Abstract
Hydrogels have become one of the potential polymers used with great performance for many issues and can be promoted as biomaterials with highly innovative characteristics and different uses. Gelatin is obtained from collagen, a co-product of the meat industry. Thus, converting wastes such as cartilage, bones, and skins into gelatin would give them added value. Furthermore, biodegradability, non-toxicity, and easy cross-linking with other substances can promote polymers with high performance and low cost for many applications, turning them into sustainable products with high acceptance in society. Gelatin-based hydrogels have been shown to be useful for different applications with important and innovative characteristics. For instance, these hydrogels have been used for biomedical applications such as bone reconstruction or drug delivery. Furthermore, they have also shown substantial performance and important characteristics for remediation for removing pollutants from water, watercourse, and effluents. After its uses, gelatin-based hydrogels can easily biodegrade and, thus, can be sustainably used in the environment. In this study, gelatin was shown to be a potential polymer for hydrogel synthesis with highly renewable and sustainable characteristics and multiple uses.
Keywords: hydrogel, gelatin, sustainability, remediation
1. Introduction
Hydrogels are characterized as three-dimensional cross-linked polymeric networks that can be produced with the most variable compounds and with the most variable uses [1]. In the last decade, hydrogels have been used in many applications and with high technical and economic viability which include biomedical and environmental areas.
Gelatin-based hydrogels are one kind of hydrogel in which gelatin is used as the cross-linked polymer and give the gel characteristics such as structure and texture. Many studies have promoted this kind of hydrogel for biomedical uses with promising and important characteristics such as tissue engineering [2,3] and/or drug delivery [4,5] with a high economic impact on society, medicine, and environmental purposes with high applicability.
The market for gelatin in 2020 rose to about USD 3.18 billion and is further expected to reach USD 4.08 billion by 2024 as forecasted in the same report [6]. The market of gelatin in 2013 was the highest in the food and beverages sector (28%), followed by nutraceuticals (25.8%) and pharmaceuticals (21%), while their use in the cosmetic industry was only 5.5% [7]. Along with the advancements in drug delivery, it caused the development of new recipients as novel dosage forms to fulfill specific functions which directly or indirectly influence the extent and or rate of drug release. This enhances the development of new and modified recipient sources that continue to emerge for better drug delivery performance [8].
Several studies have shown the high potential of hydrogels as green and renewable materials, with highly efficient and promising uses [1,9]. Gelatin-based hydrogels have several advantages due to their biocompatibility, biodegradability, and nontoxic features [10]. Furthermore, gelatin is a natural protein-derived material, and it has been used for the synthesis of medical hydrogels because of its non-immunogenicity and capacity for enhancing cell adhesion apart from its excellent biocompatibility [11].
Environmental problems such as pollution and contamination of water and soil have increased over the years of industrialization, mining, and the use of natural resources. Bioremediation and remediation processes come to assuage the contamination and promote environmental sustainability. In this way, hydrogels have a high potential for remediation with a substantial capacity for the adsorption of a wide range of pollutants such as toxic metals [12,13,14,15], organic compounds [16,17], dyes [18,19,20], and others.
New materials are well accepted by scientists and society for whatever their purpose is; however, when these new materials have innovative characteristics, they are also sustainable and can easily biodegrade in the environment, these products can be accepted in many countries and societies. So, the aim of this overview is to discuss, characterize, and indicate gelatin as a renewable source for hydrogels with many innovative applications such as biomedicine and environmental applications such as remediation for decontamination of water, effluents, and soil.
2. Hydrogels
Hydrogels are three-dimensional (3D) gel-like materials with hydrophilic functional groups in a polymeric chain and are capable of holding a large volume of hydro-fluids compared to their mass [21,22]. They can also be defined as water-absorbing natural or synthetic polymeric substances that swell im water and retain a significant amount of water within the structure without dissolving [23]. Hydrogels attract water due to the polar functional groups on the skeleton of the macromolecule and inhibit dissolving due to cross-linking. Two types of cross-linking, chemical or physical, can exist in the macromolecular chain.
Generally, hydrogels swell until the thermodynamic force of swelling is compensated by the elastic and retroactive strength of the cross-links [21]. The volume of water taken up by the macromolecule varies according to the structure of the hydrogel and the environmental conditions such as the pH, the temperature, and the ionic strength of the aqueous solution to which the polymeric network is exposed [21,22].
According to the nature of their components, hydrogels can be classified as synthetic (organic or inorganic), biological, and hybrid hydrogels. The structure of the polymeric network is controlled by gelling chemistry, leading to hydrogels with custom properties for multiple uses. Depending on the charged pendant groups on the main chain, synthetic organic hydrogels can be classified into ionic, neutral, and electronic conductor polymers [24]. Common examples of neutral polymers are poly(ethylene oxide) (PEO), poly(hydroxyethyl methacrylate) (PHEMA), and poly(vinyl alcohol) (PVA), which permit the formation of hydrogels via several cross-linking methods [24,25]. Moreover, biological hydrogels are potential materials due to their capacity of imitating the physical, chemical, and biological characteristics of tissues [26]. These hydrogels can have biologically active moieties such as gelatin, collagen, and elastin that enhance cell growth, migration, and proliferation [26]. Among these active moieties, gelatin has been extensively employed. Gelatin is obtained via the hydrolysis of collagen, which is the main protein present in the extracellular matrix of most tissues and can also be degraded enzymatically due to the metalloproteinase-sensitive sequences on its matrix [26]. Thus, it can promote biodegradation since it is a natural organic product and can be one sustainable characteristic of gelatin-based hydrogels.
Hydrogels can be produced with the most variable compositions and gel characteristics such as texture and structure. Many studies on different polymers promote these materials as innovative, green, and renewable materials with high importance for many uses [1]. Furthermore, these kinds of materials have been studied for biomedical and environmental applications [3,15,27]. Hence, their wide applicability and the broad formulation possibility with different compositions and polymers can increase their performance and make their use technically and economically viable.
In the modern world, emissions of pollutants and contamination of the environment have been important problems for humanity to solve and reduce. Here, hydrogels can be important since they show great adsorption capacity for contaminants and they can also be produced with different materials and be modified.
