil. For feral dogs, CTVT is transmitted via coitus while Tasmanian devil spreads cancer via saliva entering open wounds. These animals live in partially inbred family packs, lowering the barrier against transfer of genes between individuals. Transmissible cancers in dogs were first described more than a decade ago (Rebbeck et al., 2009). It was then hypothesized that mtDNA damage during cancer progression accumulates to the point of irreversible damage to mitochondrial respiration triggering transfer of healthy mitochondria from donor to cancer cells (Rebbeck et al., 2011). While these two types of cancer are evolutionarily “old,” having developed thousands of years ago (Murchison et al., 2010; Murchison et al., 2012; Murchison et al., 2014; Strakova and Murchison, 2015), little is known about HMT in facial tumors (Kwon et al., 2020), while HMT in CTVT has been well defined with interesting caveats (Strakova et al., 2016; Strakova et al., 2020). Sequencing nDNA and mtDNA from CTVT tumors across ∼450 individual animals from countries around the world showed no homology in nDNA, while there were only five haplotypes, indicating at least five transfers of mitochondria with mtDNA over 1,500 yr. Interestingly, clade 1 haplotype bearers were found in countries as distal as Australia, India, Romania, Tanzania, and Chile, and clade 5 haplotype bearers were found only in India. Further work by this group (Strakova et al., 2020) showed that HMT in CTVT results in positive selection of the transferred mtDNA.
Mitochondrial transfer in non-cancerous systems
The notion that HMT between cells is a more general phenomenon that occurs also in normal physiology is now emerging, and HMT plays a role not only in the context of cancer and other pathologies but also under physiological conditions (Lei and Sprandling, 2016; Jayaprakashet al., 2017; Torralba et al., 2016; Valenti et al., 2021). This is supported by reports of HMT modulating in vivo adipose tissue and heart homeostasis or thermogenesis (Nicolas-Avila et al., 2020; Brestoff et al., 2021; Rosina et al., 2022).
Mitochondrial transfer in respiratory system injury and inflammation
Tissue injury and inflammation that involve considerable cellular stress may drive HMT. A decade ago, HMT from BM-derived stromal cells to pulmonary alveoli was reported, indicating that transfer of intact mitochondria can contribute to tissue repair in vivo, implying that HMT can be utilized as a beneficial approach in MSC-based therapy (Islam et al., 2012), enhancing cellular bioenergetics with ensuing improved organ function (Sinha et al., 2016). An in vivo study showed that increased expression of Miro1 in MSCs increased HMT from the stem cells into injured bronchial epithelial cells through intercellular TNTs. This resulted in attenuation of epithelial cell apoptosis, reduced infiltration of inflammatory cells, and lower collagen deposition and mucus hypersecretion in the lungs (Ahmad et al., 2014).
Further research confirmed that exposure of the respiratory system to injury or inflammation triggers HMT from MSCs to lung epithelium, using a mouse model of cigarette smoke–induced lung damage (Li et al., 2014). Lung alveolar macrophages were also shown to acquire mitochondria from MSCs by means of TNT-like structures, resulting in enhanced phagocytic activity in a model of pneumonia. This presents a novel mechanism for the anti-microbial effect of MSCs in the acute respiratory distress syndrome (Jackson et al., 2016a; Jackson et al., 2016b).
Mitochondrial transfer in the cardiovascular system
The cardiovascular system includes cell types with both high and low dependence on mitochondrial ATP production. The former cell type is represented by CMs, the latter by ECs. While generating little ATP in their mitochondria (mtATP), ECs use mitochondrial respiration to support stress resistance and biosynthesis (Magalhaes-Novais et al., 2022; Diebold et al., 2019). Despite these differences, both CMs and ECs engage HMT, suggesting that HMT is not restricted to mtATP-dependent cell types, and emphasizing the role of HMT in stress resistance. An earlier report indicated that mitochondria could move spontaneously from CMs to ECs via transient nanotube-like structures (Koyanagi et al., 2005). Mitochondria were then shown to be endocytosed by CMs and other cells in both in vitro and in vivo models of cardiomyopathy and ischemia, indicating therapeutic potential (Masuzawa et al., 2013; Cowan et al., 2016). MSCs were found to donate mitochondria, rescuing ischemia-exposed CMs and ECs from cell death (Cselenyak et al., 2010; Mahrouf-Yorgov et al., 2017).
Unidirectional mitochondrial transfer was found in the direction of CMs upon co-culture of human MSCs with rat CMs (Boukelmoune et al., 2018), or from myofibroblasts to damaged CMs to attenuate their apoptosis during hypoxia/reoxygenation (Shen et al., 2018). Mitochondria from endothelial progenitor cells can be delivered into damaged brain ECs (Hayakawa et al., 2018). Bidirectional movement of mitochondria between cardiofibroblasts and CMs (He et al., 2011), or between MSCs and CMs or ECs (Mahrouf-Yorgov et al., 2017) was reported. Mitochondrial dysfunction plays a central pathogenic role in neonatal cardiomyopathy, and HMT improves CM bioenergetics and viability in rats exposed to pre-gestational diabetes (Louwagie et al., 2021).
Recently, it was shown that cardiac tissue releases “damaged” mitochondria via extracellular vesicles (ECVs) called exospheres are taken up by macrophages via their Mertk surface receptors and disposed of (Melentijevic et al., 2017), contributing to mitochondrial homeostasis in the heart (Nicolas-Avila et al., 2020). Inter-organ HMT from adipose tissue to CMs, causing “metabolic pre-conditioning” of the heart (Crewe et al., 2021), has also been demonstrated.
Mitochondrial transfer in the nervous system
The central nervous system (CNS) controls basic physiological functions, emotional changes, and mental health (Zheng et al., 2019). Mitochondria serve not only as the “powerhouse” of neurons but also play essential roles in metabolizing neurotransmitters, buffering Ca2+, and sending signals modulating neuronal survival (Course and Wang, 2016; Misgeld and Schwarz, 2017). Vesicles containing mitochondria are transported unidirectionally between neuronal cells (Rustom et al., 2004). Contrary to our understanding that healthy cells degrade their own mitochondria, the organelles move from retinal ganglion axons to adjacent astrocytes in the optic nerve to be disposed of via trans-mitophagy (Davis et al., 2014), and neural trans-mitophagy was shown in a mouse model of Alzheimer’s disease (AD; Lampinen et al., 2022). In the hippocampus, motility of axonal mitochondria affects the pre-synaptic strength (Sun et al., 2013). Intercellular mitochondrial transport is critical in maintaining the healthy state of mitochondria in axons and homeostasis of the CNS (Nguyen et al., 2014), and this process plays an important role in various neurological and psychiatric disorders (Zheng et al., 2019; Picard et al., 2015; Gaetani et al., 2022).
Mitochondrial motility not only impacts on tissues/cells in the CNS but also in the peripheral nervous tissue. A recent experiment demonstrated that exogenous mitochondria transplanted into injured rat spinal cord contribute to acute maintenance of bioenergetics as well as functional recovery after spinal cord injury (Gollihue and Rabchevsky, 2017). Mitochondria can move from BM MSCs to oxygen and glucose-deprived neurons, improving their survival, decreasing neuronal apoptosis, and promoting locomotor function recovery in rats after spinal cord injury, indicating potential stem cell therapy (Li et al., 2019), while mitochondria from multipotent MSCs move to neurons or astrocytes, leading to restoration of respiration in recipient cells and alleviation of ischemic damage (Babenko et al., 2015; Babenko et al., 2018).
Astrocytes have been suggested as potential mitochondrial donors. An in vivo mouse model of stroke indicated that astrocytes release functional mitochondria that were delivered to damaged neurons, resulting in ischemic injury repair and neuronal recovery (Hayakawa et al., 2016; Berridge et al., 2016). Mitochondria from astrocytes transfer to cerebrospinal fluid after subarachnoid hemorrhage, both in a rat model and humans (Chou et al., 2017), and stressed astrocytes acquire functional mitochondria from healthy astrocytes via direct contact or TNTs facilitating their own recovery (Rostami et al., 2017). Astrocytes transfer healthy mitochondria to neurons after cisplatin treatment to restore “neuronal health” (English et al., 2020). Mitochondria were found to move between astrocytes and neurons by a process involving CD38/cADP-ribose signaling and mitochondrial Rho GTPases (Miro1, Miro2; Hayakawa et al., 2016; Gao et al., 2019). Mitochondrial transfer (or exogenous delivery) may open an avenue for treatment of neurological diseases such as stroke and spinal cord injury (Han et al., 2020).
Mitochondrial transfer in other systems
Mitochondria collected from murine hepatocytes improve embryonic development following transfer to fertilized murine zygotes from young and older mice (Yi et al., 2007). Cells in primordial oocyte cysts transfer mitochondria to definitive oocytes (Lei and Spradling, 2016). Also, donation of mitochondria by MSCs protects retinal ganglion cells against mitochondrial CI defect-induced degeneration (Jiang et al., 2019).
Mitochondria released from damaged somatic cells (CMs or ECs) can be engulfed by MSCs and rapidly degraded. As a consequence, elevation of heme levels in the cytosol of recipient MSCs triggered upregulation of heme oxygenase-1, a stress-inducible protein endowed with cytoprotective properties that converts toxic heme into health-promoting compounds (Gozzelino et al., 2010). Thus, heme oxygenase-1 activation increased mitochondrial biogenesis and protected against somatic cell apoptosis by stimulating HMT from MSCs to damaged cells (Mahrouf-Yorgov et al., 2017). Although the majority of studies on HMT focus on stress-linked reactions, it is of importance to investigate the potential role of HMT in maintaining tissue homeostasis (Liu et al., 2021).
In vitro studies found that intercellular transfer of mitochondria can be bidirectional. This is exemplified by exchange of mitochondria between renal tubular cells and mesenchymal multipotent stromal cells (Plotnikov et al., 2010). Bidirectional exchange of mitochondria was detected under normal culture conditions between human vascular smooth muscle cells and BM MSCs, and this process promoted MSC proliferation (Vallabhaneni et al., 2012). Human BM-derived MSCs transfer mitochondria to macrophages in vivo via TNTs, resulting in enhanced macrophage phagocytosis, a novel mechanism promoting anti-microbial function of MSCs (Jackson et al., 2016a; Jackson et al., 2016b). A report documented HMT from MSCs to corneal epithelial cells to protect them from mitochondrial damage (Jiang et al., 2016). In retinal cells, mitochondria and endocytic organelles were found inside TNTs, indicating that mitochondria may be transferred between individual cells or between retinal pigment epithelium cells and photoreceptors (Wittig et al., 2012). Mitochondria move from adipose tissue to macrophages in order to maintain white adipose tissue homeostasis, with positive impact on obesity (Brestoff et al., 2021). Consistent with this, damaged mitochondria transfer from brown adipocytes to macrophages to regulate thermogenesis (Rosina et al., 2022). Finally, several reports indicate that platelets donate mitochondria to neutrophils or MSCs, eliciting immune (Boudreau et al., 2014) or regenerative responses (Levoux et al., 2021).
Pathological and non-pathological systems in which mitochondrial transfer has been documented are summarized in Table 2.
Table 2.
Pathological and non-pathological systems featuring mitochondrial transfer
| Condition | Result | Reference | |
|---|---|---|---|
| Pathological | Lung carcinoma | Rescued mitochondrial function | Spees et al., 2006 |
| Osteosarcoma | Rescued mitochondrial function | Cho et al., 2012 | |
| Acute lung injury | Cellular protection and tissue repair | Islam et al., 2012 | |
| Ischemia | Preserving myocardial energetics, cell viability, and enhanced post-infarct cardiac function—protect the heart from ischemia-reperfusion injury | Masuzawa et al., 2013 | |
| Allergic airway inflammation | Enhanced rescue of epithelial injury | Ahmad et al., 2014 | |
| Chronic obstructive pulmonary disease | Attenuation of cigarette smoke–induced lung damage | Li et al., 2014 | |
| Ischemia | Cardioprotection from ischemia-reperfusion injury | Cowan et al., 2016 | |
| Cerebral ischemia | Amplified cell survival signals—neurorecovery | Hayakawa et al., 2016 | |
| Acute respiratory distress syndrome | Enhancement of phagocytic activity of lung alveolar macrophages | Jackson et al., 2016a; Jackson et al., 2016b | |
| Acute myeloid leukemia | Resistance to chemotherapy | Moschoi et al., 2016 | |
| Canine transmissible venereal tumor | Acquisition of functional mtDNA | Strakova et al., 2016, 2020 | |
| Bladder cancer | Increased invasiveness | Lu et al., 20178 | |
| Acute respiratory distress syndrome | Anti-inflammatory and highly phagocytic macrophage phenotype resulting in amelioration of lung injury | Morrison et al., 2017 | |
| PD | Acquisition of functional mitochondria | Rostami et al., 2017 | |
| Oxygen-glucose deprivation | Restoring brain endothelial energetics and barrier integrity | Hayakawa et al., 2018 | |
| Hypoxia/reoxygenation injury | Attenuation of CM apoptosis | Shen et al., 2018 | |
| Asthma | Alleviated asthmatic inflammation | Yao et al., 2018 | |
| Diabetic nephropathy | Structural and functional restoration of renal proximal tubular epithelial cells | Konari et al., 2019 | |
| MM | Enhanced mitochondrial metabolism, protumoral effect | Marlein et al., 2019 | |
| Neonatal cardiomyopathy | Improvement of CM bioenergetics and viability in male rats exposed to pre-gestational diabetes | Louwagie et al., 2021 | |
| Lung carcinoma | Enhancement of metastatic potential during tumor progression | Takenaga et al., 2021 | |
| Cerebral ischemia | Increased neuronal survival and improved metabolism | Tseng et al., 2021 | |
| Glioblastoma | Adaptation of non-tumor astrocytes to tumor-like metabolism and hypoxia conditions | Valdebenito et al., 2021 | |
| AD | Increased transmitophagy of defective neuronal mitochondrial, potential alleviation of AD pathology and symptoms | Lampinen et al., 2022 | |
| Non-pathological | Cardiac homeostasis | Preserved metabolic stability and organ function | Nicolas-Avila et al., 2020 |
| White adipose tissue homeostasis | Metabolic homeostasis, impairment leads to obesity | Brestoff et al., 2021 | |
| Metabolic preconditioning of the heart | Cardio-protection against lipotoxic or ischemic stresses elicited by obesity | Crewe et al., 2021 | |
| Wound healing | Promotion of pro-angiogenic activity of MSCs via their metabolic remodeling | Levoux et al., 2021 |
Modes of HMT
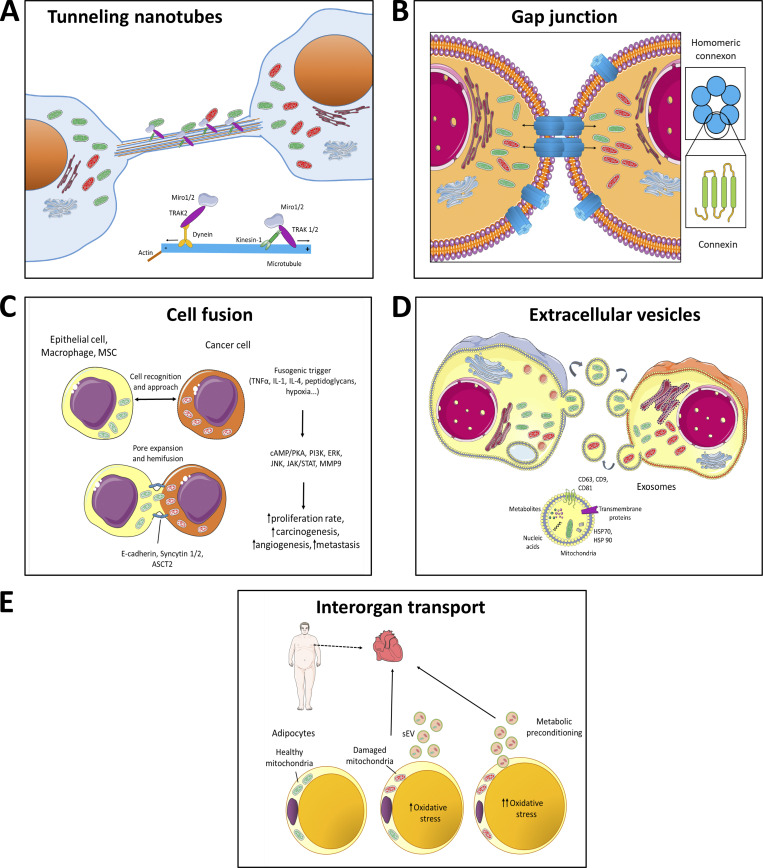
Intercellular mitochondrial transfer is important in multiple scenarios during physiology and pathophysiology. Mechanisms of HMT between cells are diverse as discussed in more detail below, including tunnelling nanotubes/cytoplasmic bridges, gap junctions, cell fusion, endocytosis of vesicles, or as free organelles (Moschoi et al., 2016; Osswald et al., 2015; Masuzawa et al., 2013; Cowan et al., 2016; Hayakawa et al., 2016; Wang and Gerdes, 2015; Herst et al., 2018). The various modes of HMT are depicted schematically in Fig. 2.
