Abstract
Schizosaccharomyces pombe cells divide by medial fission through the use of an actomyosin-based contractile ring. Constriction of the actomyosin ring is accompanied by the centripetal addition of new membranes and cell wall material. In this article, we characterize the mechanism responsible for the localization of Cps1p, a septum-synthesizing 1,3-β-glucan synthase, to the division site during cytokinesis. We show that Cps1p is an integral membrane protein that localizes to the cell division site late in anaphase. Neither F-actin nor microtubules are essential for the initial assembly of Cps1p to the medial division site. F-actin, but not microtubules, is however important for the eventual incorporation of Cps1p into the actomyosin ring. Assembly of Cps1p into the cell division ring is also dependent on the septation-inducing network (SIN) proteins that regulate division septum formation after assembly of the actomyosin ring. Fluorescence-recovery after-photobleaching experiments reveal that Cps1p does not diffuse appreciably within the plasma membrane and is retained at the division site by a mechanism that does not depend on an intact F-actin cytoskeleton. We conclude that the actomyosin ring serves as a spatial cue for Cps1p localization, whereas the maintenance of Cps1p at the division site occurs by a novel F-actin– and microtubule-independent mechanism. Furthermore, we propose that the SIN proteins ensure localization of Cps1p at the appropriate point in the cell cycle.
INTRODUCTION
Cytokinesis is the terminal stage in the cell cycle during which the boundaries between daughter cells are established and individual daughter cells are liberated. An actomyosin-based contractile ring is thought to generate the forces necessary for cell cleavage in animal cells, amoebae, and fungi (Balasubramanian et al., 2000; Gerisch and Weber 2000; Glotzer 2001). Plasma membrane barriers between the daughter cells are established in concert with actomyosin ring constriction. An additional level of complexity is seen in fungi and plant cells, where a cell wall composed of sugar polymers (α- and β-linked glucans, chitin, and other sugar polymers) is assembled concomitant with addition of the new membranes at the site of cell division (Shematek et al., 1980; Ishiguro 1998; Humbel et al., 2001). Although recent studies of cytokinesis have focused on the role of the actomyosin ring in cell division, little is known about the mechanisms regulating the assembly of cell membranes and cell wall materials at the division site.
The fission yeast Schizosaccharomyces pombe has emerged in recent years as an attractive model organism for the study of cytokinesis. S. pombe cells, like animal cells and amoebae, divide through the use of an actomyosin-based contractile ring (Chang and Nurse 1996; Le Goff et al., 1999a; Balasubramanian et al., 2000). Genetic analyses in S. pombe have identified numerous gene products that are important for positioning and assembly of the actomyosin ring, assembly of actin patches at the site of cell division, signaling onset of septum assembly, and for the physical assembly of the division septum (Nurse et al., 1976; Chang and Nurse 1996; Balasubramanian et al., 1998; Le Goff et al., 1999a; Balasubramanian et al., 2000; McCollum and Gould 2001). One of the proteins essential for division septum assembly is Cps1p, an integral membrane protein known as the putative catalytic subunit of the enzyme 1,3-β-glucan synthase (Ishiguro et al., 1997; Le Goff et al., 1999b; Liu et al., 1999). 1,3-β-Glucan is a major component of the primary septum in S. pombe (Ishiguro 1998; Humbel et al., 2001), consistent with the finding that cps1 mutants are defective in the assembly of the division septum (Le Goff et al., 1999b; Liu et al., 1999). Previous studies have shown that 1,3-β-glucan synthases are regulated by the GTPase Rho1p, which in its GTP-bound state, activates assembly of 1,3-β-glucans (Arellano et al., 1996; Drgonova et al., 1996; Qadota et al., 1996). One of the key questions pertaining to Cps1p relates to the mechanism of assembly of Cps1p to the division site, so as to ensure that the primary septum is assembled exclusively there.
In this study, we have characterized the mechanism of intracellular localization of Cps1p during cell division. We show that Cps1p accumulates at the division site in a medial ring structure overlying the actomyosin ring late in anaphase and that the incorporation of Cps1p into a ring structure is dependent on F-actin function and the septation-inducing network (SIN). Interestingly, microtubules are dispensable for the assembly of Cps1p at the division site. Fluorescence recovery after photobleaching experiments reveal that Cps1p does not diffuse freely in the plasma membrane and is retained at the division site in an F-actin–independent manner. We conclude that an F-actin–based mechanism and SIN-mediated signaling are important for targeting of Cps1p and possibly other membrane proteins during cytokinesis.
MATERIALS AND METHODS
S. pombe Strains, Media, and Reagents
The S. pombe strains used in this study are as follows: h− leu1-32 ura4-D18 (wild-type), h− GFP-cps1+::Kanr leu1-32 ura4-D18, GFP-cps1+::Kanr cdc4-8 (or cdc12-112, rng2-D5, cdc15-140), GFP-cps1+::Kanr cdc7-24 (or cdc14-118, spg1-106, sid2-250), nda3-KM113 mad2Δ, cdc16-116, GFP-cps1+::Kanr cdc25-22, and gma12-GFP::ura4+ cdc25-22. YES and EMM media were used for cell culture. GFP-cps1+::Kanr strains were constructed by fusion of green fluorescent protein (GFP) to the N-terminus of Cps1p using a PCR-based method using primers 5′-AACTTTTCTTTCTTTCAATTCTTCTTAATTT-AAGCATTAAAATTTGTCCTCTCTCCTTCTTTTGAGTCTATTTATTC-AT CGAATTCGAGCTCGTTTAAAC-3′ and 5′-GTATCATCGTCAGA-GACATAACTATTGGCATCATCTTCAAAGAGTCCACGACCCTCTT-GTTCACGCCAATACTGATCCATTTTGTATAGTTCATCCATGC-3′.gma12-GFP strain was constructed by transformation of the integration plasmid pJK210 containing the gma12 ORF fragment (without stop codon) and the GFP gene. Primers 5′-CCTCCTGGTACCTAGAACACACGAGTACTTGGACC-3′ and 5′-CCTCTCCCGGGGGATGATGGTTTCAAAAGATTTTG-3′ were used in PCR for cloning of the gma12 ORF fragment. Plasmid pREP1-GFP::CAAX was constructed by cloning a PCR fragment encoding the last 19 amino acids from Cdc42p (Miller and Johnson 1994) fused to the C terminus of GFP. This plasmid was transformed into a wild-type strain and grown in EMM medium supplemented with 5 μM thiamine. The cells growing in EMM medium were transferred to YES medium 1 h before microscopic examinations. Latrunculin A (LatA) was purchased from Molecular Probes (Eugene, OR) and used at a final concentration of 100 μM. Brefeldin A (BFA) was purchased from Sigma (St. Louis, MO) and used at a final concentration of 100 μM.
