Recent advances in microscopy and protein localization techniques have provided new insights into the remarkable complexity of the bacterial cell. Although bacteria lack discrete cellular compartments such as organelles, they possess an impressive scheme of subcellular organization at the level of protein localization. There are a growing number of examples of bacterial proteins for which specific intracellular localizations are essential for proper function and regulation. Dynamic polar localization of proteins critical for cell division, chromosome partitioning, and cell cycle control in Escherichia coli, Bacillus subtilis, and Caulobacter crescentus have recently been described (see Table 1). These exciting observations establish that bacterial polarity plays a critical cellular role and that prokaryotic organization is much more complex than previously believed.
TABLE 1.
Examples of bacterial proteins that localize to the cell pole
| Organism | Protein | Comments | Reference(s) |
|---|---|---|---|
| Bacillus subtilis | DivIVA | Sequesters MinCD to the poles; found midcell and at poles; also at poles when expressed in E. coli | 20, 21, 58 |
| MinD | Activates MinC; found at poles and midcell | 58, 59 | |
| MinC | Found at poles and midcell; localization depends on MinD | 58 | |
| Soj | ParA-like protein; oscillates from pole to pole | 57, 71 | |
| Spo0J | ParB-like protein that binds to parS sites; found in polar foci | 27, 50 | |
| Caulobacter crescentus | McpA | Methyl-accepting chemotaxis protein; localizes to flagellated pole; swarmer compartment-specific localization dependent upon proteolysis | 3 |
| FliF | Flagellar motor protein; found at swarmer pole and degraded during transition to stalked cell | 40 | |
| DivJ | Histidine kinase; localizes to stalked pole | 103 | |
| PleC | Histidine kinase; localizes to flagellated pole | 103 | |
| FlbE | Histidine kinase; regulates FlbD; midcell and stalked pole of predivisional cell | 104 | |
| Escherichia coli | MCPs | Methyl-accepting chemotaxis protein; maximal polarity depends upon CheA and CheW | 53, 54, 86 |
| CheA | Histidine kinase; maximal polarity requires MCPs and CheW | 54, 86 | |
| CheW | Linker protein; maximal polarity requires MCPs and CheA | 54 | |
| CheY | Response regulator to CheA; maximal polarity requires MCPs and CheA | 88 | |
| CheZ | Phosphatase; maximal polarity requires MCPs and CheA | 88 | |
| MinC | Oscillates between poles, requires MinD and MinE | 36, 72 | |
| MinD | Oscillates between poles, requires MinE | 73 | |
| Listeria monocytogenes | ActA | Actin-nucleating protein; enriched at old pole | 43, 87 |
| Pseudomonas aeruginosa | PilS | Histidine kinase; localized to both cell poles | 8 |
| Shigella flexneri | IcsA | Actin-nucleating protein; focused localization at old pole | 29, 90 |
Clearly, many proteins and protein complexes are able to navigate the bacterial cell and ultimately recognize their appropriate destinations. The current challenge is to uncover the mechanisms, both active and passive, by which proteins are localized and then maintained at the proper intracellular location. The goal of this minireview is to explore a variety of examples of bacterial polarity, to expand upon the current models of polar localization, and to shed light on the spectrum of ways that bacteria may distinguish the polar cellular membrane from the lateral membrane. Several excellent reviews covering recent observations of dynamic polar protein localization have recently been published (7, 37, 38, 41, 76, 82, 83, 92, 93, 102). Here we focus on other aspects of bacterial polarity, including the ultrastructural differences at the cell pole, the modes of polarity in actin-based motility and chemotaxis, and the implications of polarity in bacterial cellular function.
MORPHOLOGICAL DIFFERENCES AT CELL ENDS
The diversity of bacterial shapes extends well beyond the basic sphere, rod, and spirochete forms. Many bacteria are decorated with pili, flagella, and/or stalks, which are often found exclusively at one or both cell poles. The presence of such polar structures reveals that at least some bacteria display a complicated organization scheme, since biogenesis of polar structures clearly demands an asymmetry of their components.
In addition to these external polar structures, early ultrastructural studies revealed internal differences at the cell poles of some bacteria. One striking example is the polar organelle found at the flagellated pole of diverse gram-negative bacteria such as Aquaspirillum (65), Sphaerotilus (96), Rhodopseudomonas (95), Campylobacter (9, 75), and Helicobacter (63). In each case, the polar organelle is subpolarly located near the cytoplasmic membrane adjacent to the flagella (Fig. 1), suggesting a relationship between the polar flagella and the polar organelle. Further supporting this model, Sphaerotilus natans swarm cells have a polar organelle, whereas nonmotile cells do not (34). The polar organelle has not been identified in all polarly flagellated bacteria, although it is possible that more subtle structures take on a similar role. For example, in C. crescentus, 10-nm-diameter polar particles of unknown function have been observed at the flagellated pole of predivisional cells and occasionally observed in the tips of stalks which are derived from a flagellated pole (18). Nonflagellated cells do not have any polar particles at the cell end. Generally speaking, these ultrastructural features may represent regions of specialized activity at the poles and indicate that the cytoplasm is not of uniform composition.
FIG. 1.
