Abstract
CD63 is a lysosomal membrane protein that belongs to the tetraspanin family. Its carboxyterminal cytoplasmic tail sequence contains the lysosomal targeting motif GYEVM. Strong, tyrosine-dependent interaction of the wild-type carboxyterminal tail of CD63 with the AP-3 adaptor subunit μ3 was observed using a yeast two-hybrid system. The strength of interaction of mutated tail sequences with μ3 correlated with the degree of lysosomal localization of similarly mutated human CD63 molecules in stably transfected normal rat kidney cells. Mutated CD63 containing the cytosolic tail sequence GYEVI, which interacted strongly with μ3 but not at all with μ2 in the yeast two-hybrid system, localized to lysosomes in transfected normal rat kidney and NIH-3T3 cells. In contrast, it localized to the cell surface in transfected cells of pearl and mocha mice, which have genetic defects in genes encoding subunits of AP-3, but to lysosomes in functionally rescued mocha cells expressing the δ subunit of AP-3. Thus, AP-3 is absolutely required for the delivery of this mutated CD63 to lysosomes. Using this AP-3–dependent mutant of CD63, we have shown that AP-3 functions in membrane traffic from the trans-Golgi network to lysosomes via an intracellular route that appears to bypass early endosomes.
INTRODUCTION
Lysosomal integral membrane glycoproteins (lysosomal glycoproteins [lgps], lysosomal integral membrane proteins [LIMPs], lysosome-associated membrane proteins [LAMPs]) have short, 10–20 amino acid, cytosolic tails containing tyrosine-based or di-leucine–based motifs that are known, or thought, to determine their lysosomal targeting (Hunziker and Geuze, 1996). The tyrosine-based signals are of the form YXXØ where X is any amino acid and Ø is a bulky hydrophobic amino acid, and are clearly related to internalization signals of the same type, which interact with the μ2 subunit of the AP-2 adaptor found in clathrin-coated pits at the cell surface (Bonifacino and Dell'Angelica, 1999). Lysosomal targeting YXXØ motifs are often located at the carboxy terminus of short cytosolic tails preceded by a glycine. Their effectiveness may be modified by spacing relative to the membrane bilayer (Rohrer et al., 1996), the identity of the carboxyterminal amino acid (Gough and Fambrough, 1997; Gough et al., 1999), or proteolytic modification (Guarnieri et al., 1993; Akasaki et al., 1995). There is evidence that the lumenal (Reaves et al., 1998) and transmembrane domains (Wimer-Mackin and Granger, 1996) of some lgps also contain targeting information.
Newly synthesized lgps with GYXXØ motifs in their carboxyterminal cytosolic tails take both direct and indirect (via the plasma membrane) traffic routes from the trans-Golgi network (TGN) to lysosomes. The evidence for individual lgps taking mainly one or other of these routes has come from kinetic studies of delivery and from the endocytic uptake of anti-lgp antibodies or surface-labeled lgps from the plasma membrane. Thus, the type I integral membrane proteins LAMP-1 and LAMP-2, which between them account for >50% of the lysosomal content of lgps (Andrejewski et al., 1999), are delivered from the TGN with half-times of 30–90 min, mainly via an intracellular route (Barriocanal et al., 1986; D'Souza and August, 1986; Green et al., 1987; Carlsson and Fukuda, 1992; Akasaki et al., 1995, 1996). In contrast, lysosomal acid phosphatase, also a type I membrane protein with a cytosolic tail GYXXØ motif, is delivered with a half-time of 5–7 h, mainly via the cell surface (Braun et al., 1989).
The nature of the vesicular carriers used by lgps on the direct route from the TGN to late endocytic compartments is a matter of some dispute. Evidence that the intracellular route taken by some lgps from the TGN to lysosomes involves adaptor protein (AP)-1–associated clathrin-coated vesicles has mainly come from data showing that in vitro the LAMP-1 cytosolic domain interacts with both purified AP-1 and AP-2 and that in vivo, a small fraction, ∼3%, of intracellular LAMP-1 is present with mannose 6-phosphate receptors (MPRs) in AP-1–associated clathrin-coated vesicles budding from the TGN (Honing et al., 1996). However, more recent studies showed first, that LAMP-1 and LAMP-2 were mostly in separate TGN-derived vesicles from those containing MPR and AP-1 (Karlsson and Carlsson, 1998), and second, that in μ1A-deficient cells no change in steady-state distribution or missorting to the cell surface of LAMP-1 occurred, in contrast to the altered MPR distribution (Meyer et al., 2000). The recently discovered clathrin adaptors known as Golgi-localized, γ-ear containing, ARF-binding proteins which are the best candidates for signal-mediated sorting of MPRs at the TGN, appear to play no role in LAMP-1 delivery to lysosomes (Puertollano et al., 2001). Over the past 5 yr considerable, but mostly indirect, evidence has been obtained to suggest that AP-3 plays a role in the targeting of lgps to lysosomes and lysosome-like organelles (reviewed in Hirst and Robinson, 1998; Gu and Gruenberg, 1999; Luzio et al., 2000; Huizing et al., 2001; Mullins and Bonifacino, 2001). In yeast it has been established that two different trafficking routes from the late Golgi to the lysosome exist, which are followed by different newly synthesized proteins, and one of which is AP-3 dependent (Cowles et al., 1997; Piper et al., 1997; Stepp et al., 1997).
To obtain further information about the targeting of lgps to lysosomes and the role of AP-3, we have studied lysosomal targeting of CD63 (LIMP-I, LAMP-3), a member of the tetraspanin family of integral membrane proteins with four predicted transmembrane domains (Metzelaar et al., 1991; Maecker et al., 1997). CD63 was first described as an antigen present on the surface of activated blood platelets after transfer from dense granules (Modderman, 1989). It was later shown to colocalize with other lgps in a variety of cell types (Metzelaar et al., 1991), consistent with its predicted 10-amino acid, cytosolic, carboxyterminal tail ending in the sequence GYEVM. More recently, it has been shown to be enriched on intralumenal vesicles in late endosomes/lysosomes (Escola et al., 1998; Kobayashi et al., 2000; Piper and Luzio, 2001). The kinetics of delivery of newly synthesized CD63 from the TGN to lysosomes has shown a half-time of 2 h, implying that an intracellular delivery route is likely to be important (Barriocanal et al., 1986).
MATERIALS AND METHODS
Reagents
Unless otherwise stated reagents were purchased from Sigma Chemical (Poole, Dorset, United Kingdom). Wortmannin was purchased from Calbiochem (Nottingham, United Kingdom) and was aliquoted at −20°C as a 1 mM stock in dimethyl sulfoxide. Restriction endonucleases, shrimp alkaline phosphatase, polynucleotide kinase, and DNA polymerases were purchased from New England Biolabs (Hitchin, United Kingdom). Rat CD63 cDNA in pUEX was a gift from Drs. C. Wasmeier and J.C. Hutton (Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO). A human cDNA library from Raji cells (QUICK-Clone cDNA; CLONTECH, Palo Alto, CA) was used as a source of human CD63 cDNA, which was recovered by standard polymerase chain reaction (PCR) techniques. Human CD8 α-chain cDNA in the vector S85 was a gift from Dr. S. Munro (MRC Laboratory of Molecular Biology, Cambridge, United Kingdom), contained an AflII site at the end of the transmembrane region, and was cloned into ΔpMEP4 (Girotti and Banting, 1996) as previously described (Ihrke et al., 2000). Oligonucleotides were from R&D Systems (Abingdon, United Kingdom) and Genosys (Cambridge, United Kingdom).
