Abstract
The actomyosin purse string is an evolutionarily conserved contractile structure that is involved in cytokinesis, morphogenesis, and wound healing. Recent studies suggested that an actomyosin purse string is crucial for the closure of wounds in single cells. In the present study, morphological and pharmacological methods were used to investigate the role of this structure in the closure of wounds in the peripheral cytoplasm of sea urchin coelomocytes. These discoidal shaped cells underwent a dramatic form of actin-based centripetal/retrograde flow and occasionally opened and closed spontaneous wounds in their lamellipodia. Fluorescent phalloidin staining indicated that a well defined fringe of actin filaments assembles from the margin of these holes, and drug studies with cytochalasin D and latrunculin A indicated that actin polymerization is required for wound closure. Additional evidence that actin polymerization is involved in wound closure was provided by the localization of components of the Arp2/3 complex to the wound margin. Significantly, myosin II immunolocalization demonstrated that it is not associated with wound margins despite being present in the perinuclear region. Pharmacological evidence for the lack of myosin II involvement in wound closure comes from experiments in which a microneedle was used to produce wounds in cells in which actomyosin contraction was inhibited by treatment with kinase inhibitors. Wounds produced in kinase inhibitor-treated cells closed in a manner similar to that seen with control cells. Taken together, our results suggest that an actomyosin purse string mechanism is not responsible for the closure of lamellar wounds in coelomocytes. We hypothesize that the wounds heal by means of a combination of the force produced by actin polymerization alone and centripetal flow. Interestingly, these cells did assemble an actomyosin structure around the margin of phagosome-like membrane invaginations, indicating that myosin is not simply excluded from the periphery by some general mechanism. The results indicate that the actomyosin purse string is not the only mechanism that can mediate wound closure in single cells.
INTRODUCTION
The ability to heal physical wounds is one of the processes fundamental to the proper maintenance of cell and tissue function. The closure of wounds in layers of cells, both in vitro and in vivo, has been generally attributed to the inward migration of cells at the wound margin and the development of a circumferential contractile entity composed of an actomyosin purse string (Martin and Lewis, 1992; Conrad et al., 1993; Bement et al., 1993; Brock et al., 1996; Danjo and Gipson, 1998). This purse string consists of actin filaments interacting with myosin II bipolar filaments and is similar to the contractile ring, which mediates cytokinesis (reviewed by Rappaport, 1996), and contractile stress fibers in cells (Kazuo et al., 1998). Experiments on wounded single cells (oocytes, eggs, and embryos) indicate that membrane resealing is mediated by calcium-dependent vesicle-vesicle fusion events (McNeil and Steinhardt, 1997; Terasaki et al., 1997), and the wound margin in the cortex is again closed by means of an actomyosin purse string (Bement et al., 1999). These latter experiments on Xenopus oocytes by Bement et al. (1999) were the first demonstration of a wound-associated actomyosin purse string in a single-cell system. Taken together, the results of numerous previous studies indicate that the actomyosin purse string is conserved both mechanistically—it is involved in a wide range of processes, including wound healing, cytokinesis, and morphogenesis—as well as evolutionarily—it has been demonstrated in organisms as divergent as yeast and humans (see review by Kiehart, 1999).
In the present study, we examined the wound healing process in another single-cell system, sea urchin coelomocytes. These terminally differentiated, discoidal shaped cells have an elaborate actin cytoskeleton that undergoes an exaggerated form of centripetal/retrograde flow (Henson et al., 1999). Occasionally, spontaneous wounds appear in the lamellipodia of these cells. These wounds manifest themselves as physical holes in the lamellipod that transverse through the cell membrane and connect to the surface of the substrate (a glass coverslip). Similar wounds can also be produced by mechanical manipulation with a microneedle. Coelomocytes have the ability to close substantial spontaneous and induced wounds, and we have used immunolocalization, pharmacological inhibition, and micromanipulation methods to study the mechanism underlying this wound closure event. Our results indicate that wounds in coelomocytes are closed by actin polymerization at the wound edge and that an actomyosin purse string mechanism is not involved in this process. The lack of myosin II function is demonstrated on a morphological and pharmacological basis. These results suggest that cells have the ability to close discontinuities in their cytoplasm by at least two different mechanisms: one involves actin polymerization and is independent of myosin II function and another involves an actomyosin purse string.
MATERIALS AND METHODS
Animals, Cell Preparation, and Reagents
Sea urchins, Strongylocentrotus droebachiensis, were collected from the near shore waters surrounding the Mount Desert Island Biological Laboratory in Maine and kept in either running sea water or closed artificial sea water systems at 15°C. Coelomocytes were isolated and maintained as described by Henson et al. (1992) with the coelomocyte culture media (CCM) consisting of 0.5 M NaCl, 5 mM MgCl2, 1 mM EGTA, and 20 mM HEPES, pH 7.2. Typically the cells were used within 2–8 h of isolation. A sea urchin egg myosin II heavy-chain antiserum was generated as described by Henson et al. (1999), and antibodies against human Arp3 and p34 were the kind gift of Dr. Matthew Welch (University of California, Berkeley). A monoclonal anti-actin antibody (clone C4) was obtained from ICN (Costa Mesa, CA), fluorescent phalloidin and latrunculin A were purchased from Molecular Probes (Eugene, OR), KT5926 came from CalBioChem (Costa Mesa, CA), and the majority of other reagents and antibodies were purchased from Sigma Chemical (St. Louis, MO).
Digitally Enhanced Video Microscopy and Rate Measurements
Coelomocytes were settled onto either untreated or 0.1 mg/ml poly-l-lysine-coated glass coverslips, which were then mounted in perfusion chambers constructed of coverslip shims placed on a slide. Cells were viewed on a Optiphot 2 microscope (Nikon, Tokyo, Japan) using a 60× (NA 1.4) planapo phase-contrast objective lens. Video-enhanced images were obtained with a 70S Newvicon camera (Dage-MTI, Michigan City, IN) coupled to an Argus-10 real-time digital image processor (Hamamatsu, Bridgewater, NJ). Frame-averaged, background-subtracted, and contrast-enhanced images were recorded on a JR4500 time-lapse VCR (Javelin, Torrance, CA), and still images were printed using a P40U video copy processor (Mitsubishi, Tokyo, Japan). Time-lapse recording were typically done in the range of 6- to 12-fold time compression. Alternatively, digitized images were collected into stacks by NIH Image (National Institutes of Health, Bethesda, MD), and the stacks were saved as movie files.
