Abstract
Introduction: There have been disputes in the association between angiotensin receptor blockers (ARB) and the incidence of lung cancer. Our meta-analysis reevaluated this problem from the perspectives of race, age, drug type, comparison objects and smoking. Method: We used the following databases to carry out our literature search: Pubmed, Medline, Cochrane Library, and Ovid (From 1 January 2020 to 28 November 2021). The correlation between ARBs and the incidence rate of lung cancer was calculated by risk ratios (RRs). Confidence intervals were selected with 95% confidence intervals. Results: A total of 10 randomized controlled trials (RCTs), 18 retrospective studies and 3 case-control studies were found to satisfy the inclusion criteria. The use of ARB drugs reduced the incidence of lung cancer. The pooled results of 10 retrospective studies revealed a decreased lung cancer incidence in patients treated with ARBs, especially in patients using Valsartan. A significantly lower lung cancer incidence was found in the ARB drugs than in calcium channel blockers (CCBs) and angiotensin-converting enzyme inhibitors (ACEIs). Lung cancer occurrence was lower in Asian-based studies, especially in Mongolian-dominated and Caucasian-dominated patient populations. No significant decrease in lung cancer occurrence was found in RCTs or in patients receiving telmisartan, losartan, candesartan, irbesartan, or other placebo or in American and European-dominated patient populations. Conclusion: Compared with ACEIs and CCBs, ARBs significantly reduce the risk of lung cancer, especially in Asian and Mongolian populations. Valsartan has the best effect in reducing the risk of lung cancer in ARB drugs.
Keywords: angiotensin receptor blockers (ARB), incidence of lung cancer, risk of lung cancer, Asian, Valsartan
1. Introduction
Hypertension and cancer are the two most important fatal diseases in the world. Angiotensin receptor blockers (ARBs) are antihypertensive drugs that have complex associations with the risk of cancer and are involved in the regulation of cancer. Angiotensin II (Ang II) plays an important role in tumorigenesis by stimulating cell angiogenesis. Therefore, ARBs that block angiotensin type 2 receptors (AT2) can reduce the risk of cancer [1]. Meanwhile, ARBs may also achieve tumor growth inhibition by inhibiting lymphatic vessel growth [2] and reversing cancer-induced immunosuppression [3].
The relationship between ARBs and cancer incidence is controversial. Many meta-analyses have addressed this problem; however, small sample sizes, long publishing dates, and contradictory results limit the credibility of these studies [4,5,6,7]. Based on the analysis of randomized experiments, Sipahi et al. and the ARB Trialists Collaboration group reached different conclusions that ARBs would increase the risk of lung cancer and ARBs had no relationship with the increase in cancer incidence, respectively [6,7]. ARBs may inhibit the growth of lung cancer cells by blocking the effect of Ang II on increasing cell membrane free calcium and activating the angiotensin peptide receptor. Therefore, it is of great importance to investigate the relationship between lung cancer incidence and ARBs from the perspective of lung cancer alone. On the other hand, recently published large retrospective cohort studies may change the results.
Based on these questions, our meta-analysis focused on the relationship between lung cancer and ARB drugs, as well as the influence of race, age, drug type, comparison objects, and smoking.
2. Materials and Methods
2.1. Search Strategy
We used the following database to carry out our literature search: Pubmed, Medline, Cochrane Library, and Ovid (from 1 January 2020 to 28 November 2021). The following keywords were used: (lung cancer) AND (ARB) OR (Angiotensin Receptor Blocker).
2.2. Inclusive and Exclusive Criteria
The included studies adhered to the following criteria: (1) patients: patients without lung cancer before taking antihypertensive drugs; (2) interventions: the control group did not take ARB drugs, and the experimental group took ARB drugs; (3) outcome indicators: incidence of lung cancer; (4) study type: randomized controlled study (RCT) or retrospective cohort study or case-controlled studies. If the same study population was used, all articles with incomplete relative data and earlier publication time were excluded, and the remaining one was included. Studies that met the following criteria were excluded in this meta-analysis: (1) letters, reviews, editorials, comments, animal experiments, and duplicated studies; (2) studies in which data on the incidence of lung cancer were not available; and (3) manuscripts written in Chinese.
2.3. Quality Evaluation and Statistical Analysis
We evaluated retrospective cohort studies and case reports using the Newcastle Ottawa scale (NOS) [8], and RCTs were evaluated using the Cochrane Risk Assessment Tool [9].
Stata 14.0 (Stata Corporation, college station, TX, USA) and Review Manager (Cochrane Collaboration, Oxford, UK) were selected to perform analyses. The relationship between ARB drugs and lung cancer incidence was calculated as risk ratios (RRs) with 95% confidence intervals (95% CI).
Heterogeneity was evaluated using the I-squared (I2) test. An I2 > 50% was considered to indicate significant heterogeneity, and further analysis used the random-effects model. I2</=50% indicated acceptable heterogeneity, and a fixed-effects model was used for further analysis. If significant heterogeneity existed, sensitivity analyses were used to help determine which studies had the greatest potential impact. Heterogeneity was explained by subgroup analysis. Potential publication bias was detected using the Duval trim-and-fill method [10]. A symmetrical image indicated no publication bias. Otherwise, publication bias existed.
