Abstract
Actin is involved in the organization of the Golgi complex and Golgi-to-ER protein transport in mammalian cells. Little, however, is known about the regulation of the Golgi-associated actin cytoskeleton. We provide evidence that Cdc42, a small GTPase that regulates actin dynamics, controls Golgi-to-ER protein transport. We located GFP-Cdc42 in the lateral portions of Golgi cisternae and in COPI-coated and noncoated Golgi-associated transport intermediates. Overexpression of Cdc42 and its activated form Cdc42V12 inhibited the retrograde transport of Shiga toxin from the Golgi complex to the ER, the redistribution of the KDEL receptor, and the ER accumulation of Golgi-resident proteins induced by the active GTP-bound mutant of Sar1 (Sar1[H79G]). Coexpression of wild-type or activated Cdc42 and N-WASP also inhibited Golgi-to-ER transport, but this was not the case in cells expressing Cdc42V12 and N-WASP(ΔWA), a mutant form of N-WASP that lacks Arp2/3 binding. Furthermore, Cdc42V12 recruited GFP-N-WASP to the Golgi complex. We therefore conclude that Cdc42 regulates Golgi-to-ER protein transport in an N-WASP–dependent manner.
INTRODUCTION
The involvement of microtubules in intracellular membrane trafficking is well established (Thyberg and Moskalewski, 1999 for review), and the role of actin in membrane traffic is under extensive investigation (DePina and Langford, 1999; Qualmann et al., 2000; Apodaca, 2001 for reviews). In the secretory pathway, actin filaments are required to maintain the organization of the Golgi complex (Valderrama et al., 1998; di Campli et al., 1999) and for protein transport from the Golgi to the plasma membrane and the ER (Müsch et al., 2001; Valderrama et al., 2001). In addition, actin-binding protein spectrin/ankyrin isoforms and several myosins have been localized to the Golgi complex and implicated in transport functions (Beck et al., 1994; Müsch et al., 1997; Buss et al., 1998; Godi et al., 1998; Stow et al., 1998; Heimann et al., 1999). There is also increasing evidence implicating the Rho family of small GTPases in membrane trafficking (Ridley, 2001 for review), particularly Rho and Rac in endocytic processes (Lamaze et al., 1996; Chimini and Chavrier, 2000; Garrett et al., 2000; Jou et al., 2000; West et al., 2000; Ellis and Mellor, 2000 for review). With respect to the secretory pathway, our findings suggest that Rho does not regulate actin–Golgi interactions (Valderrama et al., 2000). However, Cdc42 is reported to be associated with Golgi membranes, and it binds to the γ component of the coatomer (Erickson et al., 1996; Wu et al., 2000). Furthermore, Cdc42 is involved in cell polarity, regulating the generation of basolateral transport vesicles from the trans-Golgi network (TGN; Kroschewski et al., 1999; Cohen et al., 2001; Müsch et al., 2001; Rojas et al., 2001). Hence, Cdc42 seems to be involved in ER-to-Golgi and post-Golgi protein transport.
Because actin is implicated in the Golgi-to-ER protein transport (Valderrama et al., 2001) and Cdc42 is located in the Golgi complex, we examined the possible regulatory role of Cdc42 in the ER/Golgi membrane dynamics in nonpolarized mammalian cells. We show that Cdc42 controls the Golgi-to-ER protein transport via N-WASP, a homologue of the Wiskott-Aldrich syndrome protein (WASP). These results are consistent with our proposal that Cdc42 exerts its effects on retrograde transport machinery by directly modulating actin dynamics at the ER/Golgi interface, probably through the Arp2/3 complex.
MATERIALS AND METHODS
Material and Expression Constructs
The GFP expression vectors for the wild-type N-WASP and N-WASP(ΔWA) forms were previously reported (Moreau et al., 2000), and those for the wild-type and the dominant-positive and dominant-negative Cdc42 forms (del Pozo et al., 1999) were kindly provided by Francisco Sánchez-Madrid (Hospital La Princesa, Madrid). Cy3-tagged native Shiga toxin fragment B was a gift from Ludger Johannes and Bruno Goud (Institute Curie, Paris). The dominant-negative Sar1 mutant (Sar1[H79G], Sar1dn) expression vector (Kuge et al., 1994) was supplied by Rainer Pepperkok (EMBL, Heidelberg). Polyclonal antibodies against KDEL receptor, Gal-T, giantin, Man II, and GFP were provided by H.-D. Soling (University of Götingen, Götingen), E. Berger (University of Zürich, Zürich), H.-P. Hauri (Biozentrum, Basel), K. Moremen (University of Georgia, Georgia), and D. Shima (Imperial Cancer Research Fund, London), respectively. Monoclonal P5D4 anti–VSV-G protein antibody was from Sigma Chemical Co. (St. Louis, MO). DMEM and fetal calf serum (FCS) were from Life Technologies/Brl Life Technologies (Paisley, UK); secondary TRITC or FITC F(ab′)2 fragments were from Boehringer Mannheim (Mannheim, Germany). Cascade blue dextran was from Molecular Probes (Eugene, OR) and Mowiol was from Calbiochem (Nottingham, UK). Unless otherwise stated, all other chemicals were from Sigma Chemical Co.
Cell Culture
HeLa and NRK cells were cultured in DMEM medium containing 10% FCS supplemented with 10 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were grown in a humidified incubator at 37°C and 5% CO2.
Microinjection Experiments
For microinjection, HeLa and NRK cells were grown for 1–2 d on Eppendorf Cellocate coverslips (Hamburg, Germany) or on normal glass coverslips. For single- or double-microinjection experiments, the GFP-Cdc42 and GFP-N-WASP constructs and the recombinant Sar1dn construct were first diluted to 50–100 ng/ml and then microinjected into the nuclei with an Automated Microinjection System (Model 5242; Eppendorf). Cells for microinjection experiments were cultured in DMEM plus 10% FCS medium containing 25 mM HEPES and supplemented with penicillin, streptomycin, and glutamine. After microinjection, the coverslips were transferred to a Petri dish containing fresh culture medium and returned to the incubator for expression. For the control Sar1dn experiments, the nuclei of cells were microinjected with the cascade blue–conjugated dextran as a microinjection marker. For comicroinjection experiments with Cdc42 or N-WASP constructs, GFP-induced fluorescence was used to identify microinjected cells.
Transient Transfection Experiments
The transfection method used was FuGENE 6 (Roche Diagnostics Corporation, Mannheim, Germany). Briefly, the cells were plated 1 day before transfection in coverslips at 50–80% confluence and incubated overnight. To prepare FuGENE 6 reagent and DNA complex, FuGENE 6 and DNA were mixed in a 3:1 proportion (μl and μg, respectively) and serum-free medium was added to a final volume of 100 μl. The complex was gently mixed and incubated for 15 min at room temperature. Meanwhile, the coverslips were washed with serum-free medium, and the appropriate volume of serum-free medium was added. Finally, the FuGENE:DNA complex and a 5% of FCS were added to the cells for overnight incubation.
VSV-G and Shiga Toxin Transport Assays
Infection with the temperature-sensitive mutant ts045 VSV was performed as described elsewhere (Valderrama et al., 1998). Indirect immunofluorescence transport of VSV-G from ER-to-Golgi complex was performed following Bonatti et al. (1989).
For the native ST-B transport experiments, HeLa cells were first incubated for 30 min in binding medium (FCS-free DMEM) and treated with Cy3-Shiga toxin B-fragment for 45 min at 4°C, and the unbound toxin was then washed for 5 min in ice-cold PBS. Thereafter, cells were incubated with DMEM at 20°C for 2 h to accumulate the internalized ST-B in early/recycling endosomes. They were then heated to 37°C to synchronize the ST-B transport to the ER via the Golgi complex.
