Abstract
Over the past decades, both 4′-modified nucleoside and carbocyclic nucleoside analogs have been under the spotlight as several compounds from either family showed anti-HIV, HCV, RSV or SARS-CoV-2 activity. Herein, we designed compounds combining these two features and report the synthesis of a series of novel 4′-substituted carbocyclic uracil derivatives along with their corresponding monophosphate prodrugs. These compounds were successfully prepared in 19 to 22 steps from the commercially available (-)-Vince lactam and were evaluated against a panel of RNA viruses including SARS-CoV-2, influenza A/B viruses and norovirus.
Keywords: antivirals, prodrugs, carbocyclic nucleosides, viral polymerase, SARS-CoV-2, influenza virus, norovirus
1. Introduction
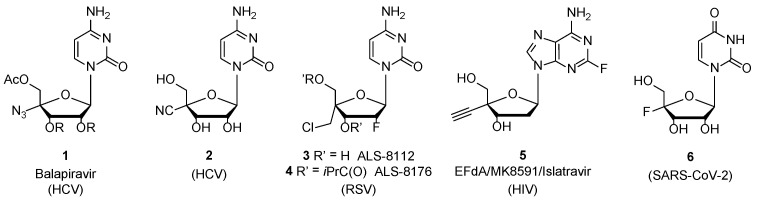
Nucleoside analogs targeting viral polymerases are the cornerstone of current antiviral therapies with numerous compounds approved for the treatment of infectious diseases such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis B virus (HBV), herpes simplex virus (HSV) or SARS-CoV-2 [1,2]. The chemistry around each position of the sugar moiety of natural ribo- or 2′-deoxyribonucleosides has been explored extensively, but more recently, synthetically more challenging 4′-modifications have received a great deal of attention [3]. For instance, 4′-azidocytidine or its methyl ester prodrug Balapiravir (1) along with its 4′-cyano analogs (2) [4], have been reported in their 5′-triphosphate form as HCV polymerase inhibitors [5]. Additionally, 4′-chloromethyl-2′-deoxy-2′-fluoro-cytidine (3) (ALS-8812) as its isopropyl ester prodrug (ALS-8176) (4) were, until recently, studied in a phase II clinical trial as a new treatment for respiratory syncytial virus (RSV) [6,7].
4′-Ethynyl-2′-deoxy-2-fluoro-adenosine (5) (EFdA/MK8591/Islatravir), the most potent in vitro anti-HIV nucleoside analog inhibitor known to date [8], is being clinically evaluated as a subdermal implant for HIV treatment and prophylaxis [9,10,11]. More recently, 4′-fluoro-uridine (6) has been reported as a potent inhibitor of RSV and SARS-CoV-2 replication [12,13] (Figure 1). Even though a number of 4′-modified ribo- or deoxyribo nucleosides have been synthesized, their carbanucleoside versions, in which the oxygen (O) in the sugar ring has been replaced by a carbon (CH2), represent an understudied family of nucleoside analogs. Several examples [14,15,16], including approved drugs entecavir and abacavir, show that this type of modification does not impact the cellular processing of the nucleoside while making compounds more resistant to nucleoside phosphorylase responsible of the N-glycosidic bond cleavage [17]. Indeed, 4′-methyl analogs (7) [18] were shown to display weak activities against a panel of RNA viruses including yellow fever (YF), dengue virus (DENV), venezuelan equine encephalis virus (VEE) and west nile virus (WNV), while 4′-ethynyl and 4′-cyano carbocyclic-2′-deoxyribonucleoside analogs (8a) and (8b) (Figure 2) displayed anti-HIV-1 activity [19] (Figure 2).
Figure 1.
Examples of 4′-substituted nucleoside analogs displaying antiviral activity.
Figure 2.
