Abstract
Simple Summary
Elongases of long-chain fatty acids (ELOs) play an important role in the function and metabolism of fatty acids of different chain lengths. However, little is known about the expression and biological function of fatty acid ELOs in insects, including in the larvae and adult blood-feeding Ae. aegypti mosquitoes. Therefore, the study of ELOs is necessary to understand the development and reproduction of insects, including mosquitoes. In this study, two novel ELO genes were identified in Ae. aegypti. We used two different RNAi methods (larval-nanoparticle feeding; adult-microinjection) to analyze the functions of AeELO2 and AeELO9 in larvae development and adult fecundity in Aedes aegypti. The results indicated that AeELO2 and AeELO9 play crucial roles in larval development and the adult fecundity of Ae. aegypti. AeELO2 mainly participated in larval molting behavior and growth, and it regulated the flexibility and elasticity of adult mosquito cuticles. AeELO9 affected the cold resistance of larvae and adult mosquitoes. It also regulated the permeability of larvae and adult mosquito cuticles and egg development, therefore affecting the reproductive ability of Ae. aegypti.
Abstract
Long-chain fatty acid elongases (ELOs) play important roles in the metabolism of fatty acids in insects. In this study, the genes for two elongases from Aedes aegypti were identified, AeELO2 and AeELO9. Quantitative real time PCR showed that AeELO2 and AeELO9 are expressed at all developmental stages and some body parts, but with different expression patterns. RNAi-mediated knockdown of AeELO2 and AeELO9 was performed to investigate their roles in the development, growth, osmotic balance, and cold tolerance of Ae. aegypti. Knockdown of AeELO2 slowed larval growth and development by causing molting abnormalities. Additionally, 33% ± 3.3% of adults died during oviposition, accompanied by an abnormal extension of cuticles in AeELO2-dsRNA knockdown mosquitos. Knockdown of AeEL09 resulted in abnormal balance of cuticular osmotic pressure and a reduction in egg production. The maximal mRNAs of AeELO2 and AeELO9 were detected in eggs at 72 h after oviposition. Moreover, AeELO2 knockdown reduced the egg hatching rates and AeELO9 knockdown larvae did not develop well. In summary, AeELO2 is involved in larval molting and growth, and its knockdown affects the flexibility and elasticity of adult mosquito cuticles. AeELO9 regulates cold tolerance, osmotic balance, and egg development in Ae. aegypti.
Keywords: Aedes aegypti, elongase, cold tolerance, cuticle, development, fecundity
1. Introduction
Fatty acids are the most important energy storage molecules in insects and act as the basic material for insect embryonic development, metamorphosis, flight, and other functions. In addition, they are vital components of insect cuticles, somatic membranes, and are the precursor of many insect sex pheromones. Some fatty acids are associated with the cold resistance of insects.
The long-chain fatty acid elongases (ELOs), the rate-limiting condensing enzymes in the first step of the elongation cycle (Figure 1), play important roles in the function and metabolism of fatty acids of different chain lengths (C18-C26) [1,2,3]. They are involved in various insect functions, including mating, reproduction, pheromone biosynthesis, and cuticle formation [4,5]. A few earlier studies have emphasized that the requirement of the elongases in growth, development, and survival is likely conserved in organisms. In Drosophila melanogaster, RNAi-mediated knockdown of a gene for a predicted elongase, CG6660, induced a lethal phenotype [6]. An essential gene, neighbor of abl (noa), encoding a very long-chain fatty acid elongase, has a somatic role in Drosophila development [7]. In Tenebrio molitor, RNAi-mediated silencing of TmELO1 increased the mortality rate indicating that TmELO1 is essential for mealworm survival [8]. Similar requirements for elongases in organism survival have been reported in other species [9,10]. An analysis of the expression pattern of the ELO gene in Apis mellifera revealed that ten ELO genes may be associated with cuticle hydrocarbon biosynthesis and have important roles in cuticle development [8]. In the brown planthopper, Nilaparvata lugens, nine NlELO genes were essential for the survival of nymphs and adults [11]. In addition, elongases are required for neonatal survival in mice [5], for the survival of the protozoan parasite, Toxoplasma gondii [10], and for the growth and survival of cancer cells [12].
Figure 1.
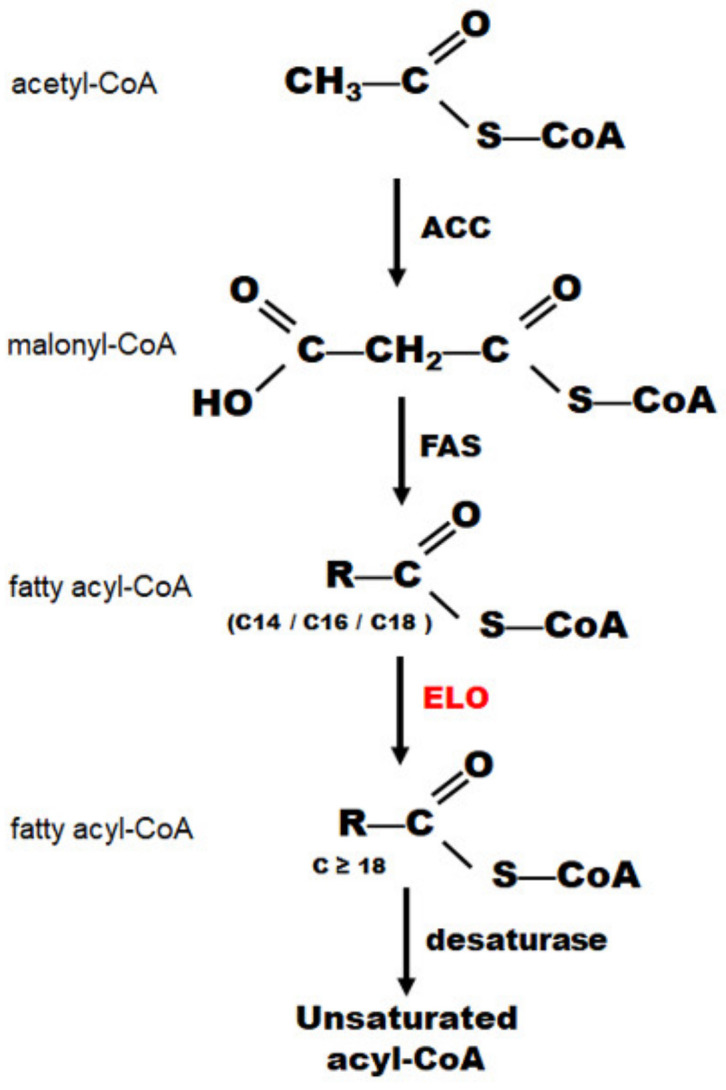
Overview of the fatty acid biosynthesis pathway. Additionally, the pathway comprises: (1) conversion of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase (ACC); (2) conversion of malonyl-CoA to fatty acyl-CoAs by fatty acid synthase (FAS); (3) elongation of fatty acyl-CoAs by long-chain fatty acid elongases (ELOs); (4) desaturase conversion of saturated fatty acids into unsaturated fatty acids.
