Abstract
Death ligands not only induce apoptosis but can also trigger necrosis with distinct biochemical and morphological features. We recently showed that in L929 cells CD95 ligation induces apoptosis, whereas TNF elicits necrosis. Treatment with anti-CD95 resulted in typical apoptosis characterized by caspase activation and DNA fragmentation. These events were barely induced by TNF, although TNF triggered cell death to a similar extent as CD95. Surprisingly, whereas the caspase inhibitor zVAD prevented CD95-mediated apoptosis, it potentiated TNF-induced necrosis. Cotreatment with TNF and zVAD was characterized by ATP depletion and accelerated necrosis. To investigate the mechanisms underlying TNF-induced cell death and its potentiation by zVAD, we examined the role of poly(ADP-ribose)polymerase-1 (PARP-1). TNF but not CD95 mediated PARP activation, whereas a PARP inhibitor suppressed TNF-induced necrosis and the sensitizing effect of zVAD. In addition, fibroblasts expressing a noncleavable PARP-1 mutant were more sensitive to TNF than wild-type cells. Our results indicate that TNF induces PARP activation leading to ATP depletion and subsequent necrosis. In contrast, in CD95-mediated apoptosis caspases cause PARP-1 cleavage and thereby maintain ATP levels. Because ATP is required for apoptosis, we suggest that PARP-1 cleavage functions as a molecular switch between apoptotic and necrotic modes of death receptor-induced cell death.
INTRODUCTION
Two forms of cell death, namely apoptosis and necrosis, are distinguished by morphological and biochemical features. Although apoptosis accounts for most of physiological cell death, necrosis is usually induced in pathological situations by accidental and acute damage to cells (Kerr et al., 1972; Wyllie et al., 1980). Necrosis is characterized by cell swelling and disruption of the cell membrane, leading to the release of the cellular content, which may result in an inflammatory response (Fiers et al., 1999). In contrast, apoptosis is a tightly regulated process controlled by a hierarchical set of molecules that were originally identified in Caenorhabditis elegans and later in mammalian cells (Cohen, 1997; Cryns and Yuan; 1998; Los et al., 1999). The apoptotic cascade has been intensively studied for death receptors such as TNF receptor-1 (TNF-R1) and CD95 (APO-1/Fas) (Schulze-Osthoff et al., 1998). After death ligands bind to their cognate receptors, several prominent biochemical events occur including the proteolytic activation of caspases as the critical executioners of apoptosis and the internucleosomal fragmentation of DNA that is mediated by cleavage of DNA fragmentation factor (DFF45/ICAD) (Los et al., 1995; Muzio et al., 1996; Liu et al., 1997; Enari et al., 1998).
Another characteristic event of apoptosis is the proteolytic cleavage of poly(ADP-ribose)polymerase-1 (PARP-1), a nuclear enzyme involved in DNA repair, DNA stability, and transcriptional regulation. Caspases, in particular caspase-3 and -7, cleave the 116-kDa form of PARP-1 at the DEVD site to generate a 85- and a 24-kDa fragment (Kaufmann et al., 1993; Lazebnik et al., 1994; Nicholson et al., 1995; Los et al., 1997, 1999). The cleavage of PARP-1 between Asp214 and Gly215 results in the separation of the two zinc-finger DNA-binding motifs from the automodification and catalytic domains, thus preventing the recruitment of the enzyme to sites of DNA damage. Although PARP-1 cleavage has been considered as a hallmark of apoptosis, its function for the regulation of cell death is largely unknown.
PARP-1 is an abundant, chromatin-associated enzyme, which upon binding to DNA strand breaks transfers long, branched poly(ADP-ribose) polymers using NAD+ as a substrate (Lindahl et al., 1995). Subsequently, it ADP-ribosylates a number of acceptor proteins including itself and components of the DNA repair machinery. The role of PARP-1 in various cell death models has been addressed previously (Leist et al., 1997a; Wang et al., 1997; de Murcia et al., 1997; Herceg and Wang, 1999; Ha and Snyder, 1999). PARP-1(−/−) cells exhibit a normal apoptotic response to various stimuli including TNF and anti-CD95 treatment, suggesting that PARP-1 is dispensable for apoptotic signaling (Leist et al., 1997a; Wang et al., 1997). Under certain experimental conditions, cells devoid of PARP-1 are more sensitive to DNA-damaging agents such as alkylating drugs (Schreiber et al., 1995; de Murcia et al., 1997; Beneke et al., 2000). PARP-1–deficient mice also showed increased sister chromatid exchange and enhanced sensitivity to ionizing irradiation, whereas they were protected against inflammation and diabetes as well as myocardial and cerebral ischemic injury (reviewed in Shall and de Murcia, 2000; Bürkle, 2001).
Until recently, PARP-1 has been considered as the sole protein capable of poly(ADP-ribosyl)ation. However, the discovery that limited modification of cellular proteins with ADP-ribose polymers still occurs in PARP-1 knockout mice suggested the presence of other proteins with PARP activity (Jacobson and Jacobson, 1999). Recently, five additional members of the PARP family have been identified based on sequence similarity with the catalytic domain of PARP-1. sPARP-1 was isolated from PARP-1(−/−) fibroblasts and represents a truncated form of 53 kDa corresponding to the catalytic domain of PARP-1 (Sallmann et al., 2000). PARP-2 is a 62-kDa family member localized on chromosome 14q11.2 that displays biochemical properties similar to those of PARP-1 (Ame et al., 1999). Despite these PARP family members lack Zn2+ finger DNA-binding and automodification domains, they still catalyze the formation of ADP-ribose polymers in a DNA-dependent manner. Three other newly identified proteins belonging to the PARP family are tankyrase (TANK-1 and -2) and VPARP which, in contrast to PARP-1, are not activated by DNA damage (Kickhoefer et al., 1998; Smith et al., 1998; Kaminker et al., 2001). Tankyrase is a telomerase complex-associated protein that binds to the telomere-binding protein TRF-1 and acts to regulate telomere length. VPARP is a component the “vault,” a large ribonucleoprotein assembly that is ubiquitously expressed in eukaryotic cells (Kong et al., 1999). The functions of the new PARP members are currently rather unknown, but their distinct cellular locations and domain structures strongly suggest that they have distinct biological roles.