Many matrices such as gelatin [28], poly(vinyl alcohol) (PVA) [1], acrylamide [29], alginate dialdehyde-gelatin (ADA-GEL) [3], dialdehyde carboxymethyl cellulose-dextrin, and gelatin [30] have been used for the synthesis of hydrogels. Additionally, many other components have been added for increasing their adsorbent capacities; these include lignin [1], montmorillonite [29], gelatin ionically modified by bacterial cellulose [4], and graphene oxide/lapndonite/gelatin hydrogels [31].
The bio-based hydrogels possessing ion exchange and/or chelating groups have attracted much attention thanks to their large water portion, 3D structural morphology, swelling property, biodegradability, and non-toxicity [32,33]. Various moieties (i.e., amine (–NH2), hydroxyl (–OH), carboxyl (–COOH), and thiol (–SH)) on the surface of hydrogels can provide active binding sites and the 3D network structure can provide diffusion pathway to exhibit superior adsorption capacities and kinetics for the water pollutants [33,34]. Numerous biomaterials have been used for the fabrication of hydrogel-based adsorbents for wastewater treatment applications [33] and for pollutant removals such as chitosan [35], starch [12], xylan [36], sugarcane bagasse cellulose [20,37], biochar [12], or ulvan from green macroalgae [38]. However, when using an individual biopolymer it is more difficult to achieve high adsorption capacity and selectivity towards a specific pollutant or multicomponent adsorption. Thus, the incorporation of other functional compounds is a common approach to achieving the aforementioned properties. Therefore, surface modification of existing biomaterial with oxygen (O)-, nitrogen (N)-, or thiol (S)-containing functional groups has received widespread attention in wastewater treatment applications [33,39].
3. Gelatin-Based Hydrogels
Traditional gelatin is produced from animal origin, and it has been made from bones, cartilage, tendons, ligaments, and skin of animals such as cattle, pigs, fish, or chickens. As aforementioned, gelatin is derived from the partial hydrolysis of collagen protein presented in the previous sources [40]. Thus, due to the process of obtaining it, it may also be referred to as hydrolyzed collagen, hydrolyzed gelatin, or collagen peptide after it has undergone hydrolysis. After this process, the final product is colorless and soluble.
Gelatin is one of the most used ingredients with non-toxic and biodegradable characteristics in food and non-food industries for many purposes: for promoting gelation, stabilizing, thickening, emulsifying, and film forming [40]. Biodegradability is today an important issue since the sustainability of each product needs to exist; otherwise, the product might have problems with its future use because sanitary treatment is expensive and delimitation of space is a large problem for cities and governments. Even after obtaining the gelatin or the desired product from it, it has been demonstrated to have biodegradable characteristics, and it can contribute to producing new materials with high biodegradability potential inside the human organism [41] and in the environment [42,43].
Gelatin is used for giving consistency and viscosity to the hydrogels and, thanks to its aforementioned biodegradable and renewable nature, it can be an important source of sustainable hydrogels. Nowadays, the use of gelatin for the synthesis of hydrogels for multiple purposes depends on its structure and texture characteristics, and the applications range from the biomedical or biotechnological areas [2,3,30,44] to the most variable water and waste treatments [15,16,27,36,45]. Thus, gelatin-based hydrogels are versatile materials, which makes them very interesting. Some useful aspects of gelatin-based hydrogels employed for all the applications together with some others such as alimentary or environmental ones are displayed in Table 1.
Table 1.
Different purposes, applicability areas, and potential uses of gelatin-based hydrogels.
| Hydrogel | Purpose | Field | Potential Use | Reference |
|---|---|---|---|---|
| Poly(vinyl) alcohol-based | Mechanical and physical resistance | Biomedical | Economically high | [28] |
| Antioxidant peptides | Protective for enzyme | Biotechnology | Economically high for food industry | [44] |
| Gelatin nanoparticle | Tissue engineering, cell culture | Biotechnology | Economically high | [2] |
| Chitosan | Drug delivery | Biomedical | Economically | [5] |
| Dopamine grafted/ 1,4-phenylenebisboronic acid and graphene oxide |
Tissue adhesives, wound dressings, and wearable devices | Biomedical | Economically high | [11] |
| Cellulose incorporation | Chromium adsorption | Environmental | Pollution control and waste treatment | [27] |
| Cellulose microcrystals incorporation | Drug delivery | Biomedical | Economically | [46] |
| Oxidized alginate | Cartilage tissue engineering | Biomedical | Economically and healthy high | [47] |
| Carrageenan and potassium sulfate | Foods, materials, and other fields | Multiple uses | Economically and industrially | [10] |
As shown in Table 1, the wide applicability of hydrogels has been studied. In the biomedical field, for instance, purposes such as tissue engineering [3,30], cell culture scaffolding for bacterial growth and further human uses [48], dental pulp regeneration [49], capsules for drug delivery [4], and the treatment of myocardial infarction [31] have been investigated. Gelatin hydrogels are usually permeable to nutrients and oxygen, which enhance the survival rate of cells and their biological functions [48]. So, hydrogels with different compositions combining gelatin with other polymers could be useful for biomedical applications.
