Abstract
Neuroinfections of the central nervous system (CNS) can be triggered by various pathogens. Viruses are the most widespread and have the potential to induce long-term neurologic symptoms with potentially lethal outcomes. In addition to directly affecting their host cells and inducing immediate changes in a plethora of cellular processes, viral infections of the CNS also trigger an intense immune response. Regulation of the innate immune response in the CNS depends not only on microglia, which are fundamental immune cells of the CNS, but also on astrocytes. These cells align blood vessels and ventricle cavities, and consequently, they are one of the first cell types to become infected after the virus breaches the CNS. Moreover, astrocytes are increasingly recognized as a potential viral reservoir in the CNS; therefore, the immune response initiated by the presence of intracellular virus particles may have a profound effect on cellular and tissue physiology and morphology. These changes should be addressed in terms of persisting infections because they may contribute to recurring neurologic sequelae. To date, infections of astrocytes with different viruses originating from genetically distinct families, including Flaviviridae, Coronaviridae, Retroviridae, Togaviridae, Paramyxoviridae, Picomaviridae, Rhabdoviridae, and Herpesviridae, have been confirmed. Astrocytes express a plethora of receptors that detect viral particles and trigger signaling cascades, leading to an innate immune response. In this review, we summarize the current knowledge on virus receptors that initiate the release of inflammatory cytokines from astrocytes and depict the involvement of astrocytes in immune functions of the CNS.
Keywords: central nervous system, astrocyte, neuroinfection, virus, immune response, pattern recognition receptor, cytokine
1. Introduction
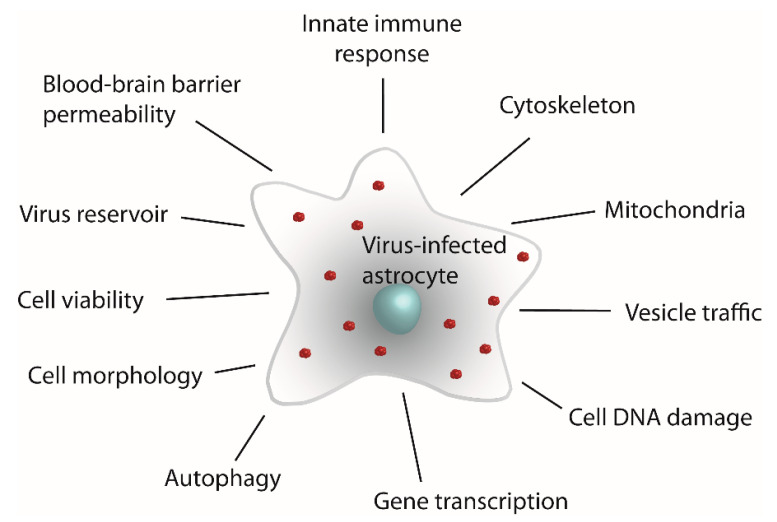
Astrocytes are glial cells with key roles in maintaining the homeostasis of the central nervous system (CNS) [1,2,3]. In addition to their well-recognized roles in sustaining and modulating functioning of neurons in normal physiologic conditions, they also participate in the development and progression of several diseases of the CNS [2,3,4,5]. An increasing amount of data on viral infections of astrocytes, in conjunction with further knowledge of cellular functions that are modified by virus infections (e.g., upregulation of cytokines, vesicular traffic, and autophagy; Figure 1) [6,7,8,9,10,11], have led to a whole new perspective on astrocytes in terms of their contribution to CNS diseases. To date, infections of astrocytes have been documented for viruses from different families, including enveloped positive-sense single-stranded RNA viruses (e.g., Flaviviridae, Coronaviridae, Retroviridae, and Togaviridae), enveloped negative-sense single-stranded RNA (e.g., Paramyxoviridae, Rhabdoviridae, and Bunyaviridae), non-enveloped viruses with a single-stranded RNA (Picomaviridae), and enveloped double-stranded DNA viruses (Herpeseviridae).
Figure 1.
Viral infection of astrocytes affects a set of cellular processes that alter the morphologic and physiologic properties of these cells. The schematic depicts an astrocyte infected with virus (red clusters) and cellular entities and processes that undergo changes triggered by viral infection.
A confirmed infection and/or the decisive role of astrocytes in the replication of various viruses, compared with other types of brain cells and endothelial cells that constitute the blood–brain barrier (BBB), have been demonstrated so far for flaviviruses, e.g., tick-borne encephalitis virus (TBEV), Japanese encephalitis virus (JEV), Zika virus (ZIKV), West Nile virus (WNV), and Kyasanur Forest disease virus (KFDV) [6,7,12,13,14,15,16,17]; retroviruses, e.g., human immunodeficiency virus-1 (HIV-1) [18]; togaviruses, e.g., western equine encephalitis virus (WEEV) [19]; paramyxoviruses, e.g., avian orthoavulavirus 1 [20], Nipah virus [21]; rhabdoviruses, e.g., rabies virus (RABV) [22]; picornaviruses, e.g., human enterovirus 71 (EV71) [23], Ljungan virus (LV) [24], Theiler’s murine encephalomyelitis virus (TMEV) [25], and vesicular stomatitis virus (VSV) [26,27]; herpesviruses, e.g., Epstein–Barr Virus (EBV) [28], human herpesvirus 6B (HH6B) [29,30]; and bunyaviruses, e.g., Crimean–Congo hemorrhagic fever virus (CCHFV) [31]. Astrocytes are also open to infection with coronaviruses, e.g., human coronavirus OC43 (HCoV-OC43), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, coronavirus infection does not always produce infectious virions in astrocytes [32,33,34,35,36]. Astrocytes are also susceptible to infection with La Crosse virus and mosquito-only flavivirus mosquito-borne pathogens, although infection of such cells is mostly non-productive [11,37]. The common denominator of infection with neurotropic viruses is the induction of a reactive astrogliosis [38]. Reactive astrogliosis ‘is the process whereby, in response to pathology, astrocytes engage in molecularly defined programs involving changes in transcriptional regulation, as well as biochemical, morphological, metabolic, and physiological remodeling, which ultimately result in gain of new function(s) or loss or upregulation of homeostatic ones [39,40]. These changes, in combination with an induction of innate immune response, trigger neurologic symptoms such as encephalitis, myelitis, postencephalitic parkinsonism associated with the loss of dopaminergic neurons, paralysis, and convulsions [9,19,20,41,42]. In this review, we describe the receptors by which astrocytes sense virus infection, and cytokines, which, when released from astrocytes, have the potential to trigger neurologic conditions that are often similar to certain neurologic disorders.
2. Immune Responses of Virus-Infected Astrocytes
2.1. Types of Viral Infections
Although most viruses cause acute self-limiting infections, some viruses are able to establish persistent infections by evolving sophisticated relationship with their hosts/host cells and by highjacking a wide array of cellular mechanisms for their own benefit.
During persistent infection, the virus is not cleared from the host after the primary infection [43]. Persistent infections include chronic focal infection (CFI), chronic diffuse infection (CDI), latent infection, and abortive infection. These types of infection differ in the quantity of infected cells and in the impact of the infection on cell viability. In a CFI, the virus is maintained in a small number of susceptible cells, which release the virus before their demise; in a CDI, all the cells are infected, and the virus continues to multiply without affecting cell viability [43]. In contrast to CFI and CDI, latent infection triggers episodes of recurrent disease, but between these episodes, the virus cannot be detected. Interestingly, various stimuli, such as superinfection by another virus, physical stress, or trauma, may reactivate the infection [43]. In contrast to these typical persistent infections, during abortive infection, cells do not produce any progeny virus [44].
Persistent infections can endure because of various mechanisms, e.g., non-productive infection (e.g., herpesvirus latency), proviral integration into the host genome (e.g., retroviruses), and/or continuous viral replication (e.g., flaviviruses, arenaviruses, and polyomaviruses (reviewed in [45]).Various viruses have evolved distinct mechanisms to enable permanent infections, yet they all share certain traits with high potential to trigger persistent infections, including (i) selection of cell subsets ideal for long-term maintenance of the viral genome, (ii) modulation of viral gene expression, (iii) viral subversion of cellular apoptotic pathways, and (iv) avoidance of clearance by the immune system (reviewed in [45]).
2.2. Viral Infections of Astrocytes
During inflammation of the CNS, astrocytes play multiple roles governed by their own intrinsic neurotoxic activities, by activation of CNS-resident microglia, and by the recruitment of peripheral inflammatory cells [46]. In general, activation of immune pathways by astrocytes is initiated after various insults, such as brain injury, ischemia, and various neuroinfections, including viral infections [8,47]. Viruses reach the CNS by various routes, including by traversing the endothelial cells of brain capillaries, penetrating through the barrier between meningeal blood and cerebrospinal fluid, and through infected peripheral and olfactory neurons [41,48,49,50]. Astrocytes are one of the earliest cell types that acquire and successfully replicate CNS-invading viruses [6,7,9,41]. These cells promptly respond to virus entry by releasing immunomodulatory molecules, thereby participating in the innate immune response, which represents the first line of defense against virus infection [51]. The contribution of astrocytes to the neurotoxic and neuroprotective roles of the innate immune response in the CNS is increasingly acknowledged.
The antiviral response of astrocytes during the early stage of viral infection acts in an opposing manner, namely, to the benefit of either the host or the virus. The role of protecting the host is mediated by the production of antiviral mediators, preventing virus replication and dissemination [8,52,53]. Conversely, astrocytes may promote replication and dissemination of viruses between cells in the CNS and act as a virus reservoir to maintain the long-term presence of the virus in the tissue [7,54].