In the present study we examined the role of PARP-1 activation and cleavage in death receptor–mediated induction of apoptosis and necrosis. Overactivation of PARP after cellular insults consumes large amounts of NAD+ and, in efforts to resynthesize NAD+, may cause massive ATP depletion (Sims et al., 1983; Szabo and Dawson, 1998). Recent studies by us and others demonstrated that intracellular ATP levels can regulate the mode of cell death (Eguchi et al., 1997; Leist et al., 1997b; Ferrari et al., 1998; Ha and Snyder, 1999). Whereas high levels of ATP enable cells to undergo apoptosis, low ATP levels shift cell death toward necrosis. It has been also found that fibroblasts from PARP-1–deficient mice are protected against ATP depletion and necrotic but not apoptotic cell death (Ha and Snyder, 1999). We previously reported that in L929 fibrosarcoma cells TNF induces necrosis, whereas in the same cells ligation of CD95 triggers apoptosis (Schulze-Osthoff et al., 1994). The biochemical mechanism underlying these distinct modes of death induction by death receptors remained largely unknown. Here, we show that PARP-1 cleavage is a critical event, a switch point that directs death receptor signaling toward either apoptosis or necrosis. TNF-triggered oxidative stress and DNA damage lead to PARP-1 activation, and thereby cause ATP depletion and subsequent induction of necrosis. In contrast, during CD95-mediated apoptosis proteolytic inactivation of PARP-1 by caspases prevents ATP depletion and thereby ensures the execution of the apoptotic process. We also demonstrate that prevention of PARP-1 cleavage is involved in the remarkable potentiation of TNF-induced necrosis by caspase inhibitors. Our results therefore suggest that the combined use of caspase and PARP inhibitors might be of therapeutic importance in pathologies, where both apoptosis and necrosis occur.
MATERIALS AND METHODS
Reagents
3-Aminobenzamide (3AB), butylated hydroxyanisole (BHA), and actinomycin D were from Sigma (Deisenhofen, Germany); the caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD) was from Enzyme Systems (Dublin, CA). Recombinant mouse TNF was a kind gift from Knoll AG (Ludwigshafen, Germany); anti-CD95 (mouse IgG3) was from BioCheck (Münster, Germany). All other chemicals were purchased either from Merck (Darmstadt, Germany) or Roth (Karlsruhe, Germany).
Cell Culture and Transfection
Cells were routinely maintained in DMEM at 37°C in 5% CO2, supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. L929 cells stably transfected with CD95 have been described previously (Schulze-Osthoff et al., 1994). Immortalized fibroblasts derived from a PARP-1(−/−) mice (Wang et al., 1995) and their derivatives stably transfected with either wild-type PARP-1 or with caspase-resistant PARP-1 (PARP-1-D214N) were described previously (Herceg and Wang, 1999). For transient transfections, cells were washed three times with Tris-buffered saline (TBS), resuspended at 1 × 108 cells in 0.4 ml TBS, transferred into cuvettes (0.4-cm electrode distance), and electroporated with 20 μg of the expression plasmids at 960 μF, 230 V. After electroporation cells were kept in the cuvettes on ice for 15 min and then seeded at 1 × 106 cells/well in 6-well plates. Dead cells were removed after 16 h by a washing step in culture medium.
Measurement of Apoptotic Hypodiploid Nuclei and Propidium Iodide Uptake
Unless otherwise indicated, for induction of cell death cells were treated with 40 ng/ml recombinant TNF or with 1 μg/ml anti-CD95 in the presence of 1 μg/ml actinomycin D (Sigma). For determination of apoptosis, 1 × 105 cells per well were seeded in 12-well plates and treated with the indicated concentrations of anti-CD95 or TNF. The leakage of fragmented DNA from apoptotic nuclei was measured by the Nicoletti method (Nicoletti et al., 1991). Briefly, apoptotic nuclei were prepared by lysing cells in cold acetone-methanol (1:1). After a washing step RNase A (0.5 mg/ml in PBS) was added, and nuclei were incubated for 30 min at 30°C. Propidium iodide (PI) was added to a final concentration of 40 μg/ml, and the nuclei were subsequently analyzed by flow cytometry. Histograms of DNA were determined by flow cytometry using a FACS Calibur and CellQuest software (Becton-Dickinson, Heidelberg, Germany). Cells to the left of the G1 peak containing hypodiploid DNA were considered as apoptotic. Necrotic cell death was assessed by PI uptake as described previously (Los et al., 1998). Cells were stained with 1 μg/ml PI and measured by flow cytometry.
DNA Fragmentation Assay
Cells, 5 × 106, were seeded into culture dishes and allowed to attach for at least 3 h. After the indicated treatments, cells were trypsinized and collected together with the floating cells in the supernatant. DNA was extracted by the salting-out technique (Murgia et al., 1992). Cells were resuspended in 1 ml culture medium and lysed in 200 μl lysis buffer (50 mM Tris-HCl, pH 8.0, 2 M NaCl, 10 mM EDTA), 50 μl 20% SDS, and 10 μl proteinase K (10 mg/ml). Samples were incubated for 30 min at 65°C, followed by an overnight incubation at 37°C. After addition of 400 μl 5 M NaCl, samples were mixed gently and centrifuged for 30 min at 400 × g. DNA from the supernatants was precipitated by the addition of 5 ml 96% ethanol. After three washing steps in 70% ethanol the DNA was dried, resuspended in 50 μl TE buffer, and incubated for 1 h with 0.1 mg/ml RNase A. DNA fragmentation was analyzed on 1.5% agarose gels in the presence of 0.5 μg/ml ethidium bromide.
Measurement of Intracellular ATP
The cellular ATP content was determined using a bioluminescence assay according to the manufacturer's instructions (Sigma). Cells, 1 × 106, were treated with the cytotoxic stimuli and inhibitors. After the indicated times, cells were washed in PBS, lysed in 0.5% Triton-X 100, 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, and incubated for 10 min on ice. After removal of cell debris by centrifugation (10 000 × g, 15 min, 4°C) the ATP content was measured with the luciferin/luciferase method in a ML2200 luminometer (Dynatech, Denkendorf, Germany). The absolute values of ATP content were determined using an internal ATP standard.