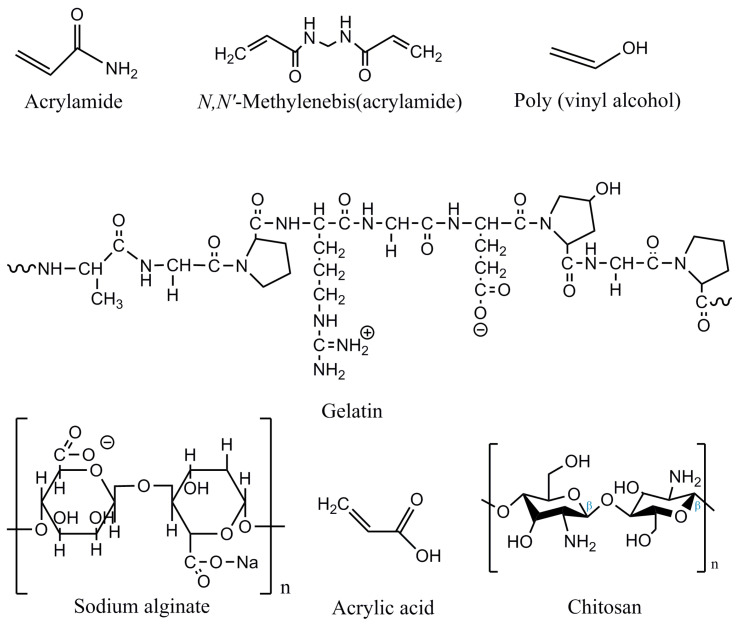
Gelatin is a protein that can easily biodegrade in the environment but has some stability limitations at high temperatures since once it has been dissolved, long durations at temperatures above 40 °C can promote protein denaturation. Moreover, gelatin is soluble in water, and this can also be a problem when synthesizing neat gelatin materials. As shown in Figure 1, gelatin is usually made from repeating units of glycine-X-Y. The high amount of the amino acids proline (12%), hydroxyproline (10%), and hydroxylysine (0.5%) make gelatin particularly special [50]. Depending on its origin, the content of proline and hydroxyproline might vary; in fact, fish gelatin is known for having a lower concentration of these amino acids compared to that coming from mammals. This affects negatively the gelling ability of fish gelatin, leading to a decrease in the gelation and melting temperatures together with a worsening of its mechanical properties, among others [51]. Thus, in order to synthesize more stable and resistant gelatin-based hydrogels, this biopolymer has been combined with many other polymers such as polyvinyl alcohol [28], alginate dialdehyde, alginate dialdehyde-gelatin reinforced with bioactive glass nanoparticles [3], dialdehyde carboxymethyl cellulose-dextrin [30], graphene oxide and laponite [31], collagen [49], chitosan [5], dopamine grafted, 1,4-phenylenebisboronic acid and graphene oxide [11], and oxidized alginate (OA) reinforced by silicon carbide nanoparticles (SiC NPs) and cross-linked with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) [52]. Figure 1 shows the chemical structure of different compounds used for the synthesis of gelatin-based hydrogels.
Figure 1.
Chemical structure of some of the compounds that enable cross-linking with gelatin for the synthesis of gelatin-based hydrogels.
3.1. Gelatin-Based Hydrogels Formulation and Preparation
Gelatin is a natural biopolymer with a high capacity for hydrogel formation with different compositions which promotes a proper cross-linking (Table 2). Green materials are really important for cleaning the environment since they have more acceptable characteristics for the sustainability of the environment. Thus, the composition of these materials is very important.
Depending on which kind of material, the concentration, composition, temperature, and time of reaction are mandatory for hydrogel formation. Additionally, if the hydrogel will be drying, the drying time and temperatures are variable (Table 2), and they are important too. Gelatin is a biodegradable protein with a temperature of denaturation starting above 40 °C [53]. So, it should be noted that all temperatures involved in the process of composition and preparation of the hydrogel should not be higher than 40 °C. Even if high temperatures may decrease the reaction time, when several components are involved in the reaction, it is still necessary to keep this temperature below 50 °C. In this way, high denaturation of the gelatin will be avoided, which is an important factor for the stability of the hydrogel. When different compounds with higher temperatures of solubilization such as PVA (around 90 °C) are used, it is important to dissolve these polymers before gelatin, then reduce the temperature, and after inserting gelatin in the formulation, avoiding premature degradation of the biopolymer. The physicochemical characteristics of the gel can influence its formation and stability and may be different for gelatins from the same source, according to the distribution of the amino acids and the extraction process used. The thermal stability of collagen is related to its content of amino acids (proline and hydroxyproline), and the higher the content of these amino acids, the greater stability of the triple helices. However, it must be highlighted that gelatin, when placed in cold water, can absorb 5 to 10 times its own mass and it swells. When this material is heated above its melting point (between 27 and 34 °C), the gelatin dissolves. This sol–gel transformation state is reversible. The thermal properties such as the melting and gelling point of gelatin are also related to the amount of proline and hydroxyproline amino acids in the original collagen [54]. Collagen denatures at temperatures above 40 °C generate a mixture of species with one, two, or three randomly coiled polypeptide chains [53]. Controlled cooling (below the melting temperature) leads to the recovery of a helical structure. Gels formed by gelatin can be considered as a partial return of molecules to an ordered state [55].
Gelatin can be the main cross-linking agent in the hydrogel; however, many other reagents can help with cross-linking, such as alginate [16,19], glutaraldehyde [19], chitosan [35,56], acrylic acid [57], N,N-methylene-bis-acrylamide [57], acrylamide [19], poly(vinyl alcohol) [58], and others. It is important to know the characteristics of the materials used in the composition of the hydrogels since they will determine the features of the synthesized hydrogel such as its stability, resistance, cross-linking, and reusability. Some materials used and listed above such as acrylamide or PVA can enhance the resistance and durability of hydrogels according to their concentration. These characteristics can determine their reusability in further adsorption processes, including the removal of other pollutants.
The reaction time is important for proper cross-linking. Many studies about hydrogels involving gelatin showed different reaction times, varying from several minutes to 24 h (Table 2). Actually, the reaction time will depend on their composition and can be modified with the different concentrations of the materials used. For instance, the cross-linking of gelatin hydrogels containing cassava flour (1%) and carbon-graphene oxide (1%) was performed in 5 min [20]. On the contrary, 24 h was needed in order to cross-link hydrogels with a more complex composition containing AM monomer (2.2%), HEMA monomer (0.2%), PVA (2.5%), cross-linking agent (EGDMA, 1.0% molar to total monomer), and TMEDA (5.0% molar to total monomer) [14].
In the molding and finishing of the hydrogels, it is necessary to remove the water and dry them via the most adequate technique and temperature (Table 2). Different forms such as oven-drying [15,37], room temperature [35], refrigerator, and lyophilizer [59] have been used. The time of drying can also be variable, ranging from 10 to 48 h, depending on the composition of the hydrogel and the method used for drying (Table 2). Temperature can also be varied, but high temperatures can initiate a partial denaturation as aforementioned, although some studies have shown drying temperatures up to 105 °C [20].
Table 2.
