Abstract
Many drug candidates are poorly water-soluble. Microenvironmental pH (pHM) modification in buccal/sublingual dosage forms has attracted increasing interest as a promising pharmaceutical strategy to enhance the oral mucosal absorption of drugs with pH-dependent solubility. Optimizing drug absorption at the oral mucosa using pHM modification is considered to be a compromise between drug solubility and drug lipophilicity (Log D)/permeation. To create a desired pHM around formulations during the dissolution process, a suitable amount of pH modifiers should be added in the formulations, and the appropriate methods of pHM measurement are required. Despite pHM modification having been demonstrated to be effective in enhancing the oral mucosal absorption of drugs, some potential risks, such as oral mucosal irritation and teeth erosion caused by the pH modifiers, should not been neglected during the formulation design process. This review aims to provide a short introduction to the pHM modification concept in buccal/sublingual dosage forms, the properties of saliva related to pHM modification, as well as suitable drug candidates and pH modifiers for pHM modifying buccal/sublingual formulations. Additionally, the methods of pHM measurement, pHM modification methods and the corresponding challenges are summarized in the present review.
Keywords: absorption enhancement, microenvironmental pH modification, buccal/sublingual dosage form, solubility
1. Introduction
Buccal/Sublingual administration is an attractive route to achieve systemic drug delivery. It offers advantages such as circumventing the hepatic first-pass metabolism and chemical/biological drug degradation associated with oral administration, the quick onset of drug action, ease of administration and relatively high level of patient compliance [1,2,3,4]. In general, formulations such as mucoadhesive films, patches, tablets, gels, etc., have been employed for buccal/sublingual drug delivery, which could increase the contact time between drugs and the oral mucosa. Among the various physicochemical properties of the drug candidates, aqueous solubility and lipophilicity (Log D)/permeation are two crucial factors affecting drug absorption at the oral mucosa. A good drug candidate should be soluble in human saliva, and possess enough lipophilicity to permeate the epithelium at the oral mucosa [3]. However, it is estimated that over 40% of drugs on the market are poorly water-soluble [5], which could lead to a slow drug release. Generally, poorly water-soluble drugs are weakly ionizable drugs, which might have pH-dependent solubility and/or pH-dependent lipophilicity (Log D)/permeation. Therefore, pH plays a crucial role in the absorption of those drugs in the oral cavity.
Microenvironmental pH (pHM) modification is widely used in oral solid dosage forms to increase the dissolution of poorly water-soluble drugs with pH-dependent solubility in the gastrointestinal (GI) tract, and this approach creates a microenvironment with an ideal pH level in the vicinity and inside the solid dosage forms by adding pH modifiers to the formulations [6,7,8,9]. It has been demonstrated that pHM modification is an effective way to increase drug dissolution and enhance drug absorption in the GI tract [7,10,11,12]. Typically, the pH rapidly changes from highly acidic in the stomach to neutral in the small intestine. Under fasted-state conditions, the gastric pH range is between 1.7 and 4.7, and the pH in the small intestine slightly increases from 5.9 in the proximal parts to 7.8 in distal parts for the human subjects [13]. The physiological environment for drug dissolution in the oral cavity is different compared to that in the GI tract. The pH range for unstimulated human saliva is 6.2 to 7.6 and the average salivary pH is 6.8 [14,15]. The neutral pH environment, without dramatic shifts, is beneficial to create a desired pHM. Additionally, the limited volume (average 0.8 to 1.1 mL) and the low secretion rate of human saliva (0.35 to 2.00 mL/min) [16,17] could lead to a slow release of the pH modifiers from the formulations and, hence, to maintaining the ideal pHM. Therefore, the oral cavity provides a suitable physiological environment for pHM modifications. pHM might not only affect drug release from formulations, but also drug permeation across the oral mucosa. Generally, pH modifiers could modulate pHM both in and in the vicinity of the buccal/sublingual formulations and affect the drug ionization, and thereby influence the drug permeation across the oral mucosa. Thus, pHM modification in buccal/sublingual dosage forms might be an effective strategy to enhance the absorption of drugs with pH-dependent solubility or/and permeation.
The aim of this review paper is to provide an overview of pHM modification in buccal/sublingual dosage forms for systemic drug delivery. In this article, the concept of pHM modification in the oral cavity is discussed. The drug candidates, pH modifiers and pHM measurement methods are summarized. Most importantly, different types of pHM modification and the corresponding cases, as well as their challenges, are highlighted. We hope that this review will be useful for the future design and development of pHM modifying buccal/sublingual formulations.
2. Concept of Microenvironmental pH (pHM) Modification in the Buccal/Sublingual Dosage Forms
pHM modification is a pharmaceutical strategy to create a microenvironment with a targeted pH in or/and around formulations during dissolution by adding pH modifiers to formulations. In this review, we will present and discuss the theories associated with pH-dependent drug dissolution/release and pH-dependent drug permeation, as well as pHM modification in buccal/sublingual dosage forms.
2.1. Theory: pH-Dependent Dissolution and Permeation
Drug dissolution/release from buccal/sublingual formulations is one of the crucial factors affecting drug absorption at the oral mucosa. The relationship between the pH, drug solubility and dissolution rate has been elucidated using the Nernst-Noyes-Whitney equation [18] (Equation (1)) and the “solubility-pH” equations (take monoacidic drugs and monobasic drugs as examples) [19,20] (Equations (2) and (3)), as described below:
| (1) |
where is the dissolution rate, D is the diffusion coefficient, S is the surface area of solid exposed, V is the volume of dissolution media, h is the thickness of the diffusion layer, Cs is the concentration (saturated) of drug at the solid surface and Cb is the concentration of drug in the bulk medium.
| (2) |
| (3) |
where Cs is the drug solubility at a given pH and CS0 is the intrinsic solubility of the drug.
According to the “Solubility-pH” equations, a slight shift in the pH might lead to a significant change in the drug solubility. Theoretically, decreasing the pH could improve the solubility of a weakly basic drug by increasing the concentration of ionized drug in the solution. When most of the dissolved drug substance remains in its ionized form, a further decrease in the pH has little effect on its solubility, and the drug solubility approaches a plateau level in the pH-solubility profile. A suitable pH level at the surface of a solid formulation exposed to dissolution media could increase the local drug concentration (Cs) and, consequently, enhance the drug dissolution and release () from the solid formulation.
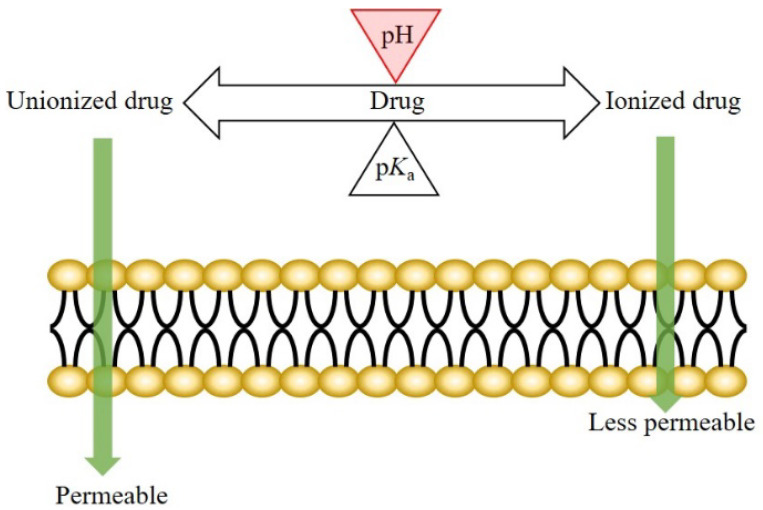
The mechanism of drug transport across the oral epithelium is similar to that across the other epithelia in the human body. Generally, both the transcellular and paracellular pathways are involved in this process [3,21,22,23,24]. For the drugs transported mainly via the transcellular route, drug permeation across the oral mucosa might be affected by the pH at the oral mucosa. According to the pH-partition theory, the neutral forms of drugs are more permeable (lipophilic) than the ionized species; therefore, a pH shift not only affects the dissociation of weakly ionizable drugs, but also the drug permeation across biological membranes (Figure 1) [25,26].
Figure 1.
Illustration of pH-partition theory.
2.2. pHmax Concept
A pHmax concept defined as the pH value at which a given drug has a maximal aqueous solubility and the sum of its ionized species and unionized species in solution is at a maximum [27]. Modulating the saliva pH at the sublingual mucosa to the pHmax by adding buffering agents in sublingual formulations was expected to lead to a maximal potential absorption. The pHmax concept was proven to be valuable in the case of a propranolol sublingual tablet with buffering agents, which achieved a higher absorption in human subjects than the conventional non-buffered tablet. However, previous studies regarding the metroprolol buccal tablet and gel did not support the pHmax concept. A specific pH level (rather than pHmax) also led to the highest buccal absorption of metroprolol [28,29].
2.3. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms
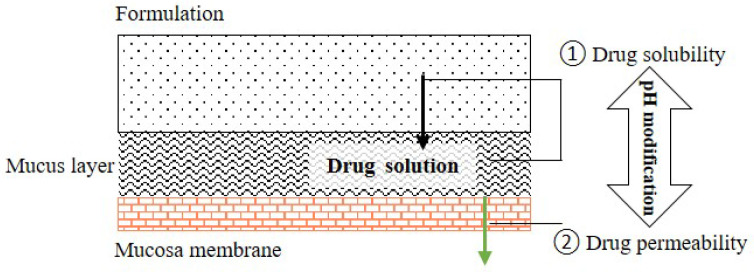
The aim of pHM modification is to enhance the absorption of a given drug by influencing its solubility and permeability. Upon pHM modification in buccal/sublingual dosage forms, a small space exists between the formulation and the mucous membrane when the formulation is attached the oral mucosa. Typically, a drug must have a sufficient aqueous solubility to be released from the formulation and dissolved in the space, before permeating through the membrane of the oral mucosa. The pH of the space and the pH inside the formulation could be modified by adding pH modifiers into the formulation, which might affect the drug release (caused by the changes in drug solubility), drug solubility in the space and drug permeation across the mucosa by influencing the drug dissociation (Figure 2). In general, the drug, pH modifier and mucoadhesive polymer are the main components of the pHM modifying buccal/sublingual formulations.