Synchronization of Cultures and Drug Treatment
The cdc25-22 mutant was used to synchronize cultures by shifting to 36°C for 4 h to arrest cells at the G2/M boundary and subsequently released from this arrest by altering the growth temperature to 24°C. LatA was added to culture at a final concentration of 100 μM immediately after release or added at the time of GFP-Cps1p ring formation. BFA was added to the culture at a final concentration of 100 μM immediately after release. Cells were collected at each time point and fixed for immunostaining. Lactose gradients were also used to synchronize cultures using protocols described elsewhere (Edwards and Carr, 1997). Briefly, 100 ml log phase cells were harvested and loaded to a lactose gradient (30% lactose at the bottom and 7% on the top) in a 15-ml centrifuge tube. The tube was centrifuged for 8 min at 1000 rpm. A fraction of 200–300 μl of synchronized cells was collected as an inoculum for setting up fresh cultures.
Immunoblotting and Protein Methods
S. pombe cells at early-log phase were harvested and broken using glass beads in lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 10% glycerol) supplemented with protease inhibitors. Buffers for protein extractions aimed at examining whether Cps1p is a membrane protein were based on those published by Harkins et al. (2001). The lysates were then spun at maximal speed in a microfuge for 30 min at 4°C. Supernatants were recovered, and pellets were resuspended in SDS-PAGE loading buffer. SDS-PAGE and immunoblotting conditions were followed as described previously (Naqvi et al., 1999). Cps1p N-terminal peptide fragment YDKNSSRDALHSDYDSS and C-terminal fragment FNLIQPATKIVYSSTKNSS were used to raise Cps1p-specific antibodies in rabbits. The antibodies were further purified using Affi-Gel (Bio-Rad, Hercules, CA) to which Cps1p-specific peptides were coupled according to the protocol provided by the manufacturer. Purified anti-Cps1p antibodies against N- and C-terminus were combined (unless otherwise mentioned) and used at a 1:100 dilution in immunoblot analysis. Anti-mouse or anti-rabbit IgG conjugated with horseradish peroxidase (Sigma) was used as secondary antibodies. The chemiluminescence was developed and captured using the horseradish peroxidase system (Sigma).
Fluorescence Microscopy and Photobleaching
Fluorescence microscopy methods used were essentially as described previously (Balasubramanian et al., 1997). Formaldehyde fixation was used before visualization of F-actin using rhodamine-conjugated phalloidin. Both formaldehyde and methanol fixations were used before the detection of Cps1p, microtubules, and GFP with anti-Cps1p antibodies (1:50 dilution, mixture of anti–N- and –C-terminus), TAT1 antibodies (1:100), and anti-GFP antibodies (1:100), respectively. Anti-mouse or anti-rabbit IgG conjugated with either Alexa-488 or -594 (Molecular Probes) was used as secondary antibodies. Photobleaching was effected via a Zeiss confocal microscope LSM system. The region of the cell to be bleached was demarcated and subjected to a laser beam, which scanned the area 200 times in less than a second. Fluorescent recovery images were processed using Adobe Photoshop (Adobe System Inc., San Jose, CA) and quantified using NIH Image (NIH, Bethesda, MD).
RESULTS
Cps1p Is a Stable Integral Membrane Protein
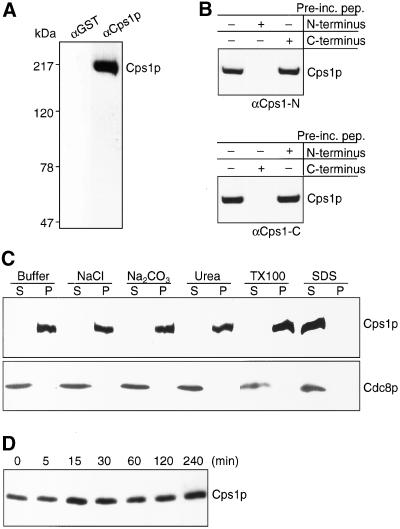
To characterize the product of the cps1 gene, which encodes a 1,3-β-glucan synthase essential for division septum assembly, we raised antibodies against Cps1p using synthetic peptides encoding the N-terminal 17 amino acids and the C-terminal 19 amino acids as immunogens. Affinity-purified antibodies against Cps1p recognized a single ∼200-kDa protein in wild-type cells, consistent with its predicted molecular weight of 199,500 Da (Figure 1A). This 200-kDa protein was not recognized by purified nonspecific rabbit IgGs (Figure 1A). To address if the 200-kDa protein was the product of the cps1 gene, we assessed the ability of the peptides used for immunizations to compete out the Cps1p immunoreactive signal on immunoblots. We separately preincubated the N-terminal and the C-terminal antiserum with either the N-terminal peptide or the C-terminal peptide before the immunoblot experiment. As shown in Figure 1B, the N-terminal peptide, but not the C-terminal peptide, was able to effectively compete with the signal on immunoblots performed with the N-terminal antibodies. Similarly, the C-terminal peptide, but not the N-terminal one, was able to compete with the signal on immunoblots performed with the C-terminal antibodies (Figure 1B). Given these competition data and the fact that the peptides used for immunizations are unique to Cps1p (based on a survey of all S. pombe proteins), we conclude that the 200-kDa protein recognized by the antibodies is the product of the cps1 gene.
Figure 1.
Cps1p is a stable integral membrane protein. (A) Cell lysates were fractionated on a 5% SDS-PAGE gel and subsequently subjected to immunoblotting with affinity purified anti–N- and C-terminal Cps1p antibodies (αCps1p) or with nonspecific antibodies (αGST) as a control. (B) Blots containing cell lysates were immunoblotted with antibodies specific to either N- or C-terminus of Cps1p. Antibodies were preincubated with antigens of N- or C-terminus peptides of Cps1p. (C) Cells were extracted after lysis in buffer containing 0.6 M NaCl, 0.1 M Na2CO3, 1.6 M urea, 4% Triton X-100, and 2% SDS. Soluble and insoluble proteins were separated by centrifugation as indicated by supernatant (s) and pellet (p), respectively. Proteins were fractionated using SDS-PAGE gels and immunoblotted using antibodies against Cps1p and Cdc8p. (D) Lysates were prepared from cells treated with 200 μM cycloheximide for the time intervals indicated and immunoblotted with antibodies against Cps1p.
We extracted total cell proteins using a variety of conditions to ascertain if Cps1p was indeed an integral membrane protein. Cells were extracted with buffer alone, buffer containing 1.6 M Urea or 0.6 M NaCl (solubilizes peripheral membrane proteins), buffer containing 0.1 M Na2CO3 (solubilizes intracellular vesicles), buffer containing the nonionic detergent Triton X-100 (solubilizes most membrane proteins), and buffer containing the strong ionic detergent SDS (solubilizes all membrane proteins). Cdc8p, a cytoplasmic protein (Balasubramanian et al., 1992), was found in the soluble supernatant fraction under all conditions (Figure 1C). Interestingly, with the exception of extraction with SDS, Cps1p was found to be insoluble under all conditions tested and was detected in the pellet fraction (Figure 1C). Thus, Cps1p is a novel integral membrane protein that is solubilized only by strong ionic detergents, but not by nonionic detergents. To test the stability of Cps1p and its half-life, S. pombe cells were treated with cycloheximide over a period of 4 h, and the relative levels of Cps1p were monitored. Interestingly, Cps1p was found to be stable throughout the time course of the experiment, and its levels did not fluctuate dramatically (Figure 1D). Thus, we conclude that Cps1p is a stable integral membrane protein that is insoluble in nonionic detergents.
