Abstract
Pulmonary fibrosis is characterized by destruction and remodeling of the lung due to an accumulation of collagen and other extracellular matrix components in the tissue. This results in progressive irreversible decreases in lung capacity, impaired gas exchange and eventually, hypoxemia. A number of inhaled and systemic toxicants including bleomycin, silica, asbestos, nanoparticles, mustard vesicants, nitrofurantoin, amiodarone, and ionizing radiation have been identified. In this article, we review the role of innate and adaptive immune cells and mediators they release in the pathogenesis of fibrotic pathologies induced by pulmonary toxicants. A better understanding of the pathogenic mechanisms underlying fibrogenesis may lead to the development of new therapeutic approaches for patients with these debilitating and largely irreversible chronic diseases.
Keywords: fibrosis, inflammation, macrophages, immune cells, fibroblasts, toxicants, mustard vesicants
1. Introduction
Pulmonary fibrosis is a common pathologic outcome of chronic lung inflammation. It is characterized by thickening and scarring of the lung parenchyma which leads to irreversible loss of lung function and often death. This is thought to be the result of persistent or repeated injury to the alveolar epithelium which leads to dysregulated wound repair. As a consequence, there is uncontrolled proliferation of fibroblasts and differentiation into myofibroblasts, and excessive production of extracellular matrix (ECM) components (Desai et al., 2018; Dowman et al., 2014; Robert et al., 2016). A variety of drugs (e.g., amiodarone, nitrofurantoin, bleomycin) and toxicants (e.g., silica dust, asbestos, mustard vesicants, nanoparticles and other particulate matter), as well as ionizing radiation, have been shown to induce pulmonary fibrosis. Evidence suggests that cells of the innate and adaptive immune system and mediators they release are key contributors to pulmonary fibrosis induced by these diverse xenobiotics (Carrington et al., 2018; Kolahian et al., 2016). In this review, we describe the role of immune cells in the development of lung fibrosis following exposure to representative pulmonary toxicants.
2. Innate and adaptive immune cells in pulmonary fibrosis
2.1. Macrophages
Macrophages are phagocytic cells of the innate immune system (Laskin et al., 2019). Resident in all tissues of the body, most prominently in the lung and liver, they function as immune sentinels, poised to defend the body against invading pathogens and insults. Largely originating during embryonic development, resident macrophages are distinct from bone-marrow derived inflammatory macrophages that accumulate in tissues in response to injury and infection. It is mainly inflammatory macrophages that are involved in the development of lung fibrosis. These cells have been broadly classified as pro-inflammatory/cytotoxic M1 and anti-inflammatory/wound repair M2 macrophages, which develop in response to signals present in the tissue microenvironment. For example, while interferon (IFN)γ alone or in concert with toll-like receptor agonists, tumor necrosis factor (TNF)α or granulocyte-macrophage colony stimulating factor (GM-CSF), induce M1 macrophage activation, interleukin (IL)-4, IL-10, and IL-13 stimulate M2 macrophage activation. Evidence suggests that excessive activation of either M1 or M2 macrophages can lead to pathology and disease progression. In this regard, while overproduction of cytotoxic and proinflammatory mediators by M1 macrophages perpetuates tissue injury and inflammation, overactivation of M2 macrophages is associated with excessive release of profibrotic mediators and the development of pulmonary fibrosis (Laskin et al., 2019; Wynn and Vannella, 2016).
Both human and animal studies support a role of overactive inflammatory macrophages in pulmonary fibrosis. In patients with idiopathic pulmonary fibrosis (IPF) and cystic fibrosis, increased numbers of macrophages have been identified in fibrotic foci, a response correlated with a worsening disease prognosis (Withana et al., 2016; Zhang et al., 2018). Macrophage-derived cytokines and growth factors linked to fibrosis (Fig. 1) are increased in patients with fibrotic lung disease and in animal models of pulmonary fibrosis (Laskin et al., 2019; Luzina et al., 2013; Wynn and Vannella, 2016). Moreover, in rodent models, overexpression or administration of M2 macrophage inducing cytokines including IL-10, IL-13 or IL-33 is associated with exacerbated responses to fibrogenic toxicants such as bleomycin, radiation and silica (Barbarin et al., 2005b; Chung et al., 2016; Li et al., 2014; Lumsden et al., 2015; Luzina et al., 2013). Conversely, collagen production and fibrosis in response to radiation, bleomycin or carbon nanotubes, are reduced in mice lacking these cytokines, or C/EBP homologous protein, which is required for M2 macrophage activation and tumor growth factor (TGF)β production (Chung et al., 2016; Laskin et al., 2019; Liu et al., 2004; Wang et al., 2014; Zhao et al., 2018). Additionally, depletion or inhibition of M2 macrophages by chlodronate liposomes or anti-colony stimulating factor (CSF)1R antibody attenuates lung fibrosis induced by bleomycin or radiation (Laskin et al., 2019; Meziani et al., 2018). Suppression of the Wnt/β-catenin signaling pathway by M2 macrophages also inhibits myofibroblast differentiation in a bleomycin-induced pulmonary fibrosis rodent model (Cao et al., 2018).
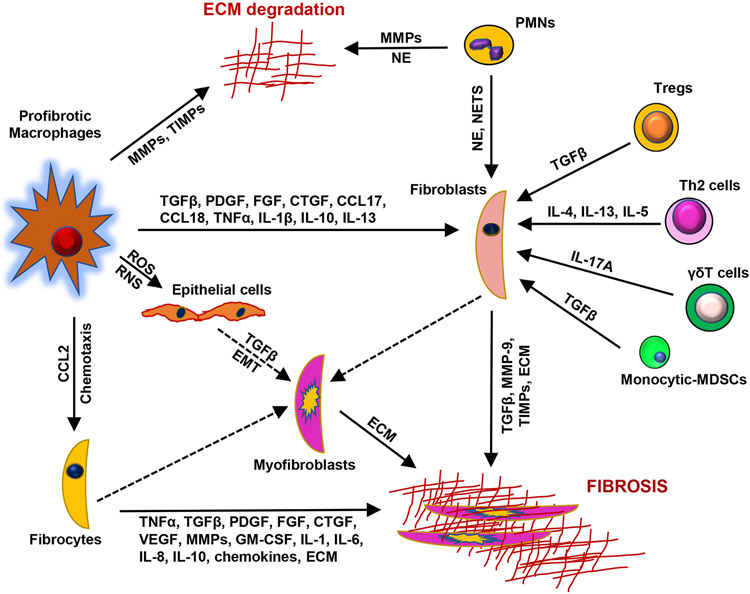
Fig. 1.
Immune cells and mediators in pulmonary fibrosis. Epithelial cell injury initiates a chain of events leading to activation and recruitment of immune cells, epithelial to mesenchymal cell transition (EMT), fibroblasts activation and differentiation into myofibroblasts, and ultimately, abnormal deposition of extracellular matrix (ECM). Macrophages contribute to fibrosis by releasing reactive oxygen and nitrogen species (ROS, RNS), cytokines (e.g., TNFα, IL-1, IL-6, IL-8, IL-13, TGFβ), chemokines (e.g., CCL2, CCCL17, CCL18), and growth factors (e.g., PDGF, CTGF, FGF, GM-CSF). ECM degradation and accumulation are mediated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), neutrophil elastase (NE) and other proteases derived from activated macrophages and neutrophils. NE and neutrophil-derived neutrophil extracellular traps (NETs) contribute to fibrosis by activating fibroblasts. TGFβ is produced by macrophages, epithelial cells, fibroblasts and T cells, and participates in fibrogenesis by inducing fibroblast activation and differentiation. IL-13, produced by Th2 cells and macrophages also upregulates TGFβ. TNFα and IL-1 induce production of TGFβ, as well as expression of MMPs, PDGF and GM-CSF which are important in tissue remodeling and fibrogenesis. Macrophage-derived chemokines promote the recruitment of fibrocytes which differentiate into ECM producing myofibroblasts. Fibrocytes also secrete profibrotic growth factors, cytokines, MMP-9, and ECM components which contribute to the progression of fibrosis. T cells modulate fibrogenesis by releasing Type II cytokines and IL-17A whereas monocytic myeloid-derived suppressor cells (MDSCs) regulate immune responses by modulating the activity of T cells in fibrosis. SMA, smooth muscle actin; Th2: T-helper cell type 2; Treg, regulatory T cell, VEGF, vascular endothelial growth factor. Dashed lines represent cell differentiation.
2.2. Neutrophils
Neutrophils or polymorphonuclear leukocytes (PMN) are short-lived bone marrow derived myeloid cells involved in nonspecific host defense and inflammation. Evidence suggests that they also contribute to the development of pulmonary fibrosis. Numbers of neutrophils and levels of the neutrophil chemoattractant CXCL8, are increased in bronchoalveolar lavage (BAL) from IPF patients (Desai et al., 2018; Kinder et al., 2008). Importantly, BAL neutrophilia is considered a prognostic marker of early mortality in IPF (Desai et al., 2018; Kinder et al., 2008). Depletion of neutrophils has also been reported to result in reduced inflammation and fibrosis in an experimental model of pulmonary fibrosis induced by bleomycin or repeated administration of Saccharopolyspora rectivirgula (Hasan et al., 2013; Leslie et al., 2020). Neutrophil elastase is also increased in BAL and serum from patients with IPF (Kolahian et al., 2016; Sugino et al., 2016). Neutrophil elastase degrades ECM components and induces fibroblast proliferation and myofibroblast differentiation (Desai et al., 2018; Gregory et al., 2015; Kolahian et al., 2016). Decreases in neutrophil elastase are associated with impaired TGFβ production and reduced numbers of inflammatory cells, fibroblasts and myofibroblasts at sites of injury; pulmonary fibrosis is also attenuated (Chua et al., 2007; Gregory et al., 2015; Takemasa et al., 2012). Neutrophils also release profibrotic matrix metalloproteinases (MMPs) including MMP-8 and MMP-9, which may contribute to their effects on ECM homeostasis (Kolahian et al., 2016).