The species-specific mouse monoclonal antibody (mAb) to rat CD63, designated AD1 (Kitani et al., 1991; Nishikata et al., 1992), was a gift from Dr. R.P. Siraganian (National Institutes of Health, Bethesda, MD). The species-specific mouse mAb to human CD63, designated CLB-gran/12, was purchased from Biodesign International (Kennebunk, ME). Fluorescein isothiocyanate-labeled CLB-gran/12 antibody used for fluorescence-activated cell sorting (FACS) analysis was purchased from Immunotech (Marseille, France). Rabbit polyclonal antisera to rat LAMP-2 (lgp110), rat ciMPR, rat TGN38, and mouse mAb to rat TGN38 were as described (Horn and Banting, 1994; Reaves et al., 1996). Rabbit polyclonal anti-rat cathepsin D was a gift from Dr. H.W. Davidson (University of Cambridge, Cambridge, United Kingdom). Rabbit polyclonal anti-γ adaptin and anti-βNAP (β3B) were gifts from Dr. M.S. Robinson (University of Cambridge). Rat monoclonal antihuman CD8α (Bindon et al., 1989) was a gift from Dr. G. Hale (University of Oxford, Oxford, United Kingdom). Rabbit polyclonal anti-human cathepsin D was purchased from DAKO (Ely, Cambridgeshire, United Kingdom). The rat mAb to mouse LAMP-1 (1D4B) developed by T. August was from the Developmental Studies Hybridoma Bank (Department of Biological Sciences, University of Iowa, Iowa City, IA). Fluorescein isothiocyanate-labeled goat anti-mouse IgG, Texas Red-labeled goat anti-mouse IgG, and Texas Red-labeled donkey anti-rabbit IgG were obtained from Amersham (Little Chalfont, Buckinghamshire, United Kingdom). Texas Red-X–labeled goat anti-rat IgG was from Molecular Probes (Eugene, OR).
Mocha, pearl, and stably transfected mocha cells expressing the δ subunit of AP-3 were as described (Peden et al., 2002) and were gifts from Dr. M.S. Robinson (University of Cambridge).
Recombinant DNA Procedures
Standard molecular biology procedures (Sambrook et al., 1989), with the variations previously described (Reaves et al., 1998), were performed unless otherwise stated. Human and rat CD63 molecules containing single amino acid replacements in the cytosolic tail were prepared by mutating cDNA with standard PCR techniques. Details of all primers used are available from the corresponding author on request.
To construct a chimera containing the lumenal and transmembrane domains of CD8 and the cytosolic tail of CD63(CD8-CD63), a corresponding piece of CD63 cDNA was amplified by PCR, introducing an AflII site at the final transmembrane/cytosolic tail boundary. The resulting expressed amino acid sequence after joining the AflII-digested CD8 cDNA and CD63PCR product was … TLYCKRLKSIRSGYEVM, with the underlined 10 residues from CD63 and all other residues from CD8.
The chimera in which the carboxyterminal cytosolic tail of CD63 was replaced by that of TGN38 (CD63-TGN38) was constructed in the same way as the lgp120-TGN38 chimeras described previously (Reaves et al., 1998). A fragment of human CD63 cDNA encoding the entire molecule except the carboxyterminal cytosolic tail, and containing a SalI site at the 3′ end, was generated by PCR. A piece of TGN38 cDNA encoding the cytosolic tail was amplified by PCR from ΔpMEP-LTT, one of the lgp120-TGN38 chimeras, so that it contained a SalI site at the transmembrane/cytosolic tail boundary. The resulting expressed amino acid sequence after joining the SalI-digested CD63 cDNA and TGN38 PCR product was … ACCLVDHNKRKIIAFALEGKRSKVTRRPKASDYQRLNLKL, with the underlined 34 residues from TGN38, the preceding D from the SalI cloning site and all other residues from CD63.
All cDNA constructs prepared as described above were ligated into the multiple cloning site in the mammalian expression vector ΔpMEP4 (Girotti and Banting, 1996) and their DNA sequences confirmed by dideoxy chain termination sequencing, with the service provided by the Department of Genetics (University of Cambridge).
Cell Culture and Transfection
Normal rat kidney (NRK), COS-7, NIH-3T3, pearl, and mocha cells were grown in tissue culture flasks or on glass coverslips as previously described for NRK cells (Reaves et al., 1998). COS-7 cells were transfected by electroporation (Reaves et al., 1998) and mocha cells with calcium phosphate coprecipitation (Robinson, 1990), as previously described. NRK cells were transfected using Transfectam reagent (Promega, Southampton, United Kingdom) or FugGENE 6 (Roche Diagnostics, Lewes, East Sussex, United Kingdom), NIH-3T3 fibroblasts with LipofectAMINE (Invitrogen, Carlsbad, CA), and pearl cells with Superfect (QIAGEN, Chatsworth, CA), according to the manufacturers' instructions. Selection and growth of stable cell lines and stimulation of protein expression in cells transfected with ΔpMEP4 constructs were as previously described (Reaves et al., 1998). Expression levels of mutant human CD63 in stably transfected NRK cells used for immunofluorescence and fluorescence-activated cell sorting (FACS) analysis were shown by quantitative immunoblotting to be between 80 and 135% of the expression level of wild-type human CD63 in the stably transfected NRK cells.
Immunofluorescence Microscopy
Indirect immunofluorescence microscopy and antibody uptake experiments were carried out as previously described (Reaves et al., 1998).
FACS Analysis
Approximately 107 stable transfected NRK cells growing in a 150-cm2 tissue culture flask were removed from the culture dish and treated essentially as described by Dell'Angelica et al. (1999), except that cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at 20°C and half was permeabilized with 0.1% Triton X-100 in PBS/1% bovine serum albumin. Intact or permeabilized cells were incubated for 45 min on ice with fluorescein-conjugated CLB-gran12 antibody to human CD63 (10 ng/ml) in 100 μl PBS/1% bovine serum albumin and subsequently analyzed on a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ). Positively fluorescent cells were identified as those with fluorescence intensities >97.5% of nontransfected cells. The ratio of positive cells in intact and permeabilized preparations was used to calculate the proportion of intracellular wild-type or mutant human CD63 in the transfected NRK cells. FACS data from each stably transfected cell line are presented relative to the amount of intracellular CD63 in the cell line expressing the wild-type human protein. This was 36% of total expressed CD63, i.e., at a level similar to that of a transfected chimeric lgp in a previous study where it was shown that lgp trafficking machinery was not saturated (Gough and Fambrough, 1997).
