Abstract
The endoplasmic reticulum (ER) of most vertebrate cells is spread out by kinesin-dependent transport along microtubules, whereas studies in Saccharomyces cerevisiae indicated that motility of fungal ER is an actin-based process. However, microtubules are of minor importance for organelle transport in yeast, but they are crucial for intracellular transport within numerous other fungi. Herein, we set out to elucidate the role of the tubulin cytoskeleton in ER organization and dynamics in the fungal pathogen Ustilago maydis. An ER-resident green fluorescent protein (GFP)-fusion protein localized to a peripheral network and the nuclear envelope. Tubules and patches within the network exhibited rapid dynein-driven motion along microtubules, whereas conventional kinesin did not participate in ER motility. Cortical ER organization was independent of microtubules or F-actin, but reformation of the network after experimental disruption was mediated by microtubules and dynein. In addition, a polar gradient of motile ER-GFP stained dots was detected that accumulated around the apical Golgi apparatus. Both the gradient and the Golgi apparatus were sensitive to brefeldin A or benomyl treatment, suggesting that the gradient represents microtubule-dependent vesicle trafficking between ER and Golgi. Our results demonstrate a role of cytoplasmic dynein and microtubules in motility, but not peripheral localization of the ER in U. maydis.
INTRODUCTION
The endoplasmic reticulum (ER) serves essential functions in biosynthesis and Ca2+ regulation within the eukaryotic cell. It is continuous with the nuclear envelope and consists of a polygonal network of tubules that extends from the cell center to the periphery. In vivo observation of the ER in various vertebrate cell systems revealed that the network is highly dynamic (Lee and Chen, 1988; Dailey and Bridgman, 1989; Sanger et al., 1989; Waterman-Storer and Salmon, 1998) with tubules undergoing branching, sliding, and ring closures (Lee and Chen, 1988; Prinz et al., 2000). The close association of ER tubules with microtubules (MTs) (Terasaki et al., 1986; Dailey and Bridgman, 1989) and impaired ER reorganization after disruption of the MT cytoskeleton (Lee et al., 1989) led to the conclusion that MT-dependent transport supports ER organization in animal cells (summarized in Allan, 1996). This notion was strongly supported by both in vivo and vitro studies showing that ER motility depends on MT polymerization (Waterman-Storer et al., 1995; Waterman-Storer and Salmon, 1998) as well as motor enzymes that hydrolyze ATP to power rapid tubule motion along MTs (Dabora and Sheetz, 1988; Vale and Hotani, 1988). It is now widely accepted that conventional kinesin provides the driving force for rapid plus end-directed ER transport toward the cell periphery of vertebrate cells (Feiguin et al., 1994). In contrast, the minus end-directed dynein motor appears to have no obvious role in ER motility in differentiated cells (summarized in Allan, 1996), but is thought to drive motion of ER tubules during early developmental stages in Xenopus eggs (Allan and Vale, 1991; Allan, 1995; Lane and Allan, 1999). This indicates that specialized cell types use different motor systems for organizing their ER.
In plants and fungi the ER network is mainly localized beneath the plasma membrane (Lancelle et al., 1987; Allen and Brown, 1988; Preuss et al., 1991; Rossanese et al., 1999) and ER motility in plant cells and in the yeast Saccharomyces cerevisiae was found to be actin based (Knebel et al., 1990; Liebe and Menzel, 1995; Prinz et al., 2000). Therefore, it was suggested that myosin motors and F-actin are involved in tubule motion and organization in plants and fungi (Allan, 1996), although the cortical localization of the network in S. cerevisiae was found to be independent of F-actin. Because, in contrast to yeast, many fungi use MTs and associated motors to support intracellular traffic (Mata and Nurse, 1997; Seiler et al., 1999; Wedlich-Söldner et al., 2000; Steinberg et al., 2001), ER organization and motility in fungi other than yeast might be MT dependent.
Herein, we set out to elucidate the role of MTs and associated motors in motility and organization of the ER in the corn smut fungus Ustilago maydis. Outside its host the dimorphic basidiomycete U. maydis exists as haploid yeast-like cells. On mating of two compatible sporidia a filamentous dikaryotic hypha is formed that invades the corn tissue and completes its life cycle (reviewed in Banuett, 1995; Kahmann et al., 2000). In its yeast-like form the fungus can easily be cultivated and is amenable to molecular genetics and cytological methods. Therefore, U. maydis is well suited to investigate the role of the cytoskeleton in growth and organization of the fungal cell (Lehmler et al., 1997; Steinberg et al., 1998, 2001; Wedlich-Söldner et al., 2000).
In this study, we demonstrate that the peripheral ER network in U. maydis is highly dynamic and that the MT-dependent motor dynein is required for this motion, whereas conventional kinesin has no obvious role in ER motility. In addition, we show that maintenance of the cortical network does not depend on an intact cytoskeleton, although MTs and dynein support the reorganization after disruption of the ER.
MATERIALS AND METHODS
Strains and Culture Conditions
U. maydis strains FB1 (a1b1) and FB2 (a2b2) have been described previously (Banuett and Herskowitz, 1989; Table 1). Transformation of plasmids was done as described (Schulz et al., 1990). In FB1EG, FB1Dyn2tsEG, FB1rDyn2EG, FB2Δkin2EG, FB1rTub1EG, and AB33EG, plasmid pERGFP was ectopically integrated into strains FB1, FB1Dyn2ts (see below), FB1rDyn2 (Straube et al., 2001), FB2Δkin2 (Lehmler et al., 1997), FB1rTub1 (Steinberg et al., 2001), and AB33 (Brachmann et al. 2001), respectively. AB33 carries two regulators for induction of filamentous growth, bE2 and bW1, under control of the nitrate reductase promoter of U. maydis (nar-promoter; see below). FB1GYPT1 contains plasmid pGFPYpt1, and FB1GAD carries pGAD integrated ectopically into wild-type strain FB1.
Table 1.
Strains and plasmids used in this study
| Strains | Genotype | Reference |
|---|---|---|
| FB1 | a1b1 | Banuett and Herskowitz, 1989 |
| FB2 | a2b2 | Banuett and Herskowitz, 1989 |
| FB1EG | a1b1 /pERGFP | This study |
| FB1rTub1 | a1b1 Pcrg-tub1, natR | Steinberg et al., 2001 |
| FB1rTub1EG | a1b1 Pcrg-tub1, natR /pERGFP | This study |
| FB2Δkin2 | a2b2 Δkin2::hphR | Lehmler et al., 1997 |
| FB2Δkin2EG | a1b1 Δkin2::hphR /pERGFP | This study |
| FB1rDyn2 | a1b1 Pcrg-dyn2, bleR | Straube et al., 2001 |
| FB1rDyn2EG | a1b1 Pcrg-dyn2, bleR /pERGFP | This study |
| FB1Dyn2ts | a1b1 Δdyn2::dyn2ts, natR | This study |
| FB1Dyn2tsEG | a1b1 Δdyn2::dyn2ts, natR /pERGFP | This study |
| FB1GVPT | a1b1 /pGFPYpt1 | This study |
| FB1GAD | a1b1 /pGFPGAD | This study |
| AB33 | a2 bE2Δ[b2I, bW2V]::[Pnar, bleR, nar(p), bW1V] | Brachmann et al., 2001 |
| AB33EG | a2 bE2 Δ[b2I, bW2V]::[Pnar, bleR, nar(p), bW1V] /pERGFP | This study |
| pERGFP | calS:egfp:hdel, natR, cbxR | This study |
| pGFPYpt1 | egfp:YPT1, cbxR | This study |
| pgfpgad | egfp:γadaptin1, cbxR | This study |
| pDyn2ts | Δdyn2::dyn2ts, natR | This study |
a, b, mating type genes; Δ, deletion; P, promoter; ::, homologous replacement; −, fusion; ts, temperature-sensitive allele; hphR, hygromycine resistance; bleR, phleomycine resistance; natR, nourseothricin resistance; cbxR, carboxin resistance; /, ectopically integrated; calS, signal sequence of calreticulin from rabbit (nt1-51); hdel, ER retention signal;
I, intergenic region of b locus;
V, variable region of b genes.
If not mentioned otherwise, strains were grown at 28°C in 2.5% potato dextrose or complete medium (CM; Holliday, 1974) supplemented with 1% arabinose (CM-A) or 1% glucose (CM-G). Solid media contained 2% (wt/vol) bacto-agar. To generate dikaryotic hyphae green fluorescent protein (GFP)-expressing strains were cospotted with a compatible wild-type strain on charcoal-containing agar plates and incubated at room temperature for 12–20 h (Banuett and Herskowitz, 1989). To investigate the ER in conditional mutant strains FB1rDyn2EG and FB2rTub1EG, cells were grown overnight in CM-A and shifted to restrictive conditions by washing them with 1 volume CM-G, followed by dilution in 10 ml of CM-G and incubation at 28°C, 200 rpm. Strain FB1Dyn2tsEG contained a temperature-sensitive allele of dyn2 (see below). Defects in the ER organization were monitored after growth at 22°C and transfer to 30–34°C for 6–7 h. To analyze the ER organization in filaments of AB33EG cells were grown in CM-G to OD600 of 0.5–0.9, washed once in water, and transferred to nutrient media supplemented with 1% glucose. This medium was modified from minimal medium but contains KNO3 as sole nitrogen source. In AB33 and its derivatives, nitrate induces the nar-promoter, resulting in the transcription of an active bE1/W2 heterodimer, which leads to filamentous growth (Brachmann et al., 2001).