Formulations of different gelatin-based hydrogels for remediation uses.
| Hydrogel Formulation | Composition | Temp. of Mixture | Time of Reaction | Removal | Drying | Reference |
|---|---|---|---|---|---|---|
| Iron oxide | 5% gelatin, 2 g FeCl2, 5.2 g FeCl3 (in 100 m) | 70 °C | 15 min | Yellow 12 (DY12) | 60 °C | [18] |
| Cassava flour extracts and carbon-graphene oxide |
1% cassava flour, 1% carbon-graphene oxide, gelatin (N.I) | NI * | 5 min | Methylene blue trihydrate (MB) and direct red 23 | 48 h at 105 °C | [20] |
| Ba(OH)2 gelatin microcapsules | Gelatin (Ba/gelatin) different batches (0.16/1.5; 0.16/2.0; 0.24/2.0; 0.24/2.5) |
80 °C | 8 min | Sulfate | NI | [10] |
| Activated carbon- alginate -cyclodextrin | 60% sodium alginate, 30% CD, 8% gelatin, 2% activated carbon | NI | 30 min | 2,4-dichlorophenol | 24 h at 55 °C | [16] |
| Starch-gelatin mixed hydrogels with ferrite@biochar@molybdenum oxide | MoO3, CoFe2O4, starch, gelatin, glutaraldehyde. Concentrations NI | Microwave | NI | Pb (II) | 50 °C | [12] |
| Chitosan | 3% chitosan, 6% gelatin | 50 °C | 2 h | Acid orange II dye | 30 °C | [56] |
| Chitosan—graphene bead | 3% chitosan/3.2% gelatin/0.1–0.2% graphene | 45 °C | Overnight | Orange II | Room temp. | [35] |
| Bentonite—bis-acrylamide—formaldehyde | Gelatin (20–40%), bentonite (1–3%), bis-acrylamide (200–500 μL), formaldehyde (50–200 μL) | NI | NI | Pb (II) | 60 °C | [13] |
| Chitosan—acrylic acid—gelatin—N,N-methylenebisacrylamide | Chitosan (1%), acrylic acid (2.3%), gelatin (2–20%), N,N-methylenebisacrylamide (2.2%) | 70 °C | 3 h | Cu2+ | 60 °C | [57] |
| P(HEMA-co-AM)—PVA | AM monomer (2.2%), HEMA monomer (0.2%), PVA (2.5%), cross-linker (EGDMA, 1.0% molar to total monomer), TMEDA (5.0% molar to total monomer) | 50 °C | 24 h | Pb (II) | 50 °C overnight | [14] |
| Sugarcane bagasse cellulose | 0.5% gelatin, bagasse (NI) | 40 °C | 1 h | Cu (II) | 50 °C in oven for 10 h | [37] |
| Alginate—acrylamide—ZnS nanocomposite | Sodium alginate and gelatin, acrylamide, glutaraldehyde (conc. varied) | 60 °C | 4 h | Biebrich scarlet and crystal violet dyes | 60 °C air oven | [19] |
| Calcium—sodium bentonite | Calcium and sodium bentonite, gelatin (5%) | 30 °C | NI | Clay turbidity, clay dosages, DBO | NI | [17] |
| Poly (vinyl alcohol) (PVA) | Poly (vinyl alcohol) (PVA)/gelatin, alginate (10% total mixture) | NI | 2 h | Pb (II) | NI | [58] |
* NI: not informed or not available in the published manuscript.
Some studies have demonstrated that natural gelatin-based hydrogels are usually soft and fragile, and hydrogels with elastic structures normally require modification of gelatin chains or amendment with chemical cross-linking agents [10]. These agents are needed to improve the structural characteristics of the hydrogels, giving them durability and/or stability.
3.2. Gelatin-Based Hydrogels for Remediation
Gelatin-based hydrogels for the adsorption of contaminants have many interesting advantages such as adaptable shapes, varying concentrations of the matrix polymer, adding charges with other materials, porosity, and permeability, for instance. Table 3 shows the variable compositions that gelatin-based hydrogels can have, containing different polymeric formulations for gel formation such as acrylamide, polyacrylamide, acrylic acid, N,N-methylenebisacrylamide and poly(vinyl alcohol) (PVA).
Many of the reported formulations for these hydrogels were used for the remediation of many organic and inorganic pollutants (Table 2). Some of the removed organic contaminants were direct yellow 12 [18], 2,4-dichlorophenol [16], acid orange II dye [56], biebrich scarlet, crystal violet [19], and methyl violet [45]. The potential of removal was high, achieving in some cases almost 99% of efficiency for the remediation of these organic compounds, most of them in aqueous solution but with potential use on water environments or effluents in waste treatment.
The adsorption of methylene blue has also been studied for water decontamination. This contaminant decreases the water and environment quality. Some studies with gelatin-based hydrogels showed high efficiency for its remediation, and high concentrations of methylene blue were removed from an aqueous solution such as 26.04 mg of methylene blue g−1 of hydrogel composed of ethylene glycol, diglycidyl, xylan, and gelatin [36].
The incorporation of other materials such as colloids with high charge change capacity has many advantages for adsorption. Some authors demonstrated that adding montmorillonite into their hydrogels based on N,N’-methylenebisacrylamide improved the charges and also the adsorption of the toluene from an aqueous solution [29]. However, in the case of gelatin-based hydrogels, other mineral clays such as bentonite have been added for a high removal efficiency of Pb (II), which was higher than 90% and 47.169 mg for Pb (II) g−1 of the hydrogel [13]. Additionally, iron oxide can be important for adsorption since it increases the resistance and recalcitrance of the hydrogel. Another study showed that the incorporation of iron oxide for the removal of 197.4 mg L−1 of direct yellow from wastewater showed a high capacity of the hydrogel to remove this organic contaminant [18].
Cellulose and cellulose derivatives have useful chemical characteristics for tailoring hydrogels with wide applicability and high efficiency [9]. The incorporation of these materials into gelatin-based hydrogels has also been studied for environmental purposes. Marciano et al. [27] incorporated cellulose extracted from wastes into a gelatin-based hydrogel and achieved an adsorption of 13 mg of Cr (VI) per g of hydrogel, which could be used in water and waste treatment from different industry effluents.
Apart from the aforementioned inorganic heavy metal ions (Pb (II) and Cr (VI)), gelatin-based hydrogels have also shown to be promising for the removal of other heavy metals such as Cu (II), Co (II), Ni (II), and Zn (II) from wastewater and or water (Table 3). Some of these studies showed high concentrations for Cu (II) removal from water with 372.5 mg g−1 of Cu (II) using a hydrogel containing TiO2 nanoparticles and branched polyethyleneimine [59], and 261.08 mg g−1 of Cu (II) using a gelatin hydrogel containing chitosan, acrylic acid, and N,N-methylene-bis-acrylamide [57]. Thus, gelatin-based hydrogels also show a high potential for copper remediation.