Figure 2.
Schematic drawing of drug permeation across oral mucosa from the buccal/sublingual formulation.
3. Properties of Saliva Associated with pH Modification
The main functions of saliva are to maintain oral health and help to build and maintain the health of hard and soft tissues. Approximately 99% of saliva is water, and the other 1% consists of a variety of electrolytes and proteins [30,31]. Regarding buccal/sublingual drug delivery, saliva provides a water-rich environment that facilitates in the drug dissolution and release from buccal/sublingual formulations before the drugs permeate through the membrane of oral mucosa [32]. To achieve a successful pHM modification in buccal/sublingual formulations, some properties of saliva should be taken into consideration during the formulation design.
3.1. pH and Buffer Capacity of Saliva
Human saliva has been reported to have a pH range of 6.2–7.6 [14]. The composition of saliva secreted from different regions differs, leading to different saliva pH. The pH in the palate, the floor of the mouth, the buccal mucosa and the tongue, in humans, have been reported to be 7.3, 6.5, 6.3 and 6.8, respectively [33]. The flow of saliva with a buffer capacity resisting pH shift could remove acidic and basic foods on the oral mucosa, maintaining the pH in the oral cavity near neutrality in a long term. The bicarbonate, the phosphate and the protein buffer systems in the whole saliva are the major systems contributing to the buffer capacity, and their concentration and buffer capacity are dependent on the secretion rate of saliva [34,35,36]. The bicarbonate system is considered to be the principal buffer of saliva and its dynamics system is complicated. The buffer capacity ranges of unstimulated human saliva and stimulated human saliva not exposed to the atmosphere (in the pH range of 4.25 to 6.75) have been reported to be 1.9–7.7 mmol H+/(L saliva × pH unit) and 2.4–9.3 mmol H+/(L saliva × pH unit), respectively. A high secretion rate of saliva implies a high concentration of bicarbonate in the saliva, which might lead to a high buffer capacity [36].
3.2. Secretion Rate of Saliva and Thickness of Salivary Film
Saliva is a complex mixture secreted by salivary glands. There are three pairs of major glands: the parotid, submandibular and sublingual glands, and numerous minor salivary glands [37]. Salivary secretion continues throughout the day, with an average total volume of 500–600 mL. Previous studies have reported that the mean flow rate of unstimulated human saliva and stimulated human saliva is 0.35 mL/min and 2 mL/min, respectively [16,38]. The function of the salivary glands is under the influence of various stimulations. For instance, tasting, smelling and chewing food can affect the properties, composition, volume and flow rate of saliva [16,37]. Additionally, some physiological factors, such as gender and age, have been reported to affect the salivary flow rate [17,39]. The volume of human saliva before swallowing is around 1.1 mL. Following a swallow, around 0.8 mL saliva stays in the mouth, and much of the saliva is present as a film on the mucosa and the surface of hard tissues in the oral cavity [17]. The estimated thickness of the human salivary films is 70–100 µm (calculated by dividing the volume of saliva by the surface area of oral mucosa) [40,41]. However, the thickness of the salivary film varies at different regions. The thicknesses of the human salivary film on the anterior tongue, buccal surface and anterior hard palate have been estimated to be 50–70 µm, 40–50 µm and 10 µm, respectively, by measuring the wetness of filter paper strips applied to different regions [16,42].
4. Drug Candidate and pH Modifier for Buccal/Sublingual Dosage Forms
4.1. Drug Candidate
The low drug loading capacity of buccal/sublingual formulations and the limited absorption area in the oral cavity are two main limitations for buccal/sublingual drug delivery. Thus, drug candidates should be high potency to achieve successful therapeutic efficacy. In addition, suitable drug candidates must not cause local irritation and toxicity at oral mucosa. Regarding physicochemical properties, high lipophilicity (log P (octanol/water) > 2), fairly good water-solubility and small molecular size (less than 800 Da) are typically considered as ideal parameters for drug candidates, as described previously [3]. The extent of different drug transport pathways across the epithelium depends on the drug physicochemical properties [43,44]. Typically, drug candidates with high lipophilicity can move across the lipid-rich epithelial cell membrane with relative ease. Fairly good water solubility allows for the fast drug release of buccal/sublingual formulations and drug diffusion across the hydrophilic cytoplasm of cells and paracellular passage. Macromolecules can be delivered via the oral mucosa, e.g., buccal insulin spray (Generex Oral-lyn®) was approved by Food and Drug Administration (FDA) for the treatment of patients under the Investigational New Drug (IND) program [45,46,47]. However, the number of marketed buccal/sublingual macromolecules is very small. Most of the marketed buccal/sublingual delivered drugs are small molecules. The selected drug products recorded in the FDA-Approved Drugs database [48] are summarized in Table 1 (only new drug applications are listed in this table and some drug products with the same active ingredients have different strengths or dosage forms).
Table 1.
Selected buccal/sublingual drug products approved by FDA for systemic drug delivery.
| Drug Name | Active Ingredients | Molecular Weight (g/mol) | Dosage Form | Administration Route | Company | Approval Date |
|---|---|---|---|---|---|---|
| Metandren® | Methyltestosterone | 302.5 | Tablet | Buccal, Sublingual | Novartis, Basel, Switzerland | - |
| Sorbitrate® | Isosorbide dinitrate | 236.1 | Tablet | Sublingual | Astrazeneca, Cambridge, UK | - |
| Isuprel® | Isoproterenol hydrochloride | 247.7 | Tablet | Sublingual, Rectal | Sanofi-Aventis US, Bridgewater (NJ), U.S. | 1948 |
| Hydergine® | Ergoloid mesylates | - | Tablet | Sublingual | Novartis, Basel, Switzerland | 1953 |
| Isordil® | Isosorbide dinitrate | 236.1 | Tablet | Sublingual | Biovail, Ontario, Canada | 1961 |
| Dentipatch® | Lidocaine | 234.3 | Film | Buccal | Noven, Miami (FL), U.S. | 1996 |
| Nitrostat® | Nitroglycerin | 227.1 | Tablet | Sublingual | Upjohn, Hastings (MI), U.S. | 2000 |
| Suboxone® | Buprenorphine hydrochloride; Naloxone hydrochloride | 504.1, 363.8 | Tablet | Sublingual | Indivior, Richmond (VA), U.S. | 2002 |
| Subutex® | Buprenorphine hydrochloride | 504.1 | Tablet | Sublingual | Indivior, Richmond (VA), U.S. | 2002 |
| Striant® | Testosterone | 288.4 | Tablet | Buccal | Auxilium Pharms, Centerbrook (CT), U.S. | 2003 |
| Fentora® | Fentanyl citrate | 528.6 | Tablet | Buccal, Sublingual | Cephalon, Frazer (PA), U.S. | 2006 |
| Edluar® | Zolpidem tartrate | 764.9 | Tablet | Sublingual | Mylan Speciality Lp, Basking Ridge (NJ), U.S. | 2009 |
| Saphris® | Asenapine maleate | 401.8 | Tablet | Sublingual | Allergan, Dublin, Ireland | 2009 |
| Onsolis® | Fentanyl citrate | 528.6 | Film | Buccal | BioDelivery Sciences International, Raleigh (NC), U.S. | 2009 |
| Suboxone® | Buprenorphine hydrochloride; Naloxone hydrochloride | 504.1, 363.8 | Film | Buccal, Sublingual | Indivior, Richmond (VA), U.S. | 2010 |
| Intermezzo® | Zolpidem tartrate | 764.9 | Tablet | Sublingual | Purdue Pharma, Stamford (CT), U.S. | 2011 |
| Abstral® | Fentanyl citrate | 528.6 | Tablet | Sublingual | Sentynl Theraps, Solana Beach (CA), U.S. | 2011 |
| Zubsolv® | Buprenorphine hydrochloride; Naloxone hydrochloride | 504.1, 363.8 | Tablet | Sublingual | Orexo US Inc., Morristown (NJ), U.S. | 2013 |
| Bunavail® | Buprenorphine hydrochloride; Naloxone hydrochloride | 504.1, 363.8 | Film | Buccal | BioDelivery Sciences International, Raleigh (NC), U.S. | 2014 |
| Belbuca® | Buprenorphine hydrochloride | 504.1 | Film | Buccal | BioDelivery Sciences International, Raleigh (NC), U.S. | 2015 |
| Dsuvia® | Sufentanil citrate | 578.7 | Tablet | Sublingual | Acelrx Pharmaceuticals, Hayward (CA), U.S. | 2018 |
| Nocdurna® | Desmopressin acetate | 1129.3 | Tablet | Sublingual | Ferring Pharmaceuticals, Saint-Prex, Switzerland | 2018 |
| Cassipa® | Buprenorphine hydrochloride; Naloxone hydrochloride | 504.1, 363.8 | Film | Sublingual | Teva Pharmaceuticals, Tel Aviv, Isreal | 2018 |
| Kynmobi® | Apomorphine hydrochloride | 303.8 | Film | Sublingual | Sunovion Pharmaceuticals, Marlborough (MA), U.S. | 2020 |
| Igalmi® | Dexmedetomidine | 200.28 | Film | Sublingual | BioXcel Therapeutics, New Haven (CT), U.S. | 2022 |
However, over 40% of marketed drugs and approximately 90% of drug candidates are reported to be poorly water-soluble [5], and most of them are weakly ionizable drugs, indicating that their solubility and/or permeability across the lipid-rich epithelium are pH-dependent [49,50,51,52]. Typically, the ionic form of a drug is more water soluble than its non-ionic form. A change in the pH might influence the ratio of the ionized form of the dissolved drug, according to the Henderson-Hasselbach equation (Equation (4)) [53]. When the difference in the water solubility (and/or lipophilicity) between the two forms is big enough, a slight pH change might have a significant effect on the drug solubility. Therefore, drug candidates suitable for pHM modification should have pH-dependent solubility and/or pH-dependent lipophilicity and be poorly soluble at physiological pH in the oral cavity. The physicochemical properties of some transmucosal delivered drugs (from DrugBank database) and the relevant literature on the buccal/sublingual delivery of the drugs are summarized in Table 2.
| (4) |
where pKa is the negative log of the drug dissociation constant; [A−] is the concentration of the base form of the drug; [HA] is the concentration of the acidic form of the drug.