Yeast Two-Hybrid System
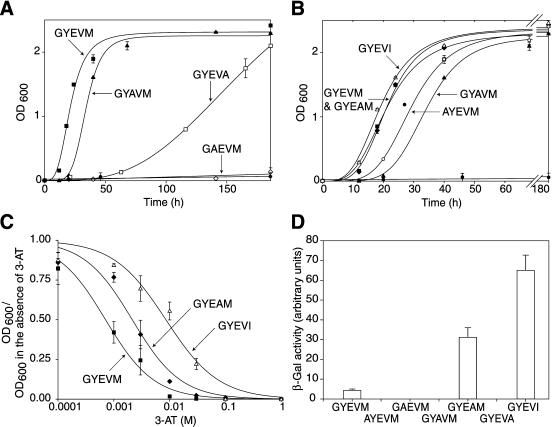
The two-hybrid system used to investigate the interactions between AP μ subunits and wild type, and mutated cytosolic domains of CD63 was as previously described (Stephens and Banting, 1998; Hirst et al., 1999). Constructs encoding μ subunits in the two-hybrid transcriptional activation domain vector pVP16 were as described (Stephens and Banting, 1998). Wild-type (KSIRSGYEVM) and mutant versions of the carboxyterminal cytosolic tail of CD63 were amplified by PCR and subcloned into the two-hybrid DNA-binding domain vector pBTM116. The yeast strain L40, maintained as previously described (Stephens and Banting, 1998), was cotransformed with two plasmids by using a polyethylene glycol-lithium acetate procedure and plated onto selective medium lacking leucine and tryptophan to select for colonies containing both plasmids. After 3–4 d, colonies were incubated in liquid cultures to perform quantitative growth assays. Liquid cultures were set up by inoculating 0.15 OD600 units of cells into 2 ml of selective medium lacking leucine, trytophan, and histidine and were assayed for growth after 0–180 h of incubation at 30°C by measurement of OD600. Each time point was assayed in triplicate. Quantitative β-galactosidase assays and determination of inhibition of cell growth with 3-amino-1,2,4-triazole (3-AT) were as previously described (Stephens and Banting, 1998). Growth curves were calculated using SigmaPlot 4.01 (Jandel Scientific, San Rafael, CA).
RESULTS
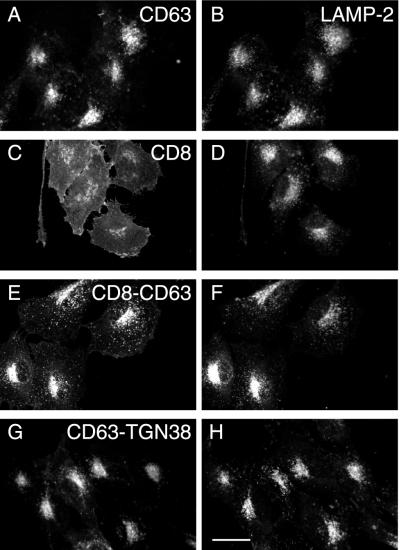
When cDNAs encoding human CD63, and a chimera of CD8 and the carboxyterminal cytosolic tail of CD63 (CD8-CD63) were transfected into NRK cells, the steady-state localization of expressed protein was essentially indistinguishable from that of endogenous LAMP-2 by fluorescence microscopy (Figure 1, A–D). CD8-CD63 was also observed to colocalize with endogenous rat CD63 (our unpublished data). As a control for CD8-CD63, the localization of expressed CD8 was examined in transfected NRK cells and found to be at the cell surface as expected (Figure 1, E and F). The intracellular localization of CD8-CD63 indicated that the carboxyterminal cytosolic tail of CD63 is sufficient to target the “neutral” plasma membrane reporter protein CD8 to lysosomes. To test whether targeting information is also present in lumenal or transmembrane domains, we examined the steady-state localization of CD63-TGN38, a chimera in which the carboxyterminal cytosolic tail of TGN38 replaced that of CD63. This TGN38 tail has been shown to localize a variety of chimeras containing the transmembrane and extracellular domains of neutral plasma membrane proteins to the TGN (Reaves et al., 1998). CD63-TGN38 expressed in transfected NRK cells colocalized with LAMP-2 (Figure 1, G and H) and not with TGN38 (our unpublished data), suggesting that as for LAMP-1 and LAMP-2 (Reaves et al., 1998), the lumenal and/or transmembrane domains of CD63 also contain lysosomal targeting information.
Figure 1.
Indirect immunofluorescence steady-state localization of wild-type human CD63, CD8, and chimeric proteins in stably transfected NRK cells. NRK cells were transfected with ΔpMEP4 constructs containing cDNA encoding either human CD63 (A and B), CD8 (C and D), CD8-CD63 (E and F), or CD63-TGN38 (G and H). Stably transfected cell lines were treated with 3 μM CdCl2 to induce protein expression and then double labeled with a mouse mAb to either CD63 (A and G) or CD8 (C and E) and a rabbit polyclonal antibody against endogenous LAMP-2 (B, D, F, and H). Bar, 20 μm.
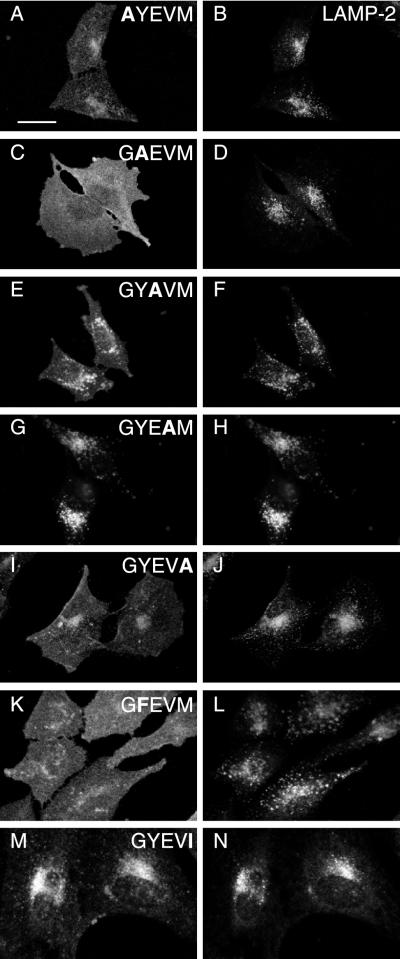
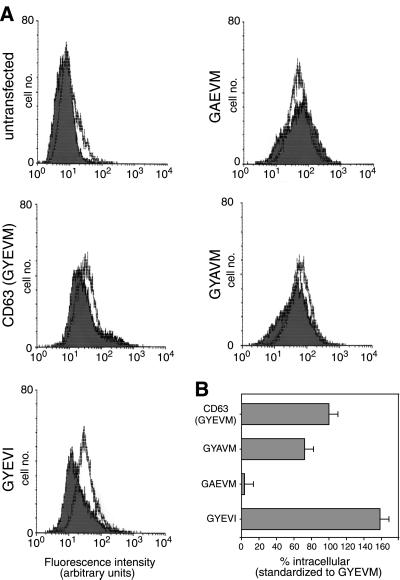
To examine the role of the GYXXØ (GYEVM) motif of the carboxyterminal cytosolic tail of CD63 in lysosomal targeting of this protein we carried out alanine scanning mutagenesis of this motif in human CD63. Stably transfected NRK cells expressing the mutant proteins were analyzed by immunofluorescence microscopy to determine the steady-state localization of the different mutated proteins (Figure 2). This showed that mutation of the G, Y, E, or M to A resulted in increased expression on the cell surface, but mutation of V to A had no effect (compare Figure 2, A–J, with wild-type in Figure 1A). Overall, the microscopy showed the order of greatest expression on the cell surface of the mutants was GAEVM > GYEVA > GYAVM > AYEVM > wild-type (GYEVM) = GYEAM. The conservative mutation of Y to F (GFEVM) resulted in increased surface localization of the mutated CD63 (Figure 2, K and L). The effect of mutating E to A (GYAVM) was particularly unexpected in the context of a GYXXØ motif and was therefore further analyzed by FACS analysis, which showed that it resulted in a reduced proportion of the protein being expressed at an intracellular location(s). In contrast, FACS analysis showed that the conservative mutation of M to I (GYEVI) increased intracellular localization (Figure 3), although this was not easily detected by immunofluorescence microscopy (Figure 2, M and N). The data obtained on the localization of alanine scan mutants of human CD63 expressed in NRK cells are not species specific because comparable data were obtained by immunofluorescence microscopy of equivalent alanine scan mutants of rat CD63 expressed in transfected COS-7 cells (our unpublished data). The sequences of the carboxyterminal cytosolic tails of rat and human wild-type CD63 are identical.
