Abstract
The Moorella thermoacetica aromatic O-demethylase was characterized as an inducible three-component system with similarity to the methanogenic methanol, methylamine, and methanethiol methyltransferases and to the O-demethylase system from Acetobacterium dehalogenans. MtvB catalyzes methyl transfer from a phenylmethylether to the cobalt center of MtvC, a corrinoid protein. MtvA catalyzes transmethylation from MtvC to tetrahydrofolate, forming methyltetrahydrofolate. Cobalamin can substitute for MtvC.
Constituting about 25% of the earth's biomass (18), lignin is a polymer composed of hydroxylated and methoxylated phenylpropanoid units linked by C C and C O C bonds that undergoes depolymerization to generate monomeric methoxylated aromatics (or phenylmethylethers) like vanillate and syringate. The anaerobic metabolism of phenylmethylethers (11) has been known since 1979 (13). Although acetogenic bacteria can be selectively isolated by growth on methoxylated aromatics like syringate (2), they do not metabolize the aromatic ring itself but use the O-methyl group as a one-carbon growth substrate (equation 1). Oxidation of one methyl group to CO2 provides the six electrons required for conversion of three methyl groups to acetate. Moorella thermoacetica can metabolize at least 20 different methoxylated aromatics (5). A 22-kDa protein and aromatic O-demethylase activity are induced when M. thermoacetica is exposed to such compounds (6, 37).
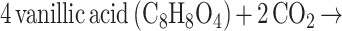 |
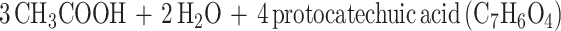 |
1 |
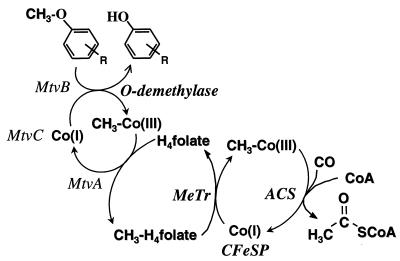
The aromatic O-demethylases of Acetobacterium woodii (3), Acetobacterium dehalogenans (27), and M. thermoacetica (8) require H4folate as the methyl acceptor, producing CH3-H4folate (8, 15, 27). Oxidation of one equivalent of CH3-H4folate to CO2 and H4folate generates six electrons (equation 2) which drive the synthesis of three equivalents of acetyl-coenzyme A (acetyl-CoA) by the Wood-Ljungdahl pathway of acetyl-CoA synthesis (Fig. 1 and reaction 3). This paper describes a three-component aromatic O-demethylase from M. thermoacetica.
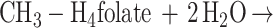 |
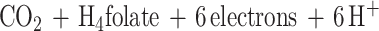 |
2 |
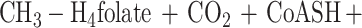 |
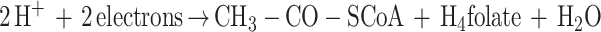 |
3 |
FIG. 1.
Scheme describing the conversion of the aromatic O-methyl group to the methyl group of acetic acid. MtvA, -B, and -C are the components of the O-demethylase system. Abbreviations: CFeSP, the corrinoid iron-sulfur protein; MeTr, the CFeSP methyltransferase; ACS, acetyl-CoA synthase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. thermoacetica was cultivated as described earlier (1) but included a vitamin mixture (36). Dicamba (2 mM) was added at an optical density at 600 nm (OD600) of 0.6. Cells were harvested at an OD600 of 2.0 using a CEPA continuous centrifuge. The cells were frozen in liquid nitrogen and stored at −80°C.
Analytical methods.
Corrinoid concentrations were measured by conversion to the dicyano derivative (23) using the extinction coefficients at 580 and 367 nm of 10.13 × 103 M−1 cm−1 and 30.82 × 103 M−1 cm−1. Metals were determined by plasma emission spectroscopy (14). Protein concentrations were determined by the Rose Bengal method (9). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done by the method of Laemmli (20), and the gels were stained as described by Blum et al. (4).
Electron paramagnetic resonance (EPR) spectra were recorded on a Bruker ESP 300E spectrometer equipped with an Oxford ITC4 temperature controller, a model 5340 automatic frequency counter (Hewlett-Packard), and a Bruker gaussmeter. The molecular mass of MtvC was determined by electrospray ionization mass spectroscopy using a Micromass Platform II instrument (Manchester, United Kingdom). N-terminal sequences were determined by automated N-terminal Edman degradation using an ABI-494 Procise Sequencer after transferring the purified protein (30 nmol) from the SDS-PAGE gel to a polyvinylidene difluoride membrane. The protein band was stained with 0.1% Amido Black, excised, and sequenced.
Enzymatic assays. (i) O-demethylase assay.