Table 2.
Physicochemical properties of transmucosal delivered drugs.
| Drug | Molecular Weight (g/mol) | pKa * | Log P | Water Solubility (mg/mL) | Relevant Literature |
|---|---|---|---|---|---|
| Fentanyl | 336.47 | 8.99 | 4.05 | 0.74 | [54,55,56] |
| Naftopidil | 392.50 | 7.35 | 3.65 | 0.07 | [57] |
| Saquinavir | 670.84 | 8.47 | 3.80 | 0.00247 | [58,59,60,61] |
| Dapoxetine hydrochloride | 305.41 | 8.96 | 4.75 | 0.00084 | [62,63] |
| Nicotine | 162.23 | 8.58 | 1.17 | 93.3 | [64,65,66] |
| Metoprolol | 267.36 | 9.67 | 2.15 | 0.402 | [29] |
| Propranolol | 259.34 | 9.42 | 3.48 | 61.7 | [27] |
* pKa values recorded in DrugBank database might be different from those reported in the literatures referenced in this review.
4.2. pH Modifier
There are a few concerns about the excipients used in pharmaceutical formulations. A pH modifier can only be considered as a pharmaceutical excipient if it has been demonstrated to be safe for human beings. So far, various pH modifiers have been applied in the food and pharmaceutical industries. The Generally Recognized as Safe (GRAS) list of the FDA lists some safe pH modifiers that have been added to food. In addition, various pH modifiers recommended for oral liquids have been collected in the United States Pharmacopeia (USP). However, the specific pH modifiers for buccal/sublingual formulations were not referenced. The pH modifiers collected in the USP [67] and their maximum potency per unit dose used in solid oral and buccal/sublingual formulations in the database of Inactive Ingredient Search for Approved Drug Products Search, provided by the FDA [68], are summarized in Table 3. The pH modifiers can be divided into three categories: acidifying agents, alkalizing agents and buffering agents. Currently, only a few pH modifiers, as shown in Table 3, were applied in the commercial buccal/sublingual formulations approved by the FDA. pH modifiers demonstrated without local irritation and toxicity to oral mucosa could also be potential choices for the buccal/sublingual dosage forms.
Table 3.
Selected pH modifiers and the maximum potency used in the drug products approved by FDA.
| pH Modifiers | Maximum Potency Per Unit Dose (mg/Unit) | |
|---|---|---|
| Oral Tablet/Capsule/Podwer | Buccal or Sublingual Tablet/Film | |
| Acetic Acid | 0.36 | - |
| Adipic Acid | - | - |
| Ammonia | 6.03 | - |
| Ammonium Carbonate | - | - |
| Ammonium Chloride | 10.67 | - |
| Diammonium Phosphate | 0.4 | 0.2 |
| Boric Acid | - | - |
| Calcium Carbonate | 550 | 145.7 |
| Calcium Hydroxide | 35 | - |
| Calcium Lactate | - | - |
| Calcium Phosphate, Tribasic | 333.3 | 99.2 |
| Citric Acid Monohydrate | 914 | 30 |
| Citric Acid, Anhydrous | 839 | 30 |
| Diethanolamine | - | - |
| Fumaric Acid | 150 | - |
| Glycine | 200 | - |
| Hydrochloric Acid | 1.72 | - |
| Alpha-Lactalbumin | - | - |
| Lactic Acid | 44 | - |
| Lysine Hydrochloride | - | - |
| Maleic Acid | 4 | - |
| Malic Acid | 315 | - |
| Methionine | NA | - |
| Monoethanolamine | 1 | - |
| Monosodium Glutamate | - | - |
| Nitric Acid | - | - |
| Phosphoric Acid | 1 | - |
| Potassium Bicarbonate | 500 | 8 |
| Potassium Citrate | NA | - |
| Potassium Hydroxide | 25.6 | - |
| Potassium Metaphosphate | NA | - |
| Potassium Phosphate, Dibasic | 30 | - |
| Potassium Phosphate, Monobasic | 25 | - |
| Propionic Acid | - | - |
| Racemethionine | - | - |
| Sodium Acetate | - | - |
| Sodium Bicarbonate | 1600 | 42 |
| Sodium Borate | - | - |
| Sodium Carbonate | 430 | 30 |
| Sodium Citrate | 1900 | 19.5 |
| Sodium Hydroxide | 60 | 1.18 |
| Sodium Lactate Solution | - | - |
| Sodium Phosphate, Dibasic | 600 | 4.07 |
| Succinic Acid | 125.3 | - |
| Sulfuric Acid | - | - |
| Tartaric Acid | 96 | 1.5 |
| Trolamine | - | - |
5. Methods for Microenvironmental pH Measurement
Typically, it is easy to determine the pH of pharmaceutical solutions potentiometrically. Analyzing the pHM in the vicinity of pharmaceutical solids or in the matrix of formulations during the drug dissolution and release process is much more challenging. Several techniques have been applied to gain information on the pHM, and to investigate the relationship between the pHM and drug dissolution behavior. pH-indicating dyes have been used to determine the pHM within and around the tablets during hydration [7,69,70]. This method only roughly estimates the pHM according to the relationship between the pH level and the color of dyes. In addition, the pH electrode has been employed to precisely investigate the pHM in the concentrated suspension of pharmaceutical solids and on the surface of hydrated tablets [71,72]. To provide the data of a quantitative nature and detailed insights into pH effects during drug dissolution and release process, fluorescence imaging [73,74], the UV/Vis imaging method [75,76] and electron paramagnetic resonance (EPR) imaging [62] have been applied to non-invasive image pH sensitive fluorescent agents, pH-indicating dyes and pH-sensitive paramagnetic compounds, respectively, giving spatial resolutions of pHM. Generally, in buccal/sublingual formulations, the surface pH (pHM) in the vicinity of the formulations during the swelling and dissolving process have been measured, and several methods are presented in the following section.
5.1. pH Electrode Approach
The most common method to determine the pHM is the pH electrode approach. As the previous studies described [57,77,78,79,80,81,82], formulations (e.g., tablets, film and patch) were allowed to swell in a limited volume of buffer solution (at neutral pH) at room temperature for a certain period. Subsequently, the pH on the surface of the formulations was determined using a pH electrode. Mucoadhesive buccal films containing ornidazole were allowed to swell in 4 mL of phosphate buffer (pH 6.8 ± 0.1) at room temperature for 120 min and the surface pH was measured using an electrode pH meter [77]. A relatively short time and low volume of medium was needed for the swelling of the rapidly dissolving films of naftopidil before the pH measurement [57]. For the polymer-based buccal tablets with a long retention time, the time period for swelling was around two hours [79,83]. Although this method is easy to operate, it is challenging to investigate the changes in the pHM adjacent to the surface of the formulations during the initial swelling process.
5.2. Computer-Enhanced Color Images Method
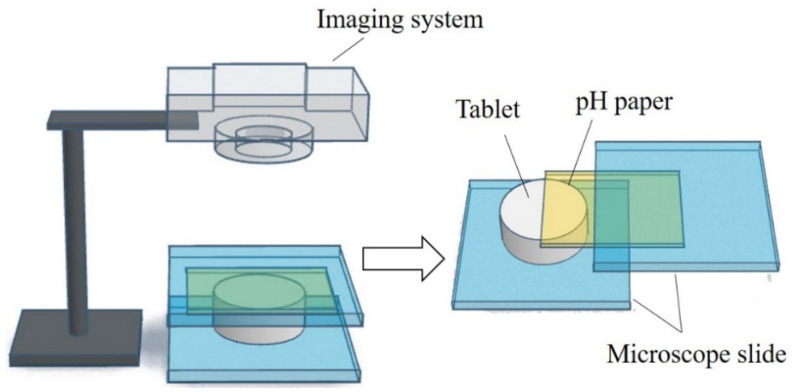
To gain more information on the pHM change during the dissolving process of the fentanyl tablet, a computer-enhanced color images (of pH paper) method was used to record the pHM as it varied over the surface of the swelling tablet [54]. The schematic view of the setup and the computer-enhanced color images of pH paper are shown in Figure 3. A piece of pH paper was placed over a tablet. The tablet with the pH paper was held between two microscope slides, and a small volume of deionized water was applied to the pH paper. The tablet was rapidly wetted by the water that permeated the pH paper. As the tablet swelled, the pH paper was digitally photographed at different time intervals. The pH over the distinct regions of the tablet surface were then determined from the digital images and in comparison to the reference pH standards. The pHM decreased from 7.0 to 5.0, and then gradually increased to around 6.0 during the first 5 min of the dissolving process [54].
Figure 3.
Schematic view of the setup of the computer-enhanced color images method.
