Abstract
Among the various dosage forms, oral medicine has extensive benefits including ease of administration and patients’ compliance, over injectable, suppositories, ocular and nasal. Despite of extensive demand and emerging advantages, over 50% of therapeutic molecules are not available in oral form due to their physicochemical properties. More importantly, most of the biologics, proteins, peptide, and large molecular drugs are mostly available in injectable form. Conventional oral drug delivery system has limitation such as degradation and lack of stability within stomach due to presence of highly acidic gastric fluid, hinders their therapeutic efficacy and demand more frequent and higher dosing. Hence, formulation for controlled, sustained, and targeted drug delivery, need to be designed with feasibility to target the specific region of gastrointestinal (GI) tract such as stomach, small intestine, intestine lymphatic, and colon is challenging. Among various oral delivery approaches, mucoadhesive vehicles are promising and has potential for improving oral drug retention and controlled absorption to treat local diseases within the GI tract, as well systemic diseases. This review provides the overview about the challenges and opportunities to design mucoadhesive formulation for oral delivery of therapeutics in a way to target the specific region of the GI tract. Finally, we have concluded with future perspective and potential of mucoadhesive formulations for oral local and systemic delivery.
Keywords: Oral medicine, Oral delivery, Mucoadhesive polymer, Gastric cancer, Inflammatory bowel disease
1. Introduction
Patients greatly prefer oral dosage form over injections and millions of individuals skipped their medications due to needle phobia and associated pain. However, most of the macromolecules are not stable in gastric environment and not absorptive due to low permeability. For instances orally administered drug faces extreme obstacles due to various conditions such as lack of stability in the acidic stomach fluids, negligible solubility, and bioavailability due to barrier associated with mucus. These limitations and obstacles make the oral delivery of bio-macromolecules impossible.
Recent advances in nanotechnology-based formulation made tremendous progress in delivery science including oral delivery (Fig. 1) [1]. Nanomaterials with size below 1000 nm can pass through the biological barriers in the intestine. Among the various nano-platforms, polymer-based nanoparticulate drug delivery system has several advantages that include flexibility of formulating various types of delivery systems (micelle, liposome, layer-by-layer, and hybrid), surface functionalization, higher payload, and protection of loaded therapeutic molecules from biological barriers [2]. Additionally, the polymer-based delivery system facilitates further coating with desired materials that may result in longer retention of the drug payload and facilitate release upon reaching to the site of action. Surface functionalization provide specificity to enhance binding ability with the targeted cell. Surface functionalization has even potential to improve cellular internalization of the nanoparticles and release the payload which offer higher therapeutic effect [3,4]. However, regarding treatment of local diseases within GI (Gastrointestinal) tract, the formulation should adhere on the intestinal lumen and retain for adequate duration for achieving effective therapy. Mucoadhesiveness is a unique property of a polymer that are promising because of their strong interaction with mucin lining within the intestinal duct. In oral delivery, mucoadhesive activity of polymeric formulation enhances the retention time in GI tract and facilitate controlled releases of drug for extended time [5].
Fig. 1.
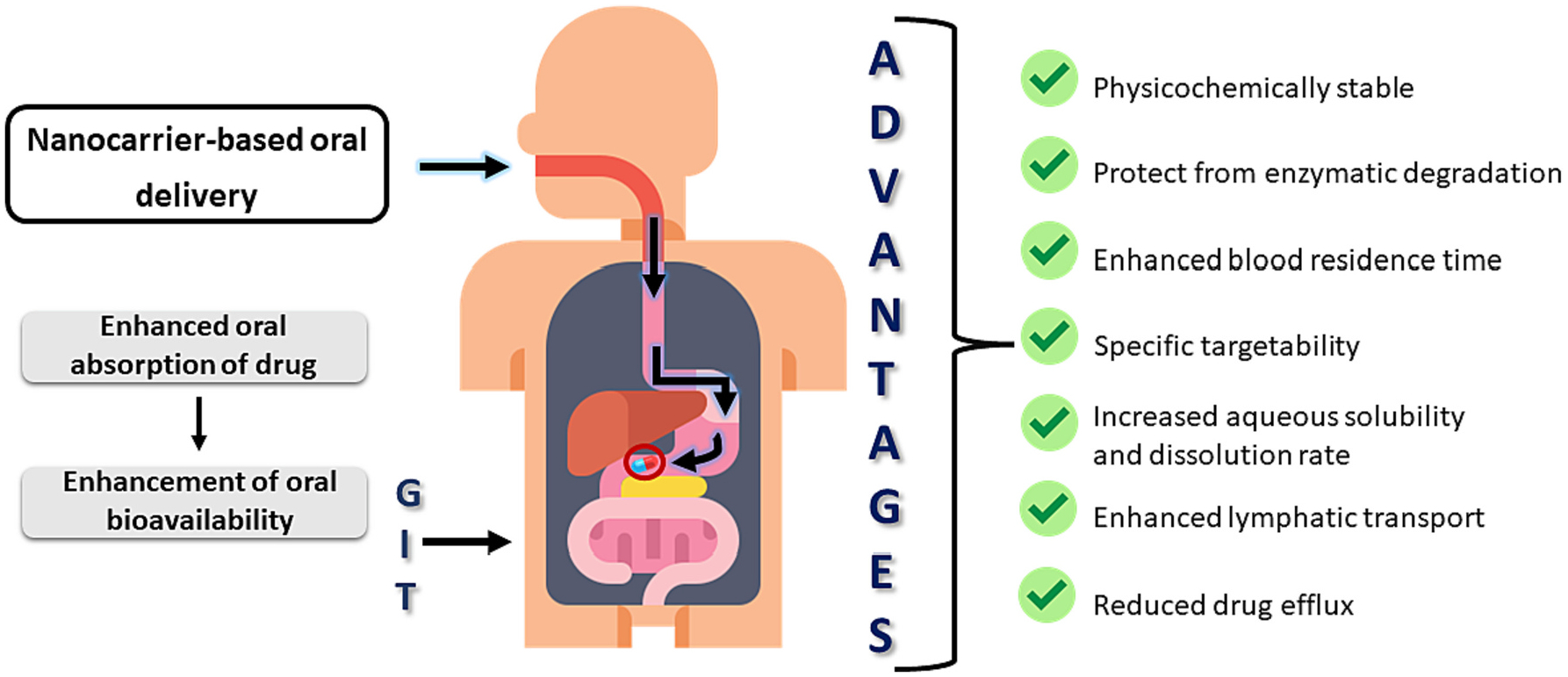
The scheme represents potential benefits of mucoadhesive-based formulation for oral drug delivery.
In the last 20 years polymer mucoadhesive oral delivery have been growing due to their interesting physicochemical properties. Among the mucoadhesive oral delivery mucoadhesive polymeric oral delivery is a most promising candidate. We have extensively reviewed the literature about polymeric mucoadhesive oral delivery. We have conducted literature search with term “mucoadhesive oral delivery” and “oral delivery mucoadhesive” and type of articles “Review articles” and publication dated range from 2015 – till date in google scholar. From the search results, we have collected the articles titles included terms “mucoadhesive”, “oral” and “polymer”. In following table, we have summarized the review articles published. Best of our knowledge we observed that, there is a space to draft a comprehensive review article focused on mucoadhesive polymers and their range of formulation for oral delivery application. More interestingly targeted delivery to stomach, small intestine, intestinal lymphatic and colon is not documented well.
From the Table 1, it is concluded that there is now publication focused on most promising mucoadhesive polymers, and their different types of formulations range from nanoparticles to composite. More interestingly, we also discussed the targeted delivery of formulation to desire site of diseases such as stomach, small intestine, intestinal lymphatic, and colon. We have discussed extensive literature comprehensively documents.
Table 1.
Review articles published 2015 to till date with title contains mucoadhesive, oral and polymer.
| Year | Title of the review articles | The theme of the review articles | Ref |
|---|---|---|---|
| 2015 | mucoadhesive oral films: the potential for unmet needs | This article focused on mucoadhesive oral films. They have reviewed the status of products in clinical trials and studied the preferable therapeutic indications and market trends of mucoadhesive oral films research. | [6] |
| 2016 | Recent advancement in mucoadhesive floating drug delivery systems: a mini review | This study specially focused on the floating mucoadhesive drug delivery systems, which increases local absorption. Also discussed the different types of formulations potential towards floating drug delivery. | [7] |
| 2017 | Mucoadhesive polymers and their mode of action: a recent update | This study reported the various mechanisms of mucoadhesion. They studied and highlighted the polymers, which are using in research and their mucoadhesive mechanism was discussed. | [8] |
| 2017 | A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems | In this study they have reported the in vivo in vitro and ex vivo methods for evaluation of mucoadhesive properties of formulation used for drug delivery. They also discussed the use of artificial and natural mucosa to study the absorption into mucus. | [9] |
| 2020 | Advances and applications of chitosan-based nanomaterials for oral delivery carriers: a review | this study comprehensively documented the chitosan-based formulation for oral delivery. They also discussed the various biotherapeutics including hydrophobic and hydrophilic drugs delivery using chitosan. | [10] |
| 2020 | Mucoadhesive electro-spun fiber-based technologies for oral medicine | This study especially focused in electro-spun mucoadhesive devices for the treatment of anti-inflammatory, local anesthesia and analgesics, and antimicrobial. | [11] |
| 2020 | Mucoadhesive formulations: innovation, merits, drawbacks, and future outlook | This article focuses on polymers involved in mucoadhesive drug delivery. the characteristics of polymers such as charge, surface groups, wettability, molecular weight and chain flexibility on mucoadhesive property and treatment potential. | [12] |
| 2021 | Mucoadhesion and mechanical assessment of oral films | This study studied the mucoadhesion and mechanical properties of film. They also discussed the various method to measure and evaluate the mucoadhesive and mechanical properties of oral films. | [13] |
| 2021 | Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: a review | This article is focused on mucoadhesive potential of food grade biopolymers nanoparticles and their potential to increase oral bioavailability of natural compounds. And their potential towards prebiotics, probiotics, and antimicrobials along the GI tact. | [14] |
| 2022 | Mucoadhesive nanocarriers as a promising strategy to enhance intracellular delivery against oral cavity carcinoma | This review discussed the potential of mucoadhesive nanocarriers towards the targeting, solubility and bioavailability enhancement and novel tumor targeted drug delivery for oral cancer treatment. | [15] |
| 2022 | Mucoadhesive formulations for oral delivery | We have discussed the mucoadhesive polymers, properties and range of formulation for targeted delivery to stomach, small intestine, intestinal lymphatic, and colon. | Present study |
This article reviews the barriers for oral drug delivery and promising role that the mucoadhesive polymers can offer to overcome several biological barriers. We have extensively reviewed hundreds of key articles related to chitosan, alginate, pectin, poly (acrylic acid) (PAA), β-glucan, and carboxymethyl cellulose (CMC). We have demonstrated the role and mechanism of mucoadhesive polymeric formulation in oral drug delivery system to target specific region of the GI tract to improve treatment of the related diseases. This article also provides the insight about gastric cancer and drug delivery system for treatment of gastric cancer (GC) and inflammatory bowel disease (IBD) using mucoadhesive polymeric system. Finally, we have concluded with further perspectives and expert opinion.
2. Major gastrointestinal diseases
2.1. Helicobacter pylori
Globally 50% of population affected by H. pylori and it is the most recalcitrant bacteria. It is a microaerophilic bacterium which reduce acidity. The treatment to clear H. pylori improved recovery of gastric ulcers and inhibits recurrence. Urease, cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VasA) are the biochemicals involved in H. pylori pathogenesis [16]. Currently antibiotics such as metronidazole, clarithromycin, and amoxicillin are the available treatment options. However, at serious condition antibiotics not effective. Maximum antibiotics consumption may lead gastric ulcers [17].
2.2. Gastric cancer
Gastric cancer is a major cancer around the globe. In 2018, 1 million new cases and 783 thousand deaths were reported. Gastric cancer is the firth most diagnosed and third leading mortality cancer [17]. The gastric cancer incidence rate high in Eastern Asia and Eastern Europe. Overall, the treatment available gastric cancer is poor and survival rate is very low. The major pathways for gastric cancer are such as gene mutations, epigenetic changes, and dysfunction of molecular signaling [18]. Currently available treatments for gastric cancers are such as Trastuzumab, Apatinib, Perbrolizumab, Ramucirumab, TAS-102, and Napabucasin. Lapatinib, Pertuzumab, Nimotuzumab are targeting to HER2/EGFR. Subitinib, Regorafenib, and Bevacizumab are targeting to VEGF/VEGER targeting. Nivolumab are targeting to PD-1/PD-L1 and Ever-olimus is targeting mTOR. miR-1179, miR-198, miR-623 and MiRNA used MiRNA treatment gastric cancer. LSD1 shRNA, Pin1 shRNA, PRSS23 shRNA, and GHET1 shRNA are used for gene therapy for gastric cancer [19].
2.3. Inflammatory bowel diseases
Inflammatory bowel disease is two types such as ulcerative colitis and Crohn’s disease. Crohn’s disease was first observed by German surgeon Wilhelm Fabry in 1623 and later described by and named US physician Burril B Crohn [20]. Ulcerative colitis first described by British physician Sir Samuel Wilks in 1859 [21]. High rate of IBD located in northern Europe, UK, and North America. IBD is also associated with race and ethnic origin. In North America, Crohn’s disease in Hispanic 4.1 per 100,000, in Asian 5.6 per 100,000, whereas in white 43.6 per 100,000 and African American 29.8 per 100,000 [22]. The treatment of IBD depends on other health issues, intense of disease, and patients’ tolerance of drugs. The most common drugs are anti-inflammatory and immunosuppressive agents such as 5-aminosalicylates and corticosteroids [23]
2.4. Colon cancer
Colon cancer or colorectal cancer is the third most common malignancy. It has high morbidity and mortality. Duo to development of colonoscopy, which is approved in 2014, colon cancer can be diagnosed even in early stage of colon cancer [24]. World Health Organization (WHO) report, 2018 informed that 1.80 million new cases of CRC were diagnosed, and 862,000 patients died worldwide. In US 145,600 cases diagnosis and 50,000 patients’ death was reported [25]. The major risk factors are environmental and genetic. Other factors are high body weight, modern lifestyle, smoking, alcohol intake, fat diet, insulin resistance, acromegaly, renal transplantation, and MMR gene mutation [26]. The common treatment strategy available is surgery. Adjuvant chemotherapy is standard care for stage III patients. Chemotherapy also improves quality of life. However, the efficacy of these strategies is less, and chemotherapy has several side effects [27]. So targeted delivery needs to be developed.
2.5. Diabetics
Diabetics is Diabetes is a chronic health issue which affects process of converting food into energy. The hormone insulin moves sugar from the blood into your cells to be stored or used for energy. With diabetes, your body either doesn’t make enough insulin or can’t effectively use the insulin it does make [28,29]. According to 2019 report, 37.3 million Americans had diabetes. Nearly 1.9 million Americans have type 1 diabetes, including about 244,000 children and adolescents. 1.4 million Americans are diagnosed with diabetes every year [30]. Common medications are insulin, insulin aspart, insulin gluilisine, insulin lispro, Tresiba, Levemir, Lantus, Toujeo [31]. However, intense and combination insulin therapy for diabetics initially not guarantee of prevention to late effects. The mechanism of glucose also contributes the diabetics which are not well understand yet [32]. So, there is no medication developed. Advanced technologies and extensive and long-term impacts need to be investigated.
There is other gastrointestinal disease such as Celiac Disease, Irritable Bowel Syndrome (IBS), Lactose Intolerance, Chronic Diarrhea, Constipation, Gastroesophageal Reflux Disease (GERD), Peptic Ulcer Disease, Crohn’s Disease, Ulcerative Colitis, Gallstones, Acute and Chronic Pancreatitis, Liver Disease, and Diverticulitis which are not prominent compared to above discussed diseases.
3. Oral drug delivery
Mucoadhesive polymeric oral drug delivery system can be designed sophistically to target a particular region within the GI tract such as stomach, small intestine, intestine lymphatic, and colon. Targeting mucoadhesive polymeric drug delivery to site of diseases enable higher absorption, higher concentration of the drug within the site of interest, and localized therapy. However, designing of the localized targeting delivery depend on various factors that includes pH value, length and surface area of the region, and enzyme activity. The physiological features of human GI tract summarized in Table 2 [33].
Table 2.
Physiological features of the human gastrointestinal tract.
| GI tract region | Length | Surface area | pH | Epithelial type | Retention time | Major enzymatic activities |
|---|---|---|---|---|---|---|
| Oral cavity | - | 0.01 | 6.5 | Stratified Squamous | - | Polysaccharides |
| Esophagus | 0.2–0.25 | 0.02 | - | Stratified Squamous | 4–8 s | - |
| Stomach | 0.25 | 3.5 | 1–3 | Secretary Columnar | 1–3 h | Proteases, lipases |
| Duodenum | 0.35 | 1.9 | 4–5.5 | Simple columnar | 30–40 min | Polysaccharides, oligosaccharides, proteases, peptidases, lipases |
| Jejunum | 2.8 | 184 | 5.5–7 | Simple columnar | 1.5–2 h | Oligosaccharides, peptidases, lipases |
| Ileum | 4.2 | 276 | 7–7.5 | Simple columnar | 5–7 h | - |
| Colon | 1.5 | 1.3 | 7.5–8 | Columnar dominated | 16–35 h | Broad spectrum of bacterial enzymes |
| Rectum | 0.12 | - | 7 | Columnar dominated | - | - |
3.1. Oral delivery system to target stomach
Stomach targeting mediated local drug delivery gained great attention due to importance of development of effective therapy for H. pylori. H. pylori infection affects 50% of population worldwide and 20% of infection result in developing gastric diseases such as gastric ulcers [34]. The major challenges in stomach targeting are gastric retention, protecting the drug molecules from acidic gastric juice, and facilitating of penetration through mucus barrier. Stomach targeting is a good choice for the drugs molecules that absorbed in the stomach, low solubility in intestine milieu due to different pH, has narrow absorption window in the stomach and upper intestine, and that undergo degradation in intestine milieu [35]. Mucoadhesive polymeric oral drug delivery system offers various strategies to overcome these existing challenges.
Mucoadhesive polymeric oral drug delivery system for effective targeting to stomach, formulation should withstand the peristaltic activity of the stomach. Gastric retention of mucoadhesive polymeric oral delivery system depends on food, fed/fasted state, and pH [36]. Hence, engineering the mucoadhesive polymeric oral drug delivery systems are needed to protect the therapeutic molecules from the harsh gastric environment. Another challenge is mucoadhesive polymeric drug delivery system need to adhere on or penetrate through mucus barrier for retention in stomach. In general, the mucus thickness in stomach ranges from 50–450 micron [37]. GI mucus protect epithelium from pathogens along with nanoparticles. Hence, efficacy of gastric mucus passage is exceptionally important for eradication of H. pylori infection [38]. Mucoadhesive polymeric oral drug delivery system showed enhance penetration of mucus barrier compared to conventional oral drug delivery systems. However, floating and therapeutics incorporated into gastro-retentive dosage form enhance gastro retention. Hence, mucus penetrating mucoadhesive polymeric oral delivery vehicle need to be achieved for stomach targeted therapy for H. pylori [39].
3.2. Targeting small intestine
In absorption of therapeutics such as nutrients, drug, electrolytes, and vitamin, small intestine plays crucial role [40]. The aim to achieve targeting to small intestine is easy through enhancement of mucoadhesive polymeric oral drug delivery system uptake and local release and absorption. The promising physiological aspects need to be considered for designing small intestine targeting mucoadhesive polymeric oral drug delivery system [41]. Mucoadhesive polymeric oral drug delivery carriers’ surface can be functionalized to improve cell specific interactions in small intestine to enhance retention time and uptake. Mucoadhesive polymeric oral drug delivery system should withstand harsh gastric milieu hence formulation should bypass the stomach. Towards this direction, polyanionic polymers having stability at acidic pH and labile to neutral pH need to be used as carrier in mucoadhesive polymeric oral drug delivery system for effective bypass of stomach [41,42]. Hence, mucoadhesive polymeric oral drug delivery system can be a potential carrier for small intestine targeted drug delivery.
The cells such as enterocytes, specialized absorptive columnar epithelial cells are the major cells present in the small intestine with various characteristic and receptor. The other cells are goblet cells, and microfold cells (M cells) [43]. Bacteria, viruses, and immunogens transcytoses from mucosal surface of the Peyer’s patches to the subepithelial dome through the M cells [44]. Hence, Peyer’s patches and M cells gave key role in transportation and absorption of therapeutics. It is more important to design the mucoadhesive polymeric oral drug delivery system with efficacy to penetrate the mucus and reach the cell surface. Enzymes, like papain or thiols have used to functionalize on the surface of mucoadhesive polymeric oral delivery vehicle to improve mucus penetration [45,46]. Further to target the mucoadhesive polymeric oral drug delivery system to specific cells, receptor-targeting ligands need to be used to functionalize the surface. Over the last decades, neonatal Fc receptors has been explored to improve oral delivery [47]. However, M cells targeted mucoadhesive polymeric oral drug delivery system got huge attention due to lack of continuous mucus coating, reduced membrane hydrolase activity, scarce glycocalyx, and drug efflux transporters are enhanced absorption of drug [48]. Vaccination and immunotherapies have been delivered successfully through targeting M cells [49,50], in pre-clinical and clinical stages. Lectins are the widely studied ligands and found very effective in targeting M cells [51].
3.3. Intestinal lymphatic targeting
The lymphatic system is a complex network of lymphatic vessels, lymph nodes, spleen, thymus, Peyer’s patches, and Tonsils [52]. Diseases like filariasis, tuberculosis, AIDS, metastasis cancers, and other chronic inflammatory diseases can be managed through mucoadhesive polymeric oral targeted delivery of therapeutics to lymphatic system. Intestinal lymphatic targeting also help circumvent first pass metabolism and reached to systemic circulation directly. This strategy is also useful to enhance oral bioavailability [53]. Intestinal lymphatics transportation pathway plays key role in oral absorption of dietary lipids. The lymphatic transportation pathway was used to study the oral absorption of drug such as halofantrine, penclomedine, ontazolast, and cyclosporine [53]. It is also proven that the chain length of the lipids affects oral absorption and fatty acids of 14 or higher carbon chain length. Prior studies showed that such peptide resulted in enhancement of transportation of fatty acid via intestinal lymphatic transport. Prodrugs mimicking triglycerides showed enhancement in lymphatic transportation compared to prodrug with mimicking to monoglycerides [54].
3.4. Colon targeting
Color targeted drug delivery can be achieved through designing the mucoadhesive polymeric oral drug delivery system with reduced absorption at GI tract prior to reaching colon. Colon targeted mucoadhesive delivery system has great potential to treat the diseases such as inflammatory bowel diseases, colon cancer, irritable bowel syndrome, diverticulitis, colon dysmotility, and parasitic disease [55]. It has reported that colon targeted mucoadhesive delivery effectively lower proteolytic activity, decreases CYP3A4 activity, diminished p-gp expression, and increased transit time in the colon compared to the small intestine [56,57]. The challenges need to address in colon targeting mucoadhesive oral delivery are protection of drug release in stomach and small intestine and feasibility of release of drug only after entering the colon.
One of the widely used strategies in colon targeting delivery have used of pH responsive polymers as carrier. Colon has pH range from 6.2 to 7.2. Among the polymer Eudragit, a methacrylic acid copolymer is extensively studies for colon targeting. Eudragit polymer dissolved at pH 6 to 7. Colon targeting Eudragit polymer are Eudragit S-100, Eudragit L-100, and Eudragit FS-30D [58,59]. However, the pH at colon changes based on diseases at colon such as ulcerative colitis pH 2.7 –5.5, Crohn’s disease pH 5.3. Eudragit polymers Eudragit L100–55 can dissolved at pH below 5.5 and Eudragit E100 dissolved at pH below 5 [60]. However, pH responsive mucoadhesive polymeric oral delivery offer further benefits of longer retention time. Various mucoadhesive pH responsive polymers have been studied for colon-targeted delivery.
4. Absorption pathways
There are various parameters effects the drug absorption in gastrointestinal tract which are presented in Table 3.
Table 3.
Factors that affect drug absorption from the gastrointestinal tract.
| Physiological factors | Physiochemical factors | Formulation factors | Miscellaneous |
|---|---|---|---|
|
|
|
|
4.1. Transcellular pathway
The absorption pathways are two types such as transcellular pathway and intracellular pathway. Various factors affect drug absorption in gastrointestinal tract and the absorption are categorized as transcellular and paracellular pathway [33]. Fig. 2 showed schematic illustrations of (A) the structure of the intestinal epithelium comprising enterocytes, goblet cells and M cells in Peyer’s patches; (B) the presumed mechanisms of the transcellular and (C) paracellular transport of therapeutics [61]. Mucoadhesive polymeric oral drug delivery system enhanced absorptions via both pathways. In transcellular pathway, therapeutics taken up by enterocytes or M cells. Particle’s size and mucoadhesive feature are the key parameters for transcellular pathway. Mucoadhesive polymeric oral drug delivery system with below 100 nm favorable for absorption by enterocytes [62]. Whereas particles 500 nm or more taken up by M cells [61]. Mucoadhesive polymeric oral drug delivery system uptake by epithelial cells can be increases by increasing mucoadhesive ability [61]. Chitosan, alginate, pectin, poly (acrylic acid), carboxymethyl cellulose are well-known mucoadhesive polymers due to electrostatic interaction with negatively charged sialic acid residues on the mucosal surface. Mucoadhesive polymers showed longer half time of its clearance from GI tract [63]. Number of studies investigated the mucoadhesive effectiveness of mucoadhesive polymeric oral drug delivery system [64,65].
Fig. 2.
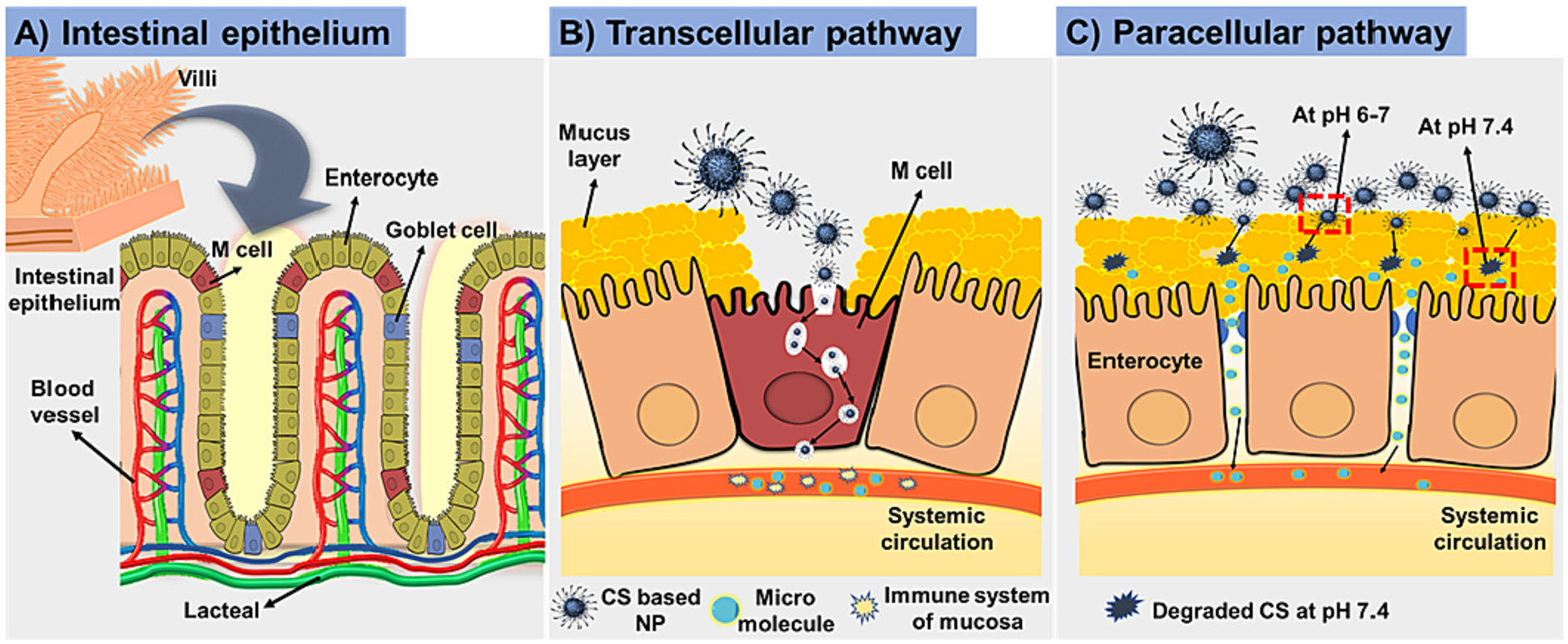
Schematic illustrations of (A) the structure of the intestinal epithelium (B) the transcellular and (C) paracellular transport of nanoparticles.
4.2. Paracellular
Paracellular absorption is normally restricted due to paracellular channels and tight junctions, which are composed of transmembrane integral proteins (Claudins), junctional adhesion molecules, plaque proteins, and regulatory proteins. Claudins forms seal between cells, plaque protein offers structural support to tight junctions, and regulatory proteins regulate signals related to tight junction permeability and cell differentiation [66]. Theoretically, paracellular uptake is infeasible because the space between epithelial cells area ranges from 0.3 to 1 nm, and it is only 20 nm when tight junctions opened fully. Several studies achieved the tight junction opening in caco-2 cells monolayer using mucoadhesive polymers [67]. Various mucoadhesive polymeric oral drug delivery system have been used to deliver the drug through opening tight junctions [68,69]. Schematic illustrations showing the mechanism of chitosan mediated reversible tight junction opening has demonstrated in Fig. 3 [67]. Mucoadhesive polymers opening tight junctions and paracellular permeability in rats has proven by microscopic and ultra-structural approaches [70]. Further transmission electron microscopic studies revealed that aggregation and retention of mucoadhesive polymeric nanoparticles occur at intestinal villi. Further, lanthanum staining also used to investigate and visualize the tight junctions opening activity of mucoadhesive polymers [67]. All these studied provided the evidence of mucoadhesive polymers activity of tight junctions opening. Due to small space between epithelial cells permeability of polymers nanoparticles anticipated release of drug from mucoadhesive polymeric oral drug delivery system. All these conclude that mucoadhesive polymeric oral drug delivery systems are effective and safe.
Fig. 3.
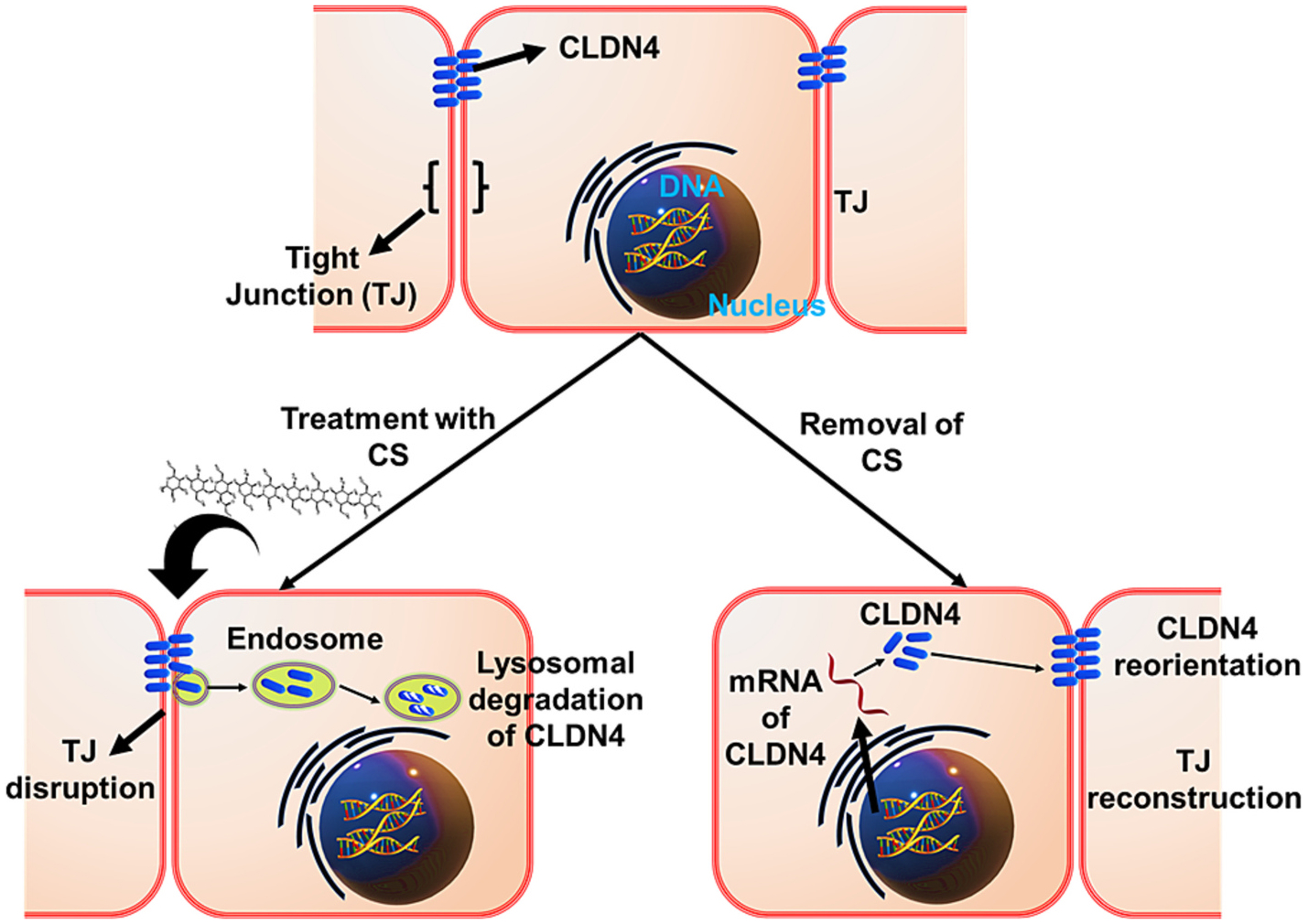
Schematic mechanism of chitosan mediated reversible tight junction (TJ) opening.
5. Mucoadhesive polymeric formulation for oral delivery
Most of the new therapeutic small molecule drugs are hydrophobic and hence limited their use due to low solubility in the gastrointestinal trace and poor permeability across intestinal biological membranes [71,72]. Hence various alternative strategies have been developed. However, the efficacy of oral drug delivery mainly depends on retention duration at site of target. Polymeric nanoparticles based oral delivery is one of the promising strategies to improve intestinal retention. In last 2 decades polymeric drug delivery systems such as polymeric nano-particles, polymeric micelles, hydrogel, and nanocomposites gains significant attention. Therapeutic agent can be delivered through various polymer based oral drug delivery vehicle which release the drug at desired site of action [73,74]. Polymeric nanoparticles can be administrated through different routes such as oral, intravenous, and transdermal. Among them, oral delivery is more favorable and feasible. Among the various polymeric oral drug delivery system, mucoadhesive polymers-based oral drug delivery has emerging potential and possibilities. The most promising mucoadhesive polymers used in oral drug delivery are chitosan, alginate, pectin, poly (acrylic acid), and carboxymethyl cellulose [75]. In following section, we have discussed the mucoadhesive polymeric oral drug delivery systems to target stomach, intestine, and colon.
The ideal characteristic of mucoadhesive polymer included- a polymer should not be toxic, non-absorbable from the GI tract, nonirritant to the mucous membrane, feasibility to form strong bond with mucin epithelial cell, should not be hindrance to drug release, good storage stability, longer shelf life, and should be cost-effective.
The most important factor effects the mucoadhesion of a polymer including molecular weight, chain length, spatial arrangement, flexibility, hydration of polymer, hydrogen bonding, charge, degree of ionization of polymers, and polymer concentration. The environmental factors are such as pH, applied strength, contact time, and swelling, as well physiological factors such as mucin turnover and disease state play critical role in mucoadhesiveness [76].
5.1. Chitosan
Chitin is one the polysaccharides produced by marine organism. Due to the properties such biocompatibility, bioactivity, biodegradability, and high mechanical strength chitin gained attention from researcher. Low solubility limited its use [77]. Researchers modified the chitin to produce chitosan. Chitosan consists of d-glucosamine and n-acetyl-d-glucosamine united with 1, 4-glycosidic linkage. Recently chitosan and its derivatives have been considering as carrier for mucoadhesive oral drug delivery to improve stability, controlled release, reduce side effect, and to enhance bioavailability [78]. Moreover, large number of studies proved the efficacy of chitosan for mucoadhesive oral drug delivery including gastric cancer.
5.1.1. Functionalization
Further to improve the physicochemical properties and to introduced functionality as per required many studies modified the chitosan. Modified chitosan showed enhance solubility and absorption at neutral pH [79]. More interestingly, immobilization of thiol groups of chitosan showed significant enhancement of mucoadhesive capability [80]. In this section, we have discussed the chitosan and modified chitosan polymer use in mucoadhesive oral drug delivery.
Due to the availability of reactive hydroxyl and amino functional group, chitosan can be easily modified with diverse array of moieties. The most common modification reported in literature are quaternization, thiolation, carboxylation, alkylation, acylation, PEGylation, and graft copolymerization to improve the beneficial physicochemical properties of chitosan such as water solubility, musoadhesion, enzymatic inhibitory, and tight junctions opening activity [81].
Quaternization mainly increases solubility of chitosan [82]. Various quaternized chitosan are synthesized such as trimethyl chitosan (TMC), dimethyl ethyl chitosan (DMEC), diethyl methyl chitosan (DEMC), and triethyl chitosan (TEC). TMC widely used in mucoadhesive polymeric oral drug delivery. The order of tight junctions opening activity is TMC > DMEC > DEMC > TEC > CS. Recently, quaternary ammonium palmitoyl glycol chitosan has been reported. It formed polymeric micelles were considered as solubilizer to enhance dissolution rate of hydro-phobic drugs [83].
Thiolated chitosan can be synthesized via coupling with sulfhydryl bearing agents such as cysteine, thioglycolic acid, and glutathione on backbone of chitosan. The most common thiolated derivatives are chitosan-thioglycolic acid (CS-TGA), chitosan-cysteine (CS-Cys), CS-glutathione, CS-4-thio butyl-amidine (CS-TBA), and chitosan-thio ethyl amidine (CS-TEA) [84]. Mucoadhesive activity increases for thiolated chitosan compared to chitosan. Due to cysteine rich glycoproteins in mucus layer, the interaction between cationic thiolated chitosan and anionic mucosal substances is strong. Mucoadhesive activity increases with increase of degree of thiolation [85]. Trimethyl chitosan – cysteine conjugate was synthesized to enhance mucoadhesion and permeation activity [86].
To increase solubility of chitosan in water, chitosan can be modified by carboxyl group. Carboxymethyl chitosan and n-succinyl chitosan have been synthesized. It has proven that carboxylate chitosan derivatives deceases transepithelial electric resistance and increases paracellular permeability of heparin in epithelial cell monolayer [61]. Carboxylate chitosan grafted poly (methyl methacrylate (PMMA)) nanoparticles showed pH sensitivity and hence pH sensitive insulin delivery was achieved [87].
Chitosan can be used to deliver both hydrophobic and hydrophilic drug through preparing derivatives by reaction with n-acylation and fatty acids, which formed nanoparticles through self-assembly in aqueous media. The acidic group interact with sialic acid and hydrophobic methyl group interact with fucose residue [88]. Insulin delivery has achieved through lauryl succinyl chitosan. Where lauryl group offers mucoadhesion and carboxyl group open the tight junctions [89].
Poly (ethylene glycol) (PEG) is a highly hydrophilic and flexible polymer makes interesting polymer. PEG offers flexibility to modify the surface to increase hydrophilicity and circulating half-life [90]. Modified PEGylated chitosan used as coating on nano capsules, which showed enhanced stability in simulated gastric fluids and reduced cytotoxicity [91].
5.1.2. Chitosan as oral delivery vehicle
Due to range of physicochemical and mechanical properties, chitosan based various types of nanocarriers have been developed such as chitosan-drug conjugate, hydrogel, micelles, microsphere, microparticles, nanocomposites, nano-capsules, and nanoparticles. Hence, chitosan is widely explored mucoadhesive polymer in oral drug delivery [92]. In vivo efficiency of drug loaded chitosan NPs in enhancing the drug absorption via the intestinal epithelium thereby increasing the drug available for absorption (Fig. 4A).
Fig. 4.