Persisting Viral Infections of Astrocytes
Persisting infections in the CNS are underestimated and an underexplored aspect of viral infections. Recently, certain viruses in the CNS, including TBEV, ZIKV, HIV-1, JEV, CCHFV, and RABV, have been attributed with the potential to maintain persisting infections of astrocytes [6,7,9,18,31,52,55,56,57,58,59,60]. For example, ZIKV-infected activated astrocytes may act as a ZIKV reservoir with the ability to replicate within foci in the mouse brain for more than a year [61]. Moreover, human fetal astrocytes can support chronic ZIKV infection and continuous viral shedding for at least 1 month [15]. In the case of ZIKV, speculation has emerged that persistent ZIKV replication and consequent surrounding inflammation of the CNS with ongoing apoptosis contribute to neurologic deficits and even worsen the long-term neurologic prognosis, yet the relative contribution of persistent inflammation in the CNS parenchyma, acting to limit the spread of the virus, remains to be evaluated [61]. In addition, it has been suggested that EBV persistently infects astrocytes; however, this also has to be further evaluated together with potential consequences on the CNS [28,62].
With the progressive research on immune functions of astrocytes, it is becoming clear that these cells are engaged in acute and persistent infections of different types. However, the role of astrocytes in infections with specific viruses remains to be addressed in detail. Particularly intriguing is persistent infection of astrocytes because that may affect the functioning of the CNS in the long run. Abortive infection of astrocytes and induction of innate immune responses may play important roles in the process of infection itself and in regulating downstream adaptive immune pathways [22,63]. Abortive infection is frequently observed in the experimental design, and for example, in infection with the cervical cancer cell line HeLa and U2OS sarcoma cells with herpes simplex virus (HSV-1), the viral genome remained in a quiescent state at least for 5 weeks [44]. Moreover, indications that astrocytes can establish persistent chronic infection comes from studies on human astrocytes infected with HIV-1; rat astrocytes infected with TBEV; and the presence of CCHFV and ZIKV in astrocytes of infected mouse brain for several weeks and up to 22 weeks, respectively [6,31,61,64]. Persistent inflammation in the CNS infected with a virus can cause tissue damage due to viral tropism in the specific brain regions, and the immune responses triggered by viral infection or persistent presence of viral RNA and neurologic symptoms may last long after viral clearance [65].
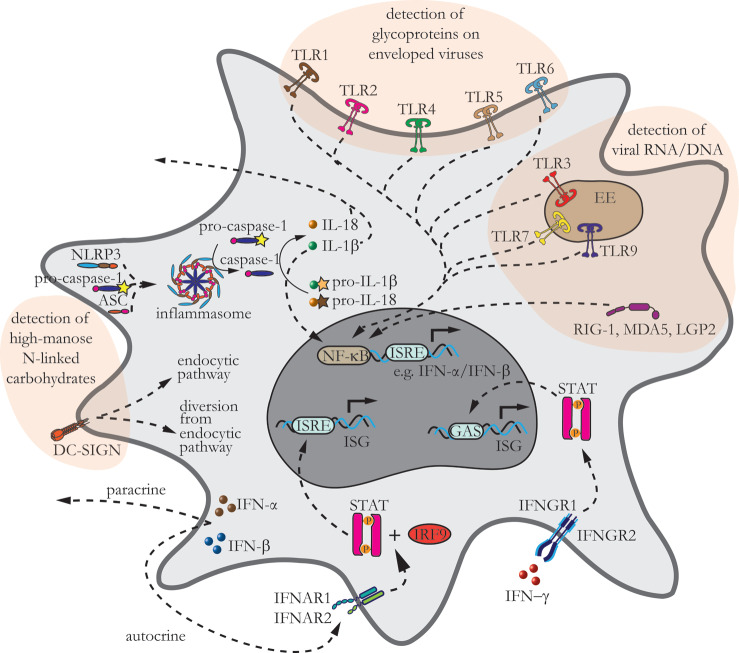
During all types of viral infections, acute and persistent, astrocytes become engaged in immune responses, and to this end, they sense the presence of viruses through a variety of receptors which, via signaling cascades, initiate the innate immune response (Figure 2).
Figure 2.
Astrocytes express several pattern recognition receptors (PRRs), which detect viral infection. The schematic depicts an astrocyte with a variety of PRRs engaged in triggering signaling cascades of the innate immune response. TLR1, TLR2, and TLRs 4-6, are located at the plasma membrane and detect glycoproteins on the viral envelope, whereas TLR3, TLR7, and TLR9 are located at the membrane of early endosomes. TLR3 detects double-stranded intermediate RNA (dsRNA), TLR7 detects single-stranded RNA (ssRNA), and TLR9 detects double-stranded DNA (dsDNA). RLRs are localized in the cytosol and sense viral RNA and DNA; they comprise RIG-I, melanoma differentiation-associated protein 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2). Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are another group of PRRs in the cytoplasm of astrocytes. NLRP3 contains pyrin N-terminal effector-binding domain (NLRPs), which together with pro-caspase-1 and ASC, make up the inflammasome; here, pro-inflammatory caspase-1 is converted to active caspase-1, which processes inactive interleukin (IL) precursors pro-IL-1β and pro-IL-18 to their active forms IL-1β and IL-18. IL-1β and IL-18 are either secreted from cells or activate nuclear factor-kappa B (NF-κB). CLRs are expressed at the cell surface, where they detect high-mannose N-linked carbohydrates on endogenous molecules and pathogens. Dendritic cell (DC)-specific ICAM-3 grabbing non-integrin (DC-SIGN) internalizes viruses that can either enter endocytotic pathway, ultimately resulting in viral degradation and antigen presentation, or can escape endocytic pathway. The CLR-related viral cycle in astrocytes has been poorly addressed thus far. PRR-triggered activation and translocation of IFN-regulatory factors (IRFs) and NF-κB initiate the production of inflammatory cytokines and type I interferons (IFNs), IFNα/β. IFNα/β act in paracrine and autocrine ways via interferon receptors (IFNARs) through which they initiate signaling cascades, leading to the transcription of interferon-stimulated genes (ISGs) acting through IFN-stimulated regulatory elements. IFN-γ secreted by antigen-presenting cells further assists IFNα/β in clearing virus from the tissue. IFN-γ acts through interferon-gamma receptor (IFNGR) protein complex IFNGR1 and IFNGR2, initiating a signaling cascade on gamma interferon activation site (GAS) elements in the DNA, inducing cytokine transcription.
2.3. Pattern Recognition Receptors in Astrocytes
Astrocytes are now also recognized as crucial mediators of innate and adaptive immune responses in the injured CNS [66]. However, the exact mechanisms by which these cells are involved in the immune response after infection with viruses are still a topic of ongoing research. In general, primary sensing of viral infection is mediated by pattern recognition receptors (PRRs) that initiate innate immune signaling by recognition of viral particles [67]. PRRs are proteins that bind to conserved patterns present in various pathogens, and they trigger signaling events that activate innate and adaptive immunity [68]. PRRs are a versatile group of receptors: Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), OAS-like receptors, and AIM-like receptors [69,70,71]. The latter two have not been described in astrocytes. Nevertheless, astrocytes modulate the innate immune response similar to professional immune cells in the CNS (microglia, monocytes, macrophages, and dendritic cells) and to other nonprofessional immune cells such as epithelial cells, endothelial cells, and fibroblasts [47,66,69,72]. PRRs that sense viral particles in astrocytes are distributed at the cell surface as well as intracellularly (Figure 2), and they recognize enveloped and non-enveloped RNA and DNA viruses through specific interactions, instigating an innate immune response (Table 1).
Table 1.
Interactions between pattern recognition receptors (PRRs) and viruses in astrocytes.
| Receptor | Virus | Cell/Tissue | Direct and Indirect Effects of Viral Binding to PRRs | References |
|---|---|---|---|---|
| TLRs (2–4) | polyI:C | human astrocytes | upregulation of TLRs (2–4), secretion of IL-6 and CXCL-10, expression of IFN-β |
[73] |
| TLR7 | EV71 | cerebral cortex in mice, mouse astrocytes | production of IL-6, apoptosis | [23] |
| TLR3, TLR4 | ZIKV | human astrocytes | increase in the release of RANTES, IP-10, IFN-β, autophagy, TLR3, TLR4 expression |
[10] |
| TLR3 | WNV | mouse brain | encephalitis, breakdown of the blood–brain barrier | [74] |
| RIG-1, MDA-5 | VSV, Sendai virus | mouse astrocytes | increase in the expression of RIG-1, MDA-5, release of IL-6, TNF-α | [27] |
| RIG-1, MDA-5 | lab-attenuated RABV | mouse astrocytes | activation of MAVS, production of TNF-α, IFN-γ, IL-6, IL-1β, IL-17, VEGF, |
[75] |
| TLR1-3 | lab-attenuated RABV | mouse brain | IFNα/β signaling pathway stimulated expression of many genes encoding inflammatory molecules such as chemokines, cytokines, TLRs (TLR1–3), and complement components | [76] |
| TLR-dependent MyD88 signaling | TMEV | Mouse astrocytes | release of IFN-β | [63] |
| DC-SIGN | HIV-1 | human astrocytes | Endocytosis of HIV-1 | [77] |
Table 1 delineates known interactions between pattern-recognition receptors (PRRs) and viruses in astrocytes. Note that interactions between NLRs and viruses are unexplored in astrocytes. poly(inosinic acid):poly(cytidylic acid) (polyI:C); human Enterovirus 71 (EV71); Japanese encephalitis virus (JEV); Zika virus (ZIKV), West Nile virus (WNV); vesicular stomatitis virus (VSV); lab-attenuated rabies virus strain (RABV), Theiler’s murine encephalomyelitis virus (TMEV), mitochondrial antiviral-signaling protein (MAVS), human immunodeficiency virus (HIV-1); myeloid differentiation primary response gene 88 (MyD88).
