Abstract
To learn more about how dyneins are targeted to specific sites in the flagellum, we have investigated a factor necessary for binding of outer arm dynein to the axonemal microtubules of Chlamydomonas. This factor, termed the outer dynein arm-docking complex (ODA-DC), previously was shown to be missing from axonemes of the outer dynein armless mutants oda1 and oda3. We have now partially purified the ODA-DC, determined that it contains equimolar amounts of Mr ∼105,000 and ∼70,000 proteins plus a third protein of Mr ∼25,000, and found that it is associated with the isolated outer arm in a 1:1 molar ratio. We have cloned a full-length cDNA encoding the Mr ∼70,000 protein; the sequence predicts a 62.5-kDa protein with potential homologs in higher ciliated organisms, including humans. Sequencing of corresponding cDNA from strain oda1 revealed it has a mutation resulting in a stop codon just downstream of the initiator ATG; thus, it is unable to make the full-length Mr ∼70,000 protein. These results demonstrate that the ODA1 gene encodes the Mr ∼70,000 protein, and that the protein is essential for assembly of the ODA-DC and the outer dynein arm onto the doublet microtubule.
INTRODUCTION
Dyneins are large, multisubunit molecular motors that generate force against microtubules. Cilia and flagella contain three major classes of dyneins: cytoplasmic dynein, of which cytoplasmic dynein 1b/2 is the retrograde motor for intraflagellar transport (Pazour et al., 1998, 1999; Porter et al. 1999); axonemal inner arm dyneins, of which there may be as many as seven different forms (Porter and Sale, 2000); and axonemal outer arm dynein, of which only one form is currently known (Witman et al., 1994). Each of these different dyneins binds with high fidelity to sites that are specific for that particular dynein. Thus, these sites must be structurally or biochemically unique in a way that ensures that the correct dynein is targeted to them. The molecular basis for this specific binding is of great interest because it literally provides the foundation for the proper functioning of the flagellum. Moreover, knowledge of how these dyneins are targeted to their correct binding sites may provide a paradigm for how other axonemal components, e.g., the radial spokes and the projections of the central pair microtubules, are correctly positioned to form one of the most complex and highly ordered macromolecular structures in the cell.
To learn more about specific targeting of dyneins, we have been studying the Chlamydomonas reinhardtii outer arm dynein, which is the most well characterized of axonemal dyneins (Witman et al., 1994; Pazour and Witman, 2000). This dynein, which produces as much as four-fifths of the force for flagellar movement (Brokaw, 1994), is attached to specific sites on the A-tubules of the flagellar doublet microtubules and repeats at 24-nm intervals along the length of the doublet. It contains three dynein heavy chains (DHCs) (termed α, β, and γ), two intermediate chains (ICs) (termed IC78 and IC69), and several light chains (LCs) (Figure 6B). The two ICs and most of the LCs are associated in an IC/LC complex located at the base of the dynein (King and Witman, 1990). One of the ICs, IC78, is in direct contact with tubulin in vivo (King et al., 1991) and is a microtubule-binding protein in vitro (King et al., 1995), so it is believed to be at least one of the dynein components that anchors the outer arm to the A-tubule.
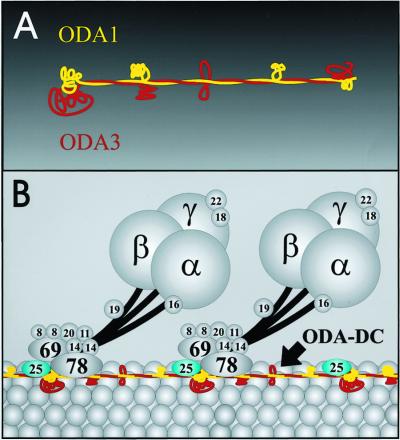
Figure 6.
Models for ODA-DC structure. (A) Diagram illustrating how the Mr ∼70,000 and ∼105,000 ODA-DC proteins might interact. In this model, the two proteins (Oda1 and Oda3, respectively) interact via their coiled-coil domains to form a rod-shaped heterodimer; noncoiled-coil regions loop out from the rod. (B) Diagram illustrating how the ODA-DCs might link up end-to-end to form a filament running the length of the A-tubule. The Mr ∼25,000 ODA-DC protein (blue subunit marked 25) is shown attached to one end of the rod-shaped ODA-DC; this placement is purely speculative. In this model, the ODA-DCs repeat with the same 24-nm periodicity as the outer dynein arms, two of which are shown attached to the ODA-DC and A-tubule. The α, β, and γ heavy chains of the outer arms are labeled α, β, and γ, respectively; other labels identify the dynein intermediate and light chains by apparent molecular weight (King and Witman, 1989).
Studies of Chlamydomonas mutants lacking the outer dynein arm (oda mutants) indicate that an additional factor is necessary for efficient assembly of the outer arm onto flagellar doublet microtubules. When the Chlamydomonas outer arm dynein is removed from the wild-type axoneme by extraction with 0.6 M KCl in the absence of Mg2+, it dissociates into a single-headed γ subunit (containing the γ DHC and two LCs) that sediments as a 12S particle, and a two-headed αβ subunit (containing the α and β DHCs, the two ICs, and all the remaining LCs) that sediments as a 21S particle (Piperno and Luck, 1979; Pfister et al., 1982; King and Witman, 1989). When these two particles were purified and added back to axonemes of the outer armless mutants oda2, oda4, oda5, or oda6, outer arms were reconstituted in their correct positions on the doublet microtubules (Takada and Kamiya, 1994). In contrast, if the subunits were added back to axonemes of the outer armless mutants oda1 or oda3, very few arms rebound. Efficient rebinding of arms to oda1 or oda3 axonemes required a factor that was present in the 0.6 M KCl extract of wild-type axonemes and sedimented at 7S. This factor apparently was missing in axonemes of oda1 and oda3, but present in axonemes of oda2, oda4, oda5, and oda6. The only discernible structural difference between axonemes of these two groups of mutants was that the latter had a small projection at the site where the outer arm normally would attach. These findings strongly suggested that the projection facilitates attachment of the dynein to the doublet microtubule, and that the 7S factor is the solubilized form of the projection. Partial purification of the factor and comparison of extracts from oda mutant axonemes showed that the factor was correlated with a polypeptide of Mr ∼70,000 (Takada and Kamiya, 1994). Because the factor can assemble onto the doublet microtubules in the absence of the outer dynein arms in vivo, and is necessary for binding of the arms to the microtubules, it has been termed the outer dynein arm-docking complex (ODA-DC).
In this report, we show that the ODA-DC contains equimolar amounts of proteins of Mr ∼105,000 and ∼70,000 plus a third protein of Mr ∼25,000, that it is present in a 1:1 stoichiometry with the outer dynein arm polypeptides, and that it remains associated with the outer dynein arm subunits when these subunits are isolated under conditions that keep them together as a three-headed αβγ complex (Takada et al., 1992). We have cloned and sequenced a full-length cDNA encoding the Mr ∼70,000 protein; the sequence predicts a novel 62-kDa polypeptide with three long coiled-coil domains. Sequencing of the corresponding DNA from the outer armless mutant oda1 reveals that it has a mutation resulting in a stop codon just downstream from the translation initiation site; hence, it is unable to make the Mr ∼70,000 ODA-DC protein. These results indicate that the Mr ∼70,000 polypeptide is the ODA1 gene product and is essential for assembly of both the outer dynein arm and the ODA-DC onto the doublet microtubule. We previously showed that the ODA3 gene product corresponds to the Mr ∼105,000 ODA-DC polypeptide and is predicted also to have three long coiled-coil regions (Koutoulis et al., 1997). Herein, we use a zero-length protein cross-linker to show that the ODA1 gene product is in direct contact with the ODA3 gene product in the soluble ODA-DC. Therefore, the ODA1 and ODA3 gene products interact with each other, possibly via their coiled-coil domains, and with an Mr ∼25,000 subunit to form a structure that targets the outer dynein arm to its correct attachment site on the doublet microtubule. Potential homologs of the ODA1 gene are expressed in higher organisms, including humans, suggesting that the ODA-DC is widespread in ciliated animals.
MATERIALS AND METHODS
Strains
C. reinhardtii strains used were wild type (137c), and outer armless mutants oda1-1, oda3-1, and oda6-1, all of which were derived from strain 137c (Kamiya, 1988).1 Cells were grown in liquid culture as described by Witman (1986).
Characterization of the ODA-DC
Flagellar axonemes from oda6 cells were isolated by the method of Witman (1986), washed with 0.5 M potassium acetate in 30 mM HEPES pH 7.5, 5 mM MgSO4, 1 mM dithiothreitol, 0.5 mM EGTA (Nakamura et al., 1997), and then extracted with 0.6 M KCl in HMDEK (30 mM HEPES pH 7.5, 5 mM MgSO4, 1 mM dithiothreitol, 0.5 mM EGTA, 25 mM potassium acetate) to solubilize the ODA-DC. Axonemes from oda3 cells, which lack the ODA-DC, were treated identically. The extracts were fractionated by 5–20% sucrose density gradient centrifugation under Mg2+-free conditions (Piperno and Luck, 1979; Pfister et al., 1982). Fractions were collected from the bottom of the tube and their proteins analyzed by SDS-PAGE.
Isolation of Three-headed Outer Arm Dynein
Axonemes from wild-type cells were isolated, washed, and extracted as described above. The dynein extract was then fractionated by centrifugation (5-ml tube, Beckman SW50.1 rotor, 39,000 rpm for 7 h) through a 5–20% sucrose density gradient containing 5 mM Mg2+ (Takada et al., 1992).