Cps1p Associates with the Actomyosin Ring Late in Anaphase
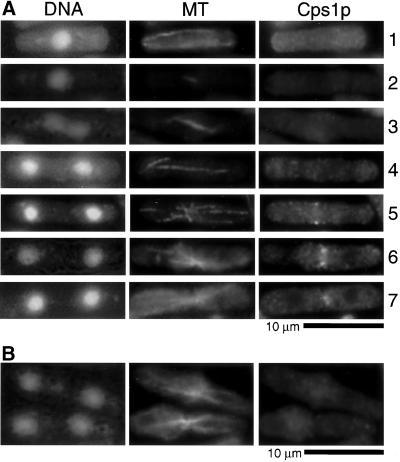
We then used Cps1p antibodies in immunofluorescence microscopy experiments to study the intracellular distribution of this protein. Staining of wild-type cells with Cps1p antibodies revealed dramatic cell cycle–dependent rearrangements of the intracellular distribution of Cps1p. No discrete Cps1p staining was detected in uninucleate interphase cells (Figure 2A, cell 1). Cps1p was also not detected in mitotic cells expected to have well-organized actomyosin rings with short, intermediate-length or elongated spindles (Figure 2A, cells 2–4). Interestingly, a medial ring-like accumulation of Cps1p was detected in cells containing a postanaphase configuration of microtubules (Figure 2A, cell 5). After this, the Cps1p ring underwent an actomyosin ring-like constriction that was accompanied by assembly of the division septum (Figure 2A, cells 6 and 7). Immunolabeling was lost when cells were stained with antibodies preincubated with the antigenic peptides used to raise the antiserum (Figure 2B). These data led to the conclusion that Cps1p localizes to a medial ring structure late in the cell cycle after completion of anaphase.
Figure 2.
Cps1p assembles into the medial actomyosin ring late in anaphase. (A) Wild-type cells were stained with DAPI, TAT1 antibodies, and affinity-purified anti-Cps1p antibodies to visualize DNA, microtubules (MT), and Cps1p, respectively. (B) Cells were stained with anti-Cps1p antibodies preincubated with Cps1p-specific antigenic peptides.
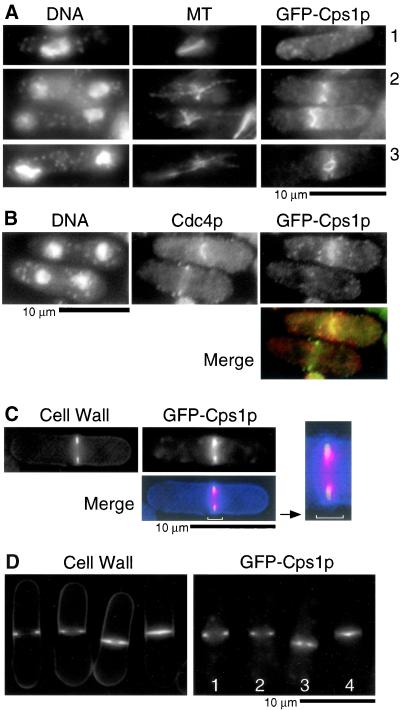
To independently confirm the localization pattern of Cps1p using a different approach, we created a strain in which the sole copy of the cps1 gene was fused at its 5′ end with the gene encoding the green fluorescent protein (GFP). GFP-Cps1p was expressed under the control of the nmt1 promoter, and this strain was maintained on thiamine-containing medium to ensure low level expression of GFP-Cps1p. Cells expressing GFP-Cps1p, under conditions of promoter repression, resembled wild-type cells. This strain was viable and formed colonies establishing that the N-terminal tag did not affect the function of Cps1p. The intracellular distribution of GFP-Cps1p (Figure 3A) was essentially very similar to that observed by immunofluorescence studies using Cps1p antibodies (Figure 2A). A notable difference was the observation that whereas Cps1p was detected at the front of the constricting actomyosin ring (by immunofluorescence microscopy using Cps1p antibodies), GFP-Cps1p was detected at the front of the constricting ring as well as in the body of the septum. Whether Cps1p in the body of the septum is a remnant of the septation process occurring at the front of the constricting ring is presently unclear.
Figure 3.
GFP-Cps1p localizes to the medial actomyosin ring late in anaphase. (A) Cells bearing GFP-cps1+ as the chromosomal copy were stained with DAPI, TAT1, and anti-GFP antibodies to visualize DNA, microtubules (MT), and GFP-Cps1p. (B) Cells were stained for DNA, Cdc4p, and GFP-Cps1p with DAPI, rabbit anti-Cdc4p antibodies, and mouse anti-GFP antibodies, respectively. In the merged image Cps1p is in red and Cdc4p in green. (C) GFP-Cps1p–expressing cells were stained for 1,3-β-glucan with aniline blue. In the merged image Cps1p is in red and septum in blue. (D) The localization of 1,3-β-glucan (aniline blue staining) and GFP-Cps1p is shown in cells at different stages of cytokinesis. Cells marked 1–4 represent those with GFP-Cps1p at different stages of constriction, with 1 being least advanced and 4 being most advanced.
The timing of Cps1p localization relative to the actomyosin ring was addressed by double labeling of GFP-Cps1p–expressing cells with antibodies against GFP and the actomyosin ring protein Cdc4p. This analysis further reinforced the conclusion that Cps1p localized to the medial ring significantly later than Cdc4p (Figure 3B). The timing of 1,3-β-glucan assembly relative to the constriction of medial Cps1p containing ring was addressed by staining GFP-Cps1p–expressing cells with aniline blue, a dye that specifically binds 1,3-β-glucan. This experiment established that the primarily 1,3-β-glucan–containing primary septum was assembled centripetally in concert with constriction of the GFP-Cps1p–containing medial ring (Figure 3, C and D). On the basis of these experiments, we conclude that Cps1p is a component of the actomyosin ring that appears to be loaded there late in mitosis. Furthermore, we conclude that constriction of the Cps1p ring happens in concert with the centripetal deposition of 1,3-β-glucan polymers.
Levels of Cps1p Do Not Fluctuate Appreciably in the Cell Cycle
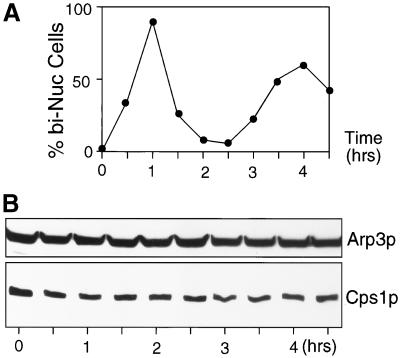
Cps1p is required for cell division, but not for cell elongation, and Cps1p is visualized only in cells undergoing mitosis and cytokinesis. We therefore considered the possibility that the level of Cps1p might be regulated in a cell cycle–dependent manner. To address this question, we prepared a synchronous population of S. pombe cells by arrest and release of the cdc25-22 mutant. The cdc25-22 mutant arrests at the G2/M boundary upon incubation at the restrictive temperature of 36°C. On release to 24°C, cdc25-22 cells underwent synchronous waves of mitosis and cytokinesis, as evident from an estimation of the septation index of the culture after release to 24°C. The first peak of synchronous septation occurred at 60 min, and the second peak of septation occurred ∼240 min after release to the permissive temperature (Figure 4A). Interestingly, the levels of Cps1p did not vary appreciably throughout the course of this experiment (Figure 4B). Thus, protein levels do not appear to determine Cps1p function. Rather, factors controlling Cps1p might be its intracellular localization and activation of the enzymatic function in the cell cycle.