3. Results
3.1. Literature Selection Results and Characteristics of the Included Studies
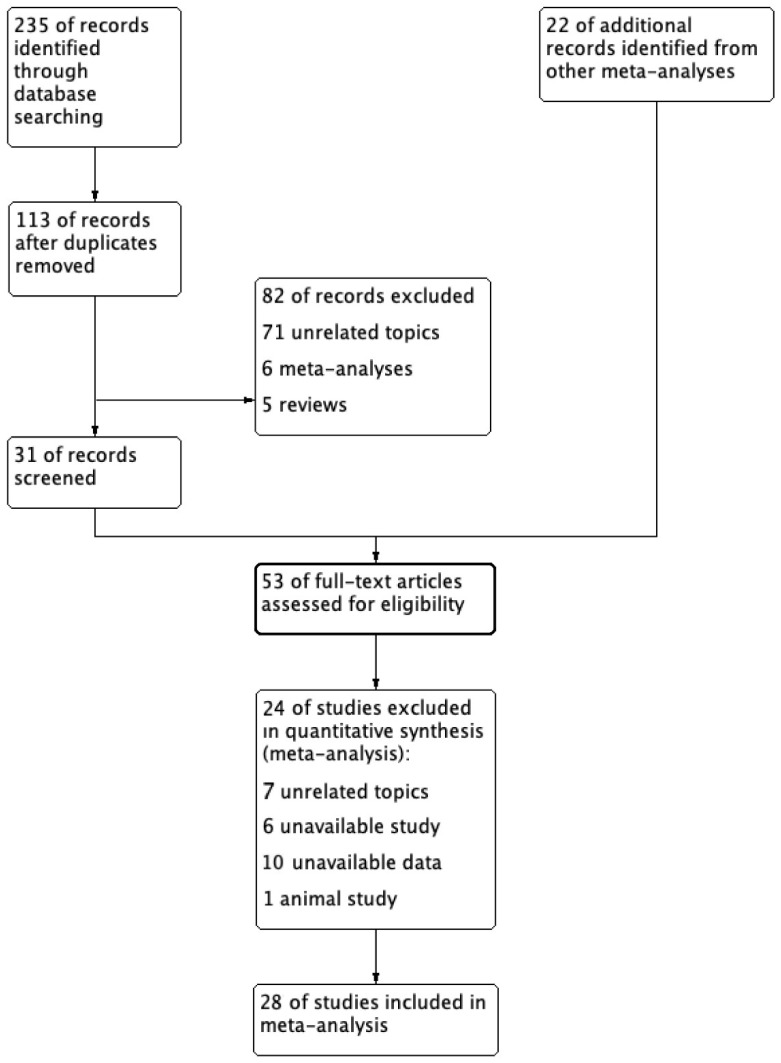
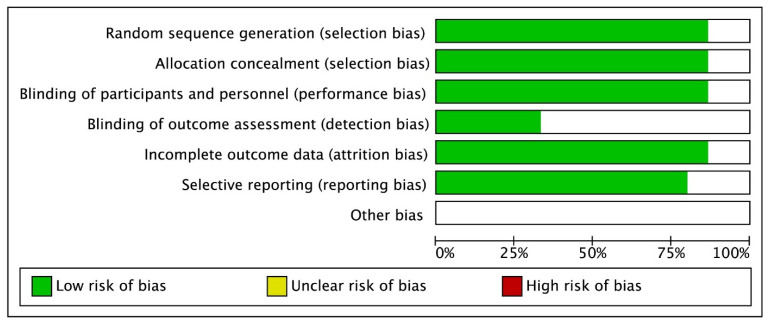
A total of 235 studies selected from database searching and 22 studies from other meta-analyses were included in the initial screening. Two reviewers screened all studies independently (Figure 1). After screening duplicated literature and reviewing abstracts and full texts, 28 studies were included in this meta-analysis. There were 10 retrospective cohort studies, 15 RCTs, and 3 case-controlled studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Among them, Jung et al. [11] included two cohort study populations, which were included. The characteristics of the studies are summarized in Table 1. Retrospective studies were evaluated using the NOS (Table 1). Bias assessment for the ten RCTs was performed using the Cochrane collaboration tool (Figure 2).
Figure 1.
Document search process.
Table 1.
The characteristics of the included studies.
| Author/Years | Study Type | Main Race | Continent | Mean Age | Study Drug | Comparison | QE |
|---|---|---|---|---|---|---|---|
| Jung 2021 (entire) [11] | RS | Yellow | Asia | 56.9 | NA | ACEI | 6 |
| Jung 2021 (new) [11] | RS | Yellow | Asia | 56.5 | NA | ACEI | 6 |
| Kumar 2021 [12] | RS | Yellow | Asia | 49.6 | NA | ACEI | 5 |
| Lin2020 [13] | RS | Yellow | Asia | 58.9 | NA | ACEI | 8 |
| Moon2020 [14] | RS | Yellow | Asia | NA | NA | CCB | 8 |
| Bhaskaran 2012 [15] | RS | White | Europe | 64 | NA | ACEI | 8 |
| Huang 2011 [16] | RS | Yellow | Asia | 58.5 | NA | non-ARB | 5 |
| Rao 2013 [17] | RS | White | America | 63 | NA | non-ARB | 8 |
| wang 2013 [18] | RS | Yellow | Asia | 62 | NA | non-ARB | 7 |
| Tascilar2016 [19] | RS | White | America | 62.5 | Telmisartan | Other ARBs | 7 |
| Pasternak2011 [20] | RS | White | Europe | 64.3 | NA | ACEI | 8 |
| ONTARGET 2008 [21] | RCT | White | America | 66.4 | Telmisartan | Ramipril | - |
| TRANSCEND 2008 [22] | RCT | White | America | 66.9 | Telmisartan | Placebo | - |
| PRoFESS 2008 [23] | RCT | White | America | 66.1 | Telmisartan | Placebo | - |
| ACTIVE 1 2011 [24] | RCT | White | America | 69.5 | Irbesartan | Placebo | - |
| I-PRESERVE 2008 [25] | RCT | White | America | 72 | Irbesartan | Placebo | - |
| IDNT 2001 [26] | RCT | White | America | 59.3 | Irbesartan | Placebo | - |
| Val-HeFT 2001 [27] | RCT | White | America | 62.7 | Valsartan | Placebo | - |
| VALIANT 2003 [28] | RCT | White | America | 64.8 | Valsartan | captopril | - |
| VALUE 2004 [29] | RCT | White | America | 67.3 | Valsartan | Amlodipine | - |
| NAVIGATOR 2010 [30] | RCT | White | America | 63.7 | Valsartan | Placebo | - |
| CHARM-Overall 2003 [31] | RCT | White | America | 65.9 | Candesartan | Placebo | - |
| TROPHY 2006 [32] | RCT | NA | America | 48.5 | Candesartan | Placebo | - |
| DIRECT (all) 2008 [33,34] | RCT | NA | America | NA | Candesartan | Placebo | - |
| SCOP 2003 [35] | RCT | White | America | 76.4 | Candesartan | Placebo | - |
| LIFE 2002 [36] | RCT | White | America | 66.9 | Losartan | Atenolol | - |
| Hallas 2012 [37] | CS | White | Europe | NA | NA | non-ARB | 6 |
| Azoulay 2012 [38] | CS | White | America | 72.4 | NA | non-ARB | 8 |
| Li 2021 [39] | CS | Yellow | Asia | NA | NA | Non-ARB | 8 |
Abbreviation: RS: retrospective study; RCT: randomized-controlled study; CS: case report. QE: quality evaluation. NA: not acquired.
Figure 2.