Figure 2.
Models of horizontal transfer of mitochondria. (A) Horizontal transfer of mitochondria by TNTs. TNTs are in general formed by F-actin filaments. In case of intercellular transfer of mitochondria, TNTs also contain microtubules and are thicker, thus able to transport bulkier structures. Transport of mitochondria along these cytoskeletal elements is propelled by dynein and kinesin motor complexes consisting of several adaptor proteins, such as Miro1 or Miro2, that are integrated in the outer mitochondrial membrane and facilitate mitochondrial transport not only along microtubules but also along actin filaments (together with Myo19). Formation of TNTs starts either as an actin-driven protrusion of the cell membranes of the two cells involved or as the dislodgement of two previously attached cells that during the partition from each other form the TNT. (B) Horizontal transfer of mitochondria by gap junctions. Gap junctions were shown to involve connexin structures, i.e., protein complexes consisting of six subunits of connexin proteins, such as Cx43. Two juxtapositioned connexon channels form pores connecting two neighboring cells, allowing for bidirectional transport of ions, signaling molecules or whole mitochondria. (C) Horizontal transfer of mitochondria by cell fusion. Cell fusion is a process that is relatively common in cancer progression and comprises several steps. At first two cells are recognized via the so-called fusogenic trigger that could involve different signaling molecules depending on the cell type (TNFα, IL-1, IL-4, and others) or specific conditions (e.g., hypoxia). The cells then approach each other and, using several cell–cell adhesion molecules, such as E-cadherin, syncytin-1 and -2, or ASCT2, which form pore expansions, “fusion” of the two neighboring cells occurs. This yields a cell that shares mitochondria from the two original cells. During this process, several signaling pathways are triggered that result in higher tumorigenesis and increased metastatic potential of the cancer cells. (D) Horizontal transfer of mitochondria by ECVs. Transfer of mitochondria via ECVs involves small double membrane structures that are formed by blebbing of plasma membrane. In contrast, exosomes are of endosomal origin and can transport various cargo including signaling molecules (different metabolites), trans-membrane proteins, nucleic acids, amino acids, and organelle fragments. (E) Inter-organ transport of mitochondria. This mode of mitochondrial horizontal transfer has been recently shown for the organelles moving from adipocytes to the heart tissue. In this particular case, damaged mitochondria from energetically stressed adipocytes of obese patients are transferred via small extracellular vesicles (sEV) to the blood circulation and are taken up by CMs of the heart tissue, triggering small ROS burst. This process results in compensatory antioxidant signaling in the heart muscle, causing metabolic pre-conditioning.
Mitochondrial transfer via TNTs
The most studied mechanism of HMT is by means of F-actin–based cytoplasmic bridges referred to as TNTs (Rustom et al., 2004; Gerdes and Carvalho, 2008; Gurke et al., 2008; Rustom, 2016; Zurzolo, 2021). TNTs are dynamic structures with a diameter of 50–200 nm and length up to several cell diameters. They are derived from plasma membrane and can form within minutes. These cell–cell connections mediate continuity between the cytoplasm of adjacent or remote cells, allowing for trafficking of mitochondria and other organelles, i.e., vesicles, individual molecules (nucleic acids), ions, and even pathogens travelling from donor to recipient cells (Rustom et al., 2004; Sowinski et al., 2008; Bukoreshtliev et al., 2009; Eugenin et al., 2009; Thayanithy et al., 2014; Roehlecke and Schmidt, 2020; Haimovich et al., 2021). Drugs that depolymerize F-actin suppress formation of TNTs (Bukoreshtliev et al., 2009). In several cases microtubules and cytokeratin filaments are present in these structures together with F-actin, their key component (Veranic et al., 2008; Wang et al., 2010).
Two basic mechanisms of TNT formation exist: actin-driven protrusion of the cell membrane that fuses with another cell or its protrusion and “cell dislodgement,” where two initially attached cells part from each other, forming a TNT containing F-actin filaments (Rustom et al., 2004; Ljubojevic et al., 2021). Existence of two different types of TNTs, with or without microtubules, implies different functions. TNTs without microtubules likely serve more as a short-distance connection, while those containing microtubules and with a wider bore serve for transport of molecules and organelles over longer distances (MacAskill and Kittler, 2010; Zampieri et al., 2021).
Mitochondria associate with actin or tubulin fibers by means of Miro proteins, playing a role in mitochondrial transport (Nahacka et al., 2021). In vitro experiments show that overexpression of Miro-1 in MSCs increased the metabolic/bioenergetic benefit of HMT following oxidative damage of recipient cells (Ahmad et al., 2014; Tseng et al., 2021). We have shown a role for TRAK-1, another adaptor protein linking mitochondria to motor proteins like kinesin (KIF5A), enabling movement of mitochondria at long range and crossing “crowded” environments (Henrichs et al., 2020). An interesting phenomenon is bidirectional transfer of mitochondria shown for several systems (see above) and as can be expected for cancer. One can envisage that mitochondria moving to the plus ends of tubulin fibers use the kinesin mobility system, while those moving in the anterograde direction use dynein as the motor protein. That these two mitochondrial transport systems do not collide can be due to a mechanism analogous to the movement of interflagellar trains in cilia (Stepanek and Pigino, 2016; Jordan et al., 2018; Pigino, 2021).
Occurrence of TNTs is a rather infrequent process taking place under a wide range of physiological conditions (development, regeneration, cell migration, etc.) and different pathological situations including tumor formation and metastasis (Lou et al., 2012; Ariazi et al., 2017; Valdebenito et al., 2021; Takenaga et al., 2021). TNTs present a component of the tumor microenvironment and can form in solid tumors and in primary cancer cells, playing an important role in cancer cell pathogenesis and invasion (Lou et al., 2012). Spontaneously formed TNTs mediate hetero-cellular exchange between various cancer and stromal cells for transfer of mitochondria, promoting tumor progression. To maintain HMT, TNTs form as previously shown in a model of ischemic vascular disease for injured cells via the so-called “find me” signals, such that phosphatidylserine exposed on the surface of ECs facilitates their connection with neighboring MSCs (Liu et al., 2014).
Stress signals generated during cellular mitochondrial damage, together with elevated levels of ROS, prompt donor cells to enhance their bioenergetics and initiate mitochondrial donation to injured recipient cells (Paliwal et al., 2018; Mahrouf-Yorgov et al., 2017; Zhang et al., 2010). Formation of TNTs via actin-driven protrusions of plasma membrane in MSCs is initiated by the cytokine TNF-α, regulating the TNFα/NF-κB/TNF-αip2 signaling pathway that leads to F-actin polymerization and TNT formation (Zhang et al., 2010; Hase et al., 2009). In 2006, Spees and colleagues reported that healthy mitochondria move from MSCs to cells with dysfunctional mitochondria, restoring their respiration (Spees et al., 2006). In a model of acute respiratory distress syndrome, TNTs were found to form between MSCs and lung macrophages, moving mitochondria across, resulting in higher phagocytic activity of lung macrophages (Jackson et al., 2016a; Jackson et al., 2016b). In a model of simulated ischemia/reperfusion injury, mitochondrial transfer occurred from MSCs to injured H9c2 cardiomyoblasts via TNTs, rescuing cardiac cells from apoptosis (Han et al., 2016). Mitochondria move in a bidirectional manner between MSCs and vascular smooth muscle cells via TNTs to initiate their proliferation (Vallabhaneni et al., 2012). BM MSCs move mitochondria to myeloma cells via TNTs, initiated by CD38 expression on myeloma cells (Marlein et al., 2019). A study of ECs exposed to chemotherapeutic stress showed mitochondrial transfer from BM MSCs via TNTs (Feng et al., 2019).
Donor cells other than MSCs also transfer mitochondria via TNTs to recipient cells. Within the same cell type, healthy cells donate mitochondria to their injured counterparts. Pheochromocytoma (PC12) cells exposed to UV light were rescued when co-cultured with untreated PC12 cells. This was promoted by TNTs formed by the stressed cells, allowing for transfer of functional mitochondria and rescuing the cells from apoptosis (Wang and Gerdes, 2015). Porcine reproductive and respiratory syndrome virus-infected cells were rescued from apoptosis/necrosis in early stages of infection by transfer of functional mitochondria via TNTs from uninfected cells (Guo et al., 2018).
TNTs also form between lung epithelial cells over distances from 1 μm to more than 100 μm (Kumar et al., 2017). In vitro and in vivo evidence shows that TNTs promote intercellular HMT between heterogeneous cancer cells, followed by increased invasiveness of bladder cancer cells (Lu et al., 2017). Pathologically stressed astrocytes with swollen ER and impaired mitochondrial dynamics signal to healthy astrocytes, which promotes HMT via TNTs (Rostami et al., 2017). A recent study showed mitochondrial transfer from astrocytes to GBM cells via TNTs as an adaptation to hypoxic and nutrient deficiencies in the tumor microenvironment (Valdebenito et al., 2021). TNTs were also found to form in retinal pigment epithelium, with HMT maintaining homeostasis of the tissue (Wittig et al., 2012; Chinnery and Keller, 2020), and in cancer spheroid 3D structures, in which cancer and stromal cells communicate via TNTs with ensuing HMT from ECs to cancer cells, contributing to chemotherapy resistance (Pasquier et al., 2013).
Mitochondrial transfer via gap junctions
Gap junctions couple nearly all cells that line external and internal surfaces of the human body, being known for transferring small molecules between cells (Goodenough and Paul, 2009). During gap junction internalization, one cell engulfs small parts of the neighboring cell (Bettadapur et al., 2020). The integral gap junction protein, connexin 43 (Cx43), is involved in ischemia/reperfusion damage of myocardial and cerebral tissue, implying HMT (Islam et al., 2012; Antanaviciute et al., 2014; Schulz et al., 2015; Norris, 2021; Qin et al., 2021; Rodriguez-Sinovas et al., 2021). Gap junction associated HMT involves Cx43 gap junctions within ovarian follicles (Norris, 2021). Mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stromal cells is required to support BM regeneration following irradiation (Golan et al., 2020). Promotion of pro-inflammatory status following bacterial LPS challenge was found to enable injured pulmonary epithelial cells to fuse with human stem cells using Cx43 during the formation of TNTs (Islam et al., 2012; Bagheri et al., 2020). Additionally, HMT via gap junctions occurs from BM MSCs to injured motor neurons to protect them from apoptosis (Li et al., 2019).
Interestingly, the role of Cx43 in the process of TNT formation was also shown in other models of HMT, for example in asthmatic inflammation, virus infection and leukemia (Smith et al., 2011; Okafo et al., 2017; Griessinger et al., 2017), indicating that the connexin system may be involved in mitochondrial transfer other than via gap junctions.
Mitochondrial transfer via cell fusion
Cell fusion is a process of two individual cells fusing their plasma membranes, sharing organelles and cytosolic compounds while their nuclei remain intact (Bagheri et al., 2020). Cytosolic constituents and organelles are hence evenly or, in some cases, partially shared between juxtaposed cells (Aguilar et al., 2013), resulting in mitochondrial delivery into recipient cells (Gomzikova et al., 2021), being common in myogenesis and placental development. An in vivo study showed that BM-derived cells fuse with neurons, CMs, and hepatocytes, raising the possibility that cell fusion may contribute to cellular homeostasis (Alvarez-Dolado et al., 2003). Stem cells can also fuse with neurons (Cusulin et al., 2012) and hepatocytes (Terada et al., 2002). Cell fusion in target organs can be driven by injury and inflammation (Weimann et al., 2003), irradiation (Alvarez-Dolado et al., 2003), or hypoxia-induced apoptosis (Noubissi et al., 2015). Cells of myeloid and lymphoid lineages fuse with cells in different tissues in response to injury or inflammation (Nygren et al., 2008). Cell fusion also occurs following co-culture of mouse CMs with human multipotent adipose-derived stem cells, resulting in HMT into CMs (Acquistapace et al., 2011).
Mitochondrial transfer via ECVs
ECVs are lipid-bound structures secreted into the extracellular space (EL Andalousi et al., 2013; Yáñez-Mó et al., 2015; Zaborowski et al., 2015). They include micro-vesicles (MVs) of 0.1–1 μm in diameter, and exosomes with diameter of 30–150 nm (Doyle and Wang, 2019; Isaac et al., 2021). The original notion was that exosomes and MVs are used by cells for disposal of unwanted material to maintain homeostasis (Yáñez-Mó et al., 2015). However, it became evident that ECVs are involved in cell-to-cell communication at a longer range, often provoking changes in the recipient cell (Isaac et al., 2021; White et al., 2006; Harding et al., 2013), also providing energy (Kumar et al., 2021). MVs can encapsulate mitochondria (∼0.5 μm in diameter) and deliver them to target cells (Vignais et al., 2017). Encapsulation of mitochondria into ECVs could be a rescue mechanism to release oxidative stress and to clear depolarized mitochondria (Gomzikova et al., 2021; Phinney et al., 2015), maintaining tissue homeostasis (Brestoff et al., 2021).
Delivery of mitochondria by ECVs participates in immune regulation, modulating the function of macrophages and neutrophils and other cells, including alveolar epithelial cells, neurons, and ECs, and contributing to intercellular communication (She et al., 2021). The first report of ECV-mediated HMT is a study showing HMT from BM MSCs to injured lung alveolar epithelial cells in a model of acute lung injury (Islam et al., 2012). ECV-mediated HMT from MSCs to macrophages, contributing to amelioration of lung injury, enhances the phagocytic capacity of M2-type alveolar macrophages and reduces secretion of TNF-α, suppressing lung inflammation (Morrison et al., 2017). Using a mouse model of focal cerebral ischemia, astrocytes were shown to use ECVs to transfer functional mitochondria to protect neurons from hypoxia and glucose deprivation (Hayakawa et al., 2016).
ECV-mediated HMT was found to occur between renal scattered tubular cells and tubular epithelial cells to alleviate renal stenosis and to recover mitochondrial respiration (Zou et al., 2018). Mitochondria derived from MSCs move to macrophages via ECVs to increase ATP production (Phinney et al., 2015). As a consequence of stimulating neutrophil activation and promoting their pro-inflammatory responses, platelets shed mitochondria in ECVs that are taken up by neutrophils in a damage-related model (Boudreau et al., 2014). Mitochondria encapsulated in ECVs can integrate into T cells to affect their mitotic processes (Hough et al., 2018). We showed that vesicular mitochondria released by platelets are internalized by MSCs, activating their pro-angiogenic properties (Levoux et al., 2021). These findings indicate that delivery of mitochondria by ECVs allows for communication between cells and participation in regulating the immune system and tissue repair processes, applicable for ECV-based mitochondrial therapy (She et al., 2021).
An interesting form of HMT between cells (or even organs) in response to stress involves release of damaged mitochondria from adipocytes in ECVs, with individual mitochondria originating from mitochondria-derived vesicles formed by budding from the mitochondrial network (Crewe et al., 2021). The function of mitochondria-derived vesicles is transport of cargo between mitochondria and other organelles, including ECVs (Todkar et al., 2021). ECVs are taken up by CMs, where they exert elevated oxidative stress that causes “metabolic” pre-conditioning of the heart (Crewe et al., 2021). We have recently shown that mitochondria move from platelets to MSCs in ECVs and as “isolated” mitochondria, ∼50% of each (Levoux et al., 2021). Both in the case of isolated mitochondria and those in ECVs, the organelles are taken up by clathrin-dependent endocytosis. Vesicles released by cancer cells contain “naked” mtDNA, which causes higher invasiveness of recipient cells (Rabas et al., 2021). While some of these reports are indicative of the existence of isolated mitochondria (or mtDNA) within the extracellular compartment, systematic studies on the mechanism by which these organelles are shed by donor cells and internalized by acceptor cells are lacking.
A novel means of HMT has recently emerged involving migrasomes (Ma et al., 2015) that form on retraction fibers of migrating cells via tetraspanin microdomains (Huang et al., 2019). These structures contain damaged mitochondria that are disposed of by the process of mitocytosis (Lu et al., 2020; Jiao et al., 2021). While migrasomes have been suggested to play a role in stroke (Schmidt-Pogoda et al., 2018), they likely deliver mitochondria (albeit damaged) to other cells (Yu and Yu, 2022).
Consequences of mitochondrial transfer
HMT has been shown to play a critical role in cell and tissue regeneration, and in damage repair, or may contribute to healing processes in brain injury, ischemic heart disease, muscle sepsis, stroke, and lung disorders (Paliwal et al., 2018). Various stress signals act as triggers of HMT, occurring primarily from MSCs to recipient cells, often in response to increased levels of ROS, damaged mitochondria/mtDNA, or inflammation (Zhang et al., 2016; Mistry et al., 2019). Cells injured by oxidative stress due to dysfunctional mitochondria send environmental cues to MSCs, triggering HMT (Islam et al., 2012; Ahmad et al., 2014). Bidirectional transfer of mitochondria occurs between MSCs and the surrounding environment where MSCs dispose of damaged mitochondria. Using rotenone to induce oxidative stress in corneal epithelial cells, HMT was initiated from MSCs, enhancing the survival capacity of the cells, paralleled by elevated mitochondrial respiration and enhanced corneal wound healing (Jiang et al., 2016). In cells derived from a patient with the mitochondrial disease MERRF (myoclonus epilepsy with ragged-red fibers), MSCs donate mitochondria leading to the rescue of injured cells via improved aerobic respiration, suppressed apoptosis and decreased mutational load and oxidative damage (Chuang et al., 2017).