Measurements of the rate of centripetal flow in cells were accomplished by tracking the movement of phase light arcs (corresponding to areas of lower actin filament density) and/or membranous structures (as described by Henson et al., 1999). To measure the rate of wound closure, the movement of the wound margin was measured. Mean rates of centripetal flow and wound closure were derived from measurements made on 20 total cells derived from three separate experiments. The differences between the means of centripetal flow rates and wound closure rates were tested for statistical significance using a two-tailed t test with the p level = 0.01.
Micromanipulation Experiments and Pharmacological Treatments
For micromanipulation experiments, glass capillary tubes were pulled on either a horizontal or vertical pipette puller and then bent into an “L” shape using a microfuge. The microneedles were then used by a Leitz micromanipulator (Leica Microsystems, Deerfield, IL) coupled with a Nikon TS100 inverted phase-contrast microscope or with an MN151 manipulator (Narashige, Tokyo, Japan) attached to a Nikon Diaphot 200 inverted phase-contrast microscope. Images were collected via a video camera (Hitachi, Tokyo, Japan) digitized using an LG-3 frame grabber (Scion, Frederick, MD) and manipulated via NIH Image.
Disruption of actin polymerization was performed by treating cells with either 0.2–1 μM cytochalasin D or 0.2 μM latrunculin A in CCM. For kinase inhibition, coelomocytes were treated with 0.5–3 μM staurosporine or 250–500 nM KT5926 in CCM. The drugs were diluted from stock solutions dissolved in dimethyl sulfoxide and the appropriate dimethyl sulfoxide alone controls were performed.
Immunoblotting and Fluorescent Localization
Affinity-purified rabbit antibodies against human Arp3 and p34 were immunoblotted against coelomocyte cytoskeletal extracts using the procedure described by Henson et al. (1999).
For fluorescent localization of filamentous actin with phalloidin, cells were fixed with 0.25% glutaraldehyde plus 0.1% Triton in buffer A (75 mM KCl, 2 mM MgCl2, 320 mM sucrose, 20 mM EGTA, 20 mM PIPES, pH 7.0) for 10 min, rinsed in phosphate-buffered saline (PBS), and then stained with rhodamine or fluorescein-conjugated phalloidin for 30 min.
For immunofluorescence staining of actin, myosin, Arp3, and p34 cells were prefixed in 0.0001% glutaraldehyde in CCM for 5 min, fixed in 1% formaldehyde and 0.5% Triton X-100 in buffer A for 10 min, and then postfixed in 100% methanol at either −20°C or room temperature for 10–30 min. After a rinse with PBS, cells were blocked with PBS plus 1% bovine serum albumin and 2% goat serum and stained with primary antibodies followed by the appropriate fluorescently labeled secondary antibodies. Western blotting of anti-Arp3 and anti-p34 was performed as described by Henson et al. (1999).
Cells mounted in antiphotobleach were viewed for conventional microscopy using a 60 (NA 1.4) planapo phase-contrast objective lens, and images were captured using either a 35-mm camera with Kodak TriX 400 film (Eastman Kodak, Rochester, NY) or a Photometrics CoolSnap digital camera (Roper Scientific, Tuscon, AZ). Confocal microscopy was performed on a Fluoview laser scanning point source instrument (Olympus, Tokyo, Japan) using a 60× (NA 1.4) planapo objective lens, and digital files were collected.
RESULTS
Filamentous Actin Assembles from the Edges of Lamellar Wounds
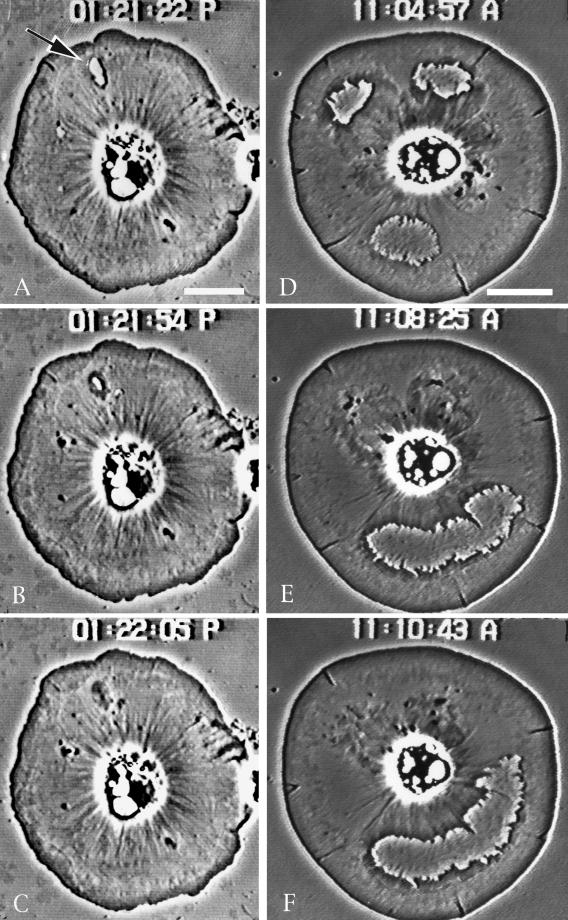
Discoidal shaped coelomocytes settled onto coverslips exhibit dramatic actin-based centripetal flow (Edds, 1993; Henson et al., 1999) and will on occasion open wounds/holes in their peripheral cytoplasm (Figure 1). These wounds traverse completely the lamellipod and represent a physical discontinuity in the cytoplasm of the cell. On opening, the membrane seals around the margin of the wound, and the edge appears highly convoluted. Within the cytoplasm of the cell a ring of phase-dense material develops around the wound and begins to exhibit flow away from the edge. The wound margin tends to smooth out coincident with the development of this phase-dense ring. Eventually the wound grows smaller and reseals, and at this point the healed membrane region exhibits centripetal flow toward the cell center. Cells have the ability to close both small and large wounds (Figure 1).