O-demethylase activity was measured using 3 mM dicamba or 1 mM vanillate as substrate in a 400-μl reaction mixture at 55°C in the anaerobic chamber. The reaction mixture contained 50 mM 2-(N-morpholino)ethanesulfonate (MES) buffer (pH 6.6), 2 mM ATP, 3 mM tetrahydrofolate, 4 mM titanium (III) citrate, 5 mM MgCl2, 5 mM NaCl, and enzyme components. The assay was quenched after 1 h by adding 0.2 M perchloric acid (final) and being centrifuged at 10,000 × g for 2 min, and substrates and products were quantified by thin-layer chromatography (TLC) and/or reverse-phase high-performance liquid chromatography (HPLC). For TLC analysis, after being quenched and centrifuged, the samples were extracted with diethyl ether (1:1 [vol/vol]), the organic phase was evaporated, and the residue was dissolved in 40 μl of diethyl ether. This solution was then chromatographed on a silica gel AL SIL G/UV (Whatman Ltd.) plate using the organic phase of a toluene-acetic acid-water (6:7:3, vol/vol/vol) mixture as mobile phase. Products were visualized with a UV lamp and by being sprayed with 1% FeCl3. The Rf values of the aromatics under these conditions were as follows: dicamba, 0.7; dichlorosalicylate, 0.54; vanillate, 0.72; catechol, 0.3; and protocatechuate, 0.05. For HPLC, samples were quenched and centrifuged as above, and then the supernatant was neutralized with NaOH and a sample (30 to 50 μl) was injected onto a μ-Bondapak C18 3.5- by 150-mm column (Waters, Milford, Mass.). To assay dicamba metabolism, the column was developed with a linear gradient from 0 to 60% methanol in 50 mM KPi buffer, pH 7.7. The 280-nm absorbance of dicamba and the 420-nm fluorescence emission (excitation wavelength, 310 nm) of dichlorosalicylate were monitored. For quantitation of vanillate and protocatechuate, the column was developed with a linear gradient from 0 to 60% methanol in 125 mM sodium acetate, pH 4.5 (solvent B), and the elution profile was monitored at 254 nm.
(ii) Vanillate:hydroxocobalamin methyl transfer assay (MtvB).
The vanillate-dependent methylation of hydroxocobalamin was performed in the dark at 55°C inside the anaerobic chamber. The reaction mixture contained 0.3 μmol of hydroxocobalamin, 0.4 μmol of vanillate, 0.8 μmol of ATP, 1.6 μmol of titanium (III) citrate, 2 μmol of MgCl2, and 2 μM NaCl in 50 mM MES buffer, pH 6.6 (final volume, 0.4 ml). The reaction was initiated by adding 0.21 nmol of MtvB. The reaction mixture was incubated for 16 h and quenched and processed for TLC analysis as described above.
(iii) Methylcobalamin:tetrahydrofolate methyl transfer assay (MtvA).
The methylcobalamin-dependent methylation of tetrahydrofolate was monitored spectrophotometrically in the dark at 55°C by a modification of a previously described procedure (12). The reaction was carried out in a 0.4-cm-path-length cuvette capped with a rubber stopper to maintain an anaerobic atmosphere. The 0.75-ml reaction mixture contained 15 nmol of methylcobalamin, 112 nmol of tetrahydrofolate, 3.75 nmol of NaCl, and 0.36 nmol of MtvA in 50 mM MES buffer, pH 6.8. The reaction was initiated with H4folate and monitored at 525 and 540 nm. In the absence of MtvA, there was no demethylation of methylcobalamin. The demethylation rate was calculated using an extinction coefficient for methylcobalamin at 540 nm of 7.7 mM−1 cm−1 (19).
(iv) Reduction of the corrinoid protein.
Dithionite stock solutions (∼1.1 M) were prepared in 0.2 M NaOH. The purified corrinoid protein was reduced chemically by adding 1 mM sodium dithionite to an argon-purged quartz cuvette containing 0.12 mg of corrinoid protein per ml in buffer A (25 mM KPi buffer, pH 7.6). The absorption spectrum was recorded 5 min after adding dithionite to the sample.
Purification of the Mtv O-demethylase components. (i) Separation of the Mtv components.
Protein purification was performed at 15°C in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) under an atmosphere of 90% nitrogen and 10% hydrogen. During purification, the O-demethylase activity was routinely determined by TLC analysis. Fifty grams (wet weight) of cells was sonicated in 100 ml of solution containing buffer A, 0.5 mg of lysozyme per ml, 2 mM dithiothreitol, and 2.5% glycerol. The sonicate was centrifuged at 30,000 × g for 90 min in a Type 35 rotor (Beckman Instruments Inc.).