Generation of a Temperature-sensitive Dynein Mutant Strain
To generate temperature-sensitive mutants in Dyn2 (Straube et al., 2001) a random mutagenesis was performed. For this purpose the last 1173 nucleotides of the open reading frame of dyn2 and additional 301 nucleotides of the 3′ untranslated region (UTR) were amplified from genomic DNA by error prone polymerase chain reaction (PCR) (modified from Spee et al., 1993). The products of all PCRs were pooled and used to replace the corresponding region of dyn2 on pNEBUH-dyn2, which is a self-replicating plasmid that contained the complete open reading frame of dyn2. The resulting plasmid pool was transformed into FB1rDyn2 and cells were grown on restrictive medium (CM-G). This was followed by replica plating on two CM-G plates that were incubated at 22 and 34°C. Colonies that were able to grow at 22°C but died at 34°C were collected and their phenotype at permissive and restrictive temperature was checked. This analysis was also done at lower temperature to minimize the effect of the temperature shift. From these investigations a strain was chosen that, at 29°C, displayed a typical FB1rDyn2 phenotype (Straube et al., 2001). The pNEBUH-dyn2ts plasmid was reisolated and the mutagenized dyn2 region was sequenced to determine the introduced point mutations (Q1373R, L1392P, D1395G, I1343N, E1452G, and E1497G). This dyn2ts allele was introduced into the wild-type locus of dyn2 by homologous integration of pDyn2ts, which was checked by Southern analysis, resulting in strain FB1Dyn2ts.
Plasmid Construction
pERGFP.
A fragment coding for the first 17 amino acids of calreticulin from rabbit (Fliegel et al., 1989) was fused to the N terminus of eGFP (CLONTECH, Palo Alto, CA), followed by the ER retention signals HDEL (Pelham, 1990). The PCR-based fusions generated an AflIII site at the 5′ end and an EagI restriction site at the 3′ end of the construct. The PCR fragment was sequenced and fused to the strong otef promoter (Spellig et al., 1996) by introducing it into plasmid p123 (Wedlich-Söldner et al., 2000) using single NcoI and NotI sites.
pGFPYpt1.
This plasmid contains YPT1 from S. cerevisiae N-terminally fused to eGFP. YPT1 was amplified from Ycplac33/GFP-YPT1 (kindly provided by Dr. B. Glick, University of Chicago, Chicago, IL), thereby generating an N-terminal NdeI site and an NotI site after the stop codon. The fragment was sequenced and inserted in plasmid potefGFPTub1 where YPT1 is under the control of the otef-promoter and replaces the tub1 gene (Steinberg et al., 2001).
pGFPGAD.
The gene encoding γ-adaptin from U. maydis (Keon et al., 1995) was amplified from genomic DNA by using specific primers that generated a NdeI site at the 5′ end and a NotI site at the 3′ end of the gene. The fragment was sequenced and inserted in plasmid potefGFPTub1 where it replaces the tub1 gene and expression of γ-adaptin is under the control of the otef promoter (Steinberg et al., 2001).
pNEBUH-dyn2.
The complete dyn2 locus, including 1.7-kb 5′ region and 1-kb 3′ region, was integrated into pNEBUH. This vector is a derivative of pNEB193 (New England Biolabs, Beverly, MA) and carries a U. maydis autonomously repeating sequence and the hygromycin resistance cassette (Müller et al., 1999).
pDyn2ts.
The plasmid carrying the temperature-sensitive allele of dyn2 (pNEBUH-dyn2ts; see above) was opened with NsiI at the end of the 3′ UTR of dyn2, and an NsiI-NsiI cassette carrying the nat-resistance cassette followed by 603 base pairs of the 3′ UTR was integrated.
Drug Treatment, Protoplast Preparation, and Staining Procedures
Stocks of 1 mM benomyl (Sigma Chemical, St. Louis, MO), nocodazole (Sigma Chemical), and cytochalasin D and E (Sigma Chemical), and 20 mM latrunculin A (LatA; kindly provided by Karen Tenney, University of California, Santa Cruz, CA) in dimethyl sulfoxide (DMSO) were stored at −20°C. Brefeldin A (BFA; Fluka, Buchs, Switzerland) was diluted in methanol at 2.5 mg/ml. For inhibitor studies these drugs were added to growing cultures at 10 μM final concentration for benomyl and cytochalasin D, 20 μM for cytochalasin E, and 200 μM for LatA and BFA. Cells were incubated for 30 min at room temperature under gentle shaking and effects on the ER were observed under epifluorescence. In control experiments corresponding amounts of DMSO and methanol were added to the cultures. Protoplasts were prepared by incubation of cells in SCS (20 mM sodium citrate, pH 5.8, 1 M sorbitol) and 3 mg/ml novozyme (Interspex Products, Foster City, CA) for 20–30 min, followed by 10-s shaking on a vortex mixer at maximum speed. For ER recovery experiments protoplasts were sedimented at 500 × g and resuspended in fresh SCS containing 1 mg/ml novozyme alone or additional inhibitors at 10 μM (benomyl, cytochalasin D, and DMSO). Protoplasts of FB1Dyn2tsEG and control strain FB1EG were prepared at 22°C and after 30–60 min of growth at 32°C. ER recovery was monitored after additional 45–60 min at 22 and 32°C, respectively. Immunofluorescence of MTs was done as described (Steinberg et al., 2001). In vivo staining of membranes and mitochondria with 3,3′-dihexylocarbocyanine iodide (Molecular Probes, Eugene, OR) was done according to published protocols (Koning et al., 1993) by using various concentrations ranging from 0.01 to 20 μg/ml. The ER was detected using ERTracker Blue-White DPX (Molecular Probes) at 10 μM according to manufacturer's instructions.
Light Microscopy and Quantitative Analysis
Cells from logarithmic cultures were embedded in 1% prewarmed low-melt agarose and immediately observed using a Zeiss Axiophot microscope and a cooled, charge-coupled device camera (C4742-95; Hamamatsu, Herrsching, Germany). Epifluorescence was observed using standard fluorescein isothiocyanate, rhodamine, and 4,6-diamidino-2-phenylindole filter sets. For colocalization studies eGFP fluorescence was detected by a specific filter set (BP 470/20, FT 493, BP 505–530; Zeiss, Oberkochen, Germany). Image processing and measurements were done with ImageProPlus (Media Cybernetics, Silver Spring, MD) and Photoshop (Adobe Systems, Mountain View, CA). Statistical analysis was performed using PRISM (GraphPad Software, San Diego, CA). All results are based on at least two independent experiments.
For quantitative analysis of ER motions within the peripheral network, sequences of 40–60 frames were taken at 400–500-ms intervals, and the number of directed motility events (tubules growth, sliding, patch movement over at least 1 μm) was determined in cells at various stages of the logarithmic growing culture. Mitotic cells that were recognized by their bud size, the absence of a nuclear envelope and the appearance of a brightly labeled bar within the daughter cell (see RESULTS; Figure 1A4) were quantified separately. Motility within these cells was also determined, but treated as an individual data set. Finally, the number of motility events per second and square micrometers was determined and the mean ± SD × 10−3 of three experiments was calculated. To avoid artifacts due to oxygen depletion all images and movies were taken within a 10 to 15-min observation. To avoid backshifting of the temperature-sensitive strain FB1Dyn2tsEG cells were not embedded and rapidly analyzed by taking two to three movies within less than a 3-min observation.
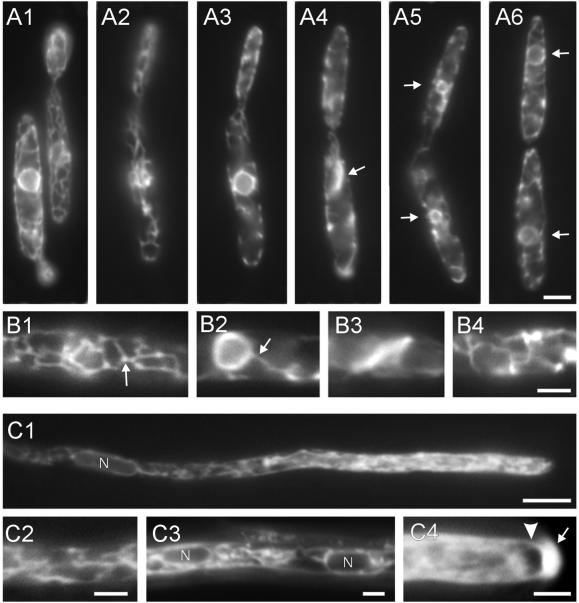
Figure 1.