Examples of bacterial polarity visualized by electron microscopy. (A) Electron micrograph showing polar staining of E. coli cells (whole mounts) with 1% uranyl acetate; (B to D) thin-section micrographs showing the polar clustering of MCPs (depicted as gold particles and indicated by arrows) in E. coli (B), polar organelle (PO) and flagellum (F) of Campylobacter jejuni (C), and polar attachment of Bdellovibrio bacteriovorus (upper right) to Erwinia carotovora (middle) (D). Micrographs C and D are courtesy of Bob Murray.
A variety of physical differences between the lateral edges of the cell and the cell poles have been observed. For example, new cell poles and sites of cell division (future cell poles) are particularly sensitive to a variety of antibiotics. Low levels of cephaloridine, penicillin, and ampicillin result in preferential spheroplast emergence at the cell poles in nondividing cells and at the center in dividing cells (60, 89). Plasmolysis vacuoles induced by the addition of sucrose are found preferentially at the cell poles in rapidly dividing cells (60). Furthermore, induction of maltose-binding protein, alkaline phosphatase, cyclic phosphodiesterase, or acid hexose monophosphatase results in the formation of large polar distortions called polar caps (15, 101). Newborn cells develop a polar cap at one pole, while elongated cells develop polar caps at two poles, indicating that the old pole is the most sensitive to distortion. Ultrastructurally, polar caps are gaps between the cytoplasm and the cell wall. This polar deformation and subsequent protein enrichment are not fixation or embedding artifacts, as these periplasmic proteins are enriched in E. coli minicells (19). Minicells are composed of two hemispherical polar ends, one derived from a recent cell division and the other derived from an old pole. The induced periplasmic proteins are incorporated into the preexisting polar cap and concentrated in the terminal minicell buds. Taken together, the localized antibiotic sensitivity and selective physiological distortions at the pole imply that the polar regions of the bacterial cell are structurally different from the lateral edges.
ICSA AND ACTA: UNIPOLAR PROTEINS INVOLVED IN ACTIN-BASED MOTILITY
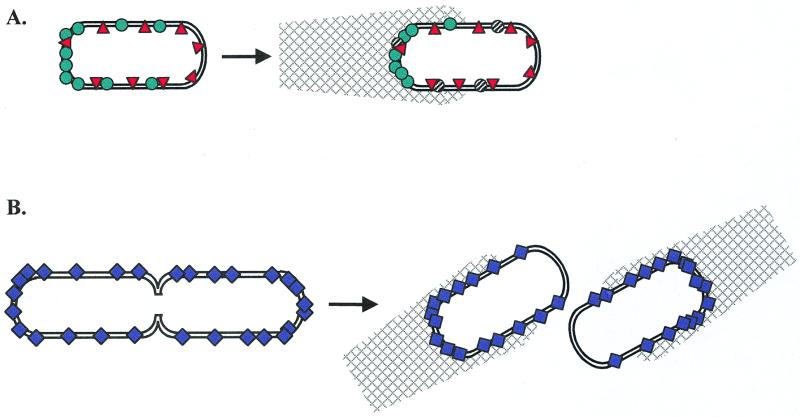
The gram-positive bacterium Listeria monocytogenes and the gram-negative bacterium Shigella flexneri are unrelated facultative intracellular pathogens that have evolved remarkably similar modes of motility dependent on the unipolar polymerization of host actin (reviewed in references 17, 45, 66, 70, and 84). In each bacterial species, a single surface protein, IcsA (VirG) in S. flexneri (5, 47, 55) and ActA in L. monocytogenes (16, 42), is required for asymmetric polymerization of host actin into a “comet tail” structure at one pole of the bacterium. IcsA and ActA are each necessary and sufficient to induce actin assembly and confer actin-based movement in cell extracts (5, 30, 44, 69). This actin-based motility facilitates cell-to-cell spread of the pathogens via bacterial projections delivering the bacteria into adjacent host cells (5, 62, 99). Despite the similar localization patterns of ActA and IcsA, it appears that the mechanisms by which their polar localizations are established are remarkably different (Fig. 2).
FIG. 2.
Comparison of polar determinants in actin-based motility systems of Shigella and Listeria. (A) IcsA (teal circles) initially accumulates at the old pole and then accumulates along the lateral edges. Protease IcsP (red triangles) cleaves and inactivates IcsA (black-and-white hatched circles) uniformly, and the balance of accumulation and proteolytic cleavage results in focused polarity and actin polymerization (mesh). (B) ActA (purple diamonds) is absent from the site of septation, and cell division results in exclusion of ActA from the new pole. Actin polymerization (mesh) at the old pole propels the cell forward.
IcsA is a 120-kDa outer membrane protein encoded on the large virulence plasmid of Shigella spp. and is anchored in the membrane by its C terminus with a 95-kDa amino-terminal domain (α-domain) exposed at the bacterial surface (94). The IcsA protein can be inactivated by proteolytic cleavage of the α-domain by IcsP (SopA), resulting in the release of a 95-kDa fragment (22, 25, 85). In wild-type cells, IcsA is predominantly found at the old cell pole (90), perhaps as a consequence of direct targeting, a mechanism by which the proteins are assembled directly into the polar membrane, rather than diffusing there from a lateral position. IcsA expressed under an inducible promoter in a IcsA− IcsP− background initially localizes to the poles, followed by lateral localization upon further induction (90), indicating that IcsA protein has an inherent tendency to localize to the poles, independent of proteolysis. Surprisingly, it appears that IcsP is capable of cleaving both polar and nonpolar IcsA indiscriminately (90). The combined activity of higher concentration of IcsA at the cell poles and random IcsP proteolysis, however, results in strong bias of active IcsA at the old cell pole.