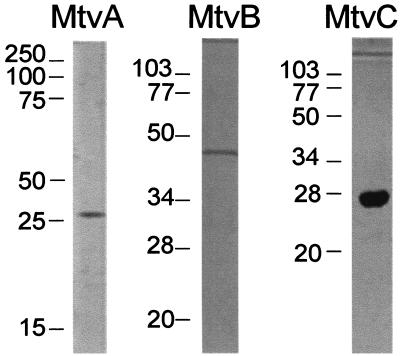
The cell extract was heat treated at 70°C for 20 min in a water bath and centrifuged at 10,000 × g for 15 min. This step gave a modest increase in specific activity but appeared to stabilize the enzyme toward further purification. The supernatant was then subjected to ammonium sulfate fractionation at 4°C. The dicamba O-demethylase activity precipitated at 40 to 70% saturation. After being centrifuged at 10,000 × g for 20 min at 4°C, the 70% pellet was solubilized in 1.2 M ammonium sulfate in buffer A and fractionated by phenyl Sepharose chromatography, which resolved the O-demethylase activity into three components: MtvA, MtvB, and MtvC. To purify each component, the assays contained two components from the phenyl Sepharose fractionation step plus column fractions that were being screened for the third component. The three components were purified to homogeneity (Fig. 2).
FIG. 2.
Polyacrylamide gel electrophoresis of purified MtvA, MtvB, and MtvC. Proteins were visualized by silver staining. The gels contained 3 μg of MtvA, 2 μg of MtvB, and 6 μg of MtvC.
The 40 to 70% ammonium sulfate fraction (above) was applied to a 2.5- by 25-cm phenyl Sepharose CL-6B (Pharmacia LKB) column equilibrated with the same solution. A linear gradient (1,200 ml) from 1.2 M to 0.3 M ammonium sulfate was run. Then, the column was washed with 5 column volumes of buffer A and further washed with 3 mM KPi buffer, pH 7.6. The dicamba O-demethylase activity separated into three components, MtvA, MtvB, and MtvC. MtvC (450 mg/265 ml) eluted at 0.94 M ammonium sulfate, MtvA (436 mg/220 ml) eluted at approximately 0.7 M, and MtvB (484 mg/213 ml) eluted in the final 3 mM KPi buffer wash. Active fractions were pooled and stored at 4°C.
(ii) MtvA purification.
The MtvA fraction from phenyl Sepharose was concentrated to 3 ml by ultrafiltration with a YM10 membrane (Amicon Division, Beverly, Mass.) and applied to a 1.5- by 68-cm Sephacryl S-100 (Amersham Pharmacia Biotech AB) gel filtration column equilibrated with buffer A containing 50 mM KCl. Active MtvA fractions (282 mg/15 ml) were pooled, applied to a 2.5- by 7-cm DEAE-Sephacel (Sigma Chemical Co., St. Louis, Mo.) column that was equilibrated with 50 mM KCl in buffer A and developed with a linear gradient from 50 to 375 mM KCl in buffer A. MtvA eluted at 130 mM KCl. The pooled fractions (12 mg/42 ml) were diluted threefold with buffer A and applied to a 2.5- by 3-cm hydroxyapatite fast-performance liquid chromatography (Calbiochem, La Jolla, Calif.) column. A linear gradient (60 ml) of 5 to 200 mM KPi buffer, pH 7.6, was applied. MtvA eluted at approximately 70 mM KPi. Active fractions were pooled (4.6 mg/10 ml) and stored at 4°C.
(iii) MtvC purification.
The phenyl Sepharose fraction containing MtvC was diluted 10-fold with buffer A containing 125 mM KCl and loaded on a 2.5- by 7-cm DEAE Sephacel column equilibrated with the same buffer. A 150-ml linear gradient from 125 to 350 mM KCl was applied. Fractions with MtvC activity eluted at approximately 270 mM KCl. The active fractions were pooled (245 mg/53 ml) and concentrated to 3 ml using a YM10 membrane. This sample was then applied to a 1.5- by 68-cm Sephacryl S-100 gel filtration column equilibrated with buffer A containing 50 mM KCl. Fractions were collected at a flow rate of 0.2 ml/min. Active MtvC fractions were pooled (8.3 mg/13 ml) and diluted 10-fold with 2 mM KPi buffer, pH 7.6. Active fractions were pooled and applied to a 2.5- by 3-cm hydroxyapatite high-performance column equilibrated with 5 mM KPi buffer, pH 7.6, which was developed with a 100-ml linear gradient from 0 to 100 mM KPi buffer, pH 7.6. MtvC activity eluted at approximately 30 mM KPi. Active MtvC fractions were pooled (3.0 mg/17 ml) and stored at 4°C.
(iv) MtvB purification.