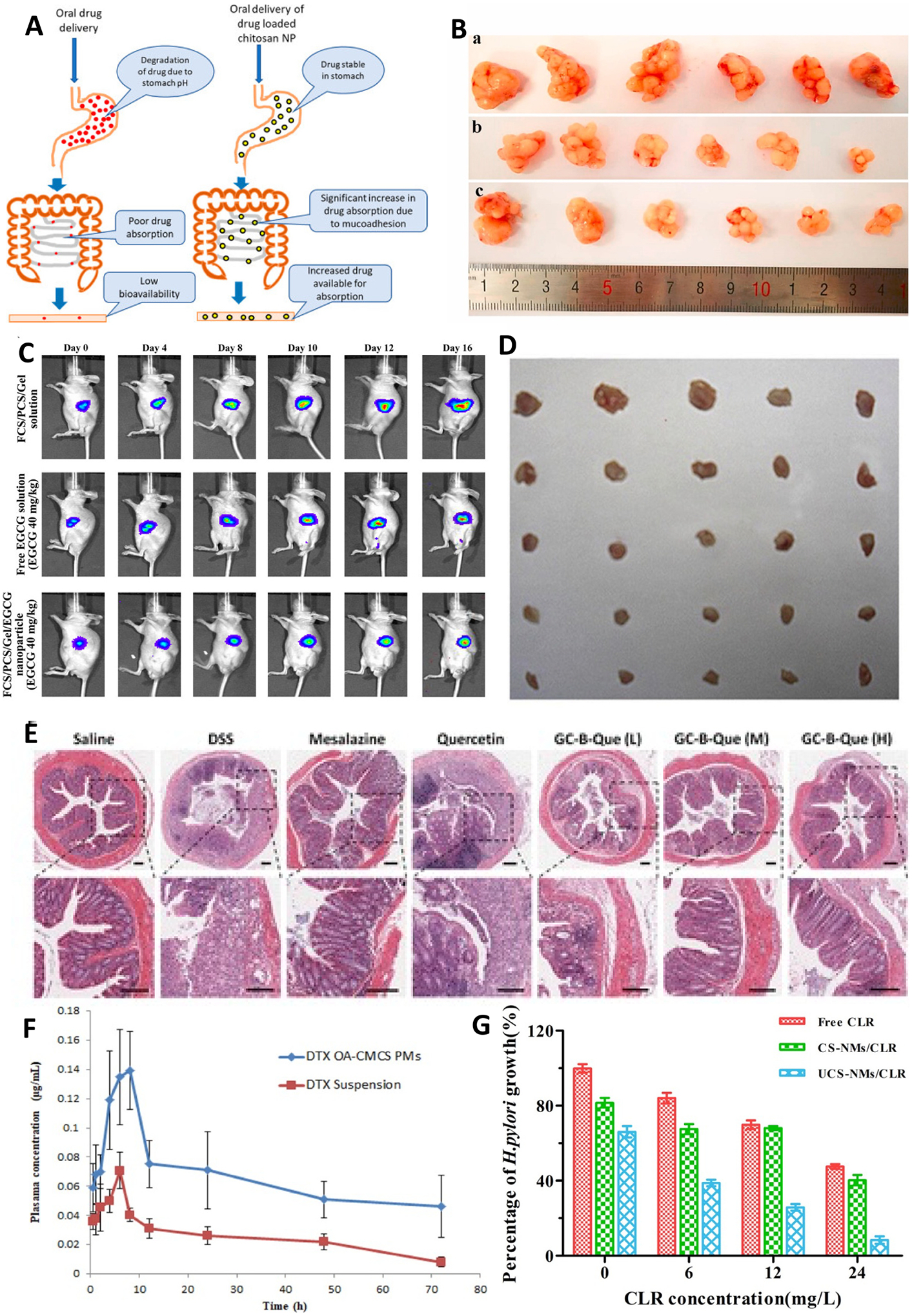
(A) in vivo efficiency of chitosan nanoparticles increasing absorption of drug through intestinal epithelium; (B) Photos of original tumor size. (a) control, (b) COS–Se (100 mg/kg), and (c) COS-Se (50 mg/kg); (C) The antitumor activities using a noninvasive in vivo imaging of Orthotopic Luc MKN45 xenograft models treated with different sample daily; (D) In vivo antitumor efficacy study (E) in vivo anti-inflammatory activity of smart responsive quercetin-conjugated glycol chitosan prodrug micelles, Histological study of inflammatory tissues after treatment by hematoxylin and eosin staining; (F) Plasma concentration vs time profile of DTX (Docetaxel) after oral administration of 10 mg/kg dose of DTX suspension and DTX loaded OA-CMCS micelles; (G) In vitro anti-bacterial activity of free clarithromycin (CLR), clarithromycin-loaded CMCS-g-SA nano-micelles (CS-NMs/CLR) and clarithromycin-loaded U-CMCS-g-SA nano-micelles (UCS-NMs/CLR) against H. pylori;. Images of tumor tissues. Reproduced with permission from ref. [93–98]. Copyright 2019 Elsevier, 2017 MDPI open access, 2020 Elsevier, 2015 American Chemical Society, 2021 American Chemical Society, 2020 Elsevier, 2019 Elsevier.
Chitosan widely used mucoadhesive polymer in oral delivery [99]. Chitosan based oral drug delivery formulation can transport through different mechanism such as transcellular route and paracellular route [100]. To improve and tune the physicochemical properties of chitosan to achieve desired drug delivery system, chitosan can be modified as quaternized chitosan, thiolated chitosan, carboxylate chitosan, amphiphilic chitosan, chitosan derivatives with chelating agents, and PEGy-lated chitosan [101]. Delivery of insulin, exendin-4, salmon calcitonin, cyclosporine, proteins, nucleic acids, and polysaccharides delivery can be achieved through chitosan [61,102].
Transcellular uptake of chitosan based oral drug delivery formulation proceeds through transcytosis, in this process formulation taken up by enterocytes (<100 nm) or M cells (<500 nm) [101]. Particle’s size and mucoadhesiveness enhance transcellular mechanism. In para-cellular, route nanoparticles can uptake through space between the epithelial cells [61,101]. Theoretically, paracellular transport is not possible. However, Chitosan has ability to open the tight junctions in intestinal cells; hence, chitosan used as paracellular permeation enhancers. Further paracellular transport of chitosan proven by microscopic and ultra-structural approaches, and visualization using electronic microscopic examination [100,103].
More interestingly, chitosan exhibits mucoadhesive which offers prolong residence time in the small intestine. Chitosan facilitate the paracellular transport of drug through opening tight junctions between epithelial cells. Therefore, many studies have focused on chitosan based nanocarriers formulation for oral delivery of drugs for gastric cancer. The features such as tight junctions opening, mucoadhesive properties, and solubility at acidic pH and aggregation at neutral pH suggested that chitosan could be an effective mucoadhesive agent and enhance absorption only an intestine lumen only at pH below its pKa i.e., 6.5 [104].
5.1.2.1. Chitosan-drug conjugate.
Due to availability of various active functional group in chitosan, provide flexibility to modify their backbone. The most common chitosan derivatives are trimethyl chitosan, carboxymethyl chitosan, and thiolated chitosan [105]. Trimethyl chitosan and carboxymethyl chitosan are water soluble polymers and forms nanoparticles through ionic gelation. Trimethyl chitosan is a partially quaternized chitosan derivative having solubility at neutral pH. Trimethyl chitosan used to conjugate with drug or peptide for delivery. Chen et al. reported the trimethyl chitosan-CSKSSDYQC peptide conjugate for delivery of gemcitabine in porcine intestine [106]. It improved the oral bioavailability of gemcitabine and anti-tumor efficacy increases 3.3-fold [106]. Carboxymethyl chitosan also showed great potential in oral delivery such as carboxymethyl chitosan/chitosan as nanocarriers to delivery extracellular products and effective absorption in intestinal mucosa. Compared to Trimethyl chitosan and carboxymethyl chitosan, thiolated chitosan promising in oral delivery due to mucoadhesive property. There are various thiolated chitosan have been developed such as chitosan-cysteine (CS-Cye), chitosan-glutathione (CS-GSH), chitosanthioglycolic acid (CS-TGA), chitosan-thiobutylamidine (CS-TBS), and chitosan-N-acetyl cysteine (CS-NAC). Among all CS-NAC showed excellent characteristic [107]. Diabetics is fast growing disease and number suggested the death rate about to double between 2009 and 2034. One of the best treatment options is insulin. The most commonly insulin administrated through subcutaneous injections. However, the major disadvantages are cause of peripheral hyperinsulinemia, which lead to hypertension and atherosclerosis [108]. The best option is oral delivery of insulin, which also reduce side effects [105]. The main issue with oral delivery of insulin is liable to GI tract and poor permeability. Hence, suitable oral delivery system is required to deliver the insulin effectively. Among the various drug delivery system, encapsulation into polymer is promising system for insulin delivery. Among the various polymer, mucoadhesive chitosan based oral drug delivery can be an effective carrier. Chitosan is biocompatible polymer with ability to protect from enzymes in GI tract [105]. Sudhakar et al. synthesized the thiolated chitosan nanoparticles (200 nm) loaded with insulin [107]. Pentaerythritol tetrakis (3-mercaptopropionate) (PETMP) used to synthesis thiolated chitosan (TC). TCNP showed sustain release of insulin at pH 5.3. It showed no effect on cell viability. The in vivo studies conducted in diabetic rats, due to mucin interactions, insulin remain prolonged period and increases biodistribution and bioavailability. FITC labelled TCNPs showed absorption and uptake in caco-2 cells [107]. This study offers better oral delivery of insulin for diabetic treatment.
Chitosan can conjugate with other polymers easily and forms chitosan-polymer conjugates. Alginate, poly-lysine, and poly (γ-glutamic acid) are the major polymers form conjugate with chitosan. PY-CAPLA copolymer has synthesized which showed excellent uptake in Caco2 cells and formed nanoparticles loaded with PTX (paclitaxel) through self-assembly. The bond between cysteine and mucin results due to mucoadhesive property [109]. It improved oral bioavailability of PTX and enhanced anti-tumor activity in Heps tumor bearing mice. Polymers along with conjugation, they also used as crosslinking to fabricate nanoparticles. Polymers such as γ-glutamic acid have used as cross-linker in formulation of chitosan-based nanoparticles. Chitosan also used as coating material to coat on the negative charge composites. Trimethyl chitosan coated PLGA (poly (lactic-co-glycolic acid)) nanoparticles used to deliver the insulin. Trimethyl chitosan-PLGA showed permeation across caco-2 cell monolayer through opening tight junctions [109].
Yearly, 990,000 incidents with 738,000 deaths are cause because of gastric cancer worldwide. 18.1 million new cases and 9.6 death with cancer were reported in 2018. Approximately 5% of patients have hereditary forms. About 30–40% of hereditary cases have identified mutations in the CDH1 gene coding E-cadherin [110]. Gastric cancer has 5-years of survival. Gastric cancer is the fourth common cancer and second according to death. Compared to male, more than double cases has reported in women. Eastern Asia and Eastern Europe are remained at top with high number of gastric cancer incidence [110]. According to WHO, gastric cancer are two types such as adenocarcinoma, carcinoma with lymphoid stroma and the hepatoid adenocarcinoma. Nearly 90% of gastric tumors are adenocarcinoma again divided as tubular, papillary, mucinous, poorly cohesive, and mixed. Pathogenesis is multi step multi factorial and complex in gastric cancer [111]. Gastric antrum cancer and gastric carcinoma are the most common types of gastric cancer. Gastric cancer has low rate of diagnosis is the reason for high rate of incidence and metastasis and mortality. Gastric cancer can determine as sporadic and familial disease. At early stage of gastric cancer is limited to mucosa and submucosa and at advanced stage it is beyond the gastric muscular layer, subserosa, and other organs [112]. Factors such as salt in diet, medication, smoking, alcohol consumption and H. pylori infections are major factors for chronic gastritis. The major risk factor in familial gastric cancer is such as H. pylori, diet habits, and gene polymorphism in pro- anti-inflammatory cytokine gene [113].
Radical surgery and chemotherapy at early-stage gastric cancer is offering nearly 90% survival. However, it is difficult to detect the gastric cancer at early stage due to lack of specific symptoms. Hence, early-stage detection rate is very low. In other hand, at advanced stage there is no chance for surgery. Due to detection of gastric cancer at advanced stage, tradition chemotherapy has limited efficacy [114]. Hence, patients leading to poor prognosis. Overall, the treatment available are poor and hence remain survival rate low. Hence, there is a high demand for research and development of treatment for gastric cancer.
Targeted delivery of therapeutics mainly two approaches such as systemic delivery of therapeutics using nanocarriers or localized delivery of therapeutics to the diseased tissue. Usually, nanocarriers are used to pack the therapeutics. Encapsulation of therapeutics candidate such as small molecule, RNAi polymer and peptide into the nanocarrier improves their solubility and bioavailability, which alter their bio distribution and can facilitate to reach site of interested [115]. This is the extensively studied approach for targeted delivery to gastric cancer.
Selenium (Se) is a potential chemotherapeutic agent against malignant tumor. The major source is selenium oligosaccharides. Artificial synthesis on demand due to lack of enough natural production. Jiang et al. synthesized the chitosan oligosaccharide (COS) conjugated selenium (COS-Se) [94]. They have studied the COS-Se toxicity, activity to improve immune function and inhibition of growth of gastric cancer. The COS-Se showed immune enhancing effect by promoting phagocytic index, spleen index and thymus index without toxicity in Kunming mice. It also showed proliferation and metastasis inhibition. COS-Se reduced CD34, VEGF and MMP-9 levels in nice mice and showed good potential as a functional food ingredient [94]. The COS-Se integrating the advantages of both entities and showing significant enhancement of immune function and blocking gastric can cancer growth (Fig. 4B). This study concluded COS-Se as a new functional food ingredient in cancer prevention.
Compared to chitosan and chitosan derivative-based nanoparticles system, conjugation of chitosan with drug is more effective for targeted delivery. Polymer strongly hold the drug due to covalent conjugation and drug release after degradation of polymer due to local conditions. Polymer-drug conjugate system widely studied for gastric cancer treatment. As chitosan has interesting properties for oral delivery, to investigate the efficacy of chitosan carboxymethyl in drug-polymer conjugate system, previous studies also reported that, NCTD showed antitumor effect in gastric cancer. However, its use in limited due to poor absorption, short half-life, and nephrotoxicity. Chi et al. conjugated NCTD to carboxymethyl chitosan and antitumor efficacy evaluated in vitro and in vivo [116]. Results suggested that conjugate more effective in triggering apoptosis of SG-7901 cell relative to free NCTD. CMCS-NCTD conjugate remarkably reduced toxicity and improve antitumor efficacy in vivo with a 59.57% tumor suppression SGC-7901 gastric tumor in mice. It also upregulates the TNF-α and Bax, and downregulate CFGF, BLC-2, and MMP-9 [116]. These results suggested that CMCS-NCTD conjugate might be promising therapeutics for gastrointestinal tumor therapy. Recently, various studied used the targeting agent conjugated polymer for targeted therapy to gastric cancer. Lin et al. synthesized the fucose-conjugated chitosan, PEG-conjugated chitosan complexes with gelatin of encapsulated green tea polyphenol extract of EGCG (epigallocatechin-3-gallate) [95]. PEG is a hydrophilic nonionic polymer with antigenic properties and non-toxic. PEG extensively used in graft formulation for oral delivery. Fucose is a deoxyhexose sugar used as targeting agent. Site-specific and target activated oral delivery can be a promising approach to treat gastric carcinoma. This formulation showed protection at gastric acid and inhibited gastric cancer cell growth, induced cell apoptosis, reduced VEGF protein expression. It also showed anti-tumor activity in in-vivo in orthotopic gastric tumor mouse model (Fig. 4C) [95]. This study opens a new window to explore further EGCG loaded nanoparticles combination with various chemotherapeutics agents needs to be investigate.
IBD is a gastrointestinal inflammatory disorder characterized by their chronic and inflammations throughout the GI tract. IBD basically two types such as Crohn’s disease (CD) and Ulcerative colitis (UC). In CD, the inflammation is discontinuous and starts from lower part of intestine and colon. In UC, inflammation is continuous from rectum to colon. Both significantly affect the quality of life of patients [117]. It also increases risk of death. IBD affected 3.5 million population globally. The major factors associated with IBD are genetic factors, environmental factors, and microbial dysbiosis. Despite of extensive research the etiology of IBD not fully understood. Drugs such as corticosteroids, amino salicylates, and antibiotics are the conventional medicine used for IBD [118]. Recently, monoclonal antibodies such as anti-tumor necrosis factors have identified for effective therapy. However, 30% of patients non-responsive to anti-TNF agents. Various drug delivery systems have been developed for IBD therapy. However, they have suffered with premature release and inability to survive with various GI barriers such as mucus layer, opening tight junctions, and ability of retention at desired site [119,117]. Hence, it is challenging to develop effective mucoadhesive oral drug delivery system for IBD therapy.
There are various types of conventional drug delivery systems have been developed for IBD treatment. Very recently, mucoadhesive polymers showed promising results with excellent physicochemical and biological properties. Among the various mucoadhesive polymers chitosan showed great potential towards IBD therapy. Chitosan oligosaccharide shows high solubility non-toxicity, and biocompatibility and hence studied extensively. Chitosan oligosaccharides also used for gastric cancer treatment. Yousef et al. investigated the chitosan oligosaccharide potential in inflammatory bowel disease therapy [120]. They have prepared chitosan oligosaccharides with 5–10K Da and more than 90% of degree of deacetylation. Oral administration of COS protected against mortality and intestinal inflammation in a mouse model of DSS (dextran sulfate sodium). The nuclear factor kappa B (NF-kB) activation and suppressed the level for tumor necrosis factor-alpha and interleukin-6 (IL-6) in colon tissue after treatment with COS to DSS and another mouse model of acute colitis induced by rectal installation of 4% acetic acid. COS also prevented the oxidative stress induced apoptosis of T84 cells. This study encourages for extensive investigation of COS activity and mechanism against IBD [120]. The prodrug approach has proven as a most successful method for colon targeted delivery of 5-ASA (5-amino salicylic acid). The prodrug of 5-ASA commercially available in the market with name sulfasalazine. However, it has several side effects such as hematuria and hepatitis. To overcome it various azo conjugated of 5-ASA investigated. However, these synthesis polymers have own disadvantages as they are no biodegradable and not biocompatible. However, mucoadhesive polymers have excellent physicochemical properties to use them as drug delivery carrier. Shen et al. explore the mucoadhesive polymer-drug conjugate system for IBD treatment [96]. They have synthesized the quercetin conjugated glycol chitosan product micelles. They used ROS responsive linker to conjugate drug to polymer. ROS (reactive oxygen species) is overexpressed at site of inflammation in colon so it can be used as stimuli for targeted delivery. At physiological pH, less than 20% and in presence of H2O2 total drug released. Bio-distribution analysis showed accumulation of micelles in colitis mice model. Micelles successfully suppressed YNF-α, IL-6, ad iNOS in DSS mice model. This study demonstrated the inflammatory targeted delivery of quercetin for improved therapeutic effect of IBD (Fig. 4D). This study encourages for smart drug delivery designing for IBD [96]. Nalinbenjapun et al. studied the 5-aminosalicylic acid conjugated with N-(4-aminobenzoyl)-chitosan for colon targeted delivery to treat IBD [121]. 4-amino benzoyl used as spacer. The drug from sulfasalazine has not release in simulated gastric fluid, simulated intestinal fluid and simulated colon fluid. However, release of 70% of drug in 24 h observed in all above mediums containing rat gastrointestinal contents. Whereas the chitosan-5-ASA conjugate release only 25% of drug in 24 h. This study proven that mucoadhesive polymer-drug conjugate system can be effective in colon targeted delivery for IBD. However further formulation of nanoparticles of mucoadhesive polymer-drug conjugated further enhanced the efficacy towards IBD [121].
5.1.2.2. Chitosan based micelles for oral medicine.
Other polymeric nanocarriers for oral delivery are micelles. Micelles are spherical core-shell nanoparticles with hydrophobic core and hydrophilic shell. Shell protects the drug loaded in core from aqueous environment [105]. Hence, Amphiphilic polymers gained great attention in drug delivery of hydrophobic drugs due to their efficiency of self-assembly structure having hydrophobic core and hydrophilic shell in aqueous media. The core can accommodate hydrophobic drug. The shell provides colloidal stability [122]. Various amphiphilic copolymers-based micelles have investigated in oral drug delivery. Recently natural polysaccharides such as chitosan gained attention in their use as carrier for oral drug delivery. Kumar et al. reported on polymeric micelles composed of amphiphilic oleic acid modified carboxymethyl chitosan (OA-CMCS) used to deliver BCS (Biopharmaceutical Classification System) Class IV drug and evaluated intestinal permeability and pharmacokinetic, where docetaxel was used as model drug [97]. Spherical docetaxel loaded OA-CMCS micelles with size 213 nm having EE of 57% formulated. The micelles showed apparent permeability of more than 6-fold. In vivo pharmacokinetics results show Cmax 1.9-fold and AUC 2.6-fold enhancement (Fig. 4E) [97]. This study demonstrated that chitosan based amphiphilic polymers can be a potential mucoadhesive oral drug delivery carrier. Wang et al. synthesized the carboxymethyl chitosan rhein polymeric micelles for oral delivery of paclitaxel and evaluated their intestinal permeation [123]. Paclitaxel loaded polymeric micelles shows size below 200 nm with drug loading capacity of 35%. The micelles enhance the absorption of paclitaxel without causing injury to intestine villi. Results reflected the micelles uptake into the enterocyte independent to P-gp. Biodistribution studies confirm the absorption of micelles at intestinal villi. It also further supported by bioimaging of tumor-bearing mice [123]. Due to ability of micelles for significant internal permeation enhancement, these micelles are promising oral delivery carrier for water insoluble drugs.
H pylori infection is a major disease at stomach. It occurs 25–50% and 70–90% of population in developed and developing countries respectively [124]. Hence, complete eradiation of H. pylori is a global challenge. Antibiotics in the stomach to cure H. pylori is not effective due to adverse gastric environment. Cong et al. developed polymeric micelles [98]. They have fabricated ureido-modified carboxymethyl chitosan graft stearic acid polymeric nano-micelles to delivery clarithromycin targeted to H. pylori [98]. The prepared nano-micelles showed 200 nm size, no cell toxicity against AGS cells, stable in simulated gastric fluid for 24 h in 1x PBS. Ureido facilitate the excellent targetability to H. pylori. Nano-micelles showed excellent drug loading efficiency and control release of clarithromycin. In-vitro studies confirmed superior anti H. pylori effect (Fig. 4F). Lin et al. has developed a stimuli pH responsive chitosan/heparin nanoparticle for stomach specific anti H. pylori therapy [125]. Through instant addition of heparin solution to chitosan generates the pH responsive nanoparticles with size of 300 nm, positive surface charge and stable over pH 1.2 to 2.5 offer protection from gastric fluids. They have demonstrated the nanoparticles adhere and infiltrate cell-cell junctions and local interaction with H. pylori. In vivo studies conducted using mouse model and results proven the localization of nanoparticles at spaces of the gastric villi [125].
5.1.2.3. Chitosan based microsphere for oral medicine.
Microspheres are one of the extensively studied nanoparticles. Microsphere of various polymers such as chitosan, chitosan derivatives, alginate derivatives, PLGA, PLA (poly (lactic acid)), and PCL (poly (caprolactone)) have widely studied in drug deliver due to their excellent physicochemical properties such as biodegradable, non-toxic, and non-immunogenic properties. Kim et al. investigated the phytic acid cross-linked chitosan microspheres for oral insulin delivery [126]. The optimum formulation achieved 97% of EE (encapsulation efficacy) and 67% insulin loading. Microsphere showed 2 h retention potential in gastric and show sustained release behavior in intestinal fluid. The permeability of microsphere in Caco-2/HT-2 monolayer showed 1.6-fold higher with negligible toxicity. The pharmacological bioavailability showed 10.6% and significantly reduced the blood glucose level with long lasting hypoglycemic effect in diabetic rats after oral administration [126]. It showed that simple ionic crosslinking could be a promising strategy for efficient oral delivery of insulin.
Selenium is an indispensable trace element need of living organism such as animal and human. The deficiency of selenium lead to acute gastric mucosal injury. Various health benefits also associated with selenium. Recently, selenium nanoparticles with bright red color gained great attention of research community. It shows free radical scavenging, immunomodulation, growth promotion, anti-tumor, antimicrobial, and anti-inflammatory effects. Moreover, selenium nanoparticles not available commercially due to lack of stability. Bai et al. developed selenium nanoparticles (60 nm) embedded chitosan microspheres and their potential on alcohol induced gastric mucosal injury in rats [127]. The microsphere enhances selenium retention in Se deficient Wistar rats. It also attenuates the ethanol induced gastric mucosal damage on pre-treatment. It also observed that reduction in lipid peroxidation and decreases aggressive nitric oxides [127]. It can take into consideration se supplement for oral delivery for prominent gastro protective effect.
Among the various APIs, curcumin also showed potential efficacy towards IBD. Various reports demonstrated the therapeutic activity of curcumin. However, poor oral bioavailability is a major concern limited its clinical use [128]. Zhang et al. developed pH responsive composite hyaluronic acid/gelatin hydrogel drug delivery system containing CMC microspheres loaded with curcumin for IBD treatment [129]. The in-vitro studies showed 65% of drug release in 50 h. In vivo pharmacokinetics study in mice showed high level the curcumin maintained in colon tissue for more than 24 h. H&E staining, myeloperoxidase and immunofluorescent staining confirm the anti-inflammatory effect of formulation. Compared to control group, formulation showed IL-6 level inhibition and TNF-α secretion was observed. Best therapeutic effect of formulation confirmed by pharmacodynamics studies. This study demonstrated that mucoadhesive polymeric microsphere could be potential nanocarriers for targeted and controlled delivery of curcumin and other drugs for IBD. This study provided a new system for delivery of hydrogel loaded microsphere system for oral delivery for long term treatment [129].
5.1.2.4. Micro/nanoparticles.
Over the last decade, chitosan-based drug insulin delivery system has shown emerging potential in oral delivery insulin with excellent bioavailability [130,131]. It has reported that chitosan coated alginate nanoparticles and beads reduced insulin release in gastric buffer whereas accelerate in intestinal buffer [132]. Zhang et al. have formulated a microparticle of chitosan/casein with bilayer shell-core for oral delivery of nattokinase [133]. Nattokinase is a thrombolytic enzyme obtained from Japanese traditional food natto [133]. It is used to treat thrombosis related cardiovascular diseases. However, oral delivery is limited due to it loss activity in gastric fluids. So, Zhang et al. developed functional oral drug delivery system [133]. Chitosan-based microparticles used to load the nattokinase through genipin crosslinking then covered by casein based protective shell via transglutaminase cross-linking [133]. Xu et al. developed microparticles based on alginate/chitosan/casein three-dimensional system for oral insulin delivery [132]. They prepared alginate/chitosan nanoparticles then coated with casein to protect NiM (NPs in MPs) (Fig. 5A), which improve the stability of the payload in stomach. They achieved 51% EE and 13% insulin release in 2 hr at simulated gastric fluid. Around 57% of insulin release slowly in-simulated intestine fluid for 10 hr (Fig. 5B). These results suggest that coating of casein on alginate/chitosan nanoparticles enhanced the stability of insulin. These microparticles significantly reduced the blood glucose levels in diabetic mice [132]. This can be a potential oral drug delivery system for insulin.
Fig. 5.
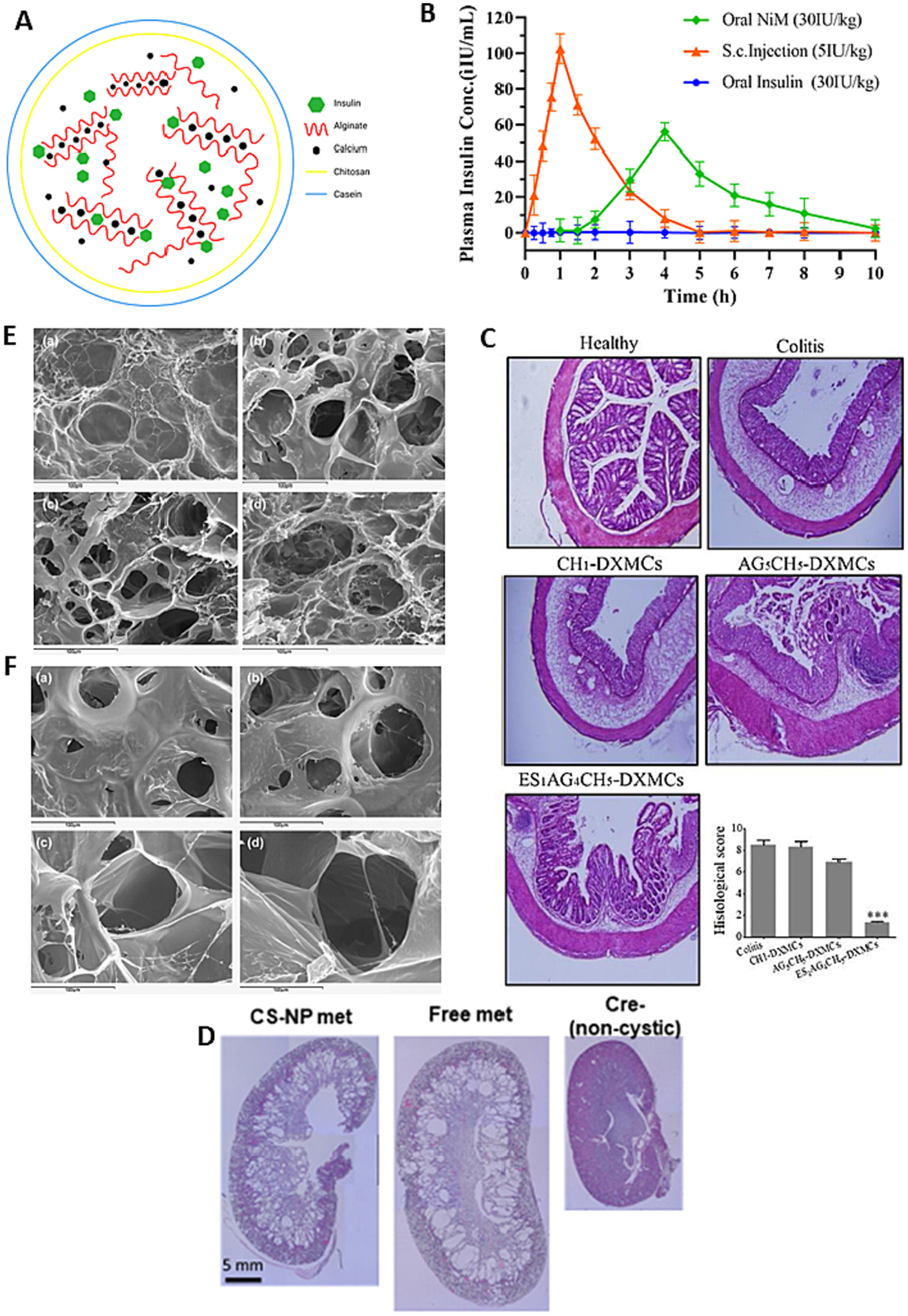
(A) Schematic representation of NiM particles; (B) Plasma insulin concentration-time curves after subcutaneous injection of insulin solution (5 IU/kg), oral administration of NiM (30 IU/kg), and oral administration of insulin solution (30 IU/kg) in diabetic mice; (C) Histological assessment of colitis in colon tissues from the different study groups shown remarkable in histological feature in mice treated with ES1AG4CH5-DXMCs; (D) H&E staining of whole kidneys shows less severe cystic phenotype in the CS-NP met group; (E) Scanning electron micrographs of CAM:[CS:CMC] 40:[25:75] (w/w) complexes. The first two micrographs depict top view of mini matrices at 30 min and pH 1.2 with low M.wt. CS (a) and high M.wt. CS (b). The other two micrographs outline surfaces at 1 h and pH 1.2: low M.wt. CS (c) and high M.wt. CS (d); (F) Scanning electron micrographs of CAM: [CS: CMC] 40:[25:75] (w/w) complexes. The first two micrographs depict top view of mini matrices at 1 h and pH 4.2 with low M.wt. CS (a) and high M.wt. CS (b). The other two micrographs outline surfaces at 4 h and pH 4.2: low M.wt. CS (c) and high M. wt. CS (d). reproduced with permission from ref. [132,134–136]. Copyright 2008, 2020, 2018 Elsevier, and 2021 Wiley.
Colon suffered with IBD. Ulcerative colitis and Crohn’s disease are the two major types of IBD. Till now etiology of IBD not elucidated. There is no such effective treatment available for IBD. Hence, it is in priority in to develop an effective therapeutic modality and strategy for better management of IBD in clinic [137]. Oshi et al. developed colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit multilayers for the treatment of IBD [136]. They fabricated nanoparticles of dexamethasone microcrystals coated with multilayers of chitosan oligosaccharide, alginate, and finally Eudragit S 100 (ES) (ES1AG4CH5-DXMCs) using a layer-by-layer coating technique. Nanoparticles showed size and surface charge 2.34 μm, and −48 mV, respectively. More interestingly, drug release was not observed in acidic pH condition of the stomach and small intestine, however adequate amount of drug release was observed in the colon pH. Nano-particles showed significant therapeutic activity in mouse model of colitis (Fig. 5C) [136]. Layer by layer protection to drug with desire and suitable mucoadhesive polymer and pH responsive polymer. This study demonstrated the layer-by-layer system has potential for colon targeted local delivery of the therapeutic modalities for efficient treatment of IBD. Very recently, Kurakula, et al published a review on progress, trends, and evaluation of colon targeted drug delivery using chitosan, progress made till 2020 [138]. Extensive reports and tremendous progress of chitosan in oral delivery confirm that chitosan is a potential mucoadhesive polymer for oral drug delivery and development.
Nanoparticle mediated drug delivery system has various benefits over small molecule therapeutics such as reduce off-target side effects and enhance drug potency. The chronic disease such as polycystic kidney disease (PKD) need continuous treatment over decades. Parenteral delivery is not found to be as effective as expected to treat PKD. Wang et al. have developed chitosan nanoparticles based oral delivery of metformin for the treatment of PKD [135]. Mucoadhesive chitosan nanoparticles was utilized to successfully deliver the metformin which showed effectiveness to treat PKD. In vitro studies confirm the permeation across intestinal barriers. The metformin loaded chitosan nanoparticles were administered orally in mice to conduct in vivo studies. The in vivo bioimaging results showed the nanoparticles are heavily accumulated within the intestine. It shows 1.3-fold higher AUC with controlled release over 24 h [135]. Moreover, the lower cyst disease has observed comparatively (Fig. 5D). The recorded blood urea nitrogen and creatinine levels were same to untreated mice, revealed that the formulation was biocompatible and nontoxic. This study demonstrated that chitosan nanoparticles could be a potential oral drug delivery system for PKD. Du et al. has developed a polylysine and cysteine functionalized chitosan nanoparticles and used as efficient platform for oral delivery of paclitaxel [109]. The amphiphilic polymer PY-CA-PLA formed nanoparticles encapsulated with paclitaxel through self-assembly with size 165 nm. The drug release follows sustained release behavior. In vitro studies conducted with caco-2 cells showed the cellular uptake profile of the formulation. In vivo studies showed oral bioavailability enhancement to 5.6-fold compared to Taxol. Bio-distribution studies confirm the improved distribution and higher tumor concentration of the drug, thereby better antitumor efficacy in Heps tumor bearing mice (Fig. 4G) [109]. It concluded that PY-CS-PLA nanoparticle could be a great oral delivery vehicle to improve oral bioavailability and therapeutic efficacy of hydrophobic anti-tumor drug.
Chitosan is a cationic mucoadhesive nontoxic, anticoagulant, and safe polymer. Heparin is anionic mucopolysaccharide with 15k Da molecular wright. Heparin consists of glucosamine and uronic acid linked by 1–4 bond. Heparin bioactivities elevate nitric oxide, which stimulate basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) and enhance gastric mucosal cell proliferation and regeneration. Yang et al formulated the nanoparticles of chitosan-heparin for drug delivery to gastric cancer [139]. Doxorubicin (DOX) used as model drug. They have studied the anticancer effects through MTT, LDH, ROS, and qPCR assays using peripheral blood mononuclear cells (PBMCs). Dox@CS-HP nanoparticles showed IC50 26.14 μg/ml. Formulated nanoparticles enhanced LDH release, intracellular ROS, mRNA levels of Bax/Bacl-2, caspase-9 and caspase-3 without altering the caspase-8 [139]. This confirmed that nanoparticles activate the intrinsic apoptotic pathway. Recently, scientific community reported that cholesterol enriched rafts play critical role in progression of gastric cancer. Several studies reported the CdtB use for cancer therapy. Based on these reports, Lai et al. fabricated the chitosan/heparin nanoparticles encapsulated CdtB for gastric cancer therapy [140]. Immunoblot analysis revealed the nanoparticles followed the p53 activation pathway. It also enhances the cell cycle arrest at G2/M, followed by apoptosis. CtdB induced cell death is mediated by ATM dependent DNA damage checkpoint response [140]. Further, detail investigation needs to explore the therapeutic activity of CtdB before clinical use.
Targeted delivery is a promising way to treat the gastric cancer because it offers tremendous advantages. Mostly in targeted delivery systems, ligand and reception binding strategy has widely used. A cyclic 9-merpeptide, GX1 (CGNSNPKSC) is exhibited high affinity and specificity with gastric cancer vasculature. Previous studies confirm the GX1 as a promising vascular marker of gastric cancer. Zhang et al used GX1 to developed multifunctional vascular targeting DTX loaded nanoparticles with N-deoxycholic acid glycol chitosan (DGC) as carrier and GX2-PEG-GPD (deoxycholic acid) conjugate as a targeting agent [141]. The synthesized particles showed 150 nm with spherical shape. The formulated GX1-DGC-DCT showed cytotoxicity to co-cultured gastric cancer cell and human umbilical vein endothelial cells than DTX 100 μM. Nano-particles showed improved cellular uptake. Nanoparticles showed 67.05% tumor inhibition rate at in-vivo in SGC791 and no weight loss in tumor bearing mice [141]. It may be potential strategy for gastric cancer therapy.
Compared to polymer-drug conjugate system, nanoparticles based oral drug delivery shows more therapeutic efficacy. Several studies have already reported the nanoparticles based oral drug delivery system has higher potential than polymer-drug conjugates. Chitosan and chitosan derivatives have been used as drug delivery carrier for IBD. Oshi et al. fabricated colon targeted dexamethasone microcrystals with pH sensitive chitosan/alginate/Eudragit S multilayers for the treatment of IBD [136]. The size of the particles is 2.3 μm with -48 mV surface charge. The particles showed sustain drug release in colon pH and protected in gastric and intestine due to coating. The therapeutic activity of the particles enhanced significantly in mouse model compared to uncoated microcrystals [136]. This study encourages the layer-by-layer coating as potential option for colon targeted delivery. However, due to multiple mucoadhesive polymer coating, the loading content of the drug becomes less. Hence, frequent dosing needed. It is also difficult to controlling the coating thickness, which directly affect the drug release kinetics. Reproducibility of coating also challenge. So smart coating technology need to be explored.
Chitosan also used for ligand/receptor mediated colon delivery system. The ligand/receptor mediated drug delivery system is more effective for localized treatment for colonic diseases. Targeted delivery can be achieved through selective interaction between ligands and surface specific receptor expressed on disease site. various ligands receptor system has been used for colon targeted delivery such as antibodies, peptides, folic acid, and hyaluronic acid [142]. The receptors overex-pressed at site of inflammatory bowel disease are such as mannose receptor, macrophage galactose lectin, transferrin receptor, folate receptor, CD98, CD44 PepT1, F4/80 [142]. Chitosan is one of the polymers widely used in colon targeted delivery. It was reported that chitosan-based nanoparticles could target the fucose receptor located on epithelial cells in vitro. Chitosan fucoidan nanoparticles fabricated through ionotropic crosslinking with loading of eggshell membrane protein. These nanoparticles showed pH specific release profile in vitro and significantly ameliorated the degree of lipopolysaccharide induced inflammations by suppressing the production of NO, TNF-alpha and IL-6 [143]. Chitosan based nanoparticles with functional properties showed excellent efficacy towards IBD. However, detailed preclinical studied need to be conducted before clinical studies.
Chitosan based ligand-receptor mediated targeted drug delivery for gastric cancer need to be explore. Clinical studies need to be conducted to for formulated having excellent in-vivo performance with feasibility of scalability and commercialization aspects.