5.3. UV/Vis Imaging Method
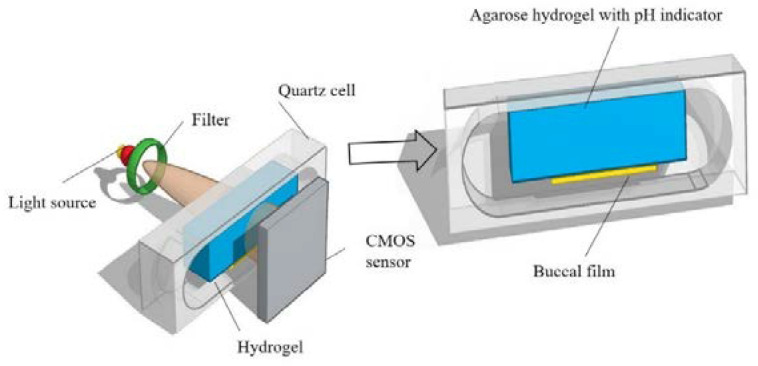
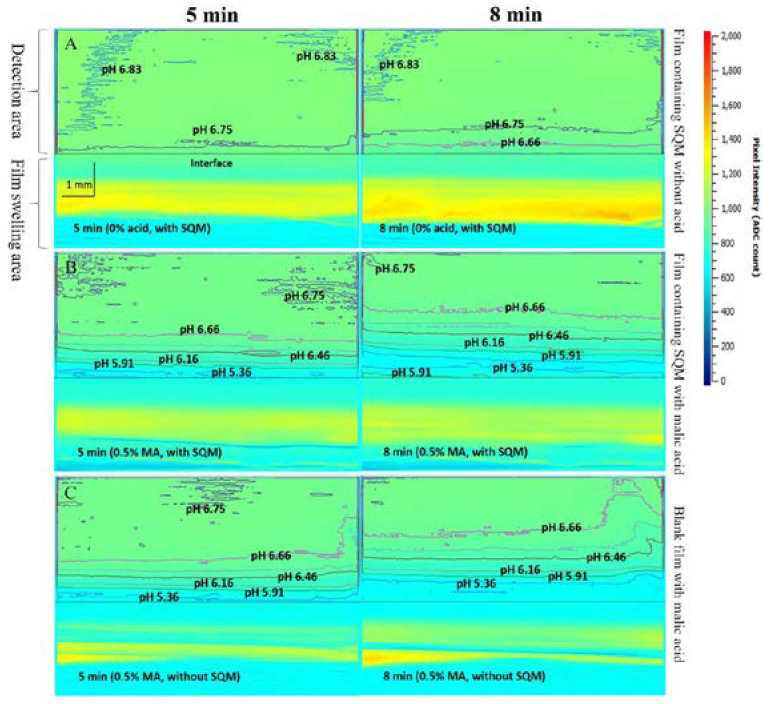
In one of our previous works, an UV/Vis imaging method with an agarose hydrogel mimicking the fluid on the surface of the buccal mucosa was constructed. The effect of the malic acid dose on the pHM during the initial dissolution of the buccal films, and the information related to film the swelling and possible drug precipitation in the films were monitored using this method [58]. The schematic view of the UV/Vis imaging setup is shown in Figure 4. The agarose hydrogel contained agarose (0.5% w/v), bromothymol blue (pH indicator, 6.29 × 10−5 M) and a buffer solution, simulating the human saliva pH and buffer capacity. A buccal film was attached on the agarose hydrogel, and the absorbance change of the pH indicator in the hydrogel at a wavelength of 610 nm was monitored during the swelling of the film. To relate the absorbance of bromothymol blue to the pH in the hydrogel, an absorbance-pH profile was constructed as a calibration curve. Based on the calibration curve, the pH during the swelling of the buccal film could be measured. The pHM in the vicinity of the buccal films at different time points were determined and selected images are shown in Figure 5. The addition of malic acid in the films led to an obvious decrease in the pHM at 5 min, whereas the pHM was increased due to the release of saquinavir from the films at 8 min (Figure 5B,C).
Figure 4.
Schematic view of the UV/V is imaging setup, reprinted with permission from [58], copyright of ©2020 Elsevier.
Figure 5.
Selected absorbance images for the buccal films with agarose hydrogel (buffer solution) containing bromothymol blue at 610 nm. The difference of color indicates different absorbance, and the contours represent the iso-absorbance/iso-pH line. A. Film containing saquinavir mesylate (SQM) without acids. B. Film containing SQM with malic acid (MA). C. Film without SQM containing MA; 0.5% is the percentage of MA (w/v) dissolved in water during the film preparation, reprinted with permission from [58], copyright of ©2020 Elsevier.
6. Microenvironmental pH (pHM) Modification Methods
According to the pH modifier classification described in Section 4.2, formulating acidifying/alkalizing agents and buffer agents are two common methods for pH modification. In addition, the application of effervescence in formulations constitutes another strategy for changing the pHM, which facilitates drug release from the formulations [54,84]. These pHM modification methods have been employed in buccal/sublingual dosage forms.
6.1. Microenvironmental pH Modification Using Acidifying/Alkalizing Agents
The most direct and effective way to change the pHM is to add acids or bases into the formulations. The pHM change might compromise the drug release from the formulations and the drug permeation, hence improving the drug absorption at the oral mucosa. Suitable pH shifts might increase the drug solubilities, despite them being poorly soluble in human saliva at the physiological pH. Previous studies have shown that the addition of organic acids leads to a significant increase in the dissolution of dapoxetine hydrochloride (DPX) particles in phosphate buffer at pH 6.8 (37 ± 0.5 °C) due to the pH-dependent solubility of DPX and the low pHM around the drug particles [62,63]. In addition, the enhanced pharmacokinetic performance of DPX via the buccal films with organic acids in male Wistar rats was observed compared to that of the marketed DPX oral tablet (Priligy®) [63]. Another study showed that the addition of citric acid led to a faster nicotine release from buccal matrix tablets, whereas it inhibited nicotine permeation across the esophageal mucosa. Conversely, the permeation of nicotine was enhanced in the oral cavity due to the incorporation of magnesium hydroxide, despite the nicotine release being retarded [64]. Nicotine is a weak base (pKa 3.04 and 7.84), the faster release of nicotine is due to the acidic pHM created by the addition of citric acid. However, the released nicotine did not readily permeate across the mucosa. The ionized nicotine at an acidic pH has a low lipophilicity, which could lead to a limited permeation for the lipid-rich mucosal membrane [65,66]. Therefore, both the solubility and lipophilicity should be taken into consideration for a drug with pH-dependent solubility and lipophilicity.
6.2. Microenvironmental pH Modification Using Buffering Agents
The pHM in the vicinity of formulations at the oral mucosa is generally affected by the release of the ingredients (particularly the acidic and basic ingredients) from the formulations. The pHM changes over time, along with the ingredients released upon dissolution. To maintain the suitable pHM and achieve optimal drug absorption at the oral mucosa, buffer agents are incorporated in formulations. The addition of buffering agents can form a buffer system in and around the matrix of the formulations and prevent the pHM from changing. This method was demonstrated to be effective in some cases. Phosphate buffer and borate buffer were used in methylcellulose-based gels to create pHM 7.4, 8.5, 9.0 and 9.5 for the buccal delivery of metoprolol in Göttingen minipigs in a previous study. A higher buccal absorption of metoprolol from the gels was observed at higher pH values, and the absolute bioavailability of metoprolol via buccal dosing was significantly higher compared to that via oral administration [29]. In this study, the metoprolol release from the gels might be similar, and the pH has little effect on metoprolol release, because the concentration of methylcellulose used in the gels was the same (1%, w/v) and metoprolol had already been dissolved in the gels. Metoprolol permeability across the buccal mucosa is the rate-limit step for the buccal absorption of metoprolol. Furthermore, metoprolol with pKa 9.56 [85] has a pH-dependent lipophilicity and permeability in vitro and ex vivo [29,86]. Thus, the pH has a crucial influence on the buccal absorption of metoprolol incorporated in gels. In another work [27], a concept of pHmax was introduced to improve the sublingual delivery of weak base compounds. To verify the applicability of this concept, propranolol with a pH-dependent solubility and a pH-permeability across porcine sublingual mucosa was chosen as the model drug, and disodium hydrogen phosphate (buffering agent) was added into sublingual propranolol tablets to make the saliva pH (pHM) close to pHmax when the tablets dissolved in saliva under the tongue. The buffered sublingual propranolol tablet and the marketed tablet (Inderal® which cannot achieve pHmax) were administered sublingually, using the same procedure in eight healthy male volunteers. The buffered tablet led to a significantly higher plasma propranolol concentration than the marketed tablet at 10–30 min, at which point the drug release profiles were similar between the two formulations [27]. Therefore, pHM modification using buffering agent is also a potential strategy to improve the buccal/sublingual delivery of drugs with pH-dependent solubility and/or permeability.
6.3. Microenvironmental pH Modification Using Effervescence
Formulations with effervescence generally contain an alkaline agent (e.g., sodium carbonate and sodium bicarbonate) and an acid that is capable of inducing the effervescence reaction during the dissolution [87]. The carbonic acid produced from the chemical reaction could decrease the pHM and rapidly convert to water and carbon dioxide. The tablet using an effervescence reaction (containing citric acid and bicarbonate) was employed to enhance the absorption of fentanyl at the buccal mucosa [54,56,88]. A dynamic shift in the pHM (pH was decreased and subsequently be increased) occurred in the microenvironment between the tablet and the buccal mucosa, and the pHM shift might be the main factor for the enhanced buccal absorption of fentanyl. The initial decrease in the pH, caused by the carbonic acid and release of citric acid from the tablet, facilitated the release of fentanyl from the tablet. The pH subsequently increased due to the dissociation of carbonic acid (into CO2 and water) and the dissipation of the CO2, which favored the formation of unionized fentanyl. The unionized fentanyl can move across the lipid-rich oral mucosal membrane with greater ease than the ionized fentanyl [54,56,88]. In addition, the thinning or tripping of the mucus layer, the disruption of the epithelial barrier, and/or the increased membrane hydrophobicity caused by the CO2 might be key factors leading to a high buccal/sublingual absorption of drugs [89,90]. In addition, effervescence led to a faster release of buspirone hydrochloride in vitro from buccal discs and a significantly higher bioavailability of buspirone hydrochloride when compared to a conventional buccal formulation without effervescence [91].