Lead is a potential heavy metal with highly toxic characteristics, and this problem increases when it is bioavailable in water by solubilization. However, gelatin-based hydrogels can remove high concentrations of this heavy metal. In fact, more than 210 mg of removed Pb (II) g−1 hydrogel have been reported for gelatin/poly(vinyl alcohol) (PVA)-based hydrogels [58].
Table 3.
Different compositions and uses of gelatin-based hydrogels for remediation.
| Gelatin-Based Hydrogel | Pollutant | Situation of Application | Potential of Removal | Reference |
|---|---|---|---|---|
| Fe3O4 | Direct yellow 12 (DY12) | Wastewater | 197.4 mg L−1 of DY12 | [18] |
| Sodium alginate, cyclodextrin, and activated carbon | 2,4-dichlorophenol (2,4-DCP) | Wastewater and contaminated water | 36.48 mg g−1 and 39.36 mg g−1 of 2,4-DCP | [16] |
| Chitosan and genipin | Acid orange II dye | Wastewater | 0.25 mmol L−1 of acid orange II dye | [56] |
| Chitosan filled with graphene bead | Orange II | Wastewater | 42.15 mg L−1 of orange II | [35] |
| ZnS, sodium alginate, and polyacrylamide | Biebrich scarlet and crystal violet | Water and wastewater | 9.7 mg L−1 of biebrich scarlet and 28.63 mg L−1 of crystal violet | [19] |
| Grafted methyl methacrylate | Methylviolet (MV) dye | Water purification | 49.5 mg L−1 of methyl violet (MV) dye | [45] |
| Ethylene glycol diglycidyl and xylan | Methylene blue | Water and wastewater | 26.04 mg g−1 of methylene blue | [36] |
| TiO2 and PEI (GTP) aerogel | Oil/water separation Properties for both oil/water-free mixtures and oil-water emulsions and Cu (II) |
Wastewater and water contaminated with oil spills | 372.5 mg g−1 of Cu (II); 99.72% of oil rejection coefficient | [59] |
| Ulva fenestrata and dialdehyde | Methylene blue, Cu (II), Co (II), Ni (II), Zn (II) | Aqueous solution | 465 mg g−1 of methylene blue; 14 mg g−1 for Cu (II), 7 mg g−1 for Co (II), and 6 mg g- 1 for Ni (II) and Zn (II) | [38] |
| Chitosan, acrylic acid, and N,N-methylene-bis-acrylamide | Cu (II) | Wastewater | 261.08 mg g−1 of Cu (II) | [57] |
| Acrylamide, acrylic acid, N,N’-methylene-bis-acrylamide, sugarcane bagasse | Cu (II) | Water and wastewater | 97.38 mg L−1 of Cu (II) | [37] |
| Bentonite | Pb (II) | Wastewater | 47.169 mg g−1 of Pb (II) | [13] |
| Poly(vinyl alcohol) (PVA) | Pb (II) | Water and wastewater | 210.43 mg g−1 of Pb (II) | [58] |
| Metal–organic frameworks UiO-66-NO2 film | Pb (II) | Water decontamination and food safety control fields | 0.98 mg L−1 of Pb (II) | [15] |
| Cellulose incorporated from waste | Cr (VI) | Water and waste treatment | 12 and 13 mg g−1 of Cr (VI) | [27] |
4. Conclusions
Hydrogels have been described as promising materials over the years for many applications in diverse fields and areas. These materials have shown many interesting characteristics such as cross-linking, shape, and elasticity that can promote them as innovative materials with novel and important applications.
Gelatin-based hydrogels have been studied in the same way since gelatin is renewable, biodegradable, and can substitute expensive and oil-derived components for gel formation, leading to the production of green and non-toxic materials for humans and the environment. However, to improve the characteristics of gelatin-based hydrogels, and depending on their final application, it is important to combine gelatin with other sustainable materials such as waste of cellulose or lignin, or chitosan or mineral clays for improving their charge and structural characteristic. The key to the process of new polymers for hydrogels with gelatin is the use of other components.
To achieve a new polymer for a gelatin-based hydrogel, the concentration of the products, time of reaction, the temperature of the reaction, and temperature for drying the material are important to ensure no denaturation of the gelatin proteins and also the earlier degradation of the hydrogel.
For environmental purposes, hydrogels have shown high performance as removers of many contaminants in aqueous solutions; however, as society is under continuous development, the type and amount of pollutants are constantly increasing and it is, therefore, still necessary to study the remediation of a wider variety of contaminants and mediums for increasing the spectrum of use of these materials in this field in the near future.
Author Contributions
Conceptualization, R.A., A.M. and S.P.; writing—original draft preparation, R.A. and S.P.; writing—review and editing, A.M. and J.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (001). The authors would like to acknowledge the financial support of the Department of Education of the Basque Government (IT1498-22). A. Morales would like to thank the University of the Basque Country (Training of Researcher Staff, PIF17/207).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Morales A., Labidi J., Gullón P. Effect of the Formulation Parameters on the Absorption Capacity of Smart Lignin-Hydrogels. Eur. Polym. J. 2020;129:109631. doi: 10.1016/j.eurpolymj.2020.109631. [DOI] [Google Scholar]
- 2.Bertsch P., Andrée L., Besheli N.H., Leeuwenburgh S.C.G. Colloidal Hydrogels Made of Gelatin Nanoparticles Exhibit Fast Stress Relaxation at Strains Relevant for Cell Activity. Acta Biomater. 2022;138:124–132. doi: 10.1016/j.actbio.2021.10.053. [DOI] [PubMed] [Google Scholar]
- 3.Monavari M., Homaeigohar S., Fuentes-Chandía M., Nawaz Q., Monavari M., Venkatraman A., Boccaccini A.R. 3D Printing of Alginate Dialdehyde-Gelatin (ADA-GEL) Hydrogels Incorporating Phytotherapeutic Icariin Loaded Mesoporous SiO2-CaO Nanoparticles for Bone Tissue Engineering. Mater. Sci. Eng. C. 2021;131:112470. doi: 10.1016/j.msec.2021.112470. [DOI] [PubMed] [Google Scholar]
- 4.Khamrai M., Banerjee S.L., Paul S., Samanta S., Kundu P.P. Curcumin Entrapped Gelatin/Ionically Modified Bacterial Cellulose Based Self-Healable Hydrogel Film: An Eco-Friendly Sustainable Synthesis Method of Wound Healing Patch. Int. J. Biol. Macromol. 2019;122:940–953. doi: 10.1016/j.ijbiomac.2018.10.196. [DOI] [PubMed] [Google Scholar]
- 5.Omer A.M., Sadik W.A.-A., El-Demerdash A.-G.M., Hassan H.S. Formulation of PH-Sensitive Aminated Chitosan-Gelatin Crosslinked Hydrogel for Oral Drug Delivery. J. Saudi Chem. Soc. 2021;25:101384. doi: 10.1016/j.jscs.2021.101384. [DOI] [Google Scholar]
- 6.Research G.V. Gelatin Market Size Expected to Reach$4.08 Billion by 2024. Grand View Research, Inc. 2016. [(accessed on 14 November 2022)]. Available online: https://www.grandviewresearch.com/press-release/global-gelatin-market.
