Abstract
The AP1 complex is a highly conserved clathrin adaptor that plays important roles in regulating cargo protein sorting and intracellular vesicle trafficking in eukaryotes. However, the functions of the AP1 complex in the plant pathogenic fungi including the devastating wheat pathogen Fusarium graminearum are still unclear. In this study, we investigated the biological functions of FgAP1σ, a subunit of the AP1 complex in F. graminearum. Disruption of FgAP1σ causes seriously impaired fungal vegetative growth, conidiogenesis, sexual development, pathogenesis, and deoxynivalenol (DON) production. The ΔFgap1σ mutants were found to be less sensitive to KCl- and sorbitol-induced osmotic stresses but more sensitive to SDS-induced stress than the wild-type PH-1. Although the growth inhibition rate of the ΔFgap1σ mutants was not significantly changed under calcofluor white (CFW) and Congo red (CR) stresses, the protoplasts released from ΔFgap1σ hyphae were decreased compared with the wild-type PH-1, suggesting that FgAP1σ is necessary for cell wall integrity and osmotic stresses in F. graminearum. Subcellular localization assays showed that FgAP1σ was predominantly localized to endosomes and the Golgi apparatus. In addition, FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP also localize to the Golgi apparatus. FgAP1β interacts with FgAP1σ, FgAP1γ, and FgAP1μ, while FgAP1σ regulates the expression of FgAP1β, FgAP1γ, and FgAP1μ in F. graminearum. Furthermore, the loss of FgAP1σ blocks the transportation of the v-SNARE protein FgSnc1 from the Golgi to the plasma membrane and delays the internalization of FM4-64 dye into the vacuole. Taken together, our results demonstrate that FgAP1σ plays vital roles in vegetative growth, conidiogenesis, sexual reproduction, DON production, pathogenicity, cell wall integrity, osmotic stress, exocytosis, and endocytosis in F. graminearum. These findings unveil the functions of the AP1 complex in filamentous fungi, most notably in F. graminearum, and lay solid foundations for effective prevention and control of Fusarium head blight (FHB).
Keywords: Fusarium graminearum, wheat scab, AP1 complex, FgAP1σ
1. Introduction
Clathrin-coated vesicles (CCVs) are among the major transport vesicles that contain two major structural components: clathrin and clathrin adaptor proteins (APs) [1,2]. Clathrin serves as a polymeric mechanical scaffold of these vesicles, and the recruitment of clathrin to membranes requires adaptors [3]. In eukaryotes, there are five distinct clathrin adaptor protein complexes, namely, AP1, AP2, AP3, AP4, and AP5, and they play important roles in vesicle assembly, recruitment of membrane cargoes and coat proteins, and vesicular transport [4,5]. Fungi possess only three functional AP complexes: AP1, AP2, and AP3; the AP4 and AP5 complexes have been lost through evolution [6].
Of the three AP complexes in fungi, the AP1 complex is the most conserved, and it is a heterotetrameric protein complex that binds membranes that are rich in phosphatidylinositol 4-phosphate, such as the membrane of the trans-Golgi network (TGN), and regulates cargo sorting between the TGN and recycling endosomes [7,8,9]. The AP1 complex consists of two large (∼100 kDa) subunits, beta-adaptin (β) and gamma-adaptin (γ), one medium-sized (∼50 kDa) subunit (μ), and one small (∼20 kDa) subunit sigma (σ) [1,10,11]. Its molecular architecture closely resembles that of AP2, the plasma-membrane-specific adaptor [12]. Previous studies have shown that the recruitment of the AP1 complex to membranes occurs following physical interaction with phosphatidyl-inositol-4-phosphate (PI4P), the HEATR5 protein Laa1 and its cofactor Laa2, and the small GTPase Arf1 [13,14,15]. Cargo recruitment is mediated by the AP1μ subunit which directly binds tyrosine motifs on the cargo proteins [16]. The ears and linkers of the β subunit specifically bind to clathrin and clathrin-accessory proteins, though the mammalian β subunit is also found to bind the dileucine motif of cargo proteins [17,18]. The σ subunit is involved in cargo selection also by binding dileucine motifs [19,20].
AP1 localizes to the TGN, and the TGN localization of this protein complex depends on the small GTPase, Arf1, and the phosphoinositide, PI-P [12]. AP1 is also required for the polarized localization of many plasma membrane proteins [21]. The AP1 protein complex participates in both clathrin-dependent and clathrin-independent processes in Saccharomyces cerevisiae [22]. The loss of the AP1 complex subunits β, γ, μ, and σ in Schizosaccharomyces pombe individually showed distinct phenotypes including growth sensitivity to drugs or temperature [23]. In Aspergillus nidulans, the AP1 complex is required for the release of the secretory vesicle, polar sorting, endosome recycling, and cytoskeleton organization [24]. AP1 functions as a heterotetrameric complex in Toxoplasma gondii, and deletion of the AP1μ subunit impaired the sorting of rhoptry and microneme proteins, parasite division, and host infection [25]. In Arabidopsis thaliana, AP1 localizes to trans-Golgi network (TGN) and regulates trafficking from the TGN to the vacuole and plasma membrane (exocytosis) [26,27]. Further study found that AP1σ (AP1σ1 and AP1σ2) is crucial for pollen wall formation by mediating the secretion of lipid transfer proteins (LTPs) and sporopollenin synthase acyl-CoA synthetase 5 (ACOS5) from tapetum into the anther locule [28]. Disruption of various subunits (γ1, β1, μ1, or σ1) of the AP1 complex in mice causes prenatal lethality and severe growth defects [29,30,31,32,33]. There are three genes encoding the AP1 small subunit σ in humans, and disruption of AP1S1 (σ1A) impaired the development of the skin and spinal cord and caused reduced pigmentation and severe motility deficits [34]. AP1B is required for the polarized distribution of many membrane proteins to the basolateral surface of LLC-PK1 kidney cells [35]. Mutations in AP1S3 (σ1C) cause a severe autoinflammatory skin disorder (called pustular psoriasis) due to defects in vesicular trafficking, and AP1S3 (σ1C) is required for Toll-like receptor homeostasis [36]. However, the role of the AP1 complex in plant pathogenic fungi is still unclear.
Fusarium graminearum is a filamentous fungus that causes an economically devastating disease called Fusarium head blight (FHB) in wheat and other cereals, and it has become one of the most serious problems for agricultural production worldwide [37,38,39,40]. In addition to enormous losses in wheat yield, mycotoxins such as deoxynivalenol (DON), nivalenol (NIV), and zearalenone (ZEA) produced by the fungus in infected grains impose serious threats to food security and human health [41,42]. Recent studies demonstrated that intracellular vesicle trafficking regulates fungal development, pathogenicity, and DON (mycotoxin) production in F. graminearum [43,44,45,46,47,48].
Although the roles of the AP1 complex in S. cerevisiae and mammals have been studied, the functions of this complex in plant pathogenic fungi are still unknown. Our previous studies revealed that the well-conserved FgAP2 complex subunits FgAP2β, FgAP2α, FgAP2mu, and FgAP2σ are essential for the polarized growth, development, and pathogenicity of F. graminearum and regulate the polar localization of the lipid flippases FgDnfA and FgDnfB [49]. In this study, we functionally characterized the AP1 complex subunit FgAP1σ in the development and pathogenesis of F. graminearum using molecular genetic techniques. Our results showed that FgAP1σ is essential for development, cell wall integrity, the response to osmotic stress, DON production, pathogenicity, and the transport of the v-SNARE FgSnc1 from the Golgi to the plasma membrane, and it causes the delay of the endocytosis process in F. graminearum. Moreover, FgAP1σ is localized to endosomes and the Golgi apparatus and regulates the expression of FgAP1β, FgAP1γ, and FgAP1μ in F. graminearum. The findings improve our understanding of the functions of the AP1 complex as well as its involvement in host–pathogen interaction and lay the foundation for effective prevention and control of FHB.
2. Materials and Methods
2.1. Fungal Strains, Media, and Culture Conditions
The wild-type PH-1 and all transformants used in this study (Table S1) were cultured at 28 °C on starch yeast media (SYM: 0.2% yeast extract, 1% starch, 0.3% sucrose, 2% agar). Vegetative growth of the various strains were assayed on starch yeast media (SYM), solid complete media (CM: 0.6% casein hydrolyzate, 1% sucrose, 0.6% yeast extract, and 2% agar), and minimal media (MM: 0.52 g KCl, 6 g NaNO3, 1.52 g KH2PO4, 10 g glucose, 0.52 g MgSO4, 0.1% trace elements (v/v), and 2% agar) at 28 °C for 3 days. Solid CM medium was supplemented with different inhibitors to test the response of the strains to different stresses. For conidiation assay, the various strains were cultivated in liquid carboxymethylcellulose (CMC) medium, cultured for 3 days to induce conidia production as described previously [50]. Conidia from PH-1 and all the mutants were visualized using an Olympus BX51 microscope. Perithecia formation assay was conducted on carrot agar medium as previously reported [51]. The vegetative growth, conidiation, and perithecia formation assays were repeated three times.