Indirect Immunofluorescence
Indirect immunofluorescence was carried out as previously described (Valderrama et al., 1998, 2000) with the following antibody dilutions: anti-KDELr, 1:1000; anti–Gal-T, 1:100; antigiantin, 1:500; anti–VSV-G, 1:50; and FITC/TRITC-conjugated secondary antibodies, 1:35. The coverslips were mounted on microscope slides using Mowiol (Calbiochem). Microscopy and imaging were performed with an Olympus BX60 epifluorescence microscope with a cooled Olympus CCD camera (Lake Success, NY) or a Leica TCS-NT confocal microscope (Heerbrugg, Switzerland). The images were processed on PCs computers using Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA).
Immunoelectron Microscopy
HeLa cells transfected with the GFP-Cdc42 expression vector constructs were processed for cryosectioning as described elsewhere (Martínez-Menárguez et al., 1999). Briefly, cells were fixed overnight with 2% paraformaldehyde plus 0.2% glutaraldehyde in 0.1 M phosphate buffer, pelleted by centrifugation, embedded in 10% gelatin, and cut into small blocks. The blocks were infused with 2.3 M sucrose, frozen in liquid nitrogen, and stored for cryoultramicrotomy. Cryosections were single-immunolabeled with rabbit polyclonal antibodies against GFP followed by protein A-gold. Samples were visualized in a Philips Tecnai 12 electron microscope (Eindhoven, The Netherlands). To establish the relative distribution of GFP-Cdc42V12 and GFP-Cdc42N17 in the Golgi area, gold particles were counted and ascribed to one of the following categories: lateral (defined as the lateral zones of the Golgi cisterna showing its characteristic dilatation), flattened central portions of the Golgi cisternae, peri-Golgi transport intermediates, and nonmembrane structures. The number of the gold particles assigned to each category was expressed as a percentage of the total labeling in the Golgi area. A total of 25 randomly selected Golgi areas were analyzed. Statistical analysis was performed using the Student's t test.
RESULTS
Cdc42 Is Located in the Lateral Rims of the Golgi Cisternae and Golgi-associated Transport Intermediates
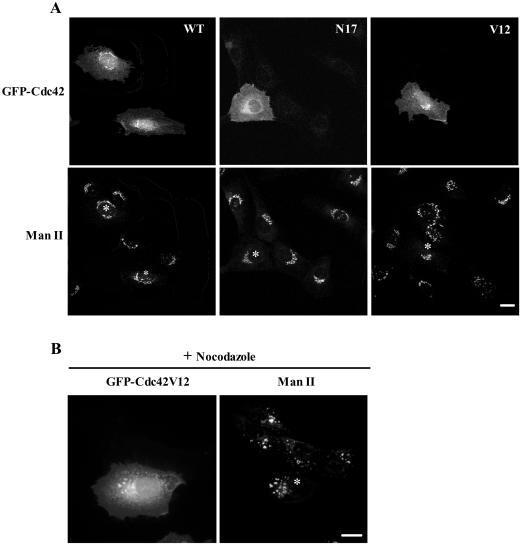
GFP-tagged Cdc42 proteins (wild-type, Cdc42WT; “activated” Cdc42, Cdc42V12; “dominant-negative” Cdc42, Cdc42N17) were observed in the cytoplasm and plasma membrane, but also in the Golgi complex 3–4 h after microinjection of cDNAs into the nucleus of NRK (Figure 1A) or HeLa cells (unpublished results). The Golgi localization was confirmed by double immunolabeling experiments with anti–Mannosidase II antibodies and by Golgi-disruption experiments with nocodazole (Figure 1B) or BFA (unpublished results). The GFP-Cdc42 signal in the Golgi complex, at the light microscopy level, was more intense for the wild-type and activated Cdc42 than the dominant-negative Cdc42N17 (Figure 1A). The presence of Cdc42 in the Golgi complex has been recently reported in astrocytes also using the GFP-tagged form of wild-type Cdc42 and in fibroblasts using polyclonal antibodies raised against a peptide mapping near the carboxy terminus of the human Cdc42 protein (Erickson et al., 1996; Etienne-Manneville and Hall, 2001).
Figure 1.
GFP-Cdc42 is localized in the Golgi complex. (A) NRK cells were microinjected into the nucleus with expressing vectors for wild-type (WT), dominant-negative (N17), and dominant-positive (V12) GFP-Cdc42 forms. After microinjection (3–4 h), the cells were processed for immunofluorescence microscopy and examined by confocal microscopy. The organization of the Golgi complex was visualized with anti–Mannosidase II (ManII) antibodies. Bar, 10 μm. (B) Microinjected NRK cells (asterisks) with GFP-Cdc42V12 construct were, after 3–4 h of expression, treated with nocodazole, which causes fragmentation and dispersal of Golgi fragments throughout the cell. The Golgi-associated Cdc42 colocalizes with ManII in small punctate Golgi structures. Bar, 10 μm.
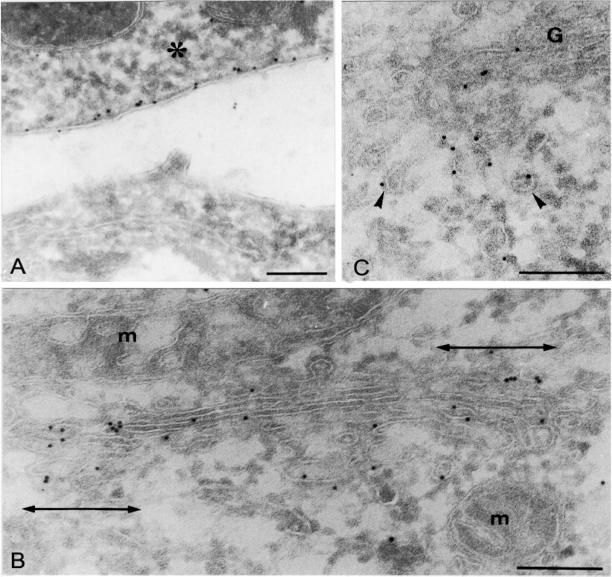
We next examined the subcellular localization of the activated and dominant-negative forms of GFP-tagged Cdc42 in HeLa cells using cryoimmunoelectron microscopy (Figure 2, A–C, and Table 1). Only transfected cells were labeled with anti-GFP antibodies demonstrating the specificity of the labeling (Figure 2A). In cells transfected with Cdc42V12, gold particles were visualized in the plasma membrane (Figure 2A) and in the Golgi area (Figure 2, B and C) but also to a lesser extent throughout the cytoplasm (nonmembrane bound; Table 1). In the Golgi region, GFP-Cdc42V12 was present in the Golgi cisternae (Figure 2A) and associated vesicles (Figure 2C). Within the Golgi stack, Cdc42 was enriched in the lateral portions of the Golgi cisternae (indicated in Figure 2B as double-headed arrows). Some of the reactive peri-Golgi transport intermediates (TIs) showed the typical 10-nm-thick COPI coat (Figure 2C). Quantitative ultrastructural analysis for Cdc42N17 transfected cells showed that the inactive Cdc42 mutant was mostly nonmembrane bound, and when observed in the Golgi stack, it was uniformly distributed along cisternae (Table 1). These ultrastructural observations suggest a role of Cdc42 in Golgi-derived intracellular trafficking.
Figure 2.
Activated GFP-Cdc42 is enriched in the lateral portions of Golgi cisternae and in peri-Golgi transport intermediates. Transfected HeLa cells with GFP-Cdc42V12 vector were fixed and processed for cryoimmunogold electron microscopy using polyclonal antibodies to GFP. Note the gold decoration for GFP-Cdc42V12 in the cytoplasmic part of the plasma membrane in a transfected cell (asterisk), whereas no gold particles are observed in the neighboring nontransfected cell (A). In the Golgi area (B and C), activated GFP-Cdc42 is predominantly located in the lateral portions of the Golgi cisternae (B) and in COPI-coated (arrowheads) and noncoated peri-Golgi transport intermediates (C). G, Golgi stack; m, mitochondria. Bars, 200 nm.