Remarkably, the polarity of IcsA does not require any of the plasmid-borne virulence genes (80). In fact, when expressed in E. coli, IcsA is found predominantly at one cell pole (44, 80), leading to the formation of comet tails and motility in Xenopus laevis extracts (44). These results confirm that IcsA is sufficient for polymerization of host actin and further demonstrate that polar localization of IcsA is an intrinsic property of the protein which is apparently recognized in E. coli as well as Shigella spp. Finally, these data provide evidence for differences between the outer membrane of the E. coli cell poles and that of the lateral edges. Clearly, IcsA targeting determinants must be able to discern the pole from the lateral edges and furthermore, distinguish the old pole from the new pole.
What are the features of the old cell pole outer membrane that distinguish it from the lateral edges or the new cell pole? Part of the answer may lie in the composition of the lipopolysaccharide (LPS). IcsA polarity is diminished in S. flexneri strains with mutations in LPS biosynthesis genes (79). Perhaps LPS is necessary to guide IcsA to the cell poles. Alternatively, defective LPS may increase the membrane fluidity, permitting lateral diffusion of polarly localized IcsA (78, 79). If this is the case, IcsA and perhaps other polar proteins in general may be maintained at the pole in part by the lower fluidity of the polar membrane.
Whereas IcsA seems to be targeted directly to the old pole of the Shigella cell, ActA polarity in Listeria appears to be established primarily as a consequence of cell division. The 70-kDa ActA protein is anchored to the bacterial membrane at its C-terminal hydrophobic region (46). ActA is concentrated at the pole from which the actin tail extends and is found along the lateral edges but is absent from the new cell pole (43) (Fig 2). How is this asymmetry generated? Upon cell division, ActA remains absent from the newborn pole, resulting in an asymmetric distribution of the protein and associated F-actin (43, 81, 98). Thus, unipolar localization of the ActA protein may involve two distinct mechanisms: exclusion of ActA from the new cell pole and perhaps enrichment of ActA at the old cell pole.
The polar distribution of ActA and the resulting actin comet tail do not require other L. monocytogenes factors. In fact, it seems that all that is required for comet tail formation and motility is that the ActA protein be asymmetrically distributed. Asymmetric attachment of ActA to dead, fixed Listeria cells (97), to nonmotile Streptococcus pneumoniae (87), or to a polystyrene bead (12) all result in effective actin-based motility. Remarkably, heterologous constitutive expression of ActA in nonpathogenic Listeria innocua also displays a polar bias, asymmetric actin assembly, and actin-based motility in Xenopus extracts (44). The mechanisms by which ActA is excluded from the newborn pole are unknown but may involve differences in the physical nature of this pole. One possibility is that the new pole lacks the features necessary for sequestration of ActA; this must be a transient state, however, since the new pole matures into the old cell pole prior to cell division. An alternate possibility is that ActA expression is cell cycle regulated and the absence of protein expression in the predivisional cell generates the asymmetry.
METHYL-ACCEPTING CHEMOTAXIS PROTEINS: TARGETING OF A COMPLEX
C. crescentus is a gram-negative bacterium which undergoes an asymmetric cell division to produce two morphologically and physiologically distinct cell types (10, 38, 39, 68). The motile swarmer cell has a single polar flagellum and polar pili and is slightly smaller than the stalked cell, which is marked by a cylindrical extension of the cell wall. Only the stalked cell can immediately undergo a new round of DNA replication and cell division; the swarmer cell must first shed its flagellum and differentiate into a stalked cell. Stalk biogenesis always takes place at the formerly flagellated pole. The stalked cell elongates, and eventually a flagellum is built at the pole opposite the stalk, so that cell division once again produces both a flagellated cell and a stalked cell. Clearly, flagellar and stalk biosynthesis machinery must be properly targeted and maintained at the correct pole in order for these processes to remain faithful through successive cycles of differentiation and division. A number of signaling proteins that facilitate cell cycle regulation have been identified. Surprisingly, many of these regulatory proteins are targeted to the cell pole in a cell cycle-dependent manner (see references 38, 39, 41, 68, 76, and 102) (Table 1).
The methyl-accepting chemotaxis protein (MCP) McpA is preferentially localized in clusters to the swarmer cell pole (3). McpA is a membrane-bound protein, with a predicted amino-terminal periplasmic ligand-binding domain and a carboxy-terminal cytoplasmic signaling domain, that is expressed just prior to cell division and turned off shortly after septation is complete (2). Proteolysis of McpA in the stalked-cell compartment of the predivisional cell results in segregation of McpA into the swarmer cell compartment (4). The 14 C-terminal amino acids of McpA are required for this degradation, and deletion of these residues results in protein localization to both the swarmer and stalked-cell poles (4). Localization of this truncated McpA protein to the stalked-cell pole indicates that swarmer to stalk cell differentiation does not render the cell pole incompetent to maintain the McpA complexes. Larger deletions of the highly conserved cytoplasmic signaling domain of McpA, however, result in a loss of polarity in the swarmer cell membrane (3), implying that the C terminus of McpA contains the sequence information required for polar localization. Removal of the methylation sites also has no effect on localization, suggesting that McpA methylation state does not dictate its polarity (3).