2.2. Gene Deletion and Complementation
We searched for the predicted F. graminearum AP1 complex proteins using S. cerevisiae AP1 complex proteins (Aps1p, Apm1p, Apl2p, and Apl4p) as a reference to perform a BLAST search against the available fungal genome and identified FGSG_10034, FGSG_17141, FGSG_06317, and FGSG_01893 genetic loci encoding the homologues of AP1 complex proteins in F. graminearum. For convenience, we named these genes FgAP1σ, FgAP1u, FgAP1β, and FgAP1γ, respectively.
F. graminearum protoplast preparation and fungal transformation were conducted as described previously [52]. Deletions of FgAP1σ (FGSG_10034) genes were achieved using a split-marker approach [53]. Table S2 presents all the primers used for gene deletion. The FgAP1σ knockout mutants were successfully generated and further verified by Southern blot. For complementation assay, green fluorescent protein (GFP) fragment was tagged at the C-terminus of the FgAP1σ gene sequence. We amplified the FgAP1σ coding sequence under the control of its native promoter. The GFP fragment that was fused with the FgAP1σ gene sequence was inserted into a pKNT plasmid to generate FgAP1σ-GFP vector using the Cloning Kit (Vazyme Biotech Co., Ltd., Nanjing, China). The resulting FgAP1σ-GFP construct was amplified using specific primer pairs and sequenced to verify its successful insertion into the plasmid. Finally, the FgAP1σ-GFP vector was transformed into the ΔFgap1σ mutant protoplasts to generate the complemented strain which was phenotypically similar to the PH-1.
2.3. Construction of Fusion Vectors
The native promoters and coding sequences of FgAP1σ-GFP, FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP constructs, as well as the GFP fragment (isolated from the FgGdt1-GFP vector) [51] were amplified using specific primer pairs (Table S2). The GFP fragment was tagged at the C-terminus of FgAP1σ, FgAP1β, FgAP1γ, and FgAP1μ and cloned into pKNT vector. The resulting FgAP1σ-GFP, FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP constructs were amplified by PCR using specific primer pairs (Table S2) and confirmed by sequencing to verify successful insertion. FgKex2 was tagged with mCherry at its C-terminus, while FgSnc1 was tagged with GFP at its N-terminus, under the control of their respective native promoters, to generate FgKex2-mCherry and GFP-FgSnc1 vectors, respectively. The constructed vectors were transformed into the wild-type PH-1 or ΔFgap1σ mutant protoplasts.
2.4. RNA Extraction and Quantitative Reverse Transcription PCR
Total RNA was extracted from mycelia of the wild type and mutants cultivated in liquid CM and incubated at 28 °C under constant shaking at 110 rpm for 2 days, or in liquid trichothecene biosynthesis induction (TBI) media and incubated at 28 °C in the dark for 3 days, using an Eastep Super Total RNA Extraction Kit (Promega, Shanghai, China ). Reverse transcription was performed to generate complementary DNA (cDNA) using a Reverse Transcription Kit (Takara, Tokyo, Japan). The expression level of each gene was quantified by qRT-PCR using a TB GREEN kit (Takara, Tokyo, Japan) with specific primers (Table S1). The Fusarium graminearum β-tubulin gene was used as an endogenous reference gene. Relative gene expressions were finally calculated using the 2−ΔΔCT method according to [54]. All qRT-PCR assays were repeated three times.
2.5. Subcellular Localization Assay
For the observation of F. graminearum mycelial morphology and cellular localization, the various strains were grown in liquid or on solid CM media at 28 °C for 24 h, and microscopic visualizations were performed using a Nikon A1R laser scanning confocal microscope (Nikon, Tokyo, Japan). Conidia from the wild-type PH-1 and ΔFgap1σ strains were stained with the cell-wall-damaging agent calcofluor white (CFW) at a final concentration of 10 μg/mL for visualization of cell walls and septa using an Olympus BX51 microscope (Olympus, Tokyo, Japan). Similarly, hyphae from the wild-type PH-1 and ΔFgap1σ strains were stained with the fluorescent dye FM4-64 at a final concentration of 4 µM, incubated, and visualized for observation of endocytosis using the Nikon A1R laser scanning confocal microscope (Nikon, Tokyo, Japan).
2.6. Yeast Two-Hybrid
For yeast two-hybrid (Y2H) assays, the full length cDNAs of FgAP1σ, FgAP1β, FgAP1γ, and FgAP1μ were amplified by PCR using specific primers (Table S2). The FgAP1σ, FgAP1β, and FgAP1μ fragments were cloned into pGBKT7 vectors pre-digested with Nde I and EcoR I as bait vectors to generate FgAP1σ-BD, FgAP1β-BD, and FgAP1μ-BD recombinant vectors, respectively. In addition, FgAP1β, FgAP1μ, and FgAP1γ fragments were cloned into pGADT7 pre-digested with Nde I and EcoR I as prey vectors to generate FgAP1β-AD, FgAP1μ-AD, and FgAP1γ-AD recombinant vectors, respectively. All prey and bait vectors were confirmed by sequencing and cotransformed into S. cerevisiae AH109 strain according to a previous study [55]. Interaction between pGBKT7-53 and pGADT7 served as positive control, while that between pGBKT7-Lam and pGADT7 served as negative control.
2.7. Pathogenicity and DON Production Assays
The pathogenicity of the various strains on flowering wheat heads was conducted as described previously [51]. For wheat spikelet infection assay, the wild-type PH-1 and ΔFgap1σ strains were inoculated in liquid CMC medium, and their conidia concentrations were adjusted to 4 × 104 cells/mL. Conidia suspensions were inoculated to fresh wheat coleoptile wounds, and disease symptoms were observed 7 days after incubation at 25 °C. For DON production assay, mycelia plugs from the strains were cultured in liquid trichothecene biosynthesis induction (TBI) media and incubated at 28 °C in the dark and without shaking for 7 days. An ELISA-based DON detection kit (FINDE, Shenzhen, China) [51] was used to measure DON levels in the liquids while the mycelia were collected, dried, and weighed. The DON production was standardized as 1 g dry weight of mycelia. The experiment was repeated three times.
3. Results
3.1. FgAP1σ Gene Deletion and Confirmation
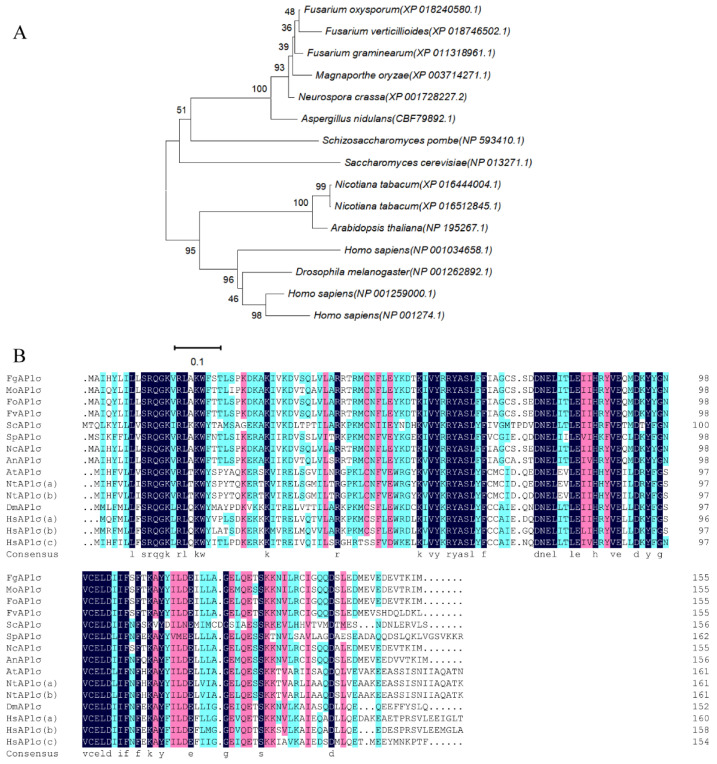
Bioinformatics analysis showed that the F. graminearum AP1 complex contains four subunits: FgAP1σ, FgAP1u, FgAP1β, and FgAP1γ. To understand the biological functions of the FgAP1 complex in F. graminearum, a targeted gene replacement strategy was used to delete FgAP1 complex subunits in PH-1 (wild type). Of the four FgAP1 genes, only FgAP1σ was deleted successfully, while FgAP1u, FgAP1β, and FgAP1γ knockout mutants could not be obtained despite multiple deletions, suggesting that they are essential for the survival of F. graminearum. FgAP1σ encodes a 181-amino-acid protein that shares 56% identity with S. cerevisiae Aps1. Phylogenetic analysis and sequence alignment of FgAP1σ and other AP1σ proteins showed that FgAP1σ is highly conserved in eukaryotes, especially in the filamentous fungi Fusarium oxysporum, Fusarium verticillioides, Manaporthe oryzae, and Neurospora crassa (Figure 1A,B). The ΔFgap1σ-14 and ΔFgap1σ-19 mutants were further confirmed by Southern blot analysis, which showed a 3.95 kb band in the FgAP1σ deletion mutants in contrast to a 5.09 kb band in the wild-type PH-1 (Figure S1A,B). In addition, FgAP1σ was tagged with GFP at its C-terminus, under the control of its native promoter to form the FgAP1σ-GFP vector. The constructed vectors were transformed into the ΔFgap1σ-14 mutant protoplasts to generate the complemented strain ΔFgap1σ-C.