Table 1.
Subcellular distribution of the gold labelling in the Golgi area of HeLa cells transfected with GFP-tagged activated and dominant-negative Cdc42 constructs (Cdc42V12 and Cdc42N17, respectively)
| Golgi cisternae
|
Peri-Golgi transport intermediates | Non–membrane bound | ||
|---|---|---|---|---|
| Lateral portions | Central portions | |||
| Cdc42V12 | 36.6 ± 3.2* | 23.8 ± 3.5* | 33.4 ± 3.2 | 6.3 ± 1.8 |
| Cdc42N17 | 16.2 ± 4.0 | 13.5 ± 4.0 | 16.6 ± 4.1 | 53.7 ± 5.3 |
Numbers represent the percentages (mean ± SEM) of the total gold labeling over the different locations of the Golgi area. See MATERIALS AND METHODS for details.
Statistical Student's t test; p ≤ 0.01.
ER-to-Golgi Protein Transport Is Cdc42 Independent
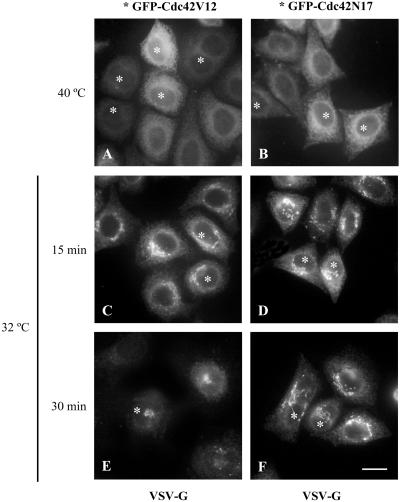
Activated Cdc42 specifically interacts with the γ subunit of coatomer, suggesting a direct role in vesicular transport (Wu et al., 2000). We examined whether the ER-to-Golgi transport of VSV-G was blocked in HeLa cells expressing Cdc42 mutants. In control cells, ts045 VSV-G mutant moves from the ER to the Golgi complex when cells are transferred from restrictive (40°C) to permissive temperature (32°C; Figure 3 C-F; GFP-Cdc42-expressing cells were detected by GFP fluorescence, marked by an asterisk). The kinetics of the ER-to-Golgi transport of VSV-G was monitored by immunofluorescence, which revealed that this transport remained unaltered in HeLa cells overexpressing the wild-type, activated or dominant negative Cdc42. The unaltered ER-to-Golgi transport was not due to the lack of signaling activity by the expressed GFP-Cdc42 proteins, because cells transfected with activated GFP-Cdc42 showed the expected filopodia formation (unpublished results).
Figure 3.
The ER-to-Golgi transport is unaltered in cells expressing Cdc42. HeLa cells were transfected with GFP-Cdc42V12 or GFP-Cdc42N17 vectors and incubated for 12 h. Subsequently, the cells were infected with the thermosensitive ts045 VSV-G mutant incubated at restrictive temperature (40°C). At this temperature VSV-G protein is retained in the ER (A and B). When cells were transferred to the permissive temperature of 32°C, the VSV-G glycoprotein exited the ER and was transported to the Golgi complex (C–F). At indicated transport times (C–F), cells were processed for indirect immunofluorescence for VSV-G glycoprotein. In this and the other panels, the GFP-Cdc42-expressing cells were detected by GFP fluorescence, as marked by asterisks (*). Note that both the dominant-positive (A, C, and E, asterisks) and dominant-negative Cdc42 mutants (B, D, and F, asterisks) show the same kinetics of transport of the VSV-G from the ER to the Golgi complex as the nontransfected neighboring cells. Bar, 10 μm.
We next tested whether the rebuilding of the Golgi complex after BFA removal is impaired by overexpression of Cdc42 mutants. Because ER-to-Golgi transport of VSV-G was unaltered, we reasoned that the rebuilding of the Golgi complex would not be affected. Transiently transfected HeLa cells expressing Cdc42 variants were first treated with BFA to induce fusion of Golgi membranes with the ER. Subsequently, BFA was withdrawn and the kinetics of the morphological appearance of the Golgi complex was examined by immunofluorescence. We found no significant differences in the reformation of perinuclear Golgi complex in these conditions (unpublished results). Thus, Cdc42 is not involved in ER-to-Golgi transport or the rebuilding of the Golgi complex.
Golgi-to-ER Membrane Flow and the Subcellular Distribution of the KDEL Receptor Are Altered in Cells Overexpressing Cdc42
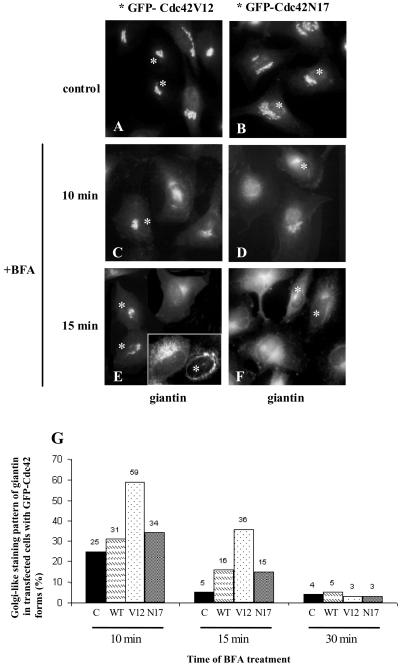
We analyzed whether Cdc42 is involved in Golgi-to-ER membrane dynamics and transport. We first monitored whether the kinetics of the Golgi complex disassembly induced by BFA was Cdc42 dependent (Figure 4). Transfected Hela cells were treated with BFA and processed for immunolabeling at various times. The kinetics of the BFA-induced Golgi membranes merging into the ER remained unaltered in cells expressing wild-type or dominant-negative forms of Cdc42 (Figure 4, D, F, and G). In contrast, in cells expressing activated Cdc42V12 the Golgi disassembly was significantly slower (Figure 4, C, E, and G).
Figure 4.
The kinetics of Golgi disassembly is slowed in cells expressing activated Cdc42. HeLa cells were incubated for 12 h after transfection with the GFP-Cdc42 vectors. The cells were treated with BFA (5 μg/ml) and the kinetics of the fusion of Golgi membranes with the ER was monitored by immunofluorescence using antibodies against the Golgi protein giantin. After BFA treatment, the pericentriolar Golgi complex can be visualized in transfected cells with GFP-Cdc42V12 (C and E, asterisks) but not in cells expressing the GFP-Cdc42N17 (D and F, asterisks) or in neighboring nontransfected cells (C–F). Inset in E shows the characteristic BFA-induced Golgi tubulation in a transfected cell with GFP-Cdc42V12 mutant (asterisk), whereas the neighbor nontransfected cells show a more ER-like staining pattern. (G) Quantitative analysis of the morphological observations. Data represent the average of two independent experiments, in which at least 200 cells were counted for each experiment. (C, nontransfected cells; WT, cells transfected with the wild-type form of Cdc42; V12, cells transfected with Cdc42V12 mutant; N17, cells transfected with Cdc42N17). Bar, 10 μm.