In insects, the cuticle has dissimilar properties at different developmental stages and in different body parts. During early developmental stages, the cuticle becomes flexible and elastic, and becomes sclerotized and melanized shortly after molting [13,14,15,16]. This process occurs periodically during the growth and development of insects. The hard exoskeleton acts as a protective barrier, while the flexible cuticle allows the insects to move [13,17]. Ae. aegypti needs to molt continuously during the larvae stage. In previous studies, it was found that 3,4-dihydroxypheny-lacetaldehyde (DOPAL) synthase is directly linked to the flexible cuticle proteins (CPs), cross-linking them through the interaction of free amino acid groups on the CPs in mosquitoes [16,18,19]. RNAi-mediated knockdown of DOPAL synthase in Ae. aegypti larvae caused procuticle, exocuticle, and endocuticle thinning and changed the cuticular surface texture, which could lead to poor flexibility of the integument [15], and significantly delay the development of larvae, which, in turn, affected larval metamorphosis. In addition, changes in the expression of a wide range of genes encoding CPs, chitin, and genes involved in lipid biosynthesis were found during transcriptome analysis of the mosquitoes after DOPAL synthase knockdown [15]. In these data, two new ELO genes, AeELO2 and AeELO9, were found to be involved in the development of larvae.
A recent study reported that acetyl-CoA carboxylase (ACC) and fatty acid synthase-1 (FAS1) are involved in lipid biosynthesis and the formation of eggshells after digestion of a blood meal in female Ae. aegypti. Their functions are different; mosquitoes deficient in ACC, but not FAS1, produced defective eggshells, and mosquitoes deficient in FAS1 had delayed blood meal digestion [20]. ACC is the first rate-limiting enzyme during fatty acid elongation and FAS is the multifunctional fatty acid synthase in eukaryotes (Figure 1). The research considered that decreased malonyl-CoA levels specifically inhibit fatty acid elongase function in mosquitoes [20]. In mammals, ELOs also have an important role in the biosynthesis of fatty acids possessing ≥ 18-carbon atoms [4,21]. However, little is known about the expression and biological function of ELOs in mosquitoes. In our study, we found that the expression of lipid biosynthesis genes was also significantly affected by RNAi-mediated knockdown of DOPAL synthase in mosquitoes [15]; among these lipid biosynthesis genes, the differential expressions of AeELO2 and AeELO9 are significant. Therefore, the functions of AeELO2 and AeELO9 were investigated using the RNAi in Ae. aegypti larvae and adults. Our data suggest that AeELO2 and AeELO9 play a vital role in regulating the mosquito development by functioning on the cuticular structure formation in larvae and fecundity in the adults of Ae. aegypti.
2. Materials and Methods
2.1. Mosquito Rearing
Ae. aegypti mosquitoes (the Rockefeller strain, provided by the Beijing Institute of Microbiology and Epidemiology, Beijing, China) were used in this study. Mosquitoes were reared as described previously [22].
2.2. Identification of AeELO2 and AeELO9
The identification of AeELO2 and AeELO9 was performed using bioinformatic approaches. The other known insect (Drosophila melanogaster; Nilaparvata lugens; Homo sapiens; Tenebrio molitor; Saccharomyces cerevisiae) ELO proteins were selected from published sources [6,8,11,23,24,25] and the corresponding protein sequences were obtained from the NCBI database. Multiple sequence alignment analysis was performed using MEGA6. The conserved histidine motif HXXHH (solid line) and the conserved motif YXYY (dotted line) are indicated.
2.3. Quantitative Real Time PCR
Spatiotemporal expression patterns of AeELO2 and AeELO9 were established by quantitative real time PCR (qPCR). Ae. aegypti eggs at different developmental stages were collected every 12 h from the beginning to the fifth day of development, and the total RNA was extracted. Then, larvae from different developmental stages, pupae, and female and male adult mosquitoes were collected. Different body parts of adults were dissected, and total RNA was extracted. The relative expression levels of AeELO2 (XM_021847806.1) and AeELO9 (XM_021837993.1) at different developmental stages and in different body parts of adults were analyzed. The following qPCR primers were used to detect AeELO2 (forward: 5′-TTG ACC ATC ATT GCC AG-3′ and reverse: 5′-CCA TCC AAG CCC TCG TA-3′) and AeELO9 (forward: 5′-CTC ACA GAC ATA CAC GA-3′ and reverse: 5′-TAA AGT AGG CAT AGA AT-3′). RSP17 was used as an internal reference gene [26].