Endoplasmic reticulum in U. maydis. (A) ER at different stages of the cell cycle. Cells of strain wild-type FB1EG expressing the ER-GFP fusion protein contain a cortical network within both the mother and daughter cell (A1). The network is located at the cell cortex (A2), and the nuclear envelope is clearly visible within the mother cell (A3, note that A2 and A3 show different focal planes of the same cell). During early mitotic stages the peripheral network persists (A4), whereas the nuclear envelope disappeared. Note that many cells contain a bar-like ER accumulation within the mother cell (A4, arrow). The nuclear envelope reappears in mother and daughter cells in telophase (A5, arrows), whereas the nuclear DNA is still condensed. Finally, both nuclei are positioned in mother and daughter cell, respectively (A6, arrows). Bar (A1–A6), 3 μm. (B) Details of the ER organization in haploid cells. ER tubules are usually branched and brightly stained dots are often visible at the branching points (B1, arrow). The peripheral network is in contact with the nuclear envelope (B2, arrow). During mitosis the ER-GFP fusion protein localizes to a bar-like structure, suggesting that ER membranes accumulate within the mother cell (B3), whereas a slightly distorted network persists at the cell periphery during mitosis (B4). Bar (B1–B4), 2 μm. (C) ER organization in dikaryotic hyphae. The ER-GFP construct stains tubular ER (C1, C2, C3) and the nuclear envelope (C1, C3, nuclei marked by “N”). In addition, an apical gradient of ER-GFP fills the apical region of the hyphae (C1). This gradient often was excluded from the apical dome (C4, arrowhead) and a very brightly region was observed at the hyphal tip (C4, arrow). Bar, 5 μm (C1) and 2 μm (C2–C4).
ER-GFP gradients in dikaryotic hyphae and hyphal AB33 cells (strain AB33EG) were observed 12–18 h after mating on charcoal containing plates or growth in nutrient media (see above) for 7–14 h, respectively. Digital images were taken and the average intensity per area was calculated using ImageProPlus software. ER-GFP intensity in areas at 2–10 and 25–30 μm away from the hyphal tip were measured and the mean value was calculated from at least 10 hyphae. Intensity of the gradient is given as a quotient of intensity near tip and within subapical region.
RESULTS
ER-GFP Fusion Protein Localizes to a Cortical Network, the Nuclear Envelope, and an Apical Vesicle Gradient in Hyphae
We used the fusion protein ER-GFP to investigate the ER in U. maydis. This fusion protein consisted of an N-terminal signal peptide of calreticulin and the C-terminal ER retention signal HDEL. Due to these signals ER-GFP was targeted to a polygonal network that was located at the cell cortex (Figure 1A1; 1A2 and 1A3, same cell at two focus planes) and mostly formed three-way junctions (77.2%, n = 57). In logarithmically growing cells of FB1EG (subsequently called “wild-type”) the network did not form cisternae, but contained mobile patches (Figure 1B1, arrow). In interphase cells ER-GFP additionally stained a sphere located in the center of the mother cell (Figure 1A1 and 1A3) that was in contact with the cortical network (1B2). The sphere surrounded the nuclear DNA (our unpublished data) and most likely represents the nuclear envelope. The peripheral network was almost not affected by fixation with formaldehyde, but disappeared under treatment with Triton X-100 or NP-40 (our unpublished data), suggesting that it consists of membranes. The tubular organization of the network was reminiscent of the ER organization in vertebrates (Lee and Chen, 1988) and S. cerevisiae (Koning et al., 1993; Prinz et al., 2000), suggesting that the network represents the ER of U. maydis. Because all attempts to stain the ER network with 3,3′-dihexylocarbocyanine iodide failed, we used the vital ER marker dye ERTracker. This dye stained structures, albeit faintly, that colocalized with the ER-GFP fusion protein (Figure 2A1–2A3).
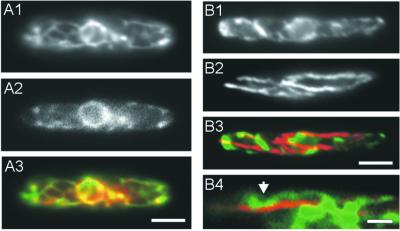
Figure 2.
Colocalization of ER-GFP, ERTracker Blue-White DPX and MTs. (A) Double-staining of ER-GFP and a vital dye specific for ER. Although staining of ERTracker Blue-White DPX was faint it clearly colocalized with ER-GFP in strain FB1EG (A1, ERTracker; A2, ER-GFP; A3, ERTracker in red, ER-GFP in green, overlay in yellow). Bar, 3 μm. (B) Double staining of ER and MTs in aldehyde-fixed cells. After fixation and preparation for immunostaining ER-GFP–containing tubules are still visible (B1). MTs, detected by anti-α-tubulin antibodies (B2) colocalize only at certain points (B3, arrow in B4; ER-GFP in green; MTs in red; overlay in yellow). Bar, 3 μm (B1–B3) and 1 μm (B4).
During mitosis the network remained at the cell periphery (Figure 1A4), but was slightly distorted (Figure 1B4). At this stage cytoplasmic MTs vanish, followed by the appearance of a short mitotic spindle within the proximal region of the bud (Steinberg et al., 2001). Interestingly, mitotic cells did not contain a nuclear envelope (Figure 1A4), but mother cells contained a brightly stained bar-like structure of 3.7 ± 0.64 μm (n = 5; Figure 1A4, 1B3, arrow). At later stages nuclear envelopes reformed (Figure 1A5, arrows), whereas the nuclear DNA was still condensed (our unpublished data), suggesting that the cells were in telophase. Finally, in G1 both nuclei enlarged (Figure 1A6, arrows), whereas cells still were not completely separated.
After fusion of two haploid cells a dikaryotic infection hypha is formed that does grow by apical tip expansion. ER organization in these hyphae was very similar to that of haploid sporidia (Figure 1C), although the network appeared to run through the interior of cell rather than being located to the periphery (Figure 1C1 and 1C2). This was most obvious from the region where both nuclei were positioned (Figure 1C1 and 1C3; nuclei marked by N). Interestingly, most hyphae contained an apical gradient of ER-GFP (Figures 1C1 and 6A1). This gradient appeared to consist of rapidly moving vesicles (Figure 3E) and extended over the apical 20–25 μm (n = 20 hyphae). In many hyphae (42%, n = 33), the apical region contained a spherical area of 1.34 ± 0.21 μm (n = 11) in length that excluded the ER-GFP marked compartment from parts of the hyphal dome (Figure 1C4, arrowhead). Finally, a brightly stained cap characterized the tip of most hyphae (Figure 1C4, arrow).
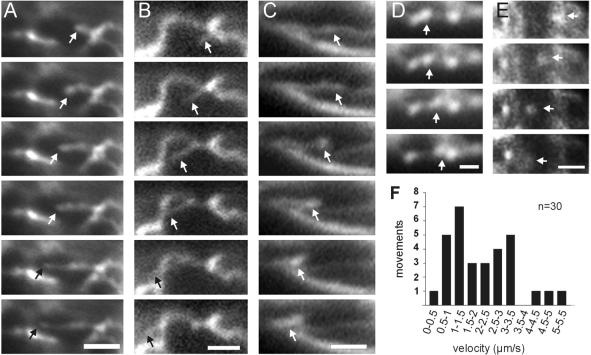
Figure 3.
Motility within the ER. (A)Tubule elongation. End of tubule marked by an arrow. Interval between frames is 0.3 s. Bar, 2 μm. (B) Lateral motion of an ER tubule. Tubule marked by an arrow. Interval between frames is 0.2 s. Bar, 2 μm. (C) Formation and motility of a wave-like structure within an ER tubule (arrow). Interval between frames is 0.2 s. Bar, 2 μm. (D) Motion of a patch along an ER tubule. Interval between frames is 0.3 s. Bar, 1 μm. (E) Motion of an ER-GFP–stained small vesicle in the apex of a dikaryotic hypha. These dots most likely represent ER-Golgi transport vesicles. Interval between frames is 0.5 s. Bar, 2 μm. (F) Velocity of ER tubule and patch motility in haploid wild-type FB1EG cells. Note that the diagram summarized all types of tubular ER motions in these cells.
MTs Support Rapid Motion of ER Tubules
We observed different types of motility within the ER network of U. maydis. Tubules rapidly extended out of the nuclear envelope or out of other tubules (Figure 3A). In addition, rapid sliding of tubules over several micrometers was observed (Figure 3B), and ER tubules occasionally formed moving waves (Figure 3C). Finally, bright patches showed rapid short-range motion along ER tubules (Figure 3D). At 28°C, which is the temperature at which U. maydis is cultivated under laboratory conditions, tubules and patches moved at an average velocity of 2.16 ± 1.28 μm/s (n = 30, range 0.48–5.13 μm/s; Figure 3F). Quantitative analysis of this motion in haploid cells reveled that this motion of tubules and patches within the network was frequent, with approximately five motility events per square micrometer and hour (Table 2). The mean number of motility events was set to 100% and used as reference for all subsequent estimates of motility rates. The observed ER motility was temperature dependent. Lowering the temperature to 22°C decreased ER motility to ∼90% of that at 28°C, whereas it increased to ∼200% at 34°C (Table 2; Figure 4, C and D).