Quality Evaluation Results of RCTs.
3.2. Overall Analysis
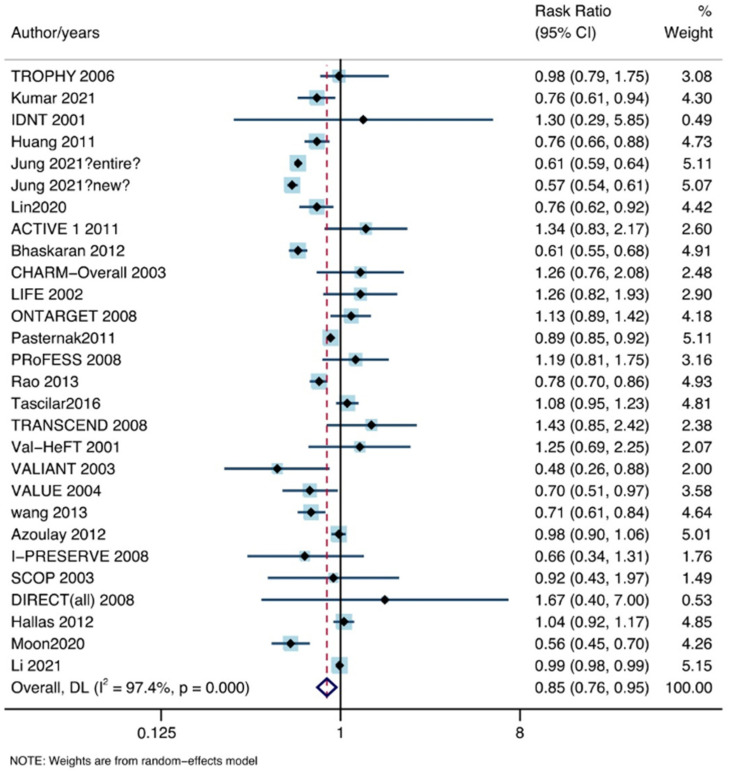
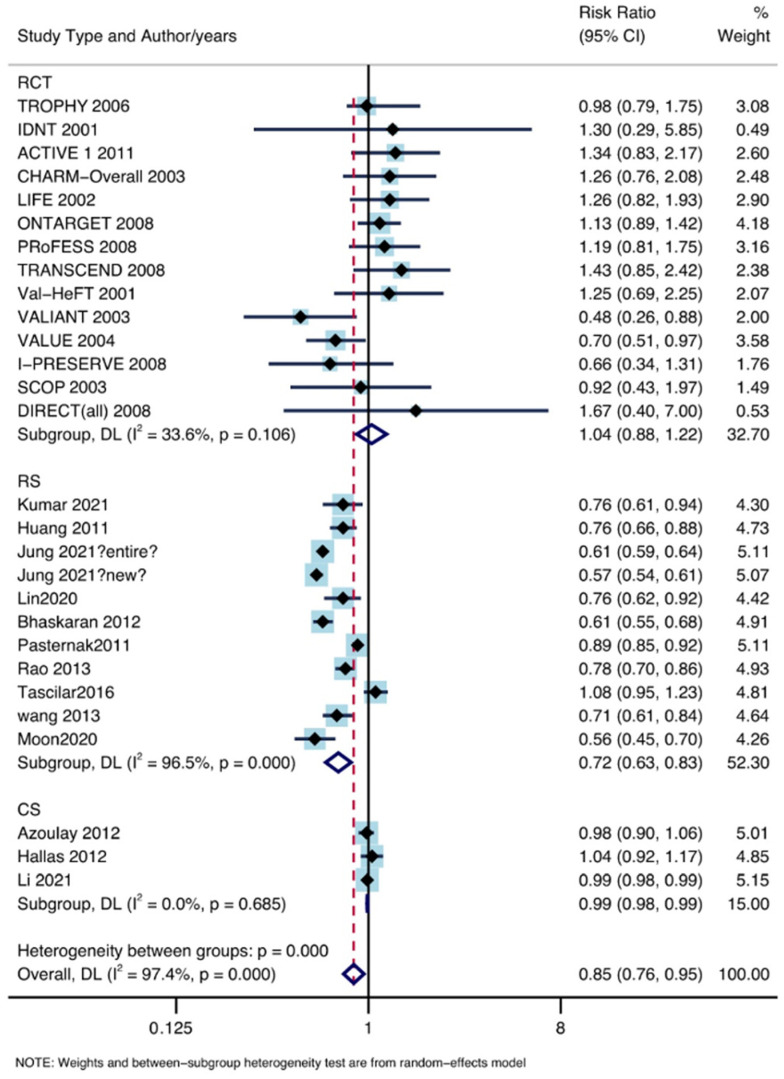
In total, 28 studies, including 6,301,712 patients, satisfied the inclusion criteria. The use of ARB drugs reduced the incidence of lung cancer (RR: 0.85, 95% CI lower: 0.76, 95% CI upper: 0.95) under the random effects model (I2 = 97.4%, P = 0.00; Figure 3).
Figure 3.