Figure 4.
Level of Cps1p is constant through the cell division cycle. (A) Plot showing accumulation of binucleate cells upon release of cdc25-22 from a G2 block. (B) Lysates prepared from a synchronous culture as in A were immunoblotted with anti-Cps1p antibodies and anti-Arp3p antibodies. Arp3p served as a loading control.
A Brefeldin A–sensitive Secretory Mechanism Is Essential for Cps1p Localization
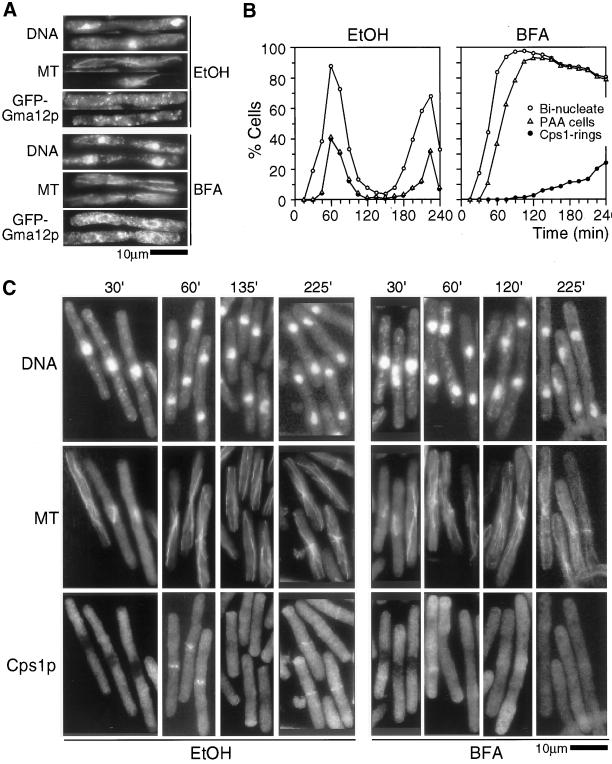
Previous studies in the nematode Caenorhabditis elegans (Skop et al., 2001) have shown that assembly of new membranes at the division site during cytokinesis takes place by a BFA-insensitive mechanism (a drug that induces fusion of Golgi structures with ER and therefore disrupts the Golgi structure). However, the final closure of the membranes in these experiments was dependent on a BFA-sensitive mechanism. We therefore addressed if the accumulation of Cps1p at the division site depended on a BFA-sensitive secretory mechanism. cdc25-22 cells expressing a Golgi marker GFP-Gma12p (Chappell et al., 1994) and a cdc25-22 strain were synchronized at the G2/M boundary and released to the permissive temperature in the presence of either BFA or ethanol (solvent used as a control). Although the cdc25-22 cells expressing GFP-Gma12p were used to visualize Golgi morphology, the untagged cdc25-22 cells were sampled at various time-points, and the localization of Cps1p relative to microtubules was monitored because Cps1p ring was normally detected in cells with postanaphase microtubule arrays. Whereas GFP-Gma12p was detected in a spotty organization throughout the cell in ethanol-treated cells, GFP-Gma12p was detected primarily in the ER network of cells treated with BFA (Figure 5A), establishing the efficiency of BFA treatment. In ethanol-treated cells, Cps1p was detected in all cells with a postanaphase array of microtubules and the peak of localization of Cps1p at the division site was detected at 60 min after release to the permissive temperature for cdc25-22. In ethanol-treated cells, the second synchronous peak of Cps1p was detected at 220 min after release to the permissive temperature for cdc25-22 (Figure 5, B and C). By contrast, in cells treated with BFA, Cps1p was not detected at the division site even 150 min after release to the permissive temperature for cdc25-22, during which ethanol-treated cells had completed the first round of division septum assembly (Figure 5, B and C). The low percentage of BFA-treated cells with Cps1p rings likely results from possible inactivation of BFA at these later time points (>180 min). Progression through mitosis and assembly of post anaphase arrays of microtubules were unaffected in cells treated with BFA, ruling out the possibility that failure to undergo mitosis might have resulted in the lack of assembly of Cps1p at the division site. Interestingly, cells treated with BFA became arrested with two nuclei and a postanaphase array of microtubules at a time point when cells treated with ethanol had undergone a second round of synchronous mitosis (see DISCUSSION). We conclude that a BFA-sensitive pathway is essential for the delivery of GFP-Cps1p to the division site.
Figure 5.
Cps1p medial ring localization at late anaphase requires the function of BFA-sensitive secretory apparatus. (A) cdc25-22 cells expressing GFP-Gma12p were synchronized at 36°C and released to 24°C in medium containing either 100 μM BFA or ethanol (control). Cells were collected and stained for DNA, microtubules (MT), and GFP-Gma12p with DAPI, TAT1 antibodies, and anti-GFP antibodies, respectively. (B) cdc25-22 cells were synchronized by arrest-and-release method. Plots shows the percentage of ethanol and BFA-treated cells with two nuclei (○), postanaphase microtubule arrays (PAA, ▵), and medial Cps1p rings (●) at times indicated after release to the permissive temperature. (C) Images of cells at different stages of cell cycle as indicated by the time after release from cdc25-22 block-and-release (as in B) that were stained for DNA, microtubules (MT), and Cps1p with DAPI, TAT1 antibodies, and anti-Cps1p antibodies, respectively.
The Actomyosin Ring Serves as a Spatial Landmark for the Assembly of Cps1p at the Division Site
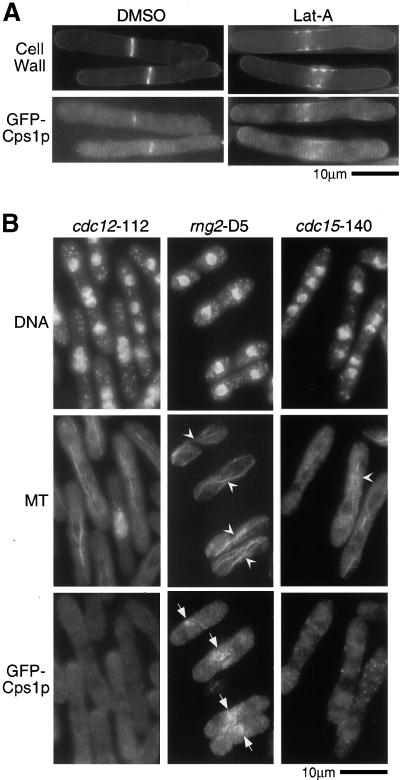
Having established that Cps1p assembles at the medial actomyosin ring using a polarized targeting mechanism, we sought to determine if F-actin and proteins important for actomyosin ring assembly were required for initial assembly of Cps1p to the division site. To test the role of F-actin in the medial assembly of Cps1p, cdc25-22 cells expressing GFP-Cps1p were synchronized by an arrest-and-release approach. On release to the permissive temperature, either LatA, an agent that sequesters monomeric actin and prevents actin polymerization, or DMSO (control) was added to the synchronous cell culture. Although cells treated with DMSO displayed prominent GFP-Cps1p rings (Figure 6A), medial Cps1p rings were not detected in cells treated with LatA. Instead, a medial band-like accumulation of GFP-Cps1p was detected in LatA-treated cells. Interestingly, improperly organized 1,3-β-glucan that somewhat overlapped with the medial accumulation of GFP-Cps1p was detected in LatA-treated cells (Figure 6A). Similar Cps1p localization was observed in cells treated with LatA when it was visualized using anti-Cps1p antibodies. Thus, an intact F-actin cytoskeleton is not essential for accumulation of Cps1p to the division site, but is important for the incorporation of Cps1p into a medial ring structure.