Figure 1.
Bioinformatics analyses of FgAP1σ homologues. (A) Phylogenetic relationship of FgAP1σ protein from different organisms. The gene names are from the Broad Institute genome annotation: Fusarium graminearum (XP_011318961.1), Magnaporthe oryzae (XP_003714271.1), Fusarium oxysporum (XP_018240580.1), Fusarium verticillioides (XP_018746502.1), Saccharomyces cerevisiae (NP_013271.1), Schizosaccharomyces pombe (NP_593410.1), Neurospora crassa (XP_001728227.2), Aspergillus nidulans (CBF79892.1), Arabidopsis thaliana (NP_195267.1), Nicotiana tabacum (XP_016444004.1), Nicotiana tabacum (XP_016512845.1), Homo sapiens (NP_001259000.1), Homo sapiens (NP_001274.1), Homo sapiens (NP_001034658.1), Drosophila melanogaster (NP_001262892.1). (B) Multiple alignment of amino acid sequences of FgAP1σ homologues.
3.2. FgAP1σ Plays an Important Role in Vegetative Growth of F. graminearum
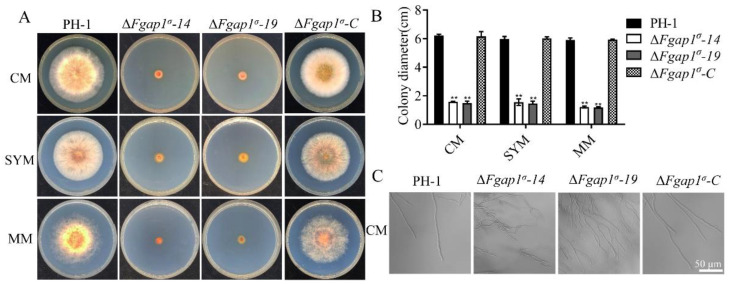
To determine whether FgAP1σ is required for the vegetative growth and colony morphology of F. graminearum, the wild-type PH-1, FgAP1σ deletion mutants (ΔFgap1σ-14, ΔFgap1σ-19), and the complementation strain ΔFgap1σ-C were cultured on CM, SYM, and MM plates for 3 days. Compared with the PH-1 and ΔFgap1σ-C strains, the ΔFgap1σ-14 and ΔFgap1σ-19 mutants showed significantly reduced vegetative and aerial hyphal growths (Figure 2A,B). Close microscopic examinations showed increased mycelial branching in the ΔFgap1σ-14 and ΔFgap1σ-19 mutants compared with the PH-1 and ΔFgap1σ-C strains. In addition, the morphology of the mutants appeared irregular (Figure 2C). These results suggest that FgAP1σ is important for F. graminearum vegetative and polarized growth.
Figure 2.
FgAP1σ is required for the vegetative growth of F. graminearum. (A) Vegetative growth of the wild-type PH-1, ΔFgap1σ mutants, and the complemented strain ΔFgap1σ-C after growth on complete media (CM), starch yeast medium (SYM), and minimal medium (MM) agar at 28 °C for 3 days. (B) Statistical analysis of the colony diameters of the PH-1, ΔFgap1σ, and ΔFgap1σ-C strains on CM, SYM, and MM media after 3 days. Level of significance was measured using an unpaired t-test (** p < 0.01). (C) Mycelial morphologies of PH-1, ΔFgap1σ, and ΔFgap1σ-C strains on CM medium. Hyperbranching was observed in the ΔFgap1σ mutants. Bar = 50 μm.
3.3. FgAP1σ Is Required for Conidiation and Sexual Development of F. graminearum
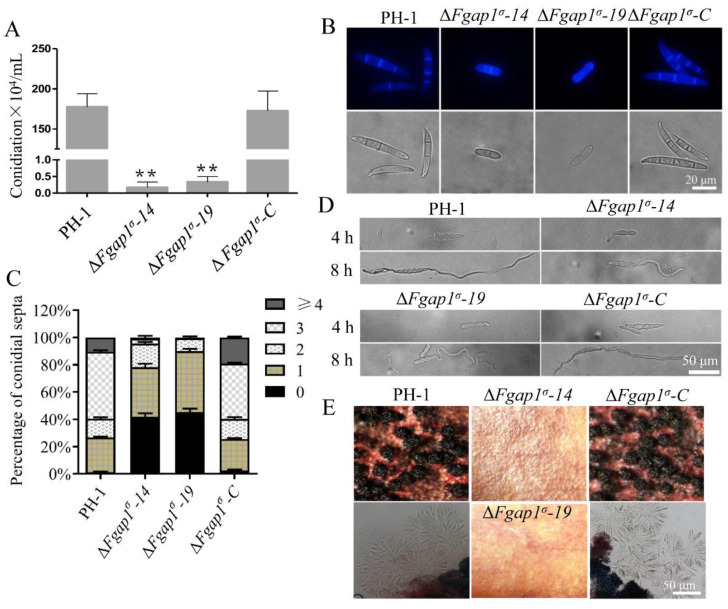
Asexual conidia and sexual ascospores produced by F. graminearum function as the important inocula that infect flowering wheat heads. We investigated the asexual reproduction of ΔFgap1σ mutants and found that the conidiation of the ΔFgap1σ-14 and ΔFgap1σ-19 mutants were significantly decreased compared with those of PH-1 and ΔFgap1σ-C strains (Figure 3A). Moreover, microscopic examinations of the conidia indicated that 95.45% of the conidia from the ΔFgap1σ-14 mutant and 99.41% of the conidia from the ΔFgap1σ-19 mutant had one or no septum, unlike most of the PH-1 and complemented strain which possessed at least three septa per conidium (Figure 3B,C). Although the conidia morphology of the ΔFgap1σ-14 and ΔFgap1σ-19 mutants was obviously abnormal, their germinations on CM media after 4 h or 8 h were not affected compared with the PH-1 and ΔFgap1σ-C strains (Figure 3D). To investigate the roles of FgAP1σ in the fungal sexual reproduction, the wild-type PH-1, ΔFgap1σ-14, ΔFgap1σ-19, and ΔFgap1σ-C strains were grown on carrot agar for perithecia and ascospore formation. As shown in Figure 3E, the loss of FgAP1σ in F. graminearum completely abolished perithecia and ascospore formation. Our results suggest that FgAP1σ plays important roles in reproduction in F. graminearum.
Figure 3.
FgAP1σ is involved in the conidiation and sexual development of F. graminearum. (A) Conidiation of the wild-type PH-1, ΔFgap1σ, and ΔFgap1σ-C strains in liquid CMC media for 3 days. Level of significance was measured using an unpaired t-test (** p < 0.01). (B) Conidial morphology was observed under a light microscope after the PH-1, ΔFgap1σ, and ΔFgap1σ-C strains were cultured in liquid CMC media for 3 days. Bar = 20 μm. (C) The number of septa in the conidia produced by the indicated strains. (D) Germination of the indicated strains in CM media after 4 h and 8 h of inoculation. Bar = 50 μm. (E) Images of the perithecia and ascospores produced by the PH-1, ΔFgap1σ, and ΔFgap1σ-C strains after 2 weeks of inoculation on carrot agar plates. Bar = 50 μm.