Neutrophil extracellular traps (NETs) are extracellular mesh composed of chromatin and neutrophil granular proteins including histones, myeloperoxidase, neutrophil elastase and α-defensins. They are released by neutrophils in response to pathogens or proinflammatory stimuli (Kruger et al., 2015; Porto and Stein, 2016). NETs have been identified in lungs of patients with fibrotic interstitial lung disease (ILD) and in mice following bleomycin administration (Chrysanthopoulou et al., 2014; Zhang et al., 2014). In the lungs of patients with ILD, NETs exist in close proximity to α-smooth muscle actin (SMA)+ fibroblasts. It has been reported that NETs induce lung fibroblast activation and differentiation into myofibroblasts; moreover, in response to NETs, myofibroblasts upregulate connective tissue growth factor (CTGF), and increase collagen production, proliferation and migration (Chrysanthopoulou et al., 2014). These responses are blunted by DNase, heparin or myeloperoxidase inhibitor which degrade NETs.
2.3. Fibrocytes
Fibrocytes are circulating bone marrow derived mesenchymal progenitor cells with an ability to produce ECM (Kolahian et al., 2016; Quan et al., 2006). These cells express stem cell and hematopoietic markers (CD34 and CD45), monocyte markers (CD11 and CD14), and various chemokine receptors; they also express collagen-1 and HLA-DR (Desai et al., 2018; Herzog and Bucala, 2010; Kolahian et al., 2016). Fibrocytes accumulate in the lung in response to CCL2, CCL12 and CXCL12 chemokines, and differentiate into fibroblasts and myofibroblasts. In IPF, increases in circulating fibrocytes are correlated with the appearance of intrapulmonary fibrocytes within fibrotic foci (Herzog and Bucala, 2010; Heukels et al., 2018; Moeller et al., 2009). Fibrocytes secrete a multitude of profibrotic growth factors (vascular endothelial growth factor [VEGF], platelet-derived growth factor [PDGF]), cytokines (TNFα, IL-10), MMP-9, as well as ECM proteins including collagen, vimentin and fibronectin which contribute to the development and progression of fibrosis (Desai et al., 2018; Herzog and Bucala, 2010; Kolahian et al., 2016).
2.4. T lymphocytes
T lymphocytes are part of the adaptive immune system. They consist of subpopulations with distinct functions including T-helper (Th) cells (Th1, Th2, Th17), regulatory T cells (Tregs), and cytotoxic T cells. Activated T lymphocytes have been observed in the lungs and BAL of patients with IPF, as well as in rodent models of pulmonary fibrosis (Daniil et al., 2005; Luzina et al., 2008; Parra et al., 2007). Moreover, depletion of T cells has been reported to reduce pulmonary fibrosis induced by bleomycin (Luzina et al., 2008). It has been suggested that fibrosis is a result of alterations in the balance between Th1 and Th2 cells and related cytokines (Luzina et al., 2008; Wynn, 2004). Th2 cytokines including IL-4, IL-13 and IL-5 are profibrotic, whereas Th1 mediators including IFNγ and IL-12 are antifibrotic (Desai et al., 2018; Luzina et al., 2008; Wynn, 2004). Overexpression of Th2 transcription factor, GATA-3 or loss of T-bet, a Th1 transcription factor, results in exacerbation of pulmonary fibrosis induced by bleomycin (Desai et al., 2018; Kolahian et al., 2016).
Tregs are known to suppress adaptive immune responses, an activity linked to fibrogenesis (Peng et al., 2014; Piccirillo, 2008). However, the precise role of Tregs in lung fibrosis is unclear as both profibrotic and antifibrotic activities have been described (Birjandi et al., 2016; Desai et al., 2018; Garibaldi et al., 2013; Re et al., 2014; Trujillo et al., 2010). Thus, while in some studies reduced numbers and lower suppressor activity of Tregs have been reported in lungs of IPF patients, in others, increases in Tregs and the TGFβ/IL-17 ratio have been described (Galati et al., 2014; Kolahian et al., 2016; Kotsianidis et al., 2009). It has been suggested that the profibrotic actions of Tregs are limited to early stages of the pathogenic process as they stimulate TGFβ1 and collagen production; conversely, at later stages they are antifibrotic (Boveda-Ruiz et al., 2013; Desai et al., 2018). This suggests that Tregs acquire fibrosis-suppressive or fibrosis-stimulatory activity depending on the local tissue environment, which changes during the progression of the disease.
2.5. Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells with potent immunosuppressive activity (Lebrun et al., 2017). MDSCs regulate immune responses by suppressing T cell activation and proliferation, and by promoting the activity of Tregs (Bronte et al., 2016; Desai et al., 2018). Monocytic-MDSCs isolated from mice treated with silica express TGFβ1; likewise, depletion of these cells reduced expression of TGFβ1 and TIMP-1, and inhibited collagen accumulation, indicating a profibrotic role of these cells (Lebrun et al., 2017). Increased numbers of circulating MDSCs have been correlated with poor lung function in IPF patients (Fernandez et al., 2016). MDSCs could therefore represent a novel therapeutic target in pulmonary fibrosis.
3. Inflammatory mediators implicated in pulmonary fibrosis
A multitude of inflammatory mediators including reactive oxygen species (ROS), reactive nitrogen species (RNS), proteases, cytokines, chemokines and growth factors regulate fibrogenesis (Table 1). These mediators have been identified in lungs of patients with fibrosis and in animal models of toxicant-induced fibrosis. Recruited inflammatory and immune cells and resident macrophages and epithelial cells not only release these mediators, they also respond to them, perpetuating the proinflammatory and/or profibrotic response (Fig. 1).
Table 1.
Inflammatory Mediators and Pulmonary Fibrosis.
Abbreviations: CCL, C-C chemokine ligand; CXCL, C-X-C chemokine ligand; CTGF, connective tissue growth factor; ECM, extracellular matrix; EMT, epithelial mesenchymal transition; GM-CSF, granulocyte-macrophage colony stimulating factor; IL, interleukin; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; SMA, smooth muscle actin; TGF, tumor growth factor; TIMP, tissue inhibitors of metalloproteinases; TNF, tumor necrosis factor.
3.1. Reactive oxygen species and reactive nitrogen species
ROS and RNS including superoxide anion, hydrogen peroxide, hydroxyl radicals, nitric oxide, and lipid peroxides are generated by macrophages and/or neutrophils during inflammatory responses (Table 1). ROS also react with nitric oxide producing peroxynitrite, a potent oxidant. ROS and RNS are known to cause DNA damage and apoptosis in lung epithelial cells; this can trigger the release of TGFβ1 (Cheresh et al., 2013). Treatment of epithelial cells with TGFβ reduces intracellular glutathione and stimulates ROS production and epithelial-mesenchymal transition (EMT), a key step in fibrogenesis (Felton et al., 2009). NADPH oxidase (NOX), which catalyzes the formation of ROS, is upregulated in lung fibroblasts isolated from patients with IPF, and in lung mesenchymal cells in response to TGFβ (Amara et al., 2010; Hecker et al., 2009). NOX4 is required for TGFβ-mediated upregulation of α-SMA and fibronectin in mesenchymal cells and induces senescence and apoptosis resistance in IPF fibroblasts (Amara et al., 2010; Hecker et al., 2014; Wynn, 2011). Inhibition of NOX4 activity by antioxidants or NOX4-specific small interfering RNA prevents myofibroblast activation and lung fibrosis (Carnesecchi et al., 2011; Hecker et al., 2009).
3.2. Proteases
MMPs and their inhibitors (e.g., tissue inhibitor of metalloproteinases [TIMPs]) have emerged as key mediators of lung fibrosis (Robert et al., 2016). They contribute to lung remodeling by stimulating ECM degradation, EMT, the development of profibrotic macrophages and their production of profibrotic mediators, and by promoting fibrocyte migration to sites of injury, and aberrant wound repair (Craig et al., 2015; Jin et al., 2014; Menou et al., 2018; Yang et al., 2007). Levels of MMP-1, MMP-2, MMP-7, MMP-9 and MMP-13 are upregulated in the lungs of IPF patients (Menou et al., 2018). Animal models of toxicant-induced lung fibrosis are associated with increases in MMP-2, MMP-9, MMP-12, MMP-13, TIMP-1 and/or TIMP-2 (Table 1). Additionally, mice lacking MMP-9 or MMP-12 are protected from bleomycin-induced lung fibrosis (Menou et al., 2018; Nkyimbeng et al., 2013). Similarly, MMP-13 knock-out mice are protected from radiation-induced pulmonary fibrosis (Flechsig et al., 2010). It appears that the precise role of MMPs and TIMPs in tissue remodeling is complex and likely depends on the nature of the toxicant, the tissue microenvironment, and the MMP/TIMP ratio.
3.3. Cytokines, chemokines and growth factors
Diverse cytokines, chemokines and growth factors have been implicated in the development of pulmonary fibrosis. Major mediators are described below and summarized in Table 1. TNFα is released mainly by macrophages; however, epithelial cells, eosinophils, and mast cells also have the capacity to release TNFα. TNFα has been reported to play a role in both the initiation and progression of fibrosis (Malaviya et al., 2017). During the initiation phase, TNF functions to activate and recruit leukocytes to inflammatory sites, stimulate the generation of inflammatory mediators, and induce oxidative and nitrosative stress. Subsequently, TNFα upregulates expression of MMPs, PDGF, GM-CSF and TGFβ which are important in tissue remodeling and fibrogenesis (Malaviya et al., 2017; Sullivan et al., 2009). Increased expression of TNFα and soluble TNF receptors have been observed in the lungs of patients with IPF and in animal models of pulmonary fibrosis (Duke and Bonner, 2018; Kawasaki, 2015; Malaviya et al., 2017; Williamson et al., 2015). Moreover, targeting TNFα inhibits TGFβ expression and attenuates pulmonary fibrosis (Malaviya et al., 2015; Zhang et al., 2008).