Figure 6.
The actomyosin ring serves as a spatial landmark for Cps1p assembly at the division site. (A) cdc25-22 cells expressing GFP-Cps1p were synchronized at 36°C, released to 24°C in medium containing either 100 μM latrunculin A (LatA) or DMSO (solvent control), and stained with aniline blue. (B) Cells bearing cdc12-112, rng2-D5, or cdc15-140 were grown at nonpermissive temperature (36°C) and stained for DNA, microtubules (MT), and GFP-Cps1p with DAPI, TAT1 antibodies, and anti-GFP antibodies, respectively. Arrowheads and arrows indicate cells with microtubule configuration resembling postanaphase array and GFP-Cps1p medial structures, respectively.
We then asked if the actomyosin ring acted as a spatial landmark for the assembly of Cps1p at the division site using a different approach. To this end, we analyzed the distribution of Cps1p in three different actomyosin ring function mutants, cdc12-112, rng2-D5, and cdc15-140. The cdc12-112 mutant fails to assemble actomyosin rings (Chang et al., 1996, 1997), whereas rng2-D5 mutants assemble improperly organized actomyosin rings and septa (Eng et al., 1998). The cdc15-140 mutant assembles medial actomyosin rings but fails to target F-actin patches and the septum-inducing kinase Sid2p to the division site (Balasubramanian et al., 1998; Sparks et al., 1999). Cells of the genotype cdc12-112, rng2-D5, and cdc15-140 were shifted to the restrictive temperature to allow expression of the mutant phenotype and were stained with antibodies against Cps1p. Cps1p was not detected at the division site in cdc12-112 and cdc15-140 cells with a postanaphase microtubular configuration (Figure 6B; postanaphase arrays marked with arrowheads). Interestingly, Cps1p was detected in improperly organized structures in the rng2-D5 mutant, consistent with the presence of aberrant actomyosin rings in this mutant (Figure 6B). Thus, we conclude that the actomyosin ring serves as a spatial landmark to target Cps1p to the division site and that Cdc15p is important for targeting Cps1p to the division site after assembly of the actomyosin ring.
F-actin–independent Maintenance of Cps1p at the Cell Division Site
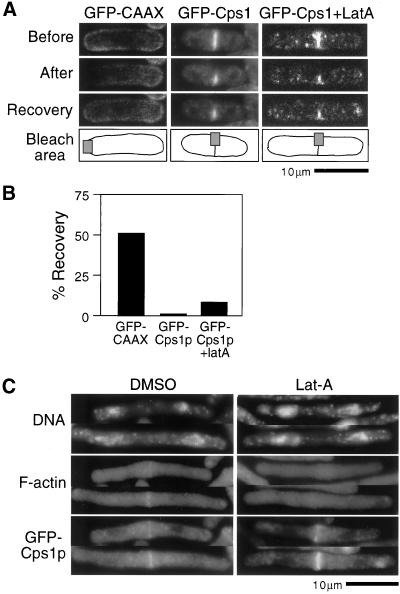
Fluorescence recovery after photobleaching (FRAP) experiments were carried out to study Cps1p dynamics and to find whether Cps1p was able to diffuse freely in the plasma membrane. In FRAP experiments, GFP-Cps1p was found to not diffuse in the medial region of the plasma membrane as evidenced by <1% of the fluorescence being recovered 2 min after photobleaching (Figure 7, A and B). In contrast, a nonspecific plasma membrane marker GFP-CAAX (the membrane localization signal from S. pombe cdc42 fused to GFP) diffused freely in the plasma membrane and recovered ∼50% of the fluorescence during the same time (Figure 7, A and B). To test if association with the actomyosin ring was responsible for the failure of GFP-Cps1p to diffuse along the plasma membrane, we sought to determine the level of recovery of GFP-Cps1p fluorescence in cells treated with LatA. In this analysis, we found that GFP-Cps1p recovered only modest levels (8%, compared with <1% in cells treated with DMSO) of fluorescence, suggesting that F-actin played a minor role in the maintenance of GFP-Cps1p at the division site (Figure 7, A and B). That F-actin played only a minor role in maintenance of the medial Cps1p ring was also established from the modest effect that was observed when cdc25-22 cells with preformed GFP-Cps1p rings were treated with LatA (Figure 7C). In these cells depleted of all detectable F-actin, GFP-Cps1p was still detected in a medial ring structure. We conclude that Cps1p does not diffuse appreciably along the long axis of the cell as well as laterally within the ring. We further conclude that F-actin–independent mechanisms are responsible for the maintenance of Cps1p at the cell division ring.
Figure 7.
Maintenance of Cps1p medial ring does not require F-actin function. (A) GFP fluorescence in cells expressing GFP-CAAX or GFP-Cps1p with or without LatA, photobleached in the area shown. Images were taken before, immediately after, and 2 min after photobleaching. (B) A plot shows the rate of recovery of GFP fluorescence 2 min after photobleaching. (C) cdc25-22 cells expressing GFP-Cps1p were grown at 36°C and released to 24°C. LatA or DMSO (control) was added into the cultures at the time when >30% cells contained GFP-Cps1p medial rings (∼1 h after release). Cells were stained for DNA, F-actin, and GFP-Cps1p with DAPI, rhodamine-conjugated phalloidin, and anti-GFP antibodies.
Microtubules Are Not Required for Assembly of Cps1p at the Division Site
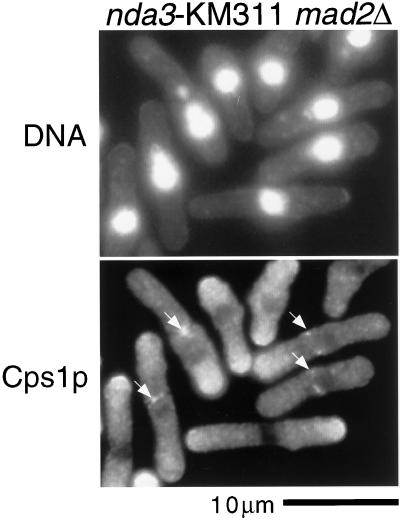
Cps1p assembles at the division site when cells assemble a postanaphase array of microtubules. In animal cells microtubules have been shown to be important for exocytosis during cytokinesis and cell division (Jesuthasan, 1998). We therefore assessed if Cps1p localization depended on microtubule integrity. Because the β-tubulin mutant nda3-KM311 is unable to execute mitosis owing to the activation of the spindle assembly checkpoint, we carried out the experiment in a nda3-KM311 mad2::ura4 strain in which the spindle assembly checkpoint is rendered inactive. Cells of the genotype nda3-KM311 mad2::ura4 were shifted to the restrictive temperature (19°C, to inactivate β-tubulin) and the localization of Cps1p was determined. Interestingly, we found that Cps1p assembled into normal looking contractile rings in these cells devoid of detectable microtubules (Figure 8). This experiment established that the postanaphase microtubule array was not important for Cps1p assembly at the division site.
