Abstract
A growing body of evidence suggested that gut microbiota is associated with liver diseases through the gut–liver axis. The imbalance of gut microbiota could be correlated with the occurrence, development, and prognosis of a series of liver diseases, including alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), viral hepatitis, cirrhosis, primary sclerosing cholangitis (PSC), and hepatocellular carcinoma (HCC). Fecal microbiota transplantation (FMT) seems to be a method to normalize the patient’s gut microbiota. This method has been traced back to the 4th century. In recent decade, FMT has been highly regarded in several clinical trials. As a novel approach to reconstruct the intestinal microecological balance, FMT has been used to treat the chronic liver diseases. Therefore, in this review, the role of FMT in the treatment of liver diseases was summarized. In addition, the relationship between gut and liver was explored through the gut–liver axis, and the definition, objectives, advantages, and procedures of FMT were described. Finally, the clinical value of FMT therapy in liver transplant (LT) recipients was briefly discussed.
Keywords: fecal microbiota transplantation, chronic liver diseases, gut microbiota, gut-liver axis, liver transplantation
1. Introduction
As a novel approach to reconstruct the intestinal microecological balance, fecal microbiota transplantation (FMT) has been gradually and widely practiced in the treatment of a variety of diseases in recent decade. This method transfers processed fecal materials from healthy donors to patients to rebuild the balance of their gut microbiota [1]. Under normal conditions, the human gastrointestinal (GI) tract, which is colonized with numerous bacterial species, individually differs and is relatively stable over time [2], while several genetic backgrounds and environmental factors, such as diet, viruses, and use of drugs can alter the balance and further cause a variety of diseases [3,4]. On the other hand, some diseases (e.g., chronic liver diseases) can also break the balance of gut microbiota. In 2013, Els et al. performed the first randomized controlled trial and demonstrated that duodenal infusion of donor feces into patients with Clostridium difficile infection (CDI) had a significant efficacy in resolving symptoms than use of antibiotics alone [5]. To date, FMT has earned endorsement of professional societies in the treatment of antibiotic-refractory CDI [6,7]. In addition, FMT has been applied to treat other diseases, such as autoimmune diseases, behavioral diseases, metabolic disorders, and organic diseases.
In fact, a great number of studies demonstrated that gut microbiota is associated with liver diseases. In 1987, it was first found that the relationship between the gut and liver was bidirectional and a cyclic process, and this physiological process was described as the gut–liver axis [1]. Accordingly, in recent two decades, researchers demonstrated that prebiotics could alleviate fat mass development and associated hepatic steatosis. Moreover, the imbalance of gut microbiota could result in the occurrence and development of a series of liver diseases, including alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), viral hepatitis, cirrhosis, primary sclerosing cholangitis (PBC), hepatocellular carcinoma (HCC), and even hepatic encephalopathy (HE) [8,9,10,11,12,13]. Based on these backgrounds, FMT has been increasingly applied in various types of chronic liver diseases [14]. As a whole, the use of FMT in liver diseases is still in the initial stage. The present review aimed to mainly concentrate on the application of FMT in the treatment of liver diseases. In addition, the relationship between gut and liver was explored through the gut–liver axis, and the definition, objectives, advantages, and technical process of FMT were described. Finally, the clinical value of FMT in liver transplant (LT) recipients was briefly discussed.
2. The Gut Microbiota and the Gut–Liver Axis
The gut microbiota consists of millions of species, with weight of approximately 1–2 kg [15,16]. The gut microbiota has been considered as an indispensable “organ” [17]. In recent twenty years, the advent of genetic tools and the metagenomics assisted scholars to realize the composition and function of gut microbiota and their association with several potential diseases. The gut microbiota has important functions in hormonal responses, inflammatory pathways, immune reactions, and metabolites [18].
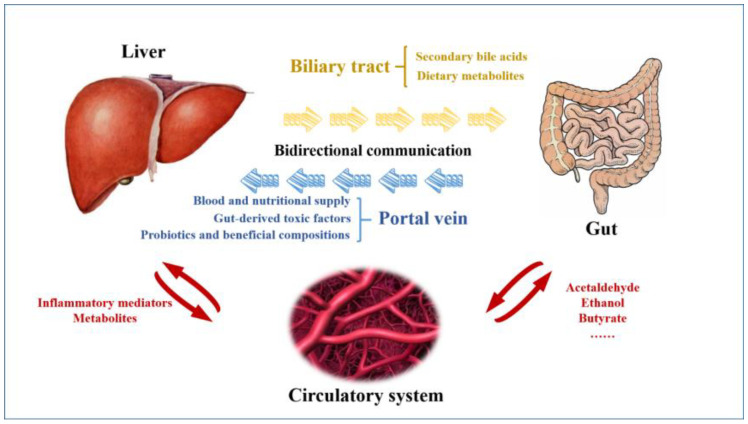
To date, alteration of gut microbiota has been reported to be associated with numerous liver diseases, which could be related to the existence of the gut–liver axis (Figure 1). Generally, the gut–liver axis refers to the bidirectional communication between GI tract and liver by biliary tract, portal vein, and systemic circulation [19]. Through portal vein, liver mainly receives almost two-thirds of its blood and nutritional supply from the gut, as well as gut-derived toxic factors, such as metabolites, damage/pathogen-associated molecular patterns, and detrimental microbiota [20]. These detrimental factors stimulate hepatocytes and hepatic immune cells, activate inflammation-related pathways, and finally cause liver diseases. On the one hand, probiotics and beneficial compositions from the gut can protect liver through the gut–liver axis [21]. On the other hand, liver regulates the intestinal function and balance of gut microbiota through circulation of bile acid. Therefore, the close interaction between gut and liver may be a very important factor in the pathogenesis of liver diseases.
Figure 1.
The diagrammatic representation of the gut–liver axis. The gut–liver axis refers to the bidirectional communication between the gastrointestinal tract and the liver by the biliary tract, the portal vein, and systemic circulation. Through portal vein, liver mainly receives blood and nutritional supply, as well as gut-derived toxic factors. Probiotics and beneficial compositions from the gut can also protect liver through the gut–liver axis. Liver regulates the intestinal function and balance of gut microbiota through the bile acid.
3. Fecal Microbiota Transplantation (FMT)
3.1. Definition, Objectives, and Advantages
FMT is also known as stool transplantation. FMT is a procedure, in which stool from a healthy donor is placed into another patient’s GI tract [22]. The components of the fecal transplants contain about 55% of microbiota and 24% of soluble components, including mucus, fat, proteins, small molecules, short chain fatty acids, etc. [23]. To date, FMT has been widely used in the treatment of recurrent CDI [24]. In addition, with the fast development of high-throughput sequencing technologies, a variety of diseases, such as diabetes, various types of cancer, and organ diseases were found to be associated with the gut microbiota [25]. Compared with using antibiotics to eliminate specific pathogenic strains and administration of specific probiotics, FMT can transfer a more complete and stable fecal microbial community of gut micro-organisms [26]. Therefore, FMT possesses greater advantages. After allogeneic organ transplantation, rejection may be caused by the immune system, triggering a response that will ultimately destroy the transplanted organ or tissue. FMT can also be a type of transplantation, while it does not exist as an immune response, which differs from other types of organ transplantation.
3.2. Preparation and Delivery
For FMT, fecal stools are mainly obtained from selected healthy donors, which can restore the balance of healthy microbiota and facilitate clear infection for patients. Before FMT, fecal stools need to be processed and prepared, and they can be then transplanted into recipients [25]. The processes of FMT preparation and delivery are briefly discussed in the following sections.
