Abstract
Transforming growth factor-β (TGF-β) is a potent regulator of cell growth and differentiation in many cell types. The Smad signaling pathway constitutes a main signal transduction route downstream of TGF-β receptors. We studied TGF-β–induced rearrangements of the actin filament system and found that TGF-β1 treatment of PC-3U human prostate carcinoma cells resulted in a rapid formation of lamellipodia. Interestingly, this response was shown to be independent of the Smad signaling pathway; instead, it required the activity of the Rho GTPases Cdc42 and RhoA, because ectopic expression of dominant negative mutant Cdc42 and RhoA abrogated the response. Long-term stimulation with TGF-β1 resulted in an assembly of stress fibers; this response required both signaling via Cdc42 and RhoA, and Smad proteins. A known downstream effector of Cdc42 is p38MAPK; treatment of the cells with the p38MAPK inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(pyridyl)1H-imidazole (SB203580), as well as ectopic expression of a kinase-inactive p38MAPK, abrogated the TGF-β–induced actin reorganization. Moreover, treatment of cells with the inhibitors of the RhoA target-protein Rho-associated coiled-coil kinase (+)-R-trans-4-(aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide (Y-27632) and 1-5(-isoquinolinesulfonyl)homopiperazine (HA-1077), as well as ectopic expression of kinase-inactive Rho coiled-coil kinase-1, abrogated the TGF-β1–induced formation of stress fibers. Collectively, these data indicate that TGF-β–induced membrane ruffles occur via Rho GTPase-dependent pathways, whereas long-term effects require cooperation between Smad and Rho GTPase signaling pathways.
INTRODUCTION
Transforming growth factor-β (TGF-β), a potent regulator of cell growth and differentiation in a variety of cell types, is a member of a large family of cytokines that, in addition to the TGF-β isoforms, includes activins, inhibins, and bone morphogenetic proteins (Heldin et al., 1997; Massagué and Chen, 2000). These soluble ligands exert their actions by binding to transmembrane receptors with intrinsic serine/threonine protein kinase activity. Receptor binding triggers a signaling cascade involving the Smad proteins, which upon receptor activation are translocated to the cell nucleus, where they are able to affect the transcription of specific genes by direct or indirect binding to their promoters (Heldin et al., 1997; Zhang and Derynck, 1999; Attisano and Wrana, 2000; Massagué and Chen, 2000; Massagué and Wotton, 2000). The Smad signaling pathway thus functions as a principal transduction expressway from the TGF-β receptors. Important TGF-β–responsive genes include genes for extracellular matrix accumulation, cell cycle control, and control of cell survival (Attisano and Wrana, 2000; Massagué and Wotton, 2000).
Studies on protein tyrosine kinase receptors such as the receptors for epidermal growth factor or platelet-derived growth factor (PDGF) have demonstrated a close correlation between transmembrane signaling and the organization of the actin filament system (Chinkers et al., 1979; Mellström et al., 1983; Rönnstrand and Heldin, 2001). Actin monomers polymerize into polar filaments by a strictly regulated process (Pollard et al., 2000). These filaments are under a continuous reconstruction and together with myosin and a huge number of actin-binding proteins they collectively form the microfilament system, which regulates the morphogenic and migratory properties of vertebrate cells (Mitchison and Cramer, 1996; Schmidt and Hall, 1998; Pollard et al., 2000). Binding of ligands such as PDGF to its receptor induces a rapid assembly of the actin filaments into peripheral membrane lamellae or lamellipodia, a phenomenon known as membrane ruffling (Mellström et al., 1983). The growth factor ligation hence constitutes a major migratory cue leading to cell locomotion (Mitchison and Cramer, 1996; Heldin et al., 1998; Rönnstrand and Heldin, 2001) and this response is mediated by members of the Rho GTPases, most notably Rac1 (Ridley et al. 1992; Nobes and Hall, 1995).
Treatment of cells in tissue culture with TGF-β has also been reported to affect the morphology of a number of different cell types such as mink lung epithelial Mv1Lu cells (Like and Massagué, 1986), Swiss 3T3 cells (Lee et al., 1999), and human epidermoid carcinoma KB cells (Koyasu et al., 1988). This effect is presumably dependent on changes in the cytoskeleton caused by altered expression of cytoskeletal elements, such as α-smooth muscle actin (Nakajima et al., 1999), vimentin and tubulin (Lomri and Marie, 1990), as well as assembly of actin filaments into stress fibers (Koyasu et al., 1988). However, this response occurred after several hours of stimulation and required new RNA and protein synthesis (Koyasu et al., 1988; Baghdassarian et al., 1993). In the present study, we investigated the effect on actin assembly occurring at short intervals after TGF-β administration. We found that treatment of rat basophilic leukemia RBL-2H3 cells, as well as human prostate carcinoma PC-3U cells, with TGF-β1 induced the formation of lamellipodia visible after 5–10 min of stimulation. Interestingly, these effects were shown to be independent on the Smad signaling pathway; instead, they where dependent on the Rho GTPases Cdc42 and RhoA. Prolonged treatment of PC-3U cells with TGF-β1 resulted in the formation of stress fibers as well as cortical actin filaments. In contrast to the initial mobilization of the actin filament system, the long-term effects were dependent on both the Smad signaling pathway and signaling via Cdc42 and RhoA involving the activity of p38MAPK and the Rho effector Rho-associated coiled-coil-containing protein kinase (ROCK). Taken together, these data indicate that TGF-β–induced membrane ruffles occurred via Rho GTPase-dependent pathways, whereas long-term effects require cooperation between Smad and Rho GTPase signaling pathways.
MATERIALS AND METHODS
Reagents, Antibodies, and DNA Work
TGF-β1 was a gift from Dr. N. Ferrara (Genentech, South San Francisco, CA) and human hepatocyte growth factor (HGF) was from R & D Systems (Minneapolis, MN). The following ROCK inhibitors were used: 1-5(-isoquinolinesulfonyl)homopiperazine (HA-1077; Alexis Biochemicals, Läufelfingen, Switzerland) and (+)-R-trans-4-(aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide (Y-27632; Biomol Research Laboratories, Plymouth Meeting, PA). The p38MAPKα and β inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(pyridyl)1H-imidazole (SB203580) were from Calbiochem (San Diego, CA). Mouse monoclonal anti-FcεRI antibody (clone BC4) was a generous gift from Dr. R. Siraganian (National Institutes of Health, Bethesda, MD), mouse monoclonal anti-TGF-β1-3 neutralizing antibody was from Genzyme (Cambridge, MA), mouse monoclonal anti-myc (9E10) was from Santa Cruz Biotechnology (Santa Cruz, CA), fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibodies were from DAKO (Glostrup, Denmark), and mouse monoclonal anti-Flag M5 was from Sigma Chemical (St. Louis, MO). Tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin was from Sigma Chemical.