7. Challenges
Despite pHM modification being a potential strategy to enhance drug absorption via buccal/sublingual formulations, the main challenge is the possible local irritation to the oral mucosa and tooth erosion due to the pH shift in the oral cavity. Studies have shown that a low pH (<5.5) in the mouth can cause the erosion of dentine and enamel [92,93]. Damage was not observed in the buccal mucosa, tongue mucosa and salivary glands of rats when drinking water with an acidic pH (pH 5.0) [94]. However, acute effects (e.g., irritation and toxicity) on the oral mucosa may be expected at a pH lower than 2.5 [95]. In fact, the damage caused by the addition of pH modifiers in formulations on the oral mucosa and teeth relates to a combination of factors, i.e., the pH, total acid content, method of delivery and duration of contact [95]. These factors should be taken into consideration, and the irritation of pHM modifying buccal/sublingual formulations to the oral mucosa should be investigated before their clinical use.
For drug delivery purposes, the mucoadhesion implies the attachment of a drug delivery system to the mucous coat on the surface of the target tissues [96,97,98]. Several simultaneous interfacial interactions are involved in the complex mucoadhesion process, and the interactions can occur through covalent bonds or supramolecular interactions (including hydrogen bond and hydrophobic/electrostatic interactions) [96,99,100,101]. In the pHM modifying buccal/sublingual formulations, polymers with a fairly good mucoadhesion are applied to extend the retention time of the formulations on the oral mucosa. Despite the potential of pHM modification to enhance drug absorption at the oral mucosa, the shift in the pHM might change the mucoadhesion and, hence, reduce the retention time of the formulations. The reason for this adverse effect may be that the dissociation of the functional groups on the polypeptide backbone in the mucin is pH-dependent. The pHM shift might influence the charge on the surface of the mucus and, hence, reduce the interactions between the mucus and the formulations [96,97]. Additionally, CO2 produced by the effervescence could hinder the process of mucoadhesion. It might also create a porous contact surface in mucoadhesive formulations, leading to a poor interaction between the formulations and the mucin [91].
pH modification by adding pH modifiers might accelerate drug degradation in mucoadhesive formulations during the application and storage. The reason is that the pH is one of the most important factors affecting drug hydrolysis [102]. In addition, there are many reports in the literature referring to the effect of pH modifiers on the chemical stability of drugs in pharmaceutical solids [8,103,104,105,106,107]. Inappropriate pH modification (unsuitable pH modifier and the use of excessive pH modifiers) may lead to poor manufacturability. A previous study has reported that the type of acids affected the manufacturability of dipyridamole granules. During the process of wet granulation, the acids with different solubilities might partially dissolve in the binder solution, affecting the formation of the granules. The granules with toluenesulfonic acid monohydrate were formed as fine granules. However, the granules with maleic acid were problematic [108].
Compendial (USP and Ph. Eur.) methods and apparatuses for conventional tablets and gels have been widely employed for drug dissolution and the release testing of buccal/sublingual dosage forms in vitro [109,110,111,112]. However, most of these methods do not sufficiently mimic the physiological conditions present in the oral cavity. Relatively large volumes of dissolution medium with stirring have been used in these methods to create sink conditions, particularly in the cases of poorly water soluble drugs. Rapidly disintegrating formulations (e.g., mucoadhesive buccal/sublingual creams and gels) would disintegrate and/or dissolve within a few minutes upon contact with large volumes of dissolution medium during stirring. Our previous study shows that the combination of the Franz diffusion cell method and the UV/Vis imaging-method provided beneficial information to the drug release study of pHM modifying mucoadhesive buccal films [59]. However, the physical light blocking caused by the polymeric matrix of the film led to a short measuring time (10 min). In the future, the UV/Vis setup should be further developed to solve the problems caused by the soluble matrix-forming polymers with low viscosity grades in buccal/sublingual formulations.
8. Conclusions
Microenvironmental pH (pHM) modification has attracted considerable interest as an effective strategy to promote systemic drug delivery across the oral mucosa, particularly for drugs with pH-dependent solubility and/or pH-dependent lipophilicity. In general, optimizing drug absorption at the oral mucosa using pHM modification is considered to be a compromise between drug solubility and drug lipophilicity. A successful pHM modification should ensure that the drugs are sufficiently soluble and lipophilic. This modification can facilitate drug releasing from the formulations and, subsequently, facilitate absorption across the oral mucosal membrane. The application of suitable pH modifiers and the monitoring of the pHM during the dissolution of formulations are crucial for the design and development of pHM modifying buccal/sublingual formulations. Among the pHM measurement methods, the computer-enhanced color images of pH paper method and the UV/Vis imaging method provided beneficial information on the pHM during the dissolution process. Buccal/Sublingual dosage forms using different pHM modification methods (including using acidifying/alkalizing agents, buffering agents and effervescence, respectively) have been found to enhance drug bioavailability in animal models and human subjects. However, inappropriate pHM modification might cause some problems, such as local irritation to the oral mucosa, tooth erosion, poor mucoadhesion and poor drug stability. Generally, pHM modification in buccal/sublingual dosage forms has great potentials to enhance drug absorption. A better understanding of the theories and challenges of pHM modification will be helpful for the future design of pHM modifying buccal/sublingual formulations for systemic drug delivery.
Author Contributions
S.H.: conceptualization and manuscript drafting. H.M.: conceptualization, writing—reviewing and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Funding Statement
This research was funded by China Scholarship Council (201708510087).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Macedo A.S., Castro P.M., Roque L., Thomé N.G., Reis C.P., Pintado M.E., Fonte P. Novel and Revisited Approaches in Nanoparticle Systems for Buccal Drug Delivery. J. Control. Release. 2020;320:125–141. doi: 10.1016/j.jconrel.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Madhav N.V.S., Shakya A.K., Shakya P., Singh K. Orotransmucosal Drug Delivery Systems: A Review. J. Control. Release. 2009;140:2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Lam J.K.W., Xu Y., Worsley A., Wong I.C.K. Oral Transmucosal Drug Delivery for Pediatric Use. Adv. Drug Deliv. Rev. 2014;73:50–62. doi: 10.1016/j.addr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H., Zhang J., Streisand J.B. Oral Mucosal Drug Delivery. Clin. Pharmacokinet. 2002;41:661–680. doi: 10.2165/00003088-200241090-00003. [DOI] [PubMed] [Google Scholar]
- 5.Loftsson T., Brewster M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi C., Kawabata Y., Wada K., Yamada S., Onoue S. Microenvironmental PH-Modification to Improve Dissolution Behavior and Oral Absorption for Drugs with PH-Dependent Solubility. Expert Opin. Drug Deliv. 2014;11:505–516. doi: 10.1517/17425247.2014.881798. [DOI] [PubMed] [Google Scholar]
- 7.Yang M., He S., Fan Y., Wang Y., Ge Z., Shan L., Gong W., Huang X., Tong Y., Gao C. Microenvironmental PH-Modified Solid Dispersions to Enhance the Dissolution and Bioavailability of Poorly Water-Soluble Weakly Basic GT0918, a Developing Anti-Prostate Cancer Drug: Preparation, Characterization and Evaluation in Vivo. Int. J. Pharm. 2014;475:97–109. doi: 10.1016/j.ijpharm.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 8.Badawy S.I.F., Hussain M.A. Microenvironmental PH Modulation in Solid Dosage Forms. J. Pharm. Sci. 2007;96:948–959. doi: 10.1002/jps.20932. [DOI] [PubMed] [Google Scholar]
- 9.Doherty C., York P. Microenvironmental PH Control of Drug Dissolution. Int. J. Pharm. 1989;50:223–232. doi: 10.1016/0378-5173(89)90126-9. [DOI] [Google Scholar]