2.4. RNA Interference in Larvae and Adult Mosquitoes
Total RNA was extracted at the developmental stage with the highest relative expression of AeELO2 and AeELO9, and cDNA was synthesized with the PrimeScript™ RT reagent kit (TaKaRa, Dalian, China). The primers (forward: 5′-ACT GTT CTA CGA GGG CTT G-3′ and reverse: 5′-CAT TTG GCA GAG TGA-3′) were used to amplify a fragment of AeELO2 and the primers (forward: 5′-AGG AAC CTA CTC GTG GGC TA-3′ and reverse: 5′-AAC TGG TGC GTG AAG A-3′) were used to amplify a fragment of AeELO9. After amplification, the amplified fragment was ligated into the pL4440 vector used for interference and transformed into HT115 (DE3) competent cells. Bacteria were cultured in LB medium until the optical density of the solution at 600 nm (OD600) was in the 0.4–0.6 range, followed by the induction by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) for 4–6 h. Bacteria were collected and harvested 4–6 h after induction, and the dsRNAs were extracted from the collected bacteria. Mosquito larvae were fed with chitosan-coated dsRNA nanoparticles, and the adult mosquitoes were microinjected with dsRNA [15].
2.5. Cold Treatment of Mosquito Larvae and Adults
The third or fourth instar larvae of the control groups and AeELO2 and AeELO9 knockdown groups were collected. A total of 20 larvae were collected from each group. Each larva was separately placed into a 1.5 mL centrifuge tube containing 500 µL sterile water. Then, these tubes were placed at 4 °C, and the larval mortality was determined every 12 h. The experiment was repeated three times. For adult mosquitoes, a total of 20 dsRNA injected adult mosquitoes were collected and placed at −20 °C for 3–4 min. Afterwards, the mosquito was placed at room temperature for 30 min and stimulated with a needle. If it did not move, it was regarded as dead. The experiment was repeated three times.
2.6. Statistical analyses
A one-way ANOVA test was performed to evaluate the significance of the differences between the groups. The threshold level for significance was p < 0.05.
3. Results
3.1. Identification of AeELO2 and AeELO9
In the Ae. aegypti ELO family, AeELO2 and AeELO9 were named after the third digits of their aliases AAEL002673 and AAEL009574. Analyses of the predicted amino acid sequences in the NCBI search indicated that AeELO2 and AeELO9 indeed encoded ELOs as they shared characteristic domains of eukaryotic ELOs, including a HXXHH motif and a YXYY motif in the domain (Figure S1) [5].
3.2. Spatiotemporal Expression Profiles of AeELO2 and AeELO9
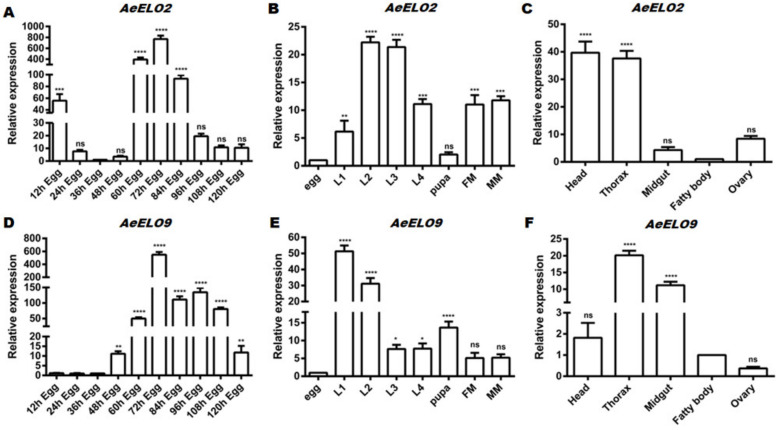
Analysis of the RNA-seq database following RNAi-mediated knockdown of DOPAL synthase in Ae. aegypti showed that AeELO2 expression was up-regulated, while that of AeELO9 was down-regulated (Figure S2A–C). At different stages of egg development, the relative expression level (the expression level was set to 1 at 36 h after oviposition) of AeELO2 was the highest at 60–84 h after oviposition, and peaked at 72 h after oviposition (Figure 2A). The egg stage of Ae. aegypti was used as the control for our studies of the complete life cycle. AeELO2 expression was the lowest at the egg and pupal stages, the highest in 2nd and 3rd instar larvae, and intermediate for the adult mosquitoes (Figure 2B). AeELO2 was predominantly expressed in the head and thorax, with relatively low expression in the ovary, fatty body, and midgut (Figure 2C). Similar to AeELO2, the relative expression of AeELO9 peaked in eggs 72 h after egg laying (Figure 2E). However, AeELO9 expression was relatively high at the late stage of egg development (from 48 h to 120 h after egg laying) (Figure 2D). The results showed that the highest AeELO9 expression was in the 1st and 2nd instar larvae, followed by the pupa stage (Figure 2E). Unlike AeELO2, AeELO9 had the highest expression level in the thorax and midgut of female adult mosquitoes, but not in the head (Figure 2F). Like AeELO2, the expression of AeELO9 in the ovary and fatty body was relatively low (Figure 2F).
Figure 2.
Expression levels of AeELO2 and AeELO9. (A) Temporal expression levels of AeELO2 at different times of egg development (12 h, 24 h, etc., represent the time after egg deposition). Eggs at 36 h were used as the control. (B) Temporal expression levels of AeELO2 at different developmental stages. Eggs at 36 h were used as the control. (C) AeELO2 expression in different body parts of adults. The fatty body was used as the control. (D) Temporal expression levels of AeELO9 at different times of egg development (12 h, 24 h, etc., represent the time after spawning). Eggs at 36 h were used as the control. (E) Temporal expression levels of AeELO9 in different developmental stages. Eggs at 36 h were used as the control. (F) AeELO9 expression in different body parts of adults. The fatty body was used as the control. Abbreviations: L1, first instar larvae; L2, second instar larvae; L3, third instar larvae; L4, fourth instar larvae; FM, female adults; MM, male adults. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns, not significant. *, **, etc., represent a statistically significant difference compared with the control.