Table 2.
Quantitative data of ER motility in U. maydis
| Strain | Temperature (°C) | No. of motility eventsa | No. of observed cells | Total area (μm2) | Total time of observation (s) | Motility events/μm2 and hourb | Amount of motilityb (%) |
|---|---|---|---|---|---|---|---|
| FB1EG | 28 | 68 | 52 | 2334.8 | 1021 | 5.04 ± 0.90 | 100 ± 20.45 |
| FB1EG | 22 | 48 | 39 | 1585.5 | 624 | 4.87 ± 1.00 | 91.49 ± 18.71 |
| FB1EG | 34 | 150 | 53 | 3084 | 870 | 10.84 ± 0.84 | 203.41 ± 15.75 |
| FB1EG (mitotic) | 28 | 3 | 13 | 1017.7 | 195 | 0.78 ± 0.68 | 14.60 ± 12.70 |
| FB1rTub1EG | 28 | 8 | 41 | 2074.6 | 800 | 0.43 ± 0.17 | 8.49 ± 3.33 |
| FB2Δkin2EG | 28 | 50 | 47 | 1893.3 | 922 | 5.09 ± 2.00 | 100.95 ± 39.74 |
| FBrDyn2EG (ara) | 28 | 16 | 48 | 2712.4 | 919 | 1.14 ± 0.88 | 22.56 ± 17.46 |
| FB1DyntsEG | 22 | 45 | 40 | 2132 | 624 | 4.63 ± 1.68 | 94.94 ± 34.42 |
| FB1DyntsEG | 34 | 20 | 47 | 4490.2 | 750 | 1.00 ± 0.46 | 18.69 ± 8.72 |
Derived from three experiments.
Mean ± standard deviation, n = 3 experiments; values compared with FB1EG, 28°C.
Figure 4.
ER organization and motility in kinesin and dynein mutants. (A) ER organization in FB2Δkin2EG, in which conventional kinesin is deleted. Neither the cortical localization (A1, arrow) nor the structure of the network (A2) is significantly altered. Bar, 3 μm (A1) and 2 μm (A2). (B) ER in the conditional dynein mutant FB1rDyn2EG after 25 h at restrictive conditions. The depletion of Dyn2, which provides the essential C-terminal part of the dynein heavy chain (Straube et al., 2001), leads to a characteristic nuclear migration and morphology defect, with many spherical nuclei clustering in an abnormal cell (B1). However, the network is still located beneath the plasma membrane (arrow in B1) and its tubular appearance is not affected (B2). Bar, 3 μm (B1) and 2 μm (B2). (C) Comparison of ER motility of wild-type strain FB1EG and several mutant strains. Motility events within the peripheral ER were counted in growing FB1EG cells (control, was set to 100%), the kinesin deletion mutant FB2Δkin2EG (Δkin2), the conditional dynein mutant FB1rDyn2EG after 25 h in CM-G (rDyn2), the conditional α-tubulin mutant strain FB1rTub1 after 8 h in CM-G (rtub1), and in mitotic cells of FB1EG (mitosis). Mean values ± SD of motility events per second and square micrometers were calculated from three experiments, including at least 40–50 cells and 1000–1500 s of observation. Although deletion of kinesin did not affect ER motility rates, depletion of Dyn2 and disruption of cytoplasmic MTs in conditional mutants or during mitosis drastically reduced ER motion. Note that FB1rDyn2EG still showed a residual activity of ∼20% of that of FB1EG. (D) ER motility in FB1EG and the temperature-sensitive dynein mutant FB1Dyn2tsEG. Motility events within the peripheral ER were counted in FB1EG cells (control) and the temperature-sensitive dynein mutant FB1DyntsEG (Dynts). At permissive temperature (22°C) both strains showed very similar motility rates. After 6–8 h at restrictive temperature (34°C) the motility rate of the control cells doubled, whereas it was drastically reduced in the dynein mutant. Mean values and SD of motility events per second and square micrometers were calculated from three experiments, including 45–50 cells and 750–860 s of observation.
Inhibitor studies demonstrated that ER motility was an MT-dependent process. Whereas treatment with cytochalasin D or E did not impair ER motility, disruption of MTs by benomyl abolished almost all tubule and patch motion (our unpublished data). The involvement of MTs was also evident from experiments with the conditional α-tubulin mutant strain FB1rTub1EG. This strain contains α-tubulin from U. maydis (tub1; Steinberg et al., 2001) under the control of the carbon source-dependent crg-promoter (Bottin et al., 1996) and the ectopically integrated ER-GFP construct. In CM-G Tub1 levels decreased, resulting in MT fragmentation within 7–8 h. ER motility was normal in CM-A (our unpublished data) but it reached only <10% of wild-type after growth in CM-G (Table 2; Figure 4C). In agreement with these results, mitotic cells, which only contain spindle and short astral MTs (Steinberg et al., 2001), showed significantly reduced ER motility (Table 2; Figure 4C). These data show that the ER of U. maydis is highly dynamic and that MTs have a central part in this motility.
Dynein Supports ER Motility
In higher eukaryotes the molecular motors conventional kinesin and cytoplasmic dynein are responsible for MT-dependent motility of ER tubules (Allan, 1996). Recently, both motors have been described for U. maydis (Lehmler et al., 1997; Straube et al., 2001). Therefore, we analyzed the role of these motors in ER motility. We made use of kinesin null mutants, as well as conditional dynein mutants, in which the C-terminal part of the split dynein heavy chain was under the control of the repressible crg-promoter (Straube et al., 2001).
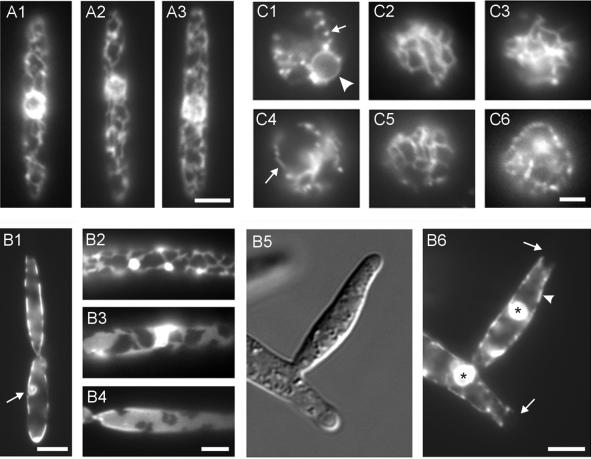
ER organization was normal in both the kinesin null mutant FB2Δkin2EG (Figure 4A1 and 4A2; arrow in 4A1 marks cortical ER) and in the conditional dynein mutant strain FBrDyn2EG at restrictive conditions (Figure 4B1 and 4B2; arrow in 4B1 marks cortical ER; 24 h in CM-G), suggesting that the cortical location and ER tubule morphology does not depend on these motors. In agreement with this, quantitative analysis of the kinesin mutant revealed that motility was not affected in the absence of conventional kinesin (Table 2; Figure 4C). In contrast, depletion of cytoplasmic dynein significantly decreased ER (Table 2; Figure 4C), indicating that cytoplasmic dynein is responsible for ER motility in U. maydis.
The ER motility remaining in the dynein mutant at restrictive conditions was significantly higher than that measured for the tubulin mutant, in which the MT tracks themselves are disrupted (see above). Because the FB1rDyn2 dynein mutant strain at restrictive conditions is comparable to a dyn2 deletion strain, this remaining activity could be due to additional motors. Alternatively, the residual ER motility could be driven by cytoplasmic dynein, because previous studies have shown that dyn2 expression is not completely repressed in the conditional dynein mutant at restrictive conditions (Straube et al., 2001). To distinguish between these possibilities, we generated a temperature-sensitive allele of dyn2 by error prone PCR (see MATERIALS AND METHODS), integrated it into the dyn2 locus and introduced the ER-GFP construct into this strain. Above 29°C the resulting temperature-sensitive dynein mutant strain FB1DyntsEG exhibited a complex temperature-sensitive phenotype, including defective nuclear migration and morphogenic defects (our unpublished data). This phenotype is characteristic for the conditional dynein mutant strain FB1rDyn2 (Straube et al., 2001), suggesting that dynein function was disturbed in this mutant at higher temperature. At 22°C ER motility in the temperature-sensitive dynein mutant was normal, whereas shift to 34°C drastically reduced ER motion (Table 2; Figure 4D). Compared with wild-type at 34°C the remaining activity was decreased to 9.18 ± 4.29% (n = 3 experiments) and not significantly different from that measured in the absence of MTs (P = 0.8377; see above). Therefore, we consider it most likely that dynein is the only MT-dependent motor for ER motility.