In cancer, chemotherapy is mostly directed at cancer cells, driving them into apoptosis. Tumor cells importing mitochondria from stromal cells are better protected from apoptosis induced by chemotherapy or radiation therapy (Osswald et al., 2015; Pasquier et al., 2013). HMT from BM MSCs to acute myeloid leukemia cells in vivo confers chemoresistance and promotes their survival (Moschoi et al., 2016). Acquisition of new mitochondria via HMT generally increases cell fitness and overall resilience (Bererdi and Fantin, 2011; Suomalainen, 2019). Therefore, an important consequence of HMT is increased stress resistance of recipient cells.
Another consequence of HMT particularly important for rapidly proliferating cancer cells relates to the ETC playing an essential role in anabolic cell proliferation (Vander Heiden et al., 2009; Koppenol et al., 2011; Birsoy et al., 2015; Sullivan et al., 2016; Spinelli and Haigis, 2018). Invasive cancer cells favor mitochondrial respiration and increased ATP formation to meet their energy demands (LeBleu et al., 2014). A subpopulation of quiescent tumor cells that self-renew slowly/infrequently are responsible for tumor relapse (Visvader and Lindeman, 2012), and are addicted to OXPHOS for their survival (Kapoor et al., 2014; Viale et al., 2014).
HMT is triggered by severely affected mitochondrial functions, such as mtDNA deletion or treatment with mitochondrial inhibitors (Cho et al., 2012; Wang and Gerdes, 2015). We showed that cancer cells without mtDNA acquire healthy mitochondria from stromal cells, recovering their respiratory ability and tumorigenic capacity (Tan et al., 2015; Dong et al., 2017). These findings demonstrate that mitochondrial respiration is indispensable for tumor formation and progression. Respiration transfers electrons to oxygen, providing a cellular redox state that allows biosynthesis. CIII/IV maintain redox cycling of coenzyme Q (CoQ; re-oxidizing its reduced form to its oxidized counterpart) needed for de novo pyrimidine synthesis via dihydroorotate dehydrogenase (DHODH; Bajzikova et al., 2019; Boukalova et al., 2020). In addition, CoQ as well as CI activity support biosynthesis of other essential metabolites, including aspartate, that feed into nucleotide synthesis pathways (Birsoy et al., 2015; Guarás et al., 2016; Sullivan et al., 2016; Bajzikova et al., 2019; Martínez-Reyes et al., 2020; Murphy and Chouchani, 2022). Conceivably, HMT plays an important role within the tumor microenvironment by maintaining metabolic homeostasis that favors biomass build up (Tan et al., 2015; Mohammadalipour et al., 2020).
Regulation of mtDNA copy number in tumorigenesis (Dickinson et al., 2013), control of respiratory complex formation and function by translational crosstalk (Couvillion et al., 2016), plus epigenetic mechanisms suggest that maintaining optimum bioenergetic balance is a dynamic and adaptive process. Emerging data indicate that, when needed, this balance is maintained via HMT (Lee et al., 2015; Sun et al., 2018). The question of mtDNA copy number and HMT is intriguing. It is unclear at present what extent of mtDNA damage provokes HMT, and the extent to which damage can be reversed by mtDNA repair. GBM cells form tumors in mice with a delay directly related to mtDNA copy number, and this is linked to animal survival (Dickinson et al., 2013). This may have connotations for translational applications, such as cancer therapy.
Mitochondria are not only the primary powerhouse of cells, but also important regulators of life, death, proliferation, motility, stemness, and other functions (Ohta, 2003). Whole mitochondria with mtDNA are “mobile elements” that move between cells, with a role in cancer development, progression, and treatment (Dong et al., 2017; Ishikawa et al., 2008). Thus, transfer of mitochondria with intact mtDNA to cancer cells increases mtDNA copy number and promotes OXPHOS to support proliferation and metastasis (Mohammadalipour et al., 2020). Mitochondrial OXPHOS, biogenesis and respiration, as well as ROS levels, elevated as a consequence of mtDNA mutations, are important for motility of cancer cells and ensuing metastasis (LeBleu et al., 2014; Ishikawa et al., 2008).
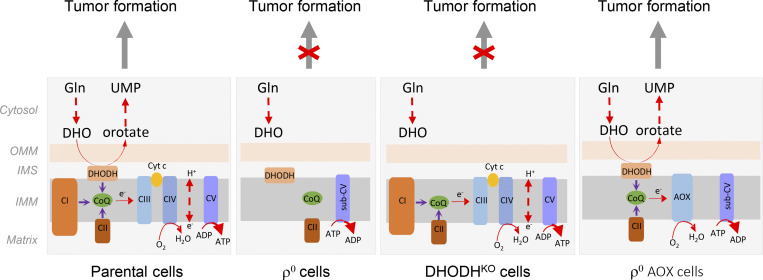
Our discovery that cancer cells lacking mtDNA acquire mitochondria from stromal cells to promote cancer progression (Tan et al., 2015) can be reconciled with a study showing that cancer cells responsible for tumor relapse rely on OXPHOS for survival (Viale et al., 2014). Using time-resolved analysis of tumor formation by mtDNA-depleted cells and genetic manipulation of OXPHOS, we showed that de novo pyrimidine biosynthesis, dependent on respiration-linked DHODH activity, is required to overcome cell-cycle arrest. Thus, DHODH-driven de novo pyrimidine biosynthesis is an essential anabolic pathway coupling respiration with tumorigenesis (Bajzikova et al., 2019). This points to mitochondrial respiration being crucial for cancer cell proliferation, while energy is supplied primarily via glycolysis. The proposed link between mitochondrial respiration and de novo pyrimidine synthesis is depicted in Fig. 3.
Figure 3.
Scheme of oxidative phosphorylation and its link to de novo pyrimidine synthesis and tumor formation. In cells with functional mitochondria, electrons are fed into the ETC by CI and CII, and by DHODH, which catalyzes conversion of dihydroorotate (DHO) to orotate in the fourth reaction of de novo pyrimidine synthesis. The electrons are intercepted by the oxidized form of CoQ, which is reduced and carries the electrons to CIII and CIV. CoQ is re-oxidized to accept more electrons from the entry points. This includes DHODH, which allows for de novo pyrimidine synthesis to occur, so that cells can transit the S-phase and eventually undergo cytokinesis, facilitating tumor initiation and progression. In cells devoid of mtDNA (ρ0 cells), respiration is completely inhibited, so that DHODH does not function, de novo pyrimidine synthesis is halted, and tumors cannot develop or progress. DHODHKO cells with functional respiration cannot transit the S-phase of the cell cycle since DHODH is inhibited. Restoration of the function of CIII and CIV, for example, by transfecting ρ0 cells with alternative oxidase (AOX), results in redox-cycling of CoQ, which restores the DHODH activity so that tumors can form and progress. Modified from Bajzikova et al. (2019). OMM, outer mitochondrial membrane; IMS inter-membrane space; IMM, inner mitochondrial space; UMP, uridine monophosphate.
Therapeutic approaches and implications of horizontal transfer of mitochondria
HMT contributes to the rescue of mitochondrial function in recipient cells. It is a “double-edged sword” that provides both benefit and harm to cells and tissues, depending on the context. Its therapeutic implications are also twofold. HMT is detrimental in cancer where new mitochondria can rejuvenate damaged cancer cells and promote tumorigenicity, thus therapeutical benefit will be provided by inhibition of the process. In situations where new mitochondria enable normal tissue homeostasis, tissue stress resistance, or wound healing, therapeutic benefit will be provided by increased HMT. While pharmacological intervention targeting HMT is in an experimental stage, we outlined approaches with potential therapeutical relevance that imply mitochondrial transfer. The priority in this context is to identify molecular targets allowing selective modulation of HMT without compromising physiology of mitochondria and the cytoskeleton. An overview of possible therapeutic implications of mitochondrial transfer is in Table 3.
Table 3.
Pathologies where mitochondrial transfer (or its blockage) has therapeutic impact
| Pathology | Experimental model | Route of administration* | Result | Reference |
|---|---|---|---|---|
| Heart | ||||
| Ischemia | Heart regional ischemia | Injection into ischemic region | Enhanced myocardial functional recovery and cell viability | McCully et al., 2009 |
| Heart regional ischemia | Injection into ischemic region | Enhancement of post-ischemic myocardial function | Masuzawa et al., 2013 | |
| Heart global ischemia | Coronary artery injection | Enhancement of post-ischemic myocardial function | Cowan et al., 2016 | |
| Heart regional ischemia | Injection into ischemic region | Enhancement of myocardial cell viability | Kaza et al., 2017 | |
| Heterotopic heart transplantation | Coronary artery injection | Enhancement of graft function and attenuation of necrosis | Moskowitzova et al., 2019 | |
| Warm global ischemia | Coronary artery injection | Enhancement of post-ischemic myocardial function | Doulamis et al., 2020 | |
| Heart regional ischemia | Pre-ischemic coronary artery injection | Enhancement of post-ischemic myocardial function | Guariento et al., 2020 | |
| Liver | ||||
| Ischemia | Partial liver ischemia | Intrasplenic injection | Attenuation of hepatic injury | Lin et al., 2013 |
| Non-alcoholic fatty liver disease | Intravenous tail injection | Attenuation of lipid accumulation and oxidative stress | Fu et al., 2017 | |
| Acetaminophen-induced liver injury | Intravenous tail injection | Attenuation of tissue injury and enhancement of hepatocyte metabolism | Shi et al., 2018 | |
| Lungs | ||||
| Experimental lung silicosis | Intravenous injection of MSCs or MSC-derived exosomes | Reduction in the size of silicotic nodules, total number of white blood cells in BALF, and expression of inflammatory and pro-fibrotic genes in the lung | Phinney et al., 2015 | |
| Acute lung ischemia-reperfusion | Pulmonary artery injection and nebulization | Improvement of lung mechanics and attenuation of tissue injury | Moskowitzova et al., 2020 | |
| Pulmonary fibrosis | Intravenous tail injection of MSCs | Mitigation of fibrotic progression | Huang et al., 2021 | |
| Kidney | ||||
| Renal artery stenosis | Intra-arterially injection | Improved perfusion and oxygenation, protective effects on injured tubular cells | Zou et al., 2018 | |
| Diabetic nephropathy | Streptozotocin-induced diabetic rats | Injection under renal capsule | Improved cellular morphology and structure of the tubular basement membrane and brush border | Konari et al., 2019 |
| CNS | ||||
| Stroke | Middle cerebral artery occlusion | Intravenous injection | Decrease of brain infarction area and partial neurological status restoration | Babenko et al., 2015 |
| Middle cerebral artery occlusion | Injection into ischemic striatum | Attenuation of brain infarction area and neuronal death, restoration of motor performance | Huang et al., 2016 | |
| Middle cerebral artery occlusion | Intravenous injection | Restoration of neural function | Babenko et al., 2018 | |
| Middle cerebral artery occlusion | Intra-arterial injection of MSCs | Improved mitochondrial function in peri-infarct area and functional recovery | Wang et al., 2019 | |
| Middle cerebral artery occlusion | Intracerebro-ventricular injection | Promotion of neuroprotection, reduced brain infarct size, induced neurogenesis | Zhang et al., 2019 | |
| PD | Neurotoxin 6-hydroxydopamine induced PD | Medial forebrain bundle injection | Attenuation of oxidative damage and degeneration of dopaminergic neurons, and improved locomotion | Chang et al., 2016 |
| Neurotoxin MPTP induced PD | Intravenous injection | Reduction of neuronal death and attenuation of damage by ROS and improved behavioral symptoms | Shi et al., 2018 | |
| Schizophrenia | Poly-I:C induced schizophrenia | Prefrontal cortex injection | Prevention of the loss of brain ∆ψm and attention deficit in adulthood | Robicsek et al., 2018 |
| AD | AD model produced by the intra-cerebro-ventricular injection of Aβ peptide | Intravenous injection (tail) | Attenuation of neuronal loss and reactive gliosis, restoration of cognitive deficits | Nitzan et al., 2019 |
| Depression | LPS-induced model of depression | Intravenous injection | Improved symptoms such as exploratory behavior and promotion of neurogenesis, antidepressant-like effects | Wang et al., 2019 |
| Aging | Aged mice (18 mo) | Intravenous injection (tail) | Significant improvement of cognitive and motor performance of aged mice | Liu et al., 2019 |
| Spinal cord | Spinal cord injury | Mediolateral gray matter of injury site | Maintenance of acute bioenergetics, functional recovery | Zhao et al., 2020 |
| Spinal cord ischemia | Intravenous injection (jugular) | Improved hindlimb motor function | Feng et al., 2019; Gollihue et al., 2018 | |
| Spinal cord injury | Injected into the epicenter of the injured spinal cord | Improved locomotor functional recovery | Li et al., 2019 | |
| Glaucoma | Optic nerve crush | Intravitreal injection | Promoted short-term neuroprotection (14 d) to retinal ganglion cells and modulated retinal oxidative metabolism; importantly, mitochondria also increased the number of axons extending ahead of the injury site in a long-term period (28 d) | McCully et al., 2017 |
| Retinal ganglion cell degeneration | Ndufs4 knockout mouse model | Vitreous cavity injection | Protection against mitochondrial damage-induced retinal ganglion cell loss | Jiang et al., 2019 |
| Corneal injury | Alkaline burn-induced corneal damage | Transplantation of MSC scaffold to the center of the cornea | Improved corneal wound healing | Jiang et al., 2016 |
| Cancer | ||||
| Melanoma lung metastasis | Intravenous tail injection | Retardation of tumor growth and prolonged animal survival | Fu et al., 2019 | |
| MM | Intravenous injection of CD38 myeloma cells | Targeting of CD38 reduced significantly mitochondrial transfer and improved animal survival | Marlein et al., 2019 | |
| Glioma cell (U87) xenograft tumors | Injection into xenograft | Inhibited glioma growth, enhanced radiosensitivity of gliomas | Sun et al., 2019 | |
| Embryonic development | In vitro blastocyst stage development | Injection into zygotes | Improved embryonic development | Yi et al., 2007 |
| Tissue injury | Full-thickness cutaneous wound and dystrophic skeletal muscle | Engraftment of MSCs and platelets into the wound area | Enhanced therapeutic efficacy of MSCs | Levoux et al., 2021 |
| BM transplantation (BMT) | Total body irradiation as a preconditioning mechanism before BMT | Intravenous tail injection of BM cells | Rapid recovery of BM microenvironment, improved hematopoietic reconstitution after BMT | Golan et al., 2020 |
administration of mitochondria unless stated otherwise
Therapeutic approaches in cancer
The primary goal of HMT-focused therapy in cancer is to curb tumorigenicity and resistance associated with mitochondrial fitness of cancer cells (Berardi and Fantin, 2011; Suomalainen, 2019; Wei et al., 2019). Cancer cells can remove damaged mitochondria and transfer them to somatic cells, while healthy mitochondria can move in the opposite direction to “fix” cancer cells. The possible approaches include (i) interference with actin filaments in order to disrupt TNTs, (ii) interference with components required for TNT formation, (iii) reducing cell adhesion to lower transfer by limiting the time two cells spend in close proximity, and (iv) suppression of signals that stimulate HMT.
Pharmacological targeting of HMT may be applied in hematologic malignancies since HMT contributes to treatment resistance. Inhibition of the adhesion molecule ICAM-1 and treatment with cytochalasin D, an inhibitor of actin polymerization that disrupts TNTs, prevents mitochondrial transfer from MSCs to leukemia cells, promoting chemotherapy-induced cell death (Wang et al., 2018). Application of microtubule/actin inhibitors (vincristine, nocodazole, latrunculin B) reduces resistance to therapy (Burt et al., 2019). Suppression of ROS production reduced HMT from BM stem cells to acute myeloid leukemia blasts stimulates apoptosis and improves survival (Marlein et al., 2017). Similar reduction was observed in multiple myeloma (MM) upon TNT disruption by cytochalasin D as well as after blocking of CD38, a surface glycoprotein that stimulates TNT formation (Marlein et al., 2019). Targeting CD38 by monoclonal antibodies has been explored as a therapeutic strategy in leukemias, but it is unclear if inhibition of HMT contributes to its benefits (Koundinya et al., 2018; Martin et al., 2019; Moreno et al., 2019). During chemotherapy, including the use of the BH3 mimetic venetoclax, MM cells acquire healthy mitochondria from BM-derived MSCs via TNTs, and damaged mitochondria are mobilized from MMs by TNTs and MVs to be disposed of by MSCs (Matula et al., 2021).