3.3. RNAi-Mediated Knockdown of AeELO2 Affects Larval Molting Behavior
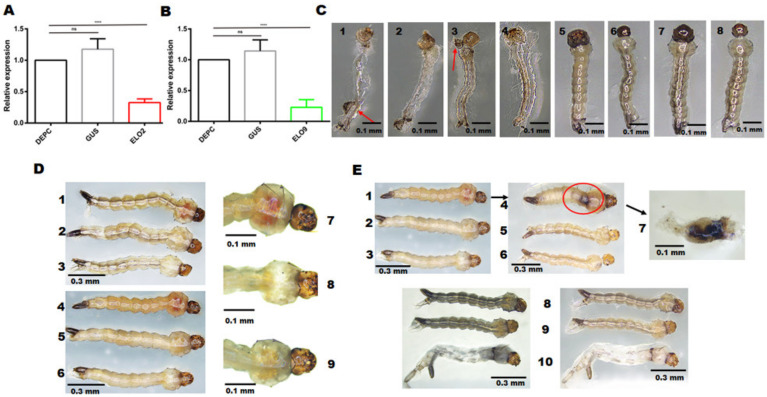
The quantitative results indicated that AeELO2 and AeELO9 mRNA levels are significantly decreased with RNAi, by 75% ± 2.3% (p < 0.0001) and 70% ± 4.5% (p < 0.0001), respectively, compared with the control groups (Figure 3A,B). Moreover, 32% ± 3.3% of the 2nd and 4th instar larvae died due to abnormal molting after AeELO2 knockdown. Specifically, the 2nd instar larvae died during ecdysis (Figure 3C). A part of the molted cuticle of the dead larvae remained in the trunk of the body (as indicated by the red arrow, showing that the old head cuticles remain on the surface of the new larva). In addition, the body contents were concentrated at the junction of the abdomen and the thorax in some abnormally molting 4th instar larvae, while both old and new cuticles were stacked at the end of the abdomen (Figure 3D). In addition, 40% ± 3.5% of the AeELO2-deficient 4th instar larvae appeared in red color on the thorax (Figure 3D). The body of larvae with a red thorax appeared swollen, and the junction between the thorax and abdomen became obviously black within 12 h after death (Figure 3E). The anatomical examination revealed that the blackened part was not the larval cuticle, but internal tissue fluid, which could be transferred to the midgut (Figure 3E). However, unlike AeELO2, AeELO9-deficient larvae did not show abnormal molting (Figure S3).
Figure 3.
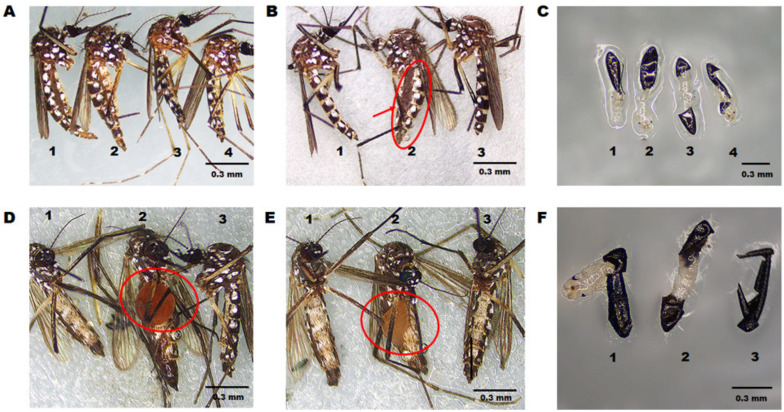
Changes in larval phenotype after RNAi-mediated AeELO2 knockdown. (A) Quantitation of AeELO2 expression in larvae after RNAi-mediated knockdown. (B) Quantitation of AeELO9 expression in larvae after RNAi-mediated knockdown. (C) Abnormal molting caused by AeELO2 knockdown. Here, 1, 2, 3, and 4 represent abnormally molting larvae in the AeELO2 knockdown group; 5 and 6 are the larvae in the gus-dsRNA treatment group; 7 and 8 represent the blank control group. (D) AeELO2 knockdown results in a red thorax. Here, 1 and 4 are the abnormal larvae after RNAi-mediated AeELO2 knockdown; 2 and 5 represent the larvae in the negative control group (gus-dsRNA treated); 3 and 6 are the larvae in the blank control group (water treated). (E) The larvae with a red thorax became swollen for a short time after death. Here, 1 represents the larvae with a red thorax in the AeELO2 knockdown group; 2 and 3 represent the larvae in the control groups; 4 represents the larvae within 12 h after death; 5 and 6 represent the larvae in the control groups at 12 h after death; 7 shows the dissected state of the larva shown in 4. In addition, 8 and 9 show larvae from the control groups; 10 represents larvae from the AeELO2 knockdown group. Abbreviations: DEPC, blank control, larvae treated with diethyl pyrocarbonate water; GUS, larvae treated with gus-dsRNA; ELO2, larvae treated with AeELO2-dsRNA; ELO9, larvae treated with AeELO9-dsRNA; **** p < 0.0001, ns, not significant.
3.4. The Effects of AeELO2 and AeELO9 on Larval Development and Freezing Tolerance
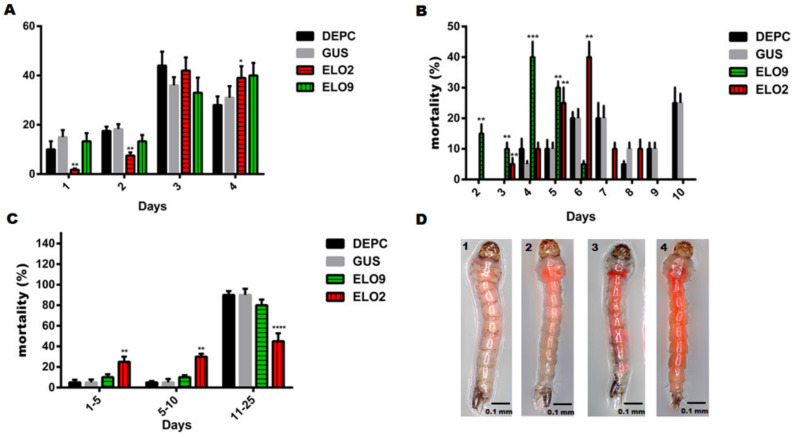
Taking the day when the larvae begin to pupate as the first day, the pupation rates of larvae in the control groups were 17% ± 2.5% on the first and second day (Figure 4A). The pupation rate in the AeELO9 knockdown group was similar to that in the control groups (Figure 4A). However, the pupation rate of AeELO2 knockdown larvae was significantly lower than that of the control larvae, at 5% ± 1.3% on the first and second days (Figure 4A). AeELO2-knockdown larvae pupation was delayed 1-2 days compared with the control larvae (Figure 4A). Therefore, AeELO2 knockdown significantly inhibited the development from larvae to pupae, whereas AeELO9 knockdown larvae were not affected.