The flag epitope-tagged mouse p38α and dominant negative p38α (DNp38) (harboring T180A and Y182F amino acid substitutions) (Han et al., 1994) in the pcDNA3 vector were generous gifts from Dr. J. Han (The Scripps Research Institute, La Jolla, CA). Enhanced green fluorescent protein (EGFP)-tagged bovine ROCK-1 and DNROCK-1 (harboring a K121G amino acid substitution) (Matsui et al., 1996) in the pBABEpuro vector were generous gifts from M. Olson (Institute for Cancer Research, London, United Kingdom), and flag epitope-tagged human Smad4 and DNSmad4 (encoding amino acid residues 1–514 only) (Hata et al., 1997) in the pCMV5 vector were generous gifts from Dr. J. Massagué (Memorial Sloan-Kettering Cancer Center, NY).
Glutathione-S-transferase (GST)-p21-activated kinase (PAK)-Cdc42/Rac interactive binding (CRIB) was created by subcloning a polymerase chain reaction (PCR)-generated fragment encoding amino acid residues 56–267 of human PAK1B (accession no. AF07188) into pGEX-2T, the generation of GST-Wiskott-Aldrich Syndrome protein (WASP)-CRIB (encoding amino acid residues 201–321 in the pGEX-KG vector) has been described (Aspenström et al., 1996). GST-rhotekin was created by subcloning a fragment generated by PCR, which encompassed the Rho-binding domain (amino acid residues 1–89) of mouse rhotekin (accession no. U54638), into pGEX-2T.
Cell Cultivation and Transfection
Rat basophilic leukemia (RBL-2H3) cells were cultured in DMEM, supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. Human prostate carcinoma (PC-3U) cells, which is a subline originating from PC-3 cells (Franzén et al., 1993), were cultured in RPMI-1640 medium, supplemented with 1% l-glutamine, 10% FBS, and penicillin/streptomycin. Cells were cultivated at 37°C in an atmosphere of 5% CO2. PC-3U cells were transiently transfected by LipofectAMINE (Invitrogen, Carlsbad, CA) by using the protocol provided by the manufacturer.
Immunohistochemistry
Cells were grown on glass coverslips and fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at 37°C and washed with PBS. The cells were thereafter permeabilized in 0.2% Triton X-100 in PBS for 5 min, washed again in PBS, and incubated in 10 mM glycine in PBS for 1 h at room temperature or overnight at 4°C. Primary as well as secondary antibodies were diluted in PBS containing 5% FBS. Cells were incubated with primary antibodies followed by secondary antibodies for intervals of 1 h with a washing step in between. The coverslips were mounted on object slides by the use of Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL). Cells were photographed by a Hamamatsu ORCA charge-coupled device digital camera by using the QED Imaging System software with a Zeiss Axioplan2 microscope.
Protein Production
For expression and purification of GST-WASP-CRIB, GST-PAK-CRIB, and GST-rhotekin, the bacteria were inoculated in 100 ml of L-broth containing 100 μg/ml ampicillin. After overnight incubation with shaking at 37°C, the culture was diluted into 900 ml of L-broth with 100 μg/ml ampicillin. The incubation proceeded at 37°C until OD600 reached 0.5. Protein expression was induced with 0.2 mM isopropyl-β-d-thiogalactopyranoside and incubated at 30°C for rhotekin or at 37°C for GST-WASP-CRIB and GST-PAK-CRIB until OD600 reached between 1.2 and 1.8. The bacteria were collected by centrifugation at 4000 rpm for 15 min in 4°C and resuspended in 5 ml of lysis buffer (50 mM Tris-HCl pH 7.5, 1% Triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol [DTT], 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride [PMSF]). The bacteria were thereafter sonicated on ice seven times for 10 s. The lysed cells were clarified by centrifugation at 14000 rpm for 30 min at 4°C. GST-fusion proteins were collected from the resulting supernatants by addition of 0.6 ml of glutathione-Sepharose beads (Amersham Biosciences AB, Uppsala, Sweden) followed by incubation end-over-end at 4°C for 60 min. The glutathione-Sepharose beads were collected by centrifugation at 2000 rpm at 4°C followed by six washes with buffer (50 mM Tris-HCl pH 7.5, 0.5% Triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1 μg/ml aprotinin, and 0.1 mM PMSF) and, finally, once with the same buffer supplemented with 10% glycerol. After the final wash, the beads containing the GST-fusion proteins were resuspended in 2 ml of wash buffer supplemented with 10% glycerol. The beads were divided into aliquots of 250 μl into prechilled tubes and stored at −70°C until used in the pull-down assay. The purity of the GST-fusion proteins was assayed by SDS-PAGE.
GST Pull-Down Assays
The cells were stimulated with TGF-β1 as indicated in the figure legends. The cells were washed with ice-cold PBS supplemented with 1 mM MgCl2. Immediately, ice-cold lysis buffer (50 mM Tris-HCl pH 7.5, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 10 μg/ml aprotinin, and 1 mM PMSF) was added. The cells were rapidly scraped off the plates and the crude lysates were transferred to prechilled Eppendorf tubes and centrifugated at 13,000 rpm for 5 min at 4°C. The supernatants were immediately supplemented with GST-PAK-CRIB, GST-WASP-CRIB, or GST-rhotekin for detection of activated Rac1, Cdc42, and RhoA, respectively. GST-fusion protein (20–30 μg) on glutathion beads was added to the supernatants and the tubes were incubated end-over-end at 4°C for 10 min. The beads were thereafter washed four times (5000 rpm for 15–20 s) with 0.5 ml of ice-cold washing buffer (50 mM Tris-HCl pH 7.5, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 10 μg/ml aprotinin, and 0.1 mM PMSF). SDS-PAGE sample buffer (containing 40 mM DTT) was added to each sample and the samples were heated at 95°C for 5 min. Samples with equal amounts of proteins were subjected to SDS-PAGE and then transferred to Immobilon-P filters (Millipore, Bedford, MA). Western blots were detected by the BM chemiluminescence blotting substrate (Roche Applied Science, Mannheim, Germany).