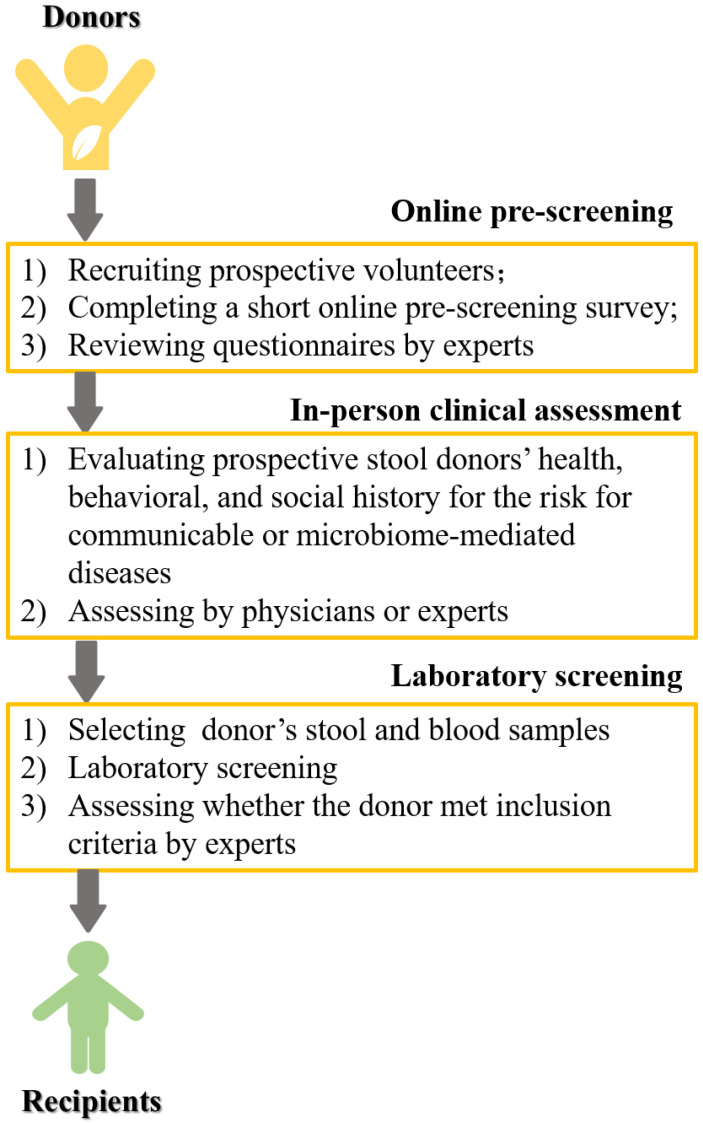
In fact, similar to organ transplantation, fecal stools for FMT are mainly obtained from healthy and selected donors. Before the emergence of stool banks, finding and screening eligible donors were challenging [27]. Stool banks have recently emerged, while their availability is limited. Generally, the process of donor screening includes online pre-screening, clinical assessment, and laboratory screening [28] (Figure 2). Before being transplanted into patients or recipients, fecal stools need to be processed and prepared. The commonly used methods have been described in Biazzo et al.’ review [25], while the detailed methods vary among different studies. In the stage of sample preparation, after obtaining stool samples from healthy donors, they mainly need to be homogenized and liquefied by blending with sterile saline. Afterwards, residual solid feces are filtered out through metal sieve. Then, homogenous liquid samples can be achieved. In the stage of microbiota preparation, fresh stools are transferred into frozen stools. Finally, these treated samples are kept at −80 °C for later FMT. When patients need FMT, frozen samples are thawed at 4 °C and reconstituted with normal saline. It is noteworthy that the standards are not widely accepted for the quality and safety control of microbiota. A previous study reported a female case who underwent FMT for recurrent and developed new-onset obesity after receiving stool from a healthy overweight donor [29]. Therefore, additional clinical studies should be performed to acquire more reliable evidence about the standard of FMT.
Figure 2.
The process of FMT donor selection.
4. Fecal Microbiota Transplantation (FMT) in the Treatment of Liver Diseases
As mentioned earlier, it was confirmed that the gut–liver axis plays an important and critical role in progression of liver diseases. Disturbances in the intestinal barrier may increase the portal influx of bacteria and their products into the liver, and further worsen a range of hepatic diseases [30,31]. Recently, a growing body of evidence demonstrated that dysfunction of gut microbiota plays a key role in the pathogenesis of ALD and NAFLD. Furthermore, other liver-associated infections, autoimmune hepatitis (AIH), and HCC have been demonstrated to be caused by dysfunction of gut microbiota. In the present study, application of FMT in the treatment of liver diseases was reviewed and discussed. The clinical trials were summarized in Table 1.
4.1. Hepatitis B Virus (HBV) Infection
HBV infection is one of the most common public health challenges in the world, and about 15–40% of HBV-infected patients may finally develop chronic liver diseases, including cirrhosis, liver failure, and even HCC [32]. The ideal endpoint of HBV-infected patients is hepatitis B surface antigen (HBsAg) loss [33]. For HBV e-antigen (HBeAg)-positive chronic hepatitis B (CHB) patients, HBeAg seroconversion is mainly the first step for treatment [34]. With the significant advances in the treatment of HBV infection, several approved therapies, including oral nucleos(t)ide analogue(s)—entecavir (ETV), tenofovir disoproxil fumarate (TDF)/tenofovir, alafenamide (TAF), and peg-interferon can be used [35]. Despite using these methods, only few patients could obtain HBeAg clearance or seroconversion, even after multiple years of antiviral therapy [36]. The specific reason has not been clearly expounded. Over the past few years, some studies demonstrated that recent therapies neglected the role of gut microbiota, and it may play a key role in immune clearance of HBV [37]. Several pilot trials with the small sample size have been conducted to explore the therapeutic effects of FMT on CHB patients. In 2017, Ren et al. first carried out a case-controlled, open-label pilot study on the application of FMT in 18 CHB patients who remained HBeAg-positive, following >3 years of ongoing ETV- or TDF-based antiviral therapy [36]. Among them, 5 patients were included in the FMT arm who received 1–7 cycles of FMT, and 40% (2/5) of patients achieved HBeAg clearance after 1–2 cycles of FMT. In 2021, Chauhan et al. performed another similar pilot study, and their results showed that in the FMT arm, 16.7% (2/12) of patients had HBeAg clearance. While in the AVT arm, no patient achieved HBeAg clearance [35]. These clinical studies confirmed the significant effects of FMT on stubborn CHB patients. However, more evidence from large-scale prospective studies is required.
Several studies attempted to explain the mechanism underlying whether the gut microbiota composition could affect the HBV infection. The CHB infection has been found to be associated with the dysfunction of HBV-specific immune responses, causing failure in the treatment of infected hepatocytes [38]. Using animal models, some risk factors, such as use of antibiotics, have been demonstrated to impair gut barrier function and increase gut permeability, leading to commensal bacterial translocation from the gut to the liver, suppressed T-cell response in the liver, and prolonged HBV infection [39]. In addition, the genetic background is a key factor in determining outcomes of patients with HBV infection. Wang et al. found that HBV infection was only persisted in C57BL/6J mice, rather than in C57BL/6N mice [40], and another study showed that in different strains of mice, duration of HBV infection was significantly different [41]. Furthermore, it has been reported that HBV infection could alter the intestinal microbiota. For instance, compared with healthy controls, the levels of Bifidobacteria and Lactobacillus were higher, and the levels of Enterococcus and Enterobacteriaceae were lower in CHB patients [42]. Moreover, compared with healthy controls, Enterobacteriaceae, Faecalibacterium prausnitzii, and Enterococcus faecalis showed a noticeable increase in asymptomatic HBV carriers, and the increased range was significantly greater in HBV patients [43]. These changes caused an increase in bacterial translocation and endotoxin load, in which activation of Toll-like receptor (TLR) facilitated immune-mediated liver injury.