2.3.1. Toll-like Receptors in Astrocytes
TLRs are archetypal type I transmembrane PRRs that sense various exogenous pathogens, including protozoa, bacteria, fungi, and viruses [78]. These proteins are among the first receptors to encounter viral constituents and are in general upregulated on internalization of the virus into host cells, including astrocytes [51,52,70,78]. Early immune responses conveyed from TLRs limit the replication and spread of the virus [52]. In cells that are involved in the innate immune response, different types of TLRs enable sensing of viruses from the extracellular space and in the host cell cytoplasm after the release of the internalized viral genetic material from the capsid [70]. In cells in general, TLRs 1, 2, 4, 5, and 6 are localized at the plasma membrane, and TLRs 3, 7, 8, and 9 are intracellular and likely signal from acidic endosomes [78]. All the plasma membrane TLRs recognize virus glycoproteins of enveloped viruses, and TLRs in endosomes or endolysosomes detect virus genetic material; these include TLR3, which detects double-stranded intermediate RNA (dsRNA), TLR7 and TLR8, which detect single-stranded RNA (ssRNA), and TLR9, which senses double-stranded DNA (dsDNA) [51,52,69].
Similar to other immune responsive cells, TLRs in astrocytes reside at the plasma membrane and in the membrane of intracellular compartments [52,70], but astrocytes do not express all TLRs (Figure 2). Under resting conditions, astrocytes express TLRs 2–4, and TLR2 and TLR4 may be upregulated on infection, as demonstrated with stimulation by lipopolysaccharide or treatment with poly(inosinic acid):poly(cytidylic acid) (polyI:C), which is a synthetic analog of dsRNA commonly used as a model of pathogen infections [79,80,81]. Human astrocytes predominantly express TLR3; hence, the most prominent response in these cells is to be expected especially from the activation of this particular TLR [73]. The expression of other TLRs in human astrocytes under resting conditions is much lower; for example, the expressions of TLRs 1, 4, 5, and 9 were moderate; those of TLRs 2, 6, 7, and 10 were detected faintly; and that of TLR8 was not detected at all [73]. It was recently revealed that plasma membrane TLR10, which is the only family member with poorly defined ligands, binds the HIV-1 envelope glycoprotein in breast milk cells [82]. However, despite its low expression in non-infected astrocytes, the possible role of this receptor in viral infection of astrocytes cannot be completely ruled out.
Activation of the innate immune response in the CNS is tailored according to the cell type and the environmental pathogen, and differences in the upregulation of TLRs in microglia and astrocytes have been observed [73]. In microglia, TLR4 is downregulated and TLR2 and TLR3 are subjected to positive feedback after TLR activation, but in astrocytes, all three TLRs (2–4) are upregulated after treatment with polyI:C [73].
The contribution of TLRs to neuroinfections in astrocytes has been studied increasingly in recent years, revealing that enveloped viruses are recognized by TLR2, TLR4, and TLR5 localized at the plasma membrane, and the genetic material in the cytoplasm is sensed by TLR3, TLR7, and TLR9, which reside in endosomal membranes [52]. In these studies, in addition to polyI:C treatments, TLR upregulation on actual virus infection has also been demonstrated in astrocytes, although these studies are scarce. For example, EV71-infected astrocytes upregulate TLR7 [23], and TLR3 and TLR7 have been demonstrated to participate in JEV-induced inflammatory responses in the brain [83]. The expressions of TLR3 and TLR4 were increased by infection with different ZIKV strains, and the expression of TLR5 appeared unaffected 2 days after infection [10]. Detailed expression patterns of these two and other TLRs and their role in infections with specific viruses in astrocytes are currently unknown (e.g., possible impacts on autophagy and on the release of cytokines) [10,83,84].
Despite the versatility in expression and localization patterns of different TLRs, activation of TLRs initiates common signaling cascades, which are also preserved in astrocytes (Figure 2). Generally, these cascades, which have been reviewed extensively elsewhere [69,70], lead to the activation of transcription factors that culminate in the production of cytokines. Viral sensing through TLRs present in endosomes and at the cell surface activate IRFs and NF-κB, respectively, and they jointly initiate the production of type I IFNs, IFNα/β, and inflammatory cytokines [51,69,85]. However, TLRs may have dual functions on neuroinfection. For example, the major function of TLR3 is promoting antiviral responses, but its activation can be also detrimental to the host. During infection with WNV, TLR3-deficient mice were found to be more resistant to the lethal WNV infection; the authors hypothesized that this was due to reversible breakdown of the BBB evoked by a TLR3-dependent inflammatory response [74]. In line with this view, compromised impermeability of the BBB was also demonstrated after infection with other viruses, as discussed in the Section 4.
2.3.2. NOD-like Receptors in Astrocytes
NLRs are a specialized group of intracellular proteins that play a critical role in the regulation of the host innate immune response. Excessive activation of NLRP3 activity is associated with the pathogenesis of various inflammatory diseases; for review, see [86]. These cytosolic receptors are scaffolding proteins that enable assembly of signaling platforms, which trigger signaling pathways (NF-κB and mitogen-activated protein kinase [MAPKs]) and control the activation of inflammatory caspases [87]. Twenty-three NLRs, which have been described in humans [87], have been categorized into five subfamilies according to their N-terminal effector-binding domain: acidic transactivation domain (NLRA); baculovirus inhibitor repeat, BIR (NLRB); caspase recruitment domain, CARD (NLRC); pyrin domain (NLRP); and NLRX1 [88]. NLRs are expressed primarily in phagocytes, including macrophages and neutrophils, although they are also expressed in epithelial cells and astrocytes [87,89].
NLRs sense viral infection by detecting ssRNA [90] and trigger the innate immune response via two pathways; however, the roles of other NLRs during virus infection are also still largely unexplored [90]. The first pathway involves the formation of a multiprotein complex termed inflammasome. This multimeric protein complex consists of NLRP; pro-caspase-1; and occasionally, the adapter apoptosis-associated speck-like protein containing a C-terminal CARD (ASC) [91]. Pro-inflammatory caspase-1 in the inflammasome is processed into active caspase-1, which when released into the cytosol, converts inactive interleukin precursors pro-IL-1β and pro-IL-18 into their mature biologically active forms IL-1β and IL-18, which can then be secreted from infected cells or, in the case of IL-1β, also activate NF-κB (Figure 2) [87,91,92].
NLRP3 is one of the most studied NLRs. NLRP3 inflammasome activation is a tightly regulated process, involving both priming and activation signals. Namely, a pathway leading to activation of NLRP3 inflammasome is initiated by the priming step., i.e., the binding of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (e.g., LPS, TNF) to PRRs trigger upregulation of NLRP3, pro-IL-1β, and pro-IL-18 transcription via NF-κB-dependent pathways and posttranslational modifications (PTMs), which appear to be the essential regulators of NLRP3 activation (for reviews, see [86,90,93]). Following priming, PAMPs and DAMPs, such as nigericin, extracellular ATP, and influenza A virus H3N2, act as second activating signal. This signal, which is essential for the formation of the NLRP3 inflammasome, triggers various intracellular events, including K+ efflux, lysosomal disruption, dispersal of the trans-Golgi network, and mitochondrial damage, leading to the release of mitochondrial (mt)DNA and the production of ROS. Priming and activation stimuli jointly induce NLRP3 oligomerization that recruits ASC, triggering the formation of the ASC speck and recruiting pro-caspase-1 (reviewed in [86]). Various RNA viruses can either activate or inhibit NLRP3 inflammasomes (for a recent review, see [94]) via PTMs such as ubiquitination (Ub), phosphorylation (P), sumoylation (S), nitrosylation (N), acetylation (Ace), alkylation (Alk), and ADP-risobylation (ADP) (for a review, see [86]). In addition, the differential DNA methylation pattern of NLRPs may be important for the fate of the cell. Different DNA methylations of NLRP2 between females and males affected SARS-CoV-2 infection and the outcome of COVID-19 disease [95]. Astrocytes have been shown to express NLRP2, NLRP3, and NLRC4 and NLRC5 [89,96,97,98]. Reactive astrocytes upregulate NLRP3 and NLRC4 [97], yet signaling patterns of inflammasome priming and activation in virus infected in astrocytes remain to be revealed.
2.3.3. Retinoic Acid-Inducible Gene I-like Receptors
RLRs are also key sensors of virus infection in the cytosol [99]. The RLR protein family comprises RIG-I, MDA5, and LGP2 receptor [99,100]. RLRs are helicases that sense viral RNA and DNA and, when activated, can trigger signaling cascades, which lead to transcriptional induction of the genes encoding type I IFNs and other immune genes that collectively establish an antiviral host response [99]. Of all the pathogens that infect mammalian cells, RLRs primarily sense viruses [70].
In astrocytes, the constitutive expression of all three RLRs (LPG2, RIG-I, and MDA-5) has been confirmed [101,102]. After virus entry, their expression is upregulated, as demonstrated for LGP2 after infection of mouse astrocytes with negative-sense RNA VSV from the Rhabdoviridae family [101]. Similarly, the expressions of RIG-I and MDA-5 are also increased in mouse astrocytes infected with VSV and another negative-sense RNA virus, Sendai virus, from the Paramyxoviridae family [101]. Both types of viruses also induce robust inflammatory response in astrocytes, as determined by increased release of IL-6 and TNF-α [27]. Finally, in field-RABV-infected human astrocytes, a strong antiviral response is triggered via RIG-I or MDA5 sensing of RABV dsRNA [22].
Similar to TLRs, RLRs are crucial for the production of IFNs; for example, non-enveloped, single-stranded positive-sense RNA TMEV from the Picornaviridae family boosted IFN-β production in astrocytes; due to its immunomodulatory effects, this virus is used as a mouse model for poliomyelitis and multiple sclerosis [63,103].
2.3.4. C-Type Lectin Receptors
CLRs are PRRs expressed at the cell surface that recognize carbohydrate structures on endogenous molecules and pathogens [67,104]. CLRs are predominantly expressed in innate immune cells such as monocytes, macrophages, DCs, and Langerhans cells (LCs) [105]. They comprise two groups: mannose receptor family group I and asialoglycoprotein receptor family group II [106]. CLRs differ from other PRRs with regard to the immediate fate of internalized pathogen. After internalization, a pathogen is degraded either by lysosomes, as demonstrated for DC-SIGN (CD209) and DEC-205 receptors, or via autophagy [105,106]. Regardless of a degradation pathway, antigens are then presented on major histocompatibility complex (MHC) [105,107].