5.1.2.5. Nanocomposite.
Lee et al. developed colon-targeted oral delivery of insulin using the ternary nanocomposite of organoclay/glycol chitosan/Eudragit S100 [144]. The nanocomposite loaded insulin and aminoclay prepared through spontaneous co-assembly then coated with glycol-chitosan and Eudragit S100. It shows more than 90% of EE (encapsulation efficiency) and pH responsive drug release behavior. Nanocomposite showed improved drug permeability 7-fold in caco-2 cells compared to free insulin and improved drug absorption in colon in rats. The insulin loaded nanocomposite (organoclay/glycol chitosan/Eudragit S100) showed significant reduction of glucose level in blood in diabetic rats. This study demonstrated the nanocomposites based colonic oral delivery of insulin could be a potential carrier. Taken together nanocomposite might be useful to enhance the bioavailability and efficacy of oral insulin [144]. Shirzadian et al. fabricated deesterified tragacanth-chitosan nanocomposite as a potential carrier for controlled and targeted oral delivery of insulin [145]. They have conducted in vitro and ex vivo studies. The nanocomposites synthesized using coacervation technique and optimized using response surface methodology. The efficacy of nanocomposite studied using in vitro release and ex vivo. The nanocomposite particles showed 20 nm size and +17 mV zeta potential. At gastrointestinal condition insulin release pH dependent manner [145]. These results encouraging that, nanocomposites can be potential carrier for oral insulin delivery. However, the nanocomposites fabrication techniques are not well developed. The controlling physicochemical properties of nanocomposites if a major challenge. The nanocomposite internal organization of composition may be different for batch to batch. Hence, reproducibility is major challenge.
5.1.2.6. Micro/nanocapsules.
Micro/Nanocapsules are vesicular structure compose of polymeric shell and aqueous oil core. Drug can load into oil core. Chitosan based oral capsules are a potential system to enhance oral absorption of drugs. Nanocapsules showed great stability over other [105]. Drug release at intestinal is challenging to developed system to pass stomach and do not enter colon. One of the best strategies is targeted delivery. There are number entities available in intestine and can be used as target to deliver the ligands. Ghaffarian et al. developed chitosan-alginate microcapsules as nanocarriers to target ICAM-1 [146]. They have formulated nanoparticles coated with antibodies against intercellular adhesion molecule – 1 (anti-ICAM) and nonspecific immunoglobulin G (IgG) encapsulated in chitosan-alginate microcapsule. They have achieved more than 95% of drug encapsulation efficiency, drug release at storage, gastric pH, and intestinal are respectably <10%, <10%, and 75–85%. Microcapsules showed 20-fold enhancement in cell targeting and 65% of improved protection in GI, reduced 40% of gastric retention, and enhance biodistribution 4 times. The nano capsules even after transit through gastric and intestinal milieus, retain stability, targetability in-vitro, cell culture, and mice [146]. This study illustrated antibody coated polymeric nanocarriers. Similar approach may have potential to developed therapy to other diseases.
Aprepitant is a selective neurokinin 1 antagonist and used to treat acute and delayed chemotherapy induced nausea and vomiting. Poor water solubility limited its use. Towards this direction, Erdogar et al. design the navel nanocapsules of chitosan-PEG coated cyclodextrins to improve oral bioavailability of aprepitant [147]. The nanocapsules are synthesized using cyclodextrins derivative with 9-carbon alkyl chain. Then coated with CS-PEG to enhance the interaction with cell membrane. Details characterization, in vitro and in vivo efficacy has evaluated. The fabricated nanocapsules showed low cytotoxicity, sustained in vitro release profile, improved intestinal permeability. Nanocapsules spewed 93% of EE of aprepitant. The nanocapsules shows sustained release profile over 24 h and nontoxic against L929 cells. Nanocapsules offer highest permeability through caco-2 cells. Oral bioavailability in rats shows great drug absorption over commercial aprepitant products in market [147]. Hence, this study revealed that these nanocapsules are potential candidate for treatment of chemotherapy induced nausea and vomiting. However, very limited number of polymers have ability to form capsules. The fabrication techniques of nanocapsules are very limited and need to be explore. Overall, the research and development of nanocapsules need to explore.
The novel system called “tablet-in-capsule” developed in 2004 by Li and Zhu [148]. It consists mini matrices inside a hard gelatin capsule. The simplicity of method and easiness of administration gained great attention in short time. Gomez-Burgaz et al. design chitosan and caroboxymethylcellulose (CMC) sodium inter polymer complex using tablet-in-capsule method (Fig. 5E–5F) [134]. They have studied the effect of molecular weight of chitosan and ratio of chitosan to CMC on physicochemical and release behavior of clarithromycin (CAM). Swelling depends on MW and ration of components. The formulation showed Fickian diffusion at pH 1.2 and non-Fickian diffusion at pH 4.2. CAM release is faster at pH 1.2 when chitosan MW is high whereas at pH 4.2 CAM release kinetics are zero order and no effect of chitosan MW. Controlled release achieved at CS/CMC 80/(75:25) (w/w) are suitable swelling and drug release profile for gastric cancer [134]. This study suggested that one should considered all these factors when designing stomach targeted delivery system.
5.1.2.7. Hydrogel.
Hydrogels are nanoscale size networks with combination of properties and features of hydrogels. Nanogels are compose of physical and chemical cross-linked polymers can be used to deliver the drug without leakage before reaching the target site [105]. Zhang et al., achieved oral drug delivery of curcumin through pH sensitive composite hyaluronic acid/gelatin HA/GE hydrogel containing carboxymethyl chitosan microsphere loaded with curcumin for inflammatory bowel disease treatment [129]. The system showed sustained drug release behavior in vitro such as 65% of drug in 50 h. In vivo studies confirm the maintenance of curcumin in the colon tissue more than 24 h. Hematoxylin and eosin, myeloperoxidase and immunofluorescence staining further confirm the therapeutic activity of curcumin. It also inhibits IL-6 and TNF-α level. Pharmacodynamics showed that the hydrogel best way to treat colitis in mice. It can be effective delivery system for curcumin [129]. Yang et al. developed hydrogel microparticles for controlled and sustained delivery of insulin [149]. Carboxymethyl β-cyclodextrin grated carboxymethyl chitosan using carbodiimide as a cross linker. The formulation shows great protection of insulin inside hydrogel in gastric environment and slowly releases at intestinal conditions. The hydrogel shows nontoxic and transported across Caco-2 cell monolayer via paracellular pathway. Formulated treated to diabetic mice and result showed significant and sustained reduction of blood glucose level. This study shows that CDCD-g-CMCs is a potential protein carrier for oral drug delivery [149]. Further, Belabassi et al. synthesized the PEGylated (mPEG2000-COOH) and fluorinated chitosan (TFB-COOH) [150]. These chitosan derivate forms nano gels presence of hyaluronic acid (HA) and tripolyphosphate (TPP) through ionic gelation method. Nano-hydrogel (CS-mPEG2000-TPP/HA) and CS-TFB-TPP/HA showed no effect on functions of cells RAW 264.7 [150]. Hence, these nano-hydrogels can be used as a targeted drug delivery to gastric cancer. Various mucoadhesive polymer chitosan based oral drug delivery literature is summarized in Table 4.
Table 4.
Chitosan based mucoadhesive oral drug delivery system.
| Mucoadhesive nanocarrier system | Other composition used | Drug | Therapy for disease | % Of Encapsulation efficiency (EE), %of drug loading (DL) and Release Profile (RB), Drug efficacy (DE), in-vitro, Ex-vivo, In-vivo, Pharmacokinetics (PK) and Pharmacodynamics (PD) | Pros | Cons | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| CS Nano particles | Alginate | Crocine (Crocus sativus L.) | Antitumor effects | %EE-91.5 %DL- 27.4 RP-In vitro release: 90% in both simulated gastric (pH 2) and intestinal pH (pH 6.8) |
|
|
Promising for oral delivery of crocine using chitosan/alginate nanovesicles | [151] |
| CS Nano particles | Poloxamer 407 Tween 80 | Insulin | Diabetics | %EE-57.23 %DL- 20.53 RP-more than 80% of insulin release in intestinal mucosa of goat at end of 6h. Ex vivo permeation-sustained permeation of insulin through Caco-2 cells up to 6 hours In vivo-showed significant hypoglycemic effect in diabetic rats |
|
|
Better efficacy of the solid liquid NP for oral insulin delivery | [152] |
| CS Nano particles | Silica NP | Bovine serum albumin (BSA) | Enhancement of the immune response | %EE-25.34 %DL- 20.21 RP- at pH 6.8 only 5% at end of 24 hr whereas at pH 7.5 at end of 12 days around 50% of BSA release. Slow-release behavior observed. In vivo-produced systemic mucosal immune response in mice |
|
|
Promising for oral vaccine delivery | [153] |
| CS Nano particles | De-esterified Tragacanth | Insulin | Diabetics | %DL- 5.39, RP- 20% of insulin released after 2 hours in gastric pH-2; 30% after 6 hours in simulated intestinal fluid of pH-6.8; 97% after 9 hours in simulated colon fluid of pH-7.4 |
|
|
Promising for oral insulin delivery | [145] |
| CS-PEG Nano particles | Cyclodextrin derivative with 9 carbons alkyl chain | Aprepitant | Acute and delayed chemotherapy-induced nausea and | %EE (nanoparticles)- 55 % %EE (Nanocapsules)- 93. RP-Sustained drug release was shown up to 24h |
|
|
Promising for oral delivery of aprepitant | [147] |
| 3 different MW CS Nano particles | Hydrogenated soybean phosphatidylcholine (HSPC)/1,2-dipalmitoyl-snglycero-3-phosphoglycerol (DPPG) liposome | Insulin | Diabetics | DL- 10.7% by weight. RP- 6% in 1h for simulated gastric fluid, 2% in two weeks in simulated intestinal fluid, and 5% in two weeks in PBS. CT- Non-toxic to L929 cells. IP- 2–3-fold increase |
|
|
The LBL CS conjugated nanoliposome carrier transports insulin via Caco-2 cells of the intestine | [154] |
| CS-stearic acid nanoparticles | L-carnitine | (PTX | Anticancer effects | %DL- 15.91±0.20 RP- only 6.6% of drug release at end of 120 h. slow-release behavior. PK- Increase 2.03-fold peak concentration (Cmax) and relative bioavailability 165.8% compared to Taxol, indicating the enhanced absorption. |
|
|
PTX loaded micelle carrier showed efficiency for oral PTX delivery via organic cation/carnitine transporter 2 (OCTN2) | [155] |
| CS nanoparticles | Solid lipid nanoparticle (SLN) | Thymoquinone (TQ) | Anti-inflammatory effect | %EE- 82.66 %DL- 9.84 RP- TQ shows controlled release profile and release nearly 80% at end of 24 h. Oral bioavailability of TQ increases many folds. |
|
|
Novel mucoadhesive CS-SLNs based platform capable to overcome the barriers for TQ oral delivery | [156] |
| TMC nanoparticles | Captex 355 Kolliphor RH40 Propylene Glycol | Amphotericin B (AmpB) | Visceral Leishmaniasis | DL- 0.71 mg/g CT- Cell viability was higher than 80% for all formulations. Formulation capable to open tight junctions and enhanced the permeability |
|
|
SNEDDS based on TMC has potentiality to transport AmpB via Caco-2 cells by loosening tight junctions between cells | [157] |
| CS nanoparticles | Tripolyphosphate (Cs-TPP) |
DNA vaccine | Disease caused by Aeromonas hydrophila in fish | %EE- 82.3%. Vaccines showed higher survival rate (76.2%) against A. hydrophila. |
|
|
CS-TPP encapsulation is promising for oral DNA vaccine delivery | [158] |
| CS nanoparticles | Poly-L-glutamic acid | Metformin | Polycystic kidney disease (PKD) | %EE- 37.3 %DL: 32.2. RP- at pH 1.2 and 2.5 the release efficiency was no more than 25%, while at pH 6.5, 7.4 it was around 50% at end of 3 h. Opening of tight junctions was observed. Formulation significantly accumulated in intestine and shows 1.3 times higher serum area under curve. PKD murine model (Pkd1fl/fl;Pax8-rtTA; Tet-O cre), a lower cyst burden was observed compared to free metformin, and was well tolerated upon repeated dosages. |
|
|
This work represents a novel CS-NP oral nano formulation for targeting PKD | [135] |
| CS nanoparticles | Snail mucin Poloxamer Poly vinyl alcohol | Insulin | Diabetics | %EE- 92.5 %DL- 23.5 RP- 20% in 1 h, and >80% release in 10 h at PBS pH 7.4. Formulation showed significant anti-hyperglycemic effects in alloxan induced diabetic rats. Enhanced the insulin absorption and reduced the glucose levels and plasma insulin level reached max in 1 h. it shows no negative effect on liver cells |
|
|
The CS-mucin based NP showed no toxicity for oral delivery of insulin | [159] |
| CS nanoparticles | PLA | Ursolic acid (UA) | Antitumor activity | EE- 97.47 RP- 53% UA was released within 144h through the diffusion mechanism. UA plasma concentration of 46 ng/mL was observed in the first 30 min after gavage. single oral dose in rats, increased the UA absorption, reduced its clearance and elimination, resulting in increased bioavailability. Half life increases 4.14. times. UA plasma |
|
|
CS-PLA NP showed potentiality in reducing the renal clearance of the drug and increased the bioavailability | [160] |
| CS nanoparticles | Alginate | Curcumin diethyl disuccinate (CDD) | Anticancer activity against MDA-MB-231 human breast cancer | %EE- 50 %DL- 5.72 RP- 86 %, 83 %, 81 %, and 64 % of CDD released from the CDD-CANPs in the SGF, STEF, SIF, and SBF, respectively, at 72 h. |
|
|
CS-AG encapsulated CDD showed better biocompatibility, cellular uptake and less cytotoxicity | [161] |
| CS nanoparticles | Hyaluronic acid (HA) Sodium tripolyphosphate (TPP) |
Insulin | Diabetics | %DL- 5.5 – 28.8 RP-After 8 h, the released amount of insulin plateaued to 75%. Strong and continuous hyperthermia effect (with a pharmacological availability (PA) of 13.8%). |
|
|
HA protected CS-TPP NP is promising for oral delivery of insulin | [162] |
| Protonated CS nanoparticles | Sodium tripolyphosphate (TPP) | Immunogenic outer membrane proteins (OMPs) Flagellin (F) protein (FP) | Salmonellosis | %EE- 70 nanoparticles were localized in ileal Peyer’s patches. The candidate |
|
|
CS based salmonella nanovaccines targets the immune cells of chicken and induces immune responses | [163] |
| CS nanoparticles | PLGA nanoparticles | Thymoquinone (TQ) | Breast cancer | %EE- 92.17 RP- formulation shows sustained release profile from 4 to 24 hr and 27% of TQ was released after 24h. Formulation showed statistically enhanced antioxidant potential and cytotoxicity against MDA-MB-231 and MCF-7. TQ-CS-PLGA-NPs exhibited about 1.92- and 3.15-fold higher Papp compared to TQ-PLGA-NPs and TQ suspension |
|
|
CS based formulation showed good permeability though the goat intestine model | [164] |
| CS nanoparticles | Coconut oil Tween-80 Lecithin | AmpB | Nephrotoxicity | RP- Slow and sustained release was observed. Improved bioavailability of AmpB compared to control. The ChiAmpB NLC presents a lower risk for nephrotoxicity and higher accumulation in the liver and spleen. |
|
|
The CS encapsulated AmpB lipid nano carrier can potentially address the challenges associated with IV administration of AmpB | [165] |
| Thiolated CS nanoparticles | Thioglycolic acid Mercaptonicotinic acid | Octreotide (OT) | Hormone therapy | %DL- 89 RP- sustained release of OT observed over 24 h. 50% release in 6h. Enhanced 4.1-fold permeation in 3 h and 7.2-fold oral bioavailability compared to OT |
|
|
This approach provided a route for oral delivery of macromolecules such as peptide | [166] |
| CS nanoparticles | Citric acid PEG Propylene glycol (PG) Glycerol | Macromolecule | N/A |
|
|
Citric acid conjugated CS could be potential for oral macromolecule delivery | [167] | |
| CS Micro particles | Alginate Casein | Insulin | Diabetics | %EE- 51.1%. RP- at Ph 6.8 insulin release 48.3% in 2 hr and at end of 8h releases 70.9%. oral Ac-NPs and NiM were gradually decreased blood glucose to 58.4% and 42.1% respectively in 4 and 6 h. NiM shows higher bioavailability and a better hypoglycemic effect on diabetic mice. |
|
|
The micro vehicle loaded with insulin showed better efficacy for lowering blood sugar level | [132] |
| CS Micro particles | Hypromellose (HPMC) Eudragit RS | Indomethacin Fluorouracil Curcumin | CRC | %EE- 58 (curcumin) and 70 (indomethacin). RP- Cur release is negligible; Indo in SGF < 5% release in 2 h, then in SIF (pH 6.8) for 6 h and SCF (pH 7.4) for 6 h resulted in release of 20% and 25%. Curcumin cytotoxicity was enhanced. |
|
|
CS_SPMC based microparticles can ensure targeted delivery of therapeutics to the colon | [168] |
| CS Micro particles | Ascorbic acid (AA) | Selenium (Se) | Alcohol induced gastric mucosal damage | LE- N/A. RP- SeNPs releases in gastric condition pH 1.2. SeNPsCM with doses (0.6–2.4 mg kg-1 bw) were safe to Wistar rats. protect rats from the ethanol-induced gastric mucosal injury. |
|
|
The CS-SE NPs can be considered as a potential agent for oral delivery of Se supplement for treating alcohol induced gastric damage | [169] |
| CS Micro particles | Casein Genipin Transglutaminase (TG) | Nattokinase | Thrombosis-related cardiovascular diseases | %EE- 2.9 mg per 100 mg. RP- 79% of the total loaded nattokinase was released in 12 h. Nattokinase microparticle significantly enhanced the oral effectiveness of nattokinase in prevention and cure of thrombosis in vivo. |
|
|
The bilayer microparticle was capable to protect nattokinase from gastric low pH and ensured controlled release of the drug | [170] |
| Carboxy methyl CS Nanocomposite | Oleic acid (OA) | DTX | Anti-cancer effect | %EE- 57.26 RP- 73 of DTX was released in 72 h at pH 6.8. Apparent permeability of DTX improved 6.57-fold. In vivo pharmacokinetic study demonstrates an increase in Cmax (1.97-fold) and AUC (2.62-fold) for DTX loaded OA-CMCS. Half-life of DTX increases 1.94 fold. |
|
|
The OA-CMCS polymeric carrier is suitable for loading highly hydrophobic water insoluble drug DTX | [97] |
| Acyl CS nanocomposite | Cinnamon oil Tween® 80 PEG 200 as oil | Cefixime (CFX) | Antibacterial activity | RP- 70 % was released at pH 6.8 within 24 h. PK- AUC and Cmax were 2-fold increased for CFX-SNEDDs, whereas 2.6 folds and 3.3 folds increase were observed for CHT-CFX-SNEDDs compared with free drug suspension. |
|
|
ACH-CFX-SNEDD particle showed prominent drug plasma concentration, and hence, better oral performance | [171] |
| Glycol-CS nanocomposite | 3-Aminopropyl-functionalized magnesium phyllosilicate (aminoclay) Eudragit ®S100 |
Insulin | Diabetics | %EE- 90%. RP- at Ph 7.4 in 15 minutes 40% of drug released. Oral EGAC-Ins significantly reduced blood glucose levels in diabetic rats. |
|
|
This work shows colonic transportation of insulin by using a glycol CS-Eudragit based NP | [144] |
| Alginate coated CS nanocomposite | Monomethoxy polyethylene glycolpoly (Lactic-co-glycolic acid) (mPEG-b-PLGA) CS coated NP | Insulin | Diabetes | %EE- 81.5 (alginate coated) and 55.2 (chitosan coated). PR- pH 1.2 release 13.91% in 4 h reches 20.68% in 6h and 47.66% in 60h.atpH 6.8 in 4h 38%, 51% in 10 h and 80.54% in 60h. Reduction in blood glucose levels (decreases 60% compared with the initial level at 12 h) in diabetic rats. Resulted in a maximum plasma concentration (41.5 ± 4.4 [μIU mL1) at 10 h. |
|
|
The doubly coated Insulin-(mPEG-b- PLGA) particles showed pH-dependent behavior upon oral administration | [172] |
5.2. β–glucan
β-glucan is a major structural component of cell walls of fungi and plants especially mushrooms, yeast, bacteria, oats, barley, seaweeds, and algae. β-glucan is monomer of polysaccharide D-glucose linked with β-glycosidic bond with different molecular weight, solubility, viscosity, ad three-dimensional shape [173]. β-glucan enhances activities of macrophages and antimicrobial activity of mononuclear cells and neutrophils [174]. β-glucan derivative β-hydroxyl- β-methyl-butyrate exhibits excellent therapeutic effect on canine colitis by reducing IL-6 and enhancing IL-10 levels. Yeast glucan exhibits positive effect on mice intestinal inflammation caused by DSS. Further properties of β-glucan area such as acts as anti-insulin resistance, anti-obesity, and antioxidant. It is also acting as anti-colitis [175]. Because of range of properties, β-glucan has gained a promising system for oral drug delivery.
5.2.1. β–glucan oral delivery
β-glucan is a biopolymer and widely used for oral drug delivery. It also has different therapeutic effect along with carrier. Various researchers investigated the therapeutic and immunological potential of β-glucan. Liu et al. conducted the study with aim to investigate the potential protective effect of oat β-glucan against DSS colitis in mice [176]. Treatment of β-glucan significantly reduced clinical symptoms with less weight loss, diarrhea and shortening of the colon. Further it reduced disease activity index and degree of histological damage in colon. It also decreases MPO, NO, MDA levels and inhibited TNF-alpha, IL-1β, IL-6, and iNOS. This study concludes that oat in diet can be provides immunity to against colitis. CD4+ T cells or T helper cells are more important in immune system. They remove tumor cells. hence, to elevate CD4+ T level is important to produce infiltrating CD4+ T cells [176]. Zou et al. investigated the inhibition of tumor growth by β-glucan through promoting CD4+ T cell immunomodulation and neutrophil-killing in mice [177]. They have extracted β-glucan from Lentinus edodes. Antitumor activity studies on mice model through intragastric, intraperitoneal and intratumoral injection (Fig. 6A). Interestingly, showed S-180 tumor suppressing ability. It also increased CD4+ T numbers which leads to tumor growth inhibition [177]. This study concluded that β-glucan can be used as effective agent for cancer immunotherapy. Nurunnabi et al. investigated the theoretical and experimentally the interfacial interactions of β-glucan with fat molecules and aqueous media [178]. The major risk factors of triggering cardiovascular, obesity and type 2 diabetics are such as excessive body fat and high cholesterol [179]. They study the dietary effect of barley-extracted on docosahexaenoic acid uptake and impact of the aqueous medium. Theoretically and experimentally their interaction are studies. Density functional theory, Monte-Carlo, molecular dynamics simulations have used for theoretical studies. DFT analysis revealed the β-glucan hydrogen bonding and nonbonding interactions with DHA. Further characterization confirms the electro-static interaction between BG and DHA (docosahexaenoic acid) experimentally. All together the mechanistic pathway is responsively for delayed fat digestion and halting the fat molecule absorption in GI tract [178]. These studies open the new window of β-glucan based oral mucoadhesive drug delivery system.
Fig. 6.
(A) representative photographs of S-180 tumors of mice were subcutaneously inoculated with S-80 cells. 2 mg/kg AG was intraperitoneally or orally administrated to mice daily after tumor inoculation for 1 week: (B) Quantitative expression of eGFP in different tissues/organs of orally administered polyplex and TAG/polyplex. TAG containing particles show duodenumspecific transportation and liver accumulation. Liver accumulation of eGFP for TAG-mediated particles was 3× higher than polyplex; (C) The signal pathway of β-glucan binding to dectin-1. Once they are bond, dectin-1 will activate Syk via double “LxxY” structures, and then trigger NF-jB mainly throughCARD9 or NIK to produce IL-10, IL-2, IL-23, IL-6 and TNF, resulting in T and B cells proliferation, DCs maturation and respiratory burst. Dectin-1 can also trigger NF-jB by Raf-pathway directly. When dectin-1 and MyD88 of TLRs are both activated, there will appear a coupling-signal that prompts the production of TNF-a, IL-10, IL-6 and IL-23, and down regulates the expression of IL-12; (D) The preparation process of YGPs/MTX and the illustration of YGPs/MTX targeting inflammatory sites and suppressing intestinal inflammation after intragastric administration. Reproduced with permission from ref. [177,180–182]. Copyright 2019 Elsevier, 2022 The Royal Society of Chemistry, 2018 Elsevier, and 2020 Wiley-Vch.
5.2.1.1. β–glucan-peptide conjugate.
β-glucan based oral drug delivery system have been studies extensively. β-glucan was used widely in oral vaccine development. Because vaccine oral delivery is a major challenge due to vaccines instability and lack of absorbability. Hence, antigen get degraded. β -glucan has active functional group which allow to conjugate the drug, peptides, and fluorescence probe for biomedical applications. Recently Nurunnabi et al. investigated the PR8 antigen oral delivery using β-glucan-GRGDS conjugate to enhance M-cell targeting ability to induce immunity [183]. For adequate microfold-cell (M-cells) targeting and uptake, they have conjugated β-glucan with glycinearginine-glycine-aspartic acid-serine (GRGDS), which protect antigen in stomach and target to M-cells. Through electrostatic interaction, β-glucan-GRGDS encapsulate antigen and forms nanoparticles size range 200–250 nm. More interestingly nanoparticles showed high cell viability and stability. In vitro M-cell model investigation confirm the M-cell targetability of nanoparticles. Superior results observed in in vivo studies with increasing antibody concentration significantly in serum, intestine, and mucus even on 21 days after oral administration [183].
5.2.1.2. β–glucan micro/nanoparticles.
β-glucan is a good carrier for inflammatory bowel diseases targeted delivery. Sun et al developed targeted delivery of methotrexate using glucan particles for IBD treatment [182]. The clinically used anti-inflammatory drug methotrexate loaded into yeast glucan particles through re-precipitation and gelation reaction. Fig. 6B presenting the yeast glucan particles loaded with methotrexate targeting inflammatory sites and suppressing intestinal inflammation after intragastric administration. These particles showed internalization into Raw 264.7 macrophages through dectin-1 and CR3 receptors. The signal pathway of β-glucan binding to dectin-1 shown in Fig. 6C. It also affectively down regulated the pro-inflammatory cytokines. More intestinally glucan particles accumulated disease site in colitis mice. Particles also suppressed the LPS induced NO production. It targets intestine macrophages and suppress their proliferation and intestinal infiltration and reduces MPO, MDA, NO, TNF-alpha, IL-6, IL-1β, CCL2 [182]. Lee et al. synthesis and functionalize the β-glucan micro/nanoparticles for effective doxorubicin delivery [184]. β-glucan is a polysaccharide biopolymer and one of the entities of cell walls of microorganisms, basidiomycetes, and plants. It can induce innate immunity and control acquired immunity. β-glucan is a biocompatibility, biodegradability high stability, and low toxicity; therefor used in drug delivery. Lee at al. designed porous, hollow β-glucan microparticles with doxorubicin. The results showed that sustained release of Dox and showed excellent antitumor activity against breast cancer cells. immunological activity was observed in culture of immune cells and cancer cells together [184]. It may be activated patients’ immune system which needs to be investigated. Enteric diseases still have an impact on healthcare system. Oral vaccination is a promising strategy to avoid the infections. So far developed vaccines limited their efficacy due to various barriers. Hence, targeted oral vaccines development is needed to overcome the limitations. Baert et al. developed β-glucan microparticles targeted to epithelial APN as oral antigen delivery [185]. They have developed aminopeptidase N (APN)-targeted β-glucan microparticles for antigen delivery. Using bio linker protein G microparticles conjugated with APN and intestinal epithelial receptor. The microparticles showed enhanced uptake by enterocytes dan dendritic cells results the great proinflammatory cytokine response. Compare to control microparticles showed higher serum antigen-specific antibody responses [185]. This study demonstrated the use of β-glucan micro or nanoparticles for antigen targeted delivery. Nanotechnology aimed to enhance immune response against modern antigens. Polysaccharides are particularly interesting candidate for oral delivery. They facilitate recognition by antigen-presenting cells. Xie et al. studied the glucan microparticles targeting M cells through oral delivery [186]. Glucan microparticles labeled with near-infrared fluorescent probe. The in vivo imaging confirms the prolong residence of glucan microparticles in GI tract over 12 Histological studies further revealed the biodistribution and no fluorescent observed in any other organs and tissues. After 12 h, liver, spleen, and lung and after 24 h al, these three showed 2.3% of accumulation. Glucan microparticles successfully transported across caco-2/Raji and Caco-2/Raji/J774A.1 co-culture monolayer. These results confirmed that glucan microparticles absorbed by M cells pathway at Peyer/s patches [186].
Li et al. developed multifunctional PLGA nanoparticles embedded carboxymethyl-β-glucan porous microcapsules for gefitinib oral delivery [187]. The gefitinib loaded FCPP (GFB/FCPP) nanoparticles showed 255 nm size and amorphous state. After encapsulation the size increases to 2.2 um. In vitro release study showed pH responsive prolonged release. Confocal imaging showed excellent cellular uptake. It showed IC50 in A549 cells as 3.82-fold lower than free drug [187]. Altogether conclude that multifunctional nanoparticles are excellent candidate for sustained drug delivery. hydrophilic taurocholic acid (TCA) as an absorption enhancing agent to increase the bioavailability of heparin and heparin derivatives through direct interaction with the bile acid transporter of the small intestine. Taurocholic acid-based formulation such as taurocholic acid linked heparin-docetaxel conjugates for anti-angiogenesis effect and taurocholic acid linked-docetaxel conjugated for cancer therapy through oral administration was investigated [188,189]. Based on these studies, Nurunnabi et al. studied the bile acid linked β-glucan nanoparticles for liver specific oral delivery of biologics. They have introduced hybrid carrier compose of taurocholic acid and β-glucan, which is more effective to protection of biologics in gastric fluid and enhance absorption and transportation through small intestine. We have used eGFP-encoded plasmid as a model biologic. After 4 h incubation, TAG showed two folds higher eGFP expression in the cell. In vivo studies revealed the TAG containing particles showed higher eGFP expression (Fig. 6D) [180]. TAG carrier effective in protection and transportation to liver. This strategy can used for effective oral delivery of biologics to liver for various types of disease treatment. Hwang et al. synthesized the β-glucan nanoparticles for single strand DNA delivery. They used DMSO and water to prepare the nanoparticles with size is 250 nm. ssDNA interested into glucan triple helix structure [190]. The interesting results suggest that β-glucan can be used as carrier for immune response enhancement.
5.3. Alginate
Alginate is natural and mucoadhesive polymer obtained from brown seaweed. Salt of alginate with sodium and calcium used in oral drug delivery [191]. Mucoadhesive activity of alginate is comparatively less. Mucoadhesive activity of alginate depends on molecular weight (MW) such as low MW alginate chains remain rigid than high MW alginate. Hence, low MW alginate results less mucoadhesive than high MW alginate [8]. Hence, in drug delivery system based on alginate cross-linked to enhance mucoadhesive activity. Alginate with combination of other mucoadhesive carriers is extensively studied in oral drug delivery [192].
Alginate is an anionic mucoadhesive polymer. It creates hydrogen bond with mucin type glycoprotein by carboxyl and hydroxyl interactions. It is a linear, water soluble, polysaccharide with 1–4 linkage of alpha-L-guluronic acid and β-D-mannuronic acid. It undergoes gelation with multivalent cations such as Ca2+, results gel [191]. Another mucoadhesive polymer is PEG. PEG is widely used in biomedical application. It is non-toxic, non-immunogenic, water soluble, rapid invivo clearance depends on molecular weight, and non-antigenic United States Food and Drug Administration (US-FDA) approved polymer. PEG has mucoadhesive properties due to feasibility to form hydrogen bond with sugar residues on glycosylated protein [193]. Davidovich-Pinhas et al. synthesized novel mucoadhesive alginate-polyethylene glycol acrylate (alginate-PEGAs) [194]. Alginate-PEGAc showed non-toxic, hence, it can used as potential polymer in many biotechnology applications. This study opens new window to enhance the mucoadhesive properties of polymer for improved mucoadhesive oral drug delivery for gastrointestinal disease treatment [195]. Further, they have synthesized alginate thiol. Alginate and alginate thiol both shown similar swelling nature. This conclude that adding thiol group to the alginate did not affect the swelling property [194]. Bernkop-Schnurch et al. attempted to improve the mucoadhesive properties of alginate through conjugation with cysteine [196]. The thiolated alginate showed 50% of enhancement of viscosity in aqueous mucus. Tablet made of thiolated alginate showed 150 min stability whereas it is just 49 min for alginate alone. All these properties encourage to thiolated alginate use in mucoadhesive oral drug delivery carrier for gastric cancer therapy with improved stability and prolonged residence time [196]. These features offer useful various dose form such as matrix, tablet, and microparticles of alginate derivative for delivery of therapeutics.
5.3.1. Alginate oral delivery
5.3.1.1. Micro/nanoparticles.
Alginate is an anionic polysaccharide extracted from marine brown algae. It forms nanoparticles by ionotropic gelation with divalent cations or cationic polymers. However, they are not stable at room temperature, so the drug loaded into polymeric nanoparticles get leak. Hence, alginate nanoparticles used to coat with suitable materials such as chitosan. Such type of alginate nanoparticles widely used in oral drug delivery. It has reported that curcumin diglutaric acid orally delivered through chitosan/alginate nanoparticles for cancer treatment. Curcumin diglutaric acid is a prodrug of curcumin, which shows good water solubility and antinociceptive activity. To improve properties further Sorasithanukarn et al encapsulated into chitosan/alginate nanoparticles [197,198]. Chitosan/alginate shows good biocompatibility, biodegradability, non-toxicity, musoadhesivity and good film formation properties. Oil-in-water emulsification and gelification method used to prepare chitosan/alginate nanoparticles. The chitosan/alginate mass ratio 0.04:1 is used. The chitosan/alginate nanoparticles showed good stability in SGF and release the CG slowly in SGF without enzymes and in body fluid. The drug release follows the Fickian diffusion and erosion of polymer mechanism. The nanoparticles showed higher cellular uptake and better anticancer activity against Caco-2, HepG2, and MDA-MD-231 cells [197,198]. It concludes that chitosan/alginate nanoparticles system is promising approach for oral administration of CG for cancer therapy. Long et al. investigated the vitamin B12 modified amphiphilic sodium alginate derivatives to enhance oral delivery of peptide drugs [199]. Peptide drugs have been used to treat various diseases. However, they suffer with poor bioavailability. Insulin loaded Vitamin B12 modified amphiphilic sodium alginate derivate (CSAS-B12) nanoparticles (50 nm) has formulated. The CSAD-B12 showed higher permeation ability through intestinal enterocytes in caco-2 cell model. In vivo studies conducted on Type 1 diabetic mice and results shows higher accumulation of nanoparticles at intestinal site, absorption, and higher activity (Fig. 7A) [199]. It indicates that it can be a promising candidate for oral peptide delivery and further studies needed before entering into clinical applications.
Fig. 7.
(A) The retention effect and absorption of FITC-insulin, CSAD/FITC-insulin, CSAD-VB12/FITC-insulin in intestine. (A) In vivo animal image system showed the fluorescent signal of FITC-insulin (A1), CSAD/FITC-insulin (A2), CSAD- VB12/FITC-insulin (A3) at the 1 h after oral administration in T1D mice. (B) Representative fluorescence imaging of small intestine from mice treated with FITC-insulin (B1), CSAD/FITC-insulin (B2), CSAD- VB12/FITC-insulin (B3); (B) Histological score of colon tissues by hematoxylin and eosin (HE) and alcian blue (AB) staining after treatment of Ax-Alg to DSS colitis mice. (C) Schematic illustration of the preparation process and the design concept of the proposed intestinal-targeted CAP carrier for pH-responsive protection and release of lactic acid bacteria. (a, c) CA beads prepared by a coextrusion method. “A” is Na-alginate solution containing Lactobacillus casei, and “B” is pure Na-alginate solution. (b, d) CAP beads prepared by adsorption of protamine molecules. (e) Ingesting CAP beads in mouth. (f) CAP beads offer improved protection for Lactobacillus in stomach. (g) CAP beads release Lactobacillus casei rapidly in the small intestine. Reproduced with permission from ref. [199–201]. Copyright 2022 Elsevier, 2019 Dove press, open access, and 2014 American Chemical Society.
Salmonella enterica serovar gallinarum cause fowl typhoid. Currently injections available but has limitations such as efficiency significantly reduced in gastric acid. Hence oral vaccines delivery to treat fowl typhoid not explored yet. Towards this direction, Onuigbo et al. developed oral delivery of fowl typhoid vaccine using chitosan/alginate microparticles [202]. They have evaluated the immune response of birds treated with fowl typhoid vaccines coated with chitosan/alginate microparticles. They have used 6 days old chicks. Vaccination done at 10 weeks and 14 weeks of age followed by challenge at 16 weeks of age. The vaccine loaded microparticles range between 0.5 to 10 μm and 60% of EE. The results shows that ELISA E-values 0.10, 0.07, and 0.02 for OCV 567, SC 634 and NEG 451, respectively after vaccination, which are 0.25, 0.19, and 0.0008, respectively after boost vaccination [202]. The results conclude that there is no significant difference the vaccine administration route. However, coating the chitosan and alginate protect the vaccines destruction in GI tract. This study demonstrated that vaccines could be delivery through oral route using mucoadhesive polymers.
In last decades, various drug delivery system such as floating, mucoadhesive, high density, and swelling have gained great attention. However, any one of the systems not able to overcome all the challenges associated with oral delivery. Hence, researchers working interdisciplinary to design the drug delivery system using combination of different systems. Floating and mucoadhesion combined approach receiving overwhelming attention [203]. In this direction, Bera et al. fabricated the core shell alginate-ghatti gum modified montmorillonite core-shell nanoparticles for stomach-specific flurbiprofen delivery for intragastric fluorbiprofen delivery through combination of floating and mucoadhesion mechanism [204]. Formulated core-shell nanoparticle at optimum condition has 91% drug encapsulation efficiency. The drug release from the nanoparticles through anomalous diffusion mechanism [204]. Alginate-AG gel membrane coated alginate modified MMT core shell nanoparticles are appropriate for intragastric delivery of flurbiprofen for long term with improved therapeutic activity. Ling et al. fabricated alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles [205]. Protein nanoparticles increases the intracellular delivery of enzymes. The gastro protective microparticles of alginate and chitosan used to deliver the protein, which offer retention of activity in SGF. Whereas in SIF it released the protein. Oral administration of microparticles reduced inflammation in DSS colitis model [205]. This suggested that alginate/chitosan micro/nanoparticles are potential oral delivery vehicle for protein delivery.
5.3.1.2. Micro/nanocapsules.
Li et al developed alginate hydrogel/gum arabic/gelatin based composite capsules and their application for oral delivery of antioxidants [206]. They have formulated composite capsule loaded with antioxidant Perinereis Aibuhitensis (PaE) loaded gum arabic/gelatin microcapsules in calcium alginate hydrogel (PaE:CA/GA/GECCs). In vitro studies confirm that it is potential to protect PaE in gastric acid. It shoes scavenging capacity 1.8-fold higher in SIF. The in vivo studies revealed that after oral administration for 30 days, the oxidative stress reduced significantly and lower malondialdehyde content in liver cells [206]. This study revealed that composite capsules could be interesting carrier for intestinal targeted delivery to enhanced absorption potential. Shamekhi et al. developed chitosan coated calcium alginate nanocapsules for oral delivery of liraglutide for diabetic patients [207]. The oral sustained delivery of lirglutide achieved through nanocapsules. Nanocapsules compose of 0.5% chitosan, 0.5% alginate, calcium chloride 0.5% in the volume ratio 3: 1:1. The nanocapsules showed 92% EE and 54% lirglutide. Nanocapsules stable over 60 days at 4 °C. In SGF 595 of lirglutide release in 6 h [207]. This study demonstrated that natural biodegradable polymer based nanocapsules are very interesting for oral drug delivery.
Huang et al. used chitosan-alginate capsules for oral probiotic vaccine expressing koi Herpesvirus ORF81 protein delivery [208]. They have demonstrated the mucoadhesive polymeric capsules as a promising strategy for mass oral vaccination of carps against KHV infection. The oral probiotic vaccine pYG-KHV-ORF81/LR CIQ249 encapsulated into chitosan-alginate capsules as oral drug delivery system for treatment of koi carp against koi herpesvirus infection. It protected vaccine from digestive system. The oral vaccination significant level of antigen specific IgM induced and showed KHV-neutralizing activity. It also offers handling stress free, cost effective, and suitable for mass oral vaccination [208]. This study confirms the mucoadhesive polymeric oral drug delivery system can be a promising candidate for vaccine delivery.