The MtvB pool from the phenyl Sepharose column was loaded on a 2.5- by 7-cm DEAE Sephacel column that had been equilibrated with buffer A. In a linear gradient (400 ml) of 0 to 400 mM KCl, MtvB eluted at 125 mM KCl. The pooled fractions (220 mg/97 ml) were then concentrated with a YM10 ultrafiltration membrane to 3 ml and loaded on a 1.5- by 68-cm Sephacryl S-100 gel filtration column equilibrated with buffer A containing 50 mM KCl. Active fractions were pooled (55 mg/12 ml) and diluted 10-fold with 20 mM Tris-HCl buffer, pH 7.6. This sample was loaded on a 2.5- by 3-cm hydroxyapatite column equilibrated with the same buffer, and a 150-ml linear gradient from 0 to 200 mM KPi buffer, pH 7.6, was applied. Active MtvB fractions eluting at 90 mM KPi were pooled (3.6 mg/12 ml) and stored at 4°C.
RESULTS AND DISCUSSION
Dicamba demethylation by M. thermoacetica.
M. thermoacetica cells were grown anaerobically with glucose and dicamba to yield approximately 55 g (wet weight) of cells. In the absence of glucose, the cells grew slowly, with a low yield. Glucose did not appear to repress the O-demethylase activity. Dicamba conversion to dichlorosalicylate was monitored by TLC and HPLC, and cells were harvested anaerobically when ∼85% of the dicamba was metabolized.
Besides metabolizing dicamba, extracts from cells grown on dicamba could demethylate vanillate to protocatechuate and further decarboxylate protocatechuate to catechol under standard assay conditions. Similarly, extracts of cells grown in the presence of vanillate could demethylate dicamba to dichlorosalicylate as well as convert vanillate to protocatechuate and catechol (not shown). Daniel et al. also found that M. thermoacetica cells grown on different methoxylated aromatic compounds catalyze the O-demethylation of the same particular methoxylated compound, suggesting the presence of a broad specificity of O-demethylase (6). As shown below, the enzyme from dicamba-grown cells utilizes vanillate much more efficiently than dicamba. To conform to the nomenclature used by Krzycki and Thauer to describe the components of the methanogenic methyltransferases involved in demethylation of methylamines, methylsulfides, and pterins (reviewed in reference 32), we designate this as the Mtv system, i.e., methyltransferase for vanillate. The reaction catalyzed by the Mtv system of M. thermoacetica is most similar to that of the four-component system from A. dehalogenans (17).
Enzymatic properties of the O-demethylase. (i) Overall reaction requirement of MtvA, MtvB, and MtvC.
The three components required for O-demethylation of dicamba and vanillate were resolved and purified to homogeneity. Omission of MtvA, -B, or -C from the O-demethylase reaction mixture results in complete loss of O-demethylase activity; thus, all three protein components are required for dicamba O-demethylase activity. Cell extracts that were bubbled with air for 0.5 h and then with nitrogen for 15 min (so that O2 was not introduced into the assay mixture) retained full O-demethylase activity. Therefore, the reaction, which involves Co(I), is O2 sensitive, not the enzyme. The demethylation reaction requires a reductant. Although dithionite can reduce Co(II) to Co(I), it inhibits the O-demethylase. On the other hand, Ti(III) citrate reduces Co(II) and drives the O-demethylase reaction. The optimal temperature is 55°C and the optimal pH is 6.6. As described above, H4folate is required as the acceptor of the methyl group from vanillate. The reaction rate is linear for up to 2 h at 55°C.
(ii) Steady-state kinetics.
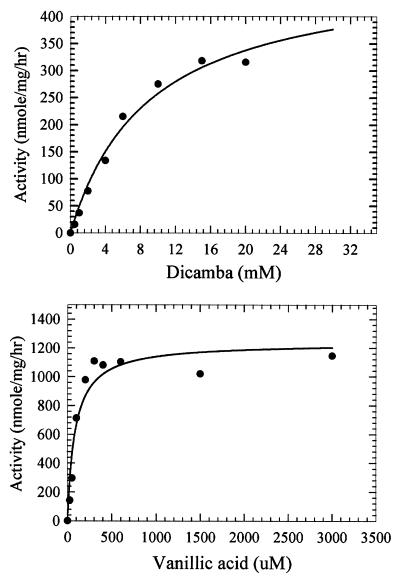
The apparent Km values for dicamba and vanillate are 9.0 mM and 85 μM, respectively, and the Vmax values with dicamba and vanillate as the methyl donors are 276 and 900 nmol mg−1 h−1, respectively (Fig. 3). The 350-fold (based on relative V/K values) preference for vanillate is striking, since the cells were grown on dicamba. These results prompted us to name the enzyme vanillate, instead of dicamba, O-demethylase. The Km value for H4folate is 0.23 mM.
FIG. 3.
Dependence of the O-demethylase reaction on dicamba and vanillate. The assay contained MtvA (3.8 μg), MtvC (2.24 μg), and MtvB (9.6 μg) in a reaction volume of 0.1 ml. The reactions were performed for 1 h under standard assay conditions, and the products formed were analyzed by reverse-phase HPLC. See Materials and Methods for details.