3.4. FgAP1σ Is Critical for F. graminearum Virulence and DON Production
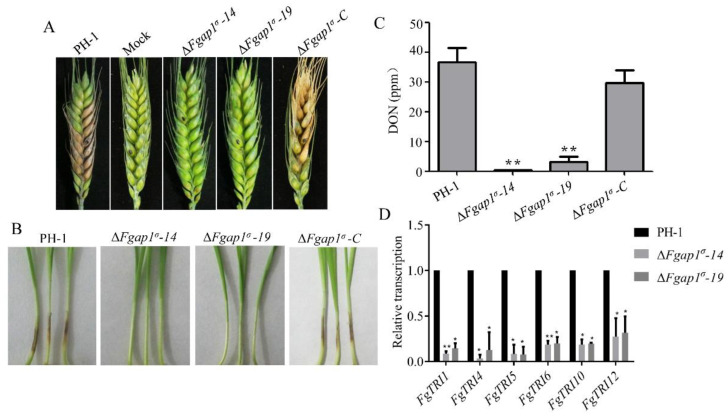
To investigate the roles of FgAP1σ in the pathogenicity of F. graminearum to its host plants, we conducted infection assays on flowering wheat heads and wheat coleoptiles. Mycelia plugs from the wild-type PH-1, ΔFgap1σ-14, ΔFgap1σ-19, and ΔFgap1σ-C strains were inoculated on flowering wheat heads under moist conditions for two weeks. The wild-type PH-1 and ΔFgap1σ-C strains caused severe head blight symptoms, while the ΔFgap1σ-14 and ΔFgap1σ-19 mutants showed significantly decreased infection capability and did not produce obvious symptoms (Figure 4A). We further conducted virulence assays of PH-1 and the ΔFgap1σ mutants on young wheat coleoptiles and obtained similar results for each strain (Figure 4B). DON is well characterized as an important mycotoxin and virulence factor in the pathogenicity of F. graminearum on wheat. To investigate whether FgAP1σ is required for DON production, we cultured the PH-1, ΔFgap1σ, and ΔFgap1σ-C strains in liquid TBI media at 28 °C for 7 days under dark conditions and checked the levels of DON production afterwards. We found that DON production was significantly decreased in the ΔFgap1σ-14 and ΔFgap1σ-19 mutants compared with the wild-type PH-1 and ΔFgap1σ-C strains (Figure 4C), which is consistent with our pathogenicity tests. Next, we examined the expression levels of the DON-biosynthesis-related genes FgTRI6, FgTRI10, FgTRI1, FgTRI4, FgTRI5, and FgTRI12 in the ΔFgap1σ mutants harvested from TBI media by quantitative reverse transcription PCR (qRT-PCR) analysis. Our results showed that the transcription levels of the various genes were significantly downregulated in the ΔFgap1σ-14 and ΔFgap1σ-19 mutants compared with those in the wild-type PH-1 (Figure 4D). Collectively, these results demonstrate that FgAP1σ regulates virulence and DON biosynthesis in F. graminearum.
Figure 4.
FgAP1σ is required for virulence. (A) Pathogenicity of ΔFgap1σ mutants on flowering wheat heads was significantly reduced compared with the wild-type PH-1 and ΔFgap1σ-C strains. Mycelia plugs from the various strains were inoculated on flowering wheat heads under moist conditions, and disease symptoms were recorded after two weeks. (B) The pathogenicity of ΔFgap1σ mutants on wheat coleoptiles was abolished. (C) Level of deoxynivalenol (DON) production by ΔFgap1σ mutants. Mycelia of the indicated strains were dried and weighed to quantify the fungal biomass. Level of significance was measured using an unpaired t-test (** p < 0.01). (D) The expression levels of some trichothecene biosynthetic genes FgTRI were significantly reduced in the ΔFgap1σ mutants. Level of significance was measured using an unpaired t-test (* p < 0.05, ** p < 0.01).
3.5. FgAP1σ Is Important for Cell Wall Integrity and Response to Osmotic Stress in F. graminearum
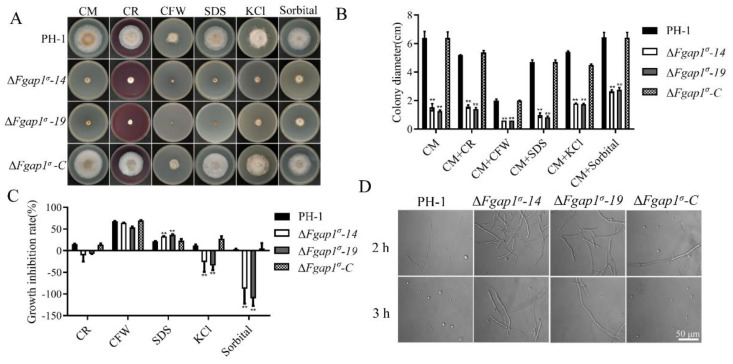
To examine the impact of FgAP1σ deletion on the responses of F. graminearum to various types of stresses, we cultured the various strains on CM media containing the cell-wall-perturbing agents Congo red (CR) and calcofluor white (CFW), the cell membrane inhibitor SDS, and the osmotic-stress-inducing agents KCl and sorbitol for 3 days and monitored growth inhibition. The results obtained from these bioassays showed that the growth inhibition rates of the ΔFgap1σ-14 and ΔFgap1σ-19 mutants under SDS-induced stress were significantly higher than in the presence of other agents, suggesting that ΔFgap1σ mutants are more sensitive to SDS-induced stress (Figure 5A–C). In addition, the growth inhibition rates of the ΔFgap1σ mutants were not significantly changed under CFW and CR stresses compared with PH-1 and ΔFgap1σ-C strains, which means that they are not sensitive to CFW and CR stresses (Figure 5A–C). Next, we examined the release of protoplasts by the ΔFgap1σ mutants in the presence of cell-wall-degrading enzymes to further confirm the integrity of the cell walls of the ΔFgap1σ mutants. Our results showed that the hyphae of the ΔFgap1σ-14 and ΔFgap1σ-19 mutants released less protoplasts than those of the wild-type PH-1 after 2 h and 3 h of incubation with cell-wall-degrading enzymes (Figure 5D), indicating that the loss of FgAP1σ affects the fungal cell wall integrity. Further study found that the ΔFgap1σ-14 and ΔFgap1σ-19 mutants were more tolerant to osmotic stress on CM media supplemented with the osmotic stress agents KCl and sorbitol than PH-1 (Figure 5A–C), indicating that the ΔFgap1σ mutants are less sensitive to KCl- and sorbitol-induced osmotic stresses.
Figure 5.
Deletion of FgAP1σ resulted in cell wall integrity and osmotic pressure defects. (A) The PH-1, ΔFgap1σ, and ΔFgap1σ-C strains were incubated on CM plates supplemented with Congo red (CR, 200 μg/mL), calcofluor white (CFW, 200 μg/mL), SDS (0.01 %), sorbitol (1 M), and KCl (1 M) at 28 °C for 3 days. (B) Colony diameter of the indicated strains under cell wall and osmotic stresses. Level of significance was measured using an unpaired t-test (** p < 0.01). (C) Growth inhibition rate of the strains due to cell wall and osmotic stresses induced by the indicated agents. Level of significance was measured using an unpaired t-test (** p < 0.01). (D) Protoplast releasing potential of the PH-1 and ΔFgap1σ mutants after treatment with cell-wall-degrading enzymes for 2 h and 3 h. Bar = 50 μm.
3.6. FgAP1σ Localizes to Endosomes and Golgi Apparatus
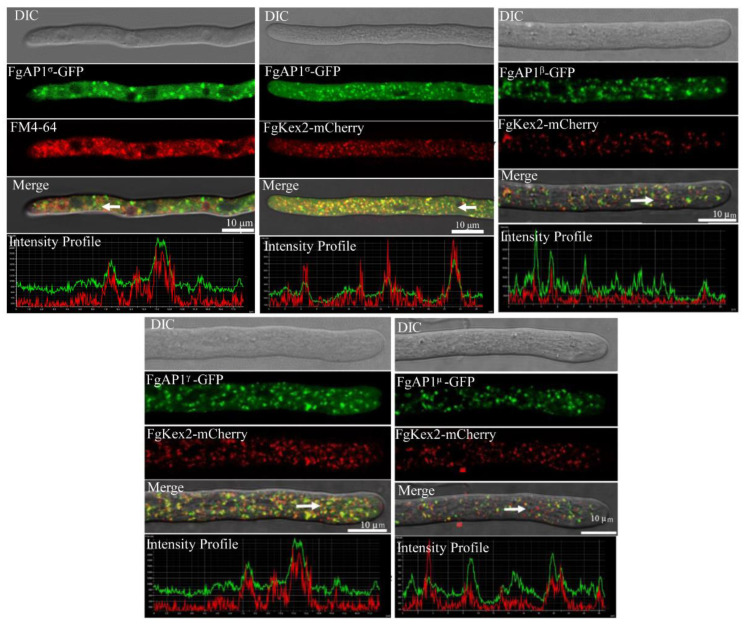
We further investigated the subcellular localization of the FgAP1 complex and found that FgAP1σ-GFP partially colocalized with FM4-64-positive endosomes in F. graminearum (Figure 6). In yeast, AP1σ localizes to the Golgi [4]. To check whether FgAP1σ-GFP is localized to the Golgi apparatus in addition to its endosome localization, we cotransformed the FgAP1σ-GFP vectors with another vector expressing the Golgi marker FgKex2-mCherry [51] into the protoplasts of PH-1. As shown in Figure 6, FgAP1σ-GFP also localized to the Golgi apparatus. To gain insight into the subcellular localization of other subunits of the FgAP1 complex, we constructed the FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP vectors and cotransformed them with the FgKex2-mCherry vector into the PH-1 protoplasts. Our results showed that FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP all colocalized with FgKex2-mCherry at the Golgi apparatus (Figure 6), suggesting that the FgAP1 complex localizes to the Golgi apparatus in F. graminearum.