IL-1 is an inducible cytokine known to be a key mediator of inflammation and fibrosis (Borthwick, 2016). In acute injury, IL-1 stimulates neutrophilia, myeloperoxidase activity and inflammation, while in chronic injury, it augments the release of TGFβ, which triggers activation, proliferation, and differentiation of fibroblasts into collagen-producing myofibroblasts. Increased production of both isoforms of IL-1 (IL1α and IL-1β) has been observed by alveolar macrophages, epithelial, and endothelial cells in lungs of IPF patients, and in rodent models of pulmonary fibrosis (Borthwick, 2016; Hoshino et al., 2009; Wilson et al., 2010). Moreover, mice deficient in IL-1 or IL-1 signaling molecules, or treated with IL-1 binding protein are resistant to bleomycin or silica-induced fibrosis (Borthwick, 2016; Gasse et al., 2007; Kolahian et al., 2016). Overexpression of IL-1β in rat lung results in upregulation of IL-6, TNFα, PDGF, TGFβ, and evidence of fibrosis characterized by the presence of myofibroblasts, fibroblast foci, and increases in collagen and fibronectin (Kolb et al., 2001).
IL-17 is a proinflammatory cytokine released by Th17 cells and γδ T cells that promotes lung injury and fibrosis by inducing neutrophilic inflammation, and airway remodeling following chronic injury. Increased levels of IL-17 have been identified in BAL of IPF patients (Wilson et al., 2010). In rodents, pulmonary fibrosis induced by bleomycin, silica or IL-1β involves IL-17A. Thus, mice lacking IL-17A have reduced fibrosis (Re et al., 2014; Wilson et al., 2010). In an experimental model of hypersensitivity pneumonitis, IL-17A is also expressed by neutrophils and macrophages; additionally, depletion of neutrophils is associated with alleviation of lung fibrosis (Hasan et al., 2013). Evidence suggests that TGFβ promotes fibrosis by upregulating IL-17A and blocking IL-17A not only attenuates bleomycin- or silica-induced lung fibrosis in mice, but also increases survival (Chen et al., 2014; Kolahian et al., 2016; Mi et al., 2011; Wilson et al., 2010).
Th2 cytokines including IL-4 and IL-13 have been shown to be important in the later phase of fibrogenesis upregulating MMPs, growth factors and chemokines, and stimulating fibroblast activation and ECM production and deposition. Levels of these cytokines and their receptors are upregulated in lungs of patients with pulmonary fibrosis, supporting a role for these cytokines in the disease (Park et al., 2009; Wynn, 2004). Fibroblasts respond to IL-4 or IL-13 by proliferating and differentiating into myofibroblasts, and by producing α-SMA and collagen (Batra et al., 2004; Hashimoto et al., 2001; Kolahian et al., 2016). Furthermore, silica- or bleomycin-induced fibrosis is inhibited in mice lacking IL-4, IL-4 receptor, IL-13, or treated with agents that block IL-13 (Ferreira et al., 2013; Lumsden et al., 2015). Both IL-4 and IL-13 are known to promote a type 2 cytokine environment and induce reprogramming of macrophages to an anti-inflammatory/profibrotic phenotype (Gessner and Rollinghoff, 2000; Laskin et al., 2019).
Generated by activated macrophages, epithelial cells, lymphocytes, fibroblasts, and endothelial cells, CC and CXC chemokines contribute to fibrosis by recruiting leukocytes, fibrocytes, and other profibrotic cells to sites of tissue injury (Palomino and Marti, 2015). Chemokines CCL2, CCL3 and CCL6 are chemotactic for mononuclear phagocytes and have been implicated in fibrosis induced by bleomycin, asbestos and radiation (Beach et al., 2020; Osafo-Addo and Herzog, 2017; Wynn, 2008). Thus, while treatment of mice with antibodies to CCL2, CCL3 or CCL6 abrogates bleomycin-induced fibrosis, mice lacking receptors for these chemokines (CCR2 and CCR1) or deficient in CCL2 are protected from bleomycin or radiation-induced lung fibrosis (Baran et al., 2007; Beach et al., 2020; Gharaee-Kermani et al., 2003; Wynn, 2008). CCL17 and CCL22 chemokines are also upregulated in lungs of patients with IPF and in rodents treated with bleomycin or radiation (Belperio et al., 2004; Inoue et al., 2004; Kolahian et al., 2016; Yogo et al., 2009). Increased levels of these chemokines correlate directly with elevations in lymphocytes and/or macrophages in the lung. High levels of chemokines including CCL2, CCL17 and CCL22 in BAL have been associated with poor outcome in patients with IPF (Shinoda et al., 2009). CXC chemokines (CXCL1, CXCL2 CXCL6, CXCL12 and CX3CL1) are also involved in promoting the trafficking of leukocytes to the site of fibrogenesis. In addition, these chemokines play a role in vascular remodeling during fibrogenesis (Strieter et al., 2007). It has been reported that fibrocytes migrate to bleomycin-injured lungs in response to CXCL12 and that this contributes to the pathogenesis of fibrosis (Phillips et al., 2004). CXCL12 also upregulates CTGF expression in pulmonary fibroblasts by binding to cell surface receptor CXCR4 (Lin et al., 2018; Luzina et al., 2015; Strieter et al., 2007). Similarly, the neutrophil chemoattractant, CXCL6, is elevated in patients with fibrosis and in lungs of bleomycin exposed mice (Besnard et al., 2013). Blocking CXCL6 in these animals is associated with reduced neutrophil and lymphocyte influx into the lung and attenuation of collagen production and fibrosis.
Growth factors, most notably TGFβ, PDGF and CTGF, are produced in the lung by a variety of cells during the development of fibrosis (Table 1). These mediators play a central role in the expansion of myofibroblasts by stimulating fibroblast proliferation, differentiation, migration and survival (Kolahian et al., 2016; Wynn, 2008). In human lungs, levels of these growth factors correlate directly with the extent of fibrosis (Wollin et al., 2015). PDGF and CTGF are mainly produced by macrophages and alveolar epithelial cells; they contribute to the recruitment of myofibroblast precursors to fibrotic lesions, myofibroblast expansion and survival. TGFβ promotes myofibroblast differentiation, induces α-SMA expression, collagen and fibronectin production, and promotes the recruitment of inflammatory cells to the sites of injury by stimulating fibroblasts to release CCL2 (Kolahian et al., 2016; Luzina et al., 2015; Williamson et al., 2015). Increased levels of TGFβ have been described in human fibrotic diseases, as well as in animal models of fibrosis where they regulate the generation of myofibroblasts and promote EMT (Jolly et al., 2018; Thomas et al., 2016). Overexpression of TGFβ in experimental rodent models results in progressive pulmonary fibrosis, upregulation of protease inhibitors (TIMP and plasminogen activator inhibitor 1) and excessive ECM accumulation in tissues (Bonniaud et al., 2004; Gauldie et al., 2006). Additionally, blocking TGFβ with soluble TGFβ receptor-Fc or by inhibiting Smad signaling mitigates fibrosis induced by IL-13 or bleomycin (Lee and Lane, 2001; Yang et al., 2019).
4. Fibrogenic pulmonary toxicants
4.1. Bleomycin
Bleomycin is a bacteria-derived glycopeptide used in cancer chemotherapy; however, its use is limited by pulmonary fibrosis, first described as an adverse effect in the 1970s (Hainan et al., 1972) Bleomycin induces DNA strand breaks in a process dependent on oxygen and iron. This leads to the production of cytotoxic ROS, lipid peroxidation, and damage to the alveolar epithelium. Inflammation is a prominent feature of bleomycin-induced injury, and a key contributor to the development of fibrosis.
Bleomycin is one of the most widely used agents for experimental induction of pulmonary fibrosis due to its ability to cause a histopathologic pattern of lung injury similar to that observed in humans undergoing chemotherapy (Della Latta et al., 2015). In rodents, the precise pathologic response and outcome varies with the route and frequency of bleomycin administration (Table 2). A commonly utilized model involves administration of a single dose of bleomycin delivered intratracheally to mice. This leads to alveolar epithelial cell damage and inflammation within 3 d - 7 d. The onset of fibrosis, characterized by EMT, fibroblast proliferation and differentiation into myofibroblasts and the synthesis of ECM proteins, is typically observed by day 14, with a maximal response at 21 d - 28 d (Carrington et al., 2018; Della Latta et al., 2015). The different stages of bleomycin-induced injury and fibrosis are associated with the appearance of distinct populations of inflammatory macrophages in the lung which produce inflammatory mediators and growth factors important in regulating the pathogenic process. Thus, during the initial acute phase (up to 7 d), characterized by pneumonitis and the appearance of proinflammatory M1 macrophages, oxidant generating enzymes and cytotoxic oxidants (e.g., NOX, hydrogen peroxide), proinflammatory cytokines (e.g. TNFα, IL-1α, IL-1β, IL-2, IL-6 and IFNγ) and chemokines (CCL2, CCL3) predominate (Table 1) (Chaudhary et al., 2006; Williamson et al., 2015). Subsequently, as anti-inflammatory/profibrotic M2 macrophages accumulate in the lung (14 d - 28 d), anti-inflammatory cytokines (IL-4, IL-13) and growth factors (TGF-β, CTGF and PDGF) are generated that promote tissue remodeling and fibrogenesis (Chaudhary et al., 2006; Della Latta et al., 2015; Williamson et al., 2015).