Pharmacological targeting of HMT has been pursued in solid tumors. Breast/lung cancer cells hijack mitochondria from tumor-resident immune cell via TNTs, dampening the anti-tumor immune response and reducing the effectiveness of immune checkpoint therapy. Pharmacological inhibition of TNT formation in syngeneic subcutaneous mouse models of lung and breast cancer by the farnesyl/geranyl-1 transferase inhibitor L-778123 (inhibits Ras/Rho GTPase activity required for TNT formation) enhanced the efficacy of immune checkpoint blockade, restored immune response, and limited tumor growth (Saha et al., 2022). HMT was observed in a mouse model of GBM undergoing radiotherapy (Osswald et al., 2015). This supports the view that interference with HMT may be used as an approach for hard-to-treat tumors and lower their resistance to therapy (van Solinge et al., 2022).
Therapeutic approaches in non-cancerous pathologies
In non-cancerous pathologies HMT is mostly beneficial. HMT improves the outcome of neurological injury and degenerative diseases affecting the brain, spinal cord, and visual system (Mohammadalipour et al., 2020), cardiovascular diseases including ischemia and cardiomyopathy (Han et al., 2016; Zhang et al., 2016), lung injury and asthma (Islam et al., 2012; Ahmad et al., 2014; Yao et al., 2018), wound healing (Levoux et al., 2021), and immune system function (Jackson et al., 2016a; Jackson et al., 2016b; Phinney et al., 2015; Morrison et al., 2017). The main objective is thus to stimulate HMT. This is a challenging task as pharmacological stimulation is usually more difficult than its inhibition. Researchers have tried to circumvent this issue, for example, by supplying healthy mitochondria to be incorporated into targeted cells, resorting to the so-called “mitotherapy.”
The therapeutic potential of mitotherapy was first explored in the cardiovascular system during ischemia/reperfusion injury. Respiration-competent mitochondria, isolated from healthy cardiac tissue, injected directly into the ischemic area of rabbit hearts during early reperfusion, enhanced myocardial functional recovery and cell viability (McCully et al., 2009). Autologous local transplantation of isolated mitochondria protected CMs from ischemia-reperfusion injury (Masuzawa et al., 2013) and offered protection during prolonged cold ischemia prior to heart transplantation (Moskowitzova et al., 2019; Moskowitzova et al., 2020). Infusion of mitochondria in the coronary artery reduced the infarct size and improved heart function (Guariento et al., 2020; Louwagie et al., 2021; Doulamis et al., 2020), and this procedure was applied in pediatric patients with congenital heart disease (Emani and McCully, 2018). It was shown that in response to energetic stress, adipocytes release ECVs containing respiration-competent mitochondria that are taken up by CMs, where they induced transient mitochondrial oxidative stress leading to pre-conditioning that protects against ischemia/reperfusion injury. This “‘metabolic pre-conditioning” was recapitulated by injection of adipocyte-derived ECVs (Crewe et al., 2021), and transplantation of mitochondria affected CM bioenergetics (Ali Pour et al., 2020). As mitotherapy also improves outcome in animal models of ischemia/reperfusion in the liver and lung (Moskowitzova et al., 2019; Moskowitzova et al., 2020; Lin et al., 2013), and in liver damage due to non-alcoholic fatty liver disease or exposure to toxic compounds (Fu et al., 2017; Shi et al., 2018), it can be concluded that mitotherapy by isolated mitochondria or mitochondria-containing ECVs has the potential to provide clinical benefit.
Mitotherapy was also extensively explored in the central and peripheral nervous system. Mitochondria derived from hamsters administered either via local intracerebral or systemic intraocular-arterial injection attenuated the area of brain infarction and neuronal death, and restored motor performance in a stroke model of middle cerebral artery occlusion (Huang et al., 2016). Administration of mitochondria by intra-cerebroventricular injection gave similar results, reducing reactive astrogliosis (Zhang et al., 2019). In a model of spinal cord injury exogenous mitochondria contributed to the maintenance of acute bioenergetics as well as functional recovery (Gollihue and Rabchevsky, 2017). In a model of Parkinson’s disease (PD), delivery of mitochondria into the medial forebrain attenuated the oxidative damage and degeneration of dopaminergic neurons, improving locomotion (Chang et al., 2016). In a model of schizophrenia, injection of mitochondria into the pre-frontal cortex of young rats prevented the loss of mitochondrial potential of brain cells and attention deficit in adulthood (Robicsek et al., 2018).
Besides mitotherapy, there are indirect approaches to stimulate HMT into damaged cells by delivery of efficient mitochondrial donors, such as MSCs that, once in close proximity, transfer mitochondria to recipient cells by ECVs or TNTs. Application of induced pluripotent stem cell-derived MSCs resulted in Miro1-dependent transfer of mitochondria, rescuing anthracycline-exposed CMs (Zhang et al., 2016). Administration of MSCs into lungs improved the outcome of LPS- and oxidative stress–induced lung injury (Islam et al., 2012; Li et al., 2018), allergic inflammation (Ahmad et al., 2014), and asthma (Yao et al., 2018). Mitochondria mobilized from mesenchymal stromal cells have beneficial effect on β cells, linking HMT and the metabolic syndrome (Rackham et al., 2020). Promising results of HMT were also observed in a model of AD (Nitzan et al., 2019) and wound healing (Levoux et al., 2021).
That HMT has a wide range of applications can be epitomized by two recent studies. Iron oxide nanoparticles enhanced formation of Cx43-containing gap junctions and increased the rate of HMT from administered MSCs in a model of pulmonary fibrosis (Huang et al., 2021); and exposure to hyperbaric oxygen led to amelioration of inflammation involving HMT (Lippert and Borlongan, 2019).
Conclusions and future directions
Since the discovery of HMT in vitro (Rustom et al., 2004) and in vivo in mouse models of lung damage (Islam et al., 2012; Ahmad et al., 2014), and in tumor models where we used mtDNA polymorphisms and somatic cells from transgenic mice with RFP-decorated mitochondria (Tan et al., 2015; Dong et al., 2017), research on HMT has gained momentum. Initial in vivo research focused on the role of HMT in cancer and its functional consequences (Tan et al., 2015; Bajzikova et al., 2019) and possible modulation of resistance to cancer therapy (Osswald et al., 2015; Moschoi et al., 2016), and later on regulation of cancer-related immune responses (Saha et al., 2022). Role of HMT has been reported for a wide array of (patho)physiological conditions such as cardiovascular diseases, metabolic syndrome–related pathologies, and wound healing (Nicolas-Avila et al., 2020; Crewe et al., 2021; Levoux et al., 2021; Rackham et al., 2020). It is likely that HMT also occurs under natural conditions, as shown for outbred mice, with the idea being that regular exchange of mitochondria between cells contributes to maintaining balanced heteroplasmy (Jayaprakash et al., 2015), or during mouse development (Marti Gutierrez et al., 2022), which is important for homeostasis (Jackson et al., 2020) that may involve mitochondrial respiration-linked remodeling (Bennett et al., 2022). More research is needed to explore the role of HMT as a process in normal development and tissue homeostasis, in particular regarding the control of mtDNA heterogeneity. This is complicated because the vast majority of experimental animals are inbred with very limited mtDNA heteroplasmy.
Heterogeneity, mutations, and deletions in mtDNA have been implicated in a wide range of diseases, frequently involving neurodegenerative/neuromuscular pathologies (Burté et al., 2015; Lightowlers et al., 2015; Area-Gomez et al., 2019; Monzio Compagnoni et al., 2020; Ng et al., 2021). It is plausible that mitochondrial therapy, by stimulation of HMT or by mitochondrial transplantation, may be effective in alleviating pathologies, with neurodegenerative or cardiovascular diseases being “hot” candidates for the emerging therapeutic modality (Emani and McCully, 2018; Picard et al., 2016; Caicedo et al., 2017; Chang et al., 2019; McCully et al., 2017; Nascimento-Dos-Santos et al., 2021; Gorman et al., 2018). Mitochondrial transplantation has been used to replace faulty maternal mitochondrial genes (Gorman et al., 2018; Jacoby et al., 2022). We have witnessed considerable progress in mitotherapy based on mitochondrial transplantation including sophisticated approaches such as transfer of mitochondria from fetal to adult MSCs using optical tweezers (Shakoor et al., 2021). Mitotherapy has been boosted by the discovery of the so called MitoCeption that allows for controlled delivery of mitochondria isolated from donor cells (Caicedo et al., 2015; Sercel et al., 2021).
Faster implementation of mitotherapy, in particular based on HMT, is hindered due to methodological limitations. Unequivocal evidence for acquisition of mitochondria in vivo was provided by stable homoplasmic polymorphisms in mtDNA (Tan et al., 2015), known to occur in inbred mouse tumor models (Bayona-Bafaluy et al., 2003). Another problem in studying the molecular mechanisms and regulation of HMT is its visualization. This is now more facile by cutting-edge microscopic techniques, such as total internal reflection fluorescence microscopy (Henrichs et al., 2020), iterative tomography (Wu et al., 2021a), or multiview confocal super-resolution microscopy (Wu et al., 2021b), in particular in combination with genetically encoded fluorescent proteins decorating mitochondria (Barrasso et al., 2018).
Almost 20 yr after the discovery of mitochondrial transfer in vitro (Rustom et al., 2004) and <10 yr after the first unequivocal report of HMT in mouse tumor models (Tan et al., 2015), there is considerable momentum indicating that this very interesting and paradigm-shifting understanding of basic cell biology will be translated into therapeutic strategies that will alleviate multiple hard-to-treat pathologies. Of these, mitochondrial diseases linked to pediatric maladies and neurological pathologies are likely to be early candidates. We anticipate that what started as intriguing basic research will soon be translated for the benefit of mankind.
Acknowledgments
We thank all former and current collaborators who have helped us in the discovery of horizontal transfer of mitochondria in mammalian systems and in understanding its relevance and mechanism.
This work was supported in part by grants from the Australian Research Council, Czech Science Foundation (20-05942S, 21-04607X, 22-34507S), and Czech Health Foundation (NU22-08-00160) to J. Neuzil, Czech Science Foundation (NU-22-34507S) to J. Rohlena, and the Health Research Council of New Zealand and Cancer Society of New Zealand to M.V. Berridge.
References
- Abounit, S., Bousset L., Loria F., Zhu S., de Chaumont F., Pieri L., Olivo-Marin J.C., Melki R., and Zurzolo C.. 2016. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 35:2120–2138. 10.15252/embj.201593411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acín-Pérez, R., Fernández-Silva P., Peleato M.L., Pérez-Martos A., and Enriquez J.A.. 2008. Respiratory active mitochondrial supercomplexes. Mol. Cell. 32:529–539. 10.1016/j.molcel.2008.10.021 [DOI] [PubMed] [Google Scholar]
- Acquistapace, A., Bru T., Lesault P.F., Figeac F., Coudert A.E., le Coz O., Christov C., Baudin X., Auber F., Yiou R., et al. 2011. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 29:812–824. 10.1002/stem.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, P.S., Baylies M.K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H., and Wong M.. 2013. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29:427–437. 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B.K., Jha K.A., Barhanpurkar A.P., et al. 2014. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33:994–1010. 10.1002/embj.201386030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Pour, P., Kenney M.C., and Kheradvar A.. 2020. Bioenergetics consequences of mitochondrial transplantation in cardiomyocytes. J. Am. Heart Assoc. 9:e014501. 10.1161/JAHA.119.014501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado, M., Pardal R., Garcia-Verdugo J.M., Fike J.R., Lee H.O., Pfeffer K., Lois C., Morrison S.J., and Alvarez-Buylla A.. 2003. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 425:968–973. 10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- Antanavičiūtė, I., Rysevaitė K., Liutkevičius V., Marandykina A., Rimkutė L., Sveikatienė R., Uloza V., and Skeberdis V.A.. 2014. Long-distance communication between laryngeal carcinoma cells. PLoS One. 9:e99196. 10.1371/journal.pone.0099196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area-Gomez, E., Guardia-Laguarta C., Schon E.A., and Przedborski S.. 2019. Mitochondria, OxPhos, and neurodegeneration: Cells are not just running out of gas. J. Clin. Invest. 129:34–45. 10.1172/JCI120848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi, J., Benowitz A., De Biasi V., Den Boer M.L., Cherqui S., Cui H., Douillet N., Eugenin E.A., Favre D., Goodman S., et al. 2017. Tunneling nanotubes and gap junctions—their role in long-range intercellular communication during development, health, and disease conditions. Front. Mol. Neurosci. 10:333. 10.3389/fnmol.2017.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryaman, J., Johnston I.G., and Jones N.S.. 2019a. Mitochondrial heterogeneity. Front. Genet. 9:718. 10.3389/fgene.2018.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryaman, J., Bowles C., Jones N.S., and Johnston I.G.. 2019b. Mitochondrial network state scales mtDNA genetic dynamics. Genetics. 212:1429–1443. 10.1534/genetics.119.302423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko, V.A., Silachev D.N., Zorova L.D., Pevzner I.B., Khutornenko A.A., Plotnikov E.Y., Sukhikh G.T., and Zorov D.B.. 2015. Improving the post-stroke therapeutic potency of mesenchymal multipotent stromal vells by cocultivation with cortical neurons: The role of crosstalk between cells. Stem Cells Transl. Med. 4:1011–1020. 10.5966/sctm.2015-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko, V.A., Silachev D.N., Popkov V.A., Zorova L.D., Pevzner I.B., Plotnikov E.Y., Sukhikh G.T., and Zorov D.B.. 2018. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 23:687. 10.3390/molecules23030687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri, H.S., Bani F., Tasoglu S., Zarebkohan A., Rahbarghazi R., and Sokullu E.. 2020. Mitochondrial donation in translational medicine; from imagination to reality. J. Transl. Med. 18:367. 10.1186/s12967-020-02529-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajzikova, M., Kovarova J., Coelho A.R., Boukalova S., Oh S., Rohlenova K., Svec D., Hubackova S., Endaya B., Judasova K., et al. 2019. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metabol. 29:399–416.e10. 10.1016/j.cmet.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris, O.R., Ederer S., Neuhaus J.F., von Kleist-Retzow J.C., Wunderlich C.M., Pal M., Wunderlich F.T., Peeva V., Zsurka G., Kunz W.S., et al. 2015. Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metabol. 21:667–677. 10.1016/j.cmet.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Barrasso, A.P., Tong X., and Poché R.A.. 2018. The mito:mKate2 mouse: A far-red fluorescent reporter mouse line for tracking mitochondrial dynamics in vivo. Genesis. 56:e23087. 10.1002/dvg.23087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayona-Bafaluy, M.P., Acín-Pérez R., Mullikin J.C., Park J.S., Moreno-Loshuertos R., Hu P., Pérez-Martos A., Fernández-Silva P., Bai Y., and Enríquez J.A.. 2003. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 31:5349–5355. 10.1093/nar/gkg739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., and Lyden D.. 2016. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell. 30:836–848. 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, C.F., Latorre-Muro P., and Puigserver P.. 2022. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 23:817–835. 10.1038/s41580-022-00506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi, M.J., and Fantin V.R.. 2011. Survival of the fittest: Metabolic adaptations in cancer. Curr. Opin. Genet. Dev. 21:59–66. 10.1016/j.gde.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Bergthorsson, U., Adams K.L., Thomason B., and Palmer J.D.. 2003. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature. 424:197–201. 10.1038/nature01743 [DOI] [PubMed] [Google Scholar]
- Berridge, M.V., Dong L., and Neuzil J.. 2015. Mitochondrial DNA in tumor initiation, progression and metastasis: Role of horizontal mtDNA transfer. Cancer Res. 75:3203–3208. 10.1158/0008-5472.CAN-15-0859 [DOI] [PubMed] [Google Scholar]
- Berridge, M.V., Schneider R.T., and McConnell M.J.. 2016. Mitochondrial transfer from astrocytes to neurons following ischemic insult: Guilt by association? Cell Metabol. 24:376–378. 10.1016/j.cmet.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Berridge, M.V., Herst P.M., and Grasso C.. 2020. Mitochondrial Movement between Cells: An Emerging Physiological Phenomenon in the Human Mitochondrial Genome: from Biology to Disease. Porcelli A.-M., Gasparre G., editors. Elsevier Books, 20:515–545 [Google Scholar]