- 10.Younes N.F., El Assasy A.E.-H.I., Makhlouf A.I.A. Microenvironmental PH-Modified Amisulpride-Labrasol Matrix Tablets: Development, Optimization and in Vivo Pharmacokinetic Study. Drug Deliv. Transl. Res. 2020;11:103–117. doi: 10.1007/s13346-019-00706-2. [DOI] [PubMed] [Google Scholar]
- 11.Onoue S., Inoue R., Taniguchi C., Kawabata Y., Yamashita K., Wada K., Yamauchi Y., Yamada S. Improved Dissolution and Pharmacokinetic Behavior of Dipyridamole Formulation with Microenvironmental PH-Modifier under Hypochlorhydria. Int. J. Pharm. 2012;426:61–66. doi: 10.1016/j.ijpharm.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Mitra A., Kesisoglou F., Beauchamp M., Zhu W., Chiti F., Wu Y. Using Absorption Simulation and Gastric PH Modulated Dog Model for Formulation Development To Overcome Achlorhydria Effect. Mol. Pharm. 2011;8:2216–2223. doi: 10.1021/mp200062a. [DOI] [PubMed] [Google Scholar]
- 13.Koziolek M., Grimm M., Becker D., Iordanov V., Zou H., Shimizu J., Wanke C., Garbacz G., Weitschies W. Investigation of PH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap® System. J. Pharm. Sci. 2015;104:2855–2863. doi: 10.1002/jps.24274. [DOI] [PubMed] [Google Scholar]
- 14.Baliga S., Muglikar S., Kale R. Salivary PH: A Diagnostic Biomarker. J. Indian Soc. Periodontol. 2013;17:461–465. doi: 10.4103/0972-124X.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciolino L.A., McCauley H.A., Fraser D.B., Wolnik K.A. The Relative Buffering Capacities of Saliva and Moist Snuff: Implications for Nicotine Absorption. J. Anal. Toxicol. 2001;25:15–25. doi: 10.1093/jat/25.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Proctor G.B. The Physiology of Salivary Secretion. Periodontology 2000. 2016;70:11–25. doi: 10.1111/prd.12116. [DOI] [PubMed] [Google Scholar]
- 17.Dawes C. Physiological Factors Affecting Salivary Flow Rate, Oral Sugar Clearance, and the Sensation of Dry Mouth in Man. J. Dent. Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 18.Dokoumetzidis A., Macheras P. A Century of Dissolution Research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006;321:1–11. doi: 10.1016/j.ijpharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Avdeef A. Solubility of Sparingly-Soluble Ionizable Drugs. Adv. Drug Deliv. Rev. 2007;59:568–590. doi: 10.1016/j.addr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Bassi P., Kaur G. PH Modulation: A Mechanism to Obtain PH-Independent Drug Release. Expert Opin. Drug Deliv. 2010;7:845–857. doi: 10.1517/17425247.2010.491508. [DOI] [PubMed] [Google Scholar]
- 21.Pather S.I., Rathbone M.J., Şenel S. Current Status and the Future of Buccal Drug Delivery Systems. Expert Opin. Drug Deliv. 2008;5:531–542. doi: 10.1517/17425247.5.5.531. [DOI] [PubMed] [Google Scholar]
- 22.Mashru R., Sutariya V., Sankalia M., Sankalia J. Transbuccal Delivery of Lamotrigine across Porcine Buccal Mucosa: In Vitro Determination of Routes of Buccal Transport. J. Pharm. Pharm. Sci. 2005;8:54–62. [PubMed] [Google Scholar]
- 23.Birudaraj R.a.j., Berner B., Shen S., Li X. Buccal Permeation of Buspirone: Mechanistic Studies on Transport Pathways. J. Pharm. Sci. 2005;94:70–78. doi: 10.1002/jps.20208. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen H.M., Rassing M.R. Nicotine Permeability across the Buccal TR146 Cell Culture Model and Porcine Buccal Mucosa in Vitro: Effect of PH and Concentration. Eur. J. Pharm. Sci. 2002;16:151–157. doi: 10.1016/S0928-0987(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Thomae A.V., Wunderli-Allenspach H., Krämer S.D. Permeation of Aromatic Carboxylic Acids across Lipid Bilayers: The PH-Partition Hypothesis Revisited. Biophys. J. 2005;89:1802–1811. doi: 10.1529/biophysj.105.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shore P.A., Brodie B.B., Hogben C.A.M. The Gastric Secretion of Drugs: A Ph Partition Hypothesis. J. Pharmacol. Exp. Ther. 1957;119:361–369. [PubMed] [Google Scholar]
- 27.Wang Y., Zuo Z., Chen X., Tomlinson B., Chow M.S.S. Improving Sublingual Delivery of Weak Base Compounds Using PHmax Concept: Application to Propranolol. Eur. J. Pharm. Sci. 2010;39:272–278. doi: 10.1016/j.ejps.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Meng-Lund E., Jacobsen J., Andersen M.B., Jespersen M.L., Karlsson J.-J., Garmer M., Jørgensen E.B., Holm R. Conscious and Anaesthetised Göttingen Mini-Pigs as an in-Vivo Model for Buccal Absorption—PH-Dependent Absorption of Metoprolol from Bioadhesive Tablets. Drug Dev. Ind. Pharm. 2014;40:604–610. doi: 10.3109/03639045.2014.884119. [DOI] [PubMed] [Google Scholar]
- 29.Holm R., Meng-Lund E., Andersen M.B., Jespersen M.L., Karlsson J.-J., Garmer M., Jørgensen E.B., Jacobsen J. In Vitro, Ex Vivo and in Vivo Examination of Buccal Absorption of Metoprolol with Varying PH in TR146 Cell Culture, Porcine Buccal Mucosa and Göttingen Minipigs. Eur. J. Pharm. Sci. 2013;49:117–124. doi: 10.1016/j.ejps.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Dodds M., Roland S., Edgar M., Thornhill M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team. 2015;2:15123. doi: 10.1038/bdjteam.2015.123. [DOI] [Google Scholar]
- 31.de Almeida P.D.V., Grégio A.M.T., Machado M.A., de Lima A.A.S., Azevedo L.R. Saliva Composition and Functions: A Comprehensive Review. J. Contemp. Dent. Pract. 2008;9:72–80. [PubMed] [Google Scholar]
- 32.Patel V.F., Liu F., Brown M.B. Advances in Oral Transmucosal Drug Delivery. J. Control. Release. 2011;153:106–116. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Aframian D., Davidowitz T., Benoliel R. The Distribution of Oral Mucosal PH Values in Healthy Saliva Secretors. Oral Dis. 2006;12:420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 34.Tenovuo J. Salivary Parameters of Relevance for Assessing Caries Activity in Individuals and Populations. Community Dent. Oral Epidemiol. 1997;25:82–86. doi: 10.1111/j.1600-0528.1997.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 35.Lazarchik D.A., Filler S.J. Effects of Gastroesophageal Reflux on the Oral Cavity. Am. J. Med. 1997;103:107S–113S. doi: 10.1016/S0002-9343(97)00334-3. [DOI] [PubMed] [Google Scholar]
- 36.Bardow A., Moe D., Nyvad B., Nauntofte B. The Buffer Capacity and Buffer Systems of Human Whole Saliva Measured without Loss of CO2. Arch. Oral Biol. 2000;45:1–12. doi: 10.1016/S0003-9969(99)00119-3. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen A.M.L., Sørensen C.E., Proctor G.B., Carpenter G.H., Ekström J. Salivary Secretion in Health and Disease. J. Oral Rehabil. 2018;45:730–746. doi: 10.1111/joor.12664. [DOI] [PubMed] [Google Scholar]
- 38.Pijpe J., Kalk W.W.I., Bootsma H., Spijkervet F.K.L., Kallenberg C.G.M., Vissink A. Progression of Salivary Gland Dysfunction in Patients with Sjögren’s Syndrome. Ann. Rheum. Dis. 2007;66:107–112. doi: 10.1136/ard.2006.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heintze U., Birkhed D., Björn H. Secretion Rate and Buffer Effect of Resting and Stimulated Whole Saliva as a Function of Age and Sex. Swed. Dent. J. 1983;7:227–238. [PubMed] [Google Scholar]
- 40.Collins L.M.C., Dawes C. The Surface Area of the Adult Human Mouth and Thickness of the Salivary Film Covering the Teeth and Oral Mucosa. J. Dent. Res. 1987;66:1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- 41.Derrien M., van Passel M.W.J., van de Bovenkamp J.H.B., Schipper R., de Vos W., Dekker J. Mucin-Bacterial Interactions in the Human Oral Cavity and Digestive Tract. Gut Microbes. 2010;1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osailan S., Pramanik R., Shirodaria S., Challacombe S., Proctor G. Investigating the Relationship between Hyposalivation and Mucosal Wetness. Oral Dis. 2011;17:109–114. doi: 10.1111/j.1601-0825.2010.01715.x. [DOI] [PubMed] [Google Scholar]
- 43.Xiang J., Fang X., Li X. Transbuccal Delivery of 2′,3′-Dideoxycytidine: In Vitro Permeation Study and Histological Investigation. Int. J. Pharm. 2002;231:57–66. doi: 10.1016/S0378-5173(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 44.Deneer V.H.M., Drese G.B., Roemelé P.E.H., Verhoef J.C., Lie-A-Huen L., Kingma J.H., Brouwers J.R.B.J., Junginger H.E. Buccal Transport of Flecainide and Sotalol: Effect of a Bile Salt and Ionization State. Int. J. Pharm. 2002;241:127–134. doi: 10.1016/S0378-5173(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 45.Easa N., Alany R.G., Carew M., Vangala A. A Review of Non-Invasive Insulin Delivery Systems for Diabetes Therapy in Clinical Trials over the Past Decade. Drug Discov. Today. 2019;24:440–451. doi: 10.1016/j.drudis.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Morales J.O., Brayden D.J. Buccal Delivery of Small Molecules and Biologics: Of Mucoadhesive Polymers, Films, and Nanoparticles. Curr. Opin. Pharmacol. 2017;36:22–28. doi: 10.1016/j.coph.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Heinemann L., Jacques Y. Oral Insulin and Buccal Insulin: A Critical Reappraisal. J. Diabetes Sci. Technol. 2009;3:568–584. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Administration FDA-Approved Drugs Database. [(accessed on 1 October 2022)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- 49.Zamora W.J., Curutchet C., Campanera J.M., Luque F.J. Prediction of PH-Dependent Hydrophobic Profiles of Small Molecules from Miertus–Scrocco–Tomasi Continuum Solvation Calculations. J. Phys. Chem. B. 2017;121:9868–9880. doi: 10.1021/acs.jpcb.7b08311. [DOI] [PubMed] [Google Scholar]