When expressed in C. crescentus, the E. coli serine receptor Tsr behaved similarly to the truncated McpA, localizing to both the stalked and flagellated pole of the predivisional cell (54). As Tsr is not subject to stalked compartment-specific degradation, the sequences required for proteolysis in McpA must not be present in Tsr (4). The high level of conservation between chemoreceptors of different bacterial species in the C-terminal region responsible for polarity raised the question of whether MCPs could be polar in bacteria with no obvious morphological asymmetries. This was indeed the case: in E. coli, MCP clusters were observed at one or both cell poles (54). MCP polarity also appears to be conserved in several other bacterial species including Rhodobacter sphaeroides, Halobacterium salinarium, Proteus mirabilis, Spirochaeta aurantia, and Vibrio furnissii (26, 33, 100), indicating that polar clustering plays a critical cellular role.
E. coli has one of the most well-studied chemotaxis systems to date (1, 23, 48, 64, 91), and its relative simplicity makes it an excellent model for dissecting the requirements for polar clustering of MCPs. E. coli has four MCPs, two high-abundance (Tsr and Tar) and two low-abundance (Trg and Tap) MCPs. All four MCPs in E. coli are membrane-bound, function as homodimers, and associate with two soluble proteins, the histidine protein kinase CheA and the linker protein CheW. The cytoplasmic proteins CheA and CheW are not associated with the pole in the absence of the MCPs, and MCP polarity is diminished in the absence of CheA and CheW (54, 86). Therefore, optimal clustering of the MCPs requires that they associate with CheA and CheW in ternary complexes. The response regulator CheY and the phosphatase CheZ are also localized to the pole in a CheA- and MCP-dependent fashion, suggesting that they, too, may be associated with the polar ternary complexes (88). Clustering may reflect higher-order interactions between ternary complexes. In fact, higher-order oligomers of ternary complexes have been observed in vitro, with approximately seven MCP dimers per CheA dimer (51). Tar bundles have been shown to dissociate when they are demethylated (51). The role methylation plays in receptor clustering in vivo is unclear. Clustering in vivo is independent of both the methyltransferase CheR and the methylesterase CheB when all four MCPs are present in the cell (52), demonstrating that neither of these adaptation proteins are necessary for clustering. Methylation state, however, affects cooperative interactions among Tsr receptors (49) and to a lesser extent, Tar receptors (6) in vitro. Clearly, the relationship between methylation state and clustering is a critical area for future study.
The precise mechanism of polar localization of MCPs has not yet been elucidated. If the ternary complexes form large oligomers, it is possible that polarity is a passive event due to diffusion limitations of the large “raft” and the curvature of the cell pole. This appears not to be the case, however, as we have shown that the low-abundance transducers when expressed as the only MCP type in the cell are polar but not particularly clustered (53). These data suggest that high levels of ternary complex clustering is not critical for their polarity, and thus, the localization may be by sequestration of complexes to the pole rather than a passive by-product of diffusion limitations.
CELL GROWTH: POLES VERSUS LATERAL EDGES
Peptidoglycan insertion in E. coli appears to be different along the lateral edges and at the poles. It has been proposed that during elongation, the lateral cell wall grows by insertion of new glycan strands between the old glycan strands, whereas during septation, new strands are synthesized and laid down side by side (13; reviewed in reference 35). After septation, the polar cell walls are regions of inactivity compared to the rest of the cell. Pulsing the growth medium with d-cysteine, which is harmless to the cell in low quantity and simple to visualize through immunodetection of reduced thiol groups, revealed that new material is incorporated into the elongating sacculus along the lateral membrane, while the polar regions remain static (14). These localization data suggest that the old pole, in particular, is a stable environment. Perhaps the stability of the old pole provides a mechanism by which proteins, once inserted, may be maintained at the pole.
The age of a cell pole may be critical for some proteins to properly localize. Each new pole will eventually become an old pole, and in an organism with obvious asymmetry, such as C. crescentus, polar maturity is easy to visualize. Following septation, the newborn poles are “bald,” having no external polar structure on the new end and having either a stalk or a flagellum at the other pole. Regardless of whether the cell is stalked or flagellated, the new pole will be the site of pili and flagellum synthesis. Finally, this flagellated pole differentiates into a stalked pole. Stalk biogenesis is the final step in C. crescentus polar maturation; once a stalked pole, always a stalked pole. It is likely that numerous other physical or physiological changes occur at these and other new bacterial poles as they mature into old poles.
The examination of E. coli minicells makes it possible to study characteristics of the poles separately from the rest of the cell. Minicells are capable of murein synthesis (74) but do so more slowly than whole cells (56). The protein compositions of minicells and whole cells differ as well (11, 31, 32, 56, 67). In addition, the polar murein composition of the peptidoglycan layer differs from that of the lateral walls in that it contains a larger percentage of short glycan strands (67). Minicell murein also contains a greater number of diaminopimelic acid cross-linkages (67), a characteristic of aged murein (28).
Subtle differences between the polar and lateral membrane composition or physiology may be critical in determining the blueprint for prokaryotic subcellular organization. Staining with specific fluorescent dyes (24, 61) or uranyl acetate (Fig. 1A) reveal differential staining of poles and septal zones. Since cell poles were once division sites, it is possible that the initial polarity is achieved as a consequence of cell division. Septation results in a nascent cell pole that may differ from other parts of the cell by protein composition, lipid composition, peptidoglycan structure, and growth rate. Any of these features may act as molecular guideposts to direct further polar development. There may also be a specific protein or protein complex acting as a “polar tag,” effectively marking the pole.