As has been found for some other methyltransferases, hydroxocobalamin or methylcobalamin could substitute for MtvC, which allowed dissection of the roles of the individual components of the Mtv system. In the first partial reaction, MtvB is the only protein required to catalyze the vanillate- or dicamba-dependent methylation of cob(I)alamin; in the second partial reaction, MtvA is the only enzyme required for the methylcobalamin-dependent methylation of H4folate.
MtrB, MtvB, a vanillate:cobalamin methyltransferase.
The function of MtvB was established by studying an O-demethylase partial reaction:transfer of the O-methyl group of vanillate to the Co(I) state of B12 to form methylcobalamin. MtvA and H4folate were not required for the demethylation of vanillate; B12 was absolutely required (in the absence of MtvC). TLC analysis of the partial reaction showed protocatechuic acid formation as the concentration of vanillate decreased, demonstrating that MtvB participates in the first part of the overall O-demethylase reaction, transferring the methyl group from the methoxylated aromatic substrate to cobalamin. The enzymatic function of MtvB is similar to that of the 36-kDa component B from A. dehalogenans (15, 17).
Purified MtvB is a homodimer with an apparent monomeric molecular mass of 48 kDa. Like MtvA, MtvB is colorless and exhibits the typical UV-visible spectrum of a protein lacking chromophoric prosthetic groups. Metals, including zinc and iron, were found in less than stoichiometric amounts (<0.1 g-atm/mol). To assess the possibility that this is a Zn protein, we determined the effect of Zn addition on the demethylation of vanillate. Even at concentrations as high as 4 mM, there was no stimulation of activity. Either this is not a zinc protein or reconstitution requires different conditions. In methionine synthase and the methyltransferases that use coenzyme M, zinc is involved in coordinating and thus activating the thiol substrate (reviewed in references 25 and 32). Zinc is also found in the methyltransferases involved in the transfer of the methyl group of methanol to cobalamin (MtaB) (31).
MtvC, a corrinoid protein.
Homogeneous preparations of MtvC contain a single subunit with an apparent molecular mass of 27 kDa based on SDS analysis and 22 kDa based on mass spectrophotometric analysis. Presumably, this is the 22-kDa protein identified by Daniel et al. to be induced when M. thermoacetica cells are exposed to syringate (6). Functionally, this enzyme is most similar to the 26-kDa corrinoid protein of A. dehalogenans called component A (15, 17). A. dehalogenans also contains a fourth component (called component C), which is an activating protein that couples the ATP-dependent reduction of Co(II) to Co(I) (17). The M. thermoacetica O-demethylase system was assayed using an artificial electron donor [Ti(III) citrate]; as was found with A. dehalogenans, with this assay the reductive activation system was not required. The N-terminal sequence of the protein, MLTDTL(S)KAMAELEEEQ(V)LA, is not significantly similar to the sequence of the A. dehalogenans corrinoid protein (component A) (16).
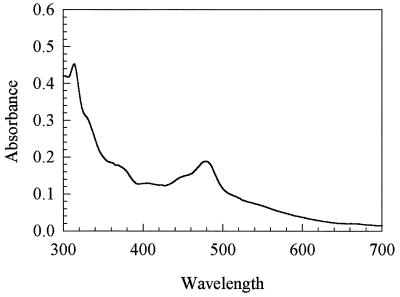
The UV-visible spectrum (Fig. 4) of the reddish brown as-isolated protein is similar to that of Co(II) corrinoids, with a major absorption peak at 474 nm (22). MtvC was calculated to contain 0.8 mol of cobalamin per mol of protein. Cobalt is the only metal found in stoichiometric amounts. There were variable amounts of zinc and iron at levels of <0.2 g-atm per mol of protein.
FIG. 4.
UV-visible spectra of purified MtvC (0.45 mg/ml).
Reduction of MtvC with 1 mM Ti(III) citrate or dithionite bleaches the 474-nm peak of cob(II)alamin as an absorption peak forms at 388 nm, which is characteristic of cob(I)alamin. However, the Co(I) state of MtvC, when generated with dithionite, was unable to demethylate dicamba or vanillate in the presence of MtvA or MtvB. Dithionite also inhibited the demethylation reaction in the standard assay. Therefore, Ti(III) citrate (4 mM) concentration was routinely used in the demethylation assays.