SDS-PAGE and Band Quantitation
Proteins from sucrose density gradient fractions were separated by electrophoresis in 5–20% polyacrylamide-SDS gels (King et al., 1986). The gels were stained with Coomassie blue and photographed on 35-mm Tech Pan film. Negatives were scanned with a Nikon Coolscan II film scanner. The resulting image files were analyzed and bands of interest integrated using one-dimensional gel analysis software (Quantity One; PDI, Huntington Station, NY).
Immunoprecipitation of the ODA-DC
In Absence of Mg2+.
Axonemes from wild-type cells were isolated, washed, and extracted as described above. The extract was dialyzed against TEDKS (30 mM Tris pH 7.5, 0.5 mM EDTA, 1 mM dithiothreitol, 25 mM KCl, 10% sucrose) containing 1 mM phenylmethylsulfonyl fluoride overnight at 4°C to dissociate outer arm dynein from the ODA-DC, and then dialyzed against phosphate-buffered saline (PBS) (6.4 mM Na2HPO4, 0.15 mM KH2PO4 pH 7.5, 137 mM NaCl, 2 mM KCl) at 4°C for 5 h. To biotinylate the proteins in the extract, sulfo-N-hydroxysuccinimide-biotin (Pierce Chemical, Rockford, IL) was added to an ∼50-fold molar excess over protein and the extract incubated at 24°C for 30 min. To remove unreacted biotin, the extract was dialyzed against PBS at 4°C overnight. The biotinylated extract was divided into two tubes (48 μg of protein/tube) and diluted to 200 μl with IP buffer 1 (3% bovine serum albumin [BSA], 1% Nonidet P-40, 10 mM Tris pH 8.0, 150 mM NaCl). Rabbit polyclonal IgG (4 μg) specific for the Mr ∼105,000 ODA-DC polypeptide (anti-DC105 antibody) (Wakabayashi et al., 2001) was added to one of the tubes and incubated on ice for 2 h. As a control, 4 μg of rabbit normal IgG was added to the other tube. Affi-Prep protein A beads (20 μl ; Bio-Rad, Hercules, CA) in 100 μl of IP buffer 1 were added and incubated at 4°C for 1 h. The beads were recovered by centrifugation and washed with IP buffer 1 at 4°C for 5 min, three times. A final wash was performed with IP buffer 1 without BSA at 4°C for 10 min. Then 50 μl of 1× SDS-PAGE sample buffer was added to the beads and the sample boiled at 100°C for 5 min. Proteins in the resulting supernatants were separated by electrophoresis in 10% SDS-polyacrylamide gels, transferred to a nitrocellulose membrane, probed with streptavidin-horseradish peroxidase (HRP) (Molecular Probes, Eugene, OR), and detected using a chemiluminescent substrate. Simultaneously, an extract from oda1 axonemes was prepared and processed identically.
In Presence of Mg2+.
Axonemes from wild-type cells were isolated and extracted with 0.6 M KCl in HMDEK as described above except that the 0.5 M potassium acetate wash was omitted. The extract was dialyzed against HMDEK for 8 h. Immunoprecipitation was performed by the method of Fowkes and Mitchell (1998) with modifications. A 0.5-ml aliquot of the 0.6 M KCl extract (2.8 mg/ml) was added to IP buffer 2 (HMDEK, 75 mM NaCl, 3% BSA, 0.1% Triton X-100, pH 7.4). This mixture was precleared by incubation with protein A-agarose (Roche Diagnostics, Tokyo, Japan) for 30 min at 4°C, followed by centrifugation. The supernatant was then incubated with the anti-DC105 antibody or normal rabbit IgG for 4 h followed by incubation with protein A-agarose for 1 h. The precipitated agarose beads were washed twice with IP washing buffer (IP buffer 2 with 0.05% Triton X-100) and twice with IP washing buffer without BSA. The immune complex was eluted by addition of an equal volume of 2× SDS-sample buffer and incubation at 100°C for 3 min. The eluted proteins were separated by electrophoresis in 6.5% SDS-polyacrylamide gels and transferred to Immobilon membranes (Millipore, Bedford, MA). Immunoreactive bands were detected using HRP-conjugated anti-rabbit IgG or anti-mouse IgG secondary antibodies and a chemiluminescent substrate.
Isolation of Mr ∼70,000 ODA-DC Protein and Peptide Sequencing
Proteins in 5–20% polyacrylamide gels of sucrose gradient fractions containing the ODA-DC from oda6 cells were transferred electrophoretically to polyvinylidene difluoride membrane (Immobilon-PSQ; Millipore) and stained with Ponceau S. A band at Mr ∼70,000, present in the 7S fraction from oda6 but absent in comparable fractions from oda3, was excised and digested with trypsin. The resulting peptides were separated by reverse phase high-performance liquid chromatography, and the amino-terminal sequences of three of the peptides (peptides 1, 2, and 3; Table 1) were determined directly using an amino acid sequencer (model 477A; Applied Biosystems, Foster City, CA).
Table 1.
Amino acid sequences of tryptic peptides from the Mr ∼70,000 ODA-DC protein and nucleotide sequences of PCR primers used to clone the protein Primers A and B were designed from the sequence PGDPFAQ of peptide 1, C from AAEKVEM of peptide 2, and D from MAQNVA of peptide 2. Nucleotide sequences were predicted with reference to Chlamydomonas codon usage (Harris, 1989; Wilkerson et al., 1994). Forward primers have additional cgcg and EcoRI sites, and reverse primers have cgcg plus BamHI sites, for subcloning into the EcoRI/BamHI site of pBluescript II.
| Amino acid sequence of peptides | |
| Peptide 1 | FSVRPGDPFAQALINR |
| Peptide 2 | AMAQNVAAEKVEMYGQAFKR |
| Peptide 3 | KAQQGTDGLAEALLAQPLTQPG |
| Nucleotide sequence of PCR primers | |
| Primer A (forward) | 5′-cgcggaattccc[gc]gg[tc]ga[tc]cc[gc]tt[tc]gc[gatc]ca[ga]-3′ |
| Primer B (reverse) | 5′-cgcgggatcc[tc]tg[gatc]gc[ag]aa[cg]gg[ag]tc[ag]cc[cg]gg-3′ |
| Primer C (forward) | 5′-cgcggaattcgc[gatc]gc[gatc]gagaaggt[gc]gagatg-3′ |
| Primer D (reverse) | 5′-cgcgggatcc[gatc]cg[gc]tgcaagac[gatc]cggta-3′ |
Amplification and Cloning of Partial cDNA Encoding the Mr ∼70,000 ODA-DC Protein
Wild-type cells were deflagellated by pH shock (Witman et al., 1972) in modified Sager and Granick Medium I (Witman, 1986), and allowed to regenerate new flagella. Total RNA was isolated from the cells ∼30 min after deflagellation (Wilkerson et al., 1994). First strand cDNA was made from the RNA by using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT as primer. cDNA fragments were amplified using specific primers (Table 1) designed from the peptide sequences of the Mr ∼70,000 protein. Polymerase chain reaction (PCR) products were subcloned between the EcoRI and BamHI sites of pBluescript II KS (−) (Stratagene, La Jolla, CA), and their ends were sequenced using Sequenase version 2.0 and the 7-deaza-dGTP sequencing kit (U.S. Biochemical, Cleveland, OH). A 750-base pair insert (pcST8-1) amplified by primers A and D was found to be correct, having additional sequences contained in peptides 1 and 2 but not used to design the primers. This cDNA was labeled with [γ-32P]dCTP by using the Stratagene random primer labeling kit.
Cloning of Full-Length cDNAs for the Mr ∼70,000 ODA-DC Protein
A λZAPII cDNA library of a C. reinhardtii wild-type strain (Wilkerson et al., 1995) was screened with the DNA probe pcST8-1. Positive phage plaques were subjected to a second round of screening. A phagemid pBluescript II SK (−) having a 2.3-kb insert (pcST737) was rescued by in vivo excision. pcST737 was digested with PstI, BamHI, SacII, and XhoI, and four fragments were subcloned. The ends of the fragments were sequenced and the data used to design internal sequencing primers. Single-stranded DNA from both strands of clone pcST737 was isolated and completely sequenced using Sequenase version 2.0 and the 7-deaza-dGTP sequencing kit (U.S. Biochemical).
Characterization of the Mutant Gene in oda1-1
oda1-1 cells were deflagellated by pH shock and allowed to regenerate flagella for 40 min under illumination, at which time total RNA was isolated from these cells and from oda1-1 and wild-type cells that had not been deflagellated. First-strand cDNA was made from the oda1-1 and wild-type RNA by using reverse transcriptase and oligo-dT primers. The PCR was then carried out with Elongase Enzyme Mix (Invitrogen), which contains Taq DNA polymerase and the proofreading Pyrococcus sp. GB-D polymerase for high fidelity, and by using two primers (Figure 2A, double underlines) designed to amplify the complete open reading frame encoding the Mr ∼70,000 ODA-DC protein. Products were cloned between the EcoRI and KpnI sites of pBluescript II KS (−) and subjected to in vitro transcription by using T7 RNA polymerase; the transcripts were then translated in vitro by using a reticulocyte lysate system (Promega, Madison, WI) containing [35S]methionine. The translation products were separated in 7.5 or 10% polyacrylamide gels and autoradiographed. Four clones obtained from oda1-1 were sequenced at their 5′ ends.
