In the twenty-first century, newly emerging viruses which are mostly zoonotic or vector-borne have continuously threatened public health and caused outbreaks of global concern. This has been highlighted by the recent COVID-19 pandemic which caused a catastrophic effect on the world’s healthcare system and the global economy [1]. Flaviviruses and alphaviruses are single-stranded RNA viruses vectored by Aedes mosquitoes that can (re)-emerge unexpectedly and cause severe viral infections in humans [2,3,4,5,6,7,8]. These flaviviruses and alphaviruses can be classified into a broader category of arboviruses, and they cause significant disease burdens and public health concerns due to the global spread and transmission over the last century [6,7,8,9,10].
The mosquito-borne flaviviruses such as dengue virus (DENV), Zika virus (ZIKV), yellow fever virus (YFV), West Nile virus (WNV), and Japanese encephalitis virus (JEV) are responsible for significant human morbidity and mortality all over the world [7,8,11,12]. In particular, DENV is estimated to cause around 400 million infections annually and 20% of infections lead to 22,000 deaths per year with more than a quarter of the world’s population now living in DENV-endemic areas [13,14,15]. The rapid geographical introduction and spread of WNV and ZIKV from the Eastern to the Western Hemisphere had caused a large number of cases with significant morbidity [16,17]. Although most ZIKV infections are asymptomatic, some ZIKV infections are associated with congenital Zika syndrome (CZS) and Guillain–Barré Syndrome (GBS) [18,19,20]. Despite the existence of a highly effective YFV vaccine, the re-emergence of YFV throughout Africa and the Americas now poses a serious public health challenge [21,22].
The alphaviruses are a genus of enveloped RNA viruses with medically important alphaviruses such as Chikungunya virus (CHIKV), Mayaro virus (MAYV), and Eastern equine encephalitis virus (EEEV) that can cause arthralgia (CHIKV and MAYV) or neuroinvasive disease (EEEV) [5,9,10,23]. Following the first identification of CHIKV in Tanzania in 1952 and subsequently, in Africa and Asia, CHIKV outbreaks became more prevalent since 2004, and CHIKV cases are now reported in over 100 countries in Asia, Africa, Europe, and the Americas [10,24,25]. For alphavirus infections, there are no specific antiviral drugs or licensed vaccines, and therefore, the current treatment is mainly symptom relief.
Despite continued threats from emerging viral diseases, the COVID-19 pandemic has demonstrated that the twenty-first century has come with a new era in vaccinology in which recombinant genetic technologies allowed remarkably rapid development of vaccines against SARS-CoV-2 in 2020 [26]. Over the last century, many vaccines based on classical platforms have played a major role in eradicating diseases such as polio and smallpox (Figure 1) [27,28]. However, the speed of the vaccine developments using the classical platforms is considerably slower than the next-generation platforms as these platforms are not often optimized for rapid large-scale production due to unavoidable limitations such as the requirement of biosafety level 3 conditions to grow large quantities of viruses for production of virus-inactivated vaccine and the extensive safety assessment and contraindications associated with administration of live-attenuated vaccines to immunocompromised and pregnant individuals [29,30]. On the other hand, the development of next-generation vaccines can go ahead as soon as the viral sequence becomes known and the DNA sequence of the whole or part of viral antigens with the critical epitopes can be used to develop vaccines using recombinant DNA technologies and thus significantly speeding up vaccine development [31,32]. These next-generation vaccine platforms include viral vectors, nucleic acid (DNA or RNA), and antigen-presenting cells (APC) (Figure 1) [26,30,33]. The potential of next-generation vaccine platforms was clearly shown during the COVID-19 pandemic where the fastest vaccine candidates that reached the phase I clinical trials were based on the next-generation platforms such as mRNA, DNA, human adenoviral vector, and chimpanzee adenoviral vector (ChAdOx1) [26]. In particular, the mRNA vaccine candidate had set a record time by reaching the clinical trial in only 69 days after the identification of the SARS-CoV-2 [26,34]. This pace of vaccine development was striking when compared to arboviral diseases caused by flaviviruses and alphaviruses such as DENV [35], CHIKV [25,36], and ZIKV [37,38,39] which reached trials in 52, 19, and 9 years after the declaration of major outbreaks by WHO, respectively as we discussed previously [26,37,40]. Looking on the bright side, the time taken for these vaccines to reach trials has progressively become shortened with the development of recombinant genetic technology. For instance, the first Zika vaccine candidate to reach the clinical trial in August 2016 was a DNA vaccine candidate which was 9 years after the ZIKV outbreak in Micronesia [37], 3 years after the major epidemic in French Polynesia in 2013 [41] but just 6 months after WHO declared Zika-related microcephaly as a Public Health Emergency of International Concern (PHEIC) in February 2016 [42] highlighting the advances in the modern vaccine development in urgent need [26].
Figure 1.
