Abstract
Objective: The effects of ROM manipulation on muscle strength and hypertrophy response remain understudied in long-term interventions. Thus, we compared the changes in strength and regional muscle hypertrophy after training in protocols with different ranges of motion (ROM) in the seated dumbbell preacher curl exercise using a within-participant experimental design. Design and methods: Nineteen young women had one arm randomly assigned to train in the initial ROM (INITIALROM: 0°–68°; 0° = extended elbow) while the contralateral arm trained in the final ROM (FINALROM: 68°–135°), three times per week over an eight-week study period. Pre- and post-training assessments included one repetition maximum (1RM) testing in the full ROM (0°–135°), and measurement of biceps brachii cross-sectional area (CSA) at 50% and 70% of humerus length. Paired t-tests were used to compare regional CSA changes between groups, the sum of CSA changes at 50% and 70% (CSAsummed), and the strength response between the training protocols. Results: The INITIALROM protocol displayed a greater CSA increase than FINALROM protocol at 70% of biceps length (p = 0.001). Alternatively, we observed similar increases between the protocols for CSA at 50% (p = 0.311) and for CSAsummed (p = 0.111). Moreover, the INITIALROM protocol displayed a greater 1RM increase than FINALROM (p < 0.001). Conclusions: We conclude that training in the initial angles of elbow flexion exercise promotes greater distal hypertrophy of the biceps brachii muscle in untrained young women. Moreover, the INITIALROM condition promotes a greater dynamic strength increase when tested at a full ROM compared to the FINALROM.
Keywords: muscle hypertrophy, partial angular, partial range of motion, partial angular displacement, muscle strength
1. Introduction
The effects of manipulating resistance training (RT) variables on muscle hypertrophy response has been an ongoing focus of investigation [1,2,3]. Among the RT variables, range of motion (ROM) was generally overlooked in past studies targeting training prescription recommendations [3,4]. However, its influence on neuromuscular responses is becoming increasingly recognized as a potential area of research interest [5,6]. ROM may alter the length at which the working muscle contracts. At the beginning of a concentric action (INITIALROM), the muscle length is longer than at the final angles of the action (FINALROM). Several investigations have reported greater metabolic stress and IGF-1 release after contractions performed at longer vs. shorter muscle lengths [7,8,9,10,11], which have been associated with muscle hypertrophy [12,13]. This raises the possibility that training exclusively in the INITIALROM, where the muscle is in a lengthened state, may promote a greater hypertrophic response than training in the FINALROM.
Several studies have compared the hypertrophic response to training in the INITIALROM and FINALROM [7,14,15]. For the quadriceps, results showed greater increases in muscle cross-sectional area (CSA) at the distal muscle regions of the rectus femoris [14] and vastus lateralis [7,14] for the group that performed the INITIALROM compared to the FINALROM training group. These findings suggest that the ROM trained may influence regional muscle growth on quadriceps muscle. Similar to results for the quadriceps, Sato et al. [16] found a greater increase in muscle thickness of the distal biceps brachii plus brachialis after preacher unilateral arm curl resistance training at INITIALROM compared to FINALROM. However, the training occurred two times per week across five weeks, raising the question as to whether differences would be maintained after a longer training period. According to Halperin et al. [17], it is possible that the initial improvements were due to the novel stimulus rather than an inherent superiority of the program. Conceivably, results may have been different if the study period lasted longer. Therefore, it is necessary to conduct further research to provide greater insight into the effect of training in various ROMs on the muscle hypertrophic response over longer time frames.
Kassiano et al. [15] compared changes in muscle thickness of the medial and lateral heads of the gastrocnemius across an eight-week RT program involving the calf raise exercise when performed in the INITIALROM vs FINALROM. Results showed the INITIALROM training elicited greater increases in muscle thickness of the medial and lateral gastrocnemius compared to the FINALROM, providing evidence that training exclusively at longer muscle lengths may be superior to training exclusively at shorter muscle lengths for enhancing muscle hypertrophy of the plantar flexors. However, these authors assessed hypertrophy at a single point along the muscle, and it is known that resistance training in different ROM configuration may induce non-homogeneous hypertrophic adaptations across the length of the muscle [14,18]. Furthermore, the change in muscle volume analyzed via magnetic resonance imaging (MRI) is considered the gold standard for estimating muscle hypertrophy [19]. However, this procedure is expensive [19]. A more economical alternative to analyzing muscle volume by MRI is to assess muscle CSA by B-mode ultrasound [19], with measurements taken at different sites along the length of the muscle [14,20]. This approach provides a proxy for muscle volume, imparting a better comprehension of the influence of ROM manipulation on muscle hypertrophy.
In addition to its effects on muscle hypertrophy, RT also promotes increases in maximal strength. Research generally shows that strength increases are specific to the given ROM trained [21,22]. Accordingly, training in the INITIALROM and FINALROM could lead to a greater strength increase in the specific angles trained [23]. Taking into account that the sticking point is a primary determinant of performance in the 1RM test [24], it can be expected that training in the INITIALROM, which promotes greater strength increases in this region, would also cause greater increases in the 1RM test performed in full ROM in comparison to training in the FINALROM. In line with this reasoning, Pedrosa et al. [14] found that 12 weeks training in the INITIALROM promoted a greater 1RM increase at full ROM than training in the FINALROM using the knee extension machine. Werkhausen et al. [2] compared the isokinetic strength after training in the INITIALROM and the full ROM in the leg press exercise. Results showed similar changes between training in the INITIALROM and a full ROM, suggesting the strength adaptations across a full ROM are influenced by the initial training angle. These findings further the rationale that training in the INITIALROM could be superior to training in the FINALROM in a full ROM strength test. However, this hypothesis has not yet been objectively tested in other muscles such as the elbow flexors.