5.3.1.3. Microspheres.
Astaxanthin is a carotenoid pigment having antioxidant activity. It is found in algae, shrimp, and yeast. The major benefits of it for human health is anti-lipid peroxidation, anti-inflammation, anti-cancer and anti-diabetes, and immunomodulation. But use of it is limited due to poor water solubility and highly unsaturated structure [209]. Zhang et al. fabricated astaxanthin-enriched colon targeted alginate microsphere for effective drug delivery to dextran sulfate sodium-induced ulcerative colitis in mice [200]. Through high-pressure spraying and ionic gelation method, they fabricated Astaxanthin rich alginate microsphere, and most of the particles are in the rage of 0.5–3.2 μm in a diameter. Microsphere tolerated in mouth, stomach, and small intestine, and release Astaxanthin in colon due to fermentation of gut microbiota. DSS colitis model mice treated with microsphere through oral gavage add highly showed reduction of DSS colitis, weight loss, oxidative damage, inflammation, colon mucosal integrity, and disease activity index. H&E staining analysis further confirm the reducing the histological score in treatment group (Fig. 7B) [200]. More interestingly, microsphere regulated the gut microbiota composition. This approach is promising for colon targeted delivery for therapy to colon diseases. It is safe, simple, and inexpensive. However, further detailed in vivo studies need to conduct. Icariin is an active flavonoid glucoside isolated from Herba epimedii. Icariin’s pharmacological activities such as anti-aging, anti-tumor and anti-inflammatory has confirmed. However, solubility is the major challenge to limit its use [210]. Wang et al. icariin loaded alginate-chitosan microsphere formulated for treatment of ulcerative colitis [211]. The therapeutic activity of formulation has studied in trinitrobenzene sulfonic acid/ethanol induced colonic mucosal injured rat model. Microsphere able to protect the icariin and release only 10% in simulated gastric fluid and 65% of icariin releases in 12 h, at colon. Formulated significantly reduced the colonic injury, but also showed reduce inflammatory responses through decreasing the production and gene expression of inflammatory mediators and cytokines in colon mucosa [211]. These studies showed that there are plenty of opportunities to treat for oral delivery to treat different disease at in-vivo and in vivo studies. However, clinical studies are very limited and most of them may have various limitation. Clinical studies need to increase research organization to make the productive research toward public healthcare.
5.3.1.4. Beads.
The ulcer and chronic gastritis are the results of poor diet, spicy food, stress, smoking, and other lifestyles. Hence, development of controlled drug delivery nanoparticles with gastroretentive ability promising approach to enhance bioavailability and activity for treatment of H pylori. Raafat et al. formulated gastroretentive amoxicillin trihydrate floating alginate-based beads for treatment of H. pylori using radiation [212]. Alginate has modified with N, N-dimethyl amino ethyl methacrylate achieved through radiation. Alg-g-DMAEMA copolymer-based amoxicillin-trihydrate floating hollow beads formulated using calcium chloride and calcium carbonate have used as cross linker and gas forming components. Nanoparticles showed excellent retention time in gastric fluid 24 h, 97% of drug encapsulation, and controlled release of drug for 15 h. More interestingly, in vitro studies confirm the 95% of eradication of H. pylori [212].
Probiotic lactic acid bacteria have many health benefits on the human health because they improve intestinal flora, strengthening immunity, inhibiting tumor growth, and promoting nutrient absorption. However, lactic acid bacteria will die before they reach to small intestine when it contacts with the gastric acid in stomach [213]. Hence, for intestine targeted delivery, formulation need to protect from acidic environment. Mei et al. developed novel intestine targeted calcium alginate beads carrier for pH sensitive protection and release of lactic acid bacteria [201]. The fabricated Ca-alginate/protamine (CAP) shell and lactobacillus-casei-encapsulated ca-alginate (CA) core. The CAP provide protection to lactobacillus casei but also offer intestinal targetability. When beads reach to small intestine, carrier dissolved in neutral environment and cooperation between protamine and trypsin (Fig. 7C) [201]. The beads at pH 2.5 simulated gastric acid after 2 h immersion bead prepared with 25% (w/v) Na-alginate has 46% of lactobacillus casei. It is 60 times low than ordinary CA bead. In 50 minutes, 100% release of drug achieved in pH 7 simulated intestinal fluid, and it is 380 min for normal ordinary CA bead [201]. This carrier provides novel efficiencies of protection and controlled release and targetability.
The colon targeted drug delivery is important to treat colon diseases such as ulcerative colitis, Crohn,s disease, amebiasis, colonic cancer, etc. Number colon targeted drug delivery systems have been developed such as drug-polymer conjugates, pH responsive polymers such as Eudragit polymers, mucoadhesive polymers such as chitosan, alginate, biodegradable polymers [214]. Recently novel systems such as pressure-controlled drug delivery, CODESTM (combined approach of pH dependent and microbial triggered drug delivery), osmotic pressure-controlled drug delivery, and multi particulate system [215]. Agarwal et al. explored pH responsive swelling, musoadhesivity, colon microflora-catered biodegradable drug delivery system for colon targeted delivery [216]. They fabricated calcium alginate carboxymethyl cellulose (CA-CMC) beads through ionic gelation for colon targeted drug delivery. The formulated beads show slow degradation in colonic fluid and nearly 90% of drug (5-fluorouracil) release presence of colonic enzymes. Further studies such as cytotoxicity, nuclear condensation-fragmentation and apoptosis analysis studies against colon adenocarcinoma cells proven the therapeutic potential of formulated beads [216].
5.3.1.5. Hydrogel.
Hydrogels are three-dimensional network of polymer having great potential to use as drug delivery carrier, scaffold in tissue engineer, biocomponents, and food systems. Biocompatibility, biodegradability, and ability to absorb biological fluid, and more amount of water offer their use in controlled delivery system. Recently natural and synthetic polymers gained interest in hydrogel. The most widely used polymers are PEG, PVA, PHEMA (poly (2-hydroxyethyl methacrylate)) and natural polymer such as chitosan, alginate, agarose, hyaluronan, collagen, fibrin, pectin, xanthan gum, guar gum, and gelatin [217,218]. In recent years, hydrogel showed wide variety of application. Pharmaceutical and medical industries also showing an interest to use the biopolymers. Cikrikci et al. developed pH sensitive alginate/gum tragacanth-based hydrogel for oral insulin delivery [219]. Fig. 8A presenting the release and absorption of insulin from intestine following oral administration of hydrogel-based systems. Insulin loaded alginate-gum tragacanth hydrogel prepared through ionotropic gelation method and chitosan polyelectrolyte complexation. Hydrogel showed nearly 100% of insulin retention in simulated gastric environment and released insulin sustain manner in simulated intestinal buffer. This showed the pH sensitivity of hydrogel [219]. The detail characterization and investigation suggest the ALG-GT gel formation at optimum concentrations can be a potential carrier for insulin oral administration. This study concluded that hydrogel of biocompatible and biodegradable, nontoxic natural polymers can be a good choice of oral inulin delivery. Yin et al. investigated the potential of agar and alginate composite hydrogel in oral drug delivery [221]. They attempted to enhance the mechanical strength of calcium alginate hydrogel for sustained drug delivery. They prepared pH responsive composite gel beads of agar and alginate in the presence of agar. The detail characterization of hydrogel revealed the enhancement of mechanical strength compared to alginate beads without agar. The content influences the swelling and release behavior. At higher agar content lower the rate of swelling and released the drug in 720 min. In vitro studies further, confirm the potential of hydrogel for controlled drug delivery [221]. Ilgin et al. synthesized the pH responsive alginate-based hydrogel for oral drug delivery [220]. They have developed pH responsive alginate hydrogel for oral colon targeted delivery. Water absorption efficiency of hydrogel investigated under the influence of various monomer composition and tuning the conditions such as salt, pH, and temperature. In vitro and in vivo studies of diclofenac sodium has studied at gastric pH 1.2 and intestinal pH 7.0. Antibacterial potential studied using gram-positive and gram-negative bacteria. The formulated hydrogel showed 22.8% drug loading and drug release profile showed 4.5% of diclofenac sodium release at pH 1.2 in 3 h and 95% at pH 7 at 20 hr. Antibacterial studies revealed that hydrogel is not harmful (Fig. 8B) [220]. Various mucoadhesive alginate polymer based oral drug delivery literature is summarized in Table 5.
Fig. 8.
(A) Schematic diagram showing the release and absorption of insulin from intestine following oral administration of hydrogel-based systems; (B) Digital photographs of antimicrobial activity tests of drug-loaded and unloaded semiIPN hydrogels (a) Schematic representation of the test application for control disc, H1, H2 and H3 hydrogels (top to bottom), or the bacteria applied (b) E. coli. (c) B. subtilus (d) S. aureus. Reproduced with permission from ref. [218,220,220]. Copyright 2012 Elsevier, 2020 the Polymer Society, Taipei.
Table 5.
Alginate based mucoadhesive oral drug delivery system.
| Mucoadhesive drug delivery system | Other composition used | Drug | Therapy for disease | % Of Encapsulation efficiency (EE), % of drug loading (DL) and Release Profile (RB), Drug efficacy (DE), in-vitro, Ex-vivo, In-vivo, Pharmacokinetics (PK) and Pharmacodynamics (PD) | Pros | Cons | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| Alginate nano particles | Casein NP | Curcumin | Anti-tumor effect | %EE- 70 RP- curcumin releases 77.5% at 24 h in SIF. PK- in vivo pharmacokinetic parameters (Cmax, Tmax and AUC0–24) for Alg-ch@CurCasNPs were significantly better than those for free curcumin. Oral administration of Alg-ch@CurCasNPs (6 doses/2 weeks-600 mg/kg) in mice showed higher therapeutic efficacy against Ehrlich carcinoma. |
High encapsulation efficiency
|
The synergistic effect of the NP composition was not analyzed | Suitability for oral delivery of curcumin by using biodegradable polymers | [222] |
| Alginate nano particles | CaCl2 | l-Cysteine (Cys) | Antioxidant effect | %EE- 79.49 RP- The Cys release was 94.33% in PBS pH 7.4, whereas it was 11.78% in HCl pH 1.2 within 720 min. |
|
|
The Ca-ALG carrier has better swelling efficiency which made it profound for oral Cys delivery | [223] |
| Alginate nano particles | CS | Lactobacillus expressing pYG-KHV-ORF81/LR CIQ249 | Koi herpesvirus (KHV) | Chitosan-alginate capsules protected the probiotic vaccine pYG-KHV-ORF81/LR CIQ249 from harsh digestive environments. Vaccine has a good immunogenicity and displayed effective KHV-neutralizing activity. |
|
|
The oral probiotic vaccination encapsulated with ALG-CS has been proposed for treating KHV | [208] |
| Alginate nano particles | PCL Pluronic127 (F127) CS |
Insulin | Diabetes | %EE- 60 RP- SGF (Ph 1.2) 46% and 62% of insulin released from PCL/CS/ALG and PCL/CS respectively. When the particles were transferred to SIF, the release rate increased briskly to about 48 % within the first 2 h and 60 % after 6h. |
|
|
The ALG-CS utilizes the pH responsive properties of the polymer to release insulin | [224] |
| Alginate nano particles | CS Deoxycholic acid (DCA) |
Insulin | Diabetes | %EE- 61.14 % DL- 3.36 RP- 28% insulin was released in 0.1 M HCl after 2 h. SIF insulin released 70.8% after 12 h (pH 6.8 PBS) and 92.9% after 36 h (pH7.4 PBS). Oral bioavailability of 15%. The CDA NPs (40 U/kg) improved the hypoglycemic effect of insulin by approximately 7.2 fold when compared to oral insulin solution at the same dose |
|
No in vivo toxicity effect was tested | pH responsive CS-DCA-ALG carrier is capable to protect insulin from degradation in the gastric environment and can enhance NP adhesion in the intestinal villi | [225] |
| Alginate nano particles | Aloe vera | Insulin | Diabetes | %EE- 47.3 |
|
Release profile not studied. In vivo or in vitro toxicity study was not done |
Biocompatible ALG-aloe vera nanocarrier for oral delivery of insulin shows promise for enhanced intestinal cell transportation | [226] |
| Alginate nano particles | DNA-nanocube Poly-L-Lysine |
Vildagliptin (VI) | Diabetes | %EE- 89.23 (DNA) and 83.35 (VI). It shows superior oral antidiabetic effects with improved GLP1 and glycemic levels. In mice, nanoparticles reduced adverse events and better control of glycemic levels |
|
|
ALG-DNA nano cube carrier showed uniform size distribution, less toxicity and better therapeutic delivery effect | [227] |
| Alginate nano particles | Layered double hydroxide nanoparticles (LDHs) CS Tripolyphosphate (TPP) CaCl2 |
BSA | %EE- approximately 60–65% (0.75 mg/mg). RP- 40% of BSA-FITC was released from ALG-CHT-LDH rapidly in 10 min and then retained at pH 1.5 for 2 h. The ALG-CHT coating protected protein release at the acidic condition (pH 1.2). |
|
|
ALG-CS coated LDH exhibited better protein internalization by the Caco-2 and macrophage cells | [228] | |
| Alginate Hydrogel | [3-(Methacryloylamino) propyl] trimethylammonium chloride (MAPTAC) Methacrylic acid (MA) 2-Hydroxyethyl methacrylate (HEMA) N,N' –Methylenebisacrylamide (MBA) N,N,N ',N'-Tetramethylethylenediamine (TEMED) Polyvinyl alcohol (PVA) |
Diclofenac sodium | Anti-inflammatory | %EE- 22.8%. RP- at pH 1.2, 4.5% of drug release in 3 h and at pH 7.0 95% of drug releases in 20 h. Formulation also showed antibacterial effect. |
|
|
ALG based pH responsive carrier showed great promises for oral drug delivery | [220] |
| Alginate Hydrogel | N/A | N/A | N/A | RP- The synthesized beads completely dissolved in 3h time |
|
|
|
[191] |
| Alginate Hydrogel | Gum Arabic (GA) Gelatin (GE) Stearic acid Tween-80 Tannic acid |
Fucoxanthin (FX) | Anti-obese Anti-oxidation Anti-cancer |
%EE- 71.11 (beads) and 80.62 (microcapsules) %DL- 0.32 (beads) and 0.93 (microcapsules). RP- in SGF microcapsules released 31.93 and beads releases only 3%. In SIF, 90% of the drug was released in the SIF in 4h. |
|
|
FX loaded GA-GE microcapsule was prepared via sonication-mixing process which was further encapsulated in ALG hydrogel which showed better efficacy for oral administration of FX |
[229] |
| Ca-alginate Hydrogel | GA, Gelatin (GE) Stearic acid Tween-80 Tannic acid |
Perinereis aibuhitensis extract (PaE) | Antioxidant | CA hydrogel could protect PaE in stomach and sustain releases in intestine and make it maintain high antioxidant activity while incubated in SGF. After, 30 days of administration, the mice of PaE:CA/GA/GE-CCs group suffered significantly lower oxidative stress level than those of other groups, |
|
|
Showed profound efficiency for decreasing oxidation stress after 30 days of treatment | [206] |
| Alginate nanocomposite | Carboxymethyl CS | Purple sweet potato extract containing high anthocyanin | Type 2 diabetes | – |
|
|
CMCS-ALG carrier is suitable for delivering purple sweet potato extract to the circulatory system | [230] |
| Alginate Nanocomposite | Whey | Iron sulfate Bovine spray-dried blood cells (BC) | Anemia | %DL- 3 to 34 mg per matrix. RP- Iron was releases 2–11% in SGF, whereas it was in SIF 83–100% in 2h. |
|
|
The ALG-whey based carrier system could be potentially effective for oral iron supplement | [231] |
| Amphiphilic alginate | Vitamin B12 N, N’-dicyclohexylcarbodiimide Cholesterol |
Insulin | Diabetes | %DL- 34%. RP- in SGF and SDF insulin releases less than 15% and in SIF it was 15%. Nontoxic to caco-2 cells. CSAD-VB12/INS nanoparticles induced more obvious reduction of blood glucose level by 54% compared with 25% for CSAD/INS group within 12 h at the same insulin dose of 70 IU/kg |
|
|
Amphiphilic ALG-VB12 carrier could be promising oral drug administration because of its better intestinal transporting efficiency | [232] |
| Alginate microparticles | Cyperus esculentus (Tiger nut) CaCl2 |
Ibuprofen | Gastrointestinal irritation |
%EE- 46.05–89.86%. RP- <8% in SGF in 6h. however, in SIF in 6h 83% of drug released. |
|
|
The cross-linked starch-ALG carrier has the potentiality for sustain release of the drug in the GI tract | [233] |
| Alginate microparticles | CaCl2 | Losartan Potassium |
Hypertension | %EE- 73.06–79.06 %DL- 12.05 to 18.2 RB- 98.51% of the drug was released from F3 formulation in 12h. |
|
|
This study represents the formation ALG based microbead particle for oral delivery of hypertension drug | [234] |
| Alginate Microparticles | Arginine CS |
Ivermectin Praziquantel |
N/A | An in vivo study of oral administration to teleost fish revealed that the bioparticles attain the intestine mucus and further, the interaction with the intestinal mucosa is timely dependent. The in vivo study endorsed that the bioparticle provides high compliance |
|
|
The polygonal structure of the carrier could be useful in drug delivery | [235] |
| Alginate Microparticles | Folic acid (FA) Eudragit (S100) |
Irinotecan hydrochloride trihydrate (IHT) | CRC | %EE- 76 (IRSLN3) and 72.6 (IRSLNF3). %DL- 36.3 (IRSLN3) and 35.2) (IRSLNF3) RP- In the case of EuBIRSLN3, the IHT release was detected to be 30.66% and for EuBIRSLNF3, it was 26.29 after 12h. The release of the drug in the intestinal region only (i.e., pH>7.0). The FA-coupled microbeads (99mTcEuBIRSLNF3) distributed higher (19.62 ± 0.78%) amount of drug (i.e., 99mTc-IHT/g of tissue) as compared to uncoupled microbeads (99mTc-EuBIRSLN3, 7.63 ± 0.49%) in the colon tumor after 48 h. |
|
|
IHT conjugated FA-SLNs encapsulated ALG-Eudragit carrier is highly efficient for delivering drug to colorectal tumor region | [236] |
| Hybrid Alginate nanoparticle | PEG cholesteryl methacrylate (CMA) Palmitoyl-2-oleoyl-snglycero- 3-phosphocholine (POPC) 1-palmitoyl-2-oleoyl-sn-glycero-3- phospho-L-serine sodium (POPS) |
N/A | N/A | – |
|
|
PEG-lipid hybrid vesicle entrapped in ALG carrier can be a suitable cargo for oral drug delivery | [237] |
5.4. Pectin
Pectin is a natural and mucoadhesive polymer with biodegradability, biocompatibility, non-toxic and heterogeneous polysaccharide. Pectin can be extracted from citrus peel and apple pomace [238]. Henri Braconnot first isolated pectin in 1825 [239]. It is a part of human diet but do not contribute nutrition significantly. It is a linear chain polymer with 1–4 linkage of d-galacturonic acid residues, which have carboxyl group [240]. Pectin follows two mechanisms of mucoadhesion, first is hydrogen bonding (carboxylic group of pectin) with mucin and electrostatic interaction between pectin and mucin molecule [238]. Interestingly, Jorgensen et al. investigated effect of MW of pectin on mucin layer penetration efficiency and reported that low MW pectin has more penetration on mucin layer easily [241], Pectin with low degree of esterification shows higher mucoadhesion activity than high degree of esterification. To improve the mucoadhesion activity of pectin, researchers studied the combination of pectin with other polymers such as pectin-gellan gum beads, modified pectin-acrylate combined carrier, pectin – jackfruit seed starch beads [8]. Recently, atomic force microscope (AFM) used to study and visualize the absorption of pectin on mucosal cell surface [238]. Thirawong et al. studied the pectin-mucin interaction using viscosity in various media [242]. After extensive investigation, scientist found pectin as a promising mucoadhesive polymer for oral drug delivery.
5.4.1. Pectin mediated oral delivery
Pectin has various functional group such as hydroxyl, carboxyl, carbomethoxy, and acylamino which area capable to synthesize broad spectrum of derivatives. Fig. 9 showed the schematic of potential reactions and products of pectin [243]. Due to above properties pectin-based hydride materials for drug delivery applications. Solid lipid nanoparticles stabilized by emulsifier with particles size below 1000 nm made great success in last 30 years. Various drug delivery system and products have been developed based on SLNs. SLNs widely used in oral delivery [244,245]. Due to thickening, gelling and degradation nature, pectin can be a potential polymer for coating to enhance stabilization of SLNs. Pectin coated SLNs showed enhanced stability and encapsulation efficiency. SLNs showed lower melting point due to Kelvin effect [246]. Drug release has controlled in pectin coated SLNs where as normal SLNs showed burst release. Pectin also used in targeted delivery to colon due to pH and enzymatic activity. Pro-drug approach can be used for colon targeted delivery. EDC/DMAP used as cross linker to prepared pectindihydroartemisinic (DHA) conjugates (PDC) which undergo self-assembly with hydroxy camptothecin (HCPT). The formed pectin-DHA/HCPT nanoparticles (70 nm) achieved 20% of DHA loading and 14% of HCPT encapsulation. Nanoparticles showed 4.8-fold and 6.8-fold higher retention in blood respectively compared to DHA and HCPT. Survival rate of 4T1 tumor bearing mice increasing compared to free DHA and HCPT (Fig. 10A) [247].
Fig. 9.
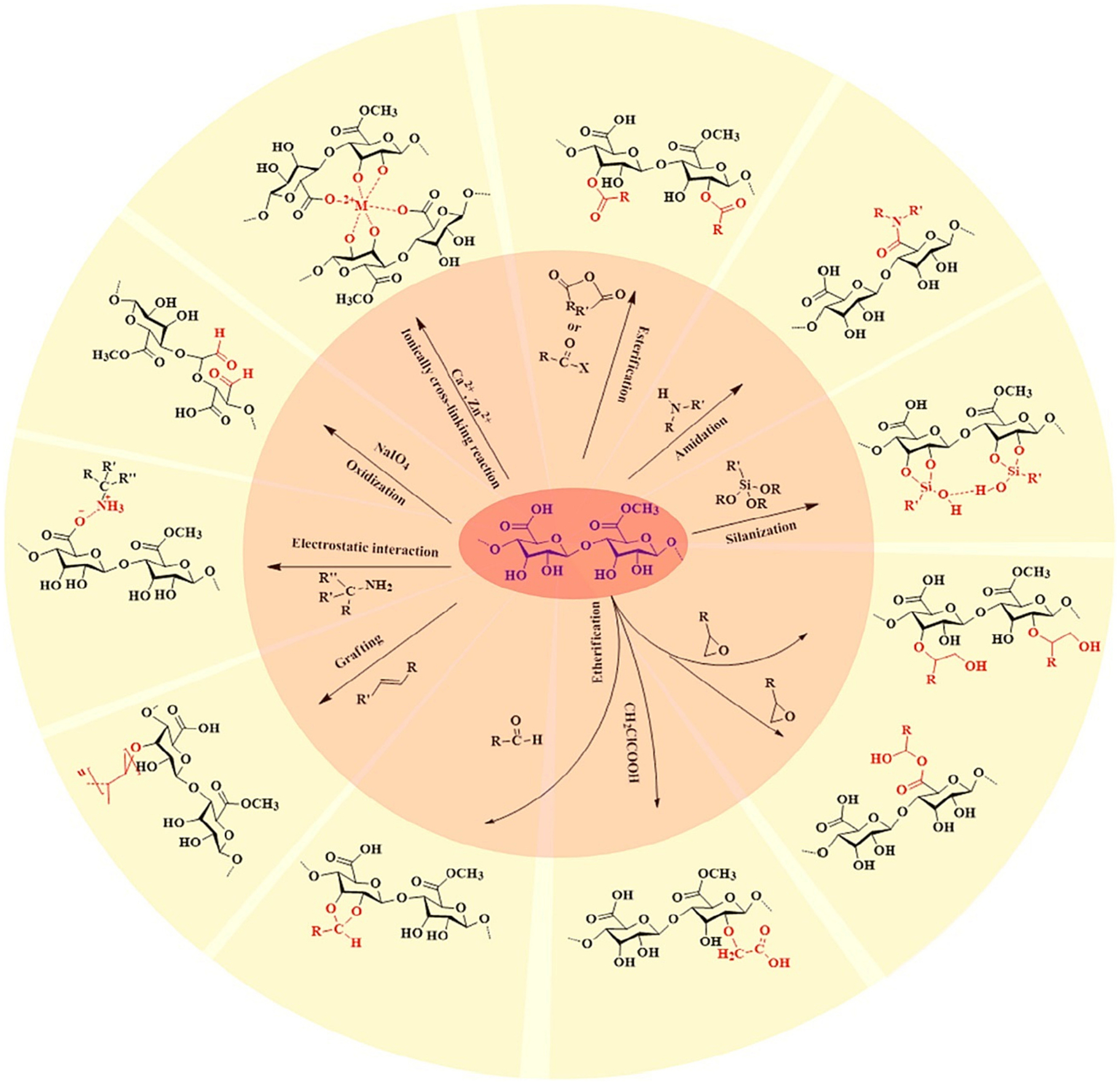
Potential reactions of pectin for synthesis of various derivatives. Reproduced with permission from the ref [243]. Copyright 2021 Elsevier.
Fig. 10.
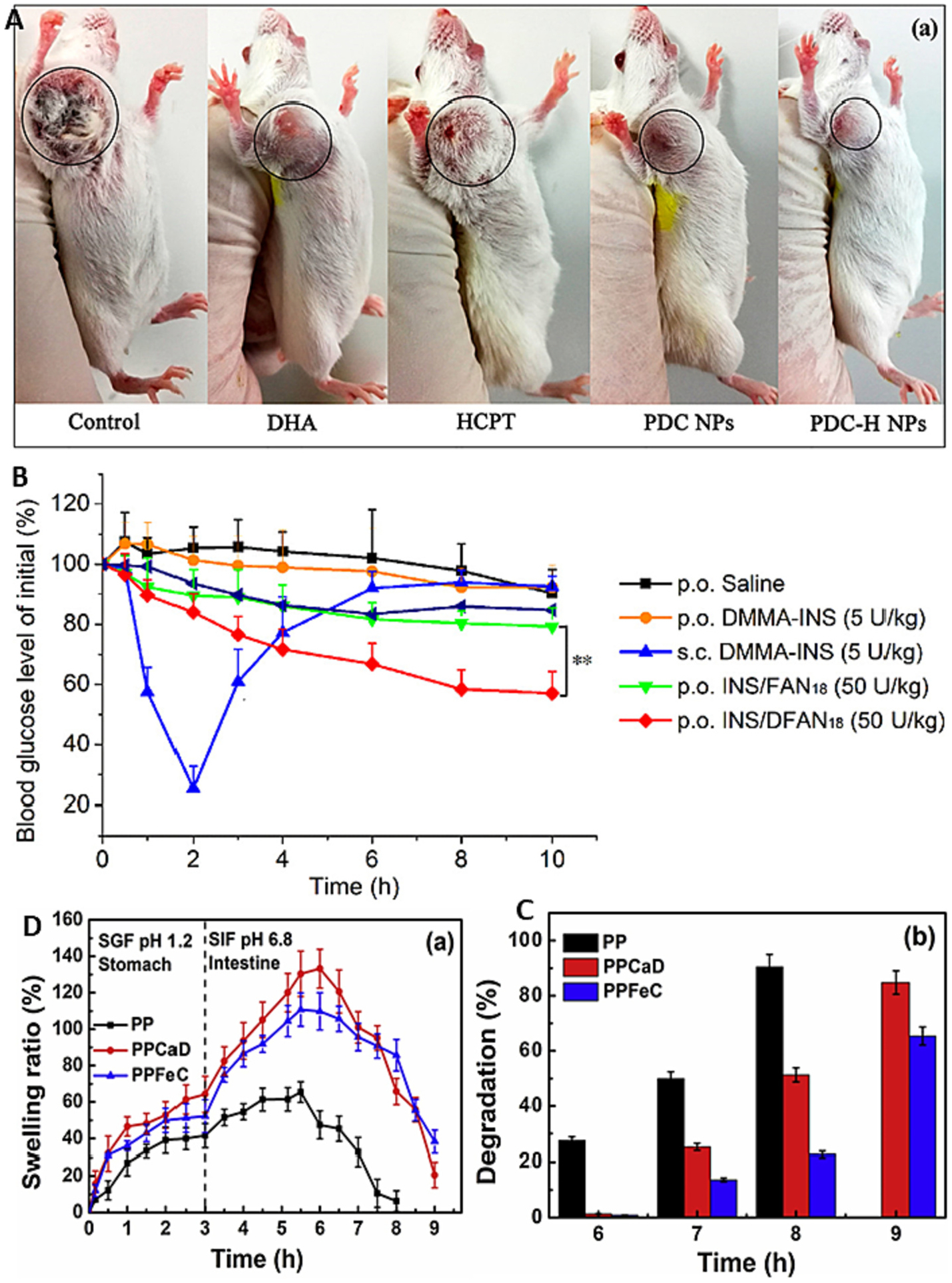
(A) Tumor photographs of each treatment group on day 24 of In vivo antitumor activity of free DHA, free HCPT, and nanoparticles in the subcutaneous mouse model of 4T1 cells. Tumor photographs of each treatment group on day 24; (B) Blood glucose level vs. time profiles of STZ-induced diabetic rats administrated with insulin solution, INS/FAN18 and INS/DFAN18 through different routes; (C) (a) Swelling ratio of the hydrogels PP, PPCaD and PPFeC in SGF at pH 1.2 and in SIF at pH 6.8 (b) degradation behavior in SIF at pH 6.8 for various hydrogels. Reproduced with permission from ref. [247–249]. Copyright 2022, 2019 Elsevier, and 2017 American Chemical Society.
5.4.1.1. Pectin-based nanoparticles.
Over the past decade, great progress has devoted on oral drug delivery system. However, only few formulations show therapeutic promise in clinical trials. Zang et al. developed oral insulin delivery system through dual crosslinking of folic acid modified pectin nanoparticles [250]. Pectin has excellent gelling property, good mucoadhesive nature, and high stability in gastrointestinal tract; it offers great potential for oral drug delivery with having capacity to hold drug. However, pectin alone cannot be able to target the enterocytes. Toward this direction, Zhang et al. developed folic acid modified pectin nanoparticles (INS/DFAN) for insulin oral delivery using cross linker calcium ions and adipic di hydrazide [250]. The in vitro studies revealed that the release of insulin depends on COOH/ADH molar ratio. INS/DFAN with FA graft ration of 18.2 showed small size of particles, high encapsulation efficiency, and high stability and cellular uptake. The formulated nanoparticle administered orally to type I diabetic rats, which showed considerable amount of blood glucose level reduction, and improved oral bioavailability (Fig. 10B) [250]. Altogether, this study conclude that dual crosslinking and FA modification is an effective strategy to develop pectin-based nanocarriers for insulin oral delivery. Prezotti et al. developed gellan gum/pectin nanoparticles for oral colon targeted delivery of resveratrol [251]. They used nebulization/ionotropic gelation method for synthesis of nanoparticles having size 330 nm, spherical share shape and more than 80% of drug loading. Drug release and permeability studies using caco-2 cell model and mucus secreting triple co-culture model. The mucoadhesive polymeric nanoparticles showed 3% of resveratrol release in 2 h in gastric acid media and 85% in 30 h in pH 6.8. The permeability achieved 5.5% [251]. This study indicated that mucoadhesive pectin-based nano-particles are safe and promising carrier for controlled and targeted delivery of resveratrol to the colon.
5.4.1.2. Pectin-based microparticles.
Deshmukh et al. formulated microparticles of amidated pectin for controlled delivery of sulfasalazine for inflammatory bowel disease [252]. They have used ionic gelation method to fabricate amidated pectin microparticles. The microparticles loaded in Eudragit S 100 coated hard gelatin capsules for pH and time dependent drug delivery to colon for IBD treatment. The optimum formulation showed 463 nm size, -32 zeta potential, 91% yield and 95% EE. The swelling index are 0.88 and 0.98 at pH 6.8 and 7.4, respectively. Drug releases 91% in simulated colonic fluid and 98% in rat cecal content for 24 h. The capsule dissolved at colonic pH 7.4 and release the drug from microparticles when administered to rabbit orally. The microparticles showed great stability and exhibited 3.3 Years of shelf life [252]. This study concluded that amidated pectin microparticles filled with Eudragit S 100 coated hard gelatin capsules can be potential delivery system for IBD management.
5.4.1.3. Pectin-based microspheres.
Parietal cells secrete the hydrochloric acid hence gastric pH always 1.7 – 4.7 but vary based on fast and fed state. It increases gradually in small intestine to pH 5.9 – 6.3 and in the proximal segment it is 7.4 – 7.8. The colon pH is 5 to 8 [253]. Several drug delivery systems used pH for colon delivery. Hence pectin coated nanoparticles system used as colon targeted delivery. The enteric coating protects in stomach and release in colon. Several enteric coating-based products available such as Mesren® MR, Salofalk®, Asacol® MR, Ipocol®, budenofalk®, Entocort® commercialized [254]. Vaidya et al. formulated metronidazole loaded pectin microsphere for colon targeted delivery [255]. Microspheres fabricated through emulsion-dehydration method and coated with Eudragit S-100. In-vitro studied showed no release of drug at gastric pH and release continuously at colon pH. The release increases presence of rat caecal contents. Biodistribution in-vivo studies confirm the accumulation of microsphere in colon [255]. This study provided a Eudragit coated pectin microsphere can be a potential colon targeted drug delivery nanocarrier. Pectin extensively studied for colon targeted and excellent in-vitro and in-vivo therapeutic activity was reported widely in literature [256]. The major limitation is that not suitable for stomach, small intestine, and intestine lymphatic targeting. This is challenging and we strongly believe that scientific community can achieve it soon.
5.4.1.4. Pectin-based beads.
Lack of toxicity and low cost of production get great attention towards formulation of controlled release dosage. It used in matrix tablets, gel beads and gel-coated pellets. Drug bioavailability and absorption can be increases significantly using pectin based floating drug delivery approach. Pectin is interesting for colon targeted drug delivery due to it degraded by colonic pectinolytic enzymes [240]. Iron deficiency is the most common nutritional deficit worldwide. Iron is essential metal for all organisms. The major key role of iron in human is binding oxygen to hemoglobin and catalysis in enzymes. Iron required 5–30 mg per day depending on age. Various fortified food available in market containing iron. They are in different color. However, iron may react with food components and color. There are other disadvantages of iron products [257]. Ionic gelation is the alternative method to prevent such undesirable effect of fortified foods. Pectin is a low degree of esterification and useful for ionic gelation. Pectin has resistant of hydrolyzation in the upper part of GI tract. Hence pectin widely used for colon targeted delivery [258]. Ghibaudo et al. fabricated iron-pectin beads for iron delivery. The beads were spherical shape with diameter 1–2 mm, density 1.29 g/ml and 93% of porosity proven the high permeability [259]. Beads get swell in simulated intestine medium than simulate gastric medium. After 4 h of treatment transport of iron higher from the bead than FeSO4. This study described the interesting iron transport system [259]. It may be an excellent method to enrich food products with iron without affecting sensory performance. Based on these studies, there is feasibility to fabricate the complex of iron with organic compounds and natural fibers as a functional ingredient in food products. It has great potential for pharmaceutical and food industrial applications.
5.4.1.5. Pectin-based nanocomposite.
Wang et al. developed novel and simple oral colon specific drug delivery system based on pectin/modified nano-carbon sphere nanocomposite gel film [260]. 3-aminopropyltriethoxysilane modified nano-carbon sphere added into pectin Ca2+film to improve pectin based oral colon targeted delivery 4-fluorouracil. They achieved 30 to 52% of EE. All the composite fluid showed better release rate such as 32, 22, 635 in SGF, SIF, SCF, respectively. The in vitro cytotoxicity studies confirm the biocompatibility of nanocomposite [260]. Hence, further studies need to be conduct before conducting clinical trials.
5.4.1.6. Pectin-based nanocomplex.
Biopolymer polyelectrolyte complexes have attracted increasing attention in recent years. PECs formed through electrostatic interactions between two or more opposite charged polyelectrolytes. The dimension and shape depend on bio-polymers and fabrication conditions. Recently it has reported that protein and polysaccharides-based PECs showed interesting results in food and pharmaceutical applications [261]. Towards this direction, Luo et al prepared nanocomplex of casein/pectin for oral delivery application [261]. They prepared nanocomplex of sodium caseinate and pectin in ration 1:1 with smaller and uniform size. Heating increases the yield and encapsulation efficiency of rutin. Nanocomplex showed potential to release the rutin in simulated intestinal conditions [261]. This system can also use for nutrients and medicines oral delivery. Hua et al. also made casein-pectin nanocomplex for oral delivery of curcumin [262]. They have prepared curcumin loaded casein micelles and surface coated with pectin through electrostatic interaction. It showed excellent performance at pH 4 then pH 2 and pH 3. The size of nanocomplex is 266 nm and 93% loading capacity with spherical shape. The anti-oxidant activity of nanocomplex showed tremendously keep bioactivity of curcumin. Delay release has observed due to surface coating confirmed by in vitro studies in SGF and controlled release in SIF [262]. This study demonstrated the oral delivery of bioactive ingredients. Potential application needs to be explored and conduct the comprehensive studies.
5.4.1.7. Pectin-based hydrogel.
Gautam and Santhiya et al. developed pectin/OEG food grade hydrogel blend for the targeted oral co-delivery of nutrients [248]. Ca2+ with vitamin D/Fe2+ with vitamin C is entrapped in edible pectin/PEG polymer blend matrix to formulate PPCaD and PPFeC hydrogel. The metal ions showed the electrostatic interaction and hydrogen bonding with pectin. In vitro studies confirm that lowest release of metal ions and vitamins in SGF at pH 1.2 for 3 h concluded that efficacy of pectin protection. However, in SIF at pH 6.8 showed highest swelling of hydrogel and highest release of nutrients at intestinal site. Fig. 10C–10D showed swelling ratio of the hydrogels and degradation behavior in SIF at pH 6.8 for various hydrogels. [248]. Zhou et al. prepared alginate hydrogel beads as a carrier of low-density lipoprotein/pectin nanogels for potential oral delivery applications [263]. Alginate beads widely studies due to their potential stability in gastric conditions. Zhou et al. utilized alginate hydrogel fabricated with Ca2+ or Fe2+ to serve as carrier for egg yolk low-density lipoprotein/pectin nanogels [263]. Beads showed great protection in gastric conditions and release at intestinal. Beads made of low viscous alginate and Ca2+ showed limited swelling and sustained release of curcumin compared to bead made of high viscous alginate and Fe2+. They also studied the dissociation of nanogels from beads using TEM (transmission electron microscope) [263]. This investigation demonstrated that potential pf alginate beads to protect and delivery the nanogels at desire site by passing gastric conditions.
5.5. Poly (acrylic acid)
PAA (poly (acrylic acid)) is a derivative of acrylic acid. PAA is a homopolymer and forms variety of co-polymer and cross-linked polymers. It is anionic polymer and may loss proton from side chain of PAA results negative charge. Deprotonated PAA showed ability to absorb water and swell many times than its original volume. PAA can be synthesized by free radical polymerization using potassium persulfate and AIBN [264]. It also used as dispersants. PAA is consider as mucoadhesive polymers because at protonated form at acidic pH is responsive for mucoadhesion. It forms H-bond between its COOH and sialic COOH of mucin glycoprotein. This bond formation enhanced viscosity [265]. Due to high viscosity the PAA based hydrogels can used in various biomedical applications such as to treat ocular irritations. PAA has various application including hygiene products, ultrafiltration, hemodialysis membrane and controlled drug delivery devices [266]. PAA based mucoadhesive oral drug delivery system have been extensively investigated. Various mucoadhesive pectin polymer based oral drug delivery literature is summarized in Table 6.
Table 6.