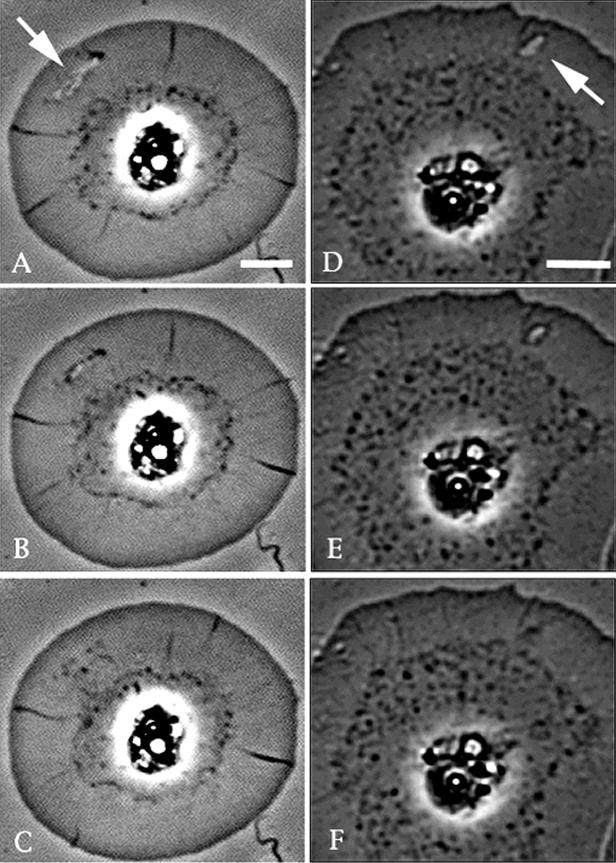
Figure 1.
Video-enhanced phase-contrast microscopy of the closure of spontaneous wounds in coelomocytes. (A–C) Closure of a small wound (arrow in A); (D–F) process in cells containing large wounds. Note that as the wounds close the margins tend to smooth out coincident with the development of a phase-dense fringe. After closing completely, this area exhibits centripetal flow toward the cell center. Bar, 10 μm.
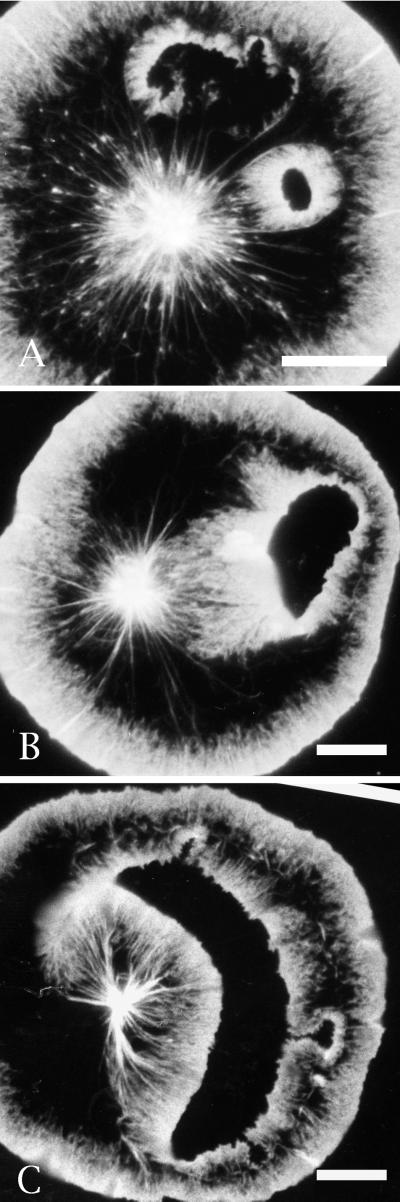
Fluorescent phalloidin staining of cells with spontaneous wounds demonstrates that the phase-dense material seen surrounding holes in video-enhanced microscopy corresponds to filamentous actin (Figure 2). The actin appears to polymerize from the wound edge in a manner reminiscent of that seen at the periphery of the cell. The outer edge of the wound margin develops less actin than seen in the inner margin. Actin flow from the outer edge is against the prevailing centripetal flow of the cell, whereas flow from the inner edge is parallel to the flow. A ridge of accumulated actin builds up in regions where the actin-based flow from the wound edge and the cell edge are in opposite directions. Note that the holes/wounds tend to form in the intermediate region of the cytoplasm located just proximal to the brush-work of actin seen at the cell edge and just distal of the pronounced radial fibers of actin seen in the cell center (Figure 2; see also Henson et al., 1999, for figures showing the ultrastructural organization of actin in coelomocytes).
Figure 2.
Phalloidin staining of filamentous actin in cells containing spontaneous wounds clearly indicates that actin assembles from the margin of small (A) and large (B and C) wounds and that the phase-dense fringe surrounding holes visualized with video-enhanced microscopy (Figure 1) represents newly polymerized actin. Note in A that the hole at the top has just opened and started to recruit actin polymerization, whereas the hole on the right is more “mature” and is in the process of closing. Bar, 10 μm.
Wound Closure Is Inhibited by Drugs That Interfere with Actin Polymerization
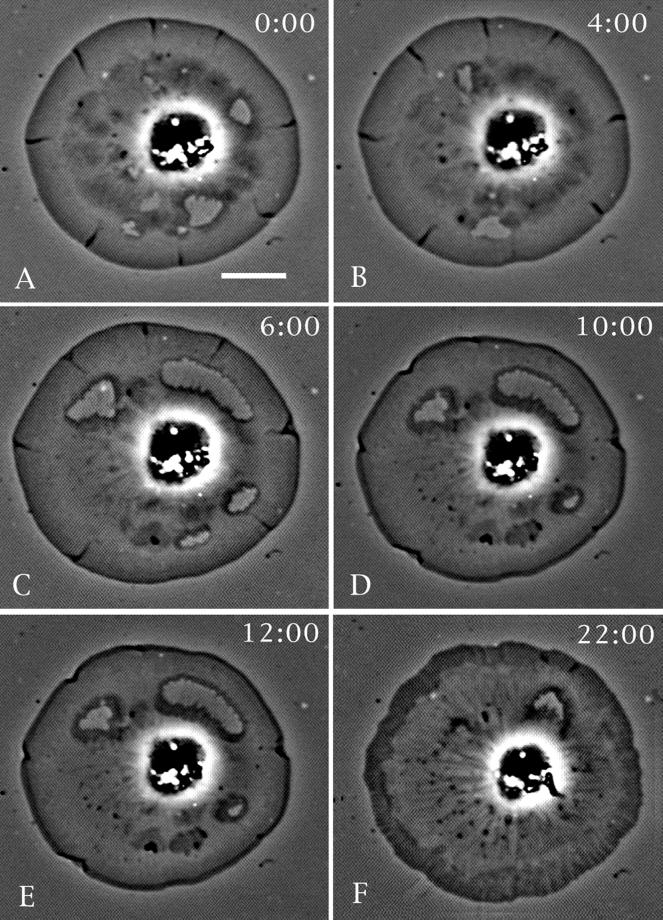
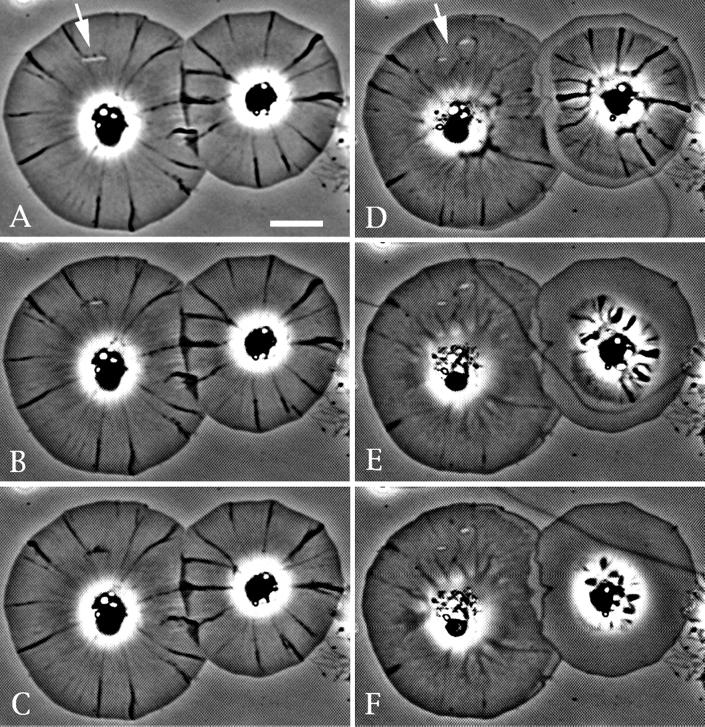
The closure of spontaneous and mechanically induced wounds in cells is inhibited by treatment with either cytochalasin D or latrunculin A (Figures 3 and 4). Figure 3 shows the treatment of a cell displaying spontaneous hole production with a low concentration of cytochalasin D (0.2 μM). The drug treatment arrests wound closure and centripetal flow, whereas upon drug wash out both of these processes resume. Lamellar wounds created by microneedles in control cells readily undergo the accumulation of phase-dense material and subsequent closure (Figure 4). Importantly, this closure of mechanical wounds can be arrested in cells treated with cytochalasin D or latrunculin A (Figure 4) immediately after the wounding event. Because these drugs interfere with actin polymerization via different mechanisms, lamellar wound healing probably involves this process.