Therefore, this study aimed to compare dynamic strength and regional muscle hypertrophy changes of the elbow flexors in young women after performing the arm curl exercise for eight weeks while training in INITIALROM and FINALROM. We hypothesized that the INITIALROM protocol would elicit greater increases in dynamic strength and distal muscle hypertrophy than FINALROM protocol.
2. Methods
We employed a within-participant experimental design whereby the right and left arms of 21 untrained women trained in INITIALROM or FINALROM for eight weeks. We chose the seated dumbbell preacher curl exercise to ensure that participants maintained strict form throughout exercise performance. Pre- and post-study strength was assessed by the 1RM test in the arm curl, and B-mode ultrasound was employed to assess CSA changes in the mid- and distal regions of the biceps.
The sample size was estimated a priori following the recommendations of Beck [25] using the software G*Power (version 3.1.9.2; Heinrich Heine Universität Düsseldorf, DE, Germany). We used the t-statistic with an alpha of 0.05, power of 0.8, and a relatively moderate Cohen’s d effect size (ES) of 0.7 and determined that 19 subjects were required for adequate statistical power. We recruited two additional subjects to account for the possibility of dropouts, expected to be ~10% of the sample.
Participants were untrained women who had not performed any physical activity for at least six months prior to the onset of the study. Two participants withdrew for personal reasons; therefore, 19 women completed the study (mean age = 22.8 ± 10.5 years; mean body mass = 64.5 ± 8.05 kg; mean height = 164.1 ± 4.7 cm). Each participant’s upper limb was allocated in a randomized fashion according to upper limb dominance. The order of training was counterbalanced whereby half of the participants performed the INITIALROM protocol with their preferred limb, while the other half performed the FINALROM protocol with their preferred limb. Before participation, written consent was obtained from each participant after being informed of the procedures, risks, and benefits of the investigation. The study followed the standards established in the Declaration of Helsinki and was approved by the ethics committee of the Federal University of Minas Gerais (approval #CAAE 91438418.4.0000.5149).
In the first pre-training session, after the anthropometric data assessment, we obtained measures of biceps brachii CSA at 50% and 70% of the distance from the acromion to the lateral epicondyle of each humerus via B-mode ultrasound (MindRay® DC-7, Shenzhen, China). It should be noted that muscle hypertrophy assessed by B-mode ultrasound imaging is highly correlated with MRI, which is considered the gold standard for measuring changes in muscle mass [26]. Images were acquired at a frequency of 21 frames/s, using a 4–10 MHz linear transducer with a depth ranging from 1 to 6 cm and gain between 50 and 64 db. The settings were individually adjusted to produce a clear image of the entire muscle for extended field-of-view, and replication at post-training. The same trained technician performed all ultrasound scans, moving the transducer in a line parallel to the humeral epicondyles at a relatively constant speed for approximately 7s at each site. The images were saved to hard drive and coded for blinded CSA calculation using the Horos® software. We averaged the two CSA measurements in each region to obtain the final values used for analyses. Moreover, we summed the CSA at 50%, and 70% of biceps length (CSAsummed) to produce an estimate of overall biceps hypertrophy. Previous studies have employed similar formulas in an attempt to produce a hypertrophy measure more representative of the whole muscle in comparison to a single muscle region [1,20]. The intraclass correlation coefficients (ICC3,1) in our laboratory for CSA at 50% and 70% of biceps length were 0.94 and 0.92, respectively.
After ultrasound imaging, we assessed participants 1RM in the seated dumbbell preacher curl exercise. The 1RM test was performed alternately on each arm throughout a full ROM, with a 3-min recovery interval between the limbs and between attempts. A final value was obtained within 5 attempts on each arm. Each attempt started with the elbow fully extended (0°), and the shoulder angle (humerus and trunk) fixed at 45°. The dumbbell was handed to the participant in this initial position, who then performed a concentric muscle action until 135° of elbow flexion (forearm perpendicular to the ground). The attempt was considered successful if the participant was able to perform the full range of elbow flexion (0° to 135°) without assistance from auxiliary movements. The dumbbell load was progressively increased (minimum of 0.5 kg) until the participant was unable to perform the concentric action with proper form. Hence, the 1RM value corresponded to the weight lifted in the previous successful attempt. This initial test was considered a familiarization session to the 1RM assessment. The 1RM test was repeated 48 h later (pre-training session 2) and the value obtained in this session was used for statistical analysis. Between 48 h and 72 h after the last training session, the ultrasound imaging and the 1RM test in a full ROM were repeated using the procedures previously described.
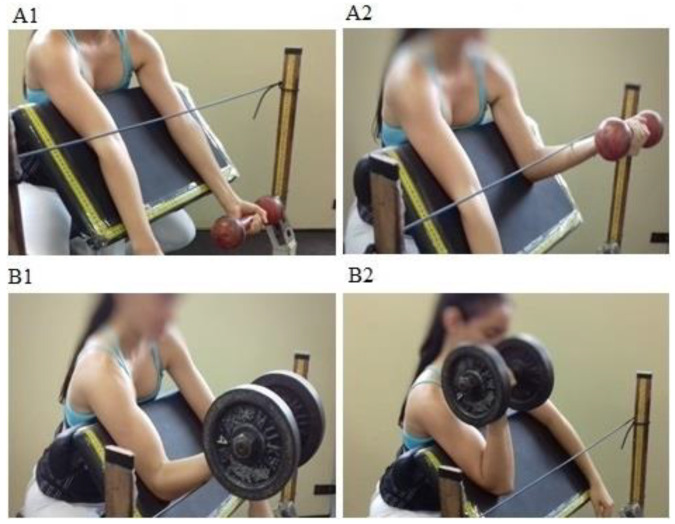
Training sessions consisted of the seated dumbbell preacher curl performed in a specific ROM for each limb. The shoulder angle was fixed at 45° (as in the 1RM test). The INITIALROM protocol was trained from 0° to 68° of elbow flexion, and the FINALROM protocol was trained from 68° to 135° of elbow flexion (Figure 1). The ROMs were individually checked by a manual goniometer (axis fixed in the elbow, and the rules fixed in the arm and forearm) at the beginning of each training session. An elastic cord was placed in front of the machine to serve as a mechanical stop ensuring training was limited to the prescribed ROMs (Figure 1). In the INITIALROM, participants began the eccentric muscle action when their forearm touched the string (68° of elbow flexion) and they continued lowering the load until full extension. In the FINALROM protocol, the participants began the concentric action when their forearm touched the string (68° of elbow flexion) and they continued raising the load until their forearm was perpendicular to the ground (135° of elbow flexion).