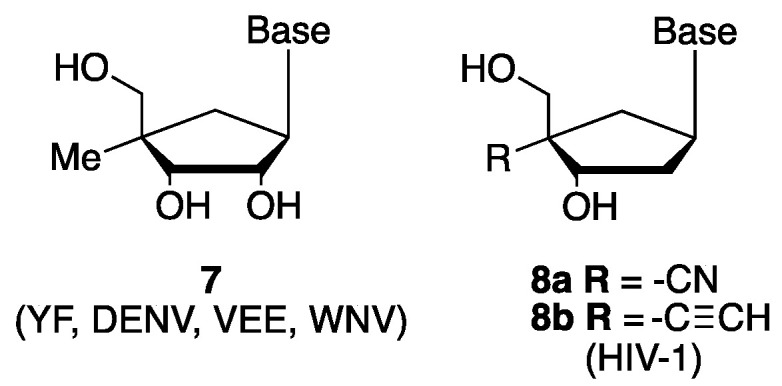
Examples of 4′-substituted carbocyclic ribo- and 2′-deoxyribo nucleoside analogs displaying antiviral activity.
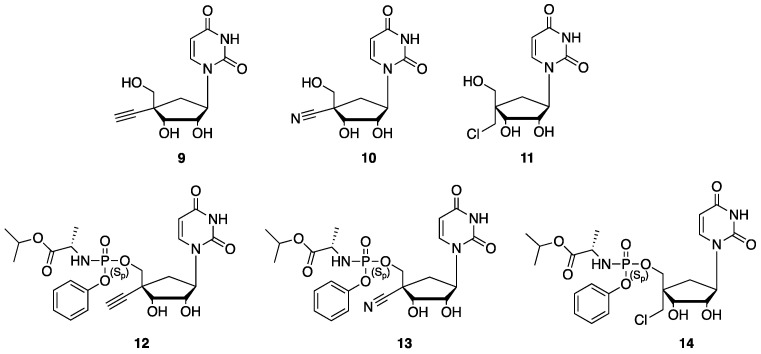
Based on these precedents, we wish to report herein the synthesis of novel 4′-substituted carbocyclic uridine analogs (9–11) and their corresponding monophosphate prodrugs (12–14) and report their evaluation against a small panel of RNA viruses including Norovirus, Influenza A, Influenza B viruses and SARS-CoV-2 (Figure 3).
Figure 3.
Targeted nucleosides (9–11) and their corresponding monophosphate prodrugs (12–14).
2. Material and Methods
2.1. Antiviral Assays
2.1.1. SARS-CoV-2 Antiviral Assays
The anti-SARS-CoV-2 activity of compounds herein prepared was evaluated at 10 μM following previously reported methods [20]. Briefly, a monolayer of Vero cells in a 96-well cell culture microplate was treated with 10 μM of each compound for 1 h followed by infection with SARS-CoV-2 (Washington Strain) at 0.1 MOI. After 1 h adsorption at 37 °C, the virus inoculum was removed, and the compound or vehicle-containing medium was added to the respected wells. Resultant virus progeny yield was measured 2 days post-treatment from the supernatant of treated infected cells by specific quantitative RT-PCR.
2.1.2. Norovirus Antiviral Assays
The anti-NoV activity of compounds herein prepared was evaluated at 10 μM following previously reported methods [21,22]. Briefly, HG23 replicon cells, kindly provided by Kyeong-Ok Chang, Kansas State University (Manhattan, KS, USA), were seeded at a density of 1.6 × 104 cells/well in 96-well plates and incubated at 37 °C and 5% CO2 overnight. Compounds were tested at 10 µM. Compounds were added in triplicate to 80–90% confluent monolayers and incubated at 37 °C and 5% CO2. Untreated cells were incubated in each plate. At 24, 48, 72 and 96 h post-treatment, total RNA was extracted using the Mag-Max Total RNA Isolation kit (Ambion, Austin, TX, USA) and NV replicon RNA was quantified by GI NoV Taqman real-time RT-PCR (NoV RT-qPCR). Protein expression levels were monitored by western blot analysis.