Figure 4.
Effects of AeELO2 and AeELO9 knockdown on the growth rate and cold tolerance of larvae and pupae. (A) Larvae to pupae development rate. The days after treatment and the percentage of pupae are shown on the x- and y-axes, respectively. Each treatment group contained 120 larvae, and the experiment was repeated three times. (B) Mortality rate of the 4th instar larvae at 4 °C. The days after treatment and mortality rate (%) are shown on the x- and y-axes, respectively. (C) Mortality rate of pupae at 4 °C. The days after treatment and mortality rate (%) are shown on the x- and y-axes, respectively. (D) The osmotic balance of larval cuticle. The larvae of different treatment groups were soaked with Eosin Y dye for 5 min. Here, 1 represents the larvae of blank control; 2 represents the larvae treated with gus-dsRNA; 3 represents the larvae in the AeELO2-dsRNA treatment group; 4 represents the larvae treated with AeELO2-dsRNA. Each treatment group contained 30 larvae, and the experiment was repeated three times. Abbreviations: DEPC, blank control, larvae treated with diethyl pyrocarbonate water; GUS, larvae treated with gus-dsRNA; ELO2, larvae treated with AeELO2-dsRNA; ELO9, larvae treated with AeELO9-dsRNA; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns, not significant. *, **, etc., represent a significant difference compared with the blank control (DEPC water-treated).
Next, survival at 4 °C was determined for larvae (3rd–4th instar) and pupae in the AeELO2 and AeELO9 knockdown and control groups. The mortality rate of AeELO9 knockdown larvae was 15% ± 4.2% on the second day at 4 °C, and the mortality rate peaked on the fourth day. All the larvae died on the sixth day (Figure 4B). Unlike AeELO9 knockdown, AeELO2 knockdown larvae and pupae started dying after 3 days at 4 °C. Their mortality rate gradually increased in the following days, with the highest mortality observed on the sixth day. Compared with AeELO9 knockdown group, all AeELO2 knockdown larvae died on the tenth day (Figure 4B). In addition, pupae were also collected and kept at 4 °C. A total of 90% ± 2.3% of the control and AeELO9 knockdown pupae were still alive after 11 days at 4 °C, while only 45% ± 5.3% of AeELO2 knockdown pupae survived after 11 days (Figure 4C).
The 4th instar larvae of different treatment groups were soaked with Eosin Y dye for 5 min and observed under a microscope after washing with deionized water (five times). The larvae of the AeELO2-dsRNA and AeELO9-dsRNA treated groups contained more of the Eosin Y dye, relative to the control larvae. This indicated that the osmotic balance of AeELO2-dsRNA and AeELO9-dsRNA treated larvae (52% ± 3.3%) was destroyed (Figure 4D).
3.5. Decreased AeELO2 and AeELO9 Expression Inhibited Egg Hatching
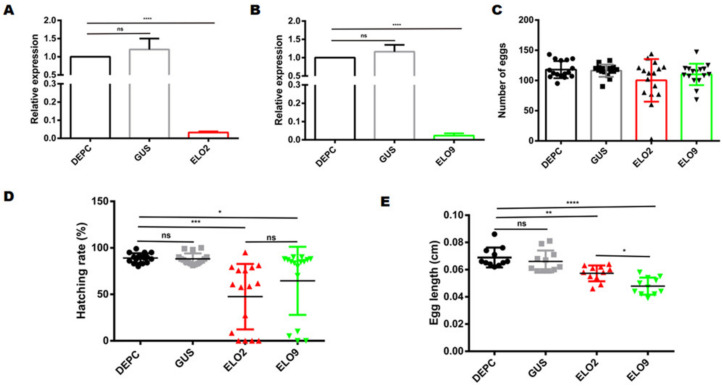
Quantitative PCR analysis showed that RNAi-mediated knockdown AeELO2 and AeELO9 in adult mosquitoes reduced expression level to 90% ± 3.3%, when compared with the control (Figure 5A,B). dsRNAs targeting AeELO2 and AeELO9 were injected into adult mosquitoes 12 h before blood feeding, and eggs were laid three days after a blood meal. There was no significant difference in the number of eggs laid between the control groups (adult mosquitoes injected with DEPC water and gus-dsRNA) and the treatment groups (adult mosquitoes injected with dsRNAs targeting AeELO2 and AeELO9) (Figure 5C). However, the hatching rate of eggs in the AeELO2 and AeELO9 knockdown groups was significantly reduced. The hatching rate of AeELO9 knockdown mosquitoes was 65% ± 2.3%, and that of AeELO2 knockdown mosquitoes was only 47, while that of the control mosquitoes was more than 90% ± 5.3% (Figure 5D). Compared with the control groups, AeELO2 and AeELO9 knockdown significantly decreased the egg hatching rate, and the effect of AeELO2 knockdown was greater than that of AeELO9 knockdown.
Figure 5.
Effects of RNAi-mediated knockdown of AeELO2 and AeELO9 on egg production and hatchability. (A) RNAi-mediated knockdown of AeELO2. (B) RNAi-mediated knockdown of AeELO9. (C) Changes of oviposition in adult mosquitoes after injection with dsRNAs targeting AeELO2 and AeELO9. The statistical unit is a single mosquito. (D) Effect of RNAi-mediated knockdown of AeELO2 and AeELO9 on egg hatching rates. (E) RNAi-mediated knockdown of AeELO2 and AeELO9 reduced egg length. Abbreviations: DEPC, blank control, adult mosquitoes treated with diethyl pyrocarbonate water; GUS, adult mosquitoes treated with gus-dsRNA; ELO2, adult mosquitoes treated with AeELO2-dsRNA; ELO9, adult mosquitoes treated with AeELO9-dsRNA; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns, not significant. *, **, etc., represent a significant difference compared with the blank control (adult mosquitoes treated with DEPC water). Red represents AeELO2 and green represents AeELO9; The black dot, gray box, red triangle, and green inverted triangle represent the number of eggs laid by DEPC water, gus-dsRNA, AeELO2, and AeELO9, respectively.