Figure 1.
Training protocols range of motion. (A1,A2) = starting and finishing the concentric action of INITIALROM protocol (0°–68° of elbow flexion), respectively. (B1,B2) = starting and finishing the concentric action of FINALROM protocol (68°–135° of elbow flexion), respectively.
Each protocol was trained three times per week, in the same session, separated by 48–72 h over the eight-week study period. We alternated the session-to-session order in which the training protocols were performed: i.e., if the INITIALROM protocol was the first to be performed in a given training session, the FINALROM protocol would be trained first in the next session. If the participant was assigned to start with INITIALROM protocol, after completing each set, 1 min interval was allowed before initiating the FINALROM protocol with the contra lateral limb. The next set for the starting protocol was only initiated after 3 min of the completion of the previous set of the INITIALROM; all recovery periods were timed with a stopwatch to ensure accuracy.
Participants performed four sets per session in both the INITIALROM and the FINALROM protocols. In an effort to standardize the training stimulus for the development of hypertrophy and muscle strength, all sets were carried out until volitional failure [27,28]. When the last set was performed with more than 10 or less than 8 repetitions, the load was increased or reduced diminished in 1 kg at the next training session, respectively. Each repetition was performed with a 2 s concentric action and a 2 s eccentric action (timed by metronome). Five sets were performed from the fifth week on, following the same previously described procedures.
The Shapiro–Wilk test confirmed the normality of data distribution, and all variables presented similar baseline values between training protocols. We analyzed the absolute difference values (post–pre-values) between training protocols by paired t-test for all variables of interest. We reported 95% confidence intervals (CI) around the point estimate. Cohen’s d effect sizes (ES) were calculated (post-pre/pooled standard deviation) with the following interpretation: trivial: <0.20; small: 0.20–0.60; moderate: 0.61–1.20; large: 1.21–2.0; very large: >2.0) [29]. All statistical procedures were performed using JASP statistics packages, version 0.14 (Wagenmakers, Amsterdam). We considered statistical significance when α < 0.05.
3. Results
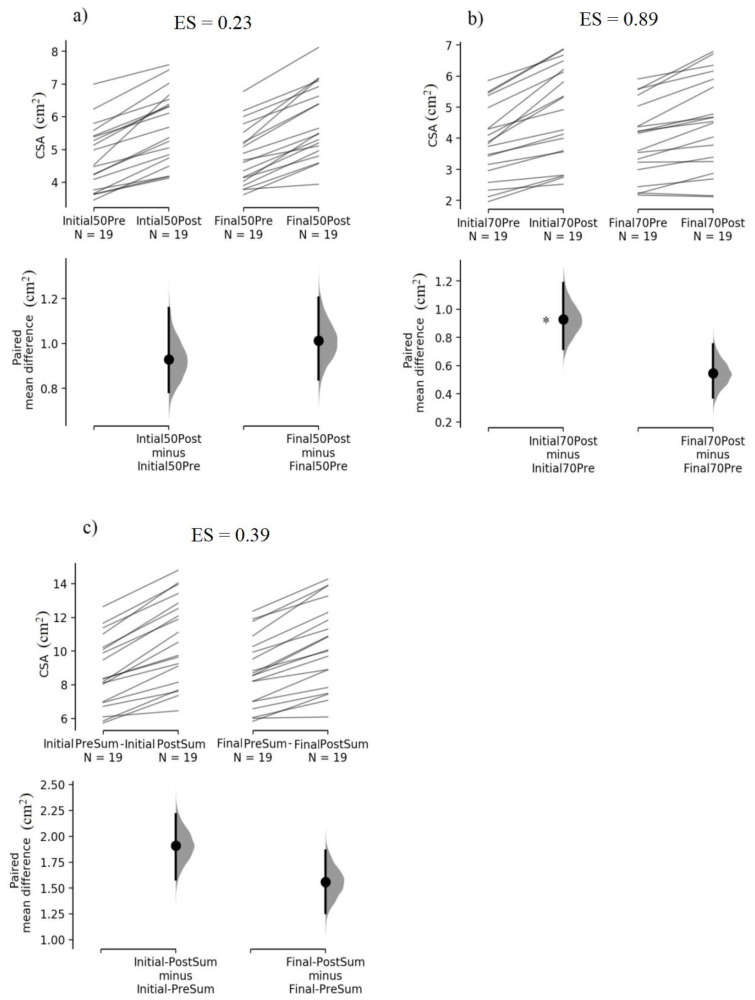
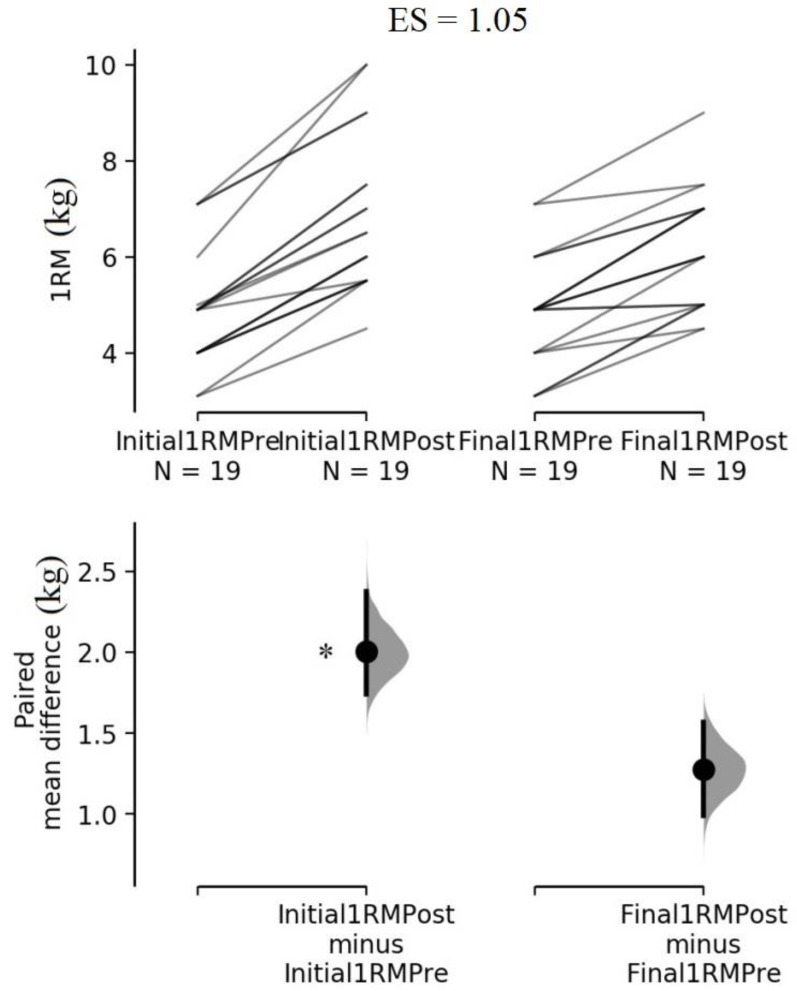
When comparing regional CSA between the training protocols, analysis showed the INITIALROM protocol displayed a greater CSA increase than the FINALROM protocol at 70% of biceps brachii length (p = 0.001; 95% CI = 0.18 to 0.59 cm2; ES = 0.89), and a relatively similar CSA increase at 50% (p = 0.331; 95% CI = −0.10 to 0.34 cm2; ES = 0.23). Analysis showed the CSAsummed increase was not statistically different between the training protocols (p = 0.111; 95% CI = −0.08 to 0.67 cm2; ES = 0.39), as shown in Figure 2. Regarding the 1RM test, analysis showed the INITIALROM protocol presented a statistically greater increase than the FINALROM protocol (p < 0.001; 95% CI = 0.39 to 1.06 kg; ES = 1.05), as shown in Figure 3.
Figure 2.