RESULTS
TGF-β Induces Rapid Rearrangement of Actin Filament System, Resulting in Membrane Ruffling
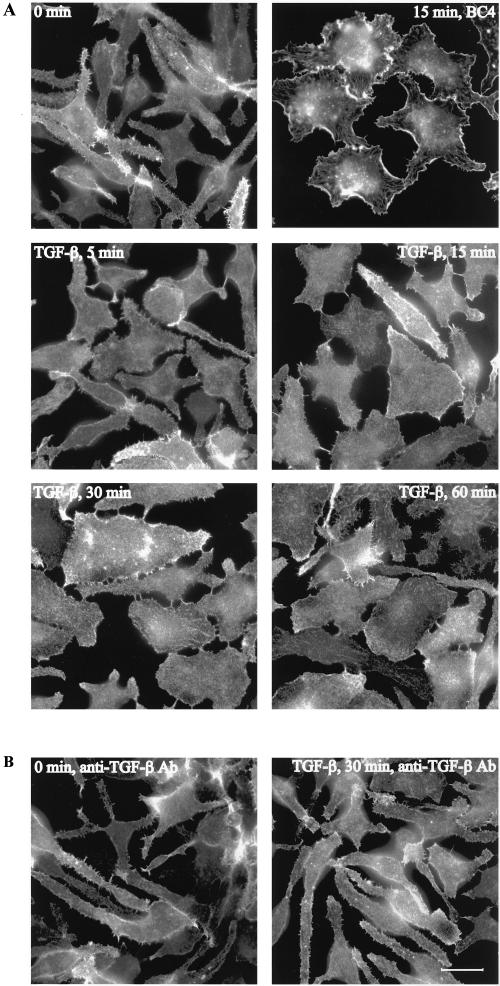
The effect on cell morphology by TGF-β1 treatment was examined using rat basophilic leukemia RBL-2H3 cells. Serum-starved, untreated cells had a rounded-up morphology, and often long protrusions, with characteristic serrated cell edges, extending from the central cell body. These protrusions were rich in actin filaments, which were visualized by staining the cells with fluorescently labeled phalloidin (Figure 1A). Stimulation of the RBL-2H3 cells with 25 ng/ml TGF-β1 induced a rapid morphological change concomitant with a robust reorganization of the actin cytoskeleton. Five minutes after TGF-β1 stimulation the cells flattened out and actin filaments started to accumulate at the cell edges, in a process highly reminiscent of membrane ruffling (Figure 1A). The ruffling activity was most intense 15–30 min after TGF-β1 administration. We compared the TGF-β1 effect on the actin filament system with the one elicited by activation of the high-affinity IgE receptor FcεRI. Activation of this receptor has previously been shown to trigger a reorganization of the actin cytoskeleton, resulting in membrane ruffling (Guillemot et al., 1997). Treating RBL-2H3 cells with BC4, which is an antibody against the extracellular domain of FcεRI, resulted in receptor aggregation and activation. BC4-treated cells flattened out and started to form membrane ruffles resembling the TGF-β1–induced membrane ruffles (Figure 1A). In addition, BC4 treatment resulted in formation of actin plaques, a response that was less apparent after TGF-β1 treatment (Figure 1A). Membrane ruffles were seen at TGF-β1 concentrations of 10 ng/ml and higher. The effect reached a maximum at 25 ng/ml after which no further activation could be detected (our unpublished data). To exclude that contaminating factors in the TGF-β1 preparation caused the actin reorganization, RBL-2H3 cells were preincubated with a TGF-β–specific antibody before stimulation with the ligand. This neutralizing antibody entirely abrogated the TGF-β1–induced actin reorganization (Figure 1B), strongly suggesting that the membrane ruffling and morphological change were dependent on TGF-β1.
Figure 1.
TGF-β1–induced actin reorganization and membrane ruffling in RBL-2H3 cells. (A) RBL-2H3 cells were serum-starved for 12 h and treated with 25 ng/ml TGF-β1 for 5, 15, 30, and 60 min. FcεRI activation was induced by addition of the cross-linking antibody BC4. (B) Monoclonal mouse anti-TGF-β1-3 neutralizing antibody was added to RBL-2H3 cells at a concentration of 25 μg/ml 1 h before stimulation with 25 ng/ml TGF-β1 for 15 min. Filamentous actin was visualized by TRITC-labeled phalloidin. Bar, 20 μm.
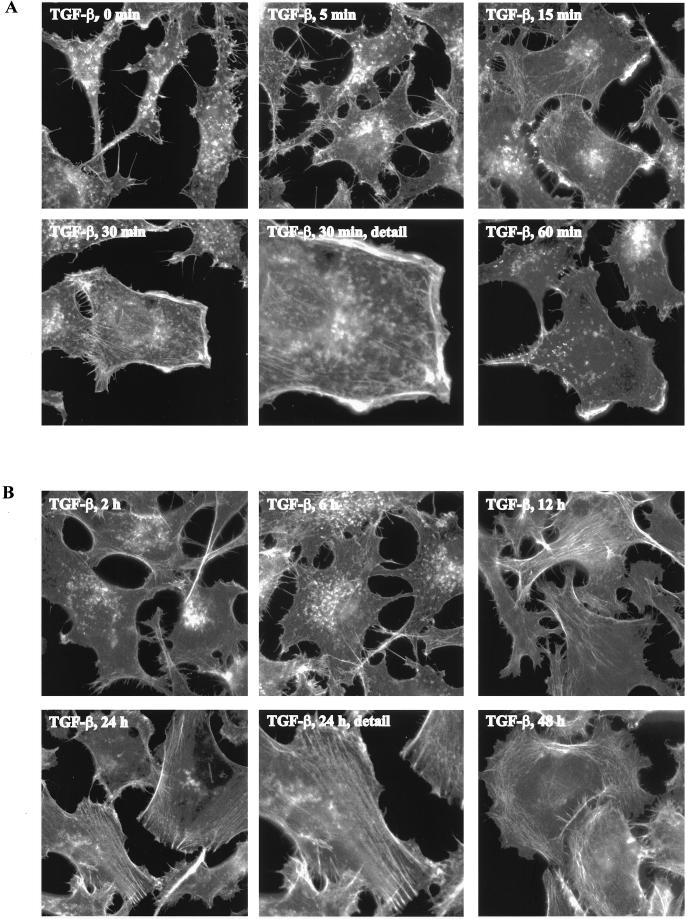
We tested whether a TGF-β1–induced actin reorganization also occurred in other cell types. To this end, human prostate epithelial cancer PC-3U cells were serum-starved for 12 h and stimulated with TGF-β1 for increasing time periods after which the cells were fixed and the actin filaments were visualized. The serum-starved cells exhibited a retracted phenotype with few distinguishable filamentous actin-containing structures (Figure 2A). On TGF-β1 stimulation, however, the cells flattened out and actin filaments accumulated at the cell edges into membrane ruffles. These membrane ruffles emerged after 5 min and became more pronounced after 15–30 min (Figure 2A). The PC-3U cells were responsive to lower concentrations of TGF-β1 compared with RBL-2H3 cells and reorganizations of the actin filament system were detected already at concentrations of 5 ng/ml (our unpublished data), but for the studies described in this work a concentration of 10 ng/ml was used.
Figure 2.