The internalization of viruses via DC-SIGN has been the most studied in DCs, where it has been revealed that, after entry into the host cell, the virus may move along two pathways: the endocytotic pathway, which results in viral degradation and antigen presentation, or the virus is diverted from the endocytotic pathway and avoids degradation [105]. Some viruses target CLRs to evade immune surveillance by suppressing or modulating type I IFNs that play a central role in the innate and adaptive defense against viruses [105]. It is currently unclear how various viruses are engaged in the endocytotic pathway or if they avoid it, and which factors determine the fate of the virus [105]. So far, in astrocytes, only HIV-1 endocytosis has been liked to DC-SIGN receptor [77].
Endocytosed viruses travel via endosomal/lysosomal pathways, where their trafficking is regulated by Ras-related proteins in brain (Rabs) at several levels of the vesicle cycle, for example, at early endosomes (Rabs 4 and 5), at late endosomes and lysosomes (Rab7), and at vesicle-recycling endosomes (Rabs 4 and 11) [6,77,108]. In astrocytes, Rab4, Rab5, and their regulator proteins, guanine nucleotide-dissociated inhibitors (GDIs), crucially affect not only fusion events in endocytosis and recycling but also their molecular interactions with the cytoskeleton, which determine directional vesicle trafficking [109,110,111]. For example, GDI-1 is implicated in all steps of the replication cycle of dengue virus (DENV) in a vascular endothelial cell-like line [112]. Endocytotic trafficking in astrocytes can be diverted on stimulation by IFN, for example, with IFN-γ, which enhances the speed of endosomal and lysosomal vesicles [113]. Such enhanced trafficking could either promote the spread of virus throughout the tissue, thus acting pro-viral, or enhance entry into the degradation pathway in the case of binding to CRLs in astrocytes, resulting in an antiviral outcome. The role of Rabs and their adaptor proteins in viral infections of astrocytes remains to be explored in detail. Moreover, Rabs are also involved in the formation and release of multivesicular body (MVB)-derived exosomal vesicles (EVs) [114,115]. In neurologic disorders, EVs are engaged in transferring various pro-inflammatory or neurotoxic cargos, and they have also been demonstrated to be important for the propagation and release of ZIKV in astrocytes [116,117].
MVB could also function as a virus reservoir in astrocytes, as proposed for HIV-1 in human macrophages [118]. In astrocytes, HIV-1 is predominantly degraded in endosomes, and only a few virions are released intact to spread infection, therefore minutely contributing to the overall viral load in the brain, but may render astrocytes as long-term viral reservoirs [119]. Interconnection between DC-SIGN and MVB in astrocytes certainly deserves further attention. The DC-SIGN receptor binds numerous enveloped viruses via their high-mannose N-linked carbohydrates [120,121]. DI-SIGN was demonstrated to bind DENV, hepatitis C virus, and HIV-1 in human DCs, and WNV in various non-neuronal mammalian cells [106,120,122,123]. In astrocytes, the binding of neurotropic viruses to DC-SIGN, with the exception of HIV-1 [124], has not been explored.
3. Viral Infection Triggers Cytokine Signaling and Their Release from Astrocytes
Astrocytes express and release a broad variety of cytokines. Among them are type I IFNs, which are constitutively produced at low quantities in a variety of organs, including the CNS [125,126]. Together with IRFs, IFNs are essential molecules that modulate immune responses after pathogen infection [127]. Astrocytes have high basal expression levels of type I IFNs (IFN-α and IFN-β) together with proteins encoded by ISGs; which allow these cells’ rapid IFN response to restrict viral spread [8]. Astrocytes are the main producers of IFN-β in the CNS after acute infections by various neurotropic viruses and during abortive infection [63]. Such a production of IFN-β was demonstrated during abortive infection with TMEV, VSV, and RABV [63]. Type I IFNs restrict viral growth via autocrine and paracrine signaling through IFNARs, as demonstrated in astrocytes infected with TBEV, JEV, WNV, and ZIKV [8]. Type I IFN mRNA in astrocytes is upregulated quickly on infection, in a matter of hours, and this upregulation has been shown to enhance cell survival [8]. In general, the production of type I IFNs is regulated by the expression of ISG and ISG-linked antiviral activity [128,129]. The principle of type I IFN signaling through autocrine and paracrine binding to IFNARs is conserved and has been extensively reviewed elsewhere [127,128]. Briefly, binding of type I IFNs to IFNARs activates the JAK-STAT signaling pathway, and the transcription of ISG is initiated via a cascade of signaling molecules [128,130]. Antiviral action of ISG encompasses different processes, for example, amplification of IFN signaling, production of cytokines that activate adaptive immunity, direct hindering of virus entry and replication, and degradation of viral RNA and proteins [131].
Although type I IFNs are among the first essential molecules that reduce the virus spread, IFN-γ further assists in virus clearance acting via IFNGR protein complex IFNGR1 and IFNGR2. Both IFNGR complexes initiate the signaling cascade acting on GAS elements in the DNA, thereby inducing cytokine transcription [132] (Figure 2). IFN-γ is secreted predominantly by activated lymphocytes, and natural killer cells, B cells, and professional antigen-presenting cells, including microglia in the CNS [133,134]. IFN-γ stimulates the expression of MHC class II molecules, including in astrocytes, which are transported within late endosomes/lysosomes [113,135]. In astrocytes, MHC class II molecule-transporting vesicles gain speed upon treatment with IFN-γ [113]. This transport is affected by the expression of intermediate filaments (Ifs), glial fibrillary acidic protein (GFAP), and vimentin [113], implying that in reactive astrocytes with boosted IF expression, such traffic is faster. This notion is important from the point of view that, in reactive astrocytes in the neurodegenerative state and during neuroinflammation, the expression of GFAP is increased [136,137]. In addition to GFAP and vimentin, the delivery of MHC class II molecules to the cell surface and fusion of MHC class II vesicles with the plasma membrane are also affected by an increase in [Ca2+]i [113]. Enhanced Ca2+ signaling in reactive astrocytes has deleterious roles in disease progression [138].
PPRs and IFN-mediated signaling in virus-infected cells evoke the expression of cytokines, including chemokines. Cytokines and chemokines released from virus-infected astrocytes are summarized in Table 2. The differences in chemokines released from human astrocytes compared with mouse astrocytes have been reported, although different developmental stages of astrocytes have not been tested yet in this regard [25].
Table 2.
Expression of cytokines and interferons in virus-infected astrocytes.
| Virus | Cell/Tissue | Inflammatory Cytokines and Chemokines | Chemokines | References |
|---|---|---|---|---|
| TBEV | primary human astrocytes | TNF α, IFN α, IL-1β, IL-6, IL-8 | CCL4/MIP-1β, CXCL10 | [9] |
| WNV | U373 astrocytic cell line | IL-1β | CXCL10, CCL2 | [139] |
| WNV | primary human astrocytes | N/A | CXCL10, CCL5 | [140] |
| ZIKV | primary human brain cortical astrocytes | IL-6, IL-8, IL-12, | CXCL-10, CCL5 | [16] |
| ZIKV | primary human brain cortical astrocytes | IL-6, IL-1α, IL-4, TGF-β1 | CXCL-10, CCL5 | [10] |
| JEV | mouse astrocytes (in situ) | N/A | CXCL10 | [141] |
| JEV | primary rat astrocytes | IL-6, TNF-α, IL-1β | CCL5 | [142] |
| JEV | primary human astrocytes | IL-6 | CXCL10, CCL2/3/4 | [12] |
| JEV | human fetal astrocyte cell line SVG | IL-18, IL-1β | [143] | |
| SeV | primary mouse astrocytes | IL-6, TNF-α | N/A | [27] |
| RABV | mouse astrocytes (in situ) | IFN-β | N/A | [63] |
| SARS-CoV-2 | astrocytes differentiated from hiPSCs | N/A *** | N/A | [32] |
| HSV-1 | mouse perivascular astrocytes (in situ) and primary astrocytes | CXCL-1 | [144] | |
| HIV-1 | primary human astrocytes **** | IL-6, IL-8 | [145] | |
| HIV-1 | primary human fetal astrocytes | TNF-α, IL-6, IL-8 | [146] | |
| VSV | primary mouse astrocytes | IL-6, TNF-α, IFN-β | [27,63] | |
| EV71 | mouse brain astrocytes, in situ | IL-6 | [23] | |
| TEMV | primary human astrocytes | IL-8 | MCP-1 | [25] |
| TEMV | mouse astrocytes (in situ) | IFN-β | [63] | |
| JHMV ** | spinal cord astrocytes (in situ) | CXCL10 | [147] | |
| MHV-A59 * | astrocyte cell line | IL-1α, IL-1β, IL-2, IL-15, IL-13, IL-17 | CXCL10 | [148] |
| TLR3 ligation | human astrocytes | IL-6, IFN-α | CXCL-10 | [73] |
hiPSCs, human-induced pluripotent stem cells; N/A, not applicable.* Experimental murine coronavirus, murine coronavirus mouse hepatitis virus A-59 (MHV-A59). ** JHM variant V2.2-1 of mouse hepatitis virus (JHMV). *** Not tested in astrocytes, but TLR3/7 and IL-6 were upregulated in neurons. **** Cells transfected with plasmid encoding HIV-1 Nef.