Once at the pole, polar proteins must also be maintained there. Proteins may be retained at the poles by the association with specific protein complexes. Alternatively, there may be a physical barrier, such as the periseptal annulus that confines proteins to the polar region (77). Perhaps the stability of the old pole or the sheer inactivity of the polar region provides a mechanism by which proteins, once inserted, are maintained at the pole (14).
CONCLUSIONS
Polar localization in many bacteria is complex. Most cases of polar localization appear to rely on a combination of strategies, and similar localization patterns may result from disparate mechanisms. For example, IcsA and ActA, the proteins critical for comet tail formation and actin-based motility in S. flexneri and L. monocytogenes, respectively, both achieve polar localization but by very different means. Polar localization of IcsA seems to be the combined result of direct targeting to the old pole and nonlocalized degradation by IcsP that causes a greater proportional decrease in nonpolar IcsA because of its lower concentration in the lateral membrane. In contrast, the polar localization of ActA appears to be primarily a consequence of exclusion from the new septal region and thus, the new cell poles, with possible further enrichment by unknown mechanisms. Although these two bacteria have different strategies for achieving polar localization, the end result is essentially the same: the critical player in actin nucleation is properly localized to the cell end to facilitate propulsion of the bacteria through the host cell. C. crescentus and E. coli MCPs slightly differ in their localization strategies as well. Although MCPs of both may reach the pole by a direct targeting method, C. crescentus additionally relies upon proteolysis to limit localization to only one cell type.
What is the function of bacterial polarity? In some cases, the cellular advantage of a polar structure is apparent. For example, polar localization of the flagellum optimizes cell swimming for a uniflagellated cell. Polar localization of the actin-nucleating proteins is essential for actin-based motility and thus, cell-to-cell spread of L. monocytogenes and S. flexneri. Stalk development at a single pole supports unidirectional growth of cells in a biofilm. In the case of C. crescentus, attachment of the stalked-cell end to the surface contributes to the asymmetric character to the biofilm itself and permits release of newborn cells in the direction away from the biofilm and into the aquatic environment. Polar attachment or adhesion of pathogens to the host cell could allow for directed invasion as seen in Bdellovibrio spp. (Fig. 1). The concentration of secretion and adhesion machinery to the cell pole may more efficiently facilitate host invasion. In other cases, the polarity of proteins or complexes is not understood. Is the polarity of the chemoreceptor proteins important for optimal clustering and hence optimal signal transduction? Is the polarity of the C. crescentus histidine kinases CckA and DivJ necessary for effective signaling or simply a means to ensure appropriate compartmentalization? Regardless of the specific roles bacterial polarity plays, it is clear that the cell poles harbor a unique microenvironment distinct from the rest of the cell. Further understanding of the molecular mechanisms of cell polarity will shed light on the overall subcellular complexity of the prokaryotic cell.
REFERENCES
- 1.Aizawa S I, Harwood C S, Kadner R J. Signaling components in bacterial locomotion and sensory reception. J Bacteriol. 2000;182:1459–1471. doi: 10.1128/jb.182.6.1459-1471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alley M R K, Gomes S L, Alexander W, Shapiro L. Genetic analysis of a temporally transcribed chemotaxis gene cluster in Caulobacter crescentus. Genetics. 1991;129:333–341. doi: 10.1093/genetics/129.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alley M R K, Maddock J R, Shapiro L. Polar localization of a bacterial chemoreceptor. Genes Dev. 1992;6:825–836. doi: 10.1101/gad.6.5.825. [DOI] [PubMed] [Google Scholar]
- 4.Alley M R K, Maddock J R, Shapiro L. Requirement of the carboxyl terminus of a bacterial chemoreceptor for its targeted proteolysis. Science. 1993;259:1754–1757. doi: 10.1126/science.8456303. [DOI] [PubMed] [Google Scholar]
- 5.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornhorst J A, Falke J J. Attractant regulation of the aspartate receptor-kinase complex: limited cooperative interactions between receptors and effects of the receptor modification state. Biochemistry. 2000;39:9486–9493. doi: 10.1021/bi0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouche J P, Pichoff S. On the birth and fate of bacterial division sites. Mol Microbiol. 1998;29:19–26. doi: 10.1046/j.1365-2958.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyd J M. Localization of the histidine kinase PilS to the poles of Pseudomonas aeruginosa and identification of a localization domain. Mol Microbiol. 2000;36:153–162. doi: 10.1046/j.1365-2958.2000.01836.x. [DOI] [PubMed] [Google Scholar]
- 9.Brock F M, Murray R G. The ultrastructure and ATPase nature of polar membrane in Campylobacter jejuni. Can J Microbiol. 1988;34:594–604. doi: 10.1139/m88-099. [DOI] [PubMed] [Google Scholar]