4.2. Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is one of the most common liver diseases, influencing 10–24% of world’s population [18]. NAFLD includes simple steatosis, nonalcoholic steatohepatitis (NASH), more severe cirrhosis as end-stage organ failure, and HCC. Recent studies have demonstrated that NAFLD was associated with a series of metabolic diseases, including obesity, type 2 diabetes mellitus (T2DM), and even cancer [44,45,46]. FMT has been reported as a potential therapeutic method for several metabolic diseases. In 2013, Roy et al. first found that NAFLD could be transmitted by FMT in mice [47]. Subsequently, Zhou et al. established a mouse model of high-fat diet (HFD)-induced steatohepatitis. After an eight-week HFD, FMT was carried out for eight weeks. This experiment demonstrated that the bacterial antigen translocation caused systemic inflammation in NAFLD patients [48]. FMT intervention could correct the gut microbiota disturbance and reverse steatohepatitis in mice fed with HFD [49]. In addition, Gomez-Hurtado et al. concentrated on the application of FMT in patients with NAFLD [50]. In this randomized clinical trial, a total of 47 patients with NAFLD were randomly assigned to FMT group who received FMT from healthy donors. Compared with patients in the non-FMT group (N = 28) who received original treatment, the clinical symptoms of NAFLD in patients undergoing FMT were significantly improved, confirming that the FMT has potential therapeutic effects on NAFLD. In addition, numerous ongoing trials concentrated on FMT and its potential in NAFLD and NASH (Identifiers: NCT02496390 and NCT02469272, respectively).
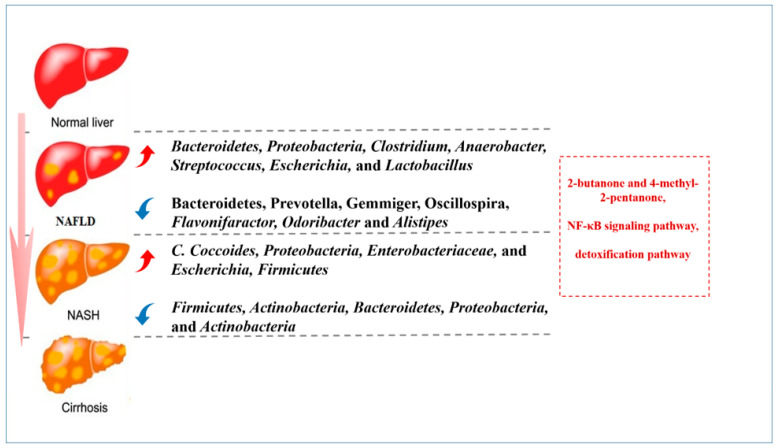
The potential mechanism indicating how the gut microbiota could affect NAFLD has been widely discussed in several studies (Figure 3). It has been reported that patients with NAFLD had significantly increased levels of Clostridium, Anaerobacter, Streptococcus, Escherichia, and Lactobacillus, and reduced levels of Flavonifaractor, Odoribacter, Alistipes, etc. [51]. While in patients with NASH, the levels of Proteobacteria, Enterobacteriaceae, Escherichia, etc., were higher [52]. These alterations can induce the levels of 2-butanone and 4-methyl-2-pentanone, which are associated with hepatocellular toxicity [53]. Additionally, a review expounded that enrichment of ethanol-producing bacteria could cause abundant ethanol in the body of patients with NALFD, which could activate nuclear factor-κB (NF-κB) signaling pathway and cause liver damage [54]. Furthermore, detoxification pathway was weakened in patients with NALFD, which could cause oxidative injury to the hepatocytes and induce inflammation and steatohepatitis [55].
Figure 3.
The potential signaling pathways and alteration of gut microbiota in patients with NAFLD/NASH. The figure shows the alteration of gut microbiota and potential signaling pathways in the process from normal liver to NAFLD/NASH, and finally to cirrhosis.
4.3. Alcoholic Liver Disease (ALD)
ALD is a spectrum of diseases, ranging from asymptomatic liver steatosis to the development of fibrosis, cirrhosis, and alcoholic hepatitis [56]. According to epidemiological data, about 20–30% of patients with a history of alcohol misuse progress to liver damage, and even liver cirrhosis or alcoholic hepatitis [57]. Recently, ensuring lasting alcohol abstinence is the key to prevent the occurrence and development of ALD. Only one-third of patients with alcoholic hepatitis are eligible for steroid therapy. In the late stage of ALD, LT is necessary, while it remains controversial whether it may be effective in the risk of infections, post-transplant recurrence, and long-time transplant waitlist. Over the past few years, studies demonstrated that gut microbiota played a key role in the progression of ALD [58,59]. Ciocan et al. enrolled patients with various degrees of ALD and assessed their structure of intestinal microbiota and function in bile acid homeostasis. They found that patients with cirrhosis and ALD had a higher level of total plasma bile acid, whereas levels of total and secondary bile acids in these cases were lower than those in healthy donors. Furthermore, ALD patients had a higher abundance of Actinobacteria and a lower level of Bacteroidetes [60]. Bajaj et al. summarized investigations into ALD and microbiota composition and function in humans in a review on ALD and the gut microbiota [56]. Using this review it was confirmed that patients with different degrees of ALD have varying structures and levels of microbiota, proving a close association between the gut microbiota and ALD. Moreover, numerous studies revealed the special mechanism indicating how the specific intestinal microbiota could affect ALD. For instance, using a mouse model of ALD, Wrzosek et al. found that tryptophan metabolism induced aryl hydrocarbon receptor activation and improved alcohol-induced liver injury [61]. In addition, ursolic acid has been reported to ameliorate intestinal oxidative stress and barrier dysfunction induced by alcohol [62].
According to these positive results from animal studies, in the last five years, some scholars attempted to certify whether FMT could treat ALD in clinic. In 2017, Philips et al. first reported a patient with severe alcohol hepatitis who was a steroid non-responder and underwent FMT [63]. In this case, the patient’s clinical, biochemical, and liver disease severity scores were significantly improved after FMT, which demonstrated that a distinct bacterial population changed before and after FMT. Subsequently, another open-label study was performed with follow-up of 3 months to compare the outcomes in patients with severe alcoholic hepatitis using different methods, including nutritional therapy (n = 17), corticosteroid therapy (n = 8), pentoxifylline therapy (n = 10), and FMT (n = 16) from healthy donors [64]. This clinical trial finally indicated that FMT for severe alcoholic hepatitis could improve survival beyond what is suggested by other therapies. After 1–2 years, the relative abundance of Porphyromonas was significantly lower and that of Bifidobacterium was higher in patients who underwent FMT than in patients who underwent corticosteroid therapy [65]. Furthermore, FMT could function as a cost-effective bridge to LT or to improve survival without transplantation. FMT was also demonstrated as a safe therapeutic approach to reduce the incidence of ALD [66]. Based on the clinical evidence, FMT is suggested as a safe and efficient therapy for ALD, especially for non-corticosteroid-responsive patients and without history of undergoing LT.