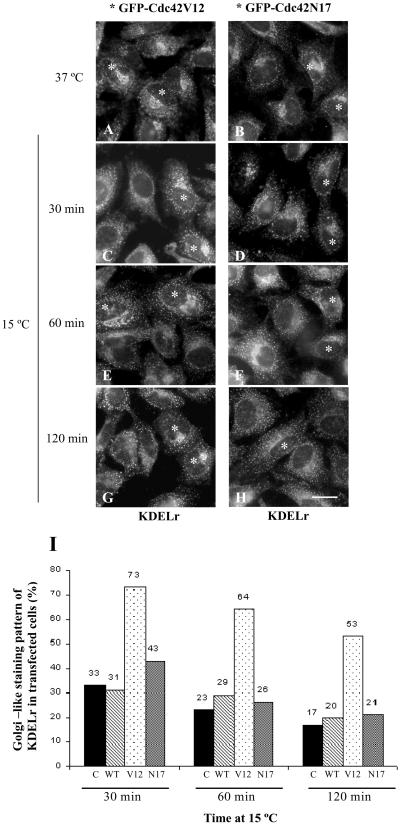
To confirm that Cdc42 is involved in Golgi-to-ER membrane dynamics, we examined the subcellular redistribution of KDEL receptor (Figure 5). The steady state distribution of this protein changed from mainly Golgi-like (Figure 5, A and B) to a punctate cytoplasmic staining pattern when HeLa cells were transferred from 37–15°C (Figure 5, C–H). This is because the KDEL receptor is trapped in the intermediate compartment at this temperature. When cells expressing activated Cdc42 were incubated at 15°C, a larger percentage of KDELr molecules remained in a juxtanuclear Golgi-like compartment (Figure 5, C, E, and G, asterisks). In contrast, their nontransfected neighboring cells (Figure 5, C, E, and G) or cells transfected with the dominant-negative Cdc42 (Figure 5, D, F, and H, asterisks) showed no delay (Figure 5I for a quantitative analysis). These results show that Cdc42 is involved in the retrograde arm of bidirectional transport between the ER and the Golgi complex.
Figure 5.
Cdc42 blocks the redistribution of the KDEL receptor when cells are incubated at 15°C. HeLa cells were transfected as described in the legends of Figures 3 and 4. At 37°C, the steady state distribution of the KDEL receptor (KDELr) shows both the Golgi-like and a diffuse punctate staining. When cells were incubated at 15°C for various times, the steady state distribution of KDELr changes to exclusive punctate staining in nontransfected (C–H) and GFP-Cdc42N17–transfected cells (asterisks in D, F, and H). In contrast, in GFP-Cdc42V12–transfected cells, the KDELr shows the Golgi-like staining pattern (C, E, and G; asterisks). (I) A quantitative visual analysis of the percentage of neighboring nontransfected (control, C) and GFP-Cdc42–transfected cells (WT, N17, and V12 forms) with a Golgi-like staining pattern. Results are the average of two independent experiments, in which at least 200 cells were counted for each experiment. Bar, 10 μm.
Golgi-to-ER Transport of Shiga Toxin and Golgi Enzymes Is Blocked by the Overexpression of Cdc42
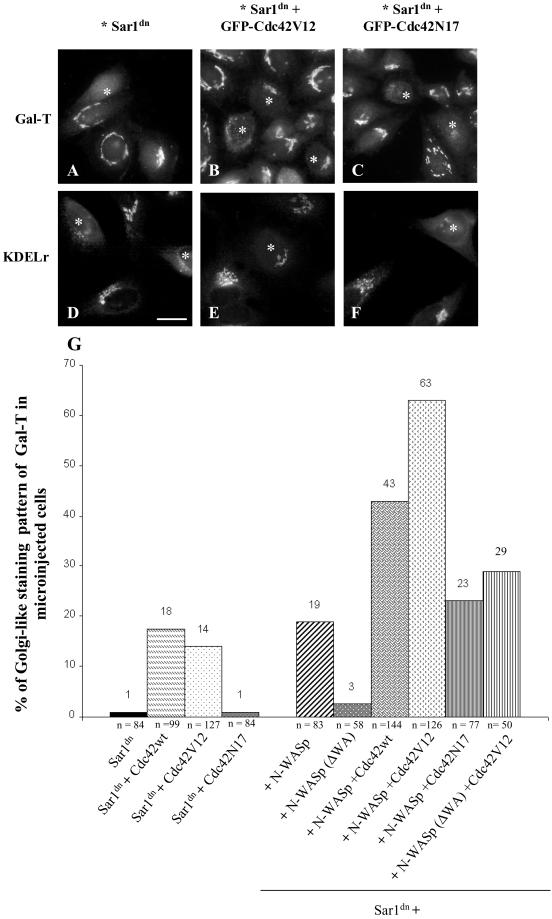
From the previous experiments, we hypothesized that Cdc42 regulates protein recycling from Golgi to the ER. To confirm this, we studied the Golgi-to-ER trafficking of the Golgi-resident protein galactosyltransferase (Gal-T) and a cargo marker, Shiga toxin. Microinjection of the GTPase-deficient Sar1 mutant protein (Sar1[H79G], Sar1dn) into living cells blocks the recycling of Golgi proteins and traps them in the ER (Aridor et al., 1995; Storrie et al., 1998; Seemann et al., 2000). We expressed Sar1dn either alone or together with GFP-Cdc42 constructs (Figure 6). After microinjection of the expression constructs, cells were incubated at 37°C for 6–7 h and processed for indirect immunofluorescence. Expression of Sar1dn led to the expected ER accumulation of Gal-T (Figure 6A, asterisk) and the mixed ER-like and punctate staining patterns for the KDEL receptor (Figure 6D, asterisk). Similar results were obtained when cells were comicroinjected into the nucleus with vectors expressing Sar1dn and the negative mutant GFP-Cdc42N17 (Figure 6, C and F, asterisks). In contrast, microinjected cells coexpressing Sar1dn and the wild-type GFP-Cdc42WT or activated GFP-Cdc42V12 mutant showed the characteristic Golgi-like staining pattern for Gal-T (Figure 6B, asterisks) and the KDEL receptor (Figure 6E, asterisk). Both staining patterns were similar to that shown by neighboring noninjected cells. A quantitative analysis of these morphological results is shown in Figure 6G.
Figure 6.
Cdc42 blocks the Sar1dn-induced ER accumulation of Golgi enzymes and the KDEL receptor. Sar1dn construct alone (A and D; asterisks) and Sar1dn plus GFP-Cdc42V12 (B, E; asterisks) or GFP-Cdc42N17 mutant constructs (C and F; asterisks) were microinjected into the nucleus of HeLa cells. After 7 h of expression, the cells were fixed and processed for indirect immunofluorescence with antibodies against galactosyltransferase (Gal-T; A–C) or the KDEL receptor (KDELr; D–F). In comicroinjected cells with Sar1dn plus activated GFP-Cdc42, Gal-T (B, asterisks), and KDELr (E, asterisk) reveal a Golgi-like staining pattern like the neighboring control (nonmicroinjected) cells. In contrast, cells microinjected with Sar1dn alone (A and D; asterisks) or with Sar1dn plus the dominant-negative GFP-Cdc42N17 (C and F; asterisks) show the characteristic ER accumulation of both Gal-T and KDELr induced by Sar1dn. (G) Microinjected cells were visually quantified for the presence of Gal-T in the Golgi complex. Results are the mean of two independent experiments, and the number of counted microinjected cells is indicated (n). Bar, 10 μm.