2.1.3. Influenza A/B Antiviral Assays
The anti- Influenza A/B activity of compounds herein prepared was evaluated at 40 μM following previously reported methods [23]. Briefly, A549 cells were seeded at a density of 35,000 cells/well and incubated at 37 °C and 5% CO2 overnight. Cells were treated with test compound at 40 µM, then incubated at 37 °C and 5% CO2 for 1 h before being inoculated with 0.1 MOI (PR8-PB2-Gluc) or 1.0 MOI (Ya88-PB1-NanoLuc).
2.2. Cytotoxicity Assays
The cytotoxicity of the compounds was evaluated using previously reported methods assays [24]. Briefly, in vitro cytotoxicity was determined using the CellTiter 96 non-radioactive cell proliferation colorimetric assay (MTT assay, Promega, Madison, WI, USA) in primary human peripheral blood mononuclear (PBM), human T lymphoblast (CEM), human hepatocellular carcinoma (Huh7) and kidney epithelial (Vero) cell lines. Toxicity levels were measured as the concentration of test compound that inhibited cell proliferation by 50% (CC50).
3. Results
Compounds (9–11) along with their corresponding monophosphate prodrugs (12–14) were successfully synthesized, characterized chemically, and evaluated for antiviral activity and cytotoxicity. Their toxicity profile was assessed and none of them displayed toxicities up to 100 μM in primary human PBM cells, CEM cells, Vero cells and human liver cells (Huh7). Furthermore, none of them exhibited anti- SARS-CoV-2, Influenza A/B viruses and Norovirus. activities at concentration up to 10 μM. Appropriate positive controls exhibited significant activity (SARS-CoV-2: Remdesivir; Influenza A/B viruses: baloxavir; Norovirus: 2’-C-methylcytidine).
4. Discussion
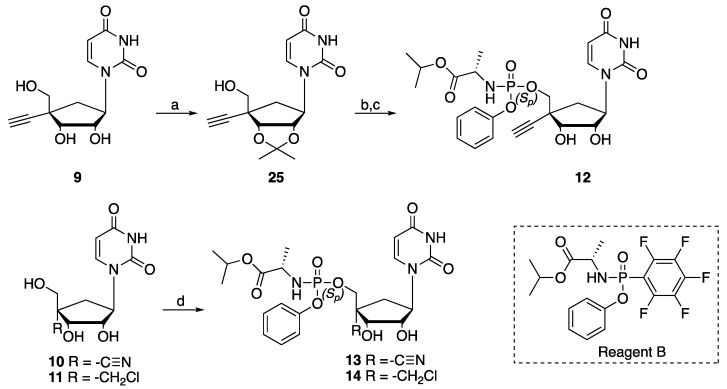
The nucleosides (9–11) and their corresponding monophosphate prodrugs (12–14) were synthesized by following the chemistry described in Scheme 1, Scheme 2 and Scheme 3 (see Supplementary Materials for detailed protocols and full characterizations).
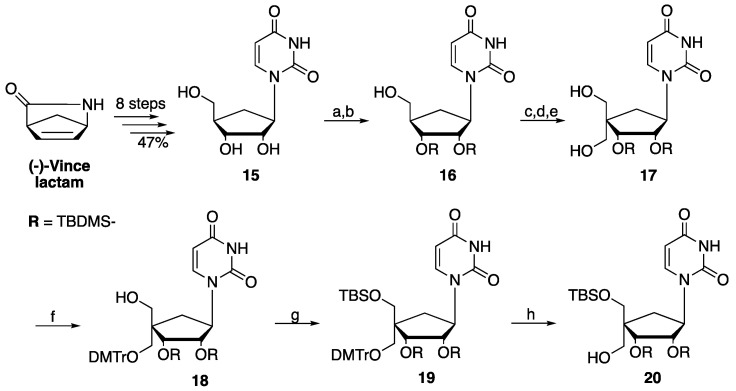
Scheme 1.