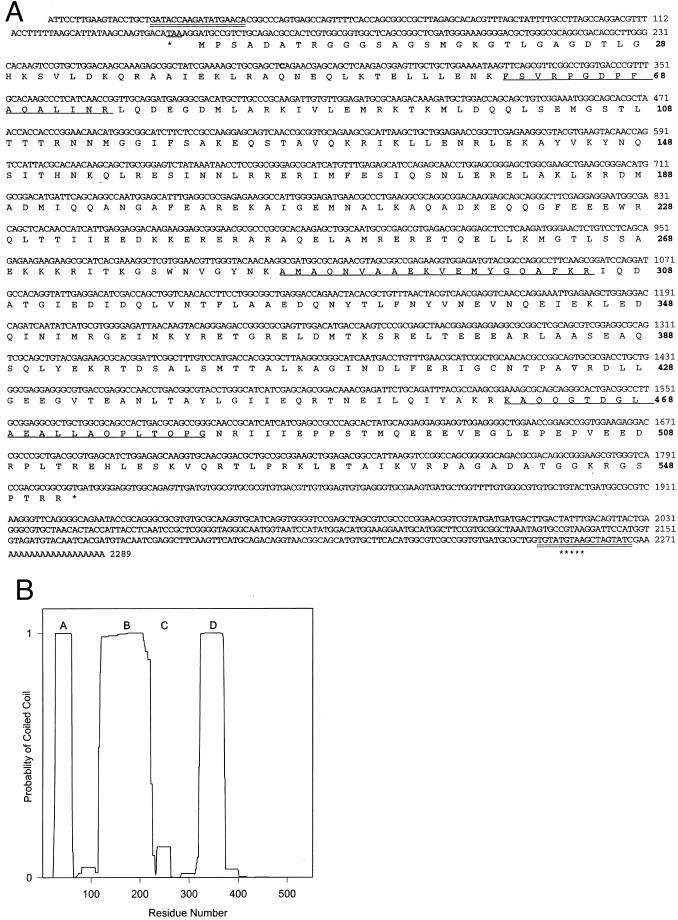
Figure 2.
Sequence and predicted coiled-coil structure of the Mr ∼70,000 ODA-DC protein. (A) Nucleotide sequence of a cDNA clone encoding the protein, and its deduced amino acid sequence. An in-frame stop codon just upstream of the predicted translation initiator ATG is indicated by an underline and an asterisk; the stop codon at the end of the long open reading frame is marked by an asterisk. Lines under the deduced amino acids indicate sequence that exactly matches that obtained by direct microsequencing of three tryptic peptides from the Mr ∼70,000 protein. Two nucleotide sequences that were used for PCR primers to amplify the complete protein coding region from oda1-1 and wild-type first-strand cDNA are indicated by double underlines in the 5′- and 3′-untranslated regions. A complete Chlamydomonas polyadenylation signal sequence (TGTAA) is marked by a row of asterisks. The C at nucleotide position 283, which is changed to a T in oda1-1, is shown in bold. These sequence data are available from GenBank/European Molecular Biology Laboratory/DNA Data Bank of Japan under accession no. AY039618. (B) The deduced amino acid sequence of the Mr ∼70,000 protein was analyzed using the program COILS (MTIDK matrix, with a 2.5 weighting of hydrophobic positions a and d), which estimates the probability that a region of polypeptide will form a coiled-coil structure (Lupas, 1996a). Regions A, B, and D (amino acids 27–60, 120–215, and 323–370, respectively) have a high probability (>99%) of forming coiled-coils. Region C (amino acids 234–262) is predicted to be α-helical, but when the NEWCOILS program is run with weighting of hydrophobic positions a and d, this region is not predicted to form a coiled coil.
Computational Analysis
The GCG suite of programs (Devereux et al., 1984) was used for sequence assembly and protein structure predictions. The program COILS (Lupas et al., 1991; Lupas, 1996a) was used to predict regions of coiled-coil structure. The PSI-BLAST program (Altschul et al., 1997) was used to search databases for related sequences. The program CLUSTAL W (Thompson et al., 1994) was used to align homologs to the Chlamydomonas sequence. The PROSITE database was used to determine possible sites for post-translational modifications (Bairoch et al., 1995).
Protein Cross-Linking
Preparation of 0.6 M KCl extracts of wild-type axonemes was as described under “Characterization of the ODA-DC” but without the 0.5 M potassium acetate wash. The zero-length cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Sigma Chemical, St. Louis, MO) was added to aliquots of the extract to various final concentrations and the mixtures incubated at 24°C for 1 h. The cross-linking reactions were stopped by addition of equal volumes of 2× SDS-PAGE sample buffer. The cross-linked products were separated by electrophoresis on 6% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, probed with a rabbit polyclonal antibody specific for the Mr ∼70,000 ODA-DC polypeptide (anti-DC70 antibody) (Wakabayashi et al., 2001), and immunoreactive bands were detected with HRP-conjugated anti-rabbit IgG and a chemiluminescent substrate. The membrane was then stripped and reprobed with the anti-DC105 antibody.
RESULTS
ODA-DC Contains Three Polypeptides
The ODA-DC previously was shown to be absent from axonemes of the outer dynein armless mutants oda1 and oda3, but to be present and functional in axonemes of mutants oda2, oda4, oda5, and oda6 (Takada and Kamiya, 1994). The complex also was shown to be present in 0.6 M KCl extracts of oda6 axonemes, and to sediment at 7S in Mg2+-free sucrose density gradients. Therefore, to partially purify the complex and positively identify its polypeptide components, axonemes of oda3 and oda6 were isolated, pre-extracted with 0.5 M potassium acetate to remove proteins that otherwise would contaminate the 0.6 M KCl fraction (Nakamura et al., 1997), and then extracted with 0.6 M KCl. The 0.6 M KCl extracts were fractionated by sucrose density gradient centrifugation, and the polypeptide composition of the fractions examined by SDS-PAGE. Polypeptides of Mr ∼105,000, ∼70,000, and ∼25,000 cosedimented at 7S in the oda6 fractions but were uniquely missing from the oda3 fractions (cf. Figure 1, A and B). The Mr ∼70,000 polypeptide previously had been identified as a component of the ODA-DC (Takada and Kamiya, 1994). The present results provide evidence that the factor contains additional proteins of Mr ∼105,000 and ∼25,000.
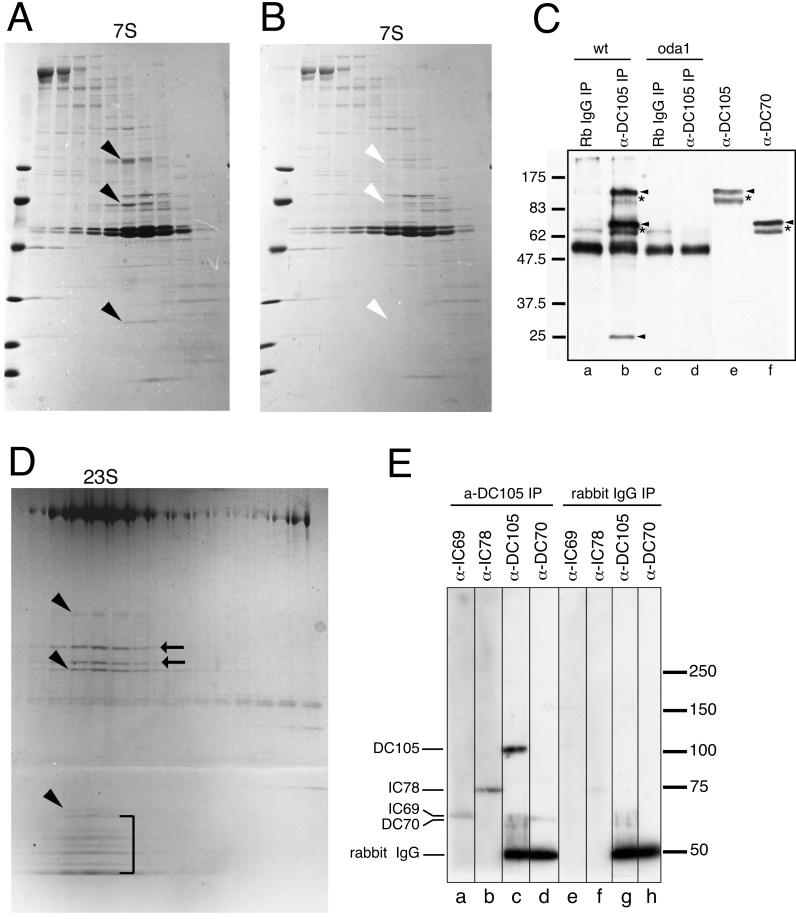
Figure 1.