- 7.Global Industry Analysts Gelatin Growing Applications in Food, Pharmaceutical and Nutritional Solutions to Drive Demand for Gelatin. [(accessed on 14 November 2022)]. Available online: https://www.marketresearch.com/Global-Industry-Analysts-v1039/Gelatin-31468046/
- 8.Mohamed F.A.A., Roberts M., Seton L., Ford J.L., Levina M., Rajabi-Siahboomi A.R. The Effect of HPMC Particle Size on the Drug Release Rate and the Percolation Threshold in Extended-Release Mini-Tablets. Drug Dev. Ind. Pharm. 2015;41:70–78. doi: 10.3109/03639045.2013.845843. [DOI] [PubMed] [Google Scholar]
- 9.Thakur A., Kaur H. Synthetic Chemistry of Cellulose Hydrogels—A Review. Mater. Today Proc. 2021;48:1431–1438. doi: 10.1016/j.matpr.2021.09.201. [DOI] [Google Scholar]
- 10.Chen H., Wu D., Ma W., Wu C., Tian Y., Wang S., Du M. Strong Fish Gelatin Hydrogels Enhanced by Carrageenan and Potassium Sulfate. Food Hydrocoll. 2021;119:106841. doi: 10.1016/j.foodhyd.2021.106841. [DOI] [Google Scholar]
- 11.Han K., Bai Q., Wu W., Sun N., Cui N., Lu T. Gelatin-Based Adhesive Hydrogel with Self-Healing, Hemostasis, and Electrical Conductivity. Int. J. Biol. Macromol. 2021;183:2142–2151. doi: 10.1016/j.ijbiomac.2021.05.147. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud M.E., Abouelanwar M.E., Mahmoud S.E.M., Salam M.A. Doping Starch-Gelatin Mixed Hydrogels with Magnetic Spinel Ferrite@biochar@molybdenum Oxide as a Highly Efficient Nanocomposite for Removal of Lead (II) Ions. J. Environ. Chem. Eng. 2021;9:106682. doi: 10.1016/j.jece.2021.106682. [DOI] [Google Scholar]
- 13.Pal P., Syed S.S., Banat F. Gelatin-Bentonite Composite as Reusable Adsorbent for the Removal of Lead from Aqueous Solutions: Kinetic and Equilibrium Studies. J. Water Process Eng. 2017;20:40–50. doi: 10.1016/j.jwpe.2017.09.010. [DOI] [Google Scholar]
- 14.Tanan W., Panpinit S., Saengsuwan S. Comparison of Microwave-Assisted and Thermal-Heated Synthesis of P(HEMA-Co-AM)/PVA Interpenetrating Polymer Network (IPN) Hydrogels for Pb(II) Removal from Aqueous Solution: Characterization, Adsorption and Kinetic Study. Eur. Polym. J. 2021;143:110193. doi: 10.1016/j.eurpolymj.2020.110193. [DOI] [Google Scholar]
- 15.Yang W., Wang J., Han Y., Luo X., Tang W., Yue T., Li Z. Robust MOF Film of Self-Rearranged UiO-66-NO2 Anchored on Gelatin Hydrogel via Simple Thermal-Treatment for Efficient Pb(II) Removal in Water and Apple Juice. Food Control. 2021;130:108409. doi: 10.1016/j.foodcont.2021.108409. [DOI] [Google Scholar]
- 16.Alsohaimi I.H., El-Aassar M.R., Elzain A.A., Alshammari M.S., Ali A.S.M. Development of Activated Carbon-Impregnated Alginate*β-Cyclodextrin/Gelatin Beads for Highly Performance Sorption of 2,4-Dichlorophenol from Wastewater. J. Mater. Res. Technol. 2020;9:5144–5153. doi: 10.1016/j.jmrt.2020.03.031. [DOI] [Google Scholar]
- 17.Shamsuddin R.M., Verbeek C.J.R., Lay M.C. Settling of Bentonite Particles in Gelatin Solutions for Stickwater Treatment. Procedia Eng. 2016;148:194–200. doi: 10.1016/j.proeng.2016.06.570. [DOI] [Google Scholar]
- 18.Mir A.A., Amooey A.A., Ghasemi S. Adsorption of Direct Yellow 12 from Aqueous Solutions by an Iron Oxide-Gelatin Nanoadsorbent; Kinetic, Isotherm and Mechanism Analysis. J. Clean. Prod. 2018;170:570–580. doi: 10.1016/j.jclepro.2017.09.101. [DOI] [Google Scholar]
- 19.Priya, Sharma A.K., Kaith B.S., Tanwar V., Bhatia J.K., Sharma N., Bajaj S., Panchal S. RSM-CCD Optimized Sodium Alginate/Gelatin Based ZnS-Nanocomposite Hydrogel for the Effective Removal of Biebrich Scarlet and Crystal Violet Dyes. Int. J. Biol. Macromol. 2019;129:214–226. doi: 10.1016/j.ijbiomac.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Ndagijimana P., Liu X., Xu Q., Lai D., Wang G., Pan B., Wang Y. Cassava Flour Extracts Solution to Induce Gelatin Cross-Linked Activated Carbon-Graphene Oxide Composites: The Adsorption Performance of Dyes from Aqueous Media. Environ. Adv. 2021;5:100079. doi: 10.1016/j.envadv.2021.100079. [DOI] [Google Scholar]
- 21.Sannino A., Demitri C., Madaghiele M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials. 2009;2:353–373. doi: 10.3390/ma2020353. [DOI] [Google Scholar]
- 22.Haque M.O., Mondal M.I.H. Synthesis and Characterization of Cellulose-Based Eco-FriendlyHydrogels. Rajshahi Univ. J. Sci. Eng. 2016;44:45–53. doi: 10.3329/rujse.v44i0.30386. [DOI] [Google Scholar]
- 23.Mao L., Hu Y., Piao Y., Chen X., Xian W., Piao D. Structure and Character of Artificial Muscle Model Constructed from Fibrous Hydrogel. Curr. Appl. Phys. 2005;5:426–428. doi: 10.1016/j.cap.2004.11.003. [DOI] [Google Scholar]
- 24.Guo Y., Bae J., Fang Z., Li P., Zhao F., Yu G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020;120:7642–7707. doi: 10.1021/acs.chemrev.0c00345. [DOI] [PubMed] [Google Scholar]