Synthesis of key intermediate (20). Reagents and conditions: (a) TBDMSCl, imidazole, DMF, 50 °C, 3 d. (b) TFA/H2O/THF (1:1:2), 0 °C, 6 h, 62% (over 2 steps). (c) DMP, pyridine, DCM, rt, on. (d) 37% CHOaq, 2 M NaOHaq, 1,4-dioxane, rt, on. (e) NaBH4, 0 °C to rt, 1.5 h, 70% (over 3 steps). (f) DMTrCl, DCM/pyridine (3:1), rt, 24 h, 56%. (g) TBDMSCl, imidazole, DMF, 50 °C, 24 h, 95%. (h) AcOH 80%aq, THF, rt, 3 h, 59%.
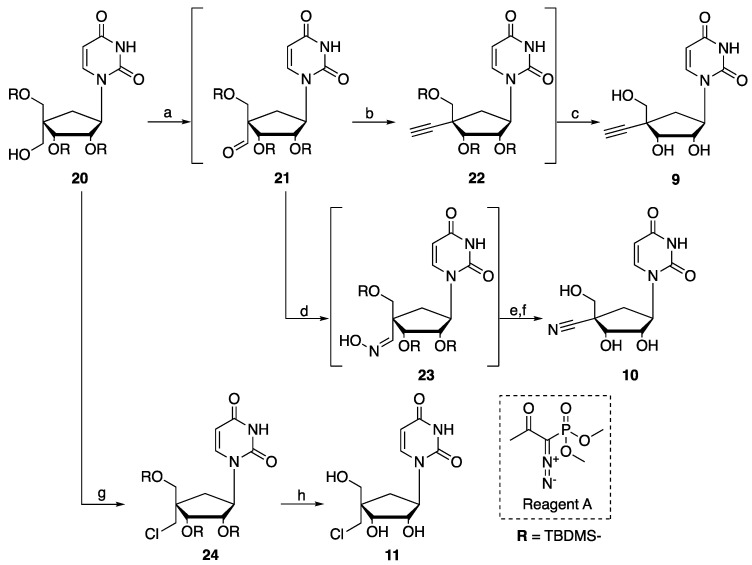
Scheme 2.
Synthesis of compounds (9), (10), (11). Reagents and conditions: (a) DMP, pyridine, DCM, rt, 16 h. (b) Dimethyl (1-diazo-2-oxopropyl)phosphonate (Reagent A), K2CO3, MeOH, rt, 16 h. (c) TBAF 1M, THF, rt, on, 49% (over 3 steps). (d) NH2OH.HCl, pyridine, rt, 2 h. (e) Burgess reagent, toluene, 1.5 h, 100 °C. (f) TBAF 1M, THF, rt, on, 44% (over 4 steps). (g) PPh3, CCl4, DCE, MW, 130 °C, 0.5 h, 67%. (h) TBAF 1M, THF, rt, on, 89%.
Scheme 3.
Synthesis of prodrug analog (12), (13), (14). Reagents and conditions. (a) Conc. H2SO4, dimethoxypropane, acetone, rt, 3 h, 65%. (b) t-BuMgCl, Reagent B, THF, 0 °C to rt, on. (c) HCl, 0 °C, to rt, 5 h, 34% (over 2 steps). (d) t-BuMgCl, Reagent B, THF, 0 °C, on; (13): 13%, (14): 5%.