Figure 6.
The localization of AP1 complex in F. graminearum. Colocalization of FgAP1σ-GFP with FM4-64 and trans-Golgi network (TGN, FgKex2-mCherry) as well as that of FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP with FgKex2-mCherry in F. graminearum. Images were captured from laser scanning confocal microscope. Bar = 10 μm.
3.7. The Relationship of FgAP1σ with Other Subunits of AP1 Complex
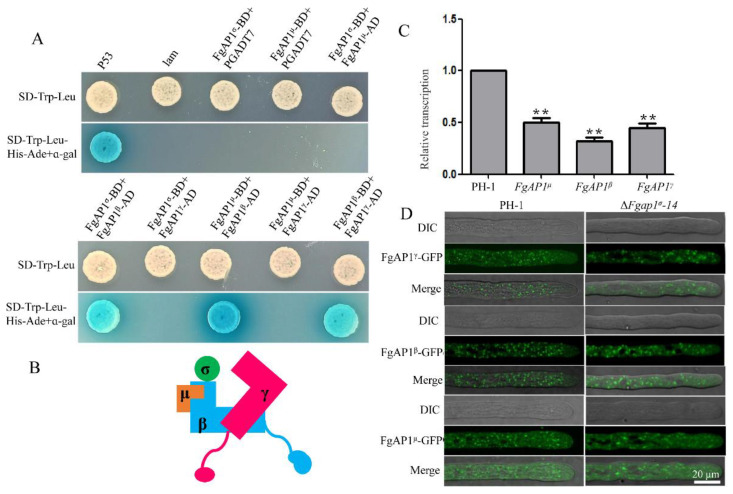
To investigate the relationship of the various subunits in the AP1 complex, we conducted yeast two-hybrid assays and found that FgAP1β interacts with FgAP1σ, FgAP1γ, and FgAP1μ, but we could not find any positive interaction between FgAP1σ and FgAP1γ nor between the former and FgAP1μ (Figure 7A). Therefore, a proposed model depicting the relationship between the AP1 complex subunits is depicted in Figure 7B, which suggests that FgAP1β, FgAP1σ, FgAP1γ, and FgAP1μ function as a complex in F. graminearum.
Figure 7.
The relationship between FgAP1 complex subunits and the localizations of the various subunits in ΔFgap1σ mutant. (A) Yeast two-hybrid assay showing the interactions of the AP1 complex subunits. There were positive interactions between FgAP1β and FgAP1σ and between FgAP1γ and FgAP1μ. The interaction between pGBKT7-Lam and pGADT7-T was used as a negative control while that between pGBKT7-53 and pGADT7-T served as a positive control. (B) The interaction model of FgAP1σ with the other subunits of FgAP1 complex. FgAP1σ interacts with FgAP1β, and FgAP1β interacts with FgAP1γ and FgAP1μ, which indicates that FgAP1σ, FgAP1β, FgAP1γ, and FgAP1μ function as a complex in F. graminearum. (C) The expression levels of FgAP1β, FgAP1γ, and FgAP1μ were significantly downregulated in ΔFgap1σ mutant. Level of significance was measured using an unpaired t-test (** p < 0.01). (D) The localizations of FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP were not affected in ΔFgap1σ mutant. Bar = 20 μm.
To further investigate the regulatory role of FgAP1σ with the other three subunits, we tested the transcription levels of FgAP1β, FgAP1γ, and FgAP1μ in the ΔFgap1σ mutants by qRT-PCR and found that they are all downregulated in the ΔFgap1σ-14 and ΔFgap1σ-19 mutants compared with the wild-type PH-1 (Figure 7C). To examine whether FgAP1σ regulates the subcellular localization of FgAP1β, FgAP1γ, and FgAP1μ, we transformed FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP vectors into the ΔFgap1σ protoplasts and checked the localizations of their protein products in the absence of FgAP1σ. Our results showed that the punctate localizations of FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP were not affected in the ΔFgap1σ mutant (Figure 7D).
3.8. FgAP1σ Is Required for Plasma Membrane Localization of the v-SNARE FgSnc1
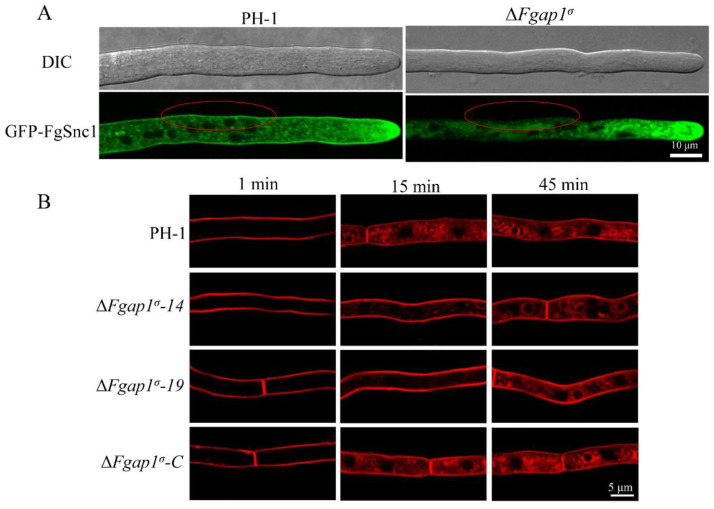
Previous studies have shown that FgSnc1 regulates the polarized secretion and fusion of vesicles in F. graminearum. To understand the roles of FgAP1σ in vesicle transport processes, we constructed the GFP-FgSnc1 vector and transformed it into the wild-type PH-1 and ΔFgap1σ protoplasts and subsequently observed the cellular localizations of GFP-FgSnc1 by confocal microscopy. In PH-1, GFP-FgSnc1 was observed to be clearly localized to the plasma membrane, and it also concentrated at the Spitzenkörper (SPK) of growing hyphal cells in PH-1 (Figure 8A). However, in the ΔFgap1σ mutant expressing GFP-FgSnc1, the hyphal tip localization of this protein was still present but not its plasma membrane localization (Figure 8A). These results suggest that FgAP1σ regulates the secretion of FgSnc1 from the Golgi to the plasma membrane.
Figure 8.
FgAP1σ is involved in exocytosis and endocytosis processes. (A) The plasma membrane localization of GFP-FgSnc1 is disrupted in the ΔFgap1σ mutant, as indicated by red circles. Bar = 10 μm. (B) FgAP1σ delays internalization of FM4-64 into the vacuole membrane. Hyphae of the wild-type PH-1, ΔFgap1σ-14, ΔFgap1σ-19, and complement strain ΔFgap1σ-C were cultured in liquid CM for 24 h and then stained with FM4-64 and observed at different time points using a fluorescence confocal microscope. Bar = 5 μm.
3.9. Loss of FgAP1σ Results in Delayed Internalization of FM4-64 into Vacuole Membrane
To investigate whether FgAP1σ is involved in endocytosis in F. graminearum, we monitored the endocytic uptake of the fluorescent dye FM4-64 by the wild type and mutants at different time points. As shown in Figure 8B, the FM4-64 signal was detected localized on the plasma membrane of all the strains within 1 min of staining. After 15 min, endosomes (FM4-64-labeled vesicles) appeared inside the wild-type PH-1 and the complemented strain ΔFgap1σ-C, indicating normal endocytosis. However, a large amount of FM4-64 remained in the membrane of the ΔFgap1σ-14 and ΔFgap1σ-19 mutants. At 45 min after staining, FM4-64 was found to internalize into the vacuole membrane through the endosomes of hyphal cells in the wild-type PH-1 and ΔFgap1σ mutants. These results collectively reveal that FgAP1σ delays the internalization of FM4-64 into the vacuole membrane in F. graminearum.