4.4. Autoimmune Hepatitis (AIH)
AIH is an entity of chronic and immune-mediated hepatitis characterized by hepatocyte injury, with the presence of circulating autoantibodies and elevated level of serum immunoglobulin G (IgG) [67]. AIH can occur globally in all ethnicities and affect both children and adults with an increasing morbidity [68]. At present, the main pathogenetic mechanism of AIH is considered as a loss of tolerance against the patient’s own liver antigens, which is potentially triggered by both genetic and environmental risk factors, such as xenobiotics and pathogens [69,70]. AIH may develop to liver cirrhosis and HCC, and it may even lead to fulminant hepatic failure. AIH can be classified into juvenile AIH (including AIH-1 and AIH-2) and adult AIH (mainly AIH-1) on the basis of the age profile [68]. In fact, triggers of AIH are very complex and have not yet been identified. For treatment, AIH patients favorably respond to corticosteroids, while some patients who are irresponsive to standard treatment may quickly develop to fibrosis and cirrhosis [71]. Therefore, development of effective therapies for patients who are irresponsive to corticosteroids is essential.
In recent years, evidence from murine models exhibited that the gut microbiota is an important environmental risk factor, participating in the pathogenesis of AIH [72,73,74]. Wei et al. performed a cross-sectional study on 91 patients with AIH and 98 healthy controls by 16S rRNA gene sequencing, and the results showed that compared with healthy controls, the gut microbiome of patients with AIH before steroid treatment was accompanied by a lower alpha-diversity and a distinct microbial composition [75]. The research provided evidence for compositional and functional alterations of gut microbiome in AIH, which suggested the potential of using gut microbiota as a biomarker to assess the incidence of AIH. In addition, some similar studies also proved that gut microbiota dysfunction had a functional association with the incidence of AIH [76,77]. Accordingly, Liang et al. established a mouse model of AIH to analyze the therapeutic effects of FMT [78]. This study showed that FMT could modulate imbalance of T follicular regulatory (TFR) and T follicular helper (TH) cells and gut microbiota composition by immune pathways in vivo. Furthermore, metabolite pathways (e.g., short-chain fatty acid, amino acid, and bile acid) and receptor pathways (e.g., Toll-like receptor 4 (TLR4), and G protein-coupled receptors (GRP41/GPR43, GPR109a) in the intestine, while nucleotide-binding and oligomerization domain NOD-like receptors (NLRs), TLR4, TLR9, and GPBAR1 in liver) were found as the influential mechanisms of altered gut microbiota in AIH [79]. The above-mentioned evidence verified the underlying therapeutic value of FMT for AIH. However, to our knowledge, no clinical study has yet assessed the therapeutic value of FMT for AIH.
4.5. Primary Sclerosing Cholangitis (PSC)
PSC is a chronic immune-related cholestatic liver disease, which can lead to cholestasis, bile duct stenosis, and hepatic fibrosis [80]. A previous study demonstrated that PSC is closely associated with inflammatory bowel disease (IBD) [81], suggesting that gut microbiota plays a key role in the PSC. Compared with healthy controls, PSC and PSC-IBD patients have significantly distinct gut microbial profiles with decreasing expression of Prevotella copri (P. copri) [82]. Several studies demonstrated that P. copri could improve glucose homeostasis with GI resection by enhancing the bile acid metabolism and signaling [83], and promote immune tolerance [84]. On the other hand, genome-wide association studies have identified that some loci are correlated with both microbiome composition and PSC, including fucosyltransferase 2 (FUT2) [85]. The protective role of gut microbiota in PSC has been demonstrated using germ-free multidrug resistance 2 knockout (mdr2−/−) mouse models [86]. Of these, the gut microbiota has emerged as a key environmental risk factor for PSC as an inflammatory disease [87].
For the treatment of PSC, there is no effective therapy, and LT seems to be the only therapeutic option [88]. However, there is still a risk of recurrent PSC after LT. According to the protective role of gut microbiota in PSC, over the past few years, additional studies have concentrated on the therapeutic effects of gut microbiota on PSC. In addition to oral antibiotics (vancomycin, metronidazole, and minocycline), few studies have assessed the therapeutic effects of FMT on PSC. In 2019, Allegretti et al. performed an open-label pilot study on 10 patients with PSC-IBD. These patients underwent FMT, and 30% of them experienced a more than 50% decrease in alkaline phosphatase (ALP) levels. Moreover, no relevant adverse event occurred [89]. Philips et al. reported a single case which received FMT for recurrent bacterial cholangitis in PSC [90]. Following FMT, the patient’s liver biochemistry, bile acid, and bacterial community were significantly improved, suggesting the applicability of FMT in the treatment of PSC. However, further evidence is required to verify the above-mentioned findings.
Table 1.
Studies related to the application of FMT in the treatment of liver diseases (without case reports).
| Diseases | Clinical Trials (Published) | Participants | Outcomes |
|---|---|---|---|
| HBV infection | [35] | AVT: 15 FMT: 12 |
(1) In the FMT arm, 16.7% (2/12) patients had HBeAg clearance in comparison to none in the AVT arm (p = 0.188). (2) None of the patients in either arm had HBsAg loss. (3) The FMT was tolerated well, 42.8% (6/14) patients reported one or more minor adverse events. |
| [36] | Control: 13 FMT: 5 |
(1) A significant decline in HBeAg titer rather than HBsAg titer was observed in the FMT arm compared to that at the baseline. HBeAg titer declined gradually after each time of FMT. (2) None of the control patients achieved HBeAg clearance (0/13) at the conclusion of the study. (3) ALT and HBV DNA were also detected at 4 weeks after each time of FMT and remained under the lower limit of detection. (4) No HBeAg seroconversion was observed at the end of follow-up. |
|
| NAFLD | [51] | Oral probiotics: 28 FMT: 47 |
(1) FMT can decrease the fat accumulation in the liver by improving the gut microbiota dysbiosis. (2) Significant differences in the clinical features and gut microbiota between lean and obese NAFLD patients. (3) FMT had better effects on gut microbiota reconstruction in lean NAFLD than in obese NAFLD patients |
| ALD | [65] | Treated with corticosteroids: 8 Nutritional support only: 17 Pentoxifylline: 10 FMT: 16 |
(1) The proportions of patients surviving at the end of 1 and 3 months in the steroids, nutrition, pentoxifylline, and FMT group were 63%, 47%, 40%, and 75% (p = 0.179) and 38%, 29%, 30%, and 75% (p = 0.036), respectively. (2) Compared with FMT, relative risk and hazard ratios for death were higher in all the other groups. |
| [67] | Control: 10 FMT: 10 |
(1) This phase 1 trial shows that FMT is safe and associated with short-term reduction in alcohol craving and consumption with favorable microbial changes versus placebo in patients with alcohol-related cirrhosis with alcohol misuse. (2) There was also a reduction in AUD-related events over 6 months in patients assigned to FMT. |
|
| PSC | [90] | FMT:10 without controls | (1) Abundance of engrafter operational taxonomic units in patients post-FMT correlated with decreased ALP levels (p = 0.02). (2) 30% (3/10) experienced a ≥50% decrease in ALP levels. (3) The diversity increased in all patients post-FMT, as early as week 1 (p < 0.01). |
ALD, alcoholic liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AUD, alcohol use disorder; AVT, antiviral therapy; DNA, deoxyribonucleic acid; FMT, fecal microbiota transplantation; HBeAg, HBV e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; NAFLD, non-Alcoholic fatty liver disease; PSC, primary sclerosing cholangitis.