TGF-β1–induced actin reorganization and membrane ruffling in PC-3U cells. PC-3U cells were starved in 1% FBS for 12 h and stimulated with 10 ng/ml TGF-β1 for 5, 15, 30, and 60 min (A) and 2, 12, 24, and 48 h (B). Filamentous actin was visualized by TRITC-labeled phalloidin. Bar, 20 μm.
TGF-β Induces Formation of Stress Fibers
When the PC-3U cells were stimulated for longer time periods, a distinct and dramatic reorganization of the actin cytoskeleton occurred. Twelve hours after TGF-β1 was added, scattered bundles of actin filaments organized in stress fibers became visible, and after 24 and 48 h, the PC-3U cells became large and well spread with fully developed stress fibers as well as cortical bundles of actin filaments (Figure 2B). Treatment with TGF-β1 for 10 min only, after which the ligand was removed by extensive washing, also induced a formation of stress fibers, demonstrating that a short pulse of TGF-β1 stimulation is sufficient to induce the long-term response (our unpublished data). Taken together, these data demonstrated that TGF-β1 was able to induce both a short-term and a long-term reorganization of the actin filament system seen as the formation of membrane ruffles and stress fibers, respectively.
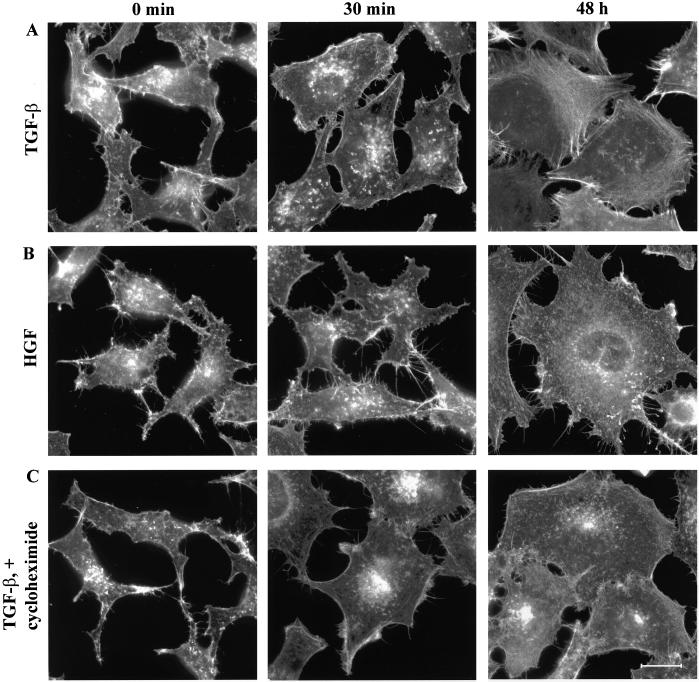
To study whether this effect on the formation of stress fibers was unique for TGF-β1 or whether it could be elicited also by other growth factors, PC-3U cells were stimulated with 25 ng/ml HGF. The HGF-stimulated PC-3U cells formed initial edge ruffles and upon prolonged stimulation they flattened out, however, no stress fibers were visible under the course of the experiment; thus, TGF-β and HGF have distinct effects on the actin filament system in PC-3U cells (Figure 3, A and B). We also tested whether the TGF-β1–induced formation of stress fibers required protein synthesis by pretreating the PC-3U cells with the protein synthesis inhibitor cycloheximide before administration of TGF-β1. The drug did not interfere with TGF-β1–induced membrane ruffles, but the cycloheximide treatment abrogated the formation of stress fibers and cortical actin filament bundles, indicating that these actin rearrangements are dependent on protein synthesis (Figure 3C).
Figure 3.
TGF-β1–induced actin reorganization: dependence on protein synthesis and comparison with the effect of HGF. PC-3U cells were starved in 1% FBS for 12 h and stimulated with 10 ng/ml TGF-β1 for 30 min or 48 h (A), 25 ng/ml HGF for 30 min or 48 h (B), or pretreated with 10 μg/ml cycloheximide for 1 h before TGF-β1 stimulation (C). Filamentous actin was visualized by TRITC-labeled phalloidin. Bar, 20 μm.
TGF-β–induced Actin Reorganization Requires Activity of Rho GTPases
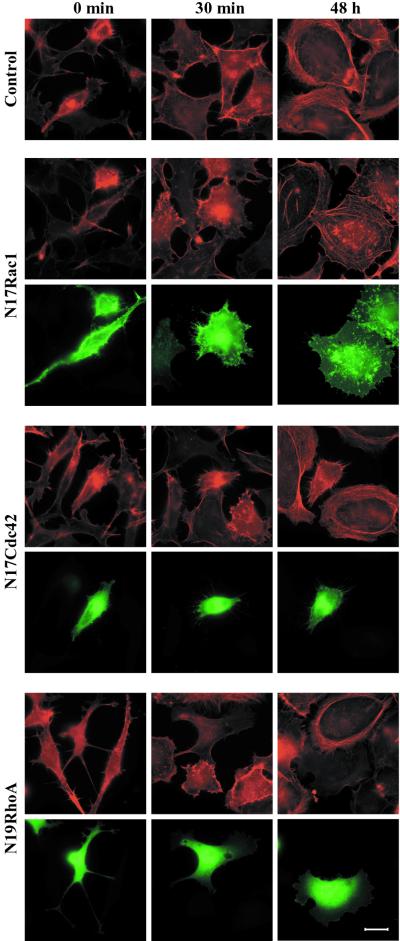
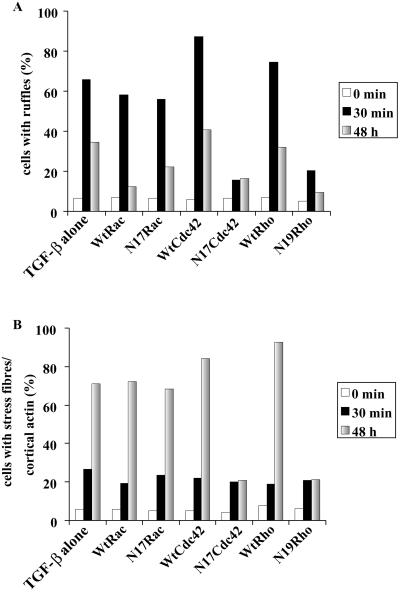
The Rho GTPases have been shown to be pivotal regulators of the actin filament system downstream of tyrosine protein kinase receptors (Van Aelst and D'Souza-Schorey, 1997; Takai et al., 2001). This prompted us to investigate the involvement of the Rho GTPases in the TGF-β1–induced actin reorganization. To this end, we transiently transfected wild-type as well as dominant negative mutants of Rac1, Cdc42, and RhoA (N17Rac1, N17Cdc42, and N19RhoA) into PC-3U cells, which were stimulated with TGF-β1 as before. Transfected cells were analyzed and scored for formation of membrane ruffles and stress fibers or cortical actin (Figures 4; 5). Cells expressing wild-type Rac1 formed membrane ruffles and stress fibers at a normal ratio (Figure 5). The N17Rac1-expressing cells formed membrane ruffles at a normal rate (Figures 4 and 5), and upon prolonged stimulation, N17Rac1 expressing cells flattened out and stress fibers formed at a normal ratio (Figures 4 and 5). These observations indicate that Rac1 is not needed for the TGF-β1–induced reorganization of the actin filament system. Cells transfected with wild-type Cdc42 formed membrane ruffles as well as stress fibers; in contrast, cells transfected with N17Cdc42 exhibited a retracted morphology with numerous protrusions. These cells neither formed membrane ruffles nor stress fibers in response to TGF-β1 (Figures 4 and 5), strongly suggesting that Cdc42 is required both for the short-term and long-term effects of TGF-β1 on the actin filament system. Cells expressing wild-type RhoA responded to TGF-β1 in a normal manner. Cells transfected with N19RhoA, however, flattened out but did not form membrane ruffles. In addition, no stress fibers formed upon TGF-β1 stimulation, indicating that RhoA is partially involved in short-term response and indispensable for the long-term response (Figures 4 and 5).