The targeted 4′-alkyno, 4′-cyano and 4′-chloromethyl carbocyclic ribonucleoside analogs (9–11) along with their corresponding prodrugs (12–14) were prepared from the same carbocyclic uridine precursor (15), which was synthetized from the (-)-Vince lactam in 8 steps (47% yield) following reported procedures [25,26,27]. Compound (15) was first per-silylated with tert-butyldimethylsilyl chloride (TBDMSCl) in presence of imidazole before selective 5′-deprotection, in presence of trifluoroacetic acid (TFA) and water, to afford compound (16) in 62% yield over 2 steps. Compound (16) was then oxidized with Dess–Martin periodinane (DMP) to form the corresponding aldehyde intermediate followed by further reaction with 37% in water paraformaldehyde in presence of sodium hydroxide and final reduction with NaBH4 to afford 4′-diol intermediate (17) in 70% yield over 3 steps. Compound (20) was synthesized from (17) through a 3-step process by selective protection of the 4′-α-hydroxymethyl group with 4,4’-dimethoxytrityl chloride (DMTrCl), followed by protection of the 4′-β-hydroxymethyl group with TBDMSCl, and then selective 5′-DMTr deprotection under acidic conditions (32% yield over 3 steps) (Scheme 1).
It is worth noting that the selectivity of this protection/deprotection sequence was later confirmed indirectly via NOESY 1H-NMR experiments of the final 4′-ethynyl carbanucleoside (9) (Figure 4). Thus, key NOE effects were observed with one of the H6′ which couples with H2′, H3′ and one of the H5′, validating the 4′-α-orientation of the alkyne group in compound (9).
Figure 4.
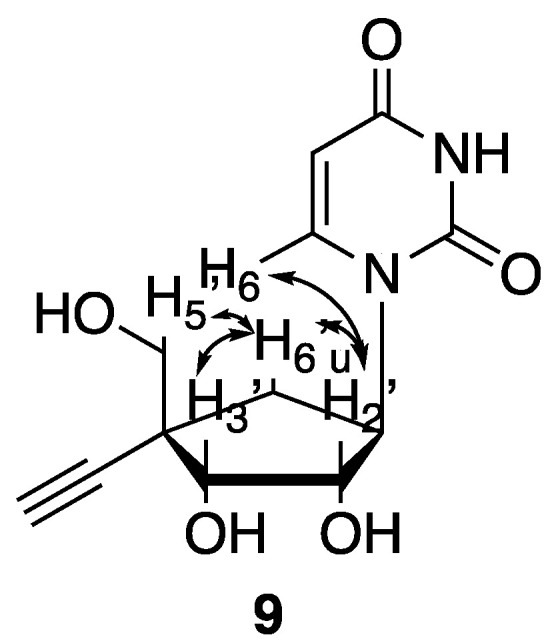
Key couplings observed in NOESY 1H-NMR experiment for compound (9).
The desired 4′-alkyno, 4′-cyano and 4′-chloromethyl carbonucleoside analogs (9–11) were prepared from compound (20) according to the chemistry reported in Scheme 2. Intermediate (20) was first oxidized with DMP to form the corresponding 5′-aldehyde intermediate (21) before being engaged in a Seyferth-Gilbert homologation in presence of dimethyl (1-diazo-2-oxopropyl)phosphonate (reagent A) and K2CO3 to give intermediate (22). After 2′-,3′-TBDMS deprotection in the presence of tetra-n-butylammonium fluoride (TBAF), the 4′-ethynyl carbocyclic uridine (9) was obtained in 49% yield over 3 steps. From the same 5′-aldehyde intermediate (21), reaction with hydroxylamine hydrochloride in pyridine followed by dehydration of intermediate (23) in presence of the Burgess reagent and final deprotection with TBAF afforded compound (10) in 44% in yield over 4 steps. Finally, 4′-chloromethyl uridine analog (11) was obtained from (20) by first, microwave-activated chlorination with PPh3 and CCl4 in DCE to afford compound (24) in 67% in yield and further deprotection using TBAF.