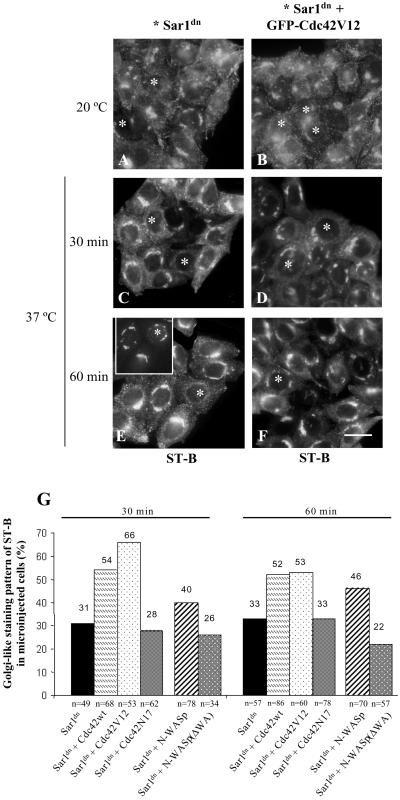
Similar experiments were performed using Shiga toxin (Figure 7), a well-established cargo marker of the Golgi-to-ER protein transport pathway (Sandvig et al., 1992). Sar1dn was microinjected into the nucleus of HeLa cells and incubated at 37°C for 90 min. Thereafter, cells were incubated with native cy3-Shiga toxin (ST-B) for 2 h at 20°C, the result of which was that the internalized toxin accumulated to early/recycling endosomes (Mallard et al., 1998). Cells were subsequently transferred to 37°C to synchronize the ST-B transport to the ER. In Sar1dn microinjected cells, the Golgi localization of ST-B was replaced by a diffuse cytoplasmic staining pattern, which is characteristic of the ER (Figure 7, C and E, asterisks). This is not the case of the neighboring nonmicroinjected cells, which showed a permanent steady state Golgi localization for ST-B (Figure 7, C and E). This is because native ST-B cycles continuously between the Golgi and the ER, but its passage through the ER is rapid (Johannes and Goud, 1998). Notice that, after 3 h of expression of Sar1dn, the Golgi complex was virtually unaltered as assessed by Gal-T staining (Figure 7E, inset and asterisk). This illustrates that the appearance of ST-B in the ER is caused by its transport from the Golgi and not merely the merging of Golgi and ER membranes induced by Sar1dn. Once in the ER, ST-B is retained by the blocking effect of Sar1dn protein on the COPII-dependent ER export machinery (Barlowe, 1998, for review). When cells were comicroinjected with Sar1dn and GFP-Cdc42V12 (asterisks in Figure 7, D and F), ST-B remained in the Golgi with a steady state distribution indistinguishable from that of the neighboring nonmicroinjected cells. This was not observed when cells were comicroinjected with Sar1dn and the dominant-negative Cdc42N17 (see Figure 7G for the quantitative analysis of morphological observations). These data indicate that overexpression of Cdc42WT or Cdc42V12 impairs the Golgi-to-ER membrane transport.
Figure 7.
Cdc42 blocks transport of native Shiga toxin B-fragment from Golgi to the ER. HeLa cells were microinjected as described in Figure 6. After 1.5 h of expression, cells were incubated with cy3-tagged native Shiga toxin (ST-B) for 45 min at 37°C, rinsed, and transferred to 20°C for 2 h to allow internalized ST-B to be retained in the early/recycling endosomes (A and B). Cells were then shifted to 37°C to elicit retrograde transport to the ER via the Golgi complex (C–F). Unlike Sar1dn-expressing cells (C and E; asterisks), ST-B remained accumulated in the Golgi complex in GFP-Cdc42V12 expressing cells (D and F; asterisks). After 60 min of transport, the Golgi complex (stained to giantin) remained virtually unaltered in Sar1dn-expressing cells (E, insert; asterisk), but ST-B (asterisk in E) showed the expected ER-like staining pattern as a consequence of its transport from Golgi to the ER. Cells shown in E and its inset were double-stained with antibodies to giantin and ST-B. (G) Quantitative validation of the morphological data in cells microinjected with different vectors. Results are the mean of two independent experiments and the number of counted microinjected cells is indicated (n). Bar, 10 μm.
Activated Cdc42 Recruits N-WASP to the Golgi Complex
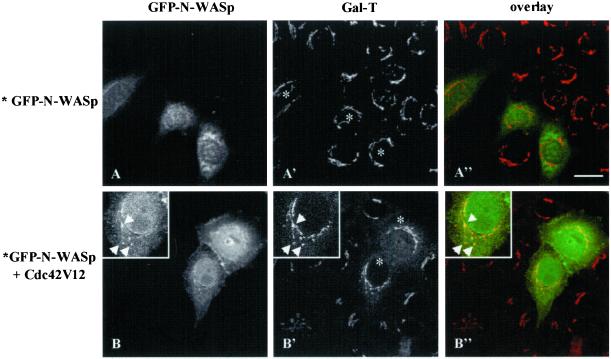
WASP and its ubiquitous form N-WASP bind, among others, to Cdc42 and PIP2, thus integrating and coordinating the signaling pathways that control actin nucleation/polymerization (Snapper and Rosen, 1999 for review; Rohatgi et al., 2000). Hence, we examined whether the regulatory effect of Cdc42 on the retrograde Golgi-to-ER membrane transport requires N-WASP. Cells were coinjected with the GFP-tagged wild-type form of N-WASP and untagged forms of Cdc42. In cells microinjected with GFP-N-WASP alone or together with Cdc42N17, no colabeling of N-WASP (visualized by the GFP fluorescence signal) with Gal-T in the Golgi complex was observed (Figure 8A, A′, and A"). However, GFP-N-WASP was located in the Golgi complex in cells coinjected with Cdc42V12 (Figure 8, B, B′, and B"). These morphological observations indicate that activated Cdc42 induces the recruitment of N-WASP to the Golgi complex.
Figure 8.
Activated Cdc42 recruits N-WASP to the Golgi complex. HeLa cells were coinjected with GFP-tagged wild-type N-WASP vector alone (A) or together with untagged Cdc42V12 construct (B). After 4 h of expression, cells were stained with antibodies to Gal-T and examined by confocal microscopy. Unlike cells expressing GFP-N-WASP alone (A, A′, and A"), GFP fluorescence corresponding to N-WASP (B) in cells that coexpress Cdc42V12 partially colocalize with Gal-T in the Golgi complex (B′) by the appearance of yellow color when images were superimposed (B", overlay). Bars, 10 μm.
N-WASP Mediates Golgi-to-ER Transport Inhibition Induced by Cdc42
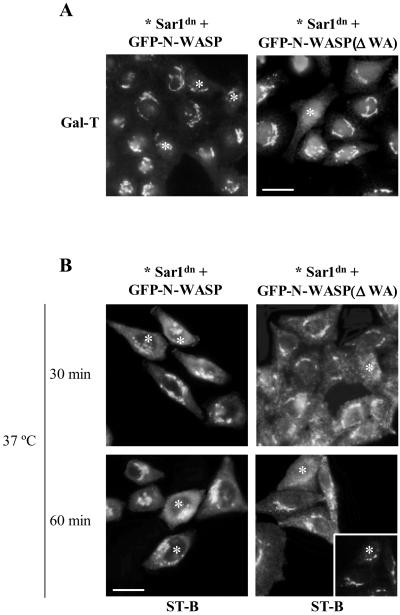
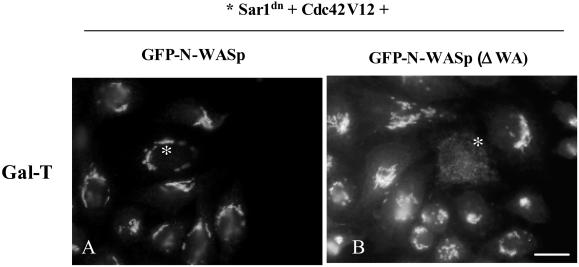
To examine the functional involvement of N-WASP in the Golgi complex and in retrograde protein transport, we coinjected GFP-N-WASP and Sar1dn and monitored the accumulation of Gal-T in the ER by immunofluorescence. In cells coexpressing Sar1dn and the GFP-N-WASP, Gal-T was retained in the Golgi complex (Figure 9A, left, asterisks). In contrast, cells expressing Sar1dn and GFP-N-WASP(ΔWA), a mutant that lacks the Arp2/3 binding domain and thus blocks endogenous N-WASP for membrane binding and target(s), showed no inhibitory effect on the Sar1dn-induced ER accumulation of Gal-T (Figure 9A, right, asterisk). We found a much higher percentage of cells that showed inhibition in the retrograde transport of Golgi enzymes when the cells coexpressed N-WASP with wild-type Cdc42 or with dominant positive Cdc42V12 (Figure 6G). This was not the case for cells coexpressing N-WASP and the dominant negative Cdc42N17, which showed a similar inhibitory effect to N-WASP alone (Figure 6G). Nonetheless, this was expected because, unlike Cdc42V12, Cdc42N17 does not bind to WASP/N-WASP (Burbelo et al., 1995). The coexpression of Cdc42V12 and N-WASP(ΔWA) also resulted in the accumulation of Gal-T in the ER (Figure 10). Therefore, N-WASP(ΔWA) alleviate the expected negative regulation of the retrograde transport by activated Cdc42 (Figure 6G).
Figure 9.
N-WASP blocks the Sar1dn-induced ER accumulation of Golgi enzymes and the retrograde transport of Shiga toxin from Golgi to the ER. (A) Sar1dn plus wild-type GFP-N-WASP (left; asterisks) or Sar1dn plus GFP-N-WASP(ΔWA) (right; asterisks) constructs were coinjected into the nucleus of HeLa cells. After 3–4 h, cells were processed for indirect immunofluorescence with anti–Gal-T antibodies. Unlike N-WASP(ΔWA), N-WASP prevents the Sar1dn-induced ER accumulation of Gal-T. These morphological observations were quantified and results are shown in Figure 7G. Bar, 10 μm. (B) The experimental procedure is described in the legend to Figure 7. N-WASP blocks transport of ST-B from Golgi to the ER (left; asterisks). However, in cells expressing GFP-N-WASP(ΔWA), ST-B accumulates in the ER (right; asterisks). Nonmicroinjected cells show the characteristic steady state Golgi localization of ST-B. The inset shows the double immunostaining to giantin, which reveals of the presence of a virtually intact Golgi complex at this time of Sar1dn expression. Bar, 10 μm.
Figure 10.
N-WASP(ΔWA) alleviates the blocking effect of activated Cdc42 on the Sar1dn-induced ER accumulation of Gal-T. Sar1dn plus untagged Cdc42V12 plus wild-type GFP-N-WASP or Sar1dn plus untagged Cdc42V12 plus GFP-N-WASP(ΔWA) constructs were coinjected into the nucleus of HeLa cells (A and B, respectively; asterisks). After 7 h, cells were processed for indirect immunofluorescence with anti–Gal-T antibodies. Unlike cells that overexpress wild-type N-WASP (A), those expressing the mutant form of N-WASP lacking the Arp2/3 binding domain (N-WASP(ΔWA)) showed that Gal-T was redistributed to the ER by Sar1dn despite Cdc42V12 (B). These morphological observations were quantified and results are shown in Figure 6G. Bar, 10 μm.
Similar results were also obtained when the retrograde transport of ST-B was examined. The coexpression of Sar1dn plus GFP-N-WASP blocked the transport of ST-B from Golgi to the ER (Figure 9B, left, asterisks). This did not occur when cells coexpressed Sar1dn plus GFP-N-WASP(ΔWA) (Figure 9B, right, asterisks). A quantitative validation of these morphological observations is shown in Figure 7G. Together, results indicate that Cdc42 regulates the Golgi-to-ER protein transport via N-WASP.
DISCUSSION
Cdc42 participates in the maintenance and establishment of cell polarity (Adams et al., 1990; Stowers et al., 1995; Etienne-Manneville and Hall, 2001; Gotta et al., 2001), and it is also involved in sorting at the TGN by regulating post-Golgi trafficking and generation of vesicles in polarized MDCK cells (Kroschewski et al., 1999; Cohen et al., 2001; Müsch et al., 2001; Rojas et al., 2001). However, the involvement of the Rho family of GTPases in the early steps of the secretory pathway is unknown. We have previously demonstrated that the Golgi-associated actin filaments are not regulated by Rho A (Valderrama et al., 2000). However, recent results suggest that Cdc42 is a key regulator in secretory pathway trafficking because (1) both Cdc42 and its binding partner IQGAP are Golgi-associated in an ARF-dependent manner (Erickson et al., 1996; McCallum et al., 1998; Etienne-Manneville and Hall, 2001); and (2) Cdc42 governs Golgi complex polarization in wounded cells (Nobes and Hall, 1999), and it binds to the γ component of the COPI coatomer (Wu et al., 2000). We have previously demonstrated that actin is involved in Golgi-to-ER transport but not in the ER-to-Golgi protein transport or in the Golgi rebuilding (which occurs after BFA withdrawal; Valderrama et al., 2001). We here show that Cdc42 is not required for ER-to-Golgi transport either. These results are at variance with those of Wu et al. (2000), who reported that a rapid cycling mutant of Cdc42 that spends more time in the GTP-bound form (Cdc42F28L) interacts with γCOP. In addition, the expression of Cdc42F28L modestly stimulates secretory protein transport as measured by acquisition of carbohydrate modification after release of the VSV-G from the ER. In accordance with the biochemical observations of Wu et al., (2000), our ultrastructural data show that Cdc42V12 is located to COPI-coated transport intermediates, which more likely are involved only in retrograde Golgi-to-ER transport (Letourneur et al., 1994; Martínez-Menárguez et al., 2001). Our results with Cdc42V12 are consistent with this idea. Unfortunately, Wu et al. (2000) only examined anterograde transport. Finally, the discrepancy of the results in the anterograde transport could simply be attributable to the use of different Cdc42 mutants in the two studies.
Molecular Mechanisms Regulating Actin-Golgi Membranes Interaction
The dissection of the actin-membrane interface is critical for understanding the events that occur at the Golgi membranes during transport and signaling. Endogenous vesicles in extracts occasionally nucleate actin polymerization (Taunton, 2001 for review). Small GTPases of the Rho family regulate actin dynamics through numerous downstream effectors (Hall, 1998) such as members of the Wiskott-Aldrich syndrome protein family (WASP/N-WASP; Aspenstrom et al., 1996; Symons et al., 1996), phospholipase D (PLD; Han et al., 1998), phosphatidylinositide 3-kinase (PI3K; Zheng et al., 1994), and IQGAPs (McCallum et al., 1998) among others. Both PLD and PI3K are involved in the generation of transport carriers and post-Golgi trafficking, respectively (Corvera and Czech, 1998, Roth et al., 1999 for reviews). In this respect, we have reported that PI3K seems to regulate the association of actin microfilaments with the Golgi complex (di Campli et al., 1999), which could be complementary to the effects of N-WASP (see below). A much higher percentage of microinjected cells showed an inhibition in the retrograde Sar1dn-induced ER accumulation of Golgi enzymes when N-WASP was coexpressed with Cdc42WT or Cdc42V12. These results indicate that N-WASP transduces signals from Cdc42 to the nucleation/polymerization of actin and it could give rise to the following situations: (1) The Golgi membranes nucleate and polymerize actin as do phagosomes (Defacque et al., 2000), endosomes, and lysosomes (Taunton et al., 2000). Preliminary data indicate that Golgi membranes promote actin nucleation (T. Babià and G. Egea, unpublished observations); (2) retrograde transport intermediates form actin comet tails for their propulsion through Arp2/3 complex, similar to those induced by Listeria, Shigella, and Vaccinia and in raft-enriched secretory and endocytic vesicles (Frischknecht et al., 1999a, 1999b; Taunton et al., 2000; Rozelle et al., 2000). However, the coexistence of an actin-independent mechanism for Cdc42 in the Golgi complex cannot be ruled out, as suggested by the findings that the release of TGN-derived apical transport vesicles is inhibited by latrunculin B and stimulated by activated Cdc42 (Müsch et al., 2001). In fact, PLD and PI3K, both targets of Cdc42, are involved in the generation of transport carriers and in post-Golgi trafficking in vitro, respectively, as previously mentioned.