Figure 3.
Cytochalasin D inhibits wound closure. (A and B) A cell undergoing closure of spontaneous wounds. Cytochalasin D (0.2 μM) was applied at the 6-min time point shown in C, and this causes the eventual cessation of hole closure and centripetal flow (10-min time point shown in D). The drug also induces a characteristic clearing of the cytoplasm and an accumulation of a phase-dense ring at the cell periphery. At the 12-min time point (E) the drug is washed out and the cell resumes hole closure and centripetal flow (22-min time point in F). Bar, 10 μm.
Figure 4.
Latrunculin A inhibits wound closure. (A–C) Closure of a hole produced by a microneedle (arrow in A) in a control cell; note the development of the characteristic phase-dense fringe (total elapsed time = 2 min). (D–F) Effect of latrunculin A perfusion immediately after the mechanical production of two new holes in the same cells shown in A–C. The holes were made and the drug was perfused ∼10 s before the capture of D. Note that the hole margins do not develop a phase-dense fringe and do not close, whereas the central cytoskeleton tends to undergo a retraction (total elapsed time = 10 min). Bar, 10 μm.
The Wound Margin Contains the Arp2/3 Complex but Not Myosin II
The Arp2/3 complex has recently been shown to be an essential component of actin polymerization dynamics and structural organization at the leading edge (reviewed by Bear et al., 2001). If actin is indeed polymerizing de novo from the newly formed edges of lamellar holes in coelomocytes, one would predict the presence of the Arp2/3 complex is this same area. Western blotting of anti-human Arp3 and p34 antibodies demonstrates strong cross-reactivity with coelomocyte proteins of the appropriate molecular masses (Figure 5). Immunolocalization clearly shows that actin and the Arp3 and p34 components of the Arp2/3 complex are found both at the expected cell edge and the margins of wounds (Figure 6). These results complement the cytochalasin D and latrunculin A drug experiments and reinforce the idea that the wound edge is the site of new actin polymerization in coelomocytes.
Figure 5.
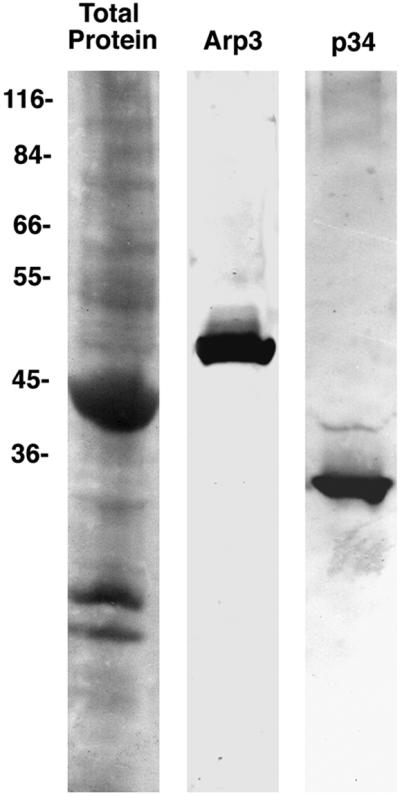
Immunoblot of anti-Arp3 and anti-p34 against cytoskeletal extracts of coelomocytes. Both the Arp3 and the p34 antibodies exhibit strong labeling of bands that migrate at the appropriate molecular mass (50 kDa for Arp3 and 34 kDa for p34). Molecular masses of marker proteins are given along the left margin.
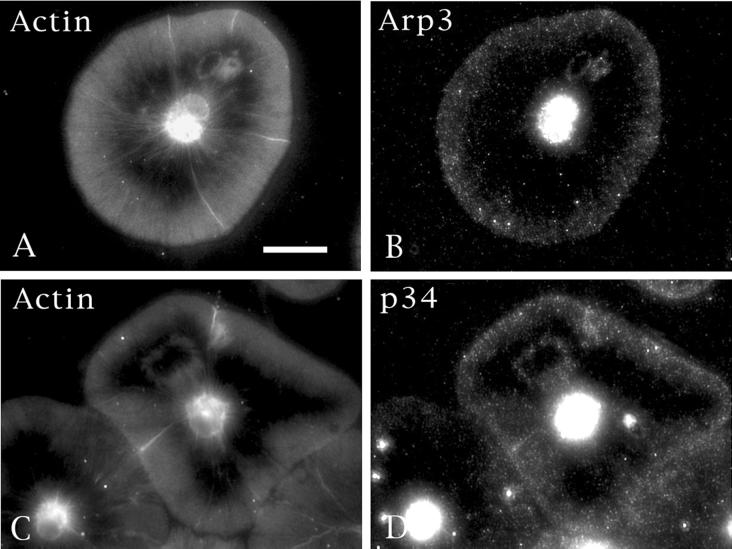
Figure 6.