4. Viral Infections of Astrocytes Trigger Neurologic Symptoms
4.1. Neurologic Symptoms Induced by Cytokines Released from Astrocytes
On the one hand, IFN-α/β signaling in astrocytes limits early spread of viruses in the CNS [8,149]; on the other hand, cytokines released from astrocytes may act detrimentally to the proper functioning of the CNS. One such harmful effect is closely linked to altered permeability properties of the BBB, where astrocytes together with cerebral microvascular endothelium, pericytes, neurons, and the extracellular matrix form a neurovascular unit [150]. Increased permeability of the BBB on viral infection can be triggered by inflammatory cytokines released from astrocytes and/or by virus-infection-induced downregulation of tight junction (TJ) genes together with the upregulation of metalloproteinases that degrade TJ proteins; these changes have been demonstrated after infections with WNV, TBEV, RABV, and HIV-1 [9,151,152,153,154]. Similar disruptions of TJs are observed in various CNS diseases, such as stroke, multiple sclerosis, cerebral infection, brain tumors, Parkinson disease, and Alzheimer diseases [155,156,157]. Moreover, the dysregulated activation of caspase-1 and IL-1β secretion, as detected in virus-infected astrocytes, is observed in several inflammatory diseases [158]. In addition, NF-κB, which induces the expression of pro-inflammatory genes encoding cytokines and chemokines after viral infection, is also involved in the regulation of CNS neuroinflammation through the regulation of cell survival and the activation and differentiation of innate immune cells [159]. An imbalance in reactive oxygen species, mitochondrial defects, and DNA breakage, which have been previously linked to neurologic disorders, have recently been described after ZIKV infection of induced pluripotent stem cell-derived astrocytes [38]. Along with a deregulation of cellular processes, glial reactivity induced by ZIKV infection has been described in mice and in post-mortem neonatal brain [38].
4.2. Effect of Viral Infection on the Glymphatic System and Survival of Neurons
Viral infection of astrocytes may play an important role in the functioning of the glymphatic system. The glymphatic system comprises perivascular tunnels, shaped by astrocytes, which function as a waste clearance system for the elimination of soluble proteins and metabolites from the CNS and facilitate the distribution of glucose, lipids, amino acids, growth factors, and neuromodulators in the brain [160,161]. Proper functioning of the glymphatic system is supported by astrocytic endfeet that surround cerebral endothelial cells in the BBB [160]. One of the hallmarks of astrocyte endfeet is condensed localization of aquaporin-4 (AQP4) water channels that promote the exchange of interstitial and cerebrospinal fluid [160,162,163]. Interestingly, HIV-1 infection instigates a decrease in the expression of AQP4 and its mislocalization, which results in decreased interstitial flow and accumulation of extracellular waste products [160]. This effect is also present after CNS infection with other neurotropic viruses. For example, mislocalization of AQP4 has been demonstrated in an EV71 infection of a mouse brain [164]. On a similar note, DENV infection has been associated with the development of neuromyelitis optica spectrum disorder; the serum of patients tested positive for AQP4 antibody [165]. The pathophysiologic mechanism behind this has not been described yet. Although DENV infection of astrocytes has not been confirmed [166,167], AQP4 antibodies affecting AQP4 localization and function in astrocytes exposes this cell type as a possible contributor to the neurologic symptoms of neuromyelitis optica spectrum disorder.
Virus-infected astrocytes can affect the survival of neurons in contradictory ways. On the one hand, it has been demonstrated that the innate immune response in astrocytes plays a vital role in the injury of dopaminergic neurons, thereby initiating Parkinson disease-like pathology, for example, in cases of infections with WEEV and WNV [19,168,169]. On the other hand, astrocytes, by amplifying the type I IFN response, can limit viral spread in astrocytes and other CNS cells, including neurons, and improve cell survival [8]. A recent study conducted on MHV-A59-infected astrocyte permanent cell lines revealed that astrocytes with various morphology differently produced pro-inflammatory cytokines in response to infection [148]. In reactive astrogliosis, a common consequence of astrocyte infection [139,142,170], astrocytes may adopt multiple phenotypes, which should be defined by a combination of molecular markers and functional readouts [40], and this will be important to take into account in future research on astrocyte infection with viruses that trigger neurologic symptoms.
5. Conclusions
To date, a limited number of studies have addressed morphologic and functional changes in astrocytes during progression of neurodegenerative diseases and effects on the neurovascular unit in the CNS, and none of them have focused on viral infections. The involvement of astrocytes in the innate immune response on viral infection is gaining attention as new neurotropic viruses are emerging among the human population and as previously considered non-neurotropic viruses are increasingly being associated with neurologic symptoms. In addition to the immediate consequences of the release of immunomodulatory molecules from astrocytes in the early stages after infection, the implications in chronic inflammation of the CNS tissue during long-term infections also deserves further attention. Moreover, the changes already identified in astrocytes regarding the expression of viral receptors, PPRs, cytokines and their relationship with the surrounding cells need to be revisited from the point of view of direct neurotoxic effects versus indirect immunomodulatory effects and correlated with the accompanying impairments of CNS functions.
Abbreviations
| BBB | Blood–brain barrier |
| CCHFV | Crimean–Congo hemorrhagic fever virus |
| CCL2 | Monocyte chemoattractant protein-1; MCP-1 |
| CCL4/MIP-1β | C-C motif chemokine ligand 4/Macrophage inflammatory protein 1β |
| CCL5/RANTES | C-C motif chemokine ligand 5/Regulated upon activation, normal T cell expressed and presumably secreted |
| CDI | Chronic diffuse infection |
| CFI | Chronic focal infection |
| CNS | Central nervous system |
| CXCL10/IP10 | Gamma-interferon-inducible protein-10; IP-10 |
| EBV | Epstein–Barr Virus |
| EV71 | Enterovirus 71 |
| GAS | Gamma interferon activation site |
| GDI | Guanine nucleotide-dissociated inhibitor |
| GFAP | Glial fibrillary acidic protein |
| HCoV-OC43 | Human coronavirus OC43 |
| HIV-1 | Human immunodeficiency virus 1 |
| HSV-1 | Herpes simplex virus-1 |
| IFN | Interferon |
| IFNAR | IFNα/β receptor |
| IL | Interleukin |
| IRF | IFN-regulatory factor |
| ISG | Interferon-stimulated gene |
| JEV | Japanese encephalitis virus |
| MERS-CoV | Middle East respiratory syndrome virus |
| MHC | Major histocompatibility complex |
| MVB | Multivesicular body |
| NLR | NOD-like receptor |
| PRR | Pattern-recognition receptor |
| RABV | Rabies virus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TBEV | Tick-borne encephalitis virus |
| TGF-β1 | Transforming growth factor β1 |
| TI | Tiht junction |
| TMEV | Theiler’s murine encephalomyelitis virus |
| VSV | Vesicular stomatitis virus |
| WEEV | Western equine encephalitis virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
Author Contributions
Conceptualization, M.P. and J.J.; Writing—Original Draft, M.P. and J.J; Writing—Review and Editing, M.P. and J.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Slovenian Research Agency grant P3 310. A4L_ACTIONS project funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 964997.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Parpura V., Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res. Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zorec R., Parpura V., Verkhratsky A. Astroglial vesicular network: Evolutionary trends, physiology and pathophysiology. Acta Physiol. 2018;222:e12915. doi: 10.1111/apha.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen M.K., Mestre H., Nedergaard M. Fluid Transport in the Brain. Physiol. Rev. :2021. doi: 10.1152/physrev.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parpura V., Heneka M.T., Montana V., Oliet S.H., Schousboe A., Haydon P.G., Stout R.F., Spray D.C., Reichenbach A., Pannicke T., et al. Glial cells in (patho)physiology. J. Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potokar M., Korva M., Jorgačevski J., Avšič-Županc T., Zorec R. Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS ONE. 2014;9:e86219. doi: 10.1371/journal.pone.0086219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgačevski J., Korva M., Potokar M., Lisjak M., Avšič-Županc T., Zorec R. ZIKV Strains Differentially Affect Survival of Human Fetal Astrocytes versus Neurons and Traffic of ZIKV-Laden Endocytotic Compartments. Sci. Rep. 2019;9:8069. doi: 10.1038/s41598-019-44559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindqvist R., Mundt F., Gilthorpe J.D., Wölfel S., Gekara N.O., Kröger A., Överby A.K. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflammation. 2016;13:277. doi: 10.1186/s12974-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palus M., Bílý T., Elsterová J., Langhansová H., Salát J., Vancová M., Růžek D. Infection and injury of human astrocytes by tick-borne encephalitis virus. J. Gen. Virol. 2014;95:2411–2426. doi: 10.1099/vir.0.068411-0. [DOI] [PubMed] [Google Scholar]