- Bettadapur, A., Miller H.W., and Ralston K.S.. 2020. Biting off what can be chewed: Trogocytosis in health, infection, and disease. Infect. Immun. 88:e00930-19. 10.1128/IAI.00930-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya, D., and Scimè A.. 2020. Mitochondrial function in muscle stem cell fates. Front. Cell Dev. Biol. 8:480. 10.3389/fcell.2020.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky, C.W., Jr. 2001. The inheritance of genes in mitochondria and chloroplasts: Laws, mechanisms, and models. Annu. Rev. Genet. 35:125–148. 10.1146/annurev.genet.35.102401.090231 [DOI] [PubMed] [Google Scholar]
- Birsoy, K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., and Sabatini D.M.. 2015. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 162:540–551. 10.1016/j.cell.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens, M.C., Wu T.J., Nabet B.Y., Xu B., Qiu Y., Yoon T., Azzam D.J., Twyman-Saint Victor C., Wiemann B.Z., Ishwaran H., et al. 2014. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 159:499–513. 10.1016/j.cell.2014.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschetti, C., Carr A., Crisp A., Eyres I., Wang-Koh Y., Lubzens E., Barraclough T.G., Micklem G., and Tunnacliffe A.. 2012. Biochemical diversification through foreign gene expression in bdelloid rotifers. PLoS Genet. 8:e1003035. 10.1371/journal.pgen.1003035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau, L.H., Duchez A.C., Cloutier N., Soulet D., Martin N., Bollinger J., Paré A., Rousseau M., Naika G.S., Lévesque T., et al. 2014. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 124:2173–2183. 10.1182/blood-2014-05-573543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukalova, S., Hubackova S., Milosevic M., Ezrova Z., Neuzil J., and Rohlena J.. 2020. Dihydroorotate dehydrogenase in oxidative phosphorylation and cancer. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165759. 10.1016/j.bbadis.2020.165759 [DOI] [PubMed] [Google Scholar]
- Boukelmoune, N., Chiu G.S., Kavelaars A., and Heijnen C.J.. 2018. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol. Commun. 6:139. 10.1186/s40478-018-0644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff, J.R., Wilen C.B., Moley J.R., Li Y., Zou W., Malvin N.P., Rowen M.N., Saunders B.T., Ma H., Mack M.R., et al. 2021. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. CellMetabol. 33:270–282.e8. 10.1016/j.cmet.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, M.D., O’Sullivan D., Klein Geltink R.I., Curtis J.D., Chang C.H., Sanin D.E., Qiu J., Kretz O., Braas D., van der Windt G.J., et al. 2016. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 166:63–76. 10.1016/j.cell.2016.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukoreshtliev, N.V., Wang X., Hodneland E., Gurke S., Barroso J.F., and Gerdes H.H.. 2009. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 583:1481–1488. 10.1016/j.febslet.2009.03.065 [DOI] [PubMed] [Google Scholar]
- Burgstaller, J.P., Johnston I.G., Jones N.S., Albrechtová J., Kolbe T., Vogl C., Futschik A., Mayrhofer C., Klein D., Sabitzer S., et al. 2014. MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep. 7:2031–2041. 10.1016/j.celrep.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt, R., Dey A., Aref S., Aguiar M., Akarca A., Bailey K., Day W., Hooper S., Kirkwood A., Kirschner K., et al. 2019. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 134:1415–1429. 10.1182/blood.2019001398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burté, F., Carelli V., Chinnery P.F., and Yu-Wai-Man P.. 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 11:11–24. 10.1038/nrneurol.2014.228 [DOI] [PubMed] [Google Scholar]
- Caicedo, A., Fritz V., Brondello J.M., Ayala M., Dennemont I., Abdellaoui N., de Fraipont F., Moisan A., Prouteau C.A., Boukhaddaoui H., et al. 2015. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep. 5:9073. 10.1038/srep09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo, A., Aponte P.M., Cabrera F., Hidalgo C., and Khoury M.. 2017. Artificial mitochondria transfer: Current challenges, advances, and future applications. Stem Cells Int. 2017:7610414. 10.1155/2017/7610414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J.C., Wu S.L., Liu K.H., Chen Y.H., Chuang C.S., Cheng F.C., Su H.L., Wei Y.H., Kuo S.J., and Liu C.S.. 2016. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res 170:40–56.e3. 10.1016/j.trsl.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Chang, C.-Y., Liang M.-Z., and Chen L.. 2019. Current progress of mitochondrial transplantation that promotes neuronal regeneration. Transl. Neurodegener. 8:17. 10.1186/s40035-019-0158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.H., Su C.C., Deng W., Lock L.F., Donovan P.J., Kayala M.A., Baldi P., Lee H.C., Chen Y., and Wang P.H.. 2019. Mitochondrial Akt signaling modulated reprogramming of somatic cells. Sci. Rep. 9:9919. 10.1038/s41598-019-46359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery, H.R., and Keller K.E.. 2020. Tunneling nanotubes and the eye: Intercellular communication and implications for ocular health and disease. Biomed. Res. Int. 2020:7246785. 10.1155/2020/7246785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y.M., Kim J.H., Kim M., Park S.J., Koh S.H., Ahn H.S., Kang G.H., Lee J.B., Park K.S., and Lee H.K.. 2012. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One. 7:e32778. 10.1371/journal.pone.0032778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, S.H., Lan J., Esposito E., Ning M., Balaj L., Ji X., Lo E.H., and Hayakawa K.. 2017. Extracellular mitochondria in cerebrospinal fluid and neuro-logical recovery after subarachnoid hemorrhage. Stroke. 48:2231–2237. 10.1161/STROKEAHA.117.017758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, Y.C., Liou C.W., Chen S.D., Wang P.W., Chuang J.H., Tiao M.M., Hsu T.Y., Lin H.Y., and Lin T.K.. 2017. Mitochondrial transfer from Wharton’s jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid. Med. Cell. Longev. 2017:5691215. 10.1155/2017/5691215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci, E., and Meldolesi J.. 2015. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 25:364–372. 10.1016/j.tcb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Conner, S.D., and Schmid S.L.. 2003. Regulated portals of entry into the cell. Nature. 422:37–44. 10.1038/nature01451 [DOI] [PubMed] [Google Scholar]
- Course, M.M., and Wang X.. 2016. Transporting mitochondria in neurons. F1000 Res. 5:1735. 10.12688/f1000research.7864.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillion, M.T., Soto I.C., Shipkovenska G., and Churchman L.S.. 2016. Synchronized mitochondrial and cytosolic translation programs. Nature. 533:499–503. 10.1038/nature18015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, D.B., Yao R., Akurathi V., Snay E.R., Thedsanamoorthy J.K., Zurakowski D., Ericsson M., Friehs I., Wu Y., Levitsky S., et al. 2016. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One. 11:e0160889. 10.1371/journal.pone.0160889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewe, C., Funcke J.B., Li S., Joffin N., Gliniak C.M., Ghaben A.L., An Y.A., Sadek H.A., Gordillo R., Akgul Y., et al. 2021. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metabol. 33:1853–1868.e11. 10.1016/j.cmet.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyak, A., Pankotai E., Horvath E.M., Kiss L., and Lacza Z.. 2010. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 11:29. 10.1186/1471-2121-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusulin, C., Monni E., Ahlenius H., Wood J., Brune J.C., Lindvall O., and Kokaia Z.. 2012. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells. 30:2657–2671. 10.1002/stem.1227 [DOI] [PubMed] [Google Scholar]
- D'Souza, A., Burch A., Dave K.M., Sreeram A., Reynolds M.J., Dobbins D.X., Kamte Y.S., Zhao W., Sabatelle C., Joy J.M., et al. 2021. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Contr. Release. 338:505–526. 10.1016/j.jconrel.2021.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C.H., Kim K.Y., Bushong E.A., Mills E.A., Boassa D., Shih T., Kinebuchi M., Phan S., Zhou Y., Bihlmeyer N.A., et al. 2014. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA. 111:9633–9638. 10.1073/pnas.1404651111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana, E. 2004. Endothelial cell-cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 5:261–270. 10.1038/nrm1357 [DOI] [PubMed] [Google Scholar]
- Dickinson, A., Yeung K.Y., Donoghue J., Baker M.J., Kelly R.D., McKenzie M., Johns T.G., and St John J.C.. 2013. The regulation of mitochondrial DNA copy number in glioblastoma cells. Cell Death Differ. 20:1644–1653. 10.1038/cdd.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold, L.P., Gil H.J., Gao P., Martinez C.A., Weinberg S.E., and Chandel N.S.. 2019. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat. Metab. 1:158–171. 10.1038/s42255-018-0011-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domhan, S., Ma L., Tai A., Anaya Z., Beheshti A., Zeier M., Hlatky L., and Abdollahi A.. 2011. Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells. PLoS One. 6:e21283. 10.1371/journal.pone.0021283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, L.F., Kovarova J., Bajzikova M., Bezawork-Geleta A., Svec D., Endaya B., Schaphibulkij K., Coelho A., Sebkova N., Ruzickova A., et al. 2017. Horizontal transfer of whole mitochondria recovers tumorigenic potential in mtDNA-deficient cells. Elife. 6:e22187. 10.7554/eLife.22187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulamis, I.P., Guariento A., Duignan T., Orfany A., Kido T., Zurakowski D., Del Nido P.J., and McCully J.D.. 2020. Mitochondrial transplantation for myocardial protection in diabetic hearts. J. Cardio-Thoracic Surg. 57:836–845. 10.1093/ejcts/ezz326 [DOI] [PubMed] [Google Scholar]
- Doyle, L.M., and Wang M.Z.. 2019. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 8:727. 10.3390/cells8070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL Andaloussi, S., Mäger I., Breakefield X.O., and Wood M.J.A.. 2013. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12:347–357. 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- Emani, S.M., and McCully J.D.. 2018. Mitochondrial transplantation: Applications for pediatric patients with congenital heart disease. Transl. Pediatr. 7:169–175. 10.21037/tp.2018.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English, K., Shepherd A., Uzor N.E., Trinh R., Kavelaars A., and Heijnen C.J.. 2020. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol. Commun. 8:36. 10.1186/s40478-020-00897-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin, E.A., Gaskill P.J., and Berman J.W.. 2009. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: A potential mechanism for intercellular HIV trafficking. Cell. Immunol. 254:142–148. 10.1016/j.cellimm.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg, M., Larsson N.G., and Gustafsson C.M.. 2007. DNA replication and transcription in mammalian mitochondria. Annu. Rev. Biochem. 76:679–699. 10.1146/annurev.biochem.76.060305.152028 [DOI] [PubMed] [Google Scholar]
- Feng, Y., Zhu R., Shen J., Wu J., Lu W., Zhang J., Zhang J., and Liu K.. 2019. Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 28:674–682. 10.1089/scd.2018.0248 [DOI] [PubMed] [Google Scholar]
- Fu, A., Shi X., Zhang H., and Fu B.. 2017. Mitotherapy for fatty liver by intravenous administration of exogenous mitochondria in male mice. Front. Pharmacol. 8:241. 10.3389/fphar.2017.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, A., Hou Y., Yu Z., Zhao Z., and Liu Z.. 2019. Healthy mitochondria inhibit the metastatic melanoma in lungs. Int. J. Biol. Sci. 15:2707–2718. 10.7150/ijbs.38104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani, S., Galzignati L., Marcati M., Durazzi P., Cianella A., Mocheggiani V., Monaco F., Bracci M., Neuzil J., Tomasetti M., et al. 2022. Mitochondrial function as related to psychological distress in health care professionals. Psychosom. Med. 84:40–49. 10.1097/PSY.0000000000001000 [DOI] [PubMed] [Google Scholar]
- Gao, L., Zhang Z., Lu J., and Pei G.. 2019. Mitochondria are dynamically transferring between human neural cells and Alexander disease-associated GFAP mutations impair the astrocytic transfer. Front. Cell. Neurosci. 13:316. 10.3389/fncel.2019.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, H.H., and Carvalho R.N.. 2008. Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20:470–475. 10.1016/j.ceb.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Gerdes, H.H., Bukoreshtliev N.V., and Barroso J.F.. 2007. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Lett. 581:2194–2201. 10.1016/j.febslet.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Gladyshev, E.A., Meselson M., and Arkhipova I.R.. 2008. Massive horizontal gene transfer in bdelloid rotifers. Science. 320:1210–1213. 10.1126/science.1156407 [DOI] [PubMed] [Google Scholar]
- Golan, K., Singh A.K., Kollet O., Bertagna M., Althoff M.J., Khatib-Massalha E., Petrovich-Kopitman E., Wellendorf A.M., Massalha H., Levin-Zaidman S., et al. 2020. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood. 136:2607–2619. 10.1182/blood.2020005399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue, J.L., and Rabchevsky A.G.. 2017. Prospects for therapeutic mitochondrial transplantation. Mitochondrion. 35:70–79. 10.1016/j.mito.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue, J.L., Patel S.P., Eldahan K.C., Cox D.H., Donahue R.R., Taylor B.K., Sullivan P.G., and Rabchevsky A.G.. 2018. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J. Neurotrauma. 35:1800–1818. 10.1089/neu.2017.5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomzikova, M.O., James V., and Rizvanov A.A.. 2021. Mitochondria donation by mesenchymal stem cells: Current understanding and mitochondria transplantation strategies. Front. Cell Dev. Biol. 9:653322. 10.3389/fcell.2021.653322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough, D.A., and Paul D.L.. 2009. Gap junctions. Cold Spring Harb. Perspect. Biol. 1:a002576. 10.1101/cshperspect.a002576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman, G.S., McFarland R., Stewart J., Feeney C., and Turnbull D.M.. 2018. Mitochondrial donation: From test tube to clinic. Lancet. 392:1191–1192. 10.1016/S0140-6736(18)31868-3 [DOI] [PubMed] [Google Scholar]
- Gousset, K., and Zurzolo C.. 2009. Tunnelling nanotubes: A highway for prion spreading? Prion. 3:94–98. 10.4161/pri.3.2.8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset, K., Schiff E., Langevin C., Marijanovic Z., Caputo A., Browman D.T., Chenouard N., de Chaumont F., Martino A., Enninga J., et al. 2009. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11:328–336. 10.1038/ncb1841 [DOI] [PubMed] [Google Scholar]
- Gozzelino, R., Jeney V., and Soares M.P.. 2010. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50:323–354. 10.1146/annurev.pharmtox.010909.105600 [DOI] [PubMed] [Google Scholar]
- Gray, M.W., Burger G., and Lang B.F.. 1999. Mitochondrial evolution. Science. 283:1476–1481. 10.1126/science.283.5407.1476 [DOI] [PubMed] [Google Scholar]
- Griessinger, E., Moschoi R., Biondani G., and Peyron J.F.. 2017. Mitochondrial transfer in the leukemia microenvironment. Trends Cancer. 3:828–839. 10.1016/j.trecan.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Gu, D., Zou X., Ju G., Zhang G., Bao E., and Zhu Y.. 2016a. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016:2093940. 10.1155/2016/2093940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J., Wu M., Guo R., Yan K., Lei J., Gao N., and Yang M.. 2016b. The architecture of the mammalian respirasome. Nature. 537:639–643. 10.1038/nature19359 [DOI] [PubMed] [Google Scholar]
- Guarás, A., Perales-Clemente E., Calvo E., Acín-Pérez R., Loureiro-Lopez M., Pujol C., Martínez-Carrascoso I., Nuñez E., García-Marqués F., Rodríguez-Hernández M.A., et al. 2016. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 15:197–209. 10.1016/j.celrep.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Guariento, A., Blitzer D., Doulamis I., Shin B., Moskowitzova K., Orfany A., Ramirez-Barbieri G., Staffa S.J., Zurakowski D., Del Nido P.J., and McCully J.D.. 2020. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J. Thorac. Cardiovasc. Surg. 160:e15–e29. 10.1016/j.jtcvs.2019.06.111 [DOI] [PubMed] [Google Scholar]
- Guha, M., Srinivasan S., Ruthel G., Kashina A.K., Carstens R.P., Mendoza A., Khanna C., Van Winkle T., and Avadhani N.G.. 2014. Mitochondrial retrograde signaling induces epithelial-mesenchymal transition and generates breast cancer stem cells. Oncogene. 33:5238–5250. 10.1038/onc.2013.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, R., Davis D., and Fang Y.. 2018. Intercellular transfer of mitochondria rescues virus-induced cell death but facilitates cell-to-cell spreading of porcine reproductive and respiratory syndrome virus. Virology. 517:122–134. 10.1016/j.virol.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Gurke, S., Barroso J.F., and Gerdes H.H.. 2008. The art of cellular communication: Tunneling nanotubes bridge the divide. Histochem. Cell Biol. 129:539–550. 10.1007/s00418-008-0412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, C.M., Falkenberg M., and Larsson N.G.. 2016. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 85:133–160. 10.1146/annurev-biochem-060815-014402 [DOI] [PubMed] [Google Scholar]