Figure 2.
Indirect immunofluorescence steady-state localization of mutated human CD63-LIMP-1 in stably transfected NRK cells. NRK cells were transfected with ΔpMEP4 constructs containing cDNA encoding human CD63 with point mutations in the cytosolic tail, either AYEVM (A and B), GAEVM (C and D), GYAVM (E and F), GYEAM (G and H), GYEVA (I and J), GYEVI (K and L), or GFEVM (M and N). Stably transfected cell lines were treated with 3 μM CdCl2 to induce protein expression and then double labeled with a mouse mAb to CD63 (A, C, E, G, I, K, and M) and a rabbit polyclonal antibody against endogenous LAMP-2 (B, D, F, H, J, L, and N). Bar, 20 μm.
Figure 3.
Fluorescence-activated cell sorting analysis of human CD63-LIMP-I and mutated proteins in stably transfected NRK cells. NRK cells were transfected with ΔpMEP4 constructs containing cDNA encoding human CD63 and constructs with point mutations in the cytosolic tail. After induction of protein expression the distribution of the wild-type and mutated human CD63 proteins between the cell surface and intracellular structures was determined by FACS analysis. (A) Fluorescence intensity for untransfected NRK cells (top left) and cells transfected with wild-type CD63 (GYEVM), and mutated constructs (GYEVI, GAEVM, and GYAVM) are shown. The filled traces represent intact cells and the unfilled traces the permeabilized cells. A larger shift to the right of the unfilled trace compared with the filled trace represents a larger proportion of the CD63 at intracellular sites. (B) Histogram showing the amount of intracellular mutated CD63 in each cell line relative to the amount of intracellular wild-type CD63 in the cell line expressing this construct.
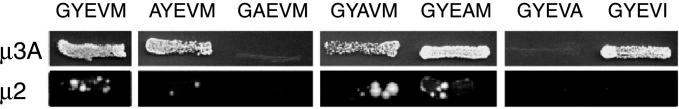
The observation that mutation of E to A within the GYEVM motif resulted in greater cell surface localization of CD63 was initially surprising given the consensus sequence for the motif as GYXXØ. However, data obtained by screening the interaction of adaptor μ subunits with a combinatorial XXXYXXØ library, by using the yeast two-hybrid system, suggested that an E at the Y + 1 position results in a YXXØ motif with preferential binding to μ3 (Ohno et al., 1998). We therefore decided to investigate the interaction of wild-type and mutant carboxyterminal cytosolic tails of CD63 with μ subunits in the yeast two-hybrid system. The μ subunits were cloned into the two-hybrid transcriptional activation domain vector pVP16, and wild-type and mutated carboxyterminal cytosolic tails of CD63 were cloned into the two-hybrid DNA-binding domain vector pBTM116. Pairs of constructs were then transformed into yeast cells, and interaction between the cytosolic tails and μ subunits assayed by the ability of cells coexpressing them 1) to grow on agar or in liquid medium lacking histidine; 2) to induce β-galactosidase expression; or, for strong interactors, 3) to grow in the presence of 3-AT, a competitive inhibitor of histidine biosynthesis. By using this two-hybrid system and measuring interaction by growth in medium lacking histidine, it has previously been shown that the wild-type carboxyterminal cytosolic tail of CD63, containing GYEVM, interacts most strongly with μ3A, weakly with μ2 and μ4, and not at all with μ1 (Hirst et al., 1999). There was an equally strong interaction with the brain-specific isoform μ3B (our unpublished data). The interaction of mutated CD63 tails with μ3A and μ2 was shown by growth on agar lacking histidine (Figure 4). Clearly, the tails containing Y-to-A and M-to-A mutations interacted very poorly with μ3A because no growth was observed. Interactions with μ2 occurred only with three constructs (GYEVM, GYAVM, and GYEAM) and were all weak, resulting in poor growth on the agar (Figure 4). To characterize the relative strengths of interaction of the mutated tails with μ3A in more detail, growth of cotransformed yeast was measured in medium lacking histidine >180 h (Figure 5, A and B). The strengths of interaction of the weakly interacting mutated tails with μ3A were easily distinguished by this method. The relative strengths of interaction of the strongly interacting wild-type and mutated tails were distinguished better by plotting dose-response curves of 3-AT inhibition of growth of cotransformed yeast in medium lacking histidine (Figure 5C) or measurement of β-galactosidase activity after growth in medium containing histidine (Figure 5D). Taking these data together, the strength of interaction of wild-type and mutated tails of CD63 with μ3A was determined to be wild-type (GYEVM) = GYEAM > AYEVM > GYAVM ≫ GYEVA ≫ GAEVM. This order is the exact reverse of that describing cell surface expression of CD63 molecules containing these mutated tails in transfected NRK cells (Figures 2 and 3).
Figure 4.
Interaction of the cytosolic domains of CD63 and mutated CD63 with μ2 and μ3A in the yeast two-hybrid system. Yeast cells were transformed with bait constructs (LexA fusion) containing the C-terminal cytosolic domains of CD63, and the mutant forms AYEVM, GAEVM, GYAVM, GYEVA, GYAVM, GYEAM, GYEVA, and GYEVI and a prey construct (either VP16-μ2, VP16-μ3A). Transformed yeast were grown on agar plates in the absence of histidine for 3 d (μ3A) or 10 d (μ2). All transformed yeast grew in the presence of histidine and no transformed yeast containing “empty” VP16 grew in the absence of histidine.
Figure 5.
Characterization of the interaction of the cytosolic domains of CD63 and mutated CD63 with μ3A in the yeast two-hybrid system. Yeast cells were transformed as described in Figure 4. (A and B) Cultures were set up containing 0.05 OD600 units of transfected cells into 2 ml of selective medium lacking histidine and grown at 30°C with shaking. OD600 measurements were made up to 180 h (B shows an expanded time scale). The control interaction between the C-terminal cytosolic domain of wild-type CD63 and empty VP16 (filled circles) is shown in each figure. Vector controls for other tails also showed no significant growth. Each point represents the mean ± SEM of the activity of three separate cultures. (C) Cultures of transformed yeast were established in selective medium lacking histidine, but containing 0–1 M 3-AT, and grown at 30°C with shaking. After incubation for 48 h, OD600 was measured and the ratio in the presence versus absence of 3-AT recorded. Each point represents the mean ± SEM of the activity of three separate cultures. (D) Cultures of transformed yeast were grown in medium containing histidine to an OD600 of ∼1.2. The β-galactosidase activity of the cultures was then measured. Each bar in the histogram represents the mean ± SEM of the activity of five separate cultures.