Formation of Transport Intermediates in the Golgi Complex via Cdc42→N-WASP→(?)Arp2/3
WASP/N-WASP is activated by the lipid second-messenger phosphatidylinositol 4,5-biphosphate (PIP2) and the GTP-bound, prenylated Cdc42 (Zigmond et al., 1997; Ma et al., 1998a, 1998b; Rohatgi et al., 1999; Higgs and Pollard, 2000; see Zigmond, 2000 and Higgs and Pollard, 2001 for reviews). Cdc42 and PIP2 can synergize to activate N-WASP, which in turns triggers actin polymerization via the Arp3/3 complex depending on the localization of both activators on the membrane surface (Prehoda et al., 2000; Rohatgi et al., 2001). Unlike dominant-negative Cdc42N17, activated Cdc42V12 is particularly enriched in the lateral portions of the Golgi cisternae, where most of peri-Golgi transport intermediates are formed. Furthermore, the enzymes responsible for the synthesis of PIP2 phosphatidylinositol-4-OH kinase-β and type I phosphatidylinositol 4-phosphate 5-kinase are both recruited in an ARF-dependent manner to isolated Golgi membranes (Godi et al., 1999; Jones et al., 2000). Thus, there could be a molecular interaction in the lateral portions of Golgi cisternae among activated Cdc42, the aforementioned PI and PIP kinases and PIP2, whose primary role in the signaling pathway may be to activate GTP/GDP nucleotide exchange on Cdc42 (Rohatgi et al., 2000). This interaction could locally and simultaneously regulate protein transport through the formation of transport intermediates or through the peri-Golgi actin assembly. It could be argued that this process does not occur in the Golgi complex because at steady state WASP/N-WASP and Arp2/3 are not located at the Golgi membranes. In fact, most of WASP/N-WASP is not associated with the actin cytoskeleton or membranes, indicating that WASP molecules are not bound to GTP-Cdc42 or PIP2 because both are associated with membranes (Regazzi et al., 1992; Nomanbhoy and Cerione, 1999). However, we found that N-WASP is localized to the Golgi complex in cells overexpressing active Cdc42. This suggests that at physiological conditions such a process, albeit transient and local, also occurs in the Golgi complex. Unlike N-WASP, N-WASP(ΔWA), a mutant that lacks Arp2/3 binding domain, did not alter retrograde transport. Furthermore, when it was coexpressed with Cdc42V12, the expected negative regulation of the retrograde transport by GTP-Cdc42 was also inhibited (Figure 10). This result strongly suggests that N-WASP mediates the Cdc42 response via an interaction with Arp2/3. This complex has in addition to its actin nucleation activity the ability to cross-link actin filaments into a characteristic dendritic network (Mullins et al., 1998). Thus, both the disassembly of microfilaments (Valderrama et al., 2001) and the possible formation of a dense peri-Golgi dendritic actin network by N-WASP-Arp2/3 impair Golgi-to-ER protein transport. We speculate that when microfilaments are disassembled transport intermediates do not interact with them. As a result, they are not transported to the ER either directly or after translocation to microtubules. In contrast, the dense peri-Golgi dendritic actin network induced by N-WASp-Arp2/3 could form a physical barrier that acts as a cage that obstructs the formation or the transport of transport intermediates. This is analogous to the effect of F-actin located underneath the plasma membrane in secretory cells (Morales et al., 2000; Trifaro et al., 2000 for review). Therefore, from our previous findings (Valderrama et al., 2001) and from those reported here, we propose that changes in the peri-Golgi actin dynamics (for example, imbalance in the peri-Golgi G-/F-actin ratio) have direct consequence on the efficacy of retrograde protein transport.
Finally, the regulation of retrograde pathway by Cdc42 and N-WASP equally affects the COPI-dependent and -independent pathways in the Golgi-to-ER protein transport, because overexpression of both Cdc42 and N-WASP hindered the Sar1dn-induced ER accumulation of Gal-T and the Golgi-to-ER transport of native ST-B (COPI-independent), and the subcellular distribution of the KDEL receptor (COPI-dependent; Storrie et al., 2000, for review). These results are consistent with those obtained when actin microfilaments were disassembled by latrunculin B and botulinum C2 toxin (Valderrama et al., 2001).
Why Does the Cdc42 Dominant Negative Form Have No Effect in the Golgi-to-ER Pathway?
We show that, unlike Cdc42V12, Cdc42N17 is mostly located in the cytoplasm (6% vs. 54%, respectively; Table 1), and therefore it cannot interfere in the signaling route(s) activated by Cdc42V12. Hence, the physiological function of Cdc42 in ER/Golgi trafficking can only be revealed when this GTPase is attached to membranes that only happens when, like other GTPases, it is in GTP state. What likely happens in physiological conditions? Most of the endogenous pool of Cdc42 is in GDP-bound form and more probably bound to Rho-GDP dissociation inhibitor protein (Rho-GDI; Nomanbhoy et al., 1999; Faure and Dagher, 2001 for review). Consequently, Cdc42 is located in the cytoplasm and therefore inactive. At steady state, only a small pool of this Cdc42 is activated, which is recruited to plasma membrane and Golgi membranes, where as expected it will activate its cognate downstream targets. We assume that the activation of Cdc42 is rapidly and locally produced (for example, in the lateral portions of the Golgi cisternae). Immediately after, GTP-Cdc42 is also rapidly inactivated by local GAPs. We rationalize that when we overexpress a mutant that encodes GTP-Cdc42, the effects of this GTP-Cdc42 will be much more apparent. In contrast, we cannot reveal a role when we overexpress a mutant that encodes a constitutively GDP-bound Cdc42, which is inactive and located in the cytoplasm. In other words, the overexpression of Cdc42N17 has no physiological effect in these processes, which involves a prior membrane attachment and activation of the GTPase for carrying out its biological function. On the other hand, there is now evidence demonstrating that the effects of a constitutively active GTPase may not necessarily be antagonistic to the effects of a dominant inhibitory form of the same GTPase (Arozarena et al., 2001; Müsch et al., 2001).
In conclusion, the regulatory mechanism involved in the role of actin filaments in the Golgi-to-ER membrane transport is mediated via Cdc42 and N-WASP. Our findings also raise the possibility that motile transport intermediates propelled by actin comets could mediate Golgi-to-ER protein transport.
ACKNOWLEDGMENTS
The authors thank Piero Crespo (IIB, UAM-CSIC, Madrid), Ferran Valderrama (ICRF, London) and Teresa Babià (Kelly Científico, Barcelona) for helpful discussions; Maite Muñoz for technical support; Anna Bosch (Serveis Científico-Tècnics, University of Barcelona) for advice with the confocal microscope; Robin Rycroft for editorial assistance; and Francisco Sánchez-Madrid, Hans-Peter Hauri, Hans-Dieter Soling, David Shima, Rainer Pepperkok, Eric Berger, Kelly Moremen, Ludger Johannes, and Bruno Goud for reagents and antibodies. This study was funded by CICYT grants SAF00–0042 and CIRIT AGP2000 to G.E.; CICYT grants PM99–0137 and BFI2000–0156 to J.B.; and IDIBAPS, CIRIT, and FIS predoctoral fellowships to A.L., O.M., and J.M.D., respectively.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0579. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0579.