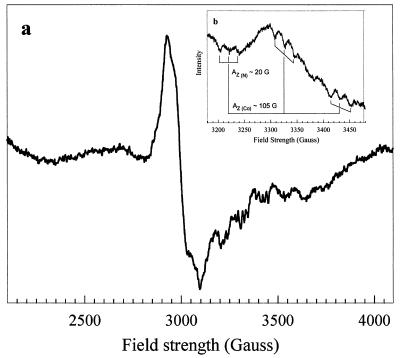
The EPR spectrum of purified as-isolated MtvC (Fig. 5) is characteristic of a Co(II) corrinoid (29). The g∥ resonance is split into eight lines by hyperfine interactions between the unpaired electrons and the cobalt nucleus, which has a nuclear spin, I, of 7/2. The strength of the interaction is described by the hyperfine coupling constant, AZ (Co) ≈ 105 G. Each of these hyperfine lines is split into a triplet superhyperfine splitting pattern [AZ (N) ≈ 20 G] (Fig. 5, inset) that is characteristic of corrinoid proteins containing a coordinated axial nitrogen ligand. This ligand could be a histidine residue from the protein, as in methionine synthase, methanogenic corrinoid proteins involved in methyl transfer, and some adenosylcobalamin enzymes (reviewed in reference 24). Proteins that have the histidine ligand generally contain a conserved motif, DxHxG-(41-42)-gxSxL-(21/22)-GG (24), although there are exceptions (32). Component A of the A. dehalogenans O-demethylase contains this “his” binding motif (16). Alternatively, the ligand could be from the benzimidazole group appended to the corrin ring, as in ribonucleotide reductase (21) and diol dehydrase (35).
FIG. 5.
EPR spectra of the Co(II) in as-isolated MtvC. The spectrum of 90 μM MtvC in 25 mM KPi buffer, pH 7.6, was recorded using the following EPR conditions: temperature, 100 K; gain, 104; power, 100 mW; modulation amplitude, 10 G; microwave frequency, 9.47 GHz; scans, 16. The coupling constants calculated were Az(Co) = 105 G and Az(N) = 20 G. The inset was recorded under identical conditions, except that the temperature was 85 K.
Properties of MtvA, a methylcobalamin: H4folate methyltransferase.
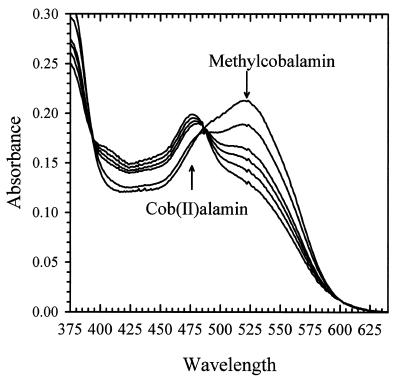
MtvA was found to catalyze the H4folate-dependent demethylation of methyl-cob(III)alamin, which was followed photometrically at 525 nm (Fig. 6). The 525-nm peak decreased as a peak at 475 nm formed, which is characteristic of cob(II)alamin. Based on the published extinction coefficient for methylcobalamin (ɛ540 = 7.70 mM−1 cm−1 [19]), the rate of demethylation is 72 nmol min−1 (mg of MtvA)−1. Under the conditions of the assay, which lacked reductant, cob(I)alamin is rapidly oxidized to cob(II)alamin. MtvA showed no activity in the vanillate- or dicamba-dependent methylation of cob(I)alamin.
FIG. 6.
Methylcobalamin-tetrahydrofolate methyl transfer catalyzed by MtvA. The reaction was performed at 55°C under anaerobic conditions as described in Materials and Methods. Spectral changes occurring during the methyl group transfer from methylcobalamin (20 μM) to H4folate (140 μM) were recorded at 0, 5, 10, 20, 40, and 60 min after addition of H4folate.
Functionally, MtvA is similar to the A. dehalogenans component D (15, 17). The reaction of MtvA (equation 4) is also analogous to that of AcsE (equation 5), the CH3-H4folate- and corrinoid-dependent methyltransferase involved in transferring the methyl group of the CH3-H4folate to the corrinoid iron-sulfur protein in the Wood-Ljungdahl pathway (33, 34). However, MtvA is unable to substitute for AcsE, and AcsE cannot substitute for MtvA. Similarly, in methanogens, the cognate corrinoid proteins and the methyltransferases involved in dimethylsulfide, trimethylamine, dimethylamine, and monomethylamine metabolism (28) are discrete gene products that cannot substitute for each other (10, 26, 28).
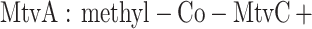 |
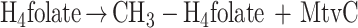 |
4 |
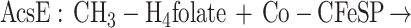 |
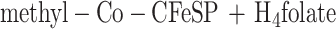 |
5 |
Based on SDS-PAGE analysis, MtvA is a 29.5-kDa protein. Similarly, component D from A. dehalogenans and AcsE from M. thermoacetica have monomeric molecular masses of 30 kDa (15, 17) and 28.6 kDa (30), respectively. The UV-visible absorption spectrum of MtvA does not reveal any chromophores other than the amino acids. AcsE also lacks prosthetic groups (30).
