Abstract
Sickle cell disease (SCD) is a genetic disease influenced by ethnicity and regional differences in its clinical course. Recent advances in the management of SCD with newer therapies are being introduced to the Western population. However, many of these treatments are yet to be used in the Arabic SCD population. Understanding the genetic variations of SCD regionally is essential to anticipate the utilization of new treatments. This systematic review’s main objective is to pool the available data on the genetic composition of SCD in the Arabic population. Data for 44,034 patients was extracted from 184 studies (11 case reports, 8 case series, 56 retrospectives, 107 prospective observational studies, and 2 clinical trials) using PubMed, Scopus, and Google Scholar. Male (49%) and female (51%) patients were equally reported wherever gender was available (N=13105). Various SCD genotypes were reported in a total of 14,257 patients, including Hb SS (77%) Hb Sβ0 (9.9%), and Hb Sβ+ (7.2%), while the rest of the genotypes, including HbSC, HbSD, HbSE, HbSO Arab, Hb S/α-Thal, Hb Sβ0 + α-Thal, and HBS Oman were individually reported in <4% of the cases. Major SCD complications in the Arab population included pain crises (48.25%) followed by neurological complications (33.46%), hepatobiliary complications (25.53%), musculoskeletal complications (24.73%), and hemolytic anemia (23.57%). The treatments reported for SCD included hydroxyurea (20%), blood transfusion (14.32%), and Deferasirox (3.03%). We did not find the use of stem cell transplantation or newer treatments such as L-Glutamine, Voxelotor, Crizanlizumab, or gene therapy reported in any of the studies included in our review. This review highlights the genetic makeup of SCD in Arab countries and its common phenotypic manifestations and will help direct further research on SCD in this region, especially concerning genetic therapy.
Systematic Review Registration
The protocol has been registered in the International Prospective Register of Systematic Reviews(PROSPERO):CRD42020218,666. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=218666.
Keywords: sickle cell disease, genotypes, SCD, sickle cell anemia, Arab
Introduction
The first description of sickle cell anemia (SCA) like disorder was provided by Dr. Africanus Horton in his book “The disease of Tropical Climates and their treatment (1872). However, in 1910, Dr. James B Herrick and Dr. Ernest Irons reported sickle-shaped red blood cells in a dental student.1 Sickle cell disease (SCD) refers to various groups of hemoglobinopathies characterized by different autosomal recessive genetic mutations in the hemoglobin beta-subunit.1–3 As a consequence of these genetic mutations, deformed red blood cells (RBCs) are produced that are not suitable for the optimal supply of oxygen to the tissues.2 The RBCs become sickled and rigid and are unable to pass through small vascular channels, resulting in hemolysis and intravascular RBC clumping, causing tissue hypoxia, ischemia, infarction, and necrosis.2 There are potentially severe complications that carry high morbidity and mortality, such as delayed growth,4,5 delayed sexual maturation,6 acute chest syndrome, acute stroke,7 chronic kidney disease,8 splenic infarction, pulmonary artery hypertension, leg ulcers, myocardial infarction, and other body organ damage.3,9–11
Within the umbrella of SCD, subgroups exist such as sickle cell anemia (HbSS), hemoglobin SC disease (HbSC), and other combined mutations including hemoglobin sickle-beta-thalassemia (beta-thalassemia positive or beta-thalassemia negative).1–3 The highest prevalence of SCD is among the people of Sub-Saharan Africa, South Asia, the Middle East, and the Mediterranean.12 The incidence is estimated to be between 300,000 to 400,000 neonates globally each year.3 In a meta-analysis of the prevalence and associated mortality in children under five years of age, Wastnedge et al reported a global birth prevalence of homozygous sickle cell disease of 112 per 100,000 live births but 1125/100,000 in Africa compared to 43.12/100,000 in Europe.13 It has been estimated that the global burden of SCD will increase by up to 30% by 2050.14
The management of SCD includes universal newborn screening programs for early diagnosis and intervention.15,16 Current medical management includes hydroxyurea, which has been proven to reduce sickling of RBCs and thereby provide mortality benefits,17,18 L-glutamine for the prevention of acute pain episodes of SCD in patients five years of age or older,19 and more recently crizanlizumab and voxelotor.17,20 Additional supportive management includes blood transfusion, pain management, and avoidance of triggering factors.17 There is no current cure for the disease in adults, but bone marrow transplantation in children has been shown to be curative in selected patients.15 Gene therapy is still in the clinical trial stage but is very promising.17,19 In anticipation of the future implementation of gene therapy for SCD and to improve SCD care overall, it is important to understand the genotypic and phenotypic SCD landscape in different regions of the world. Although many studies have reported genotypic and phenotypic variants of SCD in specific countries, the last updated review in Arab world countries was published in 2011 by El-Hazmi eta. more than a decade ago and before newer interventions and testing were so widely available.21 With this systematic review, we aim to report the various genotypes, phenotypes, mutational variations, haplotypes, and associations between phenotypes and treatments in SCD in 22 Arab countries through a rigorous search of multiple research databases and thus contribute to the current literature for better future perspectives in SCD management.