- Haimovich, G., Dasgupta S., and Gerst J.E.. 2021. RNA transfer through tunneling nanotubes. Biochem. Soc. Trans. 49:145–160. 10.1042/BST20200113 [DOI] [PubMed] [Google Scholar]
- Han, H., Hu J., Yan Q., Zhu J., Zhu Z., Chen Y., Sun J., and Zhang R.. 2016. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13:1517–1524. 10.3892/mmr.2015.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D., Zheng X., Wang X., Jin T., Cui L., and Chen Z.. 2020. Mesenchymal stem/stromal cell-mediated mitochondrial transfer and the therapeutic potential in treatment of neurological diseases. Stem Cells Int. 2020:8838046. 10.1155/2020/8838046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, C.V., Heuser J.E., and Stahl P.D.. 2013. Exosomes: Looking back three decades and into the future. J. Cell Biol. 200:367–371. 10.1083/jcb.201212113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase, K., Kimura S., Takatsu H., Ohmae M., Kawano S., Kitamura H., Ito M., Watarai H., Hazelett C.C., Yeaman C., and Ohno H.. 2009. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11:1427–1432. 10.1038/ncb1990 [DOI] [PubMed] [Google Scholar]
- Hayakawa, K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X., and Lo E.H.. 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 535:551–555. 10.1038/nature18928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa, K., Chan S.J., Mandeville E.T., Park J.H., Bruzzese M., Montaner J., Arai K., Rosell A., & Lo E.H.. 2018. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 36:1404-1410. 10.1002/stem.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, K., Shi X., Zhang X., Dang S., Ma X., Liu F., Xu M., Lv Z., Han D., Fang X., and Zhang Y.. 2011. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 92:39–47. 10.1093/cvr/cvr189 [DOI] [PubMed] [Google Scholar]
- Henrichs, V., Grycova L., Barinka C., Nahacka Z., Neuzil J., Diez S., Rohlena J., Braun M., and Lansky Z.. 2020. Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat. Commun. 11:3123. 10.1038/s41467-020-16972-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herst, P.M., Dawson R.H., and Berridge M.V.. 2018. Intercellular communication in tumor biology: A role for mitochondrial transfer. Front. Oncol. 8:344. 10.3389/fonc.2018.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough, K.P., Trevor J.L., Strenkowski J.G., Wang Y., Chacko B.K., Tousif S., Chanda D., Steele C., Antony V.B., Dokland T., et al. 2018. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 18:54–64. 10.1016/j.redox.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, P.J., Kuo C.C., Lee H.C., Shen C.I., Cheng F.C., Wu S.F., Chang J.C., Pan H.C., Lin S.Z., Liu C.S., and Su H.L.. 2016. Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transpl. 25:913–927. 10.3727/096368915X689785 [DOI] [PubMed] [Google Scholar]
- Huang, Y., Zucker B., Zhang S., Elias S., Zhu Y., Chen H., Ding T., Li Y., Sun Y., Lou J., et al. 2019. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 21:991–1002. 10.1038/s41556-019-0367-5 [DOI] [PubMed] [Google Scholar]
- Huang, T., Zhang T., Jiang X., Li A., Su Y., Bian Q., Wu H., Lin R., Li N., Cao H., et al. 2021. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci. Adv. 7:eabj0534. 10.1126/sciadv.abj0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito, L., Morandi A., Taddei M.L., Parri M., Comito G., Iscaro A., Raspollini M.R., Magherini F., Rapizzi E., Masquelier J., et al. 2019. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 38:5339–5355. 10.1038/s41388-019-0805-7 [DOI] [PubMed] [Google Scholar]
- Isaac, R., Reis F.C.G., Ying W., and Olefsky J.M.. 2021. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 33:1744–1762. 10.1016/j.cmet.2021.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., and Hayashi J.. 2008. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 320:661–664. 10.1126/science.1156906 [DOI] [PubMed] [Google Scholar]
- Islam, M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., and Bhattacharya J.. 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18:759–765. 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M.V., Morrison T.J., Doherty D.F., McAuley D.F., Matthay M.A., Kissenpfennig A., O’Kane C.M., and Krasnodembskaya A.D.. 2016a. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 34:2210–2223. 10.1002/stem.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M.V., Morrison T.J., McAuley D.F., Matthay M.A., O’Kane C.M., and Krasnodembskaya A.D.. 2016b. Mitochondrial transfer via tunnelling nanotubes (TNT) is a novel mechanism by which mesenchymal stromal cells enhance macrophage phagocytosis in in vivo models of acute lung injury. Am. J. Respir. Crit. Care Med. 193:A6607 [Google Scholar]
- Jackson, C.B., Turnbull D.M., Minczuk M., and Gammage P.A.. 2020. Therapeutic manipulation of mtDNA heteroplasmy: A shifting perspective. Trends Mol. Med. 26:698–709. 10.1016/j.molmed.2020.02.006 [DOI] [PubMed] [Google Scholar]
- Jacoby, E., Bar-Yosef O., Gruber N., Lahav E., Varda-Bloom N., Bolkier Y., Bar D., Blumkin M.B., Barak S., Eisenstein E., et al. 2022. Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci. Transl. Med. 14:eabo3724. 10.1126/scitranslmed.abo3724 [DOI] [PubMed] [Google Scholar]
- Jayaprakash, A.D., Benson E.K., Gone S., Liang R., Shim J., Lambertini L., Toloue M.M., Wigler M., Aaronson S.A., and Sachidanandam R.. 2015. Stable heteroplasmy at the single-cell level is facilitated by intercellular exchange of mtDNA. Nucleic Acids Res. 43:2177–2187. 10.1093/nar/gkv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, D., Gao F., Zhang Y., Wong D.S., Li Q., Tse H.F., Xu G., Yu Z., and Lian Q.. 2016. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 7:e2467. 10.1038/cddis.2016.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, D., Xiong G., Feng H., Zhang Z., Chen P., Yan B., Chen L., Gandhervin K., Ma C., Li C., et al. 2019. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 9:2395–2410. 10.7150/thno.29422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, H., Jiang D., Hu X., Du W., Ji L., Yang Y., Li X., Sho T., Wang X., Li Y., et al. 2021. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 184:2896–2910.e13. 10.1016/j.cell.2021.04.027 [DOI] [PubMed] [Google Scholar]
- Jordan, M.A., Diener D.R., Stepanek L., and Pigino G.. 2018. The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 20:1250–1255. 10.1038/s41556-018-0213-1 [DOI] [PubMed] [Google Scholar]
- Kadiu, I. and Gendelman H.E.. 2011a. Human immunodeficiency virus type 1 endocytic trafficking through macrophage bridging conduits facilitates spread of infection. J. Neuroimmune Pharmacol. 6:658–675. 10.1007/s11481-011-9298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu, I. and Gendelman H.E.. 2011b. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J. Proteome Res. 10:3225–3238. 10.1021/pr200262q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer, G., Courthéoux T., Peterka M., Meier S., Soste M., Melnik A., Reis K., Aspenström P., Peter M., Picotti P., and Kornmann B.. 2015. Mitotic redistribution of the mitochondrial network by Miro and Cenp-F. Nat. Commun. 6:8015. 10.1038/ncomms9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, A., Yao W., Ying H., Hua S., Liewen A., Wang Q., Zhong Y., Wu C.J., Sadanandam A., Hu B., et al. 2014. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 158:185–197. 10.1016/j.cell.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnkowska, A., Vacek V., Zubáčová Z., Treitli S.C., Petrželková R., Eme L., Novák L., Žárský V., Barlow L.D., Herman E.K., et al. 2016. A eukaryote without a mitochondrial organelle. Curr. Biol. 26:1274–1284. 10.1016/j.cub.2016.03.053 [DOI] [PubMed] [Google Scholar]
- Katrangi, E., D’Souza G., Boddapati S.V., Kulawiec M., Singh K.K., Bigger B., and Weissig V.. 2007. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. 10:561–570. 10.1089/rej.2007.0575 [DOI] [PubMed] [Google Scholar]
- Kaza, A.K., Wamala I., Friehs I., Kuebler J.D., Rathod R.H., Berra I., Ericsson M., Yao R., Thedsanamoorthy J.K., Zurakowski D., et al. 2017. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg. 153:934–943. 10.1016/j.jtcvs.2016.10.077 [DOI] [PubMed] [Google Scholar]
- Keeling, P.J., and Palmer J.D.. 2008. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9:605–618. 10.1038/nrg2386 [DOI] [PubMed] [Google Scholar]
- King, M.P., and Attardi G.. 1989. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 246:500–503. 10.1126/science.2814477 [DOI] [PubMed] [Google Scholar]
- Konari, N., Nagaishi K., Kikuchi S., and Fujimiya M.. 2019. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci. Rep. 9:5184. 10.1038/s41598-019-40163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol, W.H., Bounds P.L., and Dang C.V.. 2011. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 11:325–337. 10.1038/nrc3038 [DOI] [PubMed] [Google Scholar]
- Koundinya, M., Sudhalter J., Courjaud A., Lionne B., Touyer G., Bonnet L., Menguy I., Schreiber I., Perrault C., Vougier S., et al. 2018. Dependence on the pyrimidine biosynthetic enzyme DHODH is a synthetic lethal vulnerability in mutant KRAS-driven cancers. Cell Chem. Biol. 25:705–717.e11. 10.1016/j.chembiol.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Koyanagi, M., Brandes R.P., Haendeler J., Zeiher A.M., and Dimmeler S.. 2005. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: A novel mechanism for cell fate changes? Circ. Res. 96:1039–1041. 10.1161/01.RES.0000168650.23479.0c [DOI] [PubMed] [Google Scholar]
- Krishnan, K.J., Reeve A.K., Samuels D.C., Chinnery P.F., Blackwood J.K., Taylor R.W., Wanrooij S., Spelbrink J.N., Lightowlers R.N., and Turnbull D.M.. 2008. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 40:275–279. 10.1038/ng.f.94 [DOI] [PubMed] [Google Scholar]
- Kukat, C., and Larsson N.G.. 2013. mtDNA makes a U-turn for the mitochondrial nucleoid. Trends Cell Biol. 23:457–463. 10.1016/j.tcb.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Kukat, C., Davies K.M., Wurm C.A., Spåhr H., Bonekamp N.A., Kühl I., Joos F., Polosa P.L., Park C.B., Posse V., et al. 2015. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc. Natl. Acad. Sci. USA. 112:11288–11293. 10.1073/pnas.1512131112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., Kim J.H., Ranjan P., Metcalfe M.G., Cao W., Mishina M., Gangappa S., Guo Z., Boyden E.S., Zaki S., et al. 2017. Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Sci. Rep. 7:40360. 10.1038/srep40360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Karmacharya M., Michael I.J., Choi Y., Kim J., Kim I., and Cho Y.. 2021. Programmed exosome fusion for energy generation in living cells. Nat. Catal. 4:763–774. 10.1038/s41929-021-00669-z [DOI] [Google Scholar]
- Kwon, Y.M., Gori K., Park N., Potts N., Swift K., Wang J., Stammnitz M.R., Cannell N., Baez-Ortega A., Comte S., et al. 2020. Evolution and lineage dynamics of a transmissible cancer in Tasmanian devils. PLoS Biol. 18:e3000926. 10.1371/journal.pbio.3000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampinen, R., Belaya I., Saveleva L., Liddell J.R., Rait D., Huuskonen M.T., Giniatullina R., Sorvari A., Soppela L., Mikhailov N., et al. 2022. Neuron-astrocyte transmitophagy is altered in Alzheimer’s disease. Neurobiol. Dis. 170:105753. 10.1016/j.nbd.2022.105753 [DOI] [PubMed] [Google Scholar]
- Lapuente-Brun, E., Moreno-Loshuertos R., Acín-Pérez R., Latorre-Pellicer A., Colás C., Balsa E., Perales-Clemente E., Quirós P.M., Calvo E., Rodríguez-Hernández M.A., et al. 2013. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 340:1567–1570. 10.1126/science.1230381 [DOI] [PubMed] [Google Scholar]
- Latorre-Pellicer, A., Lechuga-Vieco A.V., Johnston I.G., Hämäläinen R.H., Pellico J., Justo-Méndez R., Fernández-Toro J.M., Clavería C., Guaras A., Sierra R., et al. 2019. Regulation of mother-to-offspring transmission of mtDNA heteroplasmy. Cell Metab. 30:1120–1130.e5. 10.1016/j.cmet.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu, V.S., O’Connell J.T., Gonzalez Herrera K.N., Wikman H., Pantel K., Haigis M.C., de Carvalho F.M., Damascena A., Domingos Chinen L.T., Rocha R.M., et al. 2014. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16:992–1003: 1–15. 10.1038/ncb3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W., Johnson J., Gough D.J., Donoghue J., Cagnone G.L., Vaghjiani V., Brown K.A., Johns T.G., and St John J.C.. 2015. Mitochondrial DNA copy number is regulated by DNA methylation and demethylation of POLGA in stem and cancer cells and their differentiated progeny. Cell Death Dis. 6:e1664. 10.1038/cddis.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, L., and Spradling A.C.. 2016. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 352:95–99. 10.1126/science.aad2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoux, J., Prola A., Lafuste P., Gervais M., Chevallier N., Koumaiha Z., Kefi K., Braud L., Schmitt A., Yacia A., et al. 2021. Platelets enhance pro-angiogenic activity of mesenchymal stem cells via mitochondrial transfer and metabolic remodeling. Cell Metab. 33:283–299. 10.1016/j.cmet.2020.12.006 [DOI] [PubMed] [Google Scholar]
- Li, X., Zhang Y., Yeung S.C., Liang Y., Liang X., Ding Y., Ip M.S.M., Tse H.F., Mak J.C.W., and Lian Q.. 2014. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 51:455–465. 10.1165/rcmb.2013-0529OC [DOI] [PubMed] [Google Scholar]
- Li, X., Michaeloudes C., Zhang Y., Wiegman C.H., Adcock I.M., Lian Q., Mak J.C.W., Bhavsar P.K., and Chung K.F.J.. 2018. Mesenchymal stem cells alleviate oxidative stress-induced mitochondrial dysfunction in the airways. J. Allergy Clin. Immunol. 141:1634–1645.e5. 10.1016/j.jaci.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Li, H., Wang C., He T., Zhao T., Chen Y.Y., Shen Y.L., Zhang X., and Wang L.L.. 2019. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 9:2017–2035. 10.7150/thno.29400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowlers, R.N., Taylor R.W., and Turnbull D.M.. 2015. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 349:1494–1499. 10.1126/science.aac7516 [DOI] [PubMed] [Google Scholar]
- Lin, H.C., Liu S.Y., Lai H.S., and Lai I.R.. 2013. Isolated mitochondria infusion mitigates ischemia-reperfusion injury of the liver in rats. Shock. 39:304–310. 10.1097/SHK.0b013e318283035f [DOI] [PubMed] [Google Scholar]
- Lippert, T., and Borlongan C.V.. 2019. Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neurosci. Ther. 25:815–823. 10.1111/cns.13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., and Butow R.A.. 2006. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40:159–185. 10.1146/annurev.genet.40.110405.090613 [DOI] [PubMed] [Google Scholar]
- Liu, K., Ji K., Guo L., Wu W., Lu H., Shan P., and Yan C.. 2014. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92:10–18. 10.1016/j.mvr.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Liu, K., Guo L., Zhou Z., Pan M., and Yan C.. 2019. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 123:74–80. 10.1016/j.mvr.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Liu, D., Gao Y., Liu J., Huang Y., Yin J., Feng Y., Shi L., Meloni B.P., Zhang C., Zheng M., and Gao J.. 2021. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 6:65. 10.1038/s41392-020-00440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojevic, N., Henderson J.M., and Zurzolo C.. 2021. The ways of actin: Why tunneling nanotubes are unique cell protrusions. Trends Cell Biol. 31:130–142. 10.1016/j.tcb.2020.11.008 [DOI] [PubMed] [Google Scholar]
- Lou, E., Fujisawa S., Morozov A., Barlas A., Romin Y., Dogan Y., Gholami S., Moreira A.L., Manova-Todorova K., and Moore M.A.. 2012. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 7:e33093. 10.1371/journal.pone.0033093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie, E.J., Larsen T.D., Wachal A.L., Gandy T.C.T., and Baack M.L.. 2021. Mitochondrial transfer improves cardiomyocyte bioenergetics and viability in male rats exposed to pregestational diabetes. Int. J. Mol. Sci. 22:2382. 10.3390/ijms22052382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J., Zheng X., Li F., Yu Y., Chen Z., Liu Z., Wang Z., Xu H., and Yang W.. 2017. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 8:15539–15552. 10.18632/oncotarget.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P., Liu R., Lu D., Xu Y., Yang X., Jiang Z., Yang C., Yu L., Lei X., and Chen Y.. 2020. Chemical screening identifies ROCK1 as a regulator of migrasome formation. Cell Discov. 6:51. 10.1038/s41421-020-0179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L., Li Y., Peng J., Wu D., Zhao X., Cui Y., Chen L., Yan X., Du Y., and Yu L.. 2015. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 25:24–38. 10.1038/cr.2014.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, S.L.N., Breakefield X.O., and Weaver A.M.. 2017. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol. 27:172–188. 10.1016/j.tcb.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill, A.F., and Kittler J.T.. 2010. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 20:102–112. 10.1016/j.tcb.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Magalhaes-Novais, S., Blecha J., Naraine R., Mikesova J., Abaffy P., Pecinova A., Milosevic M., Bohuslavova R., Prochazka J., Khan S., et al. 2022. Mitochondrial respiration supports autophagy to provide stress resistance during quiescence. Autophagy. 18:2409–2426. 10.1080/15548627.2022.2038898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrouf-Yorgov, M., Augeul L., Da Silva C.C., Jourdan M., Rigolet M., Manin S., Ferrera R., Ovize M., Henry A., Guguin A., et al. 2017. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 24:1224–1238. 10.1038/cdd.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlein, C.R., Zaitseva L., Piddock R.E., Robinson S.D., Edwards D.R., Shafat M.S., Zhou Z., Lawes M., Bowles K.M., and Rushworth S.A.. 2017. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood. 130:1649–1660. 10.1182/blood-2017-03-772939 [DOI] [PubMed] [Google Scholar]