- 10.Brun Y V, Marczynski G, Shapiro L. The expression of asymmetry during Caulobacter cell differentiation. Annu Rev Biochem. 1994;63:419–450. doi: 10.1146/annurev.bi.63.070194.002223. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan C E. Topographical distribution of penicillin-binding proteins in the Escherichia coli membrane. J Bacteriol. 1981;145:1293–1298. doi: 10.1128/jb.145.3.1293-1298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron L A, Footer M J, van Oudenaarden A, Theriot J A. Motility of ActA protein-coated microspheres driven by actin polymerization. Proc Natl Acad Sci USA. 1999;96:4908–4913. doi: 10.1073/pnas.96.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jonge B L, Wientjes F B, Jurida I, Driehuis F, Wouters J T, Nanninga N. Peptidoglycan synthesis during the cell cycle of Escherichia coli: composition and mode of insertion. J Bacteriol. 1989;171:5783–5794. doi: 10.1128/jb.171.11.5783-5794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Pedro M A, Quintela J C, Holtje J V, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietzel I, Kolb V, Boos W. Pole cap formation in Escherichia coli following induction of the maltose-binding protein. Arch Microbiol. 1978;118:207–218. doi: 10.1007/BF00415731. [DOI] [PubMed] [Google Scholar]
- 16.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Driks A, Schoenlein P V, DeRosier D J, Shapiro L, Ely B. A Caulobacter gene involved in polar morphogenesis. J Bacteriol. 1990;172:2113–2123. doi: 10.1128/jb.172.4.2113-2123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvorak H F, Wetzel B K, Heppel L A. Biochemical and cytochemical evidence for the polar concentration of periplasmic enzymes in a “minicell” strain of Escherichia coli. J Bacteriol. 1970;104:543–548. doi: 10.1128/jb.104.1.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards D H, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- 21.Edwards D H, Thomaides H B, Errington J. Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J. 2000;19:2719–2727. doi: 10.1093/emboj/19.11.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 23.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fishov I, Woldringh C L. Visualization of membrane domains in Escherichia coli. Mol Microbiol. 1999;32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda I, Suzuki T, Munakata H, Hayashi N, Katayama E, Yoshikawa M, Sasakawa C. Cleavage of Shigella surface protein VirG occurs at a specific site, but the secretion is not essential for intracellular spreading. J Bacteriol. 1995;177:1719–1726. doi: 10.1128/jb.177.7.1719-1726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gestwicki J E, Lamanna A C, Harshey R M, McCarter L L, Kiessling L L, Adler J. Evolutionary conservation of methyl-accepting chemotaxis protein location in Bacteria and Archaea. J Bacteriol. 2000;182:6499–6502. doi: 10.1128/jb.182.22.6499-6502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser P, Sharpe M E, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- 28.Glauner B, Holtje J V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990;265:18988–18996. [PubMed] [Google Scholar]
- 29.Goldberg M B, Barzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shingella flexneri protein involved in intracellular movement. Infect Agents Dis. 1993;2:210–211. [PubMed] [Google Scholar]
- 30.Goldberg M B, Theriot J A. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc Natl Acad Sci USA. 1995;92:6572–6576. doi: 10.1073/pnas.92.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodell E W, Schwarz U. Enzymes synthesizing and hydrolyzing murein in Escherichia coli. Topographical distribution over the cell envelope. Eur J Biochem. 1977;81:205–210. doi: 10.1111/j.1432-1033.1977.tb11942.x. [DOI] [PubMed] [Google Scholar]
- 32.Goodell E W, Schwarz U, Teather R M. Cell envelope composition of Escherichia coli K-12: a comparison of the cell poles and the lateral wall. Eur J Biochem. 1974;47:567–572. doi: 10.1111/j.1432-1033.1974.tb03727.x. [DOI] [PubMed] [Google Scholar]
- 33.Harrison D M, Skidmore J, Armitage J P, Maddock J R. Localization and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol Microbiol. 1999;31:885–892. doi: 10.1046/j.1365-2958.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoeniger J F, Tauschel H D, Stokes J L. The fine structure of Sphaerotilus natans. Can J Microbiol. 1973;19:309–313. doi: 10.1139/m73-051. [DOI] [PubMed] [Google Scholar]
- 35.Holtje J V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34:82–90. doi: 10.1046/j.1365-2958.1999.01575.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs C, Shapiro L. Bacterial cell division: a moveable feast. Proc Natl Acad Sci USA. 1999;96:5891–5893. doi: 10.1073/pnas.96.11.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs C, Shapiro L. Microbial asymmetric cell division: localization of cell fate determinants. Curr Opin Genet Dev. 1998;8:386–391. doi: 10.1016/s0959-437x(98)80107-x. [DOI] [PubMed] [Google Scholar]
- 39.Jenal U. Signal transduction mechanisms in Caulobacter crescentus development and cell cycle control. FEMS Microbiol Rev. 2000;24:177–191. doi: 10.1016/S0168-6445(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 40.Jenal U, Shapiro L. Cell cycle-controlled proteolysis of a flagellar motor protein that is asymmetrically distributed in the Caulobacter predivisional cell. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen R B, Shapiro L. Chromosome segregation during the prokaryotic cell division cycle. Curr Opin Cell Biol. 1999;11:726–731. doi: 10.1016/s0955-0674(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 42.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 43.Kocks C, Hellio R, Gounon P, Ohayon H, Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993;105:699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- 44.Kocks C, Marchand J B, Gouin E, d'Hauteville H, Sansonetti P J, Carlier M F, Cossart P. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol Microbiol. 1995;18:413–423. doi: 10.1111/j.1365-2958.1995.mmi_18030413.x. [DOI] [PubMed] [Google Scholar]