ODA-DC composition and association with dynein. (A and B) SDS-PAGE analyses of fractions from sucrose density gradient centrifugations of high-salt extracts from axonemes of mutant strains oda6 (A) and oda3 (B). Oda6 axonemes have ODA-DCs but lack outer arms; oda3 axonemes lack both ODA-DCs and outer arms. Three proteins (Mr ∼105,000, ∼70,000, and ∼25,000, black arrowheads in A) cosediment at 7S in the sucrose gradient fractionation of the oda6 extract, but are specifically missing in comparable fractions from the mutant oda3 (B, white arrowheads). The left lane in each gel was loaded with molecular weight standards (97.4, 66.2, 45.0, 31.0, 21.5, and 14.4 kDa). Gradients shown in A and B were centrifuged under standard conditions in the absence of Mg2+ (Piperno and Luck, 1979; Pfister et al., 1982). (C) Immunoprecipitation of the ODA-DC in the absence of Mg2+. The anti-DC105 antibody was used to immunoprecipitate the complex from biotinylated 0.6 M KCl extracts of wild-type (wt) and oda1 (oda1) axonemes. The immunoprecipitated proteins were then separated by SDS-PAGE, transferred to nitrocellulose, and probed with streptavidin-HRP (lanes a–d). The anti-DC105 antibody immunoprecipitated three proteins (arrowheads, Mr ∼105,000, ∼70,000, ∼25,000) from wild-type axonemal extracts (lane b). None of these three proteins were immunoprecipitated from wild-type extracts by using rabbit normal IgG (lane a), or from oda1 axonemal extracts by using the anti-DC105 antibody (lane d) or rabbit normal IgG (lanes c). The wild-type biotinylated 0.6 M KCl extract also was analyzed in Western blots probed with antibodies specific for the Mr ∼105,000 and ∼70,000 proteins to confirm the relative mobilities of the biotinylated proteins (lanes e and f). During the two overnight dialyses necessary to prepare the 0.6 M KCl extracts for immunoprecipitation, some proteolysis of the Mr ∼105,000 and ∼70,000 proteins occurred, resulting in minor immunoreactive fragments (*, lane b) running just below the intact proteins. The samples used for the Western blots were stored at 4°C for several days longer to accentuate the proteolytic fragments (*, lanes e and f). Numbers on left indicate molecular weight markers. (D) SDS-PAGE analyses of extract of wild-type axonemes centrifuged in a sucrose density gradient at low hydrostatic pressure in the presence of Mg2+; under these conditions the three ODA-DC proteins (arrowheads) cosediment with the three-headed outer arm dynein at 23S. Outer arm dynein intermediate chains (IC69 and IC78) and light chains are indicated by arrows and a bracket, respectively. A, B, and D are 5–20% acrylamide gradient gels (5–20%); Coomassie blue stain. (E) Dynein coimmunoprecipitates with the ODA-DC in the presence of Mg2+. Wild-type axonemes were extracted with 0.6 M KCl in HMDEK, the ODA-DC immunoprecipitated by using the rabbit polyclonal anti-DC105 antibody, and the immunoprecipitate analyzed by Western blotting. Antibodies specific for the Mr ∼105,000 (α-DC105, lane c) and Mr ∼70,000 (α-DC70, lane d) ODA-DC polypeptides and the outer dynein arm intermediate chains IC69 (α-IC69, lane a) and IC78 (α-IC78, lane b) each recognized a protein of the appropriate size in the immunoprecipitate. None of these proteins were immunoprecipitated by the normal rabbit IgG (lanes e–h). The anti-rabbit IgG secondary antibody used to probe lanes c, d, g, and h also detected the rabbit IgG used for the immunoprecipitation (dark bands at Mr ∼50,000 in lanes c, d, g, and h); the anti-mouse IgG secondary antibodies used to probe lanes a, b, e, and f did not detect the rabbit IgG. Numbers on right indicate molecular weight markers.
To confirm that the Mr ∼105,000, ∼70,000, and ∼25,000 polypeptides occur together as a complex, and to investigate whether the wild-type complex contains additional polypeptides not identified in the above-mentioned sucrose density gradient analyses, an antibody specific for the Mr ∼105,000 polypeptide (Wakabayashi et al., 2001) was used to immunoprecipitate the ODA-DC from an 0.6 M KCl extract of wild-type axonemes. Before the immunoprecipitation, the extract was dialyzed against a Mg2+-free buffer to dissociate the ODA-DC from the outer arm dynein, and then the proteins in the extract were biotinylated. The immunoprecipitated proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected using streptavidin-HRP. Three major bands corresponding to the Mr ∼105,000, ∼70,000, and ∼25,000 polypeptides were detected in the wild-type immunoprecipitate (Figure 1C, lane b). Except for two proteolytic fragments and bands present in the normal IgG control (Figure 1C, lane a), no additional bands were observed. These three polypeptides were specifically missing in immunoprecipitates prepared identically from 0.6 M KCl extracts of oda1 axonemes (Figure 1C, lane d), which lack the ODA-DC. Western blot analyses of the wild-type 0.6 M KCl extract verified that the biotinylated Mr ∼105,000 and ∼70,000 ODA-DC polypeptides comigrated with the two major high-molecular-weight proteins in the immunoprecipitate (Figure 1C, lanes e and f). These results confirm that the three putative ODA-DC polypeptides occur together in a complex in wild-type axonemes. Moreover, because a functional ODA-DC can be isolated from the 0.6 M KCl extract of wild-type axonemes (Takada and Kamiya, 1994), it is likely that these three polypeptides are sufficient for ODA-DC function and constitute the entire ODA-DC.
The ODA-DC Is Associated with Soluble Three-headed Outer Arm Dynein
When the outer dynein arm is removed from the wild-type axoneme by extraction with 0.6 M KCl and then purified by sucrose density gradient centrifugation in the presence of Mg2+, the α, β, and γ subunits remain associated as a “three-headed” complex that sediments at 23S (Takada et al., 1992). In contrast to the “two-headed” αβ subunit and “single-headed” γ subunit isolated in the absence of Mg2+, this three-headed dynein is competent to bind to the correct sites on oda1 axonemes without addition of exogenous ODA-DC (Takada et al., 1992). This raised the question of whether the ODA-DC might remain associated with the soluble three-headed dynein under these conditions. To investigate this, the three-headed dynein was prepared by the method of Takada et al. (1992), with the sucrose gradient centrifugation carried out at relatively low hydrostatic pressure to prevent dissociation of the outer arm dynein subunits (Nakamura et al., 1997). SDS-PAGE analysis of the resulting sucrose density gradient fractions revealed that the Mr ∼105,000, ∼70,000, and ∼25,000 polypeptides now cosedimented with each other and with the outer arm polypeptides at 23S (Figure 1D). Therefore, in the presence of Mg2+ and low hydrostatic pressure, the ODA-DC remains associated with the α, β, and γ outer arm dynein subunits. This is the first demonstration that the ODA-DC and the outer dynein arm directly interact with each other. That all three ODA-DC polypeptides now sediment together at 23S provides additional evidence that they are all part of the same complex.
To obtain further evidence for the direct interaction of the ODA-DC and outer arm dynein in the presence of Mg2+, a 0.6 M KCl extract in HMDEK was prepared from wild-type axonemes. The ODA-DC was immunoprecipitated from the extract by using the antibody specific for the Mr ∼105,000 ODA-DC polypeptide. Western blot analysis indicated that, as expected, the immunoprecipitate contained both the Mr ∼105,000 and ∼70,000 ODA-DC polypeptides (Figure 1E, lanes c and d). To determine whether dynein was coimmunoprecipitated with the ODA-DC, the blots were probed with mouse monoclonal antibodies 1869A and 1878A specific for the outer arm dynein intermediate chains IC69 and IC78, respectively (King et al., 1991). Both intermediate chains were detected (Figure 1E, lanes a and b). These results confirm that the ODA-DC is associated with outer arm dynein in the presence of Mg2+.
Molar Ratios of ODA-DC Proteins
To determine the stoichiometries of the ODA-DC polypeptides relative to themselves and to the outer dynein arm, quantitative densitometry was carried out on gels such as that shown in Figure 1D. The amount of Coomassie blue dye bound to a protein is approximately proportional to the number of positive charges on the protein (Tal et al., 1980). The combined mole percentages of arginine, lysine, and histidine in the Mr ∼105,000 protein, the Mr ∼70,000 protein, IC78, and IC69 are 18, 16, 15, and 13, respectively. Therefore, one would expect the values obtained by quantitative densitometry of a Coomassie blue-stained gel to accurately reflect the relative amounts of these four proteins. From the gel shown in Figure 1D, the molar ratios of the Mr ∼105,000 protein, IC69, and the Mr ∼70,000 protein relative to IC78 were determined to be 0.92, 1.05, and 1.05, respectively (Table 2). Similar results were obtained with other gels. These results indicate that the Mr ∼70,000 ODA-DC protein is present in an equimolar amount with the Mr ∼105,000 ODA-DC protein. Moreover, because the outer dynein arm contains one copy of each IC (King and Witman, 1989), there appears to be one ODA-DC for each outer arm dynein.
Table 2.
Relative stoichiometries of ODA-DC and outer dynein arm polypeptides The relative amounts of the outer dynein arm intermediate chains (IC78 and IC69) and of the Mr ∼70,000 and Mr ∼105,000 polypeptides of the ODA-DC were determined by quantitation of the Coomassie-blue stained SDS-polyacrylamide gel of sucrose density gradient fractions shown in Figure 1D. From these values and the masses predicted from the proteins' sequences (the mass of the Mr ∼70,000 polypeptide is predicted to be 62.2 kDa; see text), the molar ratios relative to IC78 were calculated.
| Polypeptide | Relative amounta | Mass (kDa) | Molar ratio |
|---|---|---|---|
| Mr∼105,000 | 1.00 | 83.4 | 0.92 (1) |
| IC78 | 1 | 76.5 | 1 (1) |
| IC69 | 0.87 | 63.4 | 1.05 (1) |
| Mr∼70,000 | 0.85 | 62.2 | 1.05 (1) |
Average of two lanes.
Visual examination of gels indicated that the Mr ∼25,000 ODA-DC protein is present in about the same amount as the Mr ∼20,000 outer dynein arm LC, but in only about one-half the amount of the Mr ∼22,000 outer dynein arm LC. The outer dynein arm is estimated to contain one copy of the Mr ∼20,000 LC and two copies of the Mr ∼22,000 LC (King and Witman, 1989). Therefore, it is likely that the three ODA-DC proteins are present in the complex in a 1:1:1 molar ratio.
Sequence and Structure of the Mr ∼70,000 ODA-DC Polypeptide
The Mr ∼70,000 ODA-DC polypeptide from gels such as that shown in Figure 1A was transferred to polyvinylidene difluoride membrane, stained with Ponceau S, excised, and digested with trypsin. The resulting fragments were isolated by high-performance liquid chromatography and their amino-terminal sequences determined directly (Table 1). From these sequences, PCR primers were designed (Table 1) for reverse transcription-PCR; total RNA from wild-type cells that were regenerating their flagella was used as template. Primer A designed from the sequence PGDPFAQ in peptide 1 and primer D designed from the sequence MAQNVA in peptide 2 yielded a 750-base pair product (pcST8-1). Sequencing the ends of this product revealed that it encoded the sequence PGDPFAQALINR—-AMAQNVA, which included sequence from both peptides that was not used in designing the primers. Insert pcST8-1 was then used to screen a wild-type cDNA library constructed in λZAPII (Wilkerson et al., 1995). A phagemid with a 2.3-kb insert (pcST737) was isolated and both strands sequenced.