Western Blot Analysis
Cell lysates were prepared by lysing PBS-washed cells in a high-salt buffer containing 1% NP-40, 20 mM HEPES, pH 7.9, 350 mM NaCl, 20% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 0.5 mM DTT, supplemented with 3 μg/ml aprotinin, 3 μg/ml leupeptin, and 2 mM PMSF. After SDS-PAGE, separated proteins were transferred onto a PVDF membrane (Amersham, Braunschweig, Germany) by electroblotting. The loading and transfer of equal amounts of protein were determined by staining the membrane with Ponceau S. Membranes were blocked for 1 h with 5% nonfat dry milk powder in TBS and then immunoblotted for 1 h with the appropriate antibody. Subsequently, membranes were washed four to six times with TBS/0.05% Tween-20 and incubated with peroxidase-conjugated affinity-purified rabbit anti-mouse or goat anti-rabbit IgG for 1 h. After extensive washing, the specific signal was detected by enhanced chemiluminescent (ECL) staining. Rabbit anti–PARP-1 (1:2000, Roche Diagnostics), rabbit anti–PARP-2 antiserum (1:1000; Alexis, Grünberg, Germany) and mouse anti-caspase-3 (1:1000, R&D Systems, Wiesbaden, Germany) were used. Proteins were visualized with horseradish peroxidase–conjugated secondary antibodies (Sigma) and ECL reagents (Amersham).
Fluorescent Measurement of Intracellular Hydrogen Peroxide
Formation of hydrogen peroxide was measured using dichlorofluorescin diacetate (DFCH) as described previously (Los et al., 1998). A 10 mM stock solution of DFCH (Molecular Probes, Eugene, OR) was prepared in DMSO and stored under nitrogen at −70°C. Cells were preloaded with 5 μM DFCH in culture medium for 0.5 h at 37°C. Measurements were performed in duplicates by flow cytometry. Arbitrary units were calculated as the ratio of fluorescence in stimulated cells relative to the fluorescent value in untreated control cells.
Immunohistochemical Detection of PARP Activity
Poly(ADP-ribose) polymer formation was detected as described (Wang et al., 1995). Briefly, cells were cultured on coverslips and stimulated as indicated. Cells were then washed and immediately fixed in ice-cold 10% trichloroacetic acid in PBS, followed by dehydration through graded dilutions of ethanol. Samples were incubated with an mAb 10H raised against poly(ADP-ribose) polymers (Kawamitsu et al., 1984) and Cy3-labeled goat anti-mouse IgG (Dianova, Hamburg, Germany) and then inspected by fluorescence microscopy. Formation of DNA strand breaks by treatment of cells with 2 mM H2O2 served as a positive control.
RESULTS
TNF Induces Necrosis, whereas CD95 Triggers Apoptosis in L929 Cells
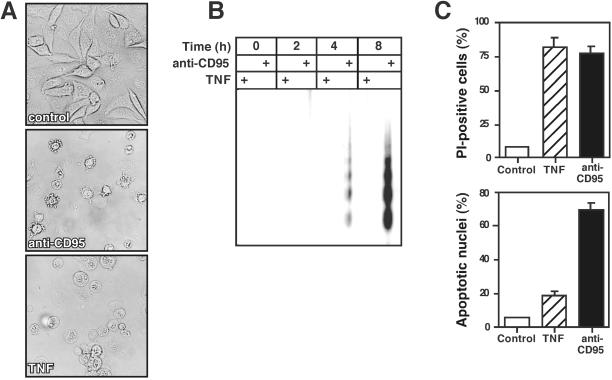
CD95-induced death is characterized by a fast kinetic with typical apoptotic morphology that can be efficiently blocked by caspase inhibitors. In contrast, triggering of TNF-R1 causes delayed cell death that can be modulated in some cell types by agents affecting the cellular redox state (Schulze-Osthoff et al., 1992, 1994). Inhibition of mRNA or protein synthesis often sensitizes cells to efficient killing by TNF or anti-CD95. We used L929 murine fibrosarcoma cells stably transfected with CD95 to study the mechanism of TNF- and CD95-induced death. Treatment of cells with TNF in the presence of actinomycin D–triggered cell death with predominant necrotic features as evident by the appearance of cytoplasmic vacuolization and swelling (Figure 1A). Actinomycin D alone was not toxic under these conditions. Unlike TNF, anti-CD95 antibody in the presence of actinomycin D killed L929 cells with typical apoptotic morphology. In this case, cells revealed membrane blebbing and shrinkage of the cytoplasm and nucleus (Figure 1A).
Figure 1.
Distinct forms of cell death induced by either anti-CD95 or TNF in L929 fibroblasts. L929 cells were treated with either anti-CD95 (1 μg/ml) or TNF (40 ng/ml) in the presence of actinomycin D. (A) Different morphological alterations after 6-h treatment of cells with anti-CD95 or TNF (magnification, ×200). (B) Detection of apoptotic DNA laddering after TNF or anti-CD95 treatment. Cells were incubated with either anti-CD95 or TNF; after the indicated time genomic DNA was separated on a 1.5% agarose gel. (C) Differences between flow cytometric measurement of cell death by PI uptake as a marker for increased cell membrane permeability and apoptotic hypodiploid nuclei 6 h after death induction by TNF and anti-CD95. Data represents the mean of four independent experiments.
To confirm these morphological observations, we prepared DNA extracts from cells treated with TNF or anti-CD95 and resolved them on agarose gels. As shown in Figure 1B, triggering of CD95 induced apoptotic DNA laddering already after 4 h, whereas after TNF incubation minimal DNA cleavage occurred not earlier than after 8 h. These differences in the death morphology were further confirmed by flow cytometric analysis. Viability staining by PI uptake 6 h after death induction showed that TNF killed L929 cells as efficiently as anti-CD95 (Figure 1C, top panel). In contrast, assessment of cell death by the apoptotic formation of hypodiploid nuclei revealed much stronger DNA fragmentation after CD95 triggering than after TNF treatment (Figure 1C, bottom panel). These results indicated that within the same cell line TNF predominantly triggered necrosis, whereas anti-CD95 induced apoptotic cell death. L929 cells therefore provide a useful cell model to dissect the pathways of necrosis and apoptosis during death receptor ligation.
Different Role of Caspase Activation upon Death Induction by TNF and CD95
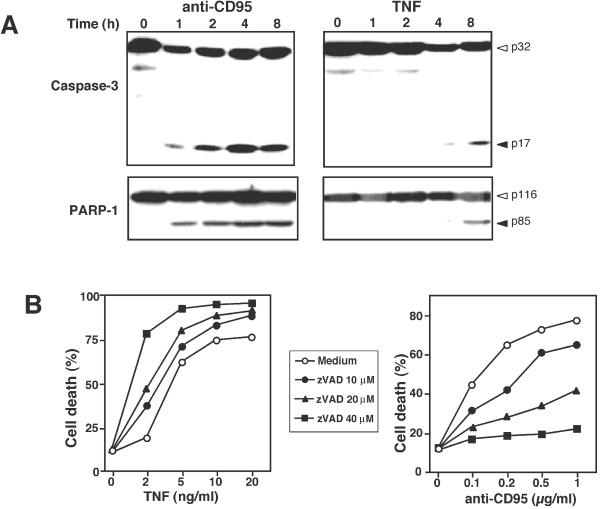
Because caspases contribute to the overall apoptotic morphology by cleavage of various cellular substrates, we examined the time course of caspase-3 activation and proteolytic cleavage of PARP-1. Engagement of CD95 caused a rapid activation of the caspase cascade. The p17 active subunit of caspase-3 and the cleaved form of PARP-1, a typical caspase-3 substrate, were detected already 1 h after triggering of CD95. In contrast, TNF-induced caspase-3 activation was much delayed, despite the fact that both stimuli killed L929 cells with a similar kinetic. Weak caspase-3 activation and corresponding PARP-1 cleavage to the 85-kDa fragment could be first seen 8 h after death induction by TNF (Figure 2A). Furthermore, we did not detect a 50-kDa fragment of PARP-1 that might be generated by lysosomal proteases, as recently observed in H2O2-induced necrosis of Jurkat cells (Gobeil et al., 2001).
Figure 2.
Time course of caspase activation and the effect of caspase inhibition on TNF- and CD95-mediated cell death. (A) L929 cells were incubated with TNF or anti-CD95 as described in Figure 1. After the indicated time cells were harvested and analyzed by immunoblotting for the processing of procaspase-3 into the active p17 active subunit (top panel) and PARP-1 cleavage (bottom panel). (B) Effect of the caspase inhibitor zVAD: Cells were preincubated for 30 min with the indicated concentrations of zVAD or the medium control and then stimulated with either TNF or anti-CD95. Cell death was determined in triplicates by PI staining and flow cytometry 5 h after cell treatment. SDs were <9%.
We then examined the effect of caspase inhibition on the cytotoxic activity of TNF and anti-CD95. Incubation of L929 cells with the broad-spectrum caspase inhibitor zVAD revealed unexpected results. Although increasing concentrations of zVAD were protective in a dose-dependent manner against CD95-mediated apoptosis, the opposite was true for death induction by TNF. Not only that zVAD did not protect cells, it even potentiated TNF-induced cell death (Figure 2B). Microscopic inspection revealed that cells treated with the combination of TNF and zVAD exhibited a more pronounced necrotic morphology than cells treated with TNF alone (our unpublished results). Thus, in this experimental system caspase inhibition rather influences the form of cell death than effectively protects against it.
Caspase-resistant PARP-1 Potentiates TNF Cytotoxicity
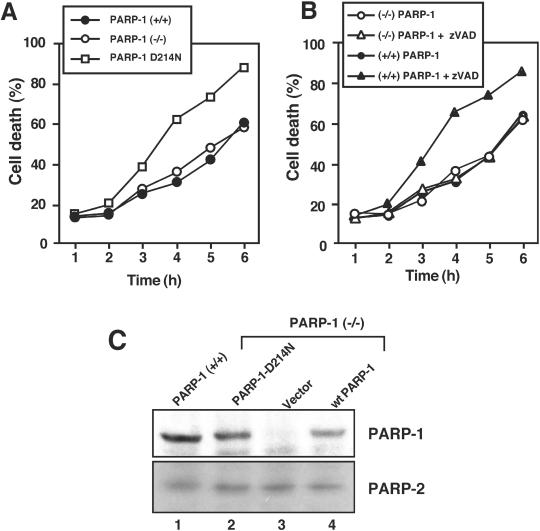
To get insights into the mechanism of the sensitizing effect of zVAD, we next examined the role of PARP-1 cleavage in TNF-mediated cell death. The experiments were performed because inhibition of PARP-1 enzymatic activity can protect against oxidative stress– and TNF-induced death (Schulze-Osthoff et al., 1992; Herceg and Wang, 1999; Ha and Snyder, 1999). We used immortalized fibroblasts from PARP-1(−/−) mice and variants stably expressing a caspase-resistant PARP-1 mutant. PARP-1-D214N carried a mutation in which the aspartate residue at position 214 was substituted by an asparagine, thus lacking the critical aspartate required for cleavage and inactivation by caspases (Herceg and Wang, 1999). As reported earlier for primary murine embryonic fibroblasts (Wang et al., 1997), upon TNF incubation PARP-1–deficient cells displayed apoptosis similar to cells expressing wild-type PARP-1 (Figure 3A). Interestingly, fibroblasts expressing the PARP-1-D214N mutant were significantly more sensitive to TNF than wild-type or PARP-1–deficient cells. The same results were obtained using PARP-1(−/−) fibroblasts transiently transfected with both forms of PARP-1 (our unpublished results). Unfortunately, however, the different immortalized fibroblast clones were not sensitive to apoptosis by CD95 ligation, and neither anti-CD95 (Jo2) nor recombinant CD95 ligand efficiently induced cell death. The resistance of immortalized fibroblasts toward CD95-mediated apoptosis might be due to a very low expression of CD95, as determined by FACS analysis (our unpublished results).
Figure 3.
Inhibition of PARP-1 cleavage potentiates TNF-induced death. Immortalized murine fibroblasts were triggered with TNF for 8 h. (A) Cells expressing the caspase-resistant PARP-1 D214N mutant were more sensitive to TNF-induced death than wild-type or PARP-1–deficient cells. (B) Effect of the caspase inhibitor zVAD on TNF-induced cell death in the different immortalized cells. Potentiation of TNF-induced death by zVAD was only observed in PARP-1(+/+) fibroblasts but not in PARP-1–deficient cells. SDs were <11%. (C) Expression of PARP-1 and -2 in immortalized wild-type fibroblasts and PARP knockout cells retransfected the caspase-resistant PARP D214N mutant, the vector control or wild-type (wt) PARP-1 cDNA. Cell lysates were separated by SDS polyacrylamide electrophoresis and immunoblotted with antibodies specific for PARP-1 and PARP-2, respectively. The immunoblots show the full-length form of PARP–1 (116 kDa) and -2 (62 kDa). No compensatory upregulation of PARP-2 expression was observed in the different cell clones.