- 10.Ojha C.R., Rodriguez M., Karuppan M.K.M., Lapierre J., Kashanchi F., El-Hage N. Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS ONE. 2019;14:e0208543. doi: 10.1371/journal.pone.0208543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavčar Verdev P., Potokar M., Korva M., Resman Rus K., Kolenc M., Avšič Županc T., Zorec R., Jorgačevski J. In human astrocytes neurotropic flaviviruses increase autophagy, yet their replication is autophagy-independent. Cell Mol. Life Sci. 2022;79:566. doi: 10.1007/s00018-022-04578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patabendige A., Michael B.D., Craig A.G., Solomon T. Brain microvascular endothelial-astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood-brain barrier model. Mol. Cell. NeuroSci. 2018;89:60–70. doi: 10.1016/j.mcn.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels B.P., Jujjavarapu H., Durrant D.M., Williams J.L., Green R.R., White J.P., Lazear H.M., Gale M., Diamond M.S., Klein R.S. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J. Clin. Investig. 2017;127:843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindqvist R., Kurhade C., Gilthorpe J.D., Överby A.K. Cell-type- and region-specific restriction of neurotropic flavivirus infection by viperin. J. Neuroinflamm. 2018;15:80. doi: 10.1186/s12974-018-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limonta D., Jovel J., Kumar A., Airo A.M., Hou S., Saito L., Branton W., Ka-Shu Wong G., Mason A., Power C., et al. Human Fetal Astrocytes Infected with Zika Virus Exhibit Delayed Apoptosis and Resistance to Interferon: Implications for Persistence. Viruses. 2018;10:646. doi: 10.3390/v10110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanik M., Formanova P., Bily T., Vancova M., Eyer L., Palus M., Salat J., Braconi C.T., Zanotto P.M.A., Gould E.A., et al. Characterisation of Zika virus infection in primary human astrocytes. BMC NeuroSci. 2018;19:5. doi: 10.1186/s12868-018-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawatsky B., McAuley A.J., Holbrook M.R., Bente D.A. Comparative pathogenesis of Alkhumra hemorrhagic fever and Kyasanur forest disease viruses in a mouse model. PLoS Negl. Trop. Dis. 2014;8:e2934. doi: 10.1371/journal.pntd.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson C.E., Tomlinson G.S., Pauly B., Brannan F.W., Chiswick A., Brack-Werner R., Simmonds P., Bell J.E. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol. Appl. Neurobiol. 2003;29:378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 19.Bantle C.M., Rocha S.M., French C.T., Phillips A.T., Tran K., Olson K.E., Bass T.A., Aboellail T., Smeyne R.J., Tjalkens R.B. Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis. Exp. Neurol. 2021;346:113845. doi: 10.1016/j.expneurol.2021.113845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt S.L., Moura V.M.B.D., Susta L., Miller P.J., Hutcheson J.M., Cardenas-Garcia S., Brown C.C., West F.D., Afonso C.L., Stanton J.B. Tropism of Newcastle disease virus strains for chicken neurons, astrocytes, oligodendrocytes, and microglia. BMC Vet. Res. 2019;15:317. doi: 10.1186/s12917-019-2053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Coffin K.M., Johnston S.C., Babka A.M., Bell T.M., Long S.Y., Honko A.N., Kuhn J.H., Zeng X. Nipah virus persists in the brains of nonhuman primate survivors. JCI Insight. 2019:4. doi: 10.1172/jci.insight.129629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potratz M., Zaeck L., Christen M., Te Kamp V., Klein A., Nolden T., Freuling C.M., Müller T., Finke S. Astrocyte Infection during Rabies Encephalitis Depends on the Virus Strain and Infection Route as Demonstrated by Novel Quantitative 3D Analysis of Cell Tropism. Cells. 2020;9:412. doi: 10.3390/cells9020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Z., Su R., Wang W., Liang Y., Zeng X., Shereen M.A., Bashir N., Zhang Q., Zhao L., Wu K., et al. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog. 2019;15:e1008142. doi: 10.1371/journal.ppat.1008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niklasson B., Lindquist L., Klitz W., Englund E., Bank N.B. Picornavirus Identified in Alzheimer’s Disease Brains: A Pathogenic Path? J. Alzheimer’s Dis. Rep. 2020;4:141–146. doi: 10.3233/ADR-200174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon D., Fuller A.C., Palma J.P., Choi I.H., Kim B.S. Induction of chemokines in human astrocytes by picornavirus infection requires activation of both AP-1 and NF-kappa B. Glia. 2004;45:287–296. doi: 10.1002/glia.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi Z., Barna M., Komatsu T., Reiss C.S. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan V.S., Furr S.R., Sterka D.G., Nelson D.A., Moerdyk-Schauwecker M., Marriott I., Grdzelishvili V.Z. Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology. 2010;400:187–196. doi: 10.1016/j.virol.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Jakhmola S., Jha H.C. Glial cell response to Epstein-Barr Virus infection: A plausible contribution to virus-associated inflammatory reactions in the brain. Virology. 2021;559:182–195. doi: 10.1016/j.virol.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N., Oka N., Takahashi M., Shimada K., Ishii A., Tatebayashi Y., Shigeta M., Yanagisawa H., Kondo K. Human Herpesvirus 6B Greatly Increases Risk of Depression by Activating Hypothalamic-Pituitary -Adrenal Axis during Latent Phase of Infection. iScience. 2020;23:101187. doi: 10.1016/j.isci.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fotheringham J., Akhyani N., Vortmeyer A., Donati D., Williams E., Oh U., Bishop M., Barrett J., Gea-Banacloche J., Jacobson S. Detection of active human herpesvirus-6 infection in the brain: Correlation with polymerase chain reaction detection in cerebrospinal fluid. J. Infect. Dis. 2007;195:450–454. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
- 31.Spengler J.R., Kelly Keating M., McElroy A.K., Zivcec M., Coleman-McCray J.D., Harmon J.R., Bollweg B.C., Goldsmith C.S., Bergeron É., Keck J.G., et al. Crimean-Congo Hemorrhagic Fever in Humanized Mice Reveals Glial Cells as Primary Targets of Neurological Infection. J. Infect. Dis. 2017;216:1386–1397. doi: 10.1093/infdis/jix215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari S.K., Wang S., Smith D., Carlin A.F., Rana T.M. Revealing Tissue-Specific SARS-CoV-2 Infection and Host Responses using Human Stem Cell-Derived Lung and Cerebral Organoids. Stem. Cell Rep. 2021;16:437–445. doi: 10.1016/j.stemcr.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C., Zhang M., Garcia G., Tian E., Cui Q., Chen X., Sun G., Wang J., Arumugaswami V., Shi Y. ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem. Cell. 2021;28:331–342.e5. doi: 10.1016/j.stem.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbour N., Côté G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J. Virol. 1999;73:3338–3350. doi: 10.1128/JVI.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson J., Mims C.A. Differential susceptibility of cultured neural cells to the human coronavirus OC43. J. Virol. 1985;53:1016–1019. doi: 10.1128/jvi.53.3.1016-1019.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y., Chen Y., Sun H., Zhang X., He L., Li J., Zhao G., Sun S. MERS-CoV infection causes brain damage in human DPP4-transgenic mice through complement-mediated inflammation. J. Gen. Virol. 2021;102:001667. doi: 10.1099/jgv.0.001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallfass C., Ackerman A., Lienenklaus S., Weiss S., Heimrich B., Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J. Virol. 2012;86:11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledur P.F., Karmirian K., Pedrosa C.D.S.G., Souza L.R.Q., Assis-de-Lemos G., Martins T.M., Ferreira J.C.C.G., de Azevedo Reis G.F., Silva E.S., Silva D., et al. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci. Rep. 2020;10:1218. doi: 10.1038/s41598-020-57914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 40.Escartin C., Galea E., Lakatos A., O’Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhäuser C., Volterra A., Carmignoto G., Agarwal A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. NeuroSci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potokar M., Jorgacevski J., Zorec R. Astrocytes in Flavivirus Infections. Int. J. Mol. Sci. 2019;20:691. doi: 10.3390/ijms20030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stupica D., Strle F., Avšič-Županc T., Logar M., Pečavar B., Bajrović F.F. Tick borne encephalitis without cerebrospinal fluid pleocytosis. BMC Infect. Dis. 2014;14:614. doi: 10.1186/s12879-014-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron S. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 44.Cohen E.M., Avital N., Shamay M., Kobiler O. Abortive herpes simplex virus infection of nonneuronal cells results in quiescent viral genomes that can reactivate. Proc. Natl. Acad. Sci. USA. 2020;117:635–640. doi: 10.1073/pnas.1910537117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kane M., Golovkina T. Common threads in persistent viral infections. J. Virol. 2010;84:4116–4123. doi: 10.1128/JVI.01905-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linnerbauer M., Wheeler M.A., Quintana F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron. 2020;108:608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Ludlow M., Kortekaas J., Herden C., Hoffmann B., Tappe D., Trebst C., Griffin D.E., Brindle H.E., Solomon T., Brown A.S., et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogovic P., Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases. 2015;3:430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mandl C. Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis. Virus Res. 2005;111:161–174. doi: 10.1016/j.virusres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Severa M., Fitzgerald K.A. TLR-mediated activation of type I IFN during antiviral immune responses: Fighting the battle to win the war. Curr. Top Microbiol. Immunol. 2007;316:167–192. doi: 10.1007/978-3-540-71329-6_9. [DOI] [PubMed] [Google Scholar]
- 52.Li L., Acioglu C., Heary R.F., Elkabes S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 2021;91:740–755. doi: 10.1016/j.bbi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vonderstein K., Nilsson E., Hubel P., Nygård Skalman L., Upadhyay A., Pasto J., Pichlmair A., Lundmark R., Överby A.K. Viperin Targets Flavivirus Virulence by Inducing Assembly of Noninfectious Capsid Particles. J. Virol. 2018:92. doi: 10.1128/JVI.01751-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li G.H., Henderson L., Nath A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016;14:373–381. doi: 10.2174/1570162X14666161006121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knap N., Korva M., Dolinšek V., Sekirnik M., Trilar T., Avšič-Županc T. Patterns of tick-borne encephalitis virus infection in rodents in Slovenia. Vector Borne Zoonotic Dis. 2012;12:236–242. doi: 10.1089/vbz.2011.0728. [DOI] [PubMed] [Google Scholar]