The nucleotide sequence (Figure 2A) reveals that the clone contains a complete open reading frame that predicts a 552-amino acid (62,234 Da) polypeptide with a pI of 5.74. An in-frame stop codon is located at nucleotide 142; the presumed translation initiation site is located at nucleotide 148. A polyadenylation signal (TGTAA) is located just upstream of the poly(A) tail in the 3′-untranslated region. The sequence exactly predicts the three amino acid sequences obtained by direct sequencing of peptides 1, 2, and 3 (cf. Figure 2A, single underlines, and Table 1), confirming that it encodes the Mr ∼70,000 ODA-DC protein.
The Mr ∼70,000 ODA-DC protein is predicted to have a high α-helical content. The COILS program (Lupas et al., 1991; Lupas, 1996a) indicates that three regions (amino acids 27–60, 120–215, and 323–370) have a high probability (1.0) of forming coiled-coil structures (Figure 2B). In addition, there is a region between amino acids 234 and 262 that is predicted to form an α-helix but does not contain a heptad repeat; 57% of the amino acids in this region are charged. This region is similar in structure and sequence to a portion of the Mr ∼105,000 ODA-DC protein (see DISCUSSION). The amino-terminal 26 amino acids, of which eight are glycine, are predicted to form a random coil. The carboxyl-terminal 59 amino acids, of which 42% are charged, also are predicted to form a random coil; this region includes a glutamic acid-rich cluster (residues E494 to E507).
A PSI-BLAST search of the databases by using the entire predicted amino acid sequence of the Mr ∼70,000 ODA-DC protein revealed one potential homolog in Leishmania major (accession no. CAB55364), two potential homologs in Drosophila melanogaster (AAF55345 and AAF56123), two potential human homologs (AK057357 and AK057488) that appear to be variants expressed from the same gene, and one mouse homolog (BC013491) that is very similar to the human homolog AK057488. An alignment of the Leishmania homolog, one of the Drosophila homologs, and the two human homologs to the Chlamydomonas protein is shown in Figure 3; BLAST E values and predicted masses and isoelectric points for the homologs are given in Table 3. The proteins are similar in predicted size, and the identities extend throughout the proteins' predicted sequences, suggesting that they are true homologs. The human homologs are known from full-length (AK057357) or partial (AK057488) testis cDNAs; comparison of their predicted carboxyl-terminal amino acid sequences reveals that the last 11 residues of the former are replaced by 249 different residues in the latter, suggesting that the two transcripts are produced by alternative splicing. The gene encoding these proteins maps to chromosome position 19q13 (Human Genome Project Working Draft, University of California, Santa Cruz, CA). No close matches were found in yeast, Caenorhabditis elegans, or Arabidopsis, all of which lack motile cilia.
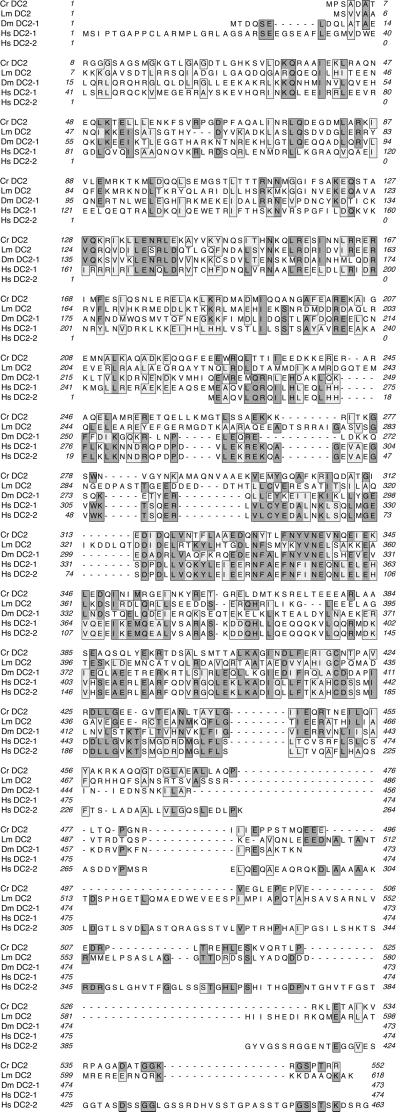
Figure 3.
Comparison of the Chlamydomonas Mr ∼70,000 ODA-DC sequence (Cr DC2) with potential homologs from L. major (Lm DC2) (accession no. CAB55364.1), D. melanogaster (Dm DC2-1) (accession no. AAF55345), and Homo sapiens (Hs DC2-1 and Hs DC2-2) (accession nos. AK057357 and AK057488, respectively). Predicted amino acid sequences from GenBank were aligned with the CLUSTAL W program. Residues identical to those in the Chlamydomonas sequence are shaded in dark gray, whereas amino acids substituted to similar residues are shaded in light gray; both types are surrounded by boxes.
Table 3.
Potential homologs of the Chlamydomonas Mr ∼70,000 ODA-DC protein The blast search was performed with BLASTP version 2.2.1 (Altschul et al., 1997) on November 9, 2001.
| Organism | Name | Accession no. | BLAST E value | BLAST identities/positives (%) | Predicted masses | Predicted pI |
|---|---|---|---|---|---|---|
| Leishmania | L1648.02 | CAB55364 | 5e-27 | 25/46 | 70,226 | 5.12 |
| Drosophila | CG14905 | AAF55345 | 3e-20 | 25/47 | 56,181 | 6.34 |
| Human | Unnamed (cDNA) | AK057357 | 3e-19 | 23/44 | 55,346 | 6.11 |
| Human | Unnamed (cDNA) | AK057488 | 3e-14 | 27/50 | N.A. | N.A. |
N.A., not applicable; complete sequence not available.
Mutant oda1-1 Has a Defect in the Gene Encoding the Mr ∼70,000 ODA-DC Protein
Axonemes of the mutants oda1 and oda3 lack the ODA-DC as well as the outer dynein arm (Takada and Kamiya, 1994), suggesting that the primary defect in these mutants involves the ODA-DC polypeptides. Indeed, the ODA3 gene previously was found to encode the Mr ∼105,000 polypeptide (Koutoulis et al., 1997), which we herein show is a component of the ODA-DC (see above). Therefore, ODA1 was a strong candidate gene for the Mr ∼70,000 protein.
As a rapid initial test to assess if the oda1-1 mutant had a defect in the gene for the Mr ∼70,000 protein, we determined whether that protein could be synthesized in vitro beginning with oda1-1 RNA. From the wild-type nucleotide sequence, we designed a pair of PCR primers to amplify the complete coding region for the Mr ∼70,000 protein. One primer used sequence >100 base pairs upstream from the initiator ATG in the 5′-untranslated region; the other used sequence next to the poly(A) tail in the 3′-untranslated region (Figure 2A, double underlines). RNA was isolated from oda1-1 cells that were actively regenerating flagella, as well as from nonregenerating wild-type and oda1-1 cells. First-strand cDNA was made using reverse transcriptase, and the PCR carried out with the above-mentioned primers. A large amount of a single product of 2.3 kb was obtained when the cDNA template was prepared from oda1-1 cells that were regenerating flagella (Figure 4A); a smaller amount of the 2.3-kb product was obtained with cDNA prepared from either wild-type cells or oda1-1 cells that were not regenerating flagella. The PCR products from wild-type and regenerating oda1-1 cells were ligated into pBluescript II KS (−) and cloned into Escherichia coli strain XL1Blue. Two wild-type clones and four oda1-1 clones were transcribed using T7 RNA polymerase, and the transcripts translated in a rabbit reticulocyte lysate containing [35S]methionine. Although all clones produced transcripts of the expected size (Figure 4B), only the wild-type transcripts yielded protein of Mr ∼70,000 (Figure 4C). With oda1-1 transcripts, smaller protein bands were detected but none had an intensity comparable to that of the Mr ∼70,000 band produced by wild-type transcripts. These results strongly suggested that strain oda1-1 has a mutation in the gene encoding the Mr ∼70,000 protein and that the complete protein is not expressed in vitro.
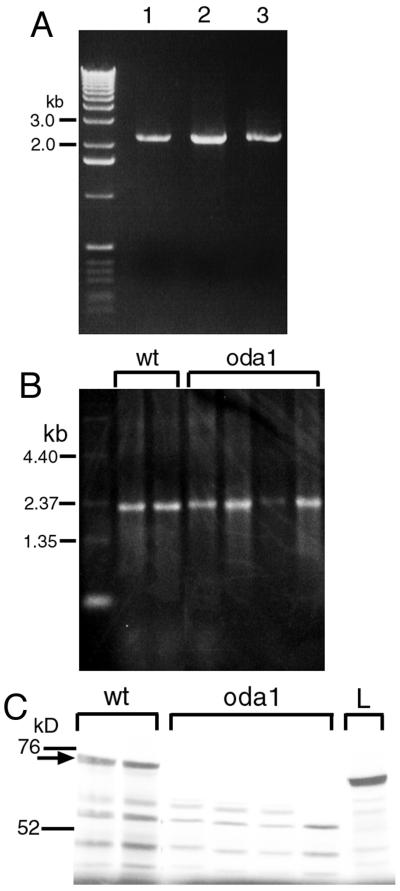
Figure 4.