These findings of TNF-induced death in cells expressing the caspase-resistant PARP-1 mutant corresponded to data obtained by pharmacological blockade of PARP-1 proteolysis. Inhibition of PARP-1 cleavage with zVAD potentiated TNF-induced death in the wild-type fibroblasts, but not in the PARP-1(−/−) cells (Figure 3B). We further investigated a potential role of PARP-2, which is most related to PARP-1. Quantitative analysis of poly(ADP-ribosyl)ation has recently shown that PARP-2 accounts for most of the residual activity in PARP-1–deficient cells which is ∼5–10% of the activity in wild-type cells (Shieh et al., 1998; Ame et al., 1999). By Western blot analysis we found a slight expression of PARP-2 that, as judged from the exposure time of the immunoblot, was considerably more weakly expressed than PARP-1 (Figure 3C). The same amount of PARP-2 was expressed in PARP-1–deficient and wild-type cells and transfectants expressing the PARP-1 mutant, indicating that there was no compensation for PARP-1 deficiency by PARP-2 upregulation. In summary, these results show that PARP-1 enzymatic activity does influence TNF cytotoxicity and that the sensitizing effect of zVAD, which may inhibit additional cysteine proteases including cathepsins (Schotte et al., 1999), was due to the inhibition of caspase-mediated PARP-1 cleavage.
PARP-1 Modulates TNF-induced Death through Changes of Intracellular ATP Levels
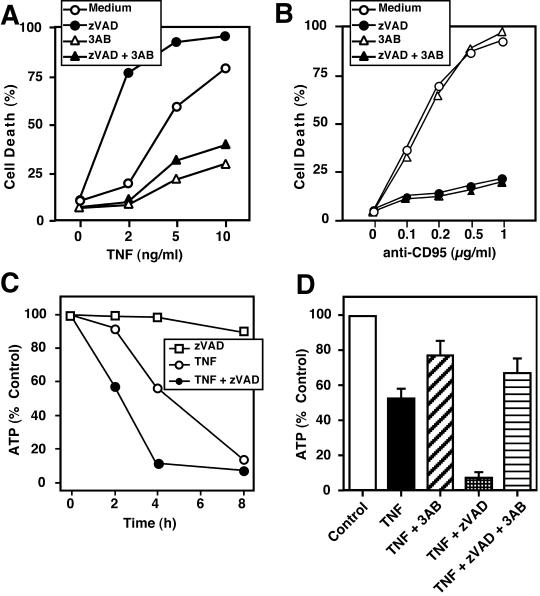
How could PARP-1 activity render cells more responsive to TNF-mediated death? PARP-1 becomes activated upon DNA damage and decorates several acceptor proteins with poly(ADP-ribose) chains, which consumes large amounts of NAD+, resulting in energy depletion. Thus, activation of PARP-1 can indirectly contribute to depletion of ATP in the cell. It has previously been shown that the PARP-1 inhibitor 3AB can significantly protect against TNF- but not CD95-mediated death (Schulze-Osthoff et al., 1992; 1994). Indeed, 3AB offered considerable protection against TNF-induced death and, moreover, was able to even counteract the sensitizing effect of caspase inhibition (Figure 4A). In contrast, anti-CD95–induced apoptosis was not inhibited by 3AB at early time points after anti-CD95 treatment, although zVAD was strongly protective (Figure 4B). A slight protective effect of 3AB in combination with zVAD, however, was noted at later time points, presumably because secondary necrosis occurred (our unpublished results).
Figure 4.
Inhibition of PARP activity counteracts potentiation of TNF-induced death and ATP depletion after caspase inhibition. (A and B) Effect of zVAD and the PARP inhibitor 3-aminobenzamide (3AB) on TNF- and anti-CD95–induced cell death. L929 cells were preincubated with 40 μM zVAD, 3 mM 3AB, or a combination thereof and then stimulated for 5 h with the indicated concentrations of TNF (A) or anti-CD95 (B). (C) Potentiation of TNF-induced ATP depletion by zVAD. Cells were stimulated with or without zVAD (40 μM) in the presence or absence of TNF. After 4 h intracellular ATP levels were determined by the luciferase method. (D) Potentiation of TNF-triggered ATP depletion by the caspase inhibitor zVAD is prevented by 3AB. Cells were treated as described in C in the presence and absence of 3AB and analyzed for the intracellular ATP content 4 h after TNF stimulation. SDs in the experiments were <10%.
It has been described previously that the depletion of cellular energy may switch apoptotic cell death that is energy dependent to necrosis (Eguchi et al., 1997; Leist et al., 1997b; Ferrari et al., 1998). Because addition of zVAD led to a more pronounced necrotic morphology in response to TNF, we examined the intracellular levels of ATP in cells treated with TNF in the absence or presence of the caspase inhibitor. TNF treatment alone caused a significant depletion of cellular ATP (Figure 4C). Cotreatment with zVAD led to an even more pronounced decrease of ATP. Because the PARP inhibitor 3AB significantly protected against TNF killing even in the presence of zVAD, we next examined the effect of 3AB on cellular ATP levels. Inhibition of PARP strongly attenuated the decrease of ATP upon TNF treatment. It also counteracted the depletion of ATP caused by TNF treatment in the presence of the caspase inhibitor (Figure 4D). The structurally related 3-aminobenzoic acid, which does not affect PARP activity, had no effect on TNF- and zVAD-induced changes (our unpublished results). Thus, protection against TNF-induced death by PARP inhibition largely correlated with the preservation of the cellular ATP pool, whereas TNF sensitization by the caspase inhibitor was associated with a dramatic ATP loss.
TNF-induced Formation of Reactive Oxygen Species Causes PARP-1 Activation
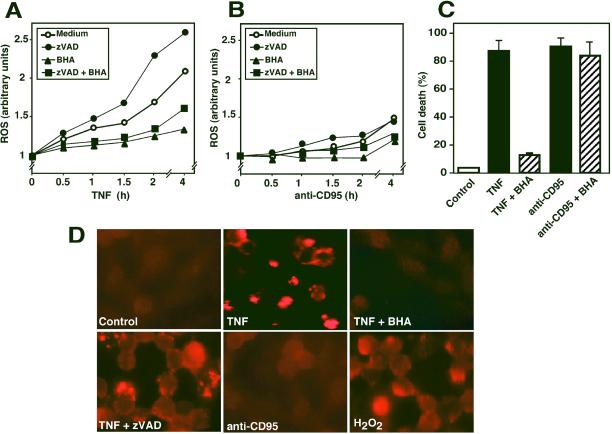
The experiments described above indicated that PARP-1 was strongly activated upon TNF-R1 triggering. Because TNF-induced kill is efficiently blocked by antioxidants (Schulze-Osthoff et al., 1993, 1994), we measured the level of reactive oxygen species (ROS) in response to TNF-R1 and CD95 triggering. TNF strongly induced the production of ROS already after 0.5 h (Figure 5A). In contrast, CD95-triggered cells largely maintained a redox state comparable to control cells, and a significant increase in the ROS level was first observed after 2 h, when the apoptotic process was already advanced (Figure 5B). Cotreatment with the caspase inhibitor zVAD potentiated TNF-induced ROS formation, whereas pretreatment with the antioxidant BHA decreased it. Parallel microscopic examination of the L929 cells (our unpublished results) and assessment of cell death by PI uptake revealed that BHA was protective against TNF-induced death (Figure 5C), whereas zVAD augmented it (see Figure 2B). In addition, analysis of PARP-1 activity by immunocytochemical detection of poly(ADP-ribose) chains demonstrated no PARP-1 activity in anti-CD95–treated L929 cells, whereas a bright nuclear staining indicative for PARP activity was observed in cells triggered with TNF (Figure 5D). The staining was more pronounced in cells with advanced morphology of cell death. It appeared granular and often with higher intensity in the peripheral regions of nuclei. Pretreatment with BHA almost completely inhibited PARP-1 activation by TNF. Similarly, a strong but more diffuse nuclear staining of PARP activity was observed upon H2O2-induced oxidative stress (Figure 5D). Thus, TNF but not anti-CD95 triggered a strong activation of PARP-1, which was prevented by antioxidants.