- 56.Valdebenito S., Castellano P., Ajasin D., Eugenin E.A. Astrocytes are HIV reservoirs in the brain: A cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. J. Neurochem. 2021;158:429–443. doi: 10.1111/jnc.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutgen V., Narasipura S.D., Barbian H.J., Richards M., Wallace J., Razmpour R., Buzhdygan T., Ramirez S.H., Prevedel L., Eugenin E.A., et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020;16:e1008381. doi: 10.1371/journal.ppat.1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He W., Zhao Z., Anees A., Li Y., Ashraf U., Chen Z., Song Y., Chen H., Cao S., Ye J. p21-Activated Kinase 4 Signaling Promotes Japanese Encephalitis Virus-Mediated Inflammation in Astrocytes. Front. Cell Infect Microbiol. 2017;7:271. doi: 10.3389/fcimb.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C.J., Chen J.H., Chen S.Y., Liao S.L., Raung S.L. Upregulation of RANTES gene expression in neuroglia by Japanese encephalitis virus infection. J. Virol. 2004;78:12107–12119. doi: 10.1128/JVI.78.22.12107-12119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray N.B., Power C., Lynch W.P., Ewalt L.C., Lodmell D.L. Rabies viruses infect primary cultures of murine, feline, and human microglia and astrocytes. Arch Virol. 1997;142:1011–1019. doi: 10.1007/s007050050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ireland D.D.C., Manangeeswaran M., Lewkowicz A.P., Engel K., Clark S.M., Laniyan A., Sykes J., Lee H.N., McWilliams I.L., Kelley-Baker L., et al. Long-term persistence of infectious Zika virus: Inflammation and behavioral sequela in mice. PLoS Pathog. 2020;16:e1008689. doi: 10.1371/journal.ppat.1008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mameli G., Poddighe L., Mei A., Uleri E., Sotgiu S., Serra C., Manetti R., Dolei A. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PLoS ONE. 2012;7:e44991. doi: 10.1371/journal.pone.0044991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfefferkorn C., Kallfass C., Lienenklaus S., Spanier J., Kalinke U., Rieder M., Conzelmann K.K., Michiels T., Staeheli P. Abortively Infected Astrocytes Appear To Represent the Main Source of Interferon Beta in the Virus-Infected Brain. J. Virol. 2016;90:2031–2038. doi: 10.1128/JVI.02979-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauhan A., Mehla R., Vijayakumar T.S., Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology. 2014;456–457:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agner S.C., Klein R.S. Viruses have multiple paths to central nervous system pathology. Curr. Opin. Neurol. 2018;31:313–317. doi: 10.1097/WCO.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colombo E., Farina C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Bottermann M., James L.C. Intracellular Antiviral Immunity. Adv. Virus Res. 2018;100:309–354. doi: 10.1016/bs.aivir.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Odendall C., Kagan J.C. Activation and pathogenic manipulation of the sensors of the innate immune system. Microbes Infect. 2017;19:229–237. doi: 10.1016/j.micinf.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 70.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kagan J.C., Barton G.M. Emerging principles governing signal transduction by pattern-recognition receptors. Cold Spring Harb. Perspect Biol. 2014;7:a016253. doi: 10.1101/cshperspect.a016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. NeuroSci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 73.Jack C.S., Arbour N., Manusow J., Montgrain V., Blain M., McCrea E., Shapiro A., Antel J.P. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 74.Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 75.Tian B., Zhou M., Yang Y., Yu L., Luo Z., Tian D., Wang K., Cui M., Chen H., Fu Z.F., et al. Lab-Attenuated Rabies Virus Causes Abortive Infection and Induces Cytokine Expression in Astrocytes by Activating Mitochondrial Antiviral-Signaling Protein Signaling Pathway. Front. Immunol. 2017;8:2011. doi: 10.3389/fimmu.2017.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z.W., Sarmento L., Wang Y., Li X.Q., Dhingra V., Tseggai T., Jiang B., Fu Z.F. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J. Virol. 2005;79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chauhan A., Khandkar M. Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: A fiery path to its destination. Microb. Pathog. 2015;78:1–6. doi: 10.1016/j.micpath.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan J., Selvarajoo K., Tsuchiya M., Lee G., Choi S. Toll-like receptor signal transduction. Exp. Mol. Med. 2007;39:421–438. doi: 10.1038/emm.2007.47. [DOI] [PubMed] [Google Scholar]
- 79.Bsibsi M., Persoon-Deen C., Verwer R.W., Meeuwsen S., Ravid R., Van Noort J.M. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 80.Mian M.F., Ahmed A.N., Rad M., Babaian A., Bowdish D., Ashkar A.A. Length of dsRNA (poly I:C) drives distinct innate immune responses, depending on the cell type. J. Leukoc. Biol. 2013;94:1025–1036. doi: 10.1189/jlb.0312125. [DOI] [PubMed] [Google Scholar]
- 81.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 82.Henrick B.M., Yao X.D., Zahoor M.A., Abimiku A., Osawe S., Rosenthal K.L. TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection. Front. Immunol. 2019;10:482. doi: 10.3389/fimmu.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashraf U., Ding Z., Deng S., Ye J., Cao S., Chen Z. Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage. Virulence. 2021;12:968–980. doi: 10.1080/21505594.2021.1899674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delgado M.A., Elmaoued R.A., Davis A.S., Kyei G., Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mogensen T.H. IRF and STAT Transcription Factors-From Basic Biology to Roles in Infection, Protective Immunity, and Primary Immunodeficiencies. Front. Immunol. 2018;9:3047. doi: 10.3389/fimmu.2018.03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKee C.M., Coll R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020;108:937–952. doi: 10.1002/JLB.3MR0720-513R. [DOI] [PubMed] [Google Scholar]
- 87.Franchi L., Warner N., Viani K., Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Negroni A., Pierdomenico M., Cucchiara S., Stronati L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018;11:49–60. doi: 10.2147/JIR.S137606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Minkiewicz J., de Rivero Vaccari J.P., Keane R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 90.Lupfer C., Kanneganti T.D. The expanding role of NLRs in antiviral immunity. Immunol. Rev. 2013;255:13–24. doi: 10.1111/imr.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 93.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choudhury S.M., Ma X., Abdullah S.W., Zheng H. Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses. J. Inflamm. Res. 2021;14:1145–1163. doi: 10.2147/JIR.S295706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konwar C., Asiimwe R., Inkster A.M., Merrill S.M., Negri G.L., Aristizabal M.J., Rider C.F., MacIsaac J.L., Carlsten C., Kobor M.S. Risk-focused differences in molecular processes implicated in SARS-CoV-2 infection: Corollaries in DNA methylation and gene expression. Epigenet. Chromatin. 2021;14:54. doi: 10.1186/s13072-021-00428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li S., Sun Y., Song M., Song Y., Fang Y., Zhang Q., Li X., Song N., Ding J., Lu M., et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021:6. doi: 10.1172/jci.insight.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sui Y., Bian L., Ai Q., Yao Y., Yu M., Gao H., Zhang A., Fu X., Zhong L., Lu D. Gastrodin Inhibits Inflammasome Through the STAT3 Signal Pathways in TNA2 Astrocytes and Reactive Astrocytes in Experimentally Induced Cerebral Ischemia in Rats. Neuromol. Med. 2019;21:275–286. doi: 10.1007/s12017-019-08544-8. [DOI] [PubMed] [Google Scholar]
- 98.Zhang L., Jiao C., Liu L., Wang A., Tang L., Ren Y., Huang P., Xu J., Mao D. NLRC5: A Potential Target for Central Nervous System Disorders. Front. Immunol. 2021;12:704989. doi: 10.3389/fimmu.2021.704989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rehwinkel J., Gack M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020;20:537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu Z., Zhang X., Wang G., Zheng H. The laboratory of genetics and physiology 2: Emerging insights into the controversial functions of this RIG-I-like receptor. Biomed. Res. Int. 2014;2014:960190. doi: 10.1155/2014/960190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furr S.R., Chauhan V.S., Sterka D., Grdzelishvili V., Marriott I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J. Neurovirol. 2008;14:503–513. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Miranda J., Yaddanapudi K., Hornig M., Lipkin W.I. Astrocytes recognize intracellular polyinosinic-polycytidylic acid via MDA-5. FASEB J. 2009;23:1064–1071. doi: 10.1096/fj.08-121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omura S., Kawai E., Sato F., Martinez N.E., Minagar A., Al-Kofahi M., Yun J.W., Cvek U., Trutschl M., Alexander J.S., et al. Theiler’s Virus-Mediated Immunopathology in the CNS and Heart: Roles of Organ-Specific Cytokine and Lymphatic Responses. Front. Immunol. 2018;9:2870. doi: 10.3389/fimmu.2018.02870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stoff M., Ebbecke T., Ciurkiewicz M., Pavasutthipaisit S., Mayer-Lambertz S., Störk T., Pavelko K.D., Baumgärtner W., Jung K., Lepenies B., et al. C-type lectin receptor DCIR contributes to hippocampal injury in acute neurotropic virus infection. Sci. Rep. 2021;11:23819. doi: 10.1038/s41598-021-03201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bermejo-Jambrina M., Eder J., Helgers L.C., Hertoghs N., Nijmeijer B.M., Stunnenberg M., Geijtenbeek T.B.H. C-Type Lectin Receptors in Antiviral Immunity and Viral Escape. Front. Immunol. 2018;9:590. doi: 10.3389/fimmu.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dengjel J., Schoor O., Fischer R., Reich M., Kraus M., Müller M., Kreymborg K., Altenberend F., Brandenburg J., Kalbacher H., et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cossart P., Helenius A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014:6. doi: 10.1101/cshperspect.a016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horgan C.P., McCaffrey M.W. Rab GTPases and microtubule motors. Biochem. Soc. Trans. 2011;39:1202–1206. doi: 10.1042/BST0391202. [DOI] [PubMed] [Google Scholar]
- 110.Potokar M., Jorgačevski J., Lacovich V., Kreft M., Vardjan N., Bianchi V., D’Adamo P., Zorec R. Impaired αGDI Function in the X-Linked Intellectual Disability: The Impact on Astroglia Vesicle Dynamics. Mol. Neurobiol. 2016;54:2458–2468. doi: 10.1007/s12035-016-9834-1. [DOI] [PubMed] [Google Scholar]
- 111.Potokar M., Lacovich V., Chowdhury H.H., Kreft M., Zorec R. Rab4 and Rab5 GTPase are required for directional mobility of endocytic vesicles in astrocytes. Glia. 2012;60:594–604. doi: 10.1002/glia.22293. [DOI] [PubMed] [Google Scholar]
- 112.Fan D., Wu N., Zhang J., Wang Z., Wang P., Gao N., An J. Effect of the Rho GTPase inhibitor-1 on the entry of dengue serotype 2 virus into EAhy926 cells. Mol. Biol. Rep. 2020;47:9739–9747. doi: 10.1007/s11033-020-05980-9. [DOI] [PubMed] [Google Scholar]