REFERENCES
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arozarena I, Matallanas D, Crespo P. Maintenance of CDC42 GDP-bound state by Rho-GDI inhibits MAP kinase activation by the exchange factor Ras-GRF. Evidence for Ras-GRF function being inhibited by Cdc42-GDP but unaffected by CDC42-GTP. J Biol Chem. 2001;276:21878–21884. doi: 10.1074/jbc.M011383200. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim Biophys Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: identification of an erythroid beta-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S, Migliaccio G, Simons K. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989;264:12590–12595. [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Buss F, Kendrick-Jones J, Lionne C, Knight AE, Cote GP, Paul LJ. The localization of myosin VI at the Golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J Cell Biol. 1998;143:1535–1545. doi: 10.1083/jcb.143.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimini G, Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- Cohen D, Müsch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- Corvera S, Czech MP. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998;8:442–446. doi: 10.1016/s0962-8924(98)01366-x. [DOI] [PubMed] [Google Scholar]
- Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, Mangeat P, Voelter W, Marriott G, Pfannstiel J, Faulstich H, Griffiths G. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–3620. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- DePina AS, Langford GM. Vesicle transport: the role of actin filaments and myosin motors. Microsc Res Tech. 1999;47:93–106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- di Campli A, Valderrama F, Babia T, De Matteis MA, Luini A, Egea G. Morphological changes in the Golgi complex correlate with actin cytoskeleton rearrangements. Cell Motil Cytoskeleton. 1999;43:334–348. doi: 10.1002/(SICI)1097-0169(1999)43:4<334::AID-CM6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Zhang C, Kahn RA, Evans T, Cerione RA. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J Biol Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCδ. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Faure J, Dagher MC. Interactions between Rho GTPases and Rho GDP dissociation inhibitor (Rho-GDI) Biochimie. 2001;83:409–414. doi: 10.1016/s0300-9084(01)01263-9. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signaling. Nature. 1999a;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- Frischknecht F, Cudmore S, Moreau V, Reckmann I, Rottger S, Way M. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr Biol. 1999b;9:89–92. doi: 10.1016/s0960-9822(99)80020-7. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Godi A, Santone I, Pertile P, Devarajan P, Stabach PR, Morrow JS, Di Tullio G, Polishchuk R, Petrucci TC, Luini A, De Matteis MA. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc Natl Acad Sci USA. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol. 2001;11:482–488. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Han JS, Kim HC, Chung JK, Kang HS, Donaldson J, Koh JK. The potential role for CDC42 protein from rat brain cytosol in phospholipase D activation. Biochem Mol Biol Int. 1998;45:1089–1103. doi: 10.1080/15216549800203312. [DOI] [PubMed] [Google Scholar]
- Heimann K, Percival JM, Weinberger R, Gunning P, Stow JL. Specific isoforms of actin-binding proteins on distinct populations of Golgi-derived vesicles. J Biol Chem. 1999;274:10743–10750. doi: 10.1074/jbc.274.16.10743. [DOI] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD. Activation by Cdc42 and PIP(2) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J Cell Biol. 2000;150:1311–1320. doi: 10.1083/jcb.150.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD. Regulation of actin filament network formation through Arp2/3 complex: Activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- Johannes L, Goud B. Surfing on a retrograde wave: how does Shiga toxin reach the endoplasmic reticulum? Trends Cell Biol. 1998;8:158–162. doi: 10.1016/s0962-8924(97)01209-9. [DOI] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Jou TS, Leung SM, Fung LM, Ruiz WG, Nelson WJ, Apodaca G. Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1. Mol Biol Cell. 2000;11:287–304. doi: 10.1091/mbc.11.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Ma L, Rohatgi R, Kirschner MW. The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc Natl Acad Sci USA. 1998a;95:15362–15367. doi: 10.1073/pnas.95.26.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Cantley LC, Janmey PA, Kirschner MW. Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J Cell Biol. 1998b;140:1125–1136. doi: 10.1083/jcb.140.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Menárguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Martínez-Menárguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, Klumperman J. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progession model of intra-Golgi transport. J Cell Biol. 2001;155:1213–1224. doi: 10.1083/jcb.200108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SJ, Erickson JW, Cerione RA. Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes. J Biol Chem. 1998;273:22537–22544. doi: 10.1074/jbc.273.35.22537. [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signaling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. cdc42 regulates the exit of apical, and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomanbhoy T, Cerione RA. Fluorescence assays of Cdc42 interactions with target/effector proteins. Biochemistry. 1999;38:15878–15884. doi: 10.1021/bi9916832. [DOI] [PubMed] [Google Scholar]
- Nomanbhoy TK, Erickson JW, Cerione RA. Kinetics of Cdc42 membrane extraction by Rho-GDI monitored by real-time fluorescence resonance energy transfer. Biochemistry. 1999;38:1744–1750. doi: 10.1021/bi982198u. [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM, Kelly RB. Molecular links between endocytosis and the actin cytoskeleton. J Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267:17512–17519. [PubMed] [Google Scholar]
- Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150:1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–26452. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- Rojas R, Ruiz WG, Leung SM, Jou TS, Apodaca G. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2001;12:2257–2274. doi: 10.1091/mbc.12.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MG, Bi K, Ktistakis NT, Yu S. Phospholipase D as an effector for ADP-ribosylation factor in the regulation of vesicular traffic. Chem Phys Lipids. 1999;98:141–152. doi: 10.1016/s0009-3084(99)00026-2. [DOI] [PubMed] [Google Scholar]
- Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Seemann J, Jokitalo E, Pypaert M, Warren G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 2000;407:1022–1026. doi: 10.1038/35039538. [DOI] [PubMed] [Google Scholar]
- Snapper SB, Rosen FS. The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Pepperkok R, Nilsson T. Breaking the COPI monopoly on Golgi recycling. Trends Cell Biol. 2000;10:385–391. doi: 10.1016/s0962-8924(00)01818-3. [DOI] [PubMed] [Google Scholar]
- Stow JL, Fath KR, Burgess DR. Budding roles for myosin II on the Golgi. Trends Cell Biol. 1998;8:138–141. doi: 10.1016/s0962-8924(98)01238-0. [DOI] [PubMed] [Google Scholar]
- Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr Opin Cell Biol. 2001;13:85–91. doi: 10.1016/s0955-0674(00)00178-2. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Trifaro J, Rose SD, Lejen T, Elzagallaai A. Two pathways control chromaffin cell cortical F-actin dynamics during exocytosis. Biochimie. 2000;82:339–352. doi: 10.1016/s0300-9084(00)00193-0. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Babia T, Ayala I, Kok JW, Renau-Piqueras J, Egea G. Actin microfilaments are essential for the cytological positioning and morphology of the Golgi complex. Eur J Cell Biol. 1998;76:9–17. doi: 10.1016/S0171-9335(98)80012-5. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Luna A, Babia T, Martinez-Menarguez JA, Ballesta J, Barth H, Chaponnier C, Renau-Piqueras J, Egea G. The Golgi-associated COPI-coated buds and vesicles contain β/γ-actin. Proc Natl Acad Sci USA. 2000;97:1560–1565. doi: 10.1073/pnas.97.4.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama F, Durán M, Babia T, Barth H, Renau-Piqueras J, Egea G. Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells. Traffic. 2001;2:717–726. doi: 10.1034/j.1600-0854.2001.21006.x. [DOI] [PubMed] [Google Scholar]
- West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–848. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Erickson JW, Lin R, Cerione RA. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405:800–804. doi: 10.1038/35015585. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- Zigmond SH, Joyce M, Borleis J, Bokoch GM, Devreotes PN. Regulation of actin polymerization in cell-free systems by GTPγS and Cdc42. J Cell Biol. 1997;138:363–374. doi: 10.1083/jcb.138.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. How WASP regulates actin polymerization. J Cell Biol. 2000;150:F117–F120. doi: 10.1083/jcb.150.6.f117. [DOI] [PMC free article] [PubMed] [Google Scholar]