Materials and Methods
Literature Search
A systematic literature search was performed for any date up to 25 November 2020 via electronic databases (PubMed, Scopus, and Google Scholar) to identify English-language articles relevant to the research question. The protocol has been described in detail and published, including the search terms and methodology.22 The following search terms were used in the literature review: “Genotype” OR “Genetics” OR “Gene” OR “Mutations” OR “Haplotype” AND “Sickle Cell Disease” OR “SCD” OR “Hemoglobin S/O” OR “Sickle cell anemia” OR “SCA” OR “sickle/ beta‐thalassemia”, “SC/SD” OR “Hb SS” OR “Hb SC” OR “Hb Sβ+” OR “Hb Sβ0” OR “HbSD” OR “HbSE” OR “HbSO Arab” OR “HBS Oman” AND “Arab” OR “Arab countries” OR “Arab Population” OR “The Middle East” OR “Algeria” OR “Bahrain” “Comoros” OR “Djibouti” OR “Egypt” OR “Iraq” OR “Jordan” OR “Kuwait” OR “Lebanon” OR “Libya” OR “Mauritania” OR “Morocco” OR “Oman” OR “Palestine” OR “Qatar” OR “Saudi Arabia” OR “Somalia” OR “Sudan” OR “Syria” OR “Tunisia” OR “the United Arab Emirates” OR “Yemen”.
Our pre-defined research question was to compile genotypes and phenotypes of all patients (pediatric and adult) with a confirmed diagnosis of SCD who had genotypes mentioned in the included studies from Arabic countries.
Study Selection
Inclusion Criteria
All English language studies from Arabic countries describing original data on patients with confirmed SCD with genotypes mentioned were included in the review. The Arab world is defined by 22 Arab countries which are members of the Arab league.23
Exclusion Criteria
Studies reporting secondary (already published) data were excluded from this review. Articles in languages other than English were also excluded from the review. Studies where original data of SCD patients was reported but genotypes were not mentioned were also excluded.
Two reviewers independently screened the finalized studies via title, abstract, and keywords. Shortlisted studies then underwent a detailed full-length review. Disagreements in the screening process were resolved by an independent review from a third member.
Bias Assessment
The Joanna Briggs Institute case report appraisal checklist for inclusion in systematic reviews and the Methodological index for non-randomized studies (MINORS) assessment tools were used for quality assessment for case reports and larger observational studies, respectively.24,25
Data Collection and Statistical Analysis
Data collected include socio-demographic variables, genetic composition, phenotypic manifestations, and various managements of SCD patients, subject to availability. Descriptive and summary statistics were used with data presented as mean (with standard deviations), median (with interquartile ranges) and numbers (with percentages) as appropriate. Data was collected by 4 reviewers independently with cross checking of data by 2 reviewers.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for the synthesis of this systematic review.26
Results
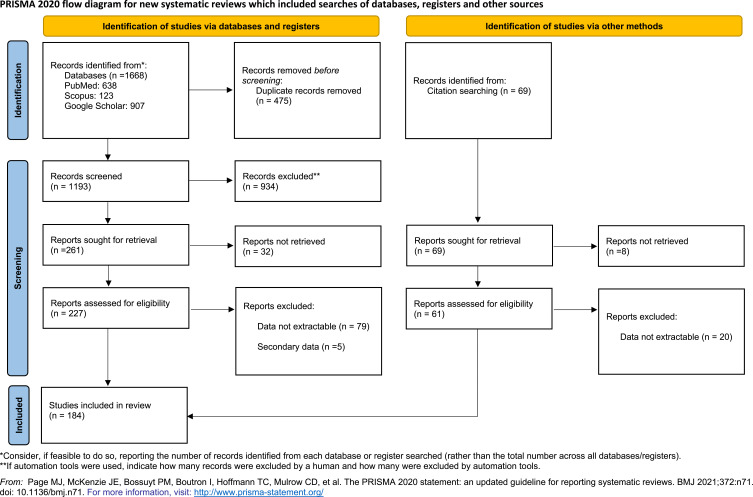
184 eligible studies reported 44,034 SCD patients in the Arabic population (Figure 1). The studies included 11 case reports, 8 case series, 56 retrospective, 107 prospective observational studies, and 2 clinical trials (one randomized and one nonrandomized) (Supplementary File 1).
Figure 1.
PRISMA flow diagram of the article screening process with the details of included and excluded studies.
As shown in Table 1, male gender represented 49% and female gender represented 51% of cases (given that the gender data was only available for 13,105 patients). More than half of the cases were reported from Saudi Arabia, followed by Bahrain with 27.24% of the cases. Fewer than 5% of the cases each were reported from Jordan, Egypt, Kuwait, and Tunisia, and less than 2% each from the remaining (Table 1). Saudi Arabia was also the leading country in the Arab region in terms of the number of SCD studies with genotype information published, followed by Kuwait then Egypt (Table 2).
Table 1.
Baseline Characteristics of the Published Data of Patients with SCD in the Arab Population (N= 44,034)
| Characteristic | N (%) |
|---|---|
|
Gender Total |
N=13105 |
| Male | 6415 (49) |
| Female | 6690 (51) |
| Country | |
| Algeria | 84 (0.19) |
| Bahrain | 12,209 (27.5) |
| Egypt | 1550 (3.5) |
| Iran | 28 (0.06) |
| Iraq | 415 (0.9) |
| Jordan | 2082 (4.7) |
| Kuwait | 1520 (3.4) |
| Lebanon | 453 (1) |
| Libya | 5 (0.01) |
| Morocco | 124 (0.2) |
| Oman | 836 (1.8) |
| Palestine | 151 (0.3) |
| Qatar | 2 (0.004) |
| Saudi Arabia | 22,708 (51.3) |
| Sudan | 476 (1) |
| Tunisia | 1104 (2.5) |
| UAE | 147 (0.3) |
| Yemen | 256 (0.5) |
| Genotypes | |
| Genotypes reported | 14,257 (32.3) |
| HbSS | 10980 (77) |
| HbSβ0 | 1415 (9.9) |
| HbSβ+ | 1037 (7.2) |
| HbSC | 502 (3.5) |
| HbS/α-Thal | 339 (2.3) |
| HBSD | 66 (0.4) |
| HbSO-Arab | 56 (0.3) |
| Hb Sβ0 + Alpha Thal | 42 (0.3) |
| HBSE | 16 (0.1) |
| HBS-Oman | 1 (0.007) |
Table 2.