Figure 8.
Microtubules are not required for Cps1p assembly to the division site. nda3-KM311 mad2::ura4 cells were shifted to nonpermissive temperature (19°C) for 4 h and stained for DNA and Cps1p with DAPI and anti-Cps1p antibodies. Arrows indicate positions of Cps1p rings.
The SIN Proteins Are Essential for Assembly of Cps1p to the Division Site
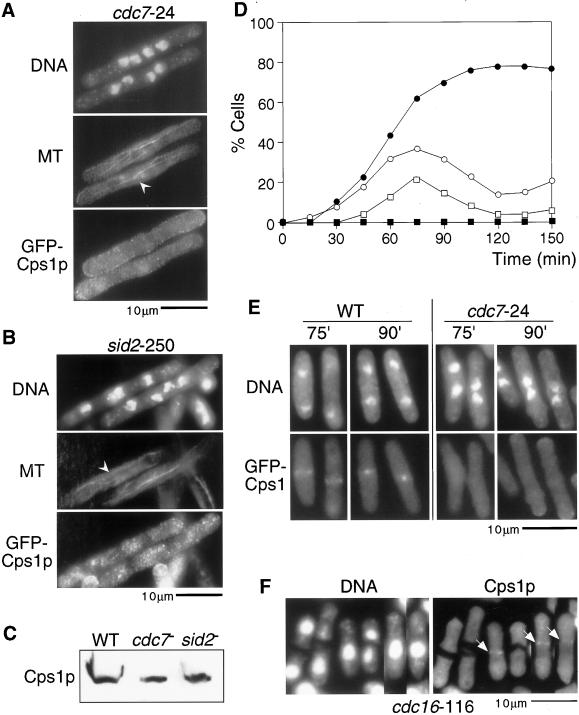
Work in S. pombe has identified a class of signaling molecules (collectively referred to as the SIN) that are essential for division septum formation after assembly of the actomyosin ring and accumulation of F-actin patches. Mutants in SIN components accumulate multiple nuclei and undergo normal actomyosin rearrangements through the cell cycle. SIN mutants display F-actin at the cell tips at interphase, and during mitosis they assemble medial actomyosin rings that disassemble rather than undergo constriction. Given that SIN mutants are defective in cytokinesis, we addressed if Cps1p localization to the division site depended on SIN function. To answer this question, SIN mutants cdc7-24 and sid2-250 expressing GFP-Cps1p were shifted to the restrictive temperature, fixed, and stained with antibodies against GFP and tubulin. Interestingly, we found that Cps1p was not detected at the division site in these heat-arrested mutants that appeared to have a postanaphase-like array (Figure 9, A and B; postanaphase-like array are marked with arrowheads and are not maintained in SIN mutants). We confirmed that Cps1p was stable in heat-arrested SIN mutants, establishing that the lack of detection of Cps1p at the division site in SIN mutants was not related to altered stability of Cps1p in these mutants (Figure 9C). We also confirmed that GFP-Cps1p localization was affected in a cdc7-24 mutant using synchronous cell cultures shifted to the restrictive temperature (Figure 9, D and E). Finally, Cps1p was also not detected at the division site in all other SIN mutants tested, namely cdc11-136, cdc14-118, sid1-239, spg1-106, and sid4-SA1. Thus, we conclude that the SIN signaling cascade is important for the assembly of Cps1p to the division site.
Figure 9.
The SIN proteins are essential for Cps1p assembly to the division site. GFP-Cps1p cells bearing either a cdc7-24 allele (A) or a sid2-250 (B) were grown at nonpermissive temperature (36°C) and stained for DNA, microtubules (MT), and GFP-Cps1p with DAPI, TAT1 antibodies, and anti-GFP antibodies. Arrowheads indicate cells with postanaphase-like microtubular arrangement. (C) Lysates from wild-type, cdc7-24, and sid2-250 cells were fractionated in SDS-PAGE gels and immunoblotted with anti-Cps1p antibodies. (D) Plot shows the percentage of cells containing two nuclei (○, ●), and GFP-Cps1p medial ring structures (□, ▪) in lactose gradient-synchronized wild-type cells (○, □) or cdc7-24 mutant cells (●, ▪) expressing GFP-Cps1p. (E) Images of lactose gradient-synchronized wild-type and cdc7-24 mutant cells expressing GFP-Cps1p. Although wild-type cells assembled a peak of GFP-Cps1p rings at 75–90 min after shift-up, no GFP-Cps1p rings were detected in cdc7-24 mutant cells after shift-up. (F) Cells bearing the cdc16-116 allele were grown at nonpermissive temperature and stained for DNA and Cps1 with DAPI and affinity-purified anti-Cps1p antibodies, respectively. Arrows indicate Cps1p rings.
To investigate a more direct role for the SIN in the localization of Cps1p, we asked if ectopic activation of the SIN in interphase cells allowed localization of Cps1p to the division site in interphase cells. Ectopic SIN activation was achieved by shifting cdc16-116 cells (Minet et al., 1979; Fankhauser et al., 1993; Fankhauser and Simanis, 1993) to the restrictive temperature. Cdc16p encodes a component of the GTPase-activating protein (GAP) complex for the SIN protein Spg1p, a rab-related GTPase (Furge et al., 1998). In cdc16-116 cells Spg1p remains in a constitutively GTP bound form, thereby constitutively activating the SIN molecules. We stained heat-arrested cdc16-116 cells with Cps1p antibodies. Interestingly, we found that Cps1p localized to the medial division site even in uninucleate cdc16-116 cells undergoing cytokinesis (Figure 9F). Collectively, these studies established that SIN activation is essential for assembly of Cps1p at the division site.
DISCUSSION
Assembly of Cps1p at the Division Site Late in Anaphase
In this study we have investigated the mechanism of assembly of the 1,3-β-glucan synthase subunit Cps1p to the division site in the fission yeast Schizosaccharomyces pombe. Using cell fractionation methods, we have shown that Cps1p is indeed an integral membrane protein. Interestingly, Cps1p was found to be insoluble in cold nonionic detergents such as Triton X-100. Studies in vertebrate cells and budding yeast have shown that Triton X-100–resistant membranes likely form microdomains in the plasma membrane and are insoluble in nonionic detergents owing to the increased concentration of cholesterol (or ergosterol in yeast) and sphingolipids (Brown and London 1998; Oliferenko et al., 1999; Bagnat et al., 2000). Future studies should address if Cps1p is indeed associated sterol-rich membranes and if lipid raft-like microdomains exist in S. pombe.