In order to express their therapeutic effect, nucleoside analogs must be phosphorylated to their corresponding 5′-triphosphate forms by three different kinases. Interestingly, the first phosphorylation is often the limiting step in this process and several monophosphate prodrugs that can be cleaved intracellularly to deliver the monophosphate form of a nucleoside have been developed [28]. Among them, phosphoramidate prodrugs (Protides) as seen in approved drugs such as sofosbuvir (HCV), tenofovir alafenamide (HIV), or remdesivir (SARS-CoV-2) have been well studied and their use has been validated clinically. Thus, the corresponding monophosphate prodrug of nucleosides (12–14) were prepared through the chemistry described in Scheme 3. Compound (9) was first protected as a 2′-,3′-acetonide intermediate (25) in 64% yield, before reacting it with isopropyl ((S)-(perfluorophenoxy)-(phenoxy)phosphoryl)-L-alaninate [29] (Reagent B) in presence of t-BuMgCl. Final deprotection under acidic conditions gave prodrug (12) in 34% yield over 2 steps. On the other hand, prodrugs (13) and (14) were prepared in 13% and 5% yields respectively, directly from the corresponding parent nucleosides (10) and (11) by reaction with Reagent B in presence of t-BuMgCl at 0 °C overnight. It is worth noting that, the protection of the 2′-3′-positions in compounds (10) and (11) (as described above for the synthesis of prodrug (12)) to form the corresponding prodrugs did not improve the overall yield as both 2′,3′-isopropylidene monophosphate prodrugs appeared to be unstable under deprotection conditions.
5. Conclusions
A series of novel 4′-substituted carbocyclic uracil derivatives containing 4′-ethynyl, 4′-cyano and 4′-chloromethyl groups were synthetized in 19 to 22 steps from the (-)-Vince lactam and evaluated against a small panel of clinically relevant RNA viruses. Unfortunately, none of these compounds, nor their corresponding monophosphate prodrugs, displayed significant antiviral activity against SARS-CoV-2, IFV-A, IFV-B or norovirus. It is worth noting though that none of them showed toxicities up to 100 μM in a panel of cell lines including PBM, CEM, Vero and Huh7 cells.
Acknowledgments
We are honored to participate in the Special Issue “Nucleos(t)ide Chemistry for Antiviral Drug Discovery: A Tribute to Antonín Holý,” our friend and colleague.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15020544/s1: 1H, 13C and 19P-NMR spectra for compounds 9–14, 16, 17, 20, 24 and 25.
Author Contributions
N.G.B.: synthesis of the compounds and writing of the original draft; R.D. and J.C.L.: SARS-CoV-2 studies. S.A.A.: IFV studies. J.D.-B.: toxicity studies. N.A. and T.M.: norovirus and toxicity studies. F.A. and R.F.S.: project conception and supervision; writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by 5RO1-MH-116695, Emory’s WSC 2020 COVID-19 (CURE Award) and in part by NIH grant 5P30-AI-50409 (CFAR).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Seley-Radtke K.L., Yates M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018;154:66–86. doi: 10.1016/j.antiviral.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin X., Liang C., Zou L., Yin Y., Wang J., Chen D., Lan W. Advance of structural modification of nucleosides scaffold. Eur. J. Med. Chem. 2021;214:113233. doi: 10.1016/j.ejmech.2021.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betson M., Allanson N., Wainwright P. A review of methods to synthesise 4′-substituted nucleosides. Org. Biomol. Chem. 2014;12:9291–9306. doi: 10.1039/C4OB01449A. [DOI] [PubMed] [Google Scholar]