Pectin based mucoadhesive oral drug delivery system.
| Mucoadhesive drug delivery system | Other composition used | Drug | Therapy for disease | % Of Encapsulation efficiency (EE), %of drug loading (DL) and Release Profile (RB), Drug efficacy (DE), in-vitro, Ex-vivo, In-vivo, Pharmacokinetics (PK) and Pharmacodynamics (PD) | Pros | Cons | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| Pectin Microparticles | High amylose starch and cellulose nanofibers | 5-aminosalicilic acid | Inflammatory bowel disease | %EE- 16–98 % DL- 1.97–26.63 RP- 25% of 5-ASA in SGF, followed by 68% in SDF. |
|
|
Encapsulation efficiency varied vary drastically | [267] |
| Pectin Microparticles | Eudragit S 100 and hard gelatin | Sulfasalazine | Inflammatory bowel disease | %EE- 95.62 ± 1.21%. RP- drug release (91.12%) in SCF (pH 7.4) and 98.07 in rat cecal content (RCC, pH 7.4) for 24 h in a sustained manner. In vivo X-ray study shows the release of radio-opaque substance from Eudragit S 100 coated hard gelatin capsules. |
|
|
|
[252] |
| Pectin Microparticles | Gellan gum | Insulin | Reducing blood glucose levels | %DL- 0.65 – 0.99%. RP- 80% insulin release after 2h Lower amount of drug releases at pH 1.2 and pH 4.5 than in phosphate buffer pH 6.8 release 67%. Due to anionic polymers on tight junctions opened. oral administration to diabetic rats showed reduction of up to 51% of blood glucose levels. |
|
|
The developed microparticle system shows very good promise for oral delivery of insulin | [268] |
| Methoxylated pectin microparticles | CS | Insulin | Reducing blood glucose levels | %EE- 62 RP- In SGF, 13% release in 120 min. In intestine at pH 6.8, 89.0% release in 120 min. |
|
|
|
[269] |
| Low methoxylated pectin microparticles |
Zinc acetate | Doxorubicin | Colorectal cancer | %EE- 906.8±90.1 μg/g bead. RP- in gastric condition in 2h nearly 60% DOX was released. |
Microbeads containing thiolated pectin-DOX conjugate exhibited reduction-responsive in reducing condition |
|
Thiolated pectin-DOX conjugate showed promising results for site specific drug delivery | [270] |
| Pectin hydrogel | Oligochitosan and Ca2+ ions | Dextran | Colon cancer | %EE- 68.1 RP- 19% of FITC-CM-Dextran released within the SGF in 2 h, in SIF 30% releases in 2h, and in SCF 94% drug was released. |
|
|
|
[271] |
| Pectin (MW 30,000–100,000) hydrogel | Ethylene glycol Di methacrylate, Methacrylic Acid, and benzoyl peroxide | 5-Fluorouracil | Colon cancer | RP- 58–60% drug release at pH 1.2 and more than 90% release at pH 7.4 after 12 h dissolution experiments. |
|
|
Prepared hydrogel showed promising results for in vivo tests | [272] |
| Pectin (esterified 60% to 70%) hydrogel | Polyethylene glycol 300 and 7-dehydrocholesterol | Ca2+ with vitamin D / Fe2+ with vitamin C | %EE- vitamin D 99.1 and vitamin C 99.3. RP- below 20% releases in SGF pH 1.2 and nearly 100% releases SIF in 6h. |
|
|
|
[248] | |
| Pectin with galacturonic acid ≥74% from citrus peel hydrogel | Alginate and egg yolk low density lipoprotein (LDL) | Curcumin | Inflammation | RP- Showing about 75% release in SGF and 100% cumulative release in 180 min. |
|
|
|
[263] |
| Pectin nanocomposite | fa | Insulin | For controlling blood glucose level | %EE- 99.8 %DL- 23 RP- INS/FAN18 presented an initial burst release of 62.1% in pH 1.2 HCl, followed by a cumulative sustained release of 75.6% in pH 6.8 PBS (2–6 h) and 96.1% in pH 7.4 PBS at the end of 24 h. Significantly lowered the blood glucose levels in type I diabetic rats, |
|
|
|
[249] |
| Pectin nanocomposite | Trimethyl chitosan, | Celastrol | Colitis | %EE- 94.45 % DL- 1.26 RP- 20% of Cel was released during the first 12 h in SCF. Survival rate of Cel/PT-LbL Lipo group was significantly enhanced, compared with the Cel/Lipo group. Cel/Lipo and Cel/ TMC Lipo, Cel/PT-LbL Lipo had better pharmacodynamic effect, which were related to the TLR4/MyD88/NF-κB signaling pathway. |
|
- |
|
[273] |
| Apple pectin nanocomposites | mucoadhesive liposomes | Amoxicillin |
Helicobacter pylori pathogenicity |
%EE- 66–83%. RP- After 1h almost 75% drug was released |
|
|
|
[274] |
| Pectin nanocomposite | Zein and Eudragit S 100 | Resveratrol | Ulcerative colitis and cancer | %EE- 3.8–4.0 % DL-84.8 RP- neutral medium with 30% ethanol 65% release in 3h. In acidic medium with 30% ethanol 84% releases in 3 h. |
|
|
|
[275] |
| Pectin nanocomposite | Gellan gum | Resveratrol (RVT) | Ulcerative colitis and cancer | %EE- >80 RP- only 3% of RES was released in acidic media over 2 h, and, in pH 6.8, the drug was released in a sustained manner, reaching 85% in 30 h. It showed high ability to interact with the intestinal tissue. |
|
|
|
[276] |
| Pectin nanospheres {Citrus pectin (MW ~ 30,000 g/mol, degree of esterification = 27.3%, and the contents of galacturonic acid N(74%)] | 3-aminopropyltriethoxysilane | 5-fluorouracil | Colorectal cancer | %EE- 31.2 – 52.6 %DL- 14.1 – 16.6 RP- The drug release profile was 32.17%, 22.77% and 63.89% in the SGF, SIF, and SCF respectively. |
|
|
|
[260] |
| Citrus pectin with degree of esterification of 27.3% | Succinic anhydride and glutaric dialdehyde | Diclofenac sodium | Inflammation | %EE- 78.81 %DL- 7.78 RP- The drug release profile was 3.04, 3.66 and 79.43% in the SGF, SIF, and SCF, respectively. |
|
|
|
[277] |
| Hybrid nanoparticle (Pectin with a galacturonic acid content of 74%) | Casein sodium | Curcumin | Inflammation | %DL- 93%. RP- in SGF, 35% of drug releases in 3h and 51.81% releases in SIF in 6h. Showed Highest antioxidant activity 46.7% |
|
|
|
[262] |
| Pectin hybrid nanoparticles (Pectin from Smilax china L.) | Fluorescein-5-thioicarbazide and Cyanine7 amine | – |
|
|
|
[278] | ||
| Pectin (Type LM-5206CS – DE < 50% | High amylose starch and CS | 5-fluorouracil | Colorectal cancer | %EE- 21.4 RP- In pH 1.2 almost 91.1% drug was released in 2h and at pH 7.4 sustained release of drug observed in 7h. In vivo biodistribution confirmed the RS/P microparticles as potential carriers for delivering drug-loaded nanoparticles to the colon. |
|
|
Very promising system for colon specific oral delivery of drugs | [279] |
| Pectin (low-methoxy) | Multi-walled carbon nanotubes | Celecoxib | Colitis | RP- at pH 1.2, <10%, at pH 6.8, ~25%, and at pH 7.4 ~90% drug releases in 2h, 6h, and 10h, respectively. In presence of pectinase undergo enzyme degradation and release drug in the colon. |
|
|
|
[280] |
5.5.1. Poly (acrylic acid) oral delivery
5.5.1.1. PAA-based hydrogels.
PAA widely used in drug delivery through formulation of hydrogel and releasing the drug through swelling nature. In stomach targeted delivery, hydrogel loaded with drug can be use as oral delivery, which swell in stomach fluid and release the drug. Mucoadhesive properties of PAA allow binding the mucin. So enhanced therapeutic efficacy [281]. Kumar et al. achieved clarithromycin delivery targeted to stomach by formulating interpenetrating polymeric network hydrogel [282]. They have prepared inter-penetrating polymeric network by crosslinking of chitosan, PAA and PVP with glutaraldehyde and N, N’- methylenebisacrylaide. The fabricated interpenetrating polymeric network shows enhanced mucoadhesive nature and higher swelling behavior. Release the drug at low pH. Hence clarithromycin showed the sustain release at stomach and maintain the antibiotic concentration [282]. However, the efficacy of the formulated polymeric network in vivo has not evaluated by these researchers. In-vitro and in-vivo studies are crucial to get therapeutic potential of formulation. Li et al recently made advancement in PAA based graft for oral drug delivery [283]. They have synthesized the xanthan gum-graft-PAA. GO-DCFP composite hydrogel. Using improved Hammers method prepared GO and used to study the absorption of mechanism of model drug Diclofenac potassium. The absorption kinetics showed pseudo second order and Freundlich model mechanism. The absorption mechanism mainly involved the pi-pi staking and hydro-phobic interaction along with electrostatic and hydrogen bonding. By in sit polymerization xanthan gum-graft-poly (acrylic acid)/GO composite hydrogel has synthesized. The hydrogel showed increase in welling with increasing pH. The in vitro release studies confirmed that controlled release behavior as it releases 96 h in artificial intestinal fluid than artificial gastric fluid. The in vivo studies exhibited the half-life of composite hydrogel as 10.7 h revealed the prolong drug release properties. The composites showed AUC0-t of DCFP 116, which is more than twice of AUC of DCFP alone [283]. The bioavailability of drug greatly enhanced. This study demonstrated the potential of composite hydrogel for sustained drug delivery. Aktas et al. developed pH resistive poly (acrylic acid-co-acrylamide) anionic hydrogel for jejunum targeted drug delivery [284]. Ionic hydrogels are favorable conduce for oral drug delivery. They have investigated the welling properties for P(Aac-co-Aam) hydrogel in the presence of 30% vol% Aac at different pH values by steady state fluorescence technique. Pyranine 4 (4sPy) a pH independent fluorescence probe has entrapped in hydrogel network during the gelation before selling. Fluorescence intensities monitored during selling process. At very high pH most of the probe release. It reached maximum swelling ration at pH 9 [284]. All results together concluded that anionic hydrogel could be a potential system for jejunum targeted drug delivery. Kunjiappan et al. enhanced the anticancer activity of 5-FU and rutin by oral delivery using pH sensitive Zein-co-acrylic acid hybrid hydrogel [285]. It is a natural and synthetic polymer combination grafted hydrogel. Both polymers and hydrogel are intrinsic biocompatibility, biodegradability, offer protection to labile rug from metabolism and controlled release. The Zein-co acrylic acid hydrogel loaded with rutin and 5-FU for effective anticancer activity. The optimum formulation showed high encapsulation efficiency and drug loading such as 12 and 10 and 89 and 81 respectively for 5-FU and rutin. Hydrogel with 52.5 ug/ml 5-FU and rutin showed 50% cell death at 34 h in cytotoxicity against MDA-MB-231 and MCF-7 [285]. Drug released at pH 7.4. Hence, this pH responsive hydrogen; can be a favorite carrier for anticancer drug oral delivery. Liu et al developed hybrid microgel of carboxymethyl starch/poly (2-isobutyl-acrylic acid) [286]. It is a pH and amylase responsive effective enteric carrier for oral insulin delivery. The hybrid microgel made through aqueous dispersion copolymerization of acrylate-grafted-carboxymethyl starch (CMS-g-AA) and 2-isobutyl acrylic acid (iBAA). The hydrogel has 13–45% of PiBAA and showed pH and amylase sensitivity. Insulin loaded microgel showed release of loaded insulin with responsive to pH change and amylase (Fig. 11C). Microgel also showed good biocompatibility and cellular update by Caco-2 cells. Further microgel shows in vitro intestinal absorption. Oral delivery of microgel to STZ induced diabetic rats led to continuous decrease of blood glucose level within 2 to 4 h and maintain hypoglycemic effect over 6 h in vivo [286]. More interestingly, pharmacological availability of insulin increases 23–38 times. It concluded that novel starch based microgel potential carrier for oral insulin delivery. Magnetic nanoparticles gained great attention and made excellent progress in last decade. US-FDA also considered magnetic nanoparticle as biocompatible and allow using in biomedical application. Mohammadi et al. further developed pH responsive hydrogel beads through formulation of interpenetrating magnetic nanocomposite of carboxymethylcellulose/PAA/Starch and Fe3O4 magnetic nanoparticles [287]. The efficacy of nanocomposite studied through loading the anticancer drugs such as doxorubicin hydrochloride and 5-fluorouracil and evaluated the performance. The loaded 5-FU successfully release in stomach due to sensitivity of pH. It also showed enhanced stability of drug I colorectal area. Cytotoxicity studies revealed the excellent cytotoxicity against SW480 cell lines [287]. It can be efficient carrier for oral drug delivery [287]. This study inspired for PAA nanocomposites a potentials carrier for colon targeted drug delivery. The thiolated PAA based inserts not soluble. So, it can be used for controlled drug delivery. Because of hydrophilic nature and reticulated structure which makes it interesting carrier for control drug release. PAA polymer extensively used in bioadhesive pharmaceutical hydrogels such as Polycarbophil, and Carbopol [288]. Used for artificial tears and dry eye syndrome therapy [289]. Ahmad et al. developed mucoadhesive bacterial cellulose-g-PAA hydrogel for oral protein delivery. The hydrogel synthesized without cross linker using electron beam irradiation, which avoid the toxicity of cross-linking agents. Bovine serum albumin used as a model protein. BSA release only 10% in SGF. Swelling and deswelling behavior and BSA release profiles shown in Fig. 11A and 11B respectively. Ex-vivo studies revealed the BSA penetration across intestinal mucosa. Further properties such as cytocompatibility, no sign of toxicity indicates the safety of hydrogel, and it can be great choice for in vivo applications. PAA based targeted delivery to small intestine and intestine lymphatic need further investigation.
Fig. 11.
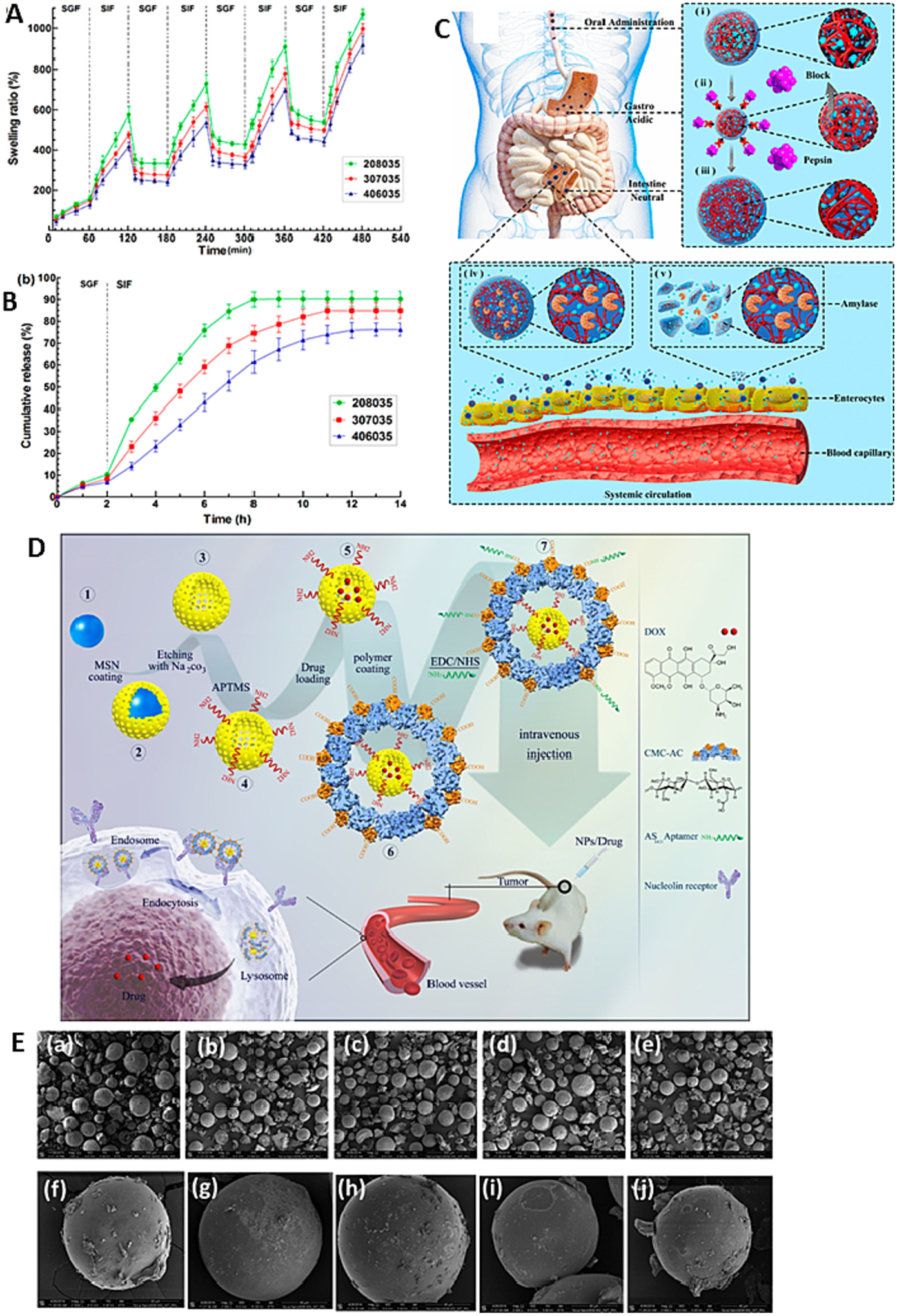
(A) Dynamic swelling/deswelling of hydrogels in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF); (B) in vitro BSA release profile of the hydrogels in SGF and SIF; (C) Proposed Schematic Illustration for the Behaviors of the Insulin-Loaded Microgels during the Delivery Process in the GI Tract; (D) Schematic presentation of the preparation of HMSNs, their coating with acetylated CMC and functionalization with AS1411 aptamer for targeting nucleolin over-expressing cancer cells; (E) SEM Micrographs of (a) MS1, (b) MS2, (c) MS3, (d) MS4, (e) MS5 microspheres at 150 magnification and single (f) MS1, (g) MS2, (h) MS3, (i) MS4, (j) MS5 microspheres at 2000 magnification. Reproduced with permission from ref. [265,286,290,291]. Copyright 2014 American Chemical Society, 2018 Elsevier, 2014 The Royal Society of chemistry, 2018 American Chemical Society.
5.5.1.2. PAA-based grafts.
Dey et al. achieved pH sensitive delivery using PAA grafted with barley. They have used 5-ASA as a model drug in this study for colon targeted [292]. As barley is fourth highest cultivated food product globally. Barley contains 95% of polysaccharide mainly starch. Grafting is the promising method for modification of polysaccharides. Dey et al. formulated PAA grafted barley through microwave assisted method. Loaded with 5-ASA an anti-inflammatory drug to treat inflammatory bowel disease. Drug release follow the Fickian diffusion mechanism at both pH 1.2 and 7.2. drug absorption rate is high in lower GIT [292]. This study encourages towards the grafting of PAA with different entities for oral delivery. By using targeting agent grafted nanoparticles can be targeted to stomach, small intestine, intestine lymphatic, and colon. Oral delivery of hydrophilic macromolecular protein delivery is challenging. Kavitha et al. synthesized PAA-grafted graphene oxide for oral intracellular protein delivery system [293]. They have used in situ atom transfer radical polymerization to synthesized graft. The graft protected the model protein BSA labeled with FITC. It releases effectively in intestine and internalized in KB cells by endocytosis and release into cytoplasm. The GO-PAA is a new class of transmembrane transporter for potential protein oral delivery [293]. Pal et al. synthesized the pH sensitive cross-linked guar gum-g-poly (acrylic acid-co-acrylonitrile) for thymoquinone against inflammation [294]. They have used microwave assisted technique for fabrication of graft. They achieved optimum formulation by tuning the various parameter and their effect on graft properties. The loaded thymoquinone maximum release at pH 7.4 and in 6 h. the graft showed goo antioxidant activity, hemocompatibility and biocompatibility against the VERO cell lines [294]. It is a cost-effective green strategy for graft formulation for sustained release of oral drug delivery. Tian et al. developed the pH responsive PAA-gated mesoporous silica nanoparticles for oral colon targeted doxorubicin delivery [295]. The used PAA brushes to anchor on pore outlets of MSNs, which can act as gatekeeper. PAA capped MSN SBA-15 (PAA/SBA-15) showed good biocompatibility, pH sensitivity and very high drug loading efficiency such as 785.7 mg/g. PAA protect doxorubicin loaded into SBA-15 in gastric condition and in colonic environment PAA opens the pores and facilitate the release of drug [295]. The formulated nanoparticles showed enhanced aqueous solubility as well. pH responsive drug delivery system can be potential for colon cancer and other colonic diseases therapies.
5.5.1.3. PAA-based microsphere.
Controlling the retention of nanoparticles between stomach and colon is critical challenge. Dexamethasone widely used in treatment of inflammatory diseases. Das et al. demonstrated the controlled delivery of β-cyclodextrins to intestine using microsphere of poly (vinyl alcohol)-poly (acrylic acid) [296]. Fig. 11E presenting the SEM images of the formulated microsphere. Dexamethasone active in the treatment of every type of B-cell malignancy and side effects associated with it. Therefore, control drug delivery is an alternative to limit or minimize the adverse effects. They compare the inclusion complexes prepared through co-precipitation and freeze drying and compare with microsphere. Results suggested no release of drug from microsphere in gastric fluid but release in intestine. Microsphere showed no cytotoxicity. This suggested that microsphere biocompatible. These microspheres can be used as pH responsive drug delivery system for targeted intestine delivery. Moreover, it minimizes the adverse effects of dexamethasone [296]. Such system can also be applied for any other drug to reduce the side effects and improved the therapeutic efficacy. Das and Subuddhi prepared microsphere of PVA-PAA containing drug-cyclodextrin complexes for controlled delivery of dexamethasone to intestine. Dexamethasone is a highly efficient treatment for every type of B-cell malignancy. However, it has various side effects. Hence, it demands controlled delivery. They have prepared hydrogel with addition of preformed solid inclusion complex of dexamethasone and β-cyclodextrin. The inclusion complex prepared through co-precipitation and freeze-drying method. Microsphere containing free drug, physical mixture and inclusion complex has formulated. Microsphere significantly protected the drug in gastric fluid and released the drug in intestinal fluid. The microsphere showed good biocompatible observed in cytotoxicity studies. Thus, microspheres are effective system for intestinal targeted drug delivery [291]. This study proposed novel system to minimize the adverse side effect of dexamethasone through controlled delivery using microsphere.
5.5.1.4. PAA-based micelles.
Zhao et al. fabricated pluronic-poly (acrylic acid)-cysteine/pluronic L121 mixed micelles improve the oral bioavailability of paclitaxel. The mixed micelles achieved 2.8% of drug loading capacity and pH sensitive release. Presence of verapamil and pluronic both improved intestinal permeability in rat. The pharmacokinetics studies revealed the AUC of mixed micelles loaded with paclitaxel is four times higher than the paclitaxel alone [297]. It proven that mixed micelles of PAA and pluronic to be potential oral drug delivery carrier for paclitaxel.
5.5.1.5. PAA based nanoparticles.
Pourjavadi et al. prepared mesoporous silica nanoparticles with bilayer coating of poly (acrylic acid-co-itaconic acid) and human serum albumin (HAS). They used to delivery gemcitabine to cancer cells. PAAIA used as pH sensitive inner shell and HAS as outer shell. Due to the electrostatic interaction between ammonium group of MCM-41 and carboxylate group of copolymers formed core-shell structure is formed. The copolymer-coated nanoparticles further coated with albumin layer. More interestingly, maximum release observed at pH 5.5, due to the collapse of bilayer [298]. This demonstrated that the potential of nanocarriers with good biocompatibility, controlled release, and pH responsive for tumor therapy. Various mucoadhesive PAA polymer based oral drug delivery literature is summarized in Table 7.
Table 7.
Poly acrylic acid based mucoadhesive oral drug delivery system.
| Mucoadhesive drug delivery system | Other composition used | Drug | Therapy for disease | % Of Encapsulation efficiency (EE), % of drug loading (DL) and Release Profile (RB), Drug efficacy (DE), in-vitro, Ex-vivo, In-vivo, Pharmacokinetics (PK) and Pharmacodynamics (PD) | Pros | Cons | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| Poly (acrylic acid-co-acrylamide) P(AAc-co-AAm) Hydrogel | Acrylamide | Pyronine 4 (4sPy) | N/A | – |
|
|
P(AAc-co-AAm) based could be efficient for jejunum targeting oral drug delivery | [284] |
| PAA Hydrogel | Graphene oxide (GO) Xanthan gum | Diclofenac potassium (DCFP) | Inflammation | DCFP release higher in SIF than SGF in 96 h. t1/2 of DCFP in formulation increases from 2.03 to 10.71h. AUC(0–t) of the DCFP increases from 53.99 mg L−1 h−1 to 116.79 mg L−1 h−1 |
pH sensitive
|
|
The PAA-XG-GO carrier has potentiality to increase the bioavailability of the drug in the intestine | [283] |
| Ion conductive PAA hydrogel | Silicone | Florescent reagent | N/A | %EE- 84 %DL- 34 RP- When voltage was increased to 3, 5, and 7 V, the amount of drug released was 53, 88, and 96%, respectively. |
|
|
This ion conductive polymeric matrix could be potential for oral drug deliveries its less cell toxicity and better targeting efficiency | [299] |
| Pluronic F127-co-poly (acrylic acid) (PF127-co-PAA) hydrogel | Pluronic F127 Ethylene glycol dimethacrylate (EGDMA) Ammonium persulfite (APS) and sodium hydrogen sulfate (SHS) |
Ivabradine | Heart failure | RP- at pH 1.2 nearly 10% of drug releases whereas at pH 6.8 100%of drug releases in 24 h. |
|
|
The PF127-PAA based pH dependent smart polymeric matrix showed controlled release of the drug upon oral administration and showed no toxicity on healthy rabbit indicating its huge potentiality for oral drug delivery | [300] |
| Acid (Zein-co-PAA) hydrogel | Zein AA N, N-methylene bisacrylamide, and ammonium persulphate |
5-FU Rutin | Colorectal cancer Antioxidant | %EE- 12 (5-FU) and 10 (Ru) %DL-89 (5-FU) and 81 (Ru) RP- At pH 7.4, 5-FU 88.73% and Ru 74.54% was releases. At Ph 1.2, 72% and 69% of 5-FU and Ru was observed. Induced 50% cancer cell death in 24h. |
|
|
The copolymer vector ensured co-delivery of 5-FU and rutin to the breast cancer site and rutin delivery could be highly potential for minimizing the doses of 5-FU to reduce drug toxicity | [285] |
| PAA-PVP hydrogel | PVP PAA di-Ethylene glycol bis-allyl carbonate |
– | – | – |
|
|
The PAA-PVP matrix is suitable for reducing cytotoxicity in mucosa cells indicating its potentiality for oral drug delivery | [301] |
| P(AAM-MAA) hydrogel | Acrylamide (AAM) Methacrylic acid (MAA) N, N-Methylenebis (acrylamide) (MBA) Pluronic F-127 |
Theophylline | Lung disease | %DL- >98% RP- 19% of drug released in 90 min, accounting for gastric emptying, and potentially possessing 45% of the remaining drug to be release once reaching the small intestines. Drug loading and release evaluation confirmed >98% drug loading capacity, with over 60% drug release in 24 h, |
|
|
The versatile oral polymeric delivery platform could be used a good candidate for oral delivery of therapeutics | [302] |
| Collagen-grafted-P(AA-co- MAA) hydrogel | AA MAA MBA TEMED |
Insulin Methylene blue (MB) |
Diabetes | %DL- approximately 50%. RP- Payload release was observed only in neutral media |
|
|
|
[303] |
| Carboxymethyl CS-grafted-PAA hydrogel | Carboxymethyl CS AA K2S2O8 |
Insulin | Diabetes | %EE- 216 mg/g RP- 16.3 ± 2.6% of INS was released at pH 1.2, while over 93.2 ± 3.8% of INS was diffused into PBS (pH 7.4). In vivo studies showed effective hypoglycemic effect. |
|
|
Shows promise as a site-specific delivery system for insulin | [304] |
| PCL-PAA hydrogel | PCL PAA Azo-isobutyronitrile N, N'-Methylene bisacrylamide |
Gliclazide (Glz) | Diabetes | RP- After 24h the release profile was 6 mg/g for pH 1.2 and 18 mg/g at pH 7.4 blood sugar level reduced to 67.14 and 57.55% showed zone of inhibition 20 mm with bacteria E. coli. |
|
|
PCL-PAA could be effective for treating type 2 diabetes by delivering Glz | [305] |
| PAA hydrogel | AA | Triamcinolone acetonide |
Skin Condition | RP- initial 60 min release percentage of drug is high and in 5h almost 95% drug was released in PBS |
|
|
A bio adhesive drug delivery system was developed that could adhere to the wet mucosa surface of a mucosa membrane | [306] |
| PF 127-PAA hydrogel | PF 127 AA MBA TEMED APS |
Epirubicin (Epi) | Colon adenocarcinoma | RP- formulation showed sustained drug release up to 96h. It showed very good inhibition efficacy of in vivo tumor growth mice. Plasma concentration (Tmax) achieved in 4.39h. |
|
|
Viable candidate for solution based oral delivery of drugs to the colon | [307] |
| PVA-PAA Bilayer hydrogel | PVA EGDMA APS SDS |
Vancomycin hydrochloride | RP- After 10h the release was nearly 60% From hydrogel drug releases at pH 2 70% and pH 9 75% and at pH 7 only 65% of drug releases. Drug releases 70% from PVA in 10h irrespective of medium. From IDN drug release percentage increases from 35% to 70% with increase of pH. |
|
|
IDN structured hydrogel could be potential for preparing soft hydrogels, hydrogel driven origami for improved drug delivery | [308] | |
| Acrylate grafted- carboxymethyl starch (CMS-g-AA) and iBAA Hybrid microgel | Carboxymethyl starch (CMS) AA iBAA α-Amylase |
Insulin | Diabetes | %DL- 2.5 – 15 RP- For all formulations 75% insulin was released in 2h presence of α-amylase. |
|
|
A systemic intestinal targeting nanocarrier has been developed which had the potential of accelerating the release of insulin in the intestine via degradation of CMS in presence of amylase in the small intestine | [286] |
| Carboxymethyl CS-co-PAA Hybrid gel | Carboxymethyl CS PAA N, N MBA Benzoylperoxide (BPO) |
5-FU | Colorectal cancer | %DL- 65–85%. RP- drug released at pH 1.2 is range from 17–24% and at pH 7.4 89–96% in 36h. Maximum absorption achieved at pH 7.4 in rabbit. |
|
|
Both in vivo and in vitro studies confirmed the potentiality of the carrier for better colonic targeting drug delivery | [309] |
| Gelatin/polyvinylpyrrolidone- co-poly (Acrylic acid) (GE/PVP-co-PAA) Hybrid hydrogel |
Gelatin PVP EGDMA Ammonium Peroxodisulphate/sodium hydrogen sulphite |
5-FU | Colorectal cancer | %EE- 361 to 443 %DL- 74 – 87 RP- In pH 1.2 solution less than 20% was released while in pH 7.4 nearly 60% drug was released after 36h |
|
|
This formulation could be used for oral administration of 5-FU | [310] |
| Carboxymethyl cellulose-PAA HYDROGEL | Carboxymethyl cellulose AA APS TEMED |
Insulin | Diabetes | %DL-16–25%. RP- Almost 70% drug release after 6h |
|
|
Good bioavailability of insulin after delivery to the rabbits makes it an excellent candidate for oral delivery of insulin | [311] |
| Barley-grafted-PAA Nanocomposite |
Barley AA Ceric ammonium nitrate (CAN) PVP |
5-Amino salicylic acid (5-ASA) |
Inflammatory bowel disease (IBD) | RP- In pH 1.2 after 12h almost 60% and in pH 7.4 after 12h nearly 90% drug was released. |
|
|
|
[292] |
| Pluronic-PAA-Cysteine-co-Pluronic L121 Micelle | PF 127 Pluronic L121 (PL 121) PAA L-Cysteine hydrochloride |
Paclitaxel (PTX) | Anti-tumor | RP- In pH 1.2 after 12h almost 60% and in pH 7.4 after 12h nearly 90% drug was released |
|
|
The designed vehicle could be a viable candidate for commercial delivery of PTX | [297] |
5.6. Carboxymethyl cellulose
CMC is a cellulose derivate. It is water soluble bio and mucoadhesive polymer. It is hydrophilicity, bioadhesive, pH-sensitivity, non-toxic, and gelation ability. CMC commonly used in drug delivery including oral delivery. It is also used in different fields such as lithium sulfur battery, water pollutants removal, conductive films, host in polymer electrolytes, catalyst, food packing, protein immobilization, and drug delivery. CMC shows interesting properties which encourages it use in oral delivery agent [312]. In 1990s, CMC has entered pharmaceutical industries. The CMC based therapeutics is inspiring in industry as well as academic research. CMC hydrogels are more interesting due to mucoadhesive responsive release features [312]. Drug delivery of CMC hydrogel can improve through functionalization, modification, and crosslink with natural and synthesis materials. The mucoadhesive polymeric oral drug delivery using CMC have discussed in following section.
5.6.1. Carboxymethyl cellulose for oral delivery
Oral drug delivery used to deliver drugs at duration, sustained and controlled frequency along with achieving the plasma concentration of drug at therapeutic levels. It can reduce the dosage rate. CMC based oral drug delivery system able to protect the drug from crystallization and degradation. CMC release the drug through diffusion, erosion/degradation so enhanced drug release time [312]. Gemici et al. studied the effect of hyaluronate-carboxymethyl-cellulose (HCMC) on the formation of postoperative adhesion in stomach visceral peritoneum damage [313]. The fabricated HCMC placed satured anterior wall of stomach of 15 rabbits. After 30 days relaparotomy was performed on the rabbits and adhesions were evaluated by an independent surgeon according to seriousness and prevalence scores. In controlled and treatment group postoperative adhesion (POA) observed in 12 and 5 respectively. The POA completely prevented 2 and 7 rabbits respectively in controlled and treatment group [313]. This study concluded that hyaluronatecarboxymethyl-cellulose could be beneficial on damaged peritoneum surfaces following surgery to reduced POA development. Among the various drug delivery system developed for targeted delivery to stomach has potential applications. Recently developed floating drug delivery system can be a potential candidate due to various advantages and it is gained great attention from the scientific community.
5.6.1.1. Hydrogel.
Khan et al developed stimuli responsive hydrogel for controlled delivery of 5-fluorouracil. Gelatin/carboxymethyl cellulose hydrogel used as carrier [314]. It is fabricated using free radical polymerization method and cross-linked with glutaraldehyde. Effect of various parameters, composition on physicochemical properties of hydrogel has investigated. Hydrogel showed maximum swelling and release at pH 1.2. in vivo pharmacokinetics showed control nature of hydrogel. MTT study confirm the biocompatibility and non-toxic nature of hydrogel. Hydrogel showed great potential against HeLa cells. Hydrogel shows safe even at 4000 mg/kg dose [314]. Altogether showed promising results of hydrogel in oral drug delivery application. Nia et al. prepared bio-nanocomposite hydrogel compose of CMC/layered double hydroxides [315]. The bio nanocomposites used for controlled amoxicillin delivery to colon to treat bacterial infections. It showed pH sensitivity. The prepared bio nanocomposite-based hydrogel beads showed excellent performance in oral delivery of amoxicillin. The toxicity studies exhibited it is safe against HUVEC cells. This hydrogel beads can be potential for oral delivery of amoxicillin [315]. However, further detail studies need to do before moving to clinical trials. From the discovery of carbon dots, in short span of time they gained great interested due to range of properties including fluorescence [316]. Rakhshaei et al prepared nanocomposite hydrogel for pH sensitive oral anticancer drug delivery with bioimaging properties [317]. They prepared graphene quantum dot cross linked CMC nanocomposite hydrogel using casting method. It showed excellent biodegradability and biocompatibility (Fig. 12B). The prepared CMC/GQDs showed pH sensitive swelling and degradation. GQDs showed fluorescence and provide the bioimaging opportunity. The doxorubicin has used as model drug. The drug loaded hydrogel showed good biocompatibility and pH sensitive drug release property [317]. It can be a promising pH triggered site-specific drug delivery system. However, further studies need to investigate. Javanbakth et al. prepare Cu cross-linked carboxymethyl cellulose/naproxen/graphene quantum dot nanocomposite’s hydrogel beads for naproxen oral delivery [318]. The used copper acetate for crosslinking agent. The prepared Cu-CMC/NPX/GQD showed controlled drug delivery characteristics. It successfully delivered the naproxen to gastrointestinal tract conditions. It effectively protected drug in gastric pH. MTT test showed the low toxicity against caco-2 cells. These beads are good choice for delivery to gastrointestinal tract [318]. Furthermore, it can used for oral delivery. Chavda et al. developed stomach specific drug delivery system using super porous hydrogel composite for sustained delivery of ranitidine hydrochloride [319]. The detail comprehensive characterization of composite using various analytical techniques confirmed that formation of interconnected pores, capillary channels, cross linked sodium carboxymethylcellulose molecules. The composites exhibited the floating and delivery of ranitidine hydrochloride for 17 h. The drug release kinetics follows the Korsmeyer-Peppas, Weibull, and Hopfenberg models. The release is anomalous non-Fickian transport [319]. This study showed that floating drug delivery can be a potential alternative for stomach targeted delivery.
Fig. 12.
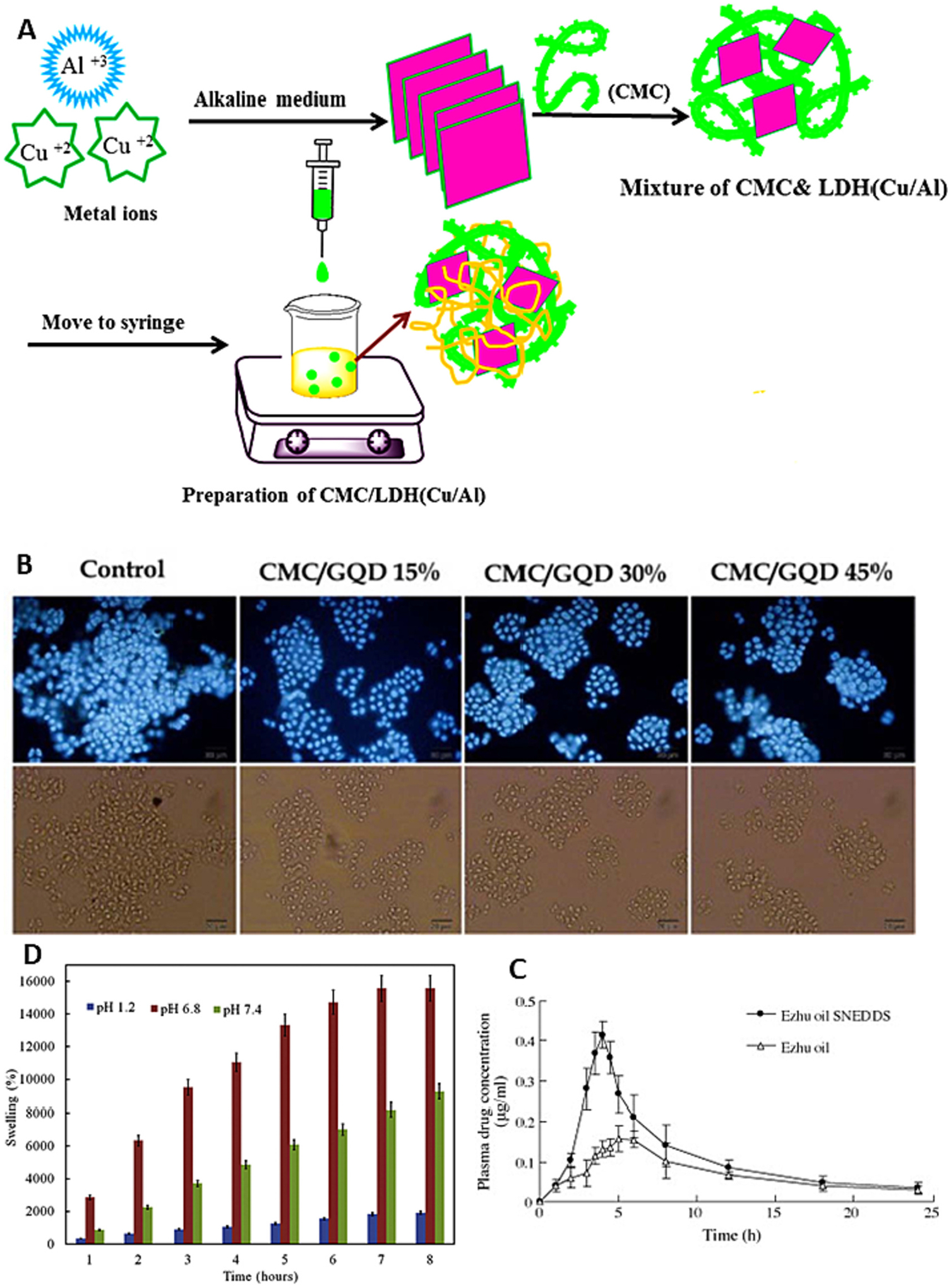
(A) Schematic procedure for preparation of CMC/LDH(Cu/Al) bio-nanocomposite hydrogel bead; (B) DAPI stained nuclei of colon cancer cells (HT29 cells) with CMC/GQDs at various GQDs percentage (15%, 30%, and 45%); (C) Plasma concentration of GM after oral administration of unformulated ZTO (500 mg/kg) or ZTO-SNEDDS (1625 mg/kg) to SD rats; (D) Swelling behavior of CMC/Cu-MOF@IBU at pH values of 1.2, 6.8 and 7.4. Reproduced with permission from ref. [315,317,320,321]. Copyright 2019, 2019, 2009, 2018 Elsevier.