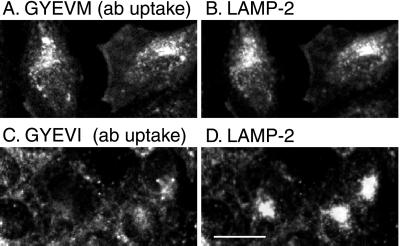
In the yeast two-hybrid experiments we also examined the strength of interaction of μ3A with a mutated CD63 tail in which M was mutated to I (GYEVI). This construct was chosen because the data from the combinatorial XXXYXXØ library presented by Ohno et al. (1998) showed that for interaction with μ3A, I is favored in the Y + 3 position. We found that the mutated CD63 tail in which M was mutated to I showed the strongest interaction with μ3A of any tail sequence we examined (Figure 5, A, C, and D) and CD63 containing this mutation had the lowest surface expression in transfected NRK cells (Figures 2K and 3). These data were not due to the creation of a dileucine-type motif of the form VI because CD63 with the mutated tail GAEVI was expressed entirely on the cell surface (our unpublished data). We also observed in the yeast two-hybrid system that the mutated CD63 tail in which M was mutated to I showed no interaction with μ2 (Figure 4). This implied that any CD63 containing this mutation delivered to the cell surface in transfected cells would be poorly internalized by clathrin-mediated endocytosis. To test this, we investigated anti-human CD63 antibody uptake in stable lines of NRK cells transfected with wild-type (GYEVM) and mutated (GYEVI) CD63. Less intracellular accumulation of antibody was observed in cells expressing the mutated (GYEVI) CD63, consistent with inefficient clathrin-mediated uptake (Figure 6).
Figure 6.
Uptake of anti-CD63 antibody by stably transfected NRK lines expressing either wild-type human CD63 (A and B) or the GYEVI construct (C and D). Protein expression was induced in stably transfected NRK cell lines with 3 μM CdCl2 for 16 h. The cells were then incubated at 37°C for 30 min in the presence of anti-CD63 mAb and then incubated for a further 30 min without antibody (i.e., chased). The cells were then fixed and immunofluorescence localization of the anti-CD63 mAb (A and C) and of endogenous LAMP-2 (B and D) carried out. Bar, 20 μm.
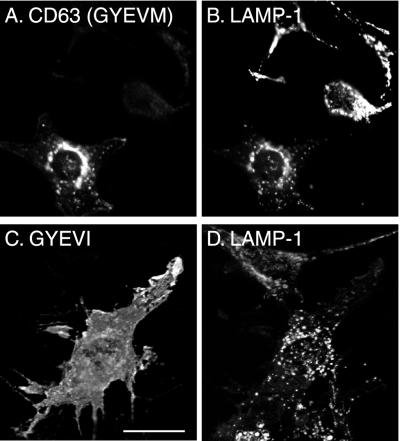
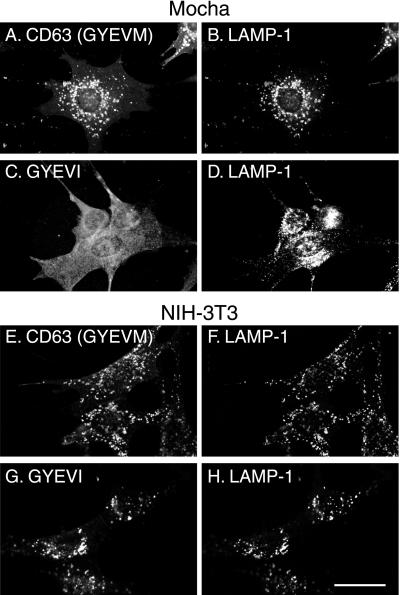
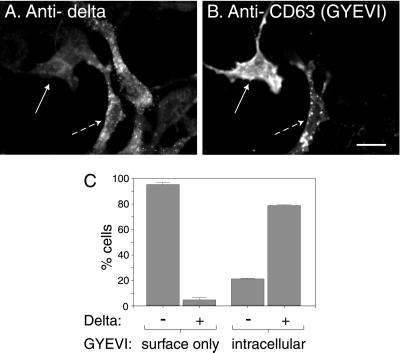
The experiments described above, together with published data on the kinetics of delivery of wild-type CD63 from TGN to lysosomes, strongly suggest that the wild-type protein traffics to lysosomes from the TGN by an intracellular AP-3–dependent route, but that any CD63 reaching the cell surface is internalized by AP-2–dependent clathrin-mediated endocytosis and delivered by the endocytic pathway to lysosomes. In contrast, mutated (GYEVI) CD63 should only reach lysosomes by an intracellular AP-3–dependent route and any delivered to the cell surface should not be efficiently internalized and delivered by endocytosis. To test this hypothesis we expressed human wild-type and mutated (GYEVI) CD63 in cells from pearl and mocha mice and, using immunofluorescence microscopy, examined the steady-state distribution of the recombinant expressed protein. As a control we also transfected wild-type mouse NIH-3T3 fibroblasts. Consistent with our hypothesis, mutated (GYEVI) CD63 was observed almost exclusively on the surface of pearl and mocha cells but colocalized with an endogenous lgp (LAMP-1) in NIH-3T3 fibroblasts (Figures 7 and 8). In contrast, wild-type (GYEVM) CD63 colocalized with endogenous LAMP-1 in transfected pearl and mocha cells (Figures 7 and 8). Similar results were obtained from both transiently transfected cells and stable transfected cell lines. To test our hypothesis further, two additional experiments were carried out. In the first, we transfected mocha cells that had been rescued by expression of the δ subunit of AP-3 with mutated (GYEVI) CD63. In the rescued cells expressing the δ subunit of AP-3 at a concentration sufficient for detection by immunofluorescence, a significant proportion of the expressed mutated (GYEVI) CD63 colocalized with intracellular, endogenous LAMP-1 (Figure 9). In the second experiment, we further transfected a stable line of pearl cells expressing mutated (GYEVI) CD63 with a cDNA construct encoding the β3B subunit of AP-3. Some intracellular localization of mutated (GYEVI) CD63 was observed in cells expressing β3B (our unpublished data; β3B was used in this experiment because of the lack of antibodies to β3A for immunofluorescence).
Figure 7.
Steady-state localization of wild-type human CD63 and the GYEVI construct in transiently transfected pearl cells. Pearl cells were transiently transfected with ΔpMEP containing cDNA encoding either wild-type CD63 (A and B) or the GYEVI construct (C and D). The transfected cells were treated with 3 μM CdCl2 to induce protein expression and then double labeled, for indirect immunofluorescence localization, with a mouse mAb to CD63 (A and D) and a rat mAb to endogenous mouse LAMP-1 (B and E). Bar, 20 μm.