The N-terminal sequence of MtvA is highly similar to that of the CH3-H4folate- and cobalamin-dependent methyltransferase from M. thermoacetica (AcsE) (Fig. 7), which folds into a TIM barrel structure containing the pterin binding site within the barrel (7). Given the strong sequence homology among the N termini of MtvA, AcsE, and component D, perhaps the O-demethylases adopt a similar fold.
FIG. 7.
Comparison of N-terminal sequences of MtvA and AcsE (the corrinoid iron-sulfur protein methyltransferase) from M. thermoacetica and component D from A. dehalogenans.
REFERENCES
- 1.Andreesen J R, Schaupp A, Neurater C, Brown A, Ljungdahl L G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO2. J Bacteriol. 1973;114:743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bache R, Pfennig N. Selective isolation of Acetobacterium woodiion methoxylated aromatic acids and determination of growth yields. Arch Microbiol. 1981;130:255–261. [Google Scholar]
- 3.Berman M H, Frazer A C. Importance of tetrahydrofolate and ATP in the anaerobic O-demethylation reaction for phenylmethylethers. Appl Environ Microbiol. 1992;58:925–931. doi: 10.1128/aem.58.3.925-931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum H, Meier H, Gross H J. Improved silver staining method of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–98. [Google Scholar]
- 5.Daniel S L, Keith E S, Yang H, Lin Y S, Drake H L. Utilization of methoxylated aromatic compounds by the acetogen Clostridium thermoaceticum: expression and specificity of the co-dependent O-demethylating activity. Biochem Biophys Res Commun. 1991;180:416–422. doi: 10.1016/s0006-291x(05)81309-9. [DOI] [PubMed] [Google Scholar]
- 6.Daniel S L, Wu Z, Drake H L. Growth of thermophilic acetogenic bacteria on methoxylated aromatic acids. FEMS Microbiol Lett. 1988;52:25–28. [Google Scholar]
- 7.Doukov T, Seravalli J, Stezowski J, Ragsdale S W. Crystal structure of a methyltetrahydrofolate and corrinoid dependent methyltransferase. Structure. 2000;8:817–830. doi: 10.1016/s0969-2126(00)00172-6. [DOI] [PubMed] [Google Scholar]
- 8.El Kasmi A, Rajasekharan S, Ragsdale S W. Anaerobic pathway for conversion of the methyl group of aromatic methyl ethers to acetic acid by Clostridium thermoaceticum. Biochemistry. 1994;33:11217–11224. doi: 10.1021/bi00203a018. [DOI] [PubMed] [Google Scholar]
- 9.Elliott J I, Brewer J M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978;190:351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson D J, Krzycki J A, Grahame D A. Specific roles of methylcobamide:coenzyme M methyltransferase isozymes in metabolism of methanol and methylamines in Methanosarcina barkeri. J Biol Chem. 1996;271:5189–5194. doi: 10.1074/jbc.271.9.5189. [DOI] [PubMed] [Google Scholar]
- 11.Frazer A C. O-demethylation and other transformations of aromatic compounds by acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 445–483. [Google Scholar]
- 12.Goulding C W, Postigo D, Matthews R G. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997;36:8082–8091. doi: 10.1021/bi9705164. [DOI] [PubMed] [Google Scholar]
- 13.Healy J B, Jr, Young L Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979;38:84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones J B., Jr Elemental analysis of soil extracts and plant tissue by plasma emission spectroscopy. Commun Soil Sci Plant Anal. 1977;8:349–365. [Google Scholar]
- 15.Kaufmann F, Wohlfarth G, Diekert G. Isolation of O-demethylase, an ether-cleaving enzyme system of the homoacetogenic strain MC. Arch Microbiol. 1997;168:136–142. doi: 10.1007/s002030050479. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann F, Wohlfarth G, Diekert G. O-demethylase from Acetobacterium dehalogenans—cloning, sequencing, and active expression of the gene encoding the corrinoid protein. Eur J Biochem. 1998;257:515–521. doi: 10.1046/j.1432-1327.1998.2570515.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann F, Wohlfarth G, Diekert G. O-demethylase from Acetobacterium dehalogenans—substrate specificity and function of the participating proteins Eur. J Biochem. 1998;253:706–711. doi: 10.1046/j.1432-1327.1998.2530706.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 19.Kreft J U, Schink B. O-demethylation by the homoacetogenic anaerobe Holophaga foetida studied by a new photometric methylation assay using electrochemically produced cob(I)alamin. Eur J Biochem. 1994;226:945–951. doi: 10.1111/j.1432-1033.1994.00945.x. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence C C, Gerfen G J, Samano V, Nitsche R, Robins M J, Retey J, Stubbe J. Binding of Cob(II)alamin to the adenosylcobalamin-dependent ribonucleotide reductase from Lactobacillus leichmannii. Identification of dimethylbenzimidazole as the axial ligand. J Biol Chem. 1999;274:7039–7042. doi: 10.1074/jbc.274.11.7039. [DOI] [PubMed] [Google Scholar]