- 50.Zamora W.J., Campanera J.M., Luque F.J. Development of a Structure-Based, PH-Dependent Lipophilicity Scale of Amino Acids from Continuum Solvation Calculations. J. Phys. Chem. Lett. 2019;10:883–889. doi: 10.1021/acs.jpclett.9b00028. [DOI] [PubMed] [Google Scholar]
- 51.Xing L., Glen R.C. Novel Methods for the Prediction of LogP, PKa, and LogD. J. Chem. Inf. Comput. Sci. 2002;42:796–805. doi: 10.1021/ci010315d. [DOI] [PubMed] [Google Scholar]
- 52.Iwanaga K., Kato S., Miyazaki M., Kakemi M. Enhancing the Intestinal Absorption of Poorly Water-Soluble Weak-Acidic Compound by Controlling Local PH. Drug Dev. Ind. Pharm. 2013;39:1887–1894. doi: 10.3109/03639045.2012.668911. [DOI] [PubMed] [Google Scholar]
- 53.Tallarida R.J., Murray R.B., editors. Manual of Pharmacologic Calculations: With Computer Programs. Springer; New York, NY, USA: 1987. Henderson—Hasselbalch Equation; pp. 74–75. [Google Scholar]
- 54.Durfee S., Messina J., Khankari R. Fentanyl Effervescent Buccal Tablets. Am. J. Drug Deliv. 2006;4:1–5. doi: 10.2165/00137696-200604010-00001. [DOI] [Google Scholar]
- 55.Darwish M., Hamed E., Messina J. Fentanyl Buccal Tablet for the Treatment of Breakthrough Pain: Pharmacokinetics of Buccal Mucosa Delivery and Clinical Efficacy. Perspect. Med. Chem. 2010;4:11–21. doi: 10.4137/PMC.S3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darwish M., Tempero K., Jiang J.G., Simonson P.G. Relative Bioavailability of Fentanyl Following Various Dosing Regimens of Fentanyl Buccal Tablet in Healthy Japanese Volunteers. Arch. Drug Inf. 2008;1:56–62. doi: 10.1111/j.1753-5174.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elagamy H.I., Essa E.A., Nouh A., El Maghraby G.M. Development and Evaluation of Rapidly Dissolving Buccal Films of Naftopidil: In Vitro and in Vivo Evaluation. Drug Dev. Ind. Pharm. 2019;45:1695–1706. doi: 10.1080/03639045.2019.1656734. [DOI] [PubMed] [Google Scholar]
- 58.He S., Østergaard J., Ashna M., Nielsen C.U., Jacobsen J., Mu H. Microenvironmental PH Modifying Films for Buccal Delivery of Saquinavir: Effects of Organic Acids on PH and Drug Release in Vitro. Int. J. Pharm. 2020;585:119567. doi: 10.1016/j.ijpharm.2020.119567. [DOI] [PubMed] [Google Scholar]
- 59.He S., Jacobsen J., Nielsen C.U., Genina N., Østergaard J., Mu H. Exploration of in Vitro Drug Release Testing Methods for Saquinavir Microenvironmental PH Modifying Buccal Films. Eur. J. Pharm. Sci. 2021;163:105867. doi: 10.1016/j.ejps.2021.105867. [DOI] [PubMed] [Google Scholar]
- 60.He S., Radeke C., Jacobsen J., Lind J.U., Mu H. Multi-Material 3D Printing of Programmable and Stretchable Oromucosal Patches for Delivery of Saquinavir. Int. J. Pharm. 2021;610:121236. doi: 10.1016/j.ijpharm.2021.121236. [DOI] [PubMed] [Google Scholar]
- 61.He S., Nielsen C.U., Mu H., Jacobsen J. In Vitro and Ex Vivo Evaluation of Bilayered Effervescent Microenvironmental PH Modifying Buccal Films with Saquinavir. J. Drug Deliv. Sci. Technol. 2022;78:103954. doi: 10.1016/j.jddst.2022.103954. [DOI] [Google Scholar]
- 62.Fouad S.A., Shamma R.N., Basalious E.B., El-Nabarawi M.A., Tayel S.A. Novel Instantly-Soluble Transmucosal Matrix (ISTM) Using Dual Mechanism Solubilizer for Sublingual and Nasal Delivery of Dapoxetine Hydrochloride: In-Vitro/in-Vivo Evaluation. Int. J. Pharm. 2016;505:212–222. doi: 10.1016/j.ijpharm.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Aldawsari H.M., Badr-Eldin S.M. Enhanced Pharmacokinetic Performance of Dapoxetine Hydrochloride via the Formulation of Instantly-Dissolving Buccal Films with Acidic PH Modifier and Hydrophilic Cyclodextrin: Factorial Analysis, in Vitro and in Vivo Assessment. J. Adv. Res. 2020;24:281–290. doi: 10.1016/j.jare.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pongjanyakul T., Kanjanabat S. Influence of PH Modifiers and HPMC Viscosity Grades on Nicotine–Magnesium Aluminum Silicate Complex-Loaded Buccal Matrix Tablets. AAPS PharmSciTech. 2012;13:674–685. doi: 10.1208/s12249-012-9790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nair M.K., Chetty D.J., Chien Y.W., Ho H. Biomembrane Permeation of Nicotine: Mechanistic Studies with Porcine Mucosae and Skin. J. Pharm. Sci. 1997;86:257–262. doi: 10.1021/js960095w. [DOI] [PubMed] [Google Scholar]
- 66.Chen L.-L.H., Chetty D.J., Chien Y.W. A Mechanistic Analysis to Characterize Oramucosal Permeation Properties. Int. J. Pharm. 1999;184:63–72. doi: 10.1016/S0378-5173(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 67.The United States . United States Pharmacopeia and National Formulary. The United States Pharmacopeial Convention; North Bethesda, MD, USA: 2016. Pharmacopeial Convention Excipients: USP and NF Excipients, Listed by Functional Category; p. 7490. [Google Scholar]
- 68.Food and Drug Administration FDA-Inactive Ingredient Search for Approved Drug Products Search. [(accessed on 1 October 2022)]; Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm.
- 69.Adhikary A., Vavia P.R. Bioadhesive Ranitidine Hydrochloride for Gastroretention with Controlled Microenvironmental PH. Drug Dev. Ind. Pharm. 2008;34:860–869. doi: 10.1080/03639040801928812. [DOI] [PubMed] [Google Scholar]
- 70.Ching A.L., Liew C.V., Chan L.W., Heng P.W.S. Modifying Matrix Micro-Environmental PH to Achieve Sustained Drug Release from Highly Laminating Alginate Matrices. Eur. J. Pharm. Sci. 2008;33:361–370. doi: 10.1016/j.ejps.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Pudipeddi M., Zannou E.A., Vasanthavada M., Dontabhaktuni A., Royce A.E., Joshi Y.M., Serajuddin A.T.M. Measurement of Surface PH of Pharmaceutical Solids: A Critical Evaluation of Indicator Dye-sorption Method and Its Comparison with Slurry PH Method. J. Pharm. Sci. 2008;97:1831–1842. doi: 10.1002/jps.21052. [DOI] [PubMed] [Google Scholar]
- 72.Siepe S., Lueckel B., Kramer A., Ries A., Gurny R. Assessment of Tailor-Made HPMC-Based Matrix Minitablets Comprising a Weakly Basic Drug Compound. Drug Dev. Ind. Pharm. 2008;34:46–52. doi: 10.1080/03639040701484106. [DOI] [PubMed] [Google Scholar]
- 73.Eisenächer F., Schädlich A., Mäder K. Monitoring of Internal PH Gradients within Multi-Layer Tablets by Optical Methods and EPR Imaging. Int. J. Pharm. 2011;417:204–215. doi: 10.1016/j.ijpharm.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Cope S.J., Hibberd S., Whetstone J., MacRae R.J., Melia C.D. Measurement and Mapping of PH in Hydrating Pharmaceutical Pellets Using Confocal Laser Scanning Microscopy. Pharm. Res. 2002;19:1554–1563. doi: 10.1023/A:1020425220441. [DOI] [PubMed] [Google Scholar]
- 75.Østergaard J., Jensen H., Larsen S.W., Larsen C., Lenke J. Microenvironmental PH Measurement during Sodium Naproxenate Dissolution in Acidic Medium by UV/Vis Imaging. J. Pharm. Biomed. Anal. 2014;100:290–293. doi: 10.1016/j.jpba.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Jensen S.S., Jensen H., Cornett C., Møller E.H., Østergaard J. Real-Time UV Imaging Identifies the Role of PH in Insulin Dissolution Behavior in Hydrogel-Based Subcutaneous Tissue Surrogate. Eur. J. Pharm. Sci. 2015;69:26–36. doi: 10.1016/j.ejps.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 77.Zhang C., Liu Y., Li W., Gao P., Xiang D., Ren X., Liu D. Mucoadhesive Buccal Film Containing Ornidazole and Dexamethasone for Oral Ulcers: In Vitro and in Vivo Studies. Pharm. Dev. Technol. 2019;24:118–126. doi: 10.1080/10837450.2018.1428814. [DOI] [PubMed] [Google Scholar]
- 78.Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive Delivery Systems. I. Evaluation of Mucoadhesive Polymers for Buccal Tablet Formulation. Drug Dev. Ind. Pharm. 2004;30:985–993. doi: 10.1081/DDC-200037245. [DOI] [PubMed] [Google Scholar]