Figure 8.
Steady-state localization of CD63 and GYEVI constructs in stably transfected mocha and 3T3 cell lines. Mocha cells (A–D) and 3T3 cells (E–H) were transfected with ΔpMEP constructs containing cDNA encoding wild-type human CD63 (A, B, E, and F) or the GYEVI construct (C, D, G, and H). Stably transfected cell lines were treated with 3 μM CdCl2 to induce protein expression and then double labeled, for indirect immunofluorescence localization, with a mouse mAb to CD63 (A, C, E, and G) and a rat mAb to endogenous mouse LAMP-1 (B, D, F, and H). Bar, 20 μm.
Figure 9.
Localization of the GYEVI construct of human CD63 in rescued mocha cells expressing the δ subunit of AP-3. A stably transfected mocha cell line expressing the δ subunit of AP-3 was transiently transfected with a ΔpMEP construct containing cDNA encoding the human CD63/LIMP-I, GYEVI construct. After treating the transfected cells with 3 μM CdCl2 to induce protein expression they were double labeled, for indirect immunofluorescence localization with a mouse mAb to human CD63 (A) and with a rabbit polyclonal antibody to the δ subunit of AP-3 (B). Bar, 20 μm. (C) Cells that were expressing the GYEVI construct were then counted and grouped according to the presence of intracellular human CD63 or only cell surface human CD63 and the presence or absence of detectable δ subunit of AP-3. The data shown in the histogram represent the mean of two experiments. In the two experiments the counted numbers of human CD63-positive cells showing only surface expression were 208 and 349. The counted numbers of CD63-positive cells also showing intracellular localization of human CD63 were 91 and 44.
The data presented above are consistent with the mutated (GYEVI) CD63 absolutely requiring functional AP-3 for targeting to lysosomes and provided us with a unique reporter protein for this pathway. As discussed in INTRODUCTION it has been reported that AP-3 is associated with endosomes as well as with the TGN (Simpson et al., 1996; Dell'Angelica et al., 1998). Because chloroquine treatment of cells causes swelling of endocytic compartments and blocks traffic through the early endosome (Reaves et al., 1998) we predicted that if mutated (GYEVI) CD63 was trafficking via this compartment it should be trapped there after chloroquine treatment along with TGN38, which normally cycles between the TGN and cell surface/early endosome (Chapman and Munro, 1994; Reaves and Banting, 1994; Banting et al., 1998). However, no mutated (GYEVI) CD63 was observed in the same structures as TGN38 in chloroquine-treated, transfected NRK cells (Figure 10, A and B). In contrast, when CD63-TGN38–expressing NRK cells were treated with chloroquine, CD63-TGN38 was observed in the same swollen structures as endogenous TGN38 (Figure 10, C and D) consistent with this molecule trafficking via an early endosome, i.e., by a different route to the AP-3–dependent route used by mutated (GYEVI) CD63. Other swollen structures depleted of TGN38 also contained CD63-TGN38, consistent with them being late endosomes and lysosomes containing CD63-TGN38 molecules that had arrived before the chloroquine was added. When NRK cells expressing wild-type human CD63 were incubated with chloroquine, this protein was mainly in separate structures to TGN38, although some colocalization was observed that presumably represents the small proportion of wild-type CD63 that traffics via the early endosome (Figure 10, E and F). These data taken together suggest that the AP-3–dependent pathway of membrane traffic from the TGN to lysosomes does not involve traffic via the early endosomal compartment in which TGN38 accumulates after chloroquine treatment.
Figure 10.
Indirect immunofluorescence steady-state localization of wild-type human CD63, GYEVI, and CD63-TGN38 constructs in stable lines of transfected NRK cells treated with chloroquine. Stably transfected NRK cell lines expressing either the GYEVI construct (A and B), CD63-TGN38 (C and D), or wild-type CD63 (E and F) were incubated for 2 h in the presence of both 3 μM CdCl2 and 100 μM chloroquine before fixation. The cells were then double labeled, for indirect immunofluorescence localization, with a mouse mAb to human CD63 (A, C, and E) and a rabbit polyclonal antibody to the lumenal domain of rat TGN38 (B, D, and F). Structures positive for TGN38, but which do not label with the anti-CD63 antibody, are indicated by single-headed arrows. Structures that label with both the TGN38 antibody and the CD63 antibody are indicated by the double-headed arrows. Bar, 10 μm.
DISCUSSION
The increased surface expression of CD63 in which G, Y, or M was mutated to A confirmed the importance of the GYEVM sequence in the localization of this protein, as is the case for the GYXXØ motif in the carboxyterminal cytoplasmic tail of other lgps, including lysosomal acid phosphatase (Peters et al., 1990; Lehmann et al., 1992), LAMP-1 (Williams and Fukuda, 1990; Harter and Mellman, 1992; Guarnieri et al., 1993), and LAMP-2 isoforms (Gough and Fambrough, 1997).
The observation that mutating E to A in the carboxyterminal cytosolic tail of CD63 produced a mutant protein that was more expressed at the cell surface than wild-type protein was unexpected because mutations at the Y + 1 position of the GYXXØ motif have been shown to have no effect on lysosomal targeting of LAMP-1 (Guarnieri et al., 1993; Honing and Hunziker, 1995; Rohrer et al., 1996). It stimulated our use of the yeast two-hybrid system to investigate the interaction of wild-type and mutated cytosolic tails of CD63 with adaptor medium chain (μ) subunits. From their screen of the interactions with μ subunits of a combinatorial peptide library, Ohno et al. (1998) observed a preference for E at the Y + 1 position of a YXXØ motif for interaction with μ3A and μ3B. They pointed out that this was “noteworthy because it is a characteristic of proteins targeted to lysosomes or lysosome-related organelles, such as … CD63.” Their data also showed a preference for G rather than A at the Y − 1 position and I rather than M at the Y + 3 position for interaction with μ3A. Our own yeast two-hybrid data are consistent with the data from Ohno et al. (1998), but in our case within the context of the actual cytosolic tail of an lgp. More importantly, we have shown for the first time that the order of cell surface versus lysosomal distribution of GYXXØ mutants of an lgp is accurately predicted by interactions with μ3A in the yeast two-hybrid system. Two other complete cytosolic tails containing YXXØ motifs have previously been shown to be capable of interacting with adaptor μ chains in the yeast two-hybrid system: those of TGN38 and LAMP-1 (Ohno et al., 1995; Stephens et al., 1997; Stephens and Banting, 1998). In both these cases, and for the tail of CD63, interaction is dependent on the Y and the Ø in the YXXØ motif.