Figure 4.
Effect of ectopic expression of dominant negative Rac1, Cdc42, and RhoA on TGF-β1–induced actin reorganization. PC-3U cells were starved in 1% FBS for 12 h and stimulated with 10 ng/ml TGF-β1 for 30 min or 48 h before and after transient transfection with pRK5mycN17Rac1, pRK5mycN17Cdc42, or pRK5mycN19RhoA. Filamentous actin was visualized by TRITC-labeled phalloidin. Myc-tagged GTPases were visualized by a myc-specific antibody followed by FITC-labeled anti-mouse antibody. Bar, 20 μm.
Figure 5.
Quantification of the effects of ectopic expression of dominant negative mutants (Figure 4) and wild-type GTPases on the formation of membrane ruffles (A) or the formation of stress fibers and cortical actin (B). The transfected cells were counted under the microscope and scored for formation of membrane ruffles and stress fibers. Each column represents the combined data from at least 250 transfected cells.
TGF-β Stimulation Results in Activation of Cdc42 and RhoA
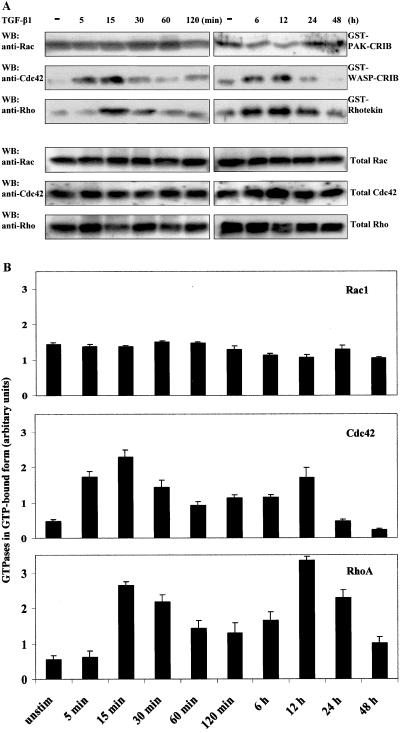
Having established the involvement of Cdc42 and RhoA in the TGF-β–induced reorganization of the actin cytoskeleton, we next studied whether activation of the TGF-β receptor could lead to an accumulation of active, GTP-loaded GTPases. For these studies, we performed GST pull-down assays with GST-fusion proteins of GTPase binding domains of different effectors for Rho GTPases. Cells were treated with TGF-β1 as before and GST-PAK-CRIB, GST-WASP-CRIB, and GST-rhotekin were used to isolate GTP-loaded Rac1, Cdc42, and RhoA, respectively, from cell lysates. The samples were subjected to SDS-PAGE followed by Western blotting with Rac1-, Cdc42-, and RhoA-specific antibodies. Rac1 was found to occur in the GTP-bound form already before stimulation, and TGF-β1 stimulation did not alter the amount of GTP-bound Rac1 (Figure 6, A and B [quantification]). In contrast, GTP-bound Cdc42 was accumulated 5 min after TGF-β1 stimulation, with a peak after 15 min; in addition, a second peak of Cdc42-GTP accumulated after 12 h of stimulation. The RhoA activation occurred slightly delayed compared with the Cdc42 response and also this activation followed a biphasic pattern with a second pronounced peak of RhoA-GTP visible after 12 h (Figure 6). Moreover, the second peak of Cdc42 and RhoA activation was dependent on synthesis of new protein, because pretreatment of the cells with cycloheximide abrogated this second pulse of GTPase activation (our unpublished data).
Figure 6.
Effects of TGF-β stimulation on GTP loading of Rac1, Cdc42, and RhoA. The amount of active, GTP-loaded Rac1, Cdc42, and RhoA was determined by GST pull-down assays with GST-PAK-CRIB, GST-WASP-CRIB, or GST-rhotekin, respectively, from TGF-β1–stimulated PC-3U cells. Rac1, Cdc42, and RhoA were detected by Western blotting by antibodies specific for the respective GTPase (A). Western blots were analyzed by densitometry and the data are combined into diagrams showing the activation of Rac1, Cdc42, and RhoA (B). Each column represents the means from three independent experiments.