Cps1p localizes to the division site as a contractile-medial ring during cytokinesis. This result was obtained from two independent approaches: immunofluorescence microscopy using Cps1p-specific antibodies and GFP epifluorescence of a strain expressing a functional GFP-Cps1p fusion protein. Interestingly, although the actomyosin ring assembled early in mitosis (in cells with short spindles), Cps1p assembled late in mitosis in cells containing a postanaphase configuration of microtubules. The Cps1p-containing actomyosin ring constricted upon completion of mitosis. The presence of Cps1p in a constricting ring suggests that the division septum is actively assembled at the constricting front of the actomyosin ring. It is likely that the primary septum composed of 1,3-α-glucan and 1,3-β-glucan is assembled by the concerted action of Cps1p and Mok1p/Ags1p, which encodes a 1,3-α-glucan synthase (Hochstenbach et al., 1998; Katayama et al., 1999). Unlike Mok1p/Ags1p, Cps1p is not detected in disk-like structures after assembly of the primary septum. Thus, consistent with recent studies of distribution of cell wall sugars by electron microscopy (Humbel et al., 2001), Cps1p might not play a major role in assembly of the secondary septa.
We have shown that the levels of Cps1p do not vary significantly throughout the cell cycle. Interestingly, however, Cps1p is detected in distinct cellular structures only late in anaphase. One possibility is that Cps1p is synthesized and is retained in the pre-Golgi compartments and perhaps some modification of Cps1p allows its localization to the cell division ring late in anaphase. Alternatively, Cps1p might be delivered to the entire plasma membrane in interphase and might be too diffuse to detect by fluorescence microscopic methods. The protein in the plasma membrane might then be taken up by endocytosis and redelivered to the division site at anaphase when proteins important for assembly of Cps1p have themselves assembled at the division site. Such a mechanism has been shown to operate in the localization of the cell wall biosynthetic enzyme Chs3p, a chitin synthase, in budding yeast (Ziman et al., 1998). Future studies should test these possibilities.
A block to BFA-dependent exocytosis, in addition to preventing localization of Cps1p, also prevented the cells from entering a second round of mitosis. Thus, these cells were arrested with two interphase nuclei and a postanaphase array of microtubules. Previous studies have shown that an F-actin–dependent checkpoint monitors completion of cytokinesis (Le Goff et al., 1999b; Liu et al., 2000; Trautmann et al., 2001). It remains to be determined whether the cytokinesis checkpoint is also activated in BFA-treated cells.
F-actin–dependent Assembly and F-actin–independent Maintenance of Cps1p at the Cell Division Ring
Studies in animal cells have shown that exocytic events during mitosis and cytokinesis are dependent on an intact microtubule cytoskeleton (Jesuthasan, 1998). Interestingly, we find that an intact microtubule cytoskeleton is dispensable for all events of cytokinesis. Actomyosin ring assembly is known to proceed in the absence of detectable microtubules (Chang et al., 1996; Naqvi et al., 1999; Motegi et al., 2000). In this study, using a β-tubulin mutant we have shown that the localization of Cps1p and division septum assembly are independent of microtubule function. Because cells with no detectable microtubules are still capable of assembling division septa, we conclude that secretion during cytokinesis is either dependent on a very small degree of microtubule function or is completely independent of it.
Through the analysis of actomyosin ring assembly mutants and cells treated with the F-actin assembly inhibitor LatA (Ayscough et al., 1997), we have identified F-actin–dependent and F-actin–independent steps in the assembly and maintenance of Cps1p at the division site. The initial accumulation of Cps1p as a broad medial band is independent of F-actin function. Interestingly, in these cells 1,3-β-glucan was detected as a series of spots and patches in the region of the broad band containing Cps1p. Thus, assembly of 1,3-β-glucan polymers can proceed in the absence of F-actin function. After initial assembly as a broad band, organization of Cps1p into a medial ring structure that overlies the actomyosin ring requires the function of an intact F-actin cytoskeleton and products of the actomyosin ring assembly genes. These observations establish the fact that the actomyosin ring serves as a spatial landmark for the incorporation of Cps1p into a medial ring structure, rather than in targeting of Cps1p to the medial region of the cell. After assembly of the actomyosin ring, Cdc15p, an SH3 domain-containing component of the actomyosin ring (Fankhauser et al., 1995) is essential for the loading of Cps1p to the medial cell division ring. Cdc15p is related to proteins such as PACSIN, a protein important for membrane transport events (Lippincott and Li, 2000). An attractive possibility is that Cdc15p function is required to target secretion during cytokinesis.
Fluorescence recovery after photobleaching experiments have shown that Cps1p does not diffuse freely in the plasma membrane. It was therefore possible that interaction with the F-actin and the actomyosin ring might account for the maintenance of Cps1p at the cell division ring. Interestingly, normal looking Cps1p rings were retained in cells with preformed Cps1p rings treated with LatA. Furthermore, only a very modest level of fluorescence was recovered when cells treated with LatA were photobleached. Thus, we conclude that an F-actin–independent mechanism is important in retaining Cps1p in the cell division ring, after assembly of Cps1p into the cell division ring. We have shown that Cps1p is a component of membranes that are insoluble in cold Triton X-100, a property shared by proteins that localize to sterol-rich membrane domains (Brown and London, 1998; Oliferenko et al., 1999; Bagnat et al., 2000). An attractive possibility is that membrane sterols play an important role in the maintenance of Cps1p at the division site, as has been shown in other organisms. However, mutants defective in sterol biosynthesis are currently unavailable in S. pombe, and these will be essential to firmly establish if the maintenance of Cps1p is sterol dependent.
The SIN Proteins Are Important for Localization of Cps1p to the Cell Division Site
Work in S. pombe has identified a class of signaling molecules (referred to as the SIN) that are important for the initiation of the division septum formation after assembly of the actomyosin ring (Le Goff et al., 1999a; Balasubramanian et al., 2000; McCollum and Gould 2001). The SIN includes protein kinases Cdc7p, Sid1p, and Sid2p (Fankhauser and Simanis 1994; Sparks et al., 1999; Guertin et al., 2000), and three novel proteins, Sid4p, Mob1p, and Cdc14p (Fankhauser and Simanis 1993; Chang and Gould 2000; Hou et al., 2000; Salimova et al., 2000). All components of the SIN cascade localize to the spindle pole bodies and are thought to regulate the timing of cell division relative to progression through anaphase. In addition, two of the SIN members, Mob1p and Sid2p, also localize to the division site during cytokinesis (Sparks et al., 1999; Hou et al., 2000; Salimova et al., 2000). However, the mechanism by which the SIN signaling pathway regulates cell division had remained unclear. In this current study, we show that the localization of Cps1p, a protein essential for division septum assembly is under control of the SIN cascade. Furthermore, we show that ectopic activation of SIN components through the use of the cdc16-116 mutant (which maintains Spg1p in its GTP bound active state) allows localization of Cps1p to the medial region in interphase cells. Thus, an intriguing possibility to have emerged from our studies is that the SIN signaling cascade might regulate cytokinesis by allowing the timely localization of Cps1p and other enzymes important for division septum assembly at the division site late in anaphase.