The association between ARB drugs and incidence of lung cancer [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
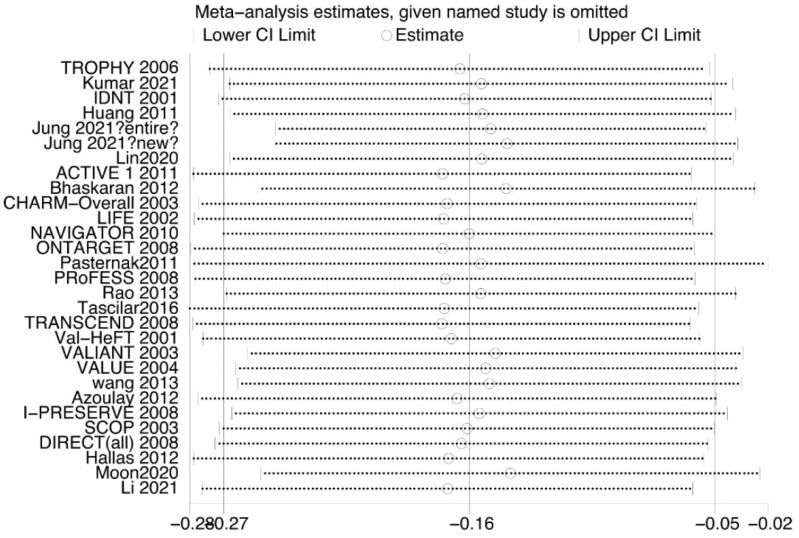
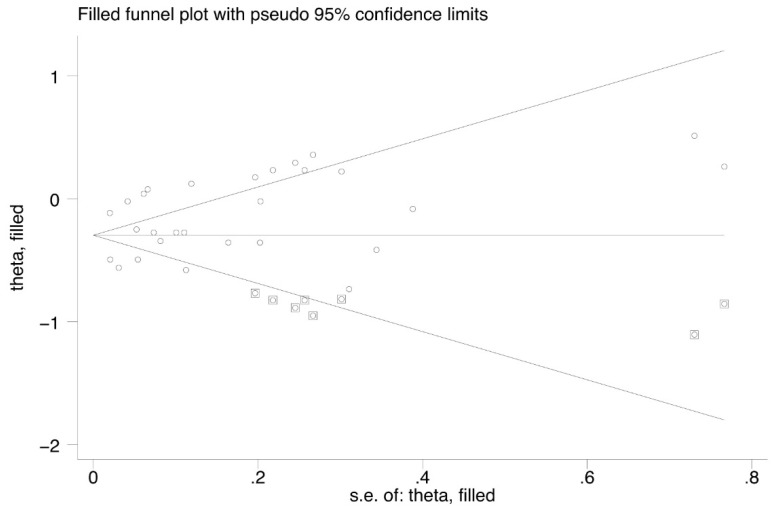
Our sensitivity analysis (Figure 4) showed that Jung et al. and Pasternak et al. significantly affect the heterogeneity. The nonparametric trim-and-fill method suggested the existence of publication bias (Figure 5).
Figure 4.
Sensitivity analysis of all studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Figure 5.
Funnel plot of nonparametric trim-and-fill method.
3.3. Subgroup Analysis by Study Type
As shown in Figure 6, 10 retrospective studies, including 5,453,716 patients; 14 randomized-controlled studies, including 126,005 patients; and 3 case reports, including 712,798 patients, reported the lung cancer incidence rate in patients using ARB drugs. The pooled results of retrospective studies revealed a reduced incidence of lung cancer in patients using ARB drugs (RR: 0.72, 95% CI lower: 0.63, 95% CI upper: 0.83). Significant heterogeneity was found (I2 = 96.5%, P = 0.00). A decreased incidence of lung cancer was found in CSs (RR: 0.99, 95% CI lower: 0.98, 95% CI upper: 0.99; I2 = 0%, P = 0.685). No significantly decreased incidence of lung cancer was found in RCTs (RR: 1.04, 95% CI lower: 0.88, 95% CI upper: 1.22; I2 = 33.6%, P = 0.106).
Figure 6.
Subgroup analysis by study type [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
3.4. Subgroup Analysis According to ARB Drugs
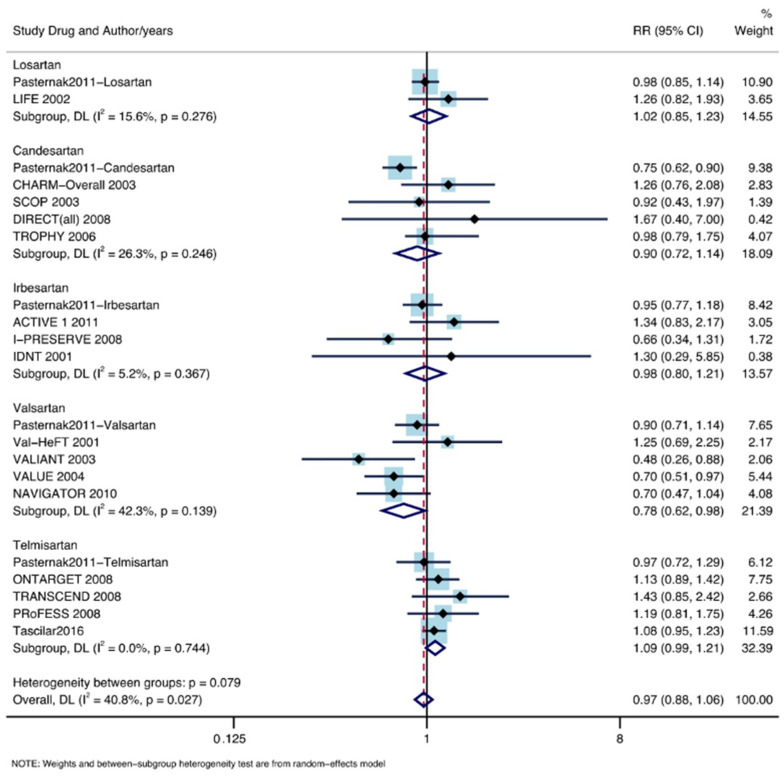
Of the patients included, 44,025 took Valsartan, 111,799 took telmisartan, 9193 took losartan, 18,008 took candesartan, and 14,284 took irbesartan. A significantly decreased lung cancer occurrence was found in patients treated with Valsartan (RR: 0.78, 95% CI lower: 0.62, 95% CI upper: 0.98; I2 = 42.3%, P = 0.139). No significant decline in the incidence of lung cancer was found in patients taking telmisartan (RR: 1.09, 95% CI lower: 0.99, 95% CI upper: 1.21; I2 = 0%, P = 0.744), losartan (RR: 1.02, 95% CI lower: 0.85, 95% CI upper: 1.23; I2 = 15.6%, P = 0.276), candesartan (RR: 0.90, 95% CI lower: 0.72, 95% CI upper: 1.14; I2 = 26.3%, P = 0.246), or irbesartan (RR: 0.98, 95% CI lower: 0.80, 95% CI upper: 1.21; I2 = 5.2%, P = 0.367). The forest plot is shown in Figure 7.
Figure 7.