- 45.Lasa I, Cossart P. Actin-based motility: towards a definition of the minimal requirements. Trends Cell Biol. 1996;6:109–113. doi: 10.1016/0962-8924(96)81001-4. [DOI] [PubMed] [Google Scholar]
- 46.Lasa I, David V, Gouin E, Marchand J B, Cossart P. The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes: the central proline-rich region acts as a stimulator. Mol Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- 47.Lett M C, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the VirG protein and determination of the complete coding sequence. J Bacteriol. 1989;171:353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levit M N, Liu Y, Stock J B. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signalling arrays. Mol Microbiol. 1998;30:459–466. doi: 10.1046/j.1365-2958.1998.01066.x. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Weis R M. Covalent modification regulates ligand binding to receptor complexes in the chemosensory system of Escherichia coli. Cell. 2000;100:357–365. doi: 10.1016/s0092-8674(00)80671-6. [DOI] [PubMed] [Google Scholar]
- 50.Lin D C H, Levin P A, Grossman A D. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc Natl Acad Sci USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Levit M, Lurz R, Surette M G, Stock J B. Receptor-mediated protein kinase activation and the mechanism of transmembrane signaling in bacterial chemotaxis. EMBO J. 1997;16:7231–7240. doi: 10.1093/emboj/16.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lybarger S R, Maddock J R. Clustering of the chemoreceptor complex in Escherichia coli is independent of the methyltransferase CheR and the methylesterase CheB. J Bacteriol. 1999;181:5527–5529. doi: 10.1128/jb.181.17.5527-5529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lybarger S R, Maddock J R. Differences in the polar clustering of the high- and low-abundance chemoreceptors of Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8057–8062. doi: 10.1073/pnas.130195397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maddock J R, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 55.Makino S, Sasakawa C, Kamata K, Kurata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 56.Markiewicz Z, Holtje J V. Failure to trigger the autolytic enzymes in minicells of Escherichia coli. FEMS Microbiol Lett. 1992;70:119–123. doi: 10.1016/0378-1097(92)90670-j. [DOI] [PubMed] [Google Scholar]
- 57.Marston A L, Errington J. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol Cell. 1999;4:673–682. doi: 10.1016/s1097-2765(00)80378-0. [DOI] [PubMed] [Google Scholar]
- 58.Marston A L, Errington J. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol. 1999;33:84–96. doi: 10.1046/j.1365-2958.1999.01450.x. [DOI] [PubMed] [Google Scholar]
- 59.Marston A L, Thomaides H B, Edwards D H, Sharpe M E, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 1998;12:3419–3430. doi: 10.1101/gad.12.21.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathys E, Van Gool A. Sensitivity of Escherichia coli to cephaloridine at different growth rates. J Bacteriol. 1979;138:642–646. doi: 10.1128/jb.138.2.642-646.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mileykovskaya E, Dowhan W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J Bacteriol. 2000;182:1172–1175. doi: 10.1128/jb.182.4.1172-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti P J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moura S B, Mendes E N, Queiroz D M, Camargos E R, Evangelina M, Fonseca F, Rocha G A, Nicoli J R. Ultrastructure of Helicobacter trogontum in culture and in the gastrointestinal tract of gnotobiotic mice. J Med Microbiol. 1998;47:513–520. doi: 10.1099/00222615-47-6-513. [DOI] [PubMed] [Google Scholar]
- 64.Mowbray S L, Sandgren M O. Chemotaxis receptors: a progress report on structure and function. J Struct Biol. 1998;124:257–275. doi: 10.1006/jsbi.1998.4043. [DOI] [PubMed] [Google Scholar]
- 65.Murray R G E, Birch-Andersen A. Specialized structure in the region of the flagella tuft in Spirillum serpens. Can J Microbiol. 1963;9:393–401. [Google Scholar]
- 66.Nhieu G T, Sansonetti P J. Mechanism of Shigella entry into epithelial cells. Curr Opin Microbiol. 1999;2:51–55. doi: 10.1016/s1369-5274(99)80009-5. [DOI] [PubMed] [Google Scholar]
- 67.Obermann W, Holtje J V. Alterations of murein structure and of penicillin-binding proteins in minicells from Escherichia coli. Microbiology. 1994;140:79–87. doi: 10.1099/13500872-140-1-79. [DOI] [PubMed] [Google Scholar]
- 68.Osteras M, Jenal U. Regulatory circuits in Caulobacter. Curr Opin Microbiol. 2000;3:171–176. doi: 10.1016/s1369-5274(00)00071-0. [DOI] [PubMed] [Google Scholar]
- 69.Pal T, Newland J W, Tall B D, Formal S B, Hale T L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989;57:477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parsot C. Shigella flexneri: genetics of entry and intercellular dissemination in epithelial cells. Curr Top Microbiol Immunol. 1994;192:217–241. doi: 10.1007/978-3-642-78624-2_10. [DOI] [PubMed] [Google Scholar]
- 71.Quisel J D, Lin D C, Grossman A D. Control of development by altered localization of a transcription factor in B. subtilis. Mol Cell. 1999;4:665–672. doi: 10.1016/s1097-2765(00)80377-9. [DOI] [PubMed] [Google Scholar]