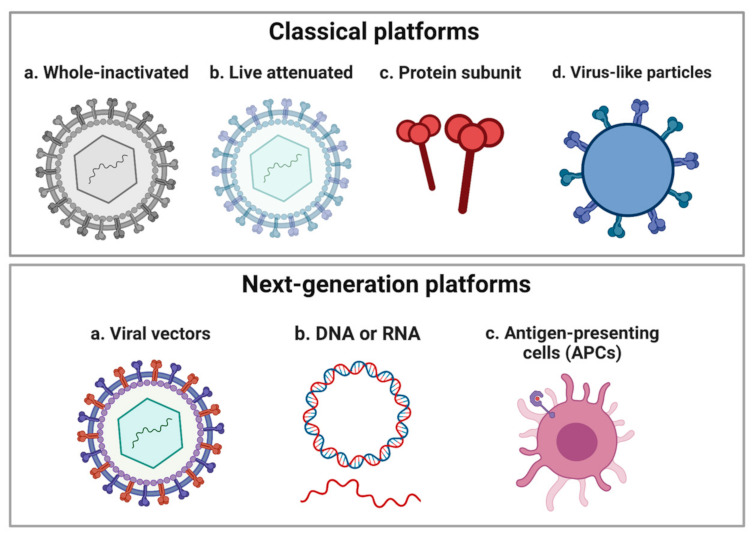
A schematic showing the classical vaccine platforms and next-generation platforms. The classical platforms include whole-inactivated, live attenuated, protein subunit, and virus-like particles (VLPs). The next-generation platforms include recombinant viral vectors, nucleic acid (DNA or RNA), and antigen-presenting cells (APC). Created with BioRender.com.
This Special Issue will feature the recent development of vaccines against flaviviruses and alphaviruses. Although licensed vaccines against flaviviruses exist such as DENV (tetravalent vaccine), JEV (inactivated), tick-borne encephalitis (inactivated), and YFV (attenuated), there are currently no vaccines against WNV and ZIKV [43]. Moreover, there may be a need for next-generation vaccines to overcome potential problems with an insufficient supply of inactivated vaccines and contraindications associated with the administration of live-attenuated vaccines to immunocompromised and pregnant individuals [22,43]. Despite slow progress in vaccine development against alphaviruses, there are currently many promising vaccines in clinical trials based on both the classical and next-generation platforms which could lead to the future licensing of vaccines against these medically important emerging arboviruses.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Department of Health through the SBRI Innovate UK grant (project Nos. 972212 and 971557) (A.R.-S.). Y.C.K. is supported by the Wellcome Trust Grant (224117/Z/21/Z).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kaye A.D., Okeagu C.N., Pham A.D., Silva R.A., Hurley J.J., Arron B.L., Sarfraz N., Lee H.N., Ghali G.E., Gamble J.W., et al. Economic Impact of COVID-19 Pandemic on Healthcare Facilities and Systems: International Perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021;35:293–306. doi: 10.1016/j.bpa.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers T.J., Hahn C.S., Galler R., Rice C.M. Flavivirus Genome Organization, Expression, and Replication. Annu. Rev. Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 3.Chambers T.J., Rice C.M. Molecular Biology of the Flaviviruses. Microbiol. Sci. 1987;4:219–223. [PubMed] [Google Scholar]
- 4.Strauss J.H., Strauss E.G. The Alphaviruses: Gene Expression, Replication, and Evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver S.C., Winegar R., Manger I.D., Forrester N.L. Alphaviruses: Population Genetics and Determinants of Emergence. Antiviral Res. 2012;94:242–257. doi: 10.1016/j.antiviral.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver S.C. Urbanization and Geographic Expansion of Zoonotic Arboviral Diseases: Mechanisms and Potential Strategies for Prevention. Trends Microbiol. 2013;21:360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver S.C., Reisen W.K. Present and Future Arboviral Threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver S.C., Charlier C., Vasilakis N., Lecuit M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver S.C. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLoS Negl. Trop. Dis. 2014;8:e2921. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver S.C., Lecuit M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 11.Pierson T.C., Diamond M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020;5:796–812. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaviviridae. Viral Hemorrhagic Fevers (VHFs). CDC. [(accessed on 24 January 2023)]; Available online: https://www.cdc.gov/vhf/virus-families/flaviviridae.html.
- 13.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The Global Distribution and Burden of Dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman M.G., Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 15.Dengue and Severe Dengue. [(accessed on 24 January 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 16.Pierson T.C., Diamond M.S. The Emergence of Zika Virus and Its New Clinical Syndromes. Nature. 2018;560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 17.Roehrig J.T. West Nile Virus in the United States—A Historical Perspective. Viruses. 2013;5:3088–3108. doi: 10.3390/v5123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Linden V., Pessoa A., Dobyns W., Barkovich A.J., van der Linden Júnior H., Filho E.L.R., Ribeiro E.M., de Carvalho Leal M., de Araújo Coimbra P.P., de Fátima Viana Vasco Aragão M., et al. Description of 13 Infants Born During October 2015-January 2016 with Congenital Zika Virus Infection without Microcephaly at Birth—Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 19.Vhp L., Aragão M.M., Pinho R.S., Hazin A.N., Paciorkowski A.R., Penalva de Oliveira A.C., Masruha M.R. Congenital Zika Virus Infection: A Review with Emphasis on the Spectrum of Brain Abnormalities. Curr. Neurol. Neurosci. Rep. 2020;20:49. doi: 10.1007/s11910-020-01072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao-Lormeau V.-M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faria N.R., Kraemer M.U.G., Hill S.C., Goes de Jesus J., Aguiar R.S., Iani F.C.M., Xavier J., Quick J., du Plessis L., Dellicour S., et al. Genomic and Epidemiological Monitoring of Yellow Fever Virus Transmission Potential. Science. 2018;361:894–899. doi: 10.1126/science.aat7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montalvo Zurbia-Flores G., Rollier C.S., Reyes-Sandoval A. Re-Thinking Yellow Fever Vaccines: Fighting Old Foes with New Generation Vaccines. Hum. Vaccin. Immunother. 2022;18:1895644. doi: 10.1080/21645515.2021.1895644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virus. Chikungunya Virus. CDC. [(accessed on 24 January 2023)]; Available online: https://www.cdc.gov/chikungunya/virus/index.html.