Amplification, in vitro transcription, and in vitro translation of cDNAs encoding the Mr ∼70,000 ODA-DC protein. (A) Amplified cDNA made from first strand cDNA by using a pair of primers (Figure 2A) designed to amplify the full-length open reading frame encoding the Mr ∼70,000 protein. Template RNAs were from wild-type cells (lane 1), oda1-1 mutant cells that had been deflagellated and were in the process of regenerating new flagella (lane 2), and oda1-1 cells that were not deflagellated (lane 3). The left lane was loaded with a 1-kb DNA ladder (Invitrogen). (B) Formaldehyde-agarose gel (1.2%) of RNA transcribed from cDNA clones derived from the PCR products shown in A. The products were cloned in pBluescript II KS (−) and transcribed in vitro by using T7 RNA polymerase. Two wild-type (wt) and four oda1-1 (oda1) clones were examined; all produced RNA of the expected size. The left lane shows an RNA ladder (0.24–9.5 kb) (Invitrogen). (C) SDS-PAGE analysis of products obtained by in vitro translation of RNAs shown in B. The wild-type RNAs (wt) produced a protein of Mr ∼70,000 (arrow), whereas the oda1-1 RNAs (oda1) failed to produce a protein of this size. The right lane (L) is a product from luciferase mRNA (61 kDa). Bars at the left indicate molecular weight markers (76,000 and 52,000).
This was confirmed by sequence analysis of the 5′ ends of the oda1-1 cDNA clones. In all four clones, the C at nucleotide position 283, 135-base pairs downstream from the initiator ATG, was changed to T (Figure 2A). This single-base replacement converts a CAG codon specifying glutamate to the stop codon TAG. This would result in the translation of only 45 of the 552 amino acids encoded by the wild-type ODA1 gene. It is likely that the minor bands <Mr ∼60,000 produced by the oda1-1 transcripts (Figure 4C) are the result of translation initiation at internal sites downstream from this stop codon.
The Mr ∼70,000 and ∼105,000 ODA-DC Polypeptides Are Cross-linked by EDC
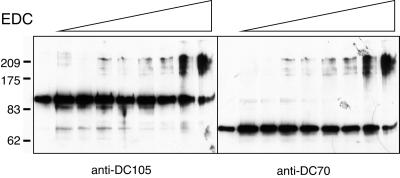
Both the Mr ∼70,000 and ∼105,000 ODA-DC polypeptides are predicted to have extended coiled-coil regions that are likely to function in dimerization or heterodimer formation (see DISCUSSION). This raised the question of whether the two proteins interact with each other to form a heterodimer that then binds the Mr ∼25,000 protein to form the ODA-DC, or whether each polypeptide associates with another copy of itself to form a homodimer, with the two types of homodimers possibly coming together during assembly of the complete ODA-DC. To investigate this, we used the zero-length cross-linker EDC to examine interactions between the ODA-DC proteins in 0.6 M KCl extracts of wild-type axonemes. KCl extracts were chosen for these experiments because interactions between the ODA-DC and tubulin should be minimized in the high-salt environment, thus simplifying the number of cross-linked products obtained. As shown in Figure 5, EDC generated a series of cross-linked products that migrated between Mr ∼175,000 and ∼210,000 and contained both the Mr ∼70,000 and ∼105,000 ODA-DC polypeptides. The apparent size of the smallest of these conjugates is of the size expected for a simple heterodimer of the Mr ∼70,000 and ∼105,000 polypeptides, but it is not clear why multiple, more slowly migrating products appeared simultaneously. One possibility is that intermolecular cross-linking at different sites produced products with different relative mobilities, a phenomenon postulated to account for a band-spreading observed for cross-linked products of α and β spectrin (Huang and Richards, 1977). In any case, all of the products appear to contain both ODA-DC polypeptides, the amounts of the monomeric proteins decrease simultaneously and concomitantly with the appearance of the cross-linked products, and there is no evidence for cross-linking of homodimers of either protein. Because EDC generates covalent linkages solely between groups that interact directly, these results indicate that the Mr ∼70,000 and ∼105,000 ODA-DC polypeptides are intimately associated with each other, and support the hypothesis that the two proteins interact with one another to form a heterodimer.
Figure 5.
Cross-linking of the ODA-DC proteins by EDC. A 0.6 M KCl extract of wild-type axonemes was incubated with increasing concentrations of EDC (0, 0.2, 0.5, 1, 2, 4, 6, 10, and 20 mM). The proteins were then separated on a 6% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The right panel shows the blot probed with the anti-DC70 antibody; the left panel shows the same blot stripped and reprobed with the anti-DC105 antibody. Multiple cross-linked products migrating between Mr ∼175,000 and ∼210,000 appear simultaneously, are recognized by both antibodies, and become progressively more prominent as the EDC concentration is increased. Numbers on left indicate molecular weight markers.
DISCUSSION
The ODA-DC Has Three Subunits
To understand the mechanism by which the outer dynein arm is targeted to its correct binding site on flagellar microtubules, we have been characterizing the C. reinhardtii ODA-DC, which originally was described as a “7S factor” when released from axonemes by high-salt extraction (Takada and Kamiya, 1994). This factor assembles onto flagellar doublet microtubules in the absence of outer arms in vivo, but is necessary for the outer dynein arm to attach to the doublet microtubules (Takada and Kamiya, 1994). Hence, the ODA-DC must be an important intermediary in the binding of outer arm dynein to its unique attachment site within the flagellar axoneme.
Herein, we present three independent lines of evidence that the ODA-DC contains polypeptides of Mr ∼105,000, ∼75,000, and ∼25,000: 1) Sucrose density gradient centrifugation was used to partially purify the ODA-DC after its release from the axoneme by a modified high-salt extraction procedure (Nakamura et al., 1997) that reduces the number of “background” polypeptides in the high-salt extract and in subsequent sucrose gradient fractions. Under these conditions, the 7S fraction from axonemes of the outer armless mutant oda6, which has the ODA-DC (Takada and Kamiya, 1994), contained polypeptides of Mr ∼105,000, ∼70,000, and ∼25,000 that were missing in equivalent fractions from the mutant oda3, which lacks the ODA-DC. These results strongly suggested that the ODA-DC contains these three polypeptides. The Mr ∼70,000 polypeptide previously was reported to be a component of the ODA-DC (Takada and Kamiya, 1994). That we were able to detect the presence or absence of the Mr ∼105,000 and ∼25,000 polypeptides in sucrose gradient fractions probably was due to the reduced number of background bands resulting from our modified extraction conditions. 2) An antibody specific for the Mr ∼105,000 protein coimmunoprecipitated the Mr ∼70,000 and ∼25,000 proteins but no other proteins from a 0.6 M KCl extract of wild-type axonemes. These results confirmed that all three proteins were part of the ODA-DC and indicated that the three proteins are likely to constitute the complete ODA-DC in wild-type cells. 3) The three polypeptides shifted simultaneously from the 7S to the 23S fraction in sucrose density gradients centrifuged under conditions where the ODA-DC remained associated with the more rapidly sedimenting outer dynein arm (see below).
The ODA-DC Interacts Directly with the Outer Dynein Arm
If the ODA-DC is directly responsible for binding the outer arm to the doublet microtubule, then it should interact with the outer arm. When the outer dynein arm was removed from the wild-type axoneme by extraction with high salt and subjected to sucrose density gradient centrifugation in the presence of Mg2+, the α, β, and γ DHCs remained associated with each other as a three-headed particle that sediments at 23S (Takada et al., 1992). We found that under these conditions the three polypeptides of the ODA-DC remained associated with the outer arm polypeptides and cosedimented with them at 23S. Therefore, the ODA-DC interacts directly with the outer arm. This interaction was confirmed by experiments in which the outer dynein arm intermediate chains were found to coimmunoprecipitate with the ODA-DC from high-salt extracts prepared in the presence of Mg2+. The association of the ODA-DC with the three-headed dynein undoubtedly explains the ability of this dynein to bind efficiently to oda1 and oda3 axonemes, which lack the ODA-DC (Takada et al., 1992). Because the entire outer arm dynein appears to be able to assemble in the cytoplasm of oda1 and oda3 mutants (Fowkes and Mitchell, 1998), the ODA-DC is not promoting outer arm assembly simply by binding the αβ and γ subunits together. More likely, it forms a direct link between the outer dynein arm and the outer doublet microtubule.
ODA-DC Polypeptide Stoichiometry
Quantitative densitometry indicated that the Mr ∼70,000 and ∼105,000 ODA-DC proteins are present in an equimolar ratio in a 23S particle consisting of the ODA-DC and the outer dynein arm; visual comparison of band intensities suggested that the Mr ∼25,000 ODA-DC protein also is present in equimolar amounts with the two larger ODA-DC subunits. Therefore, it is likely that the three ODA-DC subunits occur in a 1:1:1 molar ratio. These data alone do not distinguish whether the ODA-DC is a heterotrimer or a higher order polymer, such as a hexamer. However, we also found that there was one copy of each ODA-DC polypeptide per soluble three-headed outer arm dynein. Therefore, the simplest model is that the ODA-DC is a heterotrimer, and that each outer arm is associated with a single ODA-DC.
Structure of the Mr ∼70,000 ODA-DC Polypeptide
Beginning with peptide sequence obtained directly from microsequencing of tryptic fragments from the Mr ∼70,000 ODA-DC polypeptide, we cloned and sequenced a full-length cDNA encoding the protein. The sequence predicts a novel 62-kDa polypeptide with long regions that have a very high probability of forming coiled-coil structures, a distinctive α-helical region that probably does not form a coiled-coil, and a C terminus that is highly charged. These structural features are generally similar to those of the Mr ∼105,000 ODA-DC protein (Koutoulis et al., 1997) with which the Mr ∼70,000 protein is associated.