4.6. Hepatocellular Carcinoma (HCC)
HCC is an aggressive tumor, which is frequently diagnosed at a late stage with a median survival of about 6–20 months. By 2025, over one million cases will be globally affected by HCC [91]. LT has become a standard treatment for patients with early stage HCC in several countries [92]. However, for those patients with advanced HCC whose number and size of tumor beyond Milan criteria, the 5-year survival rate after LT remains poor [93]. Over 80% of HCC cases are associated with liver cirrhosis, representing inflammation and hepatocellular proliferation [94]. A study demonstrated that bacteria derived from gut might play a role in the recurrence of cirrhosis and HCC [95]. In addition, the latest animal studies demonstrated that gut microbiota and its metabolites could directly affect intrahepatic and peripheral inflammatory and immune responses in HCC [96,97], while immune response in HCC influences the clinical course and overall survival of the disease, mainly by impairing the functions of T cells and regulatory T cells (Tregs). To date, several investigations revealed that the gut microbiota could promote the development of HCC via the gut–liver axis [95,98,99]. Therefore, modulation of gut microbiota is deemed as a method to prevent HCC [100].
Some clinical trials related to gut microbiota in HCC have been performed or are ongoing. These studies mainly involve administration of probiotics for the treatment of HCC (Identifiers: NCT02021253, NCT05178524, and NCT03853928). However, to our knowledge, no clinical study has assessed the applicability of FMT for HCC. Only Baruch et al. reported the first human clinical trial where they found how treatment with FMT was associated with favorable changes in gene expression profiles and immune cell infiltrates in the tumor microenvironment [101]. These data indicated the benefits of FMT for the treatment of HCC [102].
5. Clinical Value of Fecal Microbiota Transplantation (FMT) for Liver Transplantation (LT) Recipients
It is widely accepted that LT is still the only therapeutic option for patients with end-stage liver disease, acute liver failure, and HCC [103]. Over the recent decades, LT has been used as a mature and conventional surgical method for liver diseases [104]. However, patients receiving LT are at a particularly higher risk of infection, such as CDI [105], cytomegalovirus (CMV) infection [106], fungi infection, recurrent HBV infection, etc. A previous cohort study demonstrated that about 19% of deaths occurred at five years after LT were related to various sources of infection [103,107]. That is mainly due to administration of immunosuppressive agents after LT attenuates immune surveillance, enabling pathogens to evade natural immunity and facilitate infection. Furthermore, pre-transplant infection and some other risk factors are also associated with post-LT infection [108]. In addition, several studies demonstrated that the types of gut microbiota may significantly change after LT [109,110,111]. Hence, restoring the gut microbiota balance by FMT may be particularly critical for LT recipients. For instance, Schneider et al. reported a case of successful FMT in a LT recipient with severe CDI that was complicated with acute kidney injury [112]. Furthermore, the safety of FMT in immunocompromised patients has been demonstrated in a meta-analysis of 44 studies [113]. Therefore, FMT may be a potential therapeutic method for CDI after LT. However, to our knowledge, no clinical study has yet assessed the applicability of FMT for infectious diseases.
6. Conclusions and Future Perspectives
Human biology is contextual on the coexisting microorganisms, with the majority living in the digestive tract, influencing various physiological functions [55]. The gut–liver axis has shown a mutual association between the intestine and liver. Hence, the close interaction between gut and liver may be a very important factor in the pathogenesis of liver diseases. In addition, a growing body of evidence demonstrated that FMT is a novel approach to reconstruct the intestinal microecological balance, therefore, FMT has been gradually and widely utilized in the treatment of several liver diseases. In this review, the clinical progress of FMT in the treatment of liver diseases was briefly summarized. However, it is noteworthy that standards related to quality and safety control of microbiota have not been widely accepted. Consequently, FMT and other microbiota-based treatments are still in the preclinical stage for patients with some liver diseases, such as AIH and HCC, thereby deserving further clinical investigation. Moreover, restoring the gut microbiota balance by FMT may be particularly critical for LT recipients.
Abbreviations
alcoholic liver disease (ALD), non-Alcoholic fatty liver disease (NAFLD), primary sclerosing cholangitis (PSC), hepatocellular carcinoma (HCC), fecal microbiota transplantation (FMT), liver transplant (LT), gastrointestinal (GI), clostridium difficile infection (CDI), hepatic encephalopathy (HE), hepatitis B virus (HBV), hepatitis B surface antigen (HBsAg), HBV e-antigen (HBeAg), chronic hepatitis B (CHB), entecavir (ETV), tenofovir disoproxil fumarate (TDF), tenofovir, alafenamide (TAF), nonalcoholic steatohepatitis (NASH), type 2 diabetes mellitus (T2D), high-fat diet (HFD), autoimmune hepatitis (AIH), follicular regulatory T (TFR), helper T (TFH), Toll-like receptor 4 (TLR4), inflammatory bowel disease (IBD), fucosyltransferase 2 (FUT2), germ-free multidrug resistance 2 (mdr2), alkaline phosphatase (ALP), regulatory T cells (Tregs), cytomegalovirus (CMV), acute kidney injury (AKI).
Author Contributions
Y.Z. and C.G. performed the work and wrote the manuscript. J.X., D.C. and B.Y. collected the data. L.W. and Z.C. obtained funding for this project. L.W. revised the manuscript, directed, and supervised the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors have no conflict of interest to declare.