5.6.1.2. Beads.
Colon targeted drug delivery system can be a potential candidate to treat colon diseases such as ulcerative colitis, Crohn’s disease, amebiosis, colonic cancer. Recently various system delivery to target colon such as polymer-drug conjugate, pH responsive polymer coated nanostructures, bioadhesive polymers, biodegradable polymers, stimuli responsive releasing systems. Agarwal et al. attempted calcium alginate-carboxymethyl cellulose beads for colon targeted drug delivery [216]. It is a pH responsive swelling, mucoadhesive and colonic microflora catered biodegradable nanocarriers system. The beads prepared through gelation process. The formulated beads show higher mucoadhesiveness and swelling and slow degradation in simulated colonic fluid. The degradation increases presence of colonic microflora. Anticancer drug 5-fluorouracil showed more than 90% of release presence of colonic enzymes. The beads efficacy evaluated against colon adenocarcinoma cells, and it showed excellent therapeutic potential [216]. This study demonstrated that CA-CMC based beads can be potential option for colon targeted delivery of therapeutics. Kim et al. fabricated alginate-carboxymethyl cellulose beads through ionic cross-linking for protein delivery [322]. Fe3+ cross linked Alginate-CMC beads prepared. With increasing the ratio of CMC, the pore size of beads increased. The beads showed tunable swelling and albumin release behaviors at different pH and different beads volume. Albumin showed controlled release kinetics in vitro studies under simulated gastric intestinal conditions over 24 h. the crosslinking protect the albumin [322]. The alginate-CMC beads showed interesting results in in vitro. However, further detail studies need to be conducted.
5.6.1.3. Microparticles.
Pharmaceuticals with low molecular weight and chemically unstable needed moderate and prolonged payload release in response to factor. Esculin, a model phytopharmaceutical. Tsirigotis-Maniecka et al. developed pH-responsive polyelectrolyte coating for carboxymethyl cellulose based microparticles in the controlled release of esculin [323]. The microparticles shows 575 of % EE with spherical shape. In vitro studies confirm that microparticles did not induce any cytotoxic effects. They have demonstrated the poly-electrolyte shell onto CMC-based microsphere may offer controlled delivery of drug with response to pH and ionic gastrointestinal conditions [323]. This study demonstrate that suitable materials need to be chose to prepare microparticles to achieve controlled delivery. Cerchiara et al formulated microparticles based on chitosan/caroboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin [324]. Using spray-drying techniques microparticles were prepared at different weight ratio of chitosan/CMC. The CH/CMC 1:3 ratio showed optimum properties based of high EE. Microparticles showed great potential to inhibit the drug degradation and showed good antibacterial activity against S. aureus. Further coating with lauric acid facilitates the drug release in colon. Microparticles showed limited release at pH 2 and improved and prolonged release at pH 7 [324]. It concluded that microparticles can be potential option for colon specific delivery of peptide drugs.
5.6.1.4. Nanoparticles.
CMC extensively studied for colon targeted delivery. Nejabat et al. fabricated acetylated carboxymethylcellulose coated how mesoporous silica hydride nanoparticles for nucleolin targeted delivery to colon adenocarcinoma [290]. DOX loaded HMSNs coated with Ac-CMC which covalently conjugated with AS1411 aptamer. These nanoparticles showed controlled, sustained drug release and enhanced blood circulation. In-vitro cytotoxicity and cellular uptake studied confirm that AS1411 targetability to nucleolin overexpressing MCF-7 and C26 cells. The formulation showed excellent therapeutic activity in vivo tumor inhibition (Fig. 11D) [290]. Even the study showed promising results, the cytotoxicity of silica nanoparticles and their clearance is a big challenge need to be address before going to clinic. SN38 poorly water soluble chemotherapeutic and other side effects limited its clinical use. CD133 is an aptamer against cancer stem cell marker. Alibolandi et al. self-assembled PEGylated CMC-SN38 nanoparticles (169 nm) for CD133 delivery to treat colorectal cancer. Nanoparticles showed enhanced cellular uptake by CD133 expressed HT29 cells. Nanoparticles showed lower IC50 in HT29 cells over-expressing CD133 [325].
5.6.1.5. Nanocomposite.
Even after great interest in the use of essential oils in food feed additives for better health there are number of factors such as oxygen, light, moisture, and acids have negative effect on stability and hence reduced biological activity. Hence, protection of essential oil is required. One of the best strategies is encapsulation [320]. Zhao et al. used self-nanoemulsifying drug delivery system for oral delivery of zedoary essential oil. They have achieved 30% of loading at optimum formulation. the loaded essential oil remained stable at 25 C more than 1 year. Oral administration to rat the AUC increases 1.7-fold and Cmax increases 2.5-fold compared to normal zedoary. Fig. 12C presents the plasma concentration after oral delivery. Ngamekaue et al. studied the effect of beeswax-carboxymethyl cellulose composite coating at various concentrations of beeswax on shelf-life and controlled release [326]. Through coacervation method they have fabrication of HBEO-loaded gelatin microcapsules. They have studied the loading content, surface oil content, crystallinity, antioxidant and antimicrobial activity, and storage stability over 3 months. Very high surface oil found even after 3-month storage of HBEO-G, antibacterial activity remains same. These microcapsules successfully delivered the essential oil to distal small intestine. CMC coating minimize the exposure to gastric fluids. More than 70% of HBEO released at distal small intestine where its antibacterial activity required [326]. This study demonstrated the microencapsulation is an ideal option for promoting essential oils with antioxidant and antibacterial activity for use in food, feed, and pharmaceutical products. This study showed promising in-vitro results. However, more details in-vitro studies need to be conducted extensively and in-vivo performance need to be evaluate.
In recent years, MOF (metal organic frameworks) gained attention to use as drug delivery carrier due to their range of properties [327]. Javanbakht et al. CMC capsulated Cu based MOF-drug monohydride as a pH sensitive nanocomposite for ibuprofen oral delivery (Fig. 12D) [321]. Oral drug delivery method commonly used due to avoid patient’s pain and discomfort. Cu based MOF loaded with IBP protected with biopolymer CMC Nanocomposite developed for oral delivery. Drug release studies showed the great protection ability of nanocomposite in stomach and extended stability and controlled release at gastrointestinal tract. The MTT studies confirm the biocompatibility of nanocomposite [321]. It concluded that nanocomposite hydrogel beads can be an innovative approach for oral drug delivery. Javanbakht et al fabricated bio-nanocomposite of CMC-coated 5-FU@MOF-5 for anticancer oral delivery [328]. 5-FU loaded into Zn based MOF and CMC used as coating to protect the drug in digestive system. This system shows promising controlled drug delivery behavior. In-vitro studies showed the notable toxicity in HeLa cells [328]. This study needs further studies in-vivo and long-term therapeutic potential and adverse effects. MOF accumulation and clearance for the body need to be studied. Various mucoadhesive CMC polymer based oral drug delivery literature is summarized in Table 8.
Table 8.
Carboxymethyl cellulose based mucoadhesive oral drug delivery system.
| Mucoadhesive drug delivery system | Other composition used | Drug | Therapy for disease | % Of Encapsulation efficiency (EE), %of drug loading (DL) and Release Profile (RB), Drug efficacy (DE), in-vitro, Ex-vivo, In-vivo, Pharmacokinetics (PK) and Pharmacodynamics (PD) | Pros | Cons | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| High purity CMC Microparticles | Chitosan and pectin | Iron from natural vegetable source | Iron deficiency | RP- Iron was released by almost 90% within 50 minutes |
|
|
|
[314] |
| CMC Microparticles | Chitosan, gelatin, or PAH and PSS | Esculin | Cytotoxicity effect on human gingival fibroblasts | %EE- 58–61 %DL- 19–20 RP- After 2h almost 50% of the drug was released when in pH 2.0 medium |
|
|
|
[323] |
| CMC 6000 fine powder of 8%, 10%, and 12% in aqueous solution Hydrogel | glycerol | Diluted red food degradable colorant | RP- 12% of drug releases in 24h |
|
|
|
[329] | |
| CMC hydrogel | Gelatin, glutaraldehyde, and acetic acid | 5-FU | Skin cancer | RP-56%, 49%, and 36% of drug releases at pH 1.2, 5.5, and 7.5 in 24h. Toxicity of drug reduced after encapsulation. Acute oral toxicity in rabbits confirmed that hydrogels is biocompatible |
|
|
|
[330] |
| CMC with CP 300–800 mPas hydrogel | Iron (II) chloride, iron (III) chloride, and acrylic acid | Doxorubicin hydrochloride and 5-fluorouracil | Different cancer cells including skin and colon cancer | %DL- 62% (5-FU) and 75% (Dox) RP- At pH 1.2 DOX and 5-FU had 20% and 25% release while at pH 7.4 both had nearly 60% release rate |
|
|
|
[287] |
| CMC and poly (methacrylic acid) hydrogel | NNMBA, TEMED, and APS | Insulin | For controlling blood glucose level | %DL- 26.4 RP- At pH 1.2 nearly 46% was released in 14h and at pH 6.8 nearly 85.86% was released in 6h. |
|
|
|
[331] |
| CMC hydrogel | Poly (lactic acid) | Curcumin | %EE- 77% RP- In 8h almost 8 mg/L drug was released in the neutral PBS. |
|
|
|
[332] | |
| CMC hydrogel | cyclohexyl isocyanide, benzaldehyde, and ethylenediamine | Gentamicin | Antibacterial activity | %DL > 90 RP- at Ph 1.2 and 6.8 drug release is less than 25% but at pH 7.4 almost 85% drug was released in 8h Formulation showed antibacterial activity toward S. aureus and E. coli. |
|
|
|
[333] |
| CMC hydrogel | Metal ions and layered double hydroxides | Amoxicillin | Colonic bacterial infections treatment |
%DL- 55–73 RP- at pH 1.2 and 6.8 the drug release ranges from 42 to 65 but at pH 7.4 59% to 88% of drug released in 8h. All drug loaded formulation showed antibacterial activity against S. aureus and E. coli. |
|
|
|
[315] |
| CMC hydrogel | Graphene quantum dots | Doxorubicin | Human colon adenocarcinoma | %DL- 2.6 RP- drug release 20% at pH 2 and 57% at pH 7.4 in 24 h. |
|
|
|
[317] |
| CMC (Mw = 250,000) hydrogel | NaOH, epichlorohydrin, chitosan, magnetic Fe3O4, and β-Cyclodextrin | Methotrexate | Cancer Treatment | %DL- 85 RP- At pH 1.2 around 15% and at pH 7.4 around 90% in 30 h. |
|
|
|
[334] |
| CMC hydrogel | layered double hydroxide (LDH) | 5-FU | Colon anticancer | %DL- 87%. RP- at pH 1.2 20%, at pH 6.8 60% and at pH 7.4 80% of drug releases after 8h. MTT analysis confirm the formulation is safe for oral delivery. |
The hydrogel system showed promising swelling, drug loading, drug- releasing, and MTT assay results |
|
Hydrogel beads system has potential for safe oral delivery system for colon cancer therapy | [335] |
| CMC Nanofiber | Fe(NO3)3-9H2O and N,N-dimethylformamide | Tetracycline | Skin treatment for harmful bacterial infection | %DL- 99.6 ± 0.5%. RP- Almost 60% drug was released in pH 7.4 after 384h. Formulation showed low cytotoxicity and sittable for drug delivery |
|
|
|
[336] |
| CMC Nanocomposite | Graphene Oxide (GO) and Zn- based MOF-5 | Doxorubicin | Cancer treatment | %DL- 6.2 RP- 0.225 mg of drug releases at pH 7.4 in 480 h. After 72h incubated, IC50 values were respectively 28.1, 15.7, 13.4, and 12.1 ng/mL of GO, CMC/MOF-5/GO, DOX@GO, and DOX@CMC/MOF-5/GO. |
|
|
|
[337] |
| CMC nanocomposite | Bilayer alginate-chitosan hydrogel | Dexamethasone | Treatment of acute and chronic ocular disorders, popliteal artery disease, inflammatory diseases such as asthma, meningitis, and rheumatoid arthritis | $DL- 0.78–5.53%; RP- Almost 25% drug was released after 12h in pH 7.4 |
|
|
|
[338] |
| CMC nanocomposites | Graphene quantum dots (GQDs) and CS | Sodium salicylate | RP- Around 70% in pH 7.4 after 8h. Formulations CS-GQD, CS-GQD/SS, SS@CMC and CS-GQD/SS@CMC shows cell viability 80.6, 71.6, 70.3 and 70.8, respectively. Hence, they are cytocompatibility. |
|
|
Promising system for oral delivery | [339] | |
| CMC nanocomposite | Graphene oxide | Methotrexate | Cancer treatment | %EE- 8–29 %DL- 16–39 RP- At pH 7.4 nearly 82% drug was released in 48 h. MTX/CMC-GO does not showed any damage to the organs of mice. MTX/CMC-GO-treated group showed inhibition rate of live metastasis was 83.3%. |
|
|
Promising for oral delivery of Methotrexate | [340] |
| CMC Hybrid nanoparticle | MOF-5 | 5-fluorouracil | Colon cancer | %EE- 84.1 RP- At pH 1.2 and 6.8, and 7.4 drug rleaase 20%, 60% and 70%, respectively in 8 h. |
|
|
|
[328] |
| CMC (MW = 90,000) hybrid nanoparticles | PEG, Fe3O4, CS, | Diclofenac | Inflammation | %EE- 55–80 RP- formulations showed pH responsive release of drug. Nearly 80% drug was released in SIF. |
|
|
|
[341] |
5.6.1.6. Miscellaneous.
Maciel et al. fabricated CMC based films enriched with natural plant extract for oral ion delivery [314]. Using tape casting method various films fabricated. The results revealed that films are fast disintegrating below 50 s. the iron release can be done within 50 minutes through Fickian diffusion mechanism [314]. It is an innovative system for iron delivery and can be used as carrier for treatment of Iron deficient disorders. Barkhordari et al. hydride system of CMC capsulated layered double hydroxide/drug for cephalexin oral delivery [342]. pH sensitive CMC bead used as protective capsule for LDH-drug (Fig. 12A). Drug cephalexin intercalated between LDH layer through co-precipitation method. The formed nanohybrid used to prepared nanocomposite hydrogel beads by association with CMC. The beads showed controlled release of drug in GI tract and protected in stomach pH [342]. This system still ne to be explored for further understanding.
Overall, chitosan is a potential mucoadhesive polymer for oral delivery with feasibility to target stomach, small intestine, intestine lymphatic, and colon. Other mucoadhesive polymers widely investigated colon targeted delivery. They have limited potential or need novel formulation to target the stomach, small intestine, and intestine lymphatic. Hence, other mucoadhesive polymers need to be explored. Pre-clinical and clinical studies need to be conducted for chitosan based oral drug delivery formulation to make it realistic. Recently ionic liquids showed promising results in oral delivery [343]. We believe ionic liquid can be an alternative system for mucoadhesive polymers in oral drug delivery applications.
6. Conclusions and Outlook
This review highlights the various potential mu coadhesive polymers-based formulation that has potential to oral delivery of macromolecules effectively and efficiently. We have put together this article after reviewing over three hundred representative research publication from the last decades. It is evident that mucoadhesive polymeric oral drug delivery systems are interesting due to desirable and physiochemical properties. Among the several types of mucoadhesive polymers, chitosan is a promising candidate for controlled, sustained, and targeted drug delivery to investigate mucoadhesive oral delivery of nanotherapeutics. Pectin is another suitable mucoadhesive polymer of choice for oral drug delivery, in terms of ease of formulation, availability of various functional groups offers range of derivatives for mucoadhesive oral delivery, and scalability. It has potential to control physico-chemical properties through desire choice of functionalization. Targeting agent can be conjugated with pectin to use as nanocarrier for targeted mucoadhesive oral drug delivery.
This review concludes that mucoadhesive polymeric oral drug delivery system is a good choice. However, sustainable mucoadhesive biopolymers need to explore. Furthermore, PAA and CMC have also recently gained great interest due to their promising properties. Mucoadhesive polymeric oral drug delivery systems are not extensively studied and well established due to limited number despite of huge potential and prospects. Moreover, toxicity of mucoadhesive polymeric nanoparticles is a major concern that need to be extensively evaluated before use of them in vivo and in pre-clinical studies.
Due to physicochemical properties, which offer the potential to survive with gastric fluids, and mucoadhesive properties, which allow the adhesion to mucus and facilitate the effective absorption of drug in the GI tract. We believe that mucoadhesive polymer based oral drug delivery systems are potential candidate for developing next generation personal medication. This review attempt to present potential of mucoadhesive polymers based oral drug delivery system to develop next generation multifunctional combination therapies, especially targeted delivery to stomach, small intestine, intestine lymphatic, and colon with control drug release. Designing of a through, extensive and realistic ex vivo experimental model is required to be able to investigate the potential of individual formulations and their physicochemical interaction with different parts of GI tract.
The impact of mucoadhesive polymer based oral drug delivery system has high significance for oral delivery science. The adverse side effects of chemotherapy can be controlled or overcome through modification with mucoadhesive polymeric oral drug delivery system. More than 5000 reports published on mucoadhesive polymer based oral drug delivery system in past decade. Despite of great development on oral formulation the existing gap and unmet needs still hindering the innovation translating from academic research to clinical application.
Acknowledgement
We acknowledge funding by Cancer Prevention Research Institute of Texas (CPRIT) through Texas Regional Excellence in Cancer Award (TREC) under Award No. PR210153, and National Institutes of Health (NIH) under Award No R03OD032624. The contents of this paper are solely the authors’ responsibility and do not necessarily represent the official views of NIH.
Data availability
No data was used for the research described in the article.
References
- [1].Agrawal U, Sharma R, Gupta M, Vyas SP, Is nanotechnology a boon for oral drug delivery? Drug Discov. Today 19 (2014) 1530–1546, 10.1016/J.DRUDIS.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [2].Ensign LM, Cone R, Hanes J, Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers, Adv. Drug Deliv. Rev 64 (2012) 557–570, 10.1016/J.ADDR.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S, Role of nanoparticle size, shape and surface chemistry in oral drug delivery, J. Control. Release 238 (2016) 176–185, 10.1016/J.JCONREL.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, Feng SS, Pharmaceutical nanotechnology for oral delivery of anticancer drugs, Adv. Drug Deliv. Rev 65 (2013) 880–890, 10.1016/J.ADDR.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [5].Bernkop-Schnürch A, Mucoadhesive systems in oral drug delivery, Drug Discov. Today Technol 2 (2005) 83–87, 10.1016/J.DDTEC.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [6].Silva BMA, Borges AF, Silva C, Coelho JFJ, Simões S, Mucoadhesive oral films: The potential for unmet needs, Int. J. Pharm 494 (2015) 537–551, 10.1016/J.IJPHARM.2015.08.038. [DOI] [PubMed] [Google Scholar]
- [7].Patil H, Tiwari R.v., Repka MA, Recent advancements in mucoadhesive floating drug delivery systems: A mini-review, J. Drug Deliv. Sci. Technol 31 (2016) 65–71, 10.1016/J.JDDST.2015.12.002. [DOI] [Google Scholar]
- [8].Chatterjee B, Amalina N, Sengupta P, Kumar Mandal U, Mucoadhesive Polymers and Their Mode of Action: A Recent Update, J. Appl. Pharm. Sci 7 (2017) 195–203, 10.7324/JAPS.2017.70533. [DOI] [Google Scholar]
- [9].Bassi da Silva J, de S. Ferreira SB, de Freitas O, Bruschi ML, A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems, Drug Dev. Ind. Pharm 43 (2017) 1053–1070, 10.1080/03639045.2017.1294600. [DOI] [PubMed] [Google Scholar]
- [10].Lang X, Wang T, Sun M, Chen X, Liu Y, Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review, Int. J. Biol. Macromol 154 (2020) 433–445, 10.1016/J.IJBIOMAC.2020.03.148. [DOI] [PubMed] [Google Scholar]
- [11].Edmans JG, Clitherow KH, Murdoch C, Hatton P.v., Spain SG, Colley HE, Mucoadhesive Electrospun Fibre-Based Technologies for Oral Medicine, Pharmaceutics 12 (2020) 504, 12 (2020) 504. doi: 10.3390/PHARMACEUTICS12060504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kumar A, Naik PK, Pradhan D, Ghosh G, Rath G, Mucoadhesive formulations: innovations, merits, drawbacks, and future outlook, Pharm. Dev. Technol 25 (2020) 797–814, 10.1080/10837450.2020.1753771. [DOI] [PubMed] [Google Scholar]
- [13].Alaei S, Omidian H, Mucoadhesion and Mechanical Assessment of Oral Films, Eur. J. Pharm. Sci 159 (2021), 105727, 10.1016/J.EJPS.2021.105727. [DOI] [PubMed] [Google Scholar]
- [14].Gómez-Guillén MC, Montero MP, Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review, Food Hydrocoll. 118 (2021), 106772, 10.1016/J.FOODHYD.2021.106772. [DOI] [Google Scholar]
- [15].Pandey M, Choudhury H, Ying JNS, Ling JFS, Ting J, Ting JSS, Hwen IKZ, Suen HW, Kamar HSS, Gorain B, Jain N, Amin MCIM, Mucoadhesive nanocarriers as a promising strategy to enhance intracellular delivery against oral cavity carcinoma, Pharmaceutics 2022 14 (2022) 795, 10.3390/PHARMACEUTICS14040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang W-Q, Lin Q, Zhuang X, Cai L-L, Ruan R-S, Lu Z-X, Tzeng C-M, Structure, Function, and Pathogenesis of SHP2 in Developmental Disorders and Tumorigenesis, (n.d.). 14 (2014) 567–588, 10.2174/1568009614666140717105001. [DOI] [PubMed] [Google Scholar]
- [17].Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ, Andersen L, Atherton J, Asaka M, Bazzoli F, Bytzer P, Chan F, Coelho LGV, de Wit N, Delchier JC, di Mario F, El-Omar E, Fock KM, Forman D, Fujioka T, Gasbarrini G, Genta R, Goh KL, Graham DY, Hirschl A, Hungin P, Hunt R, Isakov VA, Jones R, Kist M, Koletzko S, Kuipers EJ, Kupcinskas L, Ladas S, Lanas A, Machado J, Malfertheiner P, McColl KEL, Mégraud F, Michetti P, Moayyedi P, Omorain C, Pilotto A, Quina M, Rokkas T, Sharma P, Simsek Y, Sipponen P, Sollano J, Stockbrügger R, Sugano K, Vaira D, Vakil N, Vieth M, Xiao S, Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report, Gut. 56 (2007) 772–781, 10.1136/GUT.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lazar DC, Taban S, Cornianu M, Faur A, Goldis A, New advances in targeted gastric cancer treatment, World J. Gastroenterol 22 (2016) 6776, 10.3748/WJG.V22.I30.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Drugs Approved for Stomach (Gastric) Cancer - NCI, (2021). https://www.cancer.gov/about-cancer/treatment/drugs/stomach (accessed September 7, 2022).
- [20].Baumgart DC, What’s new in inflammatory bowel disease in 2008? World J Gastroenterol: WJG 14 (2008) 329, 10.3748/WJG.14.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pearce J, Wilks S, Edin HLLD, Sir Samuel Wilks (1824–1911): ‘The Most Philosophical of English Physicians’, Eur. Neurol 61 (2009) 119–123, 10.1159/000180315. [DOI] [PubMed] [Google Scholar]
- [22].Aniwan S, Harmsen WS, Tremaine WJ, Loftus E.v., Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010, Ther. Adv. Gastroenterol 12 (2019), 10.1177/1756284819827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yasmin F, Najeeb H, Shaikh S, Hasanain M, Naeem U, Moeed A, Koritala T, Hasan S, Surani S, Novel drug delivery systems for inflammatory bowel disease, World J. Gastroenterol 28 (2022) 1922, 10.3748/WJG.V28.I18.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gralnek IM, Siersema PD, Halpern Z, Segol O, Melhem A, Suissa A, Santo E, Sloyer A, Fenster J, Moons LMG, Dik VK, D’Agostino RB, Rex DK, Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial, Lancet Oncol. 15 (2014) 353–360, 10.1016/S1470-2045(14)70020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin 68 (2018) 7–30, 10.3322/CAAC.21442/FULL. [DOI] [PubMed] [Google Scholar]
- [26].Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA, Is Diabetes Mellitus an Independent Risk Factor for Colon Cancer and Rectal Cancer? Am. J. Gastroenterol 106 (2011) 1911, 10.1038/AJG.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Segal NH, Saltz LB, Evolving Treatment of Advanced Colon Cancer 60, 2009, pp. 207–219, 10.1146/Annurev.Med.60.041807.132435. [DOI] [PubMed] [Google Scholar]
- [28].Diabetes: Symptoms, Causes, Treatment, Prevention, and More, (2022). https://www.healthline.com/health/diabetes (accessed September 7, 2022).
- [29].What is diabetes? | CDC, (2022). https://www.cdc.gov/diabetes/basics/diabetes.html (accessed September 7, 2022).
- [30].Statistics About Diabetes | ADA, (2022). https://diabetes.org/about-us/statistics/about-diabetes (accessed September 7, 2022).
- [31].9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetesd2019, in: Diabetes Care 42, 2019, pp. S90–S102, 10.2337/DC19-S009. [DOI] [PubMed] [Google Scholar]
- [32].Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, Ramanunny AK, Awasthi A, Dua K, Treatment strategies against diabetes: Success so far and challenges ahead, Eur. J. Pharmacol 862 (2019), 172625, 10.1016/J.EJPHAR.2019.172625. [DOI] [PubMed] [Google Scholar]
- [33].Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ, Advances in oral drug delivery, Front. Pharmacol 12 (2021) 62, 10.3389/FPHAR.2021.618411/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kusters JG, Van Vliet AHM, Kuipers EJ, Pathogenesis of Helicobacter pylori infection, Clin. Microbiol. Rev 19 (2006) 449–490, 10.1128/CMR.00054-05/ASSET/1C3F54FF-A8CB-4449-B2EE-6B0EC9420CD2/ASSETS/GRAPHIC/ZCM0030621760007.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shishu N, Aggarwal Gupta N, Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads, in: AAPS PharmSciTech 2007. 8:2. 8, 2007, pp. E143–E149, 10.1208/PT0802048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singh BN, Kim KH, Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention, J. Control. Release 63 (2000) 235–259, 10.1016/S0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- [37].Cone RA, Barrier properties of mucus, Adv. Drug Deliv. Rev 61 (2009) 75–85, 10.1016/J.ADDR.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [38].Ji J, Yang H, Using probiotics as supplementation for Helicobacter pylori antibiotic therapy, Int. J. Mol. Sci 21 (2020) 1136, 21 (2020) 1136, 10.3390/IJMS21031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thombre NA, Gide PS, Floating-bioadhesive gastroretentive Caesalpinia pulcherrima-based beads of amoxicillin trihydrate for Helicobacter pylori eradication, Drug Deliv. 23 (2014) 405–419, 10.3109/10717544.2014.916766. [DOI] [PubMed] [Google Scholar]
- [40].Seidner DL, Schwartz LK, Winkler MF, Jeejeebhoy K, Boullata JI, Tappenden KA, Increased intestinal absorption in the era of Teduglutide and its impact on management strategies in patients with short bowel syndrome–associated intestinal failure, J. Parenter. Enter. Nutr 37 (2013) 201–211, 10.1177/0148607112472906. [DOI] [PubMed] [Google Scholar]
- [41].dos Santos AM, Carvalho SG, Meneguin AB, Sábio RM, Gremião MPD, Chorilli M, Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances and future perspectives, J. Control. Release 334 (2021) 353–366, 10.1016/J.JCONREL.2021.04.026. [DOI] [PubMed] [Google Scholar]
- [42].Anal AK, Bhopatkar D, Tokura S, Tamura H, Stevens WF, Chitosan-Alginate Multilayer Beads for Gastric Passage and Controlled Intestinal Release of Protein 29, 2003, pp. 713–724, 10.1081/DDC-120021320. [DOI] [PubMed] [Google Scholar]
- [43].Snoeck V, Goddeeris B, Cox E, The role of enterocytes in the intestinal barrier function and antigen uptake, Microbes Infect. 7 (2005) 997–1004, 10.1016/J.MICINF.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [44].Neutra MR, Kraehenbuhl JP, Transepithelial transport and mucosal defence I: the role of M cells, Trends Cell Biol. 2 (1992) 134–138, 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- [45].Mao Y, Feng S, Zhang X, Zhao Q, Fang Y, Wang S, Thiolated polymer and Cell-Penetrating Peptide dual-surface functionalization of mesoporous silicon nanoparticles to overcome intestinal barriers, J. Drug Deliv. Sci. Technol 53 (2019), 101184, 10.1016/J.JDDST.2019.101184. [DOI] [Google Scholar]
- [46].Müller C, Perera G, König V, Bernkop-Schnürch A, Development and in vivo evaluation of papain-functionalized nanoparticles, Eur. J. Pharm. Biopharm 87 (2014) 125–131, 10.1016/J.EJPB.2013.12.012. [DOI] [PubMed] [Google Scholar]
- [47].Sockolosky JT, Szoka FC, The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy, Adv. Drug Deliv. Rev 91 (2015) 109–124, 10.1016/J.ADDR.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jepson MA, Clark MA, Foster N, Mason CM, Bennett MK, Simmons NL, Hirst BH, Targeting to intestinal M cells, J. Anat 189 (1996) 507. [PMC free article] [PubMed] [Google Scholar]
- [49].KuoLee R, Chen W, M cell-targeted delivery of vaccines and therapeutics, Expert Opin. Drug Deliv 5 (2008) 693–702, 10.1517/17425247.5.6.693. [DOI] [PubMed] [Google Scholar]
- [50].Roth-Walter F, Schöll I, Untersmayr E, Fuchs R, Boltz-Nitulescu G, Weissenböck A, Scheiner O, Gabor F, Jensen-Jarolim E, M cell targeting with Aleuria aurantia lectin as a novel approach for oral allergen immunotherapy, J. Allergy Clin. Immunol 114 (2004) 1362–1368, 10.1016/J.JACI.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [51].Garinot M, Fíevez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jérôme C, Marchand-Brynaert J, Schneider YJ, Pŕeat V, PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination, J. Control. Release 120 (2007) 195–204, 10.1016/J.JCONREL.2007.04.021. [DOI] [PubMed] [Google Scholar]
- [52].Cifarelli V, Eichmann A, The Intestinal lymphatic system: functions and metabolic implications, Cell Mol. Gastroenterol. Hepatol 7 (2019) 503–513, 10.1016/J.JCMGH.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Managuli RS, Raut SY, Reddy MS, Mutalik S, Targeting the Intestinal Lymphatic System: A Versatile Path for Enhanced Oral Bioavailability of Drugs 15, 2018, pp. 787–804, 10.1080/17425247.2018.1503249. [DOI] [PubMed] [Google Scholar]
- [54].Porter CJH, Trevaskis NL, Charman WN, Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs, Nat. Rev. Drug Discov 6 (2007) 231–248, 10.1038/nrd2197, 2007 6:3. [DOI] [PubMed] [Google Scholar]
- [55].Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW, Textbook of Gastroenterology, Fifth Edition, Textbook of Gastroenterology, Fifth Edition 1–2, 2009, pp. 1–3551, 10.1002/9781444303254. [DOI] [Google Scholar]
- [56].Friend DR, Colon-specific drug delivery, Adv. Drug Deliv. Rev 7 (1991) 149–199, 10.1016/0169-409X(91)90051-D. [DOI] [Google Scholar]
- [57].Naeem M, Awan UA, Subhan F, Cao J, Hlaing SP, Lee J, Im E, Jung Y, Yoo JW, Advances in colon-targeted nano-drug delivery systems: challenges and solutions, Arch. Pharm. Res 43 (2020) 153–169, 10.1007/S12272-020-01219-0. [DOI] [PubMed] [Google Scholar]
- [58].Liu L, Yao WD, Rao YF, Lu XY, Gao JQ, pH-Responsive Carriers for Oral Drug Delivery: Challenges and Opportunities of Current Platforms 24, 2017, pp. 569–581, 10.1080/10717544.2017.1279238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jain SK, Jain AK, Rajpoot K, Expedition of Eudragit® Polymers in the Development of Novel Drug Delivery Systems, Curr. Drug Deliv 17 (2020) 448–469, 10.2174/1567201817666200512093639. [DOI] [PubMed] [Google Scholar]
- [60].Khan MZI, Prebeg Ž, Kurjakovíc N, A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100–55 and Eudragit® S100 combinations, J. Control. Release 58 (1999) 215–222, 10.1016/S0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- [61].Chen MC, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung MF, Hsu LW, Sung HW, Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules, Adv. Drug Deliv. Rev 65 (2013) 865–879, 10.1016/J.ADDR.2012.10.010. [DOI] [PubMed] [Google Scholar]
- [62].Chen MC, Sonaje K, Chen KJ, Sung HW, A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery, Biomaterials. 32 (2011) 9826–9838, 10.1016/J.BIOMATERIALS.2011.08.087. [DOI] [PubMed] [Google Scholar]
- [63].Ahuja Alka, Khar Roop K., Ali Javed, Mucoadhesive drug delivery systems, Drug Dev. Ind. Pharm 23 (5) (1997) 489–515, 10.3109/03639049709148498. [DOI] [Google Scholar]
- [64].Andrews Gavin P., Laverty Thomas P., Jones David S., Mucoadhesive polymeric platforms for controlled drug delivery, Eur. J. Pharm. Biopharm 71 (2009) 505–518, 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- [65].Ahmed A, Yadav HKS, Lakshmi SV, Namburi BVN, Shivakumar HG, Mucoadhesive Nanoparticulate System for Oral Drug Delivery: A Review, Curr. Drug Ther 7 (2012) 42–55, 10.2174/157488512800389137. [DOI] [Google Scholar]
- [66].Lemmer HJ, Hamman JH, Paracellular drug absorption enhancement through tight junction modulation, Expert Opin. Drug Deliv 10 (2012) 103–114, 10.1517/17425247.2013.745509. [DOI] [PubMed] [Google Scholar]
- [67].Yeh TH, Hsu LW, Tseng MT, Lee PL, Sonjae K, Ho YC, Sung HW, Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening, Biomaterials. 32 (2011) 6164–6173, 10.1016/J.BIOMATERIALS.2011.03.056. [DOI] [PubMed] [Google Scholar]
- [68].Yamamoto Hiromitsu, Kuno Yoshio, Sugimoto Shohei, Takeuchi Hirofumi, Kawashima Yoshiaki, Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions, J. Control. Release 101 (2) (2005) 373–381, 10.1016/j.jconrel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [69].Salama NN, Eddington ND, Fasano A, Tight junction modulation and its relationship to drug delivery, Adv. Drug Deliv. Rev 58 (2006) 15–28, 10.1016/J.ADDR.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [70].Sonaje K, Chuang EY, Lin KJ, Yen TC, Su FY, Tseng MT, Sung HW, Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: Microscopic, ultrastructural, and computed-tomographic observations, Mol. Pharm 9 (2012) 1271–1279, 10.1021/MP200572T/ASSET/IMAGES/MEDIUM/MP-2011-00572T_0006.GIF. [DOI] [PubMed] [Google Scholar]
- [71].Kumar R, Dalvi SV, Siril PF, Nanoparticle-Based Drugs and Formulations: Current Status and Emerging Applications, ACS Appl. Nano Mater 3 (2020) 4944–4961, 10.1021/acsanm.0c00606. [DOI] [Google Scholar]
- [72].Kumar R, Siril PF, Drop-by-drop solvent hot antisolvent interaction method for engineering nanocrystallization of sulfamethoxazole to enhanced water solubility and bioavailability, J. Drug Deliv. Sci. Technol (2019), 101359, 10.1016/j.jddst.2019.101359. [DOI] [Google Scholar]
- [73].Kumar R, Siril PF, Javid F, Unusual anti-leukemia activity of nanoformulated naproxen and other non-steroidal anti-inflammatory drugs, Mater. Sci. Eng. C 69 (2016) 1335–1344, 10.1016/j.msec.2016.08.024. [DOI] [PubMed] [Google Scholar]
- [74].Kumar R, Siril PF, Enhancing the Solubility of Fenofibrate by Nanocrystal Formation and Encapsulation, AAPS PharmSciTech 19 (2018) 284–292, 10.1208/s12249-017-0840-z. [DOI] [PubMed] [Google Scholar]