- 72.Raskin D M, de Boer P A. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol. 1999;181:6419–6424. doi: 10.1128/jb.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raskin D M, de Boer P A. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reeve J N. Mucopeptide biosynthesis by minicells of Escherichia coli. J Bacteriol. 1977;131:363–365. doi: 10.1128/jb.131.1.363-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritchie A E, Keeler R F, Bryner J H. Anatomical features of Vibrio fetus: electron microscopic survey. J Gen Microbiol. 1966;43:427–438. doi: 10.1099/00221287-43-3-427. [DOI] [PubMed] [Google Scholar]
- 76.Rothfield L, Justice S, Garcia-Lara J. Bacterial cell division. Annu Rev Genet. 1999;33:423–448. doi: 10.1146/annurev.genet.33.1.423. [DOI] [PubMed] [Google Scholar]
- 77.Rothfield L I, Cook W R. Periseptal annuli: organelles involved in the bacterial cell division process. Microbiol Sci. 1988;5:182–185. [PubMed] [Google Scholar]
- 78.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 79.Sandlin R C, Lampel K A, Keasler S P, Goldberg M B, Stolzer A L, Maurelli A T. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sandlin R C, Maurelli A T. Establishment of unipolar localization of IcsA in Shigella flexneri 2a is not dependent on virulence plasmid determinants. Infect Immun. 1999;67:350–356. doi: 10.1128/iai.67.1.350-356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanger J M, Sanger J W, Southwick F S. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992;60:3609–3619. doi: 10.1128/iai.60.9.3609-3619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shapiro L, Losick R. Dynamic spatial regulation in the bacterial cell. Cell. 2000;100:89–98. doi: 10.1016/s0092-8674(00)81686-4. [DOI] [PubMed] [Google Scholar]
- 83.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science. 1997;276:712–718. doi: 10.1126/science.276.5313.712. [DOI] [PubMed] [Google Scholar]
- 84.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 85.Shere K D, Sallustio S, Manessis A, D'Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 86.Skidmore J M, Ellefson D D, McNamara B P, Couto M M, Wolfe A J, Maddock J R. Polar clustering of the chemoreceptor complex in Escherichia coli occurs in the absence of complete CheA function. J Bacteriol. 2000;182:967–973. doi: 10.1128/jb.182.4.967-973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith G A, Portnoy D A, Theriot J A. Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol Microbiol. 1995;17:945–951. doi: 10.1111/j.1365-2958.1995.mmi_17050945.x. [DOI] [PubMed] [Google Scholar]
- 88.Sourjik V, Berg H C. Localization of components of the chemotaxis machinery of Escherichia coli using fluorescent protein fusions. Mol Microbiol. 2000;37:740–751. doi: 10.1046/j.1365-2958.2000.02044.x. [DOI] [PubMed] [Google Scholar]
- 89.Staugaard P, van den Berg F M, Woldringh C L, Nanninga N. Localization of ampicillin-sensitive sites in Escherichia coli by electron microscopy. J Bacteriol. 1976;127:1376–1381. doi: 10.1128/jb.127.3.1376-1381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steinhauer J, Agha R, Pham T, Varga A W, Goldberg M B. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol Microbiol. 1999;32:367–377. doi: 10.1046/j.1365-2958.1999.01356.x. [DOI] [PubMed] [Google Scholar]
- 91.Stock J, Da Re S. A receptor scaffold mediates stimulus-response coupling in bacterial chemotaxis. Cell Calcium. 1999;26:157–164. doi: 10.1054/ceca.1999.0075. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan S M, Maddock J R. Bacterial division: finding the dividing line. Curr Biol. 2000;10:R249–R252. doi: 10.1016/s0960-9822(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 93.Sullivan S M, Maddock J R. Bacterial sporulation: pole-to-pole protein oscillation. Curr Biol. 2000;10:R159–R161. doi: 10.1016/s0960-9822(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki T, Lett M C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 95.Tauschel H D. ATPase activity of the polar organelle demonstrated by cytochemical reaction in whole unstained cells of Rhodopseudomonas palustris. Arch Microbiol. 1987;148:159–161. [Google Scholar]
- 96.Tauschel H D. ATPase and cytochrome oxidase activities at the polar organelle in swarm cells of Sphaerotilus natans: an ultrastructural study. Arch Microbiol. 1985;141:303–308. doi: 10.1007/BF00428841. [DOI] [PubMed] [Google Scholar]
- 97.Theriot J A, Rosenblatt J, Portnoy D A, Goldschmidt-Clermont P J, Mitchison T J. Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell. 1994;76:505–517. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 98.Tilney L G, DeRosier D J, Tilney M S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992;118:71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wadhams G H, Martin A C, Armitage J P. Identification and localization of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol Microbiol. 2000;36:1222–1233. doi: 10.1046/j.1365-2958.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 101.Wetzel B K, Spicer S S, Dvorak H F, Heppel L A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970;104:529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wheeler R T, Gober J W, Shapiro L. Protein localization during the Caulobacter crescentus cell cycle. Curr Opin Microbiol. 1998;1:636–642. doi: 10.1016/s1369-5274(98)80108-2. [DOI] [PubMed] [Google Scholar]
- 103.Wheeler R T, Shapiro L. Differential localization of two histidine kinases controlling bacterial cell differentiation. Mol Cell. 1999;4:683–694. doi: 10.1016/s1097-2765(00)80379-2. [DOI] [PubMed] [Google Scholar]
- 104.Wingrove J A, Gober J W. Identification of an asymmetrically localized sensor histidine kinase responsible for temporally and spatially regulated transcription. Science. 1996;274:597–601. doi: 10.1126/science.274.5287.597. [DOI] [PubMed] [Google Scholar]