Components of the Arp2/3 complex localize to the wound margin. Double labeling of actin (A) and Arp3 (B) or actin (C) and p34 (D) in cells containing spontaneous holes clearly demonstrates that Arp2/3 components are concentrated both at the cell periphery and at the margins of wounds. Bar, 10 μm.
Immunolocalization of myosin II in coelomocytes shows it to be distributed within the perinuclear region where it appears to be associated with actin filaments in a ring-like array (Figure 7; see also Henson et al., 1999). Immunofluorescence localization of myosin II in cells containing spontaneous wounds indicates that myosin is found exclusively in the cell center and does not associate with wounds, despite the appearance of the actin fringe there (Figure 7). Myosin appears not to associate with the holes during any stage of the closure process. Figure 8 contrasts the localization of Arp3 and myosin with actin in cells containing spontaneous holes. Association with the edge of the wound is seen exclusively with Arp3 and not with myosin.
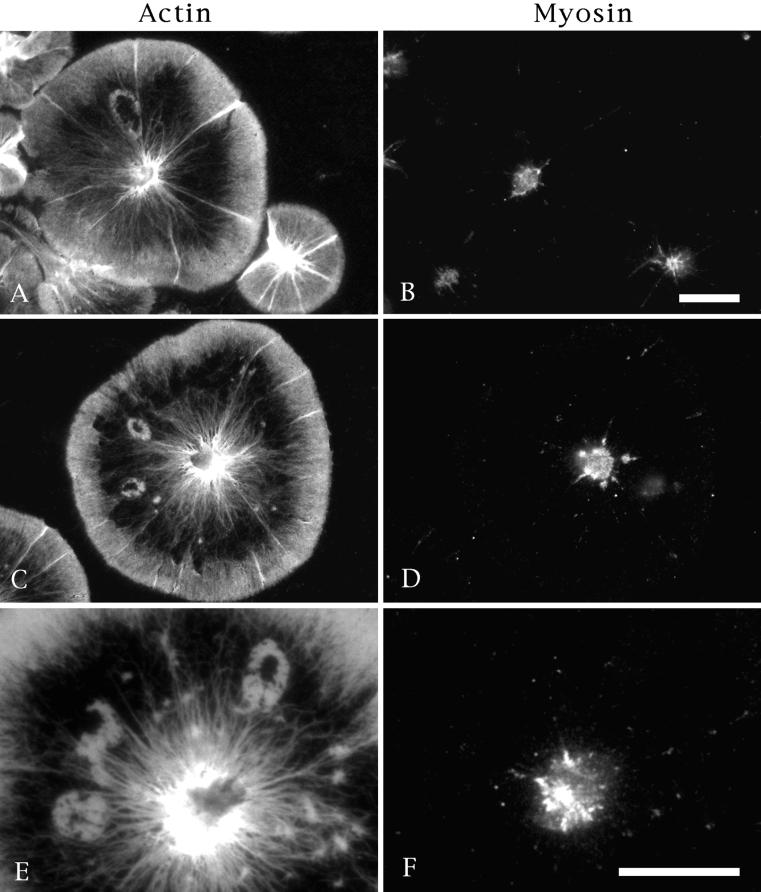
Figure 7.
Myosin II does not associate with wounds. Immunofluorescence labeling of actin (A, C, and E) and myosin II (B, D, and F) in coelomocytes containing spontaneous wounds. Although myosin is consistently localized to the perinuclear region of these cells, it is not associated with the actin fringe recruited to the wound margins. This is true for holes throughout the closure process. Bar, 10 μm.
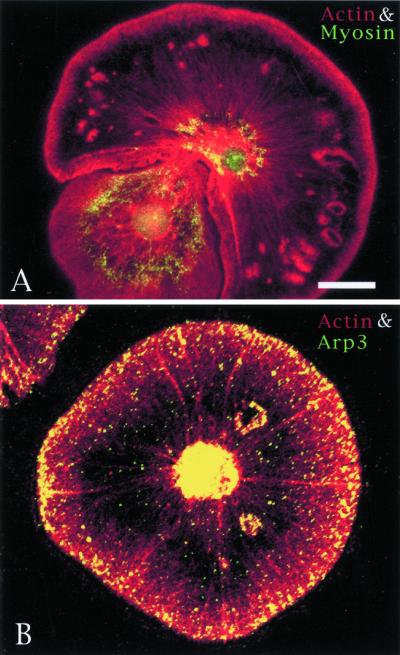
Figure 8.
Comparision of the distribution of actin (red) and myosin (green) in A with actin (red) and Arp3 (green) in B in coelomocytes containing spontaneous wounds. Arp3 associates with the wound margins, whereas myosin does not. Bar, 10 μm.
The quantitation of the rates of centripetal flow and wound closure were carried out to determine whether there were any significant differences. There was no statistically significant difference between the centripetal flow rate (mean = 3.4 μm/min) and the hole closure rate (mean = 3.2 μm/min), although the wound closure rate was more variable. Our previous studies have shown that flow rates in the periphery of cells in which actomyosin function is blocked by kinase inhibition are not different from rates in control cells (Henson et al., 1999).
Pharmacological Inhibition of Actomyosin Contraction Does Not Inhibit Wound Closure
Evidence of actomyosin-mediated tension on the coelomocyte actin cytoskeleton comes from experiments in which a high concentration of cytochalasin D causes the retraction of the central cytoskeleton away from the cell periphery (Figure 9B; Edds, 1993; Henson et al., 1999). This retraction is thought to be due to the stoppage of actin polymerization at the cell edge, the release of the peripheral actin cytoskeleton, and a subsequent myosin-mediated finite retraction (note that a similar cytoskeletal retraction phenomena occurs in coelomocytes in which actin polymerization has been inhibited by treatment with latrunculin A as evidenced in Figure 4.). Cytochalasin-induced retraction of the actin cytoskeleton has been observed in neuronal growth cones, where it has been interpreted as evidence for myosin-based tension (Forscher and Smith, 1988; Smith, 1988; Lin et al., 1996). Previous studies have indicated that actomyosin contraction can be inhibited in cells using kinase inhibitors that target the action of myosin light-chain kinase (Nakanishi et al., 1990; Kazuo et al., 1998). Interestingly, treating coelomocytes with the wide spectrum kinase inhibitor staurosporine or with the more MLCK-specific KT5926 results in an arrest of flow and a disruption of the actin cytoskeleton in the cell center, although flow continues at the cell periphery (Figure 9C; Henson et al., 1999).