Table 2.
Toxicant-induced Pulmonary Fibrosis in Rodents.
| Toxicant | Animal | Dose and Route | Frequency (x) | Time to fibrosis |
References |
|---|---|---|---|---|---|
| BLEOMYCIN | |||||
| Mouse, Rat | 1–5 mg/kg (IT) | 1× | 2–4 wk | Chaudhary et al., 2006; Della Latta et al., 2015; Gendron et al., 2017; Tanaka et al., 2017 | |
| Mouse | 0.04–5 mg/kg (IT) | 2× (day 0 and day 8), or 2×/wk., 8 doses | 2–3 wk | Degryse et al., 2010; Lin et al., 2017 | |
| Mouse | 3 mg/kg (IN) | 1× | 2–3 wk | Wollin et al., 2014 | |
| Mouse | 100–150 mg/kg (IV) | 1×−4× (day 0–21) | 4 wk | Azuma et al., 2005; Hoshino et al., 2009 | |
| Mouse, Rat | 15–100 mg/kg (IP) | 1×−;12× (day 0–28) | 4 wk | El-Medany et al., 2005; Hoshino et al., 2009 | |
| Mouse, Rat | 100–150 mg/kg (SQ) | 1–4 wk. (continuous infusion) | 2–5 wk | Aono et al., 2012; Inomata et al., 2014; Kotani et al., 2017; Liang et al., 2015 | |
| ASBESTOS | |||||
| Chrysotile | Mouse, Rat | Aerosolized 0.6–10 mg/m3 (INH) | 5–6 h/day, 5 day/wk., 3, 9, 28 or 40 days | 2–8 wk | Bernstein et al., 2018; Haegens et al., 2007; Shukla et al., 2007 |
| Chrysotile | Mouse | 5 mg/kg (IT) | 1× | 3 wk | Murthy et al., 2015 |
| Crocidolite | Mouse, Rat | 5–8 mg/kg (IT) | 1× or 2× (2 min apart) | 3–8 wk | Jablonski et al., 2017; van den Brule et al., 2014 |
| Crocidolite | Mouse | 6 mg/kg (OP) | 1× | 4–52 wk | Dymacek et al., 2018; Snyder-Talkington et al., 2016 |
| Crocidolite | Rat | Aerosolized 0.6–1.73 mg/m3 (INH) | 6 h/day, 5 day/wk., 4 wk | 2 wk | Bernstein et al., 2018 |
| SILICA | |||||
| Mouse, Rat | 15–400 mg/kg (IT) | 1× | 1–9 wk | Barbarin et al., 2005a; Lakatos et al., 2006; Li et al., 2017 | |
| Mouse | 125–800 mg/kg (IN) | 1× | 4–24 wk | Sugimoto et al., 2019; Wollin et al., 2014 | |
| Mouse | 100–125 mg/kg (OP) | 1× | 3–8 wk | Lakatos et al., 2006; Re et al., 2014 | |
| Rat | 15 mg/m3 (INH) | 6 h/day, 5 day/wk., 1–17 wk | 6–17 wk | Castranova et al., 2002 | |
| IONIZING RADIATION | |||||
| Mouse, Rat | 15–28 Gy (thorax/hemithorax) | 1× | 1–30 wk | Anscher et al., 2008; Beach et al., 2020; Bickelhaupt et al., 2017; Jiang et al., 2015; Wang et al., 2017 | |
| NANOPARTICLES | |||||
| SWCNT | Mouse | 0.2–1.0 mg/kg (OP) | 1× | 4 wk | Shvedova et al., 2008 |
| SWCNT | Mouse | 5 mg/m3 (INH) | 4× (5 h/day, 4 days) | 1 d-4 wk | Shvedova et al., 2008 |
| SWCNT | Rat | 2 mg/kg (IV) | 1×/day (1–90 days) | 4–17 wk | Qin et al., 2017 |
| MWCNT | Mouse | 0.2–4.0 mg/kg (OP) | 1× | 1 d-52 wk | Dong et al., 2015; Shvedova et al., 2008; Dymacek et al., 2018; Snyder-Talkington et al., 2016 |
| MWCNT | Mouse | 8.0–10.0 mg/kg (IT) | 1× | 4 wk | Bai et al., 2018; Ma et al., 2017; Nikota et al., 2017; van den Brule et al., 2014 |
| NiO | Mouse | 0.5–5.0 mg/kg (IT) | 1× | 4 wk | Bai et al., 2018 |
| CeO2 | Rat | 0.2–7.0 mg/kg (IT) | 1× | 1 d-4 wk | Ma et al., 2017 |
| MUSTARD VESICANTS | |||||
| NM | Mouse, Rat | 0.1–1.2 mg/kg (IT) | 1× | 4–13 wk | Ekstrand-Hammarstrom et al., 2011; Gustafsson et al., 2014; Malaviya et al., 2015; Malaviya et al., 2012; Sunil et al., 2020; Wigenstam et al., 2009 |
| SM | Rat | 150 mg/m3, 10 min; 0.4–1 mg/kg (INH) | 1× | 2–6 wk | Malaviya et al., 2020a; McGraw et al., 2018; Mishra et al., 2012 |
Abbreviations: CeO2, Cerium oxide; IN, intranasal; INH, inhalation; IP, intraperitoneal; IT, intratracheal; IV, intravenous; MWCNT, multi wall carbon nanotubes; NiO, nickel oxide; NM, nitrogen mustard; OP, oropharyngeal aspiration; SWCNT, single wall carbon nanotubes; SM, sulfur mustard; SQ, subcutaneous.
4.2. Asbestos
Asbestos is the common name for six different naturally occurring silicate mineral fibers which occur in both a serpentine shape (chrysotile) and a rigid needle-like shape known as amphiboles. Both types were highly valued for their fire proofing and thermal and acoustic insulation properties and used ubiquitously in construction and industrial insulation from the later 19th century until the mid1970's. Prolonged inhalation of asbestos (> 10 years) is associated with diffuse interstitial pulmonary fibrosis (asbestosis). Asbestos exposure related fibrosis was first described in the early 1900s, and was termed as “asbestosis” by Cooke in 1927 (Kamp, 2009). Asbestosis has been noted in occupational settings, as well as in cohabitants of exposed workers.
Various rodent models of asbestos inhalation or intratracheal instillation have been utilized to understand the mechanism of asbestosis in humans (Table 2). Exposure to asbestos is associated with activation of alveolar macrophages which phagocytize asbestos particles. This results in upregulation of inducible nitric oxide synthase (iNOS) and increased production of RNS, as well as ROS (Kamp, 2009; Liu et al., 2013). Asbestos-induced nitrosative and oxidative stress contribute to lung injury by inducing DNA damage, lipid peroxidation, protein oxidation, and activation of transcription factors including nuclear factor (NF)-κB and AP-1 in mesothelial cells, macrophages, and lung epithelial cells. This leads to upregulation of inflammatory cytokines (e.g., TNFα, IL-1α, IL-1β, IL-6 and IL-12), and chemokines (e.g., CCL2, CCL4 and CCL5) (Table 1) (Haegens et al., 2007; Shukla et al., 2007; Shukla et al., 2003). Long-term asbestos exposure results in increases in profibrotic cytokines and an accumulation of ECM proteins in lung and fibrotic lesions. This is facilitated by macrophage and epithelial cell production of fibrogenic growth factors including PDGF and TGFβ (Table 1). It has been suggested that iron on the surface of asbestos particles, and/or iron-mediated production of ROS plays a key role in inflammatory mediator release in both acute injury and chronic lung remodeling as a treatment with an oxide radical scavenger or an iron chelator attenuates the fibrotic effects of asbestos in rodent lung (Kamp, 2009; Shukla et al., 2003).
4.3. Silica
Silica or silicon dioxide (SiO2) refers to amorphous crystalline material found in many types of rock (e.g., granite and quartz). Among the most common forms are “sand” associated with sand blasting and foundry work, but only fine particles less than 2.5 μm are respirable and of toxicological concern. Occupational exposure to silica as low as 0.1 mg/m3 may cause respiratory diseases (Cohen et al., 2008). Thus, workers involved in silica flour mills, highway repair, masonry, concrete work, and abrasive blasting operations are at high risk for developing silicosis. The pathological response to silica is distinct from asbestos, as fibrosis appears in the form of nodules with a hyaline center, incorporation of surrounding airways and parenchyma with birefringent particles visible under polarized light microscopy within the nodules. These are observed as radiographically visible nodules of 1–10 mm in size. In its most common form of simple silicosis, the latency for observing visible radiographic nodules is about 10 years. Further agglomeration in accelerated silicosis produces progressively larger nodules associated with pulmonary function abnormalities and respiratory symptoms.
Exposure of rodents to silica dust through aerosol inhalation, intratracheal instillation or oropharyngeal aspiration results in the development of fibrotic nodules in the lung that resemble pulmonary lesions in humans following occupational exposure (Moore and Hogaboam, 2008). It is thought that the sticky nature of silica and its impaired clearance by phagocytic cells contributes to a persistent, nonresolving inflammatory response. Silica-induced inflammation, characterized by an accumulation of macrophages, neutrophils and lymphocytes in the lung, and the release of proteolytic enzymes and oxidants are thought to contribute to lung injury and DNA damage (Kawasaki, 2015). Macrophages and neutrophils responding to silica induced injury release proinflammatory cytokines, chemokines, and growth factors (Table 1) that amplify alveolitis and activate fibroblasts (Kawasaki, 2015; Re et al., 2014). The fibrotic response is aided by immunosuppressive Foxp3+ regulatory T lymphocytes and IL-17-producing Th17 lymphocytes (Re et al., 2014). Interestingly, mechanistic differences in the development of silicosis have been observed to vary between rodent species. Thus, in mice, silica-induced fibrosis is associated with significant increases in profibrotic cytokines including IL-4, IL-13, and IL-10 which stimulate fibroblasts to proliferate and/or produce extracellular matrix proteins. In contrast, in rat lung silicosis is a consequence of a chronic inflammatory response associated with overproduction of TNFα and IL-1, which are both proinflammatory and profibrotic (Kawasaki, 2015).