- Marlein, C.R., Piddock R.E., Mistry J.J., Zaitseva L., Hellmich C., Horton R.H., Zhou Z., Auger M.J., Bowles K.M., and Rushworth S.A.. 2019. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 79:2285–2297. 10.1158/0008-5472.CAN-18-0773 [DOI] [PubMed] [Google Scholar]
- Marti Gutierrez, N., Mikhalchenko A., Ma H., Koski A., Li Y., Van Dyken C., Tippner-Hedges R., Yoon D., Liang D., Hayama T., et al. 2022. Horizontal mtDNA transfer between cells is common during mouse development. iScience. 25:103901. 10.1016/j.isci.2022.103901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes, I., Cardona L.R., Kong H., Vasan K., McElroy G.S., Werner M., Kihshen H., Reczek C.R., Weinberg S.E., Gao P., et al. 2020. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 585:288–292. 10.1038/s41586-020-2475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, T., Strickland S., Glenn M., Charpentier E., Guillemin H., Hsu K., and Mikhael J.. 2019. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 9:41. 10.1038/s41408-019-0198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa, A., Black K.M., Pacak C.A., Ericsson M., Barnett R.J., Drumm C., Seth P., Bloch D.B., Levitsky S., Cowan D.B., and McCully J.D.. 2013. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 304:H966–H982. 10.1152/ajpheart.00883.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matula, Z., Mikala G., Lukácsi S., Matkó J., Kovács T., Monostori É., Uher F., and Vályi-Nagy I.. 2021. Stromal cells serve drug resistance for multiple myeloma via mitochondrial transfer: A study on primary myeloma and stromal cells. Cancers. 13:3461. 10.3390/cancers13143461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully, J.D., Cowan D.B., Pacak C.A., Toumpoulis I.K., Dayalan H., and Levitsky S.. 2009. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 296:H94–H105. 10.1152/ajpheart.00567.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully, J.D., Cowan D.B., Emani S.M., and Del Nido P.J.. 2017. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion. 34:127–134. 10.1016/j.mito.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Melentijevic, I., Toth M.L., Arnold M.L., Guasp R.J., Harinath G., Nguyen K.C., Taub D., Parker J.A., Neri C., Gabel C.V., et al. 2017. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature. 542:367–371. 10.1038/nature21362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld, T., and Schwarz T.L.. 2017. Mitostasis in neurons: Maintaining mitochondria in an extended cellular architecture. Neuron. 96:651–666. 10.1016/j.neuron.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry, J.J., Marlein C.R., Moore J.A., Hellmich C., Wojtowicz E.E., Smith J.G.W., Macaulay I., Sun Y., Morfakis A., Patterson A., et al. 2019. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc. Natl. Acad. Sci. USA. 116:24610–24619. 10.1073/pnas.1913278116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn, M., and Sánchez-Madrid F.. 2012. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 13:328–335. 10.1038/nrm3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadalipour, A., Dumbali S.P., and Wenzel P.L.. 2020. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front. Cell Dev. Biol. 8:603292. 10.3389/fcell.2020.603292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzio Compagnoni, G., Di Fonzo A., Corti S., Comi G.P., Bresolin N., and Masliah E.. 2020. The role of mitochondria in neurodegenerative diseases: The lesson from Alzheimer’s disease and Parkinson’s disease. Mol. Neurobiol. 57:2959–2980. 10.1007/s12035-020-01926-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, L., Perez C., Zabaleta A., Manrique I., Alignani D., Ajona D., Blanco L., Lasa M., Maiso P., Rodriguez I., et al. 2019. The mechanism of action of the anti-CD38 monoclonal antibody isatuximab in multiple myeloma. Clin. Cancer Res. 25:3176–3187. 10.1158/1078-0432.CCR-18-1597 [DOI] [PubMed] [Google Scholar]
- Moreno-Lastres, D., Fontanesi F., García-Consuegra I., Martín M.A., Arenas J., Barrientos A., and Ugalde C.. 2012. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 15:324–335. 10.1016/j.cmet.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Loshuertos, R., Acín-Pérez R., Fernández-Silva P., Movilla N., Pérez-Martos A., Rodriguez de Cordoba S., Gallardo M.E., and Enríquez J.A.. 2006. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 38:1261–1268. 10.1038/ng1897 [DOI] [PubMed] [Google Scholar]
- Morrison, T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O’Kane C.M., and Krasnodembskaya A.D.. 2017. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196:1275–1286. 10.1164/rccm.201701-0170OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschoi, R., Imbert V., Nebout M., Chiche J., Mary D., Prebet T., Saland E., Castellano R., Pouyet L., Collette Y., et al. 2016. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 128:253–264. 10.1182/blood-2015-07-655860 [DOI] [PubMed] [Google Scholar]
- Moskowitzova, K., Shin B., Liu K., Ramirez-Barbieri G., Guariento A., Blitzer D., Thedsanamoorthy J.K., Yao R., Snay E.R., Inkster J.A.H., et al. 2019. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J. Heart Lung Transpl. 38:92–99. 10.1016/j.healun.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitzova, K., Orfany A., Liu K., Ramirez-Barbieri G., Thedsanamoorthy J.K., Yao R., Guariento A., Doulamis I.P., Blitzer D., Shin B., et al. 2020. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 318:L78–L88. 10.1152/ajplung.00221.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison, E.P., Tovar C., Hsu A., Bender H.S., Kheradpour P., Rebbeck C.A., Obendorf D., Conlan C., Bahlo M., Blizzard C.A., et al. 2010. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science. 327:84–87. 10.1126/science.1180616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison, E.P., Schulz-Trieglaff O.B., Ning Z., Alexandrov L.B., Bauer M.J., Fu B., Hims M., Ding Z., Ivakhno S., Stewart C., et al. 2012. Genome sequencing and analysis of the tasmanian devil and its transmissible cancer. Cell. 148:780–791. 10.1016/j.cell.2011.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison, E.P., Wedge D.C., Alexandrov L.B., Fu B., Martincorena I., Ning Z., Tubio J.M.C., Werner E.I., Allen J., De Nardi A.B., et al. 2014. Transmissible [corrected] dog cancer genome reveals the origin and history of an ancient cell lineage. Science. 343:437–440. 10.1126/science.1247167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, M.P., and Chouchani E.T.. 2022. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 18:461–469. 10.1038/s41589-022-01004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahacka, Z., Zobalova R., Dubisova M., Rohlena J., and Neuzil J.. 2021. Miro proteins connect mitochondrial transfer and function. Crit. Rev. Mol. Cell Biol. 56:401–425. 10.1080/10409238.2021.1925216 [DOI] [PubMed] [Google Scholar]
- Nascimento-Dos-Santos, G., de-Souza-Ferreira E., Linden R., Galina A., and Petrs-Silva H.. 2021. Mitotherapy: Unraveling a promising treatment for disorders of the central nervous system and other systemic conditions. Cells. 10:1827. 10.3390/cells10071827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert, W., and Herrmann J.M.. 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76:723–749. 10.1146/annurev.biochem.76.052705.163409 [DOI] [PubMed] [Google Scholar]
- Ng, Y.S., Bindoff L.A., Gorman G.S., Klopstock T., Kornblum C., Mancuso M., McFarland R., Sue C.M., Suomalainen A., Taylor R.W., et al. 2021. Mitochondrial disease in adults: Recent advances and future promise. Lancet Neurol. 20:573–584. 10.1016/S1474-4422(21)00098-3 [DOI] [PubMed] [Google Scholar]
- Nguyen, T.T., Oh S.S., Weaver D., Lewandowska A., Maxfield D., Schuler M.H., Smith N.K., Macfarlane J., Saunders G., Palmer C.A., et al. 2014. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc. Natl. Acad. Sci. USA. 111:E3631–E3640. 10.1073/pnas.1402449111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás-Ávila, J.A., Lechuga-Vieco A.V., Esteban-Martínez L., Sánchez-Díaz M., Díaz-García E., Santiago D.J., Rubio-Ponce A., Li J.L., Balachander A., Quintana J.A., et al. 2020. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 183:94–109.e23. 10.1016/j.cell.2020.08.031 [DOI] [PubMed] [Google Scholar]
- Nitzan, K., Benhamron S., Valitsky M., Kesner E.E., Lichtenstein M., Ben-Zvi A., Ella E., Segalstein Y., Saada A., Lorberboum-Galski H., and Rosenmann H.. 2019. Mitochondrial transfer ameliorates cognitive deficits, neuronal loss, and gliosis in Alzheimer’s disease mice. J. Alzheimers Dis. 72:587–604. 10.3233/JAD-190853 [DOI] [PubMed] [Google Scholar]
- Norris, R.P. 2021. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic. 22:174–179. 10.1111/tra.12786 [DOI] [PubMed] [Google Scholar]
- Noubissi, F.K., Harkness T., Alexander C.M., and Ogle B.M.. 2015. Apoptosis-induced cancer cell fusion: A mechanism of breast cancer metastasis. FASEB J. 29:4036–4045. 10.1096/fj.15-271098 [DOI] [PubMed] [Google Scholar]
- Nygren, J.M., Liuba K., Breitbach M., Stott S., Thorén L., Roell W., Geisen C., Sasse P., Kirik D., Björklund A., et al. 2008. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat. Cell Biol. 10:584–592. 10.1038/ncb1721 [DOI] [PubMed] [Google Scholar]
- Ohta, S. 2003. A multi-functional organelle mitochondrion is involved in cell death, proliferation and disease. Curr. Med. Chem. 10:2485–2494. 10.2174/0929867033456440 [DOI] [PubMed] [Google Scholar]
- Okafo, G., Prevedel L., and Eugenin E.. 2017. Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread. Sci. Rep. 7:16660. 10.1038/s41598-017-16600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt, B., Nedvetzki S., Benninger R.K., Purbhoo M.A., Sowinski S., Hume A.N., Seabra M.C., Neil M.A., French P.M., and Davis D.M.. 2006. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177:8476–8483. 10.4049/jimmunol.177.12.8476 [DOI] [PubMed] [Google Scholar]
- Osswald, M., Jung E., Sahm F., Solecki G., Venkataramani V., Blaes J., Weil S., Horstmann H., Wiestler B., Syed M., et al. 2015. Brain tumour cells interconnect to a functional and resistant network. Nature. 528:93–98. 10.1038/nature16071 [DOI] [PubMed] [Google Scholar]
- Otsu, K., Das S., Houser S.D., Quadri S.K., Bhattacharya S., and Bhattacharya J.. 2009. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 113:4197–4205. 10.1182/blood-2008-09-176198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal, S., Chaudhuri R., Agrawal A., and Mohanty S.. 2018. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 25:31. 10.1186/s12929-018-0429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasiuk, M., Rychłowski M., Derewońko N., and Bieńkowska-Szewczyk K.. 2018. Tunneling nanotubes as a novel route of cell-to-cell spread of herpesviruses. J. Virol. 92:92. 10.1128/JVI.00090-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier, J., Guerrouahen B.S., Al Thawadi H., Ghiabi P., Maleki M., Abu-Kaoud N., Jacob A., Mirshahi M., Galas L., Rafii S., et al. 2013. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 11:94. 10.1186/1479-5876-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patananan, A.N., Wu T.-H., Chiou P.-Y., and Teitell M.A.. 2016. Modifying the mitochondrial genome. Cell Metab. 23:785–796. 10.1016/j.cmet.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros, J.A., Baumann A.K., Ruiz-Pesini E., Amin M.B., Sun C.Q., Hall J., Lim S., Issa M.M., Flanders W.D., Hosseini S.H., et al. 2005. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA. 102:719–724. 10.1073/pnas.0408894102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney, D.G., Di Giuseppe M., Njah J., Sala E., Shiva S., St Croix C.M., Stolz D.B., Watkins S.C., Di Y.P., Leikauf G.D., et al. 2015. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 6:8472. 10.1038/ncomms9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, M., Zhang J., Hancock S., Derbeneva O., Golhar R., Golik P., O’Hearn S., Levy S., Potluri P., Lvova M., et al. 2014. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. USA. 111:E4033–E4042. 10.1073/pnas.1414028111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, M., McManus M.J., Gray J.D., Nasca C., Moffat C., Kopinski P.K., Seifert E.L., McEwen B.S., and Wallace D.C.. 2015. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl. Acad. Sci. USA. 112:E6614–E6623. 10.1073/pnas.1515733112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, M., Wallace D.C., and Burelle Y.. 2016. The rise of mitochondria in medicine. Mitochondrion. 30:105–116. 10.1016/j.mito.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino, G. 2021. Intraflagellar transport. Curr. Biol. 31:R530–R536. 10.1016/j.cub.2021.03.081 [DOI] [PubMed] [Google Scholar]
- Pinto, G., Saenz-de-Santa-Maria I., Chastagner P., Perthame E., Delmas C., Toulas C., Moyal-Jonathan-Cohen E., Brou C., and Zurzolo C.. 2021. Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids. Biochem. J. 478:21–39. 10.1042/BCJ20200710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov, E.Y., Khryapenkova T.G., Vasileva A.K., Marey M.V., Galkina S.I., Isaev N.K., Sheval E.V., Polyakov V.Y., Sukhikh G.T., and Zorov D.B.. 2008. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J. Cell. Mol. Med. 12:1622–1631. 10.1111/j.1582-4934.2007.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov, E.Y., Khryapenkova T.G., Galkina S.I., Sukhikh G.T., and Zorov D.B.. 2010. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp. Cell Res. 316:2447–2455. 10.1016/j.yexcr.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Qin, Y., Jiang X., Yang Q., Zhao J., Zhou Q., and Zhou Y.. 2021. The functions, methods, and mobility of mitochondrial transfer between cells. Front. Oncol. 11:672781. 10.3389/fonc.2021.672781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabas, N., Palmer S., Mitchell L., Ismail S., Gohlke A., Riley J.S., Tait S.W.G., Gammage P., Soares L.L., Macpherson I.R., and Norman J.C.. 2021. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 220:e202006049. 10.1083/jcb.202006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackham, C.L., Hubber E.L., Czajka A., Malik A.N., King A.J.F., and Jones P.M.. 2020. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells. 38:574–584. 10.1002/stem.3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck, C.A., Thomas R., Breen M., Leroi A.M., and Burt A.. 2009. Origins and evolution of a transmissible cancer. Evolution. 63:2340–2349. 10.1111/j.1558-5646.2009.00724. [DOI] [PubMed] [Google Scholar]
- Rebbeck, C.A., Leroi A.M., and Burt A.. 2011. Mitochondrial capture by a transmissible cancer. Science. 331:303. 10.1126/science.1197696 [DOI] [PubMed] [Google Scholar]
- Reichert, D., Scheinpflug J., Karbanová J., Freund D., Bornhäuser M., and Corbeil D.. 2016. Tunneling nanotubes mediate the transfer of stem cell marker CD133 between hematopoietic progenitor cells. Exp. Hematol. 44:1092–1112.e2. 10.1016/j.exphem.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Robicsek, O., Ene H.M., Karry R., Ytzhaki O., Asor E., McPhie D., Cohen B.M., Ben-Yehuda R., Weiner I., and Ben-Shachar D.. 2018. McPHIE, D., Cohen, B.M., Ben-Yehuda, R., Weiner, I., & Ben-Shachar, D. Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues deficits in a rat model of the disorder. Schizophr. Bull. 44:432–442. 10.1093/schbul/sbx077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Sinovas, A., Sánchez J.A., Valls-Lacalle L., Consegal M., and Ferreira-González I.. 2021. Connexins in the heart: Regulation, function and involvement in cardiac disease. Int. J. Mol. Sci. 22:4413. 10.3390/ijms22094413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehlecke, C., and Schmidt M.H.H.. 2020. Tunneling nanotubes and tumor microtubes in cancer. Cancers. 12:857. 10.3390/cancers12040857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, R.S., and Bhattacharya J.. 2013. When cells become organelle donors. Physiology. 28:414–422. 10.1152/physiol.00032.2013 [DOI] [PubMed] [Google Scholar]
- Rosina, M., Ceci V., Turchi R., Chuan L., Borcherding N., Sciarretta F., Sánchez-Díaz M., Tortolici F., Karlinsey K., Chiurchiù V., et al. 2022. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 34:533–548.e12. 10.1016/j.cmet.2022.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami, J., Holmqvist S., Lindström V., Sigvardson J., Westermark G.T., Ingelsson M., Bergström J., Roybon L., and Erlandsson A.. 2017. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J. Neurosci 37:11835–11853. 10.1523/JNEUROSCI.0983-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom, A. 2016. The missing link: Does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 6:160057. 10.1098/rsob.160057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom, A., Saffrich R., Markovic I., Walther P., and Gerdes H.H.. 2004. Nanotubular highways for intercellular organelle transport. Science. 303:1007–1010. 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- Ryan, M.T., and Hoogenraad N.J.. 2007. Mitochondrial-nuclear communications. Annu. Rev. Biochem. 76:701–722. 10.1146/annurev.biochem.76.052305.091720 [DOI] [PubMed] [Google Scholar]