- 24.Staples J.E., Breiman R.F., Powers A.M. Chikungunya Fever: An Epidemiological Review of a Re-Emerging Infectious Disease. Clin. Infect. Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 25.Sergon K., Njuguna C., Kalani R., Ofula V., Onyango C., Konongoi L.S., Bedno S., Burke H., Dumilla A.M., Konde J., et al. Seroprevalence of Chikungunya Virus (CHIKV) Infection on Lamu Island, Kenya, October 2004. Am. J. Trop. Med. Hyg. 2008;78:333–337. doi: 10.4269/ajtmh.2008.78.333. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y.C., Dema B., Reyes-Sandoval A. COVID-19 Vaccines: Breaking Record Times to First-in-Human Trials. NPJ Vaccines. 2020;5:34. doi: 10.1038/s41541-020-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay A.S., Garon J., Seib K., Orenstein W.A. Polio Vaccination: Past, Present and Future. Future Microbiol. 2015;10:791–808. doi: 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- 28.Stewart A.J., Devlin P.M. The History of the Smallpox Vaccine. J. Infect. 2006;52:329–334. doi: 10.1016/j.jinf.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological Considerations for COVID-19 Vaccine Strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Riel D., de Wit E. Next-Generation Vaccine Platforms for COVID-19. Nat. Mater. 2020;19:810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 31.Deering R.P., Kommareddy S., Ulmer J.B., Brito L.A., Geall A.J. Nucleic Acid Vaccines: Prospects for Non-Viral Delivery of MRNA Vaccines. Expert Opin. Drug Deliv. 2014;11:885–899. doi: 10.1517/17425247.2014.901308. [DOI] [PubMed] [Google Scholar]
- 32.Geall A.J., Mandl C.W., Ulmer J.B. RNA: The New Revolution in Nucleic Acid Vaccines. Semin. Immunol. 2013;25:152–159. doi: 10.1016/j.smim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y.C., Reyes-Sandoval A. Chapter 7—Viral-Vectored Vaccines against SARS-CoV-2. In: Rosales-Mendoza S., Comas-Garcia M., Gonzalez-Ortega O., editors. Biomedical Innovations to Combat COVID-19. Elsevier; Amsterdam, The Netherlands: 2022. pp. 115–127. [Google Scholar]
- 34.Wang C., Horby P.W., Hayden F.G., Gao G.F. A Novel Coronavirus Outbreak of Global Health Concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashburn P.M., Craig C.F., Ashburn P.M. Commentary: Ashburn, P.M., Craig, C.F. Experimental Investigations Regarding the Etiology of Dengue. J. Infect. Dis. 1907, 4, 440–475. J. Infect. Dis. 2004;189:1747–1783. doi: 10.1093/infdis/4.3.440. discussion 1744–1746. [DOI] [PubMed] [Google Scholar]
- 36.Ross R.W. The Newala Epidemic. III. The Virus: Isolation, Pathogenic Properties and Relationship to the Epidemic. J. Hyg. 1956;54:177–191. doi: 10.1017/S0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy M.R., Chen T.-H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., Dubray C., et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 38.Dick G.W.A., Kitchen S.F., Haddow A.J. Zika Virus. I. Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 39.Dick G.W.A. Zika Virus. II. Pathogenicity and Physical Properties. Trans. R. Soc. Trop. Med. Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 40.Harrison V.R., Eckels K.H., Bartelloni P.J., Hampton C. Production and Evaluation of a Formalin-Killed Chikungunya Vaccine. J. Immunol. 1971;107:643–647. doi: 10.4049/jimmunol.107.3.643. [DOI] [PubMed] [Google Scholar]
- 41.Cao-Lormeau V.-M., Roche C., Teissier A., Robin E., Berry A.-L., Mallet H.-P., Sall A.A., Musso D. Zika Virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulland A. Zika Virus Is a Global Public Health Emergency, Declares WHO. BMJ. 2016;352:i657. doi: 10.1136/bmj.i657. [DOI] [PubMed] [Google Scholar]
- 43.FDA Vaccines Licensed for Use in the United States. [(accessed on 25 January 2023)]; Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states.