The paired mean difference for cross-sectional area in INITIALROM and FINALROM at (a) 50% humeral length; (b) 70% humeral length; and (c) summed values of 50% and 70% humeral length [30]. The raw data are plotted on the upper axes; each paired set of observations is connected by a line. On the lower axes, each paired mean difference is plotted as a bootstrap sampling distri-bution. Mean differences are depicted as dots; 95% confidence intervals are indicated by the ends of the vertical error bars. ES = effect size. * Significant differences compared with FINALROM protocol.
Figure 3.
The paired mean difference for 1 repetition maximum in INITIALROM and FINALROM [30]. The raw data are plotted on the upper axes; each paired set of observations is connected by a line. On the lower axes, each paired mean difference is plotted as a bootstrap sampling distribution. Mean differences are depicted as dots; 95% confidence intervals are indicated by the ends of the vertical error bars. ES = effect size. * Significant differences compared with FINALROM protocol.
4. Discussion
A primary finding of our study was that training in the INITIALROM elicited greater increases in CSA at 70% of biceps brachii length and in the 1RM test than the FINALROM protocol. These results are consistent with our initial hypothesis and provide further evidence that ROM manipulation impacts regional muscular adaptations across a variety of different muscles and exercises.
To our knowledge, only three previous studies compared regional hypertrophic changes after training in INITIALROM and FINALROM. McMahon et al. [7] found that the INITIALROM group achieved greater vastus lateralis hypertrophy than the FINALROM group only at the distal region after 8 weeks of knee extension training. Similarly, Pedrosa et al. [14] showed the INITIALROM group presented greater distal muscle growth of the rectus femoris and the vastus lateralis muscles than the FINALROM group after 12 weeks of knee extension training. Moreover, Sato et al. [16] demonstrated the INITIALROM training elicited greater distal biceps brachii plus brachialis muscle hypertrophy after only five weeks resistance training. These results corroborate our findings, which suggest that training in INITIALROM promotes greater distal hypertrophy of the biceps than training in FINALROM, and a similar hypertrophy response between conditions at the middle region in young, untrained women. Moreover, our study expands on the findings of McMahon et al. [7] and Pedrosa et al. [14] and supports those of Sato et al. [16] by providing evidence that training a muscle at long muscle length has a beneficial effect on muscular adaptations in the upper extremities.
Some other studies have investigated regional muscle hypertrophy between the FINALROM and the FULLROM protocols [14,18,31]. Bloomquist et al. [18] reported greater hypertrophy in the middle and distal regions of the anterior quadriceps femoris in a group of young men performing the back squat for 12 weeks in a FULLROM group compared to the FINALROM group. Similarly, McMahon et al. [31] reported greater distal vastus lateralis hypertrophy in a group of young men and women performing a variety of lower limb exercises in a FULLROM versus a FINALROM over an 8-week training period. Pedrosa et al. [14] showed greater distal muscle hypertrophy of rectus femoris and vastus lateralis muscles after training in FULLROM compared to FINALROM. Given that the main difference between training in FINALROM and FULLROM is that the FULLROM excurses the INITIALROM, it can be speculated that the greater muscle hypertrophy after training in a FULLROM results from training at longer muscle lengths.
Although speculative, a possible mechanistic explanation for the heightened regional hypertrophic response is related to the production of higher amounts of metabolic stress [10], and insulin-like growth factor (IGF)-1 release [7] when training at longer muscle lengths in comparison to training at shorter muscle lengths, which in turn may confer anabolic effects [32]. Additionally, there is evidence suggesting that both metabolic stress [33] and IGF-1 [34] concentrations may vary between muscle regions after mechanical overload, and that greater regional muscle hypertrophy occurs in regions demonstrating greater metabolic stress [35] and IGF-1 concentrations [34]. Thus, we hypothesize that training in the INITIALROM promotes a heightened physiological response at the distal portion of the muscle, thereby leading to greater muscle protein increase in this region. Previous research supports our findings [7,14,18,31]; however, no attempts were made to explore mechanisms involved, which requires further investigation.
When summing the CSA results of the two regional sites (CSAsummed), hypertrophic increases were statistically similar between training protocols. This value provides a general proxy for hypertrophy of the muscle as a whole. Our findings in this regard are consistent with previous studies on the topic [1,20]. However, although the study lasted eight weeks and is in line with previous research that aimed to measure muscle hypertrophy over this time period [7,31], we cannot necessarily infer that results would hold true over longer-term interventions nor rule out the possibility that other factors may have influenced changes [36]. Therefore, further investigation on topic using longer interventional period is recommended to confirm or refute the present findings.
In regard to the 1RM results, our study shows that training in the INITIALROM elicits greater dynamic strength improvements in a full ROM test compared to training in the FINALROM; these results were observed despite the use of heavier absolute loads when training in the FINALROM. To our knowledge, only one study to date has compared changes in dynamic strength at a FULLROM after training in the INITIALROM versus FINALROM. Pedrosa et al. [14] reported the INITIALROM group showed greater 1RM test increase at a FULLROM compared to the FINALROM after 12 weeks of knee extension training, lending support to our results.
Several studies indicate a ROM-specific strength increase after training in a FULLROM, FINALROM [18,21,22], and INITIALROM [14]. Bloomquist et al. [18] found that the training groups (FULLROM and FINALROM) presented greater 1RM increases in the trained ROM. Similarly, Martínez-Cava et al. [21] reported ROM-specific strength adaptations after 10 weeks of training in the bench press exercise at a full ROM, two-thirds ROM, and one-third-ROM). In addition, Pedrosa et al. [14] showed the INITIALROM and FINALROM groups presented greater 1RM increases in the ROM trained. Although the present study did not compare the strength performance in different ROMs, previous findings [14,18,21,22,23] support the rationale that a ROM-specific strength increase may also have occurred in the present study, and therefore influenced the results of the 1RM test in a full ROM. Accordingly, the INITIALROM training would allow a greater strength enhancement at the beginning of the concentric action of a full ROM compared to training in the FINALROM [14]. We speculate that this specific joint-angle strength adaptation (from the INITIALROM protocol) was fundamental to overcoming the sticking point and thus resulted in a superior increase in 1RM.
Changes in muscle morphology may help to provide a mechanistic explanation for the observed angular specific differences in strength increase between conditions [37]. Evidence indicates a positive association between greater increases in distal muscle CSA regions and increases in torque angles where the muscle is elongated [37]. Thus, the greater muscle hypertrophy response at 70% in the INITIALROM condition may have enhanced strength performance in a full ROM 1RM test to a greater extent than training in FINALROM. This hypothesis needs further investigation.
Additionally, it is known that the increase in maximum dynamic strength is related to alterations in neural factors, such as an increase in the number of activated motor units, improvement in inter and intra muscular synchronization, and a reduction in the activation of the antagonist muscles during exercise performance [38]. Thepaut-Mathieu et al. [39] reported that resistance training performed exclusively at a short muscle length promoted greater neural adaptations (as assessed by surface electromyography) near or at the trained angles, and this adaptation coincided with the greater observed increases in muscle strength near or at the trained angles; the response did not occur in the group that trained in long muscle length. This finding is supported by Noorkoiv et al. [37], who demonstrated a positive and significant correlation between the force increase at short muscle length angles and the increase of the surface electromyographic signal only in the group that trained at the short muscle length compared to the long length group. It therefore could be hypothesized that the contribution from the improvement of neural factors to increase the performance of the 1RM test was smaller in the FINALROM group compared to INITIALROM group. The present study did not attempt to assess neural factors; hence, further investigations are needed to better elucidate the mechanisms of strength increases associated with ROM manipulation.
This study has several limitations that should be taken into account when attempting to draw practical conclusions. First, although performing sets to volitional fatigue helps to ensure that all individuals receive a comparable hypertrophic stimulus [27], its implementation influences other variables, such as the number of repetitions performed. Future studies should seek to study ROM-induced muscular adaptations with different configurations of RT variables. Second, we only tested dynamic strength in a full ROM using the knee extension machine and thus cannot extrapolate findings to specific partial ROMs, isometric strength at different joint-angles or the transfer of these results to the activities of everyday life. Third, the findings are specific to dynamic elbow flexion exercise and thus cannot necessarily be generalized to other exercises or muscle groups. Fourth, we measured hypertrophy at only two points along the length of the biceps brachii; it would be interesting to analyze other regions or even the muscle volume to obtain a more robust perspective of hypertrophic changes. Fifth, our findings are specific to young, healthy, untrained women and thus cannot necessarily be generalized to other populations.