Localization of Cortical ER Network Is Independent of Cytoskeleton but Reconstitution of Disrupted ER Is Supported by MTs and Dynein
To analyze whether the peripheral localization of the ER in haploid cells depends on the cytoskeleton we disrupted MTs and F-actin by using benomyl or nocodazole and cytochalasins D, E, and latrunculin A, respectively. Previous studies have shown that 10 μM benomyl or nocodazole disrupts almost all MTs within 20–30 min (Steinberg et al., 2001) and cytochalasins at 10 μM, as well as LatA affect growth of U. maydis at 200 μM, respectively (our unpublished data), suggesting that these inhibitors are suitable to analyze the role of the cytoskeleton in ER organization. Surprisingly, neither treatment with cytochalasin D or E (Figure 5A1) or LatA (our unpublished data), nor incubation with benomyl (Figure 5A2) or nocodazole (our unpublished data) for 30 min affected the organization of the ER in haploid U. maydis cells. In addition, we applied benomyl and cytochalasin D simultaneously, again without effect on the ER organization (Figure 5A3). This suggests that neither actin nor MTs participate in cortical ER localization. This conclusion is also supported by double staining of MTs and ER-GFP in wild-type strain FB1EG (Figure 2B). In these experiments little colocalization between the ER network and MTs was observed (Figure 2B3 and 2B4), indicating that only transient connections between both structures might exist.
Figure 5.
Role of the cytoskeleton in cortical ER organization and ER recovery. (A) Influence of inhibitors of the cytoskeleton on the organization of the cortical ER. FB1EG cells were incubated for 30 min in 10 μM of the actin-disrupting drug cytochalasin D (A1) or in 10 μM of the MT inhibitor benomyl (A2). Neither treatment with each individual drug nor both inhibitors in combination (A3) had any detectable influence on the cortical ER. Bar, 3 μm. (B) ER organization in a conditional α-tubulin mutant strain. In strain FB1rTub1EG an α-tubulin gene, tub1, is under the control of the repressible crg-promoter (Steinberg et al., 2001). Growth for 7–8 h at restrictive conditions led to the disruption of MTs and results in condensed mitotic nuclei, which did not separate their DNA and failed to migrate into the daughter cell (B1, arrow). The ER still locates in the periphery of the cell, but beside a normal tubular appearance (B2) many cells contained ER sheets (B3 and B4). Disruption of MTs in conditional α-tubulin mutants leads to lateral budding (Steinberg et al., 2001; B5). In the absence of MTs the cortical ER is still inherited into the growing bud (B6, arrowhead), but the distal growth region lacks ER membranes (B6, arrows). Interestingly, many cells contained large accumulations of ER-GFP fusion protein (B6, asterisks; see also B2 and B3). Bar, 3 μm (B1), 2 μm (B2–B4), and 5 μm (B5 and B6). (C) Recovery of the peripheral ER after protoplast formation. Digestion of the cell wall of FB1EG cells by novozyme leads to the formation of spherical protoplasts and disrupts the peripheral ER, resulting in ER-GFP–containing vesicles (C1, arrow), whereas the nuclear envelope was not affected (C1, arrowhead). After 45 min in novozyme the ER recovered and the peripheral network reappeared (C2). This recovery was not inhibited in the presence of novozyme/10 μM cytochalsin D (C3), but was impaired in novozyme/10 μM benomyl (C4; a short tubule is marked by an arrow). In FB2Δkin2EG ER recovery was almost normal after 45 min (C5), whereas the reformation of the network was impaired in FB1rDyn2EG grown for 25 h in restrictive medium (C6). Bar, 2 μm.
These results were confirmed using the tubulin mutant strain FB1rTub1EG. After 7–8 h in CM-G the ER network was still located in the cell periphery (Figure 5B1 and 5B2), although the network occasionally lost its tubular appearance (Figure 5B3) and formed peripheral sheets (Figure 5B4). The depletion of Tub1 in CM-G efficiently disrupts MTs (Steinberg et al., 2001), again indicating that the cortical localization was not dependent on the tubulin cytoskeleton. Some large budded cells contained small ER-GFP spheres that were located in the mother cell (Figure 5B1, arrow) and colocalized with condensed DNA (our unpublished data). Therefore, these structures likely represent the nuclear envelope of mitotic nuclei that were unable to migrate into the daughter cell in the absence of MTs. In addition, the ER-GFP fusion protein accumulated in brightly stained dots (Figure 5B2, 5B3, and 5B6, asterisks). Interestingly, the ER network was still able to move into growing buds (Figure 5B5 and 5B6; arrowhead marks cortical ER), which often appeared at the lateral region of the cell in the absence of MTs (Steinberg et al., 2001). However, ER-GFP was absent from the very tips of the buds (Figure 5B6, arrows). Such an ER-free zone was not observed in cells grown at permissive conditions. This suggests that MTs have a minor role in ER inheritance during cell growth.
To check whether the cell wall might contribute to the peripheral localization of the ER network we incubated cells of strain FB1EG with novozyme. This reagent digests the cell wall of U. maydis and all subsequent experiments were done in the presence of novozyme to avoid reformation of the wall. Protoplast formation was accompanied by a disruption of the peripheral ER network, and small, stationary vesicular structures appeared at the cell periphery (Figure 5C1, arrow). The nuclear envelope remained unaffected (Figure 5C1, arrowhead). However, within 30–45 min after protoplast formation a cortical and highly mobile network reappeared (Figure 5C2). This phenomenon enabled us to investigate the role of the cytoskeleton and associated motors in the reformation of the ER. Cytochalasin D treatment at 10 μM for 45 min did not prevent the reappearance of the ER network (Figure 5C3), but disruption of MTs by using benomyl inhibited formation of a new network, and only short tubules were occasionally formed (Figure 5C4, arrow). Even after 4 h of incubation in benomyl the network was not reestablished, suggesting that MTs are needed to reorganize the cortical ER network. However, the cell wall is not required for network formation and cortical localization.
It has been proposed that the formation and distribution of the ER network depends on MT-based tubule motility (Dabora and Sheetz, 1988; Vale and Hotani, 1988; Lee et al., 1989). Therefore, we analyzed whether ER recovery needs the activity of conventional kinesin or dynein. In agreement with the results mentioned above, conventional kinesin was not required for ER network reformation after ER disruption. In protoplasts of the kinesin deletion mutant the network reappeared within 45 min (Figure 5C5). In contrast, in the conditional dynein mutant strain depletion of Dyn2 reduced the ability of protoplasts to reorganize the ER network after disruption. In these protoplasts of dynein mutant cells that were grown in CM-G for 25 h, reformation of the network was impaired (Figure 5C6). This corresponded with the described role of MTs in ER recovery. However, most protoplasts died within 2 h of incubation in novozyme, indicating that they are less viable. Therefore, we investigated ER recovery in protoplasts of the temperature-sensitive dynein mutant strain (FB1Dyn2tsEG) at permissive and restrictive temperature and compared these results with the wild-type situation. At 22°C ER recovery was not different in both strains (our unpublished data). After shift to 32°C and recovery for 40 min to 1 h the control strain contained ER tubules, although the temperature shift induced the formation of brightly labeled patches. Immediately after microscopic preparation, ER tubules were rarely observed in the temperature-sensitive dynein mutant (our unpublished data). In summary, these results argue for a role of MTs and cytoplasmic dynein in reconstruction of the ER after experimental disruption.
Gradient of Apical ER-GFP–stained Vesicles Represents ER-Golgi Recycling Vesicles
In dikaryotic hyphae derived from a cross of FB1EG and FB2 the ER-GFP construct stained a tip-ward vesicle gradient that reached over a length of ∼20–25 μm (see above; Figure 1C1). We confirmed the existence of the ER-GFP gradient by using strain AB33, in which two transcription factors that control filamentous growth are under the control of inducible promoters (Brachmann et al., 2001; see MATERIALS AND METHODS). Hyphal growth of this strain can be induced by changing the nitrogen source. Induced, AB33EG filaments contained an ER-GFP gradient similar to dikaryotic hyphae (Figure 6A1). In both dikaryotic hyphae and AB33EG the gradient appeared to consist of small dots, which occasionally exhibited directed motion at ∼0.3 μm/s (see above; Figure 3E). Because these dots carried the ER-GFP fusion protein they could be vesicles that cycle between the ER and the Golgi apparatus. To address this possibility, we investigated the effect of the drug BFA on the hyphal gradient. BFA is known to preferentially disrupt anterograde transport from ER to Golgi and leads to dispersal of the Golgi apparatus in vertebrate cells (Klausner et al., 1992).
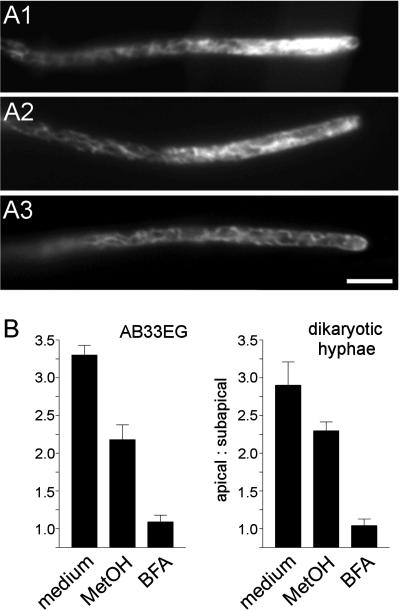
Figure 6.