4.4. Nanoparticles
Nanoparticles (ultrafine particles) range in size from 1 to 100 nm. In addition to being components of particulate air pollution, they are being engineered for broad use in medicine and many other applications. Because of their small size, nanoparticles readily localize in the lower airways and the lung, where, depending on their chemical composition and physical structure, cause acute inflammatory injury that can progress to fibrosis. The severity of the fibrogenic response to nanoparticles is determined by various physicochemical properties including residual metal catalyst content, rigidity, length, aggregation status, and surface charge (Duke and Bonner, 2018).
Multiple studies in rodents using single- or multi-wall nanotubes or metal nanoparticles have demonstrated inflammatory cell driven pulmonary fibrosis (Table 2). These particles damage the epithelial barrier and modulate the alveolar microenvironment resulting in persistent inflammation. Nanoparticle-induced fibrosis involves phagocytosis of nanoparticles, followed by alveolar macrophage activation, and the release of ROS and RNS leading to DNA damage and the production of proinflammatory cytokines (TNFα, IL-1β), chemokines (CCL3, CCL5, CXCL2), matrix metalloproteinases (MMP-2, MMP-9), and tissue inhibitor of metalloproteinases (TIMP-1 and TIMP-2) (Table 1) which together, cause lung injury and promote inflammation (Duke and Bonner, 2018; Qin et al., 2017). Profibrotic mediators (PDGF and TGFβ) released by alveolar macrophages and epithelial cells promote fibroblast activation, proliferation and differentiation, as well as EMT of alveolar epithelial cells leading to increases in lung α-SMA and fibrosis (Duke and Bonner, 2018). Other experimental models of nanoparticle-induced pulmonary fibrosis have been described (e.g., cerium oxide, nickel oxide) with similar pathogenesis (Table 2).
4.5. Mustard vesicants
Mustard vesicants including sulfur mustard (SM) and nitrogen mustard (NM) have been classified as chemical warfare agents. SM was synthesized in 1860 by Guthrie, who also observed its vesicating effect on skin (Shakarjian et al., 2010). First used during World War 1, SM has more recently been used during the Iran-Iraq war in 1980s, and in Syrian attacks on insurgents and civilians in 2016 and 2017. The lipophilic nature of SM results in rapid penetration of tissues and cells and reactions with lipids, proteins, and/or nucleic acids leading to acute tissue injury (Malaviya et al., 2015). Pulmonary toxicity of mustards is responsible for most morbidity and mortality in exposed victims; both conducting and respiratory airways are affected as well as the lower lung. Mustard-induced lung injury is progressive and leads to long-term pathologies like chronic obstructive pulmonary disease, asthma, bronchiectasis, and pulmonary fibrosis (Graham and Schoneboom, 2013).
Exposure of rodents to SM or NM causes acute bronchiolar and alveolar epithelial injury, hemorrhage, leukocyte accumulation, bronchiolization of alveolar walls and substantial increases in inflammatory proteins (iNOS, cyclooxygenase-2, TNFα, IL-6, IL-12, MMP-9) in the lung within 3 d of exposure (Malaviya et al., 2020a; Mishra et al., 2012). Subsequently, multifocal lesions characterized by perivascular and peribronchial edema, luminal accumulation of cellular debris, and multiple areas of fibrosis containing collagen fibers develop typically within 3–4 weeks of exposure (Malaviya et al., 2020b; Wigenstam et al., 2009). Mustard-induced injury and fibrosis are associated with a prominent macrophage-dominant inflammatory response. Whereas during the acute injury response, macrophages are mainly proinflammatory, during the chronic phase, enlarged and highly vacuolated anti-inflammatory/profibrotic macrophages are prominent, mainly in clusters in fibrotic foci (Malaviya et al., 2012; Mishra et al., 2012; Venosa et al., 2016). The chronic phase of respiratory injury is characterized by a predominance of profibrotic cytokines including TNFα, TGFβ, CTGF and PDGF (Table 1) which influence myofibroblast proliferation and collagen deposition (Malaviya et al., 2020b; McGraw et al., 2018; Mishra et al., 2012). This is associated with alterations in lung function and mechanics including increases in tissue elastance and decreases in compliance and static compliance (Malaviya et al., 2020b).
4.6. Ionizing radiation
First utilized as a treatment for breast cancer in early 1900s, radiation therapy (X-rays or gamma rays) is now commonly used to treat a variety of malignancies. The toxic effects of radiation on the lung were first described by Evans and Leucutia in 1925 (Hanania et al., 2019). Since then, despite significant advances in targeting, radiation-induced pulmonary injury and fibrosis remain a major complication. Radiation causes DNA damage and ROS production in lung epithelial cells (Hanania et al., 2019). This is followed by multiple phases of inflammation and aberrant wound repair which culminate in tissue remodeling and fibrosis (Table 2). The first phase, which occurs hours to days after exposure, is characterized by oxidative stress, inflammatory cell accumulation in the lung, and production of proinflammatory mediators including TNFα, IL-1α, IL-1β, IL-6 and TGFβ (Table 1). The second phase, radiation pneumonitis, follows within a few days or weeks, and lasts up to 8–16 weeks resulting in diffuse alveolar damage, edema of the interstitium and infiltration of inflammatory cells into the alveoli and alveolar septa (Beach et al., 2020; Rube et al., 2005). Late lung injury is associated with increases in profibrotic mediators (TGFβ, IL-13, PDGF and CTGF), ECM proteins, scarring within the alveolar septa resulting in widespread fibrotic lung remodeling, and loss of lung function (Abdollahi et al., 2005; Giuranno et al., 2019; Lee et al., 2015). This late phase of lung injury is also associated with the increases in chemokines (CCL2, CXCL1) and chemokine receptors (CCR2) coordinate with inflammatory cell recruitment and activation (Beach et al., 2020).
5. Antifibrotic therapies
Finding appropriate treatment options to prevent the progression of fibrosis or reverse the disease has been challenging. Clinical trials with antioxidants (e.g., N-acetylcysteine), TNFα inhibitors (e.g., etanercept), IFNγ or endothelin receptor antagonist (Bosentan) demonstrated no significant improvement in patients with IPF (King et al., 2011). Current therapies with nintedanib, a tyrosine kinase inhibitor or pirfenidone, an anti-inflammatory and antifibrotic agent, have mainly been effective in reducing acute exacerbations and hospitalizations, and in decreasing mortality (Lederer and Martinez, 2018). Nintedanib targets multiple growth factor pathways by modulating events downstream of VEGF, PDGF or FGF receptors, whereas pirfenidone acts by suppressing the release of proinflammatory and profibrotic cytokines including TNFα and TGFβ, and by inhibiting collagen synthesis. Recently, a biologic with anti-connective tissue growth factor (Lu and El-Hashash, 2019) activity (pamrevlumab) has been reported to slow the decline in forced vital capacity and to attenuate disease progression in IPF patients (Richeldi et al., 2020). Efforts are also focused on stem cell therapy to restore the integrity and function of epithelial, and alveolar cells in patients with fibrosis with some promising results (Lu and El-Hashash, 2019).
6. Summary and conclusions
Established animal models of pulmonary fibrosis have been utilized to study the role of immune and inflammatory cells in the pathogenesis of fibrosis and to test the efficacy of antifibrotic therapeutics. Macrophages, neutrophils, T lymphocytes, MDSCs and fibrocytes have been found to play important roles in pulmonary fibrogenesis by inducing oxidative stress and releasing pro-inflammatory cytokines and chemokines during the acute injury phase, whereas during fibrogenesis, they upregulate cytokines and growth factors that participate in aberrant injury and repair, myofibroblast generation and tissue remodeling. However, the process of inflammation initiation, and its progression to fibrosis is complex. It is regulated by an intricate response of multiple cell types, signaling events, changes in the tissue microenvironment and multiple feedback loops. As a consequence, effective antifibrotic therapy will likely require a multidimensional approach capable of not only targeting the activation and response of inflammatory cells but also modulating the tissue microenvironment.
Acknowledgements
This work was supported by National Institutes of Health [Grant numbers: U54AR055073, R01ES004738, and P30ES005022].