Finally, we chose to employ a within-participant design, which affords the benefit of enhancing statistical power by reducing the amount of between-participant variability [40]. While this experimental model can provide keen insights into skeletal muscle adaptations in longitudinal RT investigations [40], a potential limitation of this design is the possibility of a cross-education effect. There is evidence indicating that the cross-education effect, if it indeed occurs, would be restricted to neural parameters and muscle strength gains; morphological changes (e.g., CSA) are not materially influenced by this effect [41]. Hence, any muscle strength gains achieved in the contralateral limb should conceivably evolve from an increase in motor neuron activation without contribution from morphological adaptations. Moreover, previous studies investigating the cross-education effect for EMG amplitude have shown inconclusive results [42,43]. For example, Hortobágyi, et al. [42] found that changes in the EMG amplitude of the untrained limb depend on the training mode performed (e.g., type of muscle action). The neuromuscular changes were similar to the changes in muscle strength. Moreover, other researchers found that the cross-education effect contributes to approximately 7.8% of the muscle strength gain of the contralateral limb [44]. In the present study, the mean relative increase in the INITIALROM and FINALROM groups was 42.8 ± 14.8% and 19.0 ± 10.3%, respectively; these differences would be outside of any potential confounding from cross-education. Furthermore, it has been argued that, when both limbs of an individual are trained with different protocols, the cross-education effect is minimal or non-existent [44,45]. Thus, based on the magnitude of influence presented by Munn et al. [44], it seems likely that any difference in the strength responses between limbs would be due to training protocols with minimal confounding from cross-education.
5. Conclusions
In conclusion, the seated dumbbell preacher curl performed in the INITIALROM elicited greater biceps brachii hypertrophy at 70% length, but not at 50% nor for the CSAsummed in untrained women. Moreover, the INITIALROM protocol promoted a greater increase in the 1RM test in full ROM compared with training in the FINALROM. These findings add to the body of literature indicating that training at long muscle lengths promotes increases in hypertrophy at the distal muscle region, and these findings occur in both the upper and lower extremity musculature.
Acknowledgments
Supported by the FAPEMIG; CAPES (Brazil); and PRPq of Universidade Federal de Minas Gerais.
Author Contributions
Conceptualization, R.C.R.D., F.V.L. and M.H.C.; methodology, R.C.R.D., L.T.L. and M.G.S.; formal analysis, G.F.P., M.O.C.F., B.J.S. and M.G.S.; writing—original draft preparation, G.F.P., M.G.S. and R.C.R.D.; writing—review and editing, G.F.P., B.J.S. and R.C.R.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Federal University of Minas Gerais (protocol code CAAE-91438418.4.0000.5149 and 9 August 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data generated or analyzed during this study will are included in the published article as Table(s) and Figure(s). Any other data requirement can be directed to the corresponding author upon reasonable request.
Conflicts of Interest
B.J.S. serves on the scientific advisory board for Tonal Corporation, a manufacturer of fitness equipment. The other authors declare no conflicts of interests, financial or otherwise.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Diniz R.C.R., Tourino F.D., Lacerda L.T., Martins-Costa H.C., Lanza M.B., Lima F.V., Chagas M.H. Does the Muscle Action Duration Induce Different Regional Muscle Hypertrophy in Matched Resistance Training Protocols? J. Strength Cond. Res. 2022;36:2371–2380. doi: 10.1519/JSC.0000000000003883. [DOI] [PubMed] [Google Scholar]
- 2.Werkhausen A., Solberg C.E., Paulsen G., Bojsen-Møller J., Seynnes O.R. Adaptations to Explosive Resistance Training with Partial Range of Motion Are Not Inferior to Full Range of Motion. Scand. J. Med. Sci. Sport. 2021;31:1026–1035. doi: 10.1111/sms.13921. [DOI] [PubMed] [Google Scholar]
- 3.Morton R.W., Colenso-Semple L., Phillips S.M. Training for Strength and Hypertrophy: An Evidence-Based Approach. Curr. Opin. Physiol. 2019;10:90–95. doi: 10.1016/j.cophys.2019.04.006. [DOI] [Google Scholar]
- 4.ACSM Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 5.Pallarés J.G., Hernández-Belmonte A., Martínez-Cava A., Vetrovsky T., Steffl M., Courel-Ibáñez J. Effects of Range of Motion on Resistance Training Adaptations: A Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sport. 2021;31:1866–1881. doi: 10.1111/sms.14006. [DOI] [PubMed] [Google Scholar]
- 6.Kassiano W., Costa D., Nunes J.P., Ribeiro A.S. Which ROMs Lead to Rome? A Systematic Review of the Effects of Range of Motion on Muscle Hypertrophy. J. Strength Cond. Res. 2023. Epub ahead of printing . [DOI] [PubMed]
- 7.Mcmahon G., Morse C.I., Burden A., Winwood K., Onambélé G.L. Muscular Adaptations and Insulin-like Growth Factor-1 Responses to Resistance Training Are Stretch-Mediated. Muscle Nerve. 2014;49:108–119. doi: 10.1002/mus.23884. [DOI] [PubMed] [Google Scholar]
- 8.Russ D.W. Active and Passive Tension Interact to Promote Akt Signaling with Muscle Contraction. Med. Sci. Sports Exerc. 2008;40:88–95. doi: 10.1249/MSS.0b013e318158e450. [DOI] [PubMed] [Google Scholar]
- 9.Rindom E., Kristensen A.M., Overgaard K., Vissing K., de Paoli F.V. Activation of MTORC1 Signalling in Rat Skeletal Muscle Is Independent of the EC-Coupling Sequence but Dependent on Tension per Se in a Dose-Response Relationship. Acta Physiol. 2019;227:e13336. doi: 10.1111/apha.13336. [DOI] [PubMed] [Google Scholar]
- 10.Kooistra R.D., Blaauboer M.E., Born J.R., de Ruiter C.J., de Haan A. Knee Extensor Muscle Oxygen Consumption in Relation to Muscle Activation. Eur. J. Appl. Physiol. 2006;98:535–545. doi: 10.1007/s00421-006-0298-2. [DOI] [PubMed] [Google Scholar]
- 11.Fouré A., Ogier A.C., Guye M., Gondin J., Bendahan D. Muscle Alterations Induced by Electrostimulation Are Lower at Short Quadriceps Femoris Length. Eur. J. Appl. Physiol. 2020;120:325–335. doi: 10.1007/s00421-019-04277-5. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki H., Loenneke J.P., Buckner S.L., Abe T. Muscle Growth across a Variety of Exercise Modalities and Intensities: Contributions of Mechanical and Metabolic Stimuli. Med. Hypotheses. 2016;88:22–26. doi: 10.1016/j.mehy.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Barclay R.D., Burd N.A., Tyler C., Tillin N.A., Mackenzie R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019;6:1–9. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedrosa G.F., Lima F.V., Schoenfeld B.J., Lacerda L.T., Simões M.G., Pereira M.R., Diniz R.C.R., Chagas M.H. Partial Range of Motion Training Elicits Favorable Improvements in Muscular Adaptations When Carried out at Long Muscle Lengths. Eur. J. Sport Sci. 2022;22:1250–1260. doi: 10.1080/17461391.2021.1927199. [DOI] [PubMed] [Google Scholar]
- 15.Kassiano W., De Londrina U.E., Costa D., Kunevaliki G., Soared D., Zacarias G., Manske I., Takaki Y., Ruggiero M.F., de Lima Stavinski N.G., et al. Greater Gastrocnemius Muscle Hypertrophy after Partial Range of Motion Training Carried out at Long Muscle Lengths. J. Strengnth Cond. Res. 2022 doi: 10.1519/JSC.0000000000004460. [DOI] [PubMed] [Google Scholar]
- 16.Sato S., Yoshida R., Kiyono R., Yahata K., Yasaka K., Nunes J.P., Nosaka K., Nakamura M. Elbow Joint Angles in Elbow Flexor Unilateral Resistance Exercise Training Determine Its Effects on Muscle Strength and Thickness of Trained and Non-Trained Arms. Front. Physiol. 2021;12:734509. doi: 10.3389/fphys.2021.734509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halperin I., Vigotsky A.D., Foster C., Pyne D.B. Strengthening the Practice of Exercise and Sport-Science Research. Int. J. Sports Physiol. Perform. 2018;13:127–134. doi: 10.1123/ijspp.2017-0322. [DOI] [PubMed] [Google Scholar]
- 18.Bloomquist K., Langberg H., Karlsen S., Madsgaard S., Boesen M., Raastad T. Effect of Range of Motion in Heavy Load Squatting on Muscle and Tendon Adaptations. Eur. J. Appl. Physiol. 2013;113:2133–2142. doi: 10.1007/s00421-013-2642-7. [DOI] [PubMed] [Google Scholar]
- 19.Franchi M.V., Longo S., Mallinson J., Quinlan J.I., Taylor T., Greenhaff P.L., Narici M.V. Muscle Thickness Correlates to Muscle Cross-Sectional Area in the Assessment of Strength Training-Induced Hypertrophy. Scand. J. Med. Sci. Sport. 2018;28:846–853. doi: 10.1111/sms.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earp J.E., Newton R.U., Cormie P., Blazevich A.J. Inhomogeneous Quadriceps Femoris Hypertrophy in Response to Strength and Power Training. Med. Sci. Sports Exerc. 2015;47:2389–2397. doi: 10.1249/MSS.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Cava A., Hernández-Belmonte A., Courel-Ibáñez J., Morán-Navarro R., González-Badillo J.J., Pallarés J.G. Bench Press at Full Range of Motion Produces Greater Neuromuscular Adaptations Than Partial Executions After Prolonged Resistance Training. J. Strength Cond. Res. 2022;1:10–15. doi: 10.1519/JSC.0000000000003391. [DOI] [PubMed] [Google Scholar]
- 22.Rhea M.R., Kenn J.G., Peterson M.D., Massey D., Simão R., Marin P.J., Favero M., Cardozo D., Krein D. Joint-Angle Specific Strength Adaptations Influence Improvements in Power in Highly Trained Athletes. Hum. Mov. 2016;17:43–49. doi: 10.1515/humo-2016-0006. [DOI] [Google Scholar]
- 23.Graves J.E., Pollock l.M., Jones A., Colvin A.B., Leggett S.H. Specificity of Limited Range of Motion Variable Resistance Training. Med. Sci. Sports Exerc. 1989;21:84–89. doi: 10.1249/00005768-198902000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Brown L.E.E.E., Weir J.P. ASEP Procedures Recommendation I: Accurate Assessment of Muscular Strength and Power. J. Exerc. Physiol. 2001;4:1–21. [Google Scholar]
- 25.Beck T.W. The Importance of a Priori Sample Size Estimation in Strength and Conditioning Research. J. Strength Cond. Res. 2013;27:2323–2337. doi: 10.1519/JSC.0b013e318278eea0. [DOI] [PubMed] [Google Scholar]