Number of Studies Published per Country (N=184)
| Country | No. of Studies | Percentage |
|---|---|---|
| Saudi Arabia | 61 | 33.2 |
| Kuwait | 21 | 11.4 |
| Egypt | 20 | 10.9 |
| Oman | 15 | 82 |
| Tunisia | 15 | 82 |
| Bahrain | 14 | 7.6 |
| Sudan | 7 | 3.8 |
| Jordan | 6 | 3.3 |
| Lebanon | 6 | 3.3 |
| Yemen | 5 | 2.7 |
| Morocco | 3 | 1.6 |
| Iran | 2 | 1.1 |
| Iraq | 2 | 1.1 |
| Palestine | 2 | 1.1 |
| UAE | 2 | 1.1 |
| Algeria | 1 | 0.5 |
| Libya | 1 | 0.5 |
| Qatar | 1 | 0.5 |
Genotypes were available for 14,257 of the 44,034 cases (32.7%). The most common SCD genotype reported in this population was Hb SS, present in 77% of cases, followed by Hb Sβ0 in 9.9% and Hb Sβ+ in 7.2%, and HbSC in 3.5%, while the rest of the genotypes, including HbS/α-Thal, HbSD, HbSE, HbSO-Arab, HbSβ0 + Alpha Thal, and HBS-Oman were individually reported in <4% of the cases (N=520). (Table 1).
The most commonly reported complication of SCD was pain crisis (48.25%), followed by neurological complications (33.46%), hepatobiliary complications (25.53%), musculoskeletal complications (24.73%), and hemolytic anemia (23.57%) (Table 3). Other complications were individually reported in <15% of cases. Additionally, as shown in Table 4, treatment for SCD included hydroxyurea in around 20% of the subjects, blood transfusion in 14.32%, and Deferasirox in 3.03%. Not all studies included complication and management data.
Table 3.
Complications of Patients with SCD in the Arab Population (N= 44,034)
| Complication | Frequency | Population* | Percentage |
|---|---|---|---|
| Pain crisis | 1161 | 2407 | 48.23 |
| Neurological complications | 1526 | 4560 | 33.46 |
| Hepatobiliary complications | 1087 | 4258 | 25.53 |
| Musculoskeletal complications | 1277 | 5163 | 24.73 |
| Hemolytic anemia | 434 | 1841 | 23.57 |
| Pulmonary complications | 565 | 3857 | 14.65 |
| Acute splenic sequestration | 335 | 2826 | 11.85 |
| Renal complications | 163 | 2117 | 7.70 |
| Functional asplenia | 85 | 1498 | 5.67 |
| Hyper viscosity | 77 | 1430 | 5.38 |
| Transfusion hemosiderosis | 50 | 1920 | 2.60 |
| Ophthalmologic complications | 76 | 3329 | 2.28 |
| Dermatological complications | 10 | 1359 | 0.74 |
Notes: *Data readily available from the published studies included in the review as the complications were not reported in all the studies identified. Reported from highest to lowest frequency.
Table 4.
Treatment Options Used for Patients with SCD in the Arab Population (N= 44,034)
| Treatment | Frequency | Population* | Percentage |
|---|---|---|---|
| Blood transfusion | 1022 | 7144 | 14.3 |
| Deferasirox | 30 | 989 | 3.03 |
| Hydroxyurea | 663 | 3332 | 19.9 |
| Stem cell transplantation | 0 | ||
| L-Glutamine | |||
| Voxelotor | |||
| Crizanlizumab | |||
| Gene therapy | |||
Note: *Data readily available from the published studies included in the review as complications were not reported in all the studies identified.
Discussion
This study represents a systematic and comprehensive review of all the prior published peer-reviewed articles describing genotypes of SCD in the Arabic population. We captured 44,034 patients diagnosed with SCD in all Arab countries up to November 2020 (Table 1). The Arab world covers over 13 million km2 from North Africa to the Middle East, with a population of over 400 million.23 Arab populations exhibit high levels of genetic diversity which is mainly due to the frequent historical migrations of Arab tribes that lead to an admixture of Arabs with other populations from Asia, Europe, and Africa. Additionally, the high rate of consanguineous marriage and the large size of Arab families has contributed to a high prevalence of autosomal recessive diseases in the area, including SCD.27 Variability in genetic disease manifestations and prognosis based on ethnic differences is well-known.28 As the era of genomic medicine unfolds, it becomes imperative to delineate and understand such variations with a view to furthering personalized medicine in the clinical setting.29 As Arab ethnicity is considerably different from Asian or Western ethnic groups, genetic data from the Arab world needs to be compiled separately for a more accurate genomic assessment.