As shown in Figure 5D, the eggs laid by AeELO2 and AeELO9 knockdown mosquitoes were significantly shorter than those laid by control mosquitoes. The average egg length for the control mosquitoes was 0.67 ± 0.13 mm, while egg lengths were 0.57 ± 0.15 mm and 0.48 ± 0.11 mm in the AeELO2 and AeELO9 knockdown groups, respectively (Figure 5E). Therefore, AeELO2 and AeELO9 deficiency significantly reduced egg length compared with the controls, and AeELO9 knockdown had a greater effect on egg length than the AeELO2 knockdown (Figure 5E).
3.6. Inhibiting the Expression of AeELO2 and AeELO9 Interfered with Cuticle Elasticity and Osmotic Pressure during Adult and Egg Development
RNAi results suggested that AeELO2 and AeELO9 may not affect the number of eggs laid by a mosquito (Figure 5C), but 30% ± 3.3% of AeELO2 and AeELO9 knockdown adult mosquitoes had less oviposition (Figure S4A). Moreover, the mean egg production per AeELO2 and AeELO9 knockdown mosquito was 65, while that of the control mosquito was 110 ± 10 (Figure S4B). In addition, these adult mosquitoes exhibiting abnormal egg laying died three to five days after spawning, but control mosquitoes survived for ten days.
Microscopic observation showed that the abdomen of the control mosquitoes returned to its original state after oviposition and could support normal feeding behaviors. In contrast, the abdomens of AeELO2 knockdown adult mosquitoes with abnormal oviposition remained in the egg-laying state with limited extension and did not return to the normal state (Figure 6A). In addition to the destruction of cuticle elasticity, 25% ± 5.2% of AeELO2 knockdown adult mosquitoes died with unproduced eggs in their ovaries (Figure 6B). Whereas, AeELO9 knockdown mosquitoes did not retain unproduced eggs, nor did they die during egg laying. However, 50% ± 4.5% of AeELO9 knockdown mosquitoes died three days after oviposition with abdomens full of tissue fluid (red circle) (Figure 6D–E).
Figure 6.
Effects of RNAi-mediated knockdown of AeELO2 and AeELO9 on adult mosquito cuticles and egg development. (A) Alterations in cuticular elasticity in adult mosquitoes after AeELO2 knockdown. Here, 1, 2 show the AeELO2 knockdown group; 3 and 4 show the gus-dsRNA-treated and DEPC water-treated control groups, respectively. (B) Same as panel a, and 1 and 3 represent the gus-dsRNA-treated and DEPC-water treated control groups, respectively; 2 represents the AeELO2 knockdown group. (C) The phenotype of the hatched eggs, where 1 and 2 represent the gus-dsRNA-treated and DEPC water-treated control groups, respectively; 3 and 4 showed the AeELO2 knockdown group. (D,E) Alterations in cuticular osmotic pressure in adult mosquitoes after AeELO9 knockdown. Here, 1 and 3 represent the gus-dsRNA treated and DEPC water-treated control groups, respectively, in panels d and e; 2 AeELO9-dsRNA-treated mosquitoes. (F) Status of unhatched eggs in the AeELO9 knockdown group. Here, 1 and 2 show the gus-dsRNA treated and DEPC water-treated control groups, respectively; 3 represents the AeELO9 knockdown group. Abbreviations: DEPC, diethyl pyrocarbonate. (A,B,D,E) Each treatment group contained 30 adults, and the experiment was repeated three times. (C,F) Each treatment group dissected 60 unhatched eggs, and the experiment was repeated three times.
AeELO2 deficiency resulted in reduced egg length (Figure 4E), but no other changes in egg appearance and structure were observed. After dissection, the mature 1st instar larvae could be observed (Figure 6C). Although no obvious abnormalities were observed in the egg phenotype of AeELO9 knockdown mosquitoes, mature 1st instar larvae were not found in the eggs after dissection. As shown in Figure 6F, compared with the eggs of control mosquitoes, larvae did not develop in the eggs of AeELO9 knockdown mosquitoes.
4. Discussion
Previous studies have shown that ELOs have critical roles in the insect’s survival. For example, RNAi-mediated knockdown of CG6660 (a gene encoding a predicted elongase) in Drosophila induced a lethal phenotype [6]. In T. molitor, silencing of TmELO1 via RNAi resulted in an increased mortality rate [8], and LmELO7 deficiency caused lethal phenotypes by decreasing cuticular hydrocarbon (CHC) amounts during locust molting [27]. In this study, two novel fatty acid elongase genes (AeEL02 and AeELO9) were identified in Ae. aegypti. The results indicated that AeELO2 and AeELO9 are essential for the larva and adult survival of Ae. aegypti. AeELO2 deficiency caused abnormalities in larval molting. Moreover, AeELO2 and AeELO9 deficiency led to the rapid death of adult mosquitoes after oviposition. AeELO2 knockdown adult mosquitoes died because their abdominal cuticles were in an extremely extended state during the oviposition process. These results suggest that cuticle flexibility was affected. The innermost procuticle of insects contains the protein–chitin matrix, which is necessary for its stability. DOPAL synthase is involved in cuticular proteins cross-linking, which affects cuticular flexibility [15,16]. Following RNAi-mediated knockdown, the transcription of AeELO2 decreased significantly. These results suggest that AeELO2 may participate in the formation of flexible cuticles.