The Mr ∼70,000 protein has three regions (∼34, ∼96, and ∼48 amino acids in length) totaling 178 amino acids that are >99% likely to form a coiled-coil structure. The Mr ∼105,000 ODA-DC protein likewise has three regions (∼120, ∼70, and ∼36 amino acids) totaling ∼236 amino acids that are >99% likely to form a coiled-coil. Coiled-coils commonly function in dimerization and heterodimer formation (Lupas, 1996b). As discussed above, each ODA-DC is likely to contain just one copy of each of its component polypeptides. Moreover, experiments using the zero-length protein cross-linker EDC revealed that the Mr ∼70,000 protein is in direct contact with the Mr ∼105,000 protein, but provided no evidence that either protein interacts with itself. Therefore, we postulate that these two proteins interact via their coiled-coil domains to form a heterodimer, and that this heterodimer has an extended rod-like structure (Figure 6A). Assuming 1.5 Å/residue in a coiled-coil (Fraser and MacRae, 1973), a rod-like structure based on the presumptive coiled-coil domains of the Mr ∼70,000 protein could be up to 26.7 nm in length. This is long enough that ODA-DCs could link up end-to-end, with some overlap, to form a filament with a 24-nm repeat structure (Figure 6B; see below).
Both the Mr ∼70,000 and ∼105,000 proteins also contain a highly charged domain, following the second coiled-coil region, that is predicted to form an α-helix but does not have a heptad repeat and thus is not likely to form a coiled-coil structure (cf. region C, Figure 2B and region C, Figure 6 of Koutoulis et al., 1997). A portion of this region is 42% identical in the two proteins:
![]() In the Mr ∼105,000
protein this sequence consists of an imperfect 11-amino acid tandem
repeat (Koutoulis et al., 1997) that previously was found to
be closely related to repeats in mammalian trichohyalin, a protein that
interacts with intermediate filaments (Fietz et al., 1993;
Lee et al., 1993). In trichohyalin these repeats are
proposed to form a single-stranded α-helical rod that is stabilized
by ionic interactions between successive turns of the helix (Lee
et al., 1993); it has been proposed that these repeats
interact with charged residues on intermediate filaments. Weak repeats
with similar structure and composition also occur after a long
coiled-coil region in troponin T and caldesmon (e.g.,
K512-Q527 in chicken
caldesmon; Bryan et al., 1989); these regions are candidates
for the tropomyosin-binding sites of these proteins (Bryan et
al., 1989). Therefore, this part of the
Mr ∼70,000 protein may be involved in
protein–protein interactions.
In the Mr ∼105,000
protein this sequence consists of an imperfect 11-amino acid tandem
repeat (Koutoulis et al., 1997) that previously was found to
be closely related to repeats in mammalian trichohyalin, a protein that
interacts with intermediate filaments (Fietz et al., 1993;
Lee et al., 1993). In trichohyalin these repeats are
proposed to form a single-stranded α-helical rod that is stabilized
by ionic interactions between successive turns of the helix (Lee
et al., 1993); it has been proposed that these repeats
interact with charged residues on intermediate filaments. Weak repeats
with similar structure and composition also occur after a long
coiled-coil region in troponin T and caldesmon (e.g.,
K512-Q527 in chicken
caldesmon; Bryan et al., 1989); these regions are candidates
for the tropomyosin-binding sites of these proteins (Bryan et
al., 1989). Therefore, this part of the
Mr ∼70,000 protein may be involved in
protein–protein interactions.
The carboxyl-terminal 59 amino acids of the Mr ∼70,000 protein contain a short glutamic acid-rich region followed by a region with a high percentage of positively and negatively charged residues. Both tubulin and IC78 of outer arm dynein have highly charged basic and acidic domains, so it is possible that this portion of the Mr ∼70,000 protein interacts with those proteins. The Mr ∼105,000 protein likewise has a glutamic acid cluster near its carboxy terminus.
The size of the Mr ∼70,000 protein estimated by SDS-PAGE is greater than the 62.5-kDa mass predicted from nucleotide sequence. Disparities in this direction and of this magnitude or more are commonly observed in proteins with similar structure (e.g., chicken caldesmon, Mr 120,000–150,000 estimated vs. 87.0 kDa predicted [Bryan et al., 1989]; C. reinhardtii Mr ∼105,000 ODA-DC protein, Mr ∼105,000 estimated vs. 83.4 kDa predicted [Koutoulis et al., 1997]).
The Mr ∼70,000 ODA-DC Protein Is Encoded by the ODA1 Gene
Outer dynein armless mutants having defects at 15 different ODA loci (ODA1-ODA15) have been reported, of which 12 (oda1-oda10, oda12, and oda15) lack the complete outer arm and have a slow swimming phenotype (Kamiya, 1988; Koutoulis et al., 1997; King, 2000; Pazour and Witman, 2000). The genes ODA2, ODA4, ODA6, ODA9, ODA11, ODA12, ODA13, and ODA15 encode the γ and β DHCs, IC69, IC78, the α DHC, LC2, LC6, and LC7, respectively, of the outer arm dynein (Mitchell and Kang, 1991; Sakakibara et al., 1991, 1993; Wilkerson et al., 1994, 1995; Pazour and Witman, 2000). A sixth ODA gene (ODA3) has been cloned and found to encode the Mr ∼105,000 ODA-DC protein (Koutoulis et al., 1997). We now report that the ODA1 gene encodes the Mr ∼70,000 ODA-DC protein. Sequencing of mutant oda1-1 cDNA encoding the Mr ∼70,000 protein revealed that the mutant DNA has a point mutation that converts codon 46, which in wild-type DNA specifies a glutamine, to a stop codon. This mutation thus precludes production of the full-length Mr ∼70,000 protein in oda1-1 cells, a defect that undoubtedly is directly responsible for the loss of the ODA-DC in these cells (Takada and Kamiya, 1994). This mutant should be very useful for studies to investigate the detailed roles of the Mr ∼70,000 protein in outer arm assembly and ODA-DC function.
Functions of the Mr ∼70,000 Protein
The fact that a defect in the Mr ∼70,000 protein leads to loss of the ODA-DC in oda1-1 indicates that the Mr ∼70,000 protein is essential for assembly of the ODA-DC, and confirms that loss of the ODA-DC leads to an inability to target outer dynein arms to doublet microtubules. In contrast, loss of a major outer arm dynein structural protein in oda2, oda4, oda6, and oda9 results in failure of the outer arm to assemble, but does not lead to loss of the ODA-DC (Takada and Kamiya, 1994; Koutoulis et al., 1997). Therefore, the ODA-DC can assemble and bind to the correct position on flagellar doublet microtubules independently of the outer arm dynein.
How the ODA-DC itself assembles onto the correct site on the doublet microtubules is not known. One possibility is that the ODA-DC is a rod-like structure that links together with adjacent ODA-DCs to form a filament running longitudinally along the doublet (Figure 6B); assembly of this filament might be initiated on the correct microtubule protofilament by some structure at the base of the axoneme, or by some discontinuity in the tubulin lattice. This model is consistent with the coiled-coil nature of the Mr ∼70,000 and ∼105,000 proteins, with the direct interactions of these proteins as revealed by protein cross-linking experiments, and with the appearance of the ODA-DC in electron micrographs of cross sections of axonemes (Takada and Kamiya, 1994). Inasmuch as we observed one ODA-DC per outer arm dynein, the ODA-DCs would be expected to repeat at 24-nm intervals along the filament. Indeed, independent evidence for such a spacing has been provided by immunogold labeling of the Mr ∼105,000 ODA-DC polypeptide on outer doublet microtubules of demembranated axonemes (Wakabayashi et al., 2001). Therefore, the ODA-DC could act as a ruler to determine the 24-nm longitudinal spacing of the outer arms, although it is not known whether such a ruler is necessary in Chlamydomonas. Chlamydomonas outer arm dynein has been shown to assemble onto brain microtubules with a 24-nm periodicity (Haimo and Fenton, 1988), but it is not clear if the ODA-DC was present in the dynein preparations used for those experiments. Further studies will be necessary to determine whether purified Chlamydomonas outer arm dynein requires the ODA-DC for periodic assembly onto microtubules.
Takada and Kamiya (1997) reported that the ODA-DC has an important role in regulating the differential beat frequency of the cis- versus the trans-axoneme in demembranated, reactivated cell models of Chlamydomonas. These results indicate that the ODA-DC might regulate the activity of outer arm dynein. A potential mechanism for regulating the activity of a molecular motor is protein phosphorylation. Luck and Piperno (1989) reported that an Mr ∼73,000 phosphoprotein was missing from axonemes of oda1 and oda3 but not oda2, oda4, oda5, or oda6; this protein presumably is the same as the Mr ∼70,000 ODA-DC polypeptide. The protein had multiple isoelectric variants in two-dimensional isoelectric focusing/SDS-PAGE, suggesting that it was phosphorylated at multiple sites. We have similarly observed that the Mr ∼70,000 ODA-DC protein in axonemal extracts and purified three-headed dynein focuses in two-dimensional gels as multiple spots having different isoelectric points (Takada and Kamiya, unpublished data). Therefore, the Mr ∼70,000 protein probably is phosphorylated at multiple sites in vivo. Consistent with this, the sequence reported here has numerous potential sites for protein phosphorylation.
Our current findings will greatly facilitate studies to investigate the role of ODA-DC phosphorylation in regulating axonemal beat frequency. For example, knowledge of the sequence of the Mr ∼70,000 protein will now permit identification of those peptides and residues that are phosphorylated in vivo. Moreover, it will be possible to alter those sites by site-directed mutagenesis of the ODA1 gene, and then determine the effect of the modifications by transforming (Kindle, 1990) the altered genes back into strains containing the oda1-1 allele. These strains would be rescued for assembly of the ODA-DC and the outer dynein arm but would be defective in phosphorylation of the targeted site.