- 4.Smith D.B., Martin J.A., Klumpp K., Baker S.J., Blomgren P.A., Devos R., Granycome C., Hang J., Hobbs C.J., Jiang W.-R., et al. Design, synthesis, and antiviral properties of 4′-substituted ribonucleosides as inhibitors of hepatitis C virus replication: The discovery of R1479. Bioorg. Med. Chem. Lett. 2007;17:2570–2576. doi: 10.1016/j.bmcl.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Coats S.J., Garnier-Amblard E.C., Amblard F., Ehteshami M., Amiralaei S., Zhang H., Zhou L., Boucle S.R., Lu X., Bondada L., et al. Chutes and ladders in hepatitis C nucleoside drug development. Antivir. Res. 2014;102:119–147. doi: 10.1016/j.antiviral.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G., Deval J., Hong J., Dyatkina N., Prhavc M., Taylor J., Fung A., Jin Z., Stevens S.K., Serebryany V., et al. Discovery of 4′-chloromethyl-2′-deoxy-3′,5′-di-O-isobutyryl-2′-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J. Med. Chem. 2015;58:1862–1878. doi: 10.1021/jm5017279. [DOI] [PubMed] [Google Scholar]
- 7.Patel K., Kirkpatrick C.M., Nieforth K.A., Chanda S., Zhang Q., McClure M., Fry J., Symons J.A., Blatt L.M., Beigelman L., et al. Respiratory syncytial virus-A dynamics and the effects of lumicitabine, a nucleoside viral replication inhibitor, in experimentally infected humans. J. Antimicrob. Chemother. 2019;74:442–452. doi: 10.1093/jac/dky415. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz M., Sarafianos S.G. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591: A novel HIV-1 reverse transcriptase translocation inhibitor. Curr. Opin. HIV AIDS. 2018;4:294–299. doi: 10.1097/COH.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menéndez-Arias L., Delgad R. Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci. 2022;43:16–29. doi: 10.1016/j.tips.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Derbalah A., Karpick H., Maize H., Skersick P., Cottrell M., Rao G. Role of islatravir in HIV treatment and prevention: An update. Curr. Opin. HIV AIDS. 2022;17:240–246. doi: 10.1097/COH.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 11.Masone M.C. Islatravir implant as HIV-1 pre-exposure treatment. Nat. Rev. Urol. 2021;18:706. doi: 10.1038/s41585-021-00541-6. [DOI] [PubMed] [Google Scholar]
- 12.Painter G.R., Perryman D., Bluemling G.R. Preparation of 4′-halogen containing nucleotide and nucleoside therapeutics and uses related thereto. WO2019173602 A1. 2019 September 12;
- 13.Sourimant J., Lieber C.M., Aggarwal M., Cox R.M., Wolf J.D., Yoon J.-J., Toots M., Ye C., Sticher Z., Kolykhalov A.A., et al. 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science. 2022;375:161–167. doi: 10.1126/science.abj5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amblard F., Nolan S.P., Agrofoglio L.A. Metathesis strategy in nucleoside chemistry. Tetrahedron. 2005;61:7067–7080. doi: 10.1016/j.tet.2005.04.040. [DOI] [Google Scholar]
- 15.Bessières M., Chevrier F., Roy V., Agrofoglio L.A. Recent progress for the synthesis of selected carbocyclic nucleosides. Fut. Med. Chem. 2015;13:1809–1828. doi: 10.4155/fmc.15.105. [DOI] [PubMed] [Google Scholar]
- 16.Ojeda-Porras A.C., Roy V., Agrofoglio L.A. Chemical approaches to carbocyclic nucleosides. Chem. Rec. 2022;22:e202100307. doi: 10.1002/tcr.202100307. [DOI] [PubMed] [Google Scholar]
- 17.Boutureira O., Matheu M.I., Díaz Y., Castillón S. Advances in the enantioselective synthesis of carbocyclic nucleosides. Chem. Soc. Rev. 2013;42:5056–5072. doi: 10.1039/c3cs00003f. [DOI] [PubMed] [Google Scholar]