- [75].Andrews GP, Laverty TP, Jones DS, Mucoadhesive polymeric platforms for controlled drug delivery, Eur. J. Pharm. Biopharm 71 (2009) 505–518, 10.1016/J.EJPB.2008.09.028. [DOI] [PubMed] [Google Scholar]
- [76].Agarwal S, Aggarwal S, Mucoadhesive Polymeric Platform for Drug Delivery; A Comprehensiv…: Ingenta Connect, Curr. Drug Deliv 12 (2015) 139–156. [DOI] [PubMed] [Google Scholar]
- [77].Thanou M, Verhoef JC, Junginger HE, Oral drug absorption enhancement by chitosan and its derivatives, Adv. Drug Deliv. Rev 52 (2001) 117–126, 10.1016/S0169-409X(01)00231-9. [DOI] [PubMed] [Google Scholar]
- [78].Werle M, Takeuchi H, Bernkop-Schnürch A, Modified Chitosans for Oral Drug Delivery, J. Pharm. Sci 98 (2009) 1643–1656, 10.1002/JPS.21550. [DOI] [PubMed] [Google Scholar]
- [79].Wang J, Wang L, Yu H, Zain-ul-Abdin Y, Chen Q, Chen W, Zhou H, Chen Zhang X, Recent progress on synthesis, property and application of modified chitosan: An overview, Int. J. Biol. Macromol 88 (2016) 333–344, 10.1016/J.IJBIOMAC.2016.04.002. [DOI] [PubMed] [Google Scholar]
- [80].Werle M, Bernkop-Schnürch A, Werle M, Thiolated chitosans: useful excipients for oral drug delivery, J. Pharm. Pharmacol 60 (2008) 273–281, 10.1211/JPP.60.3.3001. [DOI] [PubMed] [Google Scholar]
- [81].Mourya VK, Inamdar NN, Chitosan-modifications and applications: Opportunities galore, React. Funct. Polym 68 (2008) 1013–1051, 10.1016/J.REACTFUNCTPOLYM.2008.03.002. [DOI] [Google Scholar]
- [82].Domard A, Rinaudo M, Terrassin C, New method for the quaternization of chitosan, Int. J. Biol. Macromol 8 (1986) 105–107, 10.1016/0141-8130(86)90007-3. [DOI] [Google Scholar]
- [83].Verma A, Tiwari A, Saraf S, Panda PK, Jain A, Jain SK, Protein and peptide delivery by chitosan systems, Chitosan Biomed. Appl (2022) 211–228, 10.1016/B978-0-12-821058-1.00006-X. [DOI] [Google Scholar]
- [84].Kumar A, Vimal A, Kumar A, Why Chitosan? From properties to perspective of mucosal drug delivery, Int. J. Biol. Macromol 91 (2016) 615–622, 10.1016/J.IJBIOMAC.2016.05.054. [DOI] [PubMed] [Google Scholar]
- [85].Jalil A, Asim MH, Le NMN, Laffleur F, Matuszczak B, Tribus M, Bernkop-Schnürch A, S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films, Int. J. Biol. Macromol 130 (2019) 148–157, 10.1016/J.IJBIOMAC.2019.02.092. [DOI] [PubMed] [Google Scholar]
- [86].Zhao X, Yin L, Ding J, Tang C, Gu S, Yin C, Mao Y, Thiolated trimethyl chitosan nanocomplexes as gene carriers with high in vitro and in vivo transfection efficiency, J. Control. Release 144 (2010) 46–54, 10.1016/J.JCONREL.2010.01.022. [DOI] [PubMed] [Google Scholar]
- [87].Cui F, Qian F, Zhao Z, Yin L, Tang C, Yin C, Preparation, characterization, and oral delivery of insulin loaded carboxylated chitosan grafted poly(methyl methacrylate) nanoparticles, Biomacromolecules. 10 (2009) 1253–1258, 10.1021/BM900035U/ASSET/IMAGES/BM900035U.SOCIAL.JPEG_V03. [DOI] [PubMed] [Google Scholar]
- [88].Harding SE, Mucoadhesive interactions, Biochem. Soc. Trans 31 (2003) 1036–1041, 10.1042/BST0311036. [DOI] [PubMed] [Google Scholar]
- [89].Rekha MR, Sharma CP, Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption, J. Control. Release 135 (2009) 144–151, 10.1016/J.JCONREL.2009.01.011. [DOI] [PubMed] [Google Scholar]
- [90].Srivastava V, Chauhan DS, Joshi PG, Maruthapandian V, Sorour AA, Quraishi MA, PEG-Functionalized Chitosan: A Biological Macromolecule as a Novel Corrosion Inhibitor, ChemistrySelect. 3 (2018) 1990–1998, 10.1002/SLCT.201701949. [DOI] [Google Scholar]
- [91].Farace C, Sánchez-Moreno P, Orecchioni M, Manetti R, Sgarrella F, Asara Y, Peula-García JM, Marchal JA, Madeddu R, Delogu LG, Immune cell impact of three differently coated lipid nanocapsules: pluronic, chitosan and polyethylene glycol, Sci. Rep 6 (2016) 1–14, 10.1038/srep18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rostami E, Progresses in targeted drug delivery systems using chitosan nanoparticles in cancer therapy: A mini-review, J. Drug Deliv. Sci. Technol 58 (2020), 101813, 10.1016/J.JDDST.2020.101813. [DOI] [Google Scholar]
- [93].Mohammed MA, Syeda JTM, Wasan KM, Wasan EK, An overview of chitosan nanoparticles and its application in non-parenteral drug delivery, Pharmaceutics 9 (2017) 53, 9 (2017) 53, 10.3390/PHARMACEUTICS9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jiang Z, Chi J, Li H, Wang Y, Liu W, Han B, Effect of chitosan oligosaccharideconjugated selenium on improving immune function and blocking gastric cancer growth, Eur. J. Pharmacol 891 (2021), 173673, 10.1016/J.EJPHAR.2020.173673. [DOI] [PubMed] [Google Scholar]
- [95].Lin YH, Chen ZR, Lai CH, Hsieh CH, Feng CL, Active Targeted Nanoparticles for Oral Administration of Gastric Cancer Therapy, Biomacromolecules. 16 (2015) 3021–3032, 10.1021/ACS.BIOMAC.5B00907/ASSET/IMAGES/LARGE/BM-2015-00907S_0010.JPEG. [DOI] [PubMed] [Google Scholar]
- [96].Shen C, Zhao L, Du X, Tian J, Yuan Y, Jia M, He Y, Zeng R, Qiao R, Li C, Smart Responsive Quercetin-Conjugated Glycol Chitosan Prodrug Micelles for Treatment of Inflammatory Bowel Diseases, Mol. Pharm 18 (2021) 1419–1430, 10.1021/ACS.MOLPHARMACEUT.0C01245/ASSET/IMAGES/LARGE/MP0C01245_0008.JPEG. [DOI] [PubMed] [Google Scholar]
- [97].Kumar R, Sirvi A, Kaur S, Samal SK, Roy S, Sangamwar AT, Polymeric micelles based on amphiphilic oleic acid modified carboxymethyl chitosan for oral drug delivery of bcs class iv compound: Intestinal permeability and pharmacokinetic evaluation, Eur. J. Pharm. Sci 153 (2020), 105466, 10.1016/J.EJPS.2020.105466. [DOI] [PubMed] [Google Scholar]
- [98].Cong Y, Geng J, Wang H, Su J, Arif M, Dong Q, Chi Z, Liu C, Ureido-modified carboxymethyl chitosan-graft-stearic acid polymeric nano-micelles as a targeted delivering carrier of clarithromycin for Helicobacter pylori: Preparation and in vitro evaluation, Int. J. Biol. Macromol 129 (2019) 686–692, 10.1016/J.IJBIOMAC.2019.01.227. [DOI] [PubMed] [Google Scholar]
- [99].Ways TMM, Lau WM, Khutoryanskiy VV, Chitosan and its derivatives for application in mucoadhesive drug delivery systems, Polymers 10 (2018) 267, 10 (2018) 267, 10.3390/POLYM10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rosenthal R, Günzel D, Finger C, Krug SM, Richter JF, Schulzke JD, Fromm M, Amasheh S, The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier, Biomaterials. 33 (2012) 2791–2800, 10.1016/J.BIOMATERIALS.2011.12.034. [DOI] [PubMed] [Google Scholar]
- [101].Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH, Advancement on modification of chitosan biopolymer and its potential applications, Int. J. Biol. Macromol 152 (2020) 681–702, 10.1016/J.IJBIOMAC.2020.02.196. [DOI] [PubMed] [Google Scholar]
- [102].Duran-Lobato Matilde, Niu Zhigao, Alonso Maria Jose, Oral delivery of biologics for precision medicine, Adv. Mater 32 (2020) 1901935, 10.1002/adma.201901935. [DOI] [PubMed] [Google Scholar]
- [103].Benediktsdóttir BE, Baldursson Ó, Másson M, Challenges in evaluation of chitosan and trimethylated chitosan (TMC) as mucosal permeation enhancers: From synthesis to in vitro application, J. Control. Release 173 (2014) 18–31, 10.1016/J.JCONREL.2013.10.022. [DOI] [PubMed] [Google Scholar]
- [104].Shafabakhsh R, Yousefi B, Asemi Z, Nikfar B, Mansournia MA, Hallajzadeh J, Chitosan: A compound for drug delivery system in gastric cancer-a review, Carbohydr. Polym 242 (2020), 116403, 10.1016/J.CARBPOL.2020.116403. [DOI] [PubMed] [Google Scholar]
- [105].Lang X, Wang T, Sun M, Chen X, Liu Y, Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review, Int. J. Biol. Macromol 154 (2020) 433–445, 10.1016/J.IJBIOMAC.2020.03.148. [DOI] [PubMed] [Google Scholar]
- [106].Chen G, Svirskis D, Lu W, Ying M, Huang Y, Wen J, N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer, J. Control. Release 277 (2018) 142–153, 10.1016/J.JCONREL.2018.03.013. [DOI] [PubMed] [Google Scholar]
- [107].Sudhakar S, Chandran SV, Selvamurugan N, Nazeer RA, Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo, Int. J. Biol. Macromol 150 (2020) 281–288, 10.1016/J.IJBIOMAC.2020.02.079. [DOI] [PubMed] [Google Scholar]
- [108].Rai VK, Mishra N, Agrawal AK, Jain S, Yadav NP, Novel drug delivery system: an immense hope for diabetics, Drug Deliv. 23 (2014) 2371–2390, 10.3109/10717544.2014.991001. [DOI] [PubMed] [Google Scholar]
- [109].Du X, Yin S, Xu L, Ma J, Yu H, Wang G, Li J, Polylysine and cysteine functionalized chitosan nanoparticle as an efficient platform for oral delivery of paclitaxel, Carbohydr. Polym 229 (2020), 115484, 10.1016/J.CARBPOL.2019.115484. [DOI] [PubMed] [Google Scholar]
- [110].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J. Clin 68 (2018) 394–424, 10.3322/CAAC.21492. [DOI] [PubMed] [Google Scholar]
- [111].Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S, Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value, World J Gastroenterol: WJG 20 (2014) 5679, 10.3748/WJG.V20.I19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M, Gastric cancer: Prevention, screening and early diagnosis, World J Gastroenterol: WJG 20 (2014) 13842, 10.3748/WJG.V20.I38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F, Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention, Cancer Epidemiol. Biomark. Prev 23 (2014) 700–713, 10.1158/1055-9965.EPI-13-1057/67649/AM/GASTRIC-CANCER-DESCRIPTIVE-EPIDEMIOLOGY-RISK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sexton RE, Al Hallak MN, Diab M, Azmi AS, Gastric cancer: a comprehensive review of current and future treatment strategies, Cancer Metastasis Rev. 39 (2020) 1179–1203, 10.1007/S10555-020-09925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Progress and challenges towards targeted delivery of cancer therapeutics, Nat. Commun 9 (2018) 1–12, 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chi J, Jiang Z, Qiao J, Peng Y, Liu W, Han B, Synthesis and anti-metastasis activities of norcantharidin-conjugated carboxymethyl chitosan as a novel drug delivery system, Carbohydr. Polym 214 (2019) 80–89, 10.1016/J.CARBPOL.2019.03.026. [DOI] [PubMed] [Google Scholar]
- [117].Wang C-PJ, Byun MJ, Kim S-N, Park W, Park HH, Kim T-H, Lee JS, Park CG, Biomaterials as therapeutic drug carriers for inflammatory bowel disease treatment, J. Control. Release 345 (2022) 1–19, 10.1016/J.JCONREL.2022.02.028. [DOI] [PubMed] [Google Scholar]
- [118].Burger Daniel, Travis Simon, Conventional medical management of inflammatory bowel disease, Gastoenterology 140 (6) (2011) 1827–1837e, 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- [119].Homayun Bahman, Kin Xueting, Choi Hyo-Jick, Challenges and recent progress in oral drug delivery systems for biopharmaceuticals, Pharmaceutics 11 (129) (2019) 1–29, 10.3390/pharmaceutics11030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Yousef M, Pichyangkura R, Soodvilai S, Chatsudthipong V, Muanprasat C, Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action, Pharmacol. Res 66 (2012) 66–79, 10.1016/J.PHRS.2012.03.013. [DOI] [PubMed] [Google Scholar]
- [121].Nalinbenjapun S, Ovatlarnporn C, Chitosan-5-aminosalicylic acid conjugates for colon-specific drug delivery: Methods of preparation and in vitro evaluations, J. Drug Deliv. Sci. Technol 57 (2020), 101397, 10.1016/J.JDDST.2019.101397. [DOI] [Google Scholar]
- [122].Kumar Raj, Mondal Kunal, Pritam Kumar Panda Ajeet Kaushik, Abolhassani Reza, Ahuja Rajeev, Rubahn Horst-Gunter, Yogendra Kumar Mishra, Core–shell nanostructures: perspectives towards drug delivery applications, J. Mater. Chem. B 8 (2020) 8992–9027, 10.1039/D0TB01559H. [DOI] [PubMed] [Google Scholar]
- [123].Wang X, Qiu L, Wang X, Ouyang H, Li T, Han L, Zhang X, Xu W, Chu K, Evaluation of intestinal permeation enhancement with carboxymethyl chitosanrhein polymeric micelles for oral delivery of paclitaxel, Int. J. Pharm 573 (2020), 118840, 10.1016/J.IJPHARM.2019.118840. [DOI] [PubMed] [Google Scholar]
- [124].Khan KH, Begum M, Saleh M, Khasru MR, Correlation between helicobacter pylori and gastric diseases: a study in King Fahad Hospital at Al-Baha of Saudi Arabia, Mymensingh Med. J 18 (2009) S113–S118. [PubMed] [Google Scholar]
- [125].Lin YH, Chang CH, Wu YS, Hsu YM, Chiou SF, Chen YJ, Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy, Biomaterials. 30 (2009) 3332–3342, 10.1016/J.BIOMATERIALS.2009.02.036. [DOI] [PubMed] [Google Scholar]
- [126].Kim JU, Shahbaz HM, Lee H, Kim T, Yang K, Roh YH, Park J, Optimization of phytic acid-crosslinked chitosan microspheres for oral insulin delivery using response surface methodology, Int. J. Pharm 588 (2020), 119736, 10.1016/J.IJPHARM.2020.119736. [DOI] [PubMed] [Google Scholar]
- [127].Bai K, Hong B, Tan R, He J, Hong Z, Selenium Nanoparticles-Embedded Chitosan Microspheres and Their Effects Upon Alcohol-Induced Gastric Mucosal Injury in Rats: Rapid Preparation, Oral Delivery, and Gastroprotective Potential of Selenium Nanoparticles, Int. J. Nanomedicine 15 (2020) 1187–1203, 10.2147/IJN.S237089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Holt Peter R., Katz Seymour, Kirshoff Robert, Curcumin therapy in inflammatory bowel disease: a pilot study, Dig. Dis. Sci 50 (11) (2005) 2191–2193, 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- [129].Zhang S, Kang L, Hu S, Hu J, Fu Y, Hu Y, Yang X, Carboxymethyl chitosan microspheres loaded hyaluronic acid/gelatin hydrogels for controlled drug delivery and the treatment of inflammatory bowel disease, Int. J. Biol. Macromol 167 (2021) 1598–1612, 10.1016/J.IJBIOMAC.2020.11.117. [DOI] [PubMed] [Google Scholar]
- [130].Seyam S, Nordin NA, Alfatama M, Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery, Pharmaceuticals. 13 (2020) 307, 10.3390/PH13100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R, Oral bioavailability of insulin contained in polysaccharide nanoparticles, Biomacromolecules. 8 (2007) 3054–3060, 10.1021/BM0703923/ASSET/IMAGES/MEDIUM/BM0703923N00001.GIF. [DOI] [PubMed] [Google Scholar]
- [132].Xu Z, Chen L, Duan X, Li X, Ren H, Microparticles based on alginate/chitosan/casein three-dimensional system for oral insulin delivery, Polym. Adv. Technol 32 (2021) 4352–4361, 10.1002/PAT.5437. [DOI] [Google Scholar]
- [133].Zhang X, Lyu X, Tong Y, Wang J, Ye J, Yang R, Chitosan/casein based microparticles with a bilayer shell–core structure for oral delivery of nattokinase, Food Funct 11 (2020) 10799–10816, 10.1039/D0FO02349C. [DOI] [PubMed] [Google Scholar]
- [134].Gómez-Burgaz M, García-Ochoa B, Torrado-Santiago S, Chitosan–carboxymethylcellulose interpolymer complexes for gastric-specific delivery of clarithromycin, Int. J. Pharm 359 (2008) 135–143, 10.1016/J.IJPHARM.2008.03.042. [DOI] [PubMed] [Google Scholar]
- [135].Wang J, Chin D, Poon C, Mancino V, Pham J, Li H, Ho PY, Hallows KR, Chung EJ, Oral delivery of metformin by chitosan nanoparticles for polycystic kidney disease, J. Control. Release 329 (2021) 1198–1209, 10.1016/J.JCONREL.2020.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Oshi MA, Naeem M, Bae J, Kim J, Lee J, Hasan N, Kim W, Im E, Jung Y, Yoo JW, Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease, Carbohydr. Polym 198 (2018) 434–442, 10.1016/J.CARBPOL.2018.06.107. [DOI] [PubMed] [Google Scholar]
- [137].Lautenschläger C, Schmidt C, Fischer D, Stallmach A, Drug delivery strategies in the therapy of inflammatory bowel disease, Adv. Drug Deliv. Rev 71 (2014) 58–76, 10.1016/J.ADDR.2013.10.001. [DOI] [PubMed] [Google Scholar]
- [138].Kurakula M, N. NR, Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020), Int. J. Biol. Macromol 165 (2020) 1924–1938, 10.1016/J.IJBIOMAC.2020.10.043. [DOI] [PubMed] [Google Scholar]
- [139].Yang H, Sun A, Yang J, Cheng H, Yang X, Chen H, Huanfei D, Falahati M, Development of doxorubicin-loaded chitosan–heparin nanoparticles with selective anticancer efficacy against gastric cancer cells in vitro through regulation of intrinsic apoptosis pathway, Arab. J. Chem 14 (2021), 103266, 10.1016/J.ARABJC.2021.103266. [DOI] [Google Scholar]
- [140].Lai CK, Lu YL, Hsieh JT, Tsai SC, Feng CL, Tsai YS, Tsai PC, Su HL, Lin YH, Lai CH, Development of chitosan/heparin nanoparticle-encapsulated cytolethal distending toxin for gastric cancer therapy, Nanomedicine (London) 9 (2014) 803–817, 10.2217/NNM.13.54. [DOI] [PubMed] [Google Scholar]
- [141].Zhang E, Xing R, Liu S, Li K, Qin Y, Yu H, Li P, Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy, Int. J. Biol. Macromol 126 (2019) 662–672, 10.1016/J.IJBIOMAC.2018.12.262. [DOI] [PubMed] [Google Scholar]
- [142].Liu P, Gao C, Chen H, Vong CT, Wu X, Tang X, Wang S, Wang Y, Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies, Acta Pharm. Sin. B 11 (2021) 2798–2818, 10.1016/J.APSB.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lee MC, Huang YC, Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells, Int. J. Biol. Macromol 131 (2019) 949–958, 10.1016/J.IJBIOMAC.2019.03.113. [DOI] [PubMed] [Google Scholar]
- [144].Lee SH, Back SY, Song JG, Han HK, Enhanced oral delivery of insulin via the colon-targeted nanocomposite system of organoclay/glycol chitosan/Eudragit®S100, J. Nanobiotechnol 18 (2020) 1–10, 10.1186/S12951-020-00662-X/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Shirzadian T, Nourbakhsh MS, Fattahi A, Bahrami G, Mohammadi G, Characterization and optimization of de-esterified Tragacanth-chitosan nanocomposite as a potential carrier for oral delivery of insulin: In vitro and ex vivo studies, J. Biomed. Mater. Res. A 109 (2021) 2164–2172, 10.1002/JBM.A.37202. [DOI] [PubMed] [Google Scholar]
- [146].Ghaffarian R, Pérez-Herrero E, Oh H, Raghavan SR, Muro S, Chitosan–alginate microcapsules provide gastric protection and intestinal release of ICAM-1-targeting nanocarriers, enabling gi targeting in vivo, Adv. Funct. Mater 26 (2016) 3382–3393, 10.1002/ADFM.201600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Erdoğar N, Akkın S, Nielsen TT, Özçelebi E, Erdoğdu B, Nemutlu E, İskit AB, Bilensoy E, Development of oral aprepitant-loaded chitosan–polyethylene glycol-coated cyclodextrin nanocapsules: formulation, characterization, and pharmacokinetic evaluation, J. Pharm. Investig 51 (2021) 297–310, 10.1007/S40005-020-00511-X/TABLES/5. [DOI] [Google Scholar]
- [148].Li B, Zhu JB, Zheng CL, Gong W, A novel system for three-pulse drug release based on “tablets in capsule” device, Int. J. Pharm 352 (2008) 159–164, 10.1016/J.IJPHARM.2007.10.043. [DOI] [PubMed] [Google Scholar]
- [149].Yang Y, Liu Y, Chen S, Cheong KL, Teng B, Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery, Carbohydr. Polym 246 (2020), 116617, 10.1016/J.CARBPOL.2020.116617. [DOI] [PubMed] [Google Scholar]
- [150].Belabassi Y, Moreau J, Gheran V, Henoumont C, Robert A, Callewaert M, Rigaux G, Cadiou C, Vander Elst L, Laurent S, Muller RN, Dinischiotu A, Voicu SN, Chuburu F, Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery, Biomacromolecules. 18 (2017) 2756–2766, 10.1021/ACS.BIOMAC.7B00668/SUPPL_FILE/BM7B00668_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- [151].Nasrpour S, Yousefi G, Niakosari M, Aminlari M, Nanoencapsulation of saffron crocin into chitosan/alginate interpolyelectrolyte complexes for oral delivery: A Taguchi approach to design optimization, J. Food Sci 87 (2022) 1148–1160, 10.1111/1750-3841.16052. [DOI] [PubMed] [Google Scholar]
- [152].Anchan RB, Koland M, Oral Insulin Delivery by Chitosan Coated Solid Lipid Nanoparticles: Ex vivo and in vivo Studies, J. Young Pharmacists 13 (2021) 43–48, 10.5530/jyp.2021.13.10. [DOI] [Google Scholar]
- [153].Wu X, Farooq MA, Li T, Geng T, Kutoka PT, Wang B, Cationic chitosanmodified silica nanoparticles for oral delivery of protein vaccine, J. Biomed. Mater. Res. A 109 (2021) 2111–2119, 10.1002/jbm.a.37198. [DOI] [PubMed] [Google Scholar]
- [154].Zhang Y, Xiong GM, Ali Y, Boehm BO, Huang YY, Venkatraman S, Layer-by-layer coated nanoliposomes for oral delivery of insulin, Nanoscale. 13 (2021) 776–789, 10.1039/d0nr06104b. [DOI] [PubMed] [Google Scholar]
- [155].Yang T, Feng J, Zhang Q, Wu W, Mo H, Huang L, Zhang W, l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel, Drug Deliv. 27 (2020) 575–584, 10.1080/10717544.2020.1748762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Rahat I, Rizwanullah M, Gilani SJ, Bin-Jummah MN, Imam SS, Kala C, Asif M, Alshehri S, Sharma SK, Thymoquinone loaded chitosan - Solid lipid nanoparticles: Formulation optimization to oral bioavailability study, J. Drug Deliv. Sci. Technol 64 (2021), 102565, 10.1016/j.jddst.2021.102565. [DOI] [Google Scholar]
- [157].Kontogiannidou E, Meikopoulos T, Virgiliou C, Bouropoulos N, Gika H, Vizirianakis IS, Müllertz A, Fatouros DG, Towards the development of Self-Nano-Emulsifying Drug Delivery Systems (SNEDDS) containing trimethyl chitosan for the oral delivery of amphotericin B: In vitro assessment and cytocompatibility studies, J. Drug Deliv. Sci. Technol 56 (2020), 101524, 10.1016/j.jddst.2020.101524. [DOI] [Google Scholar]
- [158].Thirumalaikumar E, Lelin C, Sathishkumar R, Vimal S, Anand SB, Babu MM, Citarasu T, Oral delivery of pVAX-OMP and pVAX-hly DNA vaccine using chitosan-tripolyphosphate (Cs-TPP) nanoparticles in Rohu, (Labeo rohita) for protection against Aeromonas hydrophila infection, Fish Shellfish Immunol. 115 (2021) 189–197, 10.1016/j.fsi.2021.06.004. [DOI] [PubMed] [Google Scholar]
- [159].Mumuni MA, Kenechukwu FC, Ofokansi KC, Attama AA, Díaz DD, Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment, Carbohydr. Polym 229 (2020), 115506, 10.1016/j.carbpol.2019.115506. [DOI] [PubMed] [Google Scholar]
- [160].Antonio E, dos Reis Antunes O, Marcano RGDJV Junior, Diedrich C, da Silva Santos J, Machado CS, Khalil NM, Mainardes RM, Chitosan modified poly (lactic acid) nanoparticles increased the ursolic acid oral bioavailability, Int. J. Biol. Macromol 172 (2021) 133–142, 10.1016/j.ijbiomac.2021.01.041. [DOI] [PubMed] [Google Scholar]
- [161].Sorasitthiyanukarn FN, Muangnoi C, Rojsitthisak P, Rojsitthisak P, Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate, Carbohydr. Polym 256 (2021), 117426, 10.1016/j.carbpol.2020.117426. [DOI] [PubMed] [Google Scholar]
- [162].Wu H, Guo T, Nan J, Yang L, Liao G, Park HJ, Li J, Hyaluronic-Acid-Coated Chitosan Nanoparticles for Insulin Oral Delivery: Fabrication, Characterization, and Hypoglycemic Ability 2100493, 2022, pp. 1–11, 10.1002/mabi.202100493. [DOI] [PubMed] [Google Scholar]
- [163].Renu S, Markazi AD, Dhakal S, Lakshmanappa YS, Shanmugasundaram R, Selvaraj RK, Renukaradhya GJ, Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens, 2020, pp. 761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Alshehri S, Imam SS, Rizwanullah M, Fakhri KU, Rizvi MMA, Mahdi W, Kazi M, Effect of chitosan coating on plga nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments, Coatings. 11 (2021) 1–19, 10.3390/coatings11010006. [DOI] [Google Scholar]
- [165].Tan JSL, Roberts C, Billa N, Pharmacokinetics and tissue distribution of an orally administered mucoadhesive chitosan-coated amphotericin B-Loaded nanostructured lipid carrier (NLC) in rats, J. Biomater. Sci. Polym. Ed 31 (2020) 141–154, 10.1080/09205063.2019.1680926. [DOI] [PubMed] [Google Scholar]
- [166].Maria S, Sarwar HS, Sohail MF, Imran M, Salman Qureshi O, Raza A, Ahmad NM, Iqbal A, Shahnaz G, Synthesis and characterization of pre-activated thiolated chitosan nanoparticles for oral delivery of octreotide, J. Drug Deliv. Sci. Technol 58 (2020), 10.1016/j.jddst.2020.101807. [DOI] [Google Scholar]
- [167].Ibrahim YHEY, Regdon G, Kristó K, Kelemen A, Adam ME, Hamedelniel EI, Sovány T, Design and characterization of chitosan/citrate films as carrier for oral macromolecule delivery, Eur. J. Pharm. Sci 146 (2020), 105270, 10.1016/j.ejps.2020.105270. [DOI] [PubMed] [Google Scholar]
- [168].Ma Y, Thurecht KJ, Coombes AGA, Development of enteric-coated, biphasic chitosan/HPMC microcapsules for colon-targeted delivery of anticancer drug-loaded nanoparticles, Int. J. Pharm 607 (2021), 121026, 10.1016/j.ijpharm.2021.121026. [DOI] [PubMed] [Google Scholar]
- [169].Bai K, Hong B, Tan R, He J, Hong Z, Selenium nanoparticles-embedded chitosan microspheres and their effects upon alcohol-induced gastric mucosal injury in rats: Rapid preparation, oral delivery, and gastroprotective potential of selenium nanoparticles, Int. J. Nanomedicine 15 (2020) 1187–1203, 10.2147/IJN.S237089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zhang X, Lyu X, Tong Y, Wang J, Ye J, Yang R, Chitosan/casein based microparticles with a bilayer shell-core structure for oral delivery of nattokinase, Food Funct. 11 (2020) 10799–10816, 10.1039/d0fo02349c. [DOI] [PubMed] [Google Scholar]
- [171].Saifullah S, Kanwal T, Ullah S, Kawish M, Habib SM, Ali I, Munir A, Imran M, Shah MR, Design and development of lipid modified chitosan containing muco-adhesive self-emulsifying drug delivery systems for cefixime oral delivery, Chem. Phys. Lipids 235 (2021), 105052, 10.1016/j.chemphyslip.2021.105052. [DOI] [PubMed] [Google Scholar]
- [172].Chen T, Li S, Zhu W, Liang Z, Zeng Q, Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin, J. Microencapsul 36 (2019) 96–107, 10.1080/02652048.2019.1604846. [DOI] [PubMed] [Google Scholar]
- [173].Vetvicka V, Vannucci L, Sima P, Richter J, Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials, Molecules 24 (2019) 1251, 10.3390/MOLECULES24071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Camilli G, Tabouret G, Quintin J, The complexity of fungal β-glucan in health and disease: effects on the mononuclear phagocyte system, Front. Immunol 9 (2018) 673, 10.3389/FIMMU.2018.00673/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Nie Y, Lin Q, Luo F, Effects of non-starch polysaccharides on inflammatory bowel disease, Int. J. Mol. Sci 2017 18 (2017) 1372, 10.3390/IJMS18071372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Liu B, Lin Q, Yang T, Zeng L, Shi L, Chen Y, Luo F, Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice, Food Funct. 6 (2015) 3454–3463, 10.1039/C5FO00563A. [DOI] [PubMed] [Google Scholar]
- [177].Zou S, Duan B, Xu X, Inhibition of tumor growth by β-glucans through promoting CD4+ T cell immunomodulation and neutrophil-killing in mice, Carbohydr. Polym 213 (2019) 370–381, 10.1016/J.CARBPOL.2019.03.006. [DOI] [PubMed] [Google Scholar]
- [178].Islam T, Huda MN, Ahsan MA, Afrin H, Joseph C, Salazar J, Nurunnabi M, Theoretical and experimental insights into the possible interfacial interactions between β-glucan and fat molecules in aqueous media, J. Phys. Chem. B 125 (2021) 13730–13743, 10.1021/ACS.JPCB.1C08065/ASSET/IMAGES/LARGE/JP1C08065_0010.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Nurunnabi M, Lee SA, Revuri V, Hwang YH, Kang SH, Lee M, Cho S, Cho KJ, Byun Y, Bae YH, Lee DY, kyu Lee Y, Oral delivery of a therapeutic gene encoding glucagon-like peptide 1 to treat high fat diet-induced diabetes, J. Control. Release 268 (2017) 305–313, 10.1016/J.JCONREL.2017.08.035. [DOI] [PubMed] [Google Scholar]
- [180].Chowdhury AS, Bai RG, Tamanna Islam, Muhammad Abir, Mahesh Narayan, Zehedina Khatun, Md Nurunnabi, Bile acid linked β-glucan nanoparticles for liver specific oral delivery of biologics, Biomater. Sci (2022), 10.1039/D2BM00316C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Jin Y, Li P, Wang F, β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties, Vaccine. 36 (2018) 5235–5244, 10.1016/J.VACCINE.2018.07.038. [DOI] [PubMed] [Google Scholar]
- [182].Sun Y, Duan B, Chen H, Xu X, A novel strategy for treating inflammatory bowel disease by targeting delivery of methotrexate through glucan particles, Adv. Healthc. Mater 9 (2020) 1901805, 10.1002/ADHM.201901805. [DOI] [PubMed] [Google Scholar]
- [183].Lee DY, Nurunnabi M, Kang SH, Nafiujjaman M, Huh KM, kyu Lee Y, Kim YC, Oral Gavage Delivery of PR8 Antigen with β-Glucan-Conjugated GRGDS Carrier to Enhance M-Cell Targeting Ability and Induce Immunity, Biomacromolecules. 18 (2017) 1172–1179, 10.1021/ACS.BIOMAC.6B01855/ASSET/IMAGES/LARGE/BM-2016-01855U_0006.JPEG. [DOI] [PubMed] [Google Scholar]
- [184].Lee K, Kwon Y, Hwang J, Choi Y, Kim K, Koo HJ, Seo Y, Jeon H, Choi J, Synthesis and functionalization of β-glucan particles for the effective delivery of doxorubicin molecules, ACS Omega. 4 (2019) 668–674, 10.1021/ACSOMEGA.8B02712/ASSET/IMAGES/MEDIUM/AO-2018-02712D_M003.GIF. [DOI] [Google Scholar]
- [185].Baert K, De Geest BG, De Rycke R, Da Fonseca Antunes AB, De Greve H, Cox E, B., Devriendt, β-glucan microparticles targeted to epithelial APN as oral antigen delivery system, J. Control. Release 220 (2015) 149–159, 10.1016/J.JCONREL.2015.10.025. [DOI] [PubMed] [Google Scholar]
- [186].Xie Y, Hu X, He H, Xia F, Ma Y, Qi J, Dong X, Zhao W, Lu Y, Wu W, Tracking translocation of glucan microparticles targeting M cells: implications for oral drug delivery, J. Mater. Chem. B 4 (2016) 2864–2873, 10.1039/C5TB02706C. [DOI] [PubMed] [Google Scholar]
- [187].Li X, Wang J, Li S, Liu Z, Zheng Z, Zhang Y, Development and Evaluation of Multifunctional Poly(Lactic-co-glycolic acid) Nanoparticles Embedded in Carboxymethyl β-Glucan Porous Microcapsules as a Novel Drug Delivery System for Gefitinib, Pharmaceutics 11 (2019) 469, 10.3390/PHARMACEUTICS11090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Khatun Z, Nurunnabi M, Cho KJ, Byun Y, Bae YH, Lee YK, Oral absorption mechanism and anti-angiogenesis effect of taurocholic acid-linked heparindocetaxel conjugates, J. Control. Release 177 (2014) 64–73, 10.1016/J.JCONREL.2013.12.034. [DOI] [PubMed] [Google Scholar]
- [189].Khatun Z, Nurunnabi M, Reeck GR, Cho KJ, Lee YK, Oral delivery of taurocholic acid linked heparin–docetaxel conjugates for cancer therapy, J. Control. Release 170 (2013) 74–82, 10.1016/J.JCONREL.2013.04.024. [DOI] [PubMed] [Google Scholar]
- [190].Hwang J, Lee K, Gilad AA, Choi J, Synthesis of beta-glucan nanoparticles for the delivery of single strand DNA, Biotechnol. Bioprocess Eng 23 (2018) 144–149, 10.1007/S12257-018-0003-4. [DOI] [Google Scholar]
- [191].Banks SR, Enck K, Wright M, Opara EC, Welker ME, Chemical modification of alginate for controlled oral drug delivery, J. Agric. Food Chem 67 (2019) 10481–10488, 10.1021/ACS.JAFC.9B01911/ASSET/IMAGES/ACS.JAFC.9B01911.SOCIAL.JPEG_V03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Szekalska M, Sosnowska K, Zakrzeska A, Kasacka I, Lewandowska A, Winnicka K, The influence of chitosan cross-linking on the properties of alginate microparticles with metformin hydrochloride—in vitro and in vivo evaluation, Molecules 22 (2017) 182, 10.3390/MOLECULES22010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Knop K, Hoogenboom R, Fischer D, Schubert US, Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives, Angew. Chem. Int. Ed 49 (2010) 6288–6308, 10.1002/ANIE.200902672. [DOI] [PubMed] [Google Scholar]
- [194].Davidovich-Pinhas M, Harari O, Bianco-Peled H, Evaluating the mucoadhesive properties of drug delivery systems based on hydrated thiolated alginate, J. Control. Release 136 (2009) 38–44, 10.1016/J.JCONREL.2009.01.029. [DOI] [PubMed] [Google Scholar]
- [195].Davidovich-Pinhas M, Bianco-Peled H, Alginate–PEGAc: A new mucoadhesive polymer, Acta Biomater. 7 (2011) 625–633, 10.1016/J.ACTBIO.2010.09.021. [DOI] [PubMed] [Google Scholar]
- [196].Bernkop-Schnürch A, Kast CE, Richter MF, Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine, J. Control. Release 71 (2001) 277–285, 10.1016/S0168-3659(01)00227-9. [DOI] [PubMed] [Google Scholar]
- [197].Sorasitthiyanukarn FN, Muangnoi C, Ratnatilaka Na Bhuket P, Rojsitthisak P, Rojsitthisak P, Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment, Mater. Sci. Eng. C 93 (2018) 178–190, 10.1016/J.MSEC.2018.07.069. [DOI] [PubMed] [Google Scholar]
- [198].Sorasitthiyanukarn FN, Muangnoi C, Thaweesest W, Ratnatilaka Na Bhuket P, Jantaratana P, Rojsitthisak P, Rojsitthisak P, Polyethylene glycol-chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles: a novel drug delivery system for curcumin diglutaric acid, Biomolecules 10 (2020) 73, 10.3390/BIOM10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Long L, Lai M, Mao X, Luo J, Yuan X, Zhang LM, Ke Z, Yang L, Den DYB, Investigation Of vitamin B12-modified amphiphilic sodium alginate derivatives for enhancing the oral delivery efficacy of peptide drugs, Int. J. Nanomedicine 14 (2019) 7743–7758, 10.2147/IJN.S218944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Zhang C, Xu Y, Wu S, Zheng W, Song S, Ai C, Fabrication of astaxanthin-enriched colon-targeted alginate microspheres and its beneficial effect on dextran sulfate sodium-induced ulcerative colitis in mice, Int. J. Biol. Macromol 205 (2022) 396–409, 10.1016/J.IJBIOMAC.2022.02.057. [DOI] [PubMed] [Google Scholar]
- [201].Mei L, He F, Zhou RQ, De Wu C, Liang R, Xie R, Ju XJ, Wang W, Chu LY, Novel intestinal-targeted Ca-alginate-based carrier for pH-responsive protection and release of lactic acid bacteria, ACS Appl. Mater. Interfaces 6 (2014) 5962–5970, 10.1021/AM501011J/ASSET/IMAGES/LARGE/AM-2014-01011J_0011.JPEG. [DOI] [PubMed] [Google Scholar]
- [202].Onuigbo E, Iseghohimhen J, Chah K, Gyang M, Attama A, Chitosan/alginate microparticles for the oral delivery of fowl typhoid vaccine: Innate and acquired immunity, Vaccine. 36 (2018) 4973–4978, 10.1016/J.VACCINE.2018.05.087. [DOI] [PubMed] [Google Scholar]
- [203].Patil H, Tiwari RV, Repka MA, Recent advancements in mucoadhesive floating drug delivery systems: A mini-review, J. Drug Deliv. Sci. Technol 31 (2016) 65–71, 10.1016/J.JDDST.2015.12.002. [DOI] [Google Scholar]
- [204].Bera H, Ippagunta SR, Kumar S, Vangala P, Core-shell alginate-ghatti gum modified montmorillonite composite matrices for stomach-specific flurbiprofen delivery, Mater. Sci. Eng. C 76 (2017) 715–726, 10.1016/J.MSEC.2017.03.074. [DOI] [PubMed] [Google Scholar]
- [205].Ling K, Wu H, Neish AS, Champion JA, Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles, J. Control. Release 295 (2019) 174–186, 10.1016/J.JCONREL.2018.12.017. [DOI] [PubMed] [Google Scholar]
- [206].Li Y, Liang M, Dou X, Feng C, Pang J, Cheng X, Liu H, Liu T, Wang Y, Chen X, Development of alginate hydrogel/gum Arabic/gelatin based composite capsules and their application as oral delivery carriers for antioxidant, Int. J. Biol. Macromol 132 (2019) 1090–1097, 10.1016/J.IJBIOMAC.2019.03.103. [DOI] [PubMed] [Google Scholar]
- [207].Shamekhi F, Tamjid E, Khajeh K, Development of chitosan coated calcium-alginate nanocapsules for oral delivery of liraglutide to diabetic patients, Int. J. Biol. Macromol 120 (2018) 460–467, 10.1016/J.IJBIOMAC.2018.08.078. [DOI] [PubMed] [Google Scholar]
- [208].Huang X, Ma Y, Wang Y, Niu C, Liu Z, Yao X, Jiang X, Pan R, Jia S, Li D, Guan X, Wang L, Xu Y, Oral probiotic vaccine expressing Koi Herpesvirus (KHV) ORF81 protein delivered by chitosan-alginate capsules is a promising strategy for mass oral vaccination of carps against KHV infection, J. Virol 95 (2021) 415–436, 10.1128/JVI.00415-21/ASSET/93DD6473-1970-474D-9A41-9E1AAA203775/ASSETS/IMAGES/LARGE/JVI.00415-21-F0009.JPG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Sorasitthiyanukarn FN, Muangnoi C, Rojsitthisak P, Rojsitthisak P, Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability, Food Hydrocoll. 124 (2022), 107246, 10.1016/J.FOODHYD.2021.107246. [DOI] [Google Scholar]
- [210].He C, Wang Z, Shi J, Pharmacological effects of icariin, Adv. Pharmacol 87 (2020) 179–203, 10.1016/BS.APHA.2019.10.004. [DOI] [PubMed] [Google Scholar]