TGF-β–induced Formation of Stress Fibers Requires p38MAPK Pathway
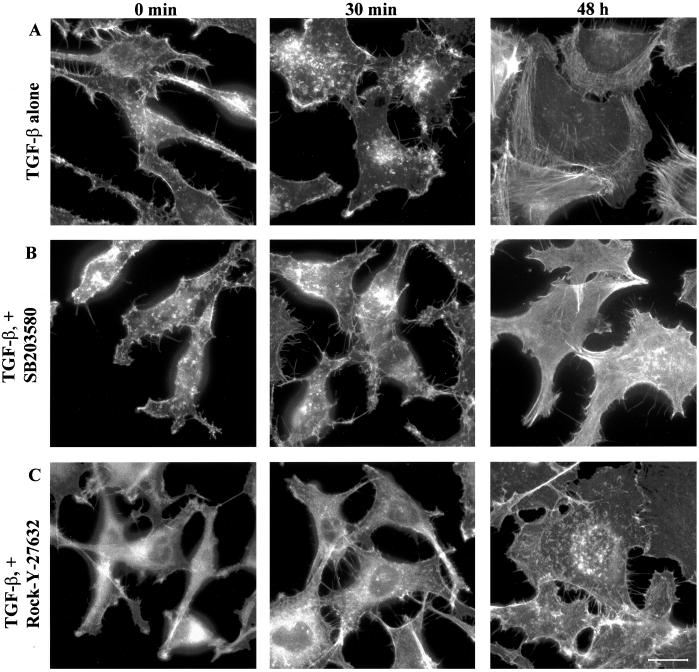
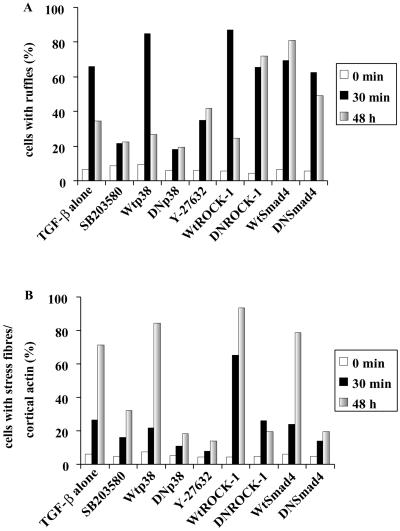
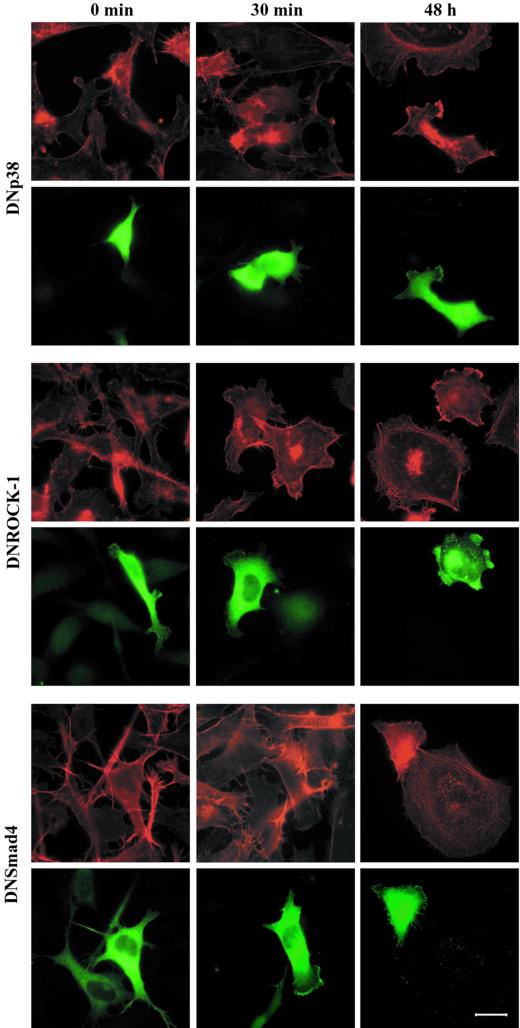
One effector downstream of Cdc42 is p38MAPK and TGF-β stimulation of PC-3U cells has been shown to lead to activation of p38MAPK but not the extracellular signal-regulated kinase (ERK) or stress-activated protein kinase/Jun NH2-terminal kinase (JNK) signaling pathways (our unpublished data). For this reason, we studied whether an inhibitor of p38MAPK, SB203580, could abrogate the reorganization of the of the actin filament system in PC-3U cells. When cells were pretreated with SB203580 and stimulated with TGF-β1 they only marginally flattened out and did not form any membrane ruffles, suggesting an involvement of p38MAPK in the early actin reorganization (Figure 7). The SB203580-treated cells eventually flattened out to some extent, but a clear reduction of the formation of actin stress fibers and, in particular, the cortical actin filaments was observed (Figures 7 and 9 [quantification]), suggesting that the p38MAPK is involved in the TGF-β–induced stress fiber formation. Recently, the SB203580 compound has been shown to inhibit the kinase activity of TGF-β type I receptors, however, at higher concentrations than those needed to inactivate the p38MAPK. We have shown that the concentration of SB203580 used in the present study do not affect the TGF-β–dependent phosphorylation of Smad2 (our unpublished data). In addition, it has been reported that 20 μM SB203580 did not prevent TGF-β–induced phosphorylation and nuclear translocation of Smad2 (Watanabe et al., 2001). To establish that the reduction of stress fibers as well as membrane ruffles was dependent on the inactivation of p38MAPK, we transiently transfected PC-3U cells with a kinase inactive mutant of p38MAPK, which has been shown to inactivate the p38MAPK signaling pathway in a DN manner. Ectopic expression of DNp38MAPK effectively interfered with TGF-β–induced actin; DNp38MAPK-expressing cells did not form stress fibers in response to TGF-β1, in contrast to the surrounding nontransfected neighbors (Figure 8). In addition, we did not observe the short-term membrane ruffle formation, indicating that p38MAPK is involved both in the long-term and short-term effects on the reorganization of the actin filament system (Figures 8 and 9).
Figure 7.
Effect of the p38MAPK inhibitor SB203580 and the ROCK inhibitor Y-27632 on TGF-β1–induced actin reorganization. PC-3U cells were starved in 1% FBS for 12 h and stimulated with 10 ng/ml TGF-β1 for 30 min or 48 h before and after pretreatment with 10 μM SB203580 or 10 μM Y-27632 for 1 h. Bar, 20 μm.
Figure 9.
Quantification of the effects of ectopic expression of dominant negative (Figure 8) and wild-type p38MAPK, Smad4, and ROCK-1 on the formation of membrane ruffles (A) or the formation of stress fibers and cortical actin (B). Transfected cells were counted under the microscope and scored for formation membrane ruffles and stress fibers. Each column represents the combined data from at least 250 transfected cells.
Figure 8.
Effect of ectopic expression of dominant negative mutants of p38MAPK, ROCK-1, and Smad4 on TGF-β1–induced actin reorganization. PC-3U cells were starved in 1% FBS for 12 h and stimulated with 10 ng/ml TGF-β1 for 30 min or 48 h before and after transient transfection with plasmids encoding flag-tagged DNp38MAPK, EGFP-tagged DNROCK-1, or flag-tagged DNSmad4. Filamentous actin was visualized by TRITC-labeled phalloidin. Flag-tagged proteins were visualized by a flag-specific antibody followed by FITC-labeled anti-mouse antibody. Bar, 20 μm.