SCD consists of a group of hemoglobinopathies, all of which contain at least one hemoglobin S allele (HbS) and another abnormal beta-hemoglobin gene (HBB), resulting in disordered hemoglobin polymerization. Two HbS genes (HbSS) is most common and accounts for 60–70% of the SCD cases in the US.30 Other pathogenic HBB variants can be combined with HbS to cause SCD such as HbC, HbE, Hbβ0, Hbβ+, HbD, and HbO Arab.31 In our review, the genotype was available for 14,482 patients which is around 32.7% of all patients in the studies. HbSS was the most common genotype reported in the Arab population at 77% of those reported, followed by HbSβ0 (9.9%) and HbSβ+ (7.2%) (Table 1). These show higher percentages of HbSS and HbSβthal and lower frequencies of HbSC than reported in the US in 2009, where SCD genotypes showed an HbSS frequency of 60%, HbSC (30%) and HbSβthal (10%). This was attributed to the large number of African American and Hispanic people in the US.30
Of interest, 16 patients had the HbSE genotype constituting around one-quarter of the cases of HbSE disease reported worldwide.32 Although HbSE has been considered to have a benign course, recent reviews have shown that HbSE can manifest with severe signs and symptoms of sickling, including acute chest syndrome. Mortality related to severe manifestations of HbSE has been reported in at least 3 cases. These patients died due to ischemia with severe sickle cell crisis, cardiopulmonary collapse secondary to sickle crisis, and massive marrow embolism during admission with pain crisis.33–35
It is important to note that some genotypes such as HbSO-Arab and HBS Oman are primarily seen in Arab ethnicities. Our review found HbSO-Arab and HBS Oman in 56 cases and one case respectively.36–43 Although their prevalence is low, attention is required as genetic therapy might differ for these genotypic variations if a curative treatment is intended.
Saudi Arabia contributed the highest number of SCD cases in our review (Table 1). This has previously been attributed to the prevalence of consanguineous marriages of up to 57.7% of all marriages.44 Several premarital screening programs and awareness programs have been instituted to decrease the prevalence of SCD in Saudi Arabia. These have been successful in decreasing the number of at-risk marriages, but the limited access to health services, the cultural stigmas, and the religious beliefs have made effective genetic counseling and screening difficult.45
Vaso-occlusive pain crisis has been reported previously as the main complication associated with SCD and the primary cause of hospitalization in SCD patients.46 Similarly in our review, vaso-occlusive pain or pain crisis was the most common reported complication (48.23%) (Table 3). This could potentially reflect different phenotypes in the Arab world. However, underreporting of complications and unpublished data could have had an impact on our results.
SCD causes several neurologic complications, such as large artery intracranial occlusive disease, thought to arise due to repeated sickling episodes causing endothelial hyperplasia and intraluminal thrombosis. It can also cause ischemic and hemorrhagic stroke, posterior cerebral encephalopathy syndrome, and cerebral fat embolism.47 In our review, 1526 patients were reported with one or more neurologic complications, indicating a high frequency in SCD patients in the Arab population (Table 3). However, neurologic complications of SCD may be rising globally and the contribution of ethnicity would require a comparative analysis of large studies of different ethnicities. Regardless, neurologic sequela of SCD can have significant impacts on quality of life in children and there have been recommendations for screening children who have poor academic performance via MRI imaging to rule out silent brain infarction (SBI) secondary to SCD.47 Similarly, transcranial Doppler ultrasound (TCD) has also been suggested to screen for new neurological events in cases with or without silent cerebral infarctions.48 This suggestion comes from a prospective cohort study on 421 children with SCD. The incidence of a new cerebral event in patients who had TCD with SBI in the background was higher (1.71 per 100 patient-years) compared to those who had TCD with no SBI (0.47 per 100 patient-years).48 Hence, TCD can be a valuable tool in neurologic risk stratification in patients with SCD.
Anemia and vaso-occlusion in SCD can lead to ischemia and end-organ damage, including musculoskeletal complications such as bone infarction, osteoporosis, and osteonecrosis.49 In our review, 1277 SCD patients were reported to have musculoskeletal complications (Table 3). Vaso-occlusion can also lead to pulmonary complications like acute chest syndrome, chronic pulmonary hypertension, and airway hyper-responsiveness.50,51 In our review, 565 SCD patients were reported to suffer from such complications (Table 3).
Sickle cells can aggregate in the spleen and can result in acute splenic sequestration crises (ASSC), a serious complication in young SCD patients.52 We found 335 Arab world SCD patients (11.85%) with this complication (Table 3), more frequent than reported in Africa, possibly because of the older age of the patients reported in the African studies.53 The studies from the African region have reported ASSC varying from 2% to 27.3%, with an overall prevalence of <10%. The single study that reported a significantly higher percentage (27.3%) compared to other added studies did not document specific details of ASSC.54 SCD patients can also suffer from hepatobiliary complications, including gallstones due to hyperbilirubinemia induced by hemolysis.55 We found 25% of the patients reported having SCD related hepatobiliary complications (Table 3), less than reported in one study from France where 40% of the children with SCD were suffering from hepatobiliary complications.56 Although this difference might be due to the different age groups in both studies (the French study included only patients <18 years of age whereas our review reports ages ranging from 1 years to 74 years), demographic variations in the patient population might also influence the phenotypic manifestations of these genotypes of SCD. Larger studies on genotypic and phenotypic correlations of SCD variants from the Arab world would serve to better answer this and validate our results.
Hydroxyurea has been approved by the FDA for the reduction of painful crises and the need for blood transfusion in homozygous SCD patients.57 It does so by increasing HbF levels, decreasing the proportion of the mutated Hb and resulting in less sickling.58 In our review, only 19.9% of the population for whom hydroxyurea use was reported took hydroxyurea (Table 4). This is low compared to the percentage reported in the USA which showed that one-third of the population included in that study had at least one hydroxyurea prescription.59 This might be attributed to the lower level of healthcare facilities and limited access to mediations in some Arab countries but it also represents an opportunity for improvement in the Arab world.
Red cell transfusion is an essential management component for SCD patients, provided as simple or exchange transfusions. There are multiple indications for red cell transfusion in SCD patients.60 However, chronic transfusion can cause iron overload, particularly in the liver and heart, for which iron chelation therapy becomes necessary.61 Only 14.3% of our study population for whom transfusion therapy was reported had received a blood transfusion, and only 3% of patients received deferasirox to treat iron overload (Table 4). This transfusion rate is much lower than the percentages reported in some African countries like Senegal (28.5%),62 and Congo (80.6%).63 Additionally, the reported use of deferasirox is low. However, it is known that chelation therapy has a low adherence rate. In the RELATH study (Registry of Latin Americans with Transfusional Hemosiderosis), around 46% of patients were reported to receive chelation therapy, out of which 20% were not compliant.64 Another potential reason is the cost of deferasirox, which is estimated to be as high as £26,061 (30 mg/kg dose) per annum for an average 70kg adult.65 Although the reason for comparatively low chelation rates in Arab countries is not apparent, it could be due to the fact that the articles included in this manuscript mainly focused on genotypes of SCD rather than its management. It would seem imperative to further explore treatment rate variations based on regional differences. Some possible reasons could include the cost of treatment, availability of treatment in various regions of the Arab world, access to healthcare, and education about the disease and its management in the general population.
The only well-established curative therapy for SCD patients currently is hematopoietic stem cell transplantation.66 Among the patient population of our review (patients who had genotypes specified), none of them reported stem cell transplantation. Newer therapies have been approved or are being studied in the management of SCD, among which gene therapy has great potential. Although there are several challenges ahead, with the rapid progress and significant research efforts in gene therapy, it is expected that it will help not only SCD but all hemoglobinopathies in the near future.67 Voxelotor is an HbS polymerization inhibitor that’s been approved by the FDA to treat SCD in patients 12 years or older. Patients taking Voxelotor 1500 mg were shown to have a rapid improvement in Hb concentration and a potential reduction in morbidity related to hemolytic anemia in SCD 72 weeks after starting the drug, without significant side effects compared to placebo.68 L-glutamine has also been approved by the FDA to treat SCD. It has been shown to decrease the frequency of pain crises in SCD patients aged 5 years and older over 48 weeks.69 More recently the FDA has approved Crizanlizumab in the treatment of SCD. This is a monoclonal antibody that binds to P-selectin on platelets and endothelial cells, helping to decrease the frequency of vaso-occlusive crises. In SCD patients aged 16 years and older it has shown a significant reduction in the frequency of vaso-occlusive crises.70 In our search, we did not find reports of any patients in the Arab countries who were treated with these newer drugs. Multiple trials are ongoing for the new therapies in SCD, such as Glutamine (NCT0537118471), Crizanlizumab (NCT03814746,72 NCT03474965,73 NCT0465782274), and Voxelotor (NCT03573882,75 NCT04188509,76 NCT02850406,77 NCT03036813,78 NCT0421808479) in SCD in the Arab countries, including Egypt, Oman, Lebanon, Saudi Arabia, and Jordan. As these newer therapies are introduced and reported on in the Arab world, we look forward to better evidence for the efficacy of these treatments in different patient populations.
Our review has limitations, some of which are inherent to the study design used. Firstly, we could only extract and analyze the readily available data from each study, which may have resulted in missing data. Secondly, the true prevalence of various genotypes and phenotypes of SCD might be different than reported as a result of unpublished and unreported data. Thirdly, we could not find any randomized controlled trials to strengthen our findings. We also note that there were no available data for four countries in the Arab world and that more than 75% of the cases reported were from Saudi Arabia and Bahrain, potentially limiting the representation and applicability across the other included countries. Also, other studies reporting data on complications and treatment may have been available but not included in this review if they did not also include genotype data. We also note that only English language articles were included despite the research population being Arabic speaking, potentially causing under-representation from countries that tend to publish in languages other than English. Nevertheless, this review represents the most updated and extensive data on genotypes and phenotypes of SCD across the Arabic world, laying a foundation for future research on the topic, especially concerning gene therapy in SCD.
Conclusion
SCD is a common hemoglobinopathy in the Arab population. Data with regards to its genotypic makeup in the region is scattered and is different from other parts of the world. Many of the approved treatment modalities are yet to be reported in this patient population. Our review highlights the genetic makeup of SCD in the Arab countries with the common phenotypic manifestations. This data will help the direction of further research on SCD in this region, especially with respect to the upcoming era of genetic therapy.
Acknowledgments
The publication of this article was funded by Qatar National Library.
An abstract of this study was presented in 64th ASH Annual Meeting & Exposition and can be found here: https://ashpublications.org/blood/article/140/Supplement%201/11127/491070/Genotypic-and-Phenotypic-Composition-of-Sickle
Funding Statement
The publication of this article was funded by Qatar National Library.
Ethics Approval and Consent to Participate
Ethical approval and consent was not required for this systematic review as only a secondary analysis is performed using already published data from the electronic databases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Mangla A, Ehsan M, Agarwal N, Maruvada S. Sickle cell anemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. [Google Scholar]
- 2.Lonergan GJ, Cline DB, Abbondanzo SL. Sickle cell anemia. Radiographics. 2001;21(4):971–994. doi: 10.1148/radiographics.21.4.g01jl23971 [DOI] [PubMed] [Google Scholar]
- 3.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10 [DOI] [PubMed] [Google Scholar]
- 4.Yassin MA, Soliman AT, De Sanctis V, et al. Statural growth and prevalence of endocrinopathies in relation to Liver Iron Content (LIC) in adult patients with Beta Thalassemia Major (BTM) and Sickle Cell Disease (SCD). Acta Biomedica Atenei Parmensis. 2018;89(2–S):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman AT, Alaaraj N, Yassin M. The effects of treatment with blood transfusion, iron chelation and hydroxyurea on puberty, growth and spermatogenesis in Sickle Cell Disease (SCD): a short update. Acta Biomed. 2021;92(4):e2021386. doi: 10.23750/abm.v92i4.11917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soliman AT, Yasin M, El-Awwa A, Abdelrahman MO, De Sanctis V. Does blood transfusion affect pituitary gonadal axis and sperm parameters in young males with sickle cell disease? Indian J Endocrinol Metab. 2013;17(6):962–968. doi: 10.4103/2230-8210.122599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wali Y, Kini V, Yassin MA. Distribution of sickle cell disease and assessment of risk factors based on transcranial Doppler values in the Gulf region. Hematology. 2020;25(1):55–62. doi: 10.1080/16078454.2020.1714113 [DOI] [PubMed] [Google Scholar]
- 8.Nashwan AJ, Yassin MA, Mohamed Ibrahim MI, Abdul Rahim HF, Shraim M. Iron Overload in Chronic Kidney Disease: less Ferritin, More T2(*)MRI. Front Med. 2022;9:865669. doi: 10.3389/fmed.2022.865669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shier A, Abdelrazek M, Soliman A, et al. Short-term Outcome AND MRI changes in three adult patients with sickle cell disease and aseptic osteonecrosis after treatment with hyperbaric oxygen therapy: a preliminary report. Case Rep Oncol. 2020;13(1):365–372. doi: 10.1159/000506330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassin M, Soliman A, De Sanctis V, et al. Liver Iron Content (LIC) in Adults with Sickle Cell Disease (SCD): correlation with serum ferritin and liver enzymes concentrations in trasfusion dependent (TD-SCD) and Non-Transfusion Dependent (NT-SCD) patients. Mediterr J Hematol Infect Dis. 2017;9(1):e2017037. doi: 10.4084/mjhid.2017.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meremikwu MM, Okomo U. Sickle cell disease. BMJ Clin Evid. 2016;2016:2402. [PMC free article] [PubMed] [Google Scholar]
- 12.David AN, Jinadu MY, Wapmuk AE, et al. Prevalence and impact of sickle cell trait on the clinical and laboratory parameters of HIV infected children in Lagos, Nigeria. Pan Afr Med J. 2018;31:113. doi: 10.11604/pamj.2018.31.113.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wastnedge E, Waters D, Patel S, et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J Glob Health. 2018;8(2):021103. doi: 10.7189/jogh.08.021103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. doi: 10.1371/journal.pmed.1001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballas SK. 7 Sickle cell disease: clinical management. Baillière’s Clin Haematol. 1998;11(1):185–214. doi: 10.1016/S0950-3536(98)80075-9 [DOI] [PubMed] [Google Scholar]
- 16.Therrell BL Jr, Lloyd-Puryear MA, Eckman JR, Mann MY. Newborn screening for sickle cell diseases in the United States: a review of data spanning 2 decades. Semin Perinatol. 2015;39(3):238–251. doi: 10.1053/j.semperi.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Tisdale JF, Thein SL, Eaton WA. Treating sickle cell anemia. Science. 2020;367(6483):1198–1199. doi: 10.1126/science.aba3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier ER. Treatment options for sickle cell disease. Pediatr Clin North Am. 2018;65(3):427–443. doi: 10.1016/j.pcl.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Kapoor S, Little JA, Pecker LH. Advances in the treatment of sickle cell disease. Mayo Clinic Proceed. 2018;93(12):1810–1824. doi: 10.1016/j.mayocp.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 20.Ali MA, Khan A, Khan SI, et al. Efficacy and safety of voxelotor in sickle cell disease: a systematic review. Blood. 2020;136(Supplement 1):35. [Google Scholar]
- 21.El-Hazmi MA, Al-Hazmi AM, Warsy AS. Sickle cell disease in Middle East Arab countries. Indian J Med Res. 2011;134(5):597–610. doi: 10.4103/0971-5916.90984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ata F, Yousaf Z, Sardar S, et al. Protocol for “Genetic composition of sickle cell disease in the Arab population: a systematic review. Health Scie Report. 2022;5(3):e450. doi: 10.1002/hsr2.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contributors W. Arab world. Wikipedia, The Free Encyclopedia; 2022. Available from: https://en.wikipedia.org/w/index.php?title=Arab_world&oldid=1093953425. Accessed June 20, 2022.