Figure 5.
Involvement of ROS formation in death receptor-induced PARP activation. (A and B) Effect of TNF and anti-CD95 on ROS formation. L929 cells were triggered with TNF (A, 40 ng/ml) or anti-CD95 (B, 1 μg/ml) for the indicated time. The antioxidant BHA (150 μM) and zVAD (50 μM) were added 15 min before treatment with the death stimuli. ROS production was measured with the dye DFCH and flow cytometry. Similar data were obtained using rhodamine-123. SDs in the above experiments were <12%. (C) Different effect of BHA on TNF- and anti-CD95–induced cell death. Cells were stimulated as described in (A) with TNF or anti-CD95 in the presence or absence of 150 μM BHA. Cell death was determined after 5 h by measurement of PI uptake. (D) Visualization of PARP activity by immunodetection of poly(ADP-ribose) chains. Cells were either stimulated with TNF (40 ng/ml) in the presence and absence of BHA or zVAD or incubated with 1 μg/ml anti-CD95 or 2 mM H2O2. PARP activation was detected with a antibody specific for poly(ADP-ribose) polymers. Note the similarity of TNF-induced and zVAD-potentiated PARP activity with the immunocytochemical signal obtained after H2O2 treatment.
DISCUSSION
In the present study, we investigated the role of PARP-1 activation, its cleavage, and PARP-1–mediated energy depletion upon cell death induction by death receptors. We and others have previously demonstrated that PARP-1–deficient cells are still able to undergo apoptosis by various apoptotic stimuli, suggesting that PARP-1 in principle is not required for apoptosis (de Murcia et al., 1997; Leist et al., 1997a; Wang et al., 1997). However, whether the cleavage and proteolytic inactivation of PARP-1 play an active role in regulation of the mode of cell death had not been addressed in detail. Most experiments in this study were performed in L929 fibrosarcoma cells. On CD95 triggering these cells died by apoptosis, whereas TNF induced a form of cell death with predominantly necrotic features (Schulze-Osthoff et al., 1994; Vercammen et al., 1998). On TNF treatment cells became round, detached from the surface, and revealed large amorphous nuclei. The morphological differences between CD95- and TNF-induced cell death were confirmed by flow cytometry and DNA laddering. CD95-triggered cells were hypodiploid and exhibited apoptotic internucleosomal DNA degradation. TNF treatment killed cells with similar efficacy; however, hypodiploid nuclei and DNA laddering were almost invisible.
The distinct modes of cell death induction were reflected by different biochemical mechanisms triggered by TNF and anti-CD95. TNF-induced necrosis is largely mediated by oxidative stress and ROS formation at the mitochondrial respiratory chain (Schulze-Osthoff et al., 1992, 1993). Antioxidants such as BHA almost completely prevented TNF-induced necrosis, but had almost no effect on CD95-mediated apoptosis. In contrast, CD95-mediated apoptosis was efficiently attenuated by the caspase inhibitor zVAD. On engagement of CD95, caspases were rapidly activated, whereas TNF induced weak caspase activation only at later time points. Interestingly, attempts to inhibit cell death with the caspase inhibitor zVAD gave opposite results in response to TNF and anti-CD95 treatment. Caspase inhibition protected against CD95-triggered apoptosis, whereas it potentiated TNF-induced death, a finding recently also made by others (Vercammen et al., 1998).
While analyzing potential differences in the biochemical mechanisms of cell death, we noticed a significant depletion of intracellular ATP in response to TNF; however, during CD95-mediated apoptosis ATP levels were maintained. Interestingly, the combination of zVAD together with TNF induced increased necrosis and also a much stronger ATP depletion as the treatment with TNF alone. A potential candidate in this scenario was PARP-1, because its inhibitors were protective against TNF-mediated death. To exclude a nonspecific effect of zVAD, we tested this hypothesis using PARP-1(−/−) cells and fibroblasts bearing a caspase-resistant PARP-1 mutant. Indeed, prevention of PARP-1 cleavage, either by pharmacological caspase inhibition or expression of the PARP-1 mutant, led to increased necrotic cell death after TNF treatment, which is consistent with previous data (Herceg and Wang, 1999). Our results also suggest that PARP-1 is responsible for the potentiation of TNF-induced necrosis after caspase inhibition. The strong depletion of ATP and consequent necrosis induced by a combined treatment with TNF and zVAD were almost completely prevented by a PARP inhibitor. Furthermore, zVAD did not potentiate TNF-induced necrosis in fibroblasts expressing the noncleavable PARP-1 mutant. This latter finding suggests that other PARP members such as PARP-2 are presumably not important for TNF-induced necrosis and its potentiation by caspase inhibition.