Subgroup analysis by different ARB drugs [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
3.5. Subgroup Analysis by Comparison Object
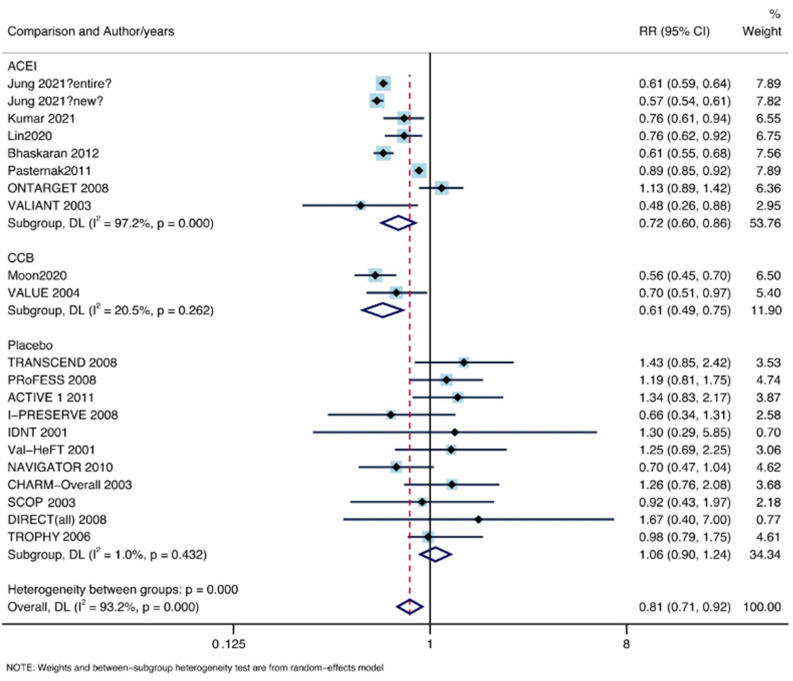
As shown in Figure 8, eight control groups, including 3,946,473 patients, received ACEIs; two control groups, including 74,207 patients, received CCBs; and 11 control groups of 72,187 patients received other types of placebo. A significant decrease in lung cancer was found in the ARBs compared with the ACEIs (RR: 0.72, 95% CI lower: 0.60, 95% CI upper: 0.86; I2 = 97.2%, P = 0.00) and CCBs (RR: 0.61, 95% CI lower: 0.49, 95% CI upper: 0.75; I2 = 20.5%, P = 0.262). No significantly decreased incidence of lung cancer was found in the other placebo group (RR: 1.06, 95% CI lower: 0.90, 95% CI upper: 1.24, I2 = 1%, P = 0.00).
Figure 8.
Subgroup analysis by comparison [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
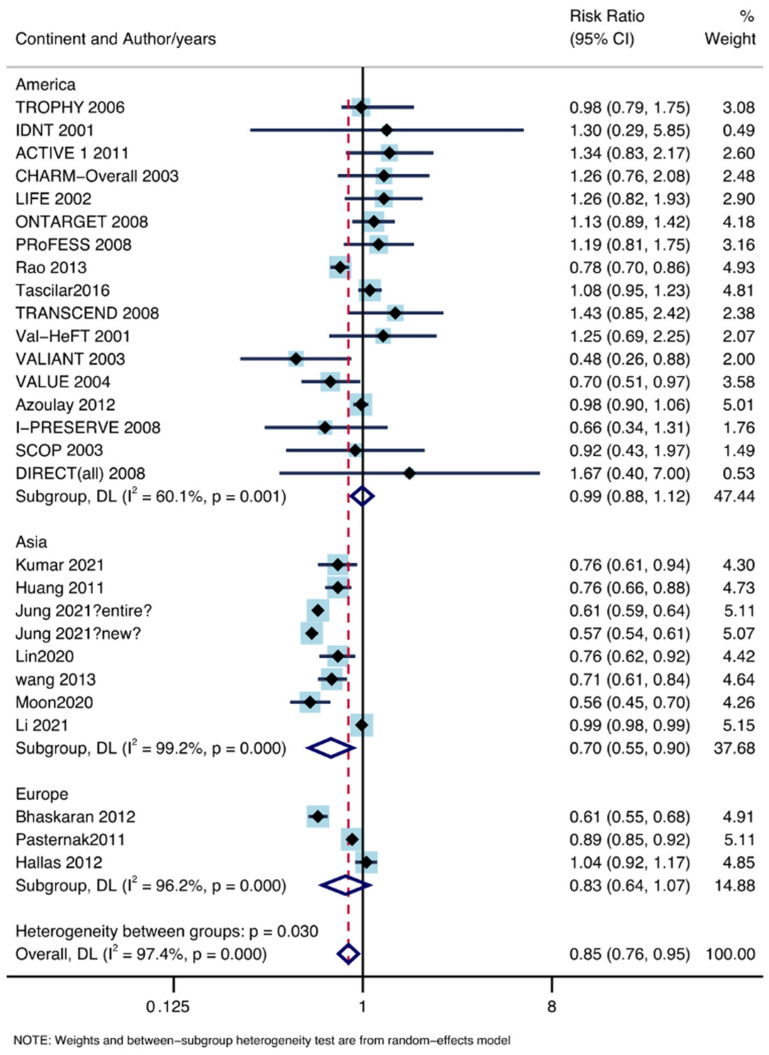
3.6. Subgroup Analysis by Continent
Eight Asian-based studies with 3,647,607 patients, 18 American-based studies with 1,878,434 patients, and three European-based studies with 775,671 patients were included. The RR value of Asian-based studies was 0.70, and the 95% CI was 0.55–0.90, which indicated decreased lung cancer incidence rate under the use of ARB drugs (I2 = 99.2%, P = 0.00). No meaningful result was obtained in the summary analysis of American-based studies (RR: 0.97, 95% CI lower: 0.87, 95% CI upper: 1.09; I2 = 59.8%, P = 0.001) and European-based studies (RR: 0.83, 95% CI lower: 0.64, 95% CI upper: 1.07; I2 = 96.2%, P = 0.00). The forest plot is shown in Figure 9.
Figure 9.