Influence of brefeldin A on the apical ER-GFP gradient in hyphal tip cells. (A) ER-GFP gradient in hyphal cells of AB33EG. Untreated cells show a prominent apical gradient (A1), which, in control experiments, was slightly affected by methanol (A2) and completely disappeared in the presence of 200 μM BFA (A3). Bar, 5 μm. (B) Quantitative effect of BFA on ER-GFP gradients in AB33EG and dikaryotic hyphae derived from crossing FB2 × FB1EG. Both filaments contain an ER-GFP gradient (medium) that was slightly sensitive to methanol, which was used as the solvent of BFA and, therefore checked for its own effect on the gradient (control). In contrast, the gradient disappeared after 30-min BFA treatment (BFA). All values are given as mean ± SEM (n = 10 hyphae).
The apical ER-GFP staining in untreated dikaryotic and AB33EG hyphae was found to be approximately 3 times as high as in the subapical region, measured 30–40 μm below the tip (apical signal/subapical signal in AB33EG: 3.30 ± 0.41, n = 10; in the dikaryon: 2.90 ± 0.98, n = 10; Figure 6B). Consistent with the idea of ER-Golgi cycling vesicles the gradient completely disappeared after incubation with 200 μM BFA for 30 min (Figure 6A3 and 6B; AB33EG: 1.09 ± 0.28, n = 10; dikaryon: 1.04 ± 0.28, n = 10). BFA was solved in methanol, and, therefore, we checked for the influence of methanol alone on the ER-GFP gradient. In these control experiments, methanol only slightly affected the gradient (Figure 6A2 and 6B; AB33EG: 2.18 ± 0.62, n = 10; dikaryon: 2.30 ± 0.29, n = 6), suggesting that BFA specifically disrupts the ER-GFP gradient.
Disperse Golgi Apparatus Localizes to Sites of Polar Secretion
If the apical gradient of ER-GFP is due to vesicles cycling between ER and Golgi, the Golgi apparatus should be located near the hyphal tip. So far, the Golgi apparatus in U. maydis had not been visualized. To analyze the subcellular localization of the Golgi we generated the strain FB1GYPT, which expressed a fusion of GFP to Ypt1p of S. cerevisiae. (Schmitt et al., 1986). This small GTPase of the Rab family is known to be specific for the Golgi apparatus in yeast (Segev et al., 1988; Preuss et al., 1992) and antibody studies indicate that Yptp-like GTPases are also located on the Golgi of mouse cells (Segev et al., 1988). In U. maydis hyphae from a cross of FB1GYPT and FB2 the GFP-Ypt1p fusion protein accumulated in the hyphal apex (Figure 7, A1 and 7A2), and at the septum (Figure 7B1 and 7B2, arrow marks the septum). Correspondingly, GFP-Ypt1p localized to the septa of dividing sporidia (Figure 7C, arrows) and in the tip of growing buds (Figure 7D). This localization is reminiscent of the distribution of the dispersed Golgi in yeast (Preuss et al., 1992), suggesting that GFP-Ypt1p indeed localizes to the Golgi apparatus in U. maydis. We could confirm this localization by using γ-adaptin from U. maydis fused to GFP (GFP-GAD). This fusion protein, which should specifically localize to trans-Golgi cisternae (Robinson, 1990; Keon et al., 1995), accumulated in the tip of the growing bud and to the hyphal apex (our unpublished data). This further supports the notion that the Golgi is localized to active growth regions in U. maydis. Finally, we applied BFA to GFP-Ypt1p-expressing hyphae and sporidia, which is known to disrupt the Golgi apparatus in animals, plants, and fungi (Klausner et al., 1992; Rutten and Knuiman, 1993; Rupes et al., 1995). Although methanol was without effect (our unpublished data), 200 μM BFA perturbed the apical GFP-Ypt1p signals in haploid cells and hyphae within 10–60 min (Figure 7, E and F), again indicating that GFP-Ypt1p localizes to the Golgi apparatus of U. maydis. In summary, our results indicate that U. maydis cells contain a disperse Golgi apparatus that localizes to the hyphal tip and growing septa. Its position within the hyphal apex supports our model of a gradient of apical vesicles that carry ER-GFP fusion protein and cycle between the ER and the Golgi apparatus.
Figure 7.
Localization of a fusion protein of GFP fused to the putative Golgi marker Ypt1p in U. maydis cells. (A) Dikaryotic hypha derived from a cross of FB1GYPT and FB2. Small and motile dots of GFP-Ypt1p are scattered over the length of the hypha (A2, arrow) and strongly accumulate in the growing tip (A1, differential interference contrast image; A2, epifluorescence of GFP-Ypt1p). Bar, 5 μm. (B) Subapical end of a dikaryotic tip cell in a hypha expressing GFP-Ypt1p. A newly formed septum (B1 and B2, arrow) separates the living tip cell from the empty sections that are left behind during hyphal growth. A strong accumulation of GFP-Ypt1 was found at this site of septation. Bar, 5 μm. (C) Dividing haploid cell of strain FB1GYPT. GFP-Ypt1p localizes to the septa (arrows). Bar, 5 μm. (D) Growing haploid cell of strain FB1GYPT. GFP-Ypt1p accumulates within the growing bud. Note that dots are scattered all over the cell (arrow). Bar, 5 μm. (E) Haploid cell of strain FB1GYPT treated with 200 μM BFA. After 1 h in BFA the apical GFP-Ypt1p accumulation disappeared and randomly distributed brightly stained dots become visible. Bar, 5 μm. (F) GFP-Ypt1p–expressing dikaryotic hypha treated with 200 μm BFA for 1 h. The apical cloud of GFP-Ypt1p staining disappeared and bright dots are scattered over the length of the hypha. Bar, 5 μm.
DISCUSSION
In the present study we analyzed the organization and dynamics of the ER in the fungus U. maydis. We made use of a GFP fusion protein (ER-GFP) that contained an N-terminal signal sequence and a C-terminal ER retention signal. Such constructs have already been successfully used to analyze the ER in plants (Roderick et al., 1997) and S. cerevisiae (Prinz et al., 2000). In U. maydis ER-GFP stained a cortical polygonal network of tubules that formed three-way junctions and were in contact with the nuclear envelope. This organization is reminiscent of the ER organization in mammalian cells (Terasaki et al., 1984), plants (Allen and Brown, 1988), and S. cerevisiae (Koning et al., 1993; Prinz et al., 2000). Moreover, the staining was sensitive to detergents (Terasaki et al., 1984) and colocalized with the ER marker ERTracker Blue-White DPX. Based on these results we propose that the network represents the ER of U. maydis.
Endoplasmic Reticulum Is Highly Motile
Motility is a characteristic feature of the ER (Lee and Chen, 1988; Prinz et al., 2000; summarized in Terasaki, 1990). The ER of U. maydis is highly dynamic, with several types of motility occurring, namely, tubule extension, tubule sliding, and patch motion. Disruption of MTs led to reduced motility, whereas F-actin appeared to have no role in ER motility in U. maydis. This suggests that MT-dependent processes support tubule motion. This is in contrast to S. cerevisiae where tubule movements need an intact actin cytoskeleton (Prinz et al., 2000). However, this yeast uses F-actin instead of MTs for most intracellular transport processes (Madden and Snyder, 1998) and might, in this respect, not be representative for fungi in general. In fact, recent studies confirm a central role of MTs in intracellular transport in Schizosaccharomyces pombe (Yaffe et al., 1996; Mata and Nurse, 1997; Sawin and Nurse, 1998) and in various filamentous fungi (Akashi et al., 1994; Seiler et al., 1999; McDaniel and Roberson, 2000; Wedlich-Söldner et al., 2000). We, therefore, consider it likely that MT-based ER motility will turn out to be a typical feature of most fungi.
At present three different mechanisms for MT-based tubule motion and formation are discussed. First, it was shown that ER networks can be generated by ATP-dependent activity of molecular motors, which either extend tubules by movement along stationary MTs (Dabora and Sheetz, 1988), or which slide MTs and attached ER membranes along stationary MTs (Vale and Hotani, 1988). ER tubules could also be attached to the plus ends of MTs and slowly extended by MT polymerization (Waterman-Storer et al., 1995; Waterman-Storer and Salmon, 1998). For U. maydis it was recently described that MTs elongate at 0.17 μm/s and that complete MTs are moved within the cell at average rates of 0.69 μm/s (Steinberg et al., 2001). However, ER motility in U. maydis was found to be a much more rapid process with a mean velocity of ∼2.2 μm/s. This rate is faster than ER motility rates of 0.4–1.7 μm/s found in other systems (Lee and Chen, 1988; Allan, 1995; Lane and Allan, 1999), and is reminiscent of in vitro transport rates of fungal motors (Steinberg and Schliwa, 1996; Steinberg, 1997; Steinberg et al., 1998). Therefore, the observed tubule motion is probably based on motors that are bound to ER membranes and move along MTs, a process called MT-dependent tethering (Dabora and Sheetz, 1988).