Funding Statement
This study was supported by grants from the Health Commission of Hubei Province (funder: ZS.C; Grant No. WJ2021C001) and Key Research and Development Plan of Hubei Province (funder: ZS.C; Grant No. 2022BCA015).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gu X., Lu Q., Zhang C., Tang Z., Chu L. Clinical Application and Progress of Fecal Microbiota Transplantation in Liver Diseases: A Review. Semin. Liver Dis. 2021;41:495–506. doi: 10.1055/s-0041-1732319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J.W., Kuo C.H., Kuo F.C., Wang Y.K., Hsu W.H., Yu F.J., Hu H.M., Hsu P.I., Wang J.Y., Wu D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019;118:S23–S31. doi: 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Tamburini S., Shen N., Wu H.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 4.Abdollahi-Roodsaz S., Abramson S.B., Scher J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016;12:446–455. doi: 10.1038/nrrheum.2016.68. [DOI] [PubMed] [Google Scholar]
- 5.van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., de Vos W.M., Visser C.E., Kuijper E.J., Bartelsman J.F., Tijssen J.G., et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 6.Surawicz C.M., Brandt L.J., Binion D.G., Ananthakrishnan A.N., Curry S.R., Gilligan P.H., McFarland L.V., Mellow M., Zuckerbraun B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S.H., Gerding D.N., Johnson S., Kelly C.P., Loo V.G., McDonald L.C., Pepin J., Wilcox M.H. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect. Control. Hosp. Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 8.Aron-Wisnewsky J., Vigliotti C., Witjes J., Le P., Holleboom A.G., Verheij J., Nieuwdorp M., Clément K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu G., Zhao Q., Wei H. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Ann. Hepatol. 2019;18:796–803. doi: 10.1016/j.aohep.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Shah A., Macdonald G.A., Morrison M., Holtmann G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am. J. Gastroenterol. 2020;115:814–822. doi: 10.14309/ajg.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Xie Y., Zhou F., Zhang B., Wu J., Yang L., Xu S., Stedtfeld R., Chen Q., Liu J., et al. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front. Microbiol. 2020;11:383. doi: 10.3389/fmicb.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia W., Rajani C., Kaddurah-Daouk R., Li H. Expert insights: The potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer’s disease and hepatic encephalopathy. Med. Res. Rev. 2020;40:1496–1507. doi: 10.1002/med.21653. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y., Cai Y., Yang Y. The Gut Microbiome and Hepatocellular Carcinoma: Implications for Early Diagnostic Biomarkers and Novel Therapies. Liver Cancer. 2022;11:113–125. doi: 10.1159/000521358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatton G., Shawcross D.L. Is treating the gut microbiome the key to achieving better outcomes in cirrhosis. Expert Rev. Gastroenterol. Hepatol. 2019;13:1–2. doi: 10.1080/17474124.2019.1543587. [DOI] [PubMed] [Google Scholar]
- 15.Kundu P., Blacher E., Elinav E., Pettersson S. Our Gut Microbiome: The Evolving Inner Self. Cell. 2017;171:1481–1493. doi: 10.1016/j.cell.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Cui X., Ye L., Li J., Jin L., Wang W., Li S., Bao M., Wu S., Li L., Geng B., et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018;8:635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cani P.D. Human gut microbiome: Hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suk K.T., Koh H. New perspective on fecal microbiota transplantation in liver diseases. J. Gastroenterol. Hepatol. 2022;37:24–33. doi: 10.1111/jgh.15729. [DOI] [PubMed] [Google Scholar]
- 19.Han H., Jiang Y., Wang M., Melaku M., Liu L., Zhao Y., Everaert N., Yi B., Zhang H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): Focusing on the gut-liver axis. Crit. Rev. Food Sci. Nutr. 2021:1–18. doi: 10.1080/10408398.2021.1966738. [DOI] [PubMed] [Google Scholar]
- 20.Miele L., Marrone G., Lauritano C., Cefalo C., Gasbarrini A., Day C., Grieco A. Gut-liver axis and microbiota in NAFLD: Insight pathophysiology for novel therapeutic target. Curr. Pharm. Des. 2013;19:5314–5324. doi: 10.2174/1381612811319290011. [DOI] [PubMed] [Google Scholar]
- 21.Song Q., Zhang X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines. 2022;10:524. doi: 10.3390/biomedicines10030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A., Khanna S. Fecal Microbiota Transplantation. JAMA. 2017;318:102. doi: 10.1001/jama.2017.6466. [DOI] [PubMed] [Google Scholar]
- 23.Bojanova D.P., Bordenstein S.R. Fecal Transplants: What Is Being Transferred. PLoS Biol. 2016;14:e1002503. doi: 10.1371/journal.pbio.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao D., Roach B., Silva M., Beck P., Rioux K., Kaplan G.G., Chang H.J., Coward S., Goodman K.J., Xu H., et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanssen N., de Vos W.M., Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: From a murky past to a bright future. Cell Metab. 2021;33:1098–1110. doi: 10.1016/j.cmet.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Biazzo M., Deidda G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022;11:4119. doi: 10.3390/jcm11144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakken J.S., Polgreen P.M., Beekmann S.E., Riedo F.X., Streit J.A. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Dubois N.E., Read C.Y., O’Brien K., Ling K. Challenges of Screening Prospective Stool Donors for Fecal Microbiota Transplantation. Biol. Res. Nurs. 2021;23:21–30. doi: 10.1177/1099800420941185. [DOI] [PubMed] [Google Scholar]
- 29.Alang N., Kelly C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015;2:ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Mouries J., Brescia P., Silvestri A., Spadoni I., Sorribas M., Wiest R., Mileti E., Galbiati M., Invernizzi P., Adorini L., et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019;71:1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitta C., Pollicino T., Raimondo G. Occult Hepatitis B Virus Infection: An Update. Viruses. 2022;14:1504. doi: 10.3390/v14071504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampertico P., Agarwal K., Berg T., Buti M., Janssen H.L., Papatheodoridis G., Zoulim F., Tacke F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Terrault N.A., Bzowej N.H., Chang K.M., Hwang J.P., Jonas M.M., Murad M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan A., Kumar R., Sharma S., Mahanta M., Vayuuru S.K., Nayak B., Kumar S. Fecal Microbiota Transplantation in Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients: A Pilot Study. Dig Dis. Sci. 2021;66:873–880. doi: 10.1007/s10620-020-06246-x. [DOI] [PubMed] [Google Scholar]
- 36.Ren Y.D., Ye Z.S., Yang L.Z., Jin L.X., Wei W.J., Deng Y.Y., Chen X.X., Xiao C.X., Yu X.F., Xu H.Z., et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765–1768. doi: 10.1002/hep.29008. [DOI] [PubMed] [Google Scholar]
- 37.Lu H., Wu Z., Xu W., Yang J., Chen Y., Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb. Ecol. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 38.Chou H.H., Chien W.H., Wu L.L., Cheng C.H., Chung C.H., Horng J.H., Ni Y.H., Tseng H.T., Wu D., Lu X., et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. USA. 2015;112:2175–2180. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo W., Zhou X., Li X., Zhu Q., Peng J., Zhu B., Zheng X., Lu Y., Yang D., Wang B., et al. Depletion of Gut Microbiota Impairs Gut Barrier Function and Antiviral Immune Defense in the Liver. Front. Immunol. 2021;12:636803. doi: 10.3389/fimmu.2021.636803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Dong Q., Li Q., Li Y., Zhao D., Sun J., Fu J., Meng F., Lin H., Luan J., et al. Dysregulated Response of Follicular Helper T Cells to Hepatitis B Surface Antigen Promotes HBV Persistence in Mice and Associates With Outcomes of Patients. Gastroenterology. 2018;154:2222–2236. doi: 10.1053/j.gastro.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Huang L.R., Wu H.L., Chen P.J., Chen D.S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA. 2006;103:17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J., Wang Y., Zhang X., Liu J., Zhang Q., Zhao Y., Peng J., Feng Q., Dai J., Sun S., et al. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front. Microbiol. 2017;8:2222. doi: 10.3389/fmicb.2017.02222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun Y., Chang Y., Kim H.N., Ryu S., Kwon M.J., Cho Y.K., Kim H.L., Cheong H.S., Joo E.J. Alterations of the Gut Microbiome in Chronic Hepatitis B Virus Infection Associated with Alanine Aminotransferase Level. J. Clin. Med. 2019;8:173. doi: 10.3390/jcm8020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantovani A., Petracca G., Beatrice G., Csermely A., Tilg H., Byrne C.D., Targher G. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut. 2022;71:778–788. doi: 10.1136/gutjnl-2021-324191. [DOI] [PubMed] [Google Scholar]