Subgroup analysis by continent [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
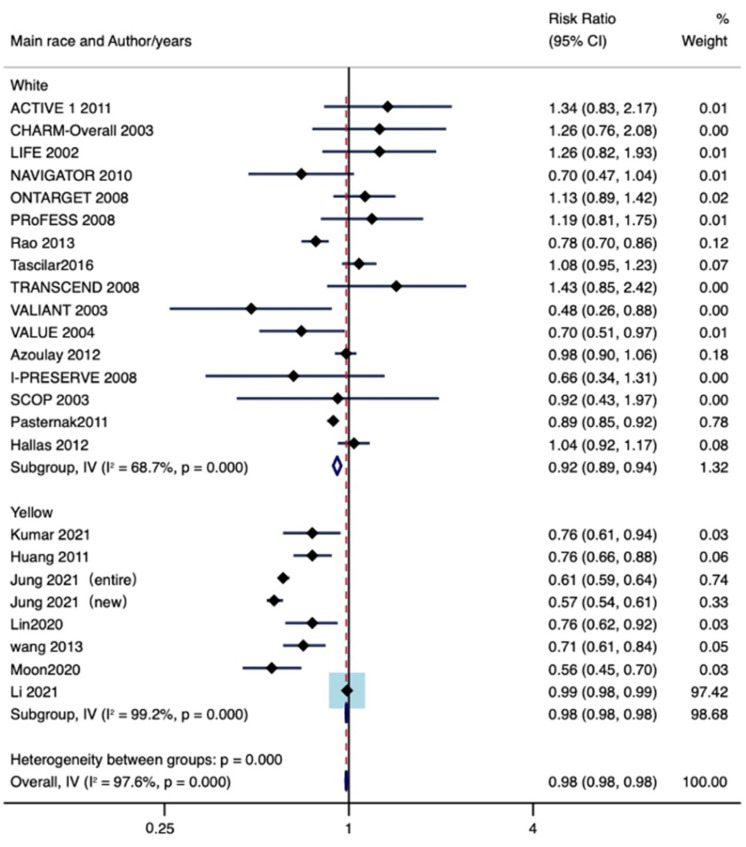
3.7. Subgroup Analysis by Main Race
The main race was defined as the race that accounts for more than half of the total study population. A total of 19 studies, including 2,648,100 patients, were mainly Caucasian, and seven studies, including 3,647,607 patients, were mainly Mongolian. As shown in Figure 10, lung cancer incidence decreased in Mongolian-dominated patient populations (RR: 0.98, 95% CI lower: 0.98, 95% CI upper: 0.98 I2 = 99.2%, P = 0.00) and in Caucasian-dominated patient populations (RR: 0.92, 95% CI lower: 0.89, 95% CI upper: 0.94; I2 = 68.7%, P = 0.00).
Figure 10.
Subgroup analysis by main race [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
4. Discussion
This meta-analysis examined a number of studies to explore the relationship between lung cancer incidence rate and ARB drug usage and compared different ARB drugs and other antihypertensive drugs in a categorical manner to explore this issue in a comprehensive way. We also conducted separate analyses according to population characteristics to explore the impact of patient characteristics on the association between drugs and disease.
To attempt to resolve the previous controversy [4,5,6,7], we included seven new large retrospective cohort studies on the basis of the original experimental studies. We found that ARB drugs are linked to a decreased incidence of lung cancer. Unlike other meta-analyses, this is the first to indicate that Valsartan significantly reduces the incidence of lung cancer in comparison with ACEIs and CCBs. A benefit in terms of reduced incidence of lung cancer was detected in Asians and Mongolians using ARB drugs for the first time.
This is the first study to link a single category of ARB drugs with the incidence of lung cancer and discuss the relationship between them. Although no significant difference was observed in all ARB drugs, the conclusion that Valsartan can reduce lung cancer risk by 22% is considerable. Sacubitril/Valsartan is an antihypertensive drug whose efficacy has been demonstrated in recent years. In comparison with enalapril, an angiotensin-converting enzyme (ACE) inhibitor, it has a better effect in reducing the incidence rate and mortality rate of heart failure patients with decreased ejection fraction [40,41,42]. However, few scholars have focused on the relationship between Valsartan and cancer, and additional clinical evidence is needed.
Compared to ACEIs and CCBs, ARB drugs were related to the reduction of lung cancer incidence. This is inconsistent with the results of the ARB trialist collaboration, which reported no significant difference between ACEIs and ARBs [7]. Unlike the statistical method of the ARB collaboration, the present ACEI control group only used ACEIs and excluded patients who used other drugs that may affect blood pressure at the same time. Therefore, the present results exclude the interference of other antihypertensive drugs and focus on the different effects of two antihypertensive drugs on lung cancer risk. In addition to the 15 RCTs included by the ARB collaboration, the present study included retrospective cohort studies published in recent years, which enriches the composition of our included articles and makes the results more reliable.
Our study indicates that the use of ARBs significantly reduces lung cancer occurrence in Asians. Similar results were reported in a meta-analysis published by Zhang et al. based on an Asian population of 298,000 individuals [43]. This study included a greater number of RCTs and retrospective cohort studies, with a total number of included people of 6 million, to make this result more convincing.
Many experiments have reported possible molecular links between ARBs and cancer. ARBs inhibit tumor cell growth by blocking signaling pathways downstream of angiotensin II Type 1 receptor (AT1R) [44,45,46], activating peroxisome proliferator-activated receptors(PPARs) [47,48,49], and inducing G0/G1 cell cycle arrest [50,51]. On the other hand, ARBs exert anti-tumor effects by increasing cancer cell autophagy through the induction of autophagy-related cell death and anti-metastatic activity [52], downregulating Bcl-2 and engaging in caspase-3-induced apoptosis pathways in cancer cells [53]. Of particular note, in lung adenocarcinoma cells, candesartan enhanced their susceptibility to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis [54]. However, the potential risk of ARBs on tumors has also been reported in certain studies. Telmisartan was found to promote tumor cell growth by improving tumor cell microcirculation [55], and cloxacin significantly increased cell adhesion and invasion on a type I collagen matrix [56]. Thus, our study demonstrates a favorable association between ARBs and lung cancer, and the molecular basis of this association remains to be investigated.
The nonparametric trim-and-fill method showed the existence of publication bias, and sensitivity analysis revealed that experiments by Jung et al. and Pasternak et al. may have caused significant heterogeneity. We reduced this bias by performing subgroup analysis to improve the reliability of the results. The present study had several limitations. The heterogeneity was high among the studies. Our sensitivity analysis showed that this could be attributed to studies by Jung et al. and Pasternak et al. However, we did not have access to raw data, which may result in data deviation. Finally, without the raw data, it was difficult to examine this issue at a comprehensive level.
5. Conclusions
The present meta-analysis indicates that, compared to ACEIs and CCBs, ARB drugs can significantly reduce the incidence of lung cancer. Among the ARBs analyzed, Valsartan was the most effective drug for reducing lung cancer incidence. ARB drugs can reduce the risk of lung cancer in Asian, Mongolian and Caucasian hypertensive patient populations.