In our yeast two-hybrid experiments we observed that a mutated CD63 tail in which M was mutated to I (GYEVI) did not interact with μ2, despite showing the strongest interaction with μ3A of all the mutated tails examined. Consistent with this observation, examination of the combinatorial XXXYXXØ peptide library screen of Ohno et al. (1998) reveals that for interaction with μ2, I is more disfavored than M at the Y + 3 position. The availability of a mutated CD63 that interacts strongly with μ3A but not with μ2 allowed us to test a number of predictions about its behavior, and the role of the AP-3–dependent trafficking pathway, when it was expressed in cultured cells. In particular, we predicted and showed that, in cells lacking functional AP-3, the GYEVI mutant of CD63 was unable to reach lysosomes because it was denied the AP-3–dependent intracellular route and could not be internalized from the cell surface. This route was recovered and the GYEVI mutant correctly delivered to lysosomes in mocha cells, which had been rescued by expression of the δ subunit of AP-3. In contrast, wild-type CD63 was still delivered to lysosomes in transfected pearl and mocha cells, consistent with the ability to be internalized efficiently from the cell surface. Our experiments have identified the first lgp, the GYEVI mutant of CD63, to be grossly mislocalized in AP-3–deficient cells. They are also consistent with previous experiments where endogenous lgps were observed to traffic to lysosomes via the cell surface in AP-3–deficient cells (Dell'Angelica et al., 1999) or in cells in which functional AP-3 concentrations were reduced by transfecting with antisense oligonucleotides to μ3A (Le Borgne et al., 1998).
The intracellular site of action of AP-3 in lysosomal targeting has not been well defined. It is unlikely that AP-3 acts at the plasma membrane because it would be difficult to reconcile such a site of action with the observation that increased quantities of lgps traffic via the cell surface in cells deficient in AP-3 (Le Borgne et al., 1998; Dell'Angelica et al., 1999). Moreover, it has been localized by microscopy at or close to the TGN (Simpson et al., 1996) and on endosomes (Dell'Angelica et al., 1998). It thus seems likely that AP-3 functions to traffic between the TGN and lysosomes on a route equivalent to the AP-3–mediated direct trafficking pathway from the Golgi complex to the vacuole proposed in yeast and used by alkaline phosphatase but not carboxypeptidase Y (Cowles et al., 1997). In the present experiments we studied the effects of chloroquine on the distribution of transfected wild-type CD63 and a chimera with the cytoplasmic tail of TGN38 to provide further insights into the intracellular site of action of AP-3. Previous work has shown that endosomal acidification is important for transport from endosomal compartments to both lysosomes and the TGN (Chapman and Munro, 1994; Reaves and Banting, 1994; Van Weert et al., 1995), such that when the pH of endosomes is raised using either a proton pump inhibitor (e.g., bafilomycin A1) or a membrane-permeant weak base (e.g., chloroquine), membrane proteins, including TGN38, furin, LAMP-1, and lysosomal acid phosphatase become trapped in early endosomal compartments (Braun et al., 1989; Chapman and Munro, 1994; Reaves and Banting 1994; Reaves et al., 1998). In addition, delivery of endocytosed horseradish peroxidase from endosomes to lysosomes is impaired (Van Weert et al., 1995). Thus, in the present study, chloroquine was used to inhibit traffic through early endosomal compartments and to see whether wild-type CD63 or the GYEVI mutant were trapped in early endosomes en route to the lysosomes. Some wild-type CD63 was trapped in early endosomes, suggesting that a proportion traffics through this compartment. In contrast, none of the GYEVI mutant was found in early endosomes, consistent with it trafficking from the TGN to lysosomes by an AP-3–dependent intracellular route that does not involve passage through early endosomes. As a control, we studied the CD63-TGN38 chimera that has a TGN38 cytosolic tail that interacts strongly with μ2, weakly with μ1, and barely detectibly with μ3 in the yeast two-hybrid system (Ohno et al., 1995; Stephens and Banting, 1998; our unpublished data). CD63-TGN38 was found in endosomal structures when chloroquine was added to the cells, suggesting that, as predicted, it does indeed traffic through early endosomes.
Why does wild-type CD63 have a GYEVM motif rather than a GYEVI motif if the latter is more efficient for AP-3–dependent delivery to the lysosome? One possible explanation is that because of the poor interaction of GYEVI with μ2 any CD63 with a GYEVI motif that is mistargeted to the cell surface would remain there; this may be detrimental to the cell and therefore unfavorable evolutionarily. Another possible explanation relates to the possible functions of CD63 at the cell surface, including involvement in cell adhesion (Vischer and Wagner, 1993; Hamamoto et al., 1994) and integrin-mediated cell migration (Berditchevski and Odintsova, 1999). Alterations in the amount of CD63 expressed in melanomas have been implicated in metastatic progression of the tumor, and this may reflect altered cell surface expression (Hara et al., 1994; Radford et al., 1997), suggesting that the amount of CD63 on the cell surface may be capable of modulation. Because the distribution of the wild-type molecule is clearly dependent on interactions of the carboxyterminal cytosolic tail with more than one adaptor protein, regulation of such interactions would provide a means of varying the cell surface concentration and may be a fruitful subject of future investigation.
ACKNOWLEDGMENTS
We thank our colleagues Margaret Robinson, Howard Davidson, Jenny Hirst, Andrew Peden, and Rachel Rudge for AP reagents, cell lines, much valuable discussion, and critical reading of the manuscript. We thank Sean Munro for the gift of a plasmid construct encoding CD8. This work was funded by the Medical Research Council and the Wellcome Trust.
Abbreviations used:
- AP
adaptor protein
- 3-AT
3-amino-1,2,4-triazole
- lgp
lysosomal glycoprotein
- MPR
mannose 6-phosphate receptor
- NRK
normal rat kidney
- TGN
trans-Golgi network
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0409. Article and publication date are at www.molbiolcell.org/cgi/10.1091/mbc.01–08–0409.