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. , 2005. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J. Exp. Med 201, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J, 2010. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax 65, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, et al. , 2008. Small molecular inhibitor of transforming growth factor-β protects against development of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys 71, 829–837. [DOI] [PubMed] [Google Scholar]
- Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, et al. , 2012. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am. J. Respir. Crit. Care Med 185, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma A, Li YJ, Abe S, Usuki J, Matsuda K, Henmi S, et al. , 2005. Interferon-β inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-β and thrombospondin. Am. J. Respir. Cell Mol. Biol 32, 93–98. [DOI] [PubMed] [Google Scholar]
- Bai KJ, Chuang KJ, Chen JK, Hua HE, Shen YL, Liao WN, et al. , 2018. Investigation into the pulmonary inflammopathology of exposure to nickel oxide nanoparticles in mice. Nanomedicine 14, 2329–2339. [DOI] [PubMed] [Google Scholar]
- Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM Jr., Hunter MG, et al. , 2007. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med 176, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin V, Nihoul A, Misson P, Arras M, Delos M, Leclercq I, et al. , 2005a. The role of pro- and anti-inflammatory responses in silica-induced lung fibrosis. Respir. Res 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin V, Xing Z, Delos M, Lison D, Huaux F, 2005b. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am. J. Phys. Lung Cell. Mol. Phys 288, L841–L848. [DOI] [PubMed] [Google Scholar]
- Batra V, Musani AI, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP, 2004. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-β1, TGF-β2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on α-smooth muscle aetin and collagen III synthesis by primary human lung fibroblasts. Clin. Exp. Allergy 34, 437–444. [DOI] [PubMed] [Google Scholar]
- Beach TA, Groves AM, Williams JP, Finkelstein JN, 2020. Modeling radiation-induced lung injury: lessons learned from whole thorax irradiation. Int. J. Radiat. Biol 96, 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Dy M, Murray L, Burdick MD, Xue YY, Strieter RM, Keane MP, 2004. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J. Immunol 173, 4692–4698. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Toth B, Rogers RA, Sepulveda R, Kunzendorf P, Phillips JI, Ernst H, 2018. Evaluation of the dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to chrysotile or crocidolite asbestos in a 28-day quantitative inhalation toxicology study. Toxicol. Appl. Pharmacol 351, 74–92. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Struyf S, Guabiraba R, Fauconnier L, Rouxel N, Proost P, et al. , 2013. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J. Leukoc. Biol 94, 1317–1323. [DOI] [PubMed] [Google Scholar]
- Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, et al. , 2017. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J. Natl. Cancer Inst 109. [DOI] [PubMed] [Google Scholar]
- Birjandi SZ, Palchevskiy V, Xue YY, Nunez S, Kern R, Weigt SS, et al. , 2016. CD4+CD25hiFoxp3+ cells exacerbate bleomycin-induced pulmonary fibrosis. Am. J. Pathol 186, 2008–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, et al. , 2004. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J. Immunol 173, 2099–2108. [DOI] [PubMed] [Google Scholar]
- Borthwick LA, 2016. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol 38, 517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveda-Ruiz D, D'Alessandro-Gabazza CN, Toda M, Takagi T, Naito M, Matsushima Y, et al. , 2013. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology 218, 245–254. [DOI] [PubMed] [Google Scholar]
- Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. , 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule S, Beckers E, Chaurand P, Liu W, Ibouraadaten S, Palmai-Pallag M, et al. , 2014. Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats. Part. Fibre Toxicol 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Wang C, Chen X, Hou J, Xiang Z, Shen Y, Han X, 2018. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep 8, 13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve 0, et al. , 2011. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal, 15, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington R, Jordan S, Pitchford SC, Page CP, 2018. Use of animal models in IPF research. Pulm. Pharmacol. Ther 51, 73–78. [DOI] [PubMed] [Google Scholar]
- Castranova V, Porter D, Millecchia L, Ma JY, Hubbs AF, Teass A, 2002. Effect of inhaled crystalline silica in a rat model: time course of pulmonary reactions. Mol. Cell. Biochem 234-235, 177–184. [PubMed] [Google Scholar]
- Chaudhary NI, Schnapp A, Park JE, 2006. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am. J. Respir. Crit. Care Med 173, 769–776. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li C, Weng D, Song L, Tang W, Dai W, et al. , 2014. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol. Appl. Pharmacol 275, 62–72. [DOI] [PubMed] [Google Scholar]
- Cheresh P, Kim SJ, Tulasiram S, Kamp DW, 2013. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 1832, 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. , 2014. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol 233, 294–307. [DOI] [PubMed] [Google Scholar]
- Chua F, Dunsmore SE, Clingen PH, Mutsaers SE, Shapiro SD, Segal AW, et al. , 2007. Mice lacking neutrophil elastase are resistant to bleomycin-induced pulmonary fibrosis. Am. J. Pathol 170, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SI, Horton JA, Ramalingam TR, White AO, Chung EJ, Hudak KE, et al. , 2016. IL-13 is a therapeutic target in radiation lung injury. Sci. Rep 6, 39714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Patel A, Green FH, 2008. Lung disease caused by exposure to coal mine and silica dust. Semin. Respir. Crit. Care Med 29, 651–661. [DOI] [PubMed] [Google Scholar]
- Craig VJ, Zhang L, Hagood JS, Owen CA, 2015. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 53, 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniil Z, Kitsanta P, Kapotsis G, Mathioudaki M, Kollintza A, Karatza M, et al. , 2005. CD8+ T lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis. Respir. Res 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, et al. , 2010. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Phys. Lung Cell. Mol. Phys 299, L442–L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Latta V, Cecchettini A, Del Ry S, Morales MA, 2015. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol. Res 97, 122–130. [DOI] [PubMed] [Google Scholar]
- Desai O, Winkler J, Minasyan M, Herzog EL, 2018. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. (Lausanne) 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Ma Q, 2017. Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part. Fibre Toxicol 14, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Porter DW, Batteli LA, Wolfarth MG, Richardson DL, Ma Q, 2015. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nano tubes. Arch. Toxicol 89, 621–633. [DOI] [PubMed] [Google Scholar]
- Dowrnan L, Hill CJ, Holland AE, 2014. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev CD006322. 10.001002/14651858.CD14006322. publ4651853. [DOI] [PubMed] [Google Scholar]
- Duke KS, Bonner JC, 2018. Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective . Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 10, e1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymacek JM, Snyder-Talkington BN, Raese R, Dong C, Singh S, Porter DW, et al. , 2018. Similar and differential canonical pathways and biological processes associated with multiwalled carbon nanotube and asbestos-induced pulmonary fibrosis: a 1-Year postexposure study. Int. J. Toxicol 37, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand-Hammarstrom B, Wigenstam E, Bucht A, 2011. Inhalation of alkylating mustard causes long-term T cell-dependent inflammation in airways and growth of connective tissue. Toxicology 280, 88–97. [DOI] [PubMed] [Google Scholar]
- El-Medany A, Hagar HH, Moursi M, At Muhammed R, El-Rakhawy FI, El-Medany G, 2005. Attenuation of bleomycin-induced lung fibrosis in rats by mesna. Eur. J. Pharmacol 509, 61–70. [DOI] [PubMed] [Google Scholar]
- Felton VM, Borok Z, Willis BC, 2009. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am. J. Physiol. Lung Cell. Mol. Physiol 297, L805–L812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez IE, Greiffo FR, Frankenberger M, Bandres J, Heinzelmann K, Neurohr C, et al. , 2016. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur. Respir. J 48, 1171–1183. [DOI] [PubMed] [Google Scholar]
- Ferreira TP, de Arantes AC, do Nascimento CV, Olsen PC, Trentin PG, Rocco PR, et al. , 2013. IL-13 immunotoxin accelerates resolution of lung pathological changes triggered by silica particles in mice. J. Immunol 191, 5220–5229. [DOI] [PubMed] [Google Scholar]
- Flechsig P, Hartenstein B, Teurich S, Dadrich M, Hauser K, Abdollahi A, et al. , 2010. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys 77, 582–590. [DOI] [PubMed] [Google Scholar]
- Galati D, De Martino M, Trotta A, Rea G, Bruzzese D, Cicchitto G, et al. , 2014. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine 66, 119–126. [DOI] [PubMed] [Google Scholar]
- Garibaldi BT, D'Alessio FR, Mock JR, Files DC, Chau E, Eto Y, et al. , 2013. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am. J. Respir. Cell Mol. Biol 48, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. , 2007. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest 117, 3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D, 2006. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc. Am. Thorac. Soc 3, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron DR, Lemay AM, Lecours PB, Perreault-Vallieres V, Huppe CA, Bosse Y, et al. , 2017. FTY720 promotes pulmonary fibrosis when administered during the remodelling phase following a bleomycin-induced lung injury. Pulm. Pharmacol. Ther 44, 50–56. [DOI] [PubMed] [Google Scholar]
- Gessner A, Rollinghoff M, 2000. Biologic functions and signaling of the interleukin-4 receptor complexes. Immunobiology 201, 285–307. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH, 2003. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 24, 266–276. [DOI] [PubMed] [Google Scholar]
- Giuranno L, lent J, De Ruysscher D, Vooijs MA, 2019. Radiation-induced lung injury (RILI). Front. Oncol 9, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Schoneboom BA, 2013. Historical perspective on effects and treatment of sulfur mustard injuries. Chem. Biol. Interact 206, 512–522. [DOI] [PubMed] [Google Scholar]
- Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, Agostini BA, et al. , 2015. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J. Leukoc. Biol 98, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson A, Svensson-Elfsmark L, Lorentzen JC, Bucht A, 2014. Strain differences influence timing and magnitude of both acute and late inflammatory reactions after intratracheal instillation of an alkylating agent in rats. J. Appl. Toxicol 34, 272–280. [DOI] [PubMed] [Google Scholar]