Figure 9.
Immunofluorescence labeling of actin in a control cell (A) and in cells treated with cytochalasin D alone (B), staurosporine alone (C), or staurosporine plus cytochalasin D sequentially (D). Cytochalasin D alone stops flow and produces a buildup of actin at the cell margin along with an actomyosin-mediated contraction of the cytoskeleton, leaving an actin-free peripheral zone. Staurosporine, a broad-spectrum kinase inhibitor that can inhibit MLCK, stops flow and disrupts the actin organization in the cell center; however, flow continues unabated at the cell periphery (C). Sequential treatment with staurosporine followed by cytochalasin D arrests flow and causes an accumulation of actin staining at the cell edge. However, the cytoskeleton does not undergo a retraction, suggesting that the kinase inhibitor is indeed inhibiting MLCK function and thereby reducing the actomyosin-mediated tension on the cytoskeleton. Bar, 10 μm.
In an attempt to determine whether kinase inhibition was indeed interfering with actomyosin-mediated tension, we treated cells sequentially with kinase inhibitors (either staurosporine or KT5926) followed by cytochalasin D. We hypothesized that cytochalasin D treatment in the presence of kinase inhibition would arrest the peripheral flow, which is based on actin polymerization, but that the lack of myosin-mediated cytoskeletal tension would prevent the retraction of the central cytoskeleton in these cells. Figure 9D indicates that retraction did not take place in kinase-inhibited cells that were treated with cytochalasin D. This result suggests that the kinase inhibition treatment does reduce myosin-mediated tension generation in these cells.
Armed with this knowledge we tested whether cells treated with kinase inhibitors were capable of closing wounds in regions where their peripheral cytoplasm was still exhibiting flow. Significantly, cells treated with kinase inhibitor retained the ability to close mechanically produced wounds in the peripheral regions where their cytoplasm was still exhibiting centripetal flow (Figure 10). This suggests that myosin II-based tension generation is not required for wound closure.
Figure 10.
Video-enhanced microscopy of a temporal sequence of two staurosporine treated cells closing mechanically-induced lamellipodial wounds (arrows in A and D). Note that there is a clear difference in the appearance of the cell center, where flow is arrested and actin organization disrupted (see Figure 5C), and the cell periphery, where flow persists. Total elapsed time for each sequence is 2 min. Bar = 10 μm.
Actin and Myosin II Are Associated with Plasma Membrane Invaginations
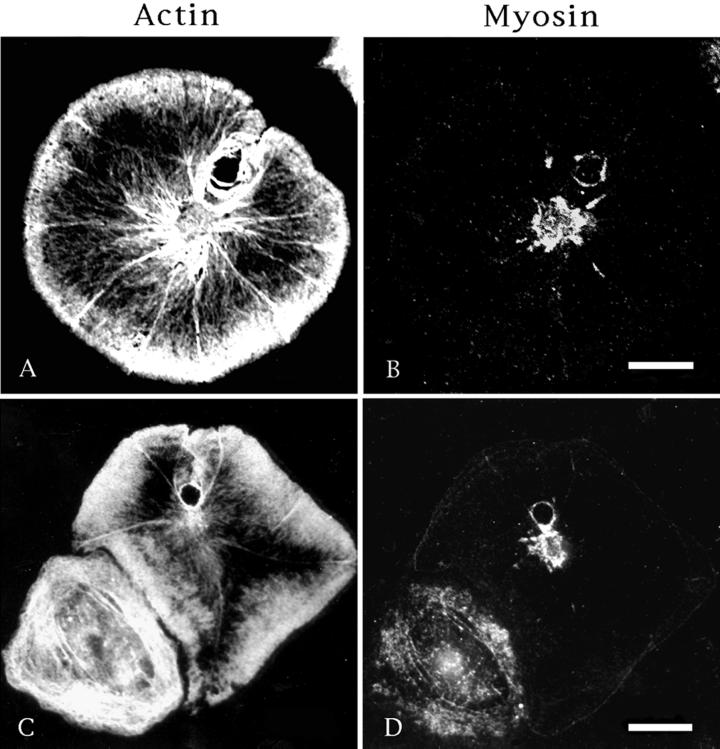
The apparent lack of myosin II association with wound closure on either a structural or functional basis implies that myosin may simply be restricted to the cell center via some type of general mechanism, such as a molecular sieving process that could result from actin-based centripetal flow. However, both actin and myosin are found surrounding phagosome-like invaginations of the plasma membrane, which occurs occasionally in the peripheral cytoplasm of coelomocytes (Figure 11). These structures result from the interaction of the coelomocyte with a smaller spherical cell type (lost during immunofluorescence processing), and there exists clear evidence of a past continuity between the hole interior and the exterior margin of the cell, indicating that these entities arose from invaginations of the plasma membrane in a manner similar to that seen with phagosomes. In cells containing spontaneous wounds (Figures 1–3 and 6–8), it is clear that the wounds develop within the cell interior and do not have any connection with the cell's exterior circumferential margin.
Figure 11.
Both actin (A and C) and myosin (B and D) localize to the margin of phagosome-like membrane invaginations seen in some cells. These invaginations appear as holes in the peripheral cytoplasm; however, unlike the holes/wounds seen previously, they have a clear connection to the outside circumference of the cell and are formed by membrane invagination. The cell seen in A and B was imaged with confocal microscopy. Bar, 10 μm.
DISCUSSION
Wound Closure in Single Cells in the Absence of an Actomyosin Purse String
The actomyosin purse string, consisting of interacting actin and myosin II filaments, is the universal mechanism invoked for constriction within cells. It is conserved structurally and phylogenetically and is known to play a role in cytokinesis, morphogenesis, epithelial sheet dynamics, and wound healing (see review by Kiehart, 1999). Within the latter category it has been implicated in the healing of wounds in cell monolayers as well as the closure of wounds created in single Xenopus oocytes. In all of these processes, the development of contractile tension is dependent on the presence of both filamentous actin and myosin II.