In AeELO9-deficient adult mosquitoes, the flexibility of the abdominal cuticles did not appear to be affected, but abdominal swelling was observed. Some studies have reported that insect CHCs are deposited in the outermost cuticle layer, forming a permeable barrier, which can effectively protect insects from the external environment. It can not only prevent water evaporation from the insect body, but effectively blocks external water, pathogens, chemical pesticides, and other harmful substances as well [3,28]. LmELO7 is involved in CHC synthesis in L. migratoria, and LmELO7 knockdown affected the water retention and epidermal permeability of locusts [27]. It has been demonstrated in migratory locusts that inhibition of epidermal hydrocarbon synthesis can increase permeability to water-soluble substances [1,29]. Thus, it can be inferred that decreased AeELO9 expression may disrupt CHC deposition in the outermost cuticles of insects. Disruptions in cuticle permeability can lead to abnormalities in water balance, abdominal swelling, and death.
Mutations of genes that affect lipid or carbohydrate metabolism can impact ovarian development, fertility, or embryogenesis [30,31]. In egg-laying animals, such as birds [32], reptiles [33], and insects, the developing embryo can only obtain nutrient input into the egg before oviposition. It is well established that lipids are the main energy source in eggs. In this report, we present data demonstrating that AeELO2 and AeELO9 are also involved in regulating egg size and hatching in mosquitoes. AeELO2 and AeELO9 knockdown significantly reduced the egg hatching rate. The mature 1st instar larvae could not hatch from AeELO2-deficient eggs. This may be due to damage to the cuticular structure. This was also evident from the abnormal molting development and growth of AeELO2-deficient larvae. Further, the effect of AeELO2 knockdown on egg size could also be due to any disruption of lipid synthesis or impact on cuticle elasticity, which could lead to the squeezing of eggs during oviposition, resulting in shorter egg length.
In addition, no larvae were found in the unhatched eggs from AeELO9 knockdown mosquitoes. Early research showed that in the process of egg development, especially during the second half, the lipid transfer from egg yolk to embryo increases [34]. Considering that AeELO9 is expressed mainly during the late stage of egg development, it is possible that AeELO9 may affect the biosynthesis of lipids from the blood meal, thereby affecting lipid transfer and egg development.
In general, the biological function of ELOs is not conserved in complete metamorphosis and incomplete metamorphosis insects. AeELO2 and AeELO9 affect the development of larvae and the flexibility or osmotic balance of adult cuticles, which not only greatly reduces the reproductive ability of Ae. aegypti, but also makes them a potential target for future pesticides.
5. Conclusions
AeELO2 and AeELO9 play crucial roles in larval development and in the adult fecundity of Ae. aegypti. AeELO2 mainly participates in larval molting and growth, and regulates the flexibility and elasticity of adult mosquito cuticles. AeELO9 affects the cold resistance of larvae and adult mosquitoes, and also regulates the permeability of adult mosquito cuticles and egg development, thereby affecting the reproduction of Ae. aegypti.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects14020189/s1, Figure S1: Amino acid alignment of AeELO2, AeELO9, and other known insect ELO proteins; Figure S2: Quantitative RCR was used to determine the relative expression levels of AeELO2 and AeELO9 after DOPAL synthase knockdown; Figure S3: Mortality of larvae molted abnormally; Figure S4: The probability of reduced oviposition and the number of eggs in adult mosquitoes after AeELO2 and AeELO9 knockdown.
Author Contributions
J.C.: Conceptualization, methodology, formal analysis, visualization, original draft, review and editing, funding acquisition. Y.-C.W.: Methodology, investigation, visualization, review and editing. J.-K.C.: methodology, data curation, review and editing. X.-J.Z.: Methodology, investigation, visualization. D.M.: review and editing. C.-H.L.: Conceptualization, resources, supervision, review and editing, funding acquisition. Q.H.: review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Funding Statement
This research was supported by the National Natural Science Foundation of China (31960703, U22A20363) and The Major Science and Technology plan of Hainan Province (ZDKJ2021035).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Qiao J.W., Fan Y.L., Wu B.J., Bai T.T., Wang Y.H., Zhang Z.F., Wang D., Liu T.X. Downregulation of NADPH-cytochrome P450 reductase via RNA interference increases the susceptibility of Acyrthosiphon pisum to desiccation and insecticides. Insect Sci. 2021;29:1105–1119. doi: 10.1111/1744-7917.12982. [DOI] [PubMed] [Google Scholar]
- 2.Chung H., Loehlin D.W., Dufour H.D., Vaccarro K., Millar J.G., Carroll S.B. A single gene affects both ecological divergence and mate choice in Drosophila. Science. 2014;343:1148–1151. doi: 10.1126/science.1249998. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y., Tittiger C., Wicker-Thomas C., Le Goff G., Young S., Wajnberg E., Fricaux T., Taquet N., Blomquist G.J., Feyereisen R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA. 2012;109:14858–14863. doi: 10.1073/pnas.1208650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillou H., Zadravec D., Martin P.G., Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Chertemps T., Duportets L., Labeur C., Ueda R., Takahashi K., Saigo K., Wicker-Thomas C. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2007;104:4273–4278. doi: 10.1073/pnas.0608142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvy J.P., Napal L., Rubin T., Poidevin M., Perrin L., Wicker-Thomas C., Montagne J. Drosophila melanogaster Acetyl-CoA-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet. 2012;8:e1002925. doi: 10.1371/journal.pgen.1002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung A., Hollmann M., Schafer M.A. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm development. J. Cell Sci. 2007;120:2924–2934. doi: 10.1242/jcs.006551. [DOI] [PubMed] [Google Scholar]