- Haegens A, Barrett TF, Gell J, Shukla A, Macpherson M, Vacek P, et al. , 2007. Airway epithelial NF-κB activation modulates asbestos-induced inflammation and mucin production in vivo. J. Immunol 178, 1800–1808. [DOI] [PubMed] [Google Scholar]
- Halnan KE, Bleehen NM, Brewin TB, Deeley TJ, Harrison DF, Howland C, et al. , 1972. Early clinical experience with bleomycin in the United Kingdom in series of 105 patients. Br. Med. J 4, 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M, 2019. Radiation-induced lung injury: assessment and management. Chest 156, 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SA, Eksteen B, Reid D, Paine HV, Alansary A, Johannson K, et al. , 2013. Role of IL-17A and neutrophils in fibrosis in experimental hypersensitivity pneumonitis. J. Allergy Clin. Immunol 131, 1663–1673. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T, 2001. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J. Allergy Clin. Immunol 107, 1001–1008. [DOI] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. , 2009. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med 15, 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. , 2014. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med 6, 231ra247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog EL, Bucala R, 2010. Fibrocytes in health and disease. Exp. Hematol 38, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukels P, van Hulst JAC, van Nimwegen M, Boorsma CE, Melgert BN, van den Toorn LM, et al. , 2018. Fibrocytes are increased in lung and peripheral blood of patients with idiopathic pulmonary fibrosis. Respir. Res 19, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Okamoto M, Sakazaki Y, Kato S, Young HA, Aizawa H, 2009. Role of proinflammatory cytokines IL-18 and IL-1β in bleomycin-induced lung injury in humans and mice. Am. J. Respir. Cell Mol. Biol 41, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, et al. , 2018. TNF-α-induced NF-κB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J. Cell. Physiol 233, 2409–2419. [DOI] [PubMed] [Google Scholar]
- Inomata M, Kamio K, Azuma A, Matsuda K, Kokuho N, Miura Y, et al. , 2014. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir. Res 15, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Fujishima S, Ikeda E, Yoshie O, Tsukamoto N, Aiso S, et al. , 2004. CCL22 and CCL17 in rat radiation pneumonitis and in human idiopathic pulmonary fibrosis. Eur. Respir. J 24, 49–56. [DOI] [PubMed] [Google Scholar]
- Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, et al. , 2017. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 31, 2520–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, Liu Y, 2015. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy 17, 560–570. [DOI] [PubMed] [Google Scholar]
- Jin X, Dai H, Ding K, Xu X, Pang B, Wang C, 2014. Rapamycin attenuates bleomycin-induced pulmonary fibrosis in rats and the expression of metalloproteinase-9 and tissue inhibitors of metalloproteinase-1 in lung tissue. Chin. Med. J 127, 1304–1309. [PubMed] [Google Scholar]
- Jin H, Kang GY, Jeon S, Kim JM, Park YN, Cho J, Lee YS, 2019. Identification of molecular signatures involved in radiation-induced lung fibrosis. J. Mol. Med. (Berl) 97, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Ward C, Eapen MS, Myers S, Hallgren O, Levine H, Sohal SS, 2018. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn 247, 346–358. [DOI] [PubMed] [Google Scholar]
- Kamp DW, 2009. Asbestos-induced lung diseases: an update. Transl. Res 153, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, 2015. A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol 27, 363–377. [DOI] [PubMed] [Google Scholar]
- Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE Jr., 2008. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133, 226–232. [DOI] [PubMed] [Google Scholar]
- King TE Jr., Pardo A, Selman M, 2011. Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961. [DOI] [PubMed] [Google Scholar]
- Kolahian S, Fernandez IE, Eickelberg O, Hartl D, 2016. Immune mechanisms in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 55, 309–322. [DOI] [PubMed] [Google Scholar]
- Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J, 2001. Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest 107, 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Masutani R, Suzuka T, Oda K, Makino S, Ii M, 2017. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci. Rep 7, 14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, et al. , 2009. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 179, 1121–1130. [DOI] [PubMed] [Google Scholar]
- Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. , 2015. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 11, e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ, 2006. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp. Lung Res 32, 181–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Malaviya R, Laskin JD, 2019. Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol. Sci 168, 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun A, Lo Re S, Chantry M, Izquierdo Carerra X, Uwambayinema F, Ricci D, et al. , 2017. CCR2+ monocytic myeloid-derived suppressor cells (M-MDSCs) inhibit collagen degradation and promote lung fibrosis by producing transforming growth factor-β1. J. Pathol 243, 320–330. [DOI] [PubMed] [Google Scholar]
- Lederer DJ, Martinez FJ, 2018. Idiopathic pulmonary fibrosis. N. Engl. J. Med 378, 1811–1823. [DOI] [PubMed] [Google Scholar]
- Lee YC, Lane KB, 2001. The many faces of transforming growth factor-β in pleural diseases. Curr. Opin. Pulm. Med 7, 173–179. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yi CO, Heo RW, Song DH, Cho YJ, Jeong YY, et al. , 2015. Clarithromycin attenuates radiation-induced lung injury in mice. PLoS One 10, e0131671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J, Millar BJ, Del Carpio Pons A, Burgoyne RA, Frost JD, Barksby BS, et al. , 2020. FPR-1 is an important regulator of neutrophil recruitment and a tissue-specific driver of pulmonary fibrosis. JCI Insight 5. 10.1172/jci.insight.125937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, et al. , 2006. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer 6. 10.1186/1471-2407-1186-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, et al. , 2014. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol 134 (1422–1432), e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yao W, Hou JY, Zhang L, Bao L, Chen HT, et al. , 2017. Crystalline silica promotes rat fibrocyte differentiation in vitro, and fibrocytes participate in silicosis in vivo. Biomed. Environ. Sci 30, 649–660. [DOI] [PubMed] [Google Scholar]
- Liang M, Lv J, Zou L, Yang W, Xiong Y, Chen X, et al. , 2015. A modified murine model of systemic sclerosis: bleomycin given by pump infusion induced skin and pulmonary inflammation and fibrosis. Lab. Investig 95, 342–350. [DOI] [PubMed] [Google Scholar]
- Lin L, Han Q, Xiong Y, Li T, Liu Z, Xu H, et al. , 2017. Krupple-like-factor 4 attenuates lung fibrosis via inhibiting epithelial-mesenchymal transition. Sci. Rep 7, 15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Shih CH, Lin YC, Yang YL, Chen BC, 2018. MEKK1, JNK, and SMAD3 mediate CXCL12-stimulated connective tissue growth factor expression in human lung fibroblasts. J. Biomed. Sci 25, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S, 2012. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5, S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Brody AR, 2001. Increased TGF-β1 in the lungs of asbestos-exposed rats and mice: reduced expression in TNF-α receptor knockout mice. J. Environ. Pathol. Toxicol. Oncol 20, 97–108. [PubMed] [Google Scholar]
- Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, et al. , 2004. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J. Immunol 173, 3425–3431. [DOI] [PubMed] [Google Scholar]
- Liu G, Cheresh P, Kamp DW, 2013. Molecular basis of asbestos-induced lung disease. Annu. Rev. Pathol 8, 161–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, El-Hashash AHK, 2019. Cell-based therapy for idiopathic pulmonary fibrosis. Stem Cell Investig. 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden RV, Worrell JC, Boylan D, Walsh SM, Cramton J, Counihan I, et al. , 2015. Modulation of pulmonary fibrosis by IL-13Rα2. Am. J. Phys. Lung Cell. Mol. Phys 308, L710–L718. [DOI] [PubMed] [Google Scholar]
- Luzina IG, Todd NW, Iacono AT, Atamas SP, 2008. Roles of T lymphocytes in pulmonary fibrosis. J. Leukoc. Biol 83, 237–244. [DOI] [PubMed] [Google Scholar]
- Luzina IG, Kopach P, Lockatell V, Kang PH, Nagarsekar A, Burke AP, et al. , 2013. Interleukin-33 potentiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol 49, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina IG, Todd NW, Sundararajan S, Atamas SP, 2015. The cytokines of pulmonary fibrosis: Much learned, much more to learn. Cytokine 74, 88–100. [DOI] [PubMed] [Google Scholar]
- Ma JY, Mercer RR, Barger M, Schwegler-Berry D, Scabilloni J, Ma JK, Castranova V, 2012. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol. Appl. Pharmacol 262, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Bishoff B, Mercer RR, Barger M, Schwegler-Berry D, Castranova V, 2017. Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol. Appl. Pharmacol 323, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Cervelli J, Anderson DR, Holmes WW, Conti ML, et al. , 2010. Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol. Appl. Pharmacol 248, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Venosa A, Hall L, Gow AJ, Sinko PJ, Laskin JD, Laskin DL, 2012. Attenuation of acute nitrogen mustard-induced lung injury, inflammation and fibrogenesis by a nitric oxide synthase inhibitor. Toxicol. Appl. Pharmacol 265, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Sunil VR, Venosa A, Verissimo VL, Cervelli JA, Vayas KN, et al. , 2015. Attenuation of nitrogen mustard-induced pulmonary injury and fibrosis by anti-tumor necrosis factor-α antibody. Toxicol. Sci 148, 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Abramova EV, Rancourt RC, Sunil VR, Napierala M, Weinstock D, et al. , 2020a. Progressive lung injury, inflammation and fibrosis in rats following inhalation of sulfur mustard. Toxicol. Sci 10.1093/toxsci/kfaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Laskin JD, Laskin DL, 2017. Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Ther 180, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Laskin JD, Laskin DL, 2020b. Long-term respiratory effects of mustard vesicants. Toxicol. Lett 319, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw MD, Dysart MM, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. , 2018. Bronchiolitis obliterans and pulmonary fibrosis after sulfur mustard inhalation in rats. Am. J. Respir. Cell Mol. Biol 58, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menou A, Duitman J, Crestani B, 2018. The impaired proteases and anti-proteases balance in idiopathic pulmonary fibrosis. Matrix Biol. 68-69, 382–403. [DOI] [PubMed] [Google Scholar]
- Meziani L, Mondini M, Petit B, Boissonnas A, Thomas de Montpreville V, Mercier O, et al. , 2018. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J 51. [DOI] [PubMed] [Google Scholar]
- Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, et al. , 2011. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J. Immunol 187, 3003–3014. [DOI] [PubMed] [Google Scholar]
- Mishra NC, Rir-Sima-Ah J, Grotendorst GR, Langley RJ, Singh SP, Gundavarapu S, et al. , 2012. Inhalation of sulfur mustard causes long-term T cell-dependent inflammation: possible role of Th17 cells in chronic lung pathology. Int. Immunopharmacol 13, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. , 2009. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 179, 588–594. [DOI] [PubMed] [Google Scholar]
- Moore BB, Hogaboam CM, 2008. Murine models of pulmonary fibrosis. Am. J. Phys. Lung Cell. Mol. Phys 294, L152–L160. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Horie M, Kobayashi N, Shinohara N, Shimada M, 2013. Inhalation toxicity assessment of carbon-based nanoparticles. Acc. Chem. Res 46, 770–781. [DOI] [PubMed] [Google Scholar]
- Mukherjee SP, Bondarenko O, Kohonen P, Andón FT, Brzicová T, Gessner I, et al. , 2018. Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors. Sci. Rep 8, 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S, Larson-Casey JL, Ryan AJ, He C, Kobzik L, Carter AB, 2015. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. FASEB J. 29, 3527–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikota J, Banville A, Goodwin LR, Wu D, Williams A, Yauk CL, et al. , 2017. Stat-6 signaling pathway and not Interleukin-1 mediates multi-walled carbon nanotube-induced lung fibrosis in mice: insights from an adverse outcome pathway framework. Part. Fibre Toxicol 14, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkyimbeng T, Ruppert C, Shiomi T, Dahal B, Lang G, Seeger W, et al. , 2013. Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis. PLoS One 8, e73279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafo-Addo AD, Herzog EL, 2017. CCL2 and T cells in pulmonary fibrosis: an old player gets a new role. Thorax 72, 967–968. [DOI] [PubMed] [Google Scholar]
- Palomino DC, Marti LC, 2015. Chemokines and immunity. Einstein (Sao Paulo) 13, 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Ahn MH, Jang HK, Jang AS, Kim DJ, Koh ES, et al. , 2009. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J. Korean Med. Sci 24, 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E, Capelozzi VL, 2007. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration 74, 159–169. [DOI] [PubMed] [Google Scholar]
- Peng X, Moore MW, Peng H, Sun H, Gan Y, Homer RJ, Herzog EL, 2014. CD4+CD25+FoxP3+ regulatory Tregs inhibit fibrocyte recruitment and fibrosis via suppression of FGF-9 production in the TGF-β1 exposed murine lung. Front. Pharmacol 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. , 2004. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest 114, 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo CA, 2008. Regulatory T cells in health and disease. Cytokine 43, 395–401. [DOI] [PubMed] [Google Scholar]
- Porto BN, Stein RT, 2016. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol 7, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Li S, Zhao G, Fu X, Xie X, Huang Y, et al. , 2017. Long-term intravenous administration of carboxylated single-walled carbon nanotubes induces persistent accumulation in the lungs and pulmonary fibrosis via the nuclear factor-kappaB pathway. Int. J. Nanomedicine 12, 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan TE, Cowper SE, Bucala R, 2006. The role of circulating fibrocytes in fibrosis. Curr. Rheumatol. Rep 8, 145–150. [DOI] [PubMed] [Google Scholar]
- Re SL, Giordano G, Yakoub Y, Devosse R, Uwambayinema F, Couillin I, et al. , 2014. Uncoupling between inflammatory and fibrotic responses to silica: evidence from MyD88 knockout mice. PLoS One 9, e99383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, Fernandez Perez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, et al. , 2020. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med 8, 25–33. [DOI] [PubMed] [Google Scholar]
- Robert S, Gicquel T, Victoni T, Valenca S, Barreto E, Bailly-Maitre B, et al. , 2016. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep 36. 10.1042/BSR20160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübe CE, Uthe D, Wilfert F, Ludwig D, Yang K, König J, et al. , 2005. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int. J. Radiat. Oncol. Biol. Phys 61, 1482–1492. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, et al. , 2010. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol. Sci 114, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda H, Tasaka S, Fujishima S, Yamasawa W, Miyamoto K, Nakano Y, et al. , 2009. Elevated CC chemokine level in broncho alveolar lavage fluid is predictive of a poor outcome of idiopathic pulmonary fibrosis. Respiration 78, 285–292. [DOI] [PubMed] [Google Scholar]
- Shukla A, Ramos-Nino M, Mossman B, 2003. Cell signaling and transcription factor activation by asbestos in lung injury and disease. Int. J. Biochem. Cell Biol 35, 1198–1209. [DOI] [PubMed] [Google Scholar]
- Shukla A, Lounsbury KM, Barrett TF, Gell J, Rincon M, Butnor KJ, et al. , 2007. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase G-δ knockout mice. Am. J. Pathol 170, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, et al. , 2008. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Phys. Lung Cell. Mol. Phys 295, L552–L565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Talkington BN, Dong C, Porter DW, Ducatman B, Wolfarth MG, Andrew M, et al. , 2016. Multiwalled carbon nano tube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: a 1-year postexposure study. J. Toxicol. Environ. Health A 79, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Gomperts BN, Keane MP, 2007. The role of CXC chemokines in pulmonary fibrosis. J. Clin. Invest 117, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Suzukawa M, Nagase H, Koizumi Y, Ro S, Kobayashi K, et al. , 2019. IL-9 blockade suppresses silica-induced lung inflammation and fibrosis in mice. Am. J. Respir. Cell Mol. Biol 60, 232–243. [DOI] [PubMed] [Google Scholar]
- Sugino K, Nakamura Y, Muramatsu Y, Hata Y, Shibuya K, Homma S, 2016. Analysis of blood neutrophil elastase, glutathione levels and pathological findings in postoperative acute exacerbation of idiopathic pulmonary fibrosis associated with lung cancer: two case reports. Mol. Clin. Oncol 5, 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR, 2009. TNF-α induces TGF-β1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J. Cell. Mol. Med 13, 1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil VR, Vayas KN, Abramova EV, Rancourt R, Cervelli JA, Malaviya R, et al. , 2020. Lung injury, oxidative stress and fibrosis in mice following exposure to nitrogen mustard. Toxicol. Appl. Pharmacol 387, 114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemasa A, Ishii Y, Fukuda T, 2012. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur. Respir. J 40, 1475–1482. [DOI] [PubMed] [Google Scholar]
- Tanaka KI, Niino T, Ishihara T, Takafuji A, Takayama T, Kanda Y, et al. , 2017. Protective and therapeutic effect of felodipine against bleomycin-induced pulmonary fibrosis in mice. Sci. Rep 7, 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BJ, Kan OK, Loveland KL, Elias JA, Bardin PG, 2016. In the shadow of fibrosis: innate immune suppression mediated by transforming growth factor-β. Am. J. Respir. Cell Mol. Biol 55, 759–766. [DOI] [PubMed] [Google Scholar]
- Trujillo G, Hartigan AJ, Hogaboam CM, 2010. T regulatory cells and attenuated bleomycin-induced fibrosis in lungs of CCR7−/− mice. Fibrogenesis Tissue Repair 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL, 2016. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol 54, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shannahan JH, Brown JM, 2014. IL-33 modulates chronic airway resistance changes induced by multi-walled carbon nanotubes. Inhal. Toxicol 26, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Y, Han J, Mei H, Yu D, Ding Q, et al. , 2017. Metformin attenuates radiation-induced pulmonary fibrosis in a murine model. Radiat. Res 188, 105–113. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Malaviya R, Sunil VR, Venosa A, Heck DE, Laskin JD, Laskin DL, 2016. Mustard vesicant-induced lung injury: advances in therapy. Toxicol. Appl. Pharmacol 305, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigenstam E, Rocksen D, Ekstrand-Hammarstrom B, Bucht A, 2009. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal. Toxicol 21, 958–964. [DOI] [PubMed] [Google Scholar]
- Williamson JD, Sadofsky LR, Hart SP, 2015. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp. Lung Res 41, 57–73. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA, 2010. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med 207, 535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withana NP, Ma X, McGuire HM, Verdoes M, van der Linden WA, Ofori LO, et al. , 2016. Non-invasive imaging of idiopathic pulmonary fibrosis using cathepsin protease probes. Sci. Rep 6, 19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B, 2014. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther 349, 209–220. [DOI] [PubMed] [Google Scholar]
- Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, Kolb M, 2015. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J 45, 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, 2004. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol 4, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, 2008. Cellular and molecular mechanisms of fibrosis. J. Pathol 214, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, 2011. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med 208, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM, 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Palm J, Konig J, Seeland U, Rosenkranz S, Feiden W, et al. , 2007. Matrix-Metallo-Proteinases and their tissue inhibitors in radiation-induced lung injury. Int. J. Radiat. Biol 83, 665–676. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen PP, Luo M, Shi WL, Hou DS, Gao Y, et al. , 2019. Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed. Pharmacother 114, 108851. [DOI] [PubMed] [Google Scholar]
- Yogo Y, Fujishima S, Inoue T, Saito F, Shiomi T, Yamaguchi K, Ishizaka A, 2009. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir. Res 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Qian J, Xing X, Kong FM, Zhao L, Chen M, Lawrence TS, 2008. Inhibition of the tumor necrosis factor-α pathway is radioprotective for the lung. Clin. Cancer Res 14, 1868–1876. [DOI] [PubMed] [Google Scholar]
- Zhang S, Shu X, Tian X, Chen F, Lu X, Wang G, 2014. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin. Exp. Immunol 177, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY, 2018. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir. Res 19, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Okamoto Y, Asano Y, Ishimaru K, Aki S, Yoshioka K, et al. , 2018. Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis through potentiating IL-13 pathway in macrophages. PLoS One 13, e0197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo WL, Zhao JM, Huang JX, Zhou W, Lei ZH, Huang YM, et al. , 2017. Effect of bosentan is correlated with MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed. Rep 6, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]