REFERENCES
- Akasaki K, Michihara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: Convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- Akasaki K, Michihara A, Fujiwara Y, Mibuka K, Tsuji H. Biosynthetic transport of a major lysosome-associated membrane glycoprotein 2, lamp-2: a significant fraction of newly synthesized lamp-2 is delivered to lysosomes by way of early endosomes. J Biochem. 1996;120:1088–1094. doi: 10.1093/oxfordjournals.jbchem.a021526. [DOI] [PubMed] [Google Scholar]
- Andrejewski N, Punnonen E-L, Guhde G, Tanaka Y, Lüllmann-Rauch R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274:12692–12701. doi: 10.1074/jbc.274.18.12692. [DOI] [PubMed] [Google Scholar]
- Banting G, Maile R, Roquemore EP. The steady state distribution of humTGN46 is not significantly altered in cells defective in clathrin-mediated endocytosis. J Cell Sci. 1998;111:3451–3458. doi: 10.1242/jcs.111.23.3451. [DOI] [PubMed] [Google Scholar]
- Barriocanal JG, Bonifacino JS, Yuan L, Sandoval IV. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986;261:16755–16763. [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes. Role of tetraspanins in integrin signaling. J Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindon CI, Hale G, Waldmann H. Synergistic complement-mediated cell lysis using pairs of antibodies. In: Knapp B, Dorken WR, Gilks EP, Rieber RE, Schmidt H, Stein H, von dem Borne AEGK, editors. Leukocyte Typing IV. Oxford, UK: Oxford University Press; 1989. pp. 349–350. [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Waheed A, Von Figura K. Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J. 1989;8:3633–3640. doi: 10.1002/j.1460-2075.1989.tb08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson SR, Fukuda M. The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch Biochem Biophys. 1992;296:630–639. doi: 10.1016/0003-9861(92)90619-8. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- D'Souza MP, August JT. A kinetic analysis of biosynthesis and localization of a lysosome-associated membrane glycoprotein. Arch Biochem Biophys. 1986;249:522–532. doi: 10.1016/0003-9861(86)90030-5. [DOI] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffin JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Girotti M, Banting G. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for rat TGN38. J Cell Sci. 1996;109:2915–2926. doi: 10.1242/jcs.109.12.2915. [DOI] [PubMed] [Google Scholar]
- Gough NR, Fambrough DM. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol. 1997;137:1161–1169. doi: 10.1083/jcb.137.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough NR, Zweifwel ME, Martinez-Augustin O, Aguilar RC, Bonifacino JS, Fambrough DM. Utilization of the indirect lysosome targeting pathway by lysosome-associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXØ targeting signals. J Cell Sci. 1999;112:4257–4269. doi: 10.1242/jcs.112.23.4257. [DOI] [PubMed] [Google Scholar]
- Green SA, Zimmer KP, Griffiths G, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;105:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Gruenberg J. Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett. 1999;452:61–66. doi: 10.1016/s0014-5793(99)00561-x. [DOI] [PubMed] [Google Scholar]
- Guarnieri FG, Arterburn LM, Penno MB, Cha Y, August JT. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J Biol Chem. 1993;268:1941–1946. [PubMed] [Google Scholar]
- Hamamoto K, Ohga S, Nomura S, Yasunaga K. Cellular distribution of CD63 antigen in platelets and in three megakaryocytic cell lines. Histochem J. 1994;26:367–375. doi: 10.1007/BF00157770. [DOI] [PubMed] [Google Scholar]
- Hara H, Lee MH, Chen H, Luo D, Jimbow K. Role of gene expression and protein synthesis of tyrosinase, TRP-1, Lamp-1, and CD63 in UVB-induced melanogenesis in human melanomas. J Invest Dermatol. 1994;102:495–500. doi: 10.1111/1523-1747.ep12373136. [DOI] [PubMed] [Google Scholar]
- Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Bright NA, Rous B, Robinson MS. Characterization of a fourth adaptor-related protein complex. Mol Biol Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Honing S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Banting G. Okadaic acid treatment leads to a fragmentation of the trans-Golgi network and an increase in expression of TGN38 at the cell surface. Biochem J. 1994;301:69–73. doi: 10.1042/bj3010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Sarangarajan R, Strovel E, Zhao Y, Gahl WA, Boissy RE. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol Biol Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Ihrke G, Gray SR, Luzio JP. Endolyn is a mucin-like type I membrane protein targeted to lysosomes by its cytoplasmic tail. Biochem J. 2000;345:287–296. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K, Carlsson SR. Sorting of lysosomal membrane glycoproteins lamp-1 and lamp-2 into vesicles distinct from mannose 6-phosphate receptor/γ-adaptin vesicles at the trans-Golgi network. J Biol Chem. 1998;273:18966–18973. doi: 10.1074/jbc.273.30.18966. [DOI] [PubMed] [Google Scholar]
- Kitani S, Berenstein E, Mergenhagen S, Tempst P, Siraganian RP. A cell surface glycoprotein of rat basophilic leukemia cells close to the high affinity IgE receptor (FceRI). Similarity to human melanoma differentiation antigen ME491. J Biol Chem. 1991;266:1903–1909. [PubMed] [Google Scholar]
- Kobayashi T, Vischer UM, Rosonoblet C, Lebrand C, Lindsay M, Parton RG, Kruithof EK, Gruenberg J. The tetraspanin CD63/lamp3 Cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell. 2000;11:1829–1843. doi: 10.1091/mbc.11.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Alconada A, Bauer U, Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J Biol Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- Lehmann LE, Eberle W, Krull S, Prill V, Schmidt B, Sander C, Von Figura K, Peters C. The internalization signal in the cytoplasmic tail of lysosomal acid phosphatase consists of the hexapeptide PGYRHV. EMBO J. 1992;11:4391–4399. doi: 10.1002/j.1460-2075.1992.tb05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113:1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- Meyer C, Zizioli D, Lausmann S, Eskelinen E-L, Hamann J, Saftig P, von Figura K, Schu P. μ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modderman PW. Cluster report: CD63. In: Knapp B, Dorken WR, Gilks EP, Rieber RE, Schmidt H, Stein H, von dem Borne AEGK, editors. Leukocyte Typing IV. Oxford, UK: Oxford University Press; 1989. , 1042. [Google Scholar]
- Mullins C, Bonifacino JS. The molecular machinery for lysosome biogenesis. BioEssays. 2001;23:333–343. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- Nishikata H, Oliver C, Mergenhagen SE, Siraganian RP. The rat mast cell antigen AD1 (homologue to human CD63 or melanoma antigen ME491) is expressed in other cells in culture. J Immunol. 1992;149:862–870. [PubMed] [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Peden, A.A., Rudge, R.E., Lui, W.W.Y., and Robinson, M.S. (2002). Assembly and function of AP-3 complexes in cells expressing mutant subunits. J. Cell Biol. (in press). [DOI] [PMC free article] [PubMed]
- Peters C, Braun M, Weber B, Wendland M, Schmidt B, Pohlmann R, Waheed A, Von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990;9:3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic. 2001;2:612–621. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- Radford KJ, Thorne RF, Hersey P. Regulation of tumor cell motility and migration by CD63 in a human melanoma cell line. J Immunol. 1997;158:3353–3358. [PubMed] [Google Scholar]
- Reaves B, Banting G. Vacuolar ATPase inactivation blocks recycling to the trans-Golgi network from the plasma membrane. FEBS Lett. 1994;345:61–66. doi: 10.1016/0014-5793(94)00437-4. [DOI] [PubMed] [Google Scholar]
- Reaves BJ, Banting G, Luzio JP. Lumenal and transmembrane domains play a role in sorting type I membrane proteins on endocytic pathways. Mol Biol Cell. 1998;9:1107–1122. doi: 10.1091/mbc.9.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localization of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Robinson MS. Cloning and expression of gamma-adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol. 1990;111:2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Simpson F, Bright NA, West MA, Newman LS, Darnell RB, Robinson MS. A novel adaptor-related protein complex. J Cell Biol. 1996;133:749–760. doi: 10.1083/jcb.133.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DJ, Banting G. Specificity of interaction between adaptor-complex medium chains and the tyrosine-based sorting motifs of TGN38 and lgp120. Biochem J. 1998;335:567–572. doi: 10.1042/bj3350567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DJ, Crump CM, Clarke AR, Banting G. Serine 331 and tyrosine 333 are both involved in the interaction between the cytosolic domain of TGN38 and the μ2 subunit of the AP2 clathrin adaptor complex. J Biol Chem. 1997;272:14104–14109. doi: 10.1074/jbc.272.22.14104. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weert AWM, Dunn KW, Geuze HJ, Maxfield FR, Stoorvogel W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer UM, Wagner DD. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1993;82:1184–1191. [PubMed] [Google Scholar]
- Williams MA, Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990;111:955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimer-Mackin S, Granger BL. Transmembrane domain mutations influence the cellular distribution of lysosomal membrane glycoprotein A. Biochem Biophys Res Commun. 1996;229:472–478. doi: 10.1006/bbrc.1996.1828. [DOI] [PubMed] [Google Scholar]