- 45.Rustgi V.K., Li Y., Gupta K., Minacapelli C.D., Bhurwal A., Catalano C., Elsaid M.I. Bariatric Surgery Reduces Cancer Risk in Adults with Nonalcoholic Fatty Liver Disease and Severe Obesity. Gastroenterology. 2021;161:171–184.e10. doi: 10.1053/j.gastro.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Salunkhe S.A., Chitkara D., Mahato R.I., Mittal A. Lipid based nanocarriers for effective drug delivery and treatment of diabetes associated liver fibrosis. Adv. Drug Deliv. Rev. 2021;173:394–415. doi: 10.1016/j.addr.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Le Roy T., Llopis M., Lepage P., Bruneau A., Rabot S., Bevilacqua C., Martin P., Philippe C., Walker F., Bado A., et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 48.Gómez-Hurtado I., Gallego-Durán R., Zapater P., Ampuero J., Aller R., Crespo J., Arias-Loste M., García-Monzón C., Bellot P., González-Rodríguez Á., et al. Bacterial antigen translocation and age as BMI-independent contributing factors on systemic inflammation in NAFLD patients. Liver Int. 2020;40:2182–2193. doi: 10.1111/liv.14571. [DOI] [PubMed] [Google Scholar]
- 49.Zhou D., Pan Q., Shen F., Cao H.X., Ding W.J., Chen Y.W., Fan J.G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue L., Deng Z., Luo W., He X., Chen Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022;12:759306. doi: 10.3389/fcimb.2022.759306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang W., Wu N., Wang X., Chi Y., Zhang Y., Qiu X., Hu Y., Li J., Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 53.Del Chierico F., Nobili V., Vernocchi P., Russo A., De Stefanis C., Gnani D., Furlanello C., Zandonà A., Paci P., Capuani G., et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 54.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 55.de Medeiros I.C., de Lima J.G. Is nonalcoholic fatty liver disease an endogenous alcoholic fatty liver disease?—A mechanistic hypothesis. Med. Hypotheses. 2015;85:148–152. doi: 10.1016/j.mehy.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Bajaj J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 57.Mathurin P., Bataller R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 2015;62:S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutlu E.A., Gillevet P.M., Rangwala H., Rangwala H., Sikaroodi M., Naqvi A., Engen P.A., Kwasny M., Lau C.K., Keshavarzian A. Colonic microbiome is altered in alcoholism. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciocan D., Voican C.S., Wrzosek L., Hugot C., Rainteau D., Humbert L., Cassard A.M., Perlemuter G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment. Pharmacol. Ther. 2018;48:961–974. doi: 10.1111/apt.14949. [DOI] [PubMed] [Google Scholar]
- 61.Wrzosek L., Ciocan D., Hugot C., Spatz M., Dupeux M., Houron C., Lievin-Le Moal V., Puchois V., Ferrere G., Trainel N., et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021;70:1299–1308. doi: 10.1136/gutjnl-2020-321565. [DOI] [PubMed] [Google Scholar]
- 62.Yan X., Ren X., Liu X., Wang Y., Ma J., Song R., Wang X., Dong Y., Fan Q., Wei J., et al. Dietary Ursolic Acid Prevents Alcohol-Induced Liver Injury via Gut-Liver Axis Homeostasis Modulation: The Key Role of Microbiome Manipulation. J. Agric. Food Chem. 2021;69:7074–7083. doi: 10.1021/acs.jafc.1c02362. [DOI] [PubMed] [Google Scholar]
- 63.Philips C.A., Phadke N., Ganesan K., Augustine P. Healthy donor faecal transplant for corticosteroid non-responsive severe alcoholic hepatitis. BMJ Case Rep. 2017;2017:bcr2017222310. doi: 10.1136/bcr-2017-222310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philips C.A., Phadke N., Ganesan K., Ranade S., Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J. Gastroenterol. 2018;37:215–225. doi: 10.1007/s12664-018-0859-4. [DOI] [PubMed] [Google Scholar]
- 65.Philips C.A.A.J., Zulfikar R.A.A.P. Three-year followup of alcohol-related hepatitis patients undergoing healthy donor fecal transplant: Analysis of clinical outcomes, relapse, gut microbiota and comparisons with standard care.Oral Abstracts. Hepatology. 2021;74:1–156. [Google Scholar]
- 66.Bajaj J.S., Gavis E.A., Fagan A., Wade J.B., Thacker L.R., Fuchs M., Patel S., Davis B., Meador J., Puri P., et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. 2021;73:1688–1700. doi: 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
- 67.Webb G.J., Hirschfield G.M., Krawitt E.L., Gershwin M.E. Cellular and Molecular Mechanisms of Autoimmune Hepatitis. Annu. Rev. Pathol. 2018;13:247–292. doi: 10.1146/annurev-pathol-020117-043534. [DOI] [PubMed] [Google Scholar]
- 68.Mieli-Vergani G., Vergani D., Czaja A.J., Manns M.P., Krawitt E.L., Vierling J.M., Lohse A.W., Montano-Loza A.J. Autoimmune hepatitis. Nat. Rev. Dis. Prim. 2018;4:18017. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 69.Manns M.P., Lohse A.W., Vergani D. Autoimmune hepatitis—Update 2015. J. Hepatol. 2015;62:S100–S111. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Liberal R., Selmi C., Gershwin M.E. Diego and Giorgina Vergani: The two hearts of translational autoimmunity. J. Autoimmun. 2016;66:1–6. doi: 10.1016/j.jaut.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Corrigan M., Hirschfield G.M., Oo Y.H., Adams D.H. Autoimmune hepatitis: An approach to disease understanding and management. Br. Med. Bull. 2015;114:181–191. doi: 10.1093/bmb/ldv021. [DOI] [PubMed] [Google Scholar]
- 72.Yuksel M., Wang Y., Tai N., Peng J., Guo J., Beland K., Lapierre P., David C., Alvarez F., Colle I., et al. A novel “humanized mouse” model for autoimmune hepatitis and the association of gut microbiota with liver inflammation. Hepatology. 2015;62:1536–1550. doi: 10.1002/hep.27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manfredo Vieira S., Hiltensperger M., Zegarra-Ruiz D., Dehner C., Khan N., Costa F.R.C., Tiniakou E., Greiling T., Ruff W., Barbieri A. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elsherbiny N.M., Rammadan M., Hassan E.A., Ali M.E., El-Rehim A.S.A., Abbas W.A., Abozaid M.A.A., Hassanin E., Hetta H.F. Autoimmune Hepatitis: Shifts in Gut Microbiota and Metabolic Pathways among Egyptian Patients. Microorganisms. 2020;8:1011. doi: 10.3390/microorganisms8071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei Y., Li Y., Yan L., Sun C., Miao Q., Wang Q., Xiao X., Lian M., Li B., Chen Y., et al. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569–577. doi: 10.1136/gutjnl-2018-317836. [DOI] [PubMed] [Google Scholar]
- 76.Liwinski T., Casar C., Ruehlemann M.C., Bang C., Sebode M., Hohenester S., Denk G., Lieb W., Lohse A.W., Franke A., et al. A disease-specific decline of the relative abundance of Bifidobacterium in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 2020;51:1417–1428. doi: 10.1111/apt.15754. [DOI] [PubMed] [Google Scholar]