- [211].Wang QS, Wang GF, Zhou J, Gao LN, Cui YL, Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis, Int. J. Pharm 515 (2016) 176–185, 10.1016/J.IJPHARM.2016.10.002. [DOI] [PubMed] [Google Scholar]
- [212].Raafat AI, Kamal H, Sharada HM, Abd Elhalim SA, Mohamed RD, Radiation development of gastroretentive amoxicillin trihydrate floating-alginate based beads for the treatment of helicobacter pylori, Radiat. Phys. Chem 179 (2021), 109268, 10.1016/J.RADPHYSCHEM.2020.109268. [DOI] [Google Scholar]
- [213].Quinto EJ, Jiménez P, Caro I, Tejero J, Mateo J, Girbés T, Probiotic lactic acid bacteria: A review, Food Nutr. Sci 05 (2014) 1765–1775, 10.4236/FNS.2014.518190. [DOI] [Google Scholar]
- [214].Amidon S, Brown JE, Dave VS, Colon-targeted oral drug delivery systems: design trends and approaches, AAPS PharmSciTech 16 (2015) 731–741, 10.1208/S12249-015-0350-9/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Philip AK, Philip B, Colon targeted drug delivery systems: a review on primary and novel approaches, Oman Med. J 25 (2010) 79, 10.5001/OMJ.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Agarwal T, Narayana SNGH, Pal K, Pramanik K, Giri S, Banerjee I, Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery, Int. J. Biol. Macromol 75 (2015) 409–417, 10.1016/J.IJBIOMAC.2014.12.052. [DOI] [PubMed] [Google Scholar]
- [217].Peppas NA, Wood KM, Blanchette JO, Hydrogels for oral delivery of therapeutic proteins, Expert. Opin. Biol. Ther 4 (2005) 881–887, 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- [218].Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM, Polymeric hydrogels for oral insulin delivery, J. Control. Release 165 (2013) 129–138, 10.1016/J.JCONREL.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [219].Cikrikci S, Mert B, Oztop MH, Development of pH sensitive alginate/gum tragacanth based hydrogels for oral insulin delivery, J. Agric. Food Chem 66 (2018) 11784–11796, 10.1021/ACS.JAFC.8B02525/ASSET/IMAGES/MEDIUM/JF-2018-02525Q_0007.GIF. [DOI] [PubMed] [Google Scholar]
- [220].Ilgin P, Ozay H, Ozay O, Synthesis and characterization of pH responsive alginate based-hydrogels as oral drug delivery carrier, J. Polym. Res 27 (2020) 1–11, 10.1007/S10965-020-02231-0/TABLES/2. [DOI] [Google Scholar]
- [221].Yin ZC, Wang YL, Wang K, A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery, J. Drug Deliv. Sci. Technol 43 (2018) 12–18, 10.1016/J.JDDST.2017.09.009. [DOI] [Google Scholar]
- [222].Elbialy NS, Mohamed N, Fabrication of the quaternary nanocomplex curcumincasein-alginate-chitosan as a potential oral delivery system for cancer nutraceutical therapy, J. Drug Deliv. Sci. Technol 70 (2022), 103226, 10.1016/j.jddst.2022.103226. [DOI] [Google Scholar]
- [223].Öztürk K, Development and in-vitro characterization of l-cysteine loaded alginate beads for oral delivery, J. Res. Pharm 26 (1) (2022) 210–219, 10.29228/jrp.119. [DOI] [Google Scholar]
- [224].Kalaycioglu GD, Elamin AA, Kinali H, Aydogan N, pH-sensitive polymeric poly (ϵ-caprolactone) core- chitosan/alginate shell particle system for oral insulin delivery, ChemistrySelect. 6 (2021) 695–704, 10.1002/slct.202004210. [DOI] [Google Scholar]
- [225].Zhang YW, Tu LL, Tang Z, Wang Q, Zheng GL, Yin LN, pH-sensitive chitosan-deoxycholic acid/alginate nanoparticles for oral insulin delivery, Pharm. Dev. Technol 26 (2021) 943–952, 10.1080/10837450.2021.1966036. [DOI] [PubMed] [Google Scholar]
- [226].Khaleel Basha S, Syed Muzammil M, Dhandayuthabani R, Sugantha Kumari V, Development of nanoemulsion of Alginate/Aloe vera for oral delivery of insulin, Mater Today Proc. 36 (2019) 357–363, 10.1016/j.matpr.2020.04.138. [DOI] [Google Scholar]
- [227].Baig MMFA, Abbas M, Naveed M, Kassim SA, Khan GJ, Sohail M, Ullah S, Hasnat M, Shah K, Ansari MT, Design, synthesis and evaluation of DNA nano-cubes as a core material protected by the alginate coating for oral administration of anti-diabetic drug, J. Food Drug Anal 27 (2019) 805–814, 10.1016/j.jfda.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Yu X, Wen T, Cao P, Shan L, Li L, Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery, J. Colloid Interface Sci 556 (2019) 258–265, 10.1016/j.jcis.2019.08.027. [DOI] [PubMed] [Google Scholar]
- [229].Li Y, Dou X, Pang J, Liang M, Feng C, Kong M, Liu Y, Cheng X, Wang Y, Chen X, Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel: Delivery system for oral efficacy enhancement of functional food ingredients, J. Funct. Foods 63 (2019), 103573, 10.1016/j.jff.2019.103573. [DOI] [Google Scholar]
- [230].I’tishom R, Wafa IA, Budi DS, Pratama NR, Oral delivery of purple sweet potato (Ipomoea batatas l.) extract-loaded carboxymethyl chitosan and alginate nanocapsule in streptozotocin-induced diabetic mice, Indian J. Pharm. Educ. Res 55 (2021) 709–714, 10.5530/ijper.55.3.143. [DOI] [Google Scholar]
- [231].Durán E, Churio O, Arias JL, Neira-Carrillo A, Valenzuela C, Preparation and characterization of novel edible matrices based on alginate and whey for oral delivery of iron, Food Hydrocoll. 98 (2020), 105277, 10.1016/j.foodhyd.2019.105277. [DOI] [Google Scholar]
- [232].Long L, Lai M, Mao X, Luo J, Yuan X, Zhang LM, Ke Z, Yang L, Den DYB, Investigation of vitamin b12-modified amphiphilic sodium alginate derivatives for enhancing the oral delivery efficacy of peptide drugs, Int. J. Nanomedicine 14 (2019) 7743–7758, 10.2147/IJN.S218944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Olayemi OJ, Apeji YE, Isimi CY, Formulation and evaluation of cyperus esculentus (tiger nut) starch-alginate microbeads in the oral delivery of ibuprofen, J. Pharm. Innov (2020), 10.1007/s12247-020-09509-2. [DOI] [Google Scholar]
- [234].Kalbhare SB, Bhandwalkar MJ, Pawar RK, Sagre AR, Sodium alginate cross-linked polymeric microbeads for oral sustained drug delivery in hypertension: Formulation and evaluation, Asian J. Res. Pharm. Sci 10 (2020) 153, 10.5958/2231-5659.2020.00029.6. [DOI] [Google Scholar]
- [235].Fernandes Patta ACM, Mathews PD, Madrid RRM, Rigoni VLS, Silva ER, Mertins O, Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications, Int. J. Biol. Macromol 148 (2020) 550–564, 10.1016/j.ijbiomac.2020.01.160. [DOI] [PubMed] [Google Scholar]
- [236].Rajpoot K, Jain SK, Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach, Int. J. Biol. Macromol 151 (2020) 830–844, 10.1016/j.ijbiomac.2020.02.132. [DOI] [PubMed] [Google Scholar]
- [237].Taipaleenmäki E, Christensen G, Brodszkij E, Mouritzen SA, Gal N, Madsen S, Hedemann MS, Knudsen TA, Jensen HM, Christiansen SL, Sparsø FV, Städler B, Mucopenetrating polymer – Lipid hybrid nanovesicles as subunits in alginate beads as an oral formulation, J. Control. Release 322 (2020) 470–485, 10.1016/j.jconrel.2020.03.047. [DOI] [PubMed] [Google Scholar]
- [238].Sriamornsak P, Wattanakorn N, Takeuchi H, Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method, Carbohydr. Polym 79 (2010) 54–59, 10.1016/J.CARBPOL.2009.07.018. [DOI] [Google Scholar]
- [239].May CD, Pectins, Thickening and Gelling Agents for Food, 1997, pp. 230–261, 10.1007/978-1-4615-2197-6_11. [DOI] [Google Scholar]
- [240].Sriamornsak P, Application of pectin in oral drug delivery, Expert Opin. Drug Deliv 8 (2011) 1009–1023, 10.1517/17425247.2011.584867. [DOI] [PubMed] [Google Scholar]
- [241].Joergensen L, Klösgen B, Simonsen AC, Borch J, Hagesaether E, New insights into the mucoadhesion of pectins by AFM roughness parameters in combination with SPR, Int. J. Pharm 411 (2011) 162–168, 10.1016/J.IJPHARM.2011.04.001. [DOI] [PubMed] [Google Scholar]
- [242].Thirawong N, Kennedy RA, Sriamornsak P, Viscometric study of pectin–mucin interaction and its mucoadhesive bond strength, Carbohydr. Polym 71 (2008) 170–179, 10.1016/J.CARBPOL.2007.05.026. [DOI] [Google Scholar]
- [243].Qiang Li D, Li J, Lin Dong H, Li X, Qi Zhang J, Ramaswamy S, Xu F, Pectin in biomedical and drug delivery applications: A review, Int. J. Biol. Macromol 185 (2021) 49–65, 10.1016/J.IJBIOMAC.2021.06.088. [DOI] [PubMed] [Google Scholar]
- [244].Kumar R, Singh A, Garg N, Acoustic cavitation-assisted formulation of solid lipid nanoparticles using different stabilizers, ACS Omega. 4 (2019) 13360–13370, 10.1021/ACSOMEGA.9B01532/SUPPL_FILE/AO9B01532_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Kumar R, Singh A, Garg N, Acoustic cavitation assisted hot melt mixing technique for solid lipid nanoparticles formulation, characterization, and controlled delivery of poorly water soluble drugs, J. Drug Deliv. Sci. Technol 54 (2019), 101277, 10.1016/J.JDDST.2019.101277. [DOI] [Google Scholar]
- [246].Kumar R, Singh A, Sharma K, Dhasmana D, Garg N, Siril PF, Preparation, characterization and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles, Mater. Sci. Eng. C 106 (2020), 110184, 10.1016/J.MSEC.2019.110184. [DOI] [PubMed] [Google Scholar]
- [247].Liu Y, Zheng D, Ma Y, Dai J, Li C, Xiao S, Liu K, Liu J, Wang L, Lei J, He J, Self-assembled nanoparticles platform based on pectin-dihydroartemisinin conjugates for codelivery of anticancer drugs, ACS Biomater. Sci. Eng 4 (2018) 1641–1650, 10.1021/ACSBIOMATERIALS.7B00842/ASSET/IMAGES/ACSBIOMATERIALS.7B00842.SOCIAL.JPEG_V03. [DOI] [PubMed] [Google Scholar]
- [248].Gautam M, Santhiya D, Pectin/PEG food grade hydrogel blend for the targeted oral co-delivery of nutrients, Colloids Surf. A Physicochem. Eng. Asp 577 (2019) 637–644, 10.1016/J.COLSURFA.2019.06.027. [DOI] [Google Scholar]
- [249].Zhang F, Pei X, Peng X, Gou D, Fan X, Zheng X, Song C, Zhou Y, Cui S, Dual crosslinking of folic acid-modified pectin nanoparticles for enhanced oral insulin delivery, Biomater. Adv (2022), 212746, 10.1016/J.BIOADV.2022.212746. [DOI] [PubMed] [Google Scholar]
- [250].Zhang F, Pei X, Peng X, Gou D, Fan X, Zheng X, Song C, Zhou Y, Cui S, Dual crosslinking of folic acid-modified pectin nanoparticles for enhanced oral insulin delivery, Biomaterials, Advances. (2022), 212746, 10.1016/J.BIOADV.2022.212746. [DOI] [PubMed] [Google Scholar]
- [251].Fabíola G, Prezotti FI, Boni NN, Ferreira D, Souza S, Almeida A, Vasconcelos T, Sarmento B, Palmira M, Gremião D, Stringhetti B, Cury F, Iola F, Prezotti G, Boni FI, Alia N, Ferreira N, Souza Silva D, Ofilo Vasconcelos T, Daflon MP, Ao G, Oral nanoparticles based on gellan gum/pectin for colon-targeted delivery of resveratrol, Drug Dev. Ind. Pharm 46 (2020) 236–245, 10.1080/03639045.2020.1716374. [DOI] [PubMed] [Google Scholar]
- [252].Deshmukh R, Harwansh RK, Das Paul S, Shukla R, Controlled release of sulfasalazine loaded amidated pectin microparticles through Eudragit S 100 coated capsule for management of inflammatory bowel disease, J. Drug Deliv. Sci. Technol 55 (2020), 101495, 10.1016/J.JDDST.2019.101495. [DOI] [Google Scholar]
- [253].Das S, Pectin based multi-particulate carriers for colon-specific delivery of therapeutic agents, Int. J. Pharm 605 (2021), 120814, 10.1016/J.IJPHARM.2021.120814. [DOI] [PubMed] [Google Scholar]
- [254].Basit AW, Advances in colonic drug delivery, Drugs 65 (2005) 1991–2007, 10.2165/00003495-200565140-00006. [DOI] [PubMed] [Google Scholar]
- [255].Vaidya A, Jain S, Agrawal RK, Jain SK, Pectin–metronidazole prodrug bearing microspheres for colon targeting, J. Saudi Chem. Soc 19 (2015) 257–264, 10.1016/J.JSCS.2012.03.001. [DOI] [Google Scholar]
- [256].Liu LS, Fishman ML, Kost J, Hicks KB, Pectin-based systems for colon-specific drug delivery via oral route, Biomaterials. 24 (2003) 3333–3343, 10.1016/S0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- [257].Abbaspour N, Hurrell R, Kelishadi R, Review on iron and its importance for human health, J. Res. Med. Sci 19 (2014) 164. [PMC free article] [PubMed] [Google Scholar]
- [258].Khotimchenko M, Pectin polymers for colon-targeted antitumor drug delivery, Int. J. Biol. Macromol 158 (2020) 1110–1124, 10.1016/J.IJBIOMAC.2020.05.002. [DOI] [PubMed] [Google Scholar]
- [259].Ghibaudo F, Gerbino E, Hugo AA, Simões MG, Alves P, Costa BFO, Campo Dall’ Orto V, Gómez-Zavaglia A, Simões PN, Development and characterization of iron-pectin beads as a novel system for iron delivery to intestinal cells, Colloids Surf. B: Biointerfaces 170 (2018) 538–543, 10.1016/J.COLSURFB.2018.06.052. [DOI] [PubMed] [Google Scholar]
- [260].Ya Wang S, Jie Meng Y, Li J, Peng Liu J, Qi Liu Z, Qiang Li D, A novel and simple oral colon-specific drug delivery system based on the pectin/modified nano-carbon sphere nanocomposite gel films, Int. J. Biol. Macromol 157 (2020) 170–176, 10.1016/J.IJBIOMAC.2020.04.197. [DOI] [PubMed] [Google Scholar]
- [261].Luo Y, Pan K, Zhong Q, Casein/pectin nanocomplexes as potential oral delivery vehicles, Int. J. Pharm 486 (2015) 59–68, 10.1016/J.IJPHARM.2015.03.043. [DOI] [PubMed] [Google Scholar]
- [262].Hua C, Yu W, Yang M, Cai Q, Gao T, Zhang S, Xu H, He H, Peng N, Liu Y, Casein-pectin nanocomplexes as a potential oral delivery system for improving stability and bioactivity of curcumin, Colloid Polym. Sci 299 (2021) 1557–1566, 10.1007/S00396-021-04858-X/FIGURES/8. [DOI] [Google Scholar]
- [263].Zhou M, Hu Q, Wang T, Xue J, Luo Y, Alginate hydrogel beads as a carrier of low density lipoprotein/pectin nanogels for potential oral delivery applications, Int. J. Biol. Macromol 120 (2018) 859–864, 10.1016/J.IJBIOMAC.2018.08.135. [DOI] [PubMed] [Google Scholar]
- [264].Arkaban H, Barani M, Akbarizadeh MR, Chauhan NPS, Jadoun S, Soltani MD, Zarrintaj P, Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications, Polymers 14 (2022) 1259, 10.3390/POLYM14061259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Ahmad N, Amin MCIM, Mahali SM, Ismail I, Chuang VTG, Biocompatible and mucoadhesive bacterial cellulose-g-poly(acrylic acid) hydrogels for oral protein delivery, Mol. Pharm 11 (2014) 4130–4142, 10.1021/MP5003015/ASSET/IMAGES/MP5003015.SOCIAL.JPEG_V03. [DOI] [PubMed] [Google Scholar]
- [266].Park H, Robinson JR, Mechanisms of Mucoadhesion of Poly(acrylic Acid) Hydrogels, Pharm. Res 4 (1987) 457–464, 10.1023/A:1016467219657. [DOI] [PubMed] [Google Scholar]
- [267].Meneguin AB, Sábio RM, de Souza MPC, Fernandes RP, de Oliveira AG, Chorilli M, Cellulose nanofibers improve the performance of retrograded starch/pectin microparticles for colon-specific delivery of 5-ASA, Pharmaceutics. 13 (2021), 10.3390/pharmaceutics13091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Meneguin AB, Beyssac E, Garrait G, Hsein H, Cury BSF, Retrograded starch/pectin coated gellan gum-microparticles for oral administration of insulin: A technological platform for protection against enzymatic degradation and improvement of intestinal permeability, Eur. J. Pharm. Biopharm 123 (2018) 84–94, 10.1016/j.ejpb.2017.11.012. [DOI] [PubMed] [Google Scholar]
- [269].Maciel VBV, Yoshida CMP, Pereira SMSS, Goycoolea FM, Franco TT, Electrostatic self-assembled chitosan-pectin nano- and microparticles for insulin delivery, Molecules. 22 (2017), 10.3390/molecules22101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Cheewatanakornkool K, Niratisai S, Manchun S, Dass CR, Sriamornsak P, Characterization and in vitro release studies of oral microbeads containing thiolated pectin–doxorubicin conjugates for colorectal cancer treatment, Asian J. Pharm. Sci 12 (2017) 509–520, 10.1016/j.ajps.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Stealey S, Guo X, Majewski R, Dyble A, Lehman K, Wedemeyer M, Steeber DA, Kaltchev MG, Chen J, Zhang W, Calcium-oligochitosan-pectin microcarrier for colonic drug delivery, Pharm. Dev. Technol 25 (2020) 260–265, 10.1080/10837450.2019.1691591. [DOI] [PubMed] [Google Scholar]
- [272].Minhas MU, Ahmad M, Anwar J, Khan S, Synthesis and characterization of biodegradable hydrogels for oral delivery of 5-fluorouracil targeted to colon: screening with preliminary in vivo studies, Adv. Polym. Technol 37 (2018) 221–229, 10.1002/adv.21659. [DOI] [Google Scholar]
- [273].Xian J, Zhong X, Gu H, Wang X, Li J, Li J, Wu Y, Zhang C, Zhang J, Colonic delivery of celastrol-loaded layer-by-layer liposomes with pectin/trimethylated chitosan coating to enhance its anti-ulcerative colitis effects, Pharmaceutics. 13 (2021), 10.3390/pharmaceutics13122005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Gottesmann M, Goycoolea FM, Steinbacher T, Menogni T, Hensel A, Smart drug delivery against Helicobacter pylori: pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo, Appl. Microbiol. Biotechnol 104 (2020) 5943–5957, 10.1007/s00253-020-10647-3. [DOI] [PubMed] [Google Scholar]
- [275].Contado C, Caselotto L, Mello P, Maietti A, Marvelli L, Marchetti N, Dalpiaz A, Design and formulation of Eudragit-coated zein/pectin nanoparticles for the colon delivery of resveratrol, Eur. Food Res. Technol 246 (2020) 2427–2441, 10.1007/s00217-020-03586-w. [DOI] [Google Scholar]
- [276].Prezotti FG, Boni FI, Ferreira NN, Silva DS, Almeida A, Vasconcelos T, Sarmento B, Gremião MPD, Cury BSF, Oral nanoparticles based on gellan gum/pectin for colon-targeted delivery of resveratrol, Drug Dev. Ind. Pharm 46 (2020) 236–245, 10.1080/03639045.2020.1716374. [DOI] [PubMed] [Google Scholar]
- [277].Ya Wang S, Li J, Zhou Y, Qiang Li D, Ming Du G, Chemical cross-linking approach for prolonging diclofenac sodium release from pectin-based delivery system, Int. J. Biol. Macromol 137 (2019) 512–520, 10.1016/j.ijbiomac.2019.07.011. [DOI] [PubMed] [Google Scholar]
- [278].Zhang Y, Liu J, Dou P, Wu Z, Zheng Z, Pan X, Zhou T, Wang K, Oral absorption characteristics and mechanisms of a pectin-type polysaccharide from Smilax china L. across the intestinal epithelium, Carbohydr. Polym 270 (2021), 118383, 10.1016/j.carbpol.2021.118383. [DOI] [PubMed] [Google Scholar]
- [279].dos Santos AM, Meneguin AB, Akhter DT, Fletcher N, Houston ZH, Bell C, Thurecht KJ, Gremião MPD, Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract, Eur. J. Pharm. Biopharm 158 (2021) 371–378, 10.1016/j.ejpb.2020.12.004. [DOI] [PubMed] [Google Scholar]
- [280].Zhu W, Han C, Dong Y, Jian B, Enzyme-responsive mechanism based on multi-walled carbon nanotubes and pectin complex tablets for oral colon-specific drug delivery system, J. Radioanal. Nucl. Chem 320 (2019) 503–512, 10.1007/s10967-019-06501-0. [DOI] [Google Scholar]
- [281].Singh B, Chauhan N, Kumar S, Radiation crosslinked psyllium and polyacrylic acid based hydrogels for use in colon specific drug delivery, Carbohydr. Polym 73 (2008) 446–455, 10.1016/J.CARBPOL.2007.12.009. [DOI] [Google Scholar]
- [282].Kumar GA, Wadood SA, Maurya SD, Ramchand D, Interpenetrating Polymeric Network Hydrogel for Stomach-Specific Drug Delivery of Clarithromycin: Preparation and Evaluation by Gupta Anish Kumar, Siddiqui Abdul Wadood, Sheo Datta Maurya, Dhakar Ramchand, SSRN, Asian J Pharm, 2010. [Google Scholar]
- [283].Li L, Zheng X, Pan C, Pan H, Guo Z, Liu B, Liu Y, A pH-sensitive and sustained-release oral drug delivery system: the synthesis, characterization, adsorption and release of the xanthan gum- graft -poly(acrylic acid)/GO–DCFP composite hydrogel, RSC Adv. 11 (2021) 26229–26240, 10.1039/D1RA01012C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Aktas DK¸, Öztekin F, pH-Sensitive poly (acrylic acid-co-acrylamide) anionic hydrogels for jejunum targeted drug delivery systems, Polym. Bull 2022 (2022) 1–13, 10.1007/S00289-022-04188-0. [DOI] [Google Scholar]
- [285].Kunjiappan S, Theivendran P, Baskararaj S, Sankaranarayanan B, Palanisamy P, Saravanan G, Arunachalam S, Sankaranarayanan M, Natarajan J, Somasundaram B, Wadhwani A, Modeling a pH-sensitive Zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery, 3, Biotech 9 (2019) 1–20, 10.1007/S13205-019-1720-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Liu L, Zhang Y, Yu S, Zhang Z, He C, Chen X, PH- and Amylase-Responsive Carboxymethyl Starch/Poly(2-isobutyl-acrylic acid) Hybrid Microgels as Effective Enteric Carriers for Oral Insulin Delivery, Biomacromolecules. 19 (2018) 2123–2136, 10.1021/ACS.BIOMAC.8B00215/ASSET/IMAGES/LARGE/BM-2018-00215X_0010.JPEG. [DOI] [PubMed] [Google Scholar]
- [287].Mohammadi R, Saboury A, Javanbakht S, Foroutan R, Shaabani A, Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system, Eur. Polym. J 153 (2021), 110500, 10.1016/J.EURPOLYMJ.2021.110500. [DOI] [Google Scholar]
- [288].Calixto G, Yoshii AC, Rocha H, Silva E, Stringhetti Ferreira Cury B, Chorilli M, Polyacrylic acid polymers hydrogels intended to topical drug delivery: preparation and characterization, Pharm. Dev. Technol 20 (2015) 490–496, 10.3109/10837450.2014.882941. [DOI] [PubMed] [Google Scholar]
- [289].Lamberts DW, Langston DP, Chu W, A Clinical Study of Slow-Releasing Artificial Tears, Ophthalmology. 85 (1978) 794–800, 10.1016/0161-6420(78)35610-4. [DOI] [PubMed] [Google Scholar]
- [290].Nejabat M, Mohammadi M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M, Fabrication of acetylated carboxymethylcellulose coated hollow mesoporous silica hybrid nanoparticles for nucleolin targeted delivery to colon adenocarcinoma, Carbohydr. Polym 197 (2018) 157–166, 10.1016/J.CARBPOL.2018.05.092. [DOI] [PubMed] [Google Scholar]
- [291].Das S, Subuddhi U, Controlled delivery of dexamethasone to the intestine from poly(vinyl alcohol)–poly(acrylic acid) microspheres containing drug-cyclodextrin complexes: influence of method of preparation of inclusion complex, RSC Adv. 4 (2014) 24222–24231, 10.1039/C4RA02736A. [DOI] [Google Scholar]
- [292].Dey KP, Mishra S, Chandra N, Colon targeted drug release studies of 5-ASA using a novel pH sensitive polyacrylic acid grafted barley, Polym. Bull 74 (2017) 3431–3453, 10.1007/S00289-016-1898-6/TABLES/5. [DOI] [Google Scholar]
- [293].Kavitha T, Kang IK, Park SY, Poly(acrylic acid)-grafted graphene oxide as an intracellular protein carrier, Langmuir. 30 (2014) 402–409, 10.1021/LA404337D/ASSET/IMAGES/MEDIUM/LA-2013-04337D_0012.GIF. [DOI] [PubMed] [Google Scholar]
- [294].Pal RR, Kumar D, Raj V, Rajpal V, Maurya P, Singh S, Mishra N, Singh N, Singh P, Tiwari N, Saraf SA, Synthesis of pH-sensitive crosslinked guar gum-gpoly(acrylic acid-co-acrylonitrile) for the delivery of thymoquinone against inflammation, Int. J. Biol. Macromol 182 (2021) 1218–1228, 10.1016/J.IJBIOMAC.2021.05.072. [DOI] [PubMed] [Google Scholar]
- [295].Tian B, Liu S, Wu S, Lu W, Wang D, Jin L, Hu B, Li K, Wang Z, Quan Z, pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin, Colloids Surf. B: Biointerfaces 154 (2017) 287–296, 10.1016/J.COLSURFB.2017.03.024. [DOI] [PubMed] [Google Scholar]
- [296].Das S, Subuddhi U, Cyclodextrin mediated controlled release of naproxen from pH-sensitive chitosan/poly(vinyl alcohol) hydrogels for colon targeted delivery, Ind. Eng. Chem. Res 52 (2013) 14192–14200, 10.1021/IE402121F/SUPPL_FILE/IE402121F_SI_001.PDF. [DOI] [Google Scholar]
- [297].Zhao Y, Li Y, Ge J, Li N, Li LB, Pluronic-poly (acrylic acid)-cysteine/Pluronic L121 mixed micelles improve the oral bioavailability of paclitaxel, Drug Dev. Ind. Pharm 40 (2014) 1483–1493, 10.3109/03639045.2013.829487. [DOI] [PubMed] [Google Scholar]
- [298].Pourjavadi A, Tehrani ZM, Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): A pH-sensitive carrier for gemcitabine delivery, Mater. Sci. Eng. C 61 (2016) 782–790, 10.1016/J.MSEC.2015.12.096. [DOI] [PubMed] [Google Scholar]
- [299].Silicone A, Drug S, Preparation and Characterization of Ionic Conductive, 2021.
- [300].Nasir N, Ahmad M, Minhas MU, Barkat K, Khalid MF, pH-responsive smart gels of block copolymer [pluronic F127-co-poly(acrylic acid)] for controlled delivery of Ivabradine hydrochloride: its toxicological evaluation, J. Polym. Res 26 (2019), 10.1007/s10965-019-1872-8. [DOI] [Google Scholar]
- [301].Oliver Urrutia C, Rosales-Ibáñez R, Dominguez García MV, Flores-Estrada J, Flores-Merino MV, Synthesis and assessment of poly(acrylic acid)/polyvinylpyrrolidone interpenetrating network as a matrix for oral mucosa cells, J. Biomater. Appl 34 (2020) 998–1008, 10.1177/0885328219883482. [DOI] [PubMed] [Google Scholar]
- [302].Hibbins AR, Kumar P, Choonara YE, Kondiah PPD, Marimuthu T, du Toit LC, Pillay V, Design of a versatile pH-responsive hydrogel for potential oral delivery of gastric-sensitive bioactives, Polymers (Basel). 9 (2017), 10.3390/polym9100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Noppakundilograt S, Choopromkaw S, Kiatkamjornwong S, Hydrolyzed collagen-grafted-poly[(acrylic acid)-co-(methacrylic acid)] hydrogel for drug delivery, J. Appl. Polym. Sci 135 (2018) 1–11, 10.1002/app.45654. [DOI] [Google Scholar]
- [304].Zhang J, Liang X, Zhang Y, Shang Q, Fabrication and evaluation of a novel polymeric hydrogel of carboxymethyl chitosan-: G -polyacrylic acid (CMC- g -PAA) for oral insulin delivery, RSC Adv. 6 (2016) 52858–52867, 10.1039/c6ra05078f. [DOI] [Google Scholar]
- [305].Bajpai SK, Chand N, Soni S, Controlled release of anti-diabetic drug Gliclazide from poly(caprolactone)/poly(acrylic acid) hydrogels, J. Biomater. Sci. Polym. Ed 26 (2015) 947–962, 10.1080/09205063.2015.1068547. [DOI] [PubMed] [Google Scholar]
- [306].Nho YC, Park JS, Lim YM, Preparation of poly(acrylic acid) hydrogel by radiation crosslinking and its application for mucoadhesives, Polymers (Basel). 6 (2014) 890–898, 10.3390/polym6030890. [DOI] [Google Scholar]
- [307].Lo YL, Hsu CY, Lin HR, PH-and thermo-sensitive pluronic/poly(acrylic acid) in situ hydrogels for sustained release of an anticancer drug, J. Drug Target 21 (2013) 54–66, 10.3109/1061186X.2012.725406. [DOI] [PubMed] [Google Scholar]
- [308].Miar S, Perez CA, Ong JL, Guda T, Polyvinyl alcohol-poly acrylic acid bilayer oral drug delivery systems: A comparison between thin films and inverse double network bilayers, J. Biomater. Appl 34 (2019) 523–532, 10.1177/0885328219861614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Ul Q, Sharif A, Sohail M, Ahmad M, Usman Minhas M, Khan S, Khan S, Kousar M, Novel polymeric composites based on carboxymethyl chitosan and poly(acrylic acid): in vitro and in vivo evaluation, J. Mater. Sci. Mater. Med 28 (2017), 10.1007/s10856-017-5952-1. [DOI] [PubMed] [Google Scholar]
- [310].Majeed A, Pervaiz F, Shoukat H, Shabbir K, Noreen S, Anwar M, Fabrication and evaluation of pH sensitive chemically cross-linked interpenetrating network [Gelatin/Polyvinylpyrrolidone-co-poly(acrylic acid)] for targeted release of 5-fluorouracil, Polym. Bull 79 (2022) 1–20, 10.1007/s00289-020-03489-6. [DOI] [Google Scholar]
- [311].Gao X, Cao Y, Song X, Zhang Z, Zhuang X, He C, Chen X, Biodegradable, pH-responsive carboxymethyl cellulose/poly(acrylic acid) hydrogels for oral insulin delivery, Macromol. Biosci 14 (2014) 565–575, 10.1002/mabi.201300384. [DOI] [PubMed] [Google Scholar]
- [312].Javanbakht S, Shaabani A, Carboxymethyl cellulose-based oral delivery systems, Int. J. Biol. Macromol 133 (2019) 21–29, 10.1016/J.IJBIOMAC.2019.04.079. [DOI] [PubMed] [Google Scholar]
- [313].Gemici K, Kucukpinar T, Cifter C, Okus A, Ay S, The effect of hyaluronatecarboxymethyl-cellulose on the formation of postoperative adhesion in stomach visceral peritoneum damage, Bratisl. Lek. Listy 115 (2014) 749–752, 10.4149/BLL_2014_144. [DOI] [PubMed] [Google Scholar]
- [314].Maciel VBV, Remedio LN, Yoshida CMP, Carvalho RA, Carboxymethyl cellulose-based orally disintegrating films enriched with natural plant extract for oral iron delivery, J. Drug Deliv. Sci. Technol 66 (2021), 102852, 10.1016/J.JDDST.2021.102852. [DOI] [Google Scholar]
- [315].Behzadi Nia S, Pooresmaeil M, Namazi H, Carboxymethylcellulose/layered double hydroxides bio-nanocomposite hydrogel: A controlled amoxicillin nanocarrier for colonic bacterial infections treatment, Int. J. Biol. Macromol 155 (2020) 1401–1409, 10.1016/J.IJBIOMAC.2019.11.115. [DOI] [PubMed] [Google Scholar]
- [316].Khatun Z, Nurunnabi M, Lee DY, Kim YJ, Byun Y, Cho KJ, Kyu Lee Y, Optical imaging, biodistribution and toxicity of orally administered quantum dots loaded heparin-deoxycholic acid, Macromol. Res 23 (2015) 686–695, 10.1007/S13233-015-3092-3. [DOI] [Google Scholar]
- [317].Rakhshaei R, Namazi H, Hamishehkar H, Rahimi M, Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties, Int. J. Biol. Macromol 150 (2020) 1121–1129, 10.1016/J.IJBIOMAC.2019.10.118. [DOI] [PubMed] [Google Scholar]
- [318].Javanbakht S, Nazari N, Rakhshaei R, Namazi H, Cu-crosslinked carboxymethylcellulose/naproxen/graphene quantum dot nanocomposite hydrogel beads for naproxen oral delivery, Carbohydr. Polym 195 (2018) 453–459, 10.1016/J.CARBPOL.2018.04.103. [DOI] [PubMed] [Google Scholar]
- [319].Chavda H, Patel C, Preparation and in vitro evaluation of a stomach specific drug delivery system based on superporous hydrogel composite, Indian J. Pharm. Sci 73 (2011) 30, 10.4103/0250-474X.89754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Zhao Y, Wang C, Chow AHL, Ren K, Gong T, Zhang Z, Zheng Y, Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies, Int. J. Pharm 383 (2010) 170–177, 10.1016/J.IJPHARM.2009.08.035. [DOI] [PubMed] [Google Scholar]
- [321].Javanbakht S, Pooresmaeil M, Hashemi H, Namazi H, Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery, Int. J. Biol. Macromol 119 (2018) 588–596, 10.1016/J.IJBIOMAC.2018.07.181. [DOI] [PubMed] [Google Scholar]
- [322].Kim MS, Park SJ, Gu BK, Kim CH, Ionically crosslinked alginate–carboxymethyl cellulose beads for the delivery of protein therapeutics, Appl. Surf. Sci 262 (2012) 28–33, 10.1016/J.APSUSC.2012.01.010. [DOI] [Google Scholar]
- [323].Tsirigotis-Maniecka M, Szyk-Warszyńska L, Lamch Ł, Weżgowiec J, Warszyński P, Wilk KA, Benefits of pH-responsive polyelectrolyte coatings for carboxymethyl cellulose-based microparticles in the controlled release of esculin, Mater. Sci. Eng. C 118 (2021), 111397, 10.1016/J.MSEC.2020.111397. [DOI] [PubMed] [Google Scholar]
- [324].Cerchiara T, Abruzzo A, Parolin C, Vitali B, Bigucci F, Gallucci MC, Nicoletta FP, Luppi B, Microparticles based on chitosan/carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin, Carbohydr. Polym 143 (2016) 124–130, 10.1016/J.CARBPOL.2016.02.020. [DOI] [PubMed] [Google Scholar]
- [325].Alibolandi M, Abnous K, Anvari S, Mohammadi M, Ramezani M, Taghdisi SM, CD133-targeted delivery of self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles to colorectal cancer, Artif Cells Nanomed, Biotechnol 46 (2018) 1159–1169, 10.1080/21691401.2018.1446969. [DOI] [PubMed] [Google Scholar]
- [326].Ngamekaue N, Chitprasert P, Effects of beeswax-carboxymethyl cellulose composite coating on shelf-life stability and intestinal delivery of holy basil essential oil-loaded gelatin microcapsules, Int. J. Biol. Macromol 135 (2019) 1088–1097, 10.1016/J.IJBIOMAC.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [327].Roth Stefaniak K, Epley CC, Novak JJ, McAndrew ML, Cornell HD, Zhu J, McDaniel DK, Davis JL, Allen IC, Morris AJ, Grove TZ, Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle, Chem. Commun 54 (2018) 7617–7620, 10.1039/C8CC01601A. [DOI] [PubMed] [Google Scholar]
- [328].Javanbakht S, Hemmati A, Namazi H, Heydari A, Carboxymethylcellulose-coated 5-fluorouracil@MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery, Int. J. Biol. Macromol 155 (2020) 876–882, 10.1016/J.IJBIOMAC.2019.12.007. [DOI] [PubMed] [Google Scholar]
- [329].Jiang X, Huang Y, Cheng Y, Zhang Z, Shi X, Qin H, Effects of lyophilization on the release profiles of 3d printed delivery systems fabricated with carboxymethyl cellulose hydrogel, Polymers (Basel). 13 (2021) 1–11, 10.3390/polym13050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Khan S, Anwar N, Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation, Carbohydr. Polym 257 (2021), 117617, 10.1016/j.carbpol.2021.117617. [DOI] [PubMed] [Google Scholar]
- [331].Li S, Chen Z, Wang J, Yan L, Chen T, Zeng Q, Fabrication and characterization of a novel semi-interpenetrating network hydrogel based on sodium carboxymethyl cellulose and poly(methacrylic acid) for oral insulin delivery, J. Biomater. Appl 35 (2020) 3–14, 10.1177/0885328220912843. [DOI] [PubMed] [Google Scholar]
- [332].Thennakoon TM, Ching YC, Chuah CH, Rahman NA, Nai-Shang L, pH-responsive poly(lactic acid)/sodium carboxymethyl cellulose film for enhanced delivery of curcumin in vitro, J. Drug Deliv. Sci. Technol 58 (2020), 101787, 10.1016/j.jddst.2020.101787. [DOI] [Google Scholar]
- [333].Javanbakht S, Nazeri MT, Shaabani A, Ghorbani M, Green one-pot synthesis of multicomponent-crosslinked carboxymethyl cellulose as a safe carrier for the gentamicin oral delivery, Int. J. Biol. Macromol 164 (2020) 2873–2880, 10.1016/j.ijbiomac.2020.08.168. [DOI] [PubMed] [Google Scholar]
- [334].Naderi Z, Azizian J, Moniri E, Farhadyar N, Synthesis and Characterization of Carboxymethyl Cellulose/β-Cyclodextrin/Chitosan Hydrogels and Investigating the Effect of Magnetic Nanoparticles (Fe3O4) on a Novel Carrier for a Controlled Release of Methotrexate as Drug Delivery, J. Inorg. Organomet. Polym. Mater 30 (2020) 1339–1351, 10.1007/s10904-019-01301-1. [DOI] [Google Scholar]
- [335].Pooresmaeil M, Behzadi Nia S, Namazi H, Green encapsulation of LDH(Zn/Al) 5-Fu with carboxymethyl cellulose biopolymer; new nanovehicle for oral colorectal cancer treatment, Int. J. Biol. Macromol 139 (2019) 994–1001, 10.1016/j.ijbiomac.2019.08.060. [DOI] [PubMed] [Google Scholar]
- [336].Darvishi S, Javanbakht S, Heydari A, Kazeminava F, Gholizadeh P, Mahdipour M, Shaabani A, Ultrasound-assisted synthesis of MIL-88(Fe) coordinated to carboxymethyl cellulose fibers: A safe carrier for highly sustained release of tetracycline, Int. J. Biol. Macromol 181 (2021) 937–944, 10.1016/j.ijbiomac.2021.04.092. [DOI] [PubMed] [Google Scholar]
- [337].Javanbakht S, Pooresmaeil M, Namazi H, Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bionanocomposite as a nanocarrier for drug delivery system, Carbohydr. Polym 208 (2019) 294–301, 10.1016/j.carbpol.2018.12.066. [DOI] [PubMed] [Google Scholar]
- [338].Karzar Jeddi M, Mahkam M, Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery, Int. J. Biol. Macromol 135 (2019) 829–838, 10.1016/j.ijbiomac.2019.05.210. [DOI] [PubMed] [Google Scholar]
- [339].Javanbakht S, Shaabani A, Encapsulation of graphene quantum dot-crosslinked chitosan by carboxymethylcellulose hydrogel beads as a pH-responsive bionanocomposite for the oral delivery agent, Int. J. Biol. Macromol 123 (2019) 389–397, 10.1016/j.ijbiomac.2018.11.118. [DOI] [PubMed] [Google Scholar]
- [340].Jiao Z, Zhang B, Li C, Kuang W, Zhang J, Xiong Y, Tan S, Cai X, Huang L, Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery, Nanotechnol. Rev 7 (2018) 291–301, 10.1515/ntrev-2018-0029. [DOI] [Google Scholar]
- [341].Sun X, Shen J, Yu D, Kun Ouyang X, Preparation of pH-sensitive Fe3O4@C/carboxymethyl cellulose/chitosan composite beads for diclofenac sodium delivery, Int. J. Biol. Macromol 127 (2019) 594–605, 10.1016/j.ijbiomac.2019.01.191. [DOI] [PubMed] [Google Scholar]
- [342].Barkhordari S, Yadollahi M, Carboxymethyl cellulose capsulated layered double hydroxides/drug nanohybrids for Cephalexin oral delivery, Appl. Clay Sci 121–122 (2016) 77–85, 10.1016/J.CLAY.2015.12.026. [DOI] [Google Scholar]
- [343].Nurunnabi M, Ibsen KN, Tanner EEL, Mitragotri S, Oral ionic liquid for the treatment of diet-induced obesity, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 25042–25047, 10.1073/PNAS.1914426116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