- 79.Patel V.M., Prajapati B.G., Patel H.V., Patel K.M. Mucoadhesive Bilayer Tablets of Propranolol Hydrochloride. AAPS PharmSciTech. 2007;8:E203–E208. doi: 10.1208/pt0803077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohamad S.A., Abdelkader H., Elrehany M., Mansour H.F. Vitamin B12 Buccoadhesive Tablets: Auspicious Non-Invasive Substitute for Intra Muscular Injection: Formulation, in Vitro and in Vivo Appraisal. Drug Dev. Ind. Pharm. 2019;45:244–251. doi: 10.1080/03639045.2018.1529787. [DOI] [PubMed] [Google Scholar]
- 81.Nafee N.A., Ismail F.A., Boraie N.A., Mortada L.M. Mucoadhesive Buccal Patches of Miconazole Nitrate: In Vitro/in Vivo Performance and Effect of Ageing. Int. J. Pharm. 2003;264:1–14. doi: 10.1016/S0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 82.Chen G., Bunt C., Wen J. Mucoadhesive Polymers-Based Film as a Carrier System for Sublingual Delivery of Glutathione. J. Pharm. Pharmacol. 2015;67:26–34. doi: 10.1111/jphp.12313. [DOI] [PubMed] [Google Scholar]
- 83.Koirala S., Nepal P., Ghimire G., Basnet R., Rawat I., Dahal A., Pandey J., Parajuli-Baral K. Formulation and Evaluation of Mucoadhesive Buccal Tablets of Aceclofenac. Heliyon. 2021;7:e06439. doi: 10.1016/j.heliyon.2021.e06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob S., Shirwaikar A., Nair A. Preparation and Evaluation of Fast-Disintegrating Effervescent Tablets of Glibenclamide. Drug Dev. Ind. Pharm. 2009;35:321–328. doi: 10.1080/03639040802337021. [DOI] [PubMed] [Google Scholar]
- 85.Avdeef A., Berger C.M. PH-Metric Solubility.: 3. Dissolution Titration Template Method for Solubility Determination. Eur. J. Pharm. Sci. 2001;14:281–291. doi: 10.1016/S0928-0987(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 86.Zur M., Gasparini M., Wolk O., Amidon G.L., Dahan A. The Low/High BCS Permeability Class Boundary: Physicochemical Comparison of Metoprolol and Labetalol. Mol. Pharm. 2014;11:1707–1714. doi: 10.1021/mp500152y. [DOI] [PubMed] [Google Scholar]
- 87.Advankar A., Maheshwari R., Tambe V., Todke P., Raval N., Kapoor D., Tekade R.K. Chapter 13—Specialized Tablets: Ancient History to Modern Developments. In: Tekade R.K., editor. Drug Delivery Systems. Academic Press; Cambridge, MA, USA: 2019. pp. 615–664. [Google Scholar]
- 88.Freye E. A New Transmucosal Drug Delivery System for Patients with Breakthrough Cancer Pain: The Fentanyl Effervescent Buccal Tablet. [(accessed on 30 September 2020)]. Available online: https://www.dovepress.com/a-new-transmucosal-drug-delivery-system-for-patients-with-breakthrough-peer-reviewed-article-JPR. [DOI] [PMC free article] [PubMed]
- 89.Eichman J.D., Robinson J.R. Mechanistic Studies on Effervescent-Induced Permeability Enhancement. Pharm. Res. 1998;15:925–930. doi: 10.1023/A:1011936901638. [DOI] [PubMed] [Google Scholar]
- 90.Pather I., Kolli C.S. Chemical Methods for Enhancing Oral Mucosal Delivery. In: Rathbone M.J., Senel S., Pather I., editors. Oral Mucosal Drug Delivery and Therapy. Springer; Boston, MA, USA: 2015. pp. 31–52. [Google Scholar]
- 91.Jaipal A., Pandey M.M., Charde S.Y., Sadhu N., Srinivas A., Prasad R.G. Controlled Release Effervescent Buccal Discs of Buspirone Hydrochloride: In Vitro and in Vivo Evaluation Studies. Drug Deliv. 2016;23:452–458. doi: 10.3109/10717544.2014.917388. [DOI] [PubMed] [Google Scholar]
- 92.Hans R., Thomas S., Garla B., Dagli R.J., Hans M.K. Effect of Various Sugary Beverages on Salivary PH, Flow Rate, and Oral Clearance Rate amongst Adults. Scientifica. 2016;2016:5027283. doi: 10.1155/2016/5027283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.West N.X., Hughes J.A., Addy M. The Effect of PH on the Erosion of Dentine and Enamel by Dietary Acids in Vitro. J. Oral Rehabil. 2001;28:860–864. doi: 10.1046/j.1365-2842.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 94.Martelli B.K.L., de Melo D.M., Nai G.A., Parizi J.L.S. Influence of Water PH in Oral Changes Caused by Cadmium Poisoning: An Experimental Study in Rats. Rev. Odontol. UNESP. 2014;43:180–184. doi: 10.1590/rou.2014.034. [DOI] [Google Scholar]
- 95.Committee on Toxicity, Food Standard Agency Home Page. [(accessed on 12 February 2023)]; Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20200803134618/https://cot.food.gov.uk/cotstatements/cotstatementsyrs/cotstatements2004/acidsweets.
- 96.Ahuja A., Khar R.K., Ali J. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1997;23:489–515. doi: 10.3109/03639049709148498. [DOI] [Google Scholar]
- 97.Asane G.S., Nirmal S.A., Rasal K.B., Naik A.A., Mahadik M.S., Rao Y.M. Polymers for Mucoadhesive Drug Delivery System: A Current Status. Drug Dev. Ind. Pharm. 2008;34:1246–1266. doi: 10.1080/03639040802026012. [DOI] [PubMed] [Google Scholar]
- 98.Pham Q.D., Nöjd S., Edman M., Lindell K., Topgaard D., Wahlgren M. Mucoadhesion: Mucin-Polymer Molecular Interactions. Int. J. Pharm. 2021;610:121245. doi: 10.1016/j.ijpharm.2021.121245. [DOI] [PubMed] [Google Scholar]
- 99.da Bassi Silva J., de Ferreira S.B.S., Reis A.V., Cook M.T., Bruschi M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers. 2018;10:254. doi: 10.3390/polym10030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Oliveira Cardoso V.M., Gremião M.P.D., Cury B.S.F. Mucin-Polysaccharide Interactions: A Rheological Approach to Evaluate the Effect of PH on the Mucoadhesive Properties. Int. J. Biol. Macromol. 2020;149:234–245. doi: 10.1016/j.ijbiomac.2020.01.235. [DOI] [PubMed] [Google Scholar]
- 101.Surendranath M., Ramesan R.M., Parameswaran R. Recent Advances in Functionally Modified Polymers for Mucoadhesive Drug Delivery. J. Mater. Chem. B. 2022;10:5913–5924. doi: 10.1039/D2TB00856D. [DOI] [PubMed] [Google Scholar]
- 102.Waterman K.C., Adami R.C., Alsante K.M., Antipas A.S., Arenson D.R., Carrier R., Hong J., Landis M.S., Lombardo F., Shah J.C., et al. Hydrolysis in Pharmaceutical Formulations. Pharm. Dev. Technol. 2002;7:113–146. doi: 10.1081/PDT-120003494. [DOI] [PubMed] [Google Scholar]
- 103.Brandl M., Magill A., Rudraraju V., Gordon M.S. Approaches for Improving the Stability of Ketorolac in Powder Blends. J. Pharm. Sci. 1995;84:1151–1153. doi: 10.1002/jps.2600841003. [DOI] [PubMed] [Google Scholar]
- 104.Tabatar T., Makino T., Kashihara T., Hirai S., Kitamori N., Toguchi H. Stabilization of a New Antiulcer Drug (Lansoprazole) in the Solid Dosage Forms. Drug Dev. Ind. Pharm. 1992;18:1437–1447. doi: 10.3109/03639049209040850. [DOI] [Google Scholar]
- 105.Chen J.-G., Chen J.-G., Markovitz D.A., Yang A.Y., Rabel S.R., Pang J., Dolinsky O., Wu L.-S., Alasandro M. Degradation of a Fluoropyridinyl Drug in Capsule Formulation: Degradant Identification, Proposed Degradation Mechanism, and Formulation Optimization. Pharm. Dev. Technol. 2000;5:561–570. doi: 10.1081/PDT-100102039. [DOI] [PubMed] [Google Scholar]
- 106.Oliyai C., Patel J.P., Carr L., Borchardt R.T. Chemical Pathways of Peptide Degradation. VII. Solid State Chemical Instability of an Aspartyl Residue in a Model Hexapeptide. Pharm. Res. 1994;11:901–908. doi: 10.1023/A:1018998312503. [DOI] [PubMed] [Google Scholar]
- 107.Strickley R.G., Anderson B.D. Solid-State Stability of Human Insulin I. Mechanism and the Effect of Water on the Kinetics of Degradation in Lyophiles from PH 2–5 Solutions. Pharm. Res. 1996;13:1142–1153. doi: 10.1023/A:1016043715791. [DOI] [PubMed] [Google Scholar]
- 108.Taniguchi C., Inoue R., Kawabata Y., Yamashita K., Wada K., Yamauchi Y., Yamada S., Onoue S. Novel Formulations of Dipyridamole with Microenvironmental PH-Modifiers for Improved Dissolution and Bioavailability under Hypochlorhydria. Int. J. Pharm. 2012;434:148–154. doi: 10.1016/j.ijpharm.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 109.Adeleke O.A., Choonara Y.E., Du Toit L.C., Pillay V. In Vivo and Ex Vivo Evaluation of a Multi-Particulate Composite Construct for Sustained Transbuccal Delivery of Carbamazepine. J. Pharm. Sci. 2014;103:1157–1169. doi: 10.1002/jps.23884. [DOI] [PubMed] [Google Scholar]
- 110.El-Mahrouk G.M., El-Gazayerly O.N., Aboelwafa A.A., Taha M.S. Chitosan Lactate Wafer as a Platform for the Buccal Delivery of Tizanidine HCl: In Vitro and in Vivo Performance. Int. J. Pharm. 2014;467:100–112. doi: 10.1016/j.ijpharm.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 111.Kassem M.A.A., ElMeshad A.N., Fares A.R. Enhanced Bioavailability of Buspirone Hydrochloride via Cup and Core Buccal Tablets: Formulation and in Vitro/in Vivo Evaluation. Int. J. Pharm. 2014;463:68–80. doi: 10.1016/j.ijpharm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Urbanova M., Gajdosova M., Steinhart M., Vetchy D., Brus J. Molecular-Level Control of Ciclopirox Olamine Release from Poly(Ethylene Oxide)-Based Mucoadhesive Buccal Films: Exploration of Structure–Property Relationships with Solid-State NMR. Mol. Pharm. 2016;13:1551–1563. doi: 10.1021/acs.molpharmaceut.6b00035. [DOI] [PubMed] [Google Scholar]