TGF-β–induced Stress Fiber Formation Requires ROCK
Because TGF-β stimulation led to the activation of RhoA we wanted to investigate whether ROCK-1, a major downstream effector of RhoA, was involved in the TGF-β1–induced actin reorganizations. PC-3U cells were therefore pretreated with the ROCK inhibitor Y-27632 and stimulated with TGF-β1 (Figure 7). Cells treated in this way exhibited the initial flattening out and formation of membrane ruffles, albeit not to the same extent as cells stimulated with TGF-β1 alone; notably, however, no stress fibers developed after prolonged exposure to TGF-β1 (Figures 7 and 9). A similar result was obtained with another ROCK inhibitor, HA-1077 (our unpublished data). Moreover, PC-3U cells transiently transfected with a cDNA expressing a kinase-inactive ROCK-1 mutant, which is known to function in a DN manner, did not form stress fibers in response to TGF-β1 treatment (Figures 8 and 9), further supporting the notion that ROCK-1 is an important component downstream of RhoA in the formation of TGF-β1–induced actin stress fibers.
Intact Smad Signaling Pathway Is Necessary for TGF-β–induced Stress Fiber Formation but Not for TGF-β–induced Membrane Ruffling
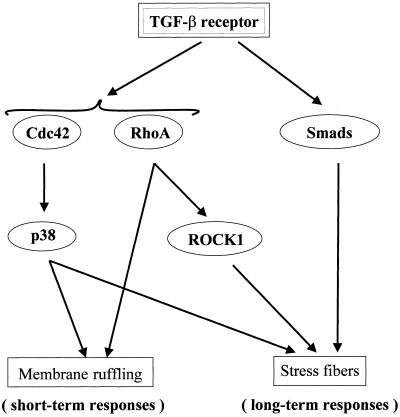
Because the Smad signaling pathway is a major avenue to transduce TGF-β–specific cellular responses we wanted to test the involvement of this pathway in the actin reorganizations. PC-3U cells were transiently transfected with a cDNA encoding a dominant negative mutant Smad4 molecule. This mutant protein did not influence the short-term membrane ruffling, but, interestingly, it abrogated the stress fiber formation (Figures 8 and 9). Thus, although the rapid reorganization of the actin filament system elicited by TGF-β requires the Rho GTPases Cdc42 and RhoA, and the Cdc42 effector p38MAPK, Smad4 is not needed. In contrast, for the long-term effects on formation of stress fibers and cortical bundles of actin Smad4 is essential together with Cdc42 and RhoA and their effectors p38MAPK and ROCK (Figure 10).
Figure 10.
Schematic representation of the tentative mediators of TGF-β–induced short-term and long-term actin reorganization.
DISCUSSION
In this study we have identified rapid as well as delayed reorganizations of the actin filament system in response to TGF-β stimulation. The rapid response did not require protein synthesis and was not affected by ectopic expression of a DNSmad4, suggesting it is independent of the Smad signaling pathway. The TGF-β–induced membrane ruffles resemble the response elicited by tyrosine kinase receptors such as the PDGF receptor (Mellström et al., 1983; Heldin et al., 1998). However, there are important differences. First, membrane ruffling mediated by the PDGF receptor occurs within a minute of stimulation, whereas the TGF-β–induced membrane ruffles did not become apparent until after 5 min of stimulation. Second, there appears to be a strong requirement of Rac1 for the formation of PDGF-induced membrane ruffles (Ridley et al., 1992); in contrast, TGF-β activation selectively activated Cdc42 and RhoA, but not Rac1. In fact, we could not record any effect on the formation of activated, GTP-bound Rac1 in response to TGF-β. These observations illustrate that similar effects on actin reorganization can be achieved using different intracellular signaling pathways.
The delayed response was visible after 24–48 h of TGF-β stimulation as a formation of actin stress fibers through a process that required Smad4 and new protein synthesis. Effects of TGF-β on actin stress fibers have also been reported by Like and Massagué (1986), Koyasu et al. (1988), and Lee et al. (1999). TGF-β–mediated effects on cell morphology occur in the process of epithelial to mesenchymal transdifferentiation. This phenomenon was first reported in mouse breast epithelial NMuMg cells, where TGF-β stimulation results in a dramatic morphological alteration: the rhomboid epithelial cells lose their cell-cell contacts, flatten out, and obtain a fibroblast-like morphology (Miettininen et al., 1994). This process occurred >48 to 72 h and was accompanied with a down-regulation of E-cadherin and a formation of stress fibers (Piek et al., 1999). This response was shown to be dependent on gene transcription via the Smad signaling pathway (Piek et al., 1999). Our PC-3U cells do not exhibit an epithelial morphology, making it difficult to compare the long-term actin reorganization with the process of epithelial to mesenchymal transdifferetion of NMuMg cells. The TGF-β–induced actin reorganization might represent a migratory cue in PC-3U cells. TGF-β has been shown to induce migration of several cell types such as epithelial cells (Boland et al., 1996; Zicha et al., 1999) and mast cells (Olsson et al., 2000).
The initial response on actin reorganization in PC-3U cells is Smad4 independent. There are several reports on Smad4-independent responses in TGF-β signaling, for instance, seen as TGF-β–induced activation of the ERK, JNK, and p38MAPK signaling pathways (reviewed in Mulder, 2000). TGF-β–induced fibronectin synthesis in human fibrosarcoma cells was found to be independent of Smad4 but to require the JNK signaling pathway (Hocevar et al., 1999). In addition, there is cross-talk between Smad and p38MAPK pathways (Engel et al., 1999; Hanafusa et al., 1999; Sano et al., 1999; Watanabe et al., 2001), and TGF-β-Smad and Ras-ERK signaling pathways (Oft et al. 1996; Kretzschmar et al., 1999; Lo et al., 2001). These reported responses appeared to depend, at least partially, on experimental conditions and cell type used in the experiments (Mulder, 2000). The previously reported Smad4-independent signals are long-term effects of TGF-β stimulation; our findings in the present study show that also rapid responses of TGF-β can be Smad4 independent.
Rho GTPases have been implicated to have roles in TGF-β signaling. One study showed that Rac1 contributes to TGF-β–mediated gene transcription (Mucsi et al., 1996). In Drosophila, the TGF-β ortholog Dpp has been implicated as an activator of Dcdc42 (Ricos et al., 1999). It has also been suggested that RhoA, but not Cdc42, has a role in epithelial to mesenchymal transdifferentiation of NMuMg cells (Bhowmick et al., 2001). Furthermore, TGF-β has been shown to induce stress fibers in HA-Ras–transformed fibroblasts in a Rho-dependent manner (Moustakas and Stournaras, 1999). In our case, we see a clear dependency of Cdc42 and RhoA, but not Rac1, in the reorganization of the actin filament system. Interestingly, the Rho-specific guanine exchange factor NET1 was recently shown to be essential for the TGF-β–mediated formation of stress fibers in Swiss 3T3 cells (Shen et al. 2001); whether NET1 is involved in the TGF-β–induced activation of RhoA in PC-3U cells is an interesting possibility that remains to be investigated. The mechanism by which the TGF-β receptor activates Cdc42 is not clear but possibly also involve activation of an exchange factor.