- 8.Zheng T., Li H., Han N., Wang S., Hackney Price J., Wang M., Zhang D. Functional Characterization of Two Elongases of Very Long-Chain Fatty Acid from Tenebrio molitor L. (Coleopt: Tenebrionidae) Sci. Rep. 2017;7:10990. doi: 10.1038/s41598-017-11134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron D.J., Tong Z., Yang Z., Kaminoh J., Kamiyah S., Chen H., Zeng J., Chen Y., Luo L., Zhang K. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int. J. Biol. Sci. 2007;3:111–119. doi: 10.7150/ijbs.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazumdar J., Wilson E.H., Masek K., Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc. Natl. Acad. Sci. USA. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D.T., Chen X., Wang X.Q., Moussian B., Zhang C.X. The fatty acid elongase gene family in the brown planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019;108:32–43. doi: 10.1016/j.ibmb.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Rohrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 13.Xie J., Peng G., Wang M., Zhong Q., Song X., Bi J., Tang J., Feng F., Gao H., Li B. RR-1 cuticular protein TcCPR69 is required for growth and metamorphosis in Tribolium castaneum. Insect Sci. 2022;29:1612–1628. doi: 10.1111/1744-7917.13038. [DOI] [PubMed] [Google Scholar]
- 14.Vannini L., Willis J.H. Localization of RR-1 and RR-2 cuticular proteins within the cuticle of Anopheles gambiae. Arthropod. Struct. Dev. 2017;46:13–29. doi: 10.1016/j.asd.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Lu H.R., Zhang L., Liao C.H., Han Q. RNA interference-mediated knockdown of 3, 4-dihydroxyphenylacetaldehyde synthase affects larval development and adult survival in the mosquito Aedes aegypti. Parasit. Vectors. 2019;12:311. doi: 10.1186/s13071-019-3568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao C., Upadhyay A., Liang J., Han Q., Li J. 3,4-Dihydroxyphenylacetaldehyde synthase and cuticle formation in insects. Dev. Comp. Immunol. 2018;83:44–50. doi: 10.1016/j.dci.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Mun S., Noh M.Y., Dittmer N.T., Muthukrishnan S., Kramer K.J., Kanost M.R., Arakane Y. Cuticular protein with a low complexity sequence becomes cross-linked during insect cuticle sclerotization and is required for the adult molt. Sci. Rep. 2015;5:10484. doi: 10.1038/srep10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotegher N., Berti G., Ferrari E., Tessari I., Zanetti M., Lunelli L., Greggio E., Bisaglia M., Veronesi M., Girotto S., et al. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017;7:40699. doi: 10.1038/srep40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vavricka C., Han Q., Huang Y., Erickson S.M., Harich K., Christensen B.M., Li J. From L-dopa to dihydroxyphenylacetaldehyde: A toxic biochemical pathway plays a vital physiological function in insects. PLoS ONE. 2011;6:e16124. doi: 10.1371/journal.pone.0016124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alabaster A., Isoe J., Zhou G., Lee A., Murphy A., Day W.A., Miesfeld R.L. Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 2011;41:946–955. doi: 10.1016/j.ibmb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobsson A., Westerberg R., Jacobsson A. Fatty acid elongases in mammals: Their regulation and roles in metabolism. Prog. Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Mysore K., Andrews E., Li P., Duman-Scheel M. Chitosan/siRNA nanoparticle targeting demonstrates a requirement for single-minded during larval and pupal olfactory system development of the vector mosquito Aedes aegypti. BMC Dev. Biol. 2014;14:9. doi: 10.1186/1471-213X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertemps T., Duportets L., Labeur C., Wicker-Thomas C. A new elongase selectively expressed in Drosophila male reproductive system. Biochem. Biophys. Res. Commun. 2005;333:1066–1072. doi: 10.1016/j.bbrc.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Schackmann M.J., Ofman R., Dijkstra I.M., Wanders R.J., Kemp S. Enzymatic characterization of ELOVL1, a key enzyme in very long-chain fatty acid synthesis. Biochim. Biophys. Acta. 2015;1851:231–237. doi: 10.1016/j.bbalip.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Oh C.S., Toke D.A., Mandala S., Martin C.E. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 1997;272:17376–17384. doi: 10.1074/jbc.272.28.17376. [DOI] [PubMed] [Google Scholar]
- 26.Dzaki N., Ramli K.N., Azlan A., Ishak I.H., Azzam G. Evaluation of reference genes at different developmental stages for quantitative real-time PCR in Aedes aegypti. Sci. Rep. 2017;7:43618. doi: 10.1038/srep43618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X., Yang Y., Niu N., Zhao Y., Liu W., Ma E., Moussian B., Zhang J. The fatty acid elongase gene LmELO7 is required for hydrocarbon biosynthesis and cuticle permeability in the migratory locust, Locusta migratoria. J. Insect Physiol. 2020;123:104052. doi: 10.1016/j.jinsphys.2020.104052. [DOI] [PubMed] [Google Scholar]
- 28.Noh M.Y., Muthukrishnan S., Kramer K.J., Arakane Y. Development and ultrastructure of the rigid dorsal and flexible ventral cuticles of the elytron of the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2017;91:21–33. doi: 10.1016/j.ibmb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Zhao X., Niu N., Zhao Y., Liu W., Moussian B., Zhang J. Two fatty acid synthase genes from the integument contribute to cuticular hydrocarbon biosynthesis and cuticle permeability in Locusta migratoria. Insect Mol. Biol. 2020;29:555–568. doi: 10.1111/imb.12665. [DOI] [PubMed] [Google Scholar]
- 30.Laws K.M., Drummond-Barbosa D. Control of Germline Stem Cell Lineages by Diet and Physiology. Results Probl. Cell Differ. 2017;59:67–99. doi: 10.1007/978-3-319-44820-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida-Oliveira F., Tuthill B.F., II, Gondim K.C., Majerowicz D., Musselman L.P. dHNF4 regulates lipid homeostasis and oogenesis in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2021;133:103569. doi: 10.1016/j.ibmb.2021.103569. [DOI] [PubMed] [Google Scholar]
- 32.Speake B.K., Murray A.M., Noble R.C. Transport and transformations of yolk lipids during development of the avian embryo. Prog. Lipid Res. 1998;37:1–32. doi: 10.1016/S0163-7827(97)00012-X. [DOI] [PubMed] [Google Scholar]
- 33.Speake B.K., Thompson M.B. Lipids of the eggs and neonates of oviparous and viviparous lizards. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000;127:453–467. doi: 10.1016/S1095-6433(00)00276-2. [DOI] [PubMed] [Google Scholar]
- 34.Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included in the article.