- 113.Vardjan N., Gabrijel M., Potokar M., Svajger U., Kreft M., Jeras M., de Pablo Y., Faiz M., Pekny M., Zorec R. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J. Neuroinflamm. 2012;9:144. doi: 10.1186/1742-2094-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blanc L., Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9:95–106. doi: 10.1080/21541248.2016.1264352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wandinger-Ness A., Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014;6:a022616. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao S., Sheng S., Wang Y., Ding L., Xu X., Xia X., Zheng J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. NeuroSci. Biobehav. Rev. 2021;125:148–159. doi: 10.1016/j.neubiorev.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 117.Huang Y., Li Y., Zhang H., Zhao R., Jing R., Xu Y., He M., Peer J., Kim Y.C., Luo J., et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018;4:19. doi: 10.1038/s41421-018-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu H., Wang J.J., Qi M., Yoon J.J., Wen X., Chen X., Ding L., Spearman P. The intracellular virus-containing compartments in primary human macrophages are largely inaccessible to antibodies and small molecules. PLoS ONE. 2012;7:e35297. doi: 10.1371/journal.pone.0035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chauhan A., Tikoo A., Patel J., Abdullah A.M. HIV-1 endocytosis in astrocytes: A kiss of death or survival of the fittest? Neurosci. Res. 2014;88:16–22. doi: 10.1016/j.neures.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ludwig I.S., Lekkerkerker A.N., Depla E., Bosman F., Musters R.J., Depraetere S., van Kooyk Y., Geijtenbeek T.B. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J. Virol. 2004;78:8322–8332. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feinberg H., Castelli R., Drickamer K., Seeberger P.H., Weis W.I. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 2007;282:4202–4209. doi: 10.1074/jbc.M609689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W., Eller M.A., Pattanapanyasat K., Sarasombath S., Birx D.L., et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis C.W., Nguyen H.Y., Hanna S.L., Sánchez M.D., Doms R.W., Pierson T.C. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deiva K., Khiati A., Hery C., Salim H., Leclerc P., Horellou P., Tardieu M. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS Res. Hum. Retrovir. 2006;22:1152–1161. doi: 10.1089/aid.2006.22.1152. [DOI] [PubMed] [Google Scholar]
- 125.Gough D.J., Messina N.L., Clarke C.J., Johnstone R.W., Levy D.E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li W., Hofer M.J., Songkhunawej P., Jung S.R., Hancock D., Denyer G., Campbell I.L. Type I interferon-regulated gene expression and signaling in murine mixed glial cells lacking signal transducers and activators of transcription 1 or 2 or interferon regulatory factor 9. J. Biol. Chem. 2017;292:5845–5859. doi: 10.1074/jbc.M116.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Negishi H., Taniguchi T., Yanai H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018;10:a028423. doi: 10.1101/cshperspect.a028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 129.Katze M.G., He Y., Gale M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 130.Lee A.J., Ashkar A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018;9:2061. doi: 10.3389/fimmu.2018.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu S.Y., Sanchez D.J., Cheng G. New developments in the induction and antiviral effectors of type I interferon. Curr. Opin. Immunol. 2011;23:57–64. doi: 10.1016/j.coi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Decker T., Kovarik P., Meinke A. GAS elements: A few nucleotides with a major impact on cytokine-induced gene expression. J. Interferon Cytokine Res. 1997;17:121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 133.Kawanokuchi J., Mizuno T., Takeuchi H., Kato H., Wang J., Mitsuma N., Suzumura A. Production of interferon-gamma by microglia. Mult. Scler. 2006;12:558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- 134.Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Popko B., Corbin J.G., Baerwald K.D., Dupree J., Garcia A.M. The effects of interferon-gamma on the central nervous system. Mol. Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pekny M., Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim. Biophys. Acta. 2016;1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 137.Pekny M., Pekna M., Messing A., Steinhäuser C., Lee J.M., Parpura V., Hol E.M., Sofroniew M.V., Verkhratsky A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 138.Shigetomi E., Saito K., Sano F., Koizumi S. Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int. J. Mol. Sci. 2019:20. doi: 10.3390/ijms20040996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Marle G., Antony J., Ostermann H., Dunham C., Hunt T., Halliday W., Maingat F., Urbanowski M.D., Hobman T., Peeling J., et al. West Nile virus-induced neuroinflammation: Glial infection and capsid protein-mediated neurovirulence. J. Virol. 2007;81:10933–10949. doi: 10.1128/JVI.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheeran M.C., Hu S., Sheng W.S., Rashid A., Peterson P.K., Lokensgard J.R. Differential responses of human brain cells to West Nile virus infection. J. Neurovirol. 2005;11:512–524. doi: 10.1080/13550280500384982. [DOI] [PubMed] [Google Scholar]
- 141.Bhowmick S., Duseja R., Das S., Appaiahgiri M.B., Vrati S., Basu A. Induction of IP-10 (CXCL10) in astrocytes following Japanese encephalitis. NeuroSci. Lett. 2007;414:45–50. doi: 10.1016/j.neulet.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 142.Chen C.J., Ou Y.C., Lin S.Y., Raung S.L., Liao S.L., Lai C.Y., Chen S.Y., Chen J.H. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J. Gen. Virol. 2010;91:1028–1037. doi: 10.1099/vir.0.013565-0. [DOI] [PubMed] [Google Scholar]
- 143.Das S., Mishra M.K., Ghosh J., Basu A. Japanese Encephalitis Virus infection induces IL-18 and IL-1beta in microglia and astrocytes: Correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J. Neuroimmunol. 2008;195:60–72. doi: 10.1016/j.jneuroim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 144.Michael B.D., Bricio-Moreno L., Sorensen E.W., Miyabe Y., Lian J., Solomon T., Kurt-Jones E.A., Luster A.D. Astrocyte- and Neuron-Derived CXCL1 Drives Neutrophil Transmigration and Blood-Brain Barrier Permeability in Viral Encephalitis. Cell Rep. 2020;32:108150. doi: 10.1016/j.celrep.2020.108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X., Kumar A. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci. Rep. 2015;5:9867. doi: 10.1038/srep09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Serramía M.J., Muñoz-Fernández M., Álvarez S. HIV-1 increases TLR responses in human primary astrocytes. Sci. Rep. 2015;5:17887. doi: 10.1038/srep17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Phares T.W., Stohlman S.A., Hinton D.R., Bergmann C.C. Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J. Virol. 2013;87:3382–3392. doi: 10.1128/JVI.03307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lavi E., Cong L. Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp. Mol. Pathol. 2020;115:104474. doi: 10.1016/j.yexmp.2020.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hwang M., Bergmann C.C. Alpha/Beta Interferon (IFN-α/β) Signaling in Astrocytes Mediates Protection against Viral Encephalomyelitis and Regulates IFN-γ-Dependent Responses. J. Virol. 2018;92:e01901-17. doi: 10.1128/JVI.01901-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Verma S., Kumar M., Gurjav U., Lum S., Nerurkar V.R. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology. 2010;397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Palus M., Vancova M., Sirmarova J., Elsterova J., Perner J., Ruzek D. Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology. 2017;507:110–122. doi: 10.1016/j.virol.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 153.Chai Q., He W.Q., Zhou M., Lu H., Fu Z.F. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 2014;88:4698–4710. doi: 10.1128/JVI.03149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McRae M. HIV and viral protein effects on the blood brain barrier. Tissue Barriers. 2016;4:e1143543. doi: 10.1080/21688370.2016.1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luissint A.C., Artus C., Glacial F., Ganeshamoorthy K., Couraud P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Edwards J.A., Denis F., Talbot P.J. Activation of glial cells by human coronavirus OC43 infection. J. Neuroimmunol. 2000;108:73–81. doi: 10.1016/S0165-5728(00)00266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017:2. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tice C., McDevitt J., Langford D. Astrocytes, HIV and the Glymphatic System: A Disease of Disrupted Waste Management? Front. Cell. Infect. Microbiol. 2020;10:523379. doi: 10.3389/fcimb.2020.523379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jessen N.A., Munk A.S., Lundgaard I., Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nagelhus E.A., Ottersen O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013;93:1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Potokar M., Jorgačevski J., Zorec R. Astrocyte Aquaporin Dynamics in Health and Disease. Int. J. Mol. Sci. 2016;17:1121. doi: 10.3390/ijms17071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang H., Yuan M., Yang E., Chen D., Su A., Wu Z. Enterovirus 71 infection induced Aquaporin-4 depolarization by increasing matrix metalloproteinase-9 activity. NeuroSci. Lett. 2021;759:136049. doi: 10.1016/j.neulet.2021.136049. [DOI] [PubMed] [Google Scholar]
- 165.Lana-Peixoto M.A., Pedrosa D., Talim N., Amaral J.M.S.S., Horta A., Kleinpaul R. Neuromyelitis optica spectrum disorder associated with dengue virus infection. J. Neuroimmunol. 2018;318:53–55. doi: 10.1016/j.jneuroim.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 166.Calderón-Peláez M.A., Velandia-Romero M.L., Bastidas-Legarda L.Y., Beltrán E.O., Camacho-Ortega S.J., Castellanos J.E. Dengue Virus Infection of Blood-Brain Barrier Cells: Consequences of Severe Disease. Front. Microbiol. 2019;10:1435. doi: 10.3389/fmicb.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li G.H., Ning Z.J., Liu Y.M., Li X.H. Neurological Manifestations of Dengue Infection. Front. Cell Infect. Microbiol. 2017;7:449. doi: 10.3389/fcimb.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sejvar J.J., Haddad M.B., Tierney B.C., Campbell G.L., Marfin A.A., Van Gerpen J.A., Fleischauer A., Leis A.A., Stokic D.S., Petersen L.R. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 169.Hart J., Tillman G., Kraut M.A., Chiang H.S., Strain J.F., Li Y., Agrawal A.G., Jester P., Gnann J.W., Whitley R.J., et al. West Nile virus neuroinvasive disease: Neurological manifestations and prospective longitudinal outcomes. BMC Infect. Dis. 2014;14:248. doi: 10.1186/1471-2334-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Myint K.S., Kipar A., Jarman R.G., Gibbons R.V., Perng G.C., Flanagan B., Mongkolsirichaikul D., Van Gessel Y., Solomon T. Neuropathogenesis of Japanese encephalitis in a primate model. PLoS Negl. Trop. Dis. 2014;8:e2980. doi: 10.1371/journal.pntd.0002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.