PARP-1(−/−) fibroblasts undergo normal apoptosis induced by various stimuli such as anti-CD95, indicating that PARP-1 itself is dispensable for apoptosis. Nevertheless, PARP-1 cleavage by caspases may be an important event for the execution of apoptosis by preventing increased NAD+ and ATP depletion (Ha and Snyder, 1999; Herceg and Wang 1999). PARP-1 activation is induced by DNA strand breaks (Lindahl et al., 1995). The enhanced necrotic death in cells expressing a noncleavable PARP-1 mutant or in cells treated with zVAD is most likely caused by excessive poly(ADP-ribosyl)ation in response to DNA damage and the consequent depletion of the intracellular energy content. Important mediators of TNF-induced DNA damage are mitochondria-derived ROS that are strongly produced upon TNF treatment. This hypothesis is supported by several lines of evidence: (1) Mitochondria are the major source of TNF-induced ROS production (Hennet et al., 1993; SchulzeOsthoff et al., 1993). (2) BHA, a lipophilic antioxidant, did not only counteract TNF toxicity, but also prevented the formation of poly(ADP-ribosyl) chains. (3) Poly(ADP-ribosyl)ation can be efficiently instigated by H2O2, a strong inducer of oxidative stress. (4) TNF, but not CD95 triggering induces a rapid prooxidant state. Finally, the considerable ROS production induced by TNF may inactivate caspases through oxidation of the critical cysteine in their active center, thus promoting necrosis (reviewed in Hampton et al., 1998). The combination of early ROS production and late caspase activation upon TNF treatment triggers high PARP activity, leading to energy depletion and necrosis. On CD95 triggering caspases proteolytically inactivate PARP-1, so that enough ATP is preserved to execute the energy-dependent apoptotic process.
Elevated poly(ADP-ribosyl)ation and subsequent depletion of NAD+ may affect cellular metabolism in several ways (Soldani et al., 2001). 1) NAD+ is a cofactor for the oxidation of glyceraldehyde-3-phosphate in the glycolytic pathway. Thus, consumption of NAD+ causes inhibition of ATP synthesis by blockade of glycolysis. 2) The loss of cellular ATP is exacerbated by reconversion of nicotinamide to NAD+ with the further consumption of four molecules of ATP per molecule of resynthesized NAD+. 3) NAD+ depletion and compromised energy metabolism may alter mitochondrial physiology and cause permeability transition (Hirsch et al., 1997). 4) At least in the yeast system, poly(ADP-ribose) polymers may impede cell cycle progression because of interference of poly-ADP-ribosylated proteins with chromatin replication (Avila et al., 1994; Collinge and Althaus, 1994). Overall, these mechanisms may be relevant for NO-induced cytotoxicity, excitotoxic damage of neurons, streptozocin-induced diabetes, and reperfusion injury of cardiomyocytes as well as inflammation and other pathological conditions (Heller et al., 1995; Eliasson et al., 1997; Endres et al., 1997; Thiemermann et al., 1997; Ha and Snyder, 1999; Oliver et al., 1999; Bürkle, 2001; Garcia Soriano et al., 2001). It will be interesting to investigate which diseases are mediated by PARP-1 activation and how this is correlated to necrosis and apoptosis.
The experiments in immortalized fibroblasts did not allow us to investigate in detail the role of PARP-1 in CD95-mediated apoptosis. Neither wild-type, PARP-1–deficient nor PARP-1 mutant-expressing cells were sensitive to anti-CD95 or recombinant CD95 ligand. The immortalized cells were also not sensitized by inhibitors of macromolecular synthesis such as cycloheximide or actinomycin D, which were sufficient to induce cell death even in the absence of CD95 ligation. The role of PARP-1 in CD95-mediated apoptosis is currently controversial. Oliver et al. (1998) reported that CD95 killing is reduced in primary fibroblasts expressing a caspase-resistant PARP-1 mutant, whereas wild-type and PARP-1–deficient cells are equally sensitive. In contrast, another study on the role of poly(ADP-ribosyl)ation found that the absence of PARP-1 rendered cells resistant to cell death after anti-CD95 treatment (Simbulan-Rosenthal et al., 1998). The apparent discrepancy between these results might be explained by the different genetic background, the process of immortalization, the technical means by which PARP-1 was introduced into the target cells, or the use of different concentrations of cycloheximide. PARP-1 deletion also failed to protect against CD95-mediated cell death of hepatocytes (Leist et al., 1997a). Furthermore, our data in L929 cells suggest that the pathway of ROS-induced PARP activation is involved in TNF-induced necrosis, but principally not important for CD95-mediated apoptosis. This is supported by the finding that antioxidants as well as a PARP inhibitor prevented TNF-induced cell death, but not CD95-mediated apoptosis.
In conclusion, the present study implies an active role of PARP-1 cleavage in determining the mode of death receptor-induced cell death. CD95 ligation leads to a rapid proteolytic inactivation of PARP-1 and maintenance of cellular energy levels. In contrast, TNF-induced ROS production, which may lead to DNA damage and subsequent PARP-mediated energy depletion, is an important factor driving cells toward necrosis. Our experiments also explain why caspase inhibitors potentiate TNF-induced necrosis. Because caspase inhibitors are being used in clinical trials, our results suggest that the combined use of caspase and PARP inhibitors might be of therapeutic importance in pathologies where both apoptosis and necrosis occur. Current trials with caspase inhibitors for clinical therapies must therefore take into account their potential effects on the mode of cell death and potential inflammatory reactions in response to necrosis.
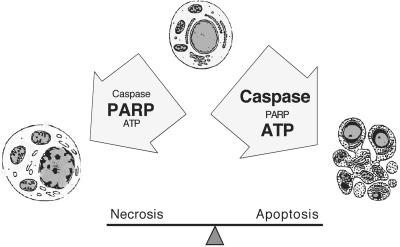
Figure 6.
Schematic representation of the role of ROS, PARP, caspases, and intracellular ATP on the mode of cell death. Cells will die by necrosis when ROS-induced DNA damage, for instance in response to TNF, causes elevated PARP activity, resulting in subsequent energy depletion. Apoptosis will occur when caspases are activated early upon death induction. Caspases proteolytically inactivate a number of proteins including PARP-1 and thereby preserve ATP levels that are required for the apoptotic process. This scenario may also explain why caspase inhibitors prevent apoptosis but increase necrotic forms of cell death after death receptor stimulation.
ACKNOWLEDGMENTS
The authors thank Dr. Reiner Jänicke for helpful comments. This work was in part supported by grants from the Deutsche Krebshilfe, the Deutsch-Israelische Projektkoordination, the Deutsche Forschungsgemeinschaft (LO 823/1-1) and the IMF Münster (LO 410119) awarded to M.L.
Abbreviations used:
- 3AB
3-aminobenzamide
- BHA
butylated hydroxyanisole
- PARP
poly(ADP-ribose)polymerase
- PI
propidium iodide
- TNF
tumor necrosis factor
- zVAD
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–05–0272. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–05–0272.