- 22.Lexa D, Savéant J-M, Zickler J. Electrochemistry of Vitamin B12. 2. Redox and acid-base equilibria in the B12a/B12rsystem. J Am Chem Soc. 1977;99:2786. doi: 10.1021/ja00450a061. [DOI] [PubMed] [Google Scholar]
- 23.Ljungdahl L G, LeGall J, Lee J-P. Isolation of a protein containing tightly bound 5-methoxybenzimidazolylcobamide (Factor IIIm) from Clostridium thermoaceticum. Biochemistry. 1973;12:1802–1808. doi: 10.1021/bi00733a022. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig M L, Matthews R G. Structure-based perspectives on B-12-dependent enzymes. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 25.Matthews R G, Goulding C W. Enzyme-catalyzed methyl transfers to thiols: the role of zinc. Curr Opin Chem Biol. 1997;1:332–339. doi: 10.1016/s1367-5931(97)80070-1. [DOI] [PubMed] [Google Scholar]
- 26.Maupin-Furlow J, Ferry J G. Characterization of the cdhD and cdhE genes encoding subunits of the corrinoid/iron-sulfur enzyme of the CO dehydrogenase complex from Methanosarcina thermophila. J Bacteriol. 1996;178:340–346. doi: 10.1128/jb.178.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meßmer M, Reinhardt S, Wohlfarth G, Diekert G. Studies on methyl chloride dehalogenase and O-demethylase in cell extracts of the homoacetogen strain MC based on a newly developed coupled enzyme assay. Arch Microbiol. 1996;165:18–25. [Google Scholar]
- 28.Paul L, Ferguson D J, Krzycki J A. The trimethylamine methyltransferase gene and multiple dimethylamine methyltransferase genes of Methanosarcina barkericontain in-frame and read-through amber codons. J Bacteriol. 2000;182:2520–2529. doi: 10.1128/jb.182.9.2520-2529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilbrow J R. EPR of B12-dependent enzyme reactions and related systems. In: Dolphin D, editor. Vitamin B12. Vol. 1. New York, N.Y: John Wiley & Sons; 1982. pp. 431–462. [Google Scholar]
- 30.Roberts D L, Zhao S, Doukov T, Ragsdale S W. The reductive acetyl coenzyme A pathway: sequence and heterologous expression of active methyltetrahydrofolate:corrinoid/iron-sulfur protein methyltransferase from Clostridium thermoaceticum. J Bacteriol. 1994;176:6127–6130. doi: 10.1128/jb.176.19.6127-6130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauer K, Thauer R K. Methanol:coenzyme M methyltransferase from Methanosarcina barkeri—Zinc dependence and thermodynamics of the methanol:cob(I)alamin methyltransferase reaction. Eur J Biochem. 1997;249:280–285. doi: 10.1111/j.1432-1033.1997.t01-1-00280.x. [DOI] [PubMed] [Google Scholar]
- 32.Sauer K, Thauer R K. The role of corrinoids in methanogenesis. In: Banerjee R, editor. Chemistry and biochemistry of B12. Vol. 1. New York, N.Y: John Wiley & Sons; 1999. pp. 655–680. [Google Scholar]
- 33.Seravalli J, Shoemaker R K, Sudbeck M J, Ragsdale S W. Binding of (6R,S)-methyltetrahydrofolate to methyltransferase from Clostridium thermoaceticum: role of protonation of methyltetrahydrofolate in the mechanism of methyl transfer. Biochemistry. 1999;38:5736–5745. doi: 10.1021/bi9824745. [DOI] [PubMed] [Google Scholar]
- 34.Seravalli J, Zhao S, Ragsdale S W. Mechanism of transfer of the methyl group from (6S)-methyltetrahydrofolate to the corrinoid/iron-sulfur protein catalyzed by the methyltransferase from Clostridium thermoaceticum: a key step in the Wood-Ljungdahl pathway of acetyl-CoA synthesis. Biochemistry. 1999;38:5728–5735. doi: 10.1021/bi982473c. [DOI] [PubMed] [Google Scholar]
- 35.Shibata N, Masuda J, Tobimatsu T, Toraya T, Suto K, Morimoto Y, Yasuoka N. A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase. Struct Fold Des. 1999;7:997–1008. doi: 10.1016/s0969-2126(99)80126-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z R, Daniel S L, Drake H L. Characterization of a CO-dependent O-demethylating enzyme system from the acetogen Clostridium thermoaceticum. J Bacteriol. 1988;170:5747–5750. doi: 10.1128/jb.170.12.5747-5750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]