- 24.Institute JB. Checklist for case reports. In: The Joanna Briggs Institute Critical Appraisal Tools for Use in. Australia: JBI Systematic Reviews; 2017. [Google Scholar]
- 25.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamamy H, Bittles AH. Genetic clinics in Arab communities: meeting individual, family and community needs. Public Health Genomics. 2009;12(1):30–40. doi: 10.1159/000153428 [DOI] [PubMed] [Google Scholar]
- 28.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine — a time for reckoning with racism. NEJM. 2021;384(5):474–480. doi: 10.1056/NEJMms2029562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chande AT, Nagar SD, Rishishwar L, et al. The impact of ethnicity and genetic ancestry on disease prevalence and risk in Colombia. Front Genet. 2021;12:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4):S512–S521. doi: 10.1016/j.amepre.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 31.Bender MA. Sickle cell disease. In: Adam MP, Ardinger HH, Pagon RA, editors. GeneReviews®. Seattle: University of Washington, Seattle; 1993. [Google Scholar]
- 32.Khamees I, Ata F, Choudry H, Soliman AT, De Sanctis V, Yassin MA. Manifestations of HbSE sickle cell disease: a systematic review. J Transl Med. 2021;19(1):262. doi: 10.1186/s12967-021-02931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith A, Cooper B, Guileyardo J, Mora A. Unrecognized hemoglobin se disease as microcytosis. Bayl Univ Med Cent Proc. 2016;29(3):309–310. doi: 10.1080/08998280.2016.11929447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayburg M, Kalinyak KA, Towbin AJ, Baker PB, Joiner CH. Fatal bone marrow embolism in a child with hemoglobin SE disease. Am J Hematol. 2010;85(3):182–184. doi: 10.1002/ajh.21605 [DOI] [PubMed] [Google Scholar]
- 35.Arbefeville EF, Tebbi CK, Chrostowski L, Adams VI. Sudden death after exercise in an adolescent with hemoglobin SE. Am J Forensic Med Pathol. 2011;32(4):341–343. doi: 10.1097/PAF.0b013e3181d8e390 [DOI] [PubMed] [Google Scholar]
- 36.Wali YA, Al-Lamki Z, Hussein SS, et al. Splenic function in Omani children with sickle cell disease: correlation with severity index, hemoglobin phenotype, iron status, and α -thalassemia trait. Pediatr Hematol Oncol. 2002;19(7):491–500. doi: 10.1080/08880010290097314 [DOI] [PubMed] [Google Scholar]
- 37.Adekile AD, Al-Sherida S, Marouf R, Mustafa N, Thomas D. The sub-phenotypes of sickle cell disease in Kuwait. Hemoglobin. 2019;43(2):83–87. doi: 10.1080/03630269.2019.1610427 [DOI] [PubMed] [Google Scholar]
- 38.Al Balushi HWM, Wali Y, Al Awadi M, et al. The super sickling haemoglobin HbS-Oman: a study of red cell sickling, K(+) permeability and associations with disease severity in patients heterozygous for HbA and HbS-Oman (HbA/S-Oman genotype). Br J Haematol. 2017;179(2):256–265. doi: 10.1111/bjh.14851 [DOI] [PubMed] [Google Scholar]
- 39.Al Jahdhamy R, Makki H, Farrell G, Al Azzawi S. A case of compound heterozygosity for Hb S and Hb S Oman. Br J Haematol. 2002;116(3):504. doi: 10.1046/j.0007-1048.2001.03284.x [DOI] [PubMed] [Google Scholar]
- 40.El-Hazmi MA, Lehmann H. Human haemoglobins and haemoglobinopathies in Arabia: hb O Arab in Saudi Arabia. Acta Haematol. 1980;63(5):268–273. doi: 10.1159/000207414 [DOI] [PubMed] [Google Scholar]
- 41.El-Shafei A, Sandhu RAO. A Pregnancy and sickle cell haemoglobinopathies in Bahrain. Saudi Med J. 1988;9(3):283–288. [Google Scholar]
- 42.Ibrahim SA, Mustafa D. Sickle-cell haemoglobin O disease in a Sudanese family. Br Med J. 1967;3(5567):715–717. doi: 10.1136/bmj.3.5567.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammed AM, Al-Hilli F, Nadkarni KV, Bhagwat GP, Bapat JP. Hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency in hospital births in Bahrain. Ann Saudi Med. 1992;12(6):536–539. doi: 10.5144/0256-4947.1992.536 [DOI] [PubMed] [Google Scholar]
- 44.El-Hazmi MA, Al-Swailem AR, Warsy AS, Al-Swailem AM, Sulaimani R, Al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. 1995;32(8):623–626. doi: 10.1136/jmg.32.8.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alotaibi MM. Sickle cell disease in Saudi Arabia: a challenge or not. J Epidemiol Glob Health. 2017;7(2):99–101. doi: 10.1016/j.jegh.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105(3):237–246. doi: 10.1111/ejh.13430 [DOI] [PubMed] [Google Scholar]
- 47.Farooq S, Testai FD. Neurologic complications of sickle cell disease. Curr Neurol Neurosci Rep. 2019;19(4):17. doi: 10.1007/s11910-019-0932-0 [DOI] [PubMed] [Google Scholar]
- 48.Jordan LC, Roberts Williams DO, Rodeghier MJ, et al. Children with sickle cell anemia with normal transcranial Doppler ultrasounds and without silent infarcts have a low incidence of new strokes. Am J Hematol. 2018;93(6):760–768. doi: 10.1002/ajh.25085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosaraju V, Harwani A, Partovi S, et al. Imaging of musculoskeletal manifestations in sickle cell disease patients. Br J Radiol. 1073;90:20160130. doi: 10.1259/bjr.20160130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khamees I, Yassin M, Rozi W. Acute chest syndrome in sickle cell disease/HBE patient, A case report; 2021. [DOI] [PMC free article] [PubMed]
- 51.Taksande A, Jameel PZ, Pujari D, Taksande B, Meshram R. Variation in pulmonary function tests among children with sickle cell anemia: a systematic review and meta-analysis. Pan Afr Med J. 2021;39(140). doi: 10.11604/pamj.2021.39.140.28755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kane I, Nagalli S. Splenic sequestration crisis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 53.Ladu AI, Aiyenigba AO, Adekile A, Bates I. The spectrum of splenic complications in patients with sickle cell disease in Africa: a systematic review. Br J Haematol. 2021;193(1):26–42. doi: 10.1111/bjh.17179 [DOI] [PubMed] [Google Scholar]