The present study indicates an important involvement of the Rho GTPases Cdc42 and RhoA in TGF-β signaling. Because Rho GTPases have such an important impact on the organization of the cytoskeleton it is pertinent to ask whether the cytoskeleton has a regulatory role in TGF-β signaling. Interestingly, nuclear entry of Smad proteins seem to be regulated by the microtubule system (Dong et al., 2000). It is an intriguing possibility, which deserves further exploration, that Rho GTPases and the cytoskeleton also have important roles in TGF-β signaling.
ACKNOWLEDGMENTS
We acknowledge generous gifts of rat basophilic leukemia RBL-2H3 cells from Dr. P. Chavrier (Institute Curie, Paris, France) and a partial clone encoding human rhotekin from Dr. T. Reid (Universite Paris-Sud, Paris, France). We thank Aris Moustakas for critical comments on this manuscript. This work was supported in part by grants from the Swedish Cancer Society (to P.A. and M.L.).
Abbreviations used:
- ERK
extracellular singnal regulated kinase
- JNK
Jun Nh2-terminal kinase
- MAPK. mitogen-activated protein kinase
PAK, p21-activated kinase
- ROCK
Rho-associated coiled-coil-containing protein kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0398. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0398.
REFERENCES
- Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich Syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Baghdassarian D, Toru-Delbauffe D, Gavaret JM, Pierre M. Effects of transforming growth factor-β1 on the extracellular matrix and cytoskeleton of cultured astrocytes. Glia. 1993;7:193–202. doi: 10.1002/glia.440070302. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland S, Boisvieux-Ulrich E, Houcine O, Baeza-Squiban A, Pouchelet M, Schoëvaërt D, Marano F. TGF-β1 promotes actin cytoskeleton reorganization and migratory phenotype in epithelial tracheal cells in primary culture. J Cell Sci. 1996;109:2207–2219. doi: 10.1242/jcs.109.9.2207. [DOI] [PubMed] [Google Scholar]
- Chinkers M, McKanna JA, Cohen S. Rapid induction of morphological changes in human carcinoma cells A-431 by epidermal growth factors. J Cell Biol. 1979;83:260–265. doi: 10.1083/jcb.83.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Microtubule binding to Smads may regulate TGFβ activity. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Franzén P, Ichijo H, Miyazono K. Different signals mediate transforming growth factor-β1-induced growth inhibition and extracellular matrix production in prostatic carcinoma cells. Exp Cell Res. 1993;207:1–7. doi: 10.1006/excr.1993.1156. [DOI] [PubMed] [Google Scholar]
- Guillemot JC, Montcourrier P, Vivier E, Davoust J, Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPases Rac1 and CDC42 in FcεRI-activated rat basophilic leukemia (RBL-2H3) cells. J Cell Sci. 1997;110:2215–2225. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- Hata A, Lo RS, Wotton D, Lagna G, Massagué J. Mutations increasing autoinhibition inactivate tumor suppressors Smad2 and Smad4. Nature. 1997;388:82–87. doi: 10.1038/40424. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Östman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochem Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S, Kadowaki T, Nishida E, Tobe K, Abe E, Kasuga M, Sakai H, Yahara I. Alteration in growth, cell morphology, and cytoskeletal structures of KB cells induced by epidermal growth factor and transforming growth factor-β. Exp Cell Res. 1988;176:107–116. doi: 10.1016/0014-4827(88)90125-5. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Park J, Kim JH, Yie SW, Chun G-T, Kim P-H, Choi EY. Reorganisation of myosin and focal adhesion proteins in Swiss 3T3 fibroblasts induced by transforming growth factor beta. Cell Biol Int. 1999;23:507–517. doi: 10.1006/cbir.1998.0401. [DOI] [PubMed] [Google Scholar]
- Like B, Massagué J. The antiproliferative effect of type beta transforming growth factor occurs at a level distal from receptors for growth-activating factors. J Biol Chem. 1986;261:13426–13429. [PubMed] [Google Scholar]
- Lo RS, Wotton D, Massagué J. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J. 2001;20:128–136. doi: 10.1093/emboj/20.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomri A, Marie PJ. Effects of transforming growth factor type beta on expression of cytoskeletal proteins in endosteal mouse osteoblastic cells. Bone. 1990;11:445–451. doi: 10.1016/8756-3282(90)90141-k. [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen Y-G. Controlling TGF-β signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mellström K, Höglund A-S, Nistér M, Heldin C-H, Westermark B, Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983;4:589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- Miettininen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Stournaras C. Regulation of actin organization by TGF-β in H-ras-transformed fibroblasts. J Cell Sci. 1999;112:1169–1179. doi: 10.1242/jcs.112.8.1169. [DOI] [PubMed] [Google Scholar]
- Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-β1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- Mulder KM. Role of ras and Mapks in TGFβ signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Yoshimura K, Nomura M, Nakamura H. Antisense oligonucleotide complementary to smooth muscle α-actin inhibits endothelial-mesenchymal transformation during chick cardinogenesis. Dev Dyn. 1999;216:489–498. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<489::AID-DVDY17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Oft M, Peli J, Rudaz C, Schwartz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-β family. J Leukoc Biol. 2000;67:350–356. doi: 10.1002/jlb.67.3.350. [DOI] [PubMed] [Google Scholar]
- Piek E, Moustakas A, Kurisaki A, Heldin C-H, ten Dijke P. TGF-β type I receptor/A.L.K-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in N.MuM.G. breast epithelial cells. J Cell Sci. 1999;112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Ricos MG, Harden N, Sem KP, Lim L, Cha W. Dcdc42 acts in TGF-β signaling during Drosophila morphogenesis: distinct roles for the Drac1/JNK and Dcdc42/TGF-cascades in cytoskeletal regulation. J Cell Sci. 1999;112:1225–1235. doi: 10.1242/jcs.112.8.1225. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L, Heldin C-H. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int J Cancer. 2001;91:757–762. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1136>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mendeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J Biol Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Shen X, Li J, Pei-chih P, Waddel D, Zhang J, Wang X-F. The activity of guanine exchange factor NET1 is essential for transforming factor-β-mediated stress fiber formation. J Biol Chem. 2001;276:15362–15368. doi: 10.1074/jbc.M009534200. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 Mitogen-activated protein kinase pathway regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276:14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Derynck R. Regulation of Smad signaling by protein associations and signaling crosstalk. Trends Cell Biol. 1999;9:274–279. doi: 10.1016/s0962-8924(99)01579-2. [DOI] [PubMed] [Google Scholar]
- Zicha D, Genot E, Dunn GA, Kramer IM. TGFβ1 induces a cell-cycle-dependent increase in motility of epithelial cells. J Cell Sci. 1999;112:447–454. doi: 10.1242/jcs.112.4.447. [DOI] [PubMed] [Google Scholar]