Motility of Endoplasmic Reticulum Is Driven by Cytoplasmic Dynein
Almost all directed ER motility in U. maydis required MTs and cytoplasmic dynein, but not conventional kinesin. This is in contrast to animal cells, where kinesin spreads the ER from the cell center to the plus ends of MTs that are located at the periphery of the cell (Lee et al., 1989; Terasaki, 1990; Allan, 1996). In U. maydis most interphase MTs are located at the cell periphery and are nucleated by cytoplasmic microtubule organizing centers. In addition, many cells in a growing culture contain antiparallel MTs (Steinberg et al., 2001). Therefore, in contrast to animal culture cells, dynein-based transport might be sufficient to generate the observed bidirectional ER motility in U. maydis. This suggests that the MT cytoskeleton largely influences the transport machinery, a notion that is supported by studies on extracts from Xenopus eggs. Similar to U. maydis, frog eggs contain a layer of cortical ER and a complex array of cortical cytoplasmic MTs (Houliston and Elinson, 1991; Larabell et al., 1996). Comparable to U. maydis, in vitro studies indicated that dynein is the major motor for ER motility in these specialized animal cells (Allan and Vale, 1991; Allan, 1995; Lane and Allan, 1999). Not much is known about MT organization of filamentous fungi, and it remains to be seen whether dynein has a central role in fungal ER motility, in general.
Cytoskeleton Is Not Required for Cortical and Tubular Organization of ER Network
It was argued that MT-based ER motility is needed to generate and position the network in vertebrate cells (Dabora and Sheetz, 1988; Lee et al., 1989; Waterman-Storer and Salmon, 1998) and destruction of MTs or inhibition of kinesin leads to a collapse of the ER (Lee et al., 1989; Feiguin et al., 1994). However, in U. maydis and S. cerevisiae (Prinz et al., 2000) the cytoskeleton is not required for maintaining the network in the cell periphery. A peripheral ER is also characteristic for plant cells and evidence exists for ER anchoring sites that tightly link the network to the plasma membrane (summarized in Staehelin, 1997). This suggests that similar sites might anchor the cortical ER in fungal cells. Both fungi and plants have a rigid cell wall, which distinguishes them from vertebrate cells. Therefore, it was tempting to speculate that this extracellular matrix could serve as an anchor of the cortical ER in both plants and fungi. To test this hypothesis, we disrupted the cell wall of U. maydis by lytic enzymes and observed the effect of this treatment on the ER. Protoplast formation indeed led to the disruption of the tubular network into vesicular structures. However, most of these vesicles remained positioned beneath the plasma membrane and the peripheral network reappeared in the absence of the wall. Therefore, we consider it most likely that the cell wall is not required for cortical network location, although it cannot be excluded that enzymatic wall digestion leaves residual cell wall patches sufficient for anchoring ER membranes.
Recovery of Endoplasmic Reticulum Requires Microtubules and Dynein
Motility rates were drastically reduced in the conditional tubulin mutant FB1rTub1EG and the dynein mutant strain FB1rDyn2EG at restrictive conditions, but ER structure was found to be almost unaffected. In addition, ER was moving into the growing bud and was located at the cell cortex in the absence of MTs, suggesting that, in contrast to vertebrate cells, ER inheritance and peripheral network formation might occur in an MT-independent manner. However, our experiments on the reappearance of the network in U. maydis protoplasts contradict this conclusion and argue that dynein- and MT-dependent ER motility participates in ER reconstruction. At present we cannot solve this contradiction. One explanation could be that the residual ER motility, still found in the tubulin and dynein mutants, might be sufficient to maintain structure and inheritance of the ER in slowly growing mutant cells, but is not enough for rapid recovery of the network in protoplasts. Alternatively, protoplast formation might have numerous effects on intracellular organelle traffic, including a disorganization of F-actin (Steinberg and Schliwa, 1993). Therefore, unknown actin-based processes might be impaired in protoplasts, resulting in more drastic effects of MT disruption in protoplasts than in the investigated mutant strains. Moreover, it was shown that ER networks can form in vitro in the absence of F-actin and MTs (Dreier and Rapoport, 2000), and it was argued that the biophysical properties of the membrane itself might determine ER structure (Terasaki, 1990). Therefore, ER inheritance and network formation in U. maydis tubulin and dynein mutants might be a consequence of self-assembly processes of the ER membrane itself. Consequently, ER network formation could be seen as a multifactorial process, and cytoskeleton-dependent motility might just be one of several mechanisms for ER construction.
Apical ER-GFP Gradient Reflects Cycling between Golgi Apparatus and ER
Hyphae of U. maydis contained a tip-ward gradient of ER-GFP fusion protein. This gradient consisted of small motile dots, suggesting that the fusion protein localized to small vesicles (Figure 8). Preliminary experiments indicate that MTs as well as dynein are involved in the directed motility of these vesicles and that benomyl disrupts the Golgi apparatus in hyphae (Steinberg, unpublished data). These observations might indicate that long-distance transport along MTs support cycling between ER and Golgi. The ER-GFP fusion protein carried the C-terminal ER retention signal HDEL that is used to recycle soluble ER proteins from the Golgi apparatus back to the ER (Pelham, 1990). This raises the possibility that the apical ER-GFP gradient consist of vesicles that cycle between these two compartments. Such a notion is supported by two findings. First, the apical gradient is sensitive to BFA treatment, which interrupts ER-to-Golgi transport (Klausner et al. 1992). Second, the cloudy Golgi apparatus of U. maydis localized to the tip of growing the hypha, to which the ER-GFP gradient is directed. Therefore, we propose that the observed hyphal accumulation of ER-GFP reflects membrane traffic between the apical Golgi and the ER.
Figure 8.
Model of ER organization and dynamics in U. maydis. The ER consists of a network that is in contact with the nuclear envelope (light gray). The cells contain antiparallel MTs (black bars; Steinberg et al., 2001) along which cytoplasmic dynein moves ER tubules. In hyphae ER-GFP–containing vesicles cycle between the ER and the apical Golgi apparatus (dark gray), which results in a gradient of ER-GFP within the first 20–25 μm of the hypha. The vesicular Golgi apparatus and other apical vesicles (in black) accumulate within the first 1–2 μm of the hypha, thereby excluding ER-GFP–stained organelles. This results in a dark area within the hyphal apex.
CONCLUSION
We observed rapid MT-dependent motility within the cortical ER network of U. maydis, and cytoplasmic dynein appears to be responsible for this motion. In contrast to animal systems, this motility is of minor importance for ER inheritance and construction, although it was required for ER recovery in protoplast. Similar to results described for S. cereviaiae (Prinz et al., 2000), the cytoskeleton was not needed for cortical localization of the ER network. This suggests that fungi have developed specialized ways to position and maintain their ER network in the cell periphery. It will be a fascinating challenge to elucidate the molecular basis for this process.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. R. Kahmann for helpful discussion and comments on the manuscript. We thank M. Artmeier for technical assistance and are grateful to Dr. B. Glick for providing a GFP-YPT1 construct. The work was supported by the Deutsche Forschungsgemeinschaft through SFB 413 and the Max-Planck-Society.
Abbreviations used:
- BFA
brefeldin A
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- LatA
latrunculin A
- MT
microtubule
Footnotes
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–10–0475. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–10–0475.