Homologs of the Mr ∼70,000 ODA-DC Protein Are Present in Higher Organisms
A BLAST search of gene and protein databases revealed potential homologs of the Chlamydomonas Mr ∼70,000 ODA-DC polypeptide in the protozoan Leishmania, in Drosophila, and in mice and humans, but not in organisms such as yeast, C. elegans, and Arabidopsis that lack motile cilia. This is the first evidence that the ODA-DC occurs in higher organisms. In humans, primary ciliary dyskinesia (PCD), an inherited disorder in which ciliary and flagellar movement is impaired, seems most frequently to be caused by loss of the outer dynein arms (Afzelius and Mossberg, 1995). As a result of this defect, PCD patients develop bronchiectasis and chronic sinusitis; male patients are infertile. Inasmuch as the Mr ∼70,000 ODA-DC gene is necessary for outer arm assembly in Chlamydomonas, its potential human homolog AK057357 is now a candidate gene for those cases of PCD in which the outer dynein arms are missing. AK057357 maps to chromosome 19q13. Interestingly, in some PCD patients lacking the outer dynein arm, the defective locus has been mapped to this same region (Meeks et al., 2000). Further studies are warranted to determine whether a defect in AK057357 causes PCD.
ACKNOWLEDGMENTS
We are grateful to Dr. John Leszyk of the UMMS Protein Microsequencing Facility for peptide sequencing, and to Kazuaki Homma for expert assistance with the protein cross-linking experiments. This work was supported by a National Institutes of Health grant (GM-30626) (to G.W.), by the Robert W. Booth Fund at the Greater Worcester Community Foundation (to G.W.), by a fellowship from the Japan Society for the Promotion of Science (to S.T.), and by a grant from the Ministry of Education, Science and Culture of Japan (to R.K.).
Abbreviations used:
- BSA
bovine serum albumin
- DHC
dynein heavy chain
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- IC
dynein IC
- HRP
horseradish peroxidase
- LC
dynein light chain
- ODA-DC
outer dynein arm-docking complex
- PCD
primary ciliary dyskinesia
- PCR
polymerase chain reaction
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–04–0201. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–04–0201.
The three oda strains previously were referred to as oda1 strain 38, oda3 strain 73, and oda6 strain 95, respectively (Kamiya, 1988).
REFERENCES
- Afzelius BA, Mossberg B. Immotile-cilia syndrome (primary ciliary dyskinesia), including Kartagener syndrome. In: Scriver CR, editor. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. New York: McGraw-Hill Book Company; 1995. pp. 3943–3954. [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1995. Nucleic Acids Res. 1995;24:189–196. doi: 10.1093/nar/24.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ. Control of flagellar bending: a new agenda based on dynein diversity. Cell Motil Cytoskeleton. 1994;28:199–204. doi: 10.1002/cm.970280303. [DOI] [PubMed] [Google Scholar]
- Bryan J, Imai M, Lee R, Moore P, Cook RG, Lin W-G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989;264:13873–13879. [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs on the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz MJ, McLaughlan CJ, Campbell MT, Rogers GE. Analysis of the sheep trichohyalin gene: potential structural and calcium-binding roles of trichohyalin in the hair follicle. J Cell Biol. 1993;121:855–865. doi: 10.1083/jcb.121.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes ME, Mitchell DR. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser RDB, MacRae TP. Conformation in fibrous proteins and related synthetic polypeptides. New York: Academic Press; 1973. [Google Scholar]
- Haimo LT, Fenton RD. Interaction of Chlamydomonasdynein with tubulin. Cell Motil Cytoskeleton. 1988;9:129–139. doi: 10.1002/cm.970090205. [DOI] [PubMed] [Google Scholar]
- Harris E. The Chlamydomonas Sourcebook. San Diego: Academic Press; 1989. [Google Scholar]
- Huang CH, Richards FM. Reaction of a lipid-soluble, unsymmetrical, cleavable, cross-linking reagent with muscle aldolase and erythrocyte membrane proteins. J Biol Chem. 1977;252:5514–5521. [PubMed] [Google Scholar]
- Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM. The dynein microtubule motor. Biochem Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- King SM, Otter T, Witman GB. Purification and characterization of Chlamydomonasflagellar dyneins. Methods Enzymol. 1986;134:291–306. doi: 10.1016/0076-6879(86)34097-7. [DOI] [PubMed] [Google Scholar]
- King SM, Patel-King RS, Wilkerson CG, Witman GB. The 78,000-Mr intermediate chain of Chlamydomonasouter arm dynein is a microtubule-binding protein. J Cell Biol. 1995;131:399–409. doi: 10.1083/jcb.131.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Wilkerson CG, Witman GB. The Mr 78,000 intermediate chain of Chlamydomonasouter arm dynein interacts with α-tubulin in situ. J Biol Chem. 1991;266:8401–8407. [PubMed] [Google Scholar]
- King SM, Witman GB. Molecular structure of Chlamydomonasouter arm dynein. In: Warner FD, Satir P, Gibbons IR, editors. Cell Movement: The Dynein ATPases. New York: Alan R. Liss; 1989. pp. 61–75. [Google Scholar]
- King SM, Witman GB. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990;265:19807–19811. [PubMed] [Google Scholar]
- Koutoulis A, Pazour GJ, Wilkerson CG, Inaba K, Sheng H, Takada S, Witman GB. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J Cell Biol. 1997;137:1069–1080. doi: 10.1083/jcb.137.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Kim IG, Marekov LN, O'Keefe EJ, Parry DA, Steinert PM. The structure of human trichohyalin. Potential multiple roles as a functional EF-hand-like calcium-binding protein, a cornified cell envelope precursor, and an intermediate filament-associated (cross-linking) protein. J Biol Chem. 1993;268:12164–12176. [PubMed] [Google Scholar]
- Luck DJL, Piperno G. Dynein arm mutants of Chlamydomonas. In: Warner FD, Satir P, Gibbons IR, editors. Cell Movement: The Dynein ATPases. New York: Alan R. Liss; 1989. pp. 49–60. [Google Scholar]
- Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996a;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996b;21:375–382. [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Meeks M, et al. A locus for primary ciliary dyskinesia maps to chromosome 19q. J Med Genet. 2000;37:241–244. doi: 10.1136/jmg.37.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Kang Y. Identification of oda6 as a Chlamydomonasdynein mutant by rescue with the wild-type gene. J Cell Biol. 1991;113:835–842. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Wilkerson CG, Witman GB. Functional interaction between Chlamydomonasouter arm dynein subunits. The γ subunit suppresses the ATPase activity of the αβ dimer. Cell Motil Cytoskeleton. 1997;37:338–345. doi: 10.1002/(SICI)1097-0169(1997)37:4<338::AID-CM5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. Forward and reverse genetic analysis of microtubule motors in Chlamydomonas. Methods. 2000;22:285–298. doi: 10.1006/meth.2000.1081. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Fay RB, Witman GB. Purification and polypeptide composition of dynein ATPases from Chlamydomonasflagella. Cell Motil Cytoskeleton. 1982;2:525–547. doi: 10.1002/cm.970020604. [DOI] [PubMed] [Google Scholar]
- Piperno G, Luck DJL. Axonemal adenosine triphosphatases from flagella of Chlamydomonas reinhardtii: purification of two dyneins. J Biol Chem. 1979;254:3084–3090. [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, Mitchell DR, Kamiya R. A Chlamydomonasouter-arm dynein mutant missing the alpha heavy chain. J Cell Biol. 1991;113:615–622. doi: 10.1083/jcb.113.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, S. Takada S, King M, Witman GB, Kamiya R. A Chlamydomonasouter arm dynein mutant with a truncated beta heavy chain. J Cell Biol. 1993;122:653–662. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Sakakibara H, Kamiya R. Three-headed outer arm dynein from Chlamydomonasthat can functionally combine with outer-arm-missing axonemes. J Biochem. 1992;111:758–762. doi: 10.1093/oxfordjournals.jbchem.a123832. [DOI] [PubMed] [Google Scholar]
- Takada S, Kamiya R. Functional reconstitution of Chlamydomonasouter dynein arms from α-β and γ subunits: requirement of a third factor. J Cell Biol. 1994;126:737–745. doi: 10.1083/jcb.126.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Kamiya R. Beat frequency difference between the two flagella of Chlamydomonasdepends on the attachment site of outer dynein arms on the outer-doublet microtubules. Cell Motil Cytoskeleton. 1997;36:68–75. doi: 10.1002/(SICI)1097-0169(1997)36:1<68::AID-CM6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tal M, Silberstein A, Nusser E. Why does Coomassie Brilliant Blue interact differently with different proteins? J Biol Chem. 1980;260:9976–9980. [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Takada S, Witman GB, Kamiya R. Transport and arrangement of the outer-dynein-arm docking complex in the flagella of Chlamydomonasmutants that lack outer dynein arms. Cell Motil Cytoskeleton. 2001;48:277–286. doi: 10.1002/cm.1015. [DOI] [PubMed] [Google Scholar]
- Wilkerson CG, King SM, Koutoulis A, Pazour GJ, Witman GB. The 78,000 Mr intermediate chain of Chlamydomonasouter arm dynein is a WD-repeat protein required for arm assembly. J Cell Biol. 1995;129:169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson CG, King SM, Witman GB. Molecular analysis of the γ heavy chain of Chlamydomonasflagellar outer-arm dynein. J Cell Sci. 1994;107:497–506. doi: 10.1242/jcs.107.3.497. [DOI] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonasflagella and axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Witman GB, Carlson K, Berliner J, Rosenbaum JL. Chlamydomonasflagella. I. Isolation and electrophoretic analysis of microtubules, membranes, matrix and mastigonemes. J Cell Biol. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB, Wilkerson CG, King SM. The biochemistry, genetics and molecular biology of flagellar dyneins. In: Hyams JS, Lloyd CW, editors. Microtubules. New York: Wiley-Liss; 1994. pp. 229–249. [Google Scholar]