4. Discussion
The AP1 complex is a conserved heterotetrameric protein complex that consists of AP1σ, AP1β, AP1γ, and AP1μ subunits in eukaryotes [8,56,57]. Previous studies in yeast, T. gondii, A. thaliana, and mammals demonstrated that the AP1 complex plays fundamental roles in vesicle assembly, cargo sorting, and intracellular vesicular transport [22,25,33,36,58]. In this study, we identified four candidate subunits (FgAP1σ, FgAP1β, FgAP1γ, and FgAP1μ) of the AP1 complex in F. graminearum and then investigated the biological functions of the small subunit FgAP1σ as well as the relationship of FgAP1σ with the other subunits in the plant fungal pathogen F. graminearum. Our results showed that FgAP1σ localizes to endosomes and the trans-Golgi network (TGN) and is critical for fungal development and pathogenicity to wheat. Further study found that FgAP1σ plays an important role in cell wall integrity and response to osmotic stresses. It also regulates the transcription levels of other AP1 subunits as well as the secretory process from the Golgi apparatus to the plasma membrane and delays the endocytosis process.
In S. pombe, Aps1p (σ) directly interacts with Apl4p (γ), while Apm1p (μ) directly interacts with Apl2p (β1). The localization of Apl4p (γ), Apm1p (μ), and Apl2p (β1) were significantly changed in the Δaps1 (Δap1σ) mutant [23]. However, the relationships between the various subunits of the AP1 complex in plant pathogenic fungi are still unclear. In this study, yeast two-hybrid assays showed that FgAP1β interacts with FgAP1σ, FgAP1γ, and FgAP1μ and form the AP1 complex in F. graminearum. However, the punctate localizations of FgAP1γ, FgAP1β, and FgAP1μ remain unchanged in the ΔFgap1σ mutant in F. graminearum (Figure 7A,B,D). This clearly demonstrates a difference in the interaction of the AP1 complex subunits between yeast and plant pathogenic fungi. In addition, FgAP1σ regulates the transcription levels of FgAP1β, FgAP1γ, and FgAP1μ (Figure 7C), suggesting that the loss of a single component of the FgAP1 complex affects the homeostasis of other components in F. graminearum. Furthermore, the loss of FgAP1σ showed clear pleiotropic phenotypes, including defects in vegetative growth, conidia production, conidia morphology, sexual reproduction, and pathogenicity. We were able to delete FgAP1σ but not the genes encoding the other subunits of the FgAP1 complex (FgAP1β, FgAP1γ, and FgAP1μ) in PH-1 (wild type), suggesting that FgAP1β, FgAP1γ, and FgAP1μ are indispensable for the survival of F. graminearum. The loss of AP1 subunits in S. pombe, T. gondii, A. thaliana, and humans results in serious phenotypic defects [23,25,26,27,28,34,35,36]. Moreover, disruption of the AP1mu1A or AP1γ genes in mice resulted in embryonic lethality [31,32]. These results suggest that the AP1 complex plays important roles in normal growth and development in eukaryotic organisms.
Fungal cell walls have a complex structure and can be remodeled by many stresses, developmental processes, and plant infection [59,60]. The endocytic cargo adaptor complex 2 (FgAP2) is required for cell wall integrity by interacting with the sensor FgWsc2B in F. graminearum [61]. In the present study, although the ΔFgap1σ-14 and ΔFgap1σ-19 mutants were not sensitive to CFW and CR stresses, the number of protoplasts released by the mutants was very low (Figure 5A–D). Moreover, the mycelial growth of the ΔFgap1σ mutants was promoted in the presence of KCl and sorbitol (Figure 5A–C). These results indicate that FgAP1σ positively regulates cell wall integrity but negatively regulates osmotic stress resistance in F. graminearum.
AP1B is required for the polarized distribution of many membrane proteins to the basolateral surface of LLC-PK1 kidney cells [35]. Polarized growth is fundamental for fungal optimal survival and infection [62]. Our results showed that FgAP1σ is required for polarized growth in F. graminearum (Figure 2C), suggesting a conserved role of the FgAP1 complex in polarized growth. AP1 binds membranes that are rich in phosphatidylinositol 4-phosphate, such as the trans-Golgi network (TGN) membrane, while AP2 associates with phosphatidylinositol 4,5-bisphosphate of the plasma membrane [9]. The FgAP2 complex colocalizes with actin patch components and regulates polarized growth in F. graminearum as shown by a previous study [49]. Here, we found that the FgAP1 complex, like FgAP2, localizes to TGN and is required for polarized growth in F. graminearum [49].
Previous studies have shown that the AP1 complex regulates cargo sorting within the TGN, endosomes, lysosomes, and plasma membrane [7,8,14,58]. In yeast, the clathrin adaptor protein complex 1 (AP1) is required for recycling of Chs3p and Tlg1p from the early endosome to the TGN [63]. A. thaliana AP1 localizes to the TGN and regulates trafficking to the vacuole and to the plasma membrane (exocytosis) [26,64,65]. In A. nidulans, AP1 is involved in anterograde sorting of both Rab11-labeled secretory vesicles and Rab5-dependent endosome recycling, as well as cytoskeleton-dependent polarized cargo traffic [24]. Soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (SNAREs) are sorted into clathrin-coated vesicles by direct interaction with clathrin adaptors [66]. FgSnc1 is a SNARE protein; it localizes to the Spitzenkörper (SPK) and plasma membrane and regulates the fusion of vesicles and polarized secretion in F. graminearum [67,68]. Snc1 mediates the fusion of vesicles from the Golgi with the plasma membrane in yeast [69]. Our results showed that the fluorescence signal of GFP-FgSnc1 could not be detected at the plasma membrane of the ΔFgap1σ mutant (Figure 8A), suggesting that FgAP1σ is involved in polarized trafficking of FgSnc1 from the Golgi to the plasma membrane and is required for the pathogenicity of F. graminearum, consistent with previous studies which demonstrated that FgMsb3, FgGyp1, and FgRab1 mediate polarized trafficking and are important for plant infection in F. graminearum [43,44,68]. Endocytosis is essential for transporting extracellular or membrane-localized macromolecules to the cytoplasm which ensures that nutrients reach the cell [5]. The AP2 complex has been demonstrated to function in endocytosis in various species [58,70,71,72,73], while the role of the AP1 complex in endocytosis is obscure. We found that the endocytic uptake of FM4-64 was delayed in the ΔFgap1σ mutants (Figure 8B), suggesting that FgAP1σ may be involved in the endocytosis process.
DON mycotoxin is known as an important virulence factor during infection of F. graminearum to wheat [74,75,76]. DON production in the ΔFgap1σ mutants was found to decrease significantly compared with PH-1 (Figure 4C). The development of full disease symptoms was also perturbed in the ΔFgap1σ mutants, probably due to the significant reduction in DON production. In summary, we demonstrated for the first time that FgAP1σ is indispensable for vegetative growth, conidiation, conidia morphology, sexual reproduction, pathogenicity, and DON biosynthesis in F. graminearum. Moreover, FgAP1σ is critical for the regulation of cell wall integrity, response to osmotic stresses, and vesicle fusion with target membranes. Therefore, our results showed that FgAP1σ is critical for growth and development, virulence, and DON biosynthesis in F. graminearum. Since the expression levels of the FgTRI genes were affected in the ΔFgap1σ mutant, then reduce DON production, further studies should examine whether the secretion of DON mycotoxin in the mutant is affected and investigate the mechanism of protein secretion as well as screening of FgAP1σ cargos so as to unveil whether the observed phenotypic defects of the ΔFgap1σ mutant are a direct result of the disruption of FgAP1σ function in vesicle transport and cargo sorting in F. graminearum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof9020145/s1, Figure S1: Southern blot analysis of the FgAP1σ deletion mutants. Genomic DNA was extracted from PH-1 and the positive transformants and digested with restriction enzymes. (A) Deletion strategy for FgAP1σ and its Southern blot confirmation. (B) NcoI DNA digestion showed a 5.09 kb band in the wild-type PH-1 and a 3.95 kb band in the ΔFgap1σ mutants. Table S1: Fungal strains used in this study. Table S2: Primers used in this study.