REFERENCES
- Akashi T, Kanbe T, Tanaka K. The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology. 1994;140:271–280. doi: 10.1099/13500872-140-2-271. [DOI] [PubMed] [Google Scholar]
- Allan V. Protein phosphatase 1 regulates the cytoplasmic dynein-driven formation of endoplasmic reticulum networks in vitro. J Cell Biol. 1995;128:879–891. doi: 10.1083/jcb.128.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan V. Role of motor proteins in organizing the endoplasmic reticulum and Golgi apparatus. Semin Cell Dev Biol. 1996;7:335–342. [Google Scholar]
- Allan VJ, Vale RD. Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J Cell Biol. 1991;113:347–359. doi: 10.1083/jcb.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NS, Brown DT. Dynamics of the endoplasmic reticulum in living onion epidermal cells in relation to microtubules, microfilaments, and intracellular particle movement. Cell Motil Cytoskeleton. 1988;10:153–163. [Google Scholar]
- Banuett F. Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu Rev Genetics. 1995;29:179–208. doi: 10.1146/annurev.ge.29.120195.001143. [DOI] [PubMed] [Google Scholar]
- Banuett F, Herskowitz I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci USA. 1989;86:5878–5882. doi: 10.1073/pnas.86.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottin A, Kamper J, Kahmann R. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol Gen Genet. 1996;253:342–352. doi: 10.1007/pl00008601. [DOI] [PubMed] [Google Scholar]
- Brachmann A, Weinzierl G, Kämper J, Kahmann R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol Microbiol. 2001;42:1047–1063. doi: 10.1046/j.1365-2958.2001.02699.x. [DOI] [PubMed] [Google Scholar]
- Dabora SL, Sheetz MP. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988;54:27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Bridgman PC. Dynamics of the endoplasmic reticulum and other membranous organelles in growth cones of cultured neurons. J Neurosci. 1989;9:1897–1909. doi: 10.1523/JNEUROSCI.09-06-01897.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F, Ferreira A, Kosik KS, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. J Cell Biol. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989;264:21522–21528. [PubMed] [Google Scholar]
- Holliday R. Ustilago maydis. In: King RC, editor. Handbook of Genetics. New York: Plenum Press; 1974. [Google Scholar]
- Houliston E, Elinson RP. Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation fertilized frog eggs. J Cell Biol. 1991;114:1017–1028. doi: 10.1083/jcb.114.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabell CA, Rowning BA, Wells J, Wu M, Gerhart JC. Confocal microscopy analysis of living Xenopus eggs and the mechanism of cortical rotation. Development. 1996;122:1281–1289. doi: 10.1242/dev.122.4.1281. [DOI] [PubMed] [Google Scholar]
- Kahmann R, Steinberg G, Basse C, Feldbrügge M, and JK. Ustilago maydis, the causative agent of corn smut disease. In: Kronstadt J, editor. Fungal Pathology. Amsterdam: Kluwer Academic Publishers; 2000. [Google Scholar]
- Keon JP, Jewitt S, Hargreaves JA. A gene encoding γ-adaptin is required for apical extension growth in Ustilago maydis. Gene. 1995;162:141–145. doi: 10.1016/0378-1119(95)00355-a. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel W, Quader H, Schnepf E. Mobile and immobile endoplasmic reticulum in onion bulb epidermis cells: short- and long-term observations with a confocal laser scanning microscope. Eur J Cell Biol. 1990;52:328–340. [PubMed] [Google Scholar]
- Koning AJ, Lum PY, Williams JM, Wright R. DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil Cytoskeleton. 1993;25:111–128. doi: 10.1002/cm.970250202. [DOI] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK. Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma. 1987;140:141–150. [Google Scholar]
- Lane JD, Allan VJ. Microtubule-based endoplasmic reticulum motility in Xenopus laevis: activation of membrane-associated kinesin during development. Mol Biol Cell. 1999;10:1909–1922. doi: 10.1091/mbc.10.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Chen LB. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Lee C, Ferguson M, Chen LB. Construction of the endoplasmic reticulum. J Cell Biol. 1989;109:2045–2055. doi: 10.1083/jcb.109.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler C, Steinberg G, Snetselaar KM, Schliwa M, Kahmann R, Bölker M. Identification of a motor protein required for filamentous growth in Ustilago maydis. EMBO J. 1997;16:3464–3473. doi: 10.1093/emboj/16.12.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebe S, Menzel D. Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol Cell. 1995;85:207–222. doi: 10.1016/0248-4900(96)85282-8. [DOI] [PubMed] [Google Scholar]
- Madden K, Snyder M. Cell polarity and morphogenesis in budding yeast. Annu Rev Microbiol. 1998;52:687–744. doi: 10.1146/annurev.micro.52.1.687. [DOI] [PubMed] [Google Scholar]
- Mata J, Nurse P. Tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- McDaniel DP, Roberson RW. Microtubules are required for motility and positioning of vesicles and mitochondria in hyphal tip cells of Allomyces macrogynus. Fungal Genet Biol. 2000;31:233–244. doi: 10.1006/fgbi.2000.1249. [DOI] [PubMed] [Google Scholar]
- Müller P, Aichinger C, Feldbrugge M, Kahmann R. The MAP kinase kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol Microbiol. 1999;34:1007–1017. doi: 10.1046/j.1365-2958.1999.01661.x. [DOI] [PubMed] [Google Scholar]
- Pelham HR. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Cloning and expression of γ-adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol. 1990;111:2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Campbell AK, Llewellyn DH. Nuclear localization of calreticulin in vivo is enhanced by its interaction with glucocorticoid receptors. FEBS Lett. 1997;405:181–185. doi: 10.1016/s0014-5793(97)00183-x. [DOI] [PubMed] [Google Scholar]
- Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O'Connor J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupes I, Mao WZ, Astrom H, Raudaskoski M. Effects of nocodazole and brefeldin A on microtubule cytoskeleton and membrane organization in the homobasidiomycete Schizophyllum commune. Protoplasma. 1995;185:212–221. [Google Scholar]
- Rutten TL, Knuiman B. Brefeldin A effects on tobacco pollen tubes. Eur J Cell Biol. 1993;61:247–55. [PubMed] [Google Scholar]
- Sanger JM, Dome JS, Mittal B, Somlyo AV, Sanger JW. Dynamics of the endoplasmic reticulum in living non-muscle and muscle cells. Cell Motil Cytoskeleton. 1989;13:301–319. doi: 10.1002/cm.970130408. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Nurse P. Regulation of cell polarity by microtubules in fission yeast. J Cell Biol. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt HD, Wagner P, Pfaff E, Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986;47:401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Schulz B, Banuett F, Dahl M, Schlesinger R, Schaefer W, Martin T, Herskowitz I, Kahmann R. The B alleles of Ustilago maydis whose combinations program pathogenic development code for polypeptides containing a homeodomain-related motif. Cell. 1990;60:295–306. doi: 10.1016/0092-8674(90)90744-y. [DOI] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Seiler S, Plamann M, Schliwa M. Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr Biol. 1999;9:779–785. doi: 10.1016/s0960-9822(99)80360-1. [DOI] [PubMed] [Google Scholar]
- Spee JH, de Vos WM, Kuipers OP. Efficient random mutagenesis method with adjustable mutation frequency by use of PCR and dITP. Nucleic Acids Res. 1993;21:777–778. doi: 10.1093/nar/21.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellig T, Bottin A, Kahmann R. Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol Gen Genet. 1996;252:503–509. doi: 10.1007/BF02172396. [DOI] [PubMed] [Google Scholar]
- Staehelin LA. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J. 1997;11:1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- Steinberg G. A kinesin-like mechanoenzyme from the zygomycete Syncephalastrum racemosum shares biochemical similarities with conventional kinesin from Neurospora crassa. Eur J Cell Biol. 1997;73:124–131. [PubMed] [Google Scholar]
- Steinberg G, Schliwa M. Organelle movements in the wild type and wall-less fz;sg;os-1 mutants of Neurospora crassa are mediated by cytoplasmic microtubules. J Cell Sci. 1993;106:555–564. doi: 10.1242/jcs.106.2.555. [DOI] [PubMed] [Google Scholar]
- Steinberg G, Schliwa M. Characterization of the biophysical and motility properties of kinesin from the fungus Neurospora crassa. J Biol Chem. 1996;271:7516–7521. doi: 10.1074/jbc.271.13.7516. [DOI] [PubMed] [Google Scholar]
- Steinberg G, Schliwa M, Lehmler C, Boelker M, Kahmann R, McIntosh JR. Kinesin from the plant pathogen Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J Cell Sci. 1998;111:2235–2246. doi: 10.1242/jcs.111.15.2235. [DOI] [PubMed] [Google Scholar]
- Steinberg G, Wedlch-Söldner R, Brill M, Schulz I. Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J Cell Sci. 2001;114:609–622. doi: 10.1242/jcs.114.3.609. [DOI] [PubMed] [Google Scholar]
- Straube A, Enard W, Berner A, Kahmann R, Steinberg G. A split motor domain in a fungal cytoplasmic dynein. EMBO J. 2001;20:5091–5100. doi: 10.1093/emboj/20.18.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M. Recent progress on structural interactions of the endoplasmic reticulum. Cell Motil Cytoskeleton. 1990;15:71–75. doi: 10.1002/cm.970150203. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Sardet C, Wong JR, Weiss MJ, Chen LB. Localization of the endoplasmic reticulum in living and glutaraledehyde fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Vale RD, Hotani H. Formation of membrane networks in vitro by kinesin-driven microtubule movement. J Cell Biol. 1988;107:2233–2242. doi: 10.1083/jcb.107.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Gregory J, F. PS, Salmon ED. Membrane/microtubule tip attachment complexes (TACs) allow the assembly dynamics of plus ends to push and pull membranes into tubulovesicular networks in interphase Xenopus egg extracts. J Cell Biol. 1995;130:1161–1169. doi: 10.1083/jcb.130.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- Wedlich-Söldner R, Bölker M, Kahmann R, Steinberg G. A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 2000;19:1974–1986. doi: 10.1093/emboj/19.9.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MP, Harata D, Verde F, Eddison M, Toda T, Nurse P. Microtubules mediate mitochondrial distribution in fission yeast. Proc Natl Acad Sci USA. 1996;93:11664–11678. doi: 10.1073/pnas.93.21.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.