- 26.Stokes T., Tripp T.R., Murphy K., Morton R.W., Oikawa S.Y., Lam Choi H., McGrath J., McGlory C., MacDonald M.J., Phillips S.M. Methodological Considerations for and Validation of the Ultrasonographic Determination of Human Skeletal Muscle Hypertrophy and Atrophy. Physiol. Rep. 2021;9:1–12. doi: 10.14814/phy2.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dankel S.J., Jessee M.B., Mattocks K.T., Mouser J.G., Counts B.R., Buckner S.L., Loenneke J.P. Training to Fatigue: The Answer for Standardization When Assessing Muscle Hypertrophy ? Sport. Med. 2016;47:1021–1027. doi: 10.1007/s40279-016-0633-7. [DOI] [PubMed] [Google Scholar]
- 28.Grgic J., Schoenfeld B.J., Orazem J., Sabol F. Effects of Resistance Training Performed to Repetition Failure or Non-Failure on Muscular Strength and Hypertrophy: A Systematic Review and Meta-Analysis. J. Sport Health Sci. 2022;11:202–211. doi: 10.1016/j.jshs.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz C.O., Morris P.E., Richler J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 30.Ho J., Tumkaya T., Aryal S., Choi H., Claridge-Chang A. Moving beyond P Values: Data Analysis with Estimation Graphics. Nat. Methods. 2019;16:565–566. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 31.McMahon G.E., Morse C.I., Burden A., Winwood K., Onambélé G.L. Impact of Range of Motion during Ecologically Valid Resistance Training Protocols on Muscle Size, Subcutaneous Fat, and Strength. J. Strength Cond. Res. 2014;28:245–255. doi: 10.1519/JSC.0b013e318297143a. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld B.J. Potential Mechanisms for a Role of Metabolic Stress in Hypertrophic Adaptations to Resistance Training. Sport. Med. 2013;43:179–194. doi: 10.1007/s40279-013-0017-1. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto N., Wakahara T., Ema R., Kawakami Y. Non-Uniform Muscle Oxygenation despite Uniform Neuromuscular Activity within the Vastus Lateralis during Fatiguing Heavy Resistance Exercise. Clin. Physiol. Funct. Imaging. 2013;33:463–469. doi: 10.1111/cpf.12054. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi A., Ikeda Y., Hirai T., Fujikawa T., Morita I. Local Changes of IGF-I MRNA, GH Receptor MRNA, and Fiber Size in Rat Plantaris Muscle Following Compensatory Overload. Jpn. J. Physiol. 2003;53:53–60. doi: 10.2170/jjphysiol.53.53. [DOI] [PubMed] [Google Scholar]
- 35.Wakahara T., Ema R., Miyamoto N., Kawakami Y. Inter- and Intramuscular Differences in Training-Induced Hypertrophy of the Quadriceps Femoris: Association with Muscle Activation during the First Training Session. Clin. Physiol. Funct. Imaging. 2017;37:405–412. doi: 10.1111/cpf.12318. [DOI] [PubMed] [Google Scholar]
- 36.Damas F., Libardi C.A., Ugrinowitsch C. The Development of Skeletal Muscle Hypertrophy through Resistance Training: The Role of Muscle Damage and Muscle Protein Synthesis. Eur. J. Appl. Physiol. 2018;118:485–500. doi: 10.1007/s00421-017-3792-9. [DOI] [PubMed] [Google Scholar]
- 37.Noorkõiv M., Nosaka K., Blazevich A.J. Effects of Isometric Quadriceps Strength Training at Different Muscle Lengths on Dynamic Torque Production. J. Sports Sci. 2015;33:1952–1961. doi: 10.1080/02640414.2015.1020843. [DOI] [PubMed] [Google Scholar]
- 38.Cormie P., McGuigan M.R., Newton R.U. Developing Maximal Neuromuscular Power. Sport. Med. 2011;41:125–146. doi: 10.2165/11538500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Thepaut-Mathieu C., Van Hoecke J., Maton B. Myoelectrical and Mechanical Changes Linked to Length Specificity during Isometric Training. J. Appl. Physiol. 1988;64:1500–1505. doi: 10.1152/jappl.1988.64.4.1500. [DOI] [PubMed] [Google Scholar]
- 40.MacInnis M.J., McGlory C., Gibala M.J., Phillips S.M. Investigating Human Skeletal Muscle Physiology with Unilateral Exercise Models: When One Limb Is More Powerful than Two. Appl. Physiol. Nutr. Metab. 2017;42:563–570. doi: 10.1139/apnm-2016-0645. [DOI] [PubMed] [Google Scholar]
- 41.Beyer K.S., Fukuda D.H., Boone C.H., Wells A.J., Townsend J.R., Jajtner A.R., Gonzalez A.M., Fragala M.S., Hoffman J.R., Stout J.R. Short-Term Unilateral Resistance Training Results in Cross Education of Strength without Changes in Muscle Size, Activation, or Endocrine Response. J. Strength Cond. Res. 2016;30:1213–1223. doi: 10.1519/JSC.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 42.Hortobágyi T., Lambert N.J., Hill J.P. Greater Cross Education Following Training with Muscle Lengthening than Shortening. Med. Sci. Sports Exerc. 1997;29:107–112. doi: 10.1097/00005768-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Lee M., Carroll T.J. Cross Education: Possible Mechanisms for the Contralateral Effects of Unilateral Resistance Training. Sport. Med. 2007;37:1–14. doi: 10.2165/00007256-200737010-00001. [DOI] [PubMed] [Google Scholar]
- 44.Munn J., Herbert R.D., Gandevia S.C. Contralateral Effects of Unilateral Resistance Training: A Meta-Analysis. J. Appl. Physiol. 2004;96:1861–1866. doi: 10.1152/japplphysiol.00541.2003. [DOI] [PubMed] [Google Scholar]
- 45.Bell Z.W., Wong V., Spitz R.W., Chatakondi R.N., Viana R., Abe T., Loenneke J.P. The Contraction History of the Muscle and Strength Change: Lessons Learned from Unilateral Training Models. Physiol. Meas. 2020;41:01TR01. doi: 10.1088/1361-6579/ab516c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study will are included in the published article as Table(s) and Figure(s). Any other data requirement can be directed to the corresponding author upon reasonable request.