Author Contributions
Conceptualization, H.Z. (Huawei Zheng) and J.Z.; methodology, C.W., M.Z., M.Y. and H.C.; software, C.W., H.C. and M.Y.; validation, H.Z. (Huawei Zheng) and J.Z.; formal analysis, C.W., M.Z. and M.Y.; investigation, M.Z., H.C., M.Y., H.Z. (Haoming Zhong) and X.C.; data curation, C.W., H.C. and M.Y.; writing—original draft, C.W. and H.Z. (Huawei Zheng); writing—review and editing, C.W., Y.S.A., W.Z., H.Z. (Huawei Zheng) and J.Z.; visualization, H.Z. (Huawei Zheng) and J.Z.; supervision, H.Z. (Huawei Zheng) and J.Z.; project administration, C.W., H.Z. (Huawei Zheng), and J.Z.; funding acquisition, C.W. and J.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31870136, No. 31670142) and the Science and technology innovation special fund project of FAFU (No. KFb22050XA) to J.Z. and the project of Fujian Medical University (YJ220009) to C.W.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schmid S.L. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annu. Rev. Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 2.Hirst J., Robinson M.S. Clathrin and adaptors. Biochim. Biophys. Acta. 1998;14:173–193. doi: 10.1016/S0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 3.Owen D.J., Collins B.M., Evans P.R. Adaptors for clathrin coats: Structure and function. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 4.Ohno H. Clathrin-associated adaptor protein complexes. J. Cell Sci. 2006;119:3719–3721. doi: 10.1242/jcs.03085. [DOI] [PubMed] [Google Scholar]
- 5.Popova N.V., Deyev I.E., Petrenko A.G. Clathrin-mediated endocytosis and adaptor proteins. Acta Nat. 2013;5:62–73. doi: 10.32607/20758251-2013-5-3-62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martzoukou O., Amillis S., Zervakou A., Christoforidis S., Diallinas G. The AP-2 complex has a specialized clathrin-independent role in apical endocytosis and polar growth in fungi. Elife. 2017;21:20083. doi: 10.7554/eLife.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Ortega B., Kim B., Welling P.A. A Common Signal Patch Drives AP-1 Protein-dependent Golgi Export of Inwardly Rectifying Potassium Channels. J. Biol. Chem. 2016;291:14963–14972. doi: 10.1074/jbc.M116.729822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Beacham G.M., Partlow E.A., Hollopeter G. Conformational regulation of AP1 and AP2 clathrin adaptor complexes. Traffic. 2019;20:741–751. doi: 10.1111/tra.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traub L.M., Kornfeld S., Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- 11.Boehm M., Bonifacino J.S. Adaptins: The final recount. Mol. Biol. Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldwein E.E., Macia E., Wang J., Yin H.L., Kirchhausen T., Harrison S.C. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA. 2004;101:14108–14113. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zysnarski C.J., Lahiri S., Javed F.T., Martínez-Márquez J.Y., Trowbridge J.W., Duncan M.C. Adaptor protein complex-1 (AP-1) is recruited by the HEATR5 protein Laa1 and its co-factor Laa2 in yeast. J. Biol. Chem. 2019;294:1410–1419. doi: 10.1074/jbc.RA118.005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaman M.N., Sowerby P.J., Robinson M.S. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J. Biol. Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- 15.Sauvageau E., McCormick P.J., Lefrancois S. In vivo monitoring of the recruitment and activation of AP-1 by Arf1. Sci Rep. 2017;7:7148. doi: 10.1038/s41598-017-07493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno H., Fournier M.C., Poy G., Bonifacino J.S. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 17.Rapoport I., Chen Y.C., Cupers P., Shoelson S.E., Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S.M., Larocque G., Wood K.M., Morris K.L., Roseman A.M., Sessions R.B., Royle S.J., Smith C.J. Multi-modal adaptor-clathrin contacts drive coated vesicle assembly. EMBO J. 2021;40:6. doi: 10.15252/embj.2021108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doray B., Lee I., Knisely J., Bu G., Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 2007;18:1887–1896. doi: 10.1091/mbc.e07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paraan M., Mendez J., Sharum S., Kurtin D., He H., Stagg S.M. The structures of natively assembled clathrin-coated vesicles. Sci. Adv. 2020;6:eaba8397. doi: 10.1126/sciadv.aba8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan M.C. New directions for the clathrin adaptor AP-1 in cell biology and human disease. Curr. Opin. Cell Biol. 2022;76:13. doi: 10.1016/j.ceb.2022.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan H.L., Finlay J.A., Chu D.S., Tan P.K., Kirchhausen T., Payne G.S. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: Evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994;13:1706–1717. doi: 10.1002/j.1460-2075.1994.tb06435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y., Takeuchi M., Sugiura R., Sio S.O., Kuno T. Deletion mutants of AP-1 adaptin subunits display distinct phenotypes in fission yeast. Genes Cells. 2009;14:1015–1028. doi: 10.1111/j.1365-2443.2009.01327.x. [DOI] [PubMed] [Google Scholar]
- 24.Martzoukou O., Diallinas G., Amillis S. Secretory Vesicle Polar Sorting, Endosome Recycling and Cytoskeleton Organization Require the AP-1 Complex in Aspergillus nidulans. Genetics. 2018;209:1121–1138. doi: 10.1534/genetics.118.301240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopal K., Werkmeister E., Barois N., Saliou J.-M., Poncet A., Huot L., Sindikubwabo F., Hakimi M.A., Langsley G., Lafont F., et al. Dual role of the Toxoplasma gondii clathrin adaptor AP1 in the sorting of rhoptry and microneme proteins and in parasite division. PLoS Pathog. 2017;13:e1006331. doi: 10.1371/journal.ppat.1006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park M., Song K., Reichardt I., Kim H., Mayer U., Stierhof Y.D., Hwang I., Jürgens G. Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. USA. 2013;110:10318–10323. doi: 10.1073/pnas.1300460110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Cai Y., Wang H., Zeng Y., Zhuang X., Li B., Jiang L. Trans-Golgi network-located AP1 gamma adaptins mediate dileucine motif-directed vacuolar targeting in Arabidopsis. Plant Cell. 2014;26:4102–4118. doi: 10.1105/tpc.114.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M., Yan X., Wang Y., Liu C., Yang Q., Tian D., Bednarek S.Y., Pan J., Wang C. ADAPTOR PROTEIN-1 complex-mediated post-Golgi trafficking is critical for pollen wall development in Arabidopsis. New Phytol. 2022;235:472–487. doi: 10.1111/nph.18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hase K., Nakatsu F., Ohmae M., Sugihara K., Shioda N., Takahashi D., Obata Y., Furusawa Y., Fujimura Y., Yamashita T., et al. AP-1B—Mediated Protein Sorting Regulates Polarity and Proliferation of Intestinal Epithelial Cells in Mice. Gastroenterology. 2013;145:625–635. doi: 10.1053/j.gastro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Glyvuk N., Tsytsyura Y., Geumann C., D’Hooge R., Hüve J., Kratzke M., Baltes J., Boening D., Klingauf J., Schu P. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010;29:1318–1330. doi: 10.1038/emboj.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zizioli D., Meyer C., Guhde G., Saftig P., von Figura K., Schu P. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
- 32.Meyer C., Zizioli D., Lausmann S., Eskelinen E.-L., Hamann J., Saftig P., Von Figura K., Schu P. μ1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson K.R., Gagnon L.H., Chang B. A hypomorphic mutation of the gamma-1 adaptin gene (Ap1g1) causes inner ear, retina, thyroid, and testes abnormalities in mice. Mamm. Genome. 2016;27:200–212. doi: 10.1007/s00335-016-9632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montpetit A., Côté S., Brustein E., Drouin C.A., Lapointe L., Boudreau M., Meloche C., Drouin R., Hudson T.J., Drapeau P., et al. Disruption of AP1S1, Causing a Novel Neurocutaneous Syndrome, Perturbs Development of the Skin and Spinal Cord. PLoS Genet. 2008;4:e1000296. doi: 10.1371/journal.pgen.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fölsch H., Pypaert M., Schu P., Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setta-Kaffetzi N., Simpson M., Navarini A.A., Patel V.M., Lu H.-C., Allen M.H., Duckworth M., Bachelez H., Burden A.D., Choon S.E., et al. AP1S3 Mutations Are Associated with Pustular Psoriasis and Impaired Toll-like Receptor 3 Trafficking. Am. J. Hum. Genet. 2014;94:790–797. doi: 10.1016/j.ajhg.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goswami R.S., Kistler H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004;5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 38.Trail F., Xu J.R., San Miguel P., Halgren R.G., Kistler H.C. Analysis of expressed sequence tags from Gibberella zeae (ana-morph Fusarium graminearum) Fungal Genet. Biol. 2003;38:187–197. doi: 10.1016/S1087-1845(02)00529-7. [DOI] [PubMed] [Google Scholar]