Concluding Remarks
In this study, we have characterized the mechanism of assembly of the integral membrane protein Cps1p to the division site in the fission yeast S. pombe. We believe that our studies might also have a bearing on understanding the mechanism of secretion during cytokinesis. We show that Cps1p might be a key target of the two major classes of cytokinesis regulatory proteins identified in S. pombe, the actomyosin ring assembly proteins and the SIN proteins. Although the actomyosin ring assembly proteins are responsible for the incorporation of Cps1p into a medially placed ring structure, the SIN components are important for the cell cycle–dependent localization of Cps1p. Finally, we have uncovered an F-actin and microtubule-independent mechanism of anchoring Cps1p to the division site, after its assembly into a medial ring structure. Future studies should investigate the molecular mechanism of retention of Cps1p at the division site.
ACKNOWLEDGMENTS
The authors thank Prof. Nam-Hai Chua for several insightful discussions on this work; Desmond Kumar for guidance on the confocal microscope usage and Dr. K. Gull for TAT1 antibodies; and Drs. Alan Munn, Robert Yang, and all members of the Institute of Molecular Agrobiology yeast laboratories, and in particular Dr. Ventris D'souza and Ms. Srividya Rajagopalan, for discussion, advice, and critical reading of the manuscript. This work was supported by research funds from the National Science and Technology Board, Singapore to M.K.B.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0581. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0581.
REFERENCES
- Arellano M, Duran A, Perez P. Rho 1 GTPase activates the (1–3)beta-d-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, McCollum D, Chang L, Wong KC, Naqvi NI, He X, Sazer S, Gould KL. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, McCollum D, Gould KL. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, McCollum D, Surana U. Tying the knot: linking cytokinesis to the nuclear cycle. J Cell Sci. 2000;113:1503–1513. doi: 10.1242/jcs.113.9.1503. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Nurse P. How fission yeast fission in the middle. Cell. 1996;84:191–194. doi: 10.1016/s0092-8674(00)80973-3. [DOI] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Chang L, Gould KL. Sid4p is required to localize components of the septation initiation pathway to the spindle pole body in fission yeast. Proc Natl Acad Sci USA. 2000;97:5249–5254. doi: 10.1073/pnas.97.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell TG, Hajibagheri MA, Ayscough K, Pierce M, Warren G. Localization of an alpha 1,2 galactosyltransferase activity to the Golgi apparatus of Schizosaccharomyces pombe. Mol Biol Cell. 1994;5:519–528. doi: 10.1091/mbc.5.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J, Drgon T, Tanaka K, Kollar R, Chen GC, Ford RA, Chan CS, Takai Y, Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Carr AM. Analysis of radiation-sensitive mutants of fission yeast. Methods Enzymol. 1997;283:471–494. doi: 10.1016/s0076-6879(97)83038-8. [DOI] [PubMed] [Google Scholar]
- Eng K, Naqvi NI, Wong KC, Balasubramanian MK. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr Biol. 1998;8:611–621. doi: 10.1016/s0960-9822(98)70248-9. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Marks J, Reymond A, Simanis V. The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 1993;12:2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol Biol Cell. 1993;4:531–539. doi: 10.1091/mbc.4.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge KA, Wong K, Armstrong J, Balasubramanian M, Albright CF. Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr Biol. 1998;8:947–954. doi: 10.1016/s0960-9822(98)70394-x. [DOI] [PubMed] [Google Scholar]
- Gerisch G, Weber I. Cytokinesis without myosin II. Curr Opin Cell Biol. 2000;12:126–132. doi: 10.1016/s0955-0674(99)00066-6. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (2001). Animal cell cytokinesis. Annu. Rev. Cell. Dev. Biol. 17. [DOI] [PubMed]
- Guertin DA, Chang L, Irshad F, Gould KL, McCollum D. The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 2000;19:1803–1815. doi: 10.1093/emboj/19.8.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins HA, Page N, Schenkman LR, De Virgilio C, Shaw S, Bussey H, Pringle JR. Bud8p and bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol Biol Cell. 2001;12:2497–2518. doi: 10.1091/mbc.12.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach F, Klis FM, van den Ende H, van Donselaar E, Peters PJ, Klausner RD. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci USA. 1998;95:9161–9166. doi: 10.1073/pnas.95.16.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou MC, Salek J, McCollum D. Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol. 2000;10:619–622. doi: 10.1016/s0960-9822(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Humbel BM, Konomi M, Takagi T, Kamasawa N, Ishijima SA, Osumi M. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast. 2001;18:433–444. doi: 10.1002/yea.694. [DOI] [PubMed] [Google Scholar]
- Ishiguro J. Genetic control of fission yeast cell wall synthesis: the genes involved in wall biogenesis and their interactions in Schizosaccharomyces pombe. Genes Genet Syst. 1998;73:181–191. doi: 10.1266/ggs.73.181. [DOI] [PubMed] [Google Scholar]
- Ishiguro J, Saitou A, Duran A, Ribas JC. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuthasan S. Furrow-associated microtubule arrays are required for the cohesion of zebrafish blastomeres following cytokinesis. J Cell Sci. 1998;111:3695–3703. doi: 10.1242/jcs.111.24.3695. [DOI] [PubMed] [Google Scholar]
- Katayama S, Hirata D, Arellano M, Perez P, Toda T. Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol. 1999;144:1173–1186. doi: 10.1083/jcb.144.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff X, Utzig S, Simanis V. Controlling septation in fission yeast: finding the middle, and timing it right [In Process Citation] Curr Genet. 1999a;35:571–584. doi: 10.1007/s002940050455. [DOI] [PubMed] [Google Scholar]
- Le Goff X, Woollard A, Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol Gen Genet. 1999b;262:163–172. doi: 10.1007/s004380051071. [DOI] [PubMed] [Google Scholar]
- Lippincott J, Li R. Involvement of PCH family proteins in cytokinesis and actin distribution. Microsc Res Technol. 2000;49:168–172. doi: 10.1002/(SICI)1097-0029(20000415)49:2<168::AID-JEMT9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Balasubramanian MK. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J Cell Sci. 2000;113:1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, McCollum D, Balasubramanian MK. Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics. 1999;153:1193–1203. doi: 10.1093/genetics/153.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D, Gould KL. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11:89–95. doi: 10.1016/s0962-8924(00)01901-2. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Johnson DI. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M, Nurse P, Thuriaux P, Mitchison JM. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1979;137:440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F, Nakano K, Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J Cell Sci. 2000;113:1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Python CP, Inoue SB, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin DE, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Salimova E, Sohrmann M, Fournier N, Simanis V. The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signaling the onset of septum formation. J Cell Sci. 2000;113:1695–1704. doi: 10.1242/jcs.113.10.1695. [DOI] [PubMed] [Google Scholar]
- Shematek EM, Braatz JA, Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980;255:888–894. [PubMed] [Google Scholar]
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CA, Morphew M, McCollum D. Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J Cell Biol. 1999;146:777–790. doi: 10.1083/jcb.146.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann S, Wolfe BA, Jorgensen P, Tyers M, Gould KL, McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]