- 18.Liu P., Sharon A., Chu C.K. Enantiomeric synthesis of carbocyclic D-4′-C-methylribonucleosides as potential antiviral agents. Tet. Asymm. 2006;17:3304–3314. doi: 10.1016/j.tetasy.2006.12.007. [DOI] [Google Scholar]
- 19.Alexandre F.-R., Rahali R., Rahali H., Guillon S., Convard T., Fillgrove K., Lai M.-T., Meillon J.-C., Xu M., Small J., et al. Synthesis and antiviral evaluation of carbocyclic nucleoside analogs of nucleoside reverse transcriptase translocation inhibitor MK-8591 (4′-ethynyl-2-fluoro-2′-deoxyadenosine) J. Med. Chem. 2018;61:9218–9228. doi: 10.1021/acs.jmedchem.8b00141. [DOI] [PubMed] [Google Scholar]
- 20.Zandi K., Amblard F., Musall K., Downs-Bowen J., Kleinbard R., Oo A., Cao D., Liang B., Russell O.O., McBrayer T., et al. Repurposing nucleoside analogs for human coronaviruses. Antimicrob. Agents Chemother. 2020;6:e01652-20. doi: 10.1128/AAC.01652-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costantini V.P., Whitaker T., Barclay L., Lee D., McBrayer T.R., Schinazi R.F., Vinjé J. Antiviral activity of nucleoside analogues against norovirus. Antivir. Ther. 2012;17:981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzzarelli K.M., Kuiper B., Spellmon N., Brunzelle J., Hackett J., Amblard F., Zhou S., Liu P., Kovari I.A., Yang Z., et al. Structural and antiviral studies of the human norovirus GII.4 protease. Biochemistry. 2019;58:900–907. doi: 10.1021/acs.biochem.8b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Zhou L., Amichai S., Zandi K., Cox B., Schinazi R., Amblard F. Novel influenza polymerase PB2 inhibitors for the treatment of influenza A infection. Bioorg. Med. Chem. Lett. 2019;29:126639. doi: 10.1016/j.bmcl.2019.126639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mengshetti S., Zhou L., Sari O., De Schutter C., Zhang H., Cho J.H., Tao S., Bassit L.C., Verma K., Domaoal R.A., et al. Discovery of a series of 2′-α-fluoro,2′-β-bromo-ribonucleosides and their phosphoramidate prodrugs as potent pan-genotypic inhibitors of hepatitis C virus. J. Med. Chem. 2019;62:1859–1874. doi: 10.1021/acs.jmedchem.8b01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Du J., Wang P., Nagarathnam D., Espiritu C.L., Bao H., Murakami E., Furman P.A., Sofia M.J. A 2′-deoxy-2′-fluoro-2′-C-methyl uridine cyclopentyl carbocyclic analog and its phosphoramidate prodrug as inhibitors of HCV NS5B polymerase. Nucleosides Nucleotides Nucleic Acids. 2012;31:277–285. doi: 10.1080/15257770.2012.658131. [DOI] [PubMed] [Google Scholar]
- 26.Sato T., Tsuzuki T., Takano S., Kohtaro K., Fukuda H., Arisawa M., Shuto S. Construction of a chiral quaternary carbon center by a radical cyclization/ring-enlargement reaction: Synthesis of 4α-azidoethyl carbocyclic ribose, a key unit for the synthesis of cyclic ADP-ribose derivatives of biological importance. Tetrahedron. 2015;71:5407–5413. doi: 10.1016/j.tet.2015.05.084. [DOI] [Google Scholar]
- 27.Akabane-Nakata M., Chickering T., Harp J.M., Schlegel M.K., Matsuda S., Egli M., Manoharan M. RNAs containing carbocyclic ribonucleotides. Org. Lett. 2022;24:525–530. doi: 10.1021/acs.orglett.1c03936. [DOI] [PubMed] [Google Scholar]
- 28.Pradere U., Garnier-Amblard E.C., Coast S.J., Amblard F., Schinazi R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014;114:9154–9218. doi: 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross B.S., Reddy P.G., Zhang H.R., Rachakonda S., Sofia M. Synthesis of diastereomerically pure nucleotide phosphoramidates. J. Org. Chem. 2011;76:8311–8319. doi: 10.1021/jo201492m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.