- 39.Figueroa M., Hammond-Kosack K.E., Solomon P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018;19:1523–1536. doi: 10.1111/mpp.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullen M., Jones R., Gallenberg D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Kistler H.C., Ma Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019;57:15–39. doi: 10.1146/annurev-phyto-082718-100318. [DOI] [PubMed] [Google Scholar]
- 42.Ferrigo D., Raiola A., Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016;21:627. doi: 10.3390/molecules21050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y., Zhang M., Li J., Yang C., Abubakar Y.S., Chen X., Zheng W., Wang Z., Zheng H., Zhou J. The Small GTPase FgRab1 Plays Indispensable Roles in the Vegetative Growth, Vesicle Fusion, Autophagy and Pathogenicity of Fusarium graminearum. Int. J. Mol. Sci. 2022;23:895. doi: 10.3390/ijms23020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Q., Yu Z., Yuan Y., Sun D., Abubakar Y.S., Zhou J., Wang Z., Zheng H. The GTPase-Activating Protein FgGyp1 Is Important for Vegetative Growth, Conidiation, and Virulence and Negatively Regulates DON Biosynthesis in Fusarium graminearium. Front Microbiol. 2021;12:621519. doi: 10.3389/fmicb.2021.621519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C., Li J., Chen X., Zhang X., Liao D., Yun Y., Zheng W., Abubakar Y.S., Li G., Wang Z., et al. FgVps9, a Rab5 GEF, Is Critical for DON Biosynthesis and Pathogenicity in Fusarium graminearum. Front. Microbiol. 2020;11:1714. doi: 10.3389/fmicb.2020.01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adnan M., Fang W., Sun P., Zheng Y., Abubakar Y.S., Zhang J., Lou Y., Zheng W., Lu G.-D. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr. Genet. 2020;66:421–435. doi: 10.1007/s00294-019-01037-y. [DOI] [PubMed] [Google Scholar]
- 47.Xie Q., Chen A., Zhang Y., Yuan M., Xie W., Zhang C., Zheng W., Wang Z., Li G., Zhou J. Component Interaction of ESCRT Complexes Is Essential for Endocytosis-Dependent Growth, Reproduction, DON Production and Full Virulence in Fusarium graminearum. Front. Microbiol. 2019;10:180. doi: 10.3389/fmicb.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B., Dong X., Zhao R., Kou R., Zheng X., Zhang H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog. 2019;15:e1007754. doi: 10.1371/journal.ppat.1007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Yun Y., Lou Y., Abubakar Y.S., Guo P., Wang S., Li C., Feng Y., Adnan M., Zhou J., et al. FgAP-2 complex is es-sential for pathogenicity and polarised growth and regulates the apical localisation of membrane lipid flippases in Fusarium graminearum. Cell Microbiol. 2019;21:17. doi: 10.1111/cmi.13041. [DOI] [PubMed] [Google Scholar]
- 50.Zheng D., Zhang S., Zhou X., Wang C., Xiang P., Zheng Q., Xu J.R. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS ONE. 2012;7:14. doi: 10.1371/journal.pone.0049495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C., Guo Z., Zhang M., Chen H., Peng M., Abubakar Y.S., Zheng H., Yun Y., Zheng W., Wang Z., et al. Golgi-localized calcium/manganese transporters FgGdt1 and FgPmr1 regulate fungal development and virulence by maintaining Ca2+ and Mn2+ homeostasis in Fusarium graminearum. Environ. Microbiol. 2022;15:1462–2920. doi: 10.1111/1462-2920.16128. [DOI] [PubMed] [Google Scholar]
- 52.Hou Z., Xue C., Peng Y., Katan T., Kistler H.C., Xu J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 2002;15:1119–1127. doi: 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- 53.Catlett N.L., Lee B.N., Yoder O.C., Turgeon B.G. Split-Marker Recombination for Efficient Targeted Deletion of Fungal Genes. Fungal Genet. Newsl. 2003;50:9–11. doi: 10.4148/1941-4765.1150. [DOI] [Google Scholar]
- 54.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 55.Clontech Laboratories Matchmaker ™ GAL4 Two-Hybrid System 3 & Libraries User Manual. Apr 3, 2007. [(accessed on 30 December 2022)]. Available online: http://www.clontech.com/images/pt/PT3247-1.PDF.
- 56.Yeung B.G., Phan H.L., Payne G.S. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanger A., Hirst J., Davies A.K., Robinson M.S. Adaptor protein complexes and disease at a glance. J. Cell Sci. 2019;132:222992. doi: 10.1242/jcs.222992. [DOI] [PubMed] [Google Scholar]
- 58.Liu C., Li Z., Tian D., Xu M., Pan J., Wu H., Wang C., Otegui M.S. AP1/2β-mediated exocytosis of tapetum-specific transporters is required for pollen development in Arabidopsis thaliana. Plant Cell. 2022;34:3961–3982. doi: 10.1093/plcell/koac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabib E., Roh D.-H., Schmidt M., Crotti L.B., Varma A. The Yeast Cell Wall and Septum as Paradigms of Cell Growth and Morphogenesis. J. Biol. Chem. 2001;276:19679–19682. doi: 10.1074/jbc.R000031200. [DOI] [PubMed] [Google Scholar]
- 60.Gow N.A.R., Latge J.P., Munro C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L., Wang M., Tang G., Ma Z., Shao W. The endocytic cargo adaptor complex is required for cell-wall integrity via interacting with the sensor FgWsc2B in Fusarium graminearum. Curr. Genet. 2019;65:1071–1080. doi: 10.1007/s00294-019-00961-3. [DOI] [PubMed] [Google Scholar]
- 62.Riquelme M., Aguirre J., Bartnicki-García S., Braus G.H., Feldbrügge M., Fleig U., Hansberg W., Herrera-Estrella A., Kämper J., Kück U., et al. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018;82:e00068-17. doi: 10.1128/MMBR.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valdivia R.H., Baggott D., Chuang J.S., Schekman R.W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2002;2:283–294. doi: 10.1016/S1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 64.Heinze L., Freimuth N., Rößling A.K., Hahnke R., Riebschläger S., Fröhlich A., Sampathkumar A., McFarlane H.E., Sauer M. EPSIN1 and MTV1 define functionally overlapping but molecularly distinct trans-Golgi network subdomains in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2020;117:25880–25889. doi: 10.1073/pnas.2004822117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu Y., Takagi J., Ito E., Ito Y., Ebine K., Komatsu Y., Goto Y., Sato M., Toyooka K., Ueda T., et al. Cargo sorting zones in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. Nat. Commun. 2021;12:1901. doi: 10.1038/s41467-021-22267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller S.E., Collins B.M., McCoy A.J., Robinson M.S., Owen D.J. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature. 2007;450:570–574. doi: 10.1038/nature06353. [DOI] [PubMed] [Google Scholar]
- 67.Zheng W., Lin Y., Fang W., Zhao X., Lou Y., Wang G., Zheng H., Liang Q., Abubakar Y.S., Olsson S., et al. The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol. 2018;219:654–671. doi: 10.1111/nph.15178. [DOI] [PubMed] [Google Scholar]
- 68.Zheng H., Li L., Yu Z., Yuan Y., Zheng Q., Xie Q., Li G., Abubakar Y.S., Zhou J., Wang Z., et al. FgSpa2 recruits FgMsb3, a Rab8 GAP, to the polarisome to regulate polarized trafficking, growth and pathogenicity in Fusarium graminearum. New Phytol. 2021;229:1665–1683. doi: 10.1111/nph.16935. [DOI] [PubMed] [Google Scholar]
- 69.Lewis M.J., Nichols B.J., Prescianotto-Baschong C., Riezman H., Pelham H.R. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canagarajah B.J., Ren X., Bonifacino J.S., Hurley J.H. The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci. 2013;22:517–529. doi: 10.1002/pro.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boucrot E., Saffarian S., Zhang R., Kirchhausen T. Roles of AP-2 in clathrin-mediated endocytosis. PLoS ONE. 2010;5:0010597. doi: 10.1371/journal.pone.0010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A Dahhan D., Reynolds G.D., Cárdenas J.J., Eeckhout D., Johnson A., Yperman K., A Kaufmann W., Vang N., Yan X., Hwang I., et al. Proteomic characterization of isolated Arabidopsis clathrin-coated vesicles reveals evolutionarily conserved and plant-specific components. Plant Cell. 2022;34:2150–2173. doi: 10.1093/plcell/koac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carroll S.Y., Stirling P.C., Stimpson H.E., Giesselmann E., Schmitt M.J., Drubin D.G. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev. Cell. 2009;17:552–560. doi: 10.1016/j.devcel.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Proctor R.H., Hohn T.M., McCormick S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995;8:593–601. doi: 10.1094/MPMI-8-0593. [DOI] [PubMed] [Google Scholar]
- 75.Desjardins A.E., Bai G.H., Plattner R.D., Proctor R.H. Analysis of aberrant virulence of Gibberella zeae following transformation-mediated complementation of a trichothecene-deficient (Tri5) mutant. Microbiology. 2000;146:2059–2068. doi: 10.1099/00221287-146-8-2059. [DOI] [PubMed] [Google Scholar]
- 76.Seong K.Y., Pasquali M., Zhou X., Song J., Hilburn K., McCormick S., Dong Y., Xu J.R., Kistler H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009;72:354–367. doi: 10.1111/j.1365-2958.2009.06649.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.