- 54.Ambe JP, Fatunde JO, Sodeinde OO. Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop Doct. 2001;31(1):26–27. doi: 10.1177/004947550103100109 [DOI] [PubMed] [Google Scholar]
- 55.Kyrana E, Rees D, Lacaille F, et al. Clinical management of sickle cell liver disease in children and young adults. Arch Dis Child. 2021;106(4):315–320. doi: 10.1136/archdischild-2020-319778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allali S, de Montalembert M, Brousse V, et al. Hepatobiliary complications in children with sickle cell disease: a retrospective review of medical records from 616 patients. J Clin Med. 2019;8(9):1481. doi: 10.3390/jcm8091481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 2014;30(2):91–96. doi: 10.1007/s12288-013-0261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: what have we learned and what questions still remain? Curr Opin Hematol. 2011;18(3):158–165. doi: 10.1097/MOH.0b013e32834521dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crego N, Douglas C, Bonnabeau E, et al. Sickle-cell disease co-management, health care utilization, and hydroxyurea use. J Am Board Fam Med. 2020;33(1):91–105. doi: 10.3122/jabfm.2020.01.190143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linder GE, Chou ST. Red cell transfusion and alloimmunization in sickle cell disease. Haematologica. 2021;106(7):1805–1815. doi: 10.3324/haematol.2020.270546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynne D, Neumayr MD, Carolyn C, Hoppe MD, Clark Brown MD. Sickle cell disease: current treatment and emerging therapies. Supplement Feature Public. 2019;25(18):S335–S343. [PubMed] [Google Scholar]
- 62.Seck M, Tall A, Faye BF, et al. Evaluation of transfusion practices in sickle cell disease in Senegal: cohort study of 1078 patients with sickle cell disease. Med Sante Trop. 2017;27(4):402–406. doi: 10.1684/mst.2017.0744 [DOI] [PubMed] [Google Scholar]
- 63.Tshilolo LM, Mukendi RK, Wembonyama SO. Blood transfusion rate in congolese patients with sickle cell anemia. Indian J Pediatr. 2007;74(8):735–738. doi: 10.1007/s12098-007-0129-4 [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro LB, Soares EA, Costa FF, Gilli SCO, Olalla Saad ST, Benites BD. The challenges of handling deferasirox in sickle cell disease patients older than 40 years. Hematology. 2019;24(1):596–600. doi: 10.1080/16078454.2019.1657667 [DOI] [PubMed] [Google Scholar]
- 65.McLeod C, Fleeman N, Kirkham J, et al. Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1. doi: 10.3310/hta13010 [DOI] [PubMed] [Google Scholar]
- 66.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41(2):109–117. doi: 10.1038/sj.bmt.1705943 [DOI] [PubMed] [Google Scholar]
- 67.Demirci S, Uchida N, Tisdale JF. Gene therapy for sickle cell disease: an update. Cytotherapy. 2018;20(7):899–910. doi: 10.1016/j.jcyt.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howard J, Ataga KI, Brown RC, et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Haematol. 2021;8(5):e323–e333. doi: 10.1016/S2352-3026(21)00059-4 [DOI] [PubMed] [Google Scholar]
- 69.Niihara Y, Miller ST, Kanter J, et al. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–235. doi: 10.1056/NEJMoa1715971 [DOI] [PubMed] [Google Scholar]
- 70.Han J, Saraf SL, Gordeuk VR. Systematic review of crizanlizumab: a new parenteral option to reduce vaso-occlusive pain crises in patients with sickle cell disease. Pharmacotherapy. 2020;40(6):535–543. doi: 10.1002/phar.2409 [DOI] [PubMed] [Google Scholar]
- 71.Ebeid FSE. Glutamine role in preventing vaso-occlusive crisis among SCD patients (Glu_SCD_Egy). ClinicalTrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05371184?term=GLUTAMINE&cond=sickle+cell+disease&cntry=EG&draw=2&rank=1. Accessed January 21, 2023. [Google Scholar]
- 72.NNP. Study of two doses of crizanlizumab versus placebo in adolescent and adult sickle cell disease patients (STAND). ClinicalTrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03814746?term=Crizanlizumab&cntry=OM&draw=2&rank=3. Accessed February 16, 2023. [Google Scholar]
- 73.Patients SoDCaSoCiPSCD. Study of dose confirmation and safety of crizanlizumab in pediatric sickle cell disease patients. ClinicalTtrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03474965?term=Crizanlizumab&cntry=OM&draw=2&rank=2. Accessed January 21, 2023. [Google Scholar]
- 74.NNP. Rollover study for patients with sickle cell disease who have completed a prior Novartis-sponsored crizanlizumab study. ClinicalTrials.gov; 2023. Available from: https://clinicaltrials.gov/ct2/show/NCT04657822?term=Crizanlizumab&cntry=OM&draw=2&rank=1. Accessed January 21, 2023. [Google Scholar]
- 75.Therapeutics GB. Study to assess the effect of long-term treatment with voxelotor in participants who have completed treatment in study GBT440-031 (034OLE); 2022. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03573882?term=voxelotor&cntry=LB&draw=2&rank=4. Accessed January 21, 2023. [Google Scholar]
- 76.Therapeutics GB. Open-label extension of voxelotor. ClinicalTrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04188509?term=voxelotor&cntry=LB&draw=2&rank=3. Accessed January 21, 2023. [Google Scholar]
- 77.Therapeutics GB. Study to evaluate the effect of GBT440 in pediatrics with sickle cell disease (HOPE Kids). ClinicalTrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02850406?term=voxelotor&cntry=LB&draw=2&rank=2. Accessed January 21, 2023. [Google Scholar]
- 78.Study to evaluate the effect of voxelotor administered orally to patients with sickle cell disease (GBT_HOPE) (GBT_HOPE). ClinicalTrials.gov; 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03036813?term=voxelotor&cntry=LB&draw=2&rank=1. Accessed January 21, 2023. [Google Scholar]
- 79.Therapeutics GB. Study to evaluate the effect of GBT440 on TCD in pediatrics with sickle cell disease (HOPE Kids 2). ClinicalTrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04218084?term=voxelotor&cntry=SA&draw=2&rank=1. Accessed January 21, 2023. [Google Scholar]