Author Contributions
Methodology, L.W.; Resources, L.W.; Data management, Z.W., C.Y.; original draft preparation, Z.W.; Writing review and editor, W.L., B.W.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflict of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Escobar E., Rodríguez-Reyna T.S., Arrieta O., Sotelo J. Angiotensin II, cell proliferation and angiogenesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2004;2:385–399. doi: 10.2174/1570161043385556. [DOI] [PubMed] [Google Scholar]
- 2.Wang L., Cai S.R., Zhang C.H., He Y.L., Zhan W.H., Wu H., Peng J.-J. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers on lymphangiogenesis of gastric cancer in a nude mouse model. Chin. Med. J. 2008;121:2167–2171. doi: 10.1097/00029330-200811010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K., Kiniwa Y., Okuyama R. CCL5 production by fibroblasts through a local renin-angiotensin system in malignant melanoma affects tumor immune responses. J. Cancer Res. Clin. Oncol. 2021;147:1993–2001. doi: 10.1007/s00432-021-03612-8. [DOI] [PubMed] [Google Scholar]
- 4.Datzmann T., Fuchs S., Andree D., Hohenstein B., Schmitt J., Schindler C. Systematic review and meta-analysis of randomised controlled clinical trial evidence refutes relationship between pharmacotherapy with angiotensin-receptor blockers and an increased risk of cancer. Eur. J. Intern. Med. 2019;64:1–9. doi: 10.1016/j.ejim.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Mc Menamin Ú C., Murray L.J., Cantwell M.M., Hughes C.M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: A systematic review. Cancer Causes Control. 2012;23:221–230. doi: 10.1007/s10552-011-9881-x. [DOI] [PubMed] [Google Scholar]
- 6.Sipahi I., Debanne S.M., Rowland D.Y., Simon D.I., Fang J.C. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARB Trialists Collaboration Effects of telmisartan, irbesartan, Valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J. Hypertens. 2011;29:623–635. doi: 10.1097/HJH.0b013e328344a7de. [DOI] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 11.Jung M.H., Lee J.H., Lee C.J., Shin J.H., Kang S.H., Kwon C.H., Kim D., Kim W., Kim H.L., Kim H.M., et al. Effect of angiotensin receptor blockers on the development of cancer: A nationwide cohort study in Korea. J. Clin. Hypertens. 2021;23:879–887. doi: 10.1111/jch.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar P., Kumar V., Murlidhar F., Fatima A., Jahangir M., Khalid D., Memon M.K., Memon S., Kumar B. Comparison Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers for Incidence of Lung Cancer: A Retrospective Study. Cureus. 2021;13:e14788. doi: 10.7759/cureus.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S.Y., Lin C.L., Lin C.C., Hsu W.H., Lin C.D., Wang I.K., Hsu C.Y., Kao C.H. Association between Angiotensin-Converting Enzyme Inhibitors and Lung Cancer-A Nationwide, Population-Based, Propensity Score-Matched Cohort Study. Cancers. 2020;12:747. doi: 10.3390/cancers12030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon S., Lee H.Y., Jang J., Park S.K. Association Between Angiotensin II Receptor Blockers and the Risk of Lung Cancer Among Patients With Hypertension From the Korean National Health Insurance Service-National Health Screening Cohort. J. Prev. Med. Public Health. 2020;53:476–486. doi: 10.3961/jpmph.20.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaskaran K., Douglas I., Evans S., van Staa T., Smeeth L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.-C., Chan W.-L., Chen Y.-C., Chen T.-J., Lin S.-J., Chen J.-W., Leu H.-B. Angiotensin II Receptor Blockers and Risk of Cancer in Patients With Systemic Hypertension. Am. J. Cardiol. 2011;107:1028–1033. doi: 10.1016/j.amjcard.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Rao G.A., Mann J.R., Shoaibi A., Pai S.G., Bottai M., Sutton S.S., Haddock K.S., Bennett C.L., Hebert J.R. Angiotensin receptor blockers: Are they related to lung cancer? J. Hypertens. 2013;31:1669–1675. doi: 10.1097/HJH.0b013e3283621ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K.-L., Liu C.-J., Chao T.-F., Huang C.-M., Wu C.-H., Chen T.-J., Chiang C.-E. Long-term use of angiotensin II receptor blockers and risk of cancer: A population-based cohort analysis. Int. J. Cardiol. 2013;167:2162–2166. doi: 10.1016/j.ijcard.2012.05.096. [DOI] [PubMed] [Google Scholar]
- 19.Tascilar K., Azoulay L., Dell’Aniello S., Bartels D.B., Suissa S. The Use of Telmisartan and the Incidence of Cancer. Am. J. Hypertens. 2016;29:1358–1365. doi: 10.1093/ajh/hpw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasternak B., Svanström H., Callréus T., Melbye M., Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation. 2011;123:1729–1736. doi: 10.1161/CIRCULATIONAHA.110.007336. [DOI] [PubMed] [Google Scholar]
- 21.Heagerty A., Yusuf S., Teo K.K., Pogue J., Dyal L., Copland I., Schumacher H., Dagenais G., Sleight P., Anderson C. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S., Teo K., Anderson C., Pogue J., Dyal L., Copland I., Schumacher H., Dagenais G., Sleight P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S., Diener H.-C., Sacco R.L., Cotton D., Ôunpuu S., Lawton W.A., Palesch Y., Martin R.H., Albers G.W., Bath P., et al. Telmisartan to Prevent Recurrent Stroke and Cardiovascular Events. N. Engl. J. Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ACTIVE I Investigators. Yusuf S., Healey J.S., Pogue J., Chrolavicius S., Flather M., Hart R.G., Hohnloser S.H., Joyner C.D., A Pfeffer M., et al. Irbesartan in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011;364:928–938. doi: 10.1056/NEJMoa1008816. [DOI] [PubMed] [Google Scholar]
- 25.Massie B.M., Carson P.E., McMurray J.J., Komajda M., McKelvie R., Zile M.R., Anderson S., Donovan M., Iverson E., Staiger C., et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N. Engl. J. Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 26.Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B., Ritz E., Atkins R.C., Rohde R., Raz I., et al. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 27.Cohn J.N., Tognoni G. A randomized trial of the angiotensin-receptor blocker Valsartan in chronic heart failure. N. Engl. J. Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer M.A., McMurray J.J., Velazquez E.J., Rouleau J.-L., Køber L., Maggioni A.P., Solomon S.D., Swedberg K., Van de Werf F., White H., et al. Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular Dysfunction, or Both. N. Engl. J. Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 29.Julius S., E Kjeldsen S., Weber M., Brunner H.R., Ekman S., Hansson L., Hua T., Laragh J., McInnes G.T., Mitchell L., et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on Valsartan or amlodipine: The VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 30.Mcmurray J.J., Holman R.R., Haffner S.M., A Bethel M., Holzhauer B., A Hua T., Belenkov Y., Boolell M., Buse J.B., Buckley B.M., et al. Effect of Valsartan on the Incidence of Diabetes and Cardiovascular Events. N. Engl. J. Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 31.A Pfeffer M., Swedberg K., Granger C.B., Held P., Mcmurray J., Michelson E.L., Olofsson B., Östergren J., Yusuf S., CHARM Investigators and Committees Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/S0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 32.Julius S., Nesbitt S.D., Egan B.M., Weber M.A., Michelson E.L., Kaciroti N., Black H.R., Grimm R.H., Messerli F.H., Oparil S., et al. Feasibility of Treating Prehypertension with an Angiotensin-Receptor Blocker. N. Engl. J. Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi N., Porta M., Klein R., Orchard T., Fuller J., Parving H.H., Bilous R., Sjølie A.K. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 34.Sjølie A.K., Klein R., Porta M., Orchard T., Fuller J., Parving H.H., Bilous R., Chaturvedi N. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 35.Lithell H., Hansson L., Skoog I., Elmfeldt D., Hofman A., Olofsson B., Trenkwalder P., Zanchetti A. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double-blind intervention trial. J. Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Dahlöf B., Devereux R.B., E Kjeldsen S., Julius S., Beevers G., de Faire U., Fyhrquist F., Ibsen H., Kristiansson K., Lederballe-Pedersen O., et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 37.Hallas J., Christensen R., Andersen M., Friis S., Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: A population-based case-control study. Br. J. Clin. Pharmacol. 2012;74:180–188. doi: 10.1111/j.1365-2125.2012.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azoulay L., Assimes T.L., Yin H., Bartels D.B., Schiffrin E.L., Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS ONE. 2012;7:e50893. doi: 10.1371/journal.pone.0050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaft J.E., Rimner A., Weder W., Azzoli C.G., Kris M.G., Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat. Rev. Clin. Oncol. 2021;18:547–557. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Docherty K.F., Vaduganathan M., Solomon S.D., McMurray J.J.V. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years after PARADIGM-HF. JACC Heart Fail. 2020;8:800–810. doi: 10.1016/j.jchf.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mcmurray J.J.V., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K., et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 42.Seferovic J.P., Claggett B., Seidelmann S.B., Seely E.W., Packer M., Zile M., Rouleau J.L., Swedberg K., Lefkowitz M., Shi V.C., et al. Effect of sacubitril/Valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W., Liang Z., Li J., Cai S. Angiotensin receptor blockers use and the risk of lung cancer: A meta-analysis. J. Renin-Angiotensin-Aldosterone Syst. 2015;16:768–773. doi: 10.1177/1470320315607391. [DOI] [PubMed] [Google Scholar]
- 44.Uemura H., Ishiguro H., Nakaigawa N., Nagashima Y., Miyoshi Y., Fujinami K., Sakaguchi A., Kubota Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: A possibility of tyrosine kinase inhibitor of growth factor. Mol. Cancer Ther. 2003;2:1139–1147. [PubMed] [Google Scholar]
- 45.Ren H., Du N., Feng J., Hu L.-J., Sun X., Sun H.-B., Zhao Y., Yang Y.-P. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol. Rep. 2012;27:1893–1903. doi: 10.3892/or.2012.1720. [DOI] [PubMed] [Google Scholar]
- 46.Kosaka T., Miyajima A., Shirotake S., Kikuchi E., Hasegawa M., Mikami S., Oya M. Ets-1 and hypoxia inducible factor-1α inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2009;70:162–169. doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimura R., Funao K., Matsuyama M., Kawahito Y., Sano H., Chargui J., Touraine J.-L., Nakatani T. Telmisartan as a peroxisome proliferator-activated receptor-γ ligand is a new target in the treatment of human renal cell carcinoma. Mol. Med. Rep. 2009;2:193–198. doi: 10.3892/mmr_00000083. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura R., Funao K., Matsuyama M., Kawahito Y., Sano H., Chargui J., Touraine J.-L., Nakatani T. Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol. Rep. 1994;20:295–300. doi: 10.3892/or_00000006. [DOI] [PubMed] [Google Scholar]
- 49.Matsuyama M., Funao K., Kuratsukuri K., Tanaka T., Kawahito Y., Sano H., Chargui J., Touraine J.-L., Yoshimura N., Yoshimura R. Telmisartan inhibits human urological cancer cell growth through early apoptosis. Exp. Ther. Med. 2010;1:301–306. doi: 10.3892/etm_00000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujihara S., Morishita A., Ogawa K., Tadokoro T., Chiyo T., Kato K., Kobara H., Mori H., Iwama H., Masaki T. The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKα/mTOR pathway in vitro and in vivo. Oncotarget. 2016;8:8536–8549. doi: 10.18632/oncotarget.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samukawa E., Fujihara S., Oura K., Iwama H., Yamana Y., Tadokoro T., Chiyo T., Kobayashi K., Morishita A., Nakahara M., et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int. J. Oncol. 2017;51:1674–1684. doi: 10.3892/ijo.2017.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woo Y., Jung Y.J. Angiotensin II receptor blockers induce autophagy in prostate cancer cells. Oncol. Lett. 2017;13:3579–3585. doi: 10.3892/ol.2017.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Araújo Júnior R.F., Leitão Oliveira A.L., de Melo Silveira R.F., de Oliveira Rocha H.A., de França Cavalcanti P., de Araújo A.A. Telmisartan induces apoptosis and regulates Bcl-2 in human renal cancer cells. Exp. Biol. Med. 2015;240:34–44. doi: 10.1177/1535370214546267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasheduzzaman M., Park S.Y. Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp. Cell Res. 2018;368:126–135. doi: 10.1016/j.yexcr.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Wadsworth B.J., Cederberg R.A., Lee C.-M., Firmino N.S., Franks S.E., Pan J., Colpo N., Lin K.-S., Benard F., Bennewith K.L. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020;493:31–40. doi: 10.1016/j.canlet.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Olschewski D.N., Hofschröer V., Nielsen N., Seidler D.G., Schwab A., Stock C. The Angiotensin II Type 1 Receptor Antagonist Losartan Affects NHE1-Dependent Melanoma Cell Behavior. Cell. Physiol. Biochem. 2018;45:2560–2576. doi: 10.1159/000488274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.