- 25.Peppas N.A., Hilt J.Z., Khademhosseini A., Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006;18:1345–1360. doi: 10.1002/adma.200501612. [DOI] [Google Scholar]
- 26.Suvarnapathaki S., Nguyen M.A., Wu X., Nukavarapu S.P., Camci-Unal G. Synthesis and Characterization of Photocrosslinkable Hydrogels from Bovine Skin Gelatin. RSC Adv. 2019;9:13016–13025. doi: 10.1039/C9RA00655A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciano J.S., Ferreira R.R., de Souza A.G., Barbosa R.F.S., de Moura Junior A.J., Rosa D.S. Biodegradable Gelatin Composite Hydrogels Filled with Cellulose for Chromium (VI) Adsorption from Contaminated Water. Int. J. Biol. Macromol. 2021;181:112–124. doi: 10.1016/j.ijbiomac.2021.03.117. [DOI] [PubMed] [Google Scholar]
- 28.Manish V., Arockiarajan A., Tamadapu G. Influence of Water Content on the Mechanical Behavior of Gelatin Based Hydrogels: Synthesis, Characterization, and Modeling. Int. J. Solids Struct. 2021;233:111219. doi: 10.1016/j.ijsolstr.2021.111219. [DOI] [Google Scholar]
- 29.Tahari N., de Hoyos-Martinez P.L., Abderrabba M., Ayadi S., Labidi J. Lignin-Montmorillonite Hydrogels as Toluene Adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2020;602:125108. doi: 10.1016/j.colsurfa.2020.125108. [DOI] [Google Scholar]
- 30.Sharma A.K., Kaith B.S., Shree B. Borax Mediated Synthesis of a Biocompatible Self-Healing Hydrogel Using Dialdehyde Carboxymethyl Cellulose-Dextrin and Gelatin. React. Funct. Polym. 2021;166:104977. doi: 10.1016/j.reactfunctpolym.2021.104977. [DOI] [Google Scholar]
- 31.Cheng Y.H., Cheng S.J., Chen H.H., Hsu W.C. Development of Injectable Graphene Oxide/Laponite/Gelatin Hydrogel Containing Wharton’s Jelly Mesenchymal Stem Cells for Treatment of Oxidative Stress-Damaged Cardiomyocytes. Colloids Surf. B Biointerfaces. 2022;209:112150. doi: 10.1016/j.colsurfb.2021.112150. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Xu J., Li R., Wang D., Li T., Yuan M., Wang J. Simple Preparation of Aminothiourea-Modified Chitosan as Corrosion Inhibitor and Heavy Metal Ion Adsorbent. J. Colloid Interface Sci. 2014;417:131–136. doi: 10.1016/j.jcis.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Godiya C.B., Cheng X., Li D., Chen Z., Lu X. Carboxymethyl Cellulose/Polyacrylamide Composite Hydrogel for Cascaded Treatment/Reuse of Heavy Metal Ions in Wastewater. J. Hazard. Mater. 2019;364:28–38. doi: 10.1016/j.jhazmat.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya R., Ray S.K. Adsorption of Industrial Dyes by Semi-IPN Hydrogels of Acrylic Copolymers and Sodium Alginate. J. Ind. Eng. Chem. 2015;22:92–102. doi: 10.1016/j.jiec.2014.06.029. [DOI] [Google Scholar]
- 35.Wu M., Chen W., Mao Q., Bai Y., Ma H. Facile Synthesis of Chitosan/Gelatin Filled with Graphene Bead Adsorbent for Orange II Removal. Chem. Eng. Res. Des. 2019;144:35–46. doi: 10.1016/j.cherd.2019.01.027. [DOI] [Google Scholar]
- 36.Seera S.D.K., Kundu D., Gami P., Naik P.K., Banerjee T. Synthesis and Characterization of Xylan-Gelatin Cross-Linked Reusable Hydrogel for the Adsorption of Methylene Blue. Carbohydr. Polym. 2021;256:117520. doi: 10.1016/j.carbpol.2020.117520. [DOI] [PubMed] [Google Scholar]
- 37.Maity J., Ray S.K. Removal of Cu (II) Ion from Water Using Sugar Cane Bagasse Cellulose and Gelatin Based Composite Hydrogels. Int. J. Biol. Macromol. 2017;97:238–248. doi: 10.1016/j.ijbiomac.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Wahlström N., Steinhagen S., Toth G., Pavia H., Edlund U. Ulvan Dialdehyde-Gelatin Hydrogels for Removal of Heavy Metals and Methylene Blue from Aqueous Solution. Carbohydr. Polym. 2020;249:116841. doi: 10.1016/j.carbpol.2020.116841. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y., Zhao W., Zhang X., Peng H., Gong Y. Thiol-Ene Synthesis of Thioether/Carboxyl-Functionalized Polymers for Selective Adsorption of Silver (I) Ions. Chem. Eng. J. 2019;375:121935. doi: 10.1016/j.cej.2019.121935. [DOI] [Google Scholar]
- 40.Ahmed M.A., Al-Kahtani H.A., Jaswir I., AbuTarboush H., Ismail E.A. Extraction and Characterization of Gelatin from Camel Skin (Potential Halal Gelatin) and Production of Gelatin Nanoparticles. Saudi J. Biol. Sci. 2020;27:1596–1601. doi: 10.1016/j.sjbs.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur K., Jindal R., Jindal D. Controlled Release of Vitamin B1 and Evaluation of Biodegradation Studies of Chitosan and Gelatin Based Hydrogels. Int. J. Biol. Macromol. 2020;146:987–999. doi: 10.1016/j.ijbiomac.2019.09.223. [DOI] [PubMed] [Google Scholar]