In the present study we have examined the process of lamellipodial wound closure in sea urchin coelomocytes, which exhibit pronounced actin-based centripetal flow. In the case of either spontaneous or mechanically induced holes, video-enhanced microscopy and fluorescent actin staining clearly indicate that actin polymerizes off the cytoplasmic face of the membrane that forms the wound margin, and as the actin-containing fringe develops, the wound edges smooth out and the hole eventually closes. Despite the presence of filamentous actin at the wound margin, our morphological and pharmacological evidence indicates that myosin II is not involved in the closure response. Immunofluorescence microscopy consistently shows that myosin II is present in the cell center but is not associated with wound margins in cells. With regard to drug treatment, wound closure was not effected in cells treated with kinase inhibitors that interfere with the actomyosin contraction, as evidenced by their ability to inhibit the retraction of the central cytoskeleton in coelomocytes treated with cytochalasin D. In growth cones, this cytochalasin d-induced retraction response has been used as evidence for the existence of a myosin-mediated contractile tension exerted on the peripheral cytoskeleton (Forscher and Smith, 1988). The lack of myosin localization and apparent function at the coelomocyte wound sites suggests that an actomyosin purse string mechanism does not mediate wound closure in these cells.
Our evidence for the lack of actomyosin contraction in coelomocyte wound repair is at odds with wound healing in single Xenopus oocytes, where there is clear evidence of the involvement of actomyosin contraction (Bement et al., 1999; Mandato and Bement, 2001). It is possible that these apparent differences in mechanism are related to the differences in the nature of the wounds in the two systems. In coelomocytes the wound involves a physical hole that extends through the entire thin lamellar region of the cell around which the membrane seals. In the oocyte experiments, physical punctures were made through the plasma membrane and into the cytoplasm of the cells. Perhaps a contractile actomyosin structure is needed to deal with these larger, more invasive wounds, whereas actin polymerization is sufficient for closing smaller, lamellar discontinuities. Given the importance of actin polymerization in coelomocyte wound repair, it is interesting to note that Xenopus wound closure has been shown to be dependent on both actomyosin contraction and polymerization (Mandato and Bement, 2001).
The absence of myosin II from coelomocyte wounds does not preclude the possible involvement of unconventional myosins in wound closure. Unconventional myosins have been implicated as being important in cell movement, membrane traffic, and signal transduction (reviewed by Mermall et al., 1998; Oliver et al., 1999) and myosin I subtypes, in particular, may be involved in the Arp2/3-regulated assembly of branched actin filaments at the leading edge (Jung et al., 2001). With regard to cellular events involving tension generation, Swanson et al. (1999) argued that myosin IC, not myosin II, is the likely mediator of a BDM-sensitive, purse-string-like contraction that closes phagosomes in mammalian macrophages. However, myosin II is the only myosin known to produce contractile tension in combination with actin, and past studies of myosin I in coelomocytes have indicated that it is associated with perinuclear cytoplasmic vesicles and not the lamellar cytoskeleton in these cells (D'Andrea et al., 1994). It should also be noted that the kinase inhibitor treatments used to interfere with actin and myosin II interaction in the present study may not inhibit the activity of unconventional myosins.
Actin-based Healing of Lamellipodial Wounds
If myosin II-based contraction is indeed not involved in coelomocyte wound healing, then how do the holes constrict? We propose that the closure of these wounds is based mainly on the force produced by actin polymerization along the entire circumference of the wound margin combined with the centripetal flow within the distal region of the cell. Significantly, the wound closure process in coelomocytes is inhibited by drugs (cytochalasin D and latrunculin A) that interfere with actin polymerization, and components of the Arp2/3 complex, known to be important in the mediation of actin polymerization, are localized to the wound margin. Actin polymerization alone has been suggested to produce protrusive force at the leading edge of cells, as well as in the motility of organelles and intracellular pathogens (Condeelis, 1993; Mitchinson and Cramer, 1996; Mogilner and Oster, 1996; Ireton and Cossart, 1997; Merrifield et al., 1999; Bear et al., 2001; Boldogh et al., 2001). The fringe of actin that develops around the margin of wounds in coelomocytes is similar in organization to the actin meshwork at the leading edge of the cell and the comet tails of actin that associate with pathogens such as Listeria. Interestingly, the coelomocyte wound closure phenomenon implies that all of the protein machinery needed for actin to bind to and begin to polymerize from a membrane-associated site is assembled very quickly onto the wound edge to allow for the rapid development of the actin assembly.
Why would it be beneficial for cells to have an actin-based wound-healing process in the lamellipodial region? Clearly, the lamellipod, which forms the leading edge of a motile cell, would be expected to be the site of potential cell injury given that it serves as the “point man” as the cell migrates into new territory. Allowing for a simple wound-healing response based on actin polymerization and centripetal flow would permit the quick closure of holes produced in these areas. Given the stationary nature and 360-degree flow pattern of the coelomocyte, why do spontaneous wounds occur in these cells? One possibility is that the coelomocyte wounds arise as a result of the cell spreading too thinly on the substrate. This can be thought of as analogous to the holes that develop in a drop of water when it is spread too thinly on a hydrophobic substrate. It is interesting to note the similarity between the appearance of the ragged edge holes in the water layer and the holes in the coelomocyte cytoplasm. The openings in coelomocytes tend to cluster in the area of lower actin filament density between the brush work of the cell's edge and the actomyosin structures in the cell center. We speculate that this zone is the area of least resistance when it comes to tension on the cell membrane and, therefore, is the site of hole appearance. Our unpublished preliminary observations suggest that we can alter the occurrence rate of spontaneous wounds by increasing the adherence of the cell with higher concentrations of poly-l-lysine and/or by subjecting the cells to osmotic shock. Further experimentation is needed to determine the exact stimulus for spontaneous hole production.
It should be pointed out that membrane-resealing events in several cell types have been shown to involve a localized decrease in membrane tension and the recruitment of internal membrane via exocytosis (Terasaki et al., 1997; Togo et al., 1999, 2000; McNeil et al., 2000; reviewed by McNeil and Steinhardt, 1997; McNeil and Terasaki, 2001). These processes involve the closure of holes in the plasma membrane of the cell, whereas the coelomocyte wound healing described in the present study involves the closure of a physical discontinuity in the thin lamellar cytoplasm around which the membrane has already sealed. The difference between these two processes is highlighted by the fact that cytochalasin D lowers membrane tension and therefore facilities membrane resealing in wounded cells (Togo et al., 1999, 2000), whereas the same drug arrests the closure of coelomocyte lamellar holes (Figure 3). Furthermore, the membrane-resealing process depends on the availability of internal membrane sources, such as endosomes, vesicles, and lysosomes. In coelomocytes, these organelles, together with microtubules, are restricted to the perinuclear region of the cell (Henson et al., 1992), and they are never found in the region of the lamellipodia where hole opening and closure take place. Therefore, although we cannot rule out the involvement of endosomes in the healing of coelomocyte wounds, they are not positioned in a manner consistent with their involvement in peripheral wound closure.
Implications of Coelomocyte Wound Closure on the Mechanism of Centripetal/Retrograde Flow
Sea urchin coelomocytes represent an excellent model system for the study of actin-based centripetal flow. We have recently shown that the flow process appears to be made up of two separable components working together: actin polymerization pushing at the cell edge combined with actomyosin contraction pulling from the cell center (Henson et al., 1999). Aspects of the wound closure process further support this dual-component model. If centripetal motility was absolutely dependent on centralized actomyosin function, then the edges of cells distal to the wound opening (and therefore cut off from the influence of the myosin-containing cell center) would be expected not to exhibit flow. However, they do show retrograde actin movement, indicating that actin polymerization from the cell edge is sufficient to drive flow in these regions. In addition retrograde flow is also seen in the actin assembled off the wound margin on the opposite side of the cell center, again emphasizing the fact that actin-based retrograde mobility can take place in an area that is physically separated from myosin II activity. These results reinforce our interpretation of pharmacological experiments in which MLCK-targeted kinase inhibitors stop flow in the cell center but maintain flow at the cell periphery.
It is also informative to compare the structural organization of actin around the wound margins relative to the remainder of the cell. The actin meshwork of the regular cell edge transforms into a region of radial actin fibers as one moves toward the cell center. However, the actin that assembles around wounds displays this type of organizational transformation only on the side of the wound that faces the cell center (Figures 2 and 6–8). The actin on the distal side of the wound opening remains as a meshwork, suggesting that actomyosin tension, concentrated in the cell center, is partially responsible for organizing the radial structures.
In the present study we have used several different approaches to demonstrate that wounds in the lamellipodia of sea urchin coelomocytes are closed by actin polymerization in the absence of the involvement of an actomyosin purse string. These results, together with published studies of Xenopus oocytes (Bement et al., 1999; Mandato and Bement, 2001), imply that single-cell wound-healing responses can involve actin polymerization alone or polymerization in combination with actomyosin contraction. Further studies are needed to determine what signals are required to initiate these critical repair processes.
ACKNOWLEDGMENTS
Special thanks are extended to Dr. Ray Rappaport for his helpful advice, thoughtful discussions, and expert assistance with the micromanipulation experiments and to Dr. Matthew Welch for generously supplying us with Arp2/3 antibodies. The authors also thank Jeanine McGreevy and Gregory Fredericks for excellent technical assistance and Dr. Calvin Simerly for his many discussions concerning wound closure phenomena. This work was supported by a National Science Foundation Young Investigator Award (MCB-9257856) to J.H.H., a National Institutes of Health grant (GM 60925) to J.H.H., a Howard Hughes Medical Institute Undergraduate Biological Sciences Program grant to Dickinson College, a Whitaker Foundation Student/Faculty Research grant to Dickinson College, and National Institute of Environmental Health Sciences Center grants (ES-03828 and ES-01247) in support of the Imaging Core at the Mount Desert Island Biological Laboratory.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc. 01–04–0167. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–04–0167.
REFERENCES
- Bear JE, Krause M, Gertler FB. Regulating cellular actin assembly. Curr Opin Cell Biol. 2001;13:158–166. doi: 10.1016/s0955-0674(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, Mandata CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Yang HC, Nowakowski WD, Karmon SL, Hays LG, Yates JR, III, Pon LA. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc Natl Acad Sci USA. 2001;98:3162–3167. doi: 10.1073/pnas.051494698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activiation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaira PT, Taylor DL. Relative distribution of actin, myosin I, and myosin II, during the wound healing response of fibroblasts. J Cell Biol. 1993;120:1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea L, Danon MA, Sgourdas GP, Bonder EM. Identification of coelomocyte unconventional myosin and its association with in vivo particle/vesicle motility. J Cell Sci. 1994;107:2081–2094. doi: 10.1242/jcs.107.8.2081. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin–mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111:3323–3331. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- Edds KT. Effects of cytochalasin and colcemid on cortical flow in coelomocytes. Cell Motil Cytoskeleton. 1993;26:262–273. doi: 10.1002/cm.970260309. [DOI] [PubMed] [Google Scholar]
- Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson JH, Nesbitt D, Wright BD, Scholey JS. Immunolocalization of kinesin in sea urchin coelomocytes: associations of kinesin with intracellular organelles. J Cell Sci. 1992;103:309–320. doi: 10.1242/jcs.103.2.309. [DOI] [PubMed] [Google Scholar]
- Henson JH, Svitkina T, Burns AR, Hughes HE, McPartland KJ, Nazarian R, Borisy GG. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol Biol Cell. 1999;10:4075–4090. doi: 10.1091/mbc.10.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Cossart P. Host-pathogen interactions during entry and actin-based movement of Listeria monocytogenes. Annu Rev Genet. 1997;31:113–138. doi: 10.1146/annurev.genet.31.1.113. [DOI] [PubMed] [Google Scholar]
- Jung G, Remmert K, Xufeng W, Volosky JM, Hammer JA., III The Dictyostelium CARMIL protein links capping protein and the Arp2/3 complex to type I myosins through their SH3 domains. J Cell Biol. 2001;153:1479–1497. doi: 10.1083/jcb.153.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuo K, Kano Y, Masuda M, Onishi H, Fujiwara K. Isolation and contraction of the stress fiber. Mol Biol Cell. 1998;9:1919–1938. doi: 10.1091/mbc.9.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP. Wound healing: the power of the purse string. Curr Biol. 1999;9:R602–R605. doi: 10.1016/s0960-9822(99)80384-4. [DOI] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Mandato CA, Bement WM. Contraction, and polymerization cooperate to assemble, and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279:527–533. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35074652. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- Mitchinson TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Yamada K, Iwahashi K, Kuroda K, Kase H. KT5926, a potent and selective inhibitor of myosin light chain kinase. Mol Pharmacol. 1990;37:482–488. [PubMed] [Google Scholar]
- Oliver TN, Berg JS, Cheney RE. Tails of unconventional myosins. Cell Mol Life Sci. 1999;56:243–257. doi: 10.1007/s000180050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in Animal Cells. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- Smith SJ. Neuronal cytomechanics: the actin-based motility of growth cones. Science. 1988;242:708–715. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N. A contractile activity that closes phagosomes in macrophages. J Cell Sci. 1999;112:307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Alderton JM, Bi G-Q, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000;11:4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]