- 77.Lou J., Jiang Y., Rao B., Li A., Ding S., Yan H., Zhou H., Liu Z., Shi Q., Cui G., et al. Fecal Microbiomes Distinguish Patients With Autoimmune Hepatitis From Healthy Individuals. Front. Cell. Infect. Microbiol. 2020;10:342. doi: 10.3389/fcimb.2020.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang M., Liwen Z., Jianguo S., Juan D., Fei D., Yin Z., Changping W., Jianping C. Fecal Microbiota Transplantation Controls Progression of Experimental Autoimmune Hepatitis in Mice by Modulating the TFR/TFH Immune Imbalance and Intestinal Microbiota Composition. Front. Immunol. 2021;12:728723. doi: 10.3389/fimmu.2021.728723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng Z., Yang L., Chu H. The Gut Microbiota: A Novel Player in Autoimmune Hepatitis. Front. Cell. Infect. Microbiol. 2022;12:947382. doi: 10.3389/fcimb.2022.947382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eaton J.E., Talwalkar J.A., Lazaridis K.N., Gores G.J., Lindor K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyson J.K., Beuers U., Jones D., Lohse A.W., Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547–2559. doi: 10.1016/S0140-6736(18)30300-3. [DOI] [PubMed] [Google Scholar]
- 82.Quraishi M.N., Acharjee A., Beggs A.D., Horniblow R., Tselepis C., Gkoutos G., Ghosh S., Rossiter A.E., Loman N., van Schaik W., et al. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease with Bile Acid Pathways. J. Crohn’s Colitis. 2020;14:935–947. doi: 10.1093/ecco-jcc/jjaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Péan N., Le Lay A., Brial F., Wasserscheid J., Rouch C., Vincent M., Myridakis A., Hedjazi L., Dumas M.E., Grundberg E., et al. Dominant gut Prevotella copri in gastrectomised non-obese diabetic Goto-Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia. 2020;63:1223–1235. doi: 10.1007/s00125-020-05122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vuillermin P.J., O’Hely M., Collier F., Allen K.J., Tang M.L.K., Harrison L.C., Carlin J.B., Saffery R., Ranganathan S., Sly P.D., et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020;11:1452. doi: 10.1038/s41467-020-14552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rupp C., Friedrich K., Folseraas T., Wannhoff A., Bode K.A., Weiss K.H., Schirmacher P., Sauer P., Stremmel W., Gotthardt D.N. Fut2 genotype is a risk factor for dominant stenosis and biliary candida infections in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2014;39:873–882. doi: 10.1111/apt.12663. [DOI] [PubMed] [Google Scholar]
- 86.Tabibian J.H., O’Hara S.P., Trussoni C.E., Tietz P.S., Splinter P.L., Mounajjed T., Hagey L.R., LaRusso N.F. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185–196. doi: 10.1002/hep.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Özdirik B., Müller T., Wree A., Tacke F., Sigal M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021;22:6975. doi: 10.3390/ijms22136975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazaridis K.N., LaRusso N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allegretti J.R., Kassam Z., Carrellas M., Mullish B.H., Marchesi J.R., Pechlivanis A., Smith M., Gerardin Y., Timberlake S., Pratt D.S., et al. Fecal Microbiota Transplantation in Patients with Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am. J. Gastroenterol. 2019;114:1071–1079. doi: 10.14309/ajg.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 90.Philips C.A., Augustine P., Phadke N. Healthy Donor Fecal Microbiota Transplantation for Recurrent Bacterial Cholangitis in Primary Sclerosing Cholangitis—A Single Case Report. J. Clin. Transl. Hepatol. 2018;6:438–441. doi: 10.14218/JCTH.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philips C.A., Rajesh S., Nair D.C., Ahamed R., Abduljaleel J.K., Augustine P. Hepatocellular Carcinoma in 2021: An Exhaustive Update. Cureus. 2021;13:e19274. doi: 10.7759/cureus.19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sapisochin G., Bruix J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017;14:203–217. doi: 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- 93.Parikh N.D., Waljee A.K., Singal A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21:1142–1152. doi: 10.1002/lt.24169. [DOI] [PubMed] [Google Scholar]
- 94.Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M., Gores G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 95.Behary J., Amorim N., Jiang X.T., Raposo A., Gong L., McGovern E., Ibrahim R., Chu F., Stephens C., Jebeili H., et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021;12:187. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loo T.M., Kamachi F., Watanabe Y., Yoshimoto S., Kanda H., Arai Y., Nakajima-Takagi Y., Iwama A., Koga T., Sugimoto Y., et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE(2)-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 97.Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M., Agdashian D., Terabe M., Berzofsky J.A., Fako V., et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dapito D.H., Mencin A., Gwak G.Y., Pradere J.P., Jang M.K., Mederacke I., Caviglia J.M., Khiabanian H., Adeyemi A., Bataller R., et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schnabl B., Brenner D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das B.K. Altered gut microbiota in hepatocellular carcinoma: Insights into the pathogenic mechanism and preclinical to clinical findings. APMIS. 2022;130:719–740. doi: 10.1111/apm.13282. [DOI] [PubMed] [Google Scholar]
- 101.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 102.Spanu D., Pretta A., Lai E., Persano M., Donisi C., Mariani S., Dubois M., Migliari M., Saba G., Ziranu P., et al. Hepatocellular carcinoma and microbiota: Implications for clinical management and treatment. World J. Hepatol. 2022;14:1319–1332. doi: 10.4254/wjh.v14.i7.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ancona G., Alagna L., Lombardi A., Palomba E., Castelli V., Renisi G., Dondossola D., Iavarone M., Muscatello A., Gori A., et al. The Interplay between Gut Microbiota and the Immune System in Liver Transplant Recipients and Its Role in Infections. Infect Immun. 2021;89:e0037621. doi: 10.1128/IAI.00376-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hübscher S.G. What is the long-term outcome of the liver allograft. J. Hepatol. 2011;55:702–717. doi: 10.1016/j.jhep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Becattini S., Taur Y., Pamer E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engelmann C., Sterneck M., Weiss K.H., Templin S., Zopf S., Denk G., Eurich D., Pratschke J., Weiss J., Braun F., et al. Prevention and Management of CMV Infections after Liver Transplantation: Current Practice in German Transplant Centers. J. Clin. Med. 2020;9:2352. doi: 10.3390/jcm9082352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Annavajhala M.K., Gomez-Simmonds A., Macesic N., Sullivan S.B., Kress A., Khan S.D., Giddins M.J., Stump S., Kim G.I., Narain R., et al. Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat. Commun. 2019;10:4715. doi: 10.1038/s41467-019-12633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abad C.L., Lahr B.D., Razonable R.R. Epidemiology and risk factors for infection after living donor liver transplantation. Liver Transpl. 2017;23:465–477. doi: 10.1002/lt.24739. [DOI] [PubMed] [Google Scholar]
- 109.Bajaj J.S., Fagan A., Sikaroodi M., White M.B., Sterling R.K., Gilles H., Heuman D., Stravitz R.T., Matherly S.C., Siddiqui M.S., et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl. 2017;23:907–914. doi: 10.1002/lt.24754. [DOI] [PubMed] [Google Scholar]
- 110.Kato K., Nagao M., Miyamoto K., Oka K., Takahashi M., Yamamoto M., Matsumura Y., Kaido T., Uemoto S., Ichiyama S. Longitudinal Analysis of the Intestinal Microbiota in Liver Transplantation. Transplant. Direct. 2017;3:e144. doi: 10.1097/TXD.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun L.Y., Yang Y.S., Qu W., Zhu Z.J., Wei L., Ye Z.S., Zhang J.R., Sun X.Y., Zeng Z.G. Gut microbiota of liver transplantation recipients. Sci. Rep. 2017;7:3762. doi: 10.1038/s41598-017-03476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider K.M., Wirtz T.H., Kroy D., Albers S., Neumann U.P., Strowig T., Sellge G., Trautwein C. Successful Fecal Microbiota Transplantation in a Patient with Severe Complicated Clostridium difficile Infection after Liver Transplantation. Case Rep. Gastroenterol. 2018;12:76–84. doi: 10.1159/000481937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shogbesan O., Poudel D.R., Victor S., Jehangir A., Fadahunsi O., Shogbesan G., Donato A. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplant for Clostridium difficile Infection in Immunocompromised Patients. Can. J. Gastroenterol. Hepatol. 2018;2018:1394379. doi: 10.1155/2018/1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.