REFERENCES
- Ame JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Menissier-de Murcia J, de Murcia G. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- Avila MA, Velasco JA, Smulson ME, Dritschilo A, Castro R, Notario V. Functional expression of human poly(ADP-ribose) polymerase in Schizosaccharomyces pombe results in mitotic delay at G1, increased mutation rate, and sensitization to radiation. Yeast. 1994;10:1003–1017. doi: 10.1002/yea.320100803. [DOI] [PubMed] [Google Scholar]
- Beneke R, Geisen C, Zevnik B, Bauch T, Muller WU, Kupper JH, Moroy T. DNA excision repair and DNA damage-induced apoptosis are linked to poly(ADP-ribosyl)ation but have different requirements for p53. Mol Cell Biol. 2000;20:6695–6703. doi: 10.1128/mcb.20.18.6695-6703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle A. Physiology and pathophysiology of poly(ADP-ribosyl)ation. Bioessays. 2001;9:795–806. doi: 10.1002/bies.1115. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge MA, Althaus FR. Expression of human poly(ADP-ribose) polymerase in Saccharomyces cerevisiae. Mol Gen Genet. 1994;245:686–693. doi: 10.1007/BF00297275. [DOI] [PubMed] [Google Scholar]
- Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Eliasson MJ, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Stepczynska A, Los M, Wesselborg S, Schulze-Osthoff K. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J Exp Med. 1998;188:979–984. doi: 10.1084/jem.188.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Garcia Soriano F, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;8:588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Fadeel B, Orrenius S. Redox regulation of the caspases during apoptosis. Ann NY Acad Sci USA. 1998;854:328–335. doi: 10.1111/j.1749-6632.1998.tb09913.x. [DOI] [PubMed] [Google Scholar]
- Heller B, Wang Z, Wagner E, Radons J, Burkle A, Fehsel K, Burkart V, Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995;270:11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- Hennet T, Richter C, Peterhans E. Tumor necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Wang ZQ. Failure of poly(ADP-ribose) polymerase cleavage by caspases leads to induction of necrosis and enhanced apoptosis. Mol Cell Biol. 1999;19:5124–5133. doi: 10.1128/mcb.19.7.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene. 1997;15:1573–1581. doi: 10.1038/sj.onc.1201324. [DOI] [PubMed] [Google Scholar]
- Jacobson MK, Jacobson EL. Discovering new ADP-ribose polymer cycles: protecting the genome and more. Trends Biochem Sci. 1999;24:415–417. doi: 10.1016/s0968-0004(99)01481-4. [DOI] [PubMed] [Google Scholar]
- Kaminker PG, Kim SH, Taylor RD, Zebarjadian Y, Funk WD, Morin GB, Yaswen P, Campisi J. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. J Biol Chem. 2001;276:35891–35899. doi: 10.1074/jbc.M105968200. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADPribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Kawamitsu H, Hoshino H, Okada H, Miwa M, Momoi H, Sugimura T. Monoclonal antibodies to poly(adenosine diphosphate ribose) recognize different structures. Biochemistry. 1984;23:3771–3777. doi: 10.1021/bi00311a032. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickhoefer VA, Rajavel KS, Scheffer GL, Dalton WS, Scheper RJ, Rome LH. Vaults are up-regulated in multidrug-resistant cancer cell lines. J Biol Chem. 1998;273:8971–8974. doi: 10.1074/jbc.273.15.8971. [DOI] [PubMed] [Google Scholar]
- Kong LB, Siva AC, Rome LH, Stewart PL. Structure of the vault, a ubiquitous cellular component. Structure Fold Des. 1999;7:371–379. doi: 10.1016/s0969-2126(99)80050-1. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Kunstle G, Volbracht C, Hentze H, Nicotera P. Apoptosis in the absence of poly-(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1997a;233:518–522. doi: 10.1006/bbrc.1997.6491. [DOI] [PubMed] [Google Scholar]
- Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997b;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Satoh MS, Poirier GG, Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995;20:405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Los M, van de Craen M, Penning CL, Schenk H, Westendorp M, Baeuerle PA, Dröge W, Krammer PH, Fiers W, Schulze-Osthoff K. Requirement of an ICE/Ced-3 protease for Fas/Apo-1-mediated apoptosis. Nature. 1995;371:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- Los M, Herr I, Friesen C, Fulda S, Schulze-Osthoff K, Debatin KM. Cross-resistance of CD95- and drug-induced apoptosis as a consequence of deficient activation of caspases (ICE/Ced-3 proteases) Blood. 1997;90:3118–3129. [PubMed] [Google Scholar]
- Los M, Khazaie K, Schulze-Osthoff K, Baeuerle PA, Schirrmacher V, Chlichlia K. Human T cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J Immunol. 1998;161:3050–3055. [PubMed] [Google Scholar]
- Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- de Murcia JM, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M, Pizzo P, Sandona D, Zanovello P, Rizzuto R, Di Virgilio F. Mitochondrial DNA is not fragmented during apoptosis. J Biol Chem. 1992;267:10939–10941. [PubMed] [Google Scholar]
- Muzio M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmann FR, Vodenicharov MD, Wang ZQ, Poirier GG. Characterization of sPARP-1. An alternative product of PARP-1 gene with poly(ADP-ribose) polymerase activity independent of DNA strand breaks. J Biol Chem. 2000;275:15504–15511. doi: 10.1074/jbc.275.20.15504. [DOI] [PubMed] [Google Scholar]
- Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Hunting D, Trucco C, Gowans B, Grunwald D, De Murcia G, De Murcia JM. A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis, and sister chromatid exchange following DNA damage. Proc Natl Acad Sci USA. 1995;92:4753–4757. doi: 10.1073/pnas.92.11.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Krammer PH, Droge W. Divergent signaling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994;13:4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Shieh WM, Ame JC, Wilson MV, Wang ZQ, Koh DW, Jacobson MK, Jacobson EL. Poly(ADP-ribose) polymerase null mouse cells synthesize ADP-ribose polymers. J Biol Chem. 1998;273:30069–30072. doi: 10.1074/jbc.273.46.30069. [DOI] [PubMed] [Google Scholar]
- Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem. 1998;273:13703–13712. doi: 10.1074/jbc.273.22.13703. [DOI] [PubMed] [Google Scholar]
- Sims JL, Berger SJ, Berger NA. Poly(ADP-ribose) polymerase inhibitors preserve nicotinamide adenine dinucleotide and adenosine 5′-triphosphate pools in DNA-damaged cells: mechanism of stimulation of unscheduled DNA synthesis. Biochemistry. 1983;22:5188–5194. doi: 10.1021/bi00291a019. [DOI] [PubMed] [Google Scholar]
- Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- Soldani C, Lazze MC, Bottone MG, Tognon G, Biggiogera M, Pellicciari CE, Scovassi AI. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res. 2001;269:193–201. doi: 10.1006/excr.2001.5293. [DOI] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner EF. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, Wagner EF. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]