- Saha, T., Dash C., Jayabalan R., Khiste S., Kulkarni A., Kurmi K., Mondal J., Majumder P.K., Bardia A., Jang H.L., and Sengupta S.. 2022. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 17:98–106. 10.1038/s41565-021-01000-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Pogoda, A., Strecker J.K., Liebmann M., Massoth C., Beuker C., Hansen U., König S., Albrecht S., Bock S., Breuer J., et al. 2018. Dietary salt promotes ischemic brain injury and is associated with parenchymal migrasome formation. PLoS One. 13:e0209871. 10.1371/journal.pone.0209871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon, E.A., DiMauro S., and Hirano M.. 2012. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 13:878–890. 10.1038/nrg3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, R., Görge P.M., Görbe A., Ferdinandy P., Lampe P.D., and Leybaert L.. 2015. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 153:90–106. 10.1016/j.pharmthera.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, B.J., Yoon S.H., and Do J.T.. 2018. Mitochondrial dynamics in stem cells and differentiation. Int. J. Mol. Sci. 19:3893. 10.3390/ijms19123893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercel, A.J., Patananan A.N., Man T., Wu T.H., Yu A.K., Guyot G.W., Rabizadeh S., Niazi K.R., Chiou P.Y., and Teitell M.A.. 2021. Stable transplantation of human mitochondrial DNA by high-throughput, pressurized isolated mitochondrial delivery. Elife. 10:e63102. 10.7554/eLife.63102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor, A., Wang B., Fan L., Kong L., Gao W., Sun J., Man K., Li G., and Sun D.. 2021. Automated optical tweezers manipulation to transfer mitochondria from fetal to adult MSCs to improve anti-aging gene expressions. Small. 17:e2103086. 10.1002/smll.202103086 [DOI] [PubMed] [Google Scholar]
- Sharma, P., and Sampath H.. 2019. Mitochondrial DNA integrity: Role in health and disease. Cells. 8:100. 10.3390/cells8020100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, N., Pasala M.S., and Prakash A.. 2019. Mitochondrial DNA: Epigenetics and environment. Environ. Mol. Mutagen. 60:668–682. 10.1002/em.22319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She, Z., Xie M., Hun M., Abdirahman A.S., Li C., Wu F., Luo S., Wan W., Wen C., and Tian J.. 2021. Immunoregulatory effects of mitochondria transferred by extracellular vesicles. Front. Immunol. 11:628576. 10.3389/fimmu.2020.628576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J., Zhang J.H., Xiao H., Wu J.M., He K.M., Lv Z.Z., Li Z.J., Xu M., and Zhang Y.Y.. 2018. Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis. 9:81. 10.1038/s41419-017-0145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X., Bai H., Zhao M., Li X., Sun X., Jiang H., and Fu A.. 2018. Treatment of acetaminophen-induced liver injury with exogenous mitochondria in mice. Transl. Res. 196:31–41. 10.1016/j.trsl.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Sinha, P., Islam M.N., Bhattacharya S., and Bhattacharya J.. 2016. Intercellular mitochondrial transfer: Bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev. 38:97–101. 10.1016/j.gde.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I.F., Shuai J., and Parker I.. 2011. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys. J 100:L37–L39. 10.1016/j.bpj.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski, S., Jolly C., Berninghausen O., Purbhoo M.A., Chauveau A., Köhler K., Oddos S., Eissmann P., Brodsky F.M., Hopkins C., et al. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10:211–219. 10.1038/ncb168 [DOI] [PubMed] [Google Scholar]
- Spees, J.L., Olson S.D., Whitney M.J., and Prockop D.J.. 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA. 103:1283–1288. 10.1073/pnas.0510511103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, J.B., and Haigis M.C.. 2018. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20:745–754. 10.1038/s41556-018-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanek, L., and Pigino G.. 2016. Microtubule doublets are double-track railways for intraflagellar transport trains. Science. 352:721–724. 10.1126/science.aaf4594 [DOI] [PubMed] [Google Scholar]
- Stewart, J.B., and Chinnery P.F.. 2021. Extreme heterogeneity of human mitochondrial DNA from organelles to populations. Nat. Rev. Genet. 22:106–118. 10.1038/s41576-020-00284-x [DOI] [PubMed] [Google Scholar]
- Stoorvogel, W., Kleijmeer M.J., Geuze H.J., and Raposo G.. 2002. The biogenesis and functions of exosomes. Traffic. 3:321–330. 10.1034/j.1600-0854.2002.30502.x [DOI] [PubMed] [Google Scholar]
- Strakova, A., and Murchison E.P.. 2015. The cancer which survived: Insights from the genome of an 11000 year-old cancer. Curr. Opin. Genet. Dev. 30:49–55. 10.1016/j.gde.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Strakova, A., Ní Leathlobhair M., Wang G.D., Yin T.T., Airikkala-Otter I., Allen J.L., Allum K.M., Bansse-Issa L., Bisson J.L., Castillo Domracheva A., et al. 2016. Mitochondrial genetic diversity, selection and recombination in a canine transmissible cancer. Elife. 5:e14552. 10.7554/eLife.14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova, A., Nicholls T.J., Baez-Ortega A., Ní Leathlobhair M., Sampson A.T., Hughes K., Bolton I.A.G., Gori K., Wang J., Airikkala-Otter I., et al. 2020. Recurrent horizontal transfer identifies mitochondrial positive selection in a transmissible cancer. Nat. Commun. 11:3059. 10.1038/s41467-020-16765-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, L.B., Gui D.Y., and Vander Heiden M.G.. 2016. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer. 16:680–693. 10.1038/nrc.2016.85 [DOI] [PubMed] [Google Scholar]
- Sun, T., Qiao H., Pan P.Y., Chen Y., and Sheng Z.H.. 2013. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4:413–419. 10.1016/j.celrep.2013.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Johnson J., and St John J.C.. 2018. Global DNA methylation synergistically regulates the nuclear and mitochondrial genomes in glioblastoma cells. Nucleic Acids Res. 46:5977–5995. 10.1093/nar/gky339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C., Liu X., Wang B., Wang Z., Liu Y., Di C., Si J., Li H., Wu Q., Xu D., et al. 2019. Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics. 9:3595–3607. 10.7150/thno.33100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen, A. 2019. Mitochondrial DNA inheritance in humans: Mix, match, and survival of the fittest. Cell Metab. 30:231–232. 10.1016/j.cmet.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Sykes, D.B., Kfoury Y.S., Mercier F.E., Wawer M.J., Law J.M., Haynes M.K., Lewis T.A., Schajnovitz A., Jain E., Lee D., et al. 2016. Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell. 167:171–186.e15. 10.1016/j.cell.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaga, K., Koshikawa N., and Nagase H.. 2021. Intercellular transfer of mitochondrial DNA carrying metastasis-enhancing pathogenic mutations from high- to low-metastatic tumor cells and stromal cells via extracellular vesicles. BMC Mol. Cell Biol. 22:52. 10.1186/s12860-021-00391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, A.S., Baty J.W., Dong L.F., Bezawork-Geleta A., Endaya B., Goodwin J., Bajzikova M., Kovarova J., Peterka M., Yan B., et al. 2015. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 21:81–94. 10.1016/j.cmet.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Tavi, P., Korhonen T., Hänninen S.L., Bruton J.D., Lööf S., Simon A., and Westerblad H.. 2010. Myogenic skeletal muscle satellite cells communicate by tunnelling nanotubes. J. Cell. Physiol. 223:376–383. 10.1002/jcp.22044 [DOI] [PubMed] [Google Scholar]
- Terada, N., Hamazaki T., Oka M., Hoki M., Mastalerz D.M., Nakano Y., Meyer E.M., Morel L., Petersen B.E., and Scott E.W.. 2002. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 416:542–545. 10.1038/nature730 [DOI] [PubMed] [Google Scholar]
- Thayanithy, V., Babatunde V., Dickson E.L., Wong P., Oh S., Ke X., Barlas A., Fujisawa S., Romin Y., Moreira A.L., et al. 2014. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 323:178–188. 10.1016/j.yexcr.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, V., Koganti R., Russell G., Sharma A., and Shukla D.. 2021. Role of tunneling nanotubes in viral infection, neurodegenerative disease, and cancer. Front. Immunol. 12:680891. 10.3389/fimmu.2021.680891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todkar, K., Chikhi L., Desjardins V., El-Mortada F., Pépin G., and Germain M.. 2021. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 12:1971. 10.1038/s41467-021-21984-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti, M., Lee W., Santarelli L., and Neuzil J.. 2017. Exosome-derived microRNAs in cancer metabolism: Possible implications in cancer diagnostics and therapy. Exp. Mol. Med. 49:e285. 10.1038/emm.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralba, D., Baixauli F., and Sánchez-Madrid F.. 2016. Mitochondria know no boundaries: Mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol 4:107. 10.3389/fcell.2016.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, N., Lambie S.C., Huynh C.Q., Sanford B., Patel M., Herson P.S., and Ormond D.R.. 2021. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J. Cereb. Blood Flow Metab. 41:761–770. 10.1177/0271678X20928147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito, S., Malik S., Luu R., Loudig O., Mitchell M., Okafo G., Bhat K., Prideaux B., and Eugenin E.A.. 2021. Tunneling nanotubes, TNT, communicate glioblastoma with surrounding non-tumor astrocytes to adapt them to hypoxic and metabolic tumor conditions. Sci. Rep. 11:14556. 10.1038/s41598-021-93775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti, D., Vacca R.A., Moro L., and Atlante A.. 2021. Mitochondria can cross cell boundaries: An overview of the biological relevance, pathophysiological implications and therapeutic perspectives of intercellular mitochondrial transfer. Int. J. Mol. Sci. 22:8312. 10.3390/ijms22158312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhaneni, K.C., Haller H., and Dumler I.. 2012. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 21:3104–3113. 10.1089/scd.2011.0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solinge, T.S., Nieland L., Chiocca E.A., and Broekman M.L.D.. 2022. Advances in local therapy for glioblastoma - taking the fight to the tumour. Nat. Rev. Neurol. 18:221–236. 10.1038/s41582-022-00621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden, M.G., Cantley L.C., and Thompson C.B.. 2009. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 324:1029–1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranic, P., Lokar M., Schütz G.J., Weghuber J., Wieser S., Hägerstrand H., Kralj-Iglic V., and Iglic A.. 2008. Different types of cell-to-cell connections mediated by nanotubular structures. Biophys. J. 95:4416–4425. 10.1529/biophysj.108.131375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale, A., Pettazzoni P., Lyssiotis C.A., Ying H., Sánchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V., et al. 2014. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 514:628–632. 10.1038/nature13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais, M.L., Caicedo A., Brondello J.M., and Jorgensen C.. 2017. Cell connections by tunneling nanotubes: Effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017:6917941. 10.1155/2017/6917941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader, J.E., and Lindeman G.J.. 2012. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell. 10:717–728. 10.1016/j.stem.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Wallace, D.C. 2012. Mitochondria and cancer. Nat. Rev. Cancer. 12:685–698. 10.1038/nrc3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., and Gerdes H.H.. 2015. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22:1181–1191. 10.1038/cdd.2014.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Veruki M.L., Bukoreshtliev N.V., Hartveit E., and Gerdes H.H.. 2010. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA. 107:17194–17199. 10.1073/pnas.1006785107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Cui J., Sun X., and Zhang Y.. 2011. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18:732–742. 10.1038/cdd.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Liu X., Qiu Y., Shi Y., Cai J., Wang B., Wei X., Ke Q., Sui X., Wang Y., et al. 2018. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 11:11. 10.1186/s13045-018-0554-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Ni J., Gao C., Xie L., Zhai L., Cui G., and Yin X.. 2019. Mitochondrial transplantation attenuates lipopolysaccharide- induced depression-like behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 93:240–249. 10.1016/j.pnpbp.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Watkins, S.C., and Salter R.D.. 2005. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 23:309–318. 10.1016/j.immuni.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Wei, W., Tuna S., Keogh M.J., Smith K.R., Aitman T.J., Beales P.L., Bennett D.L., Gale D.P., Bitner-Glindzicz M.A.K., Black G.C., et al. 2019. Germline selection shapes human mitochondrial DNA diversity. Science. 364:eaau6520. 10.1126/science.aau6520 [DOI] [PubMed] [Google Scholar]
- Weimann, J.M., Johansson C.B., Trejo A., and Blau H.M.. 2003. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5:959–966. 10.1038/ncb1053 [DOI] [PubMed] [Google Scholar]
- White, I.J., Bailey L.M., Aghakhani M.R., Moss S.E., and Futter C.E.. 2006. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 25:1–12. 10.1038/sj.emboj.7600759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig, D., Wang X., Walter C., Gerdes H.H., Funk R.H.W., and Roehlecke C.. 2012. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS One. 7:e33195. 10.1371/journal.pone.0033195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Lu Z., Jiang D., Guo Y., Qiao H., Zhang Y., Zhu T., Cai Y., Zhang X., Zhanghao K., et al. 2021a. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell. 184:3318–3332.e17. 10.1016/j.cell.2021.04.029 [DOI] [PubMed] [Google Scholar]
- Wu, Y., Han X., Su Y., Glidewell M., Daniels J.S., Liu J., Sengupta T., Rey-Suarez I., Fischer R., Patel A., et al. 2021b. Multiview confocal super-resolution microscopy. Nature. 600:279–284. 10.1038/s41586-021-04110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó, M., Siljander P.R.-M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4:27066. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y., Fan X.L., Jiang D., Zhang Y., Li X., Xu Z.B., Fang S.B., Chiu S., Tse H.F., Lian Q., and Fu Q.L.. 2018. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Rep. 11:1120–1135. 10.1016/j.stemcr.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, K., Park H.C., Ratliff B., Addabbo F., Hatzopoulos A.K., Chander P., and Goligorsky M.S.. 2010. Adriamycin nephropathy: A failure of endothelial progenitor cell-induced repair. Am. J. Pathol. 176:1685–1695. 10.2353/ajpath.2010.091071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, Y.C., Chen M.J., Ho J.Y., Guu H.F., and Ho E.S.C.. 2007. Mitochondria transfer can enhance the murine embryo development. J. Assist. Reprod. Genet. 24:445–449. 10.1007/s10815-007-9161-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S., and Yu L.. 2022. Migrasome biogenesis and functions. FEBS J. 289:7246–7254. 10.1111/febs.16183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowski, M.P., Balaj L., Breakefield X.O., and Lai C.P.-K.. 2015. (Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience. 65:783–797. 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri, L.X., Silva-Almeida C., Rondeau J.D., and Sonveaux P.. 2021. Mitochondrial transfer in cancer: A comprehensive review. Int. J. Mol. Sci. 22:3245. 10.3390/ijms22063245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., and Hauser C.J.. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 464:104–107. 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Yu Z., Jiang D., Liang X., Liao S., Zhang Z., Yue W., Li X., Chiu S.M., Chai Y.H., et al. 2016. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep. 7:749–763. 10.1016/j.stemcr.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Ma Z., Yan C., Pu K., Wu M., Bai J., Li Y., and Wang Q.. 2019. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav. Brain Res. 356:322–331. 10.1016/j.bbr.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Zhao, Z., Yu Z., Hou Y., Zhang L., and Fu A.. 2020. Improvement of cognitive and motor performance with mitotherapy in aged mice. Int. J. Biol. Sci. 16:849–858. 10.7150/ijbs.40886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y.R., Zhang X.N., and Chen Z.. 2019. Mitochondrial transport serves as a mitochondrial quality control strategy in axons: Implications for central nervous system disorders. CNS Neurosci. Ther. 25:876–886. 10.1111/cns.13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S., Victoria G.S., Marzo L., Ghosh R., and Zurzolo C.. 2015. Prion aggregates transfer through tunneling nanotubes in endocytic vesicles. Prion. 9:125–135. 10.1080/19336896.2015.1025189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, X., Kwon S.H., Jiang K., Ferguson C.M., Puranik A.S., Zhu X., and Lerman L.O.. 2018. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci. Rep. 8:1263. 10.1038/s41598-018-19750-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo, C. 2021. Tunneling nanotubes: Reshaping connectivity. Curr. Opin. Cell Biol. 71:139–147. 10.1016/j.ceb.2021.03.003 [DOI] [PubMed] [Google Scholar]