- 42.Chiou B.S., Avena-Bustillos R.J., Bechtel P.J., Jafri H., Narayan R., Imam S.H., Glenn G.M., Orts W.J. Cold Water Fish Gelatin Films: Effects of Cross-Linking on Thermal, Mechanical, Barrier, and Biodegradation Properties. Eur. Polym. J. 2008;44:3748–3753. doi: 10.1016/j.eurpolymj.2008.08.011. [DOI] [Google Scholar]
- 43.Martucci J.F., Ruseckaite R.A. Biodegradation of Three-Layer Laminate Films Based on Gelatin under Indoor Soil Conditions. Polym. Degrad. Stab. 2009;94:1307–1313. doi: 10.1016/j.polymdegradstab.2009.03.018. [DOI] [Google Scholar]
- 44.Zhang Y., Li C., Geary T., Jardim A., He S., Simpson B.K. Cold Setting of Gelatin–Antioxidant Peptides Composite Hydrogels Using a New Psychrophilic Recombinant Transglutaminase (RTGase) Food Hydrocoll. 2022;122:107116. doi: 10.1016/j.foodhyd.2021.107116. [DOI] [Google Scholar]
- 45.Chaudhary J., Thakur S., Mamba G., Prateek, Gupta R.K., Thakur V.K. Hydrogel of Gelatin in the Presence of Graphite for the Adsorption of Dye: Towards the Concept for Water Purification. J. Environ. Chem. Eng. 2021;9:104762. doi: 10.1016/j.jece.2020.104762. [DOI] [Google Scholar]
- 46.Boughriba S., Souissi N., Nasri R., Nasri M., Li S. PH Sensitive Composite Hydrogels Based on Gelatin and Reinforced with Cellulose Microcrystals: In Depth Physicochemical and Microstructural Analyses for Controlled Release of Vitamin B2. Mater. Today Commun. 2021;27:102334. doi: 10.1016/j.mtcomm.2021.102334. [DOI] [Google Scholar]
- 47.Kreller T., Distler T., Heid S., Gerth S., Detsch R., Boccaccini A.R. Physico-Chemical Modification of Gelatine for the Improvement of 3D Printability of Oxidized Alginate-Gelatine Hydrogels towards Cartilage Tissue Engineering. Mater. Des. 2021;208:109877. doi: 10.1016/j.matdes.2021.109877. [DOI] [Google Scholar]
- 48.Saotome T., Shimada N., Matsuno K., Nakamura K., Tabata Y. Gelatin Hydrogel Nonwoven Fabrics of a Cell Culture Scaffold to Formulate 3-Dimensional Cell Constructs. Regen. Ther. 2021;18:418–429. doi: 10.1016/j.reth.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leite M.L., Soares D.G., Anovazzi G., Anselmi C., Hebling J., de Souza Costa C.A. Fibronectin-Loaded Collagen/Gelatin Hydrogel Is a Potent Signaling Biomaterial for Dental Pulp Regeneration. J. Endod. 2021;47:1110–1117. doi: 10.1016/j.joen.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Hjelmgaard T., Svendsen J.Ø., Köhler B., Pawelzyk P., Lybye D., Schmücker C.M., Reiter P., Reihmann M., Thorsen P.A. Gelatin-Tannin-Based Greener Binder Technology for Stone Shot and Stone Wool Materials: A Detailed Study. ACS Omega. 2021;6:33874–33882. doi: 10.1021/acsomega.1c05153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derkach S.R., Voron’ko N.G., Kuchina Y.A., Kolotova D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers. 2020;12:3051. doi: 10.3390/polym12123051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghanbari M., Salavati-Niasari M., Mohandes F., Firouzi Z. Modified Silicon Carbide NPs Reinforced Nanocomposite Hydrogels Based on Alginate-Gelatin by with High Mechanical Properties for Tissue Engineering. Arab. J. Chem. 2022;15:103520. doi: 10.1016/j.arabjc.2021.103520. [DOI] [Google Scholar]
- 53.Hayashi A., Oh S.C. Gelation of Gelatin Solution. Agric. Biol. Chem. 1983;47:1711–1716. doi: 10.1080/00021369.1983.10865852. [DOI] [Google Scholar]
- 54.Haug I.J., Draget K.I., Smidsrød O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004;18:203–213. doi: 10.1016/S0268-005X(03)00065-1. [DOI] [Google Scholar]
- 55.de Man J.M. Principles Pf Food Chemistry. 3rd ed. Springer; New York, NY, USA: 1999. [Google Scholar]
- 56.Cui L., Xiong Z., Guo Y., Liu Y., Zhao J., Zhang C., Zhu P. Fabrication of Interpenetrating Polymer Network Chitosan/Gelatin Porous Materials and Study on Dye Adsorption Properties. Carbohydr. Polym. 2015;132:330–337. doi: 10.1016/j.carbpol.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Wang W.B., Huang D.J., Kang Y.R., Wang A.Q. One-Step in Situ Fabrication of a Granular Semi-IPN Hydrogel Based on Chitosan and Gelatin for Fast and Efficient Adsorption of Cu2+ Ion. Colloids Surf. B Biointerfaces. 2013;106:51–59. doi: 10.1016/j.colsurfb.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 58.Hui B., Zhang Y., Ye L. Structure of PVA/Gelatin Hydrogel Beads and Adsorption Mechanism for Advanced Pb(II) Removal. J. Ind. Eng. Chem. 2015;21:868–876. doi: 10.1016/j.jiec.2014.04.025. [DOI] [Google Scholar]
- 59.Jiang J., Zhang Q., Zhan X., Chen F. A Multifunctional Gelatin-Based Aerogel with Superior Pollutants Adsorption, Oil/Water Separation and Photocatalytic Properties. Chem. Eng. J. 2019;358:1539–1551. doi: